Abstract
Background
Multi-cancer early detection (MCED) testing could increase detection of cancer at early stages, when survival outcomes are better and treatment costs are lower, but is expected to increase screening costs. This study modeled an MCED test for 19 solid cancers in a US population and estimated the potential value-based price (the maximum price to meet a given willingness to pay) of the MCED test plus current single cancer screening (usual care) compared to usual care alone from a third-party payer perspective over a lifetime horizon.
Methods
A hybrid cohort-level state-transition and decision-tree model was developed to estimate the clinical and economic outcomes of annual MCED testing between age 50 and 79 years. The impact on time and stage of diagnosis was computed using an interception modeling approach, with the consequences of cancer modeled based on stage at diagnosis. The model parameters were mainly sourced from the literature, including a published case-control study to inform MCED test performance. All costs were inflated to 2021 US dollars.
Results
Multi-cancer early detection testing shifted cancer diagnoses to earlier stages, with a 53% reduction in stage IV cancer diagnoses, resulting in longer overall survival compared with usual care. Addition of MCED decreased per cancer treatment costs by $5421 and resulted in a gain of 0.13 and 0.38 quality-adjusted life-years across all individuals in the screening program and those diagnosed with cancer, respectively. At a willingness-to-pay threshold of $100,000 per quality-adjusted life-year gained, the potential value-based price of an MCED test was estimated at $1196. The projected survival of individuals diagnosed with cancer and the number of cancers detected at an earlier stage by MCED had the greatest impact on outcomes.
Conclusions
An MCED test with high specificity would potentially improve long-term health outcomes and reduce cancer treatment costs, resulting in a value-based price of $1196 at a $100,000/quality-adjusted life-year willingness-to-pay threshold.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-022-01181-3.
Key Points for Decision Makers
| Multi-cancer early detection tests present a potential paradigm shift in screening by simultaneously screening for multiple types of cancer. |
| A hybrid state-transition and decision-tree model was developed to estimate the potential value-based price of annual multi-cancer early detection test plus usual care screening versus usual care alone in US adults aged 50–79 years from a third-party payer perspective. |
| Our findings demonstrated that an earlier diagnosis with multi-cancer early detection resulting from a stage and time shift may lead to improved patient survival, increased quality-adjusted life-years, and lower per-case treatment costs when compared with usual care. |
Introduction
With 9.6 million deaths in 2018, cancer is the second leading cause of mortality worldwide [1, 2]. In that same year, more than 600,000 people died from cancer in the USA alone, while almost 2 million new cases were diagnosed [2]. From 1990 to 2015, however, the cancer mortality rate declined by 25% in the USA, with greater decreases for breast cancer (39% among women) and colorectal cancer (44% among women; 47% among men), partly owing to an increased use of screening programs [3]. The majority of cancer mortality in the USA occurs as a consequence of cancers without current recommended screening programs [4]. In 2020, the economic burden of cancer care increased from $125 billion in 2010 to $207 billion (66%) [5]. The ability to detect early-stage cancer may lead to more effective treatment and reduce the high cost of late-stage treatment.
The US Preventive Services Task Force and the National Comprehensive Cancer Network recommend various screening approaches for common cancer types [2, 6], for which mortality reduction has been established in specific groups [7, 8]. However, current screening methods are only applicable to one specific cancer type and can be invasive.
Moreover, single cancer screenings are only available for the most common cancers. Screening for a single lower incidence cancer requires screening larger numbers of individuals to detect a cancer, which can create high burdens associated with false positives for each cancer found, as well as a challenging cost-effectiveness balance. Screening for multiple cancers simultaneously may help address these challenges. Recently, multi-cancer early detection (MCED) genomic blood tests that can simultaneously screen for multiple types of cancer have been developed, and an analysis has been published suggesting the potential of these tests to be cost effective [9–12].
The objective of this study was to explore the clinical and economic implications of an MCED test for 19 solid cancers for US adults who had not previously been diagnosed with cancer and to estimate the potential value-based price (VBP) of adding the MCED test to current single cancer screening (usual care) [i.e., breast, prostate, lung, cervical, and colorectal] versus usual care alone from a third-party payer perspective over a lifetime horizon. The VBP for an intervention is the maximum price at which a specified cost-effectiveness ratio, or a willingness-to-pay (WTP) threshold, is reached. A $100,000 WTP threshold per quality-adjusted life-year (QALY) gained was considered for the base-case analysis, following studies in cancer screening [13] and estimates of US payer thresholds [14]. Other WTP thresholds were explored in scenario analyses. The impact of variations in key model parameters and assumptions were tested through sensitivity and scenario analyses.
Methods
Model Structure
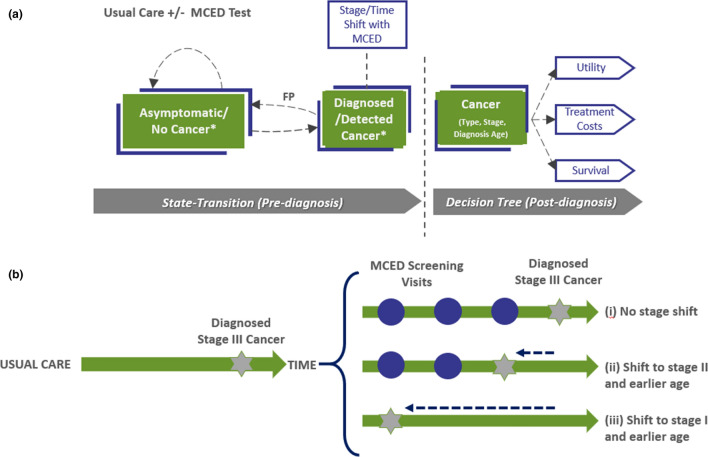
A hybrid cohort-level model with two key components was developed to efficiently capture the impact of screening on stage dynamics across multiple cancer types (Fig. 1): (1) a state-transition structure with a 1-year cycle length estimating the number of cancers diagnosed within a patient cohort over a lifetime and (2) a decision-tree component estimating the clinical and economic consequences of incident cancers diagnosed. A patient cohort entered the model at age 50 years and received annual MCED testing until age 79 years. Patient survival, cost, and quality-of-life measures were calculated both pre-diagnosis and post-diagnosis. The model approach and assumptions were validated by clinicians throughout the model’s development. Further details of the model justification can be found in the Electronic Supplementary Material (ESM). Both costs and benefits were discounted at 3% per year and all costs were inflated to 2021 US dollars using the US Bureau of Economic Analysis price index for personal consumer expenditures for healthcare [15].
Fig. 1.
Overview of the model structure with an example of stage and time shifting of diagnosed cancers due to a multi-cancer early detection (MCED) test. a *False-positive patients (in asymptomatic/no cancer group) and those misdiagnosed because of a wrong cancer signal origin (in detected cancer group) accrued additional work-up costs and disutilities before being accurately assigned to having cancer or not. b An individual who will be diagnosed with cancer at stage III under usual care (left), may (i) have the same cancer diagnosis with MCED testing, (ii) the cancer detection shifted to stage II and an earlier age, or (iii) the cancer detection shifted to stage I and an even earlier stage. Note: Patients can die from background mortality during the pre-diagnosis phase
The model estimated the fraction of the population with cancer diagnoses in the usual care arm based on age-specific and stage-specific cancer incidence rates for each cancer type. Incidence rates were derived from the Surveillance, Epidemiology, and End Results Program (SEER) [16, 17] (see Table 1 of the ESM). Recommended screening protocols, adherence to these recommendations, and subsequent diagnostic work-ups for cancers with current screening were derived from National Comprehensive Cancer Network and US Preventive Services Task Force guidelines, the literature, and the Behavioral Risk Factor Surveillance System (see Table 2 of the ESM) [18–23].
A published case-control study was mainly used to inform the inputs on test performance in the MCED arm [10]. Patients with cancer could, based on the sensitivity of the MCED test (Table 3 of the ESM), be detected earlier in time and therefore earlier in disease progression than with usual care screening alone. This earlier diagnosis with MCED is modeled by shifting the stage and time of diagnosis in the MCED arm relative to the usual care arm [24]. Here, a ‘stage shift’ refers to a shift in the stage of diagnosis from later stages to earlier stages (e.g., from stage III to I), and a ‘time shift’ refers to a diagnosis being shifted back in time to an earlier age to account for an earlier diagnosis (example shown in Fig. 1). The probability that a cancer is stage shifted (detected at an earlier stage relative to usual care) is dependent on MCED test sensitivity to that cancer type and the likelihood that the patient was screened with the MCED test while the cancer was present at an earlier stage. The extent to which a cancer that is stage shifted is shifted to an earlier age is dependent on the speed at which that cancer type progresses. The compliance rate for the MCED test was set to 90%, slightly higher than the adherence reported to regular cancer screenings based on commercial claims data [25] (e.g., breast, colorectal, cervical), reflecting the relative ease of a blood draw versus existing screening modalities, with a range of compliance tested in sensitivity analyses. More details about MCED test characteristics are discussed in the ESM.
Patients diagnosed with cancer during each cycle had their remaining survival and the cost and QALYs they accrued estimated based on their diagnosis age, cancer type, and stage. Stage-based treatment costs derived from SEER-Medicare adjusted with a cost multiplier [26, 27] as well as utilities [28] based on stage-specific utility multipliers estimated from the literature [29–39] (Tables 4–5 of the ESM) were assigned upon diagnosis and accrued for up to 5 years post-diagnosis, after which time the general population utility was assumed restored. Stage-based survival (Table 6 of the ESM) was assigned at diagnosis for the duration of the model time horizon. Background mortality was derived from US life tables, while post-diagnosis mean survival was derived from SEER [40, 41].
The model also accounted for a misdiagnosis resulting from MCED testing as well as false-positive patients resulting from screening and their corresponding work-up costs and disutility. Patients not diagnosed with cancer as well as false-positive patients stayed in the state-transition structure and continued screening and accrued QALYs based on age-based utility until the end of the screening period (i.e., when diagnosed with cancer, died, or reached the end of time horizon). Screening costs and costs associated with misdiagnosis were also considered in the model (see Table 7 of the ESM).
Key Model Components
Stage Shift and Time Shift
Stage and time shift were estimated based on a previously published interception model [24]. The interception model has a state-transition flow structure designed to compute the proportion of cancers intercepted by MCED screening. It uses inputs on the frequency of MCED screening, estimated cancer dwell times by stage, and the sensitivity of the MCED test for different cancer types and stages and generates a set of upper-triangular cancer-specific matrices on the probability of a stage shift from each cancer stage to all earlier stages. The stage shift probability matrices used in the base-case analysis are listed in Table 8 of the ESM. Figure 1 of the ESM shows an example of a stage shift with lung cancer.
After diagnosed patients were stage shifted in each model cycle, they were shifted back in time to earlier cycles to account for an earlier diagnosis with the MCED test. This shift in time was based on cancer-specific dwell times (see Table 9 of the ESM) and are consistent with results from a Delphi panel of clinical experts [24]. The time-shift distribution for single-stage transitions was based on the exponential distribution of the mean dwell time for each stage and cancer. The time-shift distribution for multi-stage transitions (i.e., stage IV to I, IV to II, III to I) was based on an empirical distribution for each cancer created by combining the exponential distribution for each stage’s dwell time.
Overdiagnosis
To understand the potential impact of overdiagnosis due to the MCED test detecting cancer in patients who would have died with undetected cancer, overdiagnosis was considered in the model. In the base case, 5% of patients who died of non-cancer mortality were assumed to have undiagnosed cancer at death based on an evidence-reported prevalence of cancer not associated with mortality in autopsy studies. [42] Cancers that remained undiagnosed at death were assumed to be distributed into stages I, II, III, and IV in proportion to observed cancer-specific and stage-specific incidence rates. These patients could have cancers stage and time shifted by MCED testing such that their cancers were diagnosed prior to the non-cancer mortality.
Sensitivity and Scenario Analyses
A series of one-way sensitivity analyses was conducted to evaluate changes in key parameter values (Table 1). In addition, several scenario analyses were performed to test specific clinical assumptions. To understand the impact of MCED among cancers with common screening versus those without, a scenario was performed excluding breast, cervical, colorectal, and prostate cancers from the list of cancers that could be detected by MCED. Lung cancer was not excluded as the majority of lung cancers occur in individuals ineligible for screening, and uptake among those eligible for screening remains low [21, 23].
Table 1.
Sensitivity analyses
| # | Key parameter changes |
|---|---|
| 1 | Compliance lowered to 80%; increased to 100% |
| 2 | Screening age 50–64 years |
| 3 | Screening age 65–79 years; use Medicare costs |
| 4 | Cancer incidence varied ±20% |
| 5 | Sensitivity associated with “other” cancers will be equal to (a) kidney and renal pelvis cancer (i.e., the cancer with the lowest mean sensitivity across all stages) and (b) lung cancer (i.e., the cancer with mid-range mean sensitivity across all stages) |
| 6 | Dwell time will be varied to (a) slow, (b) medium, and (c) fast |
| 7 | False-positive work-up costs varied ±20% |
| 8 | Disutility for false-positive work-ups varied ±50% |
| 9 | Cancer treatment costs varied ±20% |
| 10 | Disutilities due to cancer varied ±50% |
| 11 | Reduction in the difference between cancer survival and background survival by 20% |
| 12 | Lower MCED test sensitivity by 20% |
MCED multi-cancer early detection
Another scenario tested the impact of mortality during the lead time by including an option to consider iatrogenic harm in patients who were stage shifted back in time because of early detection. As the proportion of patients with iatrogenic mortality cannot exceed the proportion of patients who would have died from cancer during the lead time, this fraction was conservatively assumed the same and based on the observed mortality of cancer by type and stage in the first 3 months after diagnosis. Another scenario evaluated the implications of considering MCED test on an ever-smoking population, who have a higher cancer incidence risk in at least ten cancers (relative risks shown in Table 10 of the ESM). Additional scenarios were also explored on the number of cancer types detected by the MCED test using a stepped approach based on the incidence and sensitivity, by varying the overdiagnosis rate, and by considering alternative WTP thresholds.
Data Availability
The data generated in this study are available herein and in the ESM.
Results
Base-Case Analyses
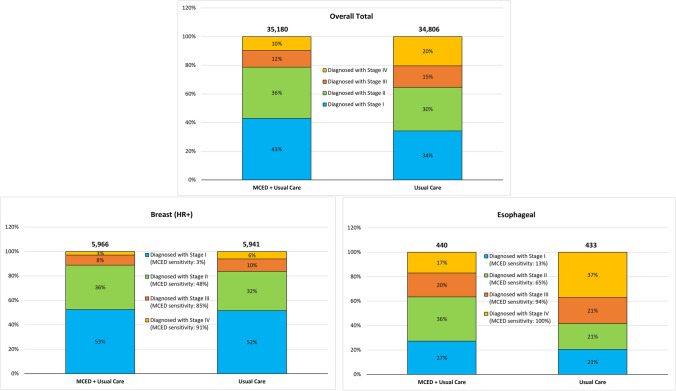
Using a lifetime analysis of a cohort of 100,000 individuals not previously diagnosed with cancer, the addition of annual screening with an MCED test led to a lower per case cancer diagnosis and per-case treatment costs, and increased QALYs when compared with usual care. Diagnosis of all cancers with MCED was shifted to earlier stages compared with usual care (see Fig. 2 and Table 11 of the ESM), and MCED testing resulted in an additional 374 diagnoses (89% from stages I and II) than usual care screening associated with overdiagnosis. For example, 3192 and 2021 more patients were diagnosed in stages I and II, respectively, and 1136 and 3704 fewer patients were diagnosed in stages III and IV, respectively, resulting in longer overall survival (0.39 additional life-years [LYs]) across individuals diagnosed with cancer, even with 8719 additional FP diagnoses and 860 inaccurate cancer signal origins associated with MCED testing. With approximately 28.9% of the population developing a cancer during the screening interval (vs 28.3% under usual care only), the overall screening population gained an average of 0.14 LYs and 0.13 QALYs.
Fig. 2.
Number of patients diagnosed with cancer (overall total and example cancers). Note: The total number of cancers diagnosed by multi-cancer early detection (MCED) plus usual care are higher than those diagnosed by the usual care arm alone as the base case considers overdiagnosis. Overall stage shift represents a combination of cancers that have a substantial stage shift due to MCED (e.g., esophageal) and those with a more modest stage shift (e.g., hormone receptor positive [HR+] breast cancer)
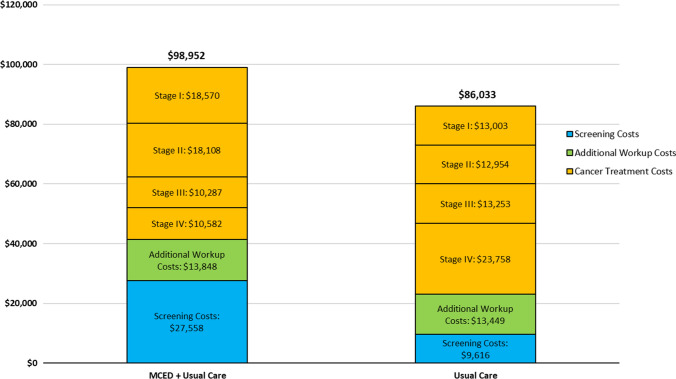
Using base-case inputs and applying a 3% health and cost discount rate across all model outcomes, the maximum value-based MCED screening test cost at WTP thresholds of $100,000/QALY was $1196/test. Using this test cost, the overall cost per person in the MCED arm was $12,919 more than in the usual care arm. The overall cost difference between the two study arms were due to higher screening costs ($17,941 additional) and additional work-up costs ($399 additional) in the MCED arm. These costs were partially offset by lower cancer treatment costs in the MCED arm compared with the usual care arm ($5421 less) due to stage and time shifting in the MCED arm (Fig. 3).
Fig. 3.
Base-case cost outcomes (lifetime cost per person). MCED multi-cancer early detection. Note: All reported cost outcomes are discounted
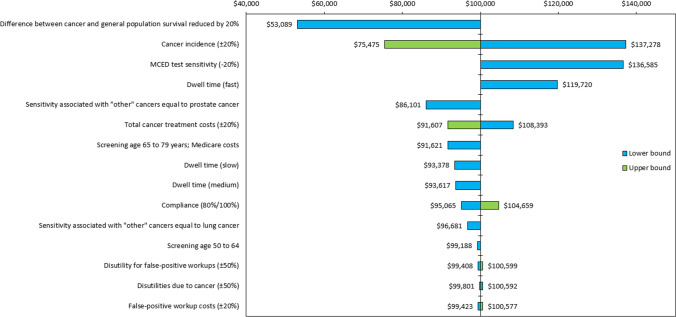
Sensitivity Analyses
The results of sensitively analyses are shown in a tornado diagram (Fig. 4). Reducing the difference between cancer survival and background mortality, cancer incidence, lowering MCED test sensitivity, and using a fast dwell time in the model resulted in the most variation in the incremental cost-effectiveness ratio (ICER). Parameters that were less influential on results were work-up costs associated with false positives (FPs), disutility due to cancer, and disutility associated with additional FP work-ups as only a small minority of FPs were related to the MCED test. Detailed results are shown in Table 12 of the ESM.
Fig. 4.
Tornado diagram of sensitivity analyses. MCED multi-cancer early detection. Note: Sorting based on the greatest to least variation in change from $100,000/quality-adjusted life-year incremental cost-effectiveness ratio
When compliance was changed from 90% in the base case to 80% and 100%, the proportion of cancers diagnosed in stages I and II reduced by 0.3% and increased by 0.3%, respectively from the base case. This percentage decreased by 1.4% when an older Medicare population was examined in the model and even fewer patients were being screened and diagnosed at earlier stages because of the older age of the population and decreased by 7.3% when screening was limited to a population aged 50–64 years. Further, more patients were diagnosed in stages I and II when the model used slower dwell times than in the base case (2.6% more with slow, 1.6% more with medium) as longer periods in each stage result in fewer interval cancers.
Scenario Analyses
As part of the scenario analyses, overdiagnosis was varied by assuming no overdiagnosis occurred in the model (0%) or a higher proportion of patients were diagnosed before death (10%). In the former scenario, overall costs per person decreased by $556 while in the latter scenario this cost increased by $556 and the resulting ICERs changed accordingly (i.e., $94,819, and $105,278 vs $100,000) (Table 13 of the ESM).
When iatrogenic harm was considered, a conservative assumption was made that all deaths within the first 3 months following diagnosis were due to iatrogenic harm and thus would be present immediately following early cancer detection. These patients did not accrue any utilities or treatment costs after this time. Compared with the base-case analysis, this led to lower overall incremental costs (−$11,940 vs −$12,919) and QALYs per person (0.12 vs 0.13 QALYs), resulting in an ICER of $101,508/QALY. When expanding this scenario to assume that iatrogenic harm only impacts QALYs gained and patients affected would nonetheless have accrued costs similar to those incurred over 5 years in the base case, the ICER increased to $109,828/QALY.
In a population of ever-smokers, more patients compared with usual care were diagnosed in earlier stages than in the base case (e.g., 7508 vs 5214 in stages I and II), which led to higher incremental LYs and QALYs (0.19 vs 0.14 LYs and 0.18 vs 0.13 QALYs), and lower overall incremental costs ($10,881 vs $12,919), resulting in an ICER of $60,282/QALY.
When MCED testing was limited to detecting only cancers not commonly screened for, fewer patients were stage/time shifted than in the base case. The scenario results showed lower incremental QALYs and higher incremental costs than in the base case (0.08 vs 0.13 QALYs and $16,163 vs $12,919 costs), resulting in a higher ICER of $205,444/QALY. When using additional thresholds of cost effectiveness at $50,000/QALY and $150,000/QALY, the VBP decreased/increased by 36% from $1196 in the base case to $767 and $1625, respectively.
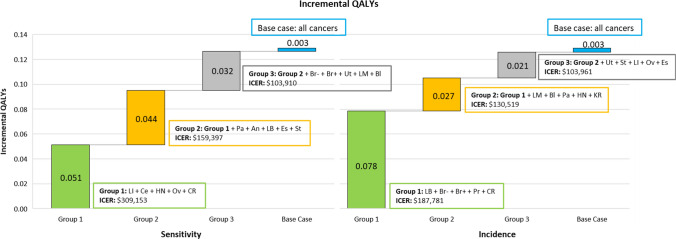
The impact of the number of cancer types detected by the MCED test was explored in more detail using a stepped approach based on test sensitivity and incidence to create hypothetical alternate versions of MCED tests (Fig. 5). These analyses found that capturing the benefit of MCED may require the ability to screen for 15 or more of the cancer groups considered here, representing a large majority of all solid cancer incidence. Specifically, the MCED test was first assumed to be sensitive only to the five cancers with the highest sensitivity associated with the MCED test. Next, the MCED test was assumed to be sensitive to ten cancers with the highest sensitivity associated with the MCED test. This stepped analysis was repeated using incidence as the determining factor for grouping cancers in sets of five. When the grouping was based on cancers associated with the highest test sensitivity, the first five cancers yielded 39.7% (0.05 QALYs) of the total incremental QALYs (0.13 QALYs) gained from all 19 cancers, while adding the next group of cancers yielded 73.6% (0.10 QALYs) of the total incremental QALYs gained in the base case. When 15 cancers were included in the analysis, an overall incremental gain of 0.13 QALYs was seen yielding 98.0% of the total incremental QALYs gained in the base case.
Fig. 5.
Results from stepped inclusion scenario analyses (sensitivity and incidence). An anus, Bl bladder, Br- breast HR-, Br+ breast HR+, Ce cervix, CR colon and rectum, Es esophagus, HN head and neck, KR kidney and renal pelvis, LI liver and intrahepatic bile duct, LB lung and bronchus, LM lymphoma, Oth other, Ov ovarian, Pa pancreas, Pr prostate, QALY quality-adjusted life-year, St stomach, Ur urothelial, Ut uterus
The first five cancers based on the highest incidence yielded 60.7% (0.08 QALYs) of the total incremental QALYs gained (0.13 QALYs) from the total cancers included in the base case. The addition of the next five cancers contributed to 81.3% (0.10 QALYs) of total incremental QALYs gained, and the top 15 cancers yielded 97.3% (0.13 QALYs) of total incremental QALYs gained. All scenarios (including grouping based on sensitivity and incidence) had higher incremental costs than the base case corresponding to the reduction in cancers detected earlier.
Discussion
This modeling study aimed to evaluate the clinical and economic outcomes of the addition of a MCED test to usual care screening and predict its potential VBP. These study results show an earlier diagnosis with MCED resulting from stage and time shifts may lead to improved patient survival and lower per-case treatment costs. Specifically, per 100,000 individuals not previously diagnosed with cancer, 4882 more patients were diagnosed with cancers in stages I and II in the MCED arm versus usual care alone. However, higher screening and work-up costs (including misdiagnosis) were found in the MCED strategy. Maximizing the breadth of cancers detected is essential to achieving the health economic benefits of MCED as capturing unscreened cancers, less prevalent cancers, and cancers missed by current screening each contribute significantly to the potential long-term benefits of MCED. At a WTP threshold of $100,000/QALY, the estimated VBP for the MCED test was $1196 from a US third-party payer perspective. Because of a lack of a standard WTP threshold for US third-party payers, alternative thresholds were explored in the scenario analysis where the lowest estimated VBP was $767/test at a $50,000/QALY threshold.
Sensitivity analyses highlighted that in the parameter ranges tested, the impact of MCED is largely driven by the number of cancers found (test sensitivity, cancer incidence), the potential survival benefits, dwell time, and the potential reductions in treatment costs following diagnosis. The largest change in the ICER was observed in the sensitivity analysis in which survival post-diagnosis improved to be closer to the background mortality rate of the general population. In this analysis, an approach was chosen such that the applied changes in survival were larger for cancers diagnosed in later stages, which had the anticipated effect of reducing the clinical benefit of early detection. Because of the increase in treatment costs associated with longer survival, however, early detection resulted in a greater reduction in treatment costs in this analysis. This dynamic was also observed in the scenario analysis in which iatrogenic harm was considered. Adding early mortality was associated with fewer incremental QALYs than the base case, but in turn also reduced the costs of treating late-stage cancers. However, the impact of the lost QALYs was more important, and the ICER rose modestly with the introduction of iatrogenic harm. Extended survival is the most important benefit of screening and expanded sensitivity analyses, including for example cure-fraction modeling, which will provide additional insight into the trade-off between clinical benefit and treatment cost changes afforded by MCED.
The ICER was strongly influenced by the number of cancers diagnosed in earlier stages, including when a variation in parameters changed the cancer incidence in the population, the sensitivity of cancers being detected by the MCED test, or tumor progression rates (which impacts the likelihood of interval cancers). In all of these analyses, changes that reduced the number of cancers detected by the MCED test corresponded to a worsening ICER, with both the clinical benefit and the offsets in treatment costs reduced. The opposite effects occurred for changes that increased the number of cancers detected, corresponding to a more favorable ICER. For example, the ICER in the ever-smoker population was improved by over 40% compared with the general population, which may highlight the magnitude of potential benefit in other high-risk populations (e.g., cancer survivors) not modeled in this study.
The results of the model were not largely changed when harms from screening were varied. In the scenario in which overdiagnosis was not considered, treatment costs decreased, but the overall ICER remained within $6000 of the base case. Similarly, in sensitivity analyses increasing the costs or quality of life penalties of false positives, ICERs worsened, but only very modestly. This reflects the characteristics of the MCED test modeled here, which has very high specificity (99.5%) and thus imposes the burden of false positives on a small fraction of screened individuals.
This study has some assumptions and limitations, and where possible, conservative assumptions have been made throughout this analysis. The model is primarily based on a previously published interception model, thus all assumptions associated with that model hold true in this analysis [24]. Specifically, invasive cancer is assumed to be a progressive disease; the raw sensitivity (the population fraction that can be detected by stage in an unmodified population) depends on the stage and is increasing by the stage; the mortality benefit from a stage shift is due to the stage at diagnosis being different than the stage at clinical presentation; and individuals cannot die before clinical presentation would have occurred. [43] FPs associated with the MCED test are in addition to the FPs based on usual care screening tests and are based on the specificity of the MCED test. The model assumes FPs will return to the cohort for additional cancer screening. Further, the model assumes the same overdiagnosis rate and cancer signal origin accuracy in the MCED test for all cancer types and stages. More detailed representation of the impact of overdiagnosis, FPs, and cancer signal origin accuracy as further data permits will allow this limitation to be avoided, but the modest impact of these inputs on the ICER in sensitivity analyses suggests that the additional detail is unlikely to meaningfully impact the results of the analyses. The model used the most relevant adherence data from regular cancer screening and specific MCED test characteristics to inform its compliance rate but there is uncertainty around this parameter, which was explored in the sensitivity analysis. The cancer treatment cost data originated from 2016 but since then several new, often expensive drugs have been introduced and utilized that could potentially underestimate the value of MCED screening in this analysis. However,, no treatment cost was assumed after 5 years, which might not be the case for less aggressive cancers. To assess the full scope of potential costs and benefits, the base-case analysis included screening from ages 50 to 79 years, which includes both commercial and Medicare payers, but the results of the two alternative screening age groups/cost source (i.e., 50–64 years, 65–79 years) indicated that the selected base-case settings result in a conservative estimate of the VBP for the MCED test.
Importantly, the model assumes historical survival from the general population is applicable to cancers detected by MCED due to a lack of available data and that changes in the stage due to screening will result in changes in mortality. While ongoing and future studies will provide new insight into these mortality effects, data on the effects of MCED on mortality are not yet available. Similarly, the real-world performance of MCED testing may also differ from the assumed performance here. Further, the model does not explicitly account for the additional post-diagnosis risk of developing cancer later in life. Cancer recurrence or patients who have multiple types of cancers are not explicitly considered, but the mean survival from the first cancer assigned to patients implicitly includes the effect of recurrence on mortality. This implicit approach does not allow consideration of the impact of resuming screening in individuals with a cancer history. Individuals with a cancer history, however, have a higher incidence of future cancers, which is associated with improved cost effectiveness of MCED screening in our sensitivity analyses. Thus, resumption of screening in the population after the initial cancer diagnoses might be anticipated to improve cost effectiveness.
Conclusions
Screening with an MCED test in addition to usual care for multiple types of cancer can lead to earlier cancer detection, which may improve patient survival and lower treatment costs. These benefits are maximized as more cancers are included in the MCED test or as test sensitivity increases. The results on VBP can provide a foundation for healthcare decision makers to understand the potential clinical and economic value of an MCED test.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
This study was funded by GRAIL LLC, a subsidiary of Illumina, Inc. GRAIL, LLC is currently held separate from Illumina Inc. under the terms of the Interim Measures Order of the European Commission dated 29 October 2021.
Conflicts of interest/competing interests
At the time of writing, Ali Tafazzoli, Alissa Shaul, Ameya Chavan, and Weicheng Ye were employed by Evidera, a healthcare research firm that provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. Evidera received funding from GRAIL LLC, a subsidiary of Illumina, Inc., to conduct the study and develop this manuscript. Anuraag R. Kansal and Josh Ofman are employees of GRAIL LLC. Scott D. Ramsey and Mark Fendrick are medical consultants who received funding from GRAIL LLC, a subsidiary of Illumina, Inc.
Ethics approval
Data used for this study were publicly available; therefore, no ethics approval was required.
Consent to participate
Data used for this study were publicly available; therefore, no informed consent was required.
Consent for publication
Not applicable.
Availability of data and material
All data used for this study are provided in the article. Additional details are available from the corresponding author on request.
Code availability
Not applicable.
Author contributions
All authors contributed to the study conception and design. AT and ARK conceptualized the study and were responsible for its overall direction and planning, with input from SDR, AS, JO, and AMF. AT, AS, WY, and AC developed the model and performed the analyses. The first draft of the manuscript was written by AT and AS and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
References
- 1.World Health Organization (WHO). Cancer: key facts. 2018. https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed Sep 2019.
- 2.National Cancer Institute (NCI). Cancer Statistics April 2018 September 2019]. https://www.cancer.gov/about-cancer/understanding/statistics. Accessed Sep 2019.
- 3.Loud JT, Murphy J. Cancer screening and early detection in the 21(st) century. Semin Oncol Nurs. 2017;33(2):121–128. doi: 10.1016/j.soncn.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Cancer Society. Estimated deaths per year in 2022. Data on file GA-2021-0065. 2022. http://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/cancer-facts-and-figures-2022.pdf. Accessed 28 Jul 2022.
- 5.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United States Preventive Services Task Force. USPSTF A and B recommendations. 2019. https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/. Accessed 28 Jul 2022.
- 7.Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Tsikouras P, Zervoudis S, Manav B, Tomara E, Iatrakis G, Romanidis C, et al. Cervical cancer: screening, diagnosis and staging. J BUON. 2016;21(2):320–325. [PubMed] [Google Scholar]
- 9.Chen M, Zhao H. Next-generation sequencing in liquid biopsy: cancer screening and early detection. Hum Genomics. 2019;13(1):34. doi: 10.1186/s40246-019-0220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV, Consortium C. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020;31(6):745–759. doi: 10.1016/j.annonc.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lennon AM, Buchanan AH, Kinde I, Warren A, Honushefsky A, Cohain AT, et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science. 2020;369(6499):eabb9601. doi: 10.1126/science.abb9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipscomb J, Horton S, Kuo A, Tomasetti C. Evaluating the impact of multicancer early detection testing on health and economic outcomes: toward a decision modeling strategy. Cancer. 2022;128(Suppl. 4):892–908. doi: 10.1002/cncr.33980. [DOI] [PubMed] [Google Scholar]
- 13.Criss SD, Cao P, Bastani M, Ten Haaf K, Chen Y, Sheehan DF, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171(11):796–804. doi: 10.7326/M19-0322. [DOI] [PubMed] [Google Scholar]
- 14.Vanness DA-O, Lomas J, Ahn H. A health opportunity cost threshold for cost-effectiveness analysis in the United States. Ann Intern Med. 2021;174(1):25–32. doi: 10.7326/M20-1392. [DOI] [PubMed] [Google Scholar]
- 15.United States (US) Bureau of Labor Statistics. Consumer Price Index. 2022. https://www.bls.gov/cpi/. Accessed 14 Jun 2022.
- 16.National Cancer Institute (NCI). Surveillance Epidemiology and End Results (SEER)*Stat Software: Surveillance Research Program. 16 May 2022; Version 8.4.0.1. https://seer.cancer.gov/seerstat/. Accessed 14 Jun 2022.
- 17.National Cancer Institute (NCI). SEER*Stat Database: incidence—SEER research data, 18 registries, Nov 2019 Sub (2000-2017)—linked to county attributes—time dependent (1990-2017) income/rurality, 1969-2018 counties. 2020 February 2021. www.seer.cancer.gov. Accessed 14 Jun 2022.
- 18.Centers for Disease Control and Prevention (CDC), National Center for Chronic Disease Prevention and Health Promotion Division of Population Health. BRFSS. Prevalence & trends data 2015. https://www.cdc.gov/brfss/brfssprevalence. Accessed 26 Feb 2019.
- 19.Force AboUSUPST. Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, Jr, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(23):2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 20.Force AboUSUPST. Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB, et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(18):1901–1913. doi: 10.1001/jama.2018.3710. [DOI] [PubMed] [Google Scholar]
- 21.Jemal A, Fedewa SA. Lung cancer screening with low-dose computed tomography in the United States: 2010 to 2015. JAMA Oncol. 2017;3(9):1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network (NCCN). https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed Feb 2021.
- 23.United States (US) Preventive Services Task Force (USPSTF). Lung cancer: screening. July 2015. 2015. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lung-cancer-screening. Accessed Sep 2019.
- 24.Hubbell E, Clarke CA, Aravanis AM, Berg CD. Modeled reductions in late-stage cancer with a multi-cancer early detection test. Cancer Epidemiol Biomarkers Prev. 2021;30(3):460–468. doi: 10.1158/1055-9965.EPI-20-1134. [DOI] [PubMed] [Google Scholar]
- 25.Compliance from BRFSS Prevalence & Trends Data except LDCT from Fedewa et al. J Natl Cancer Inst. 2021;113(8):1044–52. Data on file GA-2021-0065. 2020. https://www.cdc.gov/brfss/brfssprevalence/. Accessed 8 Dec 2021.
- 26.Reddy SR, Broder MS, Chang E, Paydar C, Chung KC, Kansal AR. Cost of cancer management by stage at diagnosis among Medicare beneficiaries. Curr Med Res Opin. 2022;38(8):1285–1294. doi: 10.1080/03007995.2022.2047536. [DOI] [PubMed] [Google Scholar]
- 27.Medicare Payment Advisory Commission. Report to the Congress: Medicare payment policy. http://medpac.gov/docs/default-source/reports/mar20_entirereport_sec.pdf. Accessed Feb 2021.
- 28.Janssen B, Szende A. Population norms for the EQ-5D. In: Szende A, Janssen B, Cabases J, editors. Self-reported population health: an international perspective based on EQ-5D. Dordrecht: Springer; 2014. pp. 19–30. [PubMed] [Google Scholar]
- 29.Bremner KE, Chong CA, Tomlinson G, Alibhai SM, Krahn MD. A review and meta-analysis of prostate cancer utilities. Med Decis Making. 2007;27(3):288–298. doi: 10.1177/0272989X07300604. [DOI] [PubMed] [Google Scholar]
- 30.Djalalov S, Rabeneck L, Tomlinson G, Bremner KE, Hilsden R, Hoch JS. A review and meta-analysis of colorectal cancer utilities. Med Decis Making. 2014;34(6):809–818. doi: 10.1177/0272989X14536779. [DOI] [PubMed] [Google Scholar]
- 31.Doherty M, Leung Y, Naik H, Patel D, Eng L, Quinn Kong Q, et al. Patient-reported symptoms and Canadian health utility scores in esophageal cancer patients. J Clin Oncol. 2015;33(15_Suppl.):6607. doi: 10.1200/jco.2015.33.15_suppl.6607. [DOI] [Google Scholar]
- 32.Endarti D, Riewpaiboon A, Thavorncharoensap M, Praditsitthikorn N, Hutubessy R, Kristina SA. Evaluation of health-related quality of life among patients with cervical cancer in Indonesia. Asian Pac J Cancer Prev. 2015;16(8):3345–3350. doi: 10.7314/APJCP.2015.16.8.3345. [DOI] [PubMed] [Google Scholar]
- 33.Gharaibeh M, McBride A, Alberts DS, Slack M, Erstad B, Alsaid N, et al. Economic evaluation for USA of systemic chemotherapies as first-line treatment of metastatic pancreatic cancer. Pharmacoeconomics. 2018;36(10):1273–1284. doi: 10.1007/s40273-018-0678-6. [DOI] [PubMed] [Google Scholar]
- 34.Havrilesky LJ, Broadwater G, Davis DM, Nolte KC, Barnett JC, Myers ER, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216–220. doi: 10.1016/j.ygyno.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hildebrandt T, Thiel FC, Fasching PA, Graf C, Bani MR, Loehberg CR, et al. Health utilities in gynecological oncology and mastology in Germany. Anticancer Res. 2014;34(2):829–835. [PubMed] [Google Scholar]
- 36.Kent EE, Ambs A, Mitchell SA, Clauser SB, Smith AW, Hays RD. Health-related quality of life in older adult survivors of selected cancers: data from the SEER-MHOS linkage. Cancer. 2015;121(5):758–765. doi: 10.1002/cncr.29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prica A, Chan K, Cheung M. Frontline rituximab monotherapy induction versus a watch and wait approach for asymptomatic advanced-stage follicular lymphoma: a cost-effectiveness analysis. Cancer. 2015;121(15):2637–2645. doi: 10.1002/cncr.29372. [DOI] [PubMed] [Google Scholar]
- 38.Schleinitz MD, DePalo D, Blume J, Stein M. Can differences in breast cancer utilities explain disparities in breast cancer care? J Gen Intern Med. 2006;21(12):1253–1260. doi: 10.1111/j.1525-1497.2006.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sturza J. A review and meta-analysis of utility values for lung cancer. Med Decis Making. 2010;30(6):685–693. doi: 10.1177/0272989X10369004. [DOI] [PubMed] [Google Scholar]
- 40.CDC. National vital statistics report. United States life tables, 2014. 2017. https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_04.pdf. Accessed 1 Feb 2021. [PubMed]
- 41.National Cancer Institute (NCI). Surveillance Epidemiology and End Results. Software: Surveillance Research Program, National Cancer Institute SEER*Stat software version 8.3.8. Data: SEER Program SEER*Stat Database: incidence—SEER Research Data, 18 registries, Nov 2019 Sub (2000-2017) - linked to county attributes—time dependent (1990-2017) income/rurality, 1969-2018 counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on the November 2019 submission. www.seer.cancer.gov. Accessed Feb 2021.
- 42.Ahlquist DA. Universal cancer screening: revolutionary, rational, and realizable. NPJ Precis Oncol. 2018;2(1):23. doi: 10.1038/s41698-018-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hubbell E, Clarke CA, Aravanis AM, Berg CD. In supplement to: modeled reductions in late-stage cancer with a multi-cancer early detection test. Cancer Epidemiol Biomark Prev. 2021;30(3):460–468. doi: 10.1158/1055-9965.EPI-20-1134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available herein and in the ESM.