Keywords: Cantú syndrome, KABCC9, KCNJ8, lymphedema, metabolic syndrome
Abstract
KATP channels function as negative regulators of active lymphatic pumping and lymph transport. This review summarizes and critiques the evidence for the expression of specific KATP channel subunits in lymphatic smooth muscle and endothelium, the roles that they play in normal lymphatic function, and their possible involvement in multiple diseases, including metabolic syndrome, lymphedema, and Cantú syndrome. For each of these topics, suggestions are made for directions for future research.
INTRODUCTION
Widely expressed throughout the body, KATP channels are voltage-independent, K+-selective channels that are activated by intracellular ADP and inhibited by intracellular ATP, thereby coupling the electrical activity of cells to their metabolic state (1, 2). Mammalian KATP channels are heterooctameric complexes of inwardly rectifying K+ channel (Kir6) pore-forming subunits and regulatory sulfonylurea receptor (SUR) subunits. Two pairs of adjacent genes (ABCC8, KCNJ11, and ABCC9, KCNJ8), on human chromosomes 11p and 12p, each encode one pair of subunits—SUR1, Kir6.2, and SUR2, Kir6.1, respectively (1). Alternate splicing of ABCC9 produces two major protein variants, SUR2A and SUR2B that differ in their final C-terminal sequences (3, 4). The expression of different combinations of Kir6 and SUR subunit genes in different cell types results in distinct KATP channel properties and functional roles in a given tissue (1, 4).
The understood roles of KATP channels in arteries/arterioles are of relevance to the present chapter on lymphatic KATP channels. Much of the literature suggests that KATP channels normally have low activity in arterial smooth muscle (SM) but become activated under ischemic conditions (1), leading to arterial SM hyperpolarization and dilatation, which in turn increases flow and oxygen supply to the ischemic tissue (4). KATP channels also contribute to the action of vasodilators that stimulate cAMP-mediated signaling (4). The literature on KATP channels in lymphatic vessels is less extensive, but almost all of it suggests that the channels are fundamentally similar to those in vascular smooth muscle and that their activation is a negative regulator of active lymphatic pumping and lymph transport. A discussion and synthesis of that literature, with speculation on the homeostatic significance of KATP channels in lymphatic vessels and suggestions for directions for future research, are the aims of this review.
SIMILARITIES AND DIFFERENCES BETWEEN LYMPHATIC AND ARTERIAL SMOOTH MUSCLE
Understanding the differences in the physiology of the lymphatic and blood vascular systems is critical to a discussion of distinct roles of lymphatic KATP channels. A primary function of the lymphatic system is the reabsorption of excess interstitial fluid and its return to the central veins. The system consists of networks of initial lymphatic capillaries intertwined among blood capillary networks in almost every tissue and organ. Lymphatic capillaries are composed of overlapping and porous lymphatic endothelial cell (LEC) junctions that facilitate absorption of excess fluid from the interstitium (5). Each lymphatic capillary network has its own specific anatomy apparently adapted to the function of a particular tissue (6). These networks coalesce into lymphatic precollectors containing luminal bileaflet valves that retard backflow. Precollectors in turn form collecting lymphatics, which also contain one-way valves and are surrounded by one or more layers of spontaneously active lymphatic smooth muscle (LSM) cells. The spontaneous contractions of these collecting vessels, in concert with this valve system, allow lymph to be actively transported “uphill” against adverse hydrostatic pressure gradients (7, 8). The contraction frequency of these vessels is exquisitely sensitive to changes in luminal pressure, enabling active lymphatic transport to be matched to the filling state of the lymphatic capillaries (9). Lymph transport can also be facilitated by passive compression of lymphatic vessels by adjacent tissue movement, e.g., skeletal muscle contraction, but the importance of active lymphatic pumping is illustrated by the observation that it accounts for 2/3 of lymphatic transport in the lower legs of humans during quiet standing (10).
Ion Channels
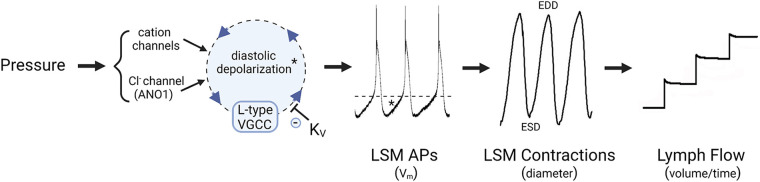
LSM exhibits significant differences from arterial SM in its electrophysiological properties. LSM cells fire spontaneous action potentials (APs) that are required to initiate large-amplitude lymphatic contractions, with the entrainment of APs among LSM cells (11–13) facilitating the effective propulsion of lymph. The membrane potential (Vm) of LSM is not stable but exhibits a gradual, spontaneous depolarization (i.e., a pacemaking potential) during the diastolic phase of the lymphatic contraction cycle (Fig. 1), bringing Vm to a threshold to trigger AP firing (14, 15); the cycle repeats after repolarization. In contrast, most arterial SM cells are quiescent with a stable resting Vm (16, 17), although some can be induced to fire APs under special conditions (18). The complement of ion channels that interacts to generate lymphatic pacemaking potentials has not been fully resolved, but substantial evidence suggests that L-type voltage-gated channels (VGCCs) carry current for the upstroke of the lymphatic AP and mediate much of the Ca2+ entry responsible for contraction (19–22), whereas background and voltage-gated K+ currents set the resting Vm and facilitate repolarization (14, 23, 24). Two studies have speculated that LSM pacemaker potentials may be driven by interstitial cells, but the evidence to date is only anatomical (25, 26). Other studies have suggested that T-type VGCCs in LSM cells are important for diastolic depolarization (14, 27), but a recent study of lymphatic vessels from Cav3.1−/−, Cav3.2−/− and double Cav3.1−/−; Cav3.2−/− knockout mice indicates that T-type VGCCs play little or no role in lymphatic pacemaking, at least in that species (28). Roles for other cation channels in LSM diastolic depolarization have also been postulated, including hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels, based on their involvement in pacemaking in other cell systems; however, a role for HCN channels in lymphatic pacemaking under physiological conditions is controversial (29–32). Voltage-gated sodium channels appear to be involved in some species under some conditions (14, 33, 34) but they are not obligatory for the LSM AP under all conditions. Smooth muscle-specific knockout of the calcium-activated Cl− channel anoctamin1 (ANO1) reduces the speed of diastolic depolarization, indicating a contribution of ANO1 to diastolic depolarization. However, ANO1-deficient LSM cells retain a residual level of spontaneous AP firing (35), suggesting the additional involvement of at least one more channel. Neither ANO1 nor most TRP cation channels are intrinsically mechanosensitive (36), so one explanation for spontaneous depolarization is that the channels providing depolarizing current are activated by inositol 1,4,5-trisphosphate receptor (IP3R)-mediated Ca2+ release downstream from mechanosensitive G-protein-coupled receptors (GPCRs) (15, 21, 35, 37). Potentially different types of ion channels may interact to initiate pacemaking in lymphatic vessels of different species (Fig. 1), explaining some of the differences between the studies cited earlier. Whether KATP channels contribute to, or modulate, lymphatic pacemaking is an important issue, as considered in depth below.
Figure 1.
Ionic mechanisms of lymphatic pacemaking. Spontaneous diastolic depolarization triggers the opening of L-type voltage-gated channels (VGCCs) that result in the firing of action potentials (APs). Increased pressure increases pacemaking frequency by increasing the rate of diastolic depolarization through regulation of one or more cation channels and the Cl− channel, anoctamin1 (ANO1), which collectively contribute to depolarizing current. After an AP fires, K+ channels repolarize the membrane and the cycle repeats. Calcium entry associated with each AP generates a twitch contraction that propels lymph centrally; lymph flow is unidirectional due to the presence of one-way luminal valves. *Diastolic depolarization phase of the AP; dotted line indicates threshold for AP firing. EDD, end diastolic diameter; ESD, end systolic diameter; LSM, lymphatic smooth muscle.
Contractile Proteins
LSM also exhibits substantial differences from arterial SM in the expression of contractile proteins. Like arterial SM, myosin light chain 20 (MLC20) phosphorylation is the predominant regulator of lymphatic muscle contraction, achieved by the balance of myosin light chain kinase/myosin light chain phosphatase (MLCK/MLCP) activity (38, 39); for this reason, lymphatic muscle is often referred to as a type of smooth muscle. Like arteries, lymphatic collectors exhibit pressure-induced basal vascular tone that can be increased or decreased by appropriate agonists. But unlike arteries, LSM cells express both smooth and striated muscle protein isoforms (40). For example, in rat, the thoracic duct (the central lymphatic trunk) expresses smooth and striated muscle isoforms of actin, tropomyosin, and myosin heavy chain (40–42). The smooth and striated muscle proteins in LSM cells are not organized into defined sarcomeres, but appear to be more aligned than the nonstriated contractile proteins in arterial SM (40). Lymphatic muscle may also express troponin C (43), which in the blood vascular system is only expressed in renal afferent arterioles (44)—vessels with the fastest constrictions of any arteries to pressure elevation (45). It is perhaps not a coincidence that LSM has a substantially higher shortening velocity than arterial SM or phasic venous SM, with maximal unloaded velocities approaching values achieved by cardiac muscle (46). The combination of striated and smooth muscle proteins presumably explains how lymphatic vessels can develop and regulate both sustained tone and phasic contractile activity, although this hypothesis has not been experimentally tested by determining the functional consequences of deleting striated muscle isoforms from lymphatic vessels. Nevertheless, for the reasons stated, some investigators have suggested that LSM may be a hybrid muscle type between cardiac muscle and arterial SM (40, 43, 46, 47).
EXPRESSION OF KATP CHANNEL ISOFORMS IN LYMPHATIC SM AND ENDOTHELIUM
Multiple studies provide evidence that LSM cells express Kir6.1 and SUR2B KATP channel subunits—the same isoforms predominantly expressed in arterial SM (4). Molecular evidence for Kir6.1 and SUR2B expression in the lymphatic system comes primarily from PCR measurements of collecting lymphatic vessels of various species, including mouse, rat, guinea pig, and human (see Table 1). Published PCR analyses are almost exclusively performed on lysates from whole lymphatic vessels, and thus potentially reflect the expression of KATP channel isoforms not only in LSM cells and LECs but in multiple other cell types found in the lymphatic wall, including dendritic cells, macrophages, mast cells, neurons, adipocytes, and adventitial fibroblasts. The evidence for expression of other KATP channel subunits, i.e., Kir6.2 and SUR1, in lymphatic vessels is complicated for this same reason. For example, Kir6.2 was not detected in guinea pig mesenteric lymphatics (62), but was detected in whole vessel lysates of human thoracic duct, although at much lower levels than Kir6.1 (24). Kir6.2, Kir6.1, and SUR2B transcripts were all detected in whole popliteal lymphatics from mouse, but only Kir6.1 and SUR2B were detected in FACS-purified popliteal LSM cells (66), suggesting that Kir6.2 message may have come from another cell type. Garner et al. (68) reported evidence for the expression of Kir6.2 in rat mesenteric lymphatics, in addition to Kir6.1 and SUR2B, using both PCR analysis and immunostaining of FACS-purified LSM cells. However, immunopositivity for Kir6.2 was only found in a subset of LSM cells. SUR1 subunits were detected by PCR but only at high cycle thresholds, and no SUR1 immunoreactivity was detected in LSM cells (68). In patch-clamp studies of FACS-purified LSM cells, the same investigators (68) found that the single-channel conductance for cromakalin-activated K+ current was 46 pS, compared with values of 29 pS for homotetrameric Kir6.1 channels and 60 pS for homotetrameric Kir6.2 channels measured in expression systems (73). This intermediate conductance level suggests that at least some LSM cells may express heterotetrameric Kir6.1/Kir6.2 channels, which also would be consistent with the postulated hybrid striated/smooth muscle phenotype of LSM (as Kir6.2 is the isoform predominantly expressed in cardiomyocytes). The expression of Kir6.1/Kir6.2 channels in LSM could result in enhanced sensitivity to intracellular ATP (74) and larger whole cell KATP currents and hyperpolarization (compared with Kir6.1 homotetrameric channels) when the channels are activated. It remains to be determined the extent to which Kir6.2 expression in LSM cells may be tissue- and species-specific.
Table 1.
Studies examining the roles of KATP channels in lymphatic vessel function
| Species | Bed | KATP Isoforms | Preparation(s)* | [Inhibitor] | [Activator] | Effects on Lymphatic Vessel Function | References# |
|---|---|---|---|---|---|---|---|
| Guinea pig | Mesentery | En face unpressurized vessel-Vm | 10 μM GLIB | GLIB had no effect on resting Vm of LECs but depol. LSMCs by 9 mV; CROM had no effect of LEC Vm; GLIB had no effect on ACh-mediated LEC biphasic hyperpol./depol. but blocked hyperpol. in LSMCs |
(48) | ||
| Guinea pig | Mesentery | Unpressurized vessel-Vm | 10 μM GLIB | 0.1–10 μM CROM | CROM hyperpol. LSMCs, blocked by GLIB; GLIB inhibited 90% of ACh/NO-induced hyperpol.; GLIB blocked hyperpol. by isoprenaline, forskolin |
(49) | |
| Rat | Mesentery | Isolated vessel-contraction | 0.1, 1 μM GLIB | 0.1–10 μM PIN | PIN inhibited spont. contractions; GLIB blocked PIN effect; GLIB had no effect on spont. contractions |
(50) | |
| Unpressurized vessel-Vm | (51) | ||||||
| Mouse (ddY) | Iliac efferent | Isolated vessel-contraction | 1, 10 μM GLIB | B16-BL6 melanoma cells released factor(s) with inhibitory action on lymphatic pump that was partially blocked by GLIB | (52) | ||
| Mouse (ddY) | Iliac efferent | Isolated vessel-contraction | 1 μM GLIB | GLIB suppressed inhibitory responses to PTH | (53) | ||
| Cow | Mesentery | 5 μM GLIB | GLIB attenuated acidosis-induced dilation | (54) | |||
| Guinea pig | Mesentery | Unpressurized vessel-Vm; in vivo-contraction | 0.1, 10 μM GLIB | GLIB caused 4 mV depol. GLIB attenuated 5-HT-induced inhibition of FREQ and hyperpol. (as did PKA inhibitor) | (55) | ||
| Rat | Iliac | Isolated vessel-contraction | 1 μM GLIB | 1 μM GLIB did not affect inhibition of contraction by the RhoK inhibitor Y-27632 | (56) | ||
| Guinea pig | Mesentery | Unpressurized vessel-Vm | 10 μM GLIB | GLIB depolarized LSMC layer; GLIB blocked hyperpol. caused by PAR2 agonists trypsin, SLIGRL-NH2 and hyperpol. by PGE2 and iloprost |
(57) | ||
| Rat | Iliac afferent | Isolated vessel-contraction | 1 μM GLIB | GLIB attenuates ATP- and adenosine-induced inhibition of contractions | (58) | ||
| Guinea pig | Mesentery | Ex vivo-perfused | 10 μM GLIB | GLIB blocks CGRP-mediated hyperpol. | (59) | ||
| Guinea pig | Mesentery | Ex vivo-perfused | 1 μM GLIB | GLIB attenuated the actions of PGE2 and iloprost | (60) | ||
| Rat | Thoracic duct | Isolated vessel-contraction | 1 μM GLIB | GLIB attenuates the effect of l-arginine on FPF | (61) | ||
| Guinea pig | Mesentery, iliac | Kir6.1, SUR2B; no Kir6.2 | PCR-whole vessel; ex vivo-perfused vessel-contraction;unpressurized vessel-Vm | 1, 10 μM GLIB | GLIB blocks VIP-induced hyperpol.; GLIB blocks VIP-induced inhibition of contraction frequency |
(62) | |
| Guinea pig | Mesentery | Kir6.1 SUR2B | PCR-whole vessel; ex vivo-perfused vessel | 1, 10 μM GLIB | CROM IC50 = 126 nM frequency; CROM IC50 = 322 nM hyperpol. |
10 μM GLIB reverses NO-induced inhibition of pumping;CROM blocks pumping GLIB rescues impaired contractions in TNBS-induced ileitis |
(63) |
| Human | Thoracic duct | Kir6.1, Kir6.2 | PCR-whole vessel; isometric strips-Vm, contraction | 10 μM GLIB | 1 μM PIN | GLIB depol. and initiates spont. activity in some quiescent vessels; GLIB increases FREQ of spont. APs, contractions and depolarizes, reduces AMP; PIN silences spont. contractions, hyperpol. by 25 mV |
(24) |
| Rat | Mesentery | Isolated vessel-contraction | 1, 10 μM GLIB | 10 μM GLIB increases basal FREQ, decreases EF 10 μM GLIB rescues lower FREQ, FPF in metabolic syndrome model | (64) | ||
| Rat | Thoracic duct | Isolated vessel-contraction | 1 μM GLIB | Hemorrhagic shock impairs lymphatic contraction through NO; GLIB attenuates NO action | (65) | ||
| Mouse (WT, Kir6.1−/−, Kir6.1 GOF, LEC, LMC reporters) | Popliteal, inguinal-axillary | Kir6.1, SUR2B in LMCs;+Kir6.2 in whole vessel; Kir6.1 without SUR in LECs | PCR-whole vessel (inguinal-axillary); PCR-sorted LMCs, LECs (inguinal-axillary);isolated vessel-contraction | 10 μM GLIB | PIN IC50 = 1.3 × 10−7 M for WT frequency; | GLIB does not significantly alter basal FREQ, AMP, FPF PIN hyperpol. and stops spont. APs; reversed by GLIB; PIN stops contractions PIN IC50 shifted to 8.3 × 10−5 M in Kir6.1−/− vessels;PIN IC50 shifted to 7 × 10−4 M in SUR2[STOP] vessels Expression of Kir6.1 GOF mutant channels in SM inhibits cont. AMP by 70%–90% at various pressures, inhibits FREQ by 40%, inhibits FPF by 40%–70% at various pressures; hyperpol. LSM by 11 mV |
(66) |
| Human | Mesentery | Vm-iso vessel | 10 μM GLIB | PIN hyperpolarizes and stops spont. APs, reversed by GLIB | (66) | ||
| Cow | Mesentery efferent | Isometric rings | 10 μM GLIB | Cocktail of ChTx, Apamin, and GLIB partially blocks H2S-mediated relaxation | (67) | ||
| Rat | Mesentery | Kir6.1, Kir6.2, SUR2 in vessels, LMCs | Whole vessel and sorted LMCs-PCR and immunostaining; isolated LMCs-patch-clamp; isolated vessel-contraction; in vivo-diameter, flow | 5 μM GLIB | CROM IC50 = 0.3 μM freq.; MINOX IC50 = 0.6 μM freq.; Diazoxide IC50 = 10 μM freq.; |
CROM activates K+ current (cell attached patch clamp of LMCs);CROM inhibits lymphatic flow in vivo; Single-channel conductance consistent with Kir6.1/6.2 heteromeric channels in LSM cells |
(68) |
| Mouse(WT, Kir6.1−/−,SUR2[stop] | Popliteal | Isolated vessel-contraction | 0.1–10 μM GLIB | 10 μM GLIB does not reverse NO-induced inhibition of FREQ.; NO inhibits contractions in Kir6.1−/− vessels; 10 μM GLIB has off-target effects on FREQ, AMP, EDD |
(69) |
Additional studies on lymph nodes not included in this table show that GLIB partially blocks the inhibitory effects of heparin, acetylcholine (ACh), sodium nitroprusside (SNP) on bovine lymph capsule contraction (70–72). AMP, amplitude; CROM, cromakalim; DIAZ, diazoxide; depol., depolarization; EDD, end diastolic diameter; EF, ejection fraction; FPF, fractional pump flow; FREQ., frequency; GLIB, glibenclamide; GOF, gain-of-function; hyperpol., hyperpolarization; LEC, lymphatic endothelial cell; LMC, lymphatic muscle cell; LSM, lymphatic smooth muscle; MINOX, minoxidil; PIN, pinacidil; SM, smooth muscle; spont., spontaneous; vip, vasoactive intestinal peptide; WT, wild type.
All Vm measurements made in LSM cells except Ref. 48;
#listed in chronological order.
Are KATP channel subunits expressed in LECs? Functional KATP channels are known to be expressed in blood vascular endothelium (75). Hypertension induced by high-salt diet + L-NG-nitro arginine methyl ester (l-NAME) administration is exacerbated by endothelial cell (EC)-specific deletion of Kir6.1, and mice deficient in both Kir6.1 and apolipoprotein E (ApoE) develop more atherosclerotic lesions than ApoE−/− mice alone (76). These results suggest that endothelial KATP channels aid in protection against hypertension and atherosclerosis. The activation of a K+ conductance hyperpolarizes ECs of arteries/arterioles, which is transmitted through myoendothelial gap junctions to the overlying SM layer to cause vasodilatation (77); however, this mechanism does not appear to operate in the lymphatic system. In lymphatic vessels, the LEC and LSM layers do not exhibit strong electrical coupling (11, 48, 78, 79) as in arteries (80); rather, the Vm of LECs is stable at ∼−70 mV (11, 48, 81), while simultaneously the Vm of LSM cells ranges from −35 to −50 mV and oscillates with the firing of APs (11, 22, 82). The KATP channel opener (KCO) cromakalim (CROM) did not elicit hyperpolarization of the endothelium of guinea pig mesenteric lymphatics, even though it hyperpolarized the overlying LSM layer, leading von der Weid and van Helden (48) to conclude that lymphatic endothelium does not express functional KATP channels. Only a single study has specifically examined the expression of KATP channel isoforms in LECs (Table 1). Message for Kir6.1 channels was detected in the absence of message for an SUR subunit in FACS-purified LECs from mouse inguinal-axillary lymphatics (66). This result suggests that only nonfunctional KATP channels may be expressed in mouse lymphatic endothelium. Further support for this idea is that overexpression of gain-of-function (GOF) Kir6.1 subunits selectively in mouse LSM caused a profound inhibition of contraction, whereas selective expression in the LEC layer was without significant effect (66). Again, such evidence may apply only to guinea pig mesenteric and mouse popliteal lymphatics and similar experiments need to be carried out on lymphatic vessels from other regions and species before concluding that KATP channels are not active in LECs.
EVIDENCE FOR FUNCTIONAL KATP CHANNELS IN LYMPHATIC VESSELS
von der Weid and van Helden (48) were first to demonstrate a functional effect of a KATP channel activator in lymphatic vessels, where CROM evoked hyperpolarization of LSM in unpressurized strips of guinea pig mesenteric lymphatic vessels; CROM (5 µM) produced 17 mV hyperpolarization, which was almost completely blocked by the KATP channel inhibitor glibenclamide (GLIB, 10 µM). Mizuno et al. (50) subsequently showed that KATP channel openers (KCOs) inhibit lymphatic contractile function. In pressurized segments of rat mesenteric lymphatic vessels studied ex vivo, 3 × 10−7 M CROM produced significant slowing of contraction frequency, whereas a 10-fold higher concentration completely stopped spontaneous contractions; these effects of CROM were blocked by GLIB (10 µM). These findings have subsequently been confirmed by a number of investigators (see Table 1) using either CROM or other KCOs, including pinacidil (PIN) or minoxidil. For mouse popliteal lymphatics studied ex vivo, the IC50 for inhibition of contraction frequency by PIN was 1.3 × 10−7 M. For rat mesenteric lymphatics studied in vivo, the IC50 for inhibition of frequency was 3 × 10−7 M for CROM and 6 × 10−7 M for minoxidil (68). In contrast, the IC50 for diazoxide, which has a preferential affinity for Kir6.2/SUR1 channels (83), was shifted some 20-fold to the right of that for CROM. It is interesting that KCOs in these studies appear to act almost exclusively on lymphatic contraction frequency (68, 69), with a lesser or inconsistent effect on contraction amplitude and tone (see examples in Refs. 50, 68, and 69 and discussion in Ref. 69), perhaps pointing to a selective contribution of KATP channels to lymphatic pacemaking rather than to modulating the calcium influx associated with contraction.
The activation of KATP channels by high concentrations of KCOs can markedly hyperpolarize LSM Vm (49, 66) and completely block lymphatic contractions, but they begin to inhibit AP firing at much lower concentrations. In both mouse popliteal and human mesenteric lymphatics, 1 µM PIN stopped spontaneous AP firing, in many cases with no overt hyperpolarization beyond the peak negative value of Vm in the pacemaking cycle (66). Thresholds of 100–300 nM for PIN, CROM, and minoxidil are reported to inhibit lymphatic contractions in mouse popliteal (66), rat mesenteric (50, 68), and guinea pig mesenteric lymphatics (63), respectively. Although CROM and PIN are considered to be relatively specific KCOs, off-target effects for both compounds on other channels have been reported, but only at concentrations higher than 1 µM (84–86). Accordingly, while the IC50 of PIN for the contraction frequency of mouse popliteal lymphatics was shifted >100-fold to the right in vessels from Kir6.1 or SUR2 knockout mice compared with wild-type (WT) controls, PIN produced significant elevations in lymphatic tone at concentrations ≤1 µM in Kir6.1 and SUR2 knockout vessels (66), consistent with an effect on a target other than KATP channels. Collectively, these observations suggest that KATP channels contribute some degree of hyperpolarizing current to the balance of currents regulating diastolic depolarization in LSM (Fig. 1), and that even slight KATP channel activation can retard AP firing.
KATP CHANNELS IN BASAL LYMPHATIC FUNCTION
Although the aforementioned studies demonstrate that functional KATP channels are present in lymphatic vessels and that their activation by KCOs leads to LSM hyperpolarization and inhibition of spontaneous lymphatic APs and contractions, the extent to which KATP channels are involved in basal (physiologic) lymphatic function is not as clear. Evidence for KATP channels normally exerting some inhibitory effects on lymphatic pacemaking and contraction is provided by the effects of GLIB on LSM Vm and on spontaneous contraction frequency. GLIB consistently causes depolarization of LSM, as demonstrated in multiple studies by von der Weid and coworkers (48, 49, 51, 55, 59). For example, 10 µM GLIB depolarized the LSM layer of guinea pig unpressurized mesenteric lymphatics by 5–9 mV (48, 49). Telinius et al. (24) found that 10 µM GLIB increased the frequency of spontaneous contractions in isometric human thoracic duct segments by 2.5-fold and initiated spontaneous contractions in 4 of 7 preparations that were quiescent. GLIB significantly increased the frequency of mouse popliteal lymphatic contractions (and decreased contraction amplitude) at concentrations ≥1 × 10−7 M (69). However, other studies have reported no significant effect of GLIB on spontaneous lymphatic contractions. For example, Mizuno et al. (50) reported that neither 1 nor 10 µM GLIB altered the frequency of spontaneous contractions of rat mesenteric lymphatics ex vivo. Only two studies have assessed the contractile behavior of lymphatic vessels in KATP channel knockout animals. Davis et al. (66) did not detect significant differences in either the frequency or amplitude of spontaneous contractions of popliteal lymphatic vessels in mice with deletion of Kir6.1 or SUR2, compared with WT controls; however, there was a trend for Kir6.1−/− vessels to have slightly a lower frequency and amplitude at most pressures, resulting in lower calculated pump flows. Likewise, Kim et al. (69) detected very little differences in the properties of spontaneous contractions between WT and Kir6.1- or SUR2-deficient mouse popliteal vessels, except that the maximal passive diameters of mouse popliteal lymphatic vessels in Kir6.1−/− mice were consistently lower than those in WT mice. In the Kir6.1 and SUR2 global knockout mice, it is possible that compensatory upregulation of other ion channels might have masked the effects of loss of KATP channel function.
There are multiple possible explanations for the discrepancies in the effects of GLIB on basal lymphatic contractions. First is the obvious possibility of tissue and/or species differences. A second explanation is that GLIB may also have off-target effects, particularly at the relatively high concentration of 10 µM most commonly used (Table 1). Patch-clamp studies have demonstrated that GLIB has inhibitory effects on various other ion channels at that concentration, including L-type VGCCs (87–89) and Cl− channels (90, 91); however, inhibitory effects on either of these channels would be predicted to slow lymphatic pacemaking (Fig. 1), opposite to what is observed in studies of lymphatic vessels. Kim et al. (69) tested concentration-response curves for GLIB in mouse popliteal lymphatics and found that concentrations of GLIB ≥ 1 × 10−6 M decreased contraction amplitude and increased frequency in WT mice, but decreased frequency with little effect on contraction amplitude in Kir6.1−/− and SUR2−/− lymphatic vessels; this mixture of effects suggests some off-target actions, at least in the mouse. However, the decrease in frequency was opposite to what was observed in WT vessels, suggesting that if anything, the effect of 10 µM GLIB on contraction frequency might have underestimated the contribution of KATP channels to lymphatic contraction frequency in this (69) and other studies. A third explanation for why only some studies find an effect of GLIB relates to the fact that most isolated lymphatic vessel preparations have no imposed pressure gradient for flow (50, 66, 69), whereas in vivo studies (68), or vessels perfused from one end in situ (55, 63), likely have higher basal flow and consequent nitric oxide (NO) production. Several studies (see Nitric Oxide) have suggested that NO production will lead to KATP channel activation such that GLIB would be predicted to have a greater effect on contraction in preparations with luminal flow.
A fourth explanation for why only some studies find an effect of GLIB on lymphatic contraction could relate to the oxygenation conditions. KATP channels in arteries and myocardium are activated under conditions of hypoxia or ischemia (1, 4), and lymphatic KATP channels presumably exhibit the same behavior. Thus, the use of highly oxygenated solutions in many ex vivo experiments on isolated lymphatic vessels (50, 55, 63) might suppress endogenous KATP channel activity. Arterial SM cells are in close contact with highly oxygenated arterial blood and therefore likely exposed to higher Po2 levels (92, 93). In contrast, most lymphatic vessels are in contact with the interstitium, where Po2 levels are often between 5 and 30 mmHg (94–96), depending on the vascular bed and the proximity of the lymphatic collectors to arteries/arterioles (93). Indeed, many microvascular studies using intravital microscopy have traditionally used deoxygenated solutions to more accurately approximate conditions in the interstitium (97, 98). Thus, inappropriately high oxygenation levels used in some lymphatic studies may have led to an underestimation of the basal activity of KATP channels, which otherwise might be predicted to have higher activity than channels in arterial SM. The pH level of the solutions used in lymphatic studies might also be important since interstitial pH may be slightly lower than that of blood and acidosis-induced inhibition lymphatic contractile activity has been shown to be mediated by KATP channels (54). These are issues that need further investigation.
ROLES FOR KATP CHANNELS IN MEDIATING LYMPHATIC VESSEL RESPONSE TO AGONISTS
The active lymph pump is modulated by numerous mechanisms, including neural, circulating, and locally produced factors that may act either to inhibit or enhance lymphatic contractile properties. The evidence to date suggests that many of these factors act in part or entirely through their effects on KATP channels (Table 1).
Nitric Oxide
Nitric oxide (NO) is the most studied of the various factors that modulate lymphatic contractions. In arteries, shear stress enhances the production of endothelium-derived NO, which diffuses to the overlying smooth muscle layer and activates various signaling pathways to inhibit basal (pressure-induced) myogenic tone and cause vasodilation (77); this process is often called flow-induced (or flow-mediated) dilation. Other EC-derived factors can contribute to flow-induced dilation but NO is the most prominent and ubiquitous factor. Gashev et al. (99) were the first to demonstrate that shear stress-induced production of NO inhibits spontaneous lymphatic contractions. In isolated, pressurized lymphatic collectors from rat mesentery, the pressure gradient between the cannulation pipettes was varied to impose different levels of forward flow while maintaining midpoint pressure approximately constant with resistance-matched pipettes. Graded increases in the pressure difference used to drive flow led to progressive inhibition of spontaneous contractions, marked by decreases in frequency, amplitude, and tone in response to flow. The responses were blocked by the endothelial nitric oxide synthase (eNOS) inhibitor l-NAME (99), indicating that they were mediated by NO. These results have been reproduced in a number of subsequent studies (100–103), with some showing that mast cell- or LEC-derived histamine release mediates a small component of the response to flow in certain lymphatic vessels (104, 105).
Inhibition of the active lymph pump is not the only effect of NO on lymphatic contractile properties. The pulsatile production of NO associated with spontaneous lymphatic contractions can enhance active lymph transport. The mechanism is as follows: NO is preferentially produced at the LEC valve regions, where shear stress is highest near the open tips of the valve leaflets (106), and its production oscillates with the lymphatic contraction cycle, as revealed by measurements with NO-sensitive electrodes (107). Under these conditions, NO production is more modest than under imposed flow and leads to a decrease in the time for relaxation following lymphatic systole, with little or no effect on contraction frequency (108). The molecular mechanism is not known, but accelerated relaxation allows more time for filling during lymphatic diastole, which enhances the contraction amplitude and ejection fraction of the subsequent contraction cycle due to a mechanism similar to the Frank–Starling effect in the heart (9). Lymph flow, calculated as the product of frequency and ejection fraction, rises if ejection fraction increases without a fall in frequency (108). Thus, NO appears to have biphasic effects on lymphatic contractions, enhancing the active lymph pump during pulsatile flow and inhibiting it at higher, nonpulsatile, flow.
NO production can also be stimulated by multiple agonists acting on the lymphatic endothelium. Mizuno et al. (109) first demonstrated that acetylcholine (ACh), or the NO donor sodium nitroprusside (SNP), inhibited spontaneous contractions and tone of isolated iliac afferent lymphatics from rat. The effects of both compounds were prevented by denudation of the endothelium. In vessels with an intact endothelium, the effects of ACh, but not SNP, were blocked by the pan-NOS inhibitor NG-nitro-l-arginine (l-NNA) (109). These findings were consistent with those from eNOS−/− mice, in which ACh-induced inhibition of spontaneous popliteal lymphatic contractions was lost (110). NO donors do not elicit hyperpolarization of the LEC layer (48, 51), but NO can readily diffuse to the LSM layer. Indeed, ACh, SNP, and another NO donor (DEA-NONOate) all hyperpolarized the LSM layer of guinea pig mesenteric lymphatics and inhibited spontaneous APs (51). Importantly, the hyperpolarizations induced by each of these compounds were substantially attenuated by 10 µM GLIB (51), suggesting that the inhibitory effects were mediated by the activation of KATP channels.
As expected from studies on arteries (111), the inhibitory effects of NO are mediated by stimulation of the cGMP/soluble guanylate cyclase (sGC) signaling pathway (49, 51, 63, 112). A number of studies have subsequently showed that KATP channels mediate, in part or in whole, the inhibitory effects of NO on lymphatic contractions (49, 58, 63, 65). It should be noted that these studies all used lymphatic vessels from rats or guinea pigs and relatively high concentrations of NO donors [e.g., 100 µM NONOate (63); 50–100 µM SNP (49); 100 µM SNP (51)]. In contrast, Kim et al. (69) found that KATP channels did not mediate the inhibitory effects of NO on the spontaneous contractions of popliteal lymphatic vessels from mice. ACh and NONOate each inhibited spontaneous contractions of WT mouse popliteal vessels, with IC50 values of 3 ×·10−8 M for ACh and 9·× 10−8 M for NONOate, respectively, on contraction frequency. However, the sensitivities to both ACh and NONOate were comparable in Kir6.1−/− vessels, with IC50 values of 2·× 10−8 M for ACh and 9·× 10−8 M for NONOate, respectively, on contraction frequency. Subsequent protocols showed that NONOate was working through stimulation of cGMP and soluble guanylate cyclase as expected, but that calcium-activated K+ channels, rather than KATP channels, mediated most (but not all) of the inhibitory effects of NO (69). This study raises questions about whether the role for KATP channels in NO-mediated inhibition of lymphatic pacemaking is unique to the rat and guinea pig, and if mouse lymphatics are the exception, or whether low and high concentrations of NO work through different mechanisms and/or on different molecular targets to modulate lymphatic contractions. Some potential mechanisms by which NO may act on KATP channels to inhibit lymphatic pacemaking are illustrated in Fig. 2. The effects of NO on human lymphatics do not appear to have been tested to date and it will be important to determine whether they are mediated by KATP channel activation—for reasons related to potential therapies for lymphatic contractile dysfunction as discussed in the KATP channels and Lymphatic Dysfunction.
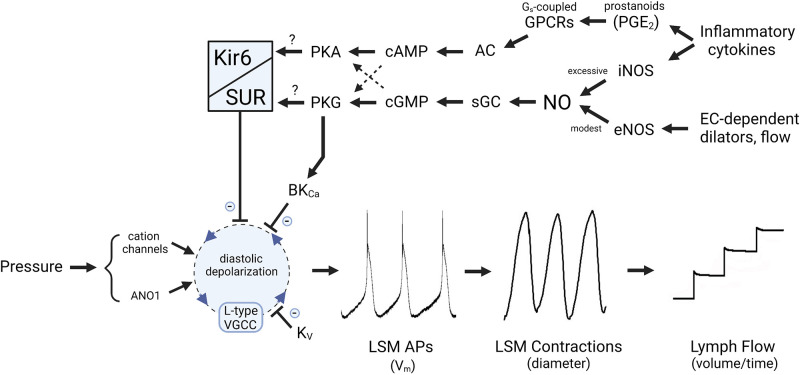
Figure 2.
Hypothesis to explain how nitric oxide (NO) and prostanoids act through KATP and/or large-conductance Ca2+-activated K+ channel (BKCa) channels to regulate the lymphatic pacemaking cycle. KATP channel activation slows the diastolic depolarization rate of the ionic pacemaker in lymphatic smooth muscle (LSM). In rat, KATP channel activity is stimulated by the NO-sGC-cGMP-PKG signaling axis; in mouse, the same pathway activates BKCa rather than KATP channels, but produces similar slowing of diastolic depolarization. Although cGMP production is associated with NO-mediated activation of KATP channels, the increased channel activity may be mediated either by channel phosphorylation by PKG or through cross-activation of PKA. Prostanoid production leads to increased cAMP levels that also activate PKA. AC, adenylate cyclase; ANO1, anoctamin1; AP, action potential; eNOS, endothelial nitric oxide synthase; iNOS, inducible NO synthase; sGC, soluble guanylate cyclase; VGCC, voltage-gated channel; GPCR, G-protein-coupled receptor.
Other Agonists
Although NO stimulates sGC-cGMP-PKG signaling, KATP channel activation appears to be largely, or additionally, through downstream activation of protein kinase A (PKA) (62). NO-sGC-cGMP-PKG signaling might activate PKA through cross-activation of PKA by cGMP (113, 114). Because cellular cGMP levels are normally much lower than cAMP levels, cGMP will compete with cAMP to activate PKA when cGMP levels rise (e.g., when NO is produced). The relative levels of the two cyclic nucleotides are also affected by the activities of phosphodiesterases (e.g., PDE3), which can degrade one nucleotide while being inhibited by the other (115). In LSM, hyperpolarizations evoked by ACh or SNP were inhibited by glibenclamide, by the sGC blocker ODQ and by the PKA inhibitor H-89 (49). SNP also inhibited spontaneous transient depolarizations, which are thought to contribute to the pacemaking potential in LSM (51), and this inhibition was attenuated by H-89 or the structurally unrelated PKA inhibitor KT-5720. The effects of several other agonists that inhibit lymphatic contractions also appear to be mediated by KATP channels, including vasoactive intestinal peptide (VIP) (62), calcitonin gene-related peptide (CGRP) (59), ATP (58), parathyroid hormone (53), and β2-adrenoreceptor signaling (49), with each study providing evidence for PKA involvement (Table 1). For example, VIP-induced hyperpolarization of LSM was almost completely blocked by GLIB or by H-89, and H-89 shifted the IC50 for VIP on contraction frequency to the right by ∼10-fold (62). The inhibitory effects of CGRP on spontaneous lymphatic contractions were also prevented by H-89 or GLIB (59). As with KCOs, the effects of these agonists were primarily on the frequency of lymphatic APs or contractions. Thus, a common link between NO or other agonists and inhibition of lymphatic contractions may be PKA-mediated activation of KATP channels, as depicted in Fig. 2. However. this conclusion relies heavily on the specificity of H-89, which inhibits at least eight other kinases, and off-target effects are likely as the typical concentration used in the earlier studies (10 µM) well exceeds the IC50 (135 nM) for PKA (116).
Prostanoids
Inflammation is known to compromise lymphatic contractile function, in part through cytokine-induced activation of inducible NO synthase (iNOS), resulting in the excessive generation of NO, and in part through production of multiple vasoactive prostanoids resulting from the metabolism of arachidonic acid by increased cyclooxygenase activity (63, 117–122). Wu et al. (123) observed dilated lymphatics with blunted spontaneous contractions occurring in a guinea pig model of ileitis; the contractile dysfunction was partially reversed by cyclooxygenase-1, -2 (COX-1 and COX-2) inhibition. Subsequent studies by the same laboratory identified PGE2 and PGI2 as the major prostanoids mediating lymphatic contractile dysfunction in both rat and guinea pig models of ileitis (60, 121). These prostanoids bind to their respective GPCRs (e.g., EP4 for PGE2, IP for PGI2), which couple to adenylate cyclase (AC) through Gs proteins (60), resulting in enhanced production of cAMP. The inhibitory effects of exogenous PGE2 and PGI2 were blocked by H-89 or GLIB (60), consistent with roles for both PKA and KATP channels. In the guinea pig ileitis model, Mathias et al. (63) also found that the impaired lymphatic contractions were partially rescued by inhibition of either KATP channels, iNOS, or sGC. Collectively, these findings suggest that NO and prostanoids generated in these models of ileitis inhibit spontaneous lymphatic pumping through the activation of LSM KATP channels.
PKA presumably activates KATP channels via phosphorylation of specific residues on one or both of the channel subunits. An initial report suggested that PKA phosphorylates one site on Kir6.1 and two sites on SUR2B (124). However, subsequent mutation of each predicted site on each subunit revealed that Kir6.1 was not phosphorylated by PKA while two residues on SUR2B were critical for mediating the effects of isoproterenol and forskolin, and only one of these (Ser 1381) was phosphorylated (125). KATP channels can also be phosphorylated by PKC (126) and PKG (127, 128), which further complicates the interpretation of many lymphatic studies, and thus, the extent to which regulation of KATP channels in LSM may be mediated or modified by any or all of these protein kinases remains to be definitively determined. Protein kinase-mediated phosphorylation of Kir6.1 in LSM would presumably require the close proximity of the kinase and Kir6.1, which might be facilitated by scaffolding proteins, as has been established for other ion channel phosphorylation mechanisms (125, 129–132). Such studies typically use a combination of patch clamp protocols, super-resolution microscopy, proximity ligation assays, site-directed mutagenesis, and/or transgenic mice to establish these relationships. As yet, none of these techniques has been applied to LSM in the context of protein kinase-mediated regulation of KATP channels.
KATP CHANNELS AND LYMPHATIC DYSFUNCTION
Cantú Syndrome
Chronic activation of KATP channels in LSM would lead to suppression of spontaneous lymphatic contractions and active lymph transport, and a clear example of SM dysfunction mediated by KATP channels is now provided by Cantú syndrome (CS). First described in 1982 by J.M. Cantú (133), and later recognized to have similarity of symptoms to those of chronic, systemic minoxidil use (134), CS is characterized by obvious physical features of congenital hypertrichosis and facial dysmorphia (133, 135). In addition, subjects with CS exhibit multiple cardiovascular abnormalities, including hypotension, cardiomegaly, patent ductus arteriosis, and dilated and tortuous cerebral arteries (135–138), as well as gastrointestinal SM problems that disrupt normal gastrointestinal (GI) motility (135). Over 50% of patients with CS develop lymphedema at some stage, especially in the lower extremities (135, 139), presumably related to hyperactive KATP channels in LSM. Other aspects of lymphatic dysfunction in patients with CS, such as localized head and neck edema (140) may be underdiagnosed. It is possible that the pericardial effusion observed in some patients with CS may be associated with depressed lymphatic contractility of the coronary lymphatics, and that CS-related neurological disorders may be related to impaired cerebral lymphatic/glymphatic drainage, as reported for other neurological diseases (141–144).
The cause of CS is now known to be gain-of-function (GOF) mutations in either the Kir6.1 or SUR2 KATP channel subunits (145, 146), with over 30 mutations identified thus far (135). Mouse models of CS engineered by knock-in CS mutations into the endogenous KCNJ8 (Kir6.1[V65M]) or ABCC9 (SUR2[A478V]) loci reproduce important features of the syndrome (147). The electrophysiological characteristics of GOF KATP channels in arterial SM are as predicted: whole cell patch-clamp recordings reveal an enhanced basal KATP conductance in vascular smooth muscle (147–149). In both Kir6.1[V65M] and SUR2[A478V] animals, this causes arterial dilation, lower diastolic and systolic blood pressures, and increased arterial compliance (147), with the severity of cardiovascular symptoms increasing with severity of the molecular GOF (147). The chronic reduction in arterial pressure results in increased sympathetic neural output to the heart (138) and activation of the renin-angiotensin system (150), resulting in high output cardiac hypertrophy. Importantly, most of the cardiovascular abnormalities in mice can be reversed with chronic, systemic GLIB treatment (151).
Lymphatic function has not yet been studied in these knock-in models. However, SM-specific expression of a transgenic GOF Kir6.1 subunit (149) resulted in severe contractile dysfunction in mouse popliteal lymphatics studied ex vivo, where contraction amplitude was reduced by 70%–90% and contraction frequency by 50% compared with those of vessels from control animals (66). The amplitude and frequency of spontaneous contractions were similarly reduced in popliteal lymphatics studied in vivo. Vm recordings from LSM cells in pressurized popliteal vessels revealed that Vm was hyperpolarized by 11 mV on average in Kir6.1 GOF vessels (66). Similar hyperpolarization and impairment of lymphatic contractile function are predicted to be observed in mice with CS-related GOF mutations in Kir6.1 or SUR2B.
Chronic KATP channel GOF in arterial SM also leads to more permanent vascular remodeling. GOF mutations in either Kir6.1 or SUR2B resulted in increases in the maximum passive diameters of multiple arteries and increased arterial compliance (147), an effect that does not appear to be reversed by GLIB treatment (151). Whether any similar remodeling of lymphatic vessel diameter occurs in CS mice remains to be tested. Interestingly, the maximal passive diameters of mouse popliteal lymphatic vessels in Kir6.1−/− mice were consistently lower than those in WT mice (69), i.e., the reverse of what happens in CS mouse blood vessels, indicating that KATP channel dysfunction may indeed have novel consequences for lymphatic vessel development.
Lymphedema
KATP channels might also contribute to lymphatic contractile dysfunction in patients with more generalized lymphedema—defined as edema with a component caused by lymphatic dysfunction and classified as either primary or secondary. Typically resulting from hereditary mutations in genes controlling lymphatic vessel and valve development, primary lymphedema in CS may be the first example caused by impaired lymphatic contractile function. Secondary lymphedema occurs as a consequence of another pathological process, e.g., congestive heart failure, chronic venous disease, or obesity. The incidence of secondary lymphedema correlates highly with basal metabolic index (BMI). Patients with BMI over 40 have a progressive risk for lymphedema (152), with morbid obesity almost always leading to the development of secondary lymphedema (153). The most common cause of lymphedema in the United States is surgical intervention and/or radiotherapy for breast cancer (154) and 20%–40% of breast cancer survivors develop lymphedema in the affected arm(s) at some point after cancer treatment due, in part, to impairment of active lymphatic transport (155–157). If left untreated, secondary lymphedema can progress through several stages, culminating in extreme interstitial fluid and protein retention, inflammation, and cytokine production, which eventually result in fibrosis and adipose deposition. All these factors can further contribute to deteriorating lymphatic function, which includes both valve incompetency and contractile defects (158–161). The only approved treatment for lymphedema is massage therapy combined with compression stockings or devices, but recent clinical studies have explored the use of anti-inflammatory treatments, including the T cell inhibitor tacrolimus (162), regulatory T cell transfer (163), and the leukotriene B4 inhibitor ketoprofen (164). Although no studies have yet linked KATP channel activation to lymphatic contractile dysfunction in secondary lymphedema, part of the rationale for anti-inflammatory therapy is to prevent excessive production of NO and/or eicosanoids through cytokine-induced activation of iNOS and/or COX2. KATP involvement in this context hinges on whether NO and prostanoids activate KATP channels in human lymphatics.
Obesity and Metabolic Syndrome
In addition to its role in the reabsorption of excess interstitial fluid, the lymphatic system also plays critical roles in dietary fat absorption and immune function. Metabolic syndrome is defined by a constellation of clinical findings that includes obesity, insulin resistance, dyslipidemia, and hypertension. The strong association between these pathologies and lymphatic dysfunction has been highlighted in a recent series of review articles (165–171). At least three lines of evidence support a causal link between lymphatic dysfunction and the development of obesity and/or metabolic syndrome.
First, several key genes and signaling pathways that control lymphatic development are linked to obesity and insulin resistance. Prox1 is the key transcription factor that determines lymphatic identity (172). Certain Prox1 gene variants are associated with increased incidences of hyperlipidemia, obesity, and type 2 diabetes in humans (173–176). Prox1−/− mice die at embryonic day 14.5 (E14.5) when the lymphatic system is developing (177). Most haplodeficient Prox1 mice die shortly after birth but those that survive to adulthood develop adult-onset obesity, with mispatterning of the lymphatic vasculature, defective barrier function, and impaired lymphatic transport (178, 179). Importantly, restoration of lymphatic function by Prox1 re-expression reverses this adult-onset obesity (179). Similarly, Vegfr3, the critical growth factor controlling lymphatic development (180, 181), and Foxc2, a transcription factor controlling lymphatic valve development (182–184), are also linked to the development of obesity and insulin resistance (185–189).
A second link between the lymphatic system and obesity/metabolic syndrome relates to the role of the lymphatic system in fat absorption and fat deposition (190, 191). Dietary fat absorption occurs through the intestinal villi, each of which contain a central lymphatic lacteal capillary with highly permeable, discontinuous, button-like LEC-LEC junctions. Lacteals drain into networks of lymphatic precollectors and collectors with less permeable zipper-like junctions (5) that transport chylomicrons through the mesentery to the intestinal lymph nodes (190). These intestinal lymphatics play a key role in the deposition of visceral adipose tissue (VAT), which, when accumulated in excess, is associated with the production of adipokines and fatty acids, leading to impaired arterial SM function, NO bioavailability, and insulin resistance (192). Adipose tissue is known to accumulate around sites of lymph leakage (178, 193, 194) and in mice, a high-fat diet stimulates the growth of mesenteric lymphatics with abnormal permeability, promoting leakage of chylomicron-rich lymph that stimulates deposition of VAT (193). Conversely, elevated levels of VEGF-A can convert button junctions in the lacteal network to zipper junctions, leading to impairment of chylomicron uptake and resistance to diet-induced obesity (195). Thus, fat deposition is closely correlated with lymph leakage and is enhanced if lymphatic permeability is abnormally elevated. Enhancing lymphatic function under these conditions may thus protect against diet-induced obesity and insulin resistance (196).
Third, there is accumulating evidence that lymphatic dysfunction may be both a cause and a consequence of the factors that contribute to obesity and metabolic syndrome (197–199). Dysfunction in this context may include several components, such as the enhanced leakiness of lymphatic collectors observed in db/db mice, a genetic model of diabetes/obesity (115), valve back-leak and closure defects in mice fed a Western diet (200), and lymphatic contractile dysfunction in animals fed high-fat or high-fructose diets as models of metabolic syndrome (199, 201–203). Lymphatic contractile dysfunction also occurs in animals with advanced age, with the major effect being a reduction in spontaneous contraction frequency (101, 102, 204). A common theme linking these conditions is metabolic stress and inflammation, and it is therefore likely that KATP channel activation may be involved. Metabolic stress may result in increased intracellular ADP/ATP ratio that would directly activate KATP channels in LSM (Fig. 3) and NO conversion to reactive oxygen species (ROS) might also facilitate KATP channel activation (205–207). Inflammation-related production of NO and prostanoids would inhibit lymphatic pumping through KATP channels, as discussed in an earlier section. To date, however, there are only two indications of KATP channel involvement in these pathologies: the profound lymphatic contractile dysfunction in the 2,4,6-trinitrobenzene sulfonic acid (TNBS) model of ileitis, due to excessive PGE2 production, and the impaired lymphatic flow and contractile function in rats fed a high-fructose diet, both of which were rescued by GLIB (63, 64). The extent to which lymphatic contractile function is compromised in other models of metabolic stress, and whether the involvement of KATP channels is a common theme, are potentially important issues that require further investigation.
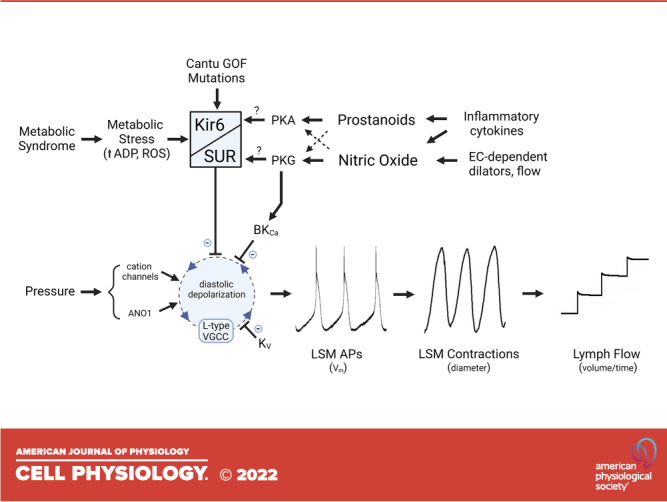
Figure 3.
Summary of mechanisms through which KATP channels regulate lymphatic pacemaking. Activation of KATP channels through the production of cAMP and cGMP results in a slower diastolic depolarization rate of the ionic pacemaker in lymphatic smooth muscle (LSM), leading to reduced lymphatic contraction frequency and reduced pumping. Gain-of-function mutations in Cantu syndrome cause constitutive activation of KATP channels and lymph pump dysfunction, eventually resulting in peripheral lymphedema. KATP channels may also be activated to varying degrees by metabolic stress in animal models of metabolic syndrome. AC, adenylate cyclase; ANO1, anoctamin1; AP, action potential; eNOS, endothelial nitric oxide synthase; GOF, gain-of-function; iNOS, inducible NO synthase; NO, nitric oxide; ROS, reactive oxygen species; sGC, soluble guanylate cyclase; GPCR, G-protein-coupled receptor.
An important related issue is whether inhibitors of mitochondrial respiration cause the activation of lymphatic KATP channels. This topic has been extensively studied in arterial SM and myocardium (1, 4), but there have been no comparable studies in lymphatic vessels. Multiple conditions related to metabolic stress could lead to KATP channel activation, both as an initiating factor and as a feed-forward mechanism to potentiate and prolong lymphatic contractile dysfunction. For example, the activation of KATP channels by metabolic stress would result in compromised active lymphatic pumping and transport, potentially leading to a buildup of metabolic products in the interstitium, exacerbating the already elevated ADP/ATP ratio in LSM cells and promoting further activation of KATP channels. Once lymphedema begins to develop, increased fibrosis and adipose deposition would result in an increased diffusion distance for O2 (and CO2) from arteries to lymphatic collectors, which could further trigger or reinforce KATP channel activation. Surprisingly, these topics have not been studied experimentally but are areas ripe for further investigation.
In summary, it is likely that a spectrum of KATP channel activation levels, ranging from modest (metabolic syndrome) to severe (Cantú syndrome), leads to the suppression of lymphatic contractile function and impaired lymph transport that contributes to multiple diseases, including obesity, metabolic syndrome, and secondary lymphedema. A better understanding of the contribution of KATP channels to these processes could potentially lead to their treatment using sulfonylurea receptor inhibitors such as glibenclamide.
GRANTS
This research was supported by National Institutes of Health Grants R01 HL-141107 and HL-122578 (to M. J. Davis) and R35 HL-140024 (to C. G. Nichols).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J.D. and C.G.N. conceived and designed research; M.J.D., H.J.K., and C.G.N. drafted manuscript; M.J.D., H.J.K., and C.G.N. edited and revised manuscript; M.J.D., H.J.K., and C.G.N. approved final version of manuscript.
ACKNOWLEDGMENTS
The figures were prepared in BioRender and an appropriate publication license was obtained.
This article is part of the special collection “Inward Rectifying K+ Channels.” Drs. Jerod Denton and Eric Delpire served as Guest Editors of this collection.
REFERENCES
- 1. Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 440: 470–476, 2006. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 2. Aittoniemi J, Fotinou C, Craig TJ, de Wet H, Proks P, Ashcroft FM. Review. SUR1: a unique ATP-binding cassette protein that functions as an ion channel regulator. Philos Trans R Soc Lond B Biol Sci 364: 257–267, 2009. doi: 10.1098/rstb.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chutkow WA, Simon MC, Le Beau MM, Burant CF. Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular KATP channels. Diabetes 45: 1439–1445, 1996. doi: 10.2337/diab.45.10.1439. [DOI] [PubMed] [Google Scholar]
- 4. Foster MN, Coetzee WA. KATP channels in the cardiovascular system. Physiol Rev 96: 177–252, 2016. doi: 10.1152/physrev.00003.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204: 2349–2362, 2007. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Breslin JW, Yang Y, Scallan JP, Sweat RS, Adderley SP, Murfee WL. Lymphatic vessel network structure and physiology. Compr Physiol 9: 207–299, 2018. doi: 10.1002/cphy.c180015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scallan JP, Zawieja SD, Castorena-Gonzalez JA, Davis MJ. Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol 594: 5749–5768, 2016. doi: 10.1113/JP272088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis MJ, Scallan JP, Wolpers JH, Muthuchamy M, Gashev AA, Zawieja DC. Intrinsic increase in lymphatic muscle contractility in response to elevated afterload. Am J Physiol Heart Circ Physiol 303: H795–H808, 2012. doi: 10.1152/ajpheart.01097.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scallan JP, Wolpers JH, Muthuchamy M, Zawieja DC, Gashev AA, Davis MJ. Independent and interactive effects of preload and afterload on the lymphatic pump. Am J Physiol Heart Circ Physiol 303: H809–H824, 2012. doi: 10.1152/ajpheart.01098.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olszewski WL, Engeset A. Intrinsic contractility of prenodal lymph vessels and lymph flow in human leg. Am J Physiol Heart Circ Physiol 239: H775–H783, 1980. doi: 10.1152/ajpheart.1980.239.6.H775. [DOI] [PubMed] [Google Scholar]
- 11. Castorena-Gonzalez JA, Zawieja SD, Li M, Srinivasan RS, Simon AM, de Wit C, de la Torre R, Martinez-Lemus LA, Hennig GW, Davis MJ. Mechanisms of connexin-related lymphedema. Circ Res 123: 964–985, 2018. doi: 10.1161/CIRCRESAHA.117.312576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hald BO, Castorena-Gonzalez JA, Zawieja SD, Gui P, Davis MJ. Electrical communication in lymphangions. Biophys J 115: 936–949, 2018. doi: 10.1016/j.bpj.2018.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castorena-Gonzalez JA, Li M, Davis MJ. Effects of elevated downstream pressure and the role of smooth muscle cell coupling through connexin45 on lymphatic pacemaking. Biomolecules 10: 1424, 2020. doi: 10.3390/biom10101424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beckett EA, Hollywood MA, Thornbury KD, McHale NG. Spontaneous electrical activity in sheep mesenteric lymphatics. Lymphat Res Biol 5: 29–43, 2007. doi: 10.1089/lrb.2007.5104. [DOI] [PubMed] [Google Scholar]
- 15. Zawieja SD, Castorena-Gonzalez JA, To KHT, Gui P, Domeier TL, Davis MJ. Electrical pacemaking in lymphatic vessels. In: Smooth Muscle Excitability, edited by Trebak M, Earley SC.. Boca Raton, FL: CRC Press, 2018, p. 324–359. [Google Scholar]
- 16. Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol Heart Circ Physiol 269: H348–H355, 1995. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- 17. Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256: 532–535, 1992. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 18. Shabir S, Borisova L, Wray S, Burdyga T. Rho-kinase inhibition and electromechanical coupling in rat and guinea-pig ureter smooth muscle: Ca2+-dependent and -independent mechanisms. J Physiol 560: 839–855, 2004. doi: 10.1113/jphysiol.2004.070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hollywood MA, Cotton KD, Thornbury KD, McHale NG. Isolated sheep mesenteric lymphatic smooth muscle cells possess both T- and L-type calcium currents (Abstract). J Physiol 501: P109–P110, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Helden DF. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J Physiol 471: 465–479, 1993. doi: 10.1113/jphysiol.1993.sp019910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imtiaz MS, Zhao J, Hosaka K, von der Weid PY, Crowe M, van Helden DF. Pacemaking through Ca2+ stores interacting as coupled oscillators via membrane depolarization. Biophys J 92: 3843–3861, 2007. doi: 10.1529/biophysj.106.095687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. von der Weid PY, Rahman M, Imtiaz MS, Van Helden DF. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol 295: H1989–H2000, 2008. doi: 10.1152/ajpheart.00007.2008. [DOI] [PubMed] [Google Scholar]
- 23. Cotton KD, Hollywood MA, McHale NG, Thornbury KD. Outward currents in smooth muscle cells isolated from sheep mesenteric lymphatics. J Physiol 503: 1–11, 1997. doi: 10.1111/j.1469-7793.1997.001bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Telinius N, Kim S, Pilegaard H, Pahle E, Nielsen J, Hjortdal V, Aalkjaer C, Boedtkjer DB. The contribution of K(+) channels to human thoracic duct contractility. Am J Physiol Heart Circ Physiol 307: H33–H43, 2014. doi: 10.1152/ajpheart.00921.2013. [DOI] [PubMed] [Google Scholar]
- 25. McCloskey KD, Hollywood MA, Thornbury KD, Ward SM, McHale NG. Kit-like immunopositive cells in sheep mesenteric lymphatic vessels. Cell Tissue Res 310: 77–84, 2002. doi: 10.1007/s00441-002-0623-y. [DOI] [PubMed] [Google Scholar]
- 26. Briggs Boedtkjer D, Rumessen J, Baandrup U, Skov Mikkelsen M, Telinius N, Pilegaard H, Aalkjaer C, Hjortdal V. Identification of interstitial Cajal-like cells in the human thoracic duct. Cells Tissues Organs 197: 145–158, 2013. doi: 10.1159/000342437. [DOI] [PubMed] [Google Scholar]
- 27. Lee S, Roizes S, von der Weid PY. Distinct roles of L- and T-type voltage-dependent Ca2+ channels in regulation of lymphatic vessel contractile activity. J Physiol 592: 5409–5427, 2014. doi: 10.1113/jphysiol.2014.280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. To KHT, Gui P, Li M, Zawieja SD, Castorena-Gonzalez JA, Davis MJ. T-type, but not L-type, voltage-gated calcium channels are dispensable for lymphatic pacemaking and spontaneous contractions. Sci Rep 10: 70, 2020. doi: 10.1038/s41598-019-56953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCloskey KD, Toland HM, Hollywood MA, Thornbury KD, McHale NG. Hyperpolarization-activated inward current in isolated sheep mesenteric lymphatic smooth muscle. J Physiol 521: 201–211, 1999. doi: 10.1111/j.1469-7793.1999.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Toland HM, McCloskey KD, Thornbury KD, Mchale NG, Hollywood MA. Ca2+-activated Cl- current in sheep lymphatic smooth muscle. Am J Physiol Cell Physiol 279: C1327–C1335, 2000. doi: 10.1152/ajpcell.2000.279.5.C1327. [DOI] [PubMed] [Google Scholar]
- 31. Negrini D, Marcozzi C, Solari E, Bossi E, Cinquetti R, Reguzzoni M, Moriondo A. Hyperpolarization-activated cyclic nucleotide-gated channels in peripheral diaphragmatic lymphatics. Am J Physiol Heart Circ Physiol 311: H892–H903, 2016. doi: 10.1152/ajpheart.00193.2016. [DOI] [PubMed] [Google Scholar]
- 32. Majgaard J, Boedtkjer D. HCN channels in lymphatics. Exp Physiol. In press. [Google Scholar]
- 33. Hollywood MA, Cotton KD, Thornbury KD, Mchale NG. Tetrodotoxin-sensitive sodium current in sheep lymphatic smooth muscle. J Physiol 503: 13–21, 1997. doi: 10.1111/j.1469-7793.1997.013bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Telinius N, Majgaard J, Kim S, Katballe N, Pahle E, Nielsen J, Hjortdal V, Aalkjaer C, Boedtkjer DB. Voltage-gated sodium channels contribute to action potentials and spontaneous contractility in isolated human lymphatic vessels. J Physiol 593: 3109–3122, 2015. doi: 10.1113/JP270166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zawieja SD, Castorena JA, Gui P, Li M, Bulley SA, Jaggar JH, Rock JR, Davis MJ. Ano1 mediates pressure-sensitive contraction frequency changes in mouse lymphatic collecting vessels. J Gen Physiol 151: 532–554, 2019. doi: 10.1085/jgp.201812294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nikolaev YA, Cox CD, Ridone P, Rohde PR, Cordero-Morales JF, Vasquez V, Laver DR, Martinac B. Mammalian TRP ion channels are insensitive to membrane stretch. J Cell Sci 132: jcs238360, 2019. doi: 10.1242/jcs.238360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davis MJ, Earley S, Li Y-S, Chien S. Vascular mechanotransduction. Physiol Rev. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang W, Nepiyushchikh Z, Zawieja DC, Chakraborty S, Zawieja SD, Gashev AA, Davis MJ, Muthuchamy M. Inhibition of myosin light chain phosphorylation decreases rat mesenteric lymphatic contractile activity. Am J Physiol Heart Circ Physiol 297: H726–H734, 2009. doi: 10.1152/ajpheart.00312.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nepiyushchikh ZV, Chakraborty S, Wang W, Davis MJ, Zawieja DC, Muthuchamy M. Differential effects of myosin light chain kinase inhibition on contractility, force development and myosin light chain 20 phosphorylation of rat cervical and thoracic duct lymphatics. J Physiol 589: 5415–5429, 2011. doi: 10.1113/jphysiol.2011.218446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muthuchamy M, Gashev A, Boswell N, Dawson N, Zawieja D. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J 17: 920–922, 2003. doi: 10.1096/fj.02-0626fje. [DOI] [PubMed] [Google Scholar]
- 41. Muthuchamy M, Zawieja DC. Molecular and cellular characterization of lymphatic muscle (Abstract). Circ Res 98: A4339, 1998. [Google Scholar]
- 42. von der Weid PY, Muthuchamy M. Regulatory mechanisms in lymphatic vessel contraction under normal and inflammatory conditions. Pathophysiology 17: 263–276, 2010. doi: 10.1016/j.pathophys.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 43. Muthuchamy M, Zawieja D. Molecular regulation of lymphatic contractility. Ann N Y Acad Sci 1131: 89–99, 2008. doi: 10.1196/annals.1413.008. [DOI] [PubMed] [Google Scholar]
- 44. Takeya K, Kathol I, Sutherland C, Wang X, Loutzenhiser R, Walsh MP. Expression of troponin subunits in the rat renal afferent arteriole. IUBMB Life 71: 1475–1481, 2019. doi: 10.1002/iub.2061. [DOI] [PubMed] [Google Scholar]
- 45. Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response: kinetic attributes and physiological role. Circ Res 90: 1316–1324, 2002. doi: 10.1161/01.res.0000024262.11534.18. [DOI] [PubMed] [Google Scholar]
- 46. Zhang R, Taucer AI, Gashev AA, Muthuchamy M, Zawieja DC, Davis MJ. Maximum shortening velocity of lymphatic muscle approaches that of striated muscle. Am J Physiol Heart Circ Physiol 305: H1494–H1507, 2013. doi: 10.1152/ajpheart.00898.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zawieja DC, von der Weid P, Gashev AA. Microlymphatic biology. In: Comprehensive Physiology, edited by Tuma R, Duran W, Ley K. Hoboken, NJ: Wiley, 2011. p. 125–158 [Google Scholar]
- 48. von der Weid PY, van Helden DF. Functional electrical properties of the endothelium in lymphatic vessels of the guinea-pig mesentery. J Physiol 504: 439–451, 1997. doi: 10.1111/j.1469-7793.1997.439be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. von der Weid PY. ATP-sensitive K+ channels in smooth muscle cells of guinea-pig mesenteric lymphatics: role in nitric oxide and β-adrenoceptor agonist-induced hyperpolarizations. Br J Pharmacol 125: 17–22, 1998. doi: 10.1038/sj.bjp.0702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mizuno R, Ono N, Ohhashi T. Involvement of ATP-sensitive K+ channels in spontaneous activity of isolated lymph microvessels in rats. Am J Physiol Heart Circ Physiol 277: H1453–H1456, 1999. doi: 10.1152/ajpheart.1999.277.4.H1453. [DOI] [PubMed] [Google Scholar]
- 51. von der Weid PY, Zhao J, Van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol Heart Circ Physiol 280: H2707–H2716, 2001. doi: 10.1152/ajpheart.2001.280.6.H2707. [DOI] [PubMed] [Google Scholar]
- 52. Nakaya K, Mizuno R, Ohhashi T. B16-BL6 melanoma cells release inhibitory factor(s) of active pump activity in isolated lymph vessels. Am J Physiol Cell Physiol 281: C1812–C1818, 2001. doi: 10.1152/ajpcell.2001.281.6.C1812. [DOI] [PubMed] [Google Scholar]
- 53. Mizuno R, Ono N, Ohhashi T. Parathyroid hormone-related protein-(1-34) inhibits intrinsic pump activity of isolated murine lymph vessels. Am J Physiol Heart Circ Physiol 281: H60–H66, 2001. doi: 10.1152/ajpheart.2001.281.1.H60. [DOI] [PubMed] [Google Scholar]
- 54. Lobov GI, Kubyshkina NA. Effect of acidosis on contractile function of mesenterial lymphatic vessels in bulls. Bull Exp Biol Med 132: 622–624, 2001. doi: 10.1023/a:1012551522465. [DOI] [PubMed] [Google Scholar]
- 55. Chan AK, von der Weid PY. 5-HT decreases contractile and electrical activities in lymphatic vessels of the guinea-pig mesentery: role of 5-HT 7-receptors. Br J Pharmacol 139: 243–254, 2003. doi: 10.1038/sj.bjp.0705264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hosaka K, Mizuno R, Ohhashi T. Rho-Rho kinase pathway is involved in the regulation of myogenic tone and pump activity in isolated lymph vessels. Am J Physiol Heart Circ Physiol 284: H2015–H2025, 2003. doi: 10.1152/ajpheart.00763.2002. [DOI] [PubMed] [Google Scholar]
- 57. Chan AK, Vergnolle N, Hollenberg MD, von der Weid PY. Proteinase-activated receptor 2 activation modulates guinea-pig mesenteric lymphatic vessel pacemaker potential and contractile activity. J Physiol 560: 563–576, 2004. doi: 10.1113/jphysiol.2004.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kousai A, Mizuno R, Ikomi F, Ohhashi T. ATP inhibits pump activity of lymph vessels via adenosine A1 receptor-mediated involvement of NO- and ATP-sensitive K+ channels. Am J Physiol Heart Circ Physiol 287: H2585–H2597, 2004. doi: 10.1152/ajpheart.01080.2003. [DOI] [PubMed] [Google Scholar]
- 59. Hosaka K, Rayner SE, von der Weid PY, Zhao J, Imtiaz MS, van Helden DF. Calcitonin gene-related peptide activates different signaling pathways in mesenteric lymphatics of guinea pigs. Am J Physiol Heart Circ Physiol 290: H13–H22, 2006. doi: 10.1152/ajpheart.00543.2005. [DOI] [PubMed] [Google Scholar]
- 60. Rehal S, Blanckaert P, Roizes S, von der Weid PY. Characterization of biosynthesis and modes of action of prostaglandin E2 and prostacyclin in guinea pig mesenteric lymphatic vessels. Br J Pharmacol 158: 1961–1970, 2009. doi: 10.1111/j.1476-5381.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang LM, Niu CY, Zhao ZG, Si YH, Zhang YP. [ATP-sensitive potassium channel involved in modulation of nitride oxide regulating contractile activity of isolated lymphatics from hemorrhagic shock rats]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 24: 457–460, 2012. [PubMed] [Google Scholar]
- 62. von der Weid PY, Rehal S, Dyrda P, Lee S, Mathias R, Rahman M, Roizes S, Imtiaz MS. Mechanisms of VIP-induced inhibition of the lymphatic vessel pump. J Physiol 590: 2677–2691, 2012. doi: 10.1113/jphysiol.2012.230599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mathias R, von der Weid PY. Involvement of the NO-cGMP-K(ATP) channel pathway in the mesenteric lymphatic pump dysfunction observed in the guinea pig model of TNBS-induced ileitis. Am J Physiol Gastrointest Liver Physiol 304: G623–634, 2013. doi: 10.1152/ajpgi.00392.2012. [DOI] [PubMed] [Google Scholar]
- 64. Zawieja SD, Wang W, Chakraborty S, Zawieja DC, Muthuchamy M. Macrophage alterations within the mesenteric lymphatic tissue are associated with impairment of lymphatic pump in metabolic syndrome. Microcirculation 23: 558–570, 2016. doi: 10.1111/micc.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang LM, Qin LP, Zhang YP, Zhao ZG, Niu CY. Nitric oxide regulates the lymphatic reactivity following hemorrhagic shock through ATP-sensitive potassium channel. Shock 45: 668–676, 2016. doi: 10.1097/SHK.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 66. Davis MJ, Kim HJ, Zawieja SD, Castorena-Gonzalez JA, Gui P, Li M, Saunders BT, Zinselmeyer BH, Randolph GJ, Remedi MS, Nichols CG. Kir6.1-dependent KATP channels in lymphatic smooth muscle and vessel dysfunction in mice with Kir6.1 gain-of-function. J Physiol 598: 3107–3127, 2020. doi: 10.1113/JP279612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lobov GI. The role of hydrogen sulfide in the dilatation of mesenteric lymphatic vessels in bulls. Bull Exp Biol Med 169: 302–305, 2020. doi: 10.1007/s10517-020-04874-x. [DOI] [PubMed] [Google Scholar]
- 68. Garner BR, Stolarz AJ, Stuckey D, Sarimollaoglu M, Liu Y, Palade PT, Rusch NJ, Mu S. KATP channel openers inhibit lymphatic contractions and lymph flow as a possible mechanism of peripheral edema. J Pharmacol Exp Ther 376: 40–50, 2021. doi: 10.1124/jpet.120.000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kim HJ, Li M, Nichols CG, Davis MJ. Large-conductance calcium-activated K+ channels, rather than KATP channels, mediate the inhibitory effects of nitric oxide on mouse lymphatic pumping. Br J Pharmacol 178: 4119–4136, 2021. doi: 10.1111/bph.15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lobov GI, Pan'kova MN. [NO-dependent. Modulation of contractile function in capsule of lymph nodes]. Ross Fiziol Zh Im I M Sechenova 96: 489–497, 2010. [PubMed] [Google Scholar]
- 71. Pan'kova MN, Lobov GI. Effect of heparin on contractile activity of lymph node capsule. Bull Exp Biol Med 159: 632–634, 2015. doi: 10.1007/s10517-015-3033-3. [DOI] [PubMed] [Google Scholar]
- 72. Lobov GI, Pan'kova MN. Atrial natriuretic peptide inhibits spontaneous contractile activity of lymph nodes. Bull Exp Biol Med 161: 221–223, 2016. doi: 10.1007/s10517-016-3380-8. [DOI] [PubMed] [Google Scholar]
- 73. Cui Y, Giblin JP, Clapp LH, Tinker A. A mechanism for ATP-sensitive potassium channel diversity: Functional coassembly of two pore-forming subunits. Proc Natl Acad Sci USA 98: 729–734, 2001. doi: 10.1073/pnas.98.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Farzaneh T, Tinker A. Differences in the mechanism of metabolic regulation of ATP-sensitive K+ channels containing Kir6.1 and Kir6.2 subunits. Cardiovasc Res 79: 621–631, 2008. doi: 10.1093/cvr/cvn138. [DOI] [PubMed] [Google Scholar]
- 75. Aziz Q, Li Y, Anderson N, Ojake L, Tsisanova E, Tinker A. Molecular and functional characterization of the endothelial ATP-sensitive potassium channel. J Biol Chem 292: 17587–17597, 2017. doi: 10.1074/jbc.M117.810325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li Y, Aziz Q, Anderson N, Ojake L, Tinker A. Endothelial ATP-sensitive potassium channel protects against the development of hypertension and atherosclerosis. Hypertension 76: 776–784, 2020. doi: 10.1161/HYPERTENSIONAHA.120.15355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Garland CJ, Dora KA. EDH: endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiol (Oxf) 219: 152–161, 2017. doi: 10.1111/apha.12649. [DOI] [PubMed] [Google Scholar]
- 78. Crowe MJ, von der Weid PY, Brock JA, van Helden DF. Co-ordination of contractile activity in guinea-pig mesenteric lymphatics. J Physiol 500: 235–244, 1997. doi: 10.1113/jphysiol.1997.sp022013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hald BO, Castorena-Gonzalez JA, Zawieja SD, Gui P, Davis MJ. Electrical communication in lymphangions. Biophys J 115: 936–949, 2018. doi: 10.1016/j.bpj.2018.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res 86: 94–100, 2000. doi: 10.1161/01.res.86.1.94. [DOI] [PubMed] [Google Scholar]
- 81. Behringer EJ, Scallan JP, Jafarnejad M, Castorena-Gonzalez JA, Zawieja SD, Moore JE Jr, Davis MJ, Segal SS. Calcium and electrical dynamics in lymphatic endothelium. J Physiol 595: 7347–7368, 2017. doi: 10.1113/JP274842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zawieja SD, Castorena-Gonzalez JA, Scallan JP, Davis MJ. Differences in L-type Ca2+ channel activity partially underlie the regional dichotomy in pumping behavior by murine peripheral and visceral lymphatic vessels. Am J Physiol Heart Circ Physiol 314: H991–H1010, 2018. doi: 10.1152/ajpheart.00499.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Matsuo M, Tanabe K, Kioka N, Amachi T, Ueda K. Different binding properties and affinities for ATP and ADP among sulfonylurea receptor subtypes, SUR1, SUR2A, and SUR2B. J Biol Chem 275: 28757–28763, 2000. doi: 10.1074/jbc.M004818200. [DOI] [PubMed] [Google Scholar]
- 84. Iguchi K, Saotome M, Yamashita K, Hasan P, Sasaki M, Maekawa Y, Watanabe Y. Pinacidil, a KATP channel opener, stimulates cardiac Na+/Ca2+ exchanger function through the NO/cGMP/PKG signaling pathway in guinea pig cardiac ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol 392: 949–959, 2019. doi: 10.1007/s00210-019-01642-1. [DOI] [PubMed] [Google Scholar]
- 85. Reslerova M, Loutzenhiser R. Divergent mechanisms of ATP-sensitive K+ channel-induced vasodilation in renal afferent and efferent arterioles. Circ Res 77: 1114–1120, 1995. doi: 10.1161/01.res.77.6.1114. [DOI] [PubMed] [Google Scholar]
- 86. Rusko J, Tanzi F, van Breemen C, Adams DJ. Calcium-activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2+ sensitivity and block. J Physiol 455: 601–621, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lee SY, Lee CO. Inhibition of Na+-K+ pump and L-type Ca2+ channel by glibenclamide in Guinea pig ventricular myocytes. J Pharmacol Exp Ther 312: 61–68, 2005. doi: 10.1124/jpet.104.074369. [DOI] [PubMed] [Google Scholar]
- 88. Bian K, Hermsmeyer K. Glyburide actions on the dihydropyridine-sensitive Ca2+ channel in rat vascular muscle. J Vasc Res 31: 256–264, 1994. doi: 10.1159/000159051. [DOI] [PubMed] [Google Scholar]
- 89. Sadraei H, Beech DJ. Ionic currents and inhibitory effects of glibenclamide in seminal vesicle smooth muscle cells. Br J Pharmacol 115: 1447–1454, 1995. doi: 10.1111/j.1476-5381.1995.tb16636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schultz BD, DeRoos AD, Venglarik CJ, Singh AK, Frizzell RA, Bridges RJ. Glibenclamide blockade of CFTR chloride channels. Am J Physiol Lung Cell Mol Physiol 271: L192–L200, 1996. doi: 10.1152/ajplung.1996.271.2.L192. [DOI] [PubMed] [Google Scholar]
- 91. Faivre JF, Rouanet S, Bril A. Comparative effects of glibenclamide, tedisamil, dofetilide, E-4031, and BRL-32872 on protein kinase A-activated chloride current in guinea pig ventricular myocytes. J Cardiovasc Pharmacol 31: 551–557, 1998. doi: 10.1097/00005344-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 92. Golub AS, Song BK, Pittman RN. The rate of O2 loss from mesenteric arterioles is not unusually high. Am J Physiol Heart Circ Physiol 301: H737–H745, 2011. doi: 10.1152/ajpheart.00353.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Torres Filho IP, Kerger H, Intaglietta M. pO2 measurements in arteriolar networks. Microvasc Res 51: 202–212, 1996. doi: 10.1006/mvre.1996.0021. [DOI] [PubMed] [Google Scholar]
- 94. Hangai-Hoger N, Tsai AG, Cabrales P, Intaglietta M. Terminal lymphatics: the potential "lethal corner" in the distribution of tissue pO2. Lymphat Res Biol 5: 159–168, 2007. doi: 10.1089/lrb.2007.5303. [DOI] [PubMed] [Google Scholar]
- 95. Barankay T, Baumgartl H, Lubbers DW, Seidl E. Oxygen pressure in small lymphatics. Pflugers Arch 366: 53–59, 1976. doi: 10.1007/BF02486560. [DOI] [PubMed] [Google Scholar]
- 96. Gladysheva NA. [Effect of hypoxia on the contractile activity of smooth muscle cells of the thoracic lymphatic duct]. Fiziol Zh SSSR Im I M Sechenova 65: 1520–1526, 1979. [PubMed] [Google Scholar]
- 97. Lombard JH, Liu YP, Fredricks KT, Bizub DM, Roman RJ, Rusch NJ. Electrical and mechanical responses of rat middle cerebral arteries to reduced Po2 and prostacyclin. Am J Physiol Heart Circ Physiol 276: H509–H516, 1999. doi: 10.1152/ajpheart.1999.276.2.H509. [DOI] [PubMed] [Google Scholar]
- 98. Shibata M, Ichioka S, Ando J, Kamiya A. Microvascular and interstitial PO2 measurements in rat skeletal muscle by phosphorescence quenching. J Appl Physiol (1985) 91: 321–327, 2001. doi: 10.1152/jappl.2001.91.1.321. [DOI] [PubMed] [Google Scholar]
- 99. Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540: 1023–1037, 2002. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation 11: 477–492, 2004. doi: 10.1080/10739680490476033. [DOI] [PubMed] [Google Scholar]
- 101. Gasheva OY, Knippa K, Nepiushchikh ZV, Muthuchamy M, Gashev AA. Age-related alterations of active pumping mechanisms in rat thoracic duct. Microcirculation 14: 827–839, 2007. doi: 10.1080/10739680701444065. [DOI] [PubMed] [Google Scholar]
- 102. Nagai T, Bridenbaugh EA, Gashev AA. Aging-associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation 18: 463–473, 2011. doi: 10.1111/j.1549-8719.2011.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tsunemoto H, Ikomi F, Ohhashi T. Flow-mediated release of nitric oxide from lymphatic endothelial cells of pressurized canine thoracic duct. Jpn J Physiol 53: 157–163, 2003. doi: 10.2170/jjphysiol.53.157. [DOI] [PubMed] [Google Scholar]
- 104. Nizamutdinova IT, Maejima D, Nagai T, Bridenbaugh E, Thangaswamy S, Chatterjee V, Meininger CJ, Gashev AA. Involvement of histamine in endothelium-dependent relaxation of mesenteric lymphatic vessels. Microcirculation 21: 640–648, 2014. doi: 10.1111/micc.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pal S, Gasheva OY, Zawieja DC, Meininger CJ, Gashev AA. Histamine-mediated autocrine signaling in mesenteric perilymphatic mast cells. Am J Physiol Regul Integr Comp Physiol 318: R590–R604, 2020. doi: 10.1152/ajpregu.00255.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bohlen HG, Gasheva OY, Zawieja DC. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am J Physiol Heart Circ Physiol 301: H1897–H1906, 2011. doi: 10.1152/ajpheart.00260.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bohlen HG, Wang W, Gashev A, Gasheva O, Zawieja D. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol 297: H1319–H1328, 2009. doi: 10.1152/ajpheart.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol 575: 821–832, 2006. doi: 10.1113/jphysiol.2006.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol Regul Integr Comp Physiol 274: R790–R796, 1998. doi: 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- 110. Scallan JP, Davis MJ. Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J Physiol 591: 2139–2156, 2013. doi: 10.1113/jphysiol.2012.250662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. SoRelle R. Nobel prize awarded to scientists for nitric oxide discoveries. Circulation 98: 2365–2366, 1998. doi: 10.1161/01.cir.98.22.2365. [DOI] [PubMed] [Google Scholar]
- 112. Gasheva OY, Gashev AA, Zawieja DC. Cyclic guanosine monophosphate and the dependent protein kinase regulate lymphatic contractility in rat thoracic duct. J Physiol 591: 4549–4565, 2013. doi: 10.1113/jphysiol.2013.258681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Keef KD, Hume JR, Zhong J. Regulation of cardiac and smooth muscle Ca2+ channels (CaV1.2a,b) by protein kinases. Am J Physiol Cell Physiol 281: C1743–C1756, 2001. doi: 10.1152/ajpcell.2001.281.6.C1743. [DOI] [PubMed] [Google Scholar]
- 114. Ono K, Trautwein W. Potentiation by cyclic GMP of beta-adrenergic effect on Ca2+ current in guinea-pig ventricular cells. J Physiol 443: 387–404, 1991. doi: 10.1113/jphysiol.1991.sp018839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Scallan JP, Hill MA, Davis MJ. Lymphatic vascular integrity is disrupted in type 2 diabetes due to impaired nitric oxide signalling. Cardiovasc Res 107: 89–97, 2015. doi: 10.1093/cvr/cvv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lochner A, Moolman JA. The many faces of H89: a review. Cardiovasc Drug Rev 24: 261–274, 2006. doi: 10.1111/j.1527-3466.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 117. Scallan JP, Bouta EM, Rahimi H, Kenney HM, Ritchlin CT, Davis MJ, Schwarz EM. Ex vivo demonstration of functional deficiencies in popliteal lymphatic vessels from TNF-transgenic mice with inflammatory arthritis. Front Physiol 12: 745096, 2021. doi: 10.3389/fphys.2021.745096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, Ruddle NH, Jain RK, Fukumura D, Padera TP. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci USA 108: 18784–18789, 2011. doi: 10.1073/pnas.1116152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chen Y, Rehal S, Roizes S, Zhu HL, Cole WC, von der Weid PY. The pro-inflammatory cytokine TNF-α inhibits lymphatic pumping via activation of the NF-κB-iNOS signaling pathway. Microcirculation 24: e12364, 2017. doi: 10.1111/micc.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Torrisi JS, Hespe GE, Cuzzone DA, Savetsky IL, Nitti MD, Gardenier JC, Garcia Nores GD, Jowhar D, Kataru RP, Mehrara BJ. Inhibition of inflammation and iNOS improves lymphatic function in obesity. Sci Rep 6: 19817, 2016. doi: 10.1038/srep19817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rehal S, von der Weid PY. Experimental ileitis alters prostaglandin biosynthesis in mesenteric lymphatic and blood vessels. Prostaglandins Other Lipid Mediat 116–117: 37–48, 2015. doi: 10.1016/j.prostaglandins.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 122. Hanley CA, Elias RM, Movat HZ, Johnston MG. Suppression of fluid pumping in isolated bovine mesenteric lymphatics by interleukin-1: interaction with prostaglandin E2. Microvasc Res 37: 218–229, 1989. doi: 10.1016/0026-2862(89)90039-3. [DOI] [PubMed] [Google Scholar]
- 123. Wu TF, Carati CJ, Macnaughton WK, von der Weid PY. Contractile activity of lymphatic vessels is altered in the TNBS model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol 291: G566–G574, 2006. doi: 10.1152/ajpgi.00058.2006. [DOI] [PubMed] [Google Scholar]
- 124. Quinn KV, Giblin JP, Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ Res 94: 1359–1366, 2004. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- 125. Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, Rojas A, Ha BT, Jiang C. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by β-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol 293: R1205–R1214, 2007. doi: 10.1152/ajpregu.00337.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shi Y, Cui N, Shi W, Jiang C. A short motif in Kir6.1 consisting of four phosphorylation repeats underlies the vascular KATP channel inhibition by protein kinase C. J Biol Chem 283: 2488–2494, 2008. doi: 10.1074/jbc.M708769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Han J, Kim N, Joo H, Kim E, Earm YE. ATP-sensitive K(+) channel activation by nitric oxide and protein kinase G in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol 283: H1545–H1554, 2002. doi: 10.1152/ajpheart.01052.2001. [DOI] [PubMed] [Google Scholar]
- 128. Han J, Kim N, Kim E, Ho WK, Earm YE. Modulation of ATP-sensitive potassium channels by cGMP-dependent protein kinase in rabbit ventricular myocytes. J Biol Chem 276: 22140–22147, 2001. doi: 10.1074/jbc.M010103200. [DOI] [PubMed] [Google Scholar]
- 129. Ali S, Solano AS, Gonzales AL, Thakore P, Krishnan V, Yamasaki E, Earley S. Nitric oxide signals through IRAG to inhibit TRPM4 channels and dilate cerebral arteries. Function (Oxf) 2: zqab051, 2021. doi: 10.1093/function/zqab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Means CK, Lygren B, Langeberg LK, Jain A, Dixon RE, Vega AL, Gold MG, Petrosyan S, Taylor SS, Murphy AN, Ha T, Santana LF, Tasken K, Scott JD. An entirely specific type I A-kinase anchoring protein that can sequester two molecules of protein kinase A at mitochondria. Proc Natl Acad Sci USA 108: E1227–E1235, 2011. doi: 10.1073/pnas.1107182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Smith FD, Omar MH, Nygren PJ, Soughayer J, Hoshi N, Lau HT, Snyder CG, Branon TC, Ghosh D, Langeberg LK, Ting AY, Santana LF, Ong SE, Navedo MF, Scott JD. Single nucleotide polymorphisms alter kinase anchoring and the subcellular targeting of A-kinase anchoring proteins. Proc Natl Acad Sci USA 115: E11465–E11474, 2018. doi: 10.1073/pnas.1816614115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Nystoriak MA, Nieves-Cintron M, Nygren PJ, Hinke SA, Nichols CB, Chen CY, Puglisi JL, Izu LT, Bers DM, Dell'acqua ML, Scott JD, Santana LF, Navedo MF. AKAP150 contributes to enhanced vascular tone by facilitating large-conductance Ca2+-activated K+ channel remodeling in hyperglycemia and diabetes mellitus. Circ Res 114: 607–615, 2014. doi: 10.1161/CIRCRESAHA.114.302168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cantú JM, Garcia-Cruz D, Sánchez-Corona J, Hernández A, Nazará Z. A distinct osteochondrodysplasia with hypertrichosis—individualization of a probable autosomal recessive entity. Hum Genet 60: 36–41, 1982. doi: 10.1007/BF00281261. [DOI] [PubMed] [Google Scholar]
- 134. Grange DK, Lorch SM, Cole PL, Singh GK. Cantu syndrome in a woman and her two daughters: Further confirmation of autosomal dominant inheritance and review of the cardiac manifestations. Am J Med Genet A 140: 1673–1680, 2006. doi: 10.1002/ajmg.a.31348. [DOI] [PubMed] [Google Scholar]
- 135. Grange DK, Roessler HI, McClenaghan C, Duran K, Shields K, Remedi MS, Knoers N, Lee JM, Kirk EP, Scurr I, Smithson SF, Singh GK, van Haelst MM, Nichols CG, van Haaften G. Cantu syndrome: findings from 74 patients in the International Cantu Syndrome Registry. Am J Med Genet C Semin Med Genet 181: 658–681, 2019. doi: 10.1002/ajmg.c.31753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Nichols CG, Singh GK, Grange DK. KATP channels and cardiovascular disease: suddenly a syndrome. Circ Res 112: 1059–1072, 2013. doi: 10.1161/CIRCRESAHA.112.300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Grange DK, Nichols CG, Singh GK. Cantu syndrome and related disorders. In: GeneReviews, edited by Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A.. Seattle, WA: University of Washington, 1993. [PubMed] [Google Scholar]
- 138. Levin MD, Singh GK, Zhang HX, Uchida K, Kozel BA, Stein PK, Kovacs A, Westenbroek RE, Catterall WA, Grange DK, Nichols CG. KATP channel gain-of-function leads to increased myocardial L-type Ca2+ current and contractility in Cantu syndrome. Proc Natl Acad Sci USA 113: 6773–6778, 2016. doi: 10.1073/pnas.1606465113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Garcia-Cruz D, Mampel A, Echeverria MI, Vargas AL, Castaneda-Cisneros G, Davalos-Rodriguez N, Patino-Garcia B, Garcia-Cruz MO, Castaneda V, Cardona EG, Marin-Solis B, Cantu JM, Nunez-Reveles N, Moran-Moguel C, Thavanati PK, Ramirez-Garcia S, Sanchez-Corona J. Cantu syndrome and lymphoedema. Clin Dysmorphol 20: 32–37, 2011. doi: 10.1097/MCD.0b013e32833d015c. [DOI] [PubMed] [Google Scholar]
- 140. Ma A, Gurnasinghani S, Kirk EP, McClenaghan C, Singh GK, Grange DK, Pandit C, Zhu Y, Roscioli T, Elakis G, Buckley M, Mehta B, Roberts P, Mervis J, Biggin A, Nichols CG. Glibenclamide treatment in a Cantu syndrome patient with a pathogenic ABCC9 gain-of-function variant: initial experience. Am J Med Genet A 179: 1585–1590, 2019. doi: 10.1002/ajmg.a.61200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Louveau A, Da Mesquita S, Kipnis J. Lymphatics in neurological disorders: a neuro-lympho-vascular component of multiple sclerosis and Alzheimer’s disease? Neuron 91: 957–973, 2016. doi: 10.1016/j.neuron.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, Viar KE, Powell RD, Baker W, Dabhi N, Bai R, Cao R, Hu S, Rich SS, Munson JM, Lopes MB, Overall CC, Acton ST, Kipnis J. Publisher correction: functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 564: E7, 2018. doi: 10.1038/s41586-018-0689-7. [DOI] [PubMed] [Google Scholar]
- 143. Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science 370: 50–56, 2020. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol 17: 1016–1024, 2018. doi: 10.1016/S1474-4422(18)30318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Cooper PE, Reutter H, Woelfle J, Engels H, Grange DK, van Haaften G, van Bon BW, Hoischen A, Nichols CG. Cantu syndrome resulting from activating mutation in the KCNJ8 gene. Hum Mutat 35: 809–813, 2014. doi: 10.1002/humu.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Harakalova M, van Harssel JJ, Terhal PA, van Lieshout S, Duran K, Renkens I, Amor DJ, Wilson LC, Kirk EP, Turner CL, Shears D, Garcia-Minaur S, Lees MM, Ross A, Venselaar H, Vriend G, Takanari H, Rook MB, van der Heyden MA, Asselbergs FW, Breur HM, Swinkels ME, Scurr IJ, Smithson SF, Knoers NV, van der Smagt JJ, Nijman IJ, Kloosterman WP, van Haelst MM, van Haaften G, Cuppen E. Dominant missense mutations in ABCC9 cause Cantu syndrome. Nat Genet 44: 793–796, 2012. doi: 10.1038/ng.2324. [DOI] [PubMed] [Google Scholar]
- 147. Huang Y, McClenaghan C, Harter TM, Hinman K, Halabi CM, Matkovich SJ, Zhang H, Brown GS, Mecham RP, England SK, Kovacs A, Remedi MS, Nichols CG. Cardiovascular consequences of KATP overactivity in Cantu syndrome. JCI Insight 3: e121153, 2018. doi: 10.1172/jci.insight.121153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. McClenaghan C, Hanson A, Sala-Rabanal M, Roessler HI, Josifova D, Grange DK, van Haaften G, Nichols CG. Cantu syndrome-associated SUR2 (ABCC9) mutations in distinct structural domains result in KATP channel gain-of-function by differential mechanisms. J Biol Chem 293: 2041–2052, 2018. doi: 10.1074/jbc.RA117.000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Li A, Knutsen RH, Zhang H, Osei-Owusu P, Moreno-Dominguez A, Harter TM, Uchida K, Remedi MS, Dietrich HH, Bernal-Mizrachi C, Blumer KJ, Mecham RP, Koster JC, Nichols CG. Hypotension due to Kir6.1 gain-of-function in vascular smooth muscle. J Am Heart Assoc 2: e000365, 2013. doi: 10.1161/JAHA.113.000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. McClenaghan C, Huang Y, Matkovich SJ, Kovacs A, Weinheimer CJ, Perez R, Broekelmann TJ, Harter TM, Lee JM, Remedi MS, Nichols CG. The mechanism of high-output cardiac hypertrophy arising from potassium channel gain-of-function in cantu syndrome. Function (Oxf) 1: zqaa004, 2020. doi: 10.1093/function/zqaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. McClenaghan C, Huang Y, Yan Z, Harter TM, Halabi CM, Chalk R, Kovacs A, van Haaften G, Remedi MS, Nichols CG. Glibenclamide reverses cardiovascular abnormalities of Cantu syndrome driven by KATP channel overactivity. J Clin Invest 130: 1116–1121, 2020. doi: 10.1172/JCI130571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Greene AK, Grant FD, Slavin SA. Lower-extremity lymphedema and elevated body-mass index. N Engl J Med 366: 2136–2137, 2012. doi: 10.1056/NEJMc1201684. [DOI] [PubMed] [Google Scholar]
- 153. Sudduth CL, Greene AK. Current overview of obesity-induced lymphedema. Adv Wound Care (New Rochelle) 11: 392–398, 2022. doi: 10.1089/wound.2020.1337. [DOI] [PubMed] [Google Scholar]
- 154. Szuba A, Rockson SG. Lymphedema: classification, diagnosis and therapy. Vasc Med 3: 145–156, 1998. doi: 10.1177/1358836X9800300209. [DOI] [PubMed] [Google Scholar]
- 155. Stanton AWB, Modi S, Britton TMB, Purushotham AD, Peters AM, Levick JR, Mortimer PS. Lymphatic drainage in the muscle and subcutis of the arm after breast cancer treatment. Breast Cancer Res Treat 117: 549–557, 2009. doi: 10.1007/s10549-008-0259-z. [DOI] [PubMed] [Google Scholar]
- 156. Stanton AW, Modi S, Mellor RH, Levick JR, Mortimer PS. Recent advances in breast cancer-related lymphedema of the arm: lymphatic pump failure and predisposing factors. Lymphat Res Biol 7: 29–45, 2009. doi: 10.1089/lrb.2008.1026. [DOI] [PubMed] [Google Scholar]
- 157. Modi S, Stanton AW, Svensson WE, Peters AM, Mortimer PS, Levick JR. Human lymphatic pumping measured in healthy and lympoedematous arms by lymphatic congestion lymphoscintography. J Physiol 583: 271–285, 2007. doi: 10.1113/jphysiol.2007.130401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Olszewski WL. Pathophysiological and clinical observations of obstructive lymphedema of the limbs. In: Lymphedema, edited by Clodius L. Stuttgart: Georg Thieme Publishers, 1977, p. 79–102. [Google Scholar]
- 159. Rockson SG. Secondary lymphedema: is it a primary disease? Lymphat Res Biol 6: 63–64, 2008. doi: 10.1089/lrb.2008.6201. [DOI] [PubMed] [Google Scholar]
- 160. Rockson SG, Rivera KK. Estimating the population burden of lymphedema. Ann N Y Acad Sci 1131: 147–154, 2008. doi: 10.1196/annals.1413.014. [DOI] [PubMed] [Google Scholar]
- 161. Olszewski WL. Contractility patterns of normal and pathologically changed human lymphatics. Ann N Y Acad Sci 979: 52–63, 2002. doi: 10.1111/j.1749-6632.2002.tb04867.x. [DOI] [PubMed] [Google Scholar]
- 162. Gardenier JC, Kataru RP, Hespe GE, Savetsky IL, Torrisi JS, Nores GD, Jowhar DK, Nitti MD, Schofield RC, Carlow DC, Mehrara BJ. Topical tacrolimus for the treatment of secondary lymphedema. Nat Commun 8: 14345, 2017. doi: 10.1038/ncomms14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Gousopoulos E, Proulx ST, Bachmann SB, Scholl J, Dionyssiou D, Demiri E, Halin C, Dieterich LC, Detmar M. Regulatory T cell transfer ameliorates lymphedema and promotes lymphatic vessel function. JCI Insight 1: e89081, 2016. doi: 10.1172/jci.insight.89081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Tian W, Rockson SG, Jiang X, Kim J, Begaye A, Shuffle EM, Tu AB, Cribb M, Nepiyushchikh Z, Feroze AH, Zamanian RT, Dhillon GS, Voelkel NF, Peters-Golden M, Kitajewski J, Dixon JB, Nicolls MR. Leukotriene B4 antagonism ameliorates experimental lymphedema. Sci Transl Med 9: eaal3920, 2017. doi: 10.1126/scitranslmed.aal3920. [DOI] [PubMed] [Google Scholar]
- 165. Norden PR, Kume T. The role of lymphatic vascular function in metabolic disorders. Front Physiol 11: 404, 2020. doi: 10.3389/fphys.2020.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Lee Y, Zawieja SD, Muthuchamy M. Lymphatic collecting vessel: new perspectives on mechanisms of contractile regulation and potential lymphatic contractile pathways to target in obesity and metabolic diseases. Front Pharmacol 13: 848088, 2022. doi: 10.3389/fphar.2022.848088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Escobedo N, Oliver G. The lymphatic vasculature: its role in adipose metabolism and obesity. Cell Metab 26: 598–609, 2017. doi: 10.1016/j.cmet.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Antoniak K, Hansdorfer-Korzon R, Mrugacz M, Zorena K. Adipose tissue and biological factors. Possible link between lymphatic system dysfunction and obesity. Metabolites 11: 617, 2021. doi: 10.3390/metabo11090617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kataru RP, Park HJ, Baik JE, Li C, Shin J, Mehrara BJ. Regulation of lymphatic function in obesity. Front Physiol 11: 459, 2020. doi: 10.3389/fphys.2020.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Jiang X, Tian W, Nicolls MR, Rockson SG. The lymphatic system in obesity, insulin resistance, and cardiovascular diseases. Front Physiol 10: 1402, 2019. doi: 10.3389/fphys.2019.01402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Chakraborty S, Davis MJ, Muthuchamy M. Emerging trends in the pathophysiology of lymphatic contractile function. Semin Cell Dev Biol 38: 55–66, 2015. doi: 10.1016/j.semcdb.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn 225: 351–357, 2002. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- 173. Horra A, Salazar J, Ferre R, Vallve JC, Guardiola M, Rosales R, Masana L, Ribalta J. Prox-1 and FOXC2 gene expression in adipose tissue: A potential contributory role of the lymphatic system to familial combined hyperlipidaemia. Atherosclerosis 206: 343–345, 2009. doi: 10.1016/j.atherosclerosis.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 174. Kim HJ, Yoo YJ, Ju YS, Lee S, Cho SI, Sung J, Kim JI, Seo JS. Combined linkage and association analyses identify a novel locus for obesity near PROX1 in Asians. Obesity (Silver Spring) 21: 2405–2412, 2013. doi: 10.1002/oby.20153. [DOI] [PubMed] [Google Scholar]
- 175. Kretowski A, Adamska E, Maliszewska K, Wawrusiewicz-Kurylonek N, Citko A, Goscik J, Bauer W, Wilk J, Golonko A, Waszczeniuk M, Lipinska D, Hryniewicka J, Niemira M, Paczkowska M, Ciborowski M, Gorska M. The rs340874 PROX1 type 2 diabetes mellitus risk variant is associated with visceral fat accumulation and alterations in postprandial glucose and lipid metabolism. Genes Nutr 10: 4, 2015. doi: 10.1007/s12263-015-0454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Adamska-Patruno E, Godzien J, Ciborowski M, Samczuk P, Bauer W, Siewko K, Gorska M, Barbas C, Kretowski A. The type 2 diabetes susceptibility PROX1 gene variants are associated with postprandial plasma metabolites profile in non-diabetic men. Nutrients 11: 882, 2019. doi: 10.3390/nu11040882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell 98: 769–778, 1999. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 178. Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet 37: 1072–1081, 2005. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 179. Escobedo N, Proulx ST, Karaman S, Dillard ME, Johnson N, Detmar M, Oliver G. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight 1: e85096, 2016. doi: 10.1172/jci.insight.85096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, Yla-Herttuala S, Alitalo K. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med 7: 199–205, 2001. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 181. Irrthum A, Karkkainen MJ, Devriendt K, Alitalo K, Vikkula M. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am J Hum Genet 67: 295–301, 2000. doi: 10.1086/303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer PS, Yla-Herttuala S, Miura N, Alitalo K. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med 10: 974–981, 2004. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 183. Sabine A, Agalarov Y, Hajjami HM-E, Jaquet M, Hagerling R, Pollmann C, Bebber D, Pfenniger A, Miura N, Dormond O, Calmes J-M, Adams RH, Makinen T, Kiefer F, Kwak B, Petrova TV. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell 22: 430–416, 2012. doi: 10.1016/j.devcel.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 184. Sabine A, Bovay E, Demir CS, Kimura W, Jaquet M, Agalarov Y, Zangger N, Scallan JP, Graber W, Gulpinar E, Kwak BR, Makinen T, Martinez-Corral I, Ortega S, Delorenzi M, Kiefer F, Davis MJ, Djonov V, Miura N, Petrova TV. FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J Clin Invest 125: 3861–3877, 2015. doi: 10.1172/JCI80454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Rutkowski JM, Markhus CE, Gyenge CC, Alitalo K, Wiig H, Swartz MA. Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am J Pathol 176: 1122–1129, 2010. doi: 10.2353/ajpath.2010.090733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Wu H, Rahman HNA, Dong Y, Liu X, Lee Y, Wen A, To KH, Xiao L, Birsner AE, Bazinet L, Wong S, Song K, Brophy ML, Mahamud MR, Chang B, Cai X, Pasula S, Kwak S, Yang W, Bischoff J, Xu J, Bielenberg DR, Dixon JB, D'Amato RJ, Srinivasan RS, Chen H. Epsin deficiency promotes lymphangiogenesis through regulation of VEGFR3 degradation in diabetes. J Clin Invest 128: 4025–4043, 2018. doi: 10.1172/JCI96063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Karaman S, Hollmen M, Robciuc MR, Alitalo A, Nurmi H, Morf B, Buschle D, Alkan HF, Ochsenbein AM, Alitalo K, Wolfrum C, Detmar M. Blockade of VEGF-C and VEGF-D modulates adipose tissue inflammation and improves metabolic parameters under high-fat diet. Mol Metab 4: 93–105, 2015. doi: 10.1016/j.molmet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 106: 563–573, 2001. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 190. Cifarelli V, Eichmann A. The intestinal lymphatic system: functions and metabolic implications. Cell Mol Gastroenterol Hepatol 7: 503–513, 2019. doi: 10.1016/j.jcmgh.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Petrova TV, Koh GY. Biological functions of lymphatic vessels. Science 369: eaax4063, 2020. doi: 10.1126/science.aax4063. [DOI] [PubMed] [Google Scholar]
- 192. Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, Taylor HA. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab 95: 5419–5426, 2010. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Cao E, Watt MJ, Nowell CJ, Quach T, Simpson JS, De Melo Ferreira V, Agarwal S, Chu H, Srivastava A, Anderson D, Gracia G, Lam A, Segal G, Hong J, Hu L, Phang KL, Escott ABJ, Windsor JA, Phillips ARJ, Creek DJ, Harvey NL, Porter CJH, Trevaskis NL. Mesenteric lymphatic dysfunction promotes insulin resistance and represents a potential treatment target in obesity. Nat Metab 3: 1175–1188, 2021. doi: 10.1038/s42255-021-00457-w. [DOI] [PubMed] [Google Scholar]
- 194. Sawane M, Kajiya K, Kidoya H, Takagi M, Muramatsu F, Takakura N. Apelin inhibits diet-induced obesity by enhancing lymphatic and blood vessel integrity. Diabetes 62: 1970–1980, 2013. doi: 10.2337/db12-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Zhang F, Zarkada G, Han J, Li J, Dubrac A, Ola R, Genet G, Boye K, Michon P, Kunzel SE, Camporez JP, Singh AK, Fong GH, Simons M, Tso P, Fernandez-Hernando C, Shulman GI, Sessa WC, Eichmann A. Lacteal junction zippering protects against diet-induced obesity. Science 361: 599–603, 2018. doi: 10.1126/science.aap9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Westcott GP, Rosen ED. Crosstalk between adipose and lymphatics in health and disease. Endocrinology 163: bqab224, 2022. doi: 10.1210/endocr/bqab224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Weitman ES, Aschen SZ, Farias-Eisner G, Albano N, Cuzzone DA, Ghanta S, Zampell JC, Thorek D, Mehrara BJ. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One 8: e70703, 2013. doi: 10.1371/journal.pone.0070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Arngrim N, Simonsen L, Holst JJ, Bulow J. Reduced adipose tissue lymphatic drainage of macromolecules in obese subjects: a possible link between obesity and local tissue inflammation? Int J Obes (Lond) 37: 748–750, 2013. doi: 10.1038/ijo.2012.98. [DOI] [PubMed] [Google Scholar]
- 199. Blum KS, Karaman S, Proulx ST, Ochsenbein AM, Luciani P, Leroux JC, Wolfrum C, Detmar M. Chronic high-fat diet impairs collecting lymphatic vessel function in mice. PLoS One 9: e94713, 2014. doi: 10.1371/journal.pone.0094713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Castorena-Gonzalez J. Lymphatic valve dysfunction in western diet-fed mice: new insights into obesity-induced lymphedema. Front Pharmacol 13: 823266, 2022. doi: 10.3389/fphar.2022.823266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Zawieja SD, Gasheva O, Zawieja DC, Muthuchamy M. Blunted flow-mediated responses and diminished nitric oxide synthase expression in lymphatic thoracic ducts of a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol 310: H385–H393, 2016. doi: 10.1152/ajpheart.00664.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Nitti MD, Hespe GE, Kataru RP, Garcia Nores GD, Savetsky IL, Torrisi JS, Gardenier JC, Dannenberg AJ, Mehrara BJ. Obesity-induced lymphatic dysfunction is reversible with weight loss. J Physiol 594: 7073–7087, 2016. doi: 10.1113/JP273061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Garcia Nores GD, Cuzzone DA, Albano NJ, Hespe GE, Kataru RP, Torrisi JS, Gardenier JC, Savetsky IL, Aschen SZ, Nitti MD, Mehrara BJ. Obesity but not high-fat diet impairs lymphatic function. Int J Obes (Lond) 40: 1582–1590, 2016. doi: 10.1038/ijo.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Karaman S, Buschle D, Luciani P, Leroux JC, Detmar M, Proulx ST. Decline of lymphatic vessel density and function in murine skin during aging. Angiogenesis 18: 489–498, 2015. doi: 10.1007/s10456-015-9479-0. [DOI] [PubMed] [Google Scholar]
- 205. Liu Y, Gutterman DD. The coronary circulation in diabetes: influence of reactive oxygen species on K+ channel-mediated vasodilation. Vascul Pharmacol 38: 43–49, 2002. doi: 10.1016/s1537-1891(02)00125-8. [DOI] [PubMed] [Google Scholar]
- 206. Avshalumov MV, Bao L, Patel JC, Rice ME. H2O2 signaling in the nigrostriatal dopamine pathway via ATP-sensitive potassium channels: issues and answers. Antioxid Redox Signal 9: 219–231, 2007. doi: 10.1089/ars.2007.9.219. [DOI] [PubMed] [Google Scholar]
- 207. Ertracht O, Malka A, Atar S, Binah O. The mitochondria as a target for cardioprotection in acute myocardial ischemia. Pharmacol Ther 142: 33–40, 2014. doi: 10.1016/j.pharmthera.2013.11.003. [DOI] [PubMed] [Google Scholar]