Abstract
Hashimoto’s thyroiditis (HT) and Graves’ disease (GD) are prevalent autoimmune disorders, representing opposite ends of the clinical spectrum of autoimmune thyroid diseases (AITD). The pathogenesis involves a complex interplay between environment and genes. Specific susceptibility genes have been discovered that predispose to AITD, including thyroid-specific and immune-regulatory genes. Growing evidence has revealed that genetic and epigenetic variants can alter autoantigen presentation during the development of immune tolerance, can enhance self-peptide binding to MHC (major histocompatibility complex), and can amplify stimulation of T- and B-cells. These gene-driven mechanistic discoveries lay the groundwork for novel treatment targets. This review summarizes recent advances in our understanding of key AITD susceptibility genes (Tg, TSHR, HLA-DR3, and CD40) and their translational therapeutic potential.
Keywords: Autoimmunity, Genes, Epigenetics, Hashimoto’s thyroiditis, Graves’ disease, thyroglobulin, CD40, HLA, MHC
A. INTRODUCTION
Autoimmune thyroid diseases (AITD) including Graves’ disease (GD) and Hashimoto’s thyroiditis (HT) represent a major public health burden. With a population prevalence of about 1-5%, AITD are not only the most prevalent disorders of the thyroid, but also the commonest autoimmune diseases [1]. While GD and HT manifest with opposing clinical presentations, hypothyroidism in HT and hyperthyroidism in GD, they share a common etiology that involves breakdown of tolerance to thyroid autoantigens [1]. In the past two decades significant progress has been made in our understanding of the mechanisms underlying the development of AITD and especially the contributions of susceptibility genes to their pathoetiology.
Mechanistically, the susceptibility genes for AITD can be classified as immune-related genes and thyroid-specific genes. The first immune-related gene triggering AITD identified was HLA-DR3, but additional immune-related susceptibility genes for AITD were later mapped, including CTLA-4, CD40, and PTPN22 [2]. Of the three genes encoding the key thyroid autoantigens, thyroglobulin (Tg), thyroid peroxidase (TPO), and the thyroid stimulating hormone receptor (TSHR), only Tg [3] and TSHR were found to be associated with AITD.
The identification of susceptibility genes for AITD has led to a much better understanding of the mechanisms triggering thyroid autoimmunity, and these, in turn, enabled us to identify new therapeutic targets. In this review we will summarize recent advances in our understanding of the mechanisms by which the key immune-related genes, HLA-DR3 and CD40, and thyroid-specific genes, Tg and TSHR, trigger AITD. We will highlight how the newly discovered mechanisms underlying the development of AITD are guiding therapeutic advancements.
B. THYROID-SPECIFIC AITD SUSCEPTIBILITY GENES: Tg AND TSHR
Two thyroid specific genes, Tg and TSHR, have been associated with AITD. The Tg gene, located on chromosome 8q24, consists of 48 exons and encodes for a large 660 kDa protein that accounts for approximatively 75 – 80% of total thyroidal protein and serves as a precursor of the active thyroid hormones, T3 (triiodothyronine) and T4 (thyroxine) [4]. The TSHR gene, located on chromosome 14q31, consists of 10 exons encoding for a G protein-coupled receptor that plays a central role in thyroid development, growth, and function [5]. Tg and TSHR are unique among AITD susceptibility genes because they are both targets of the autoimmune response: Tg has been recognized as a key antigen in both HT and GD while detectable antibodies to TSHR (TSHR-stimulating antibodies or TSAbs) are the hallmark of GD [6, 7].
Several whole-genome linkage and association studies have been performed in the last two decades and have established the unique and important roles of the Tg and TSHR genes in AITD susceptibility [8-11]. Furthermore, fine-mapping and sequencing of the TSHR [12] and Tg genes [13] have mapped unique susceptibility regions and narrowed down the number of risk-associated single nucleotide polymorphisms (SNPs) within these genes. However, less progress has been made regarding the identification of molecular/biological phenotypes triggered by the Tg and TSHR AITD-associated SNPs. Indeed, understanding the significance of risk-associated genetic variants requires functional studies focused not only on how gene-protein expression and downstream pathways are affected but on how gene-environment interactions impact disease susceptibility.
B.1. Functional significance of Tg polymorphisms: Non-coding Tg SNPs
Sequencing studies of the 5’UTR (untranslated region) of Tg by our group identified an A/G SNP located at position −1623 (rs180195) that showed strong association with AITD [13]. Transmission disequilibrium test (TDT) analysis in families with AITD confirmed this association [8]. More recently, Lahooti et al. genotyped the rs180195 variant in 529 patients with AITD and further confirmed this Tg polymorphism as a marker for thyroid autoimmunity [14]. Given the 5’UTR location of the rs180195, we postulated that the A/G polymorphism disrupts a regulatory element within the TG promoter. Bioinformatic analyses showed that the A/G SNP overlaps a binding site for the interferon regulatory factor (IRF-1); and electromobility shift assays (EMSA) and chromatin immunoprecipitation (ChIP) studies demonstrated the binding of IRF-1 to its predicted site within the Tg 5’ UTR region. Additional luciferase reporter and siRNA assays showed specific binding of IRF-1 to the disease-associated G allele which correlated with increased Tg mRNA expression only when the G allele was present at the rs180195 site [13]. Interferon alpha (IFNα), a known trigger of thyroiditis in genetically predisposed individuals [15, 16], is a potent inducer of IRF-1 expression. Indeed, we found that IFNα, by inducing IRF-1 expression, increases Tg transcription in cultured thyrocytes and up-regulated mRNA levels and active chromatin markers at both the rs180195 site and the Tg promoter [13]. These studies discovered not only a regulatory role of rs180195 for Tg gene expression but also revealed an interaction between an environmental trigger of AITD, namely viral infections which induce IFNα secretion, and a risk-associated SNP that can modulate specific molecular Tg phenotypes associated with thyroid autoimmunity. Most surprisingly, further studies from our group found that while IFNα upregulates Tg transcription, it actually decreases Tg protein levels [17]. This paradox was resolved by mechanistic studies demonstrating IFNα-activation of the unfolded protein response (UPR) and the autophagy/lysosomal degradation pathways in thyroid cells, triggering lysosomal degradation of Tg [17]. It is well-known that large proteins or protein aggregates are delivered to endo-lysosomes for clearance by the ERLAD (ER-to-lysosome-associated degradation) pathways which involve autophagy [18]. It is possible that increased IFNα-induced Tg degradation by ERLAD promotes generation of immunogenic peptides, mounting the risk of AITD development. This hypothesis is supported by our studies showing that in vitro treatment of Tg with cathepsins (CTS) generated immunogenic peptides [19, 20], including Tg.2098, a major Tg T-cell epitope in AITD [19, 21-23]. Consistent with these studies, we recently established that IFNα upregulated cathepsins CTSL and CTSS in thyrocytes and that their knockdown prevented Tg degradation and the generation of immunogenic peptides (unpublished results).
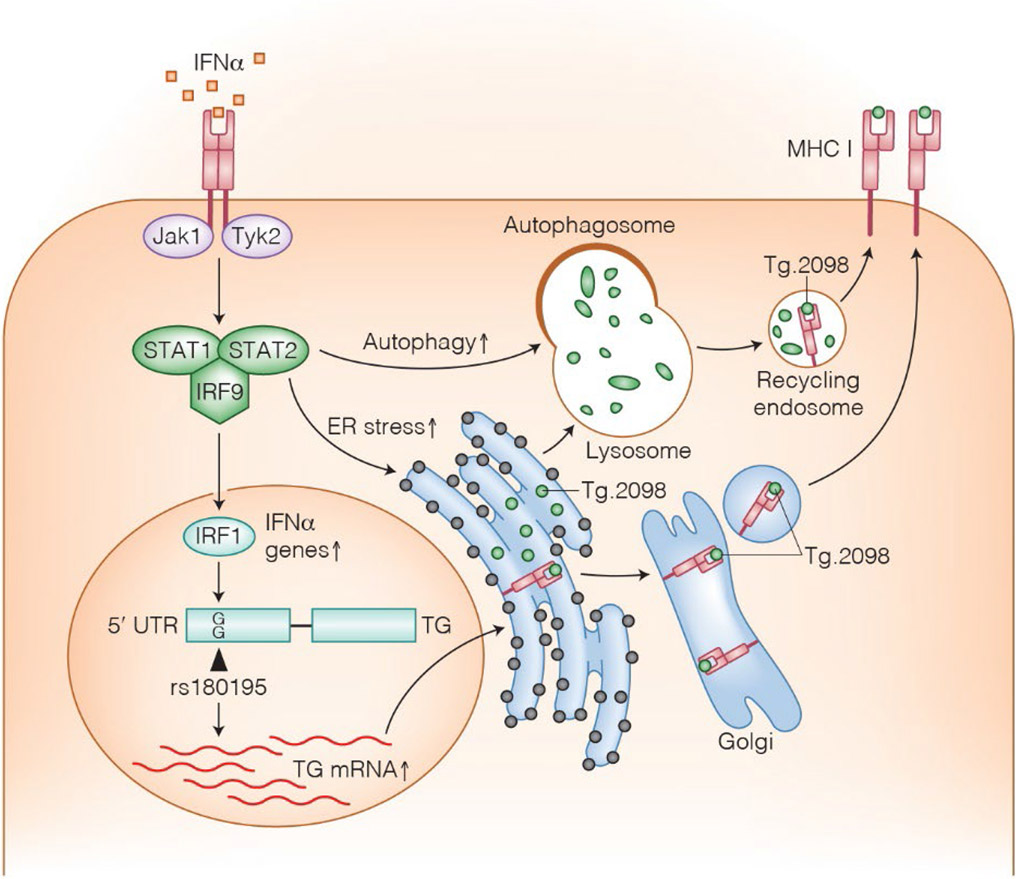
Collectively, a more comprehensive picture on how gene-environment interactions can shape the development of thyroid autoimmunity is emerging. Viral infections and other inflammatory conditions in which there is an increased production of IFNα trigger increased Tg expression in susceptible individuals carrying the G allele of the rs180195 SNP as well as ER-stress and activation of the ERLAD pathways. This leads to increased production and degradation of Tg and ultimately to the release of immunogenic peptides (Figure 1).
Figure 1:
A proposed model for environmental-genetic interaction leading to anti-Tg autoimmunity. Viral infections lead to local secretion of IFNα which in turn binds to its receptor on thyroid cells and on immune cells. Immune cell binding leads to secretion of chemokines and recruitment of immune cells. Thyroid cell binding triggers increased synthesis of Tg, as well as ER stress and increased autophagy within thyroid cells. Both lead to Tg degradation into immunogenic peptides, such as Tg.2098 that can be presented to immune cells.
B.2. Functional significance of Exonic Tg SNPs
Sequencing of all 48 exons of the Tg gene and testing them in case control association studies identified an exon 10-12 SNP cluster and an exon 33 SNP that showed strong association with AITD [3]. Three of these SNPs were missense polymorphisms that altered the amino acid sequence of the Tg protein: rs180223 (exon 10, Ser734Ala), rs853326 (exon 12, Met1028Val), and rs2076740 (exon 33, Arg1999Trp) [3, 24]. Of these, a genetic interaction between rs2076740 (Arg1999Trp) and the HLA-DRβ-Arg74 pocket variant, which binds only a select group of Tg peptides (see below, HLA-DR section) [17], was shown to significantly increase the odds ratio (OR) for GD [24]. Thus, mechanistically, it is possible that the missense Tg SNPs can trigger ERLAD degradation of Tg into peptides that inherently have increased binding affinity for HLA-DRβ-Arg74.
However, to directly assess the functional role of these variants and their impact on Tg immunogenicity, new in vivo models are warranted. We have recently generated a new mouse model of HT by immunizing mice with the cDNA of Tg in a non-replicating Adenovirus vector (Ad-Tg) [25]. This innovative EAT mouse model is a powerful tool that will enables us to manipulate the Tg cDNA (used to immunize the mice) to evaluate the contributions of these Tg exonic SNPs and their haplotypes to the development of AITD.
B.3. Functional significance of TSHR gene polymorphisms in Intron 1
Intron 1 of the TSHR gene has been mapped as a unique susceptibility region for GD by whole-genome association and fine-mapping studies in several populations [10]. Five GD-associated SNPs have been mapped to TSHR intron 1: rs179247, rs2284720, rs12101255, rs12101261, and rs2268458. Several studies have since aimed to unveil the functional significance of these variants in GD pathoetiology. To date, two distinct mechanisms have been proposed to connect intron 1 variants to increased risk of thyroid autoimmunity. One mechanism proposes that the intron 1 SNPs alter the regulation of mRNA splicing of thyroidal genes thereby resulting in decreased peripheral tolerance and increased autoimmunity to TSHR. In contrast, although not mutually exclusive, the other mechanism suggests that these disease-associated SNPs reduce TSHR expression in the thymus thereby leading to decreased central tolerance.
B.4. TSHR intron 1 SNPs and peripheral tolerance
TSHR intron 1 polymorphisms may function to predispose to AITD through variable SNP-dependent mRNA splicing. Brand et al. quantified the mRNA levels of the full length TSHR (flTSHR) and of two TSHR truncated variants, ST4 and ST5, in 12 thyroid tissues. They showed that the risk-allele variants of rs179247 and rs12101255 were associated with increased levels of ST4 and ST5 and decreased levels of flTSHR [12]. They suggested that the more abundant truncated TSHR variants would putatively encode for more antigenic TSHR extracellular A-subunits in susceptible individuals. However, more recently, another group analyzed the levels of flTSHR, ST4, and ST5 in 49 thyroid tissues and was unable to replicate these results; they found no significant effect of the rs179247 and rs12101255 genotypes on ST4 and ST5 expression [26]. The conflicting results between the two studies may be explained by the small number of thyroids sampled in the former [12], especially considering that the samples were categorized by genotype. Alternatively, it is possible that other intron 1 SNPs in linkage disequilibrium with rs179247 and rs12101255 may be responsible for the observed differential effect between the full length versus truncated TSHR mRNA levels.
B.5. TSHR intron 1 SNPs and central tolerance
Initial studies from Pujol-Borrell’s group revealed an association between intron 1 SNPs and TSHR expression in the thymus. Specifically, individuals carrying the disease-protective G allele at rs179247 had higher thymic mRNA levels of TSHR compared to individuals carrying the disease-risk A allele [27]. They then proposed that lower levels of thymic TSHR expression associated with the A allele could facilitate the escape of TSHR-reactive T-cells from negative selection predisposing to TSHR autoimmunity. The same group applied parallel sequencing to quantify the rs179247 TSHR allele in cDNA and gDNA samples from thyroid and thymus tissues of heterozygous individuals [26]. They showed a bias of overexpression of the protective rs179247 G allele in the thymus compared to the thyroid, suggesting that the protective allele is preferentially expressed in the thymus [26].
The molecular-epigenetic mechanisms by which intron 1 SNPs regulate TSHR gene expression were revealed by studies done by our group [28]. The work originated from the premise that environmental triggers modulate key interactions between genetic regulators (e.g., transcriptional factors), epigenetic changes (e.g., histone modifications), and allelic variants at noncoding regions leading to up- or down-regulation of gene expression [29]. We analyzed genome-wide modifications of histone 3 lysine 4 (K4)-monomethylation (H3K4me1), a marker of active chromatin associated with enhancer elements [30], in thyroid cells induced by IFNα and identified an open chromatin region marked by an H3K4me1 peak overlapping two adjacent SNPs in TSHR intron 1: rs1210255 and rs12101261 [28]. Functional studies identified a regulatory element overlapping the rs12101261 SNP that binds the transcriptional repressor PLZF (promyelocytic leukemia zinc finger protein). PLZF binding and transcriptional repression were restricted to the disease-associated T allele; therefore, individuals carrying the GD-risk T allele of the rs12101261 SNP had decreased mRNA expression of TSHR. Our findings strongly suggest that rs12101261 is the causative (“driver”) variant triggering GD and that the other intron 1 SNPs associated with GD (rs179247, rs2284720, rs12101255, rs2268458) are “passenger” variants in strong linkage disequilibrium with the causative SNP. Taken together these findings support an alternative disease model in which sustained production of IFNα during viral infections could, based on the specific rs12101261 SNP variants, differentially affect the regulation of TSHR expression. In the thymus, lower expression of TSHR associated with the rs12101261 risk T allele, versus the protective C allele, would then facilitate escape from central tolerance and predispose to TSHR autoimmunity.
B.6. IFNα: a new therapeutic target in AITD
IFNα represents an elemental link between the environment, immune system, and susceptibility genes in triggering thyroid autoimmunity. It ties an environmental event, such as a viral infection, to genetic susceptibility and epigenetic triggers towards the development of AITD in genetically-predisposed individuals. The aforementioned emerging data suggest that, in the context of disease-susceptible SNP variants, IFNα can adversely alter the expression of Tg and TSHR in a way that will promote thyroid autoimmunity. Other immunomodulatory and local effects of IFNα include the activation of proinflammatory cytokines and adhesion molecules, the overexpression of HLA class I proteins, and the local infiltration of lymphocytes that collectively facilitate autoantigen presentation [31]. IFNα has also been implicated in the development of a multitude of autoimmune, rheumatologic diseases such as systemic lupus erythematosus (SLE), Sjogren’s syndrome, rheumatoid arthritis (RA), type 1 diabetes mellitus, and primary biliary cholangitis, among others [31]. As a key mediator of the environment-genetic interaction and an immunomodulator with pleiotropic effects, INFα is an attractive target to potentially interrupt the progression to autoimmunity.
B.7. IFNα and IFNα Receptor MAbs: potential therapeutic agents in AITD
The growing evidence for IFNα’s fundamental role in triggering AITD in a background of genetic susceptibility raises the possibility that targeting IFNα pathways may represent a potential approach to treat AITD. Indeed, Anifrolumab, a human IgG1kappa monoclonal antibody (MAb) against the type 1 Interferon Receptor subunit 1 (IFNAR1) was recently FDA-approved in 2021 as an add-on therapy for moderate to severe SLE [32, 33]. Clinical studies demonstrated that inhibition of type 1 interferon signaling led to significant reductions in the severity of skin manifestations and in glucocorticoid dosing; moreover, a neutralization of the IFN gene signature was noted with treatment. Rontalizumab and Sifalilumab, MAbs that neutralize IFNα, also previously showed encouraging results in SLE in phase II clinical trials [34]. In AITD, however, current evidence suggests that IFNα, following a viral infection, primarily contributes to the initial trigger rather than the maintenance of the autoimmune-driven attack on the thyroid. It is, therefore, possible that therapies targeting the IFNα pathways will be less effective in AITD. Clearly, further studies are needed to determine the exact role of IFNα in both the initiation and perpetuation of thyroidal autoimmunity.
C. IMMUNE-RELATED AITD SUSCEPTIBILITY GENE: HLA-DR3
The HLA (human leukocyte antigen) complex, encoded by the MHC (major histocompatibility complex) on chromosome 6p21, represents a highly gene-dense and polymorphic region. It encodes various proteins essential in the initiation of an immune response including antigen presentation and T-cell activation and was therefore the first, salient AITD gene locus to be identified. Of the HLA subtypes, HLA-DR3 has been the most clearly linked to AITD as a susceptibility gene [35, 36]. In fact, 40-50% of GD patients harbor the HLA-DR3 gene, compared to 15-30% in the general population with an impressive estimated OR of 4.0 [37]. Although initially less conclusive, HLA-DR3’s association with HT has since also been firmly established [35]. HLA-DR3 consists of over 30 alleles, and genetic sequencing of HLA-DR3 by our group has led to the identification of a unique HLA class II variant that predisposes to both HT and GD [36, 38]. We identified a signature HLA-DRβ (beta chain of HLA-DR3) pocket variant in which an arginine at position 74 (HLA-DRβ-Arg74) conferred an increased risk for the development of AITD, compared to the protective glutamine (HLA-DRβ-Gln74) variant. Interestingly, the same HLA-DRβ-Arg74 pocket was also shown to predispose to the autoimmune polyglandular syndrome type 3 variant (APS3 variant or APS3v) [39], a clinical syndrome manifesting as concurrent thyroid autoimmunity (AITD) and islet autoimmunity (type 1 diabetes) [40, 41].
C.1. Functional Significance of the HLA-DRβ-Arg74 variant
Three-dimensional computer modeling of the HLA-DR pocket revealed that the electrostatic potential of the AITD-susceptible HLA-DRβ-Arg74 generates a more positively charged P4 HLA-DR pocket than the AITD-resistant HLA-DRβ-Gln74. Mechanistically, this positive charge differential is understood to selectively favor the binding affinity of pathogenic self-peptides to the HLA-DR pocket thereby facilitating efficient auto-antigen presentation and T-cell activation in the immunological synapse with the eventual triggering of AITD. Candidate pathogenic peptides include peptides derived from thyroid peroxidase (TPO) in HT, TSHR in GD, and Tg in both HT and GD. The interaction between pathogenic thyroidal peptides and the HLA-DRβ-Arg74 pocket therefore provides an exciting potential therapeutic target. One of the earliest steps of the autoimmune response, specifically self-antigen presentation to autoreactive T-cells that have escaped negative selection, may be halted by blocking thyroidal peptide presentation by HLA-DRβ-Arg74. Such an antigen-specific immunotherapy represents a major advance, as current treatment modalities of AITD only provide symptomatic relief through replacement of deficient thyroid hormones in HT or suppression of excess thyroid hormone production in GD. Although clinically effective, these do not reverse the underlying autoimmune disease process.
C.2. Antigen presentation by HLA-DR3: a new therapeutic target in AITD
To evaluate whether the HLA-DRβ-Arg74 pocket is a potential target for the treatment of AITD, our group investigated the effects of small molecules that bind and block HLA-DR3. Small molecule inhibitors have increasingly become an attractive approach to treat and even prevent the development of autoimmune diseases [42, 43]. Not only are small compounds inherently more stable than larger peptides and usually available in oral formulations, small molecules are also less immunogenic, less prone to elicit T-cell activation, and more likely to be absorbed with better tissue penetration. To identify candidate small molecules that block the HLA-DRβ pocket, 150,000 compounds from a large and diverse library of small molecules were virtually screened for their binding affinity to HLA-DRβ-Arg74 using molecular dynamic simulations [30]. Top hit compounds were further narrowed to only those with >50% in vitro inhibition of peptide-binding to recombinant HLA-DRβ-Arg74 by DELFIA (dissociated-enhanced lanthanide fluorescence immunoassay). We eventually identified a compound Cepharanthine, which showed significant inhibition of thyroglobulin peptide binding to HLA-DRβ-Arg74. Of note, Cepharanthine is a molecule listed on the FDA library (i.e., it has been given to humans before and has known safety data including dosage, pharmacokinetics, and toxicity profiles) [20]. Therefore, Cepharanthine was selected for further investigation to assess its translational potential in vivo using three humanized DR3 mouse models of HT [20], GD [44], and APS3v [45].
C.3. Testing Cepharanthine ex vivo and in vivo using humanized mouse models of AITD
Tg is the most abundant thyroidal protein [46] and anti-Tg antibodies serve as a hallmark of thyroid autoimmunity in humans and in mouse models of AITD [47]. Specific pathogenic Tg peptides can bind strongly and specifically to HLA-DRβ-Arg74, of which Tg.2098 has been shown to be a major human Tg epitope [19-21]. We used a humanized NOD-DR3 mouse model of HT (Experimental Autoimmune Thyroiditis or EAT) in which EAT is induced by immunizing the humanized mice with human Tg. We then tested if Cepharanthine could block T-cell responses to Tg in splenocytes isolated from EAT-induced NOD-DR3 mice. Isolated splenocytes were stimulated with Tg in the presence or absence of Cepharanthine and showed that Cepharanthine suppressed T-cell responses to Tg ex vivo. Moreover, when NOD-DR3 mice were induced with EAT and treated in vivo with Cepharanthine, there was a significant decrease in their T-cell responses to Tg. These reductions were mediated by blocking Tg peptide binding to the HLA-DRβ-Arg74 pocket [20].
As previously noted, the TSHR is the major autoantigen in GD. Similar to the key role the peptide Tg.2098 plays in HT, a TSHR peptide, designated TSHR.132, has been consistently reported to be the dominant TSHR peptide in GD [44, 48]. More so than other TSHR epitopes, TSHR.132 has been shown to specifically bind with high affinity to recombinant HLA-DRβ-Arg74 and to cells that express HLA-DRβ-Arg74. Our group also demonstrated that TSHR.132 induced strong T-cell proliferative responses when used to immunize humanized NOD-DR3 mice with increased levels of Th1 and Th2 cytokines including IFNγ, IL-2, IL-4, and IL-10 [44]. We recently created an Experimental Autoimmune Graves’ Disease (EAGD) mouse model in humanized BALB/c-DR3 mice [49] in which TSHR.132 peptide presentation within the human HLA-DR3 was confirmed. Furthermore, we showed that Cepharanthine inhibited T-cell activation by TSHR.132 ex vivo in splenocytes isolated from humanized mice induced with EAGD [44].
The co-occurrence of autoimmune thyroiditis and type 1 diabetes within the same individual is a variant of autoimmune polyglandular syndrome type 3 (APS3v) [40, 41]. As with the monoglandular HT and GD, HLA-DR3 likewise confers strong genetic susceptibility to polyglandular APS3v [39]. Indeed, the same HLA-DRβ-Arg74 flexible pocket has the ability to present both thyroid and pancreatic islet peptides, triggering APS3v [50]. In particular, we identified 3 major peptides (Tg.1571, GAD .492, and TPO.758) capable of eliciting significant T-cell and B-cell responses in NOD-DR3 mice. These peptides were then used to generate a humanized murine model of APS3v characterized by strong T-cell and humoral antibody responses to each of these peptides and by biochemical hypothyroidism. Ex vivo analyses of splenocytes isolated from the NOD-DRβ APS3v mice revealed that Cepharanthine significantly suppressed T-cell responses to GAD.492, TPO.758, and Tg.1571. In vivo, APS3v mice treated with Cepharanthine similarly showed suppressed T-cell proliferation and stimulation [45]
C.4. Cepharanthine: A potential novel therapeutic agent in AITD
Through robust virtual and in vivo screening, the small molecule Cepharanthine was uniquely identified to effectively bind HLA-DR3 and to directly interact with HLA-DRβ-Arg74, the signature pocket amino acid that is shared between HT, GD, and APS3v. In the aforementioned humanized mouse models, Cepharanthine demonstrated an inhibitory effect on T-cell stimulation and its downstream cytokine production. Cepharanthine is an alkaloid extracted from the plant Stephania cepharantha Hayata that, curiously, has been used for more than 40 years in Japan for several chronic and acute conditions [51], including immune thrombocytopenic purpura associated with multiple myeloma [52], radiation-induced leukopenia [53, 54], and even venomous snakebites [55]. Cepharanthine has also been reported to have other beneficial effects such as anti-tumor [56] and anti-allergic activities [57] and multidrug resistance reversal [58]. No major adverse effects have fortunately been reported so far, although this may be due to the limited number of studies performed.
In our humanized mouse models of HT, GD, and APS3v, we demonstrated that Cepharanthine can block the respective diseases through inhibition of T-cell activation. We hypothesize that this effect of Cepharanthine is mediated by blocking the specific HLA-DR3 pocket variant that is associated with AITD and APS3v. In fact, molecular modeling shows that Cepharanthine interacts directly with Arg74, the critical amino acid variant that facilitates and enhances autoantigenic peptide binding and presentation to T-cells (Figure 2). By blocking the HLA-DR3 pocket, Cepharanthine shows promise as a new, therapeutic agent that targets the initial step in the initiation of thyroid autoimmunity and that can be personalized to treat only patients who are positive for the HLA-DR3 allele. Furthermore, as HLA-DR3 is associated with a variety of diseases including Addison’s disease, myasthenia gravis, and SLE [59], Cepharanthine may be potentially effective in other autoimmune disorders.
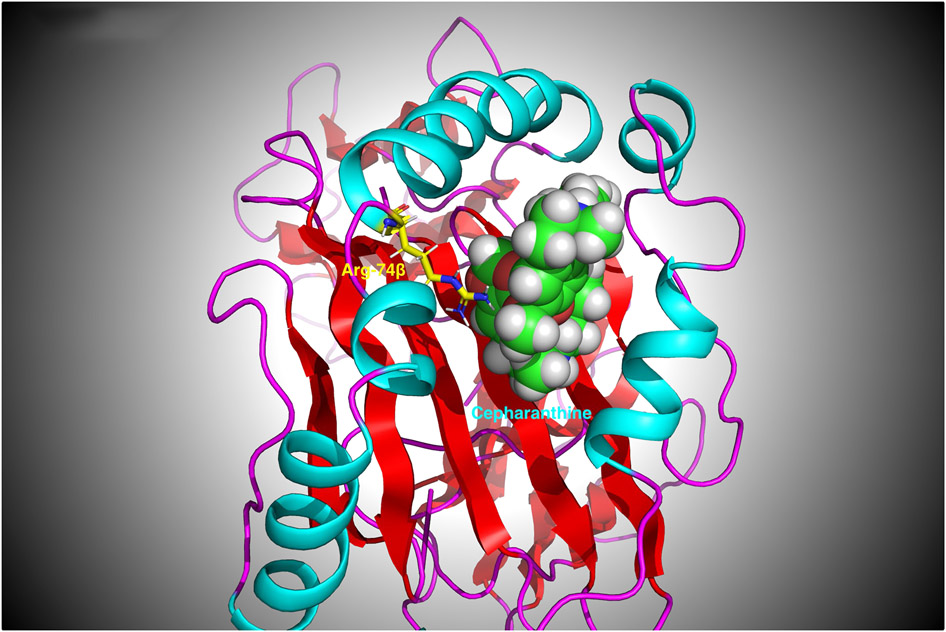
Figure 2:
3-D structure of the HLA-DRβ-Arg74 pocket with Cepharanthine bound to it. As can be seen, Cepharanthine interacts with the arginine at position β74. Arg74 (shown in yellow) is proximal to the oxygen atoms in Cepharanthine (shown in Van der Waals spheres). Therefore, Cepharanthine can prevent Tg and TSHR peptides from binding to the pocket and being presented to T-cells.
D. IMMUNE-RELATED AITD SUSCEPTIBILITY GENE: CD40
CD40 (cluster of differentiation 40), a key immune-modulating co-stimulator that regulates B-cell activity has been shown to be a major susceptibility gene for GD [2, 60]. SNPs within the CD40 gene have emerged as genetic variants predisposing to disease [61, 62]. In addition to GD, its aberrant function has been linked to a variety of other autoimmune disorders, and recently, there has been a renewed interest in CD40 as a potential therapeutic target for autoimmune diseases given its central role in a broad spectrum of immune functions.
D.1. Overview of CD40 function
CD40 is a member of the tumor necrosis factor (TNF) receptor superfamily and is a transmembrane cell surface receptor expressed classically on B lymphocytes and other antigen presenting cells (APCs). The interaction between CD40 and its ligand CD40L (CD40 ligand or CD154), expressed on activated T lymphocytes, provides essential co-stimulatory signals for proper adaptive immunity. CD40 stimulation drives B-cell proliferation, expansion, and activation. It upregulates major histocompatibility complex class II (MHC II) on B-cells, drives plasma cell differentiation, and potentiates immunoglobulin isotype class switching and antibody secretion [63]. CD40 is likewise important to the function of professional APCs; its signaling cascade enhances the maturation and survival of dendritic cells and promotes the development of activated macrophages [64]. The immunological actions of CD40 are mediated through downstream transcription pathways, such as the NF-κB cascade, and its specific effects are influenced by the culmination of proinflammatory cytokines and chemokines [65, 66]. Subsequent to CD40 binding its ligand CD40L, T-cell priming is not only optimized but CD40L undergoes cleavage, releasing a soluble CD40L that retains biological activity and is, itself, a cytokine [67].
The absence or deficiency of CD40 or CD40L results in various profound immune deficiencies as seen in Hyper IgM syndrome. Clinical manifestations include defective immunoglobulin class switching with limited IgA, IgG, and IgE, alterations in germinal center responses, and increased risk for opportunistic infections [68].
D.2. CD40 is a major GD susceptibility gene
Linkage and association studies have mapped the CD40 gene on chromosome 20q11 as a susceptibility gene-locus for GD and, interestingly, not for HT [61, 62]. Genomic sequencing studies have further identified SNPs that confer an increased risk for disease. Our group identified one of the first such CD40 polymorphisms: the −1 C/T SNP (rs1883832) located in the Kozak sequence of the 5’UTR, a consensus sequence that is essential for the initiation of protein translation [61]. In vitro studies have correlated the disease-associated C allele with approximately 30% more CD40 protein expression compared to the protective T allele. It was initially proposed that the underlying CD40 functional pathology in GD may be due to an aberrantly enhanced translational efficiency, but increased transcriptional activity most likely also plays an important role [69]. Numerous case-control association analyses have predominantly confirmed an increased risk for GD with the C allele with an estimated OR of up to 1.9 [70-73]. These findings have been replicated in a variety of geographic and ethnic populations including Caucasian American, Korean, Japanese, and Chinese Han populations. Other CD40 polymorphisms (rs11569309, rs745307, rs3765457) have been associated with reduced remission rates after withdrawal of antithyroidal therapy [74].
Underscoring a shared autoimmune pathoetiology, the CD40 gene and its genetic polymorphisms have also been implicated in other autoimmune disorders. In particular, the aforementioned rs1883832 SNP has been linked to SLE, RA, Behcet’s disease, giant cell arteritis, and immune thrombocytic purpura [75-78]. The rs4810485 SNP has likewise been associated with autoimmunity, including psoriasis, multiple sclerosis, SLE, RA, and Behcet’s disease [75, 79, 80]. In addition, increased serum levels of soluble CD40L have been reported in Sjogren’s disease as well as SLE and RA [81]. Intriguingly, studies have even shown that CD40 polymorphisms may modify the risk potential for non-autoimmune inflammatory diseases such as atherosclerosis and asthma [82, 83].
D.3. Functional significance of CD40 in GD
In addition to being expressed on APCs as an immune co-stimulatory molecule, CD40 is also expressed by a broad spectrum of non-hematopoietic cells including epithelial cells, endothelial cells, and fibroblasts [63, 84]. Thyroid follicular cells express CD40 [84] and are therefore able to provide co-stimulatory signals to locally activate T-cells. In fact, studies have demonstrated enhanced CD40 expression in thyroid tissues of GD patients [85] as well as in orbital fibroblasts of patients with Graves’ ophthalmopathy [86]. Elevated serum levels of soluble CD40L have also been associated with GD in pediatric populations [67].
We therefore hypothesized that CD40 upregulation in thyroid follicular cells, driven by a disease-associated SNP allele, may trigger GD. To test this hypothesis, we generated a transgenic mouse over-expressing CD40 in the thyroid. With induction of EAGD by a modified Nagayama protocol, these CD40-transgenic mice developed significantly higher TSHR antibody titers and thyroxine levels compared to wild-type EAGD mice [87]. Furthermore, when chimeric mice in which CD40 was knocked out in all bone marrow-derived cells, including the thyroid, underwent EAGD induction, these CD40KO mice developed significantly less TSHR antibodies than the control EAGD mice [87]. Collectively, these results support the hypothesis that thyroid-specific CD40 over-expression amplifies the production of pathogenic TSHR antibodies and can trigger and/or accelerate GD.
The exact mechanisms by which increased thyroidal CD40 expression, in the context of a risk-SNP allele, predisposes and provokes GD remains unclear. The subsequent secretion of multiple proinflammatory cytokines by thyrocytes may be a possible mechanism. In fact, both in vitro and in vivo studies have correlated CD40 activation to the secretion of an augmented cytokine profile. IL-6, IL-8, TNFα, and BAFF (B-cell activating factor) levels, among other cytokines from the canonical and non-canonical NFκB pathways, are upregulated with CD40 stimulation; and the levels are even more pronounced in GD [88]. On the other hand, CD40-CD40L blockade in murine models of GD has effectively dampened the humoral response and the degree of inflammatory thyroiditis [89, 90].
Upregulated organ-specific CD40 expression and signaling have similarly been characterized in other autoimmune diseases [91, 92]. Chondrocytes from the articular cartilage of patients with RA and animal models of collagen-induced arthritis have been shown to have higher CD40 expression [92, 93]. Endothelial cells from patients with Crohn’s disease and B-cells from kidney biopsies of patients with lupus nephritis overexpress CD40 [94, 95]. Marked CD40L upregulation has been noted in peripheral T-cells isolated from patients with SLE and psoriatic arthritis [95]. And increased levels of soluble CD40L have been described in type 1 diabetes mellitus [64, 96]. In summary, the resounding evidence indicates that amplified CD40 expression and the subsequent enhanced CD40-CD40L interaction is a shared mechanism of autoimmune dysfunction.
D.5. CD40-CD40L interaction: a new therapeutic target in GD
As mentioned, CD40 has consistently been identified as a significant player contributing to the pathogenesis of GD. The CD40-CD40L interaction provides essential signals to potentiate B-cells and prime T-cells, triggering effective antibody and cell-mediated immune responses. Normally under tight regulation, any aberrant and excessive CD40 signaling would, expectedly, disrupt the delicate balance between self-tolerance and pathogen immunity. For these reasons, the CD40-CD40L pathway is positioned as a coveted therapeutic target in GD.
D.6. CD40L and CD40 MAbs: future therapeutic agents in GD (Figure 3)
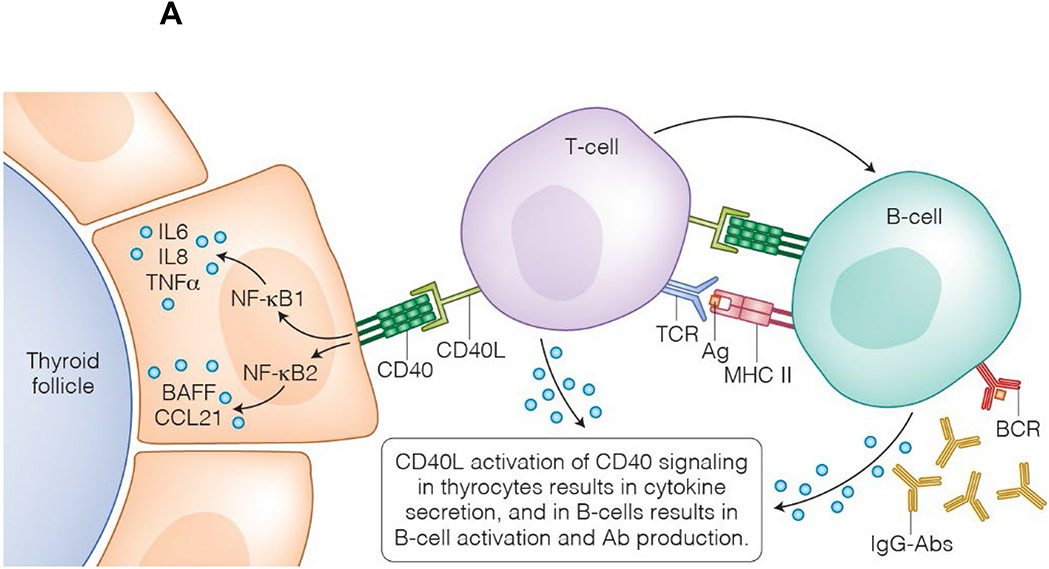
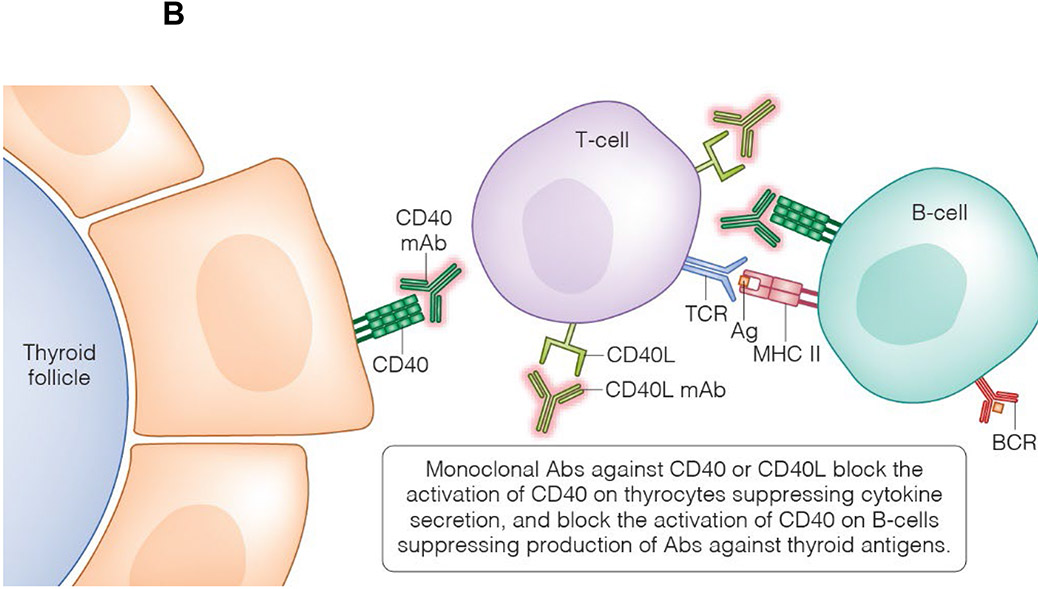
Figure 3:
Rationale for the development of CD40-targeted therapies. *A T-cell interacts with either a professional APC (i.e., B-cell) or a non-professional APC (i.e., thyroid cell); for illustrative purposes, this figure depicts both types of interactions concurrently with a single T-cell.
(A) CD40 is expressed on thyroid cells and B-cells. CD40-CD40L binding activates CD40 signaling pathways in thyroid cells, such as the canonical and non-canonical NFκB pathways, leading to the secretion of IL-6, IL-8, TNFα, and BAFF. These drive a local inflammatory response in the thyroid and, in genetically susceptible individuals, results in the activation of thyroid-reactive T-cells that have escaped tolerance. Activation of CD40 signaling pathways in B-cells leads to their differentiation into plasma cells. Autoreactive B-cells will secrete antibodies targeting the TSHR. (B) MAb’s targeting either CD40 or CD40L can block these pathways and may have a beneficial clinical effect in GD.
The earliest therapies directed against the CD40-CD40L pathway included CD40L-targeted MAbs. Unfortunately, these first generation CD40L antagonists (Ruplizumab and Toralizumab) led to significant thromboembolic events, halting further clinical trials [97]. It was determined that the thrombotic adverse effects were potentially due to the upregulated presence of CD40L and the Fc receptor (CD32a) on activated human platelets, subsequently resulting in MAb-mediated platelet aggregation [64, 95]. Second generation CD40L antagonists have since been engineered without the Fc portion and are under active preclinical or early phase investigations. Letolizumab and Dapirolizumab are currently being studied in SLE and transplant graft survival [64].
Iscalimab (CFZ533) is a humanized anti-CD40 MAb incapable of B cell depletion and with an inert Fc tail, thereby rendering it void of any potential Fc receptor-mediated effects [64, 95]. Preclinical animal models of renal transplantation showed improved renal allograft survival without any evidence of thromboembolic events. Histologically, prolonged preserved kidney morphology and an absence of splenic germinal centers were observed with treatment. Additionally, alloantibodies were notably absent and the serum cytokine levels were not increased with treatment, indicating Iscalimab’s blockade of T-cell dependent antibody responses [98]. Subsequently, the first human clinical trial to assess the safety of Iscalimab soon followed, confirming Iscalimab’s overall well-tolerated, thromboembolic-free profile. Numerous clinical trials with novel humanized anti-CD40 MAbs (Iscalimab CFZ533, Bleselumab ASKP1240, and BI 655064) are currently underway [64, 95]. The majority of these trials focus on rheumatological diseases such as Sjogren’s syndrome, RA, plaque psoriasis, and myasthenia gravis.
A recent phase II clinical trial investigated the effects of Iscalimab in patients with GD. Monthly infusions were administered over 12 weeks followed by an additional 24 week monitoring period [99]. The primary clinical outcomes measured included the levels of thyroid hormones and thyroid antibodies. Of the 15 thyrotoxic patients, seven patients responded, achieving biochemical remission by week 24, without rescue antithyroidal medications. Two of these patients even experienced improvements in orbitopathy. TSHR, TPO, and thyroglobulin antibodies were also broadly reduced with normalization of the TSHR antibodies in 4 responders. Subsequent CD40 genotyping identified that the responders share a particular CD40 haplotype (C allele of rs1883832, the G allele of rs4810485, and the T allele of rs6074033) that also correlated with significantly increased CD40 mRNA levels [100]. By targeting the underlying pathogenesis of GD, the potential therapeutic benefits of CD40 and/or CD40L blockade are compelling and promising.
E. SUMMARY
In the era of precision medicine, in which treatments are personalized to an individual’s genetic architecture and environmental history, it is necessary to comprehensively elucidate and understand the underlying drivers of disease. Studies in AITD have shown that these key mechanistic instigators include major susceptibly genes such as the thyroid-specific Tg and TSHR genes and the immunoregulatory HLA-DR3 and CD40 genes. Moreover, we have now pinpointed the functional role of SNP variants and epigenetic changes that trigger AITD and how these specifically predispose to a wide spectrum of immunological dysfunction ranging from aberrant self-peptide exposure and autoantigen presentation to T- and B-cell over-stimulation. Inhibition and circumvention at any of these pathogenic processes represent an exciting approach to target an early, underlying trigger and prevent progression to clinical disease. Current available treatments of thyroid hormone replacement and anti-thyroidal drugs for HT and GD, respectively, do not target the autoimmune process and merely provide symptomatic relief.
The future of targeted therapies in the treatment of AITD has finally arrived. In line with the various MAbs utilized for rheumatological diseases, with which AITD often shares a common pathogenesis, MAbs targeting various pathways that are altered in AITD show promise. Blocking self-peptide presentation to T-cells by MHC II complexes, an essential step in the progression to autoimmunity, with small molecules are likewise ideal targets. Such precise, targeted immune therapies potentially carry a three-fold benefit: alleviation of clinical symptoms, disease prevention, and even disease reversal.
PRACTICE POINTS.
Hashimoto’s thyroiditis (HT) and Graves’ disease (GD) are among the most prevalent autoimmune diseases.
Current treatment modalities include thyroid hormone replacement therapy in HT and suppression of excess thyroid hormone production in GD.
Both genetic and environmental determinants influence the development of autoimmune thyroid diseases (AITD). Evidence shows that epigenetic and genetic polymorphisms of the Tg, TSHR, HLA-DR3, and CD40 genes, among others, can alter the production of antigenic thyroidal self-peptides, the binding affinity of autoantigens to MHC, and the degree of T- and B-cell stimulation.
RESEARCH AGENDA.
Further research to comprehensively understand the functional genetic mechanisms of AITD’s susceptibility genes are needed. These will drive the development of novel, translational therapies in the treatment AITD.
In genetically susceptible individuals for autoimmunity, IFNα and its receptor are potential therapeutic targets for monoclonal antibodies. More studies are needed to further clarify if blocking IFNα or its receptor could be clinically useful in AITD.
Monoclonal antibodies against CD40 and CD40L are currently undergoing development and investigation for the treatment of various autoimmune disorders including GD. Future clinical trials are needed to better understand their clinical efficacy.
Small molecules that block aberrant autoantigen presentation are a class of novel therapeutics. Additional studies, including clinical trials, with Cepharanthine are needed.
ACKNOWLEDGEMENT
This work was supported in part by grants DK067555 and DK073681 from NIDDK (to YT) and by a research grant from the American Thyroid Association (to CWL).
We thank Dr. Roman Osman for creating figure 2 for the manuscript and for helpful discussions. We also want to acknowledge Katie Vicari for creating Figures 1 and 3 of the manuscript.
Footnotes
CONFLICT OF INTEREST STATEMENT
Dr. Tomer declares that he was previously (1/2015 – 6/2017) the principal investigator on a basic research project jointly funded by the Juvenile Diabetes Research Foundation and Pfizer. The current manuscript is not related to that research project. Drs. Tomer and Li declare that they submitted a patent application for Cepharanthine as a treatment for APS3v and T1D. Dr. Tomer also declares that he submitted patent applications for Cepharanthine as a treatment for AITD, for a method for predicting patient response to CD40-targeted therapies, and for peptide treatment for T1D. All other authors have no potential conflict of interest to declare.
REFERENCES
- [1].Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489–99. [DOI] [PubMed] [Google Scholar]
- [2].Jacobson EM, Tomer Y. The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: back to the future. J Autoimmun 2007;28:85–98.* [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ban Y, Greenberg DA, Concepcion E, et al. Amino acid substitutions in the thyroglobulin gene are associated with susceptibility to human and murine autoimmune thyroid disease. Proc Natl Acad Sci U S A 2003;100:15119–24.* [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Citterio CE, Rivolta CM, Targovnik HM. Structure and genetic variants of thyroglobulin: Pathophysiological implications. Mol Cell Endocrinol 2021;528:111227. [DOI] [PubMed] [Google Scholar]
- [5].Davies TF, Andersen S, Latif R, et al. Graves' disease. Nat Rev Dis Primers 2020;6:52. [DOI] [PubMed] [Google Scholar]
- [6].McLachlan SM, Rapoport B. Thyroid Autoantibodies Display both "Original Antigenic Sin" and Epitope Spreading. Front Immunol 2017;8:1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Morshed SA, Davies TF. Graves' Disease Mechanisms: The Role of Stimulating, Blocking, and Cleavage Region TSH Receptor Antibodies. Horm Metab Res 2015;47:727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hasham A, Tomer Y. Genetic and epigenetic mechanisms in thyroid autoimmunity. Immunol Res 2012;54:204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Davies TF, Yin X, Latif R. The genetics of the thyroid stimulating hormone receptor: history and relevance. Thyroid 2010;20:727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stefan M, Faustino LC. Genetics of Thyroid-Stimulating Hormone Receptor-Relevance for Autoimmune Thyroid Disease. Front Endocrinol (Lausanne) 2017;8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tomer Y, Huber A. The etiology of autoimmune thyroid disease: a story of genes and environment. J Autoimmun 2009;32:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brand OJ, Barrett JC, Simmonds MJ, et al. Association of the thyroid stimulating hormone receptor gene (TSHR) with Graves' disease. Hum Mol Genet 2009;18:1704–13. [DOI] [PubMed] [Google Scholar]
- [13].Stefan M, Jacobson EM, Huber AK, et al. Novel variant of thyroglobulin promoter triggers thyroid autoimmunity through an epigenetic interferon alpha-modulated mechanism. J Biol Chem 2011;286:31168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lahooti H, Edirimanne S, Walsh JP, et al. Single nucleotide polymorphism 1623 A/G (rs180195) in the promoter of the Thyroglobulin gene is associated with autoimmune thyroid disease but not with thyroid ophthalmopathy. Clin Ophthalmol 2017;11:1337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Menconi F, Hasham A, Tomer Y. Environmental triggers of thyroiditis: hepatitis C and interferon-alpha. J Endocrinol Invest 2011;34:78–84. [DOI] [PubMed] [Google Scholar]
- [16].Tomer Y. Hepatitis C and interferon induced thyroiditis. J Autoimmun 2010;34:J322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Faustino LC, Lombardi A, Madrigal-Matute J, et al. Interferon-alpha Triggers Autoimmune Thyroid Diseases via Lysosomal-Dependent Degradation of Thyroglobulin. J Clin Endocrinol Metab 2018;103:3678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fregno I, Molinari M. Proteasomal and lysosomal clearance of faulty secretory proteins: ER-associated degradation (ERAD) and ER-to-lysosome-associated degradation (ERLAD) pathways. Crit Rev Biochem Mol Biol 2019;54:153–63. [DOI] [PubMed] [Google Scholar]
- [19].Jacobson EM, Yang H, Menconi F, et al. Employing a recombinant HLA-DR3 expression system to dissect major histocompatibility complex II-thyroglobulin peptide dynamism: a genetic, biochemical, and reverse immunological perspective. The Journal of biological chemistry 2009;284:34231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li CW, Menconi F, Osman R, et al. Identifying a Small Molecule Blocking Antigen Presentation in Autoimmune Thyroiditis. J Biol Chem 2016;291:4079–90.* [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Menconi F, Huber A, Osman R, et al. Tg.2098 is a major human thyroglobulin T-cell epitope. J Autoimmun 2010;35:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Muixi L, Carrascal M, Alvarez I, et al. Thyroglobulin peptides associate in vivo to HLA-DR in autoimmune thyroid glands. J Immunol 2008;181:795–807. [DOI] [PubMed] [Google Scholar]
- [23].Flynn JC, McCormick DJ, Brusic V, et al. Pathogenic human thyroglobulin peptides in HLA-DR3 transgenic mouse model of autoimmune thyroiditis. Cell Immunol 2004;229:79–85. [DOI] [PubMed] [Google Scholar]
- [24].Mizuma T, Watanabe M, Inoue N, et al. Association of the polymorphisms in the gene encoding thyroglobulin with the development and prognosis of autoimmune thyroid disease. Autoimmunity 2017;50:386–92. [DOI] [PubMed] [Google Scholar]
- [25].Faustino LC, Li CW, Stefan-Lifshitz M, et al. A Novel Mouse Model of Autoimmune Thyroiditis Induced by Immunization with Adenovirus Containing Full-Length Thyroglobulin cDNA: Implications to Genetic Studies of Thyroid Autoimmunity. Thyroid 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Marin-Sanchez A, Alvarez-Sierra D, Gonzalez O, et al. Regulation of TSHR Expression in the Thyroid and Thymus May Contribute to TSHR Tolerance Failure in Graves' Disease Patients via Two Distinct Mechanisms. Front Immunol 2019;10:1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Colobran R, Armengol Mdel P, Faner R, et al. Association of an SNP with intrathymic transcription of TSHR and Graves' disease: a role for defective thymic tolerance. Hum Mol Genet 2011;20:3415–23. [DOI] [PubMed] [Google Scholar]
- [28].Stefan M, Wei C, Lombardi A, et al. Genetic-epigenetic dysregulation of thymic TSH receptor gene expression triggers thyroid autoimmunity. Proc Natl Acad Sci U S A 2014;111:12562–7.* [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Furey TS, Sethupathy P. Genetics. Genetics driving epigenetics. Science 2013;342:705–6. [DOI] [PubMed] [Google Scholar]
- [30].Local A, Huang H, Albuquerque CP, et al. Identification of H3K4me1-associated proteins at mammalian enhancers. Nat Genet 2018;50:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lombardi A, Tsomos E, Hammerstad SS, et al. Interferon alpha: The key trigger of type 1 diabetes. J Autoimmun 2018;94:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Morand EF, Furie R, Tanaka Y, et al. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N Engl J Med 2020;382:211–21. [DOI] [PubMed] [Google Scholar]
- [33].Mullard A. FDA approves AstraZeneca's anifrolumab for lupus. Nat Rev Drug Discov 2021;20:658. [DOI] [PubMed] [Google Scholar]
- [34].Psarras A, Emery P, Vital EM. Type I interferon-mediated autoimmune diseases: pathogenesis, diagnosis and targeted therapy. Rheumatology (Oxford) 2017;56:1662–75. [DOI] [PubMed] [Google Scholar]
- [35].Jacobson EM, Huber A, Tomer Y. The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology. J Autoimmun 2008;30:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ban Y, Davies TF, Greenberg DA, et al. Arginine at position 74 of the HLA-DR beta1 chain is associated with Graves' disease. Genes Immun 2004;5:203–8.* [DOI] [PubMed] [Google Scholar]
- [37].Farid NR, Stone E, Johnson G. Graves' disease and HLA: clinical and epidemiologic associations. Clinical endocrinology 1980;13:535–44. [DOI] [PubMed] [Google Scholar]
- [38].Menconi F, Monti MC, Greenberg DA, et al. Molecular amino acid signatures in the MHC class II peptide-binding pocket predispose to autoimmune thyroiditis in humans and in mice. Proc Natl Acad Sci U S A 2008;105:14034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Menconi F, Osman R, Monti MC, et al. Shared molecular amino acid signature in the HLA-DR peptide binding pocket predisposes to both autoimmune diabetes and thyroiditis. Proc Natl Acad Sci U S A 2010;107:16899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Eisenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. N Engl J Med 2004;350:2068–79. [DOI] [PubMed] [Google Scholar]
- [41].Huber A, Menconi F, Corathers S, et al. Joint genetic susceptibility to type 1 diabetes and autoimmune thyroiditis: from epidemiology to mechanisms. Endocr Rev 2008;29:697–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ji N, Somanaboeina A, Dixit A, et al. Small molecule inhibitor of antigen binding and presentation by HLA-DR2b as a therapeutic strategy for the treatment of multiple sclerosis. Journal of immunology 2013;191:5074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ostrov DA, Alkanani A, McDaniel KA, et al. Methyldopa blocks MHC class II binding to disease-specific antigens in autoimmune diabetes. The Journal of clinical investigation 2018;128:1888–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li CW, Osman R, Menconi F, et al. Cepharanthine blocks TSH receptor peptide presentation by HLA-DR3: Therapeutic implications to Graves' disease. Journal of autoimmunity 2020;108:102402.* [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li CW, Osman R, Menconi F, et al. Cepharanthine Blocks Presentation of Thyroid and Islet Peptides in a Novel Humanized Autoimmune Diabetes and Thyroiditis Mouse Model. Frontiers in immunology 2021;12:796552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shulman S. Thyroid antigens and autoimmunity. Advances in immunology 1971;14:85–185. [DOI] [PubMed] [Google Scholar]
- [47].Chen CR, Hamidi S, Braley-Mullen H, et al. Antibodies to thyroid peroxidase arise spontaneously with age in NOD.H-2h4 mice and appear after thyroglobulin antibodies. Endocrinology 2010;151:4583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Inaba H, Martin W, De Groot AS, et al. Thyrotropin receptor epitopes and their relation to histocompatibility leukocyte antigen-DR molecules in Graves' disease. J Clin Endocrinol Metab 2006;91:2286–94. [DOI] [PubMed] [Google Scholar]
- [49].Nagayama Y, Kita-Furuyama M, Ando T, et al. A novel murine model of Graves' hyperthyroidism with intramuscular injection of adenovirus expressing the thyrotropin receptor. J Immunol 2002;168:2789–94. [DOI] [PubMed] [Google Scholar]
- [50].Li CW, Osman R, Menconi F, et al. Flexible peptide recognition by HLA-DR triggers specific autoimmune T-cell responses in autoimmune thyroiditis and diabetes. Journal of autoimmunity 2017;76:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bailly C. Cepharanthine: An update of its mode of action, pharmacological properties and medical applications. Phytomedicine : international journal of phytotherapy and phytopharmacology 2019;62:152956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tabata R, Tabata C, Tazoh A, et al. Low dose cepharanthine ameliorates immune thrombocytopenic purpura associated with multiple myeloma. Int Immunopharmacol 2012;13:242–4. [DOI] [PubMed] [Google Scholar]
- [53].Ohta T, Morita K. [Effect of cepharanthin on radiotherapy induced leukopenia]. Rinsho hoshasen Clinical radiography 1990;35:471–4. [PubMed] [Google Scholar]
- [54].Suzuki R, Hara M, Shindoh J, et al. [Effects of cepharanthin on leukopenia and thrombocytopenia induced by chemotherapy in lung cancer patients]. Gan to kagaku ryoho Cancer & chemotherapy 1992;19:647–52. [PubMed] [Google Scholar]
- [55].Kimoto T, Suemitsu K, Nakayama H, et al. Therapeutic experience of venomous snakebites by the Japanese viper (Agkistrodon halys Blomhoffii) with low dose of antivenin: report of 43 consecutive cases. Nihon Geka Hokan 1997;66:71–7. [PubMed] [Google Scholar]
- [56].Harada K, Ferdous T, Itashiki Y, et al. Cepharanthine inhibits angiogenesis and tumorigenicity of human oral squamous cell carcinoma cells by suppressing expression of vascular endothelial growth factor and interleukin-8. Int J Oncol 2009;35:1025–35. [DOI] [PubMed] [Google Scholar]
- [57].Kohno H, Inoue H, Seyama Y, et al. [Mode of the anti-allergic action of cepharanthine on an experimental model of allergic rhinitis]. Nihon Yakurigaku Zasshi 1987;90:205–11. [DOI] [PubMed] [Google Scholar]
- [58].Fujimura T, Shibata H, Maekawa I, et al. Reversal of resistance to doxorubicin with cepharanthine in murine P388 leukemia cells. Jpn J Pharmacol 1990;54:464–7. [DOI] [PubMed] [Google Scholar]
- [59].Gough SC, Simmonds MJ. The HLA Region and Autoimmune Disease: Associations and Mechanisms of Action. Curr Genomics 2007;8:453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tomer Y. Mechanisms of autoimmune thyroid diseases: from genetics to epigenetics. Annu Rev Pathol 2014;9:147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Tomer Y, Concepcion E, Greenberg DA. A C/T single-nucleotide polymorphism in the region of the CD40 gene is associated with Graves' disease. Thyroid 2002;12:1129–35. [DOI] [PubMed] [Google Scholar]
- [62].Zhang QY, Liu W, Li L, et al. Genetic Study in a Large Cohort Supported Different Pathogenesis of Graves' Disease and Hashimoto's Hypothyroidism. J Clin Endocrinol Metab 2020;105. [DOI] [PubMed] [Google Scholar]
- [63].Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol 2009;21:293–300.* [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Karnell JL, Rieder SA, Ettinger R, et al. Targeting the CD40-CD40L pathway in autoimmune diseases: Humoral immunity and beyond. Adv Drug Deliv Rev 2019;141:92–103. [DOI] [PubMed] [Google Scholar]
- [65].Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol 1998;16:111–35. [DOI] [PubMed] [Google Scholar]
- [66].Bishop GA. The multifaceted roles of TRAFs in the regulation of B-cell function. Nat Rev Immunol 2004;4:775–86. [DOI] [PubMed] [Google Scholar]
- [67].Metwalley KA, Farghaly HS, Raafat DM, et al. Soluble CD40 Ligand Levels in Children with Newly Diagnosed Graves' Disease. J Clin Res Pediatr Endocrinol 2020;12:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yazdani R, Fekrvand S, Shahkarami S, et al. The hyper IgM syndromes: Epidemiology, pathogenesis, clinical manifestations, diagnosis and management. Clin Immunol 2019;198:19–30. [DOI] [PubMed] [Google Scholar]
- [69].Jacobson EM, Concepcion E, Oashi T, et al. A Graves' disease-associated Kozak sequence single-nucleotide polymorphism enhances the efficiency of CD40 gene translation: a case for translational pathophysiology. Endocrinology 2005;146:2684–91.* [DOI] [PubMed] [Google Scholar]
- [70].Kurylowicz A, Kula D, Ploski R, et al. Association of CD40 gene polymorphism (C-1T) with susceptibility and phenotype of Graves' disease. Thyroid 2005;15:1119–24. [DOI] [PubMed] [Google Scholar]
- [71].Ban Y, Tozaki T, Taniyama M, et al. Association of a C/T single-nucleotide polymorphism in the 5' untranslated region of the CD40 gene with Graves' disease in Japanese. Thyroid 2006;16:443–6. [DOI] [PubMed] [Google Scholar]
- [72].Kim TY, Park YJ, Hwang JK, et al. A C/T polymorphism in the 5'-untranslated region of the CD40 gene is associated with Graves' disease in Koreans. Thyroid 2003;13:919–25. [DOI] [PubMed] [Google Scholar]
- [73].Hsiao JY, Hsieh MC, Hsiao CT, et al. Association of CD40 and thyroglobulin genes with later-onset Graves' disease in Taiwanese patients. Eur J Endocrinol 2008;159:617–21. [DOI] [PubMed] [Google Scholar]
- [74].Wang PW, Chen IY, Juo SH, et al. Genotype and phenotype predictors of relapse of graves' disease after antithyroid drug withdrawal. Eur Thyroid J 2013;1:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Inal EE, Rustemoglu A, Inanir A, et al. Associations of rs4810485 and rs1883832 polymorphisms of CD40 gene with susceptibility and clinical findings of Behcet's disease. Rheumatol Int 2015;35:837–43. [DOI] [PubMed] [Google Scholar]
- [76].Rodriguez-Rodriguez L, Castaneda S, Vazquez-Rodriguez TR, et al. Influence of CD40 rs1883832 polymorphism in susceptibility to and clinical manifestations of biopsy-proven giant cell arteritis. J Rheumatol 2010;37:2076–80. [DOI] [PubMed] [Google Scholar]
- [77].Huang Q, Xu WD, Su LC, et al. Association of CD40 Gene Polymorphisms With Systemic Lupus Erythematosus and Rheumatoid Arthritis in a Chinese Han Population. Front Immunol 2021;12:642929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].AbdelGhafar MT, El-Kholy RA, Elbedewy TA, et al. Impact of CD40 gene polymorphisms on the risk of immune thrombocytopenic purpura. Gene 2020;736:144419. [DOI] [PubMed] [Google Scholar]
- [79].Zervou MI, Goulielmos GN, Castro-Giner F, et al. A CD40 and an NCOA5 gene polymorphism confer susceptibility to psoriasis in a Southern European population: a case-control study. Hum Immunol 2011;72:761–5. [DOI] [PubMed] [Google Scholar]
- [80].Raychaudhuri S, Remmers EF, Lee AT, et al. Common variants at CD40 and other loci confer risk of rheumatoid arthritis. Nat Genet 2008;40:1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Toubi E, Shoenfeld Y. The role of CD40-CD154 interactions in autoimmunity and the benefit of disrupting this pathway. Autoimmunity 2004;37:457–64. [DOI] [PubMed] [Google Scholar]
- [82].Sultan CS, Weitnauer M, Turinsky M, et al. Functional association of a CD40 gene single-nucleotide polymorphism with the pathogenesis of coronary heart disease. Cardiovasc Res 2020;116:1214–25. [DOI] [PubMed] [Google Scholar]
- [83].Park JH, Chang HS, Park CS, et al. Association analysis of CD40 polymorphisms with asthma and the level of serum total IgE. Am J Respir Crit Care Med 2007;175:775–82. [DOI] [PubMed] [Google Scholar]
- [84].Faure GC, Bensoussan-Lejzerowicz D, Bene MC, et al. Coexpression of CD40 and class II antigen HLA-DR in Graves' disease thyroid epithelial cells. Clin Immunol Immunopathol 1997;84:212–5. [DOI] [PubMed] [Google Scholar]
- [85].Smith TJ, Sciaky D, Phipps RP, et al. CD40 expression in human thyroid tissue: evidence for involvement of multiple cell types in autoimmune and neoplastic diseases. Thyroid 1999;9:749–55. [DOI] [PubMed] [Google Scholar]
- [86].Hwang CJ, Afifiyan N, Sand D, et al. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD40: CD154 hyperinduces IL-6, IL-8, and MCP-1. Invest Ophthalmol Vis Sci 2009;50:2262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Huber AK, Finkelman FD, Li CW, et al. Genetically driven target tissue overexpression of CD40: a novel mechanism in autoimmune disease. J Immunol 2012;189:3043–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lee HJ, Lombardi A, Stefan M, et al. CD40 Signaling in Graves Disease Is Mediated Through Canonical and Noncanonical Thyroidal Nuclear Factor kappaB Activation. Endocrinology 2017;158:410–8.* [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Chen CR, Aliesky HA, Guo J, et al. Blockade of costimulation between T cells and antigen-presenting cells: an approach to suppress murine Graves' disease induced using thyrotropin receptor-expressing adenovirus. Thyroid 2006;16:427–34. [DOI] [PubMed] [Google Scholar]
- [90].Resetkova E, Kawai K, Enomoto T, et al. Antibody to gp39, the ligand for CD40 significantly inhibits the humoral response from Graves' thyroid tissues xenografted into severe combined immunodeficient (SCID) mice. Thyroid 1996;6:267–73. [DOI] [PubMed] [Google Scholar]
- [91].Lai JH, Luo SF, Ho LJ. Targeting the CD40-CD154 Signaling Pathway for Treatment of Autoimmune Arthritis. Cells 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gotoh H, Kawaguchi Y, Harigai M, et al. Increased CD40 expression on articular chondrocytes from patients with rheumatoid arthritis: contribution to production of cytokines and matrix metalloproteinases. J Rheumatol 2004;31:1506–12. [PubMed] [Google Scholar]
- [93].Cheng T, Wang M, Chen L, et al. Increased expression of CD40/TRAF1 and activation of nuclear factor-kappakappaB-dependent proinflammatory gene expression in collagen-induced arthritis. Scand J Rheumatol 2018;47:455–60. [DOI] [PubMed] [Google Scholar]
- [94].Danese S, Sans M, Scaldaferri F, et al. TNF-alpha blockade down-regulates the CD40/CD40L pathway in the mucosal microcirculation: a novel anti-inflammatory mechanism of infliximab in Crohn's disease. J Immunol 2006;176:2617–24. [DOI] [PubMed] [Google Scholar]
- [95].Ramanujam M, Steffgen J, Visvanathan S, et al. Phoenix from the flames: Rediscovering the role of the CD40-CD40L pathway in systemic lupus erythematosus and lupus nephritis. Autoimmun Rev 2020;19:102668. [DOI] [PubMed] [Google Scholar]
- [96].Harding SA, Sommerfield AJ, Sarma J, et al. Increased CD40 ligand and platelet-monocyte aggregates in patients with type 1 diabetes mellitus. Atherosclerosis 2004;176:321–5. [DOI] [PubMed] [Google Scholar]
- [97].Kawai T, Andrews D, Colvin RB, et al. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med 2000;6:114. [DOI] [PubMed] [Google Scholar]
- [98].Espie P, He Y, Koo P, et al. First-in-human clinical trial to assess pharmacokinetics, pharmacodynamics, safety, and tolerability of iscalimab, an anti-CD40 monoclonal antibody. Am J Transplant 2020;20:463–73. [DOI] [PubMed] [Google Scholar]
- [99].Kahaly GJ, Stan MN, Frommer L, et al. A Novel Anti-CD40 Monoclonal Antibody, Iscalimab, for Control of Graves Hyperthyroidism-A Proof-of-Concept Trial. J Clin Endocrinol Metab 2020;105.* [DOI] [PubMed] [Google Scholar]
- [100].Faustino LC, Kahaly GJ, Frommer L, et al. Precision Medicine in Graves' Disease: CD40 Gene Variants Predict Clinical Response to an Anti-CD40 Monoclonal Antibody. Front Endocrinol (Lausanne) 2021;12:691781. [DOI] [PMC free article] [PubMed] [Google Scholar]