Abstract
A candidate for a vaccine against infectious bovine keratoconjunctivitis (IBK) has been cloned and characterized from Moraxella bovis. The plb gene encodes a protein of 616 amino acids (molecular mass of ∼65.8 kDa) that expresses phospholipase B activity. Amino acid sequence analysis revealed that PLB is a new member of the GDSL (Gly-Asp-Ser-Leu) family of lipolytic enzymes.
Moraxella bovis is the etiologic agent of infectious bovine keratoconjunctivitis (IBK), the most common ocular disease that occurs in cattle (2, 13, 15). IBK is highly contagious and, if left untreated, can result in corneal ulceration and temporary or permanent blindness. In vitro studies have suggested that M. bovis produces a number of virulence determinants, including type IV pili (3, 23), a secreted hemolysin (28), proteases, fibrinolysins, and phospholipases (9). To date, only type IV pili and the hemolysin have been substantively linked to the virulence of M. bovis (4, 10, 16, 17, 20, 26). Here we describe the molecular characterization of an M. bovis gene encoding a phospholipase B activity and discuss the role this enzyme may play in the virulence of this veterinary pathogen.
The Australian M. bovis strains used in this study have been defined according to their pilus serotype (25): S276R (serotype A), 3WO7 (serotype B), Dalton 2d (serotype C), R593L (serotype D), Tat849 (serotype E), 218R (serotype F), and Fleur462 (serotype G). Other M. bovis strains used, Q220 and Epp63, were isolated in the United Kingdom and the United States, respectively. All of these M. bovis strains were positive for lipase activity when tested on Tween 80 medium (30), as indicated by a zone of opalescence around areas of growth.
Cloning and sequence analysis of the M. bovis plb gene.
Genomic DNA from M. bovis strain Dalton 2d (serotype C) was purified with cetyltrimethylammonium bromide-NaCl and phenol chloroform extractions followed by isopropanol precipitation. The genomic DNA was partially digested with Sau3A under conditions that maximized the amount of DNA in the size range of 1 to 2 kb. Fragments <200 bp were removed by passing the digested DNA through a Microspin S-400 HR column (Pharmacia). The 1- to 2-kb fragments were ligated with pBR322 (5), previously digested with BamHI, and electroporated into Escherichia coli strain MC1061 (31). The partial genome library was screened on Tween 80 medium, and 28 out of 24,000 clones were found to be positive for lipase expression.
All positive clones contained a 5.4-kb fragment in common. The size of the insert DNA in one positive clone, pMB1, was reduced from 5.4 kb by restriction endonuclease digestion to 2.2 kb (pMB4, positive for lipase expression) and 2.0 kb (pMB5, negative for lipase expression). It is interesting to note that neither MC1061/pMB1 nor MC1061/pMB4 was found to be positive for protease or hemolytic activity when plated onto agar containing skim milk or erythrocytes.
Recombinant pMB4 DNA was isolated by using the Wizard Plus SV Minipreps DNA purification kit (Promega), and the nucleotide sequence of the 2.2-kb insert was determined (GenBank accession no. AY032849). An open reading frame of 1,851 bp with the potential to encode a 616-amino-acid protein with a predicted molecular mass of 65.8 kDa was identified together with a potential Shine-Dalgarno site preceded by putative promoter sequences. The sequence following the open reading frame has the potential to encode a transcriptional terminator with a ΔG value of −15 kJ mol−1. Based on subsequent protein and biochemical analyses described below, this gene was designated plb.
Identification of the plb gene product.
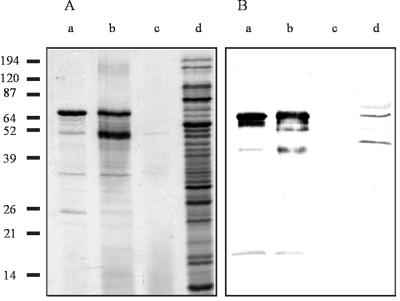
Secreted protein samples were prepared from M. bovis Dalton 2d and E. coli strains MC1061/pMB1, MC1061/pMB4, and MC1061/pMB5 by ammonium sulfate precipitation of cell supernatant fluids from overnight cultures, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12.5% polyacrylamide) (19), and visualized by staining with Coomassie blue. A dominant band running at approximately 66 kDa was identified in the extracts from MC1061/pMB1 and MC1061/pMB4, but not in those from MC1061/pMB5 (Fig. 1A, lanes a, b, and c, respectively). Due to the large number of proteins present in the M. bovis culture supernatant (contributed by the membrane blebs that slough off M. bovis during normal growth), Western blot analysis with antiserum raised in rabbits immunized with the recombinant lipase was required to confirm the presence of the lipase in the concentrated extract of M. bovis Dalton 2d-secreted proteins (Fig. 1B, lane d).
FIG. 1.
Identification of native (M. bovis) and recombinant (E. coli) lipase. (A) SDS-PAGE gel stained with Coomassie brilliant blue. (B) Western blot of the gel in panel A probed with antisera raised in rabbits against heat-inactivated recombinant lipase. Lanes: a, MC1061/pMB1; b, MC1061/pMB4; c, MC1061/pMB5; d, M. bovis Dalton 2d. Molecular mass markers are indicated in kilodaltons to the left.
The dominant 66-kDa band identified in the MC1061/pMB1 preparation (Fig. 1A, lane a) was electrotransferred to polyvinylidene difluoride membrane, excised, and subjected to automated (Edman degradation) amino-terminal sequence analysis (7). The resultant data confirmed the identity of the 66-kDa protein as the product of plb and furthermore identified the presence of a signal peptide cleavage site between residues 25 and 26 of the predicted translation product, designated PLB. This putative signal peptide has similar characteristics to classical signal peptides described by Pugsley (27), suggesting that PLB may be secreted across the cytoplasmic membrane via an s-dependent pathway.
Amino acid sequence comparisons.
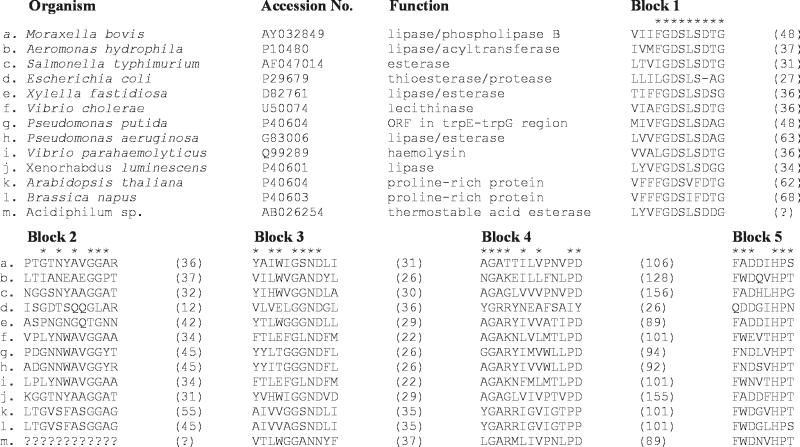
Comparison of the PLB sequence with the GenBank sequence databank by using the BLAST program (1) revealed a number of similar proteins. PLB had its closest identity (28% identity and 42% similarity) to a lipase or esterase from the plant pathogen Xylella fastidiosa (accession no. D82761). Three other proteins, including a putative autotransporter protein (PapA; accession no. CAC14200) and an esterase (EstA; accession no. G83006) from Pseudomonas aeruginosa and an outer membrane esterase from Salmonella enterica serovar Typhimurium (AAC38796), displayed 23, 23, and 31% identity and 35, 35, and 43% similarity, respectively, to PLB. M. bovis PLB was also found to be related to a group of prokaryotic and eukaryotic proteins by virtue of the presence of a highly conserved amino acid sequence motif, Gly-Asp-Ser-Leu (GDSL) (29), located in the N-terminal regions of each of these proteins (Fig. 2). While variable in size and appearing to have different functions (29, 32), the proteins aligned share five discrete blocks of relatively high sequence similarity that occur in the same relative location in each protein. The serine residue within the GDSL motif has been shown to be part of the catalytic triad of the Aeromonas hydrophila glycerophospholipid-cholesterol acyltransferase (14) and may therefore be essential for enzymatic activity, and possibly the virulence, of members of this family. We propose that the PLB from M. bovis is a member of the GDSL family and as such may play a role in the pathogenesis of the IBK infection. The precise nature of this role will be investigated by constructing a plb mutant and testing the pathogenicity of the mutant in the bovine IBK model system (20).
FIG. 2.
Conserved blocks of amino acids in the GDSL family (members b to m) of lipolytic enzymes. New family member M. bovis PLB (member a) has been compared with proteins of those organisms shown to express a protein belonging to the GDSL family. The accession number for each protein sequence is indicated, as is the function of each enzyme. Asterisks indicate residues that are conserved in six or more of the proteins. Numbers in parentheses indicate the number of amino acid residues between the conserved regions. ORF, open reading frame. The figure was adapted from data provided in the study of Upton and Buckley (29).
It has been suggested that one member of the GDSL family, the EstA esterase of P. aeruginosa, may also be a member of the autotransporter protein family (32). Autotransporters are secreted across the cytoplasmic membrane via the s-system and then secreted across the outer membrane by a unique process that does not require any energy or additional accessory factors (12, 22). The C-terminal region of PLB was found to display homology to members of the autotransporter family. Taken together with the observation that PLB probably has a classical signal peptide, we propose that PLB is a bona fide member of the autotransporter family.
Characterization of PLB enzymatic activity.
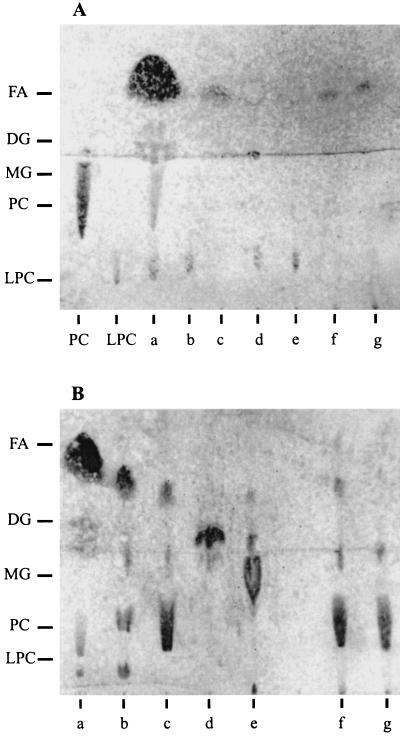
To determine the enzymatic specificity of PLB, thin-layer chromatography was used to analyze the end products of phosphatidylcholine and lysophosphatidylcholine hydrolysis (8). Ammonium sulfate-precipitated cell supernatant fluids from E. coli MC1061/pMB4 and M. bovis Dalton 2d hydrolyzed both substrates to produce free fatty acids and glycerophosphorylcholine in a manner similar to that observed for the commercial phospholipase B (Fig. 3). Based on these data, we concluded that the activity displayed by PLB was characteristic of a phospholipase B.
FIG. 3.
Thin-layer chromatographic analysis of the lipid products generated by the action of the M. bovis lipase or commercial phospholipases on two different substrates: phosphatidylcholine (PC) (A) and lysophosphatidylcholine (LPC) (B). Lanes: a, reference standards; b, phospholipase A2; c, phospholipase B; d, phospholipase C; e, phospholipase D; f, MC1061/pMB4; g, M. bovis Dalton 2d. Included as controls in panel A are unprocessed phosphatidylcholine and lysophosphatidylcholine. The other reference standards used were monoacyl glycerol (MG), diacyl glycerol (DG), and free fatty acid (FA).
Expression of PLB by M. bovis.
To investigate the frequency of expression of PLB among isolates of M. bovis, a Western blot using antisera raised in rabbits immunized with the recombinant lipase was performed with protein extracts from nine representative strains. Protein samples were made from overnight cultures, separated by SDS-PAGE (12.5% polyacrylamide), transferred to nitrocellulose, and incubated with recombinant lipase antisera. A protein band equivalent in size (∼66 kDa) to the lipase produced by E. coli MC1061/pMB4 (Fig. 4, lane j) was detected in each of the M. bovis strains (Fig. 4, lanes a to i), thus establishing the global nature of this enzyme among the species.
FIG. 4.
Distribution of PLB among M. bovis strains. Secreted proteins present in cell supernatants prepared from overnight cultures of M. bovis strains were precipitated with ammonium sulfate at a final concentration of 60%. Proteins were visualized by Western blot analysis with recombinant PLB antisera. Lanes: a, S276R; b, 3WO7; c, Dalton 2d; d, R593L; e, Tat849; f, 218R; g, Fleur462; h, Q220; i, Epp63; j, MC1061/pMB4; k, MC1061/pMB5. Molecular mass markers are indicated in kilodaltons to the left.
Previous studies have shown that the type IV pili produced by M. bovis play a fundamental role in bacterial colonization of the bovine eye and mediate the essential first step in the pathogenic process that leads to disease (20). Following adhesion, secondary virulence factors are presumed to cause the clinical damage observed as pitting on the corneal surface (18). Enzymatic activity of the M. bovis phospholipase B on membrane phospholipids could result in cell lysis and lead to such clinical symptoms. This damage could be directly caused by the presence of lysophosphatidylcholine, one of the intermediate products of PLB activity on phospholipids (6). Phospholipases are recognized as major virulence determinants in a number of bacterial species, including Listeria (11) and Corynebacterium pseudotuberculosis (24).
Until now, development of a pilus-based vaccine against IBK has been hampered by the need to construct multivalent vaccines, since the pili of specific serotypes are not cross protective against disease caused by heterologous challenge (21). The failure of such vaccines supports the need for the identification of immunogens that are antigenically conserved across M. bovis. The identification of PLB as one such conserved immunogen will likely prove important in the future development of an efficacious vaccine against IBK.
Acknowledgments
J.L.F. was a recipient of an Australian Postgraduate Award. R.A.S. and J.M.T. are members of the Cooperative Research Centre for Vaccine Technology.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baptista P J. Infectious bovine keratoconjunctivitis: a review. Br Vet J. 1981;135:225–242. doi: 10.1016/s0007-1935(17)32882-8. [DOI] [PubMed] [Google Scholar]
- 3.Beard M K M, Mattick J S, Moore L J, Mott M R, Marrs C F, Egerton J R. Morphogenetic expression of Moraxella bovis fimbriae (pili) in Pseudomonas aeruginosa. J Bacteriol. 1990;172:2601–2607. doi: 10.1128/jb.172.5.2601-2607.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billson F M, Hodgson J L, Egerton J R, Lepper A W D, Michalski W P, Schwartzkoff C L, Lehrbach P R, Tennent J M. A haemolytic cell-free preparation of Moraxella bovisconfers protection against infectious bovine keratoconjunctivitis. FEMS Microbiol Lett. 1994;124:69–74. doi: 10.1111/j.1574-6968.1994.tb07263.x. [DOI] [PubMed] [Google Scholar]
- 5.Bolivar F R, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W. Construction and characterization of new cloning vehicles. II. A(2) multipurpose cloning vehicle. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 6.Dennis A E. Phospholipases. In: Boyer PD, editor. The enzymes. 3rd ed. 16. Lipid enzymology. New York, N.Y: Academic Press, Inc.; 1983. pp. 307–353. [Google Scholar]
- 7.Edman P, Begg C. A protein sequenator. Eur J Biochem. 1967;1:80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- 8.Fifis T, Costopoulos C, Vaughan J A. Evidence for phospholipase B activity in Fusobacterium necrophorumcultures and its association with hemolysin/leucocidin activities. Vet Microbiol. 1996;49:219–233. doi: 10.1016/0378-1135(95)00195-6. [DOI] [PubMed] [Google Scholar]
- 9.Frank S K, Gerber J D. Hydrolytic enzymes of Moraxella bovis. J Clin Microbiol. 1981;13:269–271. doi: 10.1128/jcm.13.2.269-271.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gil-Turnes C. Hemagglutination, autoagglutination and pathogenicity of Moraxella bovisstrains. Can J Comp Med. 1983;47:503–504. [PMC free article] [PubMed] [Google Scholar]
- 11.Goldfine H, Knob C, Alford D, Bentz J. Membrane permeabilization by Listeria monocytogenesphosphatidylinositol-specific phospholipase C is independent of phospholipid hydrolysis and cooperative with listeriolysin O. Proc Natl Acad Sci USA. 1995;92:2979–2983. doi: 10.1073/pnas.92.7.2979. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Henderson I R, Navarro-Garcia F, Nataro J P. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:370–378. doi: 10.1016/s0966-842x(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 13.Henson J B, Grumbles L C. Infectious bovine keratoconjunctivitis. I. Etiology. Am J Vet Res. 1960;21:761–766. [PubMed] [Google Scholar]
- 14.Hilton S, Buckley J T. Studies on the reaction mechanism of a microbial lipase/acyltransferase using chemical modification and site-directed mutagenesis. J Biol Chem. 1991;266:997–1000. [PubMed] [Google Scholar]
- 15.Hughes D E, Pugh G W., Jr A five-year study of bovine infectious keratoconjunctivitis in a beef herd. J Am Vet Med Assoc. 1970;157:443–454. [PubMed] [Google Scholar]
- 16.Jackman S H, Rosenbusch R F. In vitro adherence of Moraxella bovisto intact corneal epithelium. Curr Eye Res. 1984;3:1107–1112. doi: 10.3109/02713688409000809. [DOI] [PubMed] [Google Scholar]
- 17.Jayappa H G, Lehr C. Pathogenicity and immunogenicity of piliated and nonpiliated phases of Moraxella bovisin calves. Am J Vet Res. 1986;47:2217–2221. [PubMed] [Google Scholar]
- 18.Kagonyera G M, George L W, Munn R. Light and electron microscopic changes in corneas of healthy and immunomodulated calves infected with Moraxella bovis. Am J Vet Res. 1988;49:386–395. [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Lepper A W D, Power B E. Infectivity and virulence of Australian strains of Moraxella bovisfor the murine and bovine eye in relation to pilus serogroup sub-unit size and degree of piliation. Aust Vet J. 1988;65:305–309. doi: 10.1111/j.1751-0813.1988.tb14512.x. [DOI] [PubMed] [Google Scholar]
- 21.Lepper A W D, Elleman T C, Hoyne P A, Lehrbach P R, Atwell J L, Egerton J R, Schwartzkoff L C, Tennent J M. A Moraxella bovispili vaccine produced by recombinant DNA technology for the prevention of infectious bovine keratoconjunctivitis. Vet Microbiol. 1993;36:175–183. doi: 10.1016/0378-1135(93)90138-w. [DOI] [PubMed] [Google Scholar]
- 22.Loveless B J, Saier M H J. A novel family of channel-forming autotransporting, bacterial virulence factors. Mol Membr Biol. 1997;14:113–123. doi: 10.3109/09687689709048171. [DOI] [PubMed] [Google Scholar]
- 23.Marrs C F, Schoolnik G, Koomey J M, Hardy J, Rothbard J, Falkow S. Cloning and sequencing of a Moraxella bovispilin gene. J Bacteriol. 1985;163:132–139. doi: 10.1128/jb.163.1.132-139.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNamara P J, Bradley G A, Songer J G. Targeted mutagenesis of the phospholipase D gene results in decreased virulence of Corynebacterium pseudotuberculosis. Mol Microbiol. 1994;12:921–930. doi: 10.1111/j.1365-2958.1994.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 25.Moore L J, Lepper A W D. A unified serotyping scheme for Moraxella bovis. Vet Microbiol. 1991;29:75–83. doi: 10.1016/0378-1135(91)90111-r. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen K B, Froholm L O, Bovre K. Fimbriation and colony type of Moraxella bovisin relation to conjunctival colonization and development of keratoconjunctivitis in cattle. Acta Pathol Microbiol Scand Sect B. 1972;80:911–918. doi: 10.1111/j.0365-5563.1973.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 27.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandhu T S, White F H. Production and characterization of Moraxella bovishemolysin. Am J Vet Res. 1977;38:883–885. [PubMed] [Google Scholar]
- 29.Upton C, Buckley J T. A new family of lipolytic enzymes? Trends Biochem Sci. 1995;20:178–179. doi: 10.1016/s0968-0004(00)89002-7. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Dowds B C A. Phase variation in Xenorhabdus luminescens: cloning and sequencing of the lipase gene and analysis of its expression in primary and secondary phases of the bacterium. J Bacteriol. 1993;175:1665–1673. doi: 10.1128/jb.175.6.1665-1673.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wertman K F, Wyman A R, Botstein D. Host/vector interactions which affect the viability of recombinant phage lambda clones. Gene. 1986;49:253–262. doi: 10.1016/0378-1119(86)90286-6. [DOI] [PubMed] [Google Scholar]
- 32.Wilhelm S, Tommassen J, Jaeger K-E. A novel lipolytic enzyme located in the outer membrane of Pseudomonas aeruginosa. J Bacteriol. 1999;181:6977–6986. doi: 10.1128/jb.181.22.6977-6986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]