Abstract
Background
After anterior cruciate ligament (ACL) reconstruction in clinic, firm and rapid integration of the grafted tendon into the bone tunnel remains a challenge. Exosomes from hypoxia-treated stem cells are beneficial for promoting angiogenesis and then coupling with osteogenesis. Therefore, exosomes from hypoxia-cultured bone-marrow mesenchymal stem cells (Hypo-Exos) may be a cell-free therapy for enhancing graft-bone incorporation after ACL reconstruction.
Methods
Exosomes from normoxia-cultured bone-marrow mesenchymal stem cells (Norm-Exos) or Hypo-Exos were respectively cultured with human umbilical vein endothelial cells (HUVECs) for in-vitro evaluating their functions in HUVECs proliferation, migration, and tube formation. A total of 87 rats with single-bundle ACL reconstructions in the right knee were randomly allocated into 3 different treatments: phosphate-buffered saline (PBS) with the adhesive hydrogel injection as control (Ctrl), Norm-Exos with the adhesive hydrogel injection (Norm-Exos), and Hypo-Exos with the adhesive hydrogel injection (Hypo-Exos). At postoperative weeks 2, 4, or 8, the ACL graft-bone integrations were evaluated.
Results
Hypo-Exos was a better stimulator for in-vitro HUVECs proliferation, migration, and tube formation compared to PBS or Norm-Exos. Hypo-Exos within the adhesive hydrogel could be sustained-released at least 14 days around the peri-graft site. Radiologically, at week 4 or 8, femoral or tibial bone tunnel areas (BTA), as well as bone volume/total volume ratio (BV/TV) of the femoral or tibial peri-graft bone in the Hypo-Exos group, improved significantly better than these parameters of the Ctrl and Norm-Exos groups (P<0.05 for all). Histologically, the grafted tendon-bone interface in the Hypo-Exos group showed significantly higher histologic scores at week 4 or 8 as compared with the other groups (P<0.05 for all). Immunofluorescent staining verified that type H vessels were more abundant in the Hypo-Exos group when compared to the Ctrl or Norm-Exos group at week 2. Biomechanically, the Hypo-Exos group exhibited a significantly heightened failure load compared with the Ctrl and Norm-Exos groups (P<0.05 for all) at 8 weeks. Meanwhile, the stiffness in the Hypo-Exos group was the greatest among the three groups.
Conclusion
Peri-graft Hypo-Exos injection accelerates grafted tendon-bone tunnel integration after ACL reconstruction by improving peri-graft bone microarchitecture.
Keywords: Anterior cruciate ligament reconstruction, Graft-bone integration, Exosomes, Hypoxia, Cell-free therapy
The translational potential of this article
Grafted tendon firmly and rapidly integrating with bone tunnel remains a challenge after ACL reconstruction in clinic. Exosomes from hypoxia-treated stem cells are beneficial for promoting osteogenesis and angiogenesis. The findings of this study demonstrate that Hypo-Exos may be developed as a promising cell-free approach to accelerate grafted tendon-bone integration after ACL reconstruction.
1. Introduction
Anterior cruciate ligament (ACL) plays a critical role in maintaining the normal motion and stability of the knee joint [[1], [2], [3]]. Regrettably, its rupture is one of the most common knee injuries encountered in sports medicine [[1], [2], [3]]. Due to the limited capacity to regenerate, surgical reconstruction of the ACL with an autologous tendon graft in the bone tunnel is frequently performed to restore knee integrity and stability [2,4]. After surgical ACL reconstruction, most patients showed a satisfactory outcome, while up to 25% of patients required surgical revision [5]. This high failure rate may partly be attributed to traumatic, technical, bacterial, and biological factors. More importantly, bone loss happened around the peri-graft bone directly influences the firm integration of grafted tendon to bone tunnel, thus leading to a high failure rate. Hence, reducing peri-graft bone loss could be selected as a treatment target for promoting the grafted tendon-bone tunnel healing, thus decreasing the failure rate of ACL reconstruction.
Peri-graft bone formation is closely related to the quality of grafted tendon to bone tunnel healing [6,7], which is inseparable from the angiogenesis at the early stage of post-operation. The ingrowth of blood vessels at the interface between grafted tendon and bone tunnel provides sufficient oxygen and nutrients to the cells, and eventually affects peri-graft bone formation [[8], [9], [10]]. Recently, a specific vessel subtype in the bone tissues was discovered and named type H vessels, which highly express endothelial markers (CD31 and Emcn) [11]. These special type H vessels create a special microenvironment for improving osteoprogenitors differentiating into osteoblasts, thus enhancing bone formation [11,12]. Until now, various mesenchymal stem cells (MSCs)-based therapies were developed for enhancing bone formation via the promotion of angiogenesis and osteogenesis [13,14]. However, there remain limitations and challenges that cannot be ignored, such as immune rejection, cell dedifferentiation, tumor formation, and immense care of MSCs outside the cell incubator [[15], [16], [17]]. Interestingly, an increasing number of studies indicated that MSC-secreted exosomes (MSC-Exos) can exert biological activities similar to those of MSCs [[18], [19], [20], [21]]. Exosomes (Exos) are nanosized membrane vesicles with 30–200 nm in diameter and carry a large variety of cargo molecules (proteins, lipids, and RNAs) either on their surface or within their lumen for intracellular communication [[22], [23], [24], [25], [26]]. In comparison with MSC-based therapies, MSC-Exos are more stable and storable, with a lower possibility of immune rejection [27]. Therefore, MSC-Exos-based therapies could potentially be a safer and more convenient way for peri-graft bone formation than MSC-based therapies.
Oxygen concentration has been considered a vital factor in regulating the proliferation, differentiation, and self-renewal of MSCs [28]. When MSCs are cultured in-vitro, they are in a condition of normoxia (21% O2), which is quite different from the MSCs in a hypoxic environment (2-8% O2 or even lower) in the body under natural physiological conditions [29]. A recent study isolated exosomes from MSCs under hypoxia (Hypo-Exos) similar to peripheral arterial disease (1% O2), and found that this type of exosomes is beneficial for bone formation by transferring exosomal pro-angiogenic substances [19]. Similarly, another study, using an infarcted heart model, determined that Hypo-Exos are superior to the exosomes isolated from MSCs under normoxia (Norm-Exos) for myocardial repair, which functioned by increasing vascularization of the infarcted heart tissue, reducing the apoptosis rates of cardiomyocytes as well as stimulating recruitment of cardiac progenitor cells [30]. These studies imply that the Hypo-Exos may be a stimulative substance for increasing vascularization at the peri-graft healing site.
Considering that adhesive hydrogel exhibited satisfactory retention and sustained delivery of exosomes [31,32], in this study, we established a rat ACL reconstruction model and injected Hypo-Exos into the peri-graft healing site with this adhesive hydrogel. We hypothesized that the local injected Hypo-Exos could stimulate type H vessel formation at the peri-graft site, thus promoting peri-graft bone formation, consequently enhancing the grafted tendon firmly integrated on the bone tunnel in this rat model.
2. Materials and methods
2.1. Ethics statement
The animal ethics committee of our university authorized the experimental protocol for the use of male mature SD rats in this study. According to the results of the previous study and our preliminary research [33], the number of rats required for postoperative evaluations was determined by the power analysis.
2.2. Rat BMSCs isolation
Rat bone marrow-derived MSCs (BMSCs) were isolated from adult male Sprague–Dawley rats according to the published literature [34]. Briefly, the femur bones and humerus bones were harvested from the rats immediately after sacrifice, and then the attaching muscles and residue tissues were removed aseptically. After that, the femur bone and humerus bone were cut at both ends with sharp sterile scissors, and the bone marrows were washed out with phosphate-buffered saline (PBS). The collected bone marrows were cultured with a growth medium composed of Dulbecco's modified Eagle medium (DMEM) supplemented with 20% fetal bovine serum (FBS), 100 U/mL penicillin (Gibco, USA), and 100 mg/mL streptomycin (Gibco, USA) in an incubator at 37 °C with 5% CO2. After 24 h of culture, nonadherent cells were removed carefully and a fresh medium was replaced. At approximately 90% confluency, cells were washed 3 times with PBS and then passaged. The isolated BMSCs in passage 2 were used for the following experiments.
2.3. Exosome isolation and identification
At approximately 80–90% confluency, passage 2 BMSCs were cultured in a T25 flask at 37 °C, 5% CO2, 21% O2 or 1% O2, 94% N2, 5% CO2 in exosome-depleted FBS (C38010050, Shanghai XP Biomed, China) containing media. After 3-day incubation, culture supernatants were collected for isolating exosomes using the published literature [24]. The procedure of isolating the Norm-Exos or Hypo-Exos based on differential ultracentrifugation is shown in Fig. 1. Briefly, the culture supernatant was centrifuged to remove cell debris at 4 °C with the following parameters (300g for 10 min, 2000g for 10 min, and then 10,000g for 30 min). Then, the supernatant was ultracentrifuged at 100,000g for 70 min. Exosomes accumulated on the bottom of the centrifuge tube were resuspended in PBS and centrifuged again at 100,000g for 70 min to eliminate contaminating proteins.
Fig. 1.
Schematic diagram showing the MSC-Exos purification procedure based on differential ultracentrifugation.
The morphology of Norm-Exos or Hypo-Exos was imaged by transmission electron microscopy (TEM) (HT7800, Hitachi, JPN). The size distribution and particle concentration were evaluated with a qNano platform (Flow NanoAnalyzer, NanoFCM, CHN). Western blotting was also performed on isolated Norm-Exos or Hypo-Exos to analyze the expression of CD63, CD81, and TSG101, which are common markers of exosomes.
2.4. Exosome uptake by human umbilical vein endothelial cells (HUVECs)
Human umbilical vein endothelial cells (HUVECs) were commercially purchased from ScienCell Research Laboratories (San Diego, CA, USA). PKH67 solution (5ul/mL, Sigma–Aldrich, USA) was incubated with the isolated Norm-Exos or Hypo-Exos. The excessive fluorescent dye was removed by ultracentrifugation at 100000g for 1 h at 4 °C, and the labeled exosomes were washed three times in PBS. After resuspension in PBS, these PKH67-labeled Norm-Exos or Hypo-Exos were co-cultured with HUVECs for 24 h. The uptake of PKH67-labeled Norm-Exos or Hypo-Exos by HUVECs was observed by fluorescent microscopy (ApoTome 2, Carl Zeiss, Germany).
2.5. Exosomes on HUVECs proliferation, migration, tube formation
HUVECs were seeded at a density of 2000 cells/well in a 96-well plate, and co-cultured with PBS, Norm-Exos, or Hypo-Exos at a concentration of 100 μg/mL 10 μL of CCK8 solution in a fresh culture medium was added every 24 h and incubated for 2 h at 37 °C. The optical density (OD) value at 450 nm wavelength was determined using a microplate reader (Varioskan LUX, Thermo, USA).
Transwell migration was applied to evaluate the effect of Norm-Exos or Hypo-Exos on HUVEC migration. 5 × 104 of HUVECs were seeded into the upper chamber of the transwell chamber (Corning, NY, USA; pore size 8 μm). PBS as well as Norm-Exos, and Hypo-Exos (100 μg/mL) were supplemented into the lower chamber for a 24 h culture. After gently wiping the HUVECs that did not migrate across the filter membrane, these HUVECs that migrated to the other side of the filter membrane were stained with the crystal violet solution. The mean numbers of migrated cells from five fields were counted for comparison.
To evaluate angiogenesis induced by Norm-Exos or Hypo-Exos, HUVECs were seeded at a density of 2 × 104 cells/well on Matrigel-coated 96-well plates (Matrigel, BD biosciences, CA, USA). PBS as well as Norm-Exos, and Hypo-Exos (100 μg/mL) was supplemented into the culture medium, and then the HUVECs were cultured for 30 min at 37 °C. The capillary-like structures formed by HUVECs were observed with an optical microscope after 6 h of cell seeding. The tube-forming ability was evaluated by measuring the total tube length from randomly selected five fields per well.
2.6. Exosomes loaded by an adhesive hydrogel
The preparation of adhesive hydrogel, as well as the exosomes loaded within the adhesive hydrogel, were performed in a previous study [31]. In brief, the hydrogel was composed of a specific ratio of GelMA, HA-NB, and photo-initiator (LAP). When the hydrogel solution was irradiated with a UV lamp of wavelength 365–405 nm at 37 °C for the 30s, the hydrogel could be transformed into a gel state. For the loading of exosomes with the adhesive hydrogel, Norm-Exos or Hypo-Exos suspension (100 μg/μl) were collected and then added into the hydrogel solution with a final concentration of 10 mg/ml. Then, 300 μL of the composites was received irradiation by a UV lamp to produce the Norm-Exos or Hypo-Exos loaded by the adhesive hydrogel.
2.7. Surgical technique and exosomes with the adhesive hydrogel injection
A total of 87 male Sprague–Dawley rats (12 weeks old) underwent right ACL resection following isometric ACL reconstruction by 2 authors (S.Y., Y.X.) according to an established model using an ipsilateral flexor digitorum longus tendon autograft [33]. Briefly, after the rats were anesthetized, an incision was longitudinally made on the medial aspect of the distal leg and ankle to expose the flexor digitorum longus tendon. The flexor digitorum longus tendon was harvested with an average length of 20 mm. After that, a second incision was made over the knee to excise the native ACL. Using a gauge needle with an outer diameter of 1.3 mm, bone tunnels were drilled beginning at the intra-articular portion of the proximal tibia and the distal femur corresponding to the attachment sites of the ACL. After that, the harvested flexor digitorum longus tendon was passed through the bone tunnels to reconstruct the ACL. Both ends of the graft were secured to the surrounding periosteum using 5-0 Ethibond sutures at the bone tunnel exit sites of the distal femur and proximal tibia. For the rats set as a control group (Ctrl), 300 μL of PBS with the adhesive hydrogel was uniformly injected into the tibia or femur bone tunnels at 12, 4, and 8 o'clock directions as the method shown in Fig. 2A. Meanwhile, the rats in the Norm-Exos or Hypo-Exos group received a uniform injection of Norm-Exos or Hypo-Exos with the adhesive hydrogel (300 μL, 10 mg/ml). After this, the wounds were closed and the animals were allowed free activity postoperatively.
Fig. 2.
(A) Schematic representation of rat ACL reconstruction using autogenous ipsilateral flexor digitorum longus tendon and Exos injected into peri-graft site with adhesive hydrogel. The exosomes with the adhesive hydrogel injection were injected into the peri-graft site at the positions of 12, 4, and 8 o'clock direction of the bone tunnel. (B) Experimental flowchart showing the in-vivo experiments used for evaluating the efficacy of PBS, Norm-Exos, or Hypo-Exos on grafted tendon-bone incorporation after ACL reconstruction, from which the groups, time points for sacrifice, number of animals per group, and outcome evaluations were presented.
2.8. The tracking assay of Norm-Exos or Hypo-Exos in vivo
For the tracking assay of the healing site in vivo, Norm-Exos or Hypo-Exos were labeled with 5 μM fluorescent lipophilic dye DiR (40757ES25, Yeasen, Shanghai) and embedded in the adhesive hydrogel with a volume of 300 μL and a concentration of 10 mg/mL. Subsequently, for Norm-Exos or Hypo-Exos group, an equal amount of the DiR-labeled exosome with the adhesive hydrogel was injected into the injury site around the bone tunnels at the positions of 12, 4, and 8 o'clock directions. The control group was given an equal volume of PBS hydrogel. At 1 day, 7 days, and 14 days after surgery, a non-invasive tracking system (IVIS Spectrum, PerkinElmer, USA) was used to image the DiR intensity and distribution on the knee to partially detect the degradation of pre-loaded DiR-labeled exosomes.
As shown in Fig. 2B, four knees in each of the 3 groups were harvested at postoperative week 2 for observing the number of blood vessels at the newly-formed peri-graft bone with double immunofluorescent staining; Seven knees in each of the 3 groups were used for Micro-CT and then histological analyses at postoperative week 4 or 8; Eight knees in each of the 3 groups were used for biomechanical testing at postoperative week 8.
2.9. Evaluating angiogenesis at the peri-graft bone
The blood supply around the grafted tendon is extremely important for peri-graft bone formation, which directly affects the integration quality of the graft tendon to the bone tunnel [8,9]. To investigate the effect of Norm-Exos or Hypo-Exos on angiogenesis in-vivo, PBS, Norm-Exos, or Hypo-Exos were respectively injected into the peri-graft site after ACL reconstruction to observe their effect on angiogenesis at the newly-formed peri-graft bone. Recently, CD31 and Emcn have been reported as a marker for H-type vessels, which are closely coupled with bone formation [35]. Thus, the H-type vessels in the newly-formed peri-graft bone were evaluated with immunofluorescent staining. Briefly, at postoperative week 2, the harvested knee specimens were fixed, decalcified, sliced, and then stained with anti-CD31 (ab281583, Abcam) and anti-Endomucin (Emcn) (PA5-115178, Invitrogen) overnight at 4 °C. After that, the slices were incubated with Alexa Fluor 488- and Alexa Flour 594-conjugated secondary antibodies (Abcam) for 1 h at room temperature. After triple washing with PBS, nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI, Thermo Fisher Scientific), and fluorescent images were captured using a fluorescence microscope (ApoTome 2, Carl Zeiss, Germany).
2.10. Micro-CT evaluation for peri-graft bone
For these specimens allocated to micro-CT evaluation, they were fixed in 4% paraformaldehyde, and then scanned with a high-resolution micro-CT scanner (VivaCT 40; Scanco Medical AG) with the following parameters: tube voltage, 70 kV; tube current, 114 μA; integration time, 500 ms; the number of slices, 510 (with an isotropic voxel size of 11.2 μm). The micro-CT images selected at an approximate depth of 1.5 mm from the femoral and tibial joint surface were imported into Image-Pro Plus software (Version 6.0.0; Media Cybernetics Inc) to measure the femoral and tibial bone tunnel areas (BTA). After 3-dimensional reconstructed images were obtained, a region of interest (ROI) with cylinder size (diameter = 2.00 mm, height = 4 mm) parallel to the bone tunnel was selected, which contains the newly-formed peri-graft bone in the proximal tibia and the distal femur. The bone volume/total volume (BV/TV) and trabecular thickness (Tb.Th) of the ROI were measured for comparison.
2.11. Histological evaluation for grafted tendon-bone tunnel integration
After micro-CT scanning, the samples were decalcified, embedded within paraffin, and sectioned parallel to the bone tunnels in the femur and tibia with 5-μm thickness. The slides were stained with Hematoxylin and eosin (H&E), Masson's trichrome (MT), or Safranin O/fast green (SO/FG). Histologic evaluation of the grafted tendon-bone tunnel integration was performed under light microscopy by 2 blinded observers (S.Y. and X.S.), and scored using a scoring system from published literature (Table 1) [36].
Table 1.
Scoring system for histological evaluation.
| Characteristic | Score |
|---|---|
| Fibrocartilage formation | |
|
3 |
|
2 |
|
1 |
|
0 |
| New bone formation | |
|
3 |
|
2 |
|
1 |
|
0 |
| Tendon graft integration to bone tunnel | |
|
3 |
|
2 |
|
1 |
|
0 |
| Perfect score | 9 |
2.12. Biomechanical testing
The femur-graft-tibia complex (FGTC) specimens were used for biomechanical testing by a mechanical testing machine (MTS Insight; MTS Systems Corp) immediately after harvest. After the femur and tibia were fixed by the testing apparatus, a tensile load was applied in a direction along the longitudinal axis of the ACL graft. Then, the FGTC specimens were preloaded statically by 0.5 N for 1 min, and then pulled at a crosshead speed of 5 mm/min until gross failure of the ACL occurred. The modes of failure were recorded, and the failure load (N) was obtained from the recorded load–displacement curve. Meanwhile, the stiffness (N/mm), as indicated by the slope of the curve's linear region before ACL failure, was calculated. All tests were performed at room temperature, and 0.9% saline was dropped to the specimens for avoiding dehydration.
2.13. Statistical analysis
All of our results were expressed as mean ± standard deviation (SD). Differences between 2 groups were evaluated using unpaired t-tests, while differences above 2 groups were evaluated using a one-way analysis of variance with a post hoc test. Specifically, the histological scores among the Ctrl, Norm-Exos, and Hypo-Exos groups were performed using the Kruskal–Wallis test. Fisher's exact test was used to test for differences in specimen failure mode. All statistical analyses were performed with SPSS software (version 25.0; SPSS Inc), and P <0.05 was considered significant. “∗” means P < 0.05, “∗∗” means P < 0.01, “∗∗∗” means P < 0.001.
3. Results
3.1. Identification and characterization of Norm-Exos and Hypo-Exos
To identify Norm-Exos and Hypo-Exos, TEM images showed that the isolated Norm-Exos and Hypo-Exos presented a characteristic cup-shaped morphology similar to those described by others [9,37], and had a size distribution from 50 to 150 nm. Western blotting was used to identify the exosome-specific phenotypic markers (CD63, CD81 and TSG101). As shown in Fig. 3A, the presence of exosome-specific phenotypic markers was consistent with previous literature [9,37].
Fig. 3.
(A) Identification and characterization of Norm-Exos and Hypo-Exos by observing their morphology, measuring particle size distribution, and evaluating the expression of exosomal markers. Bar = 100 nm. (B) Uptake of the green fluorescence dye PKH67-labelled Norm-Exos or Hypo-Exos into HUVECs. (C) Hypo-Exos significantly promoted the proliferation of HUVECs (n = 4). (D) Hypo-Exos significantly promoted the migration of HUVECs, as determined by the transwell assay (n = 4); Bar = 150 μm. (E) Hypo-Exos significantly enhanced the tube formation ability of HUVECs, as determined by the tube formation assay (n = 4); Bar = 150 μm. Data are presented as mean ± SD. “∗” means P<0.05, “∗∗” means P<0.01, and “∗∗∗” means P<0.001.
3.2. Hypo-Exos promoted HUVEC proliferation, migration, and angiogenic tube formation in-vitro
For the investigation of Hypo-Exos on angiogenesis, the culture medium of HUVECs was added with PBS, Norm-Exos or Hypo-Exos (100 μg/μL) to observe the difference in HUVECs proliferation, migration, and angiogenic tube formation. As shown in Fig. 3B, both Norm-Exos and Hypo-Exos can be internalized by HUVECs. CCK-8 assay results showed that both Norm-Exos and Hypo-Exos significantly promoted the proliferation of the HUVECs when compared with the PBS. However, Hypo-Exos presented better efficacy than the Norm-Exos in stimulating HUVECs proliferation (Fig. 3C). Transwell experiments (Fig. 3D) showed that both Norm-Exos and Hypo-Exos contributed to the migration of HUVECs when compared to the PBS. However, Hypo-Exos could greatly enhance the migration capability of HUVECs as compared with Norm-Exos alone. As shown in Fig. 3E, both Norm-Exos and Hypo-Exos could significantly enhance the tube formation of HUVECs at 6 h in comparison with the PBS. Furthermore, Hypo-Exos exhibited a significantly better tube formation capability when compared to the Norm-Exos.
3.3. The adhesive hydrogel sustained the release of Hypo-Exos in-vivo
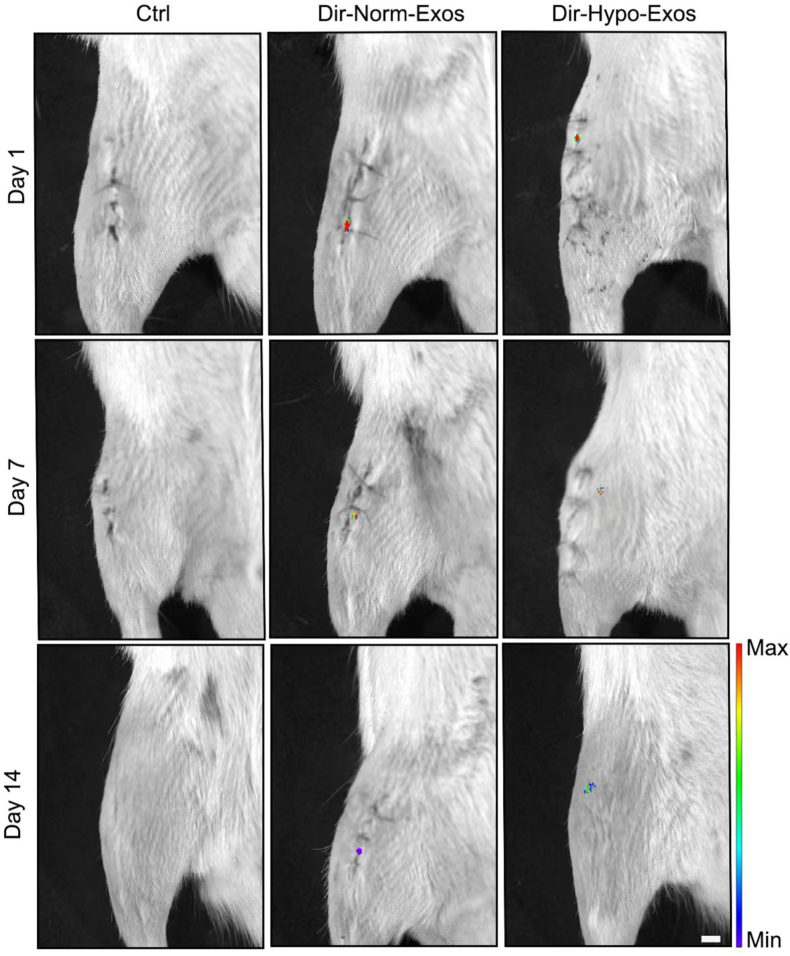
As shown in Fig. 4, Norm-Exos or Hypo-Exos hydrogel labeled with DiR was used for local injection around the bone tunnel in the operation, and the Spatio-temporal distribution of Norm-Exos or Hypo-Exos was captured by the IVIS Imaging System. The red fluorescence signals were observed at the injured site and lasted until 14 days compared with the control group, indicating a sustained release property of Norm-Exos or Hypo-Exos loaded by the adhesive hydrogel.
Fig. 4.
In-vivo tracking of the peri-graft site injected PBS, DiR-labeled Norm-Exos, or Hypo-Exos exosomes with the adhesive hydrogel. Bar = 5 mm.
3.4. Hypo-Exos promoted angiogenesis at the newly-formed peri-graft bone in-vivo
As shown in Fig. 5, immunofluorescent staining showed that Norm-Exos or Hypo-Exos promoted more CD31+/Emcn+ blood vessels formation at the newly-formed peri-graft bone as compared with the PBS. Furthermore, Hypo-Exos on stimulating the formation of CD31+/Emcn+ blood vessels were more effective than the Norm-Exos. This result confirmed that Hypo-Exos was effective for promoting the formation of H-type vessels around the grafted tendon after ACL reconstruction in rats.
Fig. 5.
Representative immunostaining images of H-type vessels (CD31+/Emcn+) in the newly-formed peri-graft bone on postoperative week 2; Bar = 200 μm.
3.5. Efficacy of Hypo-Exos on accelerating grafted tendon-bone integration after ACL reconstruction
Peri-graft bone formation and remodeling: As shown in Fig. 6, the peri-graft bone in the Hypo-Exos group presented significantly smaller femoral and tibial BTA in comparison to the Ctrl or Norm-Exos group at postoperative 4 weeks. The BV/TV and Tb.Th of the peri-graft bone in the Norm-Exos or Hypo-Exos group were significantly higher than these parameters of the Ctrl group. Especially, the Hypo-Exos group showed a significantly higher BV/TV value than the Norm-Exos groups. Similarly, the cross-sectional areas of femoral and tibial bone tunnels in the Hypo-Exos group were also significantly smaller than that of the Ctrl or Norm-Exos group at postoperative 8 weeks. As for the newly-formed peri-graft bone at the femur tunnels, the Hypo-Exos group or the Norm-Exos group showed a significantly higher BV/TV value than the Ctrl group, meanwhile, the BV/TV value of the Hypo-Exos group was significantly larger than that of the Norm-Exos group. As for the newly-formed peri-graft bone at the tibial tunnel, only the Hypo-Exos group showed a significant improvement in BV/TV value than the Ctrl group.
Fig. 6.
(A) Representative micro-computed tomography images of the distal femur and proximal tibia in the Ctrl, Norm-Exos, and Hypo-Exos groups at postoperative 4 weeks and 8 weeks (n = 7); The cross-sectional areas of the bone tunnels at a depth of 1.5 mm from the femur or tibial joint surface were measured; Bar = 1.5 mm. (B) Comparison of the femur or tibia bone tunnel areas, as well as the BV/TV and Tb·Th in the newly-formed peri-graft bone at the femur and tibia bone tunnels among the 3 groups at different time points; Data, are presented as mean ± SD. “∗” means P<0.05, “∗∗” means P<0.01, and “∗∗∗” means P<0.001.
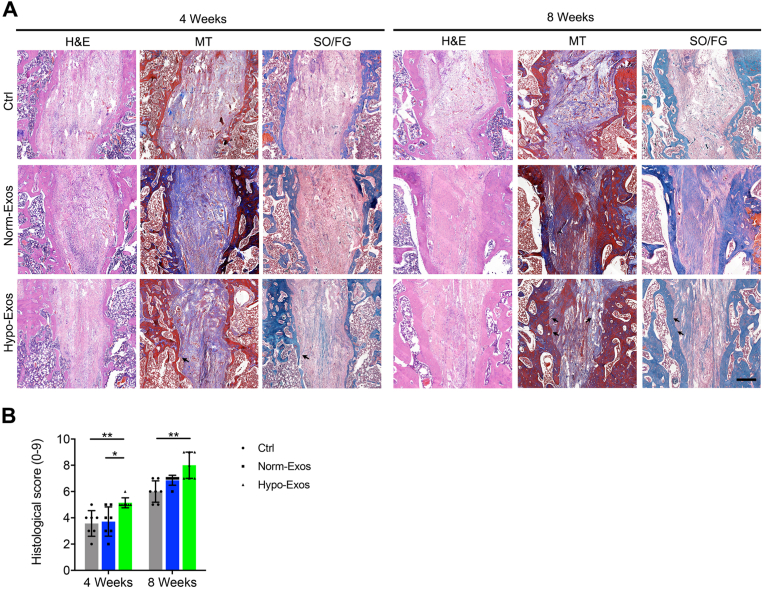
Histological evidence of graft-bone integration: Considering that no exosomes were injected into the grafted tendon of the intra-articular part, histological analysis was only conducted at the graft in the bone tunnel. At postoperative week 4 (Fig. 7A), better graft-bone integration was observed in the Hypo-Exos group, compared with that in the Ctrl or Norm-Exos group, as characterized by more chondrocyte-like cells embedded in Sharpey fibers, better bone formation and remodeling and higher graft-bone integrity at the interface tissue. Histologic scores of the graft-bone integration for the Norm-Exos group and Hypo-Exos group are significantly higher than that of the Ctrl group. Especially, the Hypo-Exos group presented a significantly higher score than the Norm-Exos group (Fig. 7B). At postoperative week 8 (Fig. 7A), in the Hypo-Exos group, the graft ACL integrated with bone tunnel significantly better than in the Ctrl and Norm-Exos group. In addition, more fibrocartilage tissues with mature cartilage cells and obvious Sharpey-like fibers were formed at the interface tissue in the Hypo-Exos group as compared with the Ctrl or Norm-Exos group, which function as a connection for the grafted tendon and bone tunnel. Similarly, the Hypo-Exos group remains to show the highest histologic scores when compared with the Ctrl or Norm-Exos group (Fig. 7B).
Fig. 7.
(A) H&E, MT and SO/FG staining results for the grafted tendon-bone tunnel integration of the Ctrl, Norm-Exos, and Hypo-Exos groups at 4 weeks and 8 weeks after ACL reconstruction (n = 7); Black arrows indicate the Sharpey fibers; Bar = 500 μm. (B) Histological scores for the grafted tendon-bone tunnel integration. Data are presented as mean ± SD. “∗” means P<0.05, “∗∗” means P<0.01, and “∗∗∗” means P<0.001.
Tensile properties: Tensile strength of FGTC specimens were loaded to failure at postoperative week 8 (n = 8 in each group). As expected, the normal FGTC specimens demonstrated a better higher tensile property than the experimental specimens. During the biomechanical test, the failure mode of the FGTC specimens was divided into ruptures at the midsubstance or pullout from the bone tunnel. In our study, all of the normal FGTC specimens were torn at the midsubstance, while 2 of 8 grafts in the Ctrl group, 4 of 8 grafts in the Norm-Exos group, 7of 8 grafts in the Hypo-Exos group failed with a mode of ruptures at the midsubstance. As shown in Fig. 8, the Hypo-Exos group appeared to show a higher tendency for midsubstance tear as compared with the Ctrl or Norm-Exos group. However, no significant difference was shown between them per the Fisher exact test (P = 0.06). The failure load was significantly higher in the Hypo-Exos group than in the Ctrl group (P<0.001) or Norm-Exos group (P<0.05). There was also a significant increase in stiffness in the Hypo-Exos group compared with the Ctrl group (P<0.05). No significant differences in stiffness were observed between the Ctrl group and the Norm-Exos group (P > 0.05).
Fig. 8.
Biomechanical testing of the femur-graft-tibia complex (FGTC) specimens at 8 weeks after surgery among the Ctrl, Norm-Exos, and Hypo-Exos groups. (A) The harvested FGTC specimens were securely mounted onto a metallic clamp, the upper clamp fastened to the femur, and a lower clamp fastened to the tibia (n = 8). (B) Failure load and ultimate stress of specimens. The black dotted line indicates the mean value of the failure load and stiffness in the native specimens. Data are presented as mean ± SD. “∗” means P<0.05, “∗∗” means P<0.01, and “∗∗∗” means P<0.001.
4. Discussions
Grafted tendon firmly and rapidly integrating with bone tunnel remains a challenge after ACL reconstruction in clinic. In this study, we elucidated the therapeutic effects of Hypo-Exos for the firm and rapid integration of grafted tendon to bone tunnel in a rat ACL reconstruction model. Micro-CT, histological staining and the biomechanical test showed that the specimens harvested from the Hypo-Exos treated group presented significantly earlier and stronger integration between ACL graft and bone tunnel compared with the specimens from Norm-Exos treated group, as characterized by more Sharpey's fibers and cartilage cells in the regenerated fibrocartilage as well as better bone formation and remodeling at the peri-graft bone. This study indicates that Hypo-Exos may be developed as a promising cell-free approach to accelerate grafted tendon-bone integration after ACL reconstruction.
The peri-graft bone loss is one of the critical reasons delaying the firm integration of tendon graft and bone tunnel [6,7]. Considering that bone loss is closely associated with local angiogenesis and its coupled osteogenesis [8,35,38], lots of strategies focused on stimulating angiogenesis and/or osteogenesis at the peri-graft site have been developed for enhancing the integration of ACL graft with the bone tunnel. After ACL reconstruction, the grafted tendons in the bone tunnel are separated from their vascular supply at the early stage. Therefore, the ingrowth of new blood vessels is the first challenge that the grafted tendon must overcome before firmly integrating with the bone tunnel. Additionally, neovascularization surrounding the grafted tendon is extremely important for the following grafted tendon-bone tunnel healing [[39], [40], [41]]. Demirag et al. reported that a higher degree of angiogenesis in the peri-graft site led to more mature histological parameters of the grafted tendon-bone healing as characterized by more Sharpey's fibers [42]. Another study found that angiogenesis in the peri-graft site increased with time from 3 to 8 weeks after ACL reconstruction using a rabbit model [43] and inhibiting angiogenesis could reduce the grafted tendon maturity and biomechanical strength [44]. Thus, the strategies capable of stimulating angiogenesis have been developed to improve the bone formation around the grafted tendon.
Stem cell-based therapies demonstrated to be effective for enhancing the integration of grafted tendon and bone [4,[45], [46], [47], [48]]. Previous studies thought that the mechanisms of transplanted stem cells are mainly attributable to their potential of directly differentiating into the desired tissue type to repair damaged tissue, while recent studies revealed that MSCs function mainly through their released bioactive factors to play a regulatory role [17,49,50]. Although the efficacy of stem cell-based therapies on the integration of grafted tendon to bone, these therapies are still facing inevitable challenges, including immune rejection, cell dedifferentiation, and tumor formation, and immense care outside the cell incubator [[15], [16], [17]]. Recent studies have determined that transplantation of MSC-Exos shows similar therapeutic effects to directly-transplanted MSCs. More importantly, compared with MSC-based therapies, MSC-Exo-based therapy is a better choice given its reduced immune rejection, improved safety, and easier storage [[18], [19], [20], [21]]. It is well known that oxygen concentration in the body under physiological conditions is quite different from the in-vitro culture condition of normoxia (21% O2). This culture condition does not mimic the hypoxia status in the body. Moreover, some studies have shown that Hypo-Exos derived from MSCs have a significantly better function in stimulating angiogenesis than Norm-Exos [19,51,52]. Similarly, our results showed that Hypo-Exos derived from rat BMSCs has stronger angiogenic capability compared with Norm-Exos, Moreover, it was also capable of inducing bone formation in the peri-graft site, thus improving the integration of ACL graft-bone tunnel.
The Hypo-Exos derived from BMSCs for the treatment of ACL reconstruction remains a new field of research; until now, the efficacy of this Hypo-Exos on the enhancement of ACL graft-bone healing has not been reported. In this study, we proved that this Hypo-Exos with the adhesive hydrogel can enhance the integration of grafted tendon to bone in a rat ACL reconstruction model by stimulating the formation of peri-graft bone, thus improving grafted tendon-bone healing. However, the mechanisms of Hypo-Exos for enhancing grafted tendon-bone integration remain unclear and still need further investigation. Recently, Wei Liu et al. found that Hypo-Exos derived from human umbilical cord MSCs promote bone fracture healing through exosomal miR-126 to promote local angiogenesis [19]. Thus, we speculate that the enhancing effects of Hypo-Exos on enhancing the integration of grafted tendon to bone after ACL reconstruction may be due in part to its promoting effect on angiogenesis. Our in-vitro results showed that Hypo-Exos can significantly promote proliferation, migration, and tube formation in HUVECs, which was consistent with published literature [19,51,52]. Chunling Xue et al. found that Hypo-Exos derived from human adipose-derived mesenchymal stem cells (ASCs) can improve angiogenesis by activating the PKA signaling pathway [51]. Similarly, Yudi Han et al. found that Hypo-Exos from human ASCs enhance angiogenesis via regulating VEGF/VEGF-R signaling [52]. To elucidate the signal pathway of Hypo-Exos derived from rat BMSCs on stimulating angiogenesis, more studies are needed in the future.
Nevertheless, several limitations should not be ignored in our study. Firstly, this study just used a small animal model. However, direct application of Hypo-Exos derived from BMSCs to the treatment of human patients is not applicable. Clinical experiment about the effects of Hypo-Exos derived from BMSCs in human subjects should be investigated. Secondly, only a single injection of set concertation of Hypo-Exos with the adhesive hydrogel was investigated in this study, it will be meaningful to further optimize the concertation of Hypo-Exos as well as injection times in the enhancement of ACL graft integrating with bone tunnel in future studies. Thirdly, this study only determined the efficacy of Hypo-Exos on graft-bone integration with radiological, histological and biomechanical evaluations, and did not describe the potential molecular mechanisms of Hypo-Exos. Previous MSC-Exo-based therapeutic studies showed that MSC-Exos enhance bone formation by delivering miR-126 to targeting cells [19]. Next step, miRNA analysis or proteomic analysis can be used to identify the active component of Hypo-Exos for graft-bone integration and to explore the angiogenic components, which may be useful in tendon-bone healing studies. Lastly, we did not include a control group that receive the adhesive hydrogel only. As reported, the use of adhesive hydrogel alone has not been found to affect the tissue healing progress, and many published papers have directly used the adhesive hydrogel as a control condition [31,32]. Despite the above-mentioned limitations, our study indicated that local injection of Hypo-Exos with the adhesive hydrogel is a therapeutic strategy for enhancing the grafted tendon-bone tunnel integration after ACL reconstruction, which may provide direction and a research basis for future clinical transplantation.
5. Conclusion
In summary, our results showed that Hypo-Exos delivered with adhesive hydrogel exhibit an angiogenic effect in the peri-graft site after ACL reconstruction, which could enhance peri-graft bone formation and remodeling, thus improving grafted tendon-bone tunnel healing. This study provides a new cell-free approach to enhancing the grafted tendon-bone integration after ACL reconstruction.
Author contributions
T.Z. designed experiments and wrote the manuscript. Y.X. and S.Y performed experiments. Y.S., B.L. D.X. and C.C. collected and analyzed the data. Y.S. and C.C. assisted in the experiments.
Declaration of competing interest
The authors declare no competing financial interests.
Acknowledgments
This work was supported by Natural Science Foundation of Changsha City (Grant No. kq2202373), the National Natural Science Foundation of China (grant 81902220), the Natural Science Foundation of Hunan Province, China (grant 2018JJ3814). Additionally, the authors would like to thank professor Hui Xie and other staff from Movement System Injury and Repair Research Center, Xiangya Hospital, Central South University, Changsha, China, for their kind assistance during the experiments.
Contributor Information
Bangbao Lu, Email: 14182832@qq.com.
Yan Xu, Email: xybs2017@163.com.
References
- 1.Siegel L., Vandenakker-Albanese C., Siegel D. Anterior cruciate ligament injuries: anatomy, physiology, biomechanics, and management. Clin J Sport Med : official journal of the Canadian Academy of Sport Medicine. 2012;22(4):349–355. doi: 10.1097/JSM.0b013e3182580cd0. [eng] [DOI] [PubMed] [Google Scholar]
- 2.Woo S.L., Wu C., Dede O., Vercillo F., Noorani S. Biomechanics and anterior cruciate ligament reconstruction. J Orthop Surg Res. 2006;1:2. doi: 10.1186/1749-799X-1-2. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y., Fu S.C., Cheuk Y.C., Ong T.Y., Feng H., Yung S.H. The effect of thermosensitive hydrogel platelet-rich-plasma complex in the treatment of partial tear of anterior cruciate ligament in rat model. J. Orthopaedic Translation. 2020;24:183–189. doi: 10.1016/j.jot.2019.12.009. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawakami Y., Takayama K., Matsumoto T., Tang Y., Wang B., Mifune Y., et al. Anterior cruciate ligament-derived stem cells transduced with BMP2 accelerate graft-bone integration after ACL reconstruction. Am J Sports Med. 2017;45(3):584–597. doi: 10.1177/0363546516671707. [DOI] [PubMed] [Google Scholar]
- 5.Kamath G.V., Redfern J.C., Greis P.E., Burks R.T. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199–217. doi: 10.1177/0363546510370929. [eng] [DOI] [PubMed] [Google Scholar]
- 6.Wen C.Y., Qin L., Lee K.M., Chan K.M. Peri-graft bone mass and connectivity as predictors for the strength of tendon-to-bone attachment after anterior cruciate ligament reconstruction. Bone. 2009;45(3):545–552. doi: 10.1016/j.bone.2008.08.112. [DOI] [PubMed] [Google Scholar]
- 7.Lui P.P.Y., Lee Y.W., Mok T.Y., Cheuk Y.C. Peri-tunnel bone loss: does it affect early tendon graft to bone tunnel healing after ACL reconstruction? Knee Surg Sports Traumatol Arthrosc. 2013;23(3):740–751. doi: 10.1007/s00167-013-2697-3. [DOI] [PubMed] [Google Scholar]
- 8.Sivaraj K.K., Adams R.H. Blood vessel formation and function in bone. Development. 2016;143(15):2706–2715. doi: 10.1242/dev.136861. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y., He B., Wang L., Yuan B., Shu H., Zhang F., et al. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res Ther. 2020;11(1):496. doi: 10.1186/s13287-020-02005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu H., Qin L., Cheung W., Lee K., Wong W., Leung K. Low-intensity pulsed ultrasound accelerated bone-tendon junction healing through regulation of vascular endothelial growth factor expression and cartilage formation. Ultrasound Med Biol. 2008;34(8):1248–1260. doi: 10.1016/j.ultrasmedbio.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–328. doi: 10.1038/nature13145. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu R., Yallowitz A., Qin A., Wu Z., Shin D.Y., Kim J.M., et al. Targeting skeletal endothelium to ameliorate bone loss. Nat Med. 2018;24(6):823–833. doi: 10.1038/s41591-018-0020-z. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan L., Zhang C., Yu Z., Shi Z., Dang X., Wang K. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and osteogenesis in rabbit femoral head osteonecrosis. Bone. 2015;81:544–553. doi: 10.1016/j.bone.2015.09.005. [eng] [DOI] [PubMed] [Google Scholar]
- 14.Ji X., Yuan X., Ma L., Bi B., Zhu H., Lei Z., et al. Mesenchymal stem cell-loaded thermosensitive hydroxypropyl chitin hydrogel combined with a three-dimensional-printed poly(ε-caprolactone)/nano-hydroxyapatite scaffold to repair bone defects via osteogenesis, angiogenesis and immunomodulation. Theranostics. 2020;10(2):725–740. doi: 10.7150/thno.39167. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeong J.O., Han J.W., Kim J.M., Cho H.J., Park C., Lee N., et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108(11):1340–1347. doi: 10.1161/CIRCRESAHA.110.239848. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mastrolia I., Foppiani E.M., Murgia A., Candini O., Samarelli A.V., Grisendi G., et al. Challenges in clinical development of mesenchymal stromal/stem cells: concise review. Stem Cells Transl Med. 2019;8(11):1135–1148. doi: 10.1002/sctm.19-0044. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toh W.S., Lai R.C., Hui J.H.P., Lim S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56–64. doi: 10.1016/j.semcdb.2016.11.008. [eng] [DOI] [PubMed] [Google Scholar]
- 18.Yuan N., Ge Z., Ji W., Li J. Exosomes secreted from hypoxia-preconditioned mesenchymal stem cells prevent steroid-induced osteonecrosis of the femoral head by promoting angiogenesis in rats. BioMed Res Int. 2021;2021 doi: 10.1155/2021/6655225. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W., Li L., Rong Y., Qian D., Chen J., Zhou Z., et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196–212. doi: 10.1016/j.actbio.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Lai R.C., Yeo R.W., Lim S.K. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82–88. doi: 10.1016/j.semcdb.2015.03.001. [eng] [DOI] [PubMed] [Google Scholar]
- 21.He C., Zheng S., Luo Y., Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237–255. doi: 10.7150/thno.21945. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X., Cai Z., Wu M., Huangfu X., Li J., Liu X. Adipose stem cell-derived exosomes recover impaired matrix metabolism of torn human rotator cuff tendons by maintaining tissue homeostasis. Am J Sports Med. 2021;49(4):899–908. doi: 10.1177/0363546521992469. [DOI] [PubMed] [Google Scholar]
- 23.Wang C., Hu Q., Song W., Yu W., He Y. Adipose stem cell-derived exosomes decrease fatty infiltration and enhance rotator cuff healing in a rabbit model of chronic tears. Am J Sports Med. 2020;48(6):1456–1464. doi: 10.1177/0363546520908847. [DOI] [PubMed] [Google Scholar]
- 24.Wang C., Song W., Chen B., Liu X., He Y. Exosomes isolated from adipose-derived stem cells: a new cell-free approach to prevent the muscle degeneration associated with torn rotator cuffs. Am J Sports Med. 2019;47(13):3247–3255. doi: 10.1177/0363546519876323. [DOI] [PubMed] [Google Scholar]
- 25.Phinney D.G., Pittenger M.F. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cell. 2017;35(4):851–858. doi: 10.1002/stem.2575. [eng] [DOI] [PubMed] [Google Scholar]
- 26.Yan L., Liu G., Wu X. Exosomes derived from umbilical cord mesenchymal stem cells in mechanical environment show improved osteochondral activity via upregulation of LncRNA H19. J. Orthopaedic Translation. 2021;26:111–120. doi: 10.1016/j.jot.2020.03.005. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connor D.E., Paulus J.A., Dabestani P.J., Thankam F.K., Dilisio M.F., Gross R.M., et al. Therapeutic potential of exosomes in rotator cuff tendon healing. J Bone Miner Metabol. 2019;37(5):759–767. doi: 10.1007/s00774-019-01013-z. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu X., Wu R., Shehadeh L.A., Zhou Q., Jiang C., Huang X., et al. Severe hypoxia exerts parallel and cell-specific regulation of gene expression and alternative splicing in human mesenchymal stem cells. BMC Genom. 2014;15:303. doi: 10.1186/1471-2164-15-303. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohyeldin A., Garzón-Muvdi T., Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7(2):150–161. doi: 10.1016/j.stem.2010.07.007. [eng] [DOI] [PubMed] [Google Scholar]
- 30.Zhu J., Lu K., Zhang N., Zhao Y., Ma Q., Shen J., et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif Cell Nanomed Biotechnol. 2018;46(8):1659–1670. doi: 10.1080/21691401.2017.1388249. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo Z., Peng W., Xu Y., Xie Y., Liu Y., Lu H., et al. Exosomal OTULIN from M2 macrophages promotes the recovery of spinal cord injuries via stimulating Wnt/β-catenin pathway-mediated vascular regeneration. Acta Biomater. 2021;136:519–532. doi: 10.1016/j.actbio.2021.09.026. [eng] [DOI] [PubMed] [Google Scholar]
- 32.Mu J., Li L., Wu J., Huang T., Zhang Y., Cao J., et al. Hypoxia-stimulated mesenchymal stem cell-derived exosomes loaded by adhesive hydrogel for effective angiogenic treatment of spinal cord injury. Biomater Sci. 2022;10(7):1803–1811. doi: 10.1039/d1bm01722e. [eng] [DOI] [PubMed] [Google Scholar]
- 33.Sun Y., Chen W., Hao Y., Gu X., Liu X., Cai J., et al. Stem cell-conditioned medium promotes graft remodeling of midsubstance and intratunnel incorporation after anterior cruciate ligament reconstruction in a rat model. Am J Sports Med. 2019;47(10):2327–2337. doi: 10.1177/0363546519859324. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y., Yu J., Zhang J., Hua Y. Simvastatin with PRP promotes chondrogenesis of bone marrow stem cells in vitro and wounded rat achilles tendon-bone interface healing in vivo. Am J Sports Med. 2019;47(3):729–739. doi: 10.1177/0363546518819108. [DOI] [PubMed] [Google Scholar]
- 35.Peng Y., Wu S., Li Y., Crane J.L. Type H blood vessels in bone modeling and remodeling. Theranostics. 2020;10(1):426–436. doi: 10.7150/thno.34126. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma P., Chen T., Wu X., Hu Y., Huang K., Wang Y., et al. Effects of bioactive strontium-substituted hydroxyapatite on osseointegration of polyethylene terephthalate artificial ligaments. J Mater Chem B. 2021 doi: 10.1039/d1tb00768h. [DOI] [PubMed] [Google Scholar]
- 37.Zhu L.P., Tian T., Wang J.Y., He J.N., Chen T., Pan M., et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8(22):6163–6177. doi: 10.7150/thno.28021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu R., Lv W.C., Xu Y., Gong M.Y., Chen X.J., Jiang N., et al. Endothelial ZEB1 promotes angiogenesis-dependent bone formation and reverses osteoporosis. Nat Commun. 2020;11(1):460. doi: 10.1038/s41467-019-14076-3. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasaki K., Kuroda R., Ishida K., Kubo S., Matsumoto T., Mifune Y., et al. Enhancement of tendon-bone osteointegration of anterior cruciate ligament graft using granulocyte colony-stimulating factor. Am J Sports Med. 2008;36(8):1519–1527. doi: 10.1177/0363546508316282. [eng] [DOI] [PubMed] [Google Scholar]
- 40.Oka S., Matsumoto T., Kubo S., Matsushita T., Sasaki H., Nishizawa Y., et al. Local administration of low-dose simvastatin-conjugated gelatin hydrogel for tendon-bone healing in anterior cruciate ligament reconstruction. Tissue Eng. 2013;19(9–10):1233–1243. doi: 10.1089/ten.TEA.2012.0325. [eng] [DOI] [PubMed] [Google Scholar]
- 41.Yoshikawa T., Tohyama H., Katsura T., Kondo E., Kotani Y., Matsumoto H., et al. Effects of local administration of vascular endothelial growth factor on mechanical characteristics of the semitendinosus tendon graft after anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2006;34(12):1918–1925. doi: 10.1177/0363546506294469. [eng] [DOI] [PubMed] [Google Scholar]
- 42.Demirag B., Sarisozen B., Ozer O., Kaplan T., Ozturk C. Enhancement of tendon-bone healing of anterior cruciate ligament grafts by blockage of matrix metalloproteinases. J Bone Joint Surg Am. 2005;87(11):2401–2410. doi: 10.2106/JBJS.D.01952. [eng] [DOI] [PubMed] [Google Scholar]
- 43.Yoshikawa T., Tohyama H., Enomoto H., Matsumoto H., Toyama Y., Yasuda K. Expression of vascular endothelial growth factor and angiogenesis in patellar tendon grafts in the early phase after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):804–810. doi: 10.1007/s00167-006-0051-8. [eng] [DOI] [PubMed] [Google Scholar]
- 44.Takayama K., Kawakami Y., Mifune Y., Matsumoto T., Tang Y., Cummins J.H., et al. The effect of blocking angiogenesis on anterior cruciate ligament healing following stem cell transplantation. Biomaterials. 2015;60:9–19. doi: 10.1016/j.biomaterials.2015.03.036. [eng] [DOI] [PubMed] [Google Scholar]
- 45.Hao Z.C., Wang S.Z., Zhang X.J., Lu J. Stem cell therapy: a promising biological strategy for tendon-bone healing after anterior cruciate ligament reconstruction. Cell Prolif. 2016;49(2):154–162. doi: 10.1111/cpr.12242. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu C.C., Ho C.J., Huang H.T., Lin S.Y., Chou S.H., Chou P.H., et al. Effect of freshly isolated bone marrow mononuclear cells and cultured bone marrow stromal cells in graft cell repopulation and tendon-bone healing after allograft anterior cruciate ligament reconstruction. Int J Mol Sci. 2021;22(6) doi: 10.3390/ijms22062791. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lui P.P., Wong O.T., Lee Y.W. Application of tendon-derived stem cell sheet for the promotion of graft healing in anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(3):681–689. doi: 10.1177/0363546513517539. [DOI] [PubMed] [Google Scholar]
- 48.Ouyang H.W., Goh J.C., Lee E.H. Use of bone marrow stromal cells for tendon graft-to-bone healing: histological and immunohistochemical studies in a rabbit model. Am J Sports Med. 2004;32(2):321–327. doi: 10.1177/0095399703258682. [DOI] [PubMed] [Google Scholar]
- 49.Sevivas N., Teixeira F.G., Portugal R., Araújo L., Carriço L.F., Ferreira N., et al. Mesenchymal stem cell secretome: a potential tool for the prevention of muscle degenerative changes associated with chronic rotator cuff tears. Am J Sports Med. 2017;45(1):179–188. doi: 10.1177/0363546516657827. [eng] [DOI] [PubMed] [Google Scholar]
- 50.Sevivas N., Teixeira F.G., Portugal R., Direito-Santos B., Espregueira-Mendes J., Oliveira F.J., et al. Mesenchymal stem cell secretome improves tendon cell viability in vitro and tendon-bone healing in vivo when a tissue engineering strategy is used in a rat model of chronic massive rotator cuff tear. Am J Sports Med. 2018;46(2):449–459. doi: 10.1177/0363546517735850. [eng] [DOI] [PubMed] [Google Scholar]
- 51.Xue C., Shen Y., Li X., Li B., Zhao S., Gu J., et al. Exosomes derived from hypoxia-treated human adipose mesenchymal stem cells enhance angiogenesis through the PKA signaling pathway. Stem Cell Dev. 2018;27(7):456–465. doi: 10.1089/scd.2017.0296. [eng] [DOI] [PubMed] [Google Scholar]
- 52.Han Y., Ren J., Bai Y., Pei X., Han Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem Cell Biol. 2019;109:59–68. doi: 10.1016/j.biocel.2019.01.017. [eng] [DOI] [PubMed] [Google Scholar]