Keywords: alcohol-associated liver disease, autophagy, ethanol, mTOR, transcription factor EB
Abstract
Mechanistic target of rapamycin (mTOR) is a serine-threonine kinase and a cellular sensor for nutrient and energy status, which is critical in regulating cell metabolism and growth by governing the anabolic (protein and lipid synthesis) and catabolic process (autophagy). Alcohol-associated liver disease (ALD) is a major chronic liver disease worldwide that carries a huge financial burden. The spectrum of the pathogenesis of ALD includes steatosis, fibrosis, inflammation, ductular reaction, and eventual hepatocellular carcinoma, which is closely associated with metabolic changes that are regulated by mTOR. In this review, we summarized recent progress of alcohol consumption on the changes of mTORC1 and mTORC2 activity, the potential mechanisms and possible impact of the mTORC1 changes on autophagy in ALD. We also discussed the potential beneficial effects and limitations of targeting mTORC1 against ALD.
INTRODUCTION
Among the chronic liver diseases, alcohol-associated liver disease (ALD) has become a major financial burden and a leading cause of liver transplantation (1, 2). Owing to the COVID-19 pandemic that led to further increased alcohol consumption, ALD-related liver transplantation and mortality are increased more significantly (3, 4). As the pandemic and increased alcohol consumption continue, the impact of ALD on health financial burden and death will be substantially increased in the near future.
The pathogenesis of ALD has been well characterized and includes a spectrum of liver changes ranging from simple steatosis to cell death, inflammation, and fibrosis with some of them eventually progressing to cirrhosis and hepatocellular carcinoma (HCC) (5, 6). Other histological and pathophysiological features of ALD include the formation of Mallory-Denk bodies, accumulation of megamitochondria, ductular reaction, and cholestasis (7–11).
Alcoholic hepatitis (AH) is an acute and severe episode in patients that have a chronic excess alcohol use history and is characterized by the rapid onset of jaundice with a high short-term mortality. Only 30% of patients with AH can survive less than 1 mo and 50% at 6 mo (12). One evident pathophysiological feature of AH is the dynamic cell-type remodeling in the liver. Increased hepatocyte damage and loss of hepatocyte identity as well as increased infiltrated immune cells such as neutrophils, accumulation of liver progenitor cells or ductular cells, and rapid progression of fibrosis in AH livers have been well documented, which may ultimately lead to liver failure in patients with AH (11, 13, 14).
Multiple mechanisms have been implicated for the pathogenesis of ALD, which include increased oxidative stress via cytochrome P450 2E1 (CYP2E1)-mediated alcohol metabolism, mitochondrial dysfunction, increased apoptotic and nonapoptotic hepatocyte death (such as necroptosis and pyroptosis), endoplasmic reticulum (ER) stress, impaired autophagy, and proteasome-mediated proteostasis and suppression of RNA-splicing factor epithelial splicing regulatory protein 2 (ESRP2) (15–19). In addition, ALD can be considered a systemic disease as alcohol consumption also increases intestinal permeability and induces changes in gut microbiota, alters innate immunity as well as increases adipose lipolysis and inflammation (20–25), all of which can contribute to the pathogenesis of ALD. Corticosteroids are currently the first-line treatment for AH but have only marginal improvement of short-term survival with risk of bacterial and fungi infections. Other previously proposed treatments such as anti-TNFα reagents infliximab and etanercept, and antioxidants vitamin E and N-acetylcysteine have been ineffective in treating patients with AH (26–30). Owing to the progress of mechanistic studies on ALD, several clinical trials for treating AH are ongoing (31). The first approach is to target gut microbiota using Lactobacillus rhamnosus GG, antibiotic augmentin (amoxicillin plus clavulanic acid), fecal transplantation, and purified hyperimmune bovine colostrum (IMM-124E) IgG antibodies against LPS. The second approach is to target inflammation using farnesoid X receptor (FXR) agonist (obeticholic acid), IL-1 receptor antagonist (anakinra), pan caspase inhibitor (Emricasan), IL-22, and ASK-1 inhibitor [Selonsertib (GS-4997)]. The third approach is to target liver regeneration using growth factors such as granulocyte colony-stimulating factor (G-CSF) (32). The fourth approach is to target oxidative stress using antioxidants such as N-acetylcysteine (NAC) and metadoxine (33, 34). We look forward to seeing the outcomes of these ongoing clinical trials in the near future.
MECHANISMS REGULATING mTOR
Mechanistic target of rapamycin (mTOR) is a serine/threonine kinase, which is critical for cell survival, cell growth, protein synthesis, and cellular homeostasis. Together with other components, mTOR forms two complexes including mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 acts as a sensor for cellular levels of nutrient and energy in regulating cell growth by coordinating signals from nutrients, growth factors, and energy levels (35, 36). There are five components in mTORC1 including mTOR, regulatory-associated protein of mTOR (RAPTOR), mammalian lethal with Sec13 protein 8 (mLST8, also known as GbL), proline-rich AKT substrate 40 kDa (PRAS40), and DEP-domain-containing mTOR-interacting protein (DEPTOR). Among the mTORC1 complex, mTOR is the catalytic subunit containing multiple domains including a kinase domain that is stabilized by mLST8. RAPTOR is a scaffold protein that is critical for the subcellular localization of mTORC1 and recruitment of mTORC1 substrates. DEPTOR directly interacts with mTOR and inhibits mTOR activation, whereas PRAS40 also negatively regulates mTOR by directly interacting with RAPTOR (36–38).
Compared with mTORC1, less is known about the molecular mechanism by which mTORC2 is activated. Unlike mTORC1, mTORC2 is not sensitive to acute rapamycin but can be inhibited by prolonged treatment with rapamycin in some cell types but not others (39), which is likely due to the different expression levels of FKBP12 and FKBP51 in different cells and tissues that determine their different responsiveness to rapamycin (40). Similar to mTORC1, mTORC2 also has five components. Besides mTOR, mLST8, and DEPTOR, mTORC2 has three different components that include rapamycin-insensitive companion of mTOR (RICTOR), protein observed with RICTOR1/2 (PROTOR1/2), and mammalian stress-activated protein kinase-interacting protein (mSIN1) (36, 41). RICTOR, a defining component of mTORC2, acts as a scaffold protein that binds with mSIN1, DEPTOR, and PROTOR1/2. mSIN1 may recruit mTORC2 to the plasma membrane through its phospholipid-binding pleckstrin homology (PH) domain in response to extracellular stimuli such as growth factors and insulin. mSIN1 inhibits mTORC2 activity by directly interacting with the mTOR kinase domain (39, 42). It is now known that the main functions of mTORC2 are regulating cytoskeleton actin reorganization and AKT activation (43).
mTORC1 regulates cell growth by integrating the signals from growth factors, oxygen, energy status, and amino acids. mTORC1 activity is regulated by phosphatidylinositol-3 kinase (PI3K)/protein-kinase B (AKT), AMPK, and the Ragulator-Rag complex in the lysosome (36, 41).
The tuberous sclerosis complex (TSC), a heterodimer that comprises TSC1 and TSC2, is one of the critical sensors in regulating mTORC1 activation. When growth factors/insulin are present, AKT is phosphorylated and activated by phosphoinositide-dependent kinase-1 (PDK1) and mTORC2, leading to TSC2 phosphorylation by AKT that disrupts the interaction of TSC1 with TSC2 and inactivates TSC1-TSC2 complex (44, 45). TSC1 and TSC2 together with Tre2-Bub2-Cdc16-1 domain family member 7 (TBC1D7) form the three-core protein complex in which TSC2 acts as a GTPase-activating protein (GAP) for the small Ras-related GTPase RHEB (Ras homolog enriched in brain). TBC1D7 knockdown decreases the association of TSC1 and TSC2 leading to decreased RHEB-GAP activity (46). The active, GTP-bound form of RHEB directly interacts with and activates mTORC1 (35, 47). As an RHEB-specific GAP, the TSC1-TSC2 complex negatively regulates mTORC1 signaling by converting RHEB into its inactive GDP-bound state (48). AKT-mediated phosphorylation of TSC2 also leads to the dissociation of TSC1-TSC2 complex from the lysosome, causing RHEB activation and resulting in the activation of mTORC1 (49).
In contrast to the growth factor, amino acids activate mTORC1 by activating the Ragulator-Rag GTPase complex independent of the TSC1-TSC2 complex (50). mTORC1 localizes on lysosomal surfaces through Ragulator, a pentameric complex composed of LAMTOR 1–5 with guanidine nucleotide exchange factor (GEF) activity, and the Rag GTPases (50, 51). Mammals have four Rag proteins: Rag A, B, C, and D proteins, which are functionally redundant GTPases (Rag A and Rag B, whereas Rag C and Rag D are functionally redundant) (50, 52). The Rag GTPase functions as heterodimers with two equivalent pairs, of which Rag A or Rag B in complex with Rag C or Rag D, respectively. Nutrient status determines the transition from the inactive form of Rag GTPase (heterodimer of GDP-bound Rag A/B and GTP-bound Rag C/D) to the active form of Rag GTPase (heterodimer of GTP-bound Rag A/B and GDP-bound Rag C/D) state or transition from the active form of Rag GTPase to the inactive form of Rag GTPase state. The active Rag GTPase heterodimer directly interacts and recruits mTORC1 to the surface of the lysosome together with the binding of mTORC1 to RHEB resulting in mTORC1 activation. In low amino acid conditions, the inactive form of Rag GTPase is maintained by GAP activity of GATOR1 (53). In the absence of amino acids, GATOR1 is recruited to RagA by the lysosomal E3 ligase RNF152-mediated ubiquitination of RagA resulting in mTORC1 inhibition (54). GATOR2 reduces the GAP activity of GATOR1 resulting in the activation of mTORC1 (48, 55, 56). Sestrins negatively regulate mTORC1 by inhibiting GATOR2 to prevent the release of GDP from Rag A/B (56). The inactive Rag GTPase recruits TSC1-TSC2 complex and inactivates RHEB (57). Upon amino acid stimulation, v-ATPase on the lysosomal surface stimulates the GEF activity of the Ragulator to promote GTP loading of Rag A/B for activation. FLCN (folliculin)-FNIP1/2 (FLCN-interacting protein 1 or 2) and leucyl-tRNA synthetase (LeuRS) act as GAP of Rag C/D, respectively, resulting in GDP-bound Rag C/D to activate mTORC1 (55, 58). The above compelling evidence strongly indicates that the regulation of mTORC1 activation is very complex, and the lysosome is a critical signaling hub for mTORC1 activation in addition to its well-known degradation function.
AMPK, the cellular glucose and energy sensor, also regulates mTORC1. In low-energy conditions, the ratio of intracellular AMP/ATP increases that leads to AMPK activation. Fructose-1,6-bisphosphate (FBP), which is a glycolytic intermediate and sensed by aldolase, binds to the v-ATPase on the lysosomal surface. In low glucose conditions, the lack of FBP from aldolase results in a change in the interaction of aldolase and v-ATPase, which promotes the translocation of AXIN:LKB1 complex to the lysosomal surface and forms a “super complex” composed of the v-ATPase, Ragulator, LKB1, and AMPK, resulting in AMPK activation via the phosphorylation of Thr172 on AMPK (59–61). Activated AMPK phosphorylates TSC2 of TSC1-TSC2 complex or phosphorylates RAPTOR in the mTORC1 to inhibit mTORC1 signaling (62, 63). Therefore, AMPK acts as the cellular sensor for glucose and ATP to regulate mTORC1 activation. In addition to regulating mTORC1, emerging evidence indicates that AMPK also regulates mTORC2 activation. During energetic stress, AMPK directly associates RICTOR and phosphorylates mTOR within mTORC2 complex leading to mTORC2 activation that promotes cell survival (64). The upstream signaling networks in regulating mTORC1 and mTORC2 are summarized in Fig. 1.
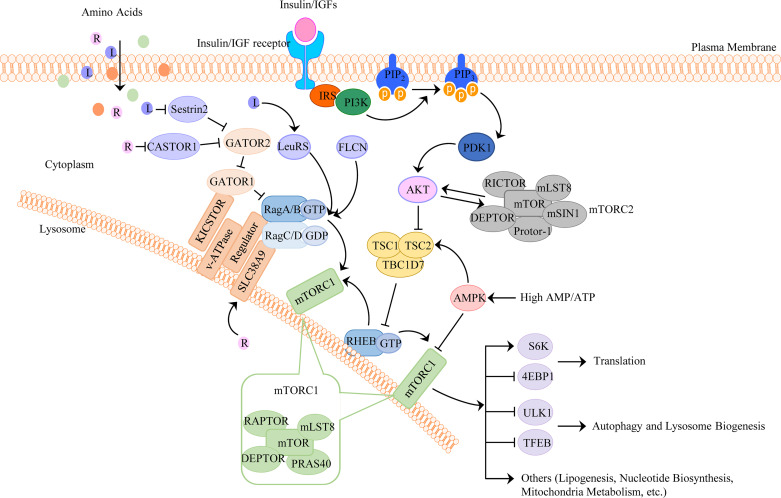
Figure 1.
The upstream signaling network regulating mTORC1 and mTORC2. mTORC1 integrates four major signals: growth factors, energy status, oxygen, and amino acids, which regulate cellular processes that are involved in the promotion of cell growth, proliferation, and metabolism. mTORC2 mainly regulates AKT activation and insulin sensitivity. 1) Growth factor-mediated mTORC1 activation. Growth factors, such as insulin/IGFs, bind to their receptors and activate PI3K to convert PtdIns(4, 5)P2 to PtdIns(3–5)P3, which subsequently activate PDK1 to promote AKT phosphorylation. AKT phosphorylates TSC2 on multiple sites to inhibit its GAP activity for the small GTPase RHEB. GTP-loaded RHEB then activates mTORC1. 2) Amino acids. Amino acids stimulate mTORC1 by promoting the conversion of small GTPase Rag heterodimers to the active form, in which Rag A or Rag B is loaded with GTP and Rag C or Rag D is loaded with GDP. The active RAG heterodimer recruits mTORC1 to the surface of the lysosome, where RHEB binds and activates mTORC1. 3) Energy status. In response to low energy (high AMP/ATP ratio) or glucose, activated AMPK phosphorylates TSC2 of TSC1/TSC2 complex, or phosphorylates RAPTOR, which leads to mTORC1 inhibition. AKT, protein kinase B; AMPK, AMP-activated protein kinase; IGFs, insulin-like growth factors; L, leucine; mTORC1, mechanistic target of rapamycin complex 1; PDK1, phosphoinositide-dependent kinase 1; PtdIns(4, 5)P2, phosphatidylinositol-4,5-bisphosphate; PtdIns(3–5)P3, phosphatidylinositol-3,4,5-triphosphate; R, arginine; Rag, RAS-related GTP-binding protein; RAPTOR, regulatory-associated protein of mTOR; RHEB, RAS homologue enriched in brain.
REGULATION OF MAMMALIAN AUTOPHAGY BY mTOR
Autophagy is an intracellular degradation pathway that involves the formation of double-membrane autophagosomes. The autophagosome enwraps intracellular proteins and organelles and traffics to lysosomes to form autolysosomes via fusion of autophagosome with lysosome. Autophagic cargos are then degraded in the autolysosomes by lysosomal proteases and lipases to provide nutrients for cell survival (65, 66). The regulation of autophagy by mTOR has been reviewed extensively, which will not be discussed in detail here. In addition to regulating the early initiation stage of autophagy, it is now known that mTORC1 negatively regulates autophagy at multiple stages of autophagy including autophagosome elongation, fusion of autophagosomes with lysosomes, and autophagosome maturation, as well as the tubulation and biogenesis of lysosomes.
In the presence of sufficient nutrients, mTORC1 negatively regulates autophagy by phosphorylating ULK1 at S757 and inhibiting its activity (67, 68). Conversely, under the energy depletion conditions, AMPK is activated and phosphorylates ULK1 at distinct sites at S317, S467, S555, T574, S637, and S777, which activates ULK1 to promote autophagy for catabolizing cellular components to generate ATP (69–71). WIPI2 (a mammalian ortholog of yeast Atg18), a WD40-repeat-containing PI3P-binding protein, is a critical protein for the elongation and growth of autophagosome membrane. mTORC1 directly phosphorylates WIPI2 at Ser395, and the phosphorylated WIPI2 can interact with HUWE1, an E3 ubiquitin ligase, to promote WIPI2 ubiquitination and proteasomal degradation resulting in the inhibition of autophagosome formation (72). Activated mTORC1 also directly interacts and phosphorylates p300, an acetyltransferase, resulting in autophagy inhibition likely by increased p300-mediated LC3 acetylation (73). In addition, mTORC1 inhibits autophagosome maturation by either phosphorylating UVRAG or Pacer (74, 75). Phosphorylated UVRAG interacts with RUBICON that enhances RUBICON-mediated inhibition on autophagosome maturation (74), whereas phosphorylated Pacer fails to interact with STX17 and the HOPSS complex resulting in impaired fusion of autophagosome with lysosome and autophagosome maturation (75). After receiving and degrading the autophagic cargos, autolysosomes can maintain the lysosome identity by morphological remodeling under a process called autophagosome-lysosome reformation (ALR) (76). mTORC1 phosphorylates UVRAG and VPS34 to induce ALR and lysosomal tubulation, which is important for cell survival during starvation (77). Lysosomes sit at the terminal stage of autophagy and are crucial components of the cellular degradation and recycling system to maintain proper cellular homeostasis (78). mTORC1 also regulates lysosomal biogenesis by phosphorylating and inhibiting the nuclear translocation of transcription factor EB (TFEB), which is a master regulator of lysosomal biogenesis and autophagy-related genes (62). During starvation, the dephosphorylated form of TFEB is able to translocate to the nucleus and induce transcription of these genes (79, 80).
mTORC1 and Rag GTPase system is the most well-studied system that phosphorylates TFEB and determines the localization and retention of TFEB in the cytosol (80–87). As we discussed earlier regarding the mechanism underlying amino acid-mediated mTORC1 activation through the Ragulator-Rag GTPase complex, TFEB undergoes a similar regulatory scenario. TFEB binds Rag GTPase through its Rag-binding site in its N-terminal (the first 30 amino acids). Deletion in this region, or S3A/R4A point mutations, forces TFEB translocation into the nucleus. TFEB lysosomal translocation relies on the active status of Rag GTPase heterodimers, which is similar for mTORC1 (87). Thus, TFEB, Rag GTPase heterodimer, mTORC1, and RAPTOR form a complex, which is required for the phosphorylation of TFEB.
As discussed earlier, TSC1-TSC2 complex negatively regulates mTORC1, and deletion of either TSC1 or TSC2 causes mTORC1 hyperactivation, which should lead to predominantly cytoplasmic retention of TFEB. Intriguingly, TFEB is hypophosphorylated at mTORC1-dependent sites in TSC1- or TSC2-deficient cells resulting in increased nuclear TFEB translocation (88, 89), suggesting that TSC1-TSC2 complex is required for the phosphorylation of TFEB by mTORC1. Overexpression of folliculin (FLCN) or constitutively active Rag C is sufficient to cause TFEB relocation to the cytoplasm in TSC2-deficient cells (89), suggesting that the Rag C/D activity is critical for TFEB phosphorylation and cellular location. Although the exact mechanisms by which loss of TSC1 or TSC2 activates TFEB remains unclear, it is likely that TSC1 or TSC2 deficiency may impair Rag C/D activity resulting in the failure of recruitment of TFEB to the lysosome where TFEB is phosphorylated by mTORC1. Nonetheless, these findings also suggest that loss of TSC1-TSC2-mediated mTORC1 activation has opposite outcomes on TFEB cellular location and activation compared with amino acid-mediated mTORC1 activation. It will be very interesting to further dissect these distinctive complex mechanisms on mTORC1 and TFEB activation in the future.
There are multiple phosphorylation sites on TFEB that may have distinctive outcomes on TFEB stability and activation. Activated mTORC1 on lysosomal surface directly interacts with and phosphorylates TFEB at residues S122, S142, and S211. When phosphorylated at S211, TFEB is released from the lysosomal surface and then binds to scaffold protein 14-3-3, which renders the retention and inactivation of TFEB in the cytosol (80, 88, 90). Interestingly, phosphorylation at S122 enhances the effect of S211 phosphorylation by mTORC1 but does not affect TFEB subcellular localization (81). In addition, degradation of TFEB is enhanced through the ubiquitin-proteasome pathway when TFEB is phosphorylated at S142 and S211 (91).
In addition to mTORC1, other serine/threonine kinases can also recognize TFEB as a substrate and modulate its nuclear-cytosolic shuttling. Phosphorylation of TFEB at S142 by extracellular-signal-regulated kinase 2 (ERK2) inhibits the nuclear entry of TFEB, and ERK inhibitors induce TFEB nuclear translocation (79, 83). Mitogen-activated protein kinase kinase kinase 3 (MAP4K3, germinal-center kinase-like kinase) physically interacts with and phosphorylates TFEB at its S3 residue, and MAP4K3 inhibition is sufficient for TFEB nuclear translocation and activation (92). TFEB is also phosphorylated at S467 residue by AKT and at S134 and S138 residues by glycogen synthase kinase 3β (GSK3β), resulting in cytosolic retention of TFEB, whereas TFEB S467A mutant displays nuclear localization (82), and GSK3 inhibitors favor TFEB nuclear translocation (93). Cyclin-dependent kinases CDK4/6 have also been reported to interact with and phosphorylate TFEB in the nucleus to promote its shutting from nucleus to cytoplasm to inactivate TFEB (94). In contrast to its cytosolic retention by phosphorylation, the phosphorylation at S461, S462, S466, and S468 residues of TFEB by protein kinase Cβ (PKCβ) stabilizes and promotes TFEB nuclear translocation in osteoclasts, suggesting a cooperative mechanism with mTORC1 (95). A mirrored mechanism of TFEB phosphorylation is played by the dephosphorylating mechanisms of phosphatases. In the case of lysosomal stress, lysosomal Ca2+ releases through the calcium channel Mucolipin 1 (MCOLN1) and activates the phosphatase calcineurin, which binds and dephosphorylates TFEB at S211 and S142 residues, thus inducing its nuclear translocation and activation (96). In response to oxidative stress induced by sodium arsenite, protein phosphatase 2 A (PP2A) is activated and dephosphorylates TFEB at S109, S114, S122, and S211 residues, resulting in TFEB activation in an mTORC1-independent fashion (97). Therefore, it appears that there are multiple different kinases/phosphatases that phosphorylate/dephosphorylate different sites of TFEB rendering either activation or inactivation of TFEB in a context-dependent manner. Whether and how these kinases/phosphatases coordinately regulate TFEB in response to various stresses remains elusive. In addition to phosphorylation (79, 81–84, 92, 93, 95, 97–100), the cellular locations of TFEB and its nuclear activity are also regulated by acetylation/deacetylation (101–103), SUMOylation (104), and alkylation (105), which have been covered elsewhere (106, 107) and will not be discussed in detail here.
Compared with the extensively studied role of mTORC1 in regulating autophagy discussed earlier, the evidence on the role of mTORC2 in the regulation of autophagy is scarce. mTORC2 negatively regulates autophagy through its activation on AKT. AKT suppresses autophagy by phosphorylating Beclin-1 and promoting its binding to intermediate filaments (108). FOXO3 is a member of the FOXO family of transcription factors and regulates the expression of a subset of autophagy-related genes in certain tissues such as muscle and liver (109, 110). Similar to TFEB, phosphorylated FOXO3 is retained in the cytosol and is inactivated. mTORC2 may inhibit autophagy indirectly via increased FOXO3 phosphorylation by AKT and serum and glucocorticoid‐induce protein kinase-1 (SGK-1) (111). Therefore, it seems that mTORC2 regulates autophagy indirectly via its activation on AKT or other kinases. Whether some of the autophagy-related proteins could be direct substrates of mTORC2 and the relevance of mTORC2-mediated autophagy in more pathophysiological settings remain to be investigated in the future.
ROLE AND MECHANISMS OF mTORC1 AND mTORC2 ACTIVATION AND AUTOPHAGY IN ALD
The effects of alcohol on hepatic mTORC1 and autophagy activity are complex and seem to be dependent on the scenario of acute or chronic alcohol consumption (112, 113). Acute alcohol administration to mice increases autophagic flux, which likely acts as an adaptive response to remove damaged mitochondria and excess lipid droplets (114, 115). Increased autophagic flux by acute alcohol is likely due to the activation of FOXO3 as well as the inhibition of both mTORC1 and mTORC2. Ethanol treatment decreases the levels of phosphorylated p70S6K and 4EBP1 in primary cultured mouse hepatocytes, which can be abolished by pharmacological inhibition of ADH and CYP2E1 or by antioxidants, suggesting that ethanol metabolism and production of oxidative stress may be involved in the inhibition of mTORC1 (114). Decreased levels of hepatic phosphorylated 4EBP1 are also observed in mice with acute ethanol binge, suggesting that this acute ethanol binge inhibits mTORC1 in mouse livers. In addition to inhibiting mTORC1, acute ethanol may also inhibit mTORC2. Acute ethanol binge or ethanol treatment decreased the levels of phosphorylated AKT at serine 473 (110, 116), the site known to be phosphorylated by mTORC2, in mouse livers and primary cultured mouse hepatocytes. Decreased AKT subsequently leads to decreased phosphorylation of FOXO3 and increased FOXO3 nuclear translocation resulting in increased expression of autophagy-related genes (110, 116). FOXO3 knockout mice have decreased expression of hepatic autophagy-related genes and numbers of autophagosomes in hepatocytes. As a result, FOXO3 knockout mice are more susceptible to acute binge alcohol-induced liver injury and steatosis, indicating FOXO3-mediated autophagy is protective against acute alcohol-induced liver injury (110). Farnesoid X receptor (FXR) is a nuclear hormone receptor that regulates the expression of genes responsible for bile acid homeostasis and autophagy (117, 118). Increased nuclear FOXO3 translocation is blunted in FXR knockout mouse livers after acute ethanol binge (119). FXR does not directly interact with FOXO3, suggesting that the regulation of FXR on FOXO3 is likely an indirect effect. Future studies are needed to determine whether the activation of mTORC1 and mTORC2 would differentially regulate FOXO3 activation in FXR knockout mice after acute ethanol binge. Nonetheless, it seems clear that both mTORC1 and mTORC2 may be involved in acute alcohol-induced autophagy by targeting distinctive autophagy-related pathways in mouse liver. Future studies to use liver-specific Rictor knockout mice will help to define the exact role of mTORC2 in acute alcohol-induced hepatic autophagy. In addition to the liver, acute alcohol also decreases the levels of phosphorylated S6K1 and 4EBP1 in mouse muscle, indicating that acute alcohol also inhibits mTORC1 in mouse muscle. Decreased mTORC1 activity leads to decreased protein synthesis that may affect muscle contraction (120). Alcohol consumption is known to cause skeletal myopathy in humans (121), and activation of autophagy can cause muscle atrophy (109). One would like to speculate that autophagy may be increased in mouse muscle as a result of decreased mTORC1 activity after acute alcohol, which may contribute to alcohol-associated myopathy.
In contrast to acute alcohol, chronic alcohol feeding or chronic feeding plus acute alcohol binge (Gao-binge) leads to increased mTORC1 activation and impairs TFEB-mediated lysosomal biogenesis in mouse livers (122). As discussed in the Mechanisms Regulating mTOR section, the lysosome sits at the end of the autophagy process, and decreased lysosome numbers by alcohol impair the overall autophagic degradation capacity, a phenomenon that we termed as insufficient autophagy (123). Pharmacological activation of ALDH2 by Alda-1 does not affect alcohol-induced mTORC1 activation and TFEB impairment, suggesting that ALDH2, a key enzyme for alcohol metabolism that converts acetaldehyde to acetate, is likely not critical for alcohol-induced mTORC1 activation and TFEB impairment (124). However, pharmacological inhibition of mTORC1 and mTORC2 by Torin 1 rescued the impaired TFEB-mediated lysosomal biogenesis and protected against Gao-binge alcohol-induced liver injury. Moreover, overexpression of TFEB in mouse livers also improves the biogenesis of both lysosomes and mitochondria that markedly ameliorates alcohol-induced liver injury (122), highlighting the critical role of mTORC1 and TFEB in ALD.
Several mechanisms have been implicated in mTORC1 activation by alcohol in mouse livers. Gao-binge alcohol increases lysosomal Rag A protein and mTOR translocation, but how alcohol increases Rag A protein and whether other Rag proteins are also involved in alcohol-induced mTOR lysosomal translocation remain unclear (122). In addition, the levels of TSC1 decreased in human alcoholic hepatitis and chronic plus binge alcohol-fed mouse livers (unpublished observation). DEPTOR, a negative regulator of mTORC1 and mTORC2, decreases in Gao-binge alcohol-fed mouse livers resulting in increased mTORC1 activation (125). Decreased hepatic DEPTOR is mediated by decreased SIRT1 caused by alcohol feeding, although the exact mechanisms of how SIRT1 regulates DEPTOR protein levels remain to be determined (125). Increased mTORC1 promotes hepatic SREBP1 activation and lipogenesis in alcohol-fed mice (125). Overexpression of DEPTOR or a kinase inactive S6K ameliorates alcohol-induced steatosis by suppressing SREBP-1 in mice (125), highlighting another important function of mTORC1 in addition to regulating TFEB and autophagy in ALD. As discussed in the Regulation of Mammalian Autophagy by mTOR section, sestrins and AMPK are negative regulators of mTORC1. Chronic alcohol feeding decreases hepatic sestrin 3 and phosphorylated AMPK in mouse livers (126). Decreased sestrin and AMPK would theoretically lead to mTORC1 activation. However, alcohol-fed mice had decreased levels of phosphorylated P70S6K, which was further decreased by overexpression of sestrin 3. As other mTORC1 substrate proteins were not determined in this study, the impact of chronic alcohol feeding on mTORC1 activity as well as how decreased sestrin 3 and AMPK would decrease P70S6K phosphorylation remains unclear (126). Moreover, as AMPK can activate mTORC2 (64), whether chronic alcohol would decrease mTORC2 activity and contribute to ALD also needs to be investigated in the future. Notably, overexpression of sestrin 3 improved alcohol-induced steatosis, although it is unclear whether overexpression of sestrin 3 would rescue impaired TFEB by alcohol (126). The possible role of mTORC1 and mTORC2 in acute and chronic alcohol-mediated autophagy and liver injury is summarized in Fig. 2.
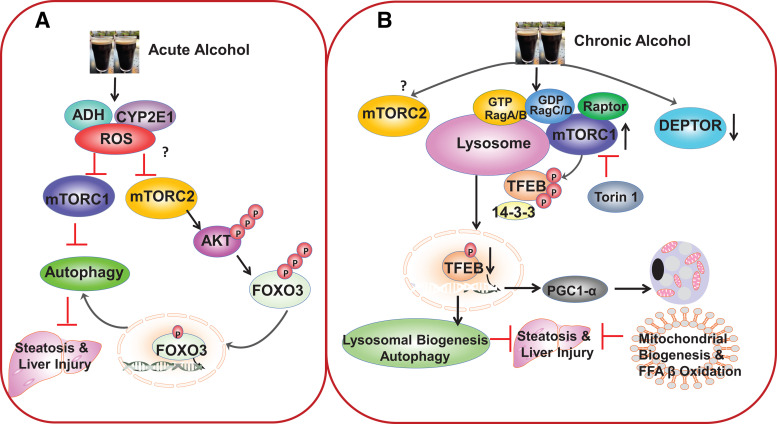
Figure 2.
Acute and chronic alcohol exposure have distinctive effects on mechanistic target of rapamycin complex 1 (mTORC1) and autophagy in mouse livers. A: acute ethanol binge induces autophagy by inhibiting mTORC1 and mechanistic target of rapamycin complex 2 (mTORC2). Ethanol is metabolized in the liver by alcohol dehydrogenase (ADH) and cytochrome P450 2E1 (CYP2E1) to increase reactive oxygen species (ROS) production, which may lead to the inhibition of both mTORC1 and mTORC2 by yet unknown mechanisms. Decreased mTORC1 then promotes hepatic autophagy induction. Decreased mTORC2 leads to decreased protein kinase B (AKT) phosphorylation and activation resulting in decreased Forkhead box O-3 (FOXO3) phosphorylation and increased FOXO3 nuclear translocation. Activated FOXO3 increases the expression of autophagy related genes and autophagy induction to protect against acute alcohol-induced liver injury. B: chronic feeding plus acute ethanol binge impairs autophagy by increasing mTORC1 activation. Chronic feeding plus acute ethanol binge increases lysosomal RagA and decreases DEP-domain-containing mTOR-interacting protein (DEPTOR) resulting in mTOR lysosomal translocation and mTORC1 activation. Increased mTORC1 activation promotes the phosphorylation and cytosolic retention of transcription factor EB (TFEB) resulting in decreased nuclear TFEB translocation. Decreased nuclear TFEB reduces the expression of lysosomal and –autophagy-related genes as well as peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) the master regulator of mitochondrial biogenesis. Torin1 inhibits mTORC1 and rescues alcohol-induced defective TFEB resulting in increased biogenesis of lysosomes and mitochondria as well as autophagy to protect against alcohol-induced liver injury. Whether chronic ethanol feeding would affect mTORC2 remains to be determined.
In contrast to the liver, chronic feeding plus acute alcohol binge decreases mTORC1 activity in white adipose tissues resulting in adipose atrophy (127). Alcohol feeding decreases p62 and LC3-II levels in white adipose tissues, implicating a possible increased autophagic flux (127). Activation of mTORC1 in 3T3-L1 adipocytes decreases the expression of ATGL and HSL, suppresses lipolysis, stimulates de novo lipogenesis, and increases lipid storage (128). Alcohol feeding increases adipose tissue lipolysis by either increasing systemic levels of catecholamine or abolishing insulin-mediated suppression of lipolysis (25, 129). Although how mTORC1 would affect systemic levels of catecholamine is unclear, an mTORC1-EGR1-ATGL regulatory pathway has been reported to be responsible of the inhibitory effects of insulin on lipolysis in adipocytes (130). Mechanistically, increased mTORC1 negatively regulates the expression of Atgl via EGR1 (130). Therefore, decreased adipose mass in alcohol-fed mice is likely due to increased lipolysis in adipocytes mediated by mTORC1 inhibition, but is less likely due to increased autophagy as adipocyte-specific deletion of Atg5 or Atg7 in mice leads to either no change or decreased adipose mass (127, 131, 132). Adipocyte-specific deletion of Raptor but not mTOR in mice leads to adipose atrophy and exacerbates alcohol-induced hepatic steatosis (127), suggesting that inhibition of mTORC1 alone but not mTORC2 is sufficient to induce adipose atrophy and contribute to alcohol-induced hepatic steatosis. Alcohol also causes insufficient autophagy in the pancreas due to impaired pancreatic TFEB induced by ERK but not mTORC1 in mice (127, 133). Therefore, it appears that alcohol may differentially regulate mTORC1 in multiple layers in different tissues with different outcomes. Nonetheless, pharmacological inhibition of mTORC1 has demonstrated beneficial effects against alcohol-induced liver injury, as administration of either Torin1 or rapamycin decreases levels of serum ALT and hepatic triglycerides (122, 125). This is partially due to improved TFEB-mediated lysosomal biogenesis and autophagy as well as inhibiting SREBP1-mediated hepatic lipogenesis (114, 122, 125). Thus, targeting mTORC1 and some of its downstream targets such as TFEB and SREBP-1 may be promising for ameliorating ALD. However, persistent inhibition of mTORC1 by chronic administration of rapamycin or liver-specific deletion of Raptor leads to increased liver inflammation and enhanced liver tumorigenesis (134). Therefore, more studies are needed to further validate the effects of mTORC1 inhibition in regulating liver steatosis and inflammation in ALD.
Alcohol consumption is a major risk of all cancer deaths and is associated with HCC (18, 135, 136). Both mTORC1 and autophagy have been implicated in HCC, although their roles in HCC development and progression are complex (137–139). Autophagy plays a dual role in tumorigenesis and cancer progression in which autophagy acts as a tumor suppressor in the tumor initiation stage but acts as a survival mechanism by providing nutrients in already transformed cancer cells (138, 139). mTORC1 is frequently activated in cancers including HCC by promoting the anabolic process and cell growth (137). Loss of function mutations of phosphatase and tensin homolog (PTEN), TSC1, or TSC2 are the major contributors to HCC with high mTORC1 activity (140, 141). In a study using a patient cohort with HCC from Hong Kong, it was found that 16.2% of these patients with HCC have mutations of TSC1 or TSC2 with persistent mTORC1 activation (141). Consistent with these human HCC data, genetic deletion of Pten or Tsc1 in mouse livers leads to spontaneous HCC (142–144), suggesting that chronic activation of mTORC1 alone is sufficient to cause HCC. Due to the hyperactivation of mTORC1 in many cancers including HCC, targeting mTORC1 has attracted a lot of interest in cancer treatment. However, rapamycin and its pharmacological analogs (rapalogs) only lead to modest or minimal beneficial effects against cancer in clinical trials (145, 146). Several potential possibilities may explain the less satisfactory effects of mTORC1 inhibitors. First, mTORC1 inhibition can activate autophagy in cancer cells that may confer cell protective effects by providing nutrients for cancer cell growth and survival. Second, inhibition of mTORC1 may also lead to TFEB activation and TFEB-mediated lysosomal and mitochondrial biogenesis favoring cancer cell survival. Third, liver-specific deletion of Raptor or Mtor in mice increases liver tumor burden in hepatic carcinogen Diethylnitrosamine (DEN) or genetic deletion of Atg5-induced mouse liver tumor models (134, 147), suggesting that both hypo and hyperactivation of mTORC1 may promote liver tumorigenesis. Therefore, a fine-tune level of mTORC1 is critical to maintain liver homeostasis. Although alcohol-induced DNA damage, increased hepatic iron deposition, oxidative stress, and epigenetic changes have been implicated in alcohol-induced liver carcinogenesis (19), increased mTORC1 activation, impaired hepatic TFEB, and insufficient autophagy may also contribute to alcohol-associated HCC. As liver-specific TFEB KO mice have attenuated liver tumorigenesis in DEN-treated and alcohol-fed mice (148), it is likely that mTORC1 and TFEB may also play dual roles in alcohol-induced liver carcinogenesis. Therefore, targeting mTORC1 and TFEB for alcohol-associated liver damage and tumorigenesis will be complex. It appears that the timing and different strategies as well as the duration to either activate or inhibit mTORC1 and TFEB is critical for intervening alcohol-associated liver damage and tumorigenesis.
SUMMARY AND FUTURE PERSPECTIVES
As a cellular nutrient sensor of amino acids and glucose, mTORC1 is critical in regulating cell metabolism and growth by governing the anabolic and catabolic process. Compelling evidence summarized in this review indicates that alcohol consumption affects mTORC1 activation in multiple tissues, and pharmacological inhibition of mTORC1 shows beneficial effects against alcohol-induced organ damage. However, the impact of long-term inhibition of mTORC1 on alcohol-induced organ damage particularly in alcohol-associated hepatitis and HCC remains unclear. Furthermore, the role of mTORC1 in different cell types in addition to hepatocytes in alcohol-associated hepatitis and HCC has not been studied. The lack of suitable mouse/animal models that can faithfully mimic human alcohol-associated hepatitis and HCC significantly halts the progress to further dissect the role of mTORC1 and its inhibitors in ALD. Moreover, studies on the role of mTORC2 in the pathogenesis of ALD have been scarce. The use of genetic deletion of Rictor to specific ablation of mTORC2 in mice will be helpful to provide better understanding of mTORC2 in ALD. Future research to develop better relevant ALD mouse models with both genetic and pharmacological manipulations on mTORC1 and mTORC2 may help to further validate and develop therapeutic options by targeting mTOR to fight this devastating disease.
GRANTS
This study was supported in part by the National Institute of Health (NIH) funds R01 DK102142, R01 AG072895, and R37 AA020518 (to W-X.D).
DISCOLSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.C. and W-X.D. conceived and designed research; X.C. prepared figures; X.C. and W-X.D. drafted manuscript; S.N.W. edited and revised manuscript; X.C., S.N.W., and W.X.D. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of X. Chao: Institute of Precision Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, 51000, China.
REFERENCES
- 1. Spillane S, Shiels MS, Best AF, Haozous EA, Withrow DR, Chen Y, Berrington de Gonzalez A, Freedman ND. Trends in alcohol-induced deaths in the United States, 2000–2016. JAMA Netw Open 3: e1921451, 2020. doi: 10.1001/jamanetworkopen.2019.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hydes T, Gilmore W, Sheron N, Gilmore I. Treating alcohol-related liver disease from a public health perspective. J Hepatol 70: 223–236, 2019. doi: 10.1016/j.jhep.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 3. Pollard MS, Tucker JS, Green HD Jr.. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open 3: e2022942, 2020. doi: 10.1001/jamanetworkopen.2020.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cholankeril G, Goli K, Rana A, Hernaez R, Podboy A, Jalal P, Da BL, Satapathy SK, Kim D, Ahmed A, Goss J, Kanwal F. Impact of COVID-19 pandemic on liver transplantation and alcohol-associated liver disease in the USA. Hepatology 74: 3316–3329, 2021. doi: 10.1002/hep.32067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141: 1572–1585, 2011. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagy LE, Ding WX, Cresci G, Saikia P, Shah VH. Linking pathogenic mechanisms of alcoholic liver disease with clinical phenotypes. Gastroenterology 150: 1756–1768, 2016. doi: 10.1053/j.gastro.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glover SC, McPhie JL, Brunt PW. Cholestasis in acute alcoholic liver disease. Lancet 2: 1305–1307, 1977. doi: 10.1016/s0140-6736(77)90360-9. [DOI] [PubMed] [Google Scholar]
- 8. Palma E, Ma X, Riva A, Iansante V, Dhawan A, Wang S, Ni HM, Sesaki H, Williams R, Ding WX, Chokshi S. Dynamin-1-like protein inhibition drives megamitochondria formation as an adaptive response in alcohol-induced hepatotoxicity. Am J Pathol 189: 580–589, 2019. doi: 10.1016/j.ajpath.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chedid A, Mendenhall CL, Tosch T, Chen T, Rabin L, Garcia-Pont P, Goldberg SJ, Kiernan T, Seeff LB, Sorrell M. Significance of megamitochondria in alcoholic liver disease. Gastroenterology 90: 1858–1864, 1986. doi: 10.1016/0016-5085(86)90253-2. [DOI] [PubMed] [Google Scholar]
- 10. Denk H, Franke WW, Eckerstorfer R, Schmid E, Kerjaschki D. Formation and involution of Mallory bodies (“alcoholic hyalin”) in murine and human liver revealed by immunofluorescence microscopy with antibodies to prekeratin. Proc Natl Acad Sci USA 76: 4112–4116, 1979. doi: 10.1073/pnas.76.8.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sancho-Bru P, Altamirano J, Rodrigo-Torres D, Coll M, Millan C, Jose Lozano J, Miquel R, Arroyo V, Caballeria J, Gines P, Bataller R. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology 55: 1931–1941, 2012. doi: 10.1002/hep.25614. [DOI] [PubMed] [Google Scholar]
- 12. Gougol A, Clemente-Sanchez A, Argemi J, Bataller R. Alcoholic hepatitis. Clin Liver Dis (Hoboken) 18: 90–95, 2021. doi: 10.1002/cld.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jung Y, Brown KD, Witek RP, Omenetti A, Yang L, Vandongen M, Milton RJ, Hines IN, Rippe RA, Spahr L, Rubbia-Brandt L, Diehl AM. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology 134: 1532–1543.e3, 2008. doi: 10.1053/j.gastro.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Argemi J, Latasa MU, Atkinson SR, Blokhin IO, Massey V, Gue JP, , et al. Defective HNF4alpha-dependent gene expression as a driver of hepatocellular failure in alcoholic hepatitis. Nat Commun 10: 3126, 2019. doi: 10.1038/s41467-019-11004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med 44: 723–738, 2008. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang S, Pacher P, De Lisle RC, Huang H, Ding WX. A mechanistic review of cell death in alcohol-induced liver injury. Alcohol Clin Exp Res 40: 1215–1223, 2016. doi: 10.1111/acer.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ji C, Kaplowitz N. Hyperhomocysteinemia, endoplasmic reticulum stress, and alcoholic liver injury. World J Gastroenterol 10: 1699–1708, 2004. doi: 10.3748/wjg.v10.i12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chao X, Ding WX. Role and mechanisms of autophagy in alcohol-induced liver injury. Adv Pharmacol 85: 109–131, 2019. doi: 10.1016/bs.apha.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Donohue TM Jr, Cederbaum AI, French SW, Barve S, Gao B, Osna NA. Role of the proteasome in ethanol-induced liver pathology. Alcohol Clin Exp Res 31: 1446–1459, 2007. doi: 10.1111/j.1530-0277.2007.00454.x. [DOI] [PubMed] [Google Scholar]
- 20. Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology 148: 30–36, 2015. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hartmann P, Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res 39: 763–775, 2015. doi: 10.1111/acer.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagy LE. The role of innate immunity in alcoholic liver disease. Alcohol Res 37: 237–250, 2015. [PMC free article] [PubMed] [Google Scholar]
- 23. Gao B, Seki E, Brenner DA, Friedman S, Cohen JI, Nagy L, Szabo G, Zakhari S. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 300: G516–G525, 2011. doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parker R, Kim SJ, Gao B. Alcohol, adipose tissue and liver disease: mechanistic links and clinical considerations. Nat Rev Gastroenterol Hepatol 15: 50–59, 2018. doi: 10.1038/nrgastro.2017.116. [DOI] [PubMed] [Google Scholar]
- 25. Kang L, Chen X, Sebastian BM, Pratt BT, Bederman IR, Alexander JC, Previs SF, Nagy LE. Chronic ethanol and triglyceride turnover in white adipose tissue in rats: inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J Biol Chem 282: 28465–28473, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, Davion T, Oberti F, Broet P, Emilie D; Foie-Alcool group of the Association Francaise pour l'Etude du Foie. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 39: 1390–1397, 2004. doi: 10.1002/hep.20206. [DOI] [PubMed] [Google Scholar]
- 27. Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, Boardman L, Gores GJ, Harmsen WS, McClain CJ, Kamath PS, Shah VH. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology 135: 1953–1960, 2008. doi: 10.1053/j.gastro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart S, Prince M, Bassendine M, Hudson M, James O, Jones D, Record C, Day CP. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol 47: 277–283, 2007. doi: 10.1016/j.jhep.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 29. Mezey E, Potter JJ, Rennie-Tankersley L, Caballeria J, Pares A. A randomized placebo controlled trial of vitamin E for alcoholic hepatitis. J Hepatol 40: 40–46, 2004. doi: 10.1016/S0168-8278(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 30. Singal AK, Kodali S, Vucovich LA, Darley-Usmar V, Schiano TD. Diagnosis and treatment of alcoholic hepatitis: a systematic review. Alcohol Clin Exp Res 40: 1390–1402, 2016. doi: 10.1111/acer.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singal AK, Shah VH. Current trials and novel therapeutic targets for alcoholic hepatitis. J Hepatol 70: 305–313, 2019. doi: 10.1016/j.jhep.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 32. Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol 109: 1417–1423, 2014. doi: 10.1038/ajg.2014.154. [DOI] [PubMed] [Google Scholar]
- 33. Nguyen-Khac E, Thevenot T, Piquet MA, Benferhat S, Goria O, Chatelain D, Tramier B, Dewaele F, Ghrib S, Rudler M, Carbonell N, Tossou H, Bental A, Bernard-Chabert B, Dupas JL. AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 365: 1781–1789, 2011. doi: 10.1056/NEJMoa1101214. [DOI] [PubMed] [Google Scholar]
- 34. Higuera-de la Tijera F, Servin-Caamano AI, Cruz-Herrera J, Serralde-Zuniga AE, Abdo-Francis JM, Gutierrez-Reyes G, Perez-Hernandez JL. Treatment with metadoxine and its impact on early mortality in patients with severe alcoholic hepatitis. Ann Hepatol 13: 343–352, 2014. [PubMed] [Google Scholar]
- 35. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12: 21–35, 2011. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol 21: 183–203, 2020. [Erratum in Nat Rev Mol Cell Biol 21: 246, 2020]. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137: 873–886, 2009. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell 25: 903–915, 2007. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 39. Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22: 159–168, 2006. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 40. Schreiber KH, Ortiz D, Academia EC, Anies AC, Liao CY, Kennedy BK. Rapamycin-mediated mTORC2 inhibition is determined by the relative expression of FK506-binding proteins. Aging Cell 14: 265–273, 2015. doi: 10.1111/acel.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 168: 960–976, 2017. [Erratum in Cell. 169: 361–371, 2017]. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu P, Gan W, Chin YR, Ogura K, Guo J, Zhang J, Wang B, Blenis J, Cantley LC, Toker A, Su B, Wei W. PtdIns(3,4,5)P3-dependent activation of the mtorc2 kinase complex. Cancer Discov 5: 1194–1209, 2015. doi: 10.1158/2159-8290.CD-15-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu GY, Sabatini DM. Author correction: mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol 21: 246, 2020. doi: 10.1038/s41580-020-0219-y. [DOI] [PubMed] [Google Scholar]
- 44. Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol 4: 658–665, 2002. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 45. Inoki K, Li Y, Zhu TQ, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4: 648–657, 2002. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 46. Dibble CC, Elis W, Menon S, Qin W, Klekota J, Asara JM, Finan PM, Kwiatkowski DJ, Murphy LO, Manning BD. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell 47: 535–546, 2012. doi: 10.1016/j.molcel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol 28: 4104–4115, 2008. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 122: 3589–3594, 2009. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Menon S, Dibble CC, Talbott G, Hoxhaj G, Valvezan AJ, Takahashi H, Cantley LC, Manning BD. Spatial control of the tsc complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell 156: 771–785, 2014. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290–303, 2010. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150: 1196–1208, 2012. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334: 678–683, 2011. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340: 1100–1106, 2013. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Deng L, Jiang C, Chen L, Jin J, Wei J, Zhao L, Chen M, Pan W, Xu Y, Chu H, Wang X, Ge X, Li D, Liao L, Liu M, Li L, Wang P. The ubiquitination of rag A GTPase by RNF152 negatively regulates mTORC1 activation. Mol Cell 58: 804–818, 2015. doi: 10.1016/j.molcel.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 55. Tsun ZY, Bar-Peled L, Chantranupong L, Zoncu R, Wang T, Kim C, Spooner E, Sabatini DM. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol Cell 52: 495–505, 2013. doi: 10.1016/j.molcel.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chantranupong L, Wolfson RL, Orozco JM, Saxton RA, Scaria SM, Bar-Peled L, Spooner E, Isasa M, Gygi SP, Sabatini DM. The sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep 9: 1–8, 2014. doi: 10.1016/j.celrep.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Parmigiani A, Nourbakhsh A, Ding BX, Wang W, Kim YC, Akopiants K, Guan KL, Karin M, Budanov AV. Sestrins inhibit mTORC1 kinase activation through the GATOR complex. Cell Rep 9: 1281–1291, 2014. doi: 10.1016/j.celrep.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 149: 410–424, 2012. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 59. Lin SC, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab 27: 299–313, 2018. doi: 10.1016/j.cmet.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 60. Zhang CS, Jiang B, Li MQ, Zhu MJ, Peng YY, Zhang YL, Wu YQ, Li TY, Liang Y, Lu ZL, Lian GL, Liu Q, Guo HL, Yin ZY, Ye ZY, Han JH, Wu JW, Yin HY, Lin SY, Lin SC. The lysosomal v-ATPase-Ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell Metab 20: 526–540, 2014. doi: 10.1016/j.cmet.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 61. Zhang CS, Hawley SA, Zong Y, Li MQ, Wang ZC, Gray A, Ma T, Cui JW, Feng JW, Zhu MJ, Wu YQ, Li TY, Ye ZY, Lin SY, Yin HY, Piao HL, Hardie DGR, Lin SC. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 548: 112–116, 2017. doi: 10.1038/nature23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 63. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kazyken D, Magnuson B, Bodur C, Acosta-Jaquez HA, Zhang DQ, Tong X, Barnes TM, Steinl GK, Patterson NE, Altheim CH, Sharma N, Inoki K, Cartee GD, Bridges D, Yin L, Riddle SM, Fingar DC. AMPK directly activates mTORC2 to promote cell survival during acute energetic stress. Sci Signal 12, 2019. doi: 10.1126/scisignal.aav3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 451: 1069–1075, 2008. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 290: 1717–1721, 2000. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Egan D, Kim J, Shaw RJ, Guan KL. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 7: 643–644, 2011. doi: 10.4161/auto.7.6.15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim J, Guan KL. Regulation of the autophagy initiating kinase ULK1 by nutrients: roles of mTORC1 and AMPK. Cell Cycle 10: 1337–1338, 2011. doi: 10.4161/cc.10.9.15291. [DOI] [PubMed] [Google Scholar]
- 69. Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331: 456–461, 2011. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141, 2011. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Loffler AS, Alers S, Dieterle AM, Keppeler H, Franz-Wachtel M, Kundu M, Campbell DG, Wesselborg S, Alessi DR, Stork B. Ulk1-mediated phosphorylation of AMPK constitutes a negative regulatory feedback loop. Autophagy 7: 696–706, 2011. doi: 10.4161/auto.7.7.15451. [DOI] [PubMed] [Google Scholar]
- 72. Wan W, You ZY, Zhou L, Xu YF, Peng C, Zhou TH, Yi C, Shi Y, Liu W. mTORC1-regulated and HUWE1-mediated WIPI2 degradation controls autophagy flux. Mol Cell 72: 303–315, 2018. doi: 10.1016/j.molcel.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 73. Wan W, You ZY, Xu YF, Zhou L, Guan ZL, Peng C, Wong CCL, Su H, Zhou TH, Xia HG, Liu W. mTORC1 phosphorylates acetyltransferase p300 to regulate autophagy and lipogenesis. Mol Cell 68: 323–335, 2017. doi: 10.1016/j.molcel.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 74. Kim YM, Jung CH, Seo M, Kim EK, Park JM, Bae SS, Kim DH. mTORC1 phosphorylates UVRAG to negatively regulate autophagosome and endosome maturation. Mol Cell 57: 207–218, 2015. doi: 10.1016/j.molcel.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cheng X, Ma X, Zhu Q, Song D, Ding XM, Li L, Jiang X, Wang XY, Tian R, Su H, Shen ZR, Chen S, Liu T, Gong WH, Liu W, Sun QM. Pacer is a mediator of mTORC1 and GSK3-TIP60 signaling in regulation of autophagosome maturation and lipid metabolism. Mol Cell 73: 788–802, 2019. doi: 10.1016/j.molcel.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 76. Yu L, McPhee CK, Zheng LX, Mardones GA, Rong YG, Peng JY, Mi N, Zhao Y, Liu ZH, Wan FY, Hailey DW, Oorschot V, Klumperman J, Baehrecke EH, Lenardo MJ. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 465: 942–6946, 2010. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Munson MJ, Allen GFG, Toth R, Campbell DG, Lucocq JM, Ganley IG. mTOR activates the VPS34-UVRAG complex to regulate autolysosomal tubulation and cell survival. EMBO J 34: 2272–2290, 2015. doi: 10.15252/embj.201590992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ballabio A. The awesome lysosome. EMBO Mol Med 8: 73–76, 2016. doi: 10.15252/emmm.201505966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. A gene network regulating lysosomal biogenesis and function. Science 325: 473–477, 2009. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 80. Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8: 903–914, 2012. doi: 10.4161/auto.19653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vega-Rubin-de-Celis S, Pena-Llopis S, Konda M, Brugarolas J. Multistep regulation of TFEB by MTORC1. Autophagy 13: 464–472, 2017. doi: 10.1080/15548627.2016.1271514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Palmieri M, Pal R, Nelvagal HR, Lotfi P, Stinnett GR, Seymour ML, Chaudhury A, Bajaj L, Bondar VV, Bremner L, Saleem U, Tse DY, Sanagasetti D, Wu SM, Neilson JR, Pereira FA, Pautler RG, Rodney GG, Cooper JD, Sardiello M. mTORC1-independent TFEB activation via Akt inhibition promotes cellular clearance in neurodegenerative storage diseases. Nat Commun 8: 14338, 2017. doi: 10.1038/ncomms14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, Ballabio A. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J 31: 1095–1108, 2012. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Roczniak-Ferguson A, Petit CS, Froehlich F, Qian S, Ky J, Angarola B, Walther TC, Ferguson SM. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci Signal 5: ra42, 2012. doi: 10.1126/scisignal.2002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Petit CS, Roczniak-Ferguson A, Ferguson SM. Recruitment of folliculin to lysosomes supports the amino acid-dependent activation of Rag GTPases. J Cell Biol 202: 1107–1122, 2013. doi: 10.1083/jcb.201307084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Di Malta C, Siciliano D, Calcagni A, Monfregola J, Punzi S, Pastore N, Eastes AN, Davis O, De Cegli R, Zampelli A, Di Giovannantonio LG, Nusco E, Platt N, Guida A, Ogmundsdottir MH, Lanfrancone L, Perera RM, Zoncu R, Pelicci PG, Settembre C, Ballabio A. Transcriptional activation of RagD GTPase controls mTORC1 and promotes cancer growth. Science 356: 1188–1192, 2017. doi: 10.1126/science.aag2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Martina JA, Puertollano R. Rag GTPases mediate amino acid-dependent recruitment of TFEB and MITF to lysosomes. J Cell Biol 200: 475–491, 2013. doi: 10.1083/jcb.201209135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pena-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC, Wolff NC, Tran TAT, Zou LH, Xie XJ, Corey DR, Brugarolas J. Regulation of TFEB and V-ATPases by mTORC1. EMBO J 30: 3242–3258, 2011. doi: 10.1038/emboj.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Alesi N, Akl EW, Khabibullin D, Liu HJ, Nidhiry AS, Garner ER, Filippakis H, Lam HC, Shi W, Viswanathan SR, Morroni M, Ferguson SM, Henske EP. TSC2 regulates lysosome biogenesis via a non-canonical RAGC and TFEB-dependent mechanism. Nat Commun 12, 2021. doi: 10.1038/s41467-021-24499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science 332: 1429–1433, 2011. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sha Y, Rao L, Settembre C, Ballabio A, Eissa NT. STUB1 regulates TFEB-induced autophagy-lysosome pathway. EMBO J 36: 2544–2552, 2017. doi: 10.15252/embj.201796699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hsu CL, Lee EX, Gordon KL, Paz EA, Shen WC, Ohnishi K, Meisenhelder J, Hunter T, La Spada AR. MAP4K3 mediates amino acid-dependent regulation of autophagy via phosphorylation of TFEB. Nat Commun 9: 942, 2018. doi: 10.1038/s41467-018-03340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li Y, Xu M, Ding X, Yan C, Song Z, Chen L, Huang X, Wang X, Jian Y, Tang G, Tang C, Di Y, Mu S, Liu X, Liu K, Li T, Wang Y, Miao L, Guo W, Hao X, Yang C. Protein kinase C controls lysosome biogenesis independently of mTORC1. Nat Cell Biol 18: 1065–1077, 2016. doi: 10.1038/ncb3407. [DOI] [PubMed] [Google Scholar]
- 94. Yin Q, Jian Y, Xu M, Huang X, Wang N, Liu Z, Li Q, Li J, Zhou H, Xu L, Wang Y, Yang C. CDK4/6 regulate lysosome biogenesis through TFEB/TFE3. J Cell Biol 219: e201911036, 2020. doi: 10.1083/jcb.201911036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ferron M, Settembre C, Shimazu J, Lacombe J, Kato S, Rawlings DJ, Ballabio A, Karsenty G. A RANKL-PKCbeta-TFEB signaling cascade is necessary for lysosomal biogenesis in osteoclasts. Genes Dev 27: 955–969, 2013. doi: 10.1101/gad.213827.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 17: 288–299, 2015. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Martina JA, Puertollano R. Protein phosphatase 2A stimulates activation of TFEB and TFE3 transcription factors in response to oxidative stress. J Biol Chem 293: 12525–12534, 2018. doi: 10.1074/jbc.RA118.003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Theeuwes WF, Gosker HR, Schols A, Langen RCJ, Remels AHV. Regulation of PGC-1alpha expression by a GSK-3beta-TFEB signaling axis in skeletal muscle. Biochim Biophys Acta Mol Cell Res 1867: 118610, 2020. doi: 10.1016/j.bbamcr.2019.118610. [DOI] [PubMed] [Google Scholar]
- 99. Young NP, Kamireddy A, Van Nostrand JL, Eichner LJ, Shokhirev MN, Dayn Y, Shaw RJ. AMPK governs lineage specification through Tfeb-dependent regulation of lysosomes. Genes Dev 30: 535–552, 2016. doi: 10.1101/gad.274142.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shin HJ, Kim H, Oh S, Lee JG, Kee M, Ko HJ, Kweon MN, Won KJ, Baek SH. AMPK-SKP2-CARM1 signalling cascade in transcriptional regulation of autophagy. Nature 534: 553–557, 2016. doi: 10.1038/nature18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang Y, Huang Y, Liu J, Zhang J, Xu M, You Z, Peng C, Gong Z, Liu W. Acetyltransferase GCN5 regulates autophagy and lysosome biogenesis by targeting TFEB. EMBO Rep 21: e48335, 2020. doi: 10.15252/embr.201948335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhang J, Wang J, Zhou Z, Park JE, Wang L, Wu S, Sun X, Lu L, Wang T, Lin Q, Sze SK, Huang D, Shen HM. Importance of TFEB acetylation in control of its transcriptional activity and lysosomal function in response to histone deacetylase inhibitors. Autophagy 14: 1043–1059, 2018. doi: 10.1080/15548627.2018.1447290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bao J, Zheng L, Zhang Q, Li X, Zhang X, Li Z, Bai X, Zhang Z, Huo W, Zhao X, Shang S, Wang Q, Zhang C, Ji J. Deacetylation of TFEB promotes fibrillar Abeta degradation by upregulating lysosomal biogenesis in microglia. Protein Cell 7: 417–433, 2016. doi: 10.1007/s13238-016-0269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Miller AJ, Levy C, Davis IJ, Razin E, Fisher DE. Sumoylation of MITF and its related family members TFE3 and TFEB. J Biol Chem 280: 146–155, 2005. doi: 10.1074/jbc.M411757200. [DOI] [PubMed] [Google Scholar]
- 105. Zhang Z, Chen C, Yang F, Zeng YX, Sun P, Liu P, Li X. Itaconate is a lysosomal inducer that promotes antibacterial innate immunity. Mol Cell 82: 2844–2857, 2022. doi: 10.1016/j.molcel.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 106. Tan A, Prasad R, Lee C, Jho EH. Past, present, and future perspectives of transcription factor EB (TFEB): mechanisms of regulation and association with disease. Cell Death Differ 29: 1433–1449, 2022.doi: 10.1038/s41418-022-01028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Vu HN, Dilshat R, Fock V, Steingrimsson E. User guide to MiT-TFE isoforms and post-translational modifications. Pigment Cell Melanoma Res 34: 13–27, 2021. doi: 10.1111/pcmr.12922. [DOI] [PubMed] [Google Scholar]
- 108. Wang RC, Wei YJ, An ZY, Zou ZJ, Xiao GH, Bhagat G, White M, Reichelt J, Levine B. Akt-mediated regulation of autophagy and tumorigenesis through beclin 1 phosphorylation. Science 338: 956–959, 2012. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6: 458–471, 2007. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 110. Ni HM, Du K, You M, Ding WX. Critical role of FoxO3a in alcohol-induced autophagy and hepatotoxicity. Am J Pathol 183: 1815–1825, 2013. doi: 10.1016/j.ajpath.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 21: 952–965, 2001. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ding WX, Manley S, Ni HM. The emerging role of autophagy in alcoholic liver disease. Exp Biol Med (Maywood) 236: 546–556, 2011. doi: 10.1258/ebm.2011.010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Williams JA, Ding WX. Role of autophagy in alcohol and drug-induced liver injury. Food Chem Toxicol 136: 111075, 2020. doi: 10.1016/j.fct.2019.111075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, Lu B, Stolz DB, Clemens DL, Yin XM. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology 139: 1740–1752, 2010. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ding WX, Li M, Yin XM. Selective taste of ethanol-induced autophagy for mitochondria and lipid droplets. Autophagy 7: 248–249, 2011. doi: 10.4161/auto.7.2.14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ni HM, Bhakta A, Wang S, Li Z, Manley S, Huang H, Copple B, Ding WX. Role of hypoxia inducing factor-1beta in alcohol-induced autophagy, steatosis and liver injury in mice. PLoS One 9: e115849, 2014. doi: 10.1371/journal.pone.0115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Seok S, Fu T, Choi SE, Li Y, Zhu R, Kumar S, Sun XX, Yoon G, Kang Y, Zhong WX, Ma J, Kemper B, Kemper JK. Transcriptional regulation of autophagy by an FXR-CREB axis. Nature 516: 108–11111, 2014. doi: 10.1038/nature13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lee JM, Wagner M, Xiao R, Kim KH, Feng D, Lazar MA, Moore DD. Nutrient-sensing nuclear receptors coordinate autophagy. Nature 516: 112–5115, 2014. doi: 10.1038/nature13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Manley S, Ni HM, Williams JA, Kong B, DiTacchio L, Guo G, Ding WX. Farnesoid X receptor regulates forkhead Box O3a activation in ethanol-induced autophagy and hepatotoxicity. Redox Biol 2: 991–1002, 2014. doi: 10.1016/j.redox.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Steiner JL, Lang CH. Alcohol impairs skeletal muscle protein synthesis and mTOR signaling in a time-dependent manner following electrically stimulated muscle contraction. J Appl Physiol (1985) 117: 1170–1179, 2014. doi: 10.1152/japplphysiol.00180.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Urbano-Marquez A, Fernandez-Sola J. Effects of alcohol on skeletal and cardiac muscle. Muscle Nerve 30: 689–707, 2004. doi: 10.1002/mus.20168. [DOI] [PubMed] [Google Scholar]
- 122. Chao X, Wang S, Zhao K, Li Y, Williams JA, Li T, Chavan H, Krishnamurthy P, He XC, Li L, Ballabio A, Ni HM, Ding WX. Impaired TFEB-mediated lysosome biogenesis and autophagy promote chronic ethanol-induced liver injury and steatosis in mice. Gastroenterology 155: 865–879, 2018. doi: 10.1053/j.gastro.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Chao X, Ni HM, Ding WX. Insufficient autophagy: a novel autophagic flux scenario uncovered by impaired liver TFEB-mediated lysosomal biogenesis from chronic alcohol-drinking mice. Autophagy 14: 1646–1648, 2018. doi: 10.1080/15548627.2018.1489170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chao XJ, Wang SG, Yang L, Ni HM, Ding WX. Trehalose activates hepatic transcription factor EB (TFEB) but fails to ameliorate alcohol-impaired TFEB and liver injury in mice. Alcohol Clin Exp Res 45: 1950–1964, 2021. doi: 10.1111/acer.14695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chen HQ, Shen F, Sherban A, Nocon A, Li Y, Wang H, Xu MJ, Rui XL, Han JY, Jiang BB, Lee D, Li N, Keyhani-Nejad F, Fan JG, Liu F, Kamat A, Musi N, Guarente L, Pacher P, Gao B, Zang MW. DEP domain-containing mTOR-interacting protein suppresses lipogenesis and ameliorates hepatic steatosis and acute-on-chronic liver injury in alcoholic liver disease. Hepatology 68: 496–514, 2018. doi: 10.1002/hep.29849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kang XQ, Petyaykina K, Tao RY, Xiong XW, Dong XC, Liangpunsakul S. The inhibitory effect of ethanol on Sestrin3 in the pathogenesis of ethanol-induced liver injury. Am J Physiol Gastrointest Liver Physiol 307: G58–G65, 2014. doi: 10.1152/ajpgi.00373.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Li Y, Chao X, Wang S, Williams JA, Ni HM, Ding WX. Role of mechanistic target of rapamycin and autophagy in alcohol-induced adipose atrophy and liver injury. Am J Pathol 190: 158–175, 2020. doi: 10.1016/j.ajpath.2019.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chakrabarti P, English T, Shi J, Smas CM, Kandror KV. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes 59: 775–781, 2010. doi: 10.2337/db09-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhao CQ, Liu YL, Xiao J, Liu LM, Chen SY, Mohammadi M, McClain CJ, Li XK, Feng WK. FGF21 mediates alcohol-induced adipose tissue lipolysis by activation of systemic release of catecholamine in mice. J Lipid Res 56: 1481–1491, 2015. doi: 10.1194/jlr.M058610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chakrabarti P, Kim JY, Singh M, Shin YK, Kim J, Kumbrink J, Wu YY, Lee MJ, Kirsch KH, Fried SK, Kandror KV. Insulin inhibits lipolysis in adipocytes via the evolutionarily conserved mTORC1-Egr1-ATGL-mediated pathway. Mol Cell Biol 33: 3659–3666, 2013. doi: 10.1128/MCB.01584-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest 119: 3329–3339, 2009. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sakane S, Hikita H, Shirai K, Myojin Y, Sasaki Y, Kudo S, Fukumoto K, Mizutani N, Tahata Y, Makino Y, Yamada R, Kodama T, Sakamori R, Tatsumi T, Takehara T. White adipose tissue autophagy and adipose-liver crosstalk exacerbate nonalcoholic fatty liver disease in mice. Cell Mol Gastroenterol Hepatol 12: 1683–1699, 2021. doi: 10.1016/j.jcmgh.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang S, Ni HM, Chao X, Ma X, Kolodecik T, De Lisle R, Ballabio A, Pacher P, Ding WX. Critical role of TFEB-mediated lysosomal biogenesis in alcohol-induced pancreatitis in mice and humans. Cell Mol Gastroenterol Hepatol 10: 59–81, 2020. doi: 10.1016/j.jcmgh.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Umemura A, Park EJ, Taniguchi K, Lee JH, Shalapour S, Valasek MA, Aghajan M, Nakagawa H, Seki E, Hall MN, Karin M. Liver damage, inflammation, and enhanced tumorigenesis after persistent mTORC1 inhibition. Cell Metab 20: 133–144, 2014. doi: 10.1016/j.cmet.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Nelson DE, Jarman DW, Rehm J, Greenfield TK, Rey G, Kerr WC, Miller P, Shield KD, Ye Y, Naimi TS. Alcohol-attributable cancer deaths and years of potential life lost in the United States. Am J Public Health 103: 641–648, 2013. doi: 10.2105/AJPH.2012.301199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology 127: S87–S96, 2004. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 137. Matter MS, Decaens T, Andersen JB, Thorgeirsson SS. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J Hepatol 60: 855–865, 2014. doi: 10.1016/j.jhep.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Chao XJ, Qian H, Wang SG, Fulte S, Ding WX. Autophagy and liver cancer. Clin Mol Hepatol 26: 606–617, 2020. doi: 10.3350/cmh.2020.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Qian H, Chao X, Williams J, Fulte S, Li T, Yang L, Ding WX. Autophagy in liver diseases: a review. Mol Aspects Med 82: 100973, 2021. doi: 10.1016/j.mam.2021.100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hu TH, Huang CC, Lin PR, Chang HW, Ger LP, Lin YW, Changchien CS, Lee CM, Tai MH. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer 97: 1929–1940, 2003. doi: 10.1002/cncr.11266. [DOI] [PubMed] [Google Scholar]
- 141. Ho DWH, Chan LK, Chiu YT, Xu IMJ, Poon RTP, Cheung TT, Tang CN, Tang VWL, Lo ILO, Lam PWY, Yau DTW, Li MX, Wong CM, Ng IOL. TSC1/2 mutations define a molecular subset of HCC with aggressive behaviour and treatment implication. Gut 66: 1496–1506, 2017. doi: 10.1136/gutjnl-2016-312734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Menon S, Yecies JL, Zhang HH, Howell JJ, Nicholatos J, Harputlugil E, Bronson RT, Kwiatkowski DJ, Manning BD. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci Signal 5, 2012. doi: 10.1126/scisignal.2002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chao X, Wang S, Fulte S, Ma X, Ahamed F, Cui W, Liu Z, Rulicke T, Zatloukal K, Zong WX, Liu W, Ni HM, Ding WX. Hepatocytic p62 suppresses ductular reaction and tumorigenesis in mouse livers with mTORC1 activation and defective autophagy. J Hepatol 76: 639–651, 2022. doi: 10.1016/j.jhep.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kenerson HL, Yeh MM, Kazami M, Jiang XY, Riehle KJ, Mcintyre RL, Park JO, Kwon S, Campbell JS, Yeung RS. Akt and mTORC1 have different roles during liver tumorigenesis in mice. Gastroenterology 144: 1055–1065, 2013. doi: 10.1053/j.gastro.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res 13: 3109–3114, 2007. doi: 10.1158/1078-0432.CCR-06-2798. [DOI] [PubMed] [Google Scholar]
- 146. Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med 13: 433–442, 2007. doi: 10.1016/j.molmed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 147. Ni HM, Chao X, Yang H, Deng F, Wang S, Bai Q, Qian H, Cui Y, Cui W, Shi Y, Zong WX, Wang Z, Yang L, Ding WX. Dual roles of mammalian target of rapamycin in regulating liver injury and tumorigenesis in autophagy-defective mouse liver. Hepatology 70: 2142–2155, 2019. doi: 10.1002/hep.30770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Chao X, Wang S, Hlobik M, Ballabio A, Ni HM, Ding WX. Loss of hepatic transcription factor EB attenuates alcohol-associated liver carcinogenesis. Am J Pathol 182: 87–103, 2021. doi: 10.1016/j.ajpath.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]