Abstract
Background
The Fibrosis-4 Index (FIB-4)non-invasively assesses fibrosis risk in chronic liver disease (CLD), but underdiagnosis limits FIB-4’s application in primary care.
Objective
To evaluate the association of FIB-4 risk with hazard of severe liver outcomes in primary care patients with and without diagnosed CLD.
Design
Retrospective cohort study of primary care data from 2007 to 2018.
Participants
Adult patients with qualifying aminotransferase and platelet count results were included and a single FIB-4 score was calculated for each patient using the first of these values. Patients with a CLD diagnosis or outcome prior to their FIB-4 score were excluded.
Measures
FIB-4 advanced fibrosis risk categorization (low, indeterminate, and high) was the primary predictor variable. Patients were followed from FIB-4 score to a severe liver outcome, a composite of cirrhosis, liver transplantation, and hepatocellular carcinoma. We analyzed the association of FIB-4 risk categories with hazard risk of a severe liver outcome using stratified Cox regression models, stratifying patients by known CLD.
Key Results
A total of 20,556 patients were followed for a mean 2,978 days (SD 1,201 days), and 4% of patients experienced a severe liver outcome. Of patients with low-, indeterminate-, and high-risk FIB-4 scores, 2%, 4%, and 20% suffered a severe liver outcome, respectively. In the overall adjusted model, high-risk FIB-4 scores were associated with hazard of severe liver disease (HR 6.64; 95% CI 5.58–7.90). High-risk FIB-4 scores were associated with severe liver outcomes for patients with known NAFLD (HR 7.32; 95% CI 3.44–15.58), other liver disease (HR 11.39; 95% CI 8.53–15.20), and no known CLD (HR 4.05; 95% CI 3.10–5.28).
Conclusions
High-risk FIB-4 scores were strongly associated with risk of severe liver outcomes in patients with and without known CLD. Comprehensive FIB-4 application in primary care may signal silently advancing liver fibrosis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-021-07341-z.
KEY WORDS: FIB-4, NAFLD, Chronic liver disease
BACKGROUND
Accuracy, simplicity, and broad availability have led to the emergence of the Fibrosis-4 Index (FIB-4) as a potentially indispensable tool for identifying patients with chronic liver disease (CLD) at high-risk for advanced fibrosis (Metavir fibrosis stages 3 and 4 [F3, F4]) in primary care1–6. FIB-4 first appeared as a non-invasive means to assess fibrosis in patients with viral hepatitis C (HCV), but its utility in fibrosis risk assessment has been explored for patients with viral hepatitis B (HBV), alcohol-related liver disease, and hemochromatosis.7–12 The application of FIB-4 to detect advanced fibrosis in nonalcoholic fatty liver disease (NAFLD) has gained significant attention as the burden of NAFLD continues to rise and increasingly contributes to severe liver disease outcomes including cirrhosis, hepatocellular carcinoma, and the need for liver transplantation.2,4,13,14 NAFLD is the CLD primary care providers are most likely to encounter, and non-invasive advanced fibrosis risk stratification is a key component in disease management since advanced fibrosis is associated with severe liver-related consequences, cardiovascular death, and all-cause mortality.1,3,5,14–19 In the recently published NAFLD Care Pathway guidelines from the American Gastroenterological Association, FIB-4 plays a central role in identifying patients with NAFLD at greatest risk for advanced fibrosis and most likely to benefit from referral to a hepatology specialist.14
Unfortunately, NAFLD and other CLDs are underdiagnosed and underrecognized in primary care.20–24 While the accuracy of FIB-4 to non-invasively assess fibrosis risk is encouraging, FIB-4’s application is severely limited by current diagnostic shortcomings. Primary care providers and practices have robust FIB-4 data due to the routine ordering of liver chemistries and platelet counts, but do not make use of the information to estimate fibrosis risk in the absence of a CLD diagnosis. Distributions of FIB-4 scores in primary care patient samples reveal a significant number of undiagnosed patients with indeterminate-risk (1.3 < FIB-4 ≤ 2.67) and high-risk (FIB-4 > 2.67) FIB-4 scores.25,26 Recent work using Swedish registry data suggests the use of FIB-4 in a general population may facilitate the prediction of future severe liver outcomes.27 We wanted to know if FIB-4 scores can help us better identify those patients at highest risk for cirrhosis, hepatocellular carcinoma, and liver transplantation in primary care patients with and without known CLD. We chose NAFLD as the CLD of emphasis to account for its rising burden and our desire to further understand the relationship between FIB-4 and severe liver disease in patients with NAFLD.
This study aims to evaluate the association of FIB-4 risk categories with severe liver disease outcomes, defined as cirrhosis, hepatocellular carcinoma, and liver transplantation, in a primary care patient population using a stratified Cox regression model. Identification of significant clinical signals, development of effective diagnostic tools, and access to decision support are all necessary to improve the recognition and management of CLD in primary care. We hypothesized that elevated FIB-4 risk assessments would be associated with future severe liver disease outcomes in a primary care sample.
METHODS
Study Design
This retrospective cohort study of electronic health record (EHR) data included primary care patients with data elements necessary for the calculation of a FIB-4 score and analyzed the association between FIB-4 risk categories (low-, indeterminate-, and high-risk) and the time to severe liver disease outcomes using a stratified Cox regression model. The Institutional Review Board at the Medical University of South Carolina (MUSC) approved this study.
Study Population
All patients receiving care from the Internal Medicine patient-centered medical home at the MUSC between January 1, 2007, and December 31, 2018, were evaluated. The practice conducts 32,000 patient visits yearly and delivers care to a diverse (39% non-white), adult (mean age 59 years) population.
Patients with aminotransferase (aspartate [AST] and alanine [ALT]) results during the study period were identified. We calculated FIB-4 scores (FIB-4=[(Age × AST)/(Platelets × √ALT)]) for each set of AST and ALT values where the AST and ALT values were less than 500 IU/L and there was a platelet count result at the time of, or within the preceding 2 months of, the aminotransferase values.7 We excluded aminotransferase values > 500 IU/L because these results were likely suggestive of an acute process not due solely to CLD, and FIB-4 calculations using markedly elevated aminotransferase levels present challenges in interpretation. The age variable in the calculation was the patient’s age at the time of the aminotransferase results. All patients with at least two FIB-4 scores were included in the patient sample to ensure included patients had more than a single data point for follow-up.
Patients with an International Classification of Diseases (ICD)-9/10 code for a CLD diagnosis (NAFLD, HCV, hemochromatosis, Wilson’s disease, etc.) or a liver disease outcome (cirrhosis, hepatocellular carcinoma, or liver transplantation) prior to their first qualifying FIB-4 score were excluded (Appendix Table A1).
Outcomes and Follow-up
The primary outcome was the time to the first occurrence of an ICD-9/10 code for a severe liver disease outcome, a composite of cirrhosis, liver transplantation, and hepatocellular carcinoma (Appendix). The occurrence of cirrhosis relies upon a combination of ICD-9/10 codes previously validated to most accurately identify cirrhosis from administrative data.28,29 A chart review of hepatology clinic notes and pathology results in the EHR was performed for the patients with a code for liver transplant to accurately identify the date of transplantation.
Patient follow-up began at the time of the calculated FIB-4 score and continued until the primary outcome event or the end of the study period (December 31, 2018). In this analysis, subjects who did not die, who dropped out, or who survived to the end of the study were considered censored.
Independent Variables
The primary predictor variables of interest were FIB-4 risk categories. Using the first FIB-4 score available for each patient, the patients were categorized into advanced fibrosis risk categories: low-risk (FIB-4 ≤ 1.3), indeterminate-risk (1.3 < FIB-4 ≤ 2.67), and high-risk (FIB-4 > 2.67). Risk thresholds were set using established values for NAFLD.30
Since the hazard of a severe liver disease outcome is expected to vary by the presence of CLD, we stratified our sample using a CLD variable. The identification of an ICD-9/10 code for CLD after the first FIB-4, but before the primary outcome or study endpoint, was used to categorize patients. This variable included 4 strata: no known CLD (None); NAFLD; other liver disease (Other Liver Dx); and NAFLD in combination with another liver disease (NAFLD + Other Liver Dx). Other CLDs included a composite of HBV, HCV, alcohol-related liver disease, autoimmune hepatitis, primary biliary cholangitis, hemochromatosis, Wilson’s disease, α-1 anti-trypsin deficiency, and drug-induced liver injury (Appendix).
Covariates
Other independent variables included demographic, vital sign, and comorbidity data. Gender was coded dichotomously as Male/Female. Race was a three-level, categorical variable coded as Black, White, and Other. Body mass index (BMI, kg/m2) was coded as a continuous variable of the patient’s BMI recorded at the time of, or just prior to, the FIB-4 score. The comorbidities of hypertension, diabetes mellitus, hyperlipidemia, cardiovascular disease, hypothyroidism, and kidney disease were identified by ICD-9/10 code placed at any time during follow-up. Diagnostic codes were composites from the Elixhauser Comorbidity Index.31
Data Sources
All data came from Medical University Hospital Authority Enterprise and EPIC© (EPIC Systems Corporation, WI) Clarity databases. Clinical, laboratory, and demographic data were obtained in the ambulatory, emergency room, and inpatient settings at the MUSC during the study period.
Statistical Analysis
Patient characteristics were reported as frequency counts and proportions for categorical variables, and mean and standard deviation for continuous variables. Patient characteristics were presented for the overall sample and by FIB-4 advanced fibrosis risk category. Continuous variables were compared across FIB-4 groups using one-way analysis of variance (ANOVA) tests and categorical variables were compared using chi-square tests. Proportions of patients with severe liver disease outcomes were calculated for the overall sample, by FIB-4 risk category, and by CLD strata. The standard deviation and 95% confidence intervals for the proportion of patients with the primary outcome were also reported.
The Kaplan-Meier analysis of time to severe liver disease was performed for the overall sample and by CLD diagnosis (NAFLD, other liver disease, NAFLD + other liver disease, and no known liver disease). Stratified Cox regression models were developed to evaluate the association of FIB-4 risk category with the hazard of severe liver disease. The models used time in days as the time scale from initial FIB-4 to the outcome of interest or the end of the study period. The models estimated the hazard ratios and 95% confidence intervals for indeterminate- and high-risk FIB-4 scores compared to low-risk FIB-4 scores (reference). The models were stratified by the 4-level, CLD variable (None, NAFLD, Other Liver Dx, and NAFLD + Other Liver Dx). An unadjusted model was performed, and then, a model adjusting for gender, race, marital status, smoking history, BMI, and multiple comorbidities was developed. Comorbidities included hypertension, diabetes, hyperlipidemia, cardiovascular disease, hypothyroidism, and kidney disease. Model assumptions of proportionality of hazard over time were first tested and confirmed, followed by residual assessments to ensure we used the most appropriate data fit and to identify any potential outliers or influential observations. The assumption of proportional hazards was tested by testing the interaction between covariates and log (time) and additional model checking was made via residual analysis. Statistical analyses were performed using SAS version 9.4 (Cary, NC).
RESULTS
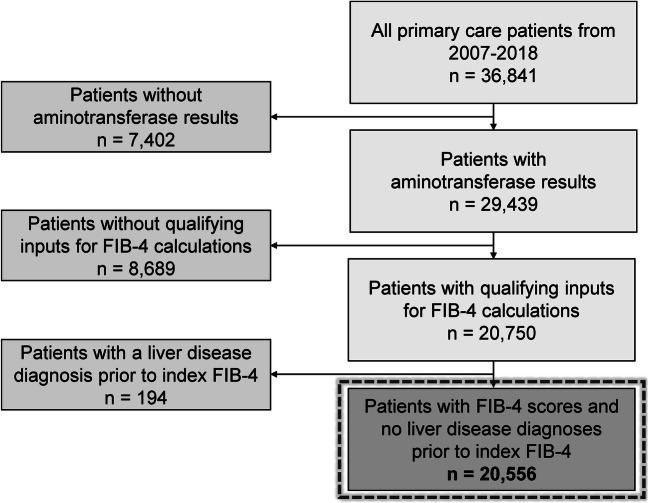
A total of 20,556 primary care patients with FIB-4 measurements met inclusion and exclusion criteria (Fig. 1). These patients had a mean age of 51 years (SD 16.6 years) at the time of the first FIB-4 result and 65% were female. Of the patient sample, 45% identified as Black. The mean BMI at the time of the FIB-4 calculation was 29.8 kg/m2 (SD 8.2) and 67% and 30% of the sample had hypertension and diabetes, respectively (Table 1).
Fig. 1.
Flow diagram of patients included in the study
Table 1.
Patient Characteristics for the Entire Cohort and by FIB-4 Risk Category
| Characteristics | Total | FIB-4 risk category | p-value | ||
|---|---|---|---|---|---|
| Low | Indeterminate | High | |||
| ≤ 1.3 | 1.31–2.67 | > 2.67 | |||
| n=20,556 | n=13,127 | n=6,012 | n=1,417 | ||
| Age (mean ± SD) | 51.0 ± 16.6 | 44.2 ± 14.6 | 62.8 ± 12.1 | 64.2 ± 15.0 | <0.001* |
| Gender (%) | <0.001† | ||||
| Female | 64.8 | 71.6 | 54.6 | 44.3 | |
| Male | 35.2 | 28.4 | 45.4 | 55.7 | |
| Race (%) | <0.001† | ||||
| Black | 45.3 | 46.1 | 43.1 | 46.4 | |
| Other | 2.6 | 3.0 | 1.7 | 2.3 | |
| White | 52.2 | 50.9 | 55.2 | 51.2 | |
| Marital status (%) | <0.001† | ||||
| Married | 45.5 | 44.4 | 49.1 | 40.2 | |
| Unmarried | 54.5 | 55.6 | 50.9 | 59.8 | |
| Current smoker (% yes) | 12.5 | 13.5 | 10.1 | 14.0 | <0.001† |
| BMI (mean ± SD) | 29.8 ± 8.2 | 30.6 ± 8.5 | 28.7 ± 7.3 | 27.5 ± 6.9 | <0.001* |
| Hypertension (%) | 67.1 | 60.3 | 78.9 | 79.8 | <0.001† |
| Diabetes (%) | 29.5 | 27.1 | 33.5 | 35.1 | <0.001† |
| Hyperlipidemia (%) | 54.4 | 47.3 | 69.4 | 56.9 | <0.001† |
| Cardiovascular disease (%) | 26.5 | 21.0 | 35.0 | 40.9 | <0.001† |
| Hypothyroidism (%) | 16.0 | 15.2 | 17.5 | 17.2 | <0.001† |
| Kidney disease (%) | 18.4 | 13.6 | 25.3 | 33.6 | <0.001† |
| Chronic liver disease (%) | <0.001† | ||||
| None | 89.0 | 91.1 | 88.2 | 73.0 | <0.001† |
| NAFLD | 2.3 | 2.4 | 2.2 | 2.5 | 0.6536† |
| Other Liver Dx | 8.2 | 6.2 | 9.1 | 23.2 | <0.001† |
| NAFLD + Other Liver Dx | 0.5 | 0.4 | 0.5 | 1.3 | <0.001† |
*One-way analysis of variance (ANOVA) test to compare means across the 3 risk categories. †Chi-square tests used to compare proportions across the FIB-4 risk categories. FIB-4, Fibrosis-4 Index; SD, standard deviation; BMI, body mass index (kg/m2); NAFLD, nonalcoholic fatty liver disease; Dx, diagnosis
After calculating the FIB-4 scores, 64% were low-risk, 29% were indeterminate-risk, and 7% were high-risk for advanced fibrosis. During the follow-up period, 2% of the sample received a diagnosis of NAFLD, 8% received a diagnosis of other CLD, and 1% received a diagnosis of NAFLD and another CLD, while 89% of the sample received no CLD diagnosis (Table 1).
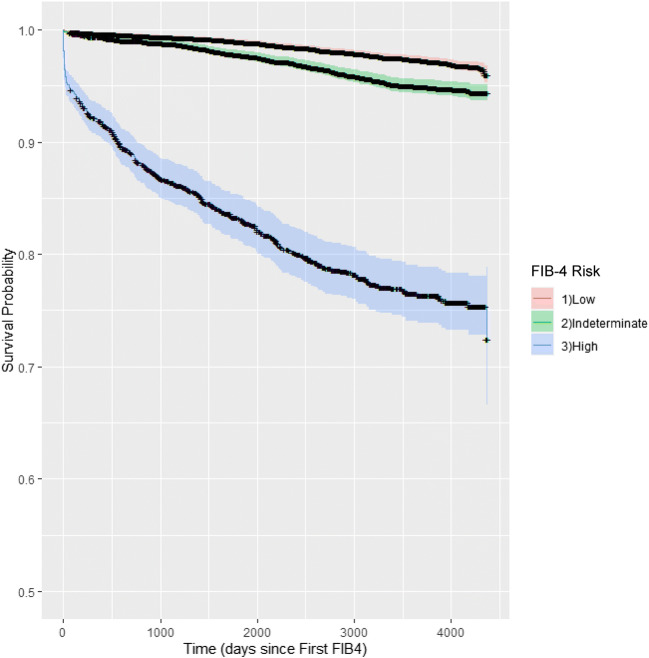
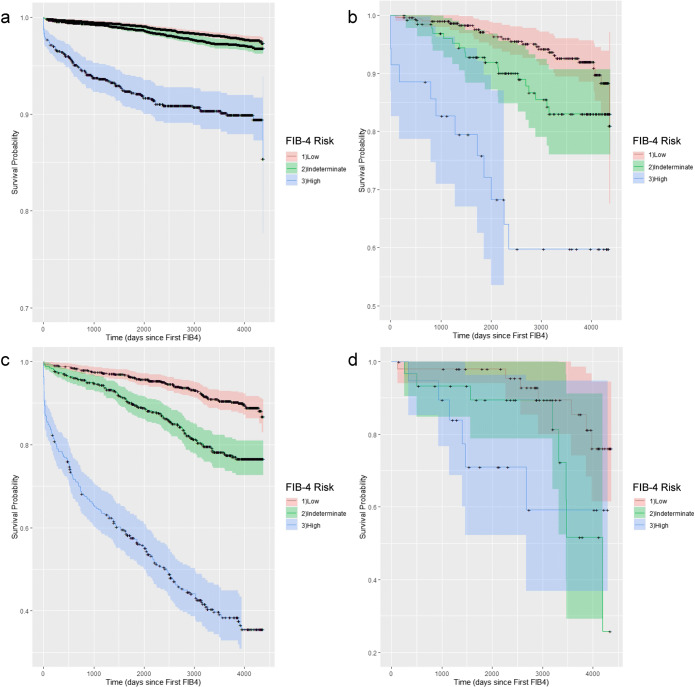
Patients were followed for a mean 2,978 days (8.2 years; SD 1,201 days). The Kaplan-Meier analysis of time to severe liver outcome is presented for the overall sample (Fig. 2), and by CLD diagnosis (Fig. 3). A total of 837 (4%) patients received a diagnostic code for a severe liver disease outcome during follow-up. Of patients with low-, indeterminate-, and high-risk FIB-4 scores, 2%, 4%, and 21% suffered a severe liver disease outcome, respectively (Table 2). In those patients with high-risk FIB-4 scores, 73% (1,034/1,417) were not diagnosed with a CLD during follow-up, and 49% (411/837) of patients with a severe liver outcome had no prior documentation of CLD.
Fig. 2.
Kaplan-Meier curve for severe liver disease risk-free survival by FIB-4 risk category for the entire sample
Fig. 3.
Kaplan-Meier curves for the severe liver disease risk-free survival by FIB-4 risk category stratified by chronic liver disease: a) no known chronic liver disease (None); b) nonalcoholic fatty liver disease (NAFLD); c) other liver disease diagnosis (Other Liver Dx); and d) known NAFLD and another chronic liver disease (NAFLD + Other Liver Dx)
Table 2.
Proportion of Patients with Severe Liver Disease Outcomes by FIB-4 Risk Category and Chronic Liver Disease Diagnosis
| FIB-4 risk category | Chronic liver disease diagnosis | n | % with severe liver disease outcome (SD) | SD % | 95% confidence interval | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Low | 13,127 | 2.2% | 14.7% | 2.0% | 2.5% | |
| None | 11,954 | 1.6% | 12.6% | 1.4% | 1.8% | |
| NAFLD | 312 | 7.4% | 26.2% | 4.5% | 10.3% | |
| Other Liver Dx | 812 | 8.3% | 27.5% | 6.4% | 10.1% | |
| NAFLD + Other Liver Dx | 49 | 14.3% | 35.3% | 4.1% | 24.4% | |
| Indeterminate | 6,012 | 4.2% | 20.0% | 3.7% | 4.7% | |
| None | 5,302 | 2.3% | 15.1% | 1.9% | 2.7% | |
| NAFLD | 131 | 13.7% | 34.6% | 7.8% | 19.7% | |
| Other Liver Dx | 548 | 18.4% | 38.8% | 15.1% | 21.7% | |
| NAFLD + Other Liver Dx | 31 | 25.8% | 44.5% | 9.5% | 42.1% | |
| High | 1,417 | 20.8% | 4.6% | 18.7% | 22.9% | |
| None | 1,034 | 9.0% | 28.6% | 7.2% | 10.7% | |
| NAFLD | 35 | 34.3% | 48.2% | 17.7% | 50.8% | |
| Other Liver Dx | 329 | 55.9% | 49.7% | 50.5% | 61.3% | |
| NAFLD + Other Liver Dx | 19 | 31.6% | 47.8% | 8.6% | 54.6% | |
| Total | 20,556 | 4.1% | 19.8% | 3.8% | 4.3% | |
FIB-4, Fibrosis-4 Index; SD, standard deviation; NAFLD, nonalcoholic fatty liver disease; Dx, diagnosis
In the unadjusted stratified Cox regression model, indeterminate-risk (HR 1.70; 95% CI 1.43–2.01) and high-risk (HR 7.37; 95% CI 6.23–8.71) FIB-4 scores were associated with a higher risk of severe liver disease outcomes (Table 3). After adjusting for patient demographics, BMI, and comorbidities, indeterminate-risk FIB-4 scores had a hazard ratio of 1.62 (95% CI 1.36–1.92) and high-risk FIB-4 scores a hazard ratio of 6.64 (95% CI 5.58–7.90) for the risk of severe liver disease (full adjusted model in the Appendix). High-risk FIB-4 scores were associated with severe liver disease outcomes for patients with known NAFLD (HR 7.32; 95% CI 3.44–15.58), other liver disease (HR 11.39; 95% CI 8.53–15.20), and a combination of NAFLD and another liver disease (HR 6.89; 95% CI 1.82–26.14). FIB-4 scores in the high-risk range had an estimated hazard ratio of 4.05 (95% CI 3.10–5.28) for the severe liver outcomes in patients with no known CLD.
Table 3.
Estimated Hazard Ratios and 95% Confidence Intervals Using Stratified Cox Regression Models for the Association between FIB-4 Risk Category and Severe Liver Disease
| Unadjusted model | Adjusted model* | |||
|---|---|---|---|---|
| FIB-4 risk (low = reference) | FIB-4 risk (low = reference) | |||
| Indeterminate | High | Indeterminate | High | |
| Overall | ||||
| HR | 1.70 | 7.37 | 1.62 | 6.64 |
| 95% CI | 1.43–2.01 | 6.23–8.71 | 1.36–1.92 | 5.58–7.90 |
| Chronic liver disease diagnosis | ||||
| None | ||||
| HR | 1.38 | 5.95 | 1.13 | 4.05 |
| 95% CI | 1.10–1.73 | 4.64–7.62 | 0.89–1.43 | 3.10–5.28 |
| NAFLD | ||||
| HR | 1.95 | 6.38 | 1.88 | 7.32 |
| 95% CI | 1.05–3.62 | 3.17–12.84 | 0.99–3.60 | 3.44–15.58 |
| Other Liver Dx | ||||
| HR | 2.39 | 10.75 | 2.65 | 11.39 |
| 95% CI | 1.76–3.26 | 8.11–14.24 | 1.93–3.63 | 8.53–15.20 |
| NAFLD + Other Liver Dx | ||||
| HR | 2.73 | 3.64 | 2.53 | 6.89 |
| 95% CI | 0.98–7.64 | 1.21–10.94 | 0.79–8.12 | 1.82–26.14 |
*Full adjusted model is in the Appendix. All models use days from the FIB-4 score as the time scale. The adjusted model controls for patient gender, race, marital status, smoking history, body mass index, and the presence of hypertension, diabetes, hyperlipidemia, cardiovascular disease, hypothyroidism, and kidney disease (full model in the Appendix). FIB-4, Fibrosis-4 Index; HR, hazard ratio; CI, confidence interval; NAFLD, nonalcoholic fatty liver disease; Dx, diagnosis
DISCUSSION
High-risk FIB-4 scores are associated with the development of severe liver outcomes in primary care patients with and without diagnosed CLD over a mean follow-up of 8.2 years. These results support the value of FIB-4 calculation and use in primary care patients with NAFLD, since the high prevalence of NAFLD may obscure a small but significant population of patients at risk for severe liver disease over the long term.2,4,5 This study suggests that high-risk FIB-4 scores should trigger investigation into the possibility of an unrecognized CLD as well as additional fibrosis assessment (e.g., vibration-controlled elastography, enhanced liver fibrosis [ELF] test) and consideration for referral to a hepatology specialist.1–6,32 Primary care can manage patients with low-risk FIB-4 scores by encouraging weight loss to prevent liver disease progression and working to reduce cardiovascular risk.14,33 Although the role of FIB-4 in the primary care management of CLDs other than NAFLD and HCV is less well-defined, our study suggests that FIB-4 is useful in identifying advanced fibrosis in these patients. FIB-4 could provide a signal for referring patients with alcohol-related liver disease to hepatology, or performing surveillance for hepatocellular carcinoma in patients previously treated for HCV.34,35
The strength of association between high-risk FIB-4 scores and severe liver outcomes in the overall stratified Cox model (HR 6.64; 95% CI 5.58–7.90) and for those patients with no previously diagnosed CLD (HR 4.05; 95% CI 3.10–5.28) supports routine calculation of FIB-4 in primary care. While momentum continues to build for the primary care utilization of FIB-4 in patients with NAFLD, FIB-4 could also play an impactful role as a herald of insidiously advancing liver disease in previously undiagnosed patients. Our data demonstrated the availability of FIB-4 score components (scores for 20,556 patients), a large proportion of patients with high-risk values (FIB-4 > 2.67) having no previously diagnosed CLD (73%), and nearly half of the patients with severe liver disease outcomes carrying no previously known CLD diagnosis (49%). Therefore, improving the recognition of NAFLD and other CLDs in primary care is imperative. While we work to address this underdiagnosis, the incorporation of FIB-4 calculation for all primary care patients can serve as a signal to intensify diagnostic efforts.
Incorporating FIB-4 into primary care will take conscientious effort and innovation. Though the variables for FIB-4 calculation are frequently available and easily accessible in an EHR, calculation currently requires manual data extraction, input to a calculator, and reintegration of the result and interpretation into the health record. These steps are unlikely to be welcomed, and consequently performed, by primary care providers given their ever-expanding list of clinical and clerical tasks.36,37 Alternatively, if prospective studies demonstrate similar associations between FIB-4 and severe liver disease, one could envision the inclusion of a FIB-4 value with any liver chemistry panel ordered if a suitable platelet count were available within the EHR during some designated acceptable period. Liver chemistries are among the most frequently ordered tests in medicine, and the utility of the information gathered from these is not always clear.38–40 By pairing a FIB-4 score with each liver test panel, one might incorporate a signal of progressing liver disease at no additional cost. Further work would need to consider how to navigate elevated scores, specifically indeterminate-risk scores, and how we communicate these scores to patients, but this warrants consideration as we strive to improve our diagnostic strategies.
We recognize limitations in this work. First, our outcome of interest is a composite of ICD-9 and ICD-10 diagnostic codes, which have not always performed well in identifying chronic and severe liver disease.41 To handle this concern, we relied upon previously validated techniques to capture cirrhosis diagnostic codes and performed a chart review on patients with an outcome for liver transplantation. This limitation also applies to the use of ICD-9/10 codes for our exclusion criteria. Also, the death data in our EHR are suboptimal. Patients dying in the hospital are accurately recorded, but those dying out of the hospital are irregularly documented. On account of this, we were unable to use death as a right censoring event, resulting in some prolonged survival periods. However, this limitation would likely result in an overestimation in our sample’s severe liver disease-free survival time, and a conservative underestimation of hazard rate. The data for FIB-4 calculation come from lab results during the study period, but we did not determine the reasons they were obtained. Also, this work focuses on a single FIB-4 value when multiple FIB-4s per patients could be calculated throughout the study period. By limiting the analysis to a single FIB-4 value, patient health details are lost, but the challenges of choosing other FIB-4 scores to interpret, when the intervals between scores and patients vary significantly, are avoided. We are working to address the relationship between FIB-4 changes over time and severe liver outcomes in future work. We attempted to restrict qualifying FIB-4 calculations by degree of AST and ALT elevation (<500 IU/L) and the timing of the platelet counts (within 2 months) to limit the number and frequency with which these data derived from acute liver diseases. Additionally, this work may not include all variables important to the progression of severe liver disease in primary care patients. Evidence of this concern appears in the adjusted regression analyses where hyperlipidemia is observed to have a lower odds of severe liver disease (OR 0.54; 95% CI 0.46–0.63). This relationship is counterintuitive, and we hypothesize it may reflect the association between statin therapy (a likely reason for coding hyperlipidemia) and the occurrence of severe liver disease, a potentially protective statin effect in CLD receiving significant attention in the literature.42 Lastly, these data come from a single center which can threaten generalizability. However, we feel that the primary care focus (compared to a hepatology clinic) and the distribution of comorbid conditions (Table 1) reflect patients throughout the USA.
CONCLUSION
High-risk FIB-4 scores were strongly associated with future severe liver disease outcomes in patients with and without a diagnosed CLD in this primary care sample. As work continues to improve diagnostic and management strategies of NAFLD and other CLDs in primary care, comprehensive application of FIB-4 in this setting could provide a critical signal of silently advancing liver fibrosis and provoke clinicians to intensify appropriate diagnostic testing.
Supplementary information
(DOCX 23 kb)
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BMI
Body mass index
- EHR
Electronic health record
- FIB-4
Fibrosis-4 Index
- ICD
International Classification of Diseases
- NAFLD
Nonalcoholic fatty liver disease
- Plt
Platelet
Author Contribution
All contributors to this work did enough to meet the threshold for authorship with all named authors contributing to the study design, interpretation of data, editing of the manuscript, and the decision to submit. Dr. Schreiner and Dr. Moran led the writing of the original draft. Dr. Gebregziabher developed the analysis plan, and Dr. Gebregziabher and Ms. Zhang performed the analyses. Ms. Zhang, Mr. Marsden, and Dr. Schreiner performed the data collection. Dr. Schreiner and Dr. Moran are co-first authors on this work.
Funding
Effort and time for this study were funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK K23DK118200 PI: Schreiner).
Data Availability
The study protocol and individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices) and in compliance with Health Insurance Portability and Accountability Act of 1996 and the Institutional Review Board at the MUSC, will be made available to investigators whose proposed use of the data has been approved by an independent review committee (“learned intermediary”) identified for this purpose.
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Andrew D. Schreiner and William P. Moran are co-first authors.
Presentations
“The Fibrosis-4 Index and severe liver disease outcomes in primary care: a stratified Cox analysis.” American Association for the Study of Liver Diseases (AASLD)- The Liver Meeting. November 2021.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md.). 2018;67(1):328-357. 10.1002/hep.29367 [DOI] [PubMed]
- 2.Davyduke T, Tandon P, Al-Karaghouli M, Abraldes JG, Ma MM. Impact of Implementing a "FIB-4 First" Strategy on a Pathway for Patients With NAFLD Referred From Primary Care. Hepatol Commun. 2019;3(10):1322–33. doi: 10.1002/hep4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava A, Gailer R, Tanwar S, Trembling P, Parkes J, Rodger A, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. 2019;71(2):371–8. doi: 10.1016/j.jhep.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 5.Tsochatzis EA, Newsome PN. Non-alcoholic fatty liver disease and the interface between primary and secondary care. Lancet Gastroenterol Hepatol. 2018;3(7):509–17. doi: 10.1016/S2468-1253(18)30077-3. [DOI] [PubMed] [Google Scholar]
- 6.Newsome PN, Cramb R, Davison SM, Dillon JF, Foulerton M, Godfrey EM, et al. Guidelines on the management of abnormal liver blood tests. Gut. 2018;67(1):6–19. doi: 10.1136/gutjnl-2017-314924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology (Baltimore, Md.) 2007;46(1):32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 8.Sonneveld MJ, Brouwer WP, Chan HL, Piratvisuth T, Jia JD, Zeuzem S, et al. Optimisation of the use of APRI and FIB-4 to rule out cirrhosis in patients with chronic hepatitis B: Results from the SONIC-B study. Lancet Gastroenterol Hepatol. 2019;4(7):538–44. doi: 10.1016/S2468-1253(19)30087-1. [DOI] [PubMed] [Google Scholar]
- 9.Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: A systemic review and meta-analysis. Hepatology (Baltimore, Md.) 2015;61(1):292–302. doi: 10.1002/hep.27382. [DOI] [PubMed] [Google Scholar]
- 10.Chang Y, Cho YK, Cho J, Jung HS, Yun KE, Ahn J, et al. Alcoholic and Nonalcoholic Fatty Liver Disease and Liver-Related Mortality: A Cohort Study. Am J Gastroenterol. 2019;114(4):620–629. doi: 10.14309/ajg.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 11.Chin J, Powell LW, Ramm LE, Hartel GF, Olynyk JK, Ramm GA. Utility of Serum Biomarker Indices for Staging of Hepatic Fibrosis Before and After Venesection in Patients With Hemochromatosis Caused by Variants in HFE. Clin Gastroenterol Hepatol. 2021;19(7):1459-1468.e5. 10.1016/j.cgh.2020.07.052 [DOI] [PubMed]
- 12.Ballestri S, Mantovani A, Baldelli E, Lugari S, Maurantonio M, Nascimbeni F, et al. Liver Fibrosis Biomarkers Accurately Exclude Advanced Fibrosis and Are Associated with Higher Cardiovascular Risk Scores in Patients with NAFLD or Viral Chronic Liver Disease. Diagnostics (Basel). 2021;11(1). 10.3390/diagnostics11010098 [DOI] [PMC free article] [PubMed]
- 13.Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020;18(12):2650-2666. 10.1016/j.cgh.2019.07.060 [DOI] [PMC free article] [PubMed]
- 14.Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, et al. Clinical Care Pathway for the Risk Stratification and Management of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2021;161(5):1657–69. doi: 10.1053/j.gastro.2021.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149(2):389–97. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology (Baltimore, Md.) 2017;65(5):1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagstrom H, Nasr P, Ekstedt M, Hammar U, Stal P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265–73. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clinical gastroenterology and hepatology : The official clinical practice journal of the American Gastroenterological Association. 2012;10(6):646–50. doi: 10.1016/j.cgh.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Taylor RS, Taylor RJ, Bayliss S, Hagstrom H, Nasr P, Schattenberg JM, et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;158(6):1611–25. doi: 10.1053/j.gastro.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 20.Cleveland ER, Ning H, Vos MB, Lewis CE, Rinella ME, Carr JJ, et al. Low Awareness of Nonalcoholic Fatty Liver Disease in a Population-Based Cohort Sample: the CARDIA Study. J Gen Intern Med. 2019;34(12):2772–8. doi: 10.1007/s11606-019-05340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loomba R, Wong R, Fraysse J, Shreay S, Li S, Harrison S, et al. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of Medicare data. Aliment Pharmacol Ther. 2020. 10.1111/apt.15679 [DOI] [PubMed]
- 22.Patel PJ, Banh X, Horsfall LU, Hayward KL, Hossain F, Johnson T, et al. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: Limited awareness of surrogate markers of fibrosis. Internal medicine journal. 2018;48(2):144–51. doi: 10.1111/imj.13667. [DOI] [PubMed] [Google Scholar]
- 23.Patel YA, Gifford EJ, Glass LM, Turner MJ, Han B, Moylan CA, et al. Identifying Nonalcoholic Fatty Liver Disease Advanced Fibrosis in the Veterans Health Administration. Dig Dis Sci. 2018;63(9):2259–66. doi: 10.1007/s10620-018-5123-3. [DOI] [PubMed] [Google Scholar]
- 24.Polanco-Briceno S, Glass D, Stuntz M, Caze A. Awareness of nonalcoholic steatohepatitis and associated practice patterns of primary care physicians and specialists. BMC Res Notes. 2016;9:157. doi: 10.1186/s13104-016-1946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreiner AD, Zhang J, Durkalski-Mauldin V, Livingston S, Marsden J, Bian J, et al. Advanced Liver Fibrosis and the Metabolic Syndrome in a Primary Care Setting. Diabetes Metab Res Rev. 2021. 10.1002/dmrr.3452 [DOI] [PMC free article] [PubMed]
- 26.Petroff D, Batz O, Jedrysiak K, Kramer J, Berg T, Wiegand J. Fibrosis-4(FIB-4) score at the primary care level: An analysis of over 160 000 blood samples. Gut. 2021;70(1):219-21. 10.1136/gutjnl-2020-320995 [DOI] [PubMed]
- 27.Hagstrom H, Talback M, Andreasson A, Walldius G, Hammar N. Repeated FIB-4 measurements can help identify individuals at risk of severe liver disease. J Hepatol. 2020;73(5):1023–9. doi: 10.1016/j.jhep.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Mapakshi S, Kramer JR, Richardson P, El-Serag HB, Kanwal F. Positive Predictive Value of International Classification of Diseases, 10th Revision, Codes for Cirrhosis and Its Related Complications. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018;16(10):1677–8. doi: 10.1016/j.cgh.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 29.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. Journal of clinical gastroenterology. 2013;47(5):e50–4. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clinical gastroenterology and hepatology : The official clinical practice journal of the American Gastroenterological Association. 2009;7(10):1104–12. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 32.Budd J, Cusi K. Nonalcoholic Fatty Liver Disease: What Does the Primary Care Physician Need to Know? Am J Med. 2020;133(5):536–43. doi: 10.1016/j.amjmed.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Younossi ZM, Noureddin M, Bernstein D, Kwo P, Russo M, Shiffman ML, et al. Role of Noninvasive Tests in Clinical Gastroenterology Practices to Identify Patients With Nonalcoholic Steatohepatitis at High Risk of Adverse Outcomes: Expert Panel Recommendations. Am J Gastroenterol. 2021;116(2):254–262. doi: 10.14309/ajg.0000000000001054. [DOI] [PubMed] [Google Scholar]
- 34.Ioannou GN, Beste LA, Green PK, Singal AG, Tapper EB, Waljee AK, et al. Increased Risk for Hepatocellular Carcinoma Persists Up to 10 Years After HCV Eradication in Patients With Baseline Cirrhosis or High FIB-4 Scores. Gastroenterology. 2019;157(5):1264–78. doi: 10.1053/j.gastro.2019.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanwal F, Kramer JR, Asch SM, Cao Y, Li L, El-Serag HB. Long-Term Risk of Hepatocellular Carcinoma in HCV Patients Treated With Direct Acting Antiviral Agents. Hepatology (Baltimore, Md.) 2020;71(1):44–55. doi: 10.1002/hep.30823. [DOI] [PubMed] [Google Scholar]
- 36.Shanafelt TD, Dyrbye LN, Sinsky C, Hasan O, Satele D, Sloan J, et al. Relationship Between Clerical Burden and Characteristics of the Electronic Environment With Physician Burnout and Professional Satisfaction. Mayo Clinic proceedings. 2016;91(7):836–48. doi: 10.1016/j.mayocp.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Arndt BG, Beasley JW, Watkinson MD, Temte JL, Tuan WJ, Sinsky CA, et al. Tethered to the EHR: Primary Care Physician Workload Assessment Using EHR Event Log Data and Time-Motion Observations. Ann Fam Med. 2017;15(5):419–26. doi: 10.1370/afm.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lilford RJ, Bentham L, Girling A, Litchfield I, Lancashire R, Armstrong D, et al. Birmingham and Lambeth Liver Evaluation Testing Strategies (BALLETS): a prospective cohort study. Health technology assessment (Winchester, England) 2013;17(28):i–xiv. doi: 10.3310/hta17280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horton S, Fleming KA, Kuti M, Looi LM, Pai SA, Sayed S, et al. The Top 25 Laboratory Tests by Volume and Revenue in Five Different Countries. Am J Clin Pathol. 2019;151(5):446–51. doi: 10.1093/ajcp/aqy165. [DOI] [PubMed] [Google Scholar]
- 40.Schreiner AD, Mauldin PD, Moran WP, Durkalski-Mauldin V, Zhang J, Schumann SO, 3rd, et al. Assessing the Burden of Abnormal LFTs and the Role of the Electronic Health Record: A Retrospective Study. Am J Med Sci. 2018;355(6):537–43. doi: 10.1016/j.amjms.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Quan H, Li B, Saunders LD, Parsons GA, Nilsson CI, Alibhai A, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424–41. doi: 10.1111/j.1475-6773.2007.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bosch J, Gracia-Sancho J, Abraldes JG. Cirrhosis as new indication for statins. Gut. 2020;69(5):953–62. doi: 10.1136/gutjnl-2019-318237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 23 kb)
Data Availability Statement
The study protocol and individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices) and in compliance with Health Insurance Portability and Accountability Act of 1996 and the Institutional Review Board at the MUSC, will be made available to investigators whose proposed use of the data has been approved by an independent review committee (“learned intermediary”) identified for this purpose.