Abstract
Objective
This pilot study evaluated the efficacy of autologous bone marrow-derived mesenchymal stem cells (BM-MSCs) versus hyaluronic acid (HA) in surgically naïve patients with knee osteoarthritis (OA).
Methods
Single-centre, single-blind randomized study of patients with knee OA. Twenty patients were randomized into groups of 10 each for intra-articular injection of cultured BM-MSCs (6 ml of BM-MSCs at 1 × 106 cells/mL) or HA (6 ml). Clinical assessments of pain, quality of life, radiographic imaging, and magnetic resonance imaging (MRI) compositional change were performed at baseline and 12 months follow-up.
Results
Compared with HA, BM-MSCs injection resulted in significant improvement in qualify of life and reduction in pain as reflected by visual analogue scale (VAS) pain score, Western Ontario and McMaster Universities Arthritis Index (WOMAC) score, and 36-Item Short Form Survey (SF-36) score collectively. T2-relaxation time tended to decrease more in the BM-MSCs group with a 38 ± 24.0% reduction in 6 out of 10 BM-MSC participants; while there was only a 12 ± 7.9% reduction in 4 out of 10 HA participants at the end of follow-up. The remaining participants showed either no response or had relaxation time increased on MRI assessment.
Conclusions
This pilot study found that autologous BM-MSCs significantly reduced pain, improved functional assessment score, and improved quality of life parameters comparing with HA at one year follow-up. Further clinical trial with larger sample size and longer follow up duration is warranted.
The Translational Potential of this Article
This pilot RCT demonstrated the feasibility and potential effectiveness of BM-MSCs advanced therapy for patients with knee OA compared to HA injection. Further multi-center clinical trial with a larger sample size and longer follow up duration in accordance with latest regulatory guidelines is warranted to ascertain the long term safety and effectiveness of MSCs therapy for cartilage regeneration in OA.
Registration
The study was registered in the Centre for Clinical Research Biostatistics - Clinical Trials Registry (CUHK_CCT00469).
Keywords: Knee osteoarthritis, Hyaluronic acid, Bone marrow derived mesenchymal stem cells, Intra-articular injection, Clinical trial
1. Introduction
Osteoarthritis (OA) is the most common degenerative joint disease which particularly affects the knee. It is characterized by progressive degeneration of articular cartilage, osteophytosis, subchondral bone sclerosis, and joint inflammation. The socioeconomic burden of OA is considerable. The annual direct and indirect costs, including health care, and lost productivity have recently been estimated as US$28.6 billion [1]. Population-wise, the prevalence for OA is predicted to increase significantly worldwide, including Asia [2,3]. OA also leads to a worsening quality of life and may lead to permanent disability. Medication, physiotherapy, and non-prosthetic surgery are altogether not effective treatment to prevent progressive destruction and pain caused by OA [4,5]. Due to a lack of clinically effective intervention to delay the progressive loss of joint tissues, total knee replacement (TKR) is generally regarded as the ultimate surgical procedure in end stage OA, resulting in enhanced mobility and pain reduction.
Articular cartilage has limited regeneration capability. The provision of exogenous cells with the ability to mediate or generate cartilage-like extracellular matrix is the main reasoning behind the current interest in cell-based therapy for OA. Autologous chondrocytes have been used for decades but the associated donor site morbidity and application limited to small chondral defects due to insufficient cell number prompted a clinical demand for an alternative cell source [6]. Mesenchymal stem cells (MSCs) are multipotent cells which can be isolated from various adult tissues and expanded in vitro. MSCs are able to differentiate into chondrogenic cells and other cell types under appropriate culture conditions [7]. MSCs also possess anti-inflammatory, immunomodulatory effects and other biological functions through the release of bioactive factors [8,9]. These properties emphasise the potential of MSCs as a cell-based therapy for articular cartilage repair in patients with knee OA. Recently, several meta-analyses attempted to summarize the therapeutic effectiveness of MSCs therapy on knee OA [[10], [11], [12]]. Using different review strategies, pooled analysis indicated improved pain relief and certain physical functions following intra-articular injection of MSCs.
Intra-articular injection of hyaluronic acid (HA) was conditionally recommended in patients with knee OA by the Osteoarthritis Research Society International (OARSI) for pain relief [13]. Previous clinical trial compared MSCs with HA in the treatment of OA, in which HA injection was also given to MSCs groups [14]. Clinical trials directly comparing MSCs and HA are limited. In this study, we conducted a randomized control clinical trial to assess the efficacy and safety of a single intra-articular injection of autologous bone marrow-derived MSCs (BM-MSCs) versus HA in patients with knee OA. Clinical and radiological outcomes over a 12 month follow-up were reported.
2. Materials and methods
2.1. Study design
This was a single-centre randomized study in patients with knee OA conducted between 2015 and 2020. The study aim was to assess the efficacy and safety of intra-articular BM-MSCs against intra-articular HA in patients with knee OA (equal randomization in a 1:1 for two groups). Once the inclusion and exclusion criteria were assessed, each participant was randomly assigned to their treatment groups by picking a sealed envelope with the treatment allocation. The prescriptions were assigned according to the randomization process. The study was performed in accordance with the approved institutional ethical protocol (CREC-2014.279-T) and registered in the Centre for Clinical Research Biostatistics - Clinical Trials Registry (CUHK_CCT00469).
Twenty eligible patients, aged between 50 and 65 years (58.00 ± 4.51 years), affected by primary OA of the knee of Kellgren-Lawrence (K-L) grade 2–3 and with a pain level equal to or higher than 5 on a Visual Analogue Scale (VAS) scale of 10 for at least 2 months were recruited with informed consent. There was no restriction on medication or any other treatment or daily activity by the recruited patients before or after intervention. Patients with the following criteria were excluded: (i) alcoholism or drug abuse; (ii) pregnancy and breast-feeding; (iii) serious pathologies such as carcinoma or autoimmune disease; (iv) hypersensitivity towards HA; (v) on-going or recent (1 month) steroid-based systemic therapy; (vi) significant hematologic disease; (vii) mechanical knee instability, ligamentous laxity or deficiency or gross knee deformity and (viii) prior knee surgery. Patients were randomized into either BM-MSCs group or HA group. All injections were given intra-articularly once into the knee joint. In the BM-MSCs group, 6 ml of BM-MSCs (1 × 106 cells per ml) was injected, while in the HA group, 6 ml of HA (Synvisc-One®: Hylan G-F 20) was injected.
2.2. Autologous MSC preparation and characterization
Human bone marrow (10 ml) was harvested from the posterior iliac crest under local anaesthesia at least 1-month before injection. Bone marrow nucleated cells were isolated and cultured with standard protocol in clean room facility [15]. In brief, 20 ml bone marrow solution diluted in Dulbecco's phosphate-buffered saline (DPBS; 1:1 ratio) was layered on Ficoll-Paque PREMIUM (GE Healthcare, Chicago, USA) before centrifugation at 400g for 30 min at 20 °C. The layer of buffy coat was collected and washed twice with 3 times the volume of DPBS. the nucleated cells were seeded at a density of 2.5 × 105 cells/cm2 in culture medium (KO-DMEM supplemented with 1% Glutamax and 10% fetal bovine serum) which was changed twice a week. At 70% confluent, the cells were collected with Tryple, then resuspended in the culture medium, and seeded at a density of 5000 cells/cm2 in culture flask. Cell culture with viability above 86% as determined by trypan blue exclusion test was used. International Society for Cellular Therapy (ISCT) recommendations were adopted for MSC characterization [7,16]. At passage 3, BM-MSCs cell suspension was washed twice with DPBS, re-suspended in 200 μl stain buffer (BD, 1 × 106 cells/ml) and incubated for 15 min at 4 °C. Primary antibodies against CD14, CD19, CD29, CD34, CD44, CD45, CD73, CD90, HLA-DR or corresponding isotype controls were added to each suspension and incubated for 15 min at 4 °C with gentle intermittent shaking before subjecting to flow cytometry. In addition, the multi-differentiation potential and clonogenicity of the BM-MSCs were evaluated with standard staining protocols. One hundred BM-MSCs were seeded per 10 cm diameter culture dish and stained with 0.5% crystal violet (Sigma-Aldrich, Missouri, USA) at day 14. The BM-MSCs at passage 3 were subjected to iosteogenic, adipogenic or chondrogenic induction using commercially available differentiation kits (StemPro, Gibco, Massachusetts, USA), followed by staining of calcium deposits by 0.5% alizarin red S (pH 4.1 adjusted with ammonia, Sigma-Aldrich, Missouri, USA), intracellular oil droplets by 0.3% freshly filtered oil red O (Sigma-Aldrich, Missouri, USA), or type II collagen in micromass culture by immunohistochemical staining with anti-type II collagen (Santa Cruz, California, USA) respectively as we previously reported [17,18]. BM-MSCs at passage 5 were used for intra-articular injection. All the reagents were purchased from Life Technologies (California, USA) unless otherwise specified.
2.3. Clinical assessment and follow-up
The study workflow was outlined in Fig. 1. Clinical evaluation was performed by orthopaedic surgeons. All patients underwent a medical examination, blood tests, radiological assessment and outcome scores documented one month before the injection (baseline). The injection was performed in the Day-Surgery ward under aseptic techniques via the lateral para-patellar entry site with the knee flexed at 90°. Follow-up evaluation and assessments were recorded at 3 months, 6 months, 9 months and 12 months post-injection. The study timetable was summarized in Table 1. VAS for pain evaluation at rest, with a burden, and during physical activity were used as primary outcome measurements. Secondary outcome measurements were the Knee Society Score (KSS), Knee Society Function Score (KSFS), magnetic resonance image (MRI) assessment, the self-administered Western Ontario and McMaster Osteoarthritis Index (WOMAC) questionnaire and the 36-Item Short Form Survey (SF-36) questionnaire.
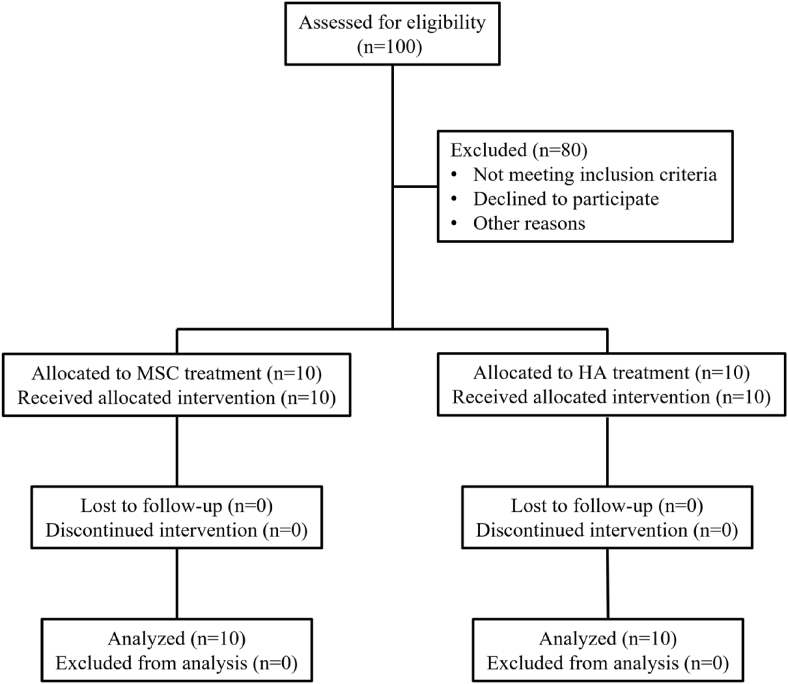
Figure 1.
Flowchart of the clinical trial.
Table 1.
Study timetable and monitoring.
| Baseline | 1 month | 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|---|---|
| MRI & X-ray | ✓ | ✓ | ||||
| Intra-articular injection of BM-MSC or HA | ✓ | |||||
| Clinical check-up | ✓ | ✓ | ✓ | ✓ | ✓ | |
| KSS & KFS | ✓ | ✓ | ✓ | ✓ | ||
| WOMAC | ✓ | ✓ | ✓ | ✓ | ✓ | |
| SF36 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| VAS | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
2.4. MRI acquisition and analysis
MRI of the knee was performed in all patients at baseline and at 12 months post-injection. Patients were scanned in the supine position on a 3.0-T system (Achieva TX, Philips Healthcare, Best, Netherlands) with a dedicated knee coil to optimize signal reception. MR sequences were as follows: proton density-weighted sagittal (FOV 160 × 90mm; TR 4000 ms; TE 30 ms; slice thickness 2.5 mm; flip angle: 90°); proton density-weighted SPAIR (spectral attenuated inversion recovery) axial (FOV 150 × 150 mm; TR 6221 ms; TE 62 ms; slice thickness 3 mm; flip angle: 90°); and T2-mapping sagittal (FOV 160 × 66mm; TR 2000 ms; TE 13 ms; slice thickness 2.5 mm; flip angle: 90°). Total scanning time was about 20–25 min. T2-mapped sagittal MR images were evaluated by one radiologist, blinded to the clinical data. ROIs (regions of interest) for cartilage T2 mapping was performed on serial sagittal images (24 slices) on a standard MR workstation (Philips Intellispace, Best, Netherlands) using a standard MR cartilage assessment tool available on this workstation. ROIs were drawn for different knee cartilage areas (retropatellar, femoral trochlear, medial femorotibial, lateral femorotibial) on consecutive sagittal images, the results summated, and an average value for T2 mapping in millisecond (ms) calculated for each knee. Lower T2-values signify healthier articular cartilage.
2.5. Statistical analysis
VAS pain score is the most reported significant benefit in MSCs therapy. According to the VAS score reported in a relevant clinical trials at the time of protocol preparation, a sample size of 10 would allow for a mean difference of 2.76 and population variance of 7.46 with 80% power and significance level of 0.05 to detect a difference between the two groups. All the enrolled participants received treatment and completed assessments at baseline and at least two follow-up time points. All the pain and functional questionnaires were answered. Participants did not come back for every follow-up assessment were still included in the statistsical analysis owing to small sample size of this pilot study. Data was presented as mean ± standard deviation or as median (lower quartile, upper quartile) where appropriate. Differences between groups at particular time points were analyzed using two-sample t test with Welch's correction and Mann-Whitney U test for normally distributed data with unequal variance and non-normally distributed data, respectively. Changes in the parameters for each subject were calculated by subtracting the baseline value from the value at each timepoint during the follow-up period. Changes in the parameters were analysed by fitting linear mixed-effects models with Restricted Maximum Likelihood (REML) estimation, where groups (BM-MSCs group Vs HA group) was considered as fixed effects and within-individual variation was adjusted as random effects. If significant effects were identified by the linear mixed-effects models, hypothesis tests for pairwise comparisons between BM-MSCs and HA groups were performed using Bonferroni's multiple comparisons test. A p-value < 0.05 was considered to be statistically significant. Statistics were performed using R 4.0.0 (The R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism 9.0 (GraphPad Software, CA, USA).
3. Results
3.1. Baseline characteristics
Baseline characteristics of the 20 subjects with knee OA, randomised into two groups, were summarized in Table 2. All subjects in both groups showed similar baseline characteristics including age and body mass index. There was no statistical difference in KL grade and clinical assessment including VAS, WOMAC, SF-36, KSS and KSFS between the two groups.
Table 2.
Baseline anthropometric and clinical characteristics.
| BM-MSCs group (n = 10) | HA group (n = 10) | P-value | |
|---|---|---|---|
| Age (years)a,c | 56.7 ± 4.83 | 59.1 ± 4.04 | 0.244 |
| Gender, female (male) | 4 (6) | 8 (2) | |
| Height (cm)a,c | 163.8 ± 9.27 | 156.6 ± 10.01 | 0.116 |
| Weight (kg)a,c | 67.6 ± 8.75 | 64.1 ± 9.93 | 0.415 |
| BMI (kg/m2)a,d | 25.4 ± 3.84 | 26.0 ± 1.95 | > 0.999 |
| Kellgren-Lawrence graded | > 0.999 | ||
| 1 | 0 | 0 | |
| 2 | 8 | 9 | |
| 3 | 2 | 1 | |
| 4 | 0 | 0 | |
| WOMAC | |||
| Total scorea,c | 40.7 ± 26.01 | 38.1 ± 20.20 | 0.806 |
| Pain scorea,c | 8.2 ± 5.37 | 7.6 ± 3.03 | 0.763 |
| Stiffness scorea,d | 3.8 ± 2.49 | 4.2 ± 1.93 | 0.682 |
| Physical functiona,d | 28.7 ± 18.73 | 32.9 ± 15.17 | 0.517 |
| Percentage scorea,c | 42.4 ± 27.09 | 39.7 ± 21.04 | 0.806 |
| SF36 | |||
| PFa,c | 49.0 ± 27.37 | 51.5 ± 21.48 | 0.823 |
| RPa,d | 11.3 ± 13.44 | 5.0 ± 6.46 | 0.385 |
| BPa,c | 47.3 ± 23.93 | 49.5 ± 16.95 | 0.594 |
| GHa,d | 50.5 ± 12.57 | 52.0 ± 7.15 | 0.691 |
| VTa,c | 30.0 ± 16.61 | 26.3 ± 18.11 | 0.635 |
| SFa,c | 70.0 ± 24.44 | 73.8 ± 20.79 | 0.716 |
| REa,d | 20.8 ± 12.58 | 11.7 ± 11.92 | 0.148 |
| MHa,c | 35.0 ± 13.21 | 29.2 ± 9.42 | 0.272 |
| PCSa,c | 39.5 ± 15.01 | 39.5 ± 9.03 | > 0.999 |
| MCSa,c | 39.0 ± 11.38 | 35.2 ± 8.01 | 0.406 |
| VAS | |||
| At restb,d | 2.5 (1.00, 3.75) | 1.5 (0.75, 3.25) | 0.591 |
| With a burdenb,d | 6.0 (3.75, 8.25) | 5.0 (3.50, 6.00) | 0.096 |
| During physical activityb,d | 6.5 (4.63, 9.00) | 5.5 (4.00, 7.25) | 0.466 |
| KSSa,d | 63.2 ± 18.40 | 63.2 ± 17.85 | 0.587 |
| KSFSa,d | 73.5 ± 14.15 | 79.0 ± 8.76 | 0.235 |
Data presented in mean ± standard deviation.
Ordinal data presented as median (lower quartile, upper quartile).
Data analyzed by tow-sample t test with Welch's correction.
Data analyzed by Mann-Whitney U test. BMI: Body Mass Index; WOMAC: Western Ontario and McMaster Universities Arthritis Index; PF: physical functioning; RP: role-physical; BP: bodily pain; GH: general health; VT: vitality; SF: social functioning; RE: role-emotional; MH: mental health; PCS: physical component summary; MCS: mental component summary; VAS: visual analogue scale; KSS: knee society score; KSFS: knee society function score; BM-MSCs, bone marrow-derived mesenchymal stem cells; HA, hyaluronic acid.
3.2. Characterization of BM-MSCs at early passage
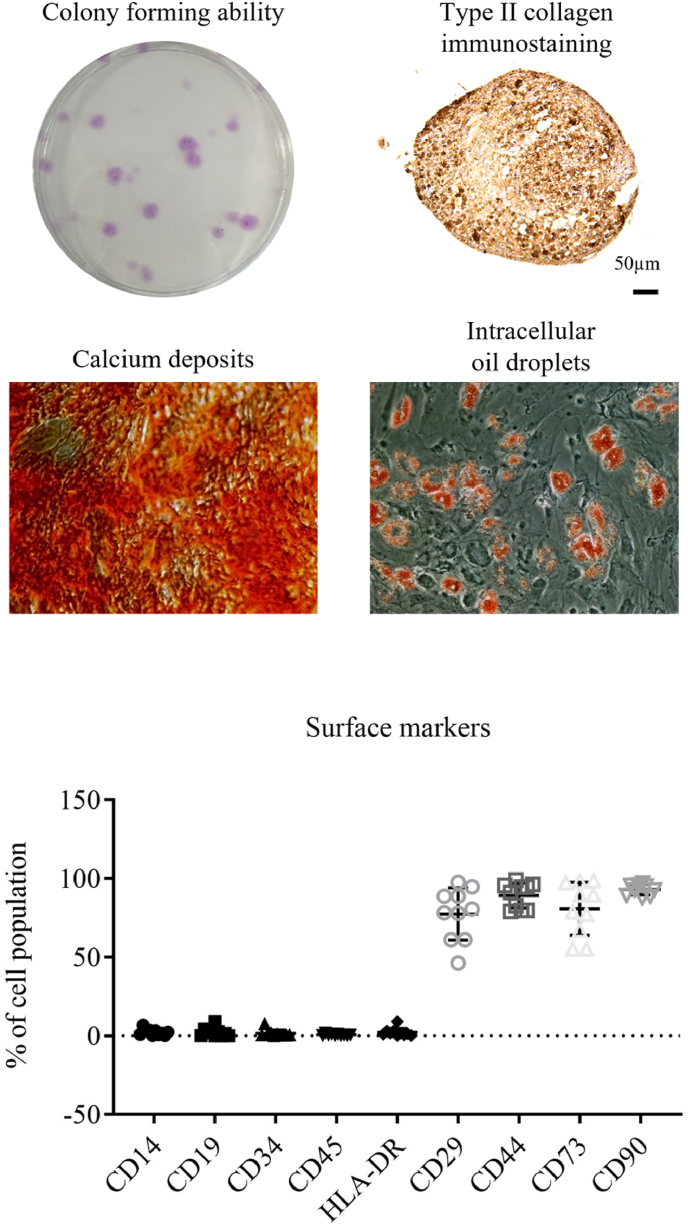
BM-MSCs at passage 3 were subjected to colony forming unit assay, multipotent differentiation assay and measurement of surface markers. Fig. 2 showed the representative images of the colonies, calcium deposits, intracellular oil droplets and expression of type II collagen in BM-MSCs upon corresponding culture condition, indicating the self-renewal activity and multipotent differentiation ability into osteogenic, adipogenic and chondrogenic cells. Meanwhile, flow cytometry showed very low expression of hematopoeitc (CD34 and 45), myelomonocyte (CD14), B cells (CD19) and MHC class II (HLA-DR) antigens, but presence of MSCs positive markers (CD29, CD44, CD73 and CD90).
Figure 2.
Representative images showing the clonogenicity and multipotent (osteogenic, adipogenic and chondrogenic) differentiation ability of BM-MSCs at passage 3.
3.3. Clinical outcomes
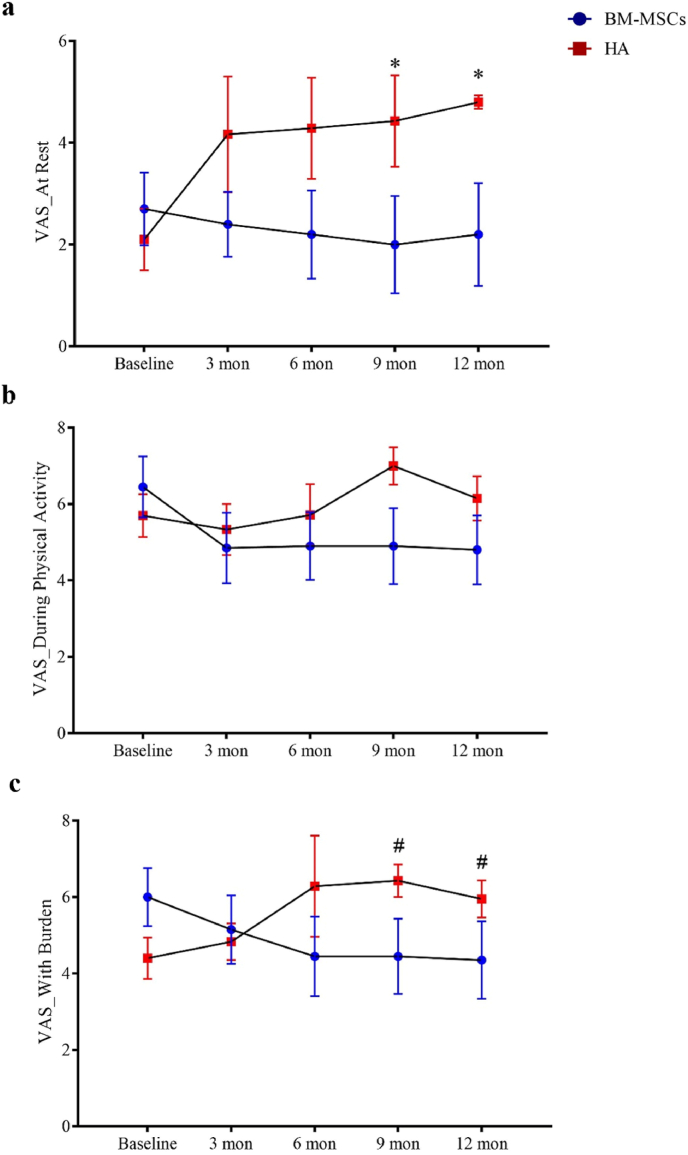
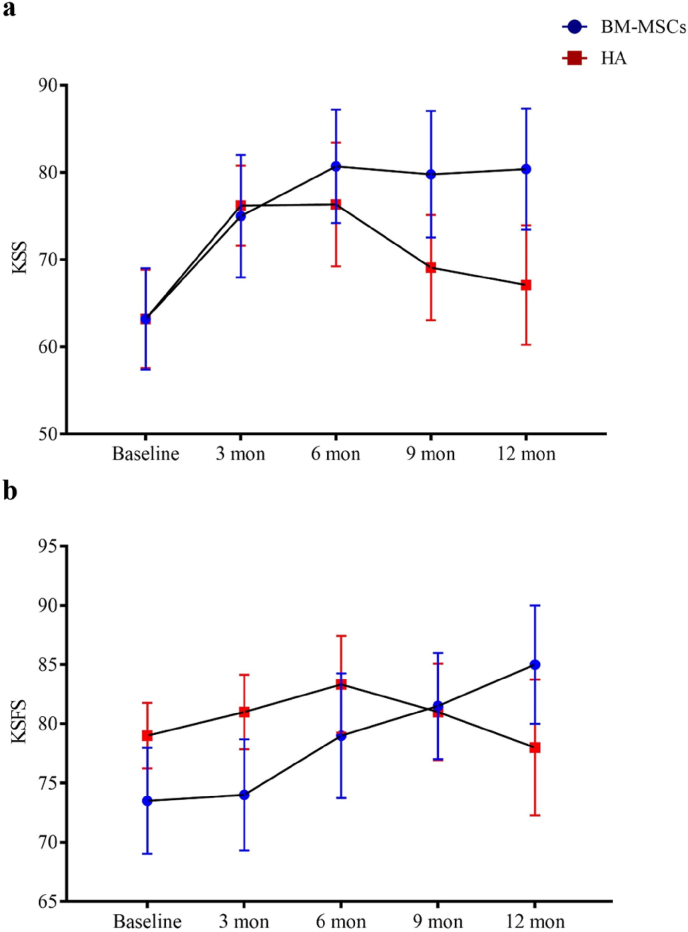
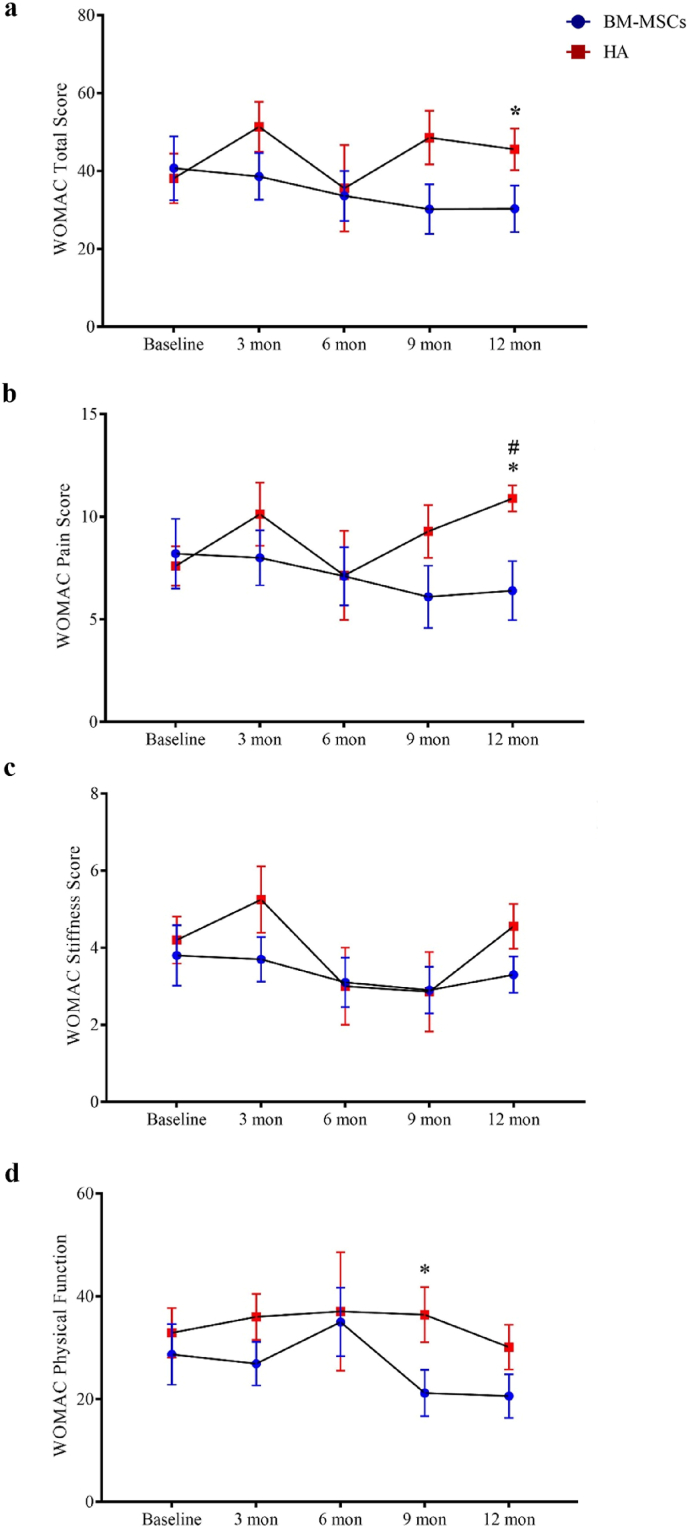
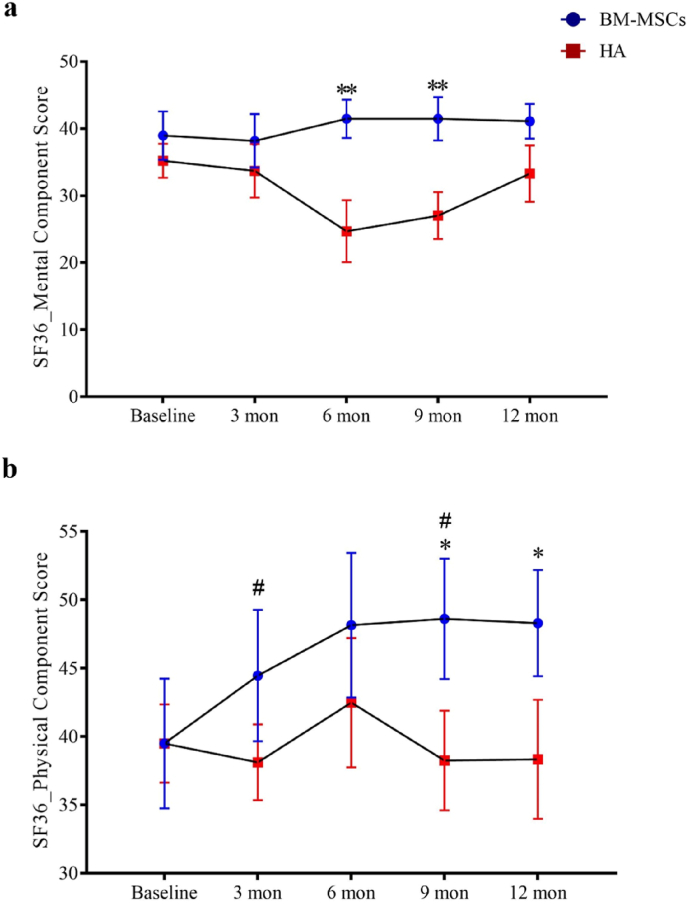
All participants completed clinical assessments before injection and at 12 months post-injection for the primary outcome. For the secondary outcome, one participant in the HA group failed to complete the WOMAC assessment at the 12 months time-point. There were no observed complications. One patient in the BM-MSCs group required a second bone marrow aspiration procedure because the first aspiration failed to reach sufficient yield of BM-MSCs for injection. BM-MSCs injection resulted in significant reduction in VAS score at rest during the 12 months follow-up periods, while HA resulted in score increase post-injection (between-group difference, −2.91; 95% CI, −4.98 to −0.84; P = 0.007). A similar trend was found for VAS score during physical activity or with burden with statistical significance reached by the differences in VAS score with burden (between-group difference, −2.93; 95% CI, −4.85 to −1.02; P = 0.003) (Fig. 3 and Table 3). KSS and KSFS scores over the 12 months study period showed an increasing trend in the BM-MSCs group, though this did not reach statistical significance compared to HA (Fig. 4 and Table 3). BM-MSCs showed a significantly decrease in WOMAC total score (between-group difference, −15.52; 95% CI, −31.00 to −0.04; P = 0.049), WOMAC pain score (between-group difference, −3.32; 95% CI, −6.52 to −0.12; P = 0.042) and WOMAC percentage score (between-group difference, −16.17; 95% CI, −32.30 to −0.04; P = 0.049) than that in HA group post intervention. WOMAC stiffness and physical function was similarly improved in the BM-MSCs group but not reaching statistical significance compared to the HA group (P = 0.641 and P = 0.444) (Fig. 5 and Table 3). For the SF-36 score, compared with the HA group, an improvement of the physical functioning score (between-group difference, 14.38; 95% CI, 4.58 to 24.18; P = 0.005), the bodily pain score (between-group difference, 25.26; 95% CI, 7.69 to 42.82; P = 0.005) and the physical component score (between-group difference, 9.03; 95% CI, 2.81 to 15.25; P = 0.005) was noticed in the BM-MSCs group (Fig. 6 and Table 3). Based on subscale analysis, the improvement in the physical component score was mainly attributed to the significantly better score for bodily pain, including more freedom from pain. Vitality and mental health were the two main features that contributed to the significant improvement in the mental component score. In addition, BM-MSCs injection significantly led to higher physical functioning at 9 month post injection.
Figure 3.
Changes of VAS pain score during the one-year follow-up period after intra-articular injection of BM-MSCs (circle) and HA (square). VAS was determined a) at rest, b) during physical activity or c) with burden at baseline, 3 months, 6 months, 9 months, and 12 months. There was a trend of pain reduction in participants which received BM-MSCs injection when compared with those who received HA injection. Significant reduction in rest pain and in pain with burden at 9 months and 12 months post injection was found in BM-MSCs group. Data shown as mean ± standard deviation. ∗P < 0.05 in Mann-Whiney U test. #P < 0.05 in Bonferroni's correction multiple comparisons test. VAS: visual analogue scale; BM-MSCs, bone marrow-derived mesenchymal stem cells; HA, hyaluronic acid; Mon, month(s).
Table 3.
Changes of WOMAC, SF36, VAS, KSS and KSFS scores from baseline to follow-ups.
| Variable | BM-MSCs |
HA |
Fixed effects (BM-MSCs Group) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 Months | 6 Months | 9 Months | 12 Months | 3 Months | 6 Months | 9 Months | 12 Months | Effect size (95% CI) | p-value | |
| ΔWOMAC a | ||||||||||
| Total score | −2.10 ± 15.54 | −7.10 ± 24.22 | −10.50 ± 18.16 | −10.40 ± 17.41 | 8.50 ± 14.81 | 0.43 ± 22.32 | 13.43 ± 19.53 | 8.33 ± 20.33 | −15.52 (−31.00, −0.04) | 0.049 |
| Pain score | −0.20 ± 4.05 | −1.10 ± 5.76 | −2.10 ± 5.26 | −1.80 ± 4.52 | 1.63 ± 2.18 | 0.43 ± 3.21 | 2.57 ± 2.37 | 3.22 ± 3.23 | −3.32 (−6.52, −0.12) | 0.042 |
| Stiffness | −0.10 ± 1.52 | −0.70 ± 2.06 | −0.90 ± 1.29 | −0.50 ± 1.58 | 1.00 ± 2.45 | −1.43 ± 2.94 | −1.57 ± 2.44 | 0.33 ± 2.55 | −0.44 (−2.30, 1.42) | 0.641 |
| Physical function | −1.80 ± 10.87 | −5.30 ± 16.9 | −7.50 ± 12.29 | −8.10 ± 12.22 | 5.88 ± 13.17 | −8.00 ± 25.77 | 3.00 ± 15.51 | −2.56 ± 18.29 | −5.18 (−18.60, 8.24) | 0.444 |
| Percetage Score | −2.19 ± 16.19 | −7.40 ± 25.23 | −10.94 ± 18.92 | −10.83 ± 18.14 | 8.85 ± 15.43 | 0.45 ± 23.25 | 13.99 ± 20.34 | 8.68 ± 21.18 | −16.17 (−32.30, −0.04) | 0.049 |
| ΔSF36a | ||||||||||
| PF | 9.00 ± 7.38 | 11.50 ± 16.17 | 11.50 ± 20.69 | 7.00 ± 19.75 | −1.25 ± 9.54 | −11.43 ± 13.14 | −11.43 ± 16.76 | 1.50 ± 11.80 | 14.38 (4.58, 24.18) | 0.005 |
| RP | 0.63 ± 14.57 | 1.88 ± 17.44 | 3.75 ± 16.46 | 6.25 ± 17.92 | 3.13 ± 10.02 | 8.03 ± 10.02 | 6.25 ± 7.22 | 3.13 ± 6.75 | −1.31 (−12.26, 9.65) | 0.812 |
| BP | 14.25 ± 20.21 | 22.25 ± 27.85 | 23.25 ± 16.75 | 22.50 ± 18.07 | −9.06 ± 8.86 | 5.00 ± 35.68 | −4.64 ± 14.75 | −8.75 ± 19.59 | 25.26 (7.69, 42.82) | 0.005 |
| GH | −4.00 ± 12.87 | −1.00 ± 8.10 | −2.00 ± 7.53 | −0.50 ± 8.32 | 3.75 ± 9.54 | 4.00 ± 10.84 | 3.00 ± 13.04 | −0.50 ± 12.35 | −1.82 (−9.87, 6.22) | 0.652 |
| VT | 1.25 ± 16.35 | 5.00 ± 18.35 | 3.75 ± 17.73 | 3.13 ± 18.69 | −6.25 ± 20.04 | −6.25 ± 18.75 | −2.68 ± 21.61 | −4.38 ± 26.52 | 10.00 (−6.84, 26.83) | 0.240 |
| SF | 5.00 ± 16.87 | 3.75 ± 18.68 | 8.75 ± 22.86 | 8.75 ± 26.39 | 0 ± 6.68 | −7.50 ± 14.25 | −10.00 ± 13.69 | −2.50 ± 9.86 | 10.16 (−3.54, 23.87) | 0.143 |
| RE | −5.00 ± 15.32 | −4.17 ± 9.00 | −4.17 ± 7.08 | −3.33 ± 11.92 | 3.12 ± 6.20 | 0 ± 4.81 | 2.38 ± 11.5 | 2.50 ± 7.91 | −5.86 (−13.16, 1.45) | 0.114 |
| MH | 0 ± 12.73 | 5.42 ± 13.18 | 1.67 ± 11.98 | 0 ± 12.73 | −9.90 ± 14.59 | −1.67 ± 15.76 | 1.19 ± 10.13 | −3.33 ± 18.72 | 6.50 (−6.22, 19.22) | 0.311 |
| PCS | 4.97 ± 4.46 | 8.66 ± 12.68 | 9.13 ± 9.27 | 8.81 ± 9.84 | −0.86 ± 3.52 | 0.97 ± 9.41 | −3.26 ± 6.62 | −1.16 ± 8.31 | 9.03 (2.81, 15.25) | 0.005 |
| MCS | 0.31 ± 11.62 | 2.50 ± 10.78 | 2.50 ± 11.14 | 2.14 ± 11.46 | −3.25 ± 7.76 | −9.60 ± 12.02 | −7.27 ± 7.94 | −1.93 ± 11.5 | 7.70 (−0.24, 15.65) | 0.057 |
| ΔVASa | ||||||||||
| At Rest | −0.30 ± 1.57 | −0.50 ± 2.99 | −0.70 ± 3.56 | −0.50 ± 3.78 | 1.67 ± 2.34 | 2.43 ± 1.99 | 2.57 ± 1.51 | 2.70 ± 2.06 | −2.91 (−4.98, −0.84) | 0.007 |
| With a burden | −0.85 ± 2.36 | −1.55 ± 3.11 | −1.55 ± 2.81 | −1.65 ± 2.81 | 1.17 ± 2.14 | 2.00 ± 2.52 | 2.14 ± 2.04 | 1.55 ± 2.17 | −2.93 (−4.85, −1.02) | 0.003 |
| During Physical Activity | −1.60 ± 2.88 | −1.55 ± 3.55 | −1.55 ± 3.32 | −1.65 ± 3.18 | −0.17 ± 0.75 | −0.57 ± 1.90 | 0.71 ± 1.25 | 0.45 ± 2.14 | −1.71 (−3,91, 0.49) | 0.126 |
| ΔKSSa | 11.80 ± 20.68 | 17.50 ± 18.48 | 16.60 ± 21.88 | 17.20 ± 22.58 | 13.00 ± 10.76 | 10.00 ± 16.48 | 5.90 ± 18.56 | 3.90 ± 21.08 | 7.10 (−7.99, 22.19) | 0.351 |
| ΔKSFSa | 0.50 ± 11.65 | 5.50 ± 11.17 | 8.00 ± 12.29 | 11.50 ± 18.57 | 2.00 ± 7.89 | 3.33 ± 11.18 | 2.00 ± 10.33 | −1.00 ± 16.63 | 4.81 (−3.88, 13.50) | 0.274 |
Changes of the scores from baseline to follow-ups.
Figure 4.
Changes of a) KSS and b) KSFS score during one-year follow-up period after intra-articular injection of BM-MSCs (circle) and HA (square) at baseline, 3 months, 6 months, 9 months, and 12 months were shown as mean ± standard deviation. Increasing trends in KSS and KSFS were noted in BM-MSCs group but not reaching statistical significance. KSS, Knee Society Score; KSFS, Knee Society Function Score; BM-MSCs, bone marrow-derived mesenchymal stem cells; HA, hyaluronic acid; Mon, month(s).
Figure 5.
Changes of WOMAC score during the one-year follow-up period after intra-articular injection of BM-MSCs (circle) and HA (square). An increasing trend in WOMAC score in the HA group indicated progression with worsening pain, stiffness and functional limitations. The WOMAC score of a) total score, b) pain score, c) stiffness score and d) physical function at baseline, 3 months, 6 months, 9 months, and 12 months were shown as mean ± standard deviation. Compared with HA, BM-MSCs injection led to significantly lower WOMAC score at the later time points of follow-up. ∗P < 0.05 in two-sample t test with Welch's correction or Mann-Whiney U test. #P < 0.05 in Bonferroni's correction multiple comparisons test. WOMAC, Western Ontario and McMaster Osteoarthritis Index; BM-MSCs, bone marrow-derived mesenchymal stem cells; HA, hyaluronic acid; Mon, month(s).
Figure 6.
Changes of SF36 score during the one-year follow-up period after intra-articular injection of BM-MSCs (circle) and HA (square). a) mental component score and b) physical component score at baseline, 3 months, 6 months, 9 months, and 12 months were shown in mean ± standard deviation. Participant satisfaction was significantly improved after BM-MSCs injection as indicated by improvement in physical component score and mental component score at the later time points of follow-up. ∗P < 0.05 and ∗∗P < 0.01 in two-sample t test with Welch's correction. #P < 0.05 in Bonferroni's correction multiple comparisons test. SF-36, 36-Item Short Form Survey; BM-MSCs, bone marrow-derived mesenchymal stem cells; HA, hyaluronic acid; Mon, month(s).
4. Radiographic assessment
A single patient in the BM-MSCs group progressed from KL Grade Ⅲ to Grade Ⅳ over the one year study period. For HA group, two cases showed a deterioration in KL grade from grade Ⅱ to Ⅲ, and from grade Ⅲ to Ⅳ respectively.
5. MR T2-cartilage mapping outcomes
Overall, for the HA group, the T2 relaxation time decreased slightly from 48.6 ± 4.4 ms pre-treatment to 47.6 ± 5.0 ms post-treatment (P = 0.922). Isubjects who received BM-MSCs injection, there was more reduction from 87.7 ± 36.6 ms pre-treatment to 70.3 ± 33.2 ms post-treatment (P = 0.375). Despite a larger variation in the BM-MSCs group, T2 values tended to decrease more in the BM-MSCs group (17.5 ± 51.9 ms) than in the HA group (1.0 ± 5.5 ms) during the 12 month follow-up period (P = 0.315) (Fig. 7). Subgroup analysis on individual participants showed that 6 out of 10 subjects receiving BM-MSCs injection showed a marked reduction in T2 value, while only 4 out of 10 subjects with HA injection showed a reduction in T2 value (−47.3 Vs −6.4 ms, respectively).
Figure 7.
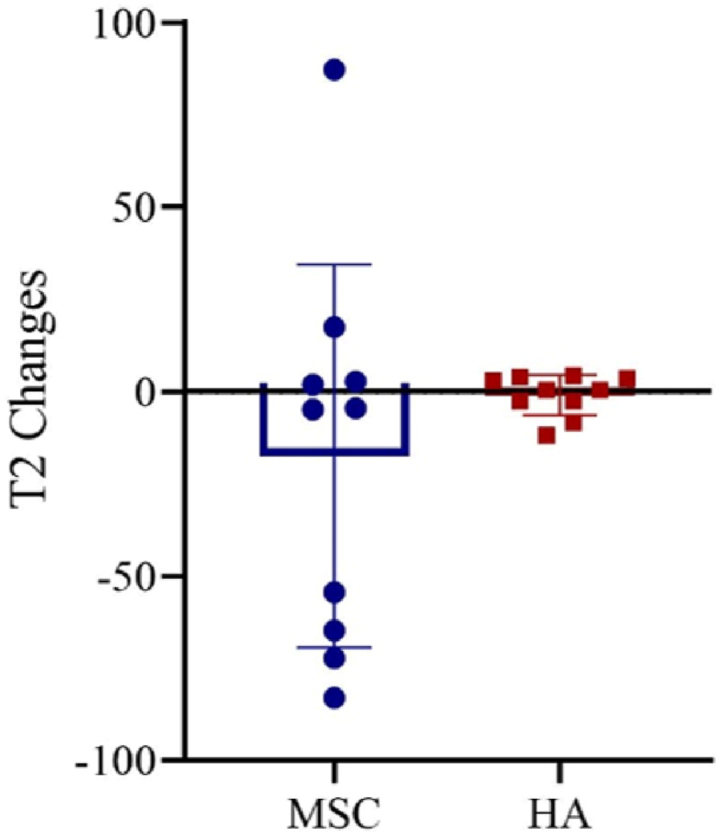
Changes of average individual MRI T2-relaxation time during the one-year follow-up period after intra-articular injection of BM-MSCs (circle) and HA (square). MRI, magnetic resonance imaging; BM-MSCs, bone marrow-derived mesenchymal stem cells; HA, hyaluronic acid.
6. Discussion
Comparing with HA injection, we found that a single intra-articular injection of BM-MSCs at 6 million cells per joint significantly reduced pain, and improved functional assessment score and certain self-reported functional and well-being parameters over a 12-month follow-up period.
Similar to previously reported studies, no adverse effects were reported by the participants. Since completion of the study, one of the 10 patients in the BM-MSCs group underwent TKR. All of the other participants have remained clinically stable. The pooled data of a previous meta-analysis [11] showed that the total rate of minor AE in MSCs group was 16.5% and 21.42% in control group, ranging from 2.5% to 60% per study, which did not reach a significant difference and the degree of variability between trials seemed to be raised by chance.
Compositional change in the articular cartilage was evaluated by T2 mapping on serial MRI examinations. MRI is regarded as a standard non-invasive imaging assessment for determining cartilage status. Change in cartilage composition including proteoglycan loss, deterioration of collagen structure, and increased cartilage water content are reflected by T2 relaxation time measurement. Higher MRI T2 values are seen in patients with mild to severe OA [19], reflecting more severe cartilage degeneration [20]. MRI analysis indicated that BM-MSCs treatment resulted in a more pronounced reduction in T2 relaxation time, except for two subjects receiving BM-MSCs injection who showed an increasing trend of T2-relaxation time, indicative of progressive cartilage degeneration. In general, our findings were in line with the results of recent meta-analysis, which showed that BM-MSCs therapy was more effective in alleviating pain and improving self-reported function with an average follow-up period of 14 months [10,12,21].
A more recent meta-analysis on MSCs therapy without adjuvant therapy reported that cultured MSCs therapy resulted in better clinical outcome in terms of pain reduction in patients with knee OA compared to uncultured MSCs [10]. As the clinical outcome of MSC therapy and the chondrogenic potential of MSCs might be associated with passage number [22,23], BM-MSCs at passage 5 were used for intervention in this study which was widely adopted by other similar RCTs for knee OA though there was yet no consensus regarding this issue. Unlike previous RCTs in which 80 ml bone marrow or more was aspirated for MSCs isolation [24,25], only 10 ml bone marrow was aspirated in this pilot study to reduce the possibility of obtaining low number of nucleated cells and potentially lower yield of MSCs in second aspirates [26]. Although this resulted in a lower MSC yield, this intervention with fewer MSCs still resulted in a similar clinical outcome compared with other RCTs that injected larger numbers of MSCs [14,25]. This suggests that a lower dose of injected MSC might not adversely affect clinical outcome. Unlike drugs or biologics which have a clearly defined and homogenous chemical structure, MSCs-based medicinal products can be regarded as a heterogeneous cell population with considerable batch-to-batch variation and, as such, variance in clinical outcome is expected. In the absence of any validated potency assay, the identity of MSCs relies on the harmonized criteria proposed by ISCT based on the plastic adherence, mutipotency, and the expression of a panel of surface markers [7]. The expression of surface markers might not be directly related to the cellular activity of MSCs or clinical outcome. Compared with younger subjects, human BM-MSCs isolated from elderly subjects usually have lower ability to form colonies and to differentiate into different lineages, and exhibit variable transcriptome and cytokine profiles, though the BM-MSCs from aged and younger donors have similar expression level of negative and positive markers [27]. In this study, we also noticed variation in the expression of positive markers (particularly CD29 and CD73), while the negative markers were found consistently absent (<2%) in all samples, indicating successful removal of hemopoietic lineage cells. A survey within the European Society of Blood and Marrow Transplantation revealed that the threshold percentage of negative (ranging from <1% to <20%) and positive (ranging from >70% to >95%) surface markers for MSCs medicinal products varied in different manufacturing centers [28]. After reviewing the clinical assessment data and the surface markers expression, it appeared that the different expression level of positive markers were not associated with clinical outcome. Allogeneic MSCs have been found to show potential benefits in patients with knee OA [25,29], which could be used as a more reliable source for MSCs therapy. On the other hand, recent studies suggest various approaches to augment the therapeutic outcome of MSCs therapy for OA, including the use of cell-free exosomes as alternative to MSCs-based therapy [30,31], or the co- or pre-treatment of small molecules to enhance the cellular activity of MSCs [32,33], which are warranted for further investigation in clinical setting to advance the clinical application of MSCs-related advanced medicinal products for OA.
There are several limitations in this study. First, subjects in the BM-MSCs group were aware of BM-MSCs treatment as they were all subjected to bone marrow aspiration. Such awareness might lead to higher follow-up compliance in the BM-MSCs group. Loss of follow-up data, particularly in the HA group, limited the continuous variables available for repeated measure analysis to assess effect change over time. As an alternative, the effect was analyzed by linear mixed-effects models. Nevertheless, retention strategy should be considered in further studies particularly when a larger sample and a longer follow up duration are involved. MRI assessment, as employed in the present study, did not provide unequivocal evidence of cartilage regeneration compared to baseline though greater reduction in T2-values with treatment were shown compared to the HA group. Arthroscopic examination and biopsy were not undertaken due to ethical concerns. BM-MSCs were injected as a suspension and, as such, the retention of the injected BM-MSCs at the OA site could not be been guaranteed. Fibrin glue or other clinically approved biomaterials should be considered in future studies to ensure more targeted delivery of the BM-MSCs to the main areas of interest. In the present study, we understood that concomitant or rescue pain medication may lead to improved patient-reported outcomes (PRO), however, considering the nature of this pilot study and cell therapy was a very new kind of treatment option to local patients, it might lead to a higher dropout rate and poor adherence during follow-up assessment if we employed more stringent recruitment criteria, such as not allowing pain medications throughout the study. In this study, provided that majority of recruited subjects are in the early stages of OA (KL grade 2) and seldom take medication for pain relief, it is reasonable to believe that the effect of concomitant pain medication in our pilot study is very limited. Nevertheless, further clinical trial in this subject area should be conducted with caution as previously recommended [34].
Overall, BM-MSCs treatment appears to result in more favorable outcomes in patients with knee OA in terms of pain reduction and some self-reported functions. The small sample size of this pilot study, however, did not enable the effect of potential confounding effect due to medication and rehabilitation programs and other potential cofounder during follow up to be assessed.
Funding source
There is no funding information to declare.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgement
The authors acknowledge Dr. Kenneth Guangpu Yang for his support on statistical analysis. This study was supported by Sports Medicine and Regenerative Technology (SMART) of Lui Che Woo Institute of Innovative Medicine, Faculty of Medicine, The Chinese University of Hong Kong.
Contributor Information
Kevin Ki-wai Ho, Email: kevinho@cuhk.edu.hk.
Gang Li, Email: gangli@cuhk.edu.hk.
References
- 1.Hunter D.J., Schofield D., Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10(7):437–441. doi: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 2.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1145–1153. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fransen M., Bridgett L., March L., Hoy D., Penserga E., Brooks P. The epidemiology of osteoarthritis in Asia. Int J Rheum Dis. 2011;14(2):113–121. doi: 10.1111/j.1756-185X.2011.01608.x. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia D., Bejarano T., Novo M. Current interventions in the management of knee osteoarthritis. J Pharm BioAllied Sci. 2013;5(1):30–38. doi: 10.4103/0975-7406.106561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W., Moskowitz R.W., Nuki G., Abramson S., Altman R.D., Arden N., et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Minas T., Ogura T., Bryant T. Autologous chondrocyte implantation. JBJS Essent Surg Tech. 2016;6(2):e24. doi: 10.2106/JBJS.ST.16.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 8.Prockop D.J. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17(6):939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong L., Zheng L.Z., Qin L., Ho K.K.W. Role of mesenchymal stem cells in osteoarthritis treatment. J Orthop Translat. 2017;9:89–103. doi: 10.1016/j.jot.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan S.H.S., Kwan Y.T., Neo W.J., Chong J.Y., Kuek T.Y.J., See J.Z.F., et al. Intra-articular injections of mesenchymal stem cells without adjuvant therapies for knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2021;49(11):3113–3124. doi: 10.1177/0363546520981704. [DOI] [PubMed] [Google Scholar]
- 11.Song Y., Zhang J., Xu H., Lin Z., Chang H., Liu W., et al. Mesenchymal stem cells in knee osteoarthritis treatment: a systematic review and meta-analysis. J Orthop Translat. 2020;24:121–130. doi: 10.1016/j.jot.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iijima H., Isho T., Kuroki H., Takahashi M., Aoyama T. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: a meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen Med. 2018;3:15. doi: 10.1038/s41536-018-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bannuru R.R., Osani M.C., Vaysbrot E.E., Arden N.K., Bennell K., Bierma-Zeinstra S.M.A., et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Lamo-Espinosa J.M., Mora G., Blanco J.F., Granero-Molto F., Nunez-Cordoba J.M., Sanchez-Echenique C., et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II) J Transl Med. 2016;14(1):246. doi: 10.1186/s12967-016-0998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee W.Y.W., Zhang T., Lau C.P.Y., Wang C.C., Chan K.M., Li G. Immortalized human fetal bone marrow-derived mesenchymal stromal cell expressing suicide gene for anti-tumor therapy in vitro and in vivo. Cytotherapy. 2013;15(12):1484–1497. doi: 10.1016/j.jcyt.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Viswanathan S., Shi Y., Galipeau J., Krampera M., Leblanc K., Martin I., et al. Mesenchymal stem versus stromal cells: international society for cell & gene therapy (ISCT(R)) mesenchymal stromal cell committee position statement on nomenclature. Cytotherapy. 2019;21(10):1019–1024. doi: 10.1016/j.jcyt.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Lee W.Y., Lui P.P., Rui Y.F. Hypoxia-mediated efficient expansion of human tendon-derived stem cells in vitro. Tissue Eng Part A. 2012;18(5–6):484–498. doi: 10.1089/ten.tea.2011.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin S., Lee W.Y.W., Feng Q., Xu L., Wang B., Man G.C.W., et al. Synergistic effects on mesenchymal stem cell-based cartilage regeneration by chondrogenic preconditioning and mechanical stimulation. Stem Cell Res Ther. 2017;8(1):221. doi: 10.1186/s13287-017-0672-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn T.C., Lu Y., Jin H., Ries M.D., Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232(2):592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasad A.P., Nardo L., Schooler J., Joseph G.B., Link T.M. T(1)rho and T(2) relaxation times predict progression of knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(1):69–76. doi: 10.1016/j.joca.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S.H., Ha C.W., Park Y.B., Nam E., Lee J.E., Lee H.J. Intra-articular injection of mesenchymal stem cells for clinical outcomes and cartilage repair in osteoarthritis of the knee: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2019;139(7):971–980. doi: 10.1007/s00402-019-03140-8. [DOI] [PubMed] [Google Scholar]
- 22.Tan A.R., Alegre-Aguaron E., O'Connell G.D., VandenBerg C.D., Aaron R.K., Vunjak-Novakovic G., et al. Passage-dependent relationship between mesenchymal stem cell mobilization and chondrogenic potential. Osteoarthritis Cartilage. 2015;23(2):319–327. doi: 10.1016/j.joca.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Bahr L., Sundberg B., Lonnies L., Sander B., Karbach H., Hagglund H., et al. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol Blood Marrow Transplant. 2012;18(4):557–564. doi: 10.1016/j.bbmt.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Lamo-Espinosa J.M., Blanco J.F., Sanchez M., Moreno V., Granero-Molto F., Sanchez-Guijo F., et al. Phase II multicenter randomized controlled clinical trial on the efficacy of intra-articular injection of autologous bone marrow mesenchymal stem cells with platelet rich plasma for the treatment of knee osteoarthritis. J Transl Med. 2020;18(1):356. doi: 10.1186/s12967-020-02530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vega A., Martin-Ferrero M.A., Del Canto F., Alberca M., Garcia V., Munar A., et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–1690. doi: 10.1097/TP.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 26.Fennema E.M., Renard A.J., Leusink A., van Blitterswijk C.A., de Boer J. The effect of bone marrow aspiration strategy on the yield and quality of human mesenchymal stem cells. Acta Orthop. 2009;80(5):618–621. doi: 10.3109/17453670903278241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Block T.J., Marinkovic M., Tran O.N., Gonzalez A.O., Marshall A., Dean D.D., et al. Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies. Stem Cell Res Ther. 2017;8(1):239. doi: 10.1186/s13287-017-0688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trento C., Bernardo M.E., Nagler A., Kuci S., Bornhauser M., Kohl U., et al. Manufacturing mesenchymal stromal cells for the treatment of graft-versus-host disease: a survey among centers affiliated with the European society for blood and marrow transplantation. Biol Blood Marrow Transplant. 2018;24(11):2365–2370. doi: 10.1016/j.bbmt.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta P.K., Chullikana A., Rengasamy M., Shetty N., Pandey V., Agarwal V., et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel(R)): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. 2016;18(1):301. doi: 10.1186/s13075-016-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni Z., Zhou S., Li S., Kuang L., Chen H., Luo X., et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8:25. doi: 10.1038/s41413-020-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan L., Liu G., Wu X. Exosomes derived from umbilical cord mesenchymal stem cells in mechanical environment show improved osteochondral activity via upregulation of LncRNA H19. J Orthop Translat. 2021;26:111–120. doi: 10.1016/j.jot.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y., Xu L., Hu L., Chen D., Yu L., Li X., et al. Stearic acid methyl ester promotes migration of mesenchymal stem cells and accelerates cartilage defect repair. J Orthop Translat. 2020;22:81–91. doi: 10.1016/j.jot.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin S., Lee W.Y.W., Xu L., Wang Y., Chen Y., Ho K.K.W., et al. Stepwise preconditioning enhances mesenchymal stem cell-based cartilage regeneration through epigenetic modification. Osteoarthritis Cartilage. 2017;25(9):1541–1550. doi: 10.1016/j.joca.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 34.McAlindon T.E., Driban J.B., Henrotin Y., Hunter D.J., Jiang G.L., Skou S.T., et al. OARSI Clinical Trials Recommendations: design, conduct, and reporting of clinical trials for knee osteoarthritis. Osteoarthritis Cartilage. 2015;23(5):747–760. doi: 10.1016/j.joca.2015.03.005. [DOI] [PubMed] [Google Scholar]