Keywords: aging, immune cells, inflammation, muscle function, sarcopenia
Abstract
Poor recovery of muscle size and strength with aging coincides with a dysregulated macrophage response during the early stages of regrowth. Immunomodulation in the form of ex vivo cytokine (macrophage-colony stimulating factor) or polarized macrophage delivery has been demonstrated to improve skeletal muscle regeneration. However, it is unclear if these macrophage-promoting approaches would be effective to improve skeletal muscle recovery following disuse in aged animals. Here, we isolated bone marrow-derived macrophages from donor mice of different ages under various experimental conditions and polarized them into proinflammatory macrophages. Macrophages were delivered intramuscularly into young adult or aged recipient mice during the early recovery period following a period of hindlimb unloading (HU). Delivery of proinflammatory macrophages from donor young adults or aged mice was sufficient to increase muscle function of aged mice during the recovery period. Moreover, proinflammatory macrophages derived from aged donor mice collected during recovery were similarly able to increase muscle function of aged mice following disuse. In addition to the delivery of macrophages, we showed that the intramuscular injection of the cytokine, macrophage-colony stimulating factor, to the muscle of aged mice following HU was able to increase muscle macrophage content and muscle force production during recovery. Together, these results suggest that macrophage immunomodulation approaches in the form of ex vivo proinflammatory macrophage or macrophage-colony stimulating factor delivery during the early recovery phase following disuse atrophy were sufficient to restore the loss of aged skeletal muscle function.
NEW & NOTEWORTHY A single intramuscular administration of polarized macrophages into muscles of aged mice following a bout of disuse atrophy was sufficient to improve functional recover similarly to young adults after disuse atrophy regardless of the age or experimental condition of the donor mice. Additionally, intramuscular delivery of macrophage-colony stimulating factor into aged mice was similarly effective. Targeting macrophage function early during the regrowth phase may be a novel tool to bolster muscle recovery in aging.
INTRODUCTION
The recovery of muscle function following a period of disuse is known to be impaired or delayed in aged populations (1, 2), leading to greater risks of falls, fractures, and subsequent bouts of disuse-induced decrements in muscle size and function (3). The mechanisms outlining this delayed recovery of aged muscle following disuse are incompletely understood, but the dysregulated recruitment and activation of immune cells to the muscle following initiation of injury appears to play an important role in the pathogenesis of delayed functional recovery of aged muscle (4, 5).
Hindlimb unloading (HU) in young and older rodents induces robust muscle atrophy, but aged rodents are unable to recover muscle function on the same time course as their young adult counterparts (6, 7). The decreased functional recovery with age corresponds with aberrant macrophage recruitment and function in the hindlimb muscles following HU (8–10). Indeed, our recent work showed that during early muscle regrowth from HU, muscle macrophages from aged mice have a blunted proinflammatory phenotype and are associated with a complimentary decrease in inflammatory and glycolytic transcriptional programs (8, 11). Similarly, if macrophage recruitment is inhibited in young adults following HU, mice exhibit a blunted recovery of muscle quality (12, 13), suggesting the importance of macrophage recruitment and function during the recovery from disuse atrophy. Not only has the abundance and function of proinflammatory macrophages shown to be diminished in muscle during the early period of reloading following HU with aging, but aged macrophages have been shown to be inherently dysfunctional compared with macrophages from young rodents (11, 14, 15). Because of the well-appreciated requisite role of infiltrating proinflammatory macrophages for effective muscle regeneration following injury (16) and regrowth following disuse (12, 13, 17), defects in the function of these cells are likely to have downstream consequential impacts on other important interstitial cells (e.g., satellite cells, fibro-adipogenic precursor cells) leading to excessive collagen deposition, smaller muscle fibers, and poor functional recovery (13, 17–21). Together, impaired or delayed muscle recovery in aging is partly attributed to macrophage dysregulation.
Since macrophages are required for muscle repair and regrowth (13), immunotherapies that target macrophages and correct age-related macrophage dysregulation may be a unique approach to restore muscle size and function in older mice following disuse. Surprisingly, this innovative idea has not been examined in the context of aging and disuse. Macrophage immunomodulation has previously been successful to improve muscle repair following ischemia-reperfusion (22–25) and laceration (26) injury in young adult mice. For example, muscle injection of proinflammatory-like macrophages during the early inflammatory phase following injury improved muscle size and function (22). And in another study, muscle delivery of macrophage-colony stimulating factor (M-CSF), a cytokine that induces recruitment, proliferation, and maturation of macrophages, into soleus muscle of young rodents during disuse, resulted in faster recovery of muscle size and strength (27). Therefore, these immunomodulation approaches may be a unique application that targets a specific cellular dysfunction noted in aged mice during recovery following disuse.
Taken together, these data propose several intriguing questions: 1) can the dysfunctional recovery of aged muscle following disuse atrophy be remedied through ex vivo macrophage immunotherapy? and 2) can reprogramming of macrophages in aged muscle with M-CSF delivery improve muscle macrophage accumulation and accelerate muscle recovery after HU? To answer these questions, we subjected young adult (3–6 mo) or aged (23–26 mo) mice to 14 days of HU followed by reloading and delivered an intramuscular injection of polarized proinflammatory macrophages derived from bone marrow progenitors, M-CSF, or PBS (Vehicle) into contralateral hindlimbs and muscle was examined during early recovery.
In this study, we showed evidence that intramuscular administration of polarized proinflammatory macrophages from donor mice increased muscle function of aged mice following HU, whereas the delivery of macrophages from a donor aged or young adult mice to young adult recipient mice following HU had negligible effects on muscle function. In addition, delivery of M-CSF increased muscle macrophage content and aided some parameters of aged muscle recovery following HU. These data add to the knowledge base of the cross talk between aged muscle and macrophages in the recovery of muscle function following a period of disuse and provide proof of concept that targeting macrophage function could help aged individuals recover more efficiently following a bout of disuse atrophy.
MATERIALS AND METHODS
Animals and Experimental Design
All mice used for this study were the C57BL/6 strain and were male. Young adult mice were from a colony maintained at the University of Utah Comparative Medicine Center and were 3–6 mo of age for all experimental procedures. Aged mice were from the National Institute of Aging mouse colony and were between 23 and 26 mo of age for all experiments. Mice were housed on a 12/12-h light/dark cycle with ad libitum access to standard chow (Envigo 2920X) and water. All experimental procedures were approved by and conducted in accordance with the guidelines set by The University of Utah Institutional Animal Care and Use Committee.
Animals underwent hindlimb unloading (HU) (2 animals/cage) using a modified unloading method as previously performed (8). This allows mice to ambulate freely on their forelimbs while their hindlimbs are suspended. Following 14 days of HU, mice were removed from the hindlimb unloading apparatus to ambulate freely for the designated recovery time. The experimental design is outlined in Fig. 1A. Briefly, mice were separated into two groups: 1) donor mice and 2) recipient mice. Donor mice were used to isolate bone marrow-derived macrophages and recipient mice received an intramuscular injection of these bone marrow-derived macrophages derived from donor mice. Macrophages from donor mice were isolated under three experimental conditions: from young adult or aged mice that were ambulatory or from aged mice that had undergone 4 days of recovery following 14 days of HU. This timepoint was chosen because previous studies from our laboratory indicated that intramuscular macrophages from aged mice are particularly dysfunctional after 4 days of recovery following HU (8, 11). Therefore, we postulated that delivery of these macrophages might further impair muscle function in aged mice. Recipient mice were young adults or aged mice that had undergone 14 days of HU and were reloaded for 1 day before the intramuscular delivery of macrophages from donors. These mice were then allowed to freely ambulate for another 3 days (RL4) before being euthanized for muscle functional testing. All experimental procedures were conducted in accordance with the guidelines set by the Institutional Animal Care and Use Committee (IACUC) at the University of Utah.
Figure 1.
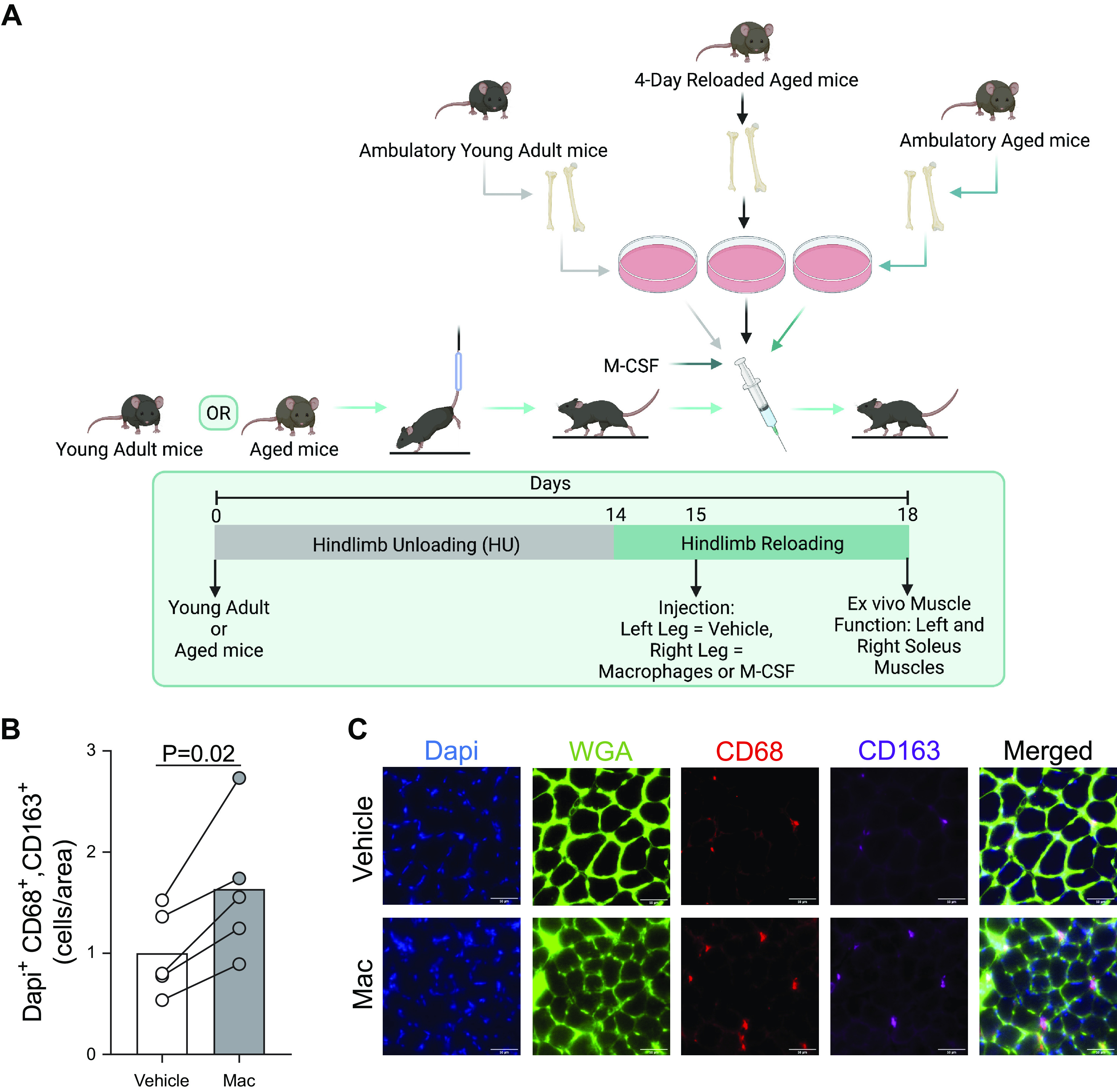
Schematic of experimental design. A: recipient young adult or aged mice were subjected to hindlimb unloading for 2 wk prior to being reloaded. After 1 day of reloading, recipient mice were injected with macrophages isolated from young adult or aged mice that were sedentary, aged mice that were hindlimb unloaded and reloaded for 4 days, or recombinant macrophage-colony stimulating factor (M-CSF). B: hindlimbs of ambulatory young (n = 2) and aged (n = 3) mice were injected with PBS (Vehicle) or macrophages (Mac) from young ambulatory mouse donors and pooled cross sections of soleus muscles were measured for the abundance of cells positive for Dapi, CD68, and CD163. C: representative image of soleus cross sections. B: individual data points from the pooled sample of ambulatory young and aged mice are shown and connected with respective contralateral limbs and the mean is represented as a bar and relative to Vehicle. A paired t test was used. n = 5/group. Figure 1A was created with BioRender.com and exported under a paid subscription.
Bone Marrow-Derived Macrophage Isolation, Polarization, and Delivery
Bone marrow-derived macrophages were isolated from respective experimental donor groups as previously described (11, 28). In brief, both tibia and femur bones from each leg of donor mice were flushed with sterile PBS and centrifuged at 400 g for 5 min before resuspension in red blood cell lysis buffer (BioLegend: 420301) for 5 min. Cells were pelleted again at 400 g for 5 min and resuspended in D10 media [high-glucose DMEM, 10% FBS, 1% Pen/Strep, 1% 1× glutamine (Gibco 25030)] supplemented with macrophage-colony stimulating factor (M-CSF, 10 ng/mL, R&D Biosystems:416-ML) and plated for 4 days before spiking with fresh D10. On day 7, cells were polarized with D10 supplemented with M-CSF, lipopolysaccharide (0.1 µg/mL, Millipore Sigma: L5543), and interferon γ (IFNγ, 40 ng/mL, Peprotech: 315-05) for 24 h. After 24 h of polarization, cells were washed two times with PBS and scraped in trypsin. Cells were pelleted and re-suspended in PBS at a concentration of 2 million cells/100 µL and rear-loaded into a syringe (MedVet International: MV1/2CCINS). The triceps surae muscle group was injected (with the needle angled in line with the muscle complex) with either 100 µL of PBS (Vehicle) while the contralateral limb was similarly injected but with 100 µL of macrophages (2 million cells), which is a proinflammatory macrophage concentration previously shown to be sufficient to alter muscle regeneration from ischemic injuries (22, 24).
Macrophage-Colony Stimulating Factor Delivery
In a final mouse experiment, we delivered M-CSF to aged mice recovering from HU using the same experimental approach as the ex vivo macrophage experiments (Fig. 1A). M-CSF (R&D Biosystems: 416ML) was reconstituted as recommended by the vendor. On day 1 of recovery following 14 days of HU, either 30 µL of M-CSF (3 µg total) or equal volume of vehicle (PBS) was injected directly into the triceps surae muscle group of the hindlimb muscle using the same injection strategy as aforementioned. Aged mice were euthanized after 4 days of reloading for the analysis of soleus function and macrophage content (immunohistochemistry).
Muscle Functional Testing
Soleus muscles were carefully sutured at each tendon and dissected from the mice while under isoflurane anesthesia before the measurement of muscle function as previously described (29, 30). Briefly, optimal length (LO) was reached through a series of pulse stimulations (0.2 ms pulse width, 20 V, 1 Hz). Rates of contraction and relaxation were measured as the average slope between 20% and 80% of the peak force of a pulse stimulation. Force-frequency relationship analyses were performed through stimulations ranging from 10 to 200 Hz (350 ms stimulation duration, 0.2 ms pulse width, 20 V) with 1 min between each stimulation. A dual-mode lever force transducer (Aurora Scientific: 300 C-LR) with DMCv5.500 or DMAv5.321 software (Aurora Scientific) was used to measure force and analyze the data, respectively.
Immunohistochemistry
Soleus muscles were frozen in liquid nitrogen-cooled 2-methylbutane and embedded in Optimal Cutting Temperature compound (Fisher Scientific; Cat 23-730-531) before sectioning at a thickness of 10 µm. Immunofluorescent staining for Dapi, CD68, CD163, and wheat germ agglutinin (WGA) was performed as previously described (8). Briefly, sections were fixed with acetone and blocked with M.O.M. Mouse IgG blocking vector (Vector Labs: MKB-2213) before overnight incubation with primary antibodies (1:100) targeting CD68 (BioRad: MCA1957GA) or CD163 (Santa Cruz: SC33560) in PBS supplemented with 2.5% horse serum. Sections were then incubated in Goat-anti-Rat IgG Cy3 (1:250, Invitrogen: A-10522) and Goat-anti-rabbit IgG AF647 (1:250, Invitrogen: A-21121) for 1 h in PBS. Prior to mounting in Vectashield mounting media (Vector Laboratories), sections were incubated in 1:10,000 Dapi and 1:50 WGA (AF488, Thermo Fisher: W11261) for 5 min. Sections were imaged on a Nikon Automated Widefield microscope and analyzed using ImageJ.
RNA Isolation and qRT-PCR
RNA isolation and reverse transcription for cDNA synthesis was performed as previously described (31). Gene expression was measured through real-time quantitative PCR with a CFX-connect Real-Time PCR thermal cycler (Bio-Rad) with SYBR Green Supermix reagent as previously described (32). All target genes were normalized to ribosomal L32 mRNA levels that did not change between groups. Primer sequences used were as follows (5'→3'): L32 (Forward) TTCCTGGTCCACAATGTCAA, (Reverse) GGCTTTTCGGTTCTTAGAGGA; IFNγ (Forward) GACAATCAGGCCATCAGCAAC, (Reverse) CGGATGAGCTCATTGAATGCTT; TNFα (Forward) CTTCTGTCTACTGAACTTCGGG, (Reverse) CACTTGGTGGTTTGCTACGAC; CD68 (Forward) CAAAGCTTCTGCTGTGGAAAT, (Reverse) GACTGGTCACGGTTGCAAG; Nos2 (Forward) CAGCACAGGAAATGTTTCAGC, (Reverse) TAGCCAGCGTACCGGATGA; Arg2 (Forward) GAAGTGGTTAGTAGAGCTGTGTC, (Reverse) GGTGAGAGGTGTATTAATGTCCG as used previously (33).
Statistical Analysis
For all bar graphs, individual data points are shown and connected to the data point that corresponds to the contralateral limb with bars representing the mean. For all scatter plots, means ± SE is shown. Paired t tests were used for analysis directly comparing the two groups. For multiple comparisons, a two-way ANOVA with Sidaks multiple-comparisons test was performed. The main effects shown above each panel indicate the main effect of treatment of Vehicle or Macrophage. P < 0.05 was considered statistically significant. GraphPad Prism 9 was used for all statistical analyses.
RESULTS
Delivery of Macrophages from Ambulatory Young Adult Donors to Reloaded Young Adult Recipients
Young adult recipient mice underwent hindlimb unloading for 2 wk before reloading (Fig. 1A). After 1 day of reloading, these mice received intramuscular injections of polarized proinflammatory macrophages that were isolated as bone marrow progenitor cells from normally ambulating young adult mice and polarized to be proinflammatory macrophages, which is a procedure we show to increase intramuscular macrophage content following macrophage delivery (Fig. 1B). After 4 days of reloading (3 days postmacrophage or PBS injection), soleus muscle function was measured. When muscles from young adult recipients were subjected to a single pulse stimulation to induce a twitch contraction, muscles that received macrophage (Mac) injection had modestly (albeit, statistically significant) higher force production compared with the contralateral muscles that received a PBS injection (Vehicle) (Fig. 2A). Similar rates of contraction and relaxation after a pulse stimulation were displayed between muscles that received Mac or Vehicle (Fig. 2, B and C), which was apparent when raw force tracings from the twitch contraction were plotted (Fig. 2D). When soleus muscles from young adult recipient mice were subjected to a wide range of stimulation frequencies the Mac or Vehicle treated muscles produced similar force, which was apparent in raw force tracings at 100 Hz (Fig. 2, E and F). Soleus muscles from young adult recipients had similar masses following the delivery of either Vehicle or Macs from ambulatory adult donors (Table 1).
Figure 2.
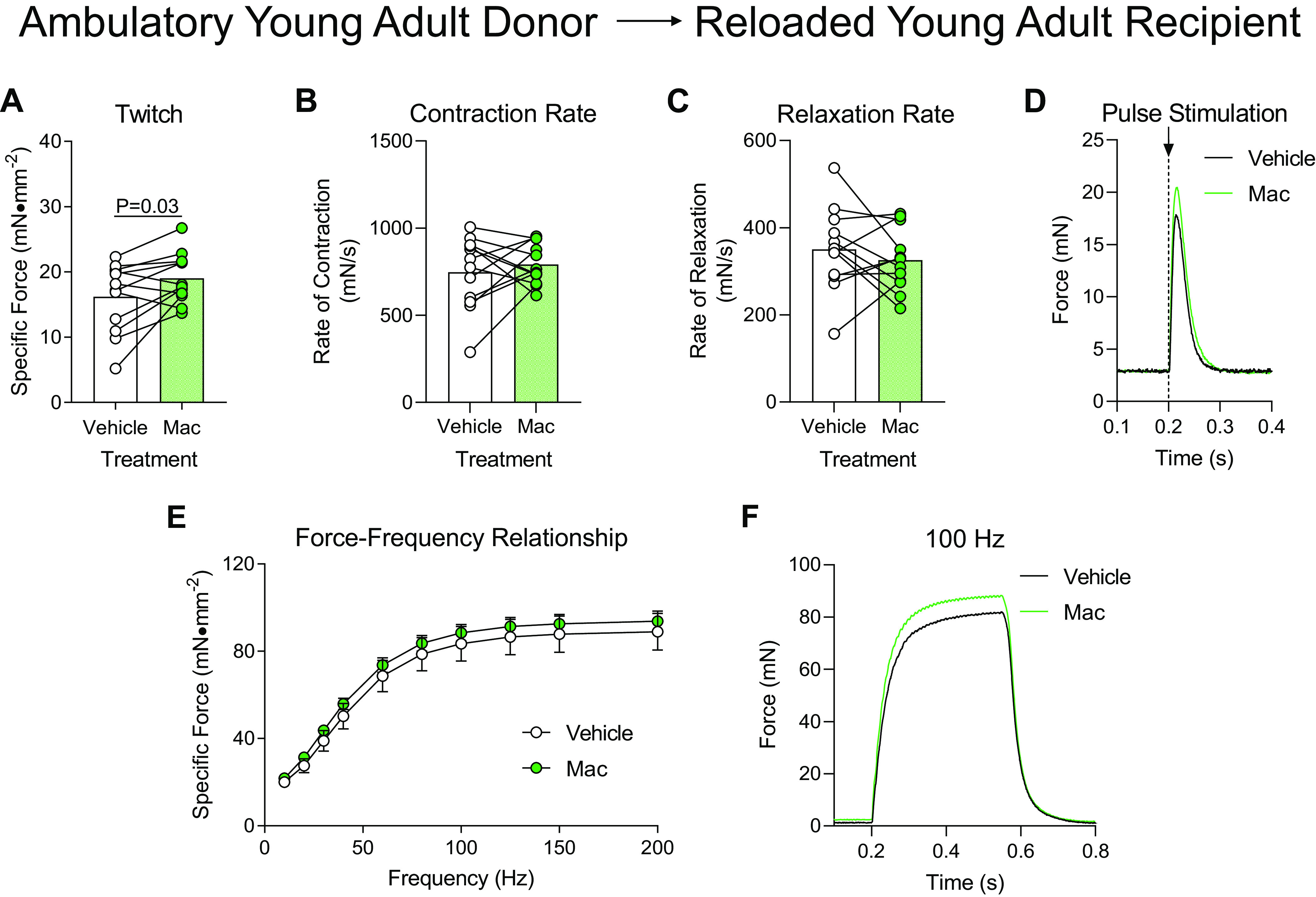
Delivery of macrophages from young adult ambulatory donors to young adult recipients following hindlimb unloading. Ex vivo force production of soleus muscles from young adult mice during early recovery (4 days) following hindlimb unloading that received intramuscular injections of macrophages from ambulatory young adult mice (Mac) or PBS (Vehicle) into contralateral hindlimbs. A–D: muscles were subjected to a single-pulse stimulation to induce a twitch contraction for the measurement of specific force (A), rate of contraction (B), or rate of relaxation (C). D: raw force tracings from a single-pulse stimulation that induced a twitch contraction. E: the specific force was measured across a range of stimulation frequencies from 10 to 200 Hz. F: raw force tracings from a 100 Hz stimulation. A–C: individual data points are shown and connected with respective contralateral limbs and the mean is represented as a bar. E: data points are shown as means ± SE. Paired t tests (A–C) or a two-way ANOVA with Sidak’s multiple-comparisons test (E) was used. n = 12/group.
Table 1.
Soleus muscle mass during recovery following hindlimb unloading following Vehicle or treatment
| Donor |
||||
|---|---|---|---|---|
| Young Adult | Aged | Reloaded Aged | M-CSF | |
| Recipients | ||||
| Young adult | ||||
| Vehicle | 8.43 ± 0.26, 12 | 7.01 ± 0.24, 7 | ||
| Treated | 8.18 ± 0.33, 12 | 7.54 ± 0.36, 7 | ||
| Aged | ||||
| Vehicle | 7.54 ± 0.47, 8 | 6.76 ± 0.33, 8 | 8.08 ± 0.24, 9 | 9.17 ± 0.59, 6 |
| Treated | 7.13 ± 0.30, 8 | 7.09 ± 0.33, 8 | 7.38 ± 0.33, 9 | 7.98 ± 0.34, 6 |
Data are represented as means ± SE, n. M-CSF, macrophage-colony stimulating factor.
Delivery of Macrophages from Ambulatory Young Adult Donors to Reloaded Aged Recipients
Donor macrophages from ambulatory young adult mice were similarly administered to aged mice following hindlimb unloading to measure muscle function after 4 days of reloading. When subjected to a single pulse stimulation to induce a twitch contraction, the aged soleus muscle that received Mac injections produced significantly more force compared with contralateral Vehicle-treated muscles (Fig. 3A) despite similar rates of contraction, relaxation, and raw force tracing curves (Fig. 3, B–D). Likewise, when the soleus muscle was subjected to a wide range of stimulation frequencies ranging from 10 to 200 Hz, the soleus muscles from the Mac-treated hindlimb produced significantly more force compared with the Vehicle-treated muscle, which was visually apparent at 100 Hz (Fig. 3, E and F). The mass of the soleus from aged recipient mice was not different in size whether they were treated with Vehicle or Macs from ambulatory young adult donor mice (Table 1).
Figure 3.
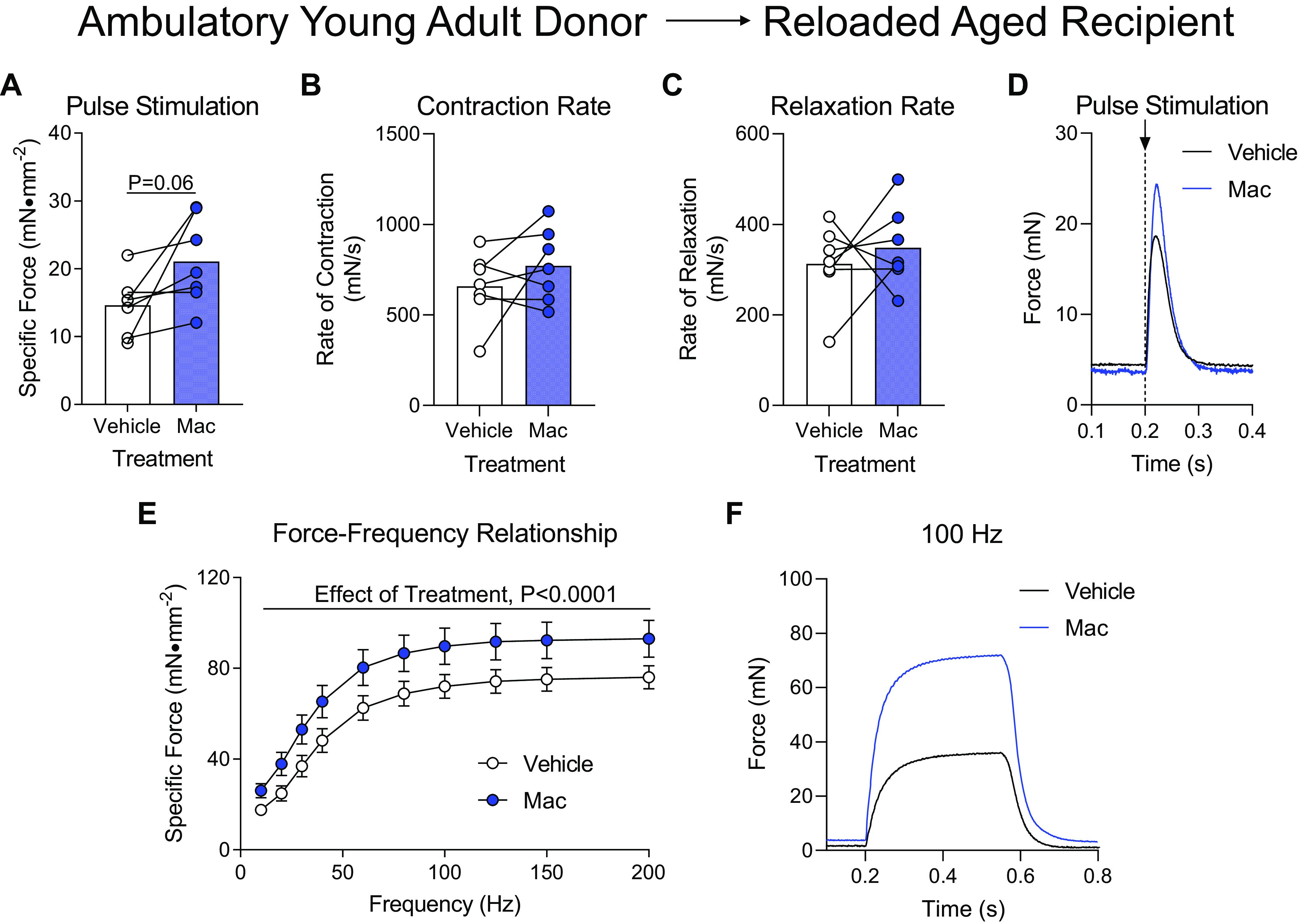
Delivery of macrophages from young adult ambulatory donors to aged recipients following hindlimb unloading. Ex vivo force production of soleus muscles from aged mice during early recovery (4 days) following hindlimb unloading that received intramuscular injections of macrophages from ambulatory young adult mice (Mac) or PBS (Vehicle) into contralateral hindlimbs. A–D: muscles were subjected to a single-pulse stimulation to induce a twitch contraction for the measurement of specific force (A), rate of contraction (B), or rate of relaxation (C). D: raw force tracings from a single-pulse stimulation that induced a twitch contraction. E: specific force was measured across a range of stimulation frequencies from 10 to 200 Hz. F: raw force tracings from a 100 Hz stimulation. A–C: individual data points are shown and connected with respective contralateral limbs and the mean is represented as a bar. E: data points are shown as means ± SE. Paired t tests (A–C) or a two-way ANOVA with Sidak’s multiple-comparisons test (E) was used. n = 8/group.
Figure 7.
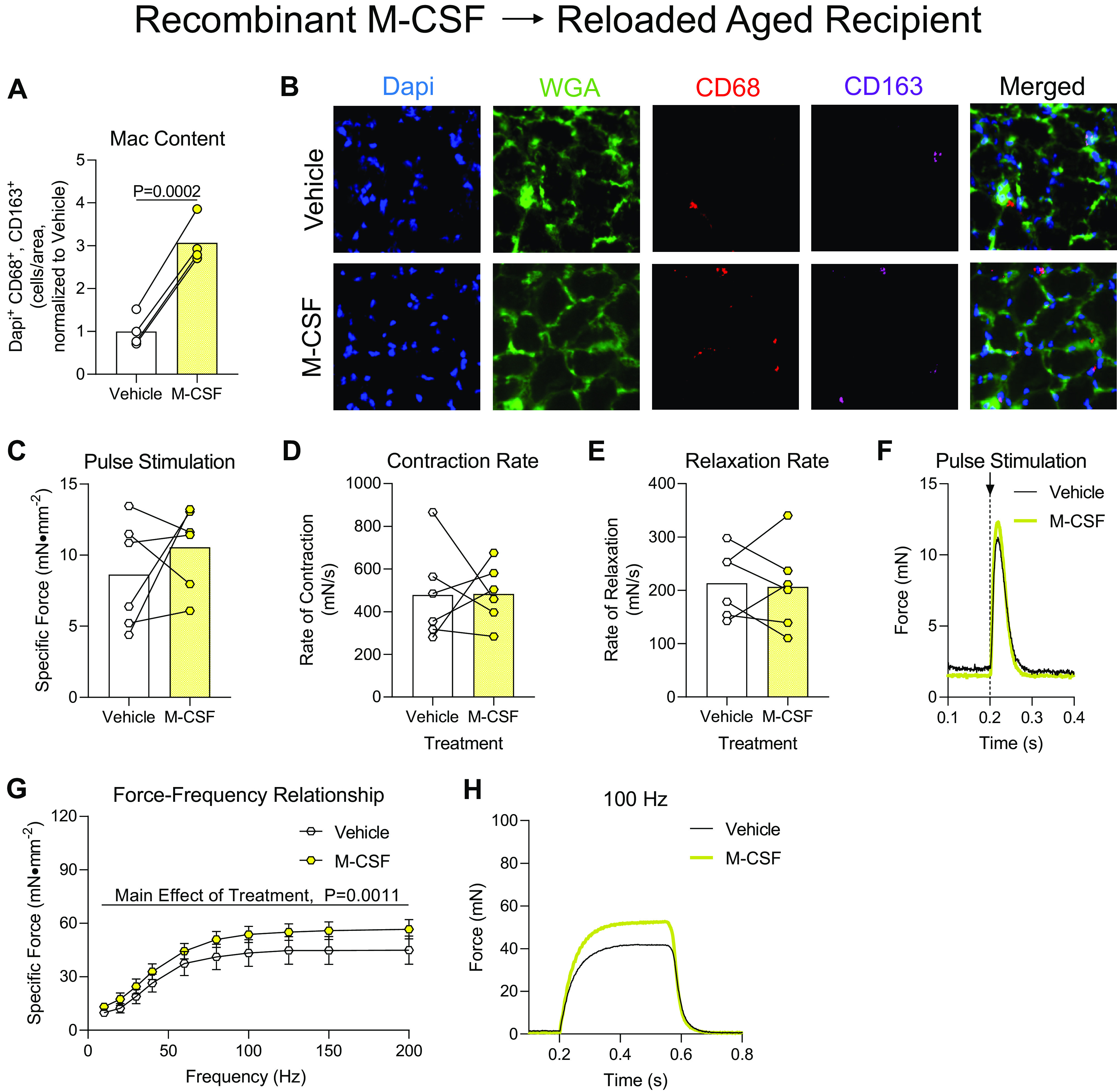
Effect of recombinant macrophage-colony stimulating factor (M-CSF) delivery to aged mice following hindlimb unloading. A: sections of soleus muscles from aged mice that received vehicle or M-CSF injections were measured for the abundance of cells positive for Dapi, CD68, and CD163. B: representative image of soleus cross sections. C–H: ex vivo force production of soleus muscles from aged mice during early recovery following hindlimb unloading that received intramuscular injections of M-CSF or PBS (Vehicle) into contralateral hindlimbs. C–F: muscles were subjected to a single pulse stimulation to induce a twitch contraction for the measurement of specific force (C), rate of contraction (D), or rate of relaxation (E). F: raw force tracings from a single-pulse stimulation that induced a twitch contraction. G: the specific force was measured across a range of stimulation frequencies from 10 to 200 Hz. H: raw force tracings from a 100 Hz stimulation. A and C–E: individual data points are shown and connected with respective contralateral limbs and the mean is represented as a bar and relative to Vehicle. G: data points are shown as means ± SE. Paired t tests (A, and C–E) or a two-way ANOVA with Sidak’s multiple-comparisons test (G) was used. C–E, and G: n = 6/group or n = 4/group (A).
Delivery of Macrophages from Ambulatory Aged Donors to Reloaded Young Adult Recipients
Young adult mice were injected with macrophages from ambulatory aged donors after 1 day of reloading and muscle function was tested after 4 days of recovery. When the soleus was subjected to a single-pulse stimulation to induce a twitch contraction, Mac- and Vehicle-treated soleus muscles produced similar maximal force, rates of contraction, and rates of relaxation (Fig. 4, A–C). Raw force tracings from the twitch contractions were also similar between Vehicle- and Mac-treated soleus muscles (Fig. 4D). When force production of the soleus muscles from young adult recipients was tested across a range of stimulation frequencies, there was a small, but statistically lower force production in the soleus muscle that received Mac injections compared with Vehicle (Fig. 4E). This defect appeared minor as raw force tracings were generally similar between Vehicle- and Mac-treated soleus muscles at 100 Hz stimulation (Fig. 4F). Nonetheless, we explored the differences in the gene expression profile of known proinflammatory markers of macrophage polarization and found that several genes were increased in macrophages from ambulatory aged donors compared with ambulatory young donors (Fig. 4G), potentially providing insight into why treating young adult mice with these hyper-proinflammatory macrophages resulted in modest defects in muscle function. The delivery of macrophages from aged ambulatory donor mice did not alter soleus mass when administered to young adult mice (Table 1).
Figure 4.
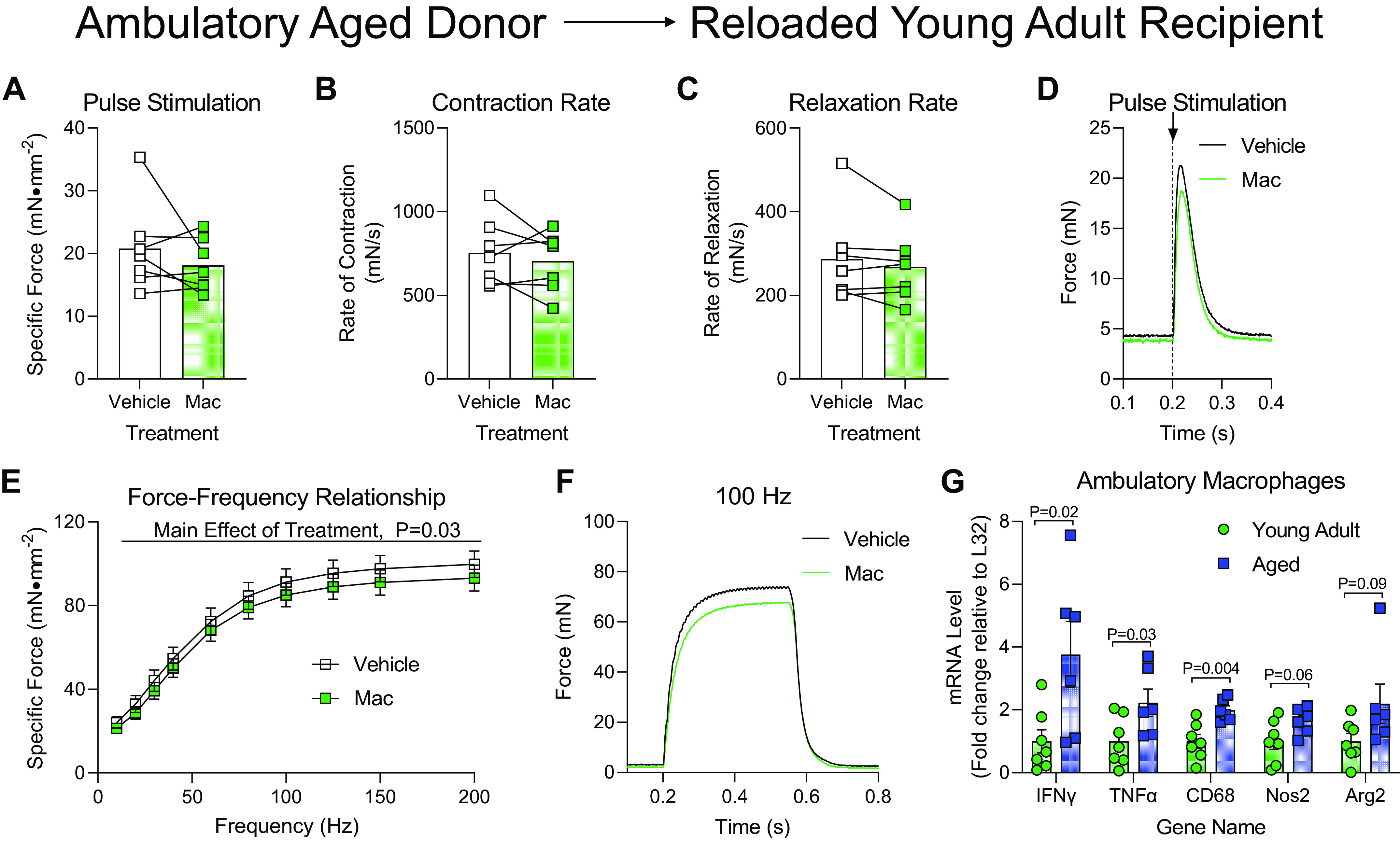
Delivery of macrophages from aged ambulatory donors to young adult recipients following hindlimb unloading. Ex vivo force production of soleus muscles from young adult mice during early recovery (4 days) following hindlimb unloading that received intramuscular injections of macrophages from ambulatory aged mice (Mac) or PBS (Vehicle) into contralateral hindlimbs. A–D: muscles were subjected to a single-pulse stimulation to induce a twitch contraction for the measurement of specific force (A), rate of contraction (B), or rate of relaxation (C). D: raw force tracings from a single-pulse stimulation that induced a twitch contraction. E: specific force was measured across a range of stimulation frequencies from 10 to 200 Hz. F: raw force tracings from a 100 Hz stimulation. G: proinflammatory gene expression from qRT-PCR of macrophages isolated from ambulatory young adult or aged mice. Individual data points are shown with means ± SE. Unpaired t tests were performed. A–C, and G: individual data points are shown and connected with respective contralateral limbs and the mean is represented as a bar. E: data points are shown as means ± SE. Paired/unpaired t tests (A–C, and G) or a two-way ANOVA with Sidak’s multiple-comparisons test (E) was used. n = 7/group.
Delivery of Macrophages from Ambulatory Aged Donors to Reloaded Aged Recipients
Reloaded aged mice were similarly administered macrophages from ambulatory aged mice before the functional analysis of their soleus muscles. Interestingly, the delivery of these Macs significantly increased maximal force, rate of contraction, and rate of relaxation (Fig. 5, A–C) in the soleus after the initiation of a twitch contraction with a single-pulse stimulation. This effect was similarly represented in raw force tracings from Vehicle- or Mac-treated soleus muscles (Fig. 5D). When solei were tested across a range of stimulation frequencies, the soleus that received Mac treatment produced significantly more force across a range of stimulation frequencies compared with Vehicle (Fig. 5E), which was also apparent when raw force tracings from a 100 Hz stimulation were plotted (Fig. 5F). The delivery of macrophages from ambulatory aged donor mice did not alter soleus mass in aged reloaded recipient mice compared with Vehicle (Table 1).
Figure 5.
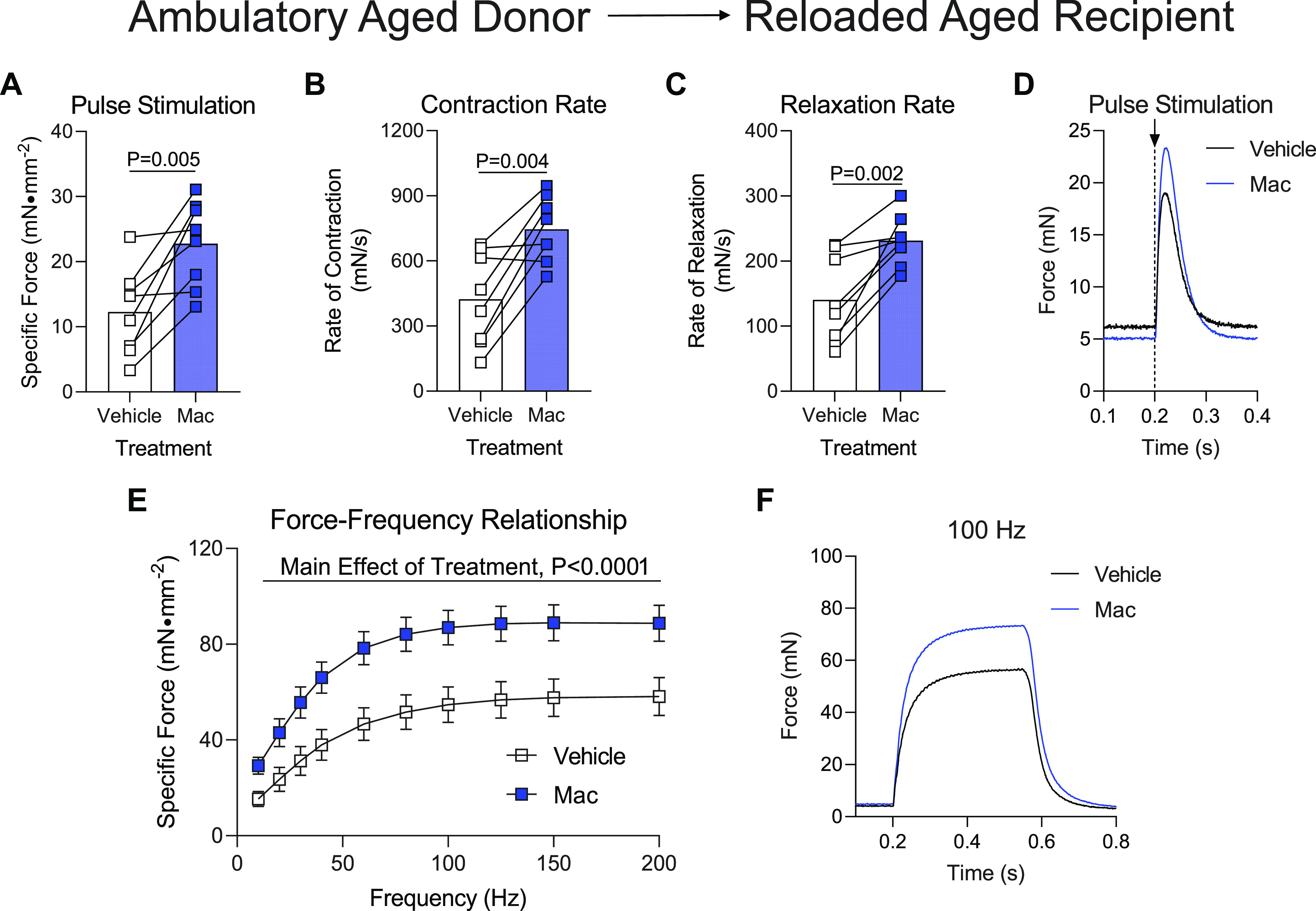
Delivery of macrophages from aged ambulatory donors to aged recipients following hindlimb unloading. Ex vivo force production of soleus muscles from aged mice during early recovery (4 days) following hindlimb unloading that received intramuscular injections of macrophages from ambulatory aged mice (Mac) or PBS (Vehicle) into contralateral hindlimbs. A–D: muscles were subjected to a single-pulse stimulation to induce a twitch contraction for the measurement of specific force (A), rate of contraction (B), or rate of relaxation (C). D: raw force tracings from a single-pulse stimulation that induced a twitch contraction. E: specific force was measured across a range of stimulation frequencies from 10 to 200 Hz. F: raw force tracings from a 100 Hz stimulation. G: soleus mass of aged mice after receiving hindlimb injection of PBS (Vehicle) or macrophages from ambulatory aged mice (Mac). A–C: individual data points are shown and connected with respective contralateral limbs and the mean is represented as a bar. E: data points are shown as means ± SE. Paired t tests (A–C) or a two-way ANOVA with Sidaks multiple-comparisons test (E) was used. n = 8/group.
Delivery of Macrophages from Aged Reloaded Donors to Aged Reloaded Recipients
Aged mice were subjected to hindlimb unloading followed by 4 days of reloading before the isolation of bone marrow-derived macrophages to test whether these macrophages were still capable of aiding in muscle recovery or were no longer beneficial after being delivered to aged donor mice following hindlimb unloading. Soleus muscles from aged reloaded mice that received Mac injections from aged reloaded donor mice exhibited a modest increase in maximal force production when stimulated to twitch (Fig. 6A), but were not different in rates of contraction or relaxation (Fig. 6, B and C). The overall increase in twitch force in Mac-treated muscles was apparent in raw force tracings after a single-pulse stimulation (Fig. 6D). When the soleus from Vehicle- or Mac-treated groups was subjected to respond across a range of stimulation frequencies, Mac-treated solei generally produced more force than the contralateral Vehicle-treated muscle (Fig. 6E), which was modestly represented in the raw force tracing from a 100 Hz stimulation (Fig. 6F). Soleus muscles that were treated with Mac had similar mass compared with contralateral muscles that were administered Vehicle (Table 1).
Figure 6.
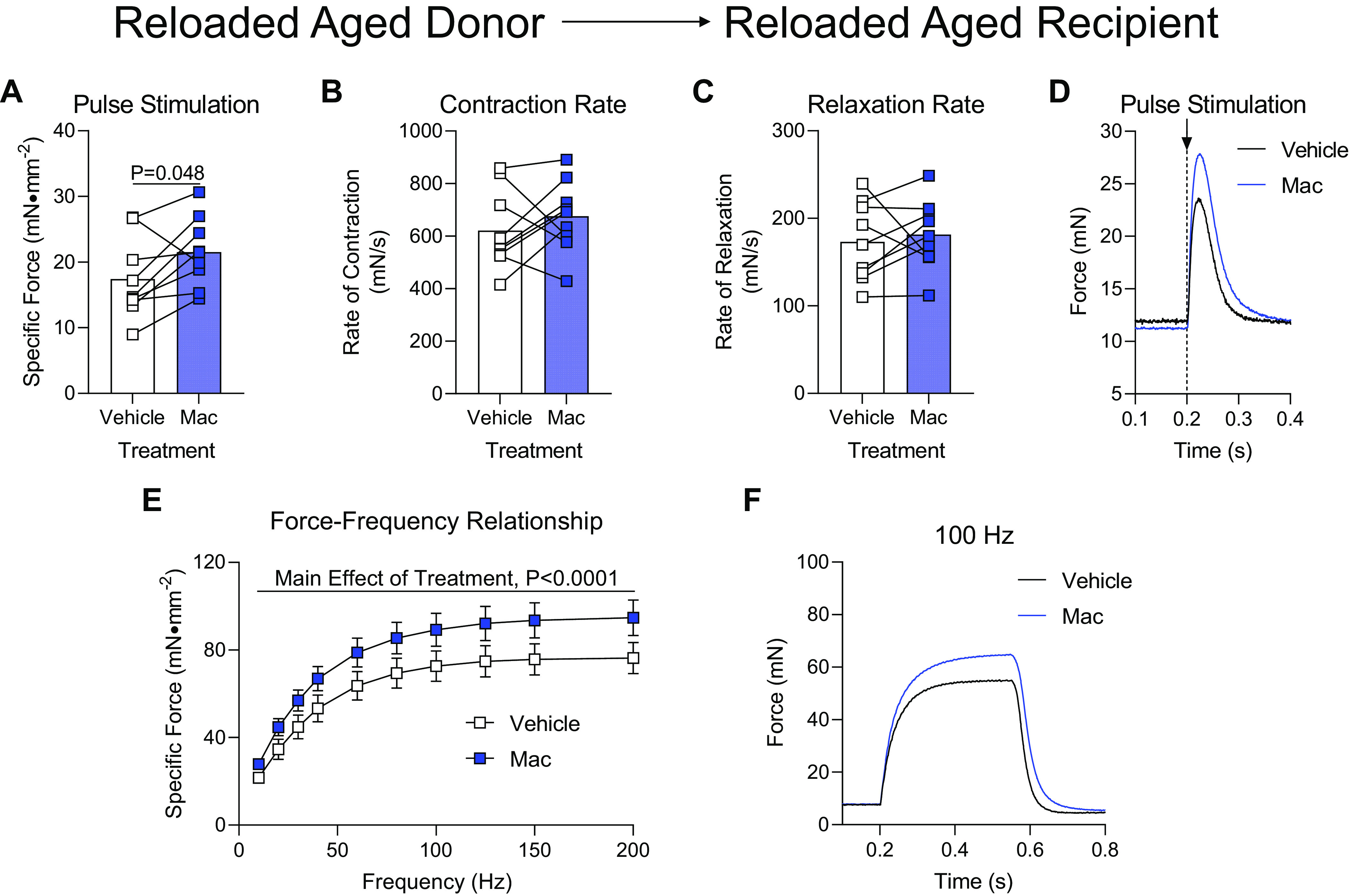
Delivery of macrophages from aged donors after hindlimb unloading/reloading to aged recipients following hindlimb unloading. Ex vivo force production of soleus muscles from aged mice during early recovery following hindlimb unloading that received intramuscular injections of macrophages from 4-day reloaded aged mice (Mac) or PBS (Vehicle) into contralateral hindlimbs. A–D: muscles were subjected to a single pulse stimulation to induce a twitch contraction for the measurement of specific force (A), rate of contraction (B), or rate of relaxation (C). D: raw force tracings from a single-pulse stimulation that induced a twitch contraction. E: specific force was measured across a range of stimulation frequencies from 10 to 200 Hz. F: raw force tracings from a 100 Hz stimulation. A–C: individual data points are shown and connected with respective contralateral limbs and the mean is represented as a bar. E: data points are shown as means ± SE. Paired t tests (A–C) or a two-way ANOVA with Sidak’s multiple-comparisons test (E) was used. n = 9/group.
Delivery of Macrophage-Colony Stimulating Factor to Reloaded Aged Mice
Next, we wanted to test the ability of directly administering M-CSF, a chemokine known to activate and promote macrophage chemotaxis, to aged mice during the recovery from disuse atrophy to aid in muscle recovery throughout the reloading process. The delivery of M-CSF after reloading promoted a dramatic increase in intramuscular macrophage content compared with Vehicle-treated soleus muscles (Fig. 7, A and B). When soleus muscles were subjected to a pulse stimulation to induce a twitch contraction they produced similar force, rates of contraction, rates of relaxation, and raw force tracings whether they received Vehicle or M-CSF (Fig. 7, C–F). Soleus muscles that were injected with M-CSF produced generally more force compared with Vehicle-treated muscles across a range of stimulation frequencies (Fig. 7G), and this modest increase was represented in raw force tracings from 100 Hz stimulations (Fig. 7H), indicating that M-CSF administration promoted the recovery of aged muscle from disuse atrophy, but to a lesser extent compared with directly administering polarized macrophages. Soleus muscles were similar in mass during this early period of reloading between Vehicle and M-CSF treatment (Table 1).
DISCUSSION
Incomplete recovery of muscle size and function is a hallmark of aged muscle following a bout of disuse (1, 34, 35), leading to greater risks for all-cause mortality and poor quality of life (36, 37). As the global aged population increases rapidly, the identification of pathways and proposal of therapeutics are important considerations that could aid in aged muscle recovery following disuse-induced dysfunction with aging. We present the novel finding that delivery of bone-marrow-derived macrophages that were activated and polarized as proinflammatory macrophages in vitro and then delivered intramuscularly to aged mice was sufficient to increase muscle function during early recovery, regardless of the age or the experimental condition of the mouse that the donor macrophages were harvested from. Similarly, we found that muscle delivery of the cytokine, macrophage-colony stimulating factor (M-CSF), increased macrophage content, and muscle force in soleus muscle of aged mice. Therefore, immunomodulation approaches that target macrophages may be a future therapeutic consideration to amplify the muscle recovery following disuse atrophy in aging.
Initially, we tested the ability of polarized proinflammatory macrophages derived from bone marrow progenitors from young adult donor mice to alter the function of soleus muscle in young adult or aged recipient mice following a bout of disuse atrophy. We found that the delivery of young adult donor polarized proinflammatory macrophages to young adult recipient mouse muscle only modestly increased the maximal force of the soleus during a twitch contraction (compared with contralateral vehicle control), but did not alter the contractile kinetics of the twitch or the maximal force across a broad range of stimulation frequencies. This modest increase in a single parameter of muscle function suggests that macrophage proinflammatory function is likely at an already optimal level in young adult mice during early recovery from disuse atrophy. On the other hand, when polarized proinflammatory macrophages from young adult donor mice were delivered to aged mice following HU, which are described to have dysregulated macrophage inflammatory phenotype in vivo (11), we observed a substantial increase in maximal force capacity of the aged soleus muscle across a range of tetanic stimuli. Importantly, the difference in maximal force production compared with vehicle in the aged muscles that received Mac treatment from young adult donors had similar force outputs as young adult mice during recovery, suggesting a rescue of aged muscle during early recovery using macrophage immunomodulation.
Since it was evident that the delivery of young adult proinflammatory macrophages was effective to increase muscle function in aged mice following disuse, the next logical question was whether delivering macrophages from aged mice could further disrupt muscle recovery following HU. Previous reports indicated that aging negatively impacts macrophage function (11, 14, 38, 39), which could explain why aged mice exhibit insufficient recovery of muscle function compared with young adults following a bout of disuse atrophy. We also tested whether Mac delivery from aged donors negatively impacted the muscle function of young adult mice following disuse atrophy. Interestingly, delivery of aged donor polarized proinflammatory macrophages into the muscle of young adult mice caused a reduction in muscle force across a range of tetanic stimulations, although this effect was very modest. However, in contrast to our initial hypothesis, the delivery of aged polarized proinflammatory macrophages to the muscle of aged mouse recipients following HU resulted in substantially higher muscle function compared with Vehicle-treated muscles across all parameters analyzed. This finding is particularly interesting as it indicates that, regardless of the age of donor, if sufficient proinflammatory polarized macrophages are delivered to the aged muscle during the early regrowth phase, muscle recovery can be improved even if the donor macrophages are partly intrinsically dysregulated. Indeed, during our in vitro macrophage characterization analysis, the macrophages from aged mouse donors were hypersensitive to a standard proinflammatory polarization cocktail (IFNγ, LPS), as noted by elevated proinflammatory gene expression compared with young adult polarized macrophages. Though aged macrophages are potentially oversensitive to stimuli in vitro, work from our laboratory showed that, in vivo, infiltrated muscle macrophages from aged mice during early recovery are absent of a proinflammatory profile compared to young adult mice (11). This infers that, in vivo, alternate nodes of dysfunction exist in the ability of aged macrophages to adopt an inflammatory phenotype and resolve muscular injuries. Some of these could be 1) insufficient volume of infiltrating macrophages to the site of damaged tissue, 2) infiltrating macrophages that are exposed to inappropriate cellular cues from the aged microenvironment thereby disrupting activation and differentiation, or 3) a combination of all of the above. Further work is warranted to test these intriguing questions.
We also considered that unloading and reloading of hindlimbs could alter bone marrow-derived macrophage function in aged mice since evidence suggests that microgravity in mice and humans dysregulates bone marrow cell homeostasis (40, 41) and hindlimb unloading has been shown to disrupt macrophage polarization (9). Therefore, we subjected aged donor mice to 14 days of HU followed by 4 days of reloading before the isolation of bone marrow-derived macrophages. We then delivered these donor-aged polarized proinflammatory macrophages to the hindlimb muscle of aged recipient mice after HU and examined early muscle recovery as in the prior experiments. Surprisingly, donor proinflammatory macrophages collected from reloaded aged mice and delivered to recipient aged muscle were comparably effective to increase soleus muscle function as the delivery of donor proinflammatory macrophages from ambulatory young adult or aged mice. Together, these data indicate that, regardless of the experimental condition of the bone-marrow-derived macrophages, aged muscle recovery can be improved with the early delivery of proinflammatory macrophages.
With the understanding that delivery of proinflammatory macrophages was sufficient to help aged muscle recovery from disuse, we postulated that, in vivo, the aged microenvironment may provide incorrect cues to mobilize and polarize macrophages during early recovery. Indeed, this concept has been documented by elegant muscle (42) and serum (43, 44) transfer studies in young and aged mice. Previously, the chemokine, M-CSF, has been used to aid in muscle recovery from several mouse injury models (27, 45, 46). In addition, blockade of M-CSF receptor activation promoted poor macrophage recruitment and inhibited muscle regeneration following damage (47). Therefore, we hypothesized that the delivery of M-CSF to aged muscle after HU may circumvent a disrupted microenvironment thereby recruiting muscle macrophages and aiding in muscle recovery following disuse. Excitingly, we found that aged muscle that was treated with M-CSF had higher macrophage content and, importantly, had greater muscle force production compared with Vehicle-treated soleus. These data support that activation of macrophages in vivo with M-CSF was capable of aiding parameters of muscle function during early recovery following disuse atrophy.
Though this study provides important insights into the biology of aging muscle and macrophages, many exciting questions remain standing. In this study, mice were only studied at early recovery, a time point in which proinflammatory macrophages are known to be abundant in young adult mice (8, 48, 49) following recovery from disuse atrophy or injury. We examined this early timepoint and capitalized on a proinflammatory macrophage immunotherapy as this was the time where we previously identified macrophage proinflammatory defects during recovery in old compared with young adult mice (11). Proinflammatory macrophages are important for not only clearing debris but also for initiating remodeling events such as activation of satellite cells and regulating fibroadipogenic progenitor cells (16, 19, 50–53). Limiting ourselves to this early timepoint is also likely why muscle mass was not different compared with Vehicle, though we did not assess fiber cross-sectional area to be confident in this observation. Future studies should also consider the delivery of anti-inflammatory polarized macrophages at a later time recovery point when these macrophage subtypes are normally abundant to determine if these cells similarly aid in muscle recovery in aging (23). We also recognize that macrophage dysfunction is presumably only one of several mechanisms that contribute to poor muscle recovery in aging since pericyte muscle delivery also was effective to improve muscle regrowth in aged mice (54). Finally, we assume that the main effect of M-CSF was to increase macrophage recruitment and activation of the skeletal muscle, but it is also likely that M-CSF could be acting directly on other tissues such as the myofiber. Indeed, skeletal muscle fibers also express the receptor for M-CSF (27, 55).
GRANTS
This work was supported by the National Institutes of Health Grants R01 AG076075, R21 AG062923 (to M. J. Drummond and R. M. O’Connell), F99AG073493 (to J. J. Petrocelli), and National Institutes of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health F32 AR072481 (to P. T. Reidy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.J.F., P.T.R., and M.J.D. conceived and designed research; P.J.F., E.M.Y., J.J.P., D.K.F., C.T.H., N.M.M.P.d.H., Z.S.M., P.T.R., and R.M.O. performed experiments; P.J.F. and E.M.Y. analyzed data; P.J.F., E.M.Y., J.J.P., P.T.R., and M.J.D. interpreted results of experiments; P.J.F., E.M.Y., and M.J.D. prepared figures; P.J.F. and M.J.D. drafted manuscript; P.J.F., E.M.Y., J.J.P., Z.S.M., P.T.R., R.M.O., and M.J.D. edited and revised manuscript; P.J.F., E.M.Y., J.J.P., D.K.F., C.T.H., N.M.M.P.d.H., Z.S.M., P.T.R., R.M.O., and M.J.D. approved final version of manuscript.
ACKNOWLEDGMENTS
Figure 1 and Graphical Abstract created with BioRender and published with permission.
REFERENCES
- 1. Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev 12: 898–906, 2013. doi: 10.1016/j.arr.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 2. Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol 5: 369, 2014. doi: 10.3389/fphys.2014.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146–M156, 2001. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 4. Tobin SW, Alibhai FJ, Wlodarek L, Yeganeh A, Millar S, Wu J, Li SH, Weisel RD, Li RK. Delineating the relationship between immune system aging and myogenesis in muscle repair. Aging Cell 20: e13312, 2021. doi: 10.1111/acel.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tidball JG, Flores I, Welc SS, Wehling-Henricks M, Ochi E. Aging of the immune system and impaired muscle regeneration: a failure of immunomodulation of adult myogenesis. Exp Gerontol 145: 111200, 2021. doi: 10.1016/j.exger.2020.111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baehr LM, West DWD, Marshall AG, Marcotte GR, Baar K, Bodine SC. Muscle-specific and age-related changes in protein synthesis and protein degradation in response to hindlimb unloading in rats. J Appl Physiol (1985) 122: 1336–1350, 2017. doi: 10.1152/japplphysiol.00703.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baehr LM, West DW, Marcotte G, Marshall AG, De Sousa LG, Baar K, Bodine SC. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany NY) 8: 127–146, 2016. doi: 10.18632/aging.100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reidy PT, McKenzie AI, Mahmassani ZS, Petrocelli JJ, Nelson DB, Lindsay CC, Gardner JE, Morrow VR, Keefe AC, Huffaker TB, Stoddard GJ, Kardon G, O'Connell RM, Drummond MJ. Aging impairs mouse skeletal muscle macrophage polarization and muscle-specific abundance during recovery from disuse. Am J Physiol Endocrinol Metab 317: E85–E98, 2019. doi: 10.1152/ajpendo.00422.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kohno S, Yamashita Y, Abe T, Hirasaka K, Oarada M, Ohno A, Teshima-Kondo S, Higashibata A, Choi I, Mills EM, Okumura Y, Terao J, Nikawa T. Unloading stress disturbs muscle regeneration through perturbed recruitment and function of macrophages. J Appl Physiol (1985) 112: 1773–1782, 2012. doi: 10.1152/japplphysiol.00103.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reidy PT, Dupont-Versteegden EE, Drummond MJ. Macrophage regulation of muscle regrowth from disuse in aging. Exerc Sport Sci Rev 47: 246–250, 2019. doi: 10.1249/JES.0000000000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fix DK, Ekiz HA, Petrocelli JJ, McKenzie AM, Mahmassani ZS, O’Connell RM, Drummond MJ. Disrupted macrophage metabolic reprogramming in aged soleus muscle during early recovery following disuse atrophy. Aging Cell 20: e13448, 2021. doi: 10.1111/acel.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dumont N, Frenette J. Macrophages protect against muscle atrophy and promote muscle recovery in vivo and in vitro: a mechanism partly dependent on the insulin-like growth factor-1 signaling molecule. Am J Pathol 176: 2228–2235, 2010. doi: 10.2353/ajpath.2010.090884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol 578: 327–336, 2007. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Wehling-Henricks M, Welc SS, Fisher AL, Zuo Q, Tidball JG. Aging of the immune system causes reductions in muscle stem cell populations, promotes their shift to a fibrogenic phenotype, and modulates sarcopenia. FASEB J 33: 1415–1427, 2019. doi: 10.1096/fj.201800973R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. J Interferon Cytokine Res 32: 18–26, 2012. doi: 10.1089/jir.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204: 1057–1069, 2007. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol 189: 3669–3680, 2012. doi: 10.4049/jimmunol.1103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang H, Melton DW, Porter L, Sarwar ZU, McManus LM, Shireman PK. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am J Pathol 184: 1167–1184, 2014. doi: 10.1016/j.ajpath.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mounier R, Theret M, Arnold L, Cuvellier S, Bultot L, Goransson O, Sanz N, Ferry A, Sakamoto K, Foretz M, Viollet B, Chazaud B. AMPKalpha1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab 18: 251–264, 2013. doi: 10.1016/j.cmet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 20. Perdiguero E, Sousa-Victor P, Ruiz-Bonilla V, Jardi M, Caelles C, Serrano AL, Munoz-Canoves P. p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J Cell Biol 195: 307–322, 2011. doi: 10.1083/jcb.201104053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21: 786–794, 2015. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- 22. Rybalko V, Hsieh PL, Merscham-Banda M, Suggs LJ, Farrar RP. The development of macrophage-mediated cell therapy to improve skeletal muscle function after injury. PLoS One 10: e0145550, 2015. doi: 10.1371/journal.pone.0145550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hammers DW, Rybalko V, Merscham-Banda M, Hsieh PL, Suggs LJ, Farrar RP. Anti-inflammatory macrophages improve skeletal muscle recovery from ischemia-reperfusion. J Appl Physiol (1985) 118: 1067–1074, 2015. doi: 10.1152/japplphysiol.00313.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hsieh PL, Rybalko V, Baker AB, Suggs LJ, Farrar RP. Recruitment and therapeutic application of macrophages in skeletal muscles after hind limb ischemia. J Vasc Surg 67: 1908–1920.e1, 2018. doi: 10.1016/j.jvs.2017.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jetten N, Donners MM, Wagenaar A, Cleutjens JP, van Rooijen N, de Winther MP, Post MJ. Local delivery of polarized macrophages improves reperfusion recovery in a mouse hind limb ischemia model. PLoS One 8: e68811, 2013. doi: 10.1371/journal.pone.0068811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Novak ML, Weinheimer-Haus EM, Koh TJ. Macrophage activation and skeletal muscle healing following traumatic injury. J Pathol 232: 344–355, 2014. [Erratum in J Pathol 233: 319, 2014]. doi: 10.1002/path.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dumont NA, Frenette J. Macrophage colony-stimulating factor-induced macrophage differentiation promotes regrowth in atrophied skeletal muscles and C2C12 myotubes. Am J Pathol 182: 505–515, 2013. doi: 10.1016/j.ajpath.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 28. O’Connell RM, Rao DS, Chaudhuri AA, Boldin MP, Taganov KD, Nicoll J, Paquette RL, Baltimore D. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med 205: 585–594, 2008. doi: 10.1084/jem.20072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petrocelli JJ, Mahmassani ZS, Fix DK, Montgomery JA, Reidy PT, McKenzie AI, de Hart NM, Ferrara PJ, Kelley JJ, Eshima H, Funai K, Drummond MJ. Metformin and leucine increase satellite cells and collagen remodeling during disuse and recovery in aged muscle. FASEB J 35: e21862, 2021. doi: 10.1096/fj.202100883R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferrara PJ, Verkerke ARP, Maschek JA, Shahtout JL, Siripoksup P, Eshima H, Johnson JM, Petrocelli JJ, Mahmassani ZS, Green TD, McClung JM, Cox JE, Drummond MJ, Funai K. Low lysophosphatidylcholine induces skeletal muscle myopathy that is aggravated by high-fat diet feeding. FASEB J 35: e21867, 2021. doi: 10.1096/fj.202101104R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferrara PJ, Rong X, Maschek JA, Verkerke AR, Siripoksup P, Song H, Green TD, Krishnan KC, Johnson JM, Turk J, Houmard JA, Lusis AJ, Drummond MJ, McClung JM, Cox JE, Shaikh SR, Tontonoz P, Holland WL, Funai K. Lysophospholipid acylation modulates plasma membrane lipid organization and insulin sensitivity in skeletal muscle. J Clin Invest 131: e135963, 2021. doi: 10.1172/JCI135963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McKenzie AI, Reidy PT, Nelson DS, Mulvey JL, Yonemura NM, Petrocelli JJ, Mahmassani ZS, Tippetts TS, Summers SA, Funai K, Drummond MJ. Pharmacological inhibition of TLR4 ameliorates muscle and liver ceramide content after disuse in previously physically active mice. Am J Physiol Regul Integr Comp Physiol 318: R503–R511, 2020. doi: 10.1152/ajpregu.00330.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Welc SS, Wehling-Henricks M, Antoun J, Ha TT, Tous I, Tidball JG. Differential effects of myeloid cell PPARdelta and IL-10 in regulating macrophage recruitment, phenotype, and regeneration following acute muscle injury. J Immunol 205: 1664–1677, 2020. doi: 10.4049/jimmunol.2000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suetta C. Plasticity and function of human skeletal muscle in relation to disuse and rehabilitation: influence of ageing and surgery. Dan Med J 64: B5377, 2017. [PubMed] [Google Scholar]
- 35. Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev 47: 123–132, 2018. doi: 10.1016/j.arr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li R, Xia J, Zhang XI, Gathirua-Mwangi WG, Guo J, Li Y, McKenzie S, Song Y. Associations of muscle mass and strength with all-cause mortality among US older adults. Med Sci Sports Exerc 50: 458–467, 2018. doi: 10.1249/MSS.0000000000001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang H, Hai S, Liu Y, Liu Y, Dong B. Skeletal muscle mass as a mortality predictor among nonagenarians and centenarians: a prospective cohort study. Sci Rep 9: 2420, 2019. doi: 10.1038/s41598-019-38893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duong L, Radley HG, Lee B, Dye DE, Pixley FJ, Grounds MD, Nelson DJ, Jackaman C. Macrophage function in the elderly and impact on injury repair and cancer. Immun Ageing 18: 4, 2021. doi: 10.1186/s12979-021-00215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Linehan E, Fitzgerald DC. Ageing and the immune system: focus on macrophages. Eur J Microbiol Immunol (Bp) 5: 14–24, 2015. doi: 10.1556/EUJMI-D-14-00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ortega MT, Pecaut MJ, Gridley DS, Stodieck LS, Ferguson V, Chapes SK. Shifts in bone marrow cell phenotypes caused by spaceflight. J Appl Physiol (1985) 106: 548–555, 2009. doi: 10.1152/japplphysiol.91138.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang P, Tian H, Zhang J, Qian J, Li L, Shi L, Zhao Y. Spaceflight/microgravity inhibits the proliferation of hematopoietic stem cells by decreasing Kit-Ras/cAMP-CREB pathway networks as evidenced by RNA-Seq assays. FASEB J 33: 5903–5913, 2019. doi: 10.1096/fj.201802413R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol Cell Physiol 256: C1262–C1266, 1989. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- 43. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433: 760–764, 2005. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 44. Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810, 2007. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 45. Martin KS, Kegelman CD, Virgilio KM, Passipieri JA, Christ GJ, Blemker SS, Peirce SM. In silico and in vivo experiments reveal M-CSF injections accelerate regeneration following muscle laceration. Ann Biomed Eng 45: 747–760, 2017. doi: 10.1007/s10439-016-1707-2. [DOI] [PubMed] [Google Scholar]
- 46. Martins L, Gallo CC, Honda TSB, Alves PT, Stilhano RS, Rosa DS, Koh TJ, Han SW. Skeletal muscle healing by M1-like macrophages produced by transient expression of exogenous GM-CSF. Stem Cell Res Ther 11: 473, 2020. doi: 10.1186/s13287-020-01992-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Segawa M, Fukada S, Yamamoto Y, Yahagi H, Kanematsu M, Sato M, Ito T, Uezumi A, Hayashi S, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Yamamoto H. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp Cell Res 314: 3232–3244, 2008. doi: 10.1016/j.yexcr.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 48. Tidball JG. Interactions between muscle and the immune system during modified musculoskeletal loading. Clin Orthop Relat Res 403 Suppl: S100–S109, 2002. doi: 10.1097/00003086-200210001-00012. [DOI] [PubMed] [Google Scholar]
- 49. Chazaud B. Macrophages: supportive cells for tissue repair and regeneration. Immunobiology 219: 172–178, 2014. doi: 10.1016/j.imbio.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 50. Yang W, Hu P. Skeletal muscle regeneration is modulated by inflammation. J Orthop Translat 13: 25–32, 2018. doi: 10.1016/j.jot.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dort J, Fabre P, Molina T, Dumont NA. Macrophages are key regulators of stem cells during skeletal muscle regeneration and diseases. Stem Cells Int 2019: 4761427, 2019. doi: 10.1155/2019/4761427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol 163: 1133–1143, 2003. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lu H, Huang D, Ransohoff RM, Zhou L.. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J 25: 3344–3355, 2011. doi: 10.1096/fj.10-178939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Munroe M, Dvoretskiy S, Lopez A, Leong J, Dyle MC, Kong H, Adams CM, Boppart MD. Pericyte transplantation improves skeletal muscle recovery following hindlimb immobilization. FASEB J 33: 7694–7706, 2019. doi: 10.1096/fj.201802580R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Okazaki T, Ebihara S, Takahashi H, Asada M, Kanda A, Sasaki H. Macrophage colony-stimulating factor induces vascular endothelial growth factor production in skeletal muscle and promotes tumor angiogenesis. J Immunol 174: 7531–7538, 2005. doi: 10.4049/jimmunol.174.12.7531. [DOI] [PubMed] [Google Scholar]


