Abstract
This review focuses on the physiology of glymphatic solute transport and waste clearance, using evidence from experimental animal models as well as from human studies. Specific topics addressed include the biophysical characteristics of fluid and solute transport in the central nervous system, glymphatic-lymphatic coupling, as well as the role of cerebrospinal fluid movement for brain waste clearance. We also discuss the current understanding of mechanisms underlying increased waste clearance during sleep.
Keywords: brain, cerebrospinal fluid, glymphatic, solute transport, waste clearance
Introduction
The ability of the brain to “wash” itself has important implications for overall brain function and health. A brief historical perspective highlighting seminal studies first describing lymphatic-like waste drainage from the central nervous system (CNS) is an important tool for understanding the present context of brain waste clearance processes discussed in this review. In the early 1970s, Cserr was to the best of our knowledge the first to introduce the concept of bulk flow driving the cerebrospinal fluid (CSF)/interstitial fluid (ISF) exchange process and clearance of waste solutes from brain parenchyma in the absence of authentic lymphatic vasculature (1–4). Notably, the existence of bulk flow in the ISF surrounding all brain cells was implied by experiments involving administering solutes of different molecular weights into the neuropil and observing that they drained from the brain at the same rate (2). These experiments also demonstrated that the solutes drained from the ISF in a pattern supporting the presence of bulk flow, i.e., along perivascular spaces (PVS) of blood vessels and parallel to white matter fiber tracks (2). In 1985 Rennels et al. (5) reported that a protein tracer, horseradish peroxidase, distributed rapidly from subarachnoid CSF into perivascular channels of the arteries and along capillaries in the cat and dog brain. A detailed histological analysis revealed that the protein tracer accumulated around penetrating arterioles and through the basal lamina of the capillary bed (5). The study by Rennels and coworkers provided the anatomical constructs of Cushing’s proclaimed “third circulation” (6) and also revealed that perivascular influx of the tracer dramatically decreased with partial occlusion of arterial pulsatile flow (5). In 2008, Carare and colleagues (7) reported that fluorescently labeled tracers such as dextran and ovalbumin administered into brain parenchyma drained out of the brain along the basement membranes of capillaries and arteries whereas larger fluorospheres (∼1-μm diameter) got stuck in the perivascular space ingested by perivascular macrophages. On the basis of these results they concluded that the capillary and artery basement membranes comprised the waste drainage routes for lower-molecular weight substance from the brain (7). Finally, in 2012 Iliff, Nedergaard, and colleagues (8) introduced the perivascular glymphatic system for the clearance of endogenous waste solutes from the central nervous system (CNS). The glymphatic system comprised the entire network of perivascular channels of the brain, and the aquaporin (AQP)4 water channels expressed on the glia end-feet were shown to play a key role in CSF/ISF exchange and brain waste clearance processes as discussed below. Intriguingly, the glymphatic clearance of waste varied in a manner dependent on the sleep/wake cycle. Like many physiological processes, the functionality and efficiency of the glymphatic waste clearance system and meningeal lymphatics decline in the aging brain. Glymphatic research has been integral to further understanding the etiology of neurodegenerative disorders. One major area of this research relates to Alzheimer’s disease (AD), which is the most common form of dementia in older adults and is currently incurable. The association of AD with poor sleep and progressive accumulation of aggregated amyloid beta (Aβ) in the brain has spurred major research efforts to understand the physiology of solute transport in the glymphatic system and how it contributes to disease states and pathology.
What Is Perivascular Brain Waste Clearance?
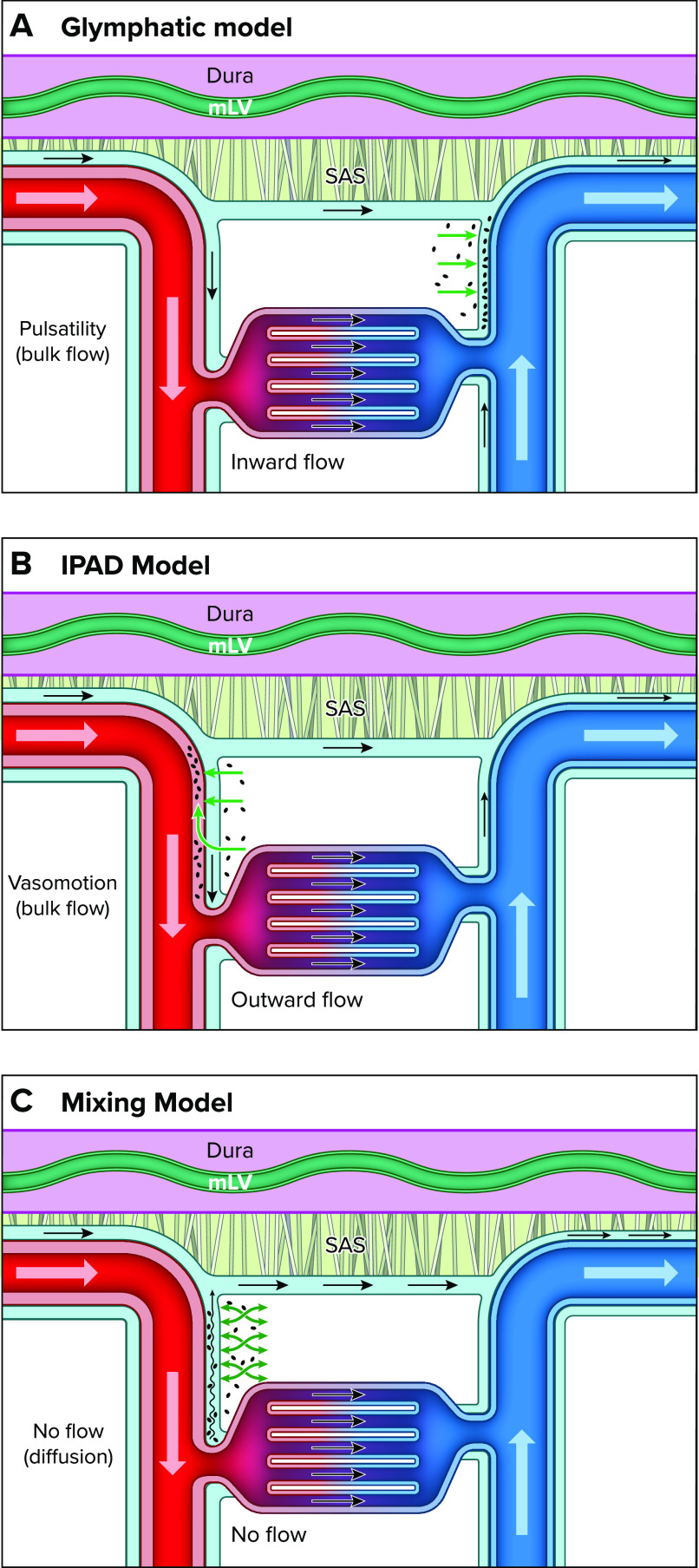
Cerebral homeostasis requires constant maintenance of cellular metabolism, fluid homeostasis, as well as waste elimination. Cerebral blood flow is highly controlled and continuously supplies nutrients and oxygen to sustain normal brain function. The blood-brain barrier (BBB) guards the brain from circulating toxins, pathogens, and inflammation as well as tightly regulating the movement of ions and molecules between the blood and brain (9–11). The tight junctions of the endothelial cells limit paracellular flux of ions and solutes across the BBB, thereby regulating water transport (11). The BBB also contains efflux transporters polarized to the luminal surface and nutrient transporters (carrier-mediated transporters) as well as transporters for transcytosis (11, 12). Although waste elimination occurs by cellular metabolism as well as at the level of the BBB, the brain also clears waste solutes through a dedicated perivascular channel network, which in the current literature is referred to as the “glymphatic system” (8, 13). The glymphatic system model is illustrated in FIGURE 1A and posits that waste solutes, including amyloid beta (Aβ) (8, 14), tau (15), and lactate (16), are cleared from the brain in the following manner:
FIGURE 1.
Schematic presentation of the three models of perivascular brain waste clearance Note that in all illustrations the dura mater is represented by a purple-colored membrane with meningeal lymphatic vasculature (mLV) in shown in green. The dura mater is separated from the subarachnoid space (SAS) by the arachnoid membrane (arachnoid trabeculae shown in gray). The cerebrospinal fluid (CSF) flowing along the periarterial and perivenous channels is illustrated in light blue. The arteries and veins are shown in red and blue, respectively. A: the glymphatic system model posits that waste solutes are cleared from the brain in the following manner: CSF flows into the brain along the perivascular spaces of the arteries (i.e., inward flow) and is propelled (via bulk flow) into the interstitial fluid (ISF) compartment, which forces waste toward the perivenous conduits, from where it merges and exits via meningeal lymphatics. B: the “intramural periarterial drainage” (IPAD) model poses that CSF flows into the brain along the periarterial channels and waste solutes from the ISF exit via walls of the arteries and are transported in a direction opposite to blood flow driven by vasomotion (outward flow). C: the mixing model poses that there is no net flow (no flow) along the penetrating cerebral vasculature. Instead, waste transport from the ISF to the periarterial perivascular spaces (PVS) is facilitated by “mixing” from physiological motion (e.g., vascular pulsatility), as illustrated by the green “mixing” arrows. Furthermore, according to the mixing model, bulk flow is present only on the surface of the brain along the large pial arteries in the subarachnoid space, which creates a favorable concentration gradient for waste egress by diffusion from the ISF to the arterial PVS in the tissue and then toward the pial surface.
1) Cerebrospinal fluid (CSF) flows (bulk flow) from the subarachnoid compartment into the perivascular spaces (PVS) associated with the penetrating arteries, known as Virchow–Robin spaces, and onward along the PVS of smaller arterioles and capillaries (8);
2) From the PVS, CSF is propelled into the interstitial fluid (ISF) space in a process facilitated by AQP4 water channels positioned on the glial end-feet (8, 17);
3) Bulk flow-driven CSF exchanges with ISF, which helps push waste solutes toward perivenous channels, which function as egress routes (8, 18, 19) that ultimately connect to meningeal lymphatic vessels.
Integrated within the glymphatic system model are four fundamental functional features. First, inflow of subarachnoid CSF into the glymphatic system occurs in the direction of blood flow along the periarterial channels and is driven by pressure (bulk flow) generated by vascular pulsatility (8, 20–22). Second, influx of CSF and CSF/ISF exchange are required for perivenous waste clearance, with both processes being dependent on AQP4 water channels (17, 20). Third, according to the glymphatic model, waste solutes in the ISF are driven toward perivenous channels, implying the existence of a pressure gradient across periarterial PVS → perivenous PVS and, consequently, advective (bulk) flow in the ISF compartment (8, 20). Finally, a crucial feature of waste transport via the glymphatic system is the dependence on the brain’s state of arousal (23, 24). This phenomenon was revealed in a pioneering study showing that perivascular CSF influx and waste clearance increased significantly during periods of sleep (or anesthesia inducing slow-wave sleep) and were largely absent during wakefulness (24). The glymphatic system for perivascular brain waste disposal continues to be viewed as a model framework, with the underlying physiology incompletely understood (25–27).
Research has yielded many opposing findings regarding perivascular solute transport including the underlying driving forces (i.e., advection or diffusion), which has given rise to different models of waste clearance (25, 28–32). There are two alternate models for perivascular waste clearance that are important to discuss, as they have direct bearing on understanding the underlying physiology and pathology of disease states. One of these, introduced by Carare et al. (7), is known as “intramural periarterial drainage” (IPAD) (29, 33, 34) and posits that although influx of CSF occurs along the periarterial channels, waste solutes do not exit via perivenous channels but instead within the walls of the arteries in a direction opposite to blood flow (FIGURE 1B). The IPAD model is rooted in the pathology observed with cerebral amyloid angiopathy (CAA), where abnormal fibrillar deposits of Aβ build up along and within the walls of the vasculature, including arteries and arterioles (type 2), or along the microvasculature (type 1) (35–38). The IPAD model also implicates vasomotion as a driving force for waste transport in the opposite direction of blood flow and toward the subarachnoid space (32, 39) (FIGURE 1B). Vasomotion is described as the spontaneous rhythmical contraction and relaxation that occurs at a frequency of ∼0.1 Hz in the vasculature (40). Presumably, vasomotion drives waste solutes from the brain by intermittently squeezing perivascular CSF toward the subarachnoid surface when the vessel periodically relaxes. Vasomotion was first observed in veins of bat wings (41) but is inherent to all vasculature and extensively studied in arteries. Vasomotion is subject to fluctuations modulated by neuronal activity (i.e., neurovascular coupling) and can be augmented if neuronal activity is synchronized, such as during engagement in a task (40, 42, 43). Recently, vasomotion in pial arterioles associated with 0.1 Hz neuronal oscillations of awake mice was shown to drive nanoparticles alongside pial arterioles in a direction opposite of blood flow, with increased transport observed during visually evoked vascular responses (32).
Another perspective of perivascular waste clearance is conceptualized in the “mixing” model (44, 45), which poses that there is no net (advective) PVS flow along the penetrating cerebral vasculature. Instead, waste transport from the ISF is directed toward the periarterial PVS, facilitated by “mixing” from physiological motion (e.g., vasomotion and vascular pulsatility) (FIGURE 1C). Furthermore, according to the mixing model, bulk flow is present only on the surface of the brain along the large pial arteries in the subarachnoid space, which creates a favorable concentration gradient for waste egress by diffusion from the ISF to the arterial PVS and then toward the pial surface (44) (FIGURE 1C). This transport pattern implies that CSF does not recirculate and instead flushes out waste along the PVS associated with pial veins (20, 46). The mixing model is a permutation of the “sink hypothesis,” formulated by Davson, who described that the rapid CSF flow and reabsorption to the blood stream served as a “sink” for transfer of interstitial solutes from the brain by simple diffusion (47). The mixing model also posits perivenous drainage of excess fluid and waste via the PVS of the pial veins, which communicates with the PVS of the pial arteries (FIGURE 1C) (44). The pial vein PVS are thought to merge with egress routes including perineural sheaths along the cranial nerves (e.g., along the olfactory nerves across the cribriform plate to draining lymphatics) or meningeal lymphatics associated with the dural sinuses. In summary, the three glymphatic perivascular waste clearance models differ in the points of view of waste egress routes and the biological forces driving the transport. An overview of the three different models of brain waste clearance is presented in Table 1.
TABLE 1.
Comparison of brain waste clearance models
| Model | Direction of Periarterial CSF Movement | Drivers of Periarterial CSF Transport | Waste Clearance Pathway(s) | Drivers of Waste Clearance |
|---|---|---|---|---|
| Glymphatic system | “Inward” in the direction of blood flow | Bulk flow | Perivenous channels | Bulk flow in ISF compartment |
| IPAD | “Outward” opposite the direction of blood flow |
Vasomotion | Inside the walls of arteries and arterioles | Vasomotion |
| Mixing | No flow | None | Periarterial channels | Diffusion gradient created by CSF bulk flow along surface vasculature |
CSF, cerebrospinal fluid; IPAD, intramural periarterial drainage; ISF, interstitial fluid.
Are the Glymphatic and Lymphatic Systems Directly Linked?
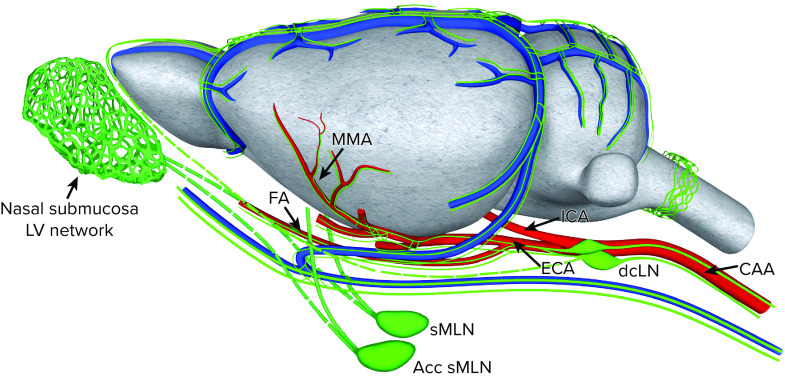
Over the last several years studies have consistently shown that macromolecules administered into the ISF of different brain regions drain through the meningeal lymphatics via afferent lymphatics primarily to the deep cervical lymph nodes (dcLNs) (48–51), implying that glymphatic waste egress routes connect to the meningeal lymphatic system. Identifying the structural and functional connections between the two systems is key to understanding perivascular waste clearance physiology. Detailed anatomical mapping of lymphatic vessels in the dura mater has been made possible by lymphatic endothelial cell-specific fluorescent reporter mice (52–54) and immunofluorescent staining for LYVE-1, PROX-1, and VEGFR-3 (55–57), revealing a dense network of bona fide lymphatic vessels in the dura mater (48, 49). The meningeal lymphatic vasculature (mLV) is embedded in the dura mater that surrounds the brain and spinal cord (58). In dura mater on the dorsal surface, mLVs are observed along the superior sagittal sinus, confluence of sinuses, transverse and sigmoid sinuses, the large retroglenoid vein, as well as along the middle meningeal artery (48, 49) (FIGURE 2). Recently, mLVs associated with the dura at the skull base, known as “basal” mLVs, were also characterized (59). In contrast to the dorsal mLVs, the basal mLVs have capillary lymphatics positioned closer to the subarachnoid space, which is thought to make them more efficient for brain waste drainage (59). However, the general consensus is that the dorsal and basal mLVs are part of the same meningeal lymphatic network and drain primarily to the dcLNs (60). A specific cluster of mLVs exists dorsal to the cribriform plate near the midline of the olfactory bulbs (61) and was recently shown to also serve as an immunoregulatory niche (62). From the point of view of mLV topology along dural including dura cavernous sinuses, the glymphatic system model positing perivenous waste efflux is better aligned with the biology compared to the IPAD model (FIGURE 1B), where waste solutes are predicted to drain along the walls of cerebral and leptomeningeal arteries. Transport from arterial walls to mLVs is difficult to explain anatomically (and functionally) but presumably would involve solutes crossing several barriers including the arachnoid membrane that is sealed by tight junctions (63, 64).
FIGURE 2.
Schematic presentation of the meningeal lymphatic network associated with dura mater of the rodent brain and their suggested connections to the different clusters of cervical lymph nodes The topology of meningeal lymphatic vasculature (mLV, green lines) is following the vasculature imbedded in the dura mater (illustrated as a gray membrane). A dense network of lymphatic vessels (LVs) is located in close proximity to the dural sinuses on the dorsal surface and large dural veins draining to the large cavernous sinuses on the ventral surface (not shown) of the brain. There is also lymphatic vasculature along the middle meningeal arteries (MMAs), which branch off the external carotid arteries. The lymphatic vasculature associated with the nasal submucosa on the other side of the cribriform plate (not shown) and olfactory bulb is also shown. The mLVs drain primarily to the deep cervical lymph nodes (dcLNs). The lymphatics in the nasal submucosa are thought to drain primarily to the submandibular lymph nodes (sMLNs) and the accessory submandibular lymph nodes (Acc sMLNs). CAA, common carotid artery; ECA, external carotid artery; FA, facial artery; ICA, internal carotid artery.
The perineural sheaths along the cranial nerves known as “perineural lymphatics” are also connected to lymphatic vasculature and drain to cervical lymphatic nodes (65, 66). The cranial nerves exit the skull together with large veins and arteries though the same foramina (e.g., stylomastoid foramen or jugular foramen) (58, 59, 65, 66). Notably, the perineural lymphatics are considered an anatomically distinct network and are not directly connected with the mLVs (49, 59, 62). The details of how the mLVs and perineural lymphatics interconnect and partake in CNS fluid homeostasis and immunological surveillance remain incompletely understood. It is possible that exits along the perineural sheath and lymphatics serve as passive outflow for excess fluid to regulate CNS fluid homeostasis. There is experimental support for regulation of CNS fluid homeostasis via perineural neural sheaths associated with olfactory nerves, for example, experiments in sheep where the cribriform plate sealed with bone wax did not result in overt increases in intracranial pressure but caused alterations in tissue compliance (67). In addition, in mutant mice with ciliopathy and olfactory bulb hypoplasia, drainage of solutes and fluid across the cribriform plate to lymphatics in the nasal submucosa was nearly abolished, and these mice were also severely hydrocephalic (68). Notably, in humans, the outflow via the cribriform plate as examined by dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) with CSF Gd contrast appeared insignificant (69).
In summary, although there is strong experimental evidence that the glymphatic system and mLVs are interconnected, the exact anatomical connection and routing of CSF in relation to glymphatic waste egress remain controversial. However, the glymphatic system model positing perivenous waste egress is best aligned with the known topology of the mLV network. Furthermore, a gap in knowledge remains on the exact topology and function of the perineuronal lymphatic vasculature versus mLVs for glymphatic waste clearance and immune surveillance in healthy and diseased CNS.
How Fast Does the Glymphatic System Remove Waste?
The amplification of solute egress from the brain during sleep (24), or with anesthetics inducing slow-wave hypnosis (70, 71), is of particular interest to the neuroscience community as it relates directly to the biological purpose of sleep and the therapeutic potential of the glymphatic system in preventing buildup of waste aggregates. However, the overall efficiency of the glymphatic system for waste disposal, even during sleep, remains poorly defined. To understand glymphatic waste clearance efficiency, data from the lymphatic system are informative. The lymphatic system is responsible for fluid homeostasis and immune surveillance in most body organs and is an efficient pathway for removal of excess fluid, proteins, and metabolites (72–74). It is estimated that the lymphatic system collects and transports up to 8 L of interstitial fluid per day from the tissues in humans and also transports and recycles proteins, i.e., the albumin concentration in interstitial fluid is very similar to its concentration in lymph (∼1.6–1.8 g/dL) (75). The larger lymph vessels and collecting lymphatic vessels have valves, and lymph flow is pulsatile (spontaneous contraction of the lymphatic muscle cells), and therefore inherently advective (73). In rodents, the mean velocity across different lymphatic vascular beds varies, with velocities of 50–600 μm/s reported in the extremities (76) and ∼1,350 μm/s in large mesenteric lymphatic vessels (77). In comparison, advective transport measured in PVS along large pial arteries on the brain surface of rodents is several orders of magnitude slower, with an average velocity of ∼20 µm/s (22, 46). To the best of our knowledge, there are no direct in vivo measurements of solute transport in the ISF of the neuropil or along perivascular spaces of the penetrating vasculature in the intact brain. The majority of studies reporting the existence of bulk flow-driven solute transport in brain are inferred from ex vivo studies using tracers with solutes of different molecular weights administered into the CSF or brain parenchyma (1, 3, 78). Unfortunately, perivascular spaces collapse ex vivo, and the validity of studies examining tracer distribution postmortem has been queried (22).
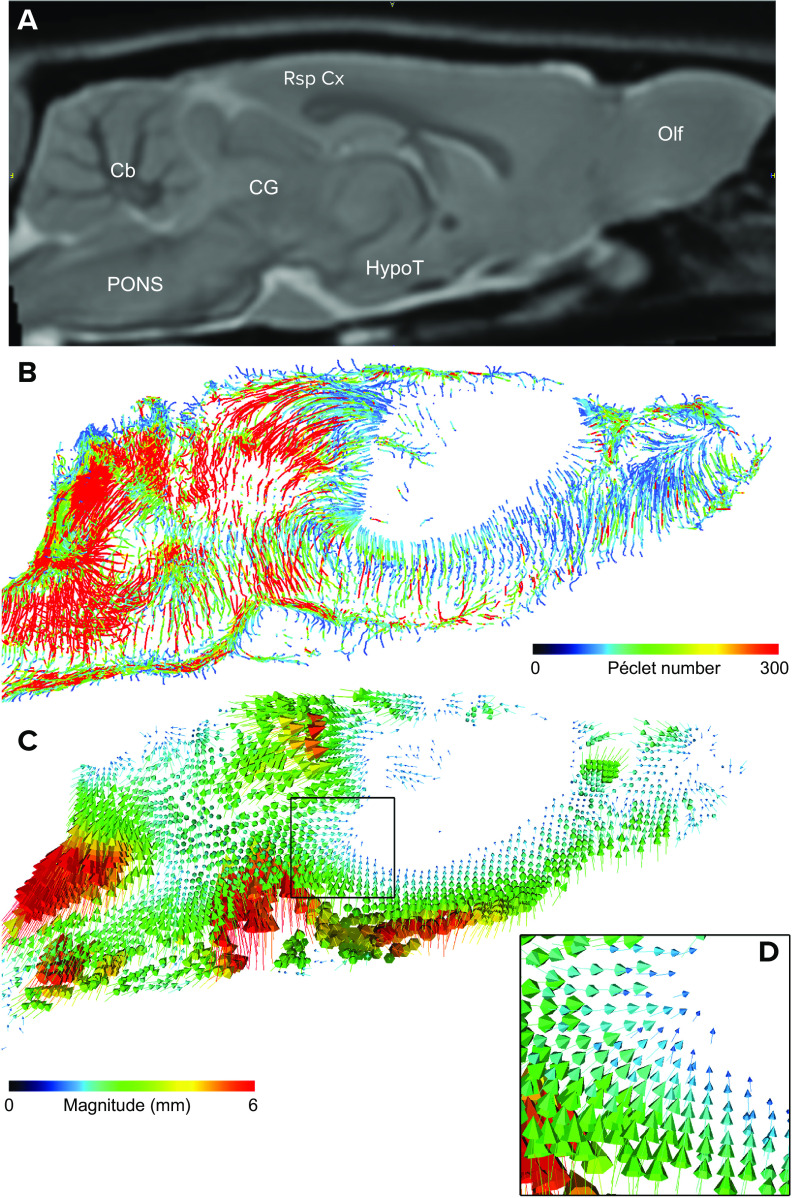
We recently reported on speed and associated Péclet (Pe) numbers of solute and fluid transport measured in the rat brain with in vivo DCE-MRI in combination with computational fluid dynamics (CFD) analysis based on regularized optimal mass transport (rOMT) theory (79–81). The rOMT model incorporates advection (bulk flow) and diffusion constraints into an energy minimization framework, to derive trajectories of fluid and solute movement, known as “pathlines,” over a given tracer circulation time; for more details see Chen et al. (79). Several metrics are derived from the rOMT analysis, including solute/fluid speed, glymphatic flux, velocity flux vectors, and the associated Pe number (79). The velocity flux vector magnitude indicates the distance traveled and direction of the solute trajectories. The Pe number is a standard, scalar, dimensionless quantity describing the ratio of the rate of advection versus that of diffusion (79). Accordingly, tissues with a higher Pe number would be dominated by advective solute and fluid transport, whereas areas with lower Pe have more diffusion. FIGURE 3 shows a two-dimensional (2-D) glymphatic transport map color coded for Pe number from a normal 3-mo-old Sprague-Dawley rat. Note that the cerebellum, pons, and retrosplenial cortex are characterized by higher Pe numbers (>200), implying advection-dominated transport. However, at the level of the hypothalamus, the glymphatic pathlines are associated with lower Pe numbers (<50), indicating more trends toward diffusion-driven transport. Although we observe higher Pe numbers in certain areas, we cannot confirm the presence of advective flow in the neuropil of these specific brain regions because of the spatial resolution of the images (300 × 300 × 300 µm3), which cannot distinguish between PVS associated with intraparenchymal vasculature and the ISF space. However, assuming volume is averaged across the ISF (∼15–20%) and vascular/PVS (∼3–5%) compartments, the rOMT data indeed suggest the presence of different degrees of advection-dominated transport in select brain regions.
FIGURE 3.
Analysis maps A: 2-dimensional (2-D) anatomical MRI map of the live rat brain in the sagittal view plane at the level of the midline (∼0.4 mm lateral to bregma) with landmarks relevant for glymphatic transport. Cb, cerebellum; CG, central gray; HypoT, hypothalamus; Olf, olfactory bulb; Rsp Cx, retrosplenial cortex. B: 2-D glymphatic transport map color coded for Péclet (Pe) number. Scale bar, 3 mm. The Pe number is a standard, scalar, dimensionless quantity describing the ratio of the rate of advection versus that of diffusion The map was generated from dynamic contrast-enhanced MRI data acquired at a voxel resolution of 0.027 mm3 from a normal 3-mo-old Sprague-Dawley rat and analyzed by regularized optimal mass transport analysis (rOMT; for more details see Ref. 79). Blue and red lines indicate glymphatic transport pathlines with low and high Pe number, respectively. Note that in the cerebellum, pons, and retrosplenial cortex glymphatic solute transport is characterized by high Pe numbers (>200), implying advection-dominated transport. However, at the level of the hypothalamus and central gray area the glymphatic pathlines are blue, with lower Pe numbers (<50) indicating more trends toward diffusion-driven transport. C: corresponding 2-D map of the corresponding velocity flux vectors also derived from the rOMT analysis. The velocity flux vectors are informative in regard to distance and direction of the solute streams. The magnitude of a given velocity flux vector indicates the distance a given particle has traveled, whereas the velocity phase shows direction. As can be seen from the velocity flux vector map, the direction of the solute streams is pointing toward the tissue (glymphatic system), and as the streams penetrate into deeper tissue regions the arrows are of smaller magnitude (blue), indicating that the travel distance and speed is slower. The black box (D) highlights a tissue area characterized by velocity flux vectors with low magnitude.
In summary, the notion of advective transport in the glymphatic system is supported by in vivo DCE-MRI data and computational rOMT analysis showing solute transport characterized by high Pe numbers within the brain tissue. However, direct in vivo evidence of advective solute transport in the neuropil or ISF in human or animal brain remains lacking. Compared with the lymphatic system, perivascular glymphatic transport and waste clearance is likely several orders of magnitude slower.
Endogenous Waste Solutes Cleared by the Glymphatic System
Only a limited number of endogenous brain waste solutes (e.g., Aβ, tau, and lactate) have been shown to clear via the glymphatic system (8, 16, 82). Waste solutes such as Aβ oligomers clear from the brain via several elimination mechanisms (83, 84). An important Aβ clearance mechanism is through endothelial cells and pericytes (85, 86) across the BBB via the low-density lipoprotein receptor-related protein 1 and other transporters in an ApoE-dependent manner (85, 86). The half-life of all Aβ isoforms (including Aβ42) was reported to be 3.8 h in 30-yr-old subjects and increased to 9.4 h in 80-yr-old subjects (87). Tau proteins also clear through the glymphatic system and are predominantly intracellular soluble protein isoforms but have also been reported to be passively released by cells even across synapses in a prionlike manner (88–91). Analysis of tau kinetics in humans revealed that the average half-life was ∼23 days (92). Lactate is another solute of interest; however, compared with Aβ and tau, the half-life of lactate is very rapid and has been estimated to be ∼1 min (93). The half-lives of these three different metabolites are representative of several elimination processes (84, 94); and the component specific to each for glymphatic transport remains unknown.
Because of the inferred connection between the glymphatic and lymphatic systems, analysis of the contents of lymph fluid has been of interest. An elegant technique for collecting lymph from the afferent lymphatic vessels draining to the dcLN was recently reported in mice (95); however, collecting lymph is technically challenging, and the literature is sparse. Assuming that solutes and fluid transported via the glymphatic system transfer to mLVs that only drain to the dcLN, this approach would potentially reveal an “undiluted” view of waste draining directly from the brain including neuropil. However, the caveat that lymph from the afferent lymphatics represents “pure brain waste” relates to the unknown contribution of proteins leaking from blood vessels in the dura (no BBB) to ISF that can also enter the mLV (96). Future experimental animal studies should be focused on comparing CSF with lymph collected from afferent draining to the dcLN and explore differences in “omics” signatures across these biofluids in health and disease states to help validate CSF biomarker profiling.
What Do CNS Fluid Homeostasis and CSF Flow Tell Us About Glymphatic Waste Clearance?
The steady state of the brain ISF milieu constitutes the essence of CNS fluid homeostasis in the healthy brain (97). The physiological balance of CSF production and efflux (reabsorption) is critical for CNS fluid homeostasis because CSF actively communicates with interstitial fluid via the glymphatic system. CSF dysregulation can occur in several conditions including trauma to the dura mater causing CSF leakage, with different types of hydrocephalus, and with pharmacological treatments interfering with CSF production. All of these conditions are associated with decreased glymphatic system transport (98–100). Tracking of CSF flow dynamics and the status of CSF production/reabsorption is therefore critical for understanding glymphatic waste clearance.
A major site of CSF production is the choroid plexus, with the rate of CSF formation first measured experimentally in goats (∼0.16 mL/min) by an invasive indirect approach known as the ventriculo-cisternal perfusion technique developed by Heisey et al. (101). CSF production in the human brain at the level of the cerebral aqueduct has been measured by noninvasive phase-contrast MRI and reported to approximate 0.35–0.40 mL/min, or ∼500–600 mL/day (102). The turnover time for total CSF volume is 5–7 h, yielding an overall turnover rate of about four times per day in the human CNS. Recently, a noninvasive approach to measure choroidal CSF “secretion” (a.k.a. blood-CSF-barrier or “BCSFB” function) was introduced by using arterial spin labeling (ASL) MRI technology to quantify the rates of water delivery from the arterial blood of the choroid plexus into ventricular CSF (103). In anesthetized mice, BCSFB function, representing CSF secretion from the choroid plexus, was measured at the level of the lateral ventricles and was shown to decrease by ∼40% in aging mice (103). The ASL MRI method was also validated by showing suppression of CSF secretion by vasopressin (i.e., antidiuretic hormone) (103). There are currently no data on BCSFB function using ASL MRI at the level of the choroid plexus from the live human brain.
There are several long-standing arguments that other sources besides choroidal CSF exist in the CNS. Given the active communication between CSF spaces and ISF, it is argued that a considerable contribution of CSF originates from the cerebral vasculature via either endothelial secretion or filtration (104–106). Indeed, studies have shown that AQP1 water channels are present on the pial arteries and arterioles in conjunction with Na-K-Cl (NKKC1) cotransporters (106), suggesting that water secretion by the cerebral vasculature actively contributes to CSF. Novel noninvasive ASL MRI studies recently uncovered brainwide signals representing water efflux from blood to the CSF compartment (e.g., subarachnoid space and cerebral ventricles) as well as in the brain tissue (107). The therapeutic implication of vascular CSF secretion is important to explore, as this alternate source of fluid might uphold CNS fluid homeostasis in neurodegenerative disease states such as aging, where choroidal CSF secretion is known to decrease (103).
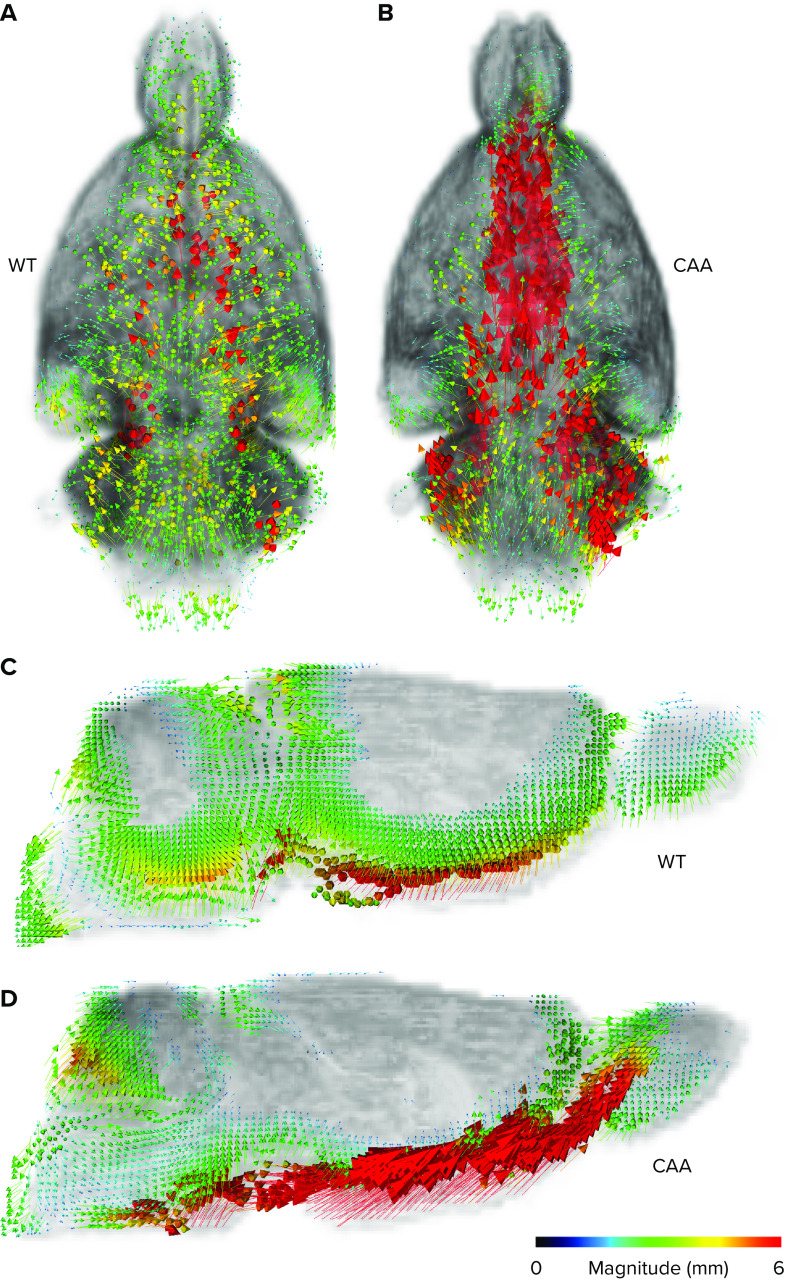
Several different MRI imaging techniques show that CSF movement in the CNS is complex and that CSF flow correlates with cardiac pulsation (108) and CSF flow in the cerebral ventricles and aqueduct can also be augmented by respiratory inspiration (109). Ultrafast blood oxygen level-dependent (BOLD) functional MRI can also track CSF movement in free CSF spaces in conjunction with neurovascular coupling in brain tissue (110, 111). In addition, several new diffusion-weighted techniques have recently been suggested and explored for measuring CSF transport across CNS barriers. Although diffusion-weighted techniques do not directly measure flow speed, the mean diffusivity and other diffusion metrics measured in free CSF spaces can perhaps indirectly inform about differences in advection or dispersion (112–114). The novel MRI techniques for capturing CSF flow dynamics and blood-to-CSF water influx are undergoing increasing investigation in human brain studies as potential surrogate end points for tracking glymphatic transport and waste clearance function. However, it is important to emphasize that the magnitude of CSF movement measured in the subarachnoid space, or CSF flow tracked in the aqueduct or basal cisterns, do not automatically inform about glymphatic system transport or perivascular waste clearance. In other words, vigorous CSF flow in the basal cistern is not predictive of glymphatic system transport unless other metrics, such as parenchymal perivascular transport and/or information of glymphatic waste egress to the dcLN, are measured in parallel. This concept is illustrated in FIGURE 4 and based on DCE-MRI and rOMT data from rat brain. In the normal rat brain, solute and fluid flow in the subarachnoid space along the skull base is moving in a symmetrical pattern around the midline and is directed into the glymphatic system; whereas in the rat with cerebral amyloid angiopathy (CAA) the CSF flow is strikingly different and directed away from the tissue, causing the glymphatic transport to decrease; for more details see Chen et al. (79). In other words, tracking only CSF flow at the skull base or in the basal cisterns could be misleading for assessing glymphatic transport in the diseased rat with CAA because it would show increased CSF flow and higher solute speed compared to the normal rat even though the “effective” CSF streaming into the glymphatic system is decreased.
FIGURE 4.
Representative examples of velocity flux vectors A and B: representative examples of velocity flux vectors (color coded for magnitude) from a 12-mo-old wild-type (WT) control rat (A) and a 12-mo-old rat with cerebral amyloid angiopathy (CAA) (B) from the viewpoint of the ventral surface. Note that in the WT rat the velocity flux vectors are configured in bilateral symmetry about the midline and point toward the tissue. In contrast, in the CAA rat the vectors are of very high magnitude (red color) and directed away from the tissue toward the olfactory fossa. C and D: corresponding 2-dimensional (2-D) maps of the WT and CAA rats shown in the sagittal view plane near the midline showing the strikingly different directional pattern of the velocity flux vector patterns across the 2 rats. In the normal WT rat the arrows are directed into the tissue (glymphatic system), whereas in the CAA rat the arrows are predominantly diverted away from the glymphatic system and redirected. Data for the figure were adapted using our data from work presented in the recently published paper by Chen et al. (79).
In summary, CSF production and transport into the glymphatic system is essential for perivascular waste egress from the CNS. CSF movement in free CSF spaces can be measured in the human brain by noninvasive MRI and is explored as a biomarker of glymphatic transport. However, without additional information on direction or drainage to the dcLN, information on CSF flow alone can potentially be misleading in regard to glymphatic transport and waste egress.
Mechanisms Underlying Increased Glymphatic Waste Clearance During Sleep
The connection between sleep and the glymphatic system was first reported by Xie et al. (24), in which they demonstrated that soluble Aβ was cleared twofold faster in naturally sleeping mice compared with awake mice. Increased glymphatic Aβ clearance was linked to increased power of 1–4 Hz delta waves on the cortical electroencephalogram during sleep compared with wakefulness (24). In the same year that the glymphatic system’s dependence on the sleep/wake cycle was reported, Spira and colleagues (115) showed that among older adults shorter sleep duration and poorer sleep quality were associated with a greater Aβ burden. It is well documented that chronic sleep loss has profound consequences on brain health and is linked to cognitive dysfunction associated with AD and tauopathies (115–118). These two hallmark studies increased the scientific interest in the importance of sustaining good sleep habits in the context of glymphatic waste clearance for brain health, as well as for preventing neurodegeneration from both acute sleep deprivation and chronic partial sleep loss.
One mechanism proposed that underlying the augmentation of glymphatic waste clearance seen during the sleep state is rapid changes in the ISF volume fraction. Specifically, Xie et al. (24) demonstrated that the cortical ISF volume fraction increased by 40–60% during sleep or during anesthesia with agents that induced slow-wave sleep compared to awake rodents, which would allow for more efficient bulk flow-driven glymphatic clearance of solutes. It was suggested that the sleep-related ISF volume changes were mediated by noradrenergic signaling because adrenergic antagonists administered into CSF induced increased both CSF tracer influx and the ISF volume fraction (24). Notably, astrocytes, key cells of the glymphatic system, have been shown to be involved in regulating the sleep/wake cycle via their response to norepinephrine, which modulates the levels of extracellular ions, ultimately driving neuronal responsiveness (119).
The increase in the ISF volume fraction during sleep compared to wakefulness has only been observed in rodents and not yet confirmed in humans. Noninvasive diffusion MRI, which measures water diffusion via the “apparent diffusion coefficient” (ADC), also known as “mean diffusivity,” can indirectly capture changes in the fraction of “free” water including the ISF volume (120–123). Diffusion MRI has therefore been applied to characterize the ISF compartment volume, as represented by changes in the ADC or mean diffusivity, under conditions of sleep deprivation (124), in sleep/wake states (125), or during anesthesia with agents inducing varying degrees of slow-wave delta activity during hypnosis (126). The mean diffusivity was shown to decrease in the awake human brain under conditions of sleep deprivation combined with task practice (124). However, Demiral et al. (125) were not able to detect robust increases in the ADC during sleep (after sleep deprivation) compared to wakefulness in human subjects, thereby not corroborating sleep-induced ISF volume increases using this metric and image modality. Similarly, another study applied diffusion MRI to measure differences in the ADCs across rats anesthetized with dexmedetomidine and low-dose isoflurane (DEXM-I) versus pure isoflurane (ISO). DEXM-I anesthesia is associated with slow-wave delta oscillation and increases glymphatic transport in comparison to ISO anesthesia (70); however, the ADC was similar across the two anesthesia groups, thereby not supporting differences in ISF volume (126). Notably, DEXM was chosen for these studies because it is an α2-adrenergic agonist that antagonizes noradrenergic signaling via presynaptic receptors in the locus coeruleus (127).
Although the diffusion MRI studies in humans and rats do not corroborate increases in the ISF volume fraction during brain states associated with sleep and/or or increased slow-wave activity, changes in the CSF compartment volume have indeed been observed. Thus, in humans an increase in the CSF compartment volume was noted during sleep compared with wakefulness (125). Furthermore, in rodents, the CSF compartment volume along the large periarterial glymphatic influx pathways increased significantly with DEXM-I compared with ISO anesthesia, which supports increased CSF influx and glymphatic transport (126). If the mechanisms underlying glymphatic transport increases during sleep as observed in mice (24) are valid in the human brain, noradrenergic modulation might be an important target of interest. During sleep, decreased norepinephrine levels influence vascular tone and increase CSF influx, and likely also increase solute clearance (128). Interestingly, a recent retrospective clinical study showed that antihypertensive treatment with beta blockers that easily cross the BBB was associated with a reduced risk of AD compared with low-BBB permeability drugs (129). Norepinephrine levels also affect the oscillatory vasomotion (130), with the vasomotion linked to neurovascular coupling during sleep documented to be stronger than in the awake state (43), which would promote enhanced waste clearance during the sleep cycle. Finally, since the choroid plexus receives adrenergic innervation, noradrenergic tone can impact CSF production. Increased stimulation of sympathetic nerves innervating the choroid plexus has been shown to decrease the net rate of CSF production (131).
In summary, multiple studies support that some sleep states are associated with increased CSF movement in the free CSF spaces in a counterbalanced pattern to neurovascular coupling, as reflected by the BOLD fMRI signal (111) and also increases in CSF compartment volume. The increased fluid movement across CNS barriers during sleep is likely to be linked to increased glymphatic transport and waste clearance; however, the underlying mechanisms for this relationship remain incompletely understood.
Summary, Open Questions, and Future Directions
There is increasing research evidence that the clearance of waste solutes through the glymphatic system plays an important role in overall brain health and function. The three models of glymphatic perivascular waste clearance each address a different aspect of waste egress routes and hypothesize as to what drives the transport. Because of the varied techniques and approaches to examining the glymphatic system, it is difficult to reach a consensus as to the specific mechanisms and drivers of solute transport and drainage. Factors such as in vivo/ex vivo experiments, anesthetics, species, and underlying pathologies can affect what is observed in the glymphatic system. For future studies, it is imperative that there is harmonization of technical approaches in order to paint a more robust picture of the glymphatic system as a whole. Moreover, because there are currently no techniques that can measure transport within the perivascular spaces or the neuropil of the intact brain in vivo, there remains much to be uncovered about the specific nuances of glymphatic solute and fluid transport. However, the topology of the mLV in close proximity to the dural venous sinuses strongly supports the glymphatic system model of perivenous waste egress and provides future research directions for understanding the coupling across the two systems.
Although mapping flow and sources of CSF in the CNS is an integral part of understanding solute transport, analysis of bulk fluid movement in the subarachnoid space or cerebral cisterns is not necessarily equated to glymphatic solute transport. The movement of fluids alone provides valuable insight into the various processes involved in CNS fluid homeostasis and CSF production/reabsorption; however, it does not provide direct insight into glymphatic waste clearance. Additional studies are needed to understand the clinical value of the novel MRI-based approaches for tracking movement of CSF as well as vascular “fluid” secretion as surrogate biomarkers of glymphatic transport and brain waste egress.
The nuances regarding the purpose of sleep have long been debated; however, the glymphatic system may shed a light on some of the protective and restorative functions that sleep has in the brain. Factors relating to sleep, such as quality and duration, are linked to a variety of different health effects, and the importance and utilization of sleep are seen ubiquitously among many species. The increased CSF flow and glymphatic transport during the sleeping state may indicate that one of the functions of sleep is to clear waste from the brain that otherwise does not occur during wakefulness. Furthermore, the augmentation of glymphatic function in sleep versus awake states underscores the importance of both adequate sleep quality and duration for long-term brain health. Vasomotion also contributes to CSF and solute transport, and given that vasomotion is modulated by neuronal activity (i.e., neurovascular coupling), it appears to be an important driver for fluid and solute transport in the glymphatic system. Notably, vasomotion is also present during wakefulness, implying that sleep states may not exclusively be required for glymphatic transport. Current knowledge is lacking as to how vasomotion influences glymphatic waste egress to the mLV and beyond. Finally, the mechanisms underlying increased glymphatic transport during sleep are still undefined, and identifying these will be essential for therapeutic strategies for sustaining brain health in aging and for prevention of neurodegenerative diseases.
Acknowledgments
The authors thank Elena Nikanorova for graphical art work for FIGURES 1 AND 2.
H.B. and A.T. are supported by grants from the National Institutes of Health (1R01 AT-011419, R01 AG-057705) and BEE consortium, Cure Alzheimer’s Fund. H.B. is supported by the Leducq Foundation.
H.B. received research support from PureTech Health LLC. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
H.B. conceived and designed research; H.B. interpreted results of experiments; H.B. prepared figures; L.Z. and H.B. drafted manuscript; L.Z., A.T., E.B., and H.B. edited and revised manuscript; L.Z., A.T., E.B., and H.B. approved final version of manuscript.
References
- 1. Cserr HF. Role of secretion and bulk flow of brain interstitial fluid in brain volume regulation. Ann NY Acad Sci 529: 9–20, 1988. doi: 10.1111/j.1749-6632.1988.tb51415.x. [DOI] [PubMed] [Google Scholar]
- 2. Cserr HF, Cooper DN, Milhorat TH. Flow of cerebral interstitial fluid as indicated by the removal of extracellular markers from rat caudate nucleus. Exp Eye Res 25, Suppl: 461–473, 1977. doi: 10.1016/s0014-4835(77)80041-9. [DOI] [PubMed] [Google Scholar]
- 3. Cserr HF, Ostrach LH. Bulk flow of interstitial fluid after intracranial injection of blue dextran 2000. Exp Neurol 45: 50–60, 1974. doi: 10.1016/0014-4886(74)90099-5. [DOI] [PubMed] [Google Scholar]
- 4. Szentistványi I, Patlak CS, Ellis RA, Cserr HF. Drainage of interstitial fluid from different regions of rat brain. Am J Physiol Renal Physiol 246: F835–F844, 1984. doi: 10.1152/ajprenal.1984.246.6.F835. [DOI] [PubMed] [Google Scholar]
- 5. Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res 326: 47–63, 1985. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 6. Cushing H. The Third Circulation. London: Oxford University Press, 1926. [Google Scholar]
- 7. Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, Weller RO. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol 34: 131–144, 2008. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 8. Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4: 147ra111, 2012. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Profaci CP, Munji RN, Pulido RS, Daneman R. The blood-brain barrier in health and disease: important unanswered questions. J Exp Med 217: e20190062, 2020. doi: 10.1084/jem.20190062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol 7: a020412, 2015. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hachinski V, Einhäupl K, Ganten D, Alladi S, Brayne C, Stephan BC, Sweeney MD, Zlokovic B, Iturria-Medina Y, Iadecola C, Nishimura N, Schaffer CB, Whitehead SN, Black SE, Østergaard L, Wardlaw J, Greenberg S, Friberg L, Norrving B, Rowe B, Joanette Y, Hacke W, Kuller L, Dichgans M, Endres M, Khachaturian ZS. Preventing dementia by preventing stroke: the Berlin Manifesto. Alzheimers Dement 15: 961–984, 2019. doi: 10.1016/j.jalz.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daneman R. The blood-brain barrier in health and disease. Ann Neurol 72: 648–672, 2012. doi: 10.1002/ana.23648. [DOI] [PubMed] [Google Scholar]
- 13. Nedergaard M. Neuroscience. Garbage truck of the brain. Science 340: 1529–1530, 2013. doi: 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, Regan S, Kasper T, Peng S, Ding F, Benveniste H, Nedergaard M, Deane R. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis 93: 215–225, 2016. doi: 10.1016/j.nbd.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, Singh I, Deane R, Nedergaard M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 34: 16180–16193, 2014. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ball KK, Cruz NF, Mrak RE, Dienel GA. Trafficking of glucose, lactate, and amyloid-beta from the inferior colliculus through perivascular routes. J Cereb Blood Flow Metab 30: 162–176, 2010. doi: 10.1038/jcbfm.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mestre H, Hablitz LM, Xavier AL, Feng W, Zou W, Pu T, Monai H, Murlidharan G, Castellanos Rivera RM, Simon MJ, Pike MM, Plá V, Du T, Kress BT, Wang X, Plog BA, Thrane AS, Lundgaard I, Abe Y, Yasui M, Thomas JH, Xiao M, Hirase H, Asokan A, Iliff JJ, Nedergaard M. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 7: e40070, 2018. doi: 10.7554/eLife.40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci (Lond) 131: 2257–2274, 2017. doi: 10.1042/CS20160381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rasmussen MK, Mestre H, Nedergaard M. Fluid transport in the brain. Physiol Rev 102: 1025–1151, 2022. doi: 10.1152/physrev.00031.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 33: 18190–18199, 2013. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76: 845–861, 2014. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mestre H, Tithof J, Du T, Song W, Peng WG, Sweeney AM, Olveda G, Thomas JH, Nedergaard M, Kelley DH. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun 9: 4878, 2018. doi: 10.1038/s41467-018-07318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Achariyar TM, Li B, Peng W, Verghese PB, Shi Y, McConnell E, Benraiss A, Kasper T, Song W, Takano T, Holtzman DM, Nedergaard M, Deane R. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener 11: 74, 2016. doi: 10.1186/s13024-016-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science 342: 373–377, 2013. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hladky SB, Barrand MA. The glymphatic hypothesis: the theory and the evidence. Fluids Barriers CNS 19: 9, 2022. doi: 10.1186/s12987-021-00282-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benveniste H, Lee H, Ozturk B, Chen X, Koundal S, Vaska P, Tannenbaum A, Volkow ND. Glymphatic cerebrospinal fluid and solute transport quantified by MRI and PET imaging. Neuroscience 474: 63–79, 2021. doi: 10.1016/j.neuroscience.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benveniste H, Elkin R, Heerdt PM, Koundal S, Xue Y, Lee H, Wardlaw J, Tannenbaum A. The glymphatic system and its role in cerebral homeostasis. J Appl Physiol (1985) 129: 1330–1340, 2020. doi: 10.1152/japplphysiol.00852.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mestre H, Mori Y, Nedergaard M. The brain’s glymphatic system: current controversies. Trends Neurosci 43: 458–466, 2020. doi: 10.1016/j.tins.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Albargothy NJ, Johnston DA, MacGregor-Sharp M, Weller RO, Verma A, Hawkes CA, Carare RO. Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol 136: 139–152, 2018. doi: 10.1007/s00401-018-1862-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aldea R, Weller RO, Wilcock DM, Carare RO, Richardson G. Cerebrovascular smooth muscle cells as the drivers of intramural periarterial drainage of the brain. Front Aging Neurosci 11: 1, 2019. doi: 10.3389/fnagi.2019.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith AJ, Verkman AS. The “glymphatic” mechanism for solute clearance in Alzheimer’s disease: game changer or unproven speculation? FASEB J 32: 543–551, 2018. doi: 10.1096/fj.201700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Veluw SJ, Hou SS, Calvo-Rodriguez M, Arbel-Ornath M, Snyder AC, Frosch MP, Greenberg SM, Bacskai BJ. Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron 105: 549–561.e5, 2020. doi: 10.1016/j.neuron.2019.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nimmo J, Johnston DA, Dodart JC, MacGregor-Sharp MT, Weller RO, Nicoll JAR, Verma A, Carare RO. Peri-arterial pathways for clearance of alpha-synuclein and tau from the brain: Implications for the pathogenesis of dementias and for immunotherapy. Alzheimers Dement (Amst) 12: e12070, 2020. doi: 10.1002/dad2.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Diem AK, MacGregor Sharp M, Gatherer M, Bressloff NW, Carare RO, Richardson G. Arterial pulsations cannot drive intramural periarterial drainage: significance for Abeta drainage. Front Neurosci 11: 475, 2017. doi: 10.3389/fnins.2017.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hecht M, Krämer LM, von Arnim CAF, Otto M, Thal DR. Capillary cerebral amyloid angiopathy in Alzheimer’s disease: association with allocortical/hippocampal microinfarcts and cognitive decline. Acta Neuropathol 135: 681–694, 2018. doi: 10.1007/s00401-018-1834-y. [DOI] [PubMed] [Google Scholar]
- 36. Richard E, Carrano A, Hoozemans JJ, van Horssen J, van Haastert ES, Eurelings LS, de Vries HE, Thal DR, Eikelenboom P, van Gool WA, Rozemuller AJ. Characteristics of dyshoric capillary cerebral amyloid angiopathy. J Neuropathol Exp Neurol 69: 1158–1167, 2010. doi: 10.1097/NEN.0b013e3181fab558. [DOI] [PubMed] [Google Scholar]
- 37. Thal DR, Ghebremedhin E, Rüb U, Yamaguchi H, Del Tredici K, Braak H. Two types of sporadic cerebral amyloid angiopathy. J Neuropathol Exp Neurol 61: 282–293, 2002. doi: 10.1093/jnen/61.3.282. [DOI] [PubMed] [Google Scholar]
- 38. De Guio F, Duering M, Fazekas F, De Leeuw FE, Greenberg SM, Pantoni L, Aghetti A, Smith EE, Wardlaw J, Jouvent E. Brain atrophy in cerebral small vessel diseases: extent, consequences, technical limitations and perspectives: the HARNESS initiative. J Cereb Blood Flow Metab 40: 231–245, 2020. doi: 10.1177/0271678X19888967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carare RO, Aldea R, Bulters D, Alzetani A, Birch AA, Richardson G, Weller RO. Vasomotion drives periarterial drainage of Abeta from the brain. Neuron 105: 400–401, 2020. doi: 10.1016/j.neuron.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 40. Das A, Murphy K, Drew PJ. Rude mechanicals in brain haemodynamics: non-neural actors that influence blood flow. Philos Trans R Soc Lond B Biol Sci 376: 20190635, 2021. doi: 10.1098/rstb.2019.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jones TW. Discovery that the veins of the bat’s wing (which are furnished with valves) are endowed with rhythmical contractility, and that the onward flow of blood is accelerated by such contraction. Edinb Med Surg J 79: 367–373, 1853. [PMC free article] [PubMed] [Google Scholar]
- 42. Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 468: 232–243, 2010. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Turner KL, Gheres KW, Proctor EA, Drew PJ. Neurovascular coupling and bilateral connectivity during NREM and REM sleep. Elife 9, 2020. doi: 10.7554/eLife.62071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bakker E, Naessens DM, VanBavel E. Paravascular spaces: entry to or exit from the brain? Exp Physiol 104: 1013–1017, 2019. doi: 10.1113/EP087424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Asgari M, de Zélicourt D, Kurtcuoglu V. Glymphatic solute transport does not require bulk flow. Sci Rep 6: 38635, 2016. doi: 10.1038/srep38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bedussi B, Almasian M, de Vos J, VanBavel E, Bakker EN. Paravascular spaces at the brain surface: low resistance pathways for cerebrospinal fluid flow. J Cereb Blood Flow Metab 38: 719–726, 2018. doi: 10.1177/0271678X17737984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Davson H. Review lecture. The blood-brain barrier. J Physiol 255: 1–28, 1976. doi: 10.1113/jphysiol.1976.sp011267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212: 991–999, 2015. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature 523: 337–341, 2015. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lundgaard I, Lu ML, Yang E, Peng W, Mestre H, Hitomi E, Deane R, Nedergaard M. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab 37: 2112–2124, 2017. doi: 10.1177/0271678X16661202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wardlaw JM, Allerhand M, Eadie E, Thomas A, Corley J, Pattie A, Taylor A, Shenkin SD, Cox S, Gow A, Starr JM, Deary IJ. Carotid disease at age 73 and cognitive change from age 70 to 76 years: a longitudinal cohort study. J Cereb Blood Flow Metab 37: 3042–3052, 2017. doi: 10.1177/0271678X16683693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Choi I, Chung HK, Ramu S, Lee HN, Kim KE, Lee S, Yoo J, Choi D, Lee YS, Aguilar B, Hong YK. Visualization of lymphatic vessels by Prox1-promoter directed GFP reporter in a bacterial artificial chromosome-based transgenic mouse. Blood 117: 362–365, 2011. doi: 10.1182/blood-2010-07-298562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Flores MV, Hall CJ, Crosier KE, Crosier PS. Visualization of embryonic lymphangiogenesis advances the use of the zebrafish model for research in cancer and lymphatic pathologies. Dev Dyn 239: 2128–2135, 2010. doi: 10.1002/dvdy.22328. [DOI] [PubMed] [Google Scholar]
- 54. Hong M, Jung E, Yang S, Jung W, Seong YJ, Park E, Bramos A, Kim KE, Lee S, Daghlian G, Seo JI, Choi I, Choi IS, Koh CJ, Kobielak A, Ying QL, Johnson M, Gardner D, Wong AK, Choi D, Hong YK. Efficient assessment of developmental, surgical and pathological lymphangiogenesis using a lymphatic reporter mouse and its embryonic stem cells. PLoS One 11: e0157126, 2016. doi: 10.1371/journal.pone.0157126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Truman LA, A-Gonzalez N, Bentley KL, Ruddle NH. Lymphatic vessel function in head and neck inflammation. Lymphat Res Biol 11: 187–192, 2013. doi: 10.1089/lrb.2013.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Petrova TV, Makinen T, Alitalo K. Signaling via vascular endothelial growth factor receptors. Exp Cell Res 253: 117–130, 1999. doi: 10.1006/excr.1999.4707. [DOI] [PubMed] [Google Scholar]
- 57. Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell 98: 769–778, 1999. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 58. Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen MH, Mathivet T, Chilov D, Li Z, Koppinen T, Park JH, Fang S, Aspelund A, Saarma M, Eichmann A, Thomas JL, Alitalo K. Development and plasticity of meningeal lymphatic vessels. J Exp Med 214: 3645–3667, 2017. doi: 10.1084/jem.20170391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I, Suh SH, Hong SP, Song JH, Hong YK, Jeong Y, Park SH, Koh GY. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572: 62–66, 2019. doi: 10.1038/s41586-019-1419-5. [DOI] [PubMed] [Google Scholar]
- 60. das Neves SP, Delivanoglou N, Da Mesquita S. CNS-draining meningeal lymphatic vasculature: roles, conundrums and future challenges. Front Pharmacol 12: 655052, 2021. doi: 10.3389/fphar.2021.655052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hsu M, Rayasam A, Kijak JA, Choi YH, Harding JS, Marcus SA, Karpus WJ, Sandor M, Fabry Z. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat Commun 10: 229, 2019. doi: 10.1038/s41467-018-08163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hsu M, Laaker C, Madrid A, Herbath M, Choi YH, Sandor M, Fabry Z. Neuroinflammation creates an immune regulatory niche at the meningeal lymphatic vasculature near the cribriform plate. Nat Immunol 23: 581–593, 2022. doi: 10.1038/s41590-022-01158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brøchner CB, Holst CB, Møllgård K. Outer brain barriers in rat and human development. Front Neurosci 9: 75, 2015. doi: 10.3389/fnins.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol 18: 123–131, 2017. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- 65. Jacob L, Boisserand LS, Geraldo LH, de Brito Neto J, Mathivet T, Antila S, Barka B, Xu Y, Thomas JM, Pestel J, Aigrot MS, Song E, Nurmi H, Lee S, Alitalo K, Renier N, Eichmann A, Thomas JL. Anatomy and function of the vertebral column lymphatic network in mice. Nat Commun 10: 4594, 2019. doi: 10.1038/s41467-019-12568-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jacob L, Brito J, Thomas JL. Three-dimensional imaging of the vertebral lymphatic vasculature and drainage using iDISCO+ and light sheet fluorescence microscopy. J Vis Exp 159: 61099, 2020. doi: 10.3791/61099. [DOI] [PubMed] [Google Scholar]
- 67. Silver I, Kim C, Mollanji R, Johnston M. Cerebrospinal fluid outflow resistance in sheep: impact of blocking cerebrospinal fluid transport through the cribriform plate. Neuropathol Appl Neurobiol 28: 67–74, 2002. doi: 10.1046/j.1365-2990.2002.00373.x. [DOI] [PubMed] [Google Scholar]
- 68. Xue Y, Gursky Z, Monte B, Koundal S, Liu X, Lee H, Michurina TV, Mellanson KA, Zhao L, Nemajerova A, Kahle KT, Takemaru KI, Enikolopov G, Peunova NI, Benveniste H. Sustained glymphatic transport and impaired drainage to the nasal cavity observed in multiciliated cell ciliopathies with hydrocephalus. Fluids Barriers CNS 19: 20, 2022. doi: 10.1186/s12987-022-00319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Melin E, Eide PK, Ringstad G. In vivo assessment of cerebrospinal fluid efflux to nasal mucosa in humans. Sci Rep 10: 14974, 2020. doi: 10.1038/s41598-020-72031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Benveniste H, Lee H, Ding F, Sun Q, Al-Bizri E, Makaryus R, Probst S, Nedergaard M, Stein EA, Lu H. Anesthesia with dexmedetomidine and low-dose isoflurane increases solute transport via the glymphatic pathway in rat brain when compared with high-dose isoflurane. Anesthesiology 127: 976–988, 2017. doi: 10.1097/ALN.0000000000001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hablitz LM, Vinitsky HS, Sun Q, Stæger FF, Sigurdsson B, Mortensen KN, Lilius TO, Nedergaard M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv 5: eaav5447, 2019. doi: 10.1126/sciadv.aav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Aukland K, Kramer GC, Renkin EM. Protein concentration of lymph and interstitial fluid in the rat tail. Am J Physiol Heart Circ Physiol 247: H74–H79, 1984. doi: 10.1152/ajpheart.1984.247.1.H74. [DOI] [PubMed] [Google Scholar]
- 73. Scallan JP, Zawieja SD, Castorena-Gonzalez JA, Davis MJ. Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol 594: 5749–5768, 2016. doi: 10.1113/JP272088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Santambrogio L. The lymphatic fluid. Int Rev Cell Mol Biol 337: 111–133, 2018. doi: 10.1016/bs.ircmb.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 75. Abdallah M, Müllertz OO, Styles IK, Mörsdorf A, Quinn JF, Whittaker MR, Trevaskis NL. Lymphatic targeting by albumin-hitchhiking: applications and optimisation. J Control Release 327: 117–128, 2020. doi: 10.1016/j.jconrel.2020.07.046. [DOI] [PubMed] [Google Scholar]
- 76. Blatter C, Meijer EF, Nam AS, Jones D, Bouma BE, Padera TP, Vakoc BJ. In vivo label-free measurement of lymph flow velocity and volumetric flow rates using Doppler optical coherence tomography. Sci Rep 6: 29035, 2016. doi: 10.1038/srep29035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13: 597–610, 2006. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- 78. Bradbury MW, Cserr HF, Westrop RJ. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am J Physiol Renal Physiol 240: F329–F336, 1981. doi: 10.1152/ajprenal.1981.240.4.F329. [DOI] [PubMed] [Google Scholar]
- 79. Chen X, Liu X, Koundal S, Elkin R, Zhu X, Monte B, Xu F, Dai F, Pedram M, Lee H, Kipnis J, Tannenbaum A, Van Nostrand WE, Benveniste H. Cerebral amyloid angiopathy is associated with glymphatic transport reduction and time-delayed solute drainage along the neck arteries. Nat Aging 2: 214–223, 2022. doi: 10.1038/s43587-022-00181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Elkin R, Nadeem S, Haber E, Steklova K, Lee H, Benveniste H, Tannenbaum A. GlymphVIS: visualizing glymphatic transport pathways using regularized optimal transport. Med Image Comput Comput Assist Interv 11070: 844–852, 2018. doi: 10.1007/978-3-030-00928-1_95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Koundal S, Elkin R, Nadeem S, Xue Y, Constantinou S, Sanggaard S, Liu X, Monte B, Xu F, Van Nostrand W, Nedergaard M, Lee H, Wardlaw J, Benveniste H, Tannenbaum A. Optimal mass transport with lagrangian workflow reveals advective and diffusion driven solute transport in the glymphatic system. Sci Rep 10: 1990, 2020. doi: 10.1038/s41598-020-59045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Patel TK, Habimana-Griffin L, Gao X, Xu B, Achilefu S, Alitalo K, McKee CA, Sheehan PW, Musiek ES, Xiong C, Coble D, Holtzman DM. Dural lymphatics regulate clearance of extracellular tau from the CNS. Mol Neurodegener 14: 11, 2019. doi: 10.1186/s13024-019-0312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hampel H, Hardy J, Blennow K, Chen C, Perry G, Kim SH, Villemagne VL, Aisen P, Vendruscolo M, Iwatsubo T, Masters CL, Cho M, Lannfelt L, Cummings JL, Vergallo A. The amyloid-beta pathway in Alzheimer’s disease. Mol Psychiatry 26: 5481–5503, 2021. doi: 10.1038/s41380-021-01249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer’s disease. CNS Neurol Disord Drug Targets 8: 16–30, 2009. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14: 133–150, 2018. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yamada K, Hashimoto T, Yabuki C, Nagae Y, Tachikawa M, Strickland DK, Liu Q, Bu G, Basak JM, Holtzman DM, Ohtsuki S, Terasaki T, Iwatsubo T. The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid beta peptides in an in vitro model of the blood-brain barrier cells. J Biol Chem 283: 34554–34562, 2008. doi: 10.1074/jbc.M801487200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Patterson BW, Elbert DL, Mawuenyega KG, Kasten T, Ovod V, Ma S, Xiong C, Chott R, Yarasheski K, Sigurdson W, Zhang L, Goate A, Benzinger T, Morris JC, Holtzman D, Bateman RJ. Age and amyloid effects on human central nervous system amyloid-beta kinetics. Ann Neurol 78: 439–453, 2015. doi: 10.1002/ana.24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Karch CM, Jeng AT, Goate AM. Extracellular tau levels are influenced by variability in tau that is associated with tauopathies. J Biol Chem 287: 42751–42762, 2012. doi: 10.1074/jbc.M112.380642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pooler AM, Phillips EC, Lau DH, Noble W, Hanger DP. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep 14: 389–394, 2013. doi: 10.1038/embor.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem 284: 12845–12852, 2009. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, Sanders DW, Cook C, Fu HJ, Boonen RA, Herman M, Nahmani E, Emrani S, Figueroa YH, Diamond MI, Clelland CL, Wray S, Duff KE. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci 19: 1085–1092, 2016. doi: 10.1038/nn.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sato C, Barthélemy NR, Mawuenyega KG, Patterson BW, Gordon BA, Jockel-Balsarotti J, Sullivan M, Crisp MJ, Kasten T, Kirmess KM, Kanaan NM, Yarasheski KE, Baker-Nigh A, Benzinger TLS, Miller TM, Karch CM, Bateman RJ. Tau kinetics in neurons and the human central nervous system. Neuron 97: 1284–1298.e7, 2018. doi: 10.1016/j.neuron.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Benveniste H, Dienel G, Jacob Z, Lee H, Makaryus R, Gjedde A, Hyder F, Rothman DL. Trajectories of brain lactate and re-visited oxygen-glucose index calculations do not support elevated non-oxidative metabolism of glucose across childhood. Front Neurosci 12: 631, 2018. doi: 10.3389/fnins.2018.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Banks WA, Kovac A, Majerova P, Bullock KM, Shi M, Zhang J. Tau proteins cross the blood-brain barrier. J Alzheimers Dis 55: 411–419, 2017. doi: 10.3233/JAD-160542. [DOI] [PubMed] [Google Scholar]
- 95. Zawieja DC, Thangaswamy S, Wang W, Furtado R, Clement CC, Papadopoulos Z, Vigano M, Bridenbaugh EA, Zolla L, Gashev AA, Kipnis J, Lauvau G, Santambrogio L. Lymphatic cannulation for lymph sampling and molecular delivery. J Immunol 203: 2339–2350, 2019. doi: 10.4049/jimmunol.1900375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J, Reich DS. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 6: e29738, 2017. doi: 10.7554/eLife.29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. De Luca LA, David RB, Menani JV. Homeostasis and body fluid regulation. In: Neurobiology of Body Fluid Homeostasis: Transduction and Integration, edited by De Luca LA, Menani JV, Johnson AK.. Boca Raton, FL: CRC Press/Taylor & Francis, 2014. [PubMed] [Google Scholar]
- 98. Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, Deane R, Nedergaard M. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci 35: 518–526, 2015. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jacobsen HH, Sandell T, Jørstad OK, Moe MC, Ringstad G, Eide PK. In vivo evidence for impaired glymphatic function in the visual pathway of patients with normal pressure hydrocephalus. Invest Ophthalmol Vis Sci 61: 24, 2020. doi: 10.1167/iovs.61.13.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Eide PK, Ringstad G. Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: a glymphatic magnetic resonance imaging study. J Cereb Blood Flow Metab 39: 1355–1368, 2019. doi: 10.1177/0271678X18760974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Heisey SR, Held D, Pappenheimer JR. Bulk flow and diffusion in the cerebrospinal fluid system of the goat. Am J Physiol 203: 775–781, 1962. doi: 10.1152/ajplegacy.1962.203.5.775. [DOI] [PubMed] [Google Scholar]
- 102. Nilsson C, Ståhlberg F, Thomsen C, Henriksen O, Herning M, Owman C. Circadian variation in human cerebrospinal fluid production measured by magnetic resonance imaging. Am J Physiol Regul Integr Comp Physiol 262: R20–R24, 1992. doi: 10.1152/ajpregu.1992.262.1.R20. [DOI] [PubMed] [Google Scholar]
- 103. Evans PG, Sokolska M, Alves A, Harrison IF, Ohene Y, Nahavandi P, Ismail O, Miranda E, Lythgoe MF, Thomas DL, Wells JA. Non-invasive MRI of blood-cerebrospinal fluid barrier function. Nat Commun 11: 2081, 2020. doi: 10.1038/s41467-020-16002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Charpak S, Smith KJ, Black SE. Reply to: Rethink the classical view of cerebrospinal fluid production. Nat Rev Neurol 17: 590–591, 2021. doi: 10.1038/s41582-021-00539-z. [DOI] [PubMed] [Google Scholar]
- 105. Roques M, De Barros A, Bonneville F. Rethink the classical view of cerebrospinal fluid production. Nat Rev Neurol 17: 590, 2021. doi: 10.1038/s41582-021-00538-0. [DOI] [PubMed] [Google Scholar]
- 106. Li Q, Aalling NN, Förstera B, Ertürk A, Nedergaard M, Møllgård K, Xavier AL. Aquaporin 1 and the Na+/K+/2Cl- cotransporter 1 are present in the leptomeningeal vasculature of the adult rodent central nervous system. Fluids Barriers CNS 17: 15, 2020. doi: 10.1186/s12987-020-0176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Petitclerc L, Hirschler L, Wells JA, Thomas DL, van Walderveen MA, van Buchem MA, van Osch MJ. Ultra-long-TE arterial spin labeling reveals rapid and brain-wide blood-to-CSF water transport in humans. Neuroimage 245: 118755, 2021. doi: 10.1016/j.neuroimage.2021.118755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yatsushiro S, Sunohara S, Hayashi N, Hirayama A, Matsumae M, Atsumi H, Kuroda K. Cardiac-driven pulsatile motion of intracranial cerebrospinal fluid visualized based on a correlation mapping technique. Magn Reson Med Sci 17: 151–160, 2018. doi: 10.2463/mrms.mp.2017-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gärtner J, Frahm J. Inspiration is the major regulator of human CSF flow. J Neurosci 35: 2485–2491, 2015. doi: 10.1523/JNEUROSCI.3246-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kiviniemi V, Wang X, Korhonen V, Keinänen T, Tuovinen T, Autio J, LeVan P, Keilholz S, Zang YF, Hennig J, Nedergaard M. Ultra-fast magnetic resonance encephalography of physiological brain activity—glymphatic pulsation mechanisms? J Cereb Blood Flow Metab 36: 1033–1045, 2016. doi: 10.1177/0271678X15622047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fultz NE, Bonmassar G, Setsompop K, Stickgold RA, Rosen BR, Polimeni JR, Lewis LD. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 366: 628–631, 2019. doi: 10.1126/science.aax5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Harrison IF, Siow B, Akilo AB, Evans PG, Ismail O, Ohene Y, Nahavandi P, Thomas DL, Lythgoe MF, Wells JA. Non-invasive imaging of CSF-mediated brain clearance pathways via assessment of perivascular fluid movement with diffusion tensor MRI. Elife 7: e34028, 2018. doi: 10.7554/eLife.34028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Horie T, Kajihara N, Matsumae M, Obara M, Hayashi N, Hirayama A, Takizawa K, Takahara T, Yatsushiro S, Kuroda K. Magnetic resonance imaging technique for visualization of irregular cerebrospinal fluid motion in the ventricular system and subarachnoid space. World Neurosurg 97: 523–531, 2017. doi: 10.1016/j.wneu.2016.07.062. [DOI] [PubMed] [Google Scholar]
- 114. Bito Y, Harada K, Ochi H, Kudo K. Low b-value diffusion tensor imaging for measuring pseudorandom flow of cerebrospinal fluid. Magn Reson Med 86: 1369–1382, 2021. doi: 10.1002/mrm.28806. [DOI] [PubMed] [Google Scholar]
- 115. Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol 70: 1537–1543, 2013. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang C, Holtzman DM. Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacology 45: 104–120, 2020. doi: 10.1038/s41386-019-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, Finn MB, Manis M, Geerling JC, Fuller PM, Lucey BP, Holtzman DM. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363: 880–884, 2019. doi: 10.1126/science.aav2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, Morris JC, Holtzman DM. Sleep quality and preclinical Alzheimer disease. JAMA Neurol 70: 587–593, 2013. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ding F, O’Donnell J, Xu Q, Kang N, Goldman N, Nedergaard M. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 352: 550–555, 2016. doi: 10.1126/science.aad4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Syková E. Extrasynaptic volume transmission and diffusion parameters of the extracellular space. Neuroscience 129: 861–876, 2004. doi: 10.1016/j.neuroscience.2004.06.077. [DOI] [PubMed] [Google Scholar]
- 121. Harris NG, Zilkha E, Houseman J, Symms MR, Obrenovitch TP, Williams SR. The relationship between the apparent diffusion coefficient measured by magnetic resonance imaging, anoxic depolarization, and glutamate efflux during experimental cerebral ischemia. J Cereb Blood Flow Metab 20: 28–36, 2000. doi: 10.1097/00004647-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 122. Verheul HB, Balázs R, Berkelbach van der Sprenkel JW, Tulleken CA, Nicolay K, Tamminga KS, van Lookeren Campagne M. Comparison of diffusion-weighted MRI with changes in cell volume in a rat model of brain injury. NMR Biomed 7: 96–100, 1994. doi: 10.1002/nbm.1940070115. [DOI] [PubMed] [Google Scholar]
- 123. Benveniste H, Hedlund LW, Johnson GA. Mechanism of detection of acute cerebral ischemia in rats by diffusion-weighted magnetic resonance microscopy. Stroke 23: 746–754, 1992. doi: 10.1161/01.str.23.5.746. [DOI] [PubMed] [Google Scholar]
- 124. Voldsbekk I, Maximov II, Zak N, Roelfs D, Geier O, Due-Tønnessen P, Elvsåshagen T, Strømstad M, Bjørnerud A, Groote I. Evidence for wakefulness-related changes to extracellular space in human brain white matter from diffusion-weighted MRI. Neuroimage 212: 116682, 2020. doi: 10.1016/j.neuroimage.2020.116682. [DOI] [PubMed] [Google Scholar]
- 125. Demiral SB, Tomasi D, Sarlls J, Lee H, Wiers CE, Zehra A, Srivastava T, Ke K, Shokri-Kojori E, Freeman CR, Lindgren E, Ramirez V, Miller G, Bandettini P, Horovitz S, Wang GJ, Benveniste H, Volkow ND. Apparent diffusion coefficient changes in human brain during sleep - Does it inform on the existence of a glymphatic system? Neuroimage 185: 263–273, 2019. doi: 10.1016/j.neuroimage.2018.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ozturk BO, Monte B, Koundal S, Dai F, Benveniste H, Lee H. Disparate volumetric fluid shifts across cerebral tissue compartments with two different anesthetics. Fluids Barriers CNS 18: 1, 2021. doi: 10.1186/s12987-020-00236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Callado LF, Stamford JA. Alpha2A- but not alpha2B/C-adrenoceptors modulate noradrenaline release in rat locus coeruleus: voltammetric data. Eur J Pharmacol 366: 35–39, 1999. doi: 10.1016/S0014-2999(98)00889-9. [DOI] [PubMed] [Google Scholar]
- 128. Plog BA, Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol 13: 379–394, 2018. doi: 10.1146/annurev-pathol-051217-111018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Beaman EE, Bonde AN, Ulv Larsen SM, Ozenne B, Lohela TJ, Nedergaard M, Gíslason GH, Knudsen GM, Holst SC. Blood-brain barrier permeable beta-blockers linked to lower risk of Alzheimer’s disease in hypertension. Brain 2022: awac076, 2022. doi: 10.1093/brain/awac076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hollenberg NK, Meyerovitz M, Harrington DP, Sandor T. Influence of norepinephrine and angiotensin II on vasomotion of renal blood supply in humans. Am J Physiol Heart Circ Physiol 252: H941–H944, 1987. doi: 10.1152/ajpheart.1987.252.5.H941. [DOI] [PubMed] [Google Scholar]
- 131. Lindvall M, Owman C. Autonomic nerves in the mammalian choroid-plexus and their influence on the formation of cerebrospinal-fluid. J Cereb Blood Flow Metab 1: 245–266, 1981. doi: 10.1038/jcbfm.1981.30. [DOI] [PubMed] [Google Scholar]