Abstract
Human lower limb activity recognition (HLLAR) has grown in popularity over the last decade mainly because to its applications in the identification and control of neuromuscular disorders, security, robotics, and prosthetics. Surface electromyography (sEMG) sensors provide various advantages over other wearable or visual sensors for HLLAR applications, including quick response, pervasiveness, no medical monitoring, and negligible infection. Recognizing lower limb activity from sEMG signals is also challenging owing to the noise in the sEMG signal. Pre- processing of sEMG signals is extremely desirable before the classification because they allow a more consistent and precise evaluation in the above applications. This article provides a segment-by-segment overview of: (1) Techniques for eliminating artifacts from sEMG signals from the lower limb. (2) A survey of existing datasets of lower limb sEMG. (3) A concise description of the various techniques for processing and classifying sEMG data for various applications involving lower limb activity. Finally, an open discussion is presented, which may result in the identification of a variety of future research possibilities for human lower limb activity recognition. Therefore, it is possible to anticipate that the framework presented in this study can aid in the advancement of sEMG-based recognition of human lower limb activity.
Keywords: Human lower limb activity recognition, Surface electromyography signal, Machine learning techniques, Biomedical signal processing, Human-machine interaction
Introduction
In recent times, Human Activity Recognition (HAR) has attracted the attention of researchers, particularly because of the advancements in computer vision, artificial intelligence approaches, availability of wearable sensors, and the Internet of Things. HAR recognises a variety of human actions, including walking, sitting, running, standing, sleeping, showering, driving, and cooking. Numerous HAR applications can be found across a variety of disciplines, including healthcare monitoring, smart homes with aided surveillance, and tele-immersion applications [1, 2].
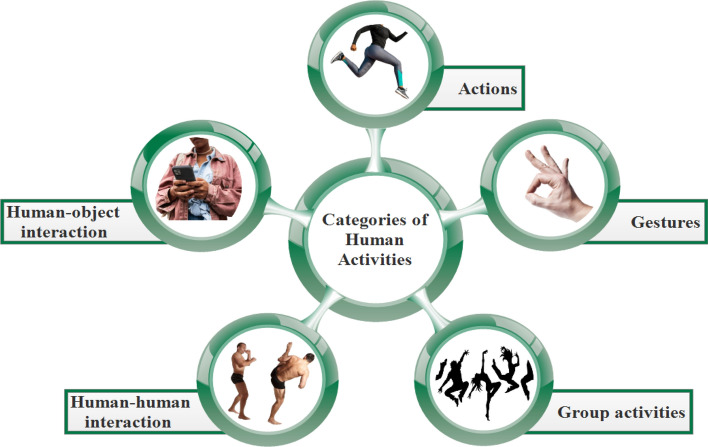
The HAR’s goal is to analyze people’s daily behaviors through observational data collected from them and their neighboring environments of living. It is a challenging problem because of the several difficulties inherent in HAR. However, the difficulty level associated with these obstacles varies according to the activity being considered. Based on the difficulty level and activity length, HAR may be categorised into five distinct types of activities, as shown in Fig. 1 [3, 4]:
Gestures based Activities involves simple activities such as the opening-closing of hands and bending of arms.
Action based Activities are single-person activities that may be composed of multiple gestures organized, such as standing, sitting, walking, cycling, etc.
Interaction based Activities involves two agents, one of that is a human and the other of that might be a person or an object. The interaction may be categorised into two types: human-object interaction and human-human interaction, depending on the nature of the agents. Human-human interactions include wrestling, embracing, and shaking hands, whereas human-object interactions include human interaction with a phone or a laptop and may be other human work’s on machine.
Group Activities are the activities performed by the group of multiple persons. For example, a group of people marching, a group having a meeting.
Fig. 1.
Representation of lower limb muscles
Two approaches can be used to observe and record the activities of the human lower limb. One is accomplished through the use of a visual sensor, while the other is accomplished through the use of wearable sensors [5] as shown in Fig. 2. In the vision-based approach, a camera is used to record the data about a human’s activities and collected data is in the form of images or video, which may then be categorised using computer vision techniques. Computer vision-based approaches provide excellent results and are also simple to implement; however, it faces numerous drawbacks for the HAR [6, 7], i.e.,
Security/privacy;
Lightning variation;
Perspective change if using single view acquisition system;
Partial occlusion of human body parts;
Limited range;
Requirement of more powerful computing machines.
Fig. 2.
Methods for human activity recognition
Improvement in sensor technology leads to the development of accelerometers, EMG, gyroscopes, and barometers, which can be used for capturing the data. Recently, these sensors are integrated with smartphones and wearables such as wristbands, smartwatches, and clothing which further improves the flexibility for a recording of the data. The EMG technique is better than the other wearable sensor techniques for detecting human activities for the application of controlling an artificial limb or exoskeleton, because it can anticipate movements and detect signal changes more quickly [8, 9]. Table 1 indicates some of the previous articles on human activity recognition. The articles are listed in chronological sequence by year of publication, and the technique is also specified in this table as Vision-Based or Sensor Based.
Table 1.
Literature Survey: founds work on human activity recognition
| Author | Year | Approach | Sensor | Activities |
|---|---|---|---|---|
| Englehart et al. [10] | 2001 | Sensor | EMG | Close/Open hand, Wrist Extension/Flexion, Radial/Ulnar Deviation |
| Pawar et al. [11] | 2007 | Sensor | ECG | Sitting on a chair, left/right and both arms simultaneously lowered and raised, Walking, Twisting Left-Right-Left body movement, Down/Up Stairs |
| Tunçel et al. [12] | 2009 | Sensor | Gyroscope | Standing, right leg lower part back movement, right leg both part forward movement with knee bending, forward/backward locomotion without bending of knee, Extending the right leg towards the opposite side of body, Squatting, the movement of both the upper and lower legs, while seated on a stool, raise just the bottom portion of the right leg. |
| Khan et al. [13] | 2010 | Sensor | Accelerometer | Combination of sitting and standing, i.e, sit-stand and vice-versa, a combo of lie and stand, i.e., lie-stand and vice-versa, normal and fast Locomotion, simple sitting/standing, upstairs/downstairs walking. |
| Wang et al. [14] | 2012 | Sensor | Triaxial Accelerometer | Task related to the household such as relax and sitting on the crouch, working on the computer by sitting at a desk, drinking/eating stuff, sleeping on the bed, locomotion in the corridor/ holding a box in arms, upstairs/downstairs, hand-washing, cleaning of windows/table/floor using cleaning rag, standing without movement and sitting. |
| Zhang et al. [15] | 2013 | Sensor | Accelerometer, Gyroscope, Magnetometer | Multiple type of locomotion tasks such as forward/right/left, upstairs/downstairs, normal, fast, jumping, stand-still and sitting. |
| Park et al. [16] | 2016 | Vision | Depth Camera | lift arms, push right, duck, goggles, shoot, bow, wind it up, throw, had enough, beat both, change weapon, and kick |
| Chen et al. [17] | 2017 | Vision | Cameras | Normal Walk, Slow Walk with Halt, Slow Lame Walk |
| Naik et al. [18] | 2018 | Sensor | EMG | Walking, Sitting, Standing |
| Hussain et al. [19] | 2019 | Sensor | Gyroscope | Locomotion at multiple speeds such as slow/normal/fast and positive/negative incline ground locomotion |
| Vijayvargiya et al. [20] | 2021 | Sensor | EMG | Walking, Sitting, Standing |
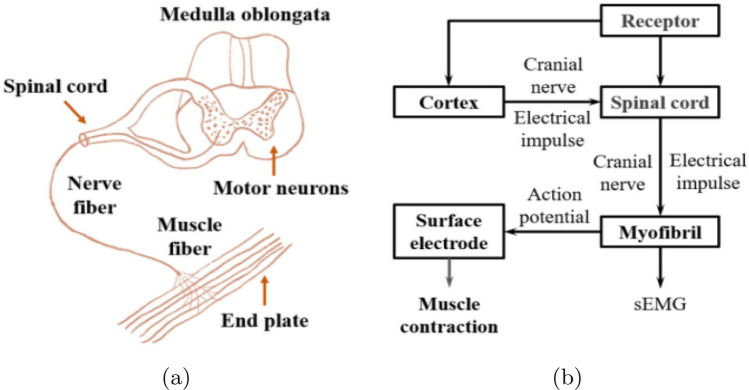
Electromyography (EMG) techniques such as surface electromyography (sEMG) and intramuscular electromyography (iEMG) are frequently employed for acquiring the EMG signals as shown in Fig. 3. sEMG has several advantages relatively to iEMG, including the fact that the electrodes can be worn without causing discomfort, and the risk of inflammation is extremely low. Long term control using surface electrodes is an easy task than the handling of iEMG needles. sEMG electrodes are classified into two types: gelled and dry sEMG electrodes. For short amounts of time, wet sEMG electrodes may provide higher-quality sEMG signals. As the gel dries, motion artefacts and high contact impedance may exacerbate the degradation of the signal. Therefore, if stability is more important than quality, dry sEMG electrodes are preferable [21]. However, the positioning of the sEMG sensors has a major impact on the recorded signal and the computational algorithm’s subsequent analysis and recognition. It is a technique in biomedical which evaluates and records the muscles generated electrical signals. The process for generating a EMG signal is as follows. Motor nerves in the cerebral cortex which is part of the central nervous system generate electrical signals. These brain signals are transmitted via axons to muscle fibers, resulting in pulse sequences that drive them to contract and generate muscular tension. Meanwhile, in the human body, a current is created, resulting in transmembrane potential. Muscles cells developed the potential difference between the internal and external potentials, by the current is created in the human body, known to be transmembrane potential. When the muscle cells are inactive, they have a polarised membrane potential. During the polarisation, a resetting potential is formed by the potential distance between the interior and outside of the membrane of the cell. Then, a cell is stimulated leads to depolarization, this propensity will continue to develop roughly. The corresponding action potential is defined as an electromyography signal, Fig. 4.
Fig. 3.

Types of EMG electrodes
Fig. 4.
The mechanism by which the EMG signal is generated: a the neuromuscular system’s structure, b a schematic representation of the nerve and muscle system’s EMG signal transduction [22]
A HAR system can monitor the activities of a human’s lower limbs by utilising a sEMG sensors . This type of human lower limb activity recognition (HLLAR) system can be beneficial in a variety of situations, including identifying jumps and lifts while performing ballet movements, identifying movements such as standing, sitting, walking, running, stair climbing and descending, vacuuming, and situps, diagnosing neuromusculoskeletal disorders that cause knee pain, composing a robotic prosthetic limb in the case of an amputee’s missing limb, and sports [23, 24]. The major contribution of this review article is as follows:
Provide a thorough investigation of the general structure and methodologies for detecting human lower limb activity using sEMG.
Describe and synthesise the sEMG datasets used in HLLAR.
Classify and evaluate the processes used in HLLAR for conventional data processing, feature engineering, and feature extraction.
Classify and analyse machine learning approaches applicable to HLLAR, with a focus on current research in deep learning in HLLAR.
Describe the difficulties and potential directions of HLLAR.
The findings from the search are presented in Fig. 5 A total of 5361 relevant records from Google Scholar, PubMed, and Scopus were evaluated during the initial phase of the search. There were only 2836 records remaining after eliminating duplicates. There were a total of 578 records considered for inclusion after being checked for their titles and abstracts. Finally, the systematic review examined 92 studies, 71 of which were published after 2010, and 21 of which were published before 2010.
Fig. 5.

PRISMA flow diagram for the systematic review
The presented research article is organised as follows: Sect. 2 discusses the overview of sEMG dataset for lower limb activities. Section 3 provides noise sources in sEMG signal during lower limb activities. Section 4 comprises the sEMG signal processing techniques. A detailed overview of machine learning techniques on the lower limb activity recognition using sEMG signal is presented in Sect. 5. Then, Sect. 6 demonstrates the applications of the lower limb activity recognition. Finally, Sect. 7 concludes the overall work and identify potential future scopes.
Overview of available sEMG datasets
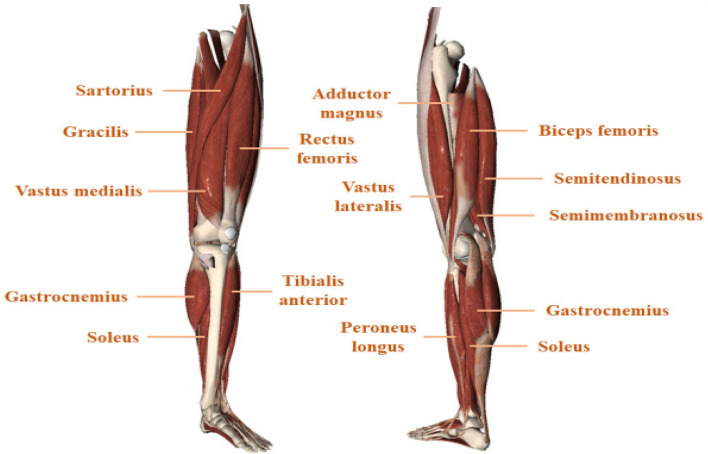
This section discusses various sEMG datasets presented in the literature, which are collected for different human lower limb activities. As per the author’s knowledge, there have been very few publicly available sEMG datasets on human lower-limb motions. The sEMG dataset for lower limb movements is presented in Table 2, which includes several subjects and their performed activities during the data collection. The sEMG signal of lower limb activities is collected from the lower limb muscles. The anatomical structure of lower limb muscles is presented in Fig. 6. Table 3 shows which limb muscles are more active or relevant for different human movements.
Table 2.
A survey of sEMG datasets for human lower limb activities
| Dataset | Year | Subjects | Activities | Sampling frequency | Instrument | Number of sensors |
|---|---|---|---|---|---|---|
| HAR-sEMG [25] | 2020 | 9 (7 Male, 2 female) | Running, Standing, Lunge Stretching, Jumping, Walking | 2000 Hz | DELSYS Trigno wireless EMG equipment | 6 |
| SUKFNN [26] | 2020 | 5 (Healthy) | walking, downstairs, upstairs, crossing obstacles and standing | 2000 Hz | Biometrics wireless sEMG sensor system | 3 |
| HHMM [27] | 2019 | 5 | Walking, Running, Jumping, Sit-to-stand | 1000 Hz | Noraxon Telemyo 2400T & 2400R | 8 |
| ENABLE3S [28] | 2018 | 10 (7 Male and 3 female) | Sitting, Standing, zero incline Walking, Stair upward/downwards, Stairs, Ramp Ascent/Descent | 1000 Hz | Delsys DE-2.1 sEMG Sensor | 7 |
| TAS [29] | 2016 | 10 | walking, sitting, standing, Stair upward/downwards | 1000 Hz | Biopac BN-EMG2s | 9 |
| MFWF [30] | 2015 | 5 | Downstairs, upstairs, downhill, and uphill | 1000 Hz | NORAXON MyoResearch XP | 9 |
| UCI-sEMG [31] | 2014 | 22 (11 Normal and 11 Abnormal) | Walking, Sitting, Standing | 1000 Hz | MWX8 by Biometrics | 4 |
Fig. 6.
Representation of lower limb muscles [22]
Table 3.
Relevant muscles involvement in movement
| Name of muscle | Types of movements |
|---|---|
| Satorius | Hip flexion and lateral rotation exercise |
| Quadriceps (Rectus femoris, Vastus medialis, Vastus lateralis, Vastus intermedius) | Hip flexion or knee extension |
| Hamstrings (biceps femoris, semitendinosus, semimembranosus) | Hip extension or knee flexion |
| Adductor magnus | Hip adduction |
| Gracilis | Hip adduction, rotation, and flexion |
| Gastrocnemius | Knee flexion and ankle plantar flexion |
| Soles | Plantar flexion of the foot |
| Tibialis anterior | Foot dorsiflexion |
The HAR-sEMG [25] dataset was built by employing the Trigno wireless biofeedback sensors to collect sEMG data from five lower limb movements, including jogging, standing, lunge stretching, walking, and jumping. To acquire the HAR-sEMG dataset, nine healthy subjects, i.e., two females and seven men with a mean age of 23.5 years took part in the study. They each provided 1800 EMG signals with a duration of 10 seconds each. A total of six major muscles were chosen for the experiment, all of which are involved in lower-limb movements and can be easily identified within the muscle group. A total of six sensors, each of which was attached to a separate muscle region, were used to collect the sEMG signals for the HAR-sEMG dataset. The dataset includes the muscles namely, tibialis anterior, gastrocnemius medialis, gastrocnemius lateralis rectus femoris, vastus lateral femoral, and semitendinosus muscles of the thigh, as well as others.
The SUKFNN [26] dataset includes 2500 sEMG recordings from five patients. The sEMG signal was obtained utilising a Biometrics UK-developed and -manufactured sEMG acquisition equipment and five common lower limb movements: plane walking, crossing of the obstacle, sit-up, ascending/descending the stairs. The medial gastrocnemius, lateral femoral muscle, and semitendinosus muscles of the lower limb were deployed for the collection of a dataset in the present study.
The HHMM [27] dataset contains the lower limb sEMG signals data of various activities for five people such as walking, jumping, running, and sit-to-stand. Eight different recording sites were used to record the sEMG dataset, the data is collected using the Noraxon Telemyo 2400T & 2400R (Noraxon Inc., AZ, USA) at 1,000-Hz sampling frequency and wet-type electrodes. The participants who were not injured were instructed to normal/fast left or right side walking across the spaces without implementation the walking and running exercises on the treadmill. A total of five sessions were conducted, with each session lasting 40 seconds and the total number of sessions being 5. Participants were instructed the alternate between the sit-to-stand action, such as the standing-up/sitting-down for the 40 seconds in each session, which was then done five times. For the counter movement leaps, participants were instructed to attempt to jump as high as they possibly could in position. The total of 8 muscles such as rectus femoris, biceps femoris, vastus lateralis, vastus medialis, peroncus longus, tibialis anterior, gastrocnemius medialis, gastrocnemius lateralis were considered in this study.
ENABLE3S dataset [28] proposed by Hu et al. consists of the neuro-mechanical lower-limb bilateral signals from 10 healthy subjects (3-female,7-male; 160–193 cm; 23–29 years; 54–95 kg) using three wearable sensors like., EMG sensors, inertial measurement unit (IMU), and goniometer. On both lower limbs, sEMG electrodes were implanted on seven muscles: gastrocnemius, vastus lateralis, semitendinus, soleus, biceps femoris, rectus femoris, and tibialis anterior. Each subject repeated two distinct sequences of the seven gait activities mentioned below for a total of 25 repetitions: Sitting Standing Level ground walking Stair/Ramp ascent flat ground walking Ramp descent/ Stair descent flat ground walking Standing Sitting. These exercises took place in a 20 x 30 foot area that contained a platform (thirty inches tall) connected to the stairs (7.7 inch rise, 10 inch run) and length of ramp (14 ft. long). The length of the chosen segment was approximately 45 feet. Following that, signals were amplified 1000 times at a sampling rate of 1 kHz and then sent through a band-pass Butterworth filter operating between 20 and 450 Hz.
The TAS dataset [29] includes ten patients with no historical musculoskeletal disorders and have aged between 26–32 years. The MP150 and six BioPac BN-EMG2s commercial available sEMG instruments were used to augment the sEMG signals. The raw data were acquired on the sEMG signal collecting device utilising a 1-kHz sampling rate with a bandpass filter of range 20–450 Hz and a 16-bit ADC converter. Nine sEMG channels were attached to the multiple muscles for collection of the dataset; namely, the biceps femoris, peroneus longus, sartorius, semitendinosus, gastrocnemius, tibialis anterior, rectus femoris, and vastus lateralis/medialis,
In the MFWF dataset [30] includes 600 sEMG signals were recorded from 5 subjects for the lower limbs activities, who have performed the four activities such as going up and down the stairs, walking up and downhill. The surface electromyography equipment NORAXON MyoResearch XP 16-channel was used to capture the sEMG signal. The sampling rate for the equipment can reach up to 2048Hz, with a bandwidth ranging from 10Hz to 1000Hz. Experiments were conducted to acquire sEMG signals from nine different muscles of lower limb: rectus femoris, vastus medialis, vastus lateris, biceps femoris, semitendinosus, tibialis anterior, medial gastrocnemius, lateral gastrocnemius, and soleus muscles.
UCI-sEMG dataset [31] proposed by Sanchez et al. concerns the three different lower limb movements performed 22 male, 11 subjects are healthy and the other 11 with knee abnormalities. Subjects were older than 18 years. Lower limb activities such as walking, sitting, and standing were performed. There was no prior case history of discomfort or damage in the knee of normal subjects. In abnormal subjects, the four had meniscus injuries, six had anterior cruciate ligament (ACL) injuries, and one had sciatic nerve injuries. The sEMG signal is collected from the left leg for the healthy person whereas the acquired signal from the abnormal subject was from the affected leg. Four surface electrodes were used for the data collection. These are placed around the muscles; namely, biceps femoris, rectus femoris, semitendinosus, and vastus medialis. A goniometer was also affixed to the outside of the knee joint for capturing the joint angle which could be used for the knee prosthetic leg application. The sample rate and resolution were 1000 Hz, and 14 bits, respectively.
Noise sources in sEMG signal during lower limb activity
Whenever a muscle’s EMG signal is recorded, it is contaminated by a variety of noise sources. The EMG signal’s characteristics are determined by the subject’s internal structure, which includes blood flow velocity, skin formation, skin temperature, measuring site, tissue structure, and other factors. Assessing and classifying EMG signals is highly difficult due to the diverse order of EMG that is influenced by the physiological/anatomical properties of muscles. In the following, the several types of electrical noise that impact EMG signals [32–34] are discussed.
Inherent noise in the EMG electrodes
Most of the electronic equipment generates the inherent or electrical noise which consists of the frequency components in the range of 0 Hz to several thousand Hz. Therefore, it is found that the silver/silver chloride electrodes (10 mm × 1 mm) has an appropriate signal-to-noise ratio (SNR) and also electrically exceptionally stable for the recording of EMG signals. On the other hand, there is an inverse relationship between the electrode size and impedance. High electrode impedance significantly lowers the signal quality and results in a poor signal-to-noise ratio. Therefore, it is necessary to consider both factors before choosing the appropriate electrodes. High electrode impedances or a large number of electrodes may be used in studies when the requirement of the statistical power is high; otherwise, use the low electrode impedances is suggested. This type of noise can be mitigated by the employment of smart circuit design with high-quality instrumentation.
Ambient noise
The human body works as an antenna, with electric and magnetic radiation assaulting its surface all the time, causing electromagnetic noise. The unwanted signal recorded from a muscle is either superimposed or canceled by electromagnetic sources in the environment. The magnitude of the noise is about one to three times the sEMG signal. It is almost impossible to avoid the interference of this type of noise in EMG signals on the earth’s surface. Power sources like Power-Line Interference (PLI) are the major cause of the generation of the ambient noise ranges in 60 Hz (or 50 Hz). It is important to understand the reasoning behind of generation of noise. It is because of the differences between the electrode impedances and stray currents flowing through the wires and patients. Off-line processing is needed to eliminate the recorded artifact [35]. In some cases, when the inference has a high-frequency component then there is a necessity for a high pass filter. However, it is necessary to understand the sEMG signal’s nature before implementing the processing of data.
Motion artifact
Motion artifacts [36] are generated by establishing the connection between the electrode to an amplifier and also contact between the electrode’s detecting surface and the body skin. In order to record the sEMG signals, there is a requirement of placing the electrodes near the chosen muscle groups. Whenever an activity is performed, the length of muscles is decreased, so the position of the muscle, skin, and electrodes moves relative to each other. At this moment, the movement artifact is detected in the electrodes. Typically, the range of noise generated is lies between 1-10 Hz, and the voltage magnitude corresponds to the sEMG signals.
Motion artifacts resemble the signal characteristics of baseline wander noise [37]. It is a low frequency artefact in the EMG that can be caused by a variety of factors, including patient movement, poor contact between electrode cables and EMG recording equipment, inadequate skin preparation where the electrode is placed, and dirty electrodes.
Inherent instability of signal
Since the magnitude of the sEMG signal is depend upon the activity performed, these dependencies make it unpredictable in nature. EMG signal is adversely impacted by the firing rate of the motor units [38]; typically, firing range has a frequency in the range 0-20 Hz. This type of noise is badly impacting the quality of the signal; therefore, it is important to mitigate this. In addition, multiple factors like the number of active motor units and mechanical contact between muscle fibers also affect the sEMG signal nature.
Cross talk
Crosstalk [39] is a special type of EMG signal which is generated from the unknown muscle group, an unwanted signal. It can contaminate the signal and lead to the erroneous interpretation of the data. Crosstalk is influenced by a variety of physiological factors; however, it may be reduced by carefully selecting electrode sizes and inter-electrode spacing.
As many forms of noise contaminate the signals, the process of analyzing and identifying EMG data becomes exceedingly challenging. So, here are some recommendations for recording high-quality EMG signals:
Surface EMG electrodes of high quality should be used with small inter-electrode spacing (1cm or less).
Ensure that the surface EMG signal is recorded at a rate of at least 1000 Hz by setting the sampling rate to 1000 Hz.
Adjusting the hardware amplification (referred to as gain) is important for EMG signal quality; a properly configured gain improves the signal-to-noise ratio. A gain of 1000 is often appropriate for surface EMG signals. Check that there is no clipping in the signal; if so, ensure that the EMG sensor and reference electrode are properly connected.
For general usage in surface EMG recording, a Butterworth filter with corner frequencies ranging from 20 to 500 Hz is suggested.
Inspect the recording to ensure that there are no instruments or electrical connections that might cause line interference during the recording.
To obtain valuable information from the EMG signal, the skin’s impedance must be minimized and skin should be completely cleaned. The dead skin was removed using an abrasive gel.
Place the EMG sensor in the muscular belly, away from innervation zones and tendon origins.
Ensure that good contact between the electrodes and the skin.
sEMG signal processing techniques
Denoising techniques
The process of evaluating and classifying EMG data gets extremely challenging as various types of noise contaminate the data. As a result, signal denoising is a necessary step that must be completed before the signals can be used for classification purposes. Many researchers have presented various approaches for the detection of muscle activity which allows for a consistent and accurate assessment of neurophysiological, rehabitational, and assistive technology findings. The noises that are not in the spectrum range of sEMG signal can be mitigated using state-of-art filters like High Pass, Low Pass, or Band Pass. However, they have faced difficulties for mitigation of white Gaussian noise present in the sEMG signal spectrum. In recent times, various researchers have implemented novel methods such as wavelet denoising, Empirical Mode Decomposition (EMD), and Independent Component Analysis (ICA), which helps in the reduction of these noises in the sEMG signal [40, 41].
As per the literature, it is beneficial to employ the wavelet denoising technique on the sEMG signal dataset consisting of upper and lower limbs. Phinyomark et al. proposed utilising the wavelet denoising approach to denoise the sEMG signal [42]. Wavelet denoising can effectively remove random disturbances such as White Gaussian noise usually present in sEMG signals. In the implementation of wavelet denoising over signals, discrete coefficients of wavelets are generated when the signal is passed via low-pass as well as high pass filters. With the help of the wavelet denoising approach, detail and approximation coefficients are acquired using signal decomposition and thresholding is accomplished. The total number of coefficients is decided by the level of decomposition. There is a total of 324 wavelet functions from 15 wavelet families. This technique requires the selection of five parameters such as mother wavelet, level of decomposition, threshold selection, threshold rescaling, and thresholding function [43].
Phinyomark et al. [44] investigated the denoising of the sEMG signal for multifunction myoelectric control using five wavelet algorithms: sym5, sym8, db2, db5, and coif5. It is discovered that scale level 4 performs the best as compared to the other scale levels based on the mean square error (MSE) parameter for processed sEMG data. In addition, it was also suggested that the order coiflet function gives the optimal reconstruction for the sEMG signal. Jiang and others [41] analysed four typical threshold estimation functions and concluded that EMG signals are insensitive to the choice of the threshold estimation function. Kumar et al. [45] quantified muscle failure using the Symlet function (Sym4 and Sym5) decomposed at levels 8 and 9. Hossain and colleagues [46] demonstrated that mother wavelet db45 had the highest contrast between 50 and 70 Hz when compared to the other four mother wavelets such as Haar, db2, sym4, and sym5. Vijayvargiya et al. [47] used the wavelet denoising technique for the detection of human knee abnormality using sEMG signal. In this study, it was calculated the value of mean absolute error, signal to noise ratio, mean squared error, and peak signal to noise ratio of the sEMG signal with different mother wavelets and level of decomposition. They have observed that sym4 originating from the family of symlet to the first level of decomposition led to the best results relatively to other mother wavelets and levels of decomposition.
In the other study of Vijayvargiya et al. [48], wavelet denoising is used with db7 from the Daubechies family till fourth level decomposition for the classification of three lower limb activities such as walking, sitting, and standing, for three different cases: 1) Knee healthy subjects, 2) Knee anomaly subjects, and 3) Pooled data (Combined of healthy and knee abnormal subjects). Dutta et al. [49] used the discrete wavelet transform of identifying the six distinct lower limb activities: full leg swing, forward leg swing, lifting knee, backward swing, squatting, and sideways leg swing using On-Body Creeping Wave Propagation.
Independent component analysis (ICA) [50] is a signal processing technique for separating additive subcomponents from a multivariate signal. This is achieved by assuming that the subcomponents are non-Gaussian signals and statistically independent. By maximising the statistical independence of the estimated components, ICA locates the independent components. There are various methods to establish a proxy for independence, and the one you choose determines the ICA algorithm. The following are the two widest techniques of independence for ICA: 1) Mutual information minimization, 2) Non-Gaussianity maximization. The Kullback-Leibler Divergence and maximal entropy metrics are used in the Minimization-of-Mutual-Information family of ICA techniques. Kurtosis and negentropy are used in the non-Gaussianity family of ICA algorithms, which are justified by the central limit theorem. It is now commonly used to identify and eliminate noise sources from EMG signals, as well as to decompose EMG signals into as many distinct components as possible. The ICA techniques such as the fast ICA, the Joint Approximate Diagonalization of Eigen-matrices (JADE), and the Infomax Estimation or maximum likelihood algorithm are commonly employed for the filtering of the EMG signal. [51–54].
The decomposition methodologies which assume that the process is stationary/linear may produce inaccurate or misleading findings because of the presence of non-stationary/non-linearity in the sEMG dataset [55]. A stationary time series signal is one in which statistical characteristics (for example, the mean and variance) do not change over time. On the other hand, non-stationary signal is a time series whose statistical features are changing throughout the span of time. Empirical Mode Decomposition is a very effective topology for the decomposition of the non-stationary/non-linear signals that have both complex spatial and temporal characteristics to their orthogonal components, which is known to be the Intrinsic Mode Functions (IMF) [56]. IMF is a single-frequency oscillatory mode or a mono-component function [57]. The signal can be subdivided into that multiple number of IMFs using the EMD approaches iteratively. On the other hand, the EMD approaches have complexity issues with the frequent appearance mode mixing because of its sensitivity to noise [58]. In order to address this problem, the EEMD was developed which is a noise-assisted data analysis method that defines the IMFs as the average of an ensemble of trials [59].
Wavelet de-noising is frequently used to remove white Gaussian noise and undesired signals such as the contributions from other muscle signals while preserving key properties of the signal. Empirical Mode Decomposition is employed to filter out sounds such as power line interference (PLI) and baseline wandering (BW). Because of its mostly independent signal-to-noise ratio and minor impacts on the frequency content, the ICA-based filtering process successfully removes power-line noise (PLI). According to the existing literature, all signal processing procedures have advantages and disadvantages, hence researchers also adopted a combination of different strategies for EMG signal processing. Zhang et al. [60] developed the noise-assisted multivariate empirical mode decomposition (NA-MEMD) approach for pre-processing multiple channel EMG signals that allow the temporal and spatial features of different muscle groups to be statistically depicted. Vijayvargiya and colleagues [20] proposed the wavelet denoising with ensemble empirical mode decomposition (WD-EEMD) pre-processing technique for denoising the sEMG signal of lower leg muscles for activity detection applications. Naik et al. [18] developed an ICA-based classification algorithm for lower limb sEMG data, which was implemented and validated with signals from healthy people and people with knee pathology.
Segmentation
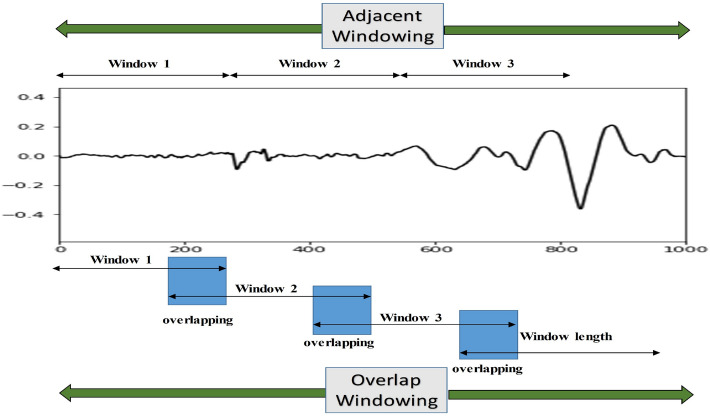
The nature of the EMG signal is random in nature, so segmented EMG signals are preferable to whole sEMG signals after the denoising. The segmentation is used as a part of pre-processing as it is a smart way to process time-series data to reduce the computational complexity. To implement the segmentation process, the windowing method is usually utilized. Two discrete methods of windowing are present to accomplish the process of segmentation: adjacent windowing and overlapping windowing [61]. The following segment overlaps the preceding segment in the overlap window; however, no segments overlap in the adjacent windowing approach as shown in Fig. 7. Englehart et al. [62] observed that the lengths of EMG data have an influence on classification accuracy. For data segmentation, two critical factors to consider are the windowing technique and the data length. Farina et al. [63] demonstrated that a window size of 250-500 ms is appropriate, and that employing a segment length smaller than 128 ms degrades classifier performance, resulting in large bias and variation of features.
Fig. 7.
Types of Windowing [48]
Machine learning techniques
Numerous noises and artifacts can be seen in a raw sEMG signal reduces the overall accuracy since the required information stays as an amalgam in the raw sEMG dataset. To improve the classification accuracy, firstly the sEMG signal is denoised, and, then the features are extracted for use as input to a computational classifier. There are three different types of feature extraction approaches are presented in the literature: time-domain (TD), frequency-domain (FD), and time-frequency domain (TFD) feature. Time-domain features are popular because they perform better signal classification in low-noise conditions and require less processing effort [64]. Therefore, in the majority of studies, time-domain features are employed to classify sEMG signals. The most commonly used features extracted from sEMG signal are indicated in Table 4.
Table 4.
Feature Extraction from sEMG signal
| Features | References | |
|---|---|---|
| Time domain | Integrated EMG | [65, 66] |
| Mean absolute value (MAV) | [20, 47, 65–68] | |
| Simple square integral (SSI) | [65, 66] | |
| Root Mean Square (RMS) | [20, 47, 65–67] | |
| Zero Crossing (ZC) | [20, 47, 65, 67] | |
| Slope Sign Change (SSC) | [20, 47, 65, 67] | |
| Variance (VAR) | [20, 47, 65–67] | |
| Wilison Amplitude (WAMP) | [47, 65, 67] | |
| Myopulse Percentage Rate (MYOP) | [47, 65, 67] | |
| Difference Absolute Standard Deviation Value (DASDV) | [20, 47, 65, 67] | |
| Skewness (Skew) | [20, 47, 65, 67, 69] | |
| Kurtosis (Kurt) | [20, 47, 65, 67, 69] | |
| Waveform Length (WL) | [65, 66, 68, 70] | |
| Histogram of EMG (HIST) | [65] | |
| Auto-regressive coefficients (AR) | [65, 66, 71] | |
| Average Amplitude Change (AAC) | [20, 47, 65, 67] | |
| Frequency Domain | Mean Frequency (MNF) | [32, 65, 66, 69, 70] |
| Median Frequency (MDF) | [32, 65, 66, 69, 70] | |
| Peak Frequency (PKF) | [32, 65] | |
| Mean Power Frequency (MNP) | [32, 65] | |
| Total Power (TTP) | [32, 65] | |
| Frequency Ration (FR) | [32, 65] | |
| Power Spectrum Ratio (PSR) | [65, 69, 70] | |
| Variation of Central Frequency (VCF) | [32, 65] | |
| Time-Frequency Domain | Discrete Wavelet Transofrm (DWT) | [65, 71] |
| Continous Wavelet Transform (CWT) | [65, 72] | |
| Emprical Mode Decomposition (EMD) | [65, 73] | |
| Short Term Fourier Transform (STFT) | [65, 71, 74] | |
| Wavelet Packet Transform (WPT) | [65, 75] | |
Many researchers have been interested in developing an efficient method for identifying electromyography signal patterns. Various kinds of classifiers, such as Artificial Neural Networks, Support Vector Machine, Linear Discriminant Analysis, Random Forest, and Decision Tree, have been efficiently employed for different EMG applications [20, 47, 67, 76]. The feature vector is utilised as an input to the classifier after the feature extraction procedure. As per the studies, dimensional reduction strategies such as principal component analysis, and linear discriminant analysis, are used to reduce the classifier’s burden and processing time. Toledo-Pérez et al. [77] utilised the principal component analysis approach to minimise the dimensionality of the retrieved features from the lower limb sEMG signal and investigate the impact of changing the number of channels or the muscles. Phinyomark et al. [78] employed linear discriminant analysis, which reduces the number of features needed in the categorization of hand movements using sEMG signals. Englehart and colleagues [10] employed principle components analysis to minimise the size of a wavelet-based feature set, which increased classification accuracy significantly in a myoelectric-controlled prosthesis application. A dimensionality reduction strategy minimises intra-class variability while increasing inter-class distance, simplifying the classification problem. After the feature reduction process, the features are employed to perform the classification task. Luan et al. [25] compared the five different feature reduction techniques such as Principal component analysis (PCA), Linear Discriminant Analysis (LDA), ) Locally Linear Embedding (LLE), Laplacian eigenmaps, and Rank preserving discriminant analysis (RPDA) for six distinct lower limb activities. There are several ways to dimensionality reduction, which are given below [79]:
Linear Discriminant Analysis;
Independent Component Analysis;
Principal Component Analysis;
General Discriminant Analysis;
High Correlation Filter;
Low Variance Filter;
Missing Value Ratio;
Backward Feature Elimination;
Random Forest;
Factor Analysis;
Projection methods;
Forward Feature Selection.
The electromyogram classification system’s effectiveness is strongly dependent on the selection and extraction of high-quality features [80]. With the feature extraction stage in a classification system, the information density of the signal is increased [81]. Naik et al. [18] made good use of a multivariate technique called entropy bound minimization (ICA-EBM) for the assortment of lower limb activities. It classifies the walking, standing, and sitting activities of 11 healthy and 11 possessed knee pathology subjects with an accuracy rate of 96.1% and 86.2% by using a linear discriminant analysis classifier. Vijayvargiya and colleagues [20] proposed a new hybrid wavelet denoising with ensemble empirical mode decomposition (WD-EEMD) pre-processing technique for the classification of three lower limb activities same as Naik et al. with an accuracy of 90.69% and 97.45% for healthy and knee abnormal subjects, respectively. In the article [29], the authors proposed a new top and slope feature extraction technique for lower limb human motion detection (Table 5).
Table 5.
Summary of lower limb activity recognition using sEMG
| Author | Preprocessing | Activities | Classifier | Accuracy |
|---|---|---|---|---|
| [18] | ICA-EBM | Walking, sitting, standing | LDA | – |
| [20] | WD-EEMD | Walking, sitting, standing | LDA | 97.45% for healthy |
| 90.69% for knee abnormal | ||||
| [48] | WD | Walking, sitting, standing | 1D CNN | 99.35% for healthy |
| 97.63% for knee abnormal | ||||
| [82] | WD | Walking, sitting, standing | MP-ANN | – |
| [83] | – | Horizontal walking, crossing obstacles, standing up, going down the stairs, and going up the stairs | Gaussian Kernel LDA | 96.00% |
| [84] | – | Sit, stand, level walk, stair ascent, stair descent, ramp ascent and ramp descent | SVM, KNN, LDA, ANN | 96.43-98.78% |
| [85] | – | Walking, sitting, standing | Long-term Recurrent Convolution Network | 98.1% for healthy |
| 92.4% for knee abnormal | ||||
| [86] | WD-SVD | Flexion of the leg up (standing), hip extension from a sitting position (sitting) and gait (walking) | SVM | 91.85% |
Deep learning algorithms have been used in several different areas in recent years. Deep learning does not need the extraction of handcrafted features. These algorithms extract features and later classify them accordingly. A Convolutional Neural Network (CNN) is a type of deep learning algorithm that can automatically extract the signal features. Vijayvargiya et al. classify three different activities for three distinct cases: healthy, knee abnormal, and pooled data, which is a combined dataset of healthy and knee abnormal participants. They employed a voting-based 1D convolutional neural network and obtained classification accuracy of 99.35, 97.63, and 97.14 percent for healthy, pathological knee, and pooled data, respectively [48]. Gautam et al. [86] developed a new classification methodology that considers the lower-limb movements when determining knee joint angle prognosis. CNN and Long Short-term Memory (LSTM) collaborate to develop an architecture for classifying activities, with CNN extracting features from sEMG signal data and LSTM predicting joint angles and interpreting the features. After that, a dense layer is connected for classification. The authors developed the MyoNet model, which uses these three blocks to predict lower-limb activities (walking, standing, and sitting) as well as joint angle. In [87], to achieve the classification of the limb movements from EMG signal, a Deep belief network (DBN) was used to get the better of local minima problems and overfitting issues. This 4-class classifier algorithm achieved better classification performance with reduced training time and was trained for each subject.
Applications
In the last decade, sEMG-based solutions have grown with an increasing number of demonstrations and attempts in three critical rehabilitation scenarios as shown in Fig. 8. First, the lower limb activity recognition based on sEMG plays an important role in accurately diagnosing the neuromuscular diseases in the lower limb so that patients could begin the rehabilitation process before their disorders progressed further. Vijayvargiya et al. [67] used sEMG signal-based lower limb activities to differentiate between diseased and healthy knees. In this study, 11 time-domain characteristics were extracted from the sEMG signal of four lower limb muscles and classified using five machine learning classifiers: support vector machine, decision tree, k-nearest neighbor, random forest, and additional tree classifiers, all with 10-fold cross-validation. The results revealed that the extra tree classifier outperforms the other classifiers with 91.3 % of accuracy. In the other study of Vijayvargiya et al. [47], the effect of imbalance of sEMG signal during the walking of knee abnormal and healthy subject was studied. The walking task takes longer for people with a knee deformity than for healthy subjects. Therefore, the length of the sEMG signal was longer than the one of healthy subjects. It resulted in an imbalance in the collected data. Thus, the oversampling was used for increasing the training minority dataset which helps in the balancing of the used dataset. The classification between myopathy and neuropathy based on sEMG signal using neural network was also presented by Swaroop et al. [88].
Fig. 8.
Application scenarios of the sEMG based activity recognition
Second, sEMG-based LLAR have been used to assess patients’ rehabilitation progress and therapy success on a broader scale. Robot-assisted rehabilitation and therapy are becoming more popular for assisting the elderly, and disabled patients. As proven by favorable clinical outcomes and recovery time, robot-assisted lower limb rehabilitation has significant benefits over standard manual therapy and training. Meng et al. [89] conducted a systematic review on the recent development of mechanisms and control techniques for robot-assisted lower limb rehabilitation, focusing on various robotic mechanisms, training modes, and control strategies.
Thirdly, sEMG-based LLAR is used to control assistive equipment such as intelligent prostheses and orthoses [90]. These auxiliary devices can be employed for aiding the patients during physical rehabilitation. It can ease the load on the physical therapy to a certain extent [91, 92]. Additionally, this technology enables these gadgets to attain a higher level of security and a more pleasant user experience. In recent times, the interface between the human-machine become a research topic for the artificial lower limb. It is because of the requirement of the deliberate human locomotion knowledge and also helps the external devices for the development of the active control mechanisms.
Conclusion and future prospective
This study delves into the fundamental structure and methodology for identifying human lower limb activity using sEMG signals which is very important due to its applications in the diagnosis of neuromuscular diseases, security, controlling of robots/prostheses, human-machine interaction, and pattern recognition. Due to the presence of undesirable signal sources or artifacts in EMG data, filtering procedures are advised to reduce the existent noise. However, while this procedure may minimise these noises, it does not ensure the originality of EMG signals. Classification of EMG data is critical for real-time control of, for example, a robotic limb. As a result, researchers are concentrating their efforts on EMG signal processing methods to develop a more accurate, simple, and dependable system for recognising lower limb motion patterns. This study discussed the methods and procedures utilised for sEMG signal pre-processing. The sEMG signal is random in nature so, features are extracted from it after denoising and used as input to a computational classifier to increase classification accuracy. The most commonly used extracted features from sEMG signal were discussed. The implemented machine learning algorithms were then indicated. Finally, an overview of different applications of lower limb activity recognition were presented.
Other significant findings that should be investigated further in the future are yet possible. To begin, the accessible dataset comprises information from a rather limited number of patients. As a result, the recommended approaches should be examined using a large number of participants to mitigate the bias imposed by small datasets. There are limited lower limb activity datasets available on abnormal people based on sEMG signals, therefore researchers can focus on this field as well. There has been relatively little research on the imbalance of sEMG signals in healthy and pathological knee individuals, consequently it is also an open topic for researchers. Feature extraction is a crucial step for the classification task using machine learning techniques. So, in further study, one may strive to minimise the extracted feature space by employing feature reduction or selection strategies. On the brighter side, this study provides a clear and concise overview of sEMG signal based human lower limb activity recognition techniques, databases, challenges, application, and its future prospective.
Acknowledgements
This work is supported by Visvesvaraya Ph.D. Scheme, Meity, Govt. of India, MEITY-PHD-2942.
Funding
The authors have not disclosed any funding.
Declarations
Conflict of interest
The authors report no declarations of interest.
Ethical approval
This article does not contain any study with human participants performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ankit Vijayvargiya, Email: ankitvijayvargiya29@gmail.com.
Bharat Singh, Email: bharatsingh1942@gmail.com.
Rajesh Kumar, Email: rkumar.ee@mnit.ac.in.
João Manuel R. S. Tavares, Email: tavares@fe.up.pt
References
- 1.Ranasinghe S, Al Machot F, Mayr HC. A review on applications of activity recognition systems with regard to performance and evaluation. Int J Distrib Sens Netw. 2016;12(8):1550147716665520. doi: 10.1177/1550147716665520. [DOI] [Google Scholar]
- 2.Jamieson A, Murray L, Stankovic L, Stankovic V, Buis A. Human activity recognition of individuals with lower limb amputation in free-living conditions: a pilot study. Sensors. 2021;21(24):8377. doi: 10.3390/s21248377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal J, Ryoo M. Human activity analysis: a review. ACM Comput Surv. 2011;43(3):1–43. doi: 10.1145/1922649.1922653. [DOI] [Google Scholar]
- 4.Dang LM, Min K, Wang H, Piran MJ, Lee CH, Moon H. Sensor-based and vision-based human activity recognition: a comprehensive survey. Pattern Recogn. 2020;108:107561. doi: 10.1016/j.patcog.2020.107561. [DOI] [Google Scholar]
- 5.Hussain Z, Sheng M, Zhang WE. Different approaches for human activity recognition: A survey (2019). arXiv preprint arXiv:1906.05074
- 6.Lara OD, Labrador MA. A survey on human activity recognition using wearable sensors. IEEE Commun Surv Tutor. 2012;15(3):1192–1209. doi: 10.1109/SURV.2012.110112.00192. [DOI] [Google Scholar]
- 7.Beddiar DR, Nini B, Sabokrou M, Hadid A. Vision-based human activity recognition: a survey. Multimed Tools Appl. 2020;79(41):30509–30555. doi: 10.1007/s11042-020-09004-3. [DOI] [Google Scholar]
- 8.Cheng J, Chen X, Shen M. A framework for daily activity monitoring and fall detection based on surface electromyography and accelerometer signals. IEEE J Biomed Health Inform. 2012;17(1):38–45. doi: 10.1109/TITB.2012.2226905. [DOI] [PubMed] [Google Scholar]
- 9.Yang B-S, Liao S-T. Fall detecting using inertial and electromyographic sensors. In: Proceedings of the 36th Annual Meeting of the American Society of Biomechanics, Gainsville, FL, USA; 2012. p. 15–18.
- 10.Englehart K, Hudgin B, Parker PA. A wavelet-based continuous classification scheme for multifunction myoelectric control. IEEE Trans Biomed Eng. 2001;48(3):302–311. doi: 10.1109/10.914793. [DOI] [PubMed] [Google Scholar]
- 11.Pawar T, Chaudhuri S, Duttagupta SP. Body movement activity recognition for ambulatory cardiac monitoring. IEEE Trans Biomed Eng. 2007;54(5):874–882. doi: 10.1109/TBME.2006.889186. [DOI] [PubMed] [Google Scholar]
- 12.Tunçel O, Altun K, Barshan B. Classifying human leg motions with uniaxial piezoelectric gyroscopes. Sensors. 2009;9(11):8508–8546. doi: 10.3390/s91108508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan AM, Lee Y-K, Lee SY, Kim T-S. A triaxial accelerometer-based physical-activity recognition via augmented-signal features and a hierarchical recognizer. IEEE Trans Inf Technol Biomed. 2010;14(5):1166–1172. doi: 10.1109/TITB.2010.2051955. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Jiang M, Hu Y, Li H. An incremental learning method based on probabilistic neural networks and adjustable fuzzy clustering for human activity recognition by using wearable sensors. IEEE Trans Inf Technol Biomed. 2012;16(4):691–699. doi: 10.1109/TITB.2012.2196440. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Sawchuk AA. Human daily activity recognition with sparse representation using wearable sensors. IEEE J Biomed Health Inform. 2013;17(3):553–560. doi: 10.1109/JBHI.2013.2253613. [DOI] [PubMed] [Google Scholar]
- 16.Park S, Park J, Al-Masni M, Al-Antari M, Uddin MZ, Kim T-S. A depth camera-based human activity recognition via deep learning recurrent neural network for health and social care services. Proc Comput Sci. 2016;100:78–84. doi: 10.1016/j.procs.2016.09.126. [DOI] [Google Scholar]
- 17.Chen Z, Zhu Q, Soh YC, Zhang L. Robust human activity recognition using smartphone sensors via ct-pca and online svm. IEEE Trans Industr Inf. 2017;13(6):3070–3080. doi: 10.1109/TII.2017.2712746. [DOI] [Google Scholar]
- 18.Naik GR, Selvan SE, Arjunan SP, Acharyya A, Kumar DK, Ramanujam A, Nguyen HT. An ica-ebm-based semg classifier for recognizing lower limb movements in individuals with and without knee pathology. IEEE Trans Neural Syst Rehabil Eng. 2018;26(3):675–686. doi: 10.1109/TNSRE.2018.2796070. [DOI] [PubMed] [Google Scholar]
- 19.Hussain T, Maqbool HF, Iqbal N, Khan M, Salman, Dehghani-Sanij AA. Computational model for the recognition of lower limb movement using wearable gyroscope sensor. Int J Sens Netw. 2019;30(1):35–45. doi: 10.1504/IJSNET.2019.099230. [DOI] [Google Scholar]
- 20.Vijayvargiya A, Gupta V, Kumar R, Dey N, Tavares JMR. A hybrid wd-eemd semg feature extraction technique for lower limb activity recognition. IEEE Sens J. 2021.
- 21.Jamal MZ. Signal acquisition using surface emg and circuit design considerations for robotic prosthesis. Comput Intell Electromyograp Analysis-A Perspect Curr Appl Fut Chall. 2012;18:427–448. [Google Scholar]
- 22.Fang C, He B, Wang Y, Cao J, Gao S. Emg-centered multisensory based technologies for pattern recognition in rehabilitation: state of the art and challenges. Biosensors. 2020;10(8):85. doi: 10.3390/bios10080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendry D, Chai K, Campbell A, Hopper L, O’Sullivan P, Straker L. Development of a human activity recognition system for ballet tasks. Sports Medicine-Open. 2020;6(1):10. doi: 10.1186/s40798-020-0237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gopura R, Kiguchi K, Mann G, Torricelli D. Robotic Prosthetic Limbs. Hindawi; 2018.
- 25.Luan Y, Shi Y, Wu W, Liu Z, Chang H, Cheng J. Har-semg: A dataset for human activity recognition on lower-limb semg. Knowl Inf Syst. 2021;63(10):2791–2814. doi: 10.1007/s10115-021-01598-w. [DOI] [Google Scholar]
- 26.Shi X, Qin P, Zhu J, Zhai M, Shi W. Feature extraction and classification of lower limb motion based on semg signals. IEEE Access. 2020;8:132882–132892. doi: 10.1109/ACCESS.2020.3008901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park S, Lee D, Chung WK, Kim K. Hierarchical motion segmentation through semg for continuous lower limb motions. IEEE Robot Autom Lett. 2019;4(4):4402–4409. doi: 10.1109/LRA.2019.2932343. [DOI] [Google Scholar]
- 28.Hu B, Rouse E, Hargrove L. Benchmark datasets for bilateral lower-limb neuromechanical signals from wearable sensors during unassisted locomotion in able-bodied individuals. Front Robot AI. 2018;5:14. doi: 10.3389/frobt.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryu J, Lee B-H, Kim D-H. semg signal-based lower limb human motion detection using a top and slope feature extraction algorithm. IEEE Signal Process Lett. 2016;24(7):929–932. doi: 10.1109/LSP.2016.2636320. [DOI] [Google Scholar]
- 30.Yu Y, Fan L, Kuang S, Sun L, Zhang F. The research of semg movement pattern classification based on multiple fused wavelet function. In: 2015 IEEE International Conference on Cyber Technology in Automation, Control, and Intelligent Systems (CYBER), IEEE; 2015. p. 487–491.
- 31.Sanchez O, Sotelo J, Gonzales M, Hernandez G. Emg dataset in lower limb data set. UCI machine learning repository. 2014;2.
- 32.Nazmi N, Abdul Rahman MA, Yamamoto S-I, Ahmad SA, Zamzuri H, Mazlan SA. A review of classification techniques of emg signals during isotonic and isometric contractions. Sensors. 2016;16(8):1304. doi: 10.3390/s16081304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reaz MBI, Hussain MS, Mohd-Yasin F. Techniques of emg signal analysis: detection, processing, classification and applications. Biol Proc Online. 2006;8(1):11–35. doi: 10.1251/bpo115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chowdhury RH, Reaz MB, Ali MABM, Bakar AA, Chellappan K, Chang TG. Surface electromyography signal processing and classification techniques. Sensors. 2013;13(9):12431–12466. doi: 10.3390/s130912431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clancy EA, Morin EL, Merletti R. Sampling, noise-reduction and amplitude estimation issues in surface electromyography. J Electromyogr Kinesiol. 2002;12(1):1–16. doi: 10.1016/S1050-6411(01)00033-5. [DOI] [PubMed] [Google Scholar]
- 36.De Luca CJ, Gilmore LD, Kuznetsov M, Roy SH. Filtering the surface emg signal: Movement artifact and baseline noise contamination. J Biomech. 2010;43(8):1573–1579. doi: 10.1016/j.jbiomech.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 37.Romero FP, Romaguera LV, Vázquez-Seisdedos CR, Costa MGF, Neto JE, et al. Baseline wander removal methods for ecg signals: A comparative study; 2018. arXiv preprint arXiv:1807.11359
- 38.Amrutha N, Arul V. A review on noises in emg signal and its removal. Int J Sci Res Publ. 2017;7(5):23–27. [Google Scholar]
- 39.Van Vugt J, Van Dijk J. A convenient method to reduce crosstalk in surface emg. Clin Neurophysiol. 2001;112(4):583–592. doi: 10.1016/S1388-2457(01)00482-5. [DOI] [PubMed] [Google Scholar]
- 40.Andrade AO, Nasuto S, Kyberd P, Sweeney-Reed CM, Van Kanijn F. Emg signal filtering based on empirical mode decomposition. Biomed Signal Process Control. 2006;1(1):44–55. doi: 10.1016/j.bspc.2006.03.003. [DOI] [Google Scholar]
- 41.Jiang C-F, Kuo S-L. A comparative study of wavelet denoising of surface electromyographic signals. In: 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, IEEE; 2007. p. 1868–1871. [DOI] [PubMed]
- 42.Phinyomark A, Phukpattaranont P, Limsakul C. Wavelet-based denoising algorithm for robust emg pattern recognition. Fluct Noise Lett. 2011;10(02):157–167. doi: 10.1142/S0219477511000466. [DOI] [Google Scholar]
- 43.Phinyomark A, Limsakul C, Phukpattaranont P. A comparative study of wavelet denoising for multifunction myoelectric control. In: 2009 International Conference on Computer and Automation Engineering, IEEE; 2009. p. 21–25.
- 44.Phinyomark A, Limsakul C, Phukpattaranont P. An optimal wavelet function based on wavelet denoising for multifunction myoelectric control. In: 2009 6th International Conference on Electrical Engineering/electronics, Computer, Telecommunications and Information Technology, vol. 2, IEEE; 2009. p. 1098–1101.
- 45.Kumar DK, Pah ND, Bradley A. Wavelet analysis of surface electromyography. IEEE Trans Neural Syst Rehabil Eng. 2003;11(4):400–406. doi: 10.1109/TNSRE.2003.819901. [DOI] [PubMed] [Google Scholar]
- 46.Hussain M, Mamun M. Effectiveness of the wavelet transform on the surface emg to understand the muscle fatigue during walk. Meas Sci Rev. 2012;12(1):28. doi: 10.2478/v10048-012-0005-x. [DOI] [Google Scholar]
- 47.Vijayvargiya A, Prakash C, Kumar R, Bansal S, Tavares JMR. Human knee abnormality detection from imbalanced semg data. Biomed Signal Process Control. 2021;66:102406. doi: 10.1016/j.bspc.2021.102406. [DOI] [Google Scholar]
- 48.Vijayvargiya A, Khimraj, Kumar R, Dey N. Tavares: Voting-based 1d cnn model for human lower limb activity recognition using semg signal. Phys Eng Sci Med. 2021;44. [DOI] [PubMed]
- 49.Dutta S, Basu B, Talukdar FA. Classification of lower limb activities based on discrete wavelet transform using on-body creeping wave propagation. IEEE Trans Instrum Meas. 2020;70:1–7. [Google Scholar]
- 50.Comon P. Independent component analysis, a new concept? Signal Process. 1994;36(3):287–314. doi: 10.1016/0165-1684(94)90029-9. [DOI] [Google Scholar]
- 51.Nakamura H, Yoshida M, Kotani M, Akazawa K, Moritani T. The application of independent component analysis to the multi-channel surface electromyographic signals for separation of motor unit action potential trains: Part i–measuring techniques. J Electromyogr Kinesiol. 2004;14(4):423–432. doi: 10.1016/j.jelekin.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Acharya D, Panda G. A review of independent component analysis techniques and their applications. IETE Tech Rev. 2008;25(6):320–332. doi: 10.4103/0256-4602.45424. [DOI] [Google Scholar]
- 53.Zhou P, Lowery MM, Rymer WZ. Extracting motor unit firing information by independent component analysis of surface electromyogram: A preliminary study using a simulation approach. Int J Comput Syst Signals. 2006;7(1):19–28. [Google Scholar]
- 54.Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7(6):1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 55.Sapsanis C, Georgoulas G, Tzes A. Emg based classification of basic hand movements based on time-frequency features. In: 21st Mediterranean Conference on Control and Automation, IEEE; 2013. p. 716–722. [DOI] [PubMed]
- 56.Huang NE, Shen Z, Long SR, Wu MC, Shih HH, Zheng Q, Yen N-C, Tung CC, Liu HH. The empirical mode decomposition and the hilbert spectrum for nonlinear and non-stationary time series analysis. Proc R Soc Lond Ser A Math Phys Eng Sci. 1998;454(1971):903–995. doi: 10.1098/rspa.1998.0193. [DOI] [Google Scholar]
- 57.Gaci S. A new ensemble empirical mode decomposition (eemd) denoising method for seismic signals. Energy Proc. 2016;97:84–91. doi: 10.1016/j.egypro.2016.10.026. [DOI] [Google Scholar]
- 58.Naik GR, Selvan SE, Nguyen HT. Single-channel emg classification with ensemble-empirical-mode-decomposition-based ica for diagnosing neuromuscular disorders. IEEE Trans Neural Syst Rehabil Eng. 2015;24(7):734–743. doi: 10.1109/TNSRE.2015.2454503. [DOI] [PubMed] [Google Scholar]
- 59.Lei Y, He Z, Zi Y. Eemd method and wnn for fault diagnosis of locomotive roller bearings. Expert Syst Appl. 2011;38(6):7334–7341. doi: 10.1016/j.eswa.2010.12.095. [DOI] [Google Scholar]
- 60.Zhang Y, Xu P, Li P, Duan K, Wen Y, Yang Q, Zhang T, Yao D. Noise-assisted multivariate empirical mode decomposition for multichannel emg signals. Biomed Eng Online. 2017;16(1):1–17. doi: 10.1186/s12938-017-0397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banos O, Galvez J-M, Damas M, Pomares H, Rojas I. Window size impact in human activity recognition. Sensors. 2014;14(4). [DOI] [PMC free article] [PubMed]
- 62.Englehart K, Hudgins B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans Biomed Eng. 2003;50(7):848–854. doi: 10.1109/TBME.2003.813539. [DOI] [PubMed] [Google Scholar]
- 63.Farina D, Merletti R. Comparison of algorithms for estimation of emg variables during voluntary isometric contractions. J Electromyogr Kinesiol. 2000;10(5):337–349. doi: 10.1016/S1050-6411(00)00025-0. [DOI] [PubMed] [Google Scholar]
- 64.Daud WMBW, Yahya AB, Horng CS, Sulaima MF, Sudirman R. Features extraction of electromyography signals in time domain on biceps brachii muscle. Int J Model Optim. 2013;3(6):515. doi: 10.7763/IJMO.2013.V3.332. [DOI] [Google Scholar]
- 65.Phinyomark A, Phukpattaranont P, Limsakul C. Feature reduction and selection for emg signal classification. Expert Syst Appl. 2012;39(8):7420–7431. doi: 10.1016/j.eswa.2012.01.102. [DOI] [Google Scholar]
- 66.Spiewak C, Islam M, Zaman A, Rahman MH. A comprehensive study on emg feature extraction and classifiers. Open Access J Biomed Eng Biosci. 2018;1(1):1–10. doi: 10.32474/OAJBEB.2018.01.000104. [DOI] [Google Scholar]
- 67.Vijayvargiya A, Kumar R, Dey N, Tavares JMR. Comparative analysis of machine learning techniques for the classification of knee abnormality. In: 2020 IEEE 5th International Conference on Computing Communication and Automation (ICCCA), IEEE; 2020. p. 1–6.
- 68.Too J, Abdullah AR, Mohd Saad N, Tee W. Emg feature selection and classification using a pbest-guide binary particle swarm optimization. Computation. 2019;7(1):12. doi: 10.3390/computation7010012. [DOI] [Google Scholar]
- 69.Javaid HA, Rashid N, Tiwana MI, Anwar MW. Comparative analysis of emg signal features in time-domain and frequency-domain using myo gesture control. In: Proceedings of the 2018 4th International Conference on Mechatronics and Robotics Engineering; 2018. p. 157–162.
- 70.Too J, Abdullah A, Zawawi TT, Saad NM, Musa H. Classification of emg signal based on time domain and frequency domain features. Int J Human Technol Interact (IJHaTI) 2017;1(1):25–30. [Google Scholar]
- 71.Oladazimi M, Molaei-Vaneghi F, Safari M, Asadi H, Aghay Kaboli S. A review for feature extraction of emg signal processing. In: 4th International Conference on Computer and Automation Engineering (ICCAE 2012), ASME Press; 2012. p. 85–94.
- 72.Ismail AR, Asfour SS. Continuous wavelet transform application to emg signals during human gait. In: Conference Record of Thirty-Second Asilomar Conference on Signals, Systems and Computers (Cat. No. 98CH36284), vol. 1, IEEE; 1998. p. 325–329.
- 73.Sapsanis C, Georgoulas G, Tzes A, Lymberopoulos D. Improving emg based classification of basic hand movements using emd. In: 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE; 2013. p. 5754–5757. [DOI] [PubMed]
- 74.Lee J-Y. Variable short-time fourier transform for vibration signals with transients. J Vib Control. 2015;21(7):1383–1397. doi: 10.1177/1077546313499389. [DOI] [Google Scholar]
- 75.Xing K, Yang P, Huang J, Wang Y, Zhu Q. A real-time emg pattern recognition method for virtual myoelectric hand control. Neurocomputing. 2014;136:345–355. doi: 10.1016/j.neucom.2013.12.010. [DOI] [Google Scholar]
- 76.Vijayvargiya A, Singh PL, Verma SM, Kumar R, Bansal S. Performance comparison analysis of different classifier for early detection of knee osteoarthritis. In: Sensors for Health Monitoring; Elsevier; 2019. p. 243–257.
- 77.Toledo-Pérez DC, Martínez-Prado MA, Gómez-Loenzo RA, Paredes-García WJ, Rodríguez-Reséndiz J. A study of movement classification of the lower limb based on up to 4-emg channels. Electronics. 2019;8(3):259. doi: 10.3390/electronics8030259. [DOI] [Google Scholar]
- 78.Phinyomark A, Hu H, Phukpattaranont P, Limsakul C. Application of linear discriminant analysis in dimensionality reduction for hand motion classification. Meas Sci Rev. 2012;12(3):82–89. doi: 10.2478/v10048-012-0015-8. [DOI] [Google Scholar]
- 79.Velliangiri S, Alagumuthukrishnan S, et al. A review of dimensionality reduction techniques for efficient computation. Proc Comput Sci. 2019;165:104–111. doi: 10.1016/j.procs.2020.01.079. [DOI] [Google Scholar]
- 80.Chan FH, Yang Y-S, Lam F, Zhang Y-T, Parker PA. Fuzzy emg classification for prosthesis control. IEEE Trans Rehabil Eng. 2000;8(3):305–311. doi: 10.1109/86.867872. [DOI] [PubMed] [Google Scholar]
- 81.Scheme E, Englehart K. Electromyogram pattern recognition for control of powered upper-limb prostheses: state of the art and challenges for clinical use. J Rehab Res Dev. 2011;48(6). [DOI] [PubMed]
- 82.Herrera-González M, Martínez-Hernández GA, Rodríguez-Sotelo JL, Avilés-Sánchez ÓF. Knee functional state classification using surface electromyographic and goniometric signals by means of artificial neural networks. Ing Univ. 2015;19(1):51–66. [Google Scholar]
- 83.Qin P, Shi X. Evaluation of feature extraction and classification for lower limb motion based on semg signal. Entropy. 2020;22(8):852. doi: 10.3390/e22080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meng L, Pang J, Wang Z, Xu R, Ming D. The role of surface electromyography in data fusion with inertial sensors to enhance locomotion recognition and prediction. Sensors. 2021;21(18):6291. doi: 10.3390/s21186291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Y, Li P, Zhu X, Su SW, Guo Q, Xu P, Yao D. Extracting time-frequency feature of single-channel vastus medialis emg signals for knee exercise pattern recognition. PLoS ONE. 2017;12(7):0180526. doi: 10.1371/journal.pone.0180526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gautam A, Panwar M, Biswas D, Acharyya A. Myonet: A transfer-learning-based lrcn for lower limb movement recognition and knee joint angle prediction for remote monitoring of rehabilitation progress from semg. IEEE J Trans Eng Health Med. 2020;8:1–10. doi: 10.1109/JTEHM.2020.3023898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang J, Ling C, Li S. Emg signals based human action recognition via deep belief networks. IFAC-PapersOnLine. 2019;52(19):271–276. doi: 10.1016/j.ifacol.2019.12.108. [DOI] [Google Scholar]
- 88.Swaroop R, Kaur M, Suresh P, Sadhu PK. Classification of myopathy and neuropathy emg signals using neural network. In: 2017 International Conference on Circuit, Power and Computing Technologies (ICCPCT), IEEE; 2017. p. 1–5
- 89.Meng W, Liu Q, Zhou Z, Ai Q, Sheng B, Xie SS. Recent development of mechanisms and control strategies for robot-assisted lower limb rehabilitation. Mechatronics. 2015;31:132–145. doi: 10.1016/j.mechatronics.2015.04.005. [DOI] [Google Scholar]
- 90.Wolf EJ, Cruz TH, Emondi AA, Langhals NB, Naufel S, Peng GC, Schulz BW, Wolfson M. Advanced technologies for intuitive control and sensation of prosthetics. Biomed Eng Lett. 2020;10(1):119–128. doi: 10.1007/s13534-019-00127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khoshdel V, Akbarzadeh A, Naghavi N, Sharifnezhad A, Souzanchi-Kashani M. semg-based impedance control for lower-limb rehabilitation robot. Intel Serv Robot. 2018;11(1):97–108. doi: 10.1007/s11370-017-0239-4. [DOI] [Google Scholar]
- 92.Singh RM, Chatterji S, Kumar A. Trends and challenges in emg based control scheme of exoskeleton robots-a review. Int J Sci Eng Res. 2012;3(9):933–940. [Google Scholar]