Abstract
Background
It is well known that appropriate mechanical stimulation benefits tendon-bone (T-B) healing, however, the mechanisms behind this are still uncovered completely. Here, we aimed to explore whether the IL-4/JAK/STAT signaling pathway mediated macrophage polarization was involved in mechanical stimulation induced T-B healing.
Method
C57BL/6 mice rotator cuff (RC) repair model was established, and the mice were randomly allocated to the following group. 1. Mice were allowed for free cage activities after surgery (FC group); 2. Mice received treadmill running initiated on postoperative day 7 (TR group); 3. Mice only received a local injection of hydrogel containing IL-4 neutralizing antibody without postoperative intervention (FC + AF-404-SP group); 4. Mice received a local injection of hydrogel containing IL-4 neutralizing antibody and postoperative treadmill running (TR + AF-404-SP group). The expression of IL-4 within supraspinatus tendon (SST) enthesis was measured by Enzyme-linked immunosorbent assay (ELISA). In addition, the activation of JAK/STAT signaling pathway in macrophages and identification of macrophage phenotype at the RC insertion site was detected by Flow cytometry and qRT-PCR. T-B healing quality in this RC repair model was evaluated by histological staining, Micro-computed tomography (Micro-CT) scanning, and biomechanical testing.
Result
In this study, using the RC repair model, we confirmed that generation of IL-4, activation of the JAK/STAT signaling pathway in macrophages, the ability of macrophages to polarize towards M2 subtype, and T-B healing quality were significantly enhanced in TR group compared to FC group. When comparing FC + AF-404-SP group with TR + AF-404-SP group, it was found that the mechanical stimulation induced this effect was depleted following the blockade of the IL-4/JAK/STAT signaling pathway.
Conclusion
Our finding suggested that mechanical stimulation could accelerate T-B healing via activating the IL-4/JAK/STAT signaling pathway that modulates macrophages to polarize towards M2 subtype.
The translational potential of this article
This is the first study to reveal a significant role of mechanical stimulation in the IL-4/JAK/STAT signaling pathway activation and macrophage polarization during RC T-B healing, which highlights the IL-4/JAK/STAT signaling pathway as a potential target to mediate macrophage M2 polarization and improves T-B healing for RC repair.
Keywords: Mechanical stimulation, Tendon-bone healing, Rotator cuff repair, Macrophage polarization, IL-4/JAK/STAT signaling Pathway
1. Introduction
Tendon-bone interface (TBI) is an ordered transitional interface structure connecting tendon and bone to mediate the load transfer and disperse concentrated stress, which is composed of four typical tissue zones: tendon, uncalcified fibrocartilage, calcified fibrocartilage, and bone [1,2]. RC tear, a common cause of shoulder pain and disability, often occurs at the T-B insertion site [3]. Surgical repair and reconstruction represent standard treatments for these injuries, with more than 270,000 RC reconstructions performed annually in the United States. However, structural failure of TBI usually results in unsatisfactory RC reconstruction and a high incidence of re-tear [3,4]. Therefore, it is imperative for RC repair to promote tendon-bone (T-B) healing.
Mechanical stimulation is one of the most important factors that may influence the process of T-B healing, as appropriate loading exercise after surgery has been found to benefit the original gradient structure regeneration and ultimate mechanical property of TBI, while postoperative immobilization exerts a detrimental effect on the T-B healing [[5], [6], [7], [8]]. Furthermore, the reduction of muscle loading can retard the development of tendon enthesis by impeding mineralized bone accumulation and fibrocartilage formation [[9], [10], [11]]. Therefore, attributing to the important effect of mechanical force on TBI development and repair after injury, loading exercise following tendon injury is often considered the crucial protocol of postoperative rehabilitation [12,13]. However, mechanisms behind the positive effect of mechanical loading on T-B healing remain poorly understood.
At the early stage of TBI healing, many inflammatory cells accumulated rapidly at the injury site. During this phase, macrophages may play a critical role in initiating and regulating TBI healing [14,15]. In response to environmental signals, macrophages could polarize into M1 or M2 subtypes. Generally, M1 macrophages generate pro-inflammatory cytokines to induce an inflammatory response, however, M2 macrophages secrete not only anti-inflammatory cytokines but also a series of growth factors to promote wound healing and tissue regeneration [16]. It has been proven that the modulation of the balance between M1 and M2 macrophages, contributing to more M2 macrophage polarization is a promising strategy for TBI repair [15,17]. Interestingly, mechanical stimulation applied to macrophages could modulate them to polarize towards M2 phenotype that produces a series of anti-inflammatory cytokines to regulate the local inflammatory microenvironment and induces osteogenesis [18], which might account for mechanical force induced T-B healing.
Mechanical stress has been shown to promote the generation of Interleukin-4 (IL-4) via T-cell-dependent manner [19]. Moreover, it is also reported that mechanical stimulation regulates the function of chondrocytes by activating the integrin-mediated release of IL-4 [20]. It is well known that IL-4 is a crucial cytokine that modulates macrophages to polarize toward M2 phenotype. The binding of IL-4 to its receptor could activate JAK (Janus kinase) 1 and JAK3. Next, the JAK activation leads to the activation of STAT6 (signal transducers and transcription activators of transcription 6) and nuclear translocation. Furthermore, activation of the JAK/STAT signaling in macrophages shifts itself from the pro-inflammatory to the anti-inflammatory phenotype to promote tissue healing [21,22].
Therefore, we hypothesized that mechanical stimulation promotes macrophages to polarize towards M2 subtype by activating the IL-4/JAK/STAT signaling pathway, which subsequently accelerates tendon-bone healing. The purpose of the present study was to explore whether the IL-4/JAK/STAT signaling pathway mediated macrophage polarization was associated with mechanical stimulation-induced tendon-bone healing.
2. Materials and methods
All animal experimental procedures were performed in accordance with U.K. Animals (Scientific Procedures) Act, 1986 and European guidelines (2010/63/EU) and approved by the Laboratory Animal Committee of Xiangya Hospital of Central South University (No. 201703222).
2.1. Study design
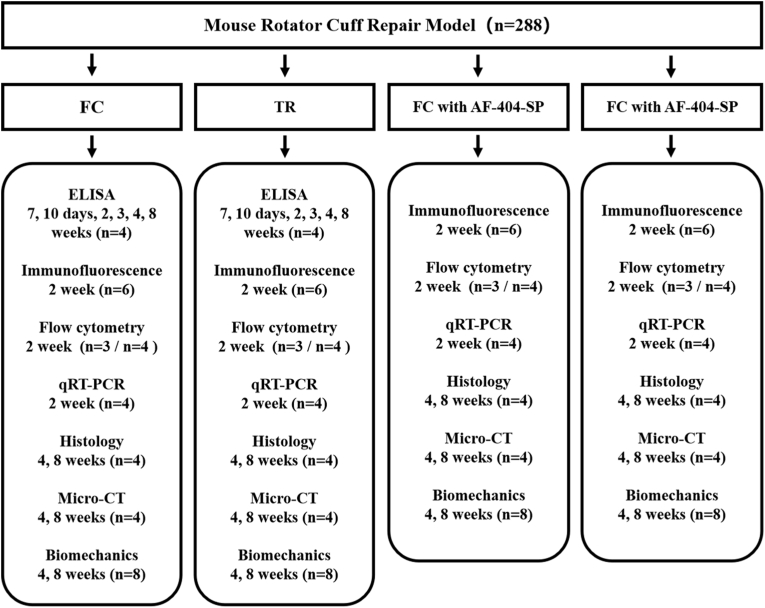
A total of 288 male C57BL/6 mice underwent acute RC injury and repair surgery (Fig. 1). The animals were randomly allocated to the following group. 1.84 mice were allowed for free cage activities after surgery (FC group); 2.84 mice received treadmill running initiated on postoperative day 7 (TR group); 3.60 mice only received a local injection of hydrogel containing IL-4 neutralizing antibody without postoperative intervention (FC + AF-404-SP group); 4.60 mice received a local injection of hydrogel containing IL-4 neutralizing antibody and postoperative treadmill running (TR + AF-404-SP group). Mice in FC group and TR group have been euthanized to harvest supraspinatus tendon (SST) enthesis for ELISA at 0 days (Normal enthesis), 7 days, 10 days, 2 weeks 3weeks 4weeks and 8 weeks after surgery. Next, the collected specimens among all groups at postoperative week 2 were tested by flow cytometry and qRT-PCR to evaluate the activation of JAK/STAT signaling pathway in macrophages and macrophage polarization. In addition, the supraspinatus-humeral head composites were harvested at postoperative week 2 for immunofluorescent staining and at postoperative week 4 and 8 for histological staining, Micro-CT scanning, and biomechanical test.
Figure 1.
Experimental design flowchart.
2.2. Animal experiment
The 8-week-old mature male C57BL6 mice were used to establish the rotator cuff (RC) repair model as previously reported [[23], [24], [25]]. As shown in Figure S1, animals were firstly anesthetized with pentobarbital, and then a skin incision was made over the anterolateral side of the left shoulder. The deltoid was identified by overlying vasculature and detached carefully to expose the ST. After the end of the RC was placed with 6–0 PDS suture in an “8” figure fashion, the supraspinatus tendon was sharply transected at its insertion on the greater tuberosity and any fibrocartilage at the enthesis was scraped until bellowing spongy bone was exposed. To block the IL-4/JAK/STAT signaling pathway, the RC insertion site was injected with 5 μl thermoresponsive hydrogel (Flexcell®, Burlington, NC, USA) containing IL-4 neutralizing antibody AF-404-SP (0.1 ug/ml, R&D system, Minneapolis, USA) by microsyringe. A bone tunnel was transversely made on the humeral head and the suture was passed through the drilled hole to tie the RC to its anatomic position. The detached deltoid and skin incision were then sutured in a layer.
2.3. Mechanical stimulation
In this study, a motor-powered treadmill was used to load mechanical stimulation to the RC TBI according to our previously designed protocol [26]. Briefly, before the operation, all groups of mice were arranged for one-week adaptive training to become familiar with the lane environment. Treadmill speed (5–10 m/min) was increased daily, and surgery was performed until all mice could be adapted to run at the speed of 10 m/min for 20 min per day. On postoperative day 7, the mice that were scheduled to acquire mechanical stimulation began with running at 10 m/min on a 0° declined lane for 20 min per day, 5 days per week. Mice were euthanized 1 day after the last time treadmill running to avoid the instant effect of mechanical stimulation.
2.4. Enzyme-linked immunosorbent assay (ELISA)
The SST enthesis specimens including the tendon (one millimeter in length) and the portion of the humeral head proximal to the growth plate near the tendon insertion site were harvested at 0 days (Normal enthesis), 7 days, 10 days, 2 weeks, 3weeks, 4weeks, and 8 weeks after surgery as previously reported [27,28]. Afterward, the tissue homogenate was generated to determine cytokine levels of IL-4 in TBI by a mouse Interleukin (IL-4) ELISA kit (CUSABIO, Wuhan, China) according to the manufacturer's instructions. The protein concentration in each sample lysate was determined by BCA kit (MultiSciences, Hangzhou, China). The IL-4 content was then normalized to the protein content.
2.5. Flow cytometry
Samples were collected, minced, and digested with α-MEM containing 10% FBS in the presence of collagenase I and collagenase II at 37 °C for 1 h. Digested material was filtered with the 70-um strainer and the filtrate was centrifuged at 300 g for 5 min. Next, the collected cells were fixed, permeabilized, and blocked with CD16/32 antibody (Biolegend, CA, USA) on ice for 10 min. To determine JAK/STAT activation in macrophages, the cells were incubated with FITC anti-mouseF4/80 (Biolegend) and primary antibody anti-Phospho-Jak1 (Bioss, Beijing, China), anti-Phospho-JAK3 (Affinity, Jiangsu, China) or anti-Phospho-STAT6 (Abcam, Cambridge, Uk) on ice for 30 min, subsequently incubated with Alexa-Fluor 647 (Abcam) conjugated secondary antibody on ice for additional 30 min. To evaluate macrophages’ phenotype, these cells were stained with FITC anti-mouse F4/80, APC anti-mouse CD86, and PE anti-mouse CD206 antibody (all from Biolegend) on ice for 30 min. Cells that were not treated with any antibody were used as negative controls. The cells were then washed twice to remove excess antibodies, resuspended in 100ul PBS containing 5% FBS, and analyzed by a BD FACSCanto II (New Jersey, USA)
2.6. qRT-PCR
The SST enthesis tissues were harvested at postoperative weeks 2 for RNA extraction using the TRIzol reagent (Invitrogen). GoScriptTM Reverse Transcription System (Promega, Madison, USA) and GoTaq® qPCR Master Mix (Promega) cDNA were respectively used for reverse transcription and PCR amplification reaction, the latter was performed on ABI PRISM® 7900HT System (Applied Biosystems, USA). The relative expression of the interest gene was calculated using the 2−ΔΔCt method and normalized to the levels of housekeeping gene β-actin. Results are presented as relative gene expression of diverse groups when in comparison with normal enthesis. The details of primer sequences employed for qRT-PCR analysis are listed in Table 1.
Table 1.
List of primer sequences used in this study.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| TNF-α | GCTCCTCCACTTGGTGGTTT | AGGCGGTGCCTATGTCTCAG |
| INOS | GTTCTCAGCCCAACAATACAAGA | GTGGACGGGTCGATGTCAC |
| CCR7 | TGTACGAGTCGGTGTGCTTC | GGTAGGTATCCGTCATGGTCTTG |
| Arg-1 | GAGCCACCGTTTTACATTGTGA | CTCGCCCACTAGGCAGTTC |
| IL-10 | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| CD206 | AGCTTCATCTTCGGGCCTTTG | GGTGACCACTCCTGCTGCTTTAG |
| β-actin | GGAGATCACAGCTCTGGCT | GTCGATTGTCGTCCTGAGG |
2.7. Histological evaluation
The shoulder specimens including supraspinatus muscle, tendon, and humerus were collected, fixated in 4% formaldehyde, decalcified in EDTA, dehydrated in ethanol solutions and embedded in paraffin. 5 μm of paraffin slices were deparaffinized, rehydrated, and stained with hematoxylin and eosin (H&E) or toluidine blue &fast green staining (TB&FG). H&E staining was used for general histology and TB&FG staining was performed to assess fibrocartilage regeneration. The histological images were semi-quantitatively analyzed in a blinded fashion by two independent observers with a previously reported modified tendon-to-bone maturing score (Table S1) [28].
2.8. Micro-computed tomography (Micro-CT) imaging
Micro-CT analysis was performed to evaluate new bone formation at SST enthesis. All specimens were fixed overnight with 4% paraformaldehyde and scanned with Micro-CT system (SkyScan 1176, Bruker, Germany). Imaging was performed with 11.5-μm voxel size, at 45 KVp, 100 μA, and a 231-ms exposure per view. The reconstruction of three-dimensional images was conducted by Amira (Visage Imaging, Australia). The trabecular bone within the humeral head near the tendon attachment (within approximately 1 mm) and proximal to the growth plate was selected for a region of interest to determine morphological parameters of the newly formed bone including bone volume/total volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp) by CT-Analyzer (CTAn, Bruker, Germany).
2.9. Biomechanical testing
As the ultimate criterion to assess T-B healing in the RC repair model, biomechanical testing was performed to determine failure load, stiffness, and ultimate strength by computer-controlled electronic material testing machine (Instron, Boston, USA). Before the test, the width and thickness of the footprint were measured by using a 0.02 mm caliper under the same tensile load (0.1 N) and their cross-sectional area (CSA) was then calculated. Subsequently, the humerus shaft was carefully gripped by a lower clamp, meanwhile, the tendon was fixed by sandpaper, glue, and upper clamp. Finally, the specimen was loaded to failure at a 1 mm/min rate.
2.10. Statistical analysis
All data were presented as mean ± standard deviation (mean ± SD) and statistically analyzed by GraphPad Prism software (San Diego, CA, USA). Data were confirmed for normal distribution by Shapiro–Wilk test and homoscedasticity by Levene's Test before analysis of variance (ANOVA). Statistical analysis of the data at a single time point was carried out using one-way ANOVA followed by Tukey's multiple comparisons test. Statistical analysis of the data at multiple time points was carried out using two-way ANOVA followed Tukey's multiple comparisons test. P < 0.05 was considered a statistically significant difference. To verify that the sample size will provide 80% power to statistically analyze the data, power calculations for ANOVA were performed by using the G∗Power software.
3. Results
3.1. The expression of IL-4 at SST enthesis following treadmill exercise
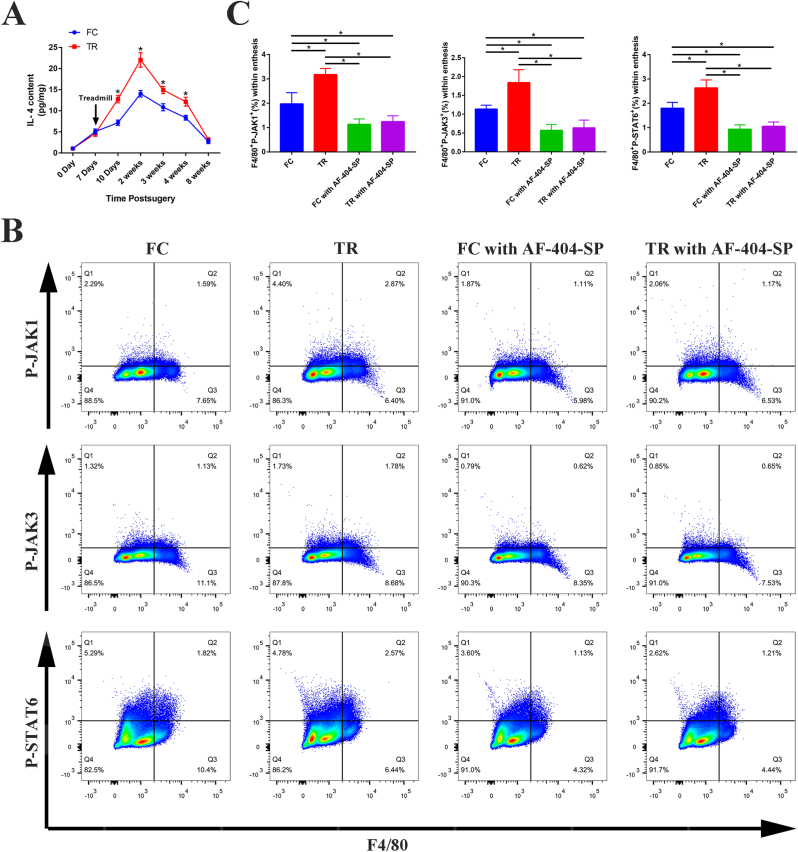
As evidenced by results from the ELISA, the expression level of IL-4 was low in normal SST enthesis and tended to increase postoperatively. At the RC healing site in both FC group and TR group, IL-4 expression level reached its highest level at postoperative week 2, then decreased gradually to near normal level at 8 weeks after surgery. More importantly, the IL-4 content of the RC healing site in TR group was significantly higher than that in FC group at 10 days, 2 weeks, 3 weeks, and 4 weeks postoperatively (P < 0.05, post hoc power >0.8). The above results suggested that mechanical stimulation can promote the regeneration of IL-4 at the TBI during the healing process (Fig. 2A).
Figure 2.
Description of the IL-4/JAK/STAT signaling axis activation in enthesis following RC repair surgery (A) IL-4 content (pg) of SST enthesis at different time points after surgery detected by ELISA normalized against protein content (mg). n = 4 per group (B) Representative flow cytometry plots showing the percentages of macrophages expressing P-JAK1 (F4/80+P-JAK1+)、P-JAK3 (F4/80+P-JAK3+) or P-STAT6 (F4/80+P-STAT6+) within enthesis tissue at postoperative week 2 (C) Quantification of flow cytometry data in (B). n = 4 per group. RC, rotator cuff; SST, supraspinatus tendon. ∗P < 0.05 represents significant differences between the indicated columns.
3.2. The activation of JAK/STAT signaling in macrophages within SST enthesis
Flow cytometry is a novel technique that has the potential to monitor routinely the expression of phosphospecific proteins, which may be more sensitive and have more accurate quantitative ability than Western blotting [29]. Throughout the remainder of the results from ELISA, we employed flow cytometry to investigate the effect of mechanical stimulation on the activation of JAK/STAT signaling pathways in macrophages within SST enthesis at 2 weeks after surgery. The results indicated that the percentages of macrophages expressing P-JAK1 (F4/80+P-JAK1+)、P-JAK3 (F4/80+P-JAK3+) and P-STAT6 (F4/80+P-STAT6+) within enthesis tissue were significantly higher in TR group than that in FC group (P < 0.05, post hoc power >0.8). Thus, we confirmed that the JAK/STAT signaling pathway in macrophages within SST enthesis is activated under mechanical stimulation. Meanwhile, the addition of thermoresponsive hydrogel with IL-4 antibody to SST enthesis, from which ∼42% and ∼67% of IL-4 antibody were released at 7 d and 15 d, could suppress the mechanical stimulation induced the activation of JAK/STAT pathway, as evidenced by no significant difference in the proportion of macrophages expressing P-JAK1、P-JAK3 and P-STAT6 was observed between FC with AF-404-SP group and TR with AF-404-SP group (Fig. 2B–C and Figure S2).
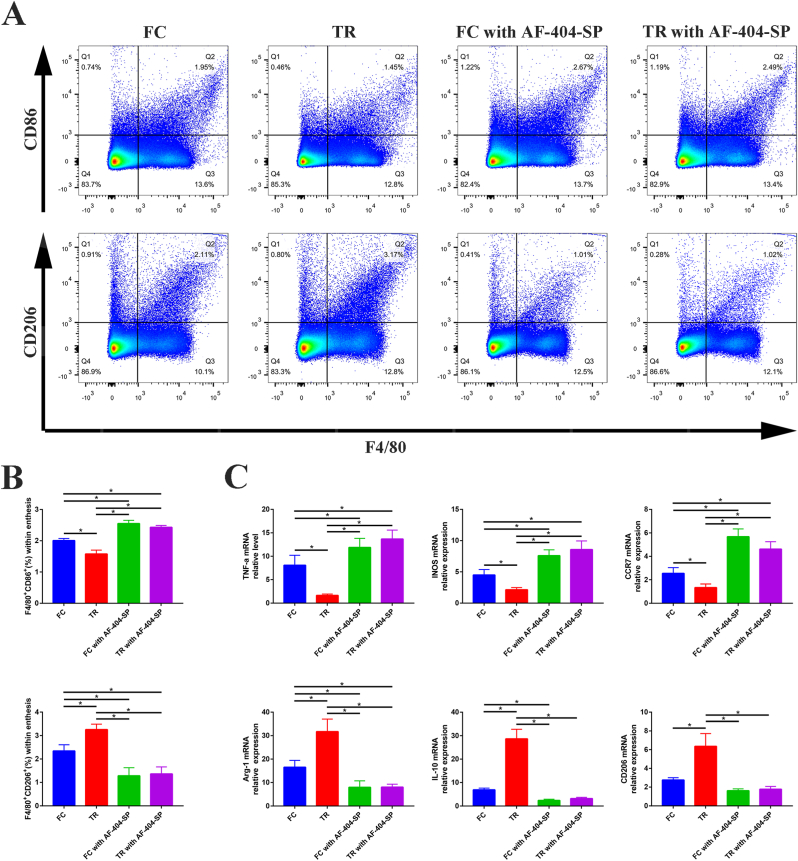
3.3. IL-4/JAK/STAT signaling pathway was involved in mechanical stimulation induced macrophage M2 polarization during tendon-bone healing
At 2 weeks after surgery, analysis of the proportion of M1 and M2 phenotypes using flow cytometry and immunofluorescent staining (Fig. 3 A-B and Figure S3) revealed that the lower M1 macrophages (F4/80 + CD86+) and more M2 macrophages (F4/80 + CD206+) were expressed at the SST insertion site of TR group in comparison with FC group (P < 0.05, post hoc power >0.8). Furthermore, the mRNA expression analysis of the injury sites (Fig. 3 C) also showed that M1-specific biomarkers (TNF-a, iNOS, and CCR7) increased and M2-specific biomarkers (Arg-1, IL-10, and CD206) decreased in TR group compared with FC group (P < 0.05, post hoc power >0.8), which has good coherence with flow cytometry and immunofluorescent staining results we mentioned above. However, there were no significant differences in expression of M1 and M2 macrophages between FC with AF-404-SP group and TR with AF-404-SP group, which revealed that mechanical stimulation induced macrophage M2 polarization was depleted following the blockade of IL-4/JAK/STAT signaling with the addition of IL-4 antagonist.
Figure 3.
Characterization of macrophage phenotypes in SST enthesis of mice at postoperative week 2 (A) Representative flow cytometry plots showing the percentages of M1 (F4/80+CD86+) and M2 (F4/80+CD206+) phenotype within SST enthesis (B) Quantification of flow cytometry data in (A). n = 3 per group (C) Gene expression profiles of M1 macrophage marker (TNF-a, iNOS, and CCR7) and M2 macrophage marker (Arg, IL-10, and CD206) in SST enthesis tissue. n = 4 per group. Expression of interest genes is normalized to β-actin and given as a relative change. SST, supraspinatus tendon. ∗P < 0.05 represents significant differences between the indicated columns.
3.4. IL-4/JAK/STAT signaling pathway was indispensable for mechanical stimulation induced tendon-bone healing in the RC repair model
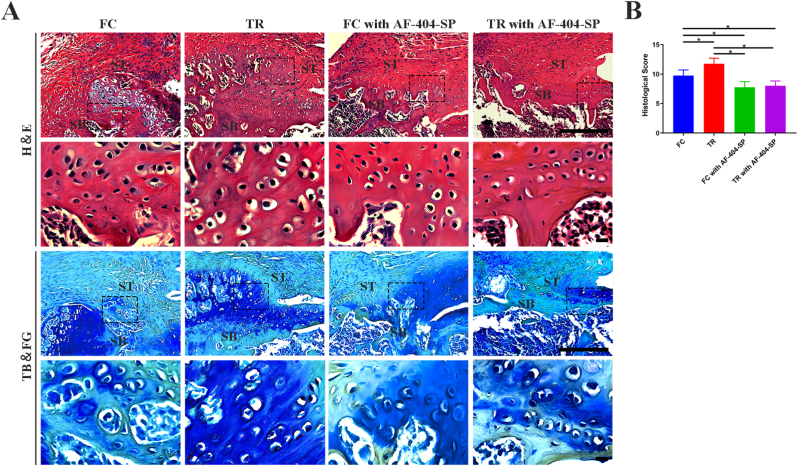
3.4.1. Histological analysis
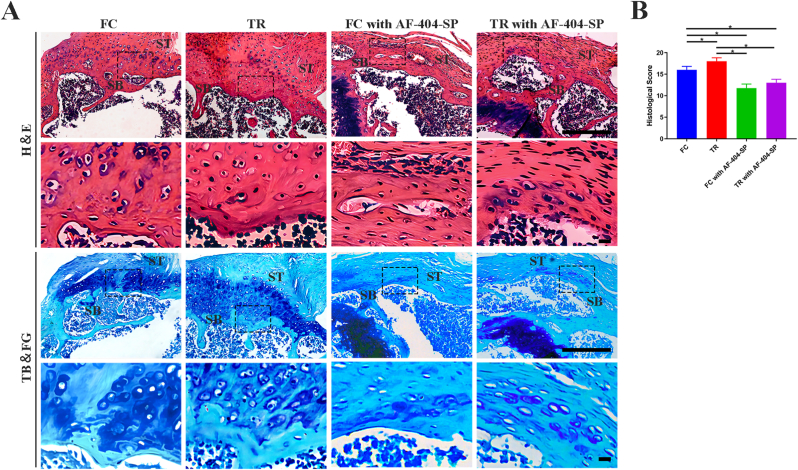
Based on H&E and TB&FG staining images (Figure 4, Figure 5), the supraspinatus tendon inserted new-born bone at the healing interface without gap in all group. In comparision to FC group, TR group showed better fibrocartilage regeneration which was characterized by more cartilage-like cells and richer proteoglycans accumulation at repaired site at postoperative week 4 and 8. Quantitatively, the histological scores for the repaired site in the TR group were lower than that in the FC group at 4 and 8 week after surgery (P < 0.05, post hoc power >0.8). Moreover, maturing scores showed no significant difference between the FC with AF-404-SP group and TR with AF-404-SP group at postoperative weeks 4 and 8. In brief, postoperative mechanical stimulation would improve the morphology of the T-B healing site, which requires the activation of IL-4/JAK/STAT signaling.
Figure 4.
Histological assessment of ST enthesis at 4 weeks postoperatively for all groups (A) Representative images of H&E and TB&FG staning. The area selected by the rectangle dashed line is the local magnified area. Scale bar = 200 μm (B) Results of the modified tendon-to-bone maturity scores in (A). n = 4 per group. SB, subchondral bone; ST, supraspinatus tendon; H&E, hematoxylin and eosin; TB&FG, toluidine blue and fast green; ∗P < 0.05 represents significant differences between the indicated columns.
Figure 5.
Histological assessment of ST enthesis at 8 weeks postoperatively for all groups (A) Representative images of H&E and TB&FG staning. The area selected by the rectangle dashed line is the local magnified area. Scale bar = 200 μm (B) Results of the modified tendon-to-bone maturity scores in (A). n = 4 per group. SB, subchondral bone; ST, supraspinatus tendon; H&E, hematoxylin and eosin; TB&FG, toluidine blue and fast green; ∗P < 0.05 represents significant differences between the indicated columns.
3.4.2. Micro-CT analysis
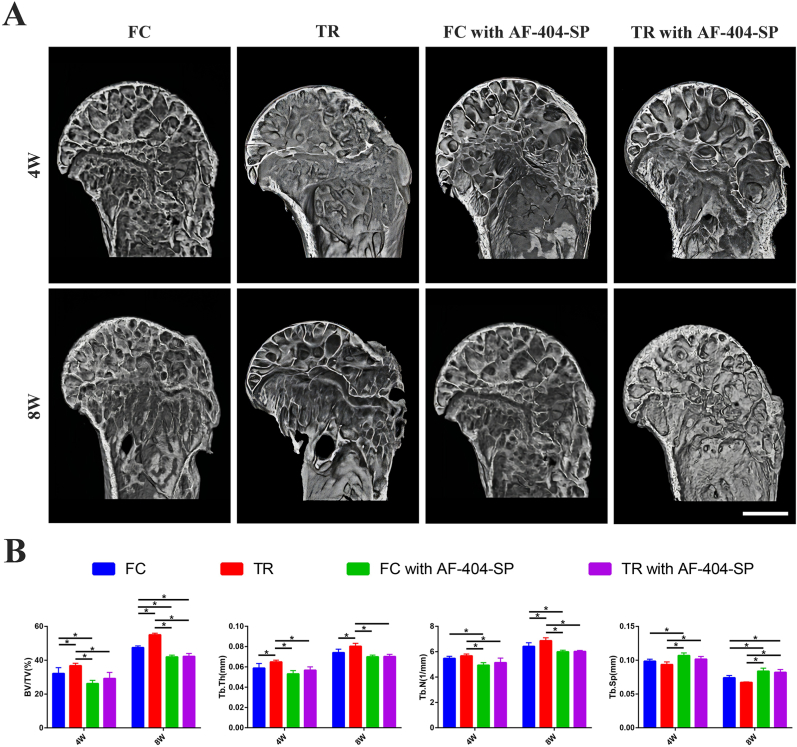
The representative 3D reconstruction images of humeral head by Micro-CT showed local bone volume fraction increased with healing time among all groups. The more regenerated bone at the healing site was observed in TR group compared with FC group at postoperative week 4 and 8. There were no differences in new bone formation between FC with AF-404-SP group and FC with AF-404-SP group under macroscopic view. Quantitatively, at postoperative week 4 and 8, BV/TV and Tb.Th of the disorganized bone in TR group showed a significant improvement over those of FC group (P < 0.05, post hoc power >0.8). At postoperative week 8, Tb.N in TR group was significantly higher than those of FC group (P < 0.05, post hoc power >0.8). Moreover, there was no difference in bone parameters between FC with AF-404-SP group and TR with AF-404-SP group at postoperative weeks 4 and 8. These results indicate that IL-4/JAK/STAT signaling was involved in the mechanical stimulation induced formation of new bone at the tendon-bone healing site (Fig. 6).
Figure 6.
Micro-CT analysis of the RC healing site defects at postoperative week 4 and 8 (A) Representative 3D reconstruction images of humeral head for all groups, Scale bar = 500 μm (B) Quantitative analysis of the bone volume/total volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp) for regenerated bone in (A). n = 4 per group. RC, rotator cuff; SST, supraspinatus tendon. All data are mean ± SD. ∗P < 0.05 represents significant differences between the indicated columns.
3.5. Biomechanical evaluation
To evaluate the ultimate T-B healing quality in the RC repair model, all failures occurred at the SST attachment site. There was no statistical difference in the cross-sectional area among the four groups at 4 and 8 weeks after surgery. At postoperative week 4 and 8, biomechanical testing indicated that failure load and ultimate strength in TR group showed significantly higher than that in FC group (P < 0.05, post hoc power >0.8), meanwhile, no significant difference in failure load and ultimate strength between FC with AF-404-SP group and TR with AF-404-SP group was observed. For Stiffness, there was no significant difference between FC group and TR group or between FC with AF-404-SP group and TR with AF-404-SP group at 4 weeks postoperatively, moreover, there was no significant difference among the four groups at 8 weeks postoperatively. These results suggested that mechanical stimulation would enhance biomechanical properties of the T-B healing site via the IL-4/JAK/STAT signaling pathway (Fig. 7).
Figure 7.
Biomechanical property of the T-B healing site at 4 and 8 weeks after rotator cuff repair surgery, as expressed by failure load, stiffness, ultimate strength, and cross-sectional area. n = 8 per group. ∗P < 0.05 represents significant differences between the indicated columns.
4. Discussion
In this study, we intended to test the hypothesis that mechanical stimulation promotes RC tendon-bone healing via activating the IL-4/JAK/STAT signaling pathway mediated macrophage polarization. Therefore, we firstly demonstrated that mechanical stimulation promoted the expression of IL-4 and activated the JAK/STAT signaling pathway in macrophages at the tendon-bone healing site in the RC repair model. Our work further confirmed that mechanical stimulation loaded on SST enthesis effectively modulates macrophages to polarize towards M2 rather than M1 phenotype, which might interfere with the inflammatory cascades and exert curative effects, thereby accelerating T-B healing. Furthermore, we illustrated that loading-induced M2 macrophage polarization and T-B healing largely depended on the activation of the IL-4/JAK/STAT signaling pathway.
The restoration of the tendon-bone interface to the native structure is essential for the functional recovery of the injured tendon. Numerous therapeutic strategies have been developed to accelerate the T-B healing process, but optimal ones are still being explored [30,31]. Nowadays, many researchers regard loading exercise as an important part of postoperative rehabilitation following a tendon injury, while to what extent the mechanical stimulation would benefit the tendon-bone healing is controversial [6,12,32]. Recent progress confirms that short-term immobilization and delayed application of moderate mechanical loading could improve the healing quality of TBI [26,33], however, premature and excessive loading exercise is detrimental to T-B healing [25,34]. This study shows the same result as the previous one, we illustrate that appropriate mechanical stimulation loaded by treadmill running promotes the T-B healing in the RC repair model, as evidenced by enhanced osteogenesis, chondrogenesis, and biomechanical property. Collectively, mechanical stimulation is indispensable for T-B healing, whereas immobilization remains a crucial treatment after RC surgery in clinic. Balance between immobilization and loading exercise is essential to optimize the healing enthesis and obtain a stronger interface that ensures successful clinical outcome.
It has been suggested that further understanding and regulation of the biological environment during the tendon-bone healing could be beneficial for the regeneration of native enthesis tissues rather than scar formation [35,36]. In the early phase after surgical reconstruction, there was a dominant inflammatory environment in the tendon-bone interface, which regulates the regenerative healing process [2,37]. Macrophages are prominent members of innate immune cells and could mediate both the initiation and resolution of inflammation. Moreover, the previous findings have revealed that macrophage polarization is closely related to T-B healing. Chen et al. confirmed the benefit of conditioned medium from human bone marrow-derived stem cells in RC tendon-bone healing, which relied on its regulation of macrophage polarization [37]. Both Shi et al. and Zhu et al. showed that exosomes derived from mesenchymal stem cells enhance the T-B Healing by promoting macrophage M2 polarization [15,38]. More importantly, macrophages have been proved to be mechanically sensitive and their phenotype can be regulated by mechanical cues [18,39], which will either facilitate or hinder tissue regeneration. Not surprisingly, apart from determining the benefit of mechanical stimulation in T-B healing, our findings also indicated that mechanical stimulation applied to TBI could induce the shift of macrophages from M1 to M2 subtype.
While numerous factors and their downstream signaling pathway have been reported to be closely related to macrophage polarization, M2 macrophage phenotype firstly arose with IL-4 stimuli and now IL-4 is widely used to induce the generation of M2 macrophages to explore their effect on the biological behaviors of other cells and animal model [16,21,40]. Moreover, it is well established that the binding of IL-4 to its receptor activates the JAK/STAT signaling pathway, which explains the mechanism behind regulation by IL-4 on macrophage phenotype [22]. Our result showed that treadmill running significantly up-regulated the level of IL-4 at SST enthesis and promoted the expression of JAK1, JAK3, and STAT6 in macrophages within the T-B healing site. Similarly, the mechanical cue has been also proved to be able to augment the generation of IL-4 by T cells and chondrocytes [19,20]. Furthermore, by using AF-404-SP to neutralize IL-4 at the enthesis, the phosphorylation of JAK1, JAK3, and STAT6 in macrophages, the modulation of macrophage polarization and T-B healing induced by mechanical stimulation were suppressed. This observation indicated that mechanical stimulation regulated macrophages phenotype and T-B healing by activating the IL-4/JAK/STAT signaling pathway.
There are some limitations to this study. Firstly, considering differences in anatomy and healing capacity between mice and human, the proposed signaling pathway should be validated in human tissue samples. Second, the dose of IL-4 neutralizing antibody is used according to the manufacturer's instructions and the result of ELISA. The optimized dose needs more research to be identified. Last but foremost, numerous signaling molecules or physiological changes may be involved in mechanical stimulation induced T-B healing. The identification of the IL-4/JAK/STAT signaling pathway explored in this study is dependent on experience and previously reported paper review, which might cause some bias. Microarray or sequencing assay could be used to disclose the whole underlying mechanisms and targets.
5. Conclusion
In summary, our findings suggest that the positive role of mechanical stimulation in tendon-bone healing is, at least partially, related to IL-4/JAK/STAT mediated macrophage M2 polarization. Targeting the IL-4/JAK/STAT signaling pathway or macrophage polarization may represent a promising therapeutic strategy for tendon-bone healing.
Author contributions
Hongbin Lu and Jianzhong Hu conceived experiments and provided funding assistance. Shengcan Li and Linfeng Wang designed experiments. Yuqian Liu and Linfeng Wang performed experiments and drafted the original manuscript. Deyi Sun assisted in the experiments and data analysis. Tao Zhang and Can Chen checked the manuscripts.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81730068), the Science and Technology Major Project of Changsha (No. kh2102015), and the Fundamental Research Funds for the Central Universities of Central South University (2022ZZTS0855).
Declaration of competing interest
The authors declare that no conflicts of interest exist.
Acknowledgments
The authors thank Professor Hui Xie and other staff from Movement System Injury and Repair Research Center, Xiangya Hospital, Central South University, Changsha, China, for their kind assistance during the experiments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2022.08.008.
Contributor Information
Deyi Sun, Email: sun950092@hotmail.com.
Hongbin Lu, Email: hongbinlu@hotmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cao Y., Yang S., Zhao D., Li Y., Cheong S.S., Han D., et al. Three-dimensional printed multiphasic scaffolds with stratified cell-laden gelatin methacrylate hydrogels for biomimetic tendon-to-bone interface engineering. J Orthop Translat. 2020;23:89–100. doi: 10.1016/j.jot.2020.01.004. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu H., Chen C., Qu J., Chen H., Chen Y., Zheng C., et al. Initiation timing of low-intensity pulsed ultrasound stimulation for tendon-bone healing in a rabbit model. Am J Sports Med. 2016;44(10):2706–2715. doi: 10.1177/0363546516651863. [eng] [DOI] [PubMed] [Google Scholar]
- 3.Ye C., Zhang W., Wang S., Jiang S., Yu Y., Chen E., et al. Icariin promotes tendon-bone healing during repair of rotator cuff tears: a biomechanical and histological study. Int J Mol Sci. 2016;17(11) doi: 10.3390/ijms17111780. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Y., He B., Wang L., Yuan B., Shu H., Zhang F., et al. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res Ther. 2020;11(1):496. doi: 10.1186/s13287-020-02005-x. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedi A., Kovacevic D., Fox A.J., Imhauser C.W., Stasiak M., Packer J., et al. Effect of early and delayed mechanical loading on tendon-to-bone healing after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2010;92(14):2387–2401. doi: 10.2106/JBJS.I.01270. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomopoulos S., Zampiakis E., Das R., Silva M.J., Gelberman R.H. The effect of muscle loading on flexor tendon-to-bone healing in a canine model. J Orthop Res. 2008;26(12):1611–1617. doi: 10.1002/jor.20689. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camp C.L., Lebaschi A., Cong G.T., Album Z., Carballo C., Deng X.H., et al. Timing of postoperative mechanical loading affects healing following anterior cruciate ligament reconstruction: analysis in a murine model. J Bone Joint Surg Am. 2017;99(16):1382–1391. doi: 10.2106/JBJS.17.00133. [eng] [DOI] [PubMed] [Google Scholar]
- 8.Hettrich C.M., Gasinu S., Beamer B.S., Fox A., Ying O., Deng X.H., et al. The effect of immobilization on the native and repaired tendon-to-bone interface. J Bone Joint Surg Am. 2013;95(10):925–930. doi: 10.2106/JBJS.K.01329. [eng] [DOI] [PubMed] [Google Scholar]
- 9.Thomopoulos S., Kim H.M., Rothermich S.Y., Biederstadt C., Das R., Galatz L.M. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. J Orthop Res. 2007;25(9):1154–1163. doi: 10.1002/jor.20418. [eng] [DOI] [PubMed] [Google Scholar]
- 10.Tatara A.M., Lipner J.H., Das R., Kim H.M., Patel N., Ntouvali E., et al. The role of muscle loading on bone (Re)modeling at the developing enthesis. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097375. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz A.G., Lipner J.H., Pasteris J.D., Genin G.M., Thomopoulos S. Muscle loading is necessary for the formation of a functional tendon enthesis. Bone. 2013;55(1):44–51. doi: 10.1016/j.bone.2013.03.010. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galloway M.T., Lalley A.L., Shearn J.T. The role of mechanical loading in tendon development, maintenance, injury, and repair. J Bone Joint Surg Am. 2013;95(17):1620–1628. doi: 10.2106/JBJS.L.01004. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peltz C.D., Dourte L.M., Kuntz A.F., Sarver J.J., Kim S.Y., Williams G.R., et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91(10):2421–2429. doi: 10.2106/JBJS.H.01121. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawamura S., Ying L., Kim H.J., Dynybil C., Rodeo S.A. Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res. 2005;23(6):1425–1432. doi: 10.1016/j.orthres.2005.01.014.1100230627. [eng] [DOI] [PubMed] [Google Scholar]
- 15.Shi Y., Kang X., Wang Y., Bian X., He G., Zhou M., et al. Exosomes derived from bone marrow stromal cells (BMSCs) enhance tendon-bone healing by regulating macrophage polarization. Med Sci Mon Int Med J Exp Clin Res. 2020;26 doi: 10.12659/MSM.923328. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He X.T., Li X., Yin Y., Wu R.X., Xu X.Y., Chen F.M. The effects of conditioned media generated by polarized macrophages on the cellular behaviours of bone marrow mesenchymal stem cells. J Cell Mol Med. 2018;22(2):1302–1315. doi: 10.1111/jcmm.13431. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu J., Chamberlain C.S., Ji M.L., Saether E.E., Leiferman E.M., Li W.J., et al. Tendon-to-Bone healing in a rat extra-articular bone tunnel model: a comparison of fresh autologous bone marrow and bone marrow-derived mesenchymal stem cells. Am J Sports Med. 2019;47(11):2729–2736. doi: 10.1177/0363546519862284. [eng] [DOI] [PubMed] [Google Scholar]
- 18.Dong L., Song Y., Zhang Y., Zhao W., Wang C., Lin H., et al. Mechanical stretch induces osteogenesis through the alternative activation of macrophages. J Cell Physiol. 2021;236(9):6376–6390. doi: 10.1002/jcp.30312. [eng] [DOI] [PubMed] [Google Scholar]
- 19.Wong V.W., Paterno J., Sorkin M., Glotzbach J.P., Levi K., Januszyk M., et al. Mechanical force prolongs acute inflammation via T-cell-dependent pathways during scar formation. Faseb J. 2011;25(12):4498–4510. doi: 10.1096/fj.10-178087. [eng] [DOI] [PubMed] [Google Scholar]
- 20.Salter D.M., Millward-Sadler S.J., Nuki G., Wright M.O. Integrin-interleukin-4 mechanotransduction pathways in human chondrocytes. Clin Orthop Relat Res. 2001;(391 Suppl):S49–S60. doi: 10.1097/00003086-200110001-00006. [eng] [DOI] [PubMed] [Google Scholar]
- 21.He Y., Gao Y., Zhang Q., Zhou G., Cao F., Yao S. IL-4 switches microglia/macrophage M1/M2 polarization and alleviates neurological damage by modulating the JAK1/STAT6 pathway following ICH. Neuroscience. 2020;437:161–171. doi: 10.1016/j.neuroscience.2020.03.008. [eng] [DOI] [PubMed] [Google Scholar]
- 22.Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebaschi A.H., Deng X.H., Camp C.L., Zong J., Cong G.T., Carballo C.B., et al. Biomechanical, histologic, and molecular evaluation of tendon healing in a new murine model of rotator cuff repair. Arthroscopy. 2018;34(4):1173–1183. doi: 10.1016/j.arthro.2017.10.045. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell R., Taub P., Cagle P., Flatow E.L., Andarawis-Puri N. Development of a mouse model of supraspinatus tendon insertion site healing. J Orthop Res. 2015;33(1):25–32. doi: 10.1002/jor.22727. [eng] [DOI] [PubMed] [Google Scholar]
- 25.Chen Y., Zhang T., Wan L., Wang Z., Li S., Hu J., et al. Early treadmill running delays rotator cuff healing via Neuropeptide Y mediated inactivation of the Wnt/β-catenin signaling. J Orthop Translat. 2021;30:103–111. doi: 10.1016/j.jot.2021.08.004. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu H., Li S., Zhang T., Wang Z., Chen C., Chen H., et al. Treadmill running initiation times and bone-tendon interface repair in a murine rotator cuff repair model. J Orthop Res. 2021;39(9):2017–2027. doi: 10.1002/jor.24863. [eng] [DOI] [PubMed] [Google Scholar]
- 27.Xiao H., Zhang T., Li C., Cao Y., Wang L., Chen H., et al. Mechanical stimulation promotes enthesis injury repair by mobilizing Prrx1(+) cells via ciliary TGF-β signaling. Elife. 2022;11 doi: 10.7554/eLife.73614. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang T., Chen Y., Chen C., Li S., Xiao H., Wang L., et al. Treadmill exercise facilitated rotator cuff healing is coupled with regulating periphery neuropeptides expression in a murine model. J Orthop Res. 2021;39(3):680–692. doi: 10.1002/jor.24678. [eng] [DOI] [PubMed] [Google Scholar]
- 29.Vafadari R., Weimar W., Baan C.C. Phosphospecific flow cytometry for pharmacodynamic drug monitoring: analysis of the JAK-STAT signaling pathway. Clin Chim Acta. 2012;413(17–18):1398–1405. doi: 10.1016/j.cca.2011.12.023. [eng] [DOI] [PubMed] [Google Scholar]
- 30.Atesok K., Fu F.H., Wolf M.R., Ochi M., Jazrawi L.M., Doral M.N., et al. Augmentation of tendon-to-bone healing. J Bone Joint Surg Am. 2014;96(6):513–521. doi: 10.2106/JBJS.M.00009. [eng] [DOI] [PubMed] [Google Scholar]
- 31.Zhu C., Qiu J., Thomopoulos S., Xia Y. Augmenting tendon-to-bone repair with functionally graded scaffolds. Adv Healthc Mater. 2021;10(9) doi: 10.1002/adhm.202002269. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Packer J.D., Bedi A., Fox A.J., Gasinu S., Imhauser C.W., Stasiak M., et al. Effect of immediate and delayed high-strain loading on tendon-to-bone healing after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2014;96(9):770–777. doi: 10.2106/JBJS.L.01354. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa Y., Lebaschi A.H., Wada S., Green S.J.E., Wang D., Album Z.M., et al. Duration of postoperative immobilization affects MMP activity at the healing graft-bone interface: evaluation in a mouse ACL reconstruction model. J Orthop Res. 2019;37(2):325–334. doi: 10.1002/jor.24177. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wada S., Lebaschi A.H., Nakagawa Y., Carballo C.B., Uppstrom T.J., Cong G.T., et al. Postoperative tendon loading with treadmill running delays tendon-to-bone healing: immunohistochemical evaluation in a murine rotator cuff repair model. J Orthop Res. 2019;37(7):1628–1637. doi: 10.1002/jor.24300. [eng] [DOI] [PubMed] [Google Scholar]
- 35.Huegel J., Williams A.A., Soslowsky L.J. Rotator cuff biology and biomechanics: a review of normal and pathological conditions. Curr Rheumatol Rep. 2015;17(1):476. doi: 10.1007/s11926-014-0476-x. [eng] [DOI] [PubMed] [Google Scholar]
- 36.Lorbach O., Baums M.H., Kostuj T., Pauly S., Scheibel M., Carr A., et al. Advances in biology and mechanics of rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2015;23(2):530–541. doi: 10.1007/s00167-014-3487-2. [eng] [DOI] [PubMed] [Google Scholar]
- 37.Chen W., Sun Y., Gu X., Cai J., Liu X., Zhang X., et al. Conditioned medium of human bone marrow-derived stem cells promotes tendon-bone healing of the rotator cuff in a rat model. Biomaterials. 2021;271 doi: 10.1016/j.biomaterials.2021.120714. [eng] [DOI] [PubMed] [Google Scholar]
- 38.Zhu D., Johnson T.K., Wang Y., Thomas M., Huynh K., Yang Q., et al. Macrophage M2 polarization induced by exosomes from adipose-derived stem cells contributes to the exosomal proangiogenic effect on mouse ischemic hindlimb. Stem Cell Res Ther. 2020;11(1):162. doi: 10.1186/s13287-020-01669-9. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ballotta V., Driessen-Mol A., Bouten C.V., Baaijens F.P. Strain-dependent modulation of macrophage polarization within scaffolds. Biomaterials. 2014;35(18):4919–4928. doi: 10.1016/j.biomaterials.2014.03.002. [eng] [DOI] [PubMed] [Google Scholar]
- 40.Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.