Abstract
The effect of age and sex on intracranial and extracranial cerebrovascular function is poorly understood. We investigated the relationships between age, sex, and cerebrovascular reactivity (CVR) to hypercapnia in 73 healthy adults (18–80 yr, n = 39 female). CVR to hypercapnia was assessed in the middle cerebral artery (MCA) using transcranial Doppler ultrasound and at the internal carotid artery (ICA) using duplex ultrasound. MCA CVR was characterized by peak MCA velocity (MCAv) response per mmHg increase in end-tidal CO2 and by using a monoexponential model to characterize the kinetics (time constant) of the MCAv response. ICA reactivity was assessed as the relative peak increase in artery diameter. Hierarchical multiple regression determined the relationships between age, sex, and the age-by-sex interaction on all baseline and CVR outcomes. There was no relationship between ICA reactivity (%) with age (P = 0.07), sex (P = 0.56), or a moderator effect of sex on the age effect (P = 0.24). MCAv CVR showed no relationship with age (P = 0.59), sex (P = 0.09), or an age-by-sex moderator effect (P = 0.90). We observed a positive relationship of MCAv CVR time constant with age (P = 0.013), such that the speed of the MCA response was slower with advancing age. The present study provides comprehensive data on age- and sex-specific relationships with intracranial and extracranial cerebrovascular responses to hypercapnia. Despite similar MCAv CVR and ICA reactivity between sexes, kinetic responses of the MCA revealed a slower rate of adjustment with advancing age.
NEW & NOTEWORTHY We observed similar MCA CVR and ICA reactivity in males and females. However, kinetic responses of the MCA to hypercapnia suggest that advancing age slows down the rate at which MCA velocity increases in response to hypercapnia. These data indicate distinct regulatory differences, and an impaired vasomotor control of the cerebrovasculature with advancing age, not detected by traditional methods.
Keywords: aging, carbon dioxide, cerebral blood flow, internal carotid artery, life span
INTRODUCTION
Cerebral blood flow (CBF) responsiveness to hypercapnia, termed cerebrovascular reactivity (CVR), is vital in stabilizing pH levels and maintaining delivery of oxygen and nutrients to the brain (1). Previous research has evidenced the clinical importance of this outcome, with a lower CVR later in life (60+ yr of age) associated with increased risk of age-associated disease including Alzheimer’s disease, cognitive decline (2), and all-cause mortality (3). Thus, it is important to understand the physiological changes in hypercapnia-induced CVR associated with advancing age.
Despite numerous studies investigating the effect of advancing age on CVR of the anterior circulation, conclusions remain unclear (4). Some studies demonstrate CVR to decline in older compared with young adults (5–8); however, other studies show CVR to remain unchanged in older adults (9–12) or even increase (13, 14). To date, research has largely ignored comparisons with middle-aged groups (∼40–60 yr), where regulatory, functional, and structural alterations to the vasculature may manifest, and the rate of decline in CVR might be greatest (5, 15). These disparate findings between studies may be underpinned by several factors including the method of assessment, nature of hypercapnia administration, or differences in the sample population, such as age, sex, and physical fitness, which may all independently influence CVR (16–19).
Burley et al. (16) showed that when they used transcranial Doppler (TCD) ultrasound, older adults had significantly greater CVR compared with younger adults. However, with blood oxygen level-dependent magnetic resonance imaging (BOLD-MRI) measures, no differences between groups were observed. Most previous studies used TCD and were limited by the reliance on velocity measures of a single intracranial vessel [middle cerebral artery (MCA)]. Previous findings using extracranial internal carotid artery (ICA) measures have shown a decreased ICA reactivity in older (68 ± 1 yr) compared with young (23 ± 11 yr) adults (20). However, given the small sample size of 20 participants, sex differences and the sex-dependent effect of age have yet to be adequately addressed.
Few studies investigate the effect of sex on CVR in aging adults (13, 14, 20). This is a vital consideration given the potential effects of estrogen and evidence that the effects of aging on the vasculature are sex-dependent (21). Carter et al. (22) demonstrated that in young females, MCAv CVR was greater compared with males; however, this sex difference was not evident in the ICA. This is consistent across some (18, 23), but not all (24, 25), studies, possibly due to the different methodologies and the use of TCD measures alone, as TCD relies on the assumption that the MCA diameter does not change (26). This, however, may not hold true during hypercapnia (27), with the magnitude of changes in dilation potentially influenced by age (6, 28) and sex (24). In contrast, Miller et al. (6), using MRI, demonstrated that decreases in intracranial artery responses to hypercapnia were evident with advancing age in males, but not females. This highlights the need to study the effects in males and females separately as not to confound interpretations on the effect of advancing age.
Recent research has highlighted the importance of investigating dynamic kinetic-based analyses on cerebrovascular regulation, in addition to traditional amplitude-based inferences (29). This can be achieved using a monoexponential model, where the time delay, time constant, and mean response time can provide additional information on the speed of the response (29, 30). These outcomes have been shown to be indicative of regulatory responses during exercise stressors (31, 32) but have yet to be applied to examine the effect of age and sex on cerebrovascular responses to hypercapnia.
The first aim of this study is to determine intracranial and extracranial CVR to hypercapnia across the healthy adult life span in males and females, exploring traditional CVR. The second aim is to investigate if any alterations in CVR with age are sex-dependent. We hypothesized that 1) cerebrovascular reactivity in the intra- and extracranial vessels would show a negative relationship with age and 2) the rate of decline would be sex-dependent, with higher CVR in both the MCA and ICA in younger females and a greater rate of decline with advancing age compared with males.
METHODS
Ethical Approval
All experimental procedures and protocols were approved by the University of Queensland Ethics Committee (No. 2019001863), and the study conformed to the standards set by the Declaration of Helsinki. Written, informed consent was obtained before participation in the study.
Participants
Participant recruitment was based on an a priori power calculation to detect differences between age groups for ICA dilation (%) in response to hypercapnia, set for large effect size (F = 0.4), power (0.8), and α (0.05) (20) (G* Power 3.1 Kiel, Germany). This resulted in a target recruitment of 20 participants per age group (young = 18–39 yr; middle age = 40–64 yr; and older = 40–64 yr). Assuming a 20% data loss due to image capture/analysis problems, we set a target of ∼70–75 participants. Seventy-three adults between the ages of 18–80 yr volunteered to take part in this study.
Exclusion criteria included diagnosed arterial hypertension, smoking, any known cardiometabolic or respiratory diseases, the use of any prescribed medications known to influence cardiovascular function (e.g., statins, thyroid medication), and a body mass index (BMI) > 35 kg/m2. Any premenopausal females with an irregular menstrual cycle or the use of progesterone-only contraceptive pill were excluded from the study. In addition, naturally menstruating premenopausal females (n = 8) were tested in the follicular phase (1–14 days) to allow better comparisons between sexes (33). Females on the combined contraceptive pill (n = 10) were tested during the inactive pill phase (days 1–7). Postmenopausal females on hormone replacement therapy were excluded from the study.
Study Design
Following baseline screening, participants completed one visit to the laboratory. They were required to fast for a minimum of 3 h and refrain from nitrate-rich foods for 12 h before testing. In addition, participants were required to avoid vigorous physical activity, caffeine, and alcohol consumption for 24 h before testing. Body mass and stature were measured according to standard procedures to the nearest 0.1 kg and 0.1 cm, respectively. BMI classifications were used to determine the weight status of participants (34). Physical activity levels were assessed via the Active Australia survey (35) and reported as METmin/wk, which accounts for time spent in different intensities of aerobic activity (36). This survey has been validated against pedometer and accelerometery data in healthy middle-aged adults (R = 0.52) (37) and compares favorably to other self-reported physical activity surveys (ICC = 0.64) (38). Female participants self-reported menopausal status via a questionnaire and were categorized into either premenopausal (regular periods), perimenopausal (irregular cycles), early postmenopausal [1–3 yr following last menstrual period (LMP)], or postmenopausal (6+ yr LMP) (39). Following initial screening and questionnaires, participants were required to rest in a darkened temperature-controlled laboratory (∼23°C) for 15 min in the supine position before instrumentation and the commencement of the protocol.
Experimental Measures
The CVR protocol was conducted in the supine position in line with recommendations (40) and to replicate existing studies, given the potential effects of body position on CBF outcomes (41). It consisted of a 2-min baseline breathing ambient room air, followed by 5 min of hypercapnia. During hypercapnia, 5% CO2 was administered with 21% O2 (balanced nitrogen). This replicates the protocol from other laboratories which have investigated the effects of hypercapnia on advancing age (8) and within the normal vasodilatory stimuli ranges of 5–7% CO2 (42). A three-way valve (Hans Rudolph, Shawne, KS) allowed inspiratory gases to be switched from ambient air to the 5% CO2 mixture (using a 170-L Douglas bag, Hans Rudolph). Participants were instructed to breathe normally during hypercapnia and the baseline periods. Cardiorespiratory parameters were simultaneously determined throughout the protocol, as described in Cardiorespiratory Measures. Hypercapnia was chosen as the stimulus because of its sensitivity to disease risk (42) and the availability of reliability data on this outcome (30).
Cardiorespiratory Measures
During the protocol, beat-by-beat blood pressure was continuously measured by finger volume-clamp method (Finapress, NOVA, The Netherlands). Participants wore a snorkel mouthpiece and nose clip (Hans Rudolph) to measure end-tidal carbon dioxide () and end-tidal oxygen concentrations () using a gas analyzer (ADInstruments, ML206, Colorado Springs, CO), which was calibrated before each participant via known concentrations of O2 and CO2. V̇E was measured using a spirometer (ADInstruments), calibrated with a 3-L syringe. Heart rate was assessed using a three-lead ECG (Finapress, NOVA, The Netherlands). All data were sampled continuously (PowerLab; Model No. 8/30, ADInstruments) and stored at 200 Hz using an analog-to-digital converter interfaced with a laptop computer (LabChart version 8, ADInstruments) for offline analysis.
Cerebrovascular Measures
Intracranial.
A 2-MHz transcranial Doppler ultrasound probe (Spencer Technologies, ST3, Redmond, WA) was used to insonate the right MCA at an initial depth of ∼50 mm through the trans-temporal window using previously described guidelines (43). The Doppler signals were acquired, optimized, and secured using an adjustable headset (adult M600 bilateral head frame; Spencer Technologies, Redmond, WA). Beat-by-beat MCAv was calculated as the mean across each cardiac cycle and exported from LabChart as second-by-second data for analysis (Version 8, ADInstruments).
Extracranial.
Diameter and mean blood velocity were measured in the right ICA using a 12-MHz linear-array Doppler probe through a high-resolution ultrasound machine (Terason, 3300, U-smart, Burlington, MA). The ICA was identified, and the image and waveform were optimized in accordance with extracranial carotid artery guidelines (40). Doppler velocity assessments were obtained using pulse-wave mode with an insonation angle ≤60°. Following optimization of the longitudinal B-mode image of the arterial walls, images of the artery and associated velocity waveforms were simultaneously recorded during the baseline and hypercapnic periods.
Data Processing
Steady-state response.
Baseline values were averaged over 120 s of supine rest. All data from LabChart were exported as 1-s averages into Excel (Microsoft, Seattle, WA). Analysis of ICA diameter and flow were performed using custom-designed, edge-detection, and wall-tracking software (BloodFlow Analysis, version 5.1). This approach is independent of investigator bias with automated wall tracking and has previously been comprehensively described and validated (44, 45). Analysis was performed blinded to the participant age and sex. From synchronized diameter and velocity data, blood flow (the product of lumen cross-sectional area and Doppler velocity), and shear rate (4 × mean blood velocity/vessel diameter) were calculated at 30 Hz (46). ICA data were then interpolated from 30 to 1 Hz and exported into an Excel spreadsheet. The LabChart and ICA vascular data were time aligned to the start of the hypercapnic protocol in LabChart and reexported to Excel for subsequent analysis using in-house, carotid shear-mediated dilation software. This automated software calculated indices of baseline (median value of the baseline), peak response following the onset of CO2, and the relative change (%) from baseline to peak, for all ICA variables (diameter, peak systolic velocity, mean blood flow, and shear rate).
Flow pulsatility index (PI) was calculated as the difference between diastolic and systolic MCAv divided by the mean MCAv (MCAvsystolic – MCAvdiastolic/MCAvmean) (47). The PI response was obtained at baseline and the final 30 s of each minute of hypercapnia. Cerebrovascular resistance index (CVRi) was calculated as mean arterial pressure (MAP) divided by MCAv (mmHg/cm/s) at baseline and the last 30 s of hypercapnia to appropriately capture the hypercapnic response, given that CVRi decreases throughout the response. The responses to hypercapnia were obtained in the final 30 s of each minute, and differences from baseline to peak during hypercapnia were calculated for HR, MAP, ICA blood flow, MCAv, V̇e, , and .
Calculation of CVR to hypercapnia was expressed as the absolute change from baseline MCAv per unit increase (mmHg) in . This response was quantified as the peak rolling 30-s average during hypercapnia, wherever this occurred (30). CVR was calculated in this way as the most reliable analysis method to address recent concerns on the variability of changes in MCAv during open-circuit breathing (30, 48).
Kinetic response.
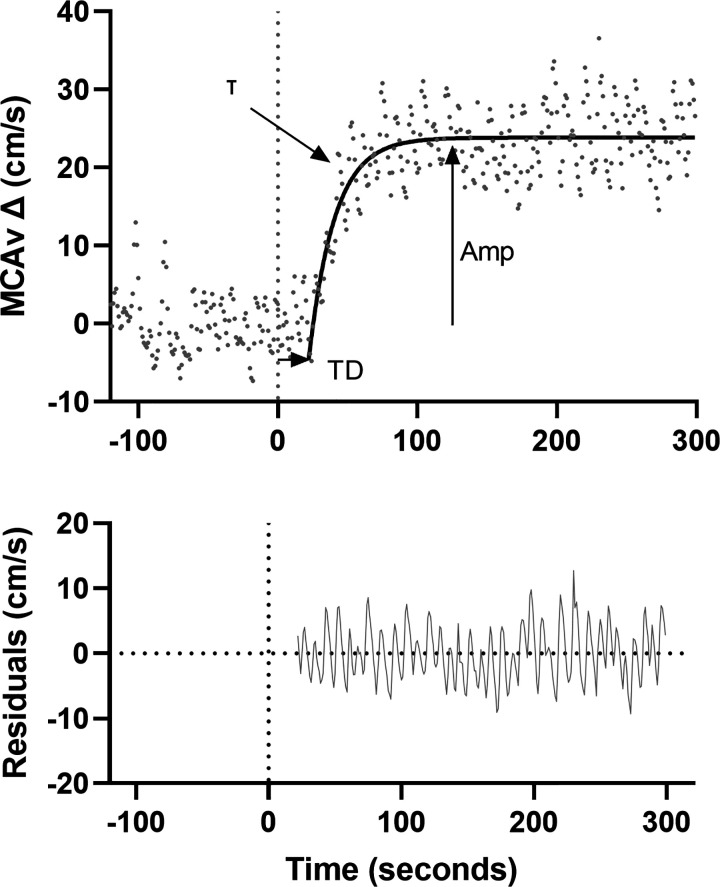
Data were baseline corrected for the 120 s preceding hypercapnia and analyzed using a monoexponential model with time delay using GraphPad Prism (Fig. 1) (GraphPad Software, San Diego, CA) as follows: MCAv(t) = ΔMCAvA[1 – e–(t − TD/τ)], where MCAv(t) is the MCAv at a given time (t), ΔMCAvA is the amplitude change of MCAv from baseline to its asymptote, TD is the time delay, and τ is the time constant, in accordance with kinetic modeling in previous work (29, 30). Mean response time (MRT) was calculated as the sum of the model derived τ and the TD. The model was fitted from the start of the exponential rise until a deviation from a subjective visual steady state was observed. All models were then checked by two independent researchers for consistency, and any disagreements were discussed until a consensus was reached. Acceptability of appropriate fit was determined as the goodness of fit R2 > 0.50 and normality of residuals. The precision of the derived τ was quantified using 95% confidence intervals.
Figure 1.
An example of MCAv trace for one participant at rest (120 s) and following onset of hypercapnia (time 0, denoted by dotted line). Residual plot is included below. Hypercapnic response characterized by a monoexponential model with a delay term shown by the solid black line. Time delay (TD) presents the time from the start of hypercapnia (0 s) to the onset of the exponential rise. Time constant (τ) presents the time taken to reach 63% of the response amplitude and it reflects the rate of increase in MCAv. Amplitude (Amp) presents the change from baseline to the peak of the exponential increase in MCAv. MCAv, middle cerebral artery velocity.
Internal carotid artery dilation.
All ICA data were passed through a two-stage filtering process; a median filter (with a rank of 7) was applied to the parameter data, followed by passage through a Savitzky–Golay finite impulse-response smoothing filter with a window size of 13 data points and a polynomial order of 1 (22). These filters removed high-frequency noise to reveal the underlying lower frequency physiological response profiles. All subsequent analyses were performed using this graphed, filtered data of variables including ICA shear rate, diameter, mean blood flow, peak blood flow, mean velocity, and peak systolic velocity. The following variables were automatically detected and calculated by the software: 1) baseline, the median value of the 2-min baseline period preceding hypercapnia; 2) peak response, the autodetected maximum value of the filtered data identified after the onset of CO2; and 3) relative change, change from baseline to peak, calculated as [(peak − baseline)/baseline] ×100. The total and initial stimulus for subsequent dilation were quantified as the shear rate (SR) area under the curve (AUC) from the time of CO2 onset to the time of peak diameter (SRAUC) and to the first 50 s after CO2 onset (SRAUC-initial). SRAUC-initial was chosen as an attempt to account for the initial shear stress stimulus, driving the potential changes in diameter. SRAUC and SRAUC-50s were calculated using the trapezoid rule (GraphPad, Prism, version 9). In addition, a thresholding algorithm was applied to each data array (e.g., ICA shear, ICA diameter), which identified threshold points. These thresholds were defined as the point at which each variable began to systematically increase, above the baseline level, after the application of the CO2 stimulus. The threshold point was calculated as follows: threshold point = (maximum value – minimum value) × %variation factor) + baseline median value (20, 22). This variation factor was chosen to ensure that the variable had increased to a point that represented a definitive deviation from baseline, which also exceeded fluctuations associated with cardiac and respiratory cycles. Once the software had automatically detected the threshold points, they were depicted on the raw data array and visually inspected to ensure they met the following criteria: 1) the algorithm-detected threshold point occurred before the peak value and 2) the variable did not decrease below the algorithm-detected threshold point before the peak value occurring (22). In cases where they did not meet the criteria (∼22%), the threshold points were manually adjusted independently to a point where it was deemed there was a clear deviation from baseline values that met the above criteria. This was then checked by two independent researchers.
ICA dilation (%) was allometrically scaled to account for differences in baseline ICA diameter as previously described (49, 50).
Statistical Analyses
Statistical analyses were conducted using SPSS (version 25; IBM, Armonk, NY). All data are presented as means ± SD. Differences in participant characteristics were explored using a two-way analysis of variance (ANOVA) with sex (male, female) and age (young, middle age, older) as the independent variables. For aim 1, hierarchical multiple regression was used to determine the relationships between age (yr; model 1) and sex (model 2) with all baseline and hypercapnic variables of interest. For aim 2, an interaction term of age × sex (model 3) was added to address whether sex moderated the effects of age by assessing the differences in regression slope coefficients between males and females on all variables of interest. The outputs for model 1 and 2 included the slope coefficient (unstandardized β) and the explained variance of the full model (R2) and the significance of the relationship (P value). For model 3, the output included slope coefficients (unstandardized β) of the interaction terms for males by age and for females by age. The P value describes whether there was a significant difference in slope coefficients between males and females with advancing age (significant interaction), and the R2 denotes the degree of explained variance of the entire regression model with the interaction term included.
To adjust for any variance explained by body mass and physical activity levels, BMI (kg/m2) and self-reported physical activity (METmin/wk) were added to the model. Finally, MAP was added to the model to adjust for any variance on cerebrovascular and ICA outcomes explained by changes in perfusion pressure. In instances where these factors (BMI, physical activity, and MAP) significantly explained any variance in the overall model response, the results are presented for the full model and whether the addition of this variable altered the effects of age, sex, and the age-by-sex interaction term. A simple linear regression was run to investigate the influence of menopause on variables of interest in a female-only model. The model investigated the relationship between early postmenopause (1–3 yr LMP) and late postmenopause (6+ yr LMP) to a reference group of premenopausal females on variables of interest. All data were normally distributed as assessed by visual inspection of Q-Q plots and homoscedasticity of the studentized residuals plotted against the predicted values. Linearity was established by visual inspection of a scatterplot. There was no evidence of multicollinearity as evidenced by no tolerance values less than 0.24. Although some data points were identified as above two to three standard deviations from the mean, none was deemed implausible and removed. Statistical significance was accepted at an α of P < 0.05.
RESULTS
Participants were recruited into young (n = 25, 12 female, age = 27.0 ± 2.6 yr, range = 22–32 yr), middle-aged (n = 30, 17 female, age = 52.9 ± 7.5 yr, range = 35–63 yr), and older (n = 18, 10 female, age = 69.8 ± 3.5 yr, range = 65–77 yr) groups. Participant characteristics of the cohort can be seen in Table 1. Intracranial kinetic analyses are presented for 71 adults (38 females), because of unacceptable model fit in two individuals. Extracranial analyses are included for 58 individuals (31 females). Reasons for data loss of ICA analyses were the inability to obtain a sufficiently clear ultrasound image in 15 individuals.
Table 1.
Participant characteristics and baseline cerebrovascular parameters
| Total |
Young |
Middle |
Older |
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Males | Females | Males | Females | Males | Females | Males | Females |
| n | 34 | 39 | 13 | 12 | 13 | 17 | 8 | 10 |
| Age, yr | 46.9 ± 18.5 | 50.6 ± 16.5 | 26.8 ± 2.9a,b | 27.2 ± 2.3a,b | 50.3 ± 8.4a,c | 54.7 ± 6.5a,c | 70.9 ± 3.3b,c | 70.0 ± 3.5b,c |
| Stature, cm | 175.7 ± 9.4* | 171.5 ± 7.9 | 179.4 ± 6.7 | 172.0 ± 7.9 | 170.2 ± 9.9 | 174.4 ± 7.5 | 178.4 ± 8.8 | 165.7 ± 5.5 |
| Weight, kg | 77.0 ± 13.7 | 73.7 ± 13.5 | 77.7 ± 13.0 | 79.0 ± 17.7 | 70.3 ± 11.1 | 72.1 ± 11.5 | 85.8 ± 14.0 | 70.2 ± 10.0 |
| BMI, kg·m2 | 21.8 ± 3.2 | 21.4 ± 3.3 | 21.6 ± 3.4 | 22.9 ± 4.3 | 20.6 ± 2.5 | 20.6 ± 2.7 | 24.0 ± 3.1 | 21.2 ± 2.9 |
| Normal weight, %Ω | 75 | 74 | 62 | 67 | 85 | 76 | 75 | 80 |
| PA, METmin/wk | 2,358 ± 1,590 | 2,029 ± 1,071 | 2,172 ± 829 | 2,309 ± 1,159 | 1,867 ± 1,076 | 1,944 ± 992 | 3,373 ± 2,579 | 1,859 ± 1,151 |
| MAP, mmHg | 96 ± 19 | 103 ± 17 | 87 ± 26a,b | 93 ± 11a,b | 97 ± 10a,c | 103 ± 15a,c | 108 ± 7b,c | 111 ± 13b,c |
| Intracranial cerebrovascular parameters (n = 73, females = 39) | ||||||||
| MCAv, cm/s | 63.7 ± 14.0* | 72.7 ± 15.9 | 70.8 ± 14.0b | 76.9 ± 10.9b | 62.8 ± 12.2c | 74.8 ± 15.0c | 53.7 ± 11.3b,c | 64.2 ± 20.2b,c |
| CVCi, cm/s/mmHg | 0.65 ± 0.19* | 0.74 ± 0.21 | 0.77 ± 0.18a,b | 0.88 ± 0.16a,b | 0.64 ± 0.15a,c | 0.73 ± 0.16a,c | 0.48 ± 0.14b,c | 0.58 ± 0.23b,c |
| CVRi, mmHg/cm/s | 1.6 ± 0.5 | 1.5 ± 0.5 | 1.3 ± 0.4a,b | 1.2 ± 0.2a,b | 1.6 ± 0.4a,c | 1.4 ± 0.3a,c | 2.1 ± 0.5b,c | 2.0 ± 0.6b,c |
| PI, AU | 0.76 ± 0.16† | 0.74 ± 0.12 | 0.82 ± 0.22 | 0.67 ± 0.10 | 0.69 ± 0.10c | 0.72 ± 0.10c | 0.78 ± 0.08c | 0.85 ± 0.11c |
| , mmHg | 41.7 ± 6.2 | 41.4 ± 8.8 | 44.6 ± 5.4 | 42.4 ± 4.6 | 42.9 ± 4.4 | 41.2 ± 4.6 | 36.7 ± 6.0 | 40.5 ± 4.9 |
| V̇e, L/min | 11.7 ± 4.2 | 10.8 ± 4.2 | 11.9 ± 4.1 | 11.4 ± 4.3 | 11.6 ± 5.2 | 11.4 ± 4.8 | 11.3 ± 2.7 | 9.2 ± 2.8 |
| Extracranial internal carotid artery parameters (n = 58, females = 31) | ||||||||
| ICA diameter, cm | 0.61 ± 0.11* | 0.52 ± 0.08 | 0.60 ± 0.10 | 0.49 ± 0.05 | 0.56 ± 0.03 | 0.54 ± 0.08 | 0.68 ± 0.16 | 0.53 ± 0.10 |
| Peak systolic velocity, cm/s | 30.9 ± 7.7 | 36.2 ± 12.5 | 29.8 ± 7.5 | 34.4 ± 9.9 | 31.4 ± 8.1 | 35.2 ± 10.6 | 31.9 ± 8.4 | 40.2 ± 18.5 |
| Mean blood flow, mL/min | 470.8 ± 235.8 | 417.1 ± 270.0 | 476.4 ± 267.2 | 266.4 ± 98.5 | 395.3 ± 221.0 | 535.7 ± 388.9 | 566.3 ± 200.8 | 412.3 ± 199.5 |
| Shear rate, s−1 | 213.1 ± 69.2* | 268.2 ± 86.5 | 207.7 ± 57.4 | 287.7 ± 104.0 | 222.4 ± 50.9 | 256.4 ± 80.6 | 206.9 ± 106.9 | 263.8 ± 78.0 |
Values are means ± SD. Data were compared using a two-way ANOVA with main effects of age (males and females) and sex (young, middle, and older). *Significant main effects of sex. When main effect of age is present, post hoc pairwise comparisons reveal where significant differences lie: ayoung vs. middle, byoung vs. older, cmiddle vs. older. †Significant age-by-sex interaction effect. ΩAccording to BMI classifications [Weir and Jan (34)], proportion of participants classified as normal weight are presented as a percentage. BMI, body mass index; CVCi, cerebrovascular conductance index; CVRi, cerebrovascular resistance index; MAP, mean arterial pressure; MCAv, Middle cerebral artery velocity; PA, physical activity; , end-tidal carbon dioxide; PI, pulsatility index; V̇e, minute ventilation.
The main effects of age and sex on participant characteristics are highlighted in Table 1. A significant age-by-sex interaction was present for PI (P = 0.006). Post hoc pairwise comparisons revealed significant differences between young males compared with females (P = 0.004). In male participants, significant differences between young compared with middle-aged adults were present (P = 0.008). In female participants, significant differences between older adults compared with young (P = 0.001) and middle-aged adults were observed (P = 0.01).
Baseline Responses
The relationships between age and baseline cerebrovascular and cardiorespiratory variables of interest can be seen in Table 2. These data are presented for age (model 1), age and sex (model 2), and the moderator effect of sex on the relationship between age and variables of interest (model 3).
Table 2.
Baseline cardiovascular and cerebrovascular variables
|
Model 1: Age |
Model 2: Age and Sex |
Model 3: Interaction (Age × Sex) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age |
Sex |
|||||||||||
|
P value |
R 2 | β |
P value |
β |
P value |
β | Combined R2 |
P value |
R 2 | β Males | β Females | |
| Intracranial cerebrovascular parameters (n = 73, females = 39) | ||||||||||||
| MCAv, cm/s | 0.005 | 0.11 | −0.29 ± 0.10 | 0.001 | −0.33 ± 0.09 | 0.003 | 10.2 ± 3.3 | 0.21 | 0.41 | 0.22 | −0.41 ± 0.13 | −0.25 ± 0.14 |
| CVCi, cm/s/mmHg | <0.001 | 0.33 | −0.006 ± 0.001 | <0.001 | −0.007 ± 0.001 | 0.006 | 0.11 ± 0.04 | 0.37 | 0.80 | 0.37 | −0.007 ± 0.002 | −0.006 ± 0.002 |
| CVRi, mmHg/cm/s | <0.001 | 0.30 | 0.016 ± 0.003 | <0.001 | 0.016 ± 0.003 | 0.09 | 0.17 ± 0.10 | 0.33 | 0.49 | 0.34 | 0.014 ± 0.004 | 0.018 ± 0.004 |
| PI, AU | 0.29 | 0.016 | 0.001 ± 0.001 | 0.25 | 0.001 ± 0.001 | 0.42 | −0.027 ± 0.033 | 0.025 | 0.002 | 0.14 | −0.001 ± 0.001 | 0.004 ± 0.001 |
| Cardiovascular parameters (n = 73, females = 39) | ||||||||||||
| MAP, mmHg | <0.001 | 0.25 | 0.49 ± 0.10 | <0.001 | 0.47 ± 0.10 | 0.15 | 5.20 ± 3.50 | 0.27 | 0.92 | 0.27 | 0.48 ± 0.14 | 0.46 ± 0.15 |
| , mmHg | 0.06 | 0.048 | −0.09 ± 0.05 | 0.07 | −0.10 ± 0.05 | 0.93 | −0.15 ± 1.7 | 0.048 | 0.16 | 0.075 | −0.16 ± 0.07 | −0.02 ± 0.07 |
| V̇e, L/min | 0.28 | 0.02 | −0.031 ± 0.03 | 0.31 | −0.029 ± 0.03 | 0.57 | −0.57 ± 0.9 | 0.02 | 0.45 | 0.03 | −0.009 ± 0.04 | −0.05 ± 0.04 |
| Extracranial internal carotid artery parameters (n = 58, females = 31) | ||||||||||||
| ICA diameter, cm | 0.09 | 0.05 | 0.001 ± 0.001 | 0.08 | 0.002 ± 0.001 | 0.001 | −0.09 ± 0.02 | 0.23 | 0.73 | 0.23 | 0.002 ± 0.001 | 0.001 ± 0.001 |
| Peak systolic velocity, cm/s | 0.31 | 0.02 | 0.08 ± 0.08 | 0.44 | 0.06 ± 0.06 | 0.07 | 5.4 ± 2.8 | 0.08 | 0.70 | 0.08 | 0.094 ± 0.12 | 0.03 ± 0.12 |
| Mean blood flow, mL/min | 0.14 | 0.04 | 2.9 ± 1.9 | 0.12 | 3.0 ± 1.9 | 0.38 | −60.5 ± 67.9 | 0.06 | 0.24 | 0.08 | 0.91 ± 2.7 | 5.5 ± 2.8 |
| Shear rate, s−1 | 0.48 | 0.009 | −0.47 ± 0.65 | 0.34 | −0.60 ± 0.62 | 0.007 | 59.2 ± 21.3 | 0.14 | 0.49 | 0.14 | −0.17 ± 0.8 | −1.0 ± 0.9 |
Values are means ± SD. Boldface indicates significant relationship (P < 0.05). Model 1 presents the relationship between age and the indicated variable. Model 2 presents the addition of sex to the model and the relationship between age and sex with the indicated variable. Model 3 indicates if sex moderates the relationship between age and the indicated variable, with individual unstandardized β Coefficients shown for males and females. In models 1–3, R2 value reflects the full model. In models 1 and 3, β represents the unstandardized β coefficient representing the change in variable units for every year increase in age. In model 2, the β coefficient provides the difference in females vs. males in the variable units. CVCi, cerebrovascular conductance index; CVRi; cerebrovascular resistance index; ICA, internal carotid artery; MAP, mean arterial pressure; MCAv, middle cerebral artery velocity; , end-tidal carbon dioxide; PI, pulsatility index; V̇e, minute ventilation.
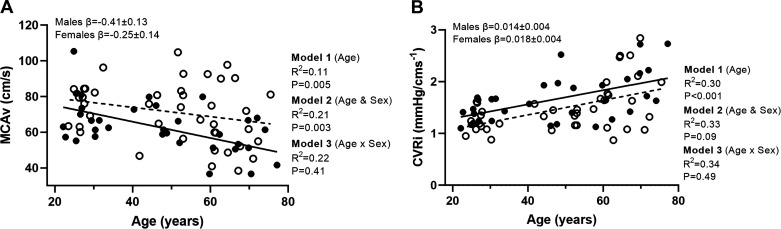
There was a negative relationship between MCAv and age (model 1). With the addition of sex to the model (model 2), this showed a relationship between MCAv and sex, explaining 21% of variance (P = 0.003) and MCAv higher in females (β = 10.2 ± 3.3 cm/s; Fig. 2A, Table 2). There was no age-by-sex interaction (model 3) for baseline MCAv (P = 0.41). There was a positive relationship between CVRi and age (P < 0.001; R2 = 0.30; Fig. 2B); with the addition of sex in model 2, this did not lead to an increase in explained variance for CVRi (P = 0.09). Sex did not moderate the effect of age on baseline CVRi (P = 0.49).
Figure 2.
A and B: linear regression analysis demonstrating the relationships between age and baseline middle cerebral artery velocity (MCAv; A) and cerebrovascular resistance index (CVRi; B; n = 73, females = 39). Solid line represents the regression fit for males, and dotted line represents the regression fit for female participants. Hierarchical regression models (P value and R2) are presented for the relationship with age (model 1), the relationship with sex (model 2), and the moderator relationship of the sex-dependent relationship with age (model 3). Slope coefficients (β) of the relationship with age are presented for males and females separately (model 3). MCAv, middle cerebral artery velocity.
Baseline ICA diameter showed no relationship with age (P = 0.09), but there was an effect of sex, with the ICA diameter greater in males and explaining 23% of variance (P < 0.001). Sex did not moderate the effect of age on baseline ICA diameter such that the slope coefficients were not different in males and females with age (P = 0.73).
Baseline ICA shear rate, peak systolic velocity, and mean blood flow all showed no relationship with age (P ≥ 0.14). With the addition of sex to the model, there was a relationship for shear rate (P = 0.007). All other ICA variables (peak systolic velocity and mean blood flow) showed no relationship with the addition of sex to the model (P ≥ 0.07). Sex did not moderate the effects of age on baseline shear rate, peak systolic velocity, and mean blood flow (P ≥ 0.24; Table 2).
For baseline , with the addition of physical activity (weekly METmin) to the model, this led to an increase in explained variance of the full model (R2 = 16, P = 0.04; β = −0.005 ± 0.002). The addition of physical activity to the model did not significantly influence the effects of age (R2 = 0.052; P = 0.05), sex (R2 = 0.052; P = 0.97), or the interaction of age and sex on baseline (R2 = 0.095; P = 0.17). For all other baseline cardiovascular and cerebrovascular outcomes, the addition of physical activity and BMI did not lead to an increase in explained variance and therefore was not included in the model (R2 ≤ 0.41; P ≥ 0.057). With the addition of MAP to the model, this led to an increase in explained variance for baseline MCAv (R2 = 0.28; P = 0.03; β = 0.25 ± 0.11). The addition of MAP to the model did not influence any of the relationships between baseline MCAv and age (R2 = 0.11; P = 0.005), sex (R2 = 0.21; P = 0.003), or the interaction of age and sex (R2 = 0.22; P = 0.41). There was no relationship between MAP and any ICA baseline responses (R2 ≤ 0.02; P ≥ 0.26).
Peak Hypercapnia Responses
The relationship between age and sex and the peak hypercapnic cerebrovascular and cardiorespiratory variables of interest can be seen in Table 3. These data are presented for age (model 1), age and sex (model 2), and the moderator effect of sex on the relationship between age and variables of interest (model 3). Data for cardiovascular and cerebrovascular responses to hypercapnia are presented in Supplemental Table S1; see https://doi.org/10.6084/m9.figshare.20422593).
Table 3.
Peak cardiovascular and cerebrovascular responses to hypercapnia
|
Model 1: Age |
Model 2: Age and Sex |
Model 3: Interaction (Age × Sex) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age |
Sex |
|||||||||||
|
P value |
R 2 | β |
P value |
β |
P value |
β | Combined R2 |
P value |
R 2 | β Males | β Females | |
| Intracranial cerebrovascular parameters | ||||||||||||
| MCAv, cm/s | 0.03 | 0.07 | −0.30 ± 0.13 | 0.007 | −0.35 ± 0.13 | 0.002 | 14.0 ± 4.4 | 0.18 | 0.28 | 0.20 | −0.49 ± 0.18 | −0.21 ± 0.18 |
| CVR, cm−1/mmHg | 0.59 | 0.004 | −0.003 ± 0.006 | 0.45 | −0.004 ± 0.006 | 0.09 | 0.34 ± 0.20 | 0.05 | 0.90 | 0.05 | −0.005 ± 0.008 | −0.004 ± 0.008 |
| PI, AU | 0.45 | 0.008 | 0.003 ± 0.004 | 0.47 | 0.003 ± 0.004 | 0.74 | 0.046 ± 0.14 | 0.01 | 0.72 | 0.01 | 0.001 ± 0.005 | 0.004 ± 0.006 |
| CVCi, cm/s/mmHg | <0.001 | 0.26 | −0.007 ± 0.001 | <0.001 | −0.007 ± 0.001 | 0.01 | 0.11 ± 0.05 | 0.32 | 0.83 | 0.32 | −0.007 ± 0.002 | −0.007 ± 0.002 |
| CVRi, mmHg/cm/s | <0.001 | 0.24 | 0.012 ± 0.003 | <0.001 | 0.013 ± 0.003 | 0.07 | 0.16 ± 0.09 | 0.27 | 0.43 | 0.28 | 0.015 ± 0.004 | 0.011 ± 0.004 |
| Cardiovascular parameters | ||||||||||||
| MAP, mmHg | <0.001 | 0.31 | 0.48 ± 0.10 | <0.001 | 0.47 ± 0.12 | 0.34 | 3.24 ± 3.32 | 0.32 | 0.30 | 0.33 | 0.36 ± 0.14 | 0.57 ± 0.13 |
| , mmHg | 0.32 | 0.01 | −0.14 ± 0.13 | 0.28 | −0.15 ± 0.14 | 0.43 | −3.9 ± 4.8 | 0.02 | 0.77 | 0.02 | −0.12 ± 0.20 | −0.19 ± 0.19 |
| V̇e, L/min | 0.74 | 0.002 | −0.017 ± 0.05 | 0.93 | −0.005 ± 0.05 | 0.04 | −3.8 ± 1.8 | 0.06 | 0.99 | 0.06 | −0.005 ± 0.05 | −0.004 ± 0.08 |
| Extracranial internal carotid artery parameters | ||||||||||||
| ICA diameter dilation, % | 0.07 | 0.06 | 0.043 ± 0.02 | 0.06 | 0.044 ± 0.02 | 0.56 | −0.47 ± 0.79 | 0.07 | 0.24 | 0.09 | 0.018 ± 0.032 | 0.072 ± 0.033 |
| Peak velocity, cm/s | 0.09 | 0.05 | 0.21 ± 0.12 | 0.13 | 0.19 ± 0.12 | 0.14 | 6.43 ± 4.29 | 0.09 | 0.41 | 0.10 | 0.29 ± 0.17 | 0.09 ± 0.17 |
| Peak velocity, Δ% | 0.21 | 0.03 | 0.27 ± 0.21 | 0.18 | 0.27 ± 0.21 | 0.43 | −6.0 ± 7.5 | 0.04 | 0.02 | 0.13 | 0.78 ± 0.29 | −0.20 ± 0.29 |
| Mean blood flow, mL/min | 0.02 | 0.11 | 7.1 ± 2.8 | 0.01 | 7.4 ± 2.8 | 0.20 | −128.7 ± 98.0 | 0.13 | 0.86 | 0.13 | 6.9 ± 3.9 | 8.0 ± 4.1 |
| Mean blood flow, Δ% | 0.03 | 0.08 | 0.50 ± 0.23 | 0.03 | 0.51 ± 0.23 | 0.56 | −4.8 ± 8.2 | 0.09 | 0.10 | 0.13 | 0.87 ± 0.32 | 0.11 ± 0.34 |
| Shear rate, s−1 | 0.97 | 0.00 | −0.036 ± 1.02 | 0.80 | −0.24 ± 0.96 | 0.008 | 91.82 ± 33.17 | 0.13 | 0.11 | 0.17 | 1.30 ± 1.33 | −1.82 ± 1.35 |
| Shear rate, Δ% | 0.10 | 0.05 | 0.38 ± 0.23 | 0.10 | 0.39 ± 0.23 | 0.57 | −4.5 ± 7.9 | 0.05 | 0.01 | 0.17 | 0.96 ± 0.31 | −1.91 ± 0.31 |
| SRAUC, s−1 | 0.17 | 0.03 | −140.7 ± 101.5 | 0.14 | −152.5 ± 101.7 | 0.25 | 4136.7 ± 3563.9 | 0.06 | 0.53 | 0.07 | −90.37 ± 141.33 | −220.80 ± 148.18 |
| SRAUC initial, s−1 | 0.83 | 0.001 | −2.74 ± 12.93 | 0.68 | −5.29 ± 12.63 | 0.05 | 894.89 ± 442.92 | 0.07 | 0.07 | 0.13 | 16.34 ± 17.09 | −29.07 ± 17.92 |
Values are means ± SD. Boldface indicates significant relationship (P < 0.05). Model 1 presents the relationship between age and the indicated variable. Model 2 presents the addition of sex to the model and the relationship between age and sex with the indicated variable. Model 3 indicates if sex moderates the relationship between age and the indicated variable, with individual β coefficients shown for males and females. In models 1–3, R2 value reflects the full model. In models 1 and 3, β represents the unstandardized β coefficient representing the change in variable units for every year increase in age. In model 2, the Δ β coefficient provides the difference in females vs. males in the variable units. CVCi, cerebrovascular conductance index; CVR, cerebrovascular reactivity; CVRi; cerebrovascular resistance index; ICA, internal carotid artery; MAP, mean arterial pressure; MCAv, middle cerebral artery velocity; , end-tidal carbon dioxide; PI, pulsatility index; SRAUC, shear rate area under the curve; V̇e, minute ventilation.
There was a negative relationship between age and peak MCAv (P = 0.03; Table 3). With the addition of sex to the model, there was a significant relationship explaining an additional 11% of variance (P = 0.002) with peak MCAv greater in females. Sex did not moderate the effect of age on peak MCAv (P = 0.28). With the addition of MAP to the model, this led to an increase in explained variance for peak MCAv (R2 = 0.26; P = 0.02). The addition of MAP to the model did not alter the relationship with age (R2 = 0.07; P = 0.03), sex (R2 = 0.18; P = 0.002), and the interaction of age and sex (R2 = 0.20; P = 0.28).
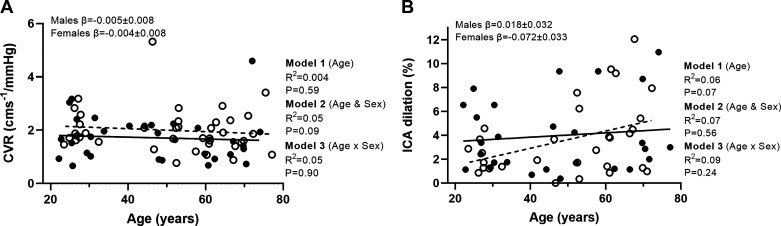
MCAv CVR (cm/mmHg) showed no relationship with age (P = 0.59), sex (P = 0.09), or any moderator effect (P = 0.90; Table 3, Fig. 3A). With the addition of physical activity (weekly METmin) to the model, this did not lead to an increase in explained variance (R2 = 0.05; P = 0.05) and did not influence the relationships with age (P = 0.84; R2 = 0.001), sex (P = 0.08; R2 = 0.05), or the moderator effect (P = 0.94; R2 = 0.05). With the addition of MAP to the model, an increase in explained variance for MCAv CVR was observed (R2 = 0.13; P = 0.01). This did not alter the relationships between MCAv CVR with age (P = 0.59; R2 = 0.004), sex (P = 0.09; R2 = 0.05), or any moderator effect (P = 0.90; R2 = 0.05). For peak , there was no increase in explained variance with the addition of physical activity (R2 = 0.07; P = 0.05).
Figure 3.
A and B: linear regression analysis demonstrating the relationships between age and CVR (n = 73, females = 39; A) and %ICA dilation and age (n = 58, females = 31; B). Solid line represents the regression fit for males, and dotted line represents the regression fit for female participants. Hierarchical regression models (P value and R2) are presented for the relationship with age (model 1), the relationship with sex (model 2), and the moderator relationship of the sex-dependent relationship with age (model 3). Slope coefficients (β) of the relationship with age are presented for males and females separately (model 3). CVR, cerebrovascular reactivity; ICA, internal carotid artery.
ICA dilation was not explained by the magnitude of the SRAUC (P = 0.60; R2 = −0.005) or SRAUC-initial (P = 0.25; R2 = 0.02); therefore, ICA dilation (%) was not normalized to shear rate. This held true at all age groups when analyses were investigated in young (P > 0.11), middle (P > 0.69), and older (P > 0.23) groups separately for SRAUC and SRAUC-initial.
Hypercapnic peak responses for the ICA are shown in Table 3. For percent dilation of the ICA (allometrically scaled), there was no relationship with age (P = 0.07; Fig. 3B). With the addition of sex to the model, there was no relationship (P = 0.56), and no significant moderator effect was observed for ICA dilation (%; P = 0.24).
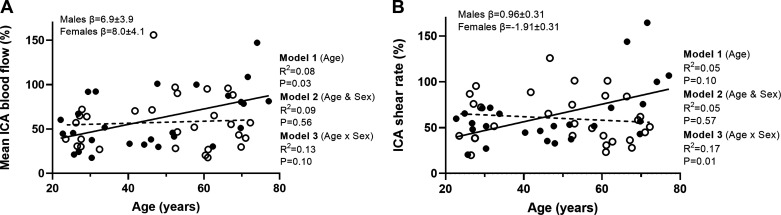
Mean ICA blood flow and the percent change in mean blood flow all showed a positive relationship with age (P ≤ 0.03). However, no sex (P ≥ 0.20) or moderator effects were observed (P ≥ 0.10).
The percent change in peak systolic ICA velocity showed no relationship with age (P = 0.21). With the addition of sex to the model, there was no relationship (P = 0.43); however, sex moderated the effect of age on percent change ICA velocity (P = 0.02). Simple slopes analyses revealed that there was a positive relationship between age and peak ICA velocity in males (β = 0.78 ± 0.29; P = 0.02) but not in female participants (β = −0.20 ± 0.29; P = 0.10).
Peak shear rate showed no relationship with age (P = 0.97; R = −0.005); however, with the addition of sex to the model, this showed a relationship with shear rate higher in female participants and the model explaining 13% of variance (P = 0.008). No significant moderator effect of age was observed for peak shear rate (P = 0.11). As a percent change in shear rate, no relationship with age (P = 0.10) or sex (P = 0.57) was observed; however, sex was shown to moderate the relationship with age (P = 0.01). Simple slopes analyses revealed that there was a positive linear relationship in males with age (P = 0.003; β = 0.96 ± 0.31) and a negative relationship with age in females for shear rate as a percent change (P = 0.01; β = −1.91 ± 0.31; Fig. 4).
Figure 4.
A and B: linear regression analysis demonstrating the relationships between age and ICA percent change in mean blood flow (A) and ICA percent change in shear rate (n = 58, females = 31; B). Solid line represents the regression fit for males, and dotted line represents the regression fit for female participants. Hierarchical regression models (P value and R2) are presented for the relationship with age (model 1), the relationship with sex (model 2), and the moderator relationship of the sex-dependent relationship with age (model 3). Slope coefficients (β) of the relationship with age are presented for males and females separately (model 3). ICA, internal carotid artery.
Intracranial Kinetics Analyses
Dynamic onset response data are shown in Table 4. The MCAv response was well fitted by an exponential model (standard error of the τ: 2.46 ± 1.43). For all kinetic analysis outcomes of interest, there was no increase in explained variance with the addition of physical activity and BMI to the regression model (R2 < 0.023; P > 0.23) and therefore were not included in the full model.
Table 4.
Intracranial responses to hypercapnia
|
Model 1: Age |
Model 2: Age and Sex |
Model 3: Interaction (Age × Sex) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age |
Sex |
|||||||||||
| P value | R 2 | β | P value | β | P value | β | Combined R2 | P value | R 2 | β Males | β Females | |
| Kinetic parameters (n = 71, females = 38) | ||||||||||||
| MCAv τ, s | 0.013 | 0.09 | 0.28 ± 0.11 | 0.023 | 0.25 ± 0.11 | 0.13 | 5.79 ± 3.77 | 0.12 | 0.77 | 0.12 | 0.28 ± 0.15 | 0.22 ± 0.15 |
| MCAv ΔAmp, cm/s | 0.49 | 0.007 | 0.035 ± 0.05 | 0.72 | 0.018 ± 0.05 | 0.03 | 3.87 ± 1.74 | 0.08 | 0.31 | 0.09 | −0.033 ± 0.071 | 0.07 ± 0.07 |
| MCAv TD, s | 0.05 | 0.05 | 0.095 ± 0.048 | 0.05 | 0.10 ± 0.049 | 0.52 | −1.12 ± 1.71 | 0.06 | 0.13 | 0.09 | 0.18 ± 0.07 | 0.026 ± 0.07 |
| MCAv MRT, s | 0.002 | 0.13 | 0.37 ± 0.12 | 0.004 | 0.35 ± 0.12 | 0.26 | 4.67 ± 4.10 | 0.15 | 0.37 | 0.16 | 0.46 ± 0.17 | 0.25 ± 0.17 |
| τ, s | 0.81 | 0.001 | 0.018 ± 0.08 | 0.79 | 0.021 ± 0.08 | 0.84 | −0.54 ± 2.63 | 0.001 | 0.52 | 0.008 | −0.03 ± 0.11 | 0.07 ± 0.11 |
| ΔAmp, mmHg | 0.004 | 0.11 | 0.042 ± 0.01 | 0.003 | 0.044 ± 0.01 | 0.41 | −0.42 ± 0.50 | 0.12 | 0.64 | 0.12 | 0.05 ± 0.02 | 0.04 ± 0.02 |
| TD, s | 0.77 | 0.001 | 0.014 ± 0.05 | 0.81 | 0.012 ± 0.05 | 0.71 | 0.63 ± 1.69 | 0.003 | 0.13 | 0.04 | 0.086 ± 0.07 | −0.062 ± 0.07 |
| MRT, s | 0.69 | 0.002 | 0.033 ± 0.08 | 0.70 | 0.032 ± 0.05 | 0.97 | −0.09 ± 2.80 | 0.002 | 0.76 | 0.004 | 0.06 ± 0.12 | 0.007 ± 0.12 |
Values are means ± SD. Boldface indicates significant relationship (P < 0.05). Model 1 presents the relationship between age and the indicated variable. Model 2 presents the addition of sex to the model and the relationship between age and sex with the indicated variable. Model 3 indicates if sex moderates the relationship between age and the indicated variable, with individual β coefficients shown for males and females. In models 1–3, the R2 value reflects the full model. In model 2, the β coefficient provides the difference in females vs. males in the variable units. Amp, amplitude; MCAv, middle cerebral artery velocity; MRT, mean response time; , end-tidal carbon dioxide; τ, time constant; TD, time delay.
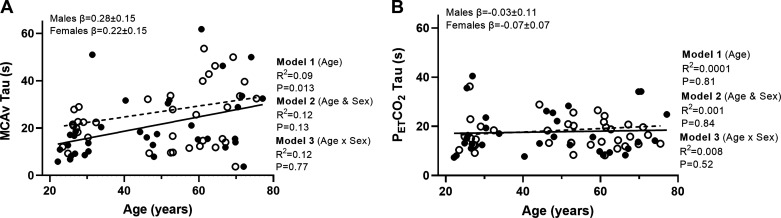
For the MCA time constant, there was a positive relationship with age, such that the speed of the MCA response was slower with advancing age (P = 0.013). With the addition of sex to the model, this did not explain any variance in the response (P = 0.13), and no moderator effect of sex was observed for the MCAv time constant (P = 0.77; Table 4, Fig. 5A). For the time constant, there was no relationship with age (P = 0.81; Fig. 5B). With the addition of sex to the model, no relationship was observed (P = 0.84) and no moderator effect (P = 0.52).
Figure 5.
A and B: linear regression analysis demonstrating the relationships between age and MCAv time constant (τ; A) and time constant (n = 71, females = 38; B). Solid line represents the regression fit for males, and dotted line represents the regression fit for female participants. Hierarchical regression models (P value and R2) are presented for the relationship with age (model 1), the relationship with sex (model 2), and the moderator relationship of the sex-dependent relationship with age (model 3). Slope coefficients (β) of the relationship with age are presented for males and females separately (model 3). MCAv, middle cerebral artery velocity; , end-tidal carbon dioxide.
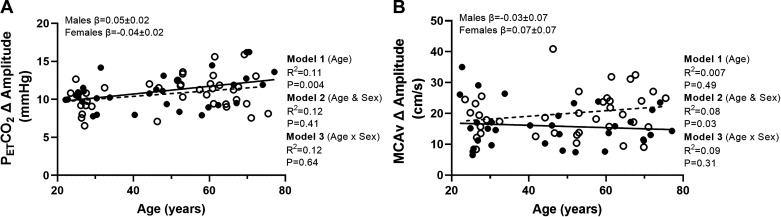
For the amplitude of the MCA response expressed as an absolute change, there was no relationship between the response and age (P = 0.49; Fig. 6B). With the addition of sex to the model, this showed a relationship with MCAv amplitude higher in female participants and the model explaining 8% the variance (P = 0.03). However, no moderator effect was observed (P = 0.31). The amplitude of the response showed a positive relationship with age (P = 0.004). There was no relationship with sex (P = 0.41) and no moderator effect (P = 0.64; Fig. 6A).
Figure 6.
A and B: linear regression analysis demonstrating the relationships between age and amplitude (A) and MCAv amplitude (n = 71, females = 38; B). Solid line represents the regression fit for males, and dotted line represents the regression fit for female participants ( ). Hierarchical regression models (P value and R2) are presented for the relationship with age (model 1), the relationship with sex (model 2), and the moderator relationship of the sex-dependent relationship with age (model 3). Slope coefficients (β) of the relationship with age are presented for males and females separately (model 3). MCAv, middle cerebral artery velocity; , end-tidal carbon dioxide.
). Hierarchical regression models (P value and R2) are presented for the relationship with age (model 1), the relationship with sex (model 2), and the moderator relationship of the sex-dependent relationship with age (model 3). Slope coefficients (β) of the relationship with age are presented for males and females separately (model 3). MCAv, middle cerebral artery velocity; , end-tidal carbon dioxide.
For the MCAv MRT, there was a positive relationship with age, such that the speed of the response was slower with advancing age (P = 0.002). With the addition of sex to the model, this did not explain any additional variance (P = 0.26), and no moderator effect of sex was observed for the MCAv MRT (P = 0.37). For the MRT, there was no relationship with age (P = 0.69). With the addition of sex to the model no relationship was observed (P = 0.97), and no moderator relationship was observed for the MRT (P = 0.76).
As a pooled data set, there was a positive relationship between the MCAv and time constants (R2 = 0.29; P = 0.013). When split into age categories, there was a positive relationship present in young adults (R2 = 0.62; P = 0.001); however, this relationship was lost in middle (R2 = 0.25; P = 0.18) and older aged adults (R2 = 0.13; P = 0.39).
Relationships between Baseline and Hypercapnic Variables and Menopausal Status
The relationships between menopausal status and variables of interest are shown in Table 5. There was a negative relationship between MCAv and menopausal stage (P = 0.03; R2 = 0.18). Simple slopes showed the decrease in MCAv was different in late postmenopausal females compared with premenopausal females (P = 0.02); however, early postmenopausal females showed no difference in slope coefficients to premenopausal females (P = 0.78).
Table 5.
Relationships between menopausal status and cerebrovascular variables
| Model |
Coefficients |
|||||
|---|---|---|---|---|---|---|
| P value | R 2 | Early postmenopausal |
Late postmenopausal |
|||
| β | P value | β | P value | |||
| n | 7 | 15 | ||||
| Baseline intracranial parameters | ||||||
| MCAv, cm/s | 0.03 | 0.18 | 2.0 ± 7.0 | 0.78 | −13.0 ± 5.2 | 0.02 |
| MAP, mmHg | 0.01 | 0.22 | 5.2 ± 6.4 | 0.42 | 15.1 ± 4.7 | 0.003 |
| CVCi, cm/s/mmHg | 0.002 | 0.30 | −0.05 ± 0.09 | 0.57 | −0.24 ± 0.06 | < 0.001 |
| CVRi, mmHg/cm/s | <0.001 | 0.35 | 0.07 ± 0.19 | 0.73 | 0.59 ± 0.14 | < 0.001 |
| Baseline cardiovascular parameters | ||||||
| , mmHg | 0.72 | 0.018 | −3.2 ± 4.2 | 0.45 | −1.6 ± 3.1 | 0.60 |
| PI, AU | 0.001 | 0.31 | −0.004 ± 0.05 | 0.94 | 0.13 ± 0.04 | 0.001 |
| Baseline extracranial internal carotid artery parameters | ||||||
| Diameter, cm | 0.08 | 0.16 | 0.08 ± 0.05 | 0.09 | 0.05 ± 0.03 | 0.07 |
| Shear rate, s−1 | 0.49 | 0.05 | −29.4 ± 54.9 | 0.60 | −39.6 ± 33.3 | 0.24 |
| Mean blood flow, mL/min | 0.21 | 0.11 | 289.6 ± 166.3 | 0.09 | 94.8 ± 103.5 | 0.37 |
| Peak velocity, cm/s | 0.83 | 0.013 | 4.2 ± 7.2 | 0.57 | 1.8 ± 4.8 | 0.70 |
| Peak intracranial parameters | ||||||
| MCAv, cm/s | 0.11 | 0.12 | −1.1 ± 9.6 | 0.89 | −15.0 ± 7.1 | 0.04 |
| MAP, mmHg | 0.009 | 0.23 | 5.4 ± 6.7 | 0.43 | 16.4 ± 5.0 | 0.002 |
| CVCi, cm/s/mmHg | 0.003 | 0.28 | −0.07 ± 0.10 | 0.48 | −0.27 ± 0.07 | 0.001 |
| CVRi, mmHg/cm/s | 0.005 | 0.21 | 0.06 ± 0.17 | 0.73 | 0.43 ± 0.13 | 0.002 |
| Peak extracranial internal carotid artery parameters | ||||||
| ICA dilation, % | 0.17 | 0.12 | 1.2 ± 1.8 | 0.51 | 2.2 ± 1.1 | 0.06 |
| ICA mean blood flow, mL/min | 0.16 | 0.13 | 447.8 ± 227.1 | 0.06 | 111.1 ± 141.4 | 0.79 |
| ICA mean blood flow, % | 0.71 | 0.03 | 12.5 ± 19.5 | 0.53 | −4.3 ± 12.1 | 0.73 |
| ICA shear rate, s−1 | 0.31 | 0.02 | −22.2 ± 83.9 | 0.79 | −79.6 ± 50.9 | 0.13 |
| Peak systolic velocity, cm/s | 0.59 | 0.04 | 9.5 ± 10.4 | 0.37 | −1.4 ± 7.0 | 0.84 |
| Peak systolic velocity, % | 0.22 | 0.04 | 9.6 ± 15.2 | 0.53 | −14.4 ± 10.2 | 0.17 |
| SR, % | 0.41 | 0.06 | 10.7 ± 17.8 | 0.55 | −11.0 ± 10.8 | 0.32 |
| SR AUC, AU | 0.18 | 0.12 | −11,279.7 ± 11,174.3 | 0.32 | −10,353.1 ± 5,689.7 | 0.08 |
| Kinetic parameters | ||||||
| MCAv τ, s | 0.78 | 0.014 | 0.74 ± 9.5 | 0.94 | 4.6 ± 6.6 | 0.12 |
| MCA ΔAmp, cm/s | 0.25 | 0.08 | −3.7 ± 3.6 | 0.32 | 2.4 ± 2.5 | 0.34 |
| τ, s | 0.99 | 0.001 | 0.8 ± 5.1 | 0.87 | 0.2 ± 3.8 | 0.96 |
| ΔAmp, mmHg | 0.25 | 0.08 | −3.7 ± 3.6 | 0.32 | 2.4 ± 2.5 | 0.34 |
Values are means ± SD. Boldface indicates significant relationship (P < 0.05). β Coefficients for early postmenopausal and late postmenopausal females are presented compared with reference group of premenopausal females. Amp, amplitude; CVR, cerebrovascular reactivity; CVRi, cerebrovascular resistance index; MAP, mean arterial pressure; MCAv, middle cerebral artery velocity; , end-tidal carbon dioxide; PI, pulsatility index; SRAUC, shear rate area under the curve; τ, time constant; TD, time delay; V̇e, minute ventilation.
DISCUSSION
This is the first study to document the cross-sectional relationships of age and sex on kinetic responses to hypercapnia. The primary findings were that 1) despite a negative relationship between age and absolute peak MCAv to hypercapnia, the relative amplitude-based responses to hypercapnia in the MCA and ICA reactivity were preserved with age; 2) a positive relationship between age and the MCAv τ kinetic response, suggesting that age slows down the speed at which MCAv increases in response to a hypercapnic challenge; and 3) reduced baseline and peak MCAv in late postmenopausal females, but no differences in ICA blood flow or CVR compared with premenopausal females.
Resting Cerebral Blood Flow with Age
Declines in resting CBF with advancing age have been well documented (51–54). The current data corroborates this, documenting decreases in baseline MCAv with advancing age by ∼3 cm/s per decade alongside an increased CVRi. Despite a higher baseline MCAv in females than males, the relationship between age and MCAv was similar between males and females. This implies that the declines observed with aging were similar in both males and females with no age-by-sex interaction evident. Previous research has documented higher MCAv in young females; however, there was a greater rate of decline with advancing age (55, 56). This is contrary to the current study where we found preserved MCAv and CVRi in early postmenopausal females with 13 cm/s lower MCAv in late menopausal females; thus, declines in females in the current sample were driven by postmenopausal females. Differences between studies may be due to a younger average female sample in the current data with prior research comparing young versus older adults at extremes of the aging spectrum.
Declines in resting MCAv with advancing age may be due to numerous factors inclusive of decreased brain volume, cerebral metabolism, increases in arterial stiffness, oxidative stress, and decreased nitric oxide (NO) bioavailability (57–60). The greater declines in females are likely related to the loss of estrogen with the onset of menopause, which plays a pivotal role in mediating vascular function and blood pressure via the production of NO (61–63). However, the effects of the menopausal transition and loss of estrogen are less well documented in the cerebral vessels (64). Future research whereby blood markers for female sex hormones and NO concentrations are sampled is required to further understand the effects of menopause disentangled from the effects of age on the cerebrovascular circulation. In addition, in premenopausal females, large intra- and interindividual differences in estrogen concentrations prevail, even when controlling for cycle phase (65). Thus, direct measures of hormone concentrations in larger sample sizes are required in future investigations to account for this potential added variability in female responses and to fully understand the effects of female sex hormones on the cerebral circulation.
Despite observing a negative relationship between MCAv and age, baseline ICA mean blood flow showed no relationship with advancing age. This is in line with previous findings (66–68). It is proposed that declines in the posterior circulation with advancing age are more marked compared with the anterior circulation (68, 69). A potential explanation for the preserved mean ICA blood flow response may be due to the high physical activity levels in the current sample (∼2,182 METmin/wk), with regular physical activity shown to attenuate the age-related decline in CBF (70, 71) and endurance exercise training shown to increase CBF and CVR responses (72). When accounted for as a covariate, physical activity did not show a relationship with ICA mean flow; however, physical activity levels in the current sample were all relatively high across the sample (89% met or exceeded the PA guidelines), and therefore, comparisons on the influence of physical activity levels are limited and do not negate the potential for physical activity to influence the current results. Aerobic fitness levels were not measured in the current study, which may be a better predictor of both endothelial function and cognitive function (73, 74). In particular, a recent study has documented a positive relationship between fitness and resting MCAv in females but not in males; however, this relationship was no longer significant when adjusted for age (75). Further research investigating the relationships of age and sex on cerebral responses should therefore include direct measures of cardiorespiratory fitness to fully understand the effects of aging and sex on the cerebral circulation and any age- and sex-specific interactions with cardiorespiratory fitness.
Cerebrovascular Reactivity and Age
Contrary to our hypotheses, MCAv CVR and %ICA reactivity showed no association with age. Interestingly, however, when included as a covariate, MAP showed a significant relationship with CVR. With the addition of MAP, this did not alter the relationships between CVR with age and sex and therefore indicates the effect of MAP is independent of the effects of age and sex. However, when blood pressure was factored into the hypercapnic response, presented as MCAv CVC, this showed a reduced response with age. It therefore seems that in older adults, there is a reliance on increased perfusion pressure to increase CBF to hypercapnia, consistent with recent data (6, 14, 28, 76). Despite similar absolute responses, current and previous data highlight regulatory differences with advancing age, inclusive of increased cerebrovascular resistance during the vasodilatory CO2 stimulus. Our findings are in line with Oudegeest-Sander et al. (9), Ito et al. (10), Murrell et al. (11), and Stefanidis et al. (12), who all observed preserved responses to hypercapnia with advancing age. However, research is conflicting with others reporting declines in CVR, in both the MCA and ICA (6, 20, 77). The study by Miller et al. (6) was able to account for MCA diameter changes and highlighted the ability of the MCA to dilate in young adults during hypercapnia, but not in older adults. This again indicates distinct regulatory differences with age. It suggests that using TCD-based measures of the MCA may underestimate flow and thus MCAv CVR in our younger adults, masking any potential alterations with age.
The current findings of no relationship between ICA reactivity and age are observed despite an increased mean blood flow and velocity in the ICA. Therefore, despite a potentially greater blood flow stimulus and perfusion pressure, no alterations in dilation of the ICA were observed with age. This may be indicative of an impaired response, with a greater stimulus required to elicit similar responses. Shear rate has been previously shown as the driving stimulus for changes in ICA dilation during hypercapnia (20). However, in the current study, there was no relationship between ICA dilation and shear stress, irrespective of age. This is in line with emerging evidence, highlighting that CVR assessed via steady-state CO2 does not reflect endothelial NO-dependent dilation (78). Given that this metric was not solely an endothelium-mediated measure of cerebrovascular function, this may explain why no alterations were found with advancing age.
Endothelial shear stress is an important regulator of vessel tone, mediating alterations in vessel structure, and function (79). In addition, declines in shear stress have been highlighted as an independent predictor of cognitive decline in older adults (80). In the present study, baseline shear rate was higher in females than males with no effect of age. We did observe a trend for a lower shear stress with older age; however, this was not significant. This is contrary to previous research that shows decreases in shear stress in cerebral arteries with age (81, 82). These decreases in shear rate with aging occur alongside increases in baseline diameter (82). However, the present study did not find increases in diameter with age and therefore may explain the preserved shear rate with age. This is in line with Iwamoto et al. (20) who observed no changes in baseline shear rate or diameter in older versus younger adults. It therefore seems that in the present sample population, vascular remodeling and increases in arterial diameter have not yet manifested, potentially because of the good health status and high physical activity levels reported. For shear rate responses to hypercapnia, no aging effect was seen, similar to previous findings (20). However, we did observe a sex-dependent effect of aging on shear rate responses, with an increase in males and a blunted response in females.
A notable difference with advancing age was the slowed MCAv speed of response (τ, MRT), observed in both male and female participants. These data indicate that despite a maintained capacity to obtain the same relative increase in MCAv, advancing age slows down the rate at which this response occurs. Reasons for this blunted MCAv τ and MRT with aging are largely speculative; however, it seems that in a healthy sample where cerebrovascular function remains intact, the time course of the response may reveal impairments in vasomotor control of the cerebrovasculature. Given the cerebrovaculature comprises numerous integrative mechanisms governing cerebrovascular control to ensure adequate CBF is maintained (83), this delayed response may be reflective of a compensatory response and reliance on differential mechanistic pathways to meet the demands of the brain. The MCAv τ was shown to be related to the τ in young adults; however, this relationship was lost with advancing age. This highlights distinct regulatory differences with advancing age and indicates the blunted response with age was not due to the ventilatory response and may be due to other cerebral factors, inclusive of decreased cerebral metabolism, increased arterial stiffness, reduced compliance, slower autoregulatory responses, and greater reliance on perfusion pressure.
This study provides a comprehensive assessment of the age- and sex-dependent cerebrovascular responses to hypercapnia, using intra- and extracranial assessments in an adequately powered study design. Despite the novelties, the limitations of the current study should be acknowledged. First, TCD measures the velocity of the MCA and not absolute flow (4). Although the two are highly correlated (84) and prior work has shown the MCA diameter to remain constant during moderate elevations in MAP and as seen in the current study (26, 85, 86), more recent evidence suggests this may not be the case (27, 87), particularly in the context of aging (6). Despite the draw backs of TCD, the higher temporal resolution provides possibilities for novel assessments of the dynamic responses to hypercapnia which the current study employs. Furthermore, the current study was unable to use prospective end-tidal targeting to standardize the end-tidal to arterial CO2 gradient (4). Therefore, the potential for differences in MCA and ICA reactivity between individuals may have been due to differing stimuli and ventilatory responses. However, we found no differences in concentrations with age or sex in the current sample and therefore believe that the lack of end-tidal targeting did not have a marked effect. Also, by not using end-tidal targeting, we were able to explore the kinetic relationships between and MCAv responses with age. A final consideration of the current study is that we did not include measures of hypocapnia-induced CVR, which may have offered additional insights into the aging response (88). Future research should therefore employ both hypo- and hypercapnia-induced CVR to discern the effects of age and sex on CVR across the entire ventilatory range. However, given there is a lack of standardization of hypocapnia measures of CVR, this should first be addressed. Future research should also include measures of the posterior circulation, given the presence of regional differences in cerebrovascular regulation (23, 89).
In conclusion, this study provides insight into the relationships between age and sex on MCA and ICA reactivity to hypercapnia. In addition, this is the first study to investigate the kinetic responses of the MCA to hypercapnia with advancing age. Our findings demonstrated that despite similar MCAv CVR and ICA reactivity, dynamic responses of MCAv were significantly blunted with aging. These novel findings highlight the need for further investigation into the effects of age and sex on CVR responses. In particular, longitudinal study designs in larger sample sizes, with direct measures of sex hormones in females and cardiorespiratory fitness across the cohort. This will aid in accounting for any individual variability in the age-related responses and advance current understanding on the influence of sex hormones and menopause on cerebrovascular responses.
SUPPLEMENTAL DATA
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.20422593.
GRANTS
J.L.K. is supported by a QUEX institute scholarship (University of Queensland and University of Exeter partnership).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L.K., B.B., A.R.B., J.S.C., and T.G.B. conceived and designed research; J.L.K., S.L.R., and F.K.P. performed experiments; J.L.K. analyzed data; J.L.K. interpreted results of experiments; J.L.K. prepared figures; J.L.K. drafted manuscript; J.L.K., B.B., A.R.B., S.L.R., F.K.P., J.S.C., and T.G.B. edited and revised manuscript; J.L.K., B.B., A.R.B., S.L.R., F.K.P., J.S.C., and T.G.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We are very grateful to all volunteers for their time in participating in this research.
REFERENCES
- 1. Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 27: 484–492, 1948. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shim Y, Yoon B, Shim DS, Kim W, An J-Y, Yang D-W. Cognitive correlates of cerebral vasoreactivity on Transcranial Doppler in older adults. J Stroke Cerebrovasc Dis 24: 1262–1269, 2015. doi: 10.1016/j.jstrokecerebrovasdis.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 3. Portegies ML, de Bruijn RF, Hofman A, Koudstaal PJ, Ikram MA. Cerebral vasomotor reactivity and risk of mortality: the Rotterdam Study. Stroke 45: 42–47, 2014. doi: 10.1161/STROKEAHA.113.002348. [DOI] [PubMed] [Google Scholar]
- 4. Hoiland RL, Fisher JA, Ainslie PN. Regulation of the cerebral circulation by arterial carbon dioxide. Compr Physiol 9: 1101–1154, 2019. doi: 10.1002/cphy.c180021. [DOI] [PubMed] [Google Scholar]
- 5. Peng SL, Chen X, Li Y, Rodrigue KM, Park DC, Lu H. Age-related changes in cerebrovascular reactivity and their relationship to cognition: a four-year longitudinal study. NeuroImage 174: 257–262, 2018. doi: 10.1016/j.neuroimage.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller KB, Howery AJ, Rivera-Rivera LA, Johnson SC, Rowley HA, Wieben O, Barnes JN. Age-related reductions in cerebrovascular reactivity using 4D flow MRI. Front Aging Neurosci 11: 281, 2019. doi: 10.3389/fnagi.2019.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barnes JN, Schmidt JE, Nicholson WT, Joyner MJ. Cyclooxygenase inhibition abolishes age-related differences in cerebral vasodilator responses to hypercapnia. J Appl Physiol (1985) 112: 1884–1890, 2012. doi: 10.1152/japplphysiol.01270.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bakker SL, de Leeuw FE, den Heijer T, Koudstaal PJ, Hofman A, Breteler MM. Cerebral haemodynamics in the elderly: the Rotterdam study. Neuroepidemiology 23: 178–184, 2004. p doi: 10.1159/000078503. [DOI] [PubMed] [Google Scholar]
- 9. Oudegeest-Sander MH, van Beek AH, Abbink K, Olde Rikkert MG, Hopman MT, Claassen JA. Assessment of dynamic cerebral autoregulation and cerebrovascular CO2 reactivity in ageing by measurements of cerebral blood flow and cortical oxygenation. Exp Physiol 99: 586–598, 2014. doi: 10.1113/expphysiol.2013.076455. [DOI] [PubMed] [Google Scholar]
- 10. Ito H, Kanno I, Ibaraki M, Hatazawa J. Effect of aging on cerebral vascular response to Paco2 changes in humans as measured by positron emission tomography. J Cereb Blood Flow Metab 22: 997–1003, 2002. doi: 10.1097/00004647-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 11. Murrell CJ, Cotter JD, Thomas KN, Lucas SJ, Williams MJ, Ainslie PN. Cerebral blood flow and cerebrovascular reactivity at rest and during sub-maximal exercise: effect of age and 12-week exercise training. Age (Dordr) 35: 905–920, 2013. doi: 10.1007/s11357-012-9414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stefanidis KB, Askew CD, Klein T, Lagopoulos J, Summers MJ. Healthy aging affects cerebrovascular reactivity and pressure-flow responses, but not neurovascular coupling: a cross-sectional study. PLoS One 14: e0217082, 2019. doi: 10.1371/journal.pone.0217082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu YS, Tarumi T, Tseng BY, Palmer DM, Levine BD, Zhang R. Cerebral vasomotor reactivity during hypo- and hypercapnia in sedentary elderly and Masters athletes. J Cereb Blood Flow Metab 33: 1190–1196, 2013. doi: 10.1038/jcbfm.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tomoto T, Riley J, Turner M, Zhang R, Tarumi T. Cerebral vasomotor reactivity during hypo- and hypercapnia across the adult lifespan. J Cereb Blood Flow Metab 40: 600–610, 2020. doi: 10.1177/0271678X19828327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, Deal JA, McKhann GM, Mosley TH, Sharrett AR, Schneider ALC, Windham BG, Wruck LM, Knopman DS. Associations between midlife vascular risk factors and 25-year incident dementia in the atherosclerosis risk in communities (ARIC) cohort. JAMA Neurol 74: 1246–1254, 2017. doi: 10.1001/jamaneurol.2017.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burley CV, Francis ST, Thomas KN, Whittaker AC, Lucas SJE, Mullinger KJ. Contrasting measures of cerebrovascular reactivity between MRI and Doppler: a cross-sectional study of younger and older healthy individuals. Front Physiol 12, 2021. doi: 10.3389/fphys.2021.656746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnes JN, Taylor JL, Kluck BN, Johnson CP, Joyner MJ. Cerebrovascular reactivity is associated with maximal aerobic capacity in healthy older adults. J Appl Physiol (1985) 114: 1383–1387, 2013. doi: 10.1152/japplphysiol.01258.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kastrup A, Thomas C, Hartmann C, Schabet M. Sex dependency of cerebrovascular CO2 reactivity in normal subjects. Stroke 28: 2353–2356, 1997. doi: 10.1161/01.STR.28.12.2353. [DOI] [PubMed] [Google Scholar]
- 19. Smith EC, Pizzey FK, Askew CD, Mielke GI, Ainslie PN, Coombes JS, Bailey TG. Effects of cardiorespiratory fitness and exercise training on cerebrovascular blood flow and reactivity: a systematic review with meta-analyses. Am J Physiol Heart Circ Physiol 321: H59–H76, 2021. doi: 10.1152/ajpheart.00880.2020. [DOI] [PubMed] [Google Scholar]
- 20. Iwamoto E, Bock JM, Casey DP. Hypercapnia-induced shear-mediated dilation in the internal carotid artery is blunted in healthy older adults. Am J Physiol Heart Circ Physiol 315: H1279–H1286, 2018. doi: 10.1152/ajpheart.00335.2018. [DOI] [PubMed] [Google Scholar]
- 21. Pabbidi MR, Kuppusamy M, Didion SP, Sanapureddy P, Reed JT, Sontakke SP. Sex differences in the vascular function and related mechanisms: role of 17β-estradiol. Am J Physiol Heart Circ Physiol 315: H1499–H1518, 2018. doi: 10.1152/ajpheart.00194.2018. [DOI] [PubMed] [Google Scholar]
- 22. Carter HH, Atkinson CL, Heinonen IH, Haynes A, Robey E, Smith KJ, Ainslie PN, Hoiland RL, Green DJ. Evidence for shear stress-mediated dilation of the internal carotid artery in humans. Hypertension 68: 1217–1224, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07698. [DOI] [PubMed] [Google Scholar]
- 23. Sato K, Sadamoto T, Hirasawa A, Oue A, Subudhi AW, Miyazawa T, Ogoh S. Differential blood flow responses to CO2 in human internal and external carotid and vertebral arteries. J Physiol 590: 3277–3290, 2012. doi: 10.1113/jphysiol.2012.230425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller KB, Howery AJ, Rivera-Rivera LA, Wieben O, Barnes JN. Sex differences in the cerebral hemodynamic response to hypercapnia in young adults. FASEB J 34: 105009, 2020. p doi: 10.1096/fasebj.2020.34.s1.01910. 30256215 [DOI] [Google Scholar]
- 25. Peltonen GL, Harrell JW, Rousseau CL, Ernst BS, Marino ML, Crain MK, Schrage WG. Cerebrovascular regulation in men and women: stimulus-specific role of cyclooxygenase. Physiol Rep 3: e12451, 2015. doi: 10.14814/phy2.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000. doi: 10.1161/01.STR.31.7.1672. [DOI] [PubMed] [Google Scholar]
- 27. Hoiland RL, Ainslie PN. CrossTalk proposal: the middle cerebral artery diameter does change during alterations in arterial blood gases and blood pressure. J Physiol 594: 4073–4075, 2016. doi: 10.1113/JP271981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coverdale NS, Badrov MB, Shoemaker JK. Impact of age on cerebrovascular dilation versus reactivity to hypercapnia. J Cereb Blood Flow Metab 37: 344–355, 2017. doi: 10.1177/0271678X15626156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tallon CM, Barker AR, Nowak-Flück D, Ainslie PN, McManus AM. The influence of age and sex on cerebrovascular reactivity and ventilatory response to hypercapnia in children and adults. Exp Physiol 105: 1090–1101, 2020. doi: 10.1113/EP088293. [DOI] [PubMed] [Google Scholar]
- 30. Koep JL, Weston ME, Barker AR, Bailey TG, Coombes JS, Lester A, Bond B. The within- and between-day reliability of cerebrovascular reactivity using traditional and novel analytical approaches. Exp Physiol 107: 29–41, 2022. doi: 10.1113/EP090031. [DOI] [PubMed] [Google Scholar]
- 31. Weston ME, Barker AR, Tomlinson OW, Coombes JS, Bailey TG, Bond B. Differences in cerebrovascular regulation and ventilatory responses during ramp incremental cycling in children, adolescents, and adults. J Appl Physiol (1985) 131: 1200–1210, 2021. doi: 10.1152/japplphysiol.00182.2021. [DOI] [PubMed] [Google Scholar]
- 32. Billinger SA, Craig JC, Kwapiszeski SJ, Sisante JV, Vidoni ED, Maletsky R, Poole DC. Dynamics of middle cerebral artery blood flow velocity during moderate-intensity exercise. J Appl Physiol (1985) 122: 1125–1133, 2017. doi: 10.1152/japplphysiol.00995.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Williams JS, Dunford EC, MacDonald MJ. Impact of the menstrual cycle on peripheral vascular function in premenopausal women: systematic review and meta-analysis. Am J Physiol Heart Circ Physiol 319: H1327–H1337, 2020. doi: 10.1152/ajpheart.00341.2020. [DOI] [PubMed] [Google Scholar]
- 34. Weir CB, Jan A. BMI classification percentile and cut off points. In: StatPearls. Treasure Island, FL: StatPearls, 2022. [PubMed] [Google Scholar]
- 35.AIWH. The Active Australia Survey: a Guide and Manual for Implementation, Analysis and Reporting. Canberra: Australian Institute of Health and Wellfare, 2003. [Google Scholar]
- 36. Armstrong T, Bauman A, Davies J. Physical Activity Patterns of Australian Adults: Results of the 1999 National Physical Activity Survey. Canberra: Australian Institute of Health and Welfare, 2000. [Google Scholar]
- 37. Brown WJ, Burton NW, Marshall AL, Miller YD. Reliability and validity of a modified self-administered version of the Active Australia physical activity survey in a sample of mid-age women. Aust N Z J Public Health 32: 535–541, 2008. doi: 10.1111/j.1753-6405.2008.00305.x. [DOI] [PubMed] [Google Scholar]
- 38. Brown WJ, Trost SG, Bauman A, Mummery K, Owen N. Test-retest reliability of four physical activity measures used in population surveys. J Sci Med Sport 7: 205–215, 2004. doi: 10.1016/S1440-2440(04)80010-0. [DOI] [PubMed] [Google Scholar]
- 39. Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ; Straw +10 Collaborative Group. Executive summary of the stages of reproductive aging workshop +10: addressing the unfinished agenda of staging reproductive aging. Climacteric 15: 105–114, 2012. doi: 10.3109/13697137.2011.650656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thomas KN, Lewis NCS, Hill BG, Ainslie PN. Technical recommendations for the use of carotid duplex ultrasound for the assessment of extracranial blood flow. Am J Physiol Regul Integr Comp Physiol 309: R707–R720, 2015. doi: 10.1152/ajpregu.00211.2015. [DOI] [PubMed] [Google Scholar]
- 41. Favre ME, Lim V, Falvo MJ, Serrador JM. Cerebrovascular reactivity and cerebral autoregulation are improved in the supine posture compared to upright in healthy men and women. PLoS One 15: e0229049, 2020. doi: 10.1371/journal.pone.0229049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fierstra J, Sobczyk O, Battisti-Charbonney A, Mandell DM, Poublanc J, Crawley AP, Mikulis DJ, Duffin J, Fisher JA. Measuring cerebrovascular reactivity: what stimulus to use? J Physiol 591: 5809–5821, 2013. doi: 10.1113/jphysiol.2013.259150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Willie CK, Colino F, Bailey D, Tzeng YC, Binsted G, Jones LW, Haykowsky M, Bellapart J, Ogoh S, Smith K, Smirl J, Day T, Lucas S, Eller LK, Ainslie P. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196: 221–237, 2011. doi: 10.1016/j.jneumeth.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 44. Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008. doi: 10.1161/HYPERTENSIONAHA.107.101014. [DOI] [PubMed] [Google Scholar]
- 45. Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol (1985) 91: 929–937, 2001. doi: 10.1152/jappl.2001.91.2.929. [DOI] [PubMed] [Google Scholar]
- 46. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gosling RG, King DH. Arterial assessment by Doppler-shift ultrasound. Proc R Soc Med 67: 447–449, 1974. doi: 10.1177/00359157740676P113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Burley CV, Lucas RAI, Whittaker AC, Mullinger K, Lucas SJE. The CO2 -stimulus duration and steady-state time-point used for data extraction alters the cerebrovascular reactivity outcome measure. Exp Physiol 105: 893–903, 2020. doi: 10.1113/EP087883. [DOI] [PubMed] [Google Scholar]
- 49. Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Heterogeneity in conduit artery function in humans: impact of arterial size. Am J Physiol Heart Circ Physiol 295: H1927–H1934, 2008. doi: 10.1152/ajpheart.00405.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carr JMJR, Hoiland RL, Caldwell HG, Coombs GB, Howe CA, Tremblay JC, Green DJ, Ainslie PN. Internal carotid and brachial artery shear-dependent vasodilator function in young healthy humans. J Physiol 598: 5333–5350, 2020. doi: 10.1113/JP280369. [DOI] [PubMed] [Google Scholar]
- 51. Chen JJ, Rosas HD, Salat DH. Age-associated reductions in cerebral blood flow are independent from regional atrophy. NeuroImage 55: 468–478, 2011. doi: 10.1016/j.neuroimage.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kety SS. Human cerebral blood flow and oxygen consumption as related to aging. J Chronic Dis 3: 478–486, 1956. doi: 10.1016/0021-9681(56)90146-1. [DOI] [PubMed] [Google Scholar]
- 53. Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Jones T, Healy MJR, Gibbs JM, Wise RJS, Hatazawa J, Herold S, Beaney RP, Brooks DJ, Spinks T, Rhodes C, Frackowiak RSJ. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 113: 27–47, 1990. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- 54. Müller M, Schimrigk K. A comparative assessment of cerebral haemodynamics in the basilar artery and carotid territory by transcranial Doppler sonography in normal subjects. Ultrasound Med Biol 20: 677–687, 1994. doi: 10.1016/0301-5629(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 55. Aanerud J, Borghammer P, Rodell A, Jónsdottir KY, Gjedde A. Sex differences of human cortical blood flow and energy metabolism. J Cereb Blood Flow Metab 37: 2433–2440, 2017. doi: 10.1177/0271678X16668536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alwatban MR, Aaron SE, Kaufman CS, Barnes JN, Brassard P, Ward JL, Miller KB, Howery AJ, Labrecque L, Billinger SA. Effects of age and sex on middle cerebral artery blood velocity and flow pulsatility index across the adult lifespan. J Appl Physiol (1985) 130: 1675–1683, 2021. doi: 10.1152/japplphysiol.00926.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tarumi T, Shah F, Tanaka H, Haley AP. Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. Am J Hypertens 24: 1108–1113, 2011. doi: 10.1038/ajh.2011.101. [DOI] [PubMed] [Google Scholar]
- 58. Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility – Reykjavik Study. Brain 134: 3398–3407, 2011. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Seals DR, Alexander LM. Vascular aging. J Appl Physiol (1985) 125: 1841–1842, 2018. doi: 10.1152/japplphysiol.00448.2018. [DOI] [PubMed] [Google Scholar]
- 60. Tarumi T, Zhang R. Cerebral blood flow in normal aging adults: cardiovascular determinants, clinical implications, and aerobic fitness. J Neurochem 144: 595–608, 2018. doi: 10.1111/jnc.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 340: 1801–1811, 1999. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 62. Rosenthal T, Oparil S. Hypertension in women. J Hum Hypertens 14: 691–704, 2000. doi: 10.1038/sj.jhh.1001095. [DOI] [PubMed] [Google Scholar]
- 63. Joyner MJ, Barnes JN, Hart EC, Wallin BG, Charkoudian N. Neural control of the circulation: how sex and age differences interact in humans. Compr Physiol 5: 193–215, 2015. doi: 10.1002/cphy.c140005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ruediger SL, Koep JL, Keating SE, Pizzey FK, Coombes JS, Bailey TG. Effect of menopause on cerebral artery blood flow velocity and cerebrovascular reactivity: Systematic review and meta-analysis. Maturitas 148: 24–32, 2021. doi: 10.1016/j.maturitas.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 65. Liu KR, Lew LA, McGarity-Shipley EC, Byrne AC, Islam H, Fenuta AM, Pyke KE. Individual variation of follicular phase changes in endothelial function across two menstrual cycles. Exp Physiol 106: 1389–1400, 2021. doi: 10.1113/EP089482. [DOI] [PubMed] [Google Scholar]
- 66. Flück D, Ainslie PN, Bain AR, Wildfong KW, Morris LE, Fisher JP. Extra- and intracranial blood flow regulation during the cold pressor test: influence of age. J Appl Physiol (1985) 123: 1071–1080, 2017. doi: 10.1152/japplphysiol.00224.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nowak-Flück D, Ainslie PN, Bain AR, Ahmed A, Wildfong KW, Morris LE, Phillips AA, Fisher JP. Effect of healthy aging on cerebral blood flow, CO2 reactivity, and neurovascular coupling during exercise. J Appl Physiol (1985) 125: 1917–1930, 2018. doi: 10.1152/japplphysiol.00050.2018. [DOI] [PubMed] [Google Scholar]
- 68. Olesen ND, Nielsen HB, Olsen NV, Secher NH. The age-related reduction in cerebral blood flow affects vertebral artery more than internal carotid artery blood flow. Clin Physiol Funct Imaging 39: 255–260, 2019. doi: 10.1111/cpf.12568. [DOI] [PubMed] [Google Scholar]
- 69. Dörfler P, Puls I, Schliesser M, Mäurer M, Becker G. Measurement of cerebral blood flow volume by extracranial sonography. J Cereb Blood Flow Metab 20: 269–271, 2000. doi: 10.1097/00004647-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 70. Bailey DM, Marley CJ, Brugniaux JV, Hodson D, New KJ, Ogoh S, Ainslie PN. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 44: 3235–3238, 2013. doi: 10.1161/STROKEAHA.113.002589. [DOI] [PubMed] [Google Scholar]
- 71. Thomas BP, Yezhuvath US, Tseng BY, Liu P, Levine BD, Zhang R, Lu H. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J Magn Reson Imaging 38: 1177–1183, 2013. doi: 10.1002/jmri.24090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bliss ES, Wong RHX, Howe PRC, Mills DE. Benefits of exercise training on cerebrovascular and cognitive function in ageing. J Cereb Blood Flow Metab 41: 447–470, 2021. doi: 10.1177/0271678X20957807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vidoni ED, Johnson DK, Morris JK, Van Sciver A, Greer CS, Billinger SA, Donnelly JE, Burns JM. Dose-response of aerobic exercise on cognition: a community-based, pilot randomized controlled trial. PLoS One 10: e0131647, 2015. doi: 10.1371/journal.pone.0131647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Barnes JN, Corkery AT. Exercise improves vascular function, but does this translate to the brain? Brain Plast 4: 65–79, 2018. doi: 10.3233/BPL-180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zeller NP, Miller KB, Zea RD, Howery AJ, Labrecque L, Aaron SE, Brassard P, Billinger SA, Barnes JN. Sex-specific effects of cardiorespiratory fitness on age-related differences in cerebral hemodynamics. J Appl Physiol (1985) 132: 1310–1317, 2022. doi: 10.1152/japplphysiol.00782.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Miller KB, Howery AJ, Harvey RE, Eldridge MW, Barnes JN. Cerebrovascular reactivity and central arterial stiffness in habitually exercising healthy adults. Front Physiol 9: 1096, 2018. doi: 10.3389/fphys.2018.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Iwamoto E, Sakamoto R, Tsuchida W, Yamazaki K, Kamoda T, Neki T, Katayose M, Casey DP. Effects of menstrual cycle and menopause on internal carotid artery shear-mediated dilation in women. Am J Physiol Heart Circ Physiol 320: H679–H689, 2021. doi: 10.1152/ajpheart.00810.2020. [DOI] [PubMed] [Google Scholar]
- 78. Hoiland RL, Caldwell HG, Carr J, Howe CA, Stacey BS, Dawkins T, Wakeham DJ, Tremblay JC, Tymko MM, Patrician A, Smith KJ, Sekhon MS, MacLeod DB, Green DJ, Bailey DM, Ainslie PN. Nitric oxide contributes to cerebrovascular shear-mediated dilatation but not steady-state cerebrovascular reactivity to carbon dioxide. J Physiol 600: 1385–1403, 2022. doi: 10.1113/JP282427. [DOI] [PubMed] [Google Scholar]
- 79. Rubanyi GM, Freay AD, Kauser K, Johns A, Harder DR. Mechanoreception by the endothelium: mediators and mechanisms of pressure- and flow-induced vascular responses. Blood Vessels 27: 246–257, 1990. doi: 10.1159/000158816. [DOI] [PubMed] [Google Scholar]
- 80. Liu Z, Zhao Y, Wang X, Zhang H, Cui Y, Diao Y, Xiu J, Sun X, Jiang G. Low carotid artery wall shear stress is independently associated with brain white-matter hyperintensities and cognitive impairment in older patients. Atherosclerosis 247: 78–86, 2016. doi: 10.1016/j.atherosclerosis.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 81. Zhao X, Zhao M, Amin-Hanjani S, Du X, Ruland S, Charbel FT. Wall shear stress in major cerebral arteries as a function of age and gender—a study of 301 healthy volunteers. J Neuroimaging 25: 403–407, 2015. doi: 10.1111/jon.12133. [DOI] [PubMed] [Google Scholar]
- 82. Irace C, Carallo C, De Franceschi MS, Scicchitano F, Milano M, Tripolino C, Scavelli F, Gnasso A. Human common carotid wall shear stress as a function of age and gender: a 12-year follow-up study. Age (Dordr) 34: 1553–1562, 2012. doi: 10.1007/s11357-011-9318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol 592: 841–859, 2014. doi: 10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brauer P, Kochs E, Werner C, Bloom M, Policare R, Pentheny S, Yonas H, Kofke WA, Schulte Am Esch J. Correlation of transcranial Doppler sonography mean flow velocity with cerebral blood flow in patients with intracranial pathology. J Neurosurg Anesthesiol 10: 80–85, 1998. doi: 10.1097/00008506-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 85. Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery 32: 737–741, 1993. [PubMed] [Google Scholar]
- 86. Poulin MJ, Robbins PA. Indexes of flow and cross-sectional area of the middle cerebral artery using doppler ultrasound during hypoxia and hypercapnia in humans. Stroke 27: 2244–2250, 1996. doi: 10.1161/01.STR.27.12.2244. [DOI] [PubMed] [Google Scholar]
- 87. Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, Dahan A, van Lieshout JJ, van Osch MJ. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol (1985) 117: 1084–1089, 2014. doi: 10.1152/japplphysiol.00651.2014. [DOI] [PubMed] [Google Scholar]
- 88. Ringelstein EB, Sievers C, Ecker S, Schneider PA, Otis SM. Noninvasive assessment of CO2-induced cerebral vasomotor response in normal individuals and patients with internal carotid artery occlusions. Stroke 19: 963–969, 1988. doi: 10.1161/01.str.19.8.963. [DOI] [PubMed] [Google Scholar]
- 89. Koep JL, Taylor CE, Coombes JS, Bond B, Ainslie PN, Bailey TG. Autonomic control of cerebral blood flow: fundamental comparisons between peripheral and cerebrovascular circulations in humans. J Physiol 600: 15–39, 2022. doi: 10.1113/JP281058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.20422593.