Abstract
Background
Vascular cell adhesion molecule‐1 (VCAM‐1) and intercellular adhesion molecule‐1 (ICAM‐1) modulate atherosclerosis by promoting leukocyte infiltration, neutrophil recruitment, endothelial cell proliferation, etc., which may directly or indirectly facilitate the occurrence of major adverse cardiac events (MACE). This study intended to investigate the value of VCAM‐1 and ICAM‐1 for predicting MACE in ST‐segment elevation myocardial infarction (STEMI) patients.
Methods
Totally, 373 STEMI patients receiving the percutaneous coronary intervention and 50 health controls (HCs) were included. Serum VCAM‐1 and ICAM‐1 were detected by ELISA. Meanwhile, MACE was recorded during a median follow‐up of 18 (range: 1–46) months in STEMI patients.
Results
Vascular cell adhesion molecule‐1 and ICAM‐1 were raised in STEMI patients compared with HCs (both p < 0.001). VCAM‐1 (p = 0.002) and ICAM‐1 (p = 0.012) high were linked with raised accumulating MACE rate in STEMI patients. Notably, VCAM‐1 high (hazard ratio [HR] = 2.339, p = 0.031), age ≥ 65 years (HR = 2.019, p = 0.039), history of diabetes mellitus (DM) (HR = 2.395, p = 0.011), C‐reactive protein (CRP) ≥ 5 mg/L (HR = 2.550, p = 0.012), multivessel disease (HR = 2.561, p = 0.007) independently predicted MACE risk in STEMI patients. Furthermore, a nomogram‐based prediction model combining these factors was established, exhibiting an acceptable value for estimating 1, 2, and 3‐year MACE risk, with AUC of 0.764, 0.716, and 0.778, respectively, in STEMI patients.
Conclusion
This study confirms the value of VCAM‐1 and ICAM‐1 measurement in predicting MACE risk in STEMI patients. Moreover, VCAM‐1 plus other traditional prognostic factors (such as age, history of DM, CRP, and multivessel disease) cloud further improve the predictive accuracy of MACE risk in STEMI patients.
Keywords: intercellular adhesion molecule‐1, major adverse cardiac events, risk factor, ST‐segment elevation myocardial infarction, vascular cell adhesion molecule‐1
This study intended to investigate the value of Vascular cell adhesion molecule‐1 (VCAM‐1) and intercellular adhesion molecule‐1 (ICAM‐1) for predicting major adverse cardiac events (MACE) in ST‐segment elevation myocardial infarction (STEMI) patients. Serum VCAM‐1 and ICAM‐1 from 373 STEMI patients and 50 health controls (HCs) were detected by ELISA. Meanwhile, MACE was recorded during a median follow‐up of 18 (range: 1–46) months. It was observed that VCAM‐1 and ICAM‐1 were raised in STEMI patients compared with HCs. In STEMI patients, VCAM‐1 and ICAM‐1 high were linked with raised accumulating MACE rate. Notably, VCAM‐1 high and other traditional prognostic factors independently predicted MACE risk. Furthermore, a nomogram‐based prediction model combining these factors assisted in estimating MACE risk. Conclusively, this study confirms the value of VCAM‐1 and ICAM‐1 in predicting MACE risk. Moreover, VCAM‐1 plus other traditional prognostic factors further improve the predictive accuracy of MACE risk in STEMI patients.
1. INTRODUCTION
ST‐segment elevation myocardial infarction (STEMI) is the most severe form of acute coronary syndrome (ACS) with impressive morbidity and mortality worldwide; meanwhile, it is generally accepted that STEMI is partly attributed to atherosclerotic plaque induced‐occlusive thrombosis. 1 , 2 , 3 At present, the treatments for STEMI patients include thrombolytic therapy, percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), etc.; among which PCI is favored if available within a reasonable timeframe. 2 , 4 , 5 However, due to the postoperative incidence of major adverse cardiac events (MACE), including all‐cause mortality, recurrent MI, and repeat revascularization, the long‐term prognosis of STEMI patients remains unsatisfying. 6 , 7 , 8 , 9 Conclusively, it is fundamental to discover new biomarkers that may predict MACE risk, then further improve the stratified management to achieve better clinical outcomes for STEMI patients.
Vascular cell adhesion molecule‐1 (VCAM‐1) and intercellular adhesion molecule‐1 (ICAM‐1) participate in the pathology and progression of cardiac diseases. 10 , 11 As stated by a previous study, VCAM‐1 inhibition suppresses inflammation and myocardial cell apoptosis, hence ameliorating cardiac functions in myocardial infarction rats. 10 Concerning ICAM‐1, it promotes proinflammatory leukocyte infiltration and fibrosis, as well as aggravates cardiac dysfunction, thus inhibiting the cardiac remodeling in the pressure overload‐induced heart failure mouse. 11 Clinically, a quite small number of studies seek the relations of VCAM‐1 and ICAM‐1 with adverse cardiovascular events in STEMI patients. 12 , 13 A previous study claims that VCAM‐1 and ICAM‐1 are linked to post‐acute myocardial infarction heart failure in STEMI patients. 13 Contrary to the above evidence, another study argues that VCAM‐1 and ICAM‐1 could not predict cardiovascular causes‐induced mortality in STEMI patients. 12 Notably, the sample sizes of these two studies are relatively insufficient, and the linkage of VCAM‐1 and ICAM‐1 with MACE‐related prognosis is conflicting in STEMI patients. As a result, it is necessary to include more STEMI patients to further confirm the values of VCAM‐1 and ICAM‐1 for forecasting MACE risk, which might be helpful for enhancing the stratified management of STEMI patients.
Accordingly, the current study detected serum levels of VCAM‐1 and ICAM‐1 in 373 STEMI patients, intending to investigate their performance on predicting accumulating MACE risk in STEMI patients.
2. METHODS
2.1. Participants
A total of 373 STEMI patients who underwent PCI between October 2017 and December 2019 were consecutively recruited. The patients' inclusion criteria were as follows: (1) diagnosed with STEMI 14 ; (2) received PCI for the first time; (3) age more than 18 years old. The patients' exclusion criteria were as follows: (1) history of previous cardiothoracic surgery; (2) complicated with systemic inflammatory disorders; (3) accompanied with systemic immune disorders; (4) had solid tumors or hematologic malignancies.
In addition, 50 healthy subjects were enrolled as health controls (HCs). The age of HCs was limited to 45–75 years old, and the male to female ratio of HCs was 4 to 1, in order to make age and gender matched to STEMI patients. The HCs' exclusion criteria were as follows: (1) history of previous cardiothoracic surgery; (2) systemic inflammatory, immune, or malignant disease. The Institutional Review Board of Cangzhou Central Hospital of Tianjin Medical University approved this study, and all participants' written informed consents were obtained.
2.2. Clinical data and sample collection
The STEMI patients' demographics and medical histories, laboratory findings, disease features, and treatment information were obtained through Electronic Health Record System. Peripheral blood (PB) samples of 373 STEMI patients were collected prior to surgery. PB samples of 50 HCs were gathered after enrollment.
2.3. Enzyme‐linked immunosorbent assay (ELISA)
After blood collection, the serum of STEMI patients and HCs was separated for ELISA. Then the levels of VCAM‐1 (ab223591) and ICAM‐1 (ab229383) were assayed by commercial ELISA Kits (Abcam). The procedures of determination referred to the manufacturer's protocols strictly.
2.4. Follow‐up and outcomes
All STEMI patients were closely followed up until September 2021. The range of follow‐up was 1–46 months, and the median of follow‐up was 18 months. The MACE over the follow‐up were evaluated by the attending physician, then the accumulating MACE rate was computed. The MACE was considered as a composite of all‐cause death, any myocardial infarction, and any repeat revascularization. 15
2.5. Statistical analysis
The statistical analyses were conducted through SPSS v26.1 (IBM Corp.). The graphs were mapped by R V.4.0.5 (ggplot2 package, available at www.r‐project.org) and GraphPad Prism v6.01 (GraphPad Software Inc.). The differences of the VCAM‐1 and ICAM‐1 between two groups were calculated through the Mann–Whitney U test. In STEMI patients, the VCAM‐1 was classified as high (≥669.1 ng/ml) and low (<669.1 ng/ml) through the median value (669.1 ng/ml); similarly, ICAM‐1 was classified as high (≥130.3 ng/ml) and low (<130.3 ng/ml) based on the median value (130.3 ng/ml) as well. The correlation of the VCAM‐1 and ICAM‐1 (high vs. low) with the accumulating MACE rate was performed via Kaplan–Meier curve and Tarone‐Ware test. After continuous variables converted to categorical variables by median or clinical cut points, the factors associated with MACE risk were evaluated through univariate and multivariate Cox's proportional hazards regression model (all factors were included, then performed via step‐forward method). The visualization of the multivariate prediction model was performed through nomogram. Further, the receiver operating characteristic (ROC) curve was performed to illustrate the models' predictive ability. A p value < 0.05 indicated statistical significance.
3. RESULTS
3.1. Clinical features
The included STEMI patients had a mean age of 61.9 ± 10.4 years with 95 (25.5%) females and 278 (74.5%) males. Meanwhile, there were 96 (25.7%) patients with a history of diabetes mellitus (DM). The median (interquartile range [IQR]) levels of laboratory findings were as follows: serum creatinine (Scr) (84.6 [73.6–100.4] μmol/L), low‐density lipoprotein cholesterol (LDL‐C) (3.1 [2.3–3.9] mmol/L), C‐reactive protein (CRP) (4.7 [3.2–6.3] mg/L), cardiac troponin I (cTnI) (4.4 [3.0–6.30] ng/ml), and creatine kinase‐myocardial band (CK‐MB) (32.5 [18.3–53.9] ng/ml). Regarding disease features, the median (IQR) value of infarct size was 23.0% (17.0%–29.0%); meanwhile, there were 158 (42.4%) patients with multivessel disease. Moreover, the median (IQR) value of total stent length was 33.0 (23.0–38.0) mm in STEMI patients. Other clinical information is listed in Table 1.
TABLE 1.
STEMI patients' characteristics
| Items | STEMI patients (N = 373) |
|---|---|
| Demographics | |
| Age (years), mean ± SD | 61.9 ± 10.4 |
| Gender, n (%) | |
| Female | 95 (25.5) |
| Male | 278 (74.5) |
| BMI (kg/m2), mean ± SD | 24.6 ± 3.3 |
| Current smoker, n (%) | 145 (38.9) |
| Medical history | |
| History of hypertension, n (%) | 258 (69.2) |
| History of hyperlipidemia, n (%) | 168 (45.0) |
| History of DM, n (%) | 96 (25.7) |
| Laboratory findings | |
| WBC (109/L), median (IQR) | 10.0 (7.6–12.7) |
| FBG (mmol/L), median (IQR) | 5.2 (4.4–6.3) |
| Scr (μmol/L), median (IQR) | 84.6 (73.6–100.4) |
| TG (mmol/L), median (IQR) | 1.8 (1.0–2.5) |
| TC (mmol/L), median (IQR) | 4.6 (3.8–5.4) |
| LDL‐C (mmol/L), median (IQR) | 3.1 (2.3–3.9) |
| HDL‐C (mmol/L), median (IQR) | 1.0 (0.9–1.2) |
| CRP (mg/L), median (IQR) | 4.7 (3.2–6.3) |
| cTnI (ng/ml), median (IQR) | 4.4 (3.0–6.30) |
| CK‐MB (ng/ml), median (IQR) | 32.5 (18.3–53.9) |
| Disease features | |
| Symptom‐to‐balloon time (min), median (IQR) | 190.0 (140.0–280.0) |
| Infarct size a (%), median (IQR) | 23.0 (17.0–29.0) |
| Culprit lesion, n (%) | |
| Left anterior descending artery | 159 (42.6) |
| Left circumflex artery | 75 (20.1) |
| Right coronary artery | 139 (37.3) |
| Multivessel disease, n (%) | 158 (42.4) |
| Treatment information | |
| Thrombus aspiration, n (%) | 82 (22.0) |
| Number of implanted stents, n (%) | |
| 1 | 285 (76.4) |
| 2 | 88 (23.6) |
| Type of stent, n (%) | |
| Sirolimus‐eluting stent | 253 (67.8) |
| Everolimus‐eluting stent | 120 (32.2) |
| Stent diameter (mm), median (IQR) | 3.0 (3.0–3.5) |
| Total stent length (mm), median (IQR) | 33.0 (23.0–38.0) |
Abbreviations: BMI, body mass index; CK‐MB, creatine kinase‐myocardial band; CRP, C‐reactive protein; cTnI, cardiac troponin I; DM, diabetes mellitus; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; Scr, serum creatinine; SD, standard deviation; STEMI, ST‐segment elevation myocardial infarction; TC, total cholesterol; TG, triglyceride; WBC, white blood cell.
Evaluated on the second or third day after PCI.
3.2. VCAM‐1 and ICAM‐1 expressions
Vascular cell adhesion molecule‐1 was raised in STEMI patients (median [IQR]: 669.1 [541.8–894.5] ng/ml) compared to HCs (median [IQR]: 388.2 [248.0–574.0] ng/ml) (p < 0.001) (Figure 1A). Further ROC curve analysis exhibited that VCAM‐1 presented a good value in distinguishing STEMI patients from HCs (area under the curve [AUC] (95% confidence interval [CI]): 0.852 [0.790–0.914]); besides, the value of VCAM‐1 at the best cut‐off point was 450.6 ng/ml (Figure 1B). Similarly, ICAM‐1 was also elevated in STEMI patients (median [IQR]: 130.3 [97.1–174.5] ng/ml) compared with HCs (median [IQR]: 72.7 [52.2–101.8] ng/ml) (p < 0.001) (Figure 1C). Subsequent ROC curve analysis showed that ICAM‐1 presented a good capacity in discriminating STEMI patients from HCs (AUC [95% CI]: 0.805 [0.734–0.875]); additionally, the value of ICAM‐1 at the best cut‐off point was 107.1 ng/ml (Figure 1D).
FIGURE 1.
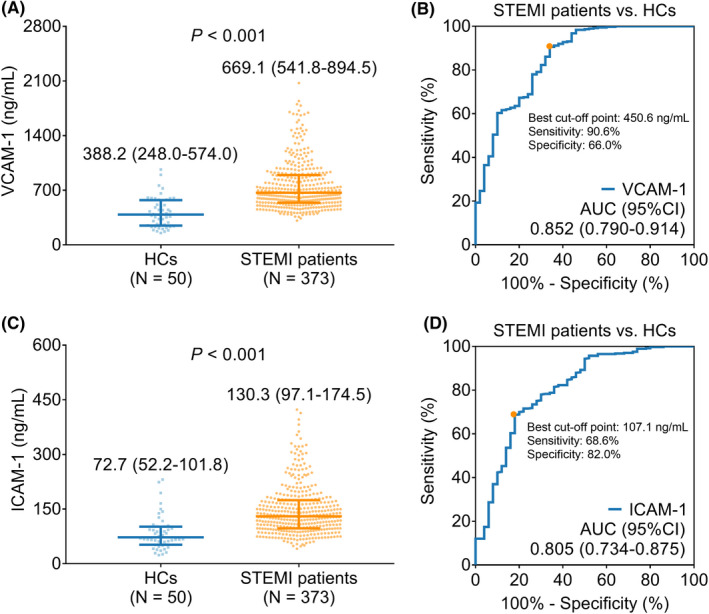
VCAM‐1 and ICAM‐1 were upregulated in STEMI patients than HCs. Comparison of VCAM‐1 in STEMI patients vs. HCs (A); the value of VCAM‐1 for distinguishing STEMI patients from HCs (B); comparison of ICAM‐1 in STEMI patients vs. HCs (C); the capacity of ICAM‐1 for discriminating STEMI patients from HCs (D).
3.3. Linkage of VCAM‐1 and ICAM‐1 with accumulating MACE rate
Vascular cell adhesion molecule‐1 high was linked to raised accumulating MACE rate in STEMI patients (p = 0.002). Specifically, the 1, 2, and 3‐year accumulating MACE rates were 9.9%, 14.9%, and 22.0% in patients with VCAM‐1 high; and they were 3.0%, 5.7%, and 17.2% in patients with VCAM‐1 low (Figure 2A).
FIGURE 2.
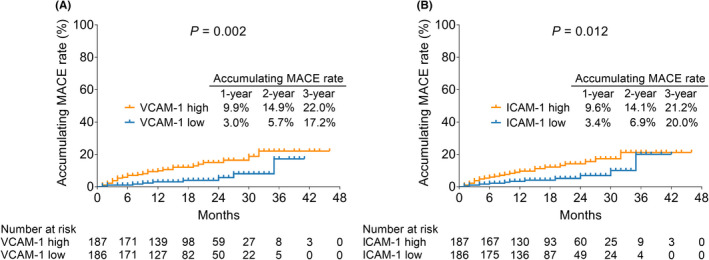
VCAM‐1 and ICAM‐1 high were correlated with raised accumulating MACE rate in STEMI patients. Association between VCAM‐1 high and accumulating MACE rate (A); linkage between ICAM‐1 high and accumulating MACE rate (B) in STEMI patients.
Regarding ICAM‐1, it was found that ICAM‐1 high was also linked with elevated accumulating MACE rate in STEMI patients (p = 0.012). Particularly, the 1, 2, and 3‐year accumulating MACE rates were 9.6%, 14.1%, and 21.2% in patients with ICAM‐1 high; whereas they were 3.4%, 6.9%, and 20.0% in patients with ICAM‐1 low (Figure 2B).
3.4. Factors related to MACE risk by univariate and multivariate Cox's proportional hazards regression analysis
Vascular cell adhesion molecule‐1 (high vs. low) (hazard ratio [HR]: 2.825, p = 0.007), ICAM‐1 (high vs. low) (HR = 2.244, p = 0.026), history of DM (yes vs. no) (HR = 2.049, p = 0.034), Scr (≥110 vs. <110 μmol/L) (HR = 2.239, p = 0.037), LDL‐C (≥3.4 vs. <3.4 mmol/L) (HR = 2.150, p = 0.024), CRP (≥5 vs. <5 mg/L) (HR = 2.388, p = 0.014), cTnI (≥median vs. <median) (HR = 2.028, p = 0.046), multivessel disease (yes vs. no) (HR = 2.407, p = 0.011), and total stent length (≥median vs. <median) (HR = 2.543, p = 0.010) were all linked with raised MACE risk in STEMI patients (Table 2).
TABLE 2.
Factors related to MACE risk by univariate Cox's proportional hazards regression analysis
| Items | p value | HR | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| VCAM‐1 (high vs. low) | 0.007 | 2.825 | 1.328 | 6.010 |
| ICAM‐1 (high vs. low) | 0.026 | 2.244 | 1.103 | 4.563 |
| Age (≥65 vs. <65 years) | 0.107 | 1.715 | 0.891 | 3.304 |
| Gender (male vs. female) | 0.098 | 2.223 | 0.864 | 5.718 |
| BMI (≥28 vs. <28 kg/m2) | 0.080 | 1.922 | 0.924 | 3.998 |
| Current smoker (yes vs. no) | 0.284 | 1.431 | 0.743 | 2.756 |
| History of hypertension (yes vs. no) | 0.247 | 1.591 | 0.724 | 3.495 |
| History of hyperlipidemia (yes vs. no) | 0.057 | 1.907 | 0.982 | 3.705 |
| History of DM (yes vs. no) | 0.034 | 2.049 | 1.056 | 3.977 |
| WBC (≥109/L vs. <109/L) | 0.060 | 1.947 | 0.973 | 3.898 |
| FBG (≥6.2 vs. <6.2 mmol/L) | 0.462 | 1.297 | 0.649 | 2.595 |
| Scr (≥110 vs. <110 μmol/L) | 0.037 | 2.239 | 1.052 | 4.767 |
| TG (≥1.7 vs. <1.7 mmol/L) | 0.082 | 1.879 | 0.924 | 3.819 |
| TC (≥5.2 vs. <5.2 mmol/L) | 0.146 | 1.643 | 0.841 | 3.213 |
| LDL‐C (≥3.4 vs. <3.4 mmol/L) | 0.024 | 2.150 | 1.107 | 4.172 |
| HDL‐C (≤0.94 vs. >0.94 mmol/L) | 0.638 | 0.844 | 0.415 | 1.715 |
| CRP (≥5 vs. <5 mg/L) | 0.014 | 2.388 | 1.193 | 4.782 |
| cTnI (≥median vs. <median) | 0.046 | 2.028 | 1.013 | 4.060 |
| CK‐MB (≥median vs. <median) | 0.143 | 1.652 | 0.844 | 3.234 |
| Symptom‐to‐balloon time (≥130 vs. <130 min) | 0.050 | 4.174 | 1.002 | 17.389 |
| Infarct size (≥median vs. <median) | 0.072 | 1.866 | 0.945 | 3.684 |
| Culprit lesion, n (%) | ||||
| Left anterior descending artery | Ref. | |||
| Left circumflex artery | 0.431 | 0.640 | 0.210 | 1.946 |
| Right coronary artery | 0.176 | 1.624 | 0.805 | 3.278 |
| Multivessel disease (yes vs. no) | 0.011 | 2.407 | 1.219 | 4.752 |
| Thrombus aspiration (yes vs. no) | 0.068 | 0.333 | 0.102 | 1.085 |
| Number of implanted stents (2 vs. 1) | 0.358 | 1.395 | 0.686 | 2.837 |
| Type of stent (Everolimus‐eluting stent vs. Sirolimus‐eluting stent) | 0.140 | 0.552 | 0.250 | 1.216 |
| Stent diameter (≥median vs. <median) | 0.210 | 0.635 | 0.312 | 1.291 |
| Total stent length (≥median vs. <median) | 0.010 | 2.543 | 1.251 | 5.170 |
Abbreviations: BMI, body mass index; CI, confidence interval; CK‐MB, creatine kinase‐myocardial band; CRP, C‐reactive protein; cTnI, cardiac troponin I; DM, diabetes mellitus; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; ICAM‐1, intercellular adhesion molecule‐1; LDL‐C, low‐density lipoprotein cholesterol; MACE, major adverse cardiac events; Scr, serum creatinine; TC, total cholesterol; TG, triglyceride; VCAM‐1, vascular cell adhesion molecule‐1; WBC, white blood cell.
Further multivariate Cox analysis showed that VCAM‐1 (high vs. low) (HR = 2.339, p = 0.031), age (≥65 vs. <65 years) (HR = 2.019, p = 0.039), history of DM (yes vs. no) (HR = 2.395, p = 0.011), CRP (≥5 vs. <5 mg/L) (HR = 2.550, p = 0.012), and multivessel disease (yes vs. no) (HR = 2.561, p = 0.007) were all independently related to elevated MACE risk in STEMI patients (Table 3).
TABLE 3.
Factors related to MACE risk by multivariate Cox's proportional hazards regression analysis
| Items | p value | HR | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| VCAM‐1 (high vs. low) | 0.031 | 2.339 | 1.081 | 5.061 |
| Age (≥65 vs. <65 years) | 0.039 | 2.019 | 1.037 | 3.932 |
| History of DM (yes vs. no) | 0.011 | 2.395 | 1.220 | 4.699 |
| CRP (≥5 vs. <5 mg/L) | 0.012 | 2.550 | 1.229 | 5.291 |
| Multivessel disease (yes vs. no) | 0.007 | 2.561 | 1.295 | 5.064 |
Abbreviations: CI, confidence interval; CRP, C‐reactive protein; DM, diabetes mellitus; HR, hazard ratio; MACE, major adverse cardiac events; VCAM‐1, vascular cell adhesion molecule‐1.
3.5. Nomogram based on multivariate prediction model
In this study, a prediction model combining these factors was performed through a nomogram (Figure 3A). The indicators on the nomogram were used to score a patient based on the patient's clinical characteristics and then those scores would be added up to calculate the total score. Finally, a functional transformation between the total score and the probability of the outcome event was used to predict the patient's prognosis. ROC curve analysis exhibited that the abovementioned prediction model had a good value for evaluating MACE risk at 1, 2, and 3‐year, with AUC of 0.764, 0.716, and 0.778, respectively, in STEMI patients (Figure 3B).
FIGURE 3.
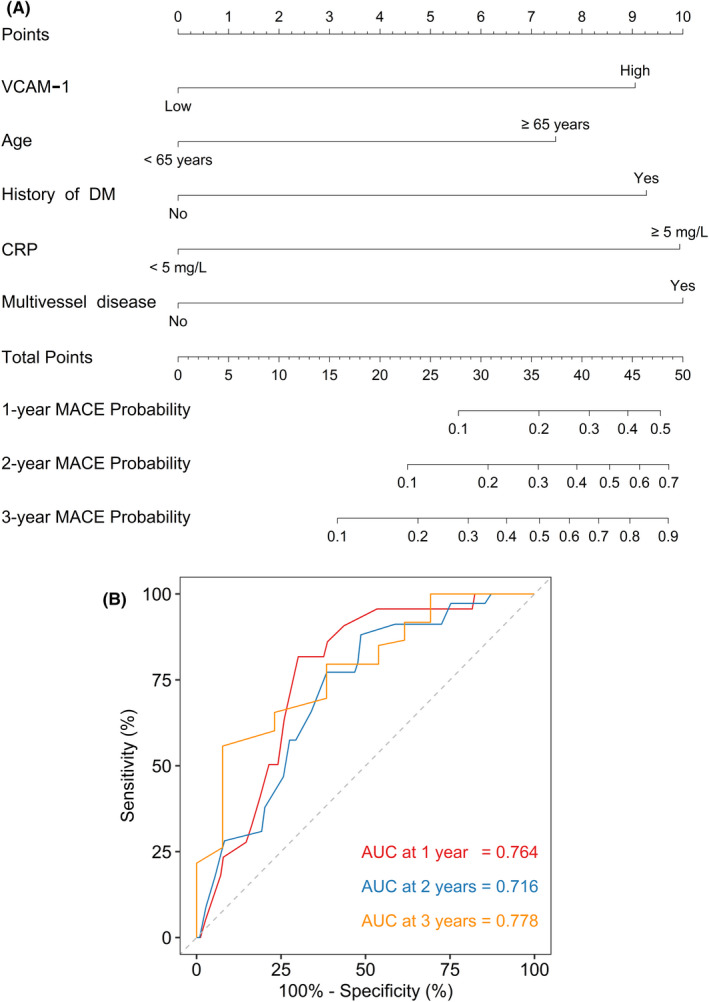
Prediction model was performed through nomogram for forecasting MACE in STEMI patients. The prediction model was performed through nomogram (A) and its ROC curve for predicting MACE risk at 1, 2, and 3 years (B) in STEMI patients.
4. DISCUSSION
Vascular cell adhesion molecule‐1 and ICAM‐1 modulate atherosclerosis in various ways, which may directly or indirectly facilitate the occurrence of MACE. 16 , 17 Concerning VCAM‐1, it is involved in the process of myeloid cell recruitment in atherosclerosis by interacting with nucleotide‐binding oligomerization domain 1 (NOD1). 17 In terms of ICAM‐1, it facilitates atherosclerosis by activating transcriptional regulation of mineralocorticoid receptors (MR). 16 With respect to the clinical implication of VCAM‐1 and ICAM‐1, they are observed to be enhanced in cardiovascular disease patients, such as ACS, stable angina, and acute ischemic stroke patients. 18 , 19 This study observed that both VCAM‐1 and ICAM‐1 were elevated in STEMI patients in contrast to HCs. Possible arguments would be that: (1) VCAM‐1 might facilitate STEMI by interacting with NOD1 to recruit myeloid cells, or promoting myocardial cell apoptosis and inflammation 10 , 17 ; (2) increased ICAM‐1 could activate the MR, as well as promote proinflammatory leukocyte infiltration, fibrosis, etc., to boost the development of atherosclerosis 11 , 16 ; hence, VCAM‐1 and ICAM‐1 were elevated in STEMI patients by comparison with HCs. Moreover, the ROC curve analysis exhibited that VCAM‐1 and ICAM‐1 possessed good capacity in discriminating STEMI patients from HCs, which revealed that VCAM‐1 and ICAM‐1 could be helpful for the diagnosis of STEMI. Subsequent studies could conduct related research to verify this finding.
Vascular cell adhesion molecule‐1 and ICAM‐1 serve as risk factors to indicate adverse cardiovascular events in several complex disease patients. 20 , 21 , 22 According to a previous study, VCAM‐1 predicts MACE in peripheral arterial disease patients. 20 At the same time, VCAM‐1 independently forecasts cardiovascular events risk in chronic obstructive pulmonary disease patients. 21 In terms of ICAM‐1, it is linked to cardiovascular event risk in obstructive sleep apnea patients. 22 The current study discovered that both VCAM‐1 and ICAM‐1 were positively linked with MACE risk. The potential explanation would be that: (1) elevated VCAM‐1 facilitated the adhesion of circulating leukocytes 23 ; meanwhile, VCAM‐1 might promote adhesion and infiltration of monocytes into the endothelium 24 ; these processes boosted the progression of cardiac diseases; therefore, VCAM‐1 could forecast elevated MACE risk in STEMI patients; (2) raised ICAM‐1 might foster fibrosis, vascular hypertrophy, macrophage infiltration, as well as reactive oxygen species production, resulting in the incidence of MACE 25 ; therefore, elevated ICAM‐1 also had the potency to predict higher MACE risk in STEMI patients.
Further multivariate Cox analysis suggested that age ≥65 years, history of DM, CRP ≥5 mg/L, and multivessel disease could all independently forecast enhanced MACE risk in STEMI patients. Possible arguments would be that: (1) regarding age, STEMI patients with age ≥65 years were more likely to suffer from myocardial infarction, heart failure, cardiac death, etc. 26 , 27 , 28 ; (2) history of DM might affect the endothelial functions, which would further cause the incidence of MACE 29 ; (3) inflammation participated in the progression of cardiovascular diseases, and CRP could reflect inflammation status 23 , 30 , 31 ; (4) STEMI patients with the multivessel disease were susceptible to MACE 32 ; therefore, abovementioned factors independently predicted MACE risk in STEMI patients. A subsequent prediction model performed by nomogram provided a reference for predicting the MACE risk at 1, 2, and 3 years; meanwhile, the ROC curve analysis suggested that this multivariate prediction model performed by nomogram may assist in MACE prediction for STEMI patients. Notably, in‐stent anti‐coagulants were applied in this study, and the corresponding information was also recorded. Specifically, approximately 70% of STEMI patients applied Rapamycin‐eluting stents since Rapamycin had been included in China's medical insurance, and the left 30% of patients used Everolimus‐eluting stents in this study. However, further confirmation is needed to evaluate whether different types of in‐sent anti‐coagulant use would affect the prognosis.
Some limitations might exist in this study: (1) this was a single‐center study; thus, selection and regional bias might exist; (2) In order to reduce the compounders, the current study only included previously PCI‐naïve STEMI patients; however, the prognostic value of VCAM‐1 and ICAM‐1 in STEMI patients who had PCI history remained unclear; (3) multi‐time‐point detection should be taken into account for evaluating the longitudinal changes of VCAM‐1 and ICAM‐1 in monitoring STEMI progression; (4) a part of STEMI patients were not local residents, which hampered the follow‐up and might affect the reliability of the findings; (5) compared with STEMI patients, the number of HCs were relatively insufficient, which might affect the statistical power.
In conclusion, this study confirms the value of VCAM‐1 and ICAM‐1 measurement in predicting MACE risk in STEMI patients. Moreover, VCAM‐1 plus other traditional prognostic factors (such as age, history of DM, CRP, and multivessel disease) could further improve the predictive accuracy of MACE risk in STEMI patients.
ACKNOWLEDGEMENTS
This study was supported by Medical Science Research Project Plan of Hebei Province in 2020 (20200176).
CONFLICT OF INTEREST
The authors have no relevant financial or non‐financial interests to disclose.
Yu J, Liu Y, Peng W, Xu Z. Serum VCAM‐1 and ICAM‐1 measurement assists for MACE risk estimation in ST‐segment elevation myocardial infarction patients. J Clin Lab Anal. 2022;36:e24685. doi: 10.1002/jcla.24685
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Vogel B, Claessen BE, Arnold SV, et al. ST‐segment elevation myocardial infarction. Nat Rev Dis Primers. 2019;5(1):39. [DOI] [PubMed] [Google Scholar]
- 2. Bhatt DL, Lopes RD, Harrington RA. Diagnosis and treatment of acute coronary syndromes: a review. JAMA. 2022;327(7):662‐675. [DOI] [PubMed] [Google Scholar]
- 3. Bergmark BA, Mathenge N, Merlini PA, Lawrence‐Wright MB, Giugliano RP. Acute coronary syndromes. Lancet. 2022;399(10332):1347‐1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Y, Tian Y, Dong P, et al. Treatment delay and reperfusion management of acute ST‐segment elevation myocardial infarction: analysis of the China STEMI care project phase 1. QJM. 2021;114(5):299‐305. [DOI] [PubMed] [Google Scholar]
- 5. Farmer D, Jimenez E. Re‐evaluating the role of CABG in acute coronary syndromes. Curr Cardiol Rep. 2020;22(11):148. [DOI] [PubMed] [Google Scholar]
- 6. Marquis‐Gravel G, Dalgaard F, Jones AD, et al. Post‐discharge bleeding and mortality following acute coronary syndromes with or without PCI. J Am Coll Cardiol. 2020;76(2):162‐171. [DOI] [PubMed] [Google Scholar]
- 7. Gouda P, Savu A, Bainey KR, Kaul P, Welsh RC. Long‐term risk of death and recurrent cardiovascular events following acute coronary syndromes. PLoS One. 2021;16(7):e0254008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang S, Wang W, Sawhney JPS, et al. Antithrombotic management and long‐term outcomes following percutaneous coronary intervention for acute coronary syndrome in Asia. Int J Cardiol. 2020;310:16‐22. [DOI] [PubMed] [Google Scholar]
- 9. Xiang D, Xiang X, Zhang W, et al. Management and outcomes of patients with STEMI during the COVID‐19 pandemic in China. J Am Coll Cardiol. 2020;76(11):1318‐1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang L, Yang A, Li X, Liu K, Tan J. Down‐regulation of VCAM‐1 in bone mesenchymal stem cells reduces inflammatory responses and apoptosis to improve cardiac function in rat with myocardial infarction. Int Immunopharmacol. 2021;101(Pt A):108180. [DOI] [PubMed] [Google Scholar]
- 11. Salvador AM, Nevers T, Velazquez F, et al. Intercellular adhesion molecule 1 regulates left ventricular leukocyte infiltration, cardiac remodeling, and function in pressure overload‐induced heart failure. J Am Heart Assoc. 2016;5(3):e003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan ZX, Hua Q, Li YP, Liu RK, Yang Z. Interleukin‐6, but not soluble adhesion molecules, predicts a subsequent mortality from cardiovascular disease in patients with acute ST‐segment elevation myocardial infarction. Cell Biochem Biophys. 2011;61(2):443‐448. [DOI] [PubMed] [Google Scholar]
- 13. Lino DOC, Freitas IA, Meneses GC, et al. Interleukin‐6 and adhesion molecules VCAM‐1 and ICAM‐1 as biomarkers of post‐acute myocardial infarction heart failure. Braz J Med Biol Res. 2019;52(12):e8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119‐177. [DOI] [PubMed] [Google Scholar]
- 15. Hong D, Choi KH, Song YB, et al. Prognostic implications of post‐percutaneous coronary intervention neutrophil‐to‐lymphocyte ratio on infarct size and clinical outcomes in patients with acute myocardial infarction. Sci Rep. 2019;9(1):9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marzolla V, Armani A, Mammi C, et al. Essential role of ICAM‐1 in aldosterone‐induced atherosclerosis. Int J Cardiol. 2017;232:233‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gonzalez‐Ramos S, Paz‐Garcia M, Rius C, et al. Endothelial NOD1 directs myeloid cell recruitment in atherosclerosis through VCAM‐1. FASEB J. 2019;33(3):3912‐3921. [DOI] [PubMed] [Google Scholar]
- 18. Wang JN, Yan YY, Guo ZY, Jiang YJ, Liu LL, Liu B. Negative Association of Circulating MicroRNA‐126 with high‐sensitive C‐reactive protein and vascular cell adhesion Molecule‐1 in patients with coronary artery disease following percutaneous coronary intervention. Chin Med J (Engl). 2016;129(23):2786‐2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al‐Rubiay HF, Al‐Kuraishy HM, Al‐Gareeb AI. Intercellular adhesive molecule 1 (ICAM‐1) and acute ischaemic stroke: role of statins. J Pak Med Assoc. 2021;71(Suppl 8)(12):S11‐S16. [PubMed] [Google Scholar]
- 20. Lee PT, Liao IC, Lee CH, Hsu LW, Liu PY. Expression of vascular cell adhesion Molecule‐1 in peripheral artery disease is enriched in patients with advanced kidney disease. Acta Cardiol Sin. 2021;37(6):591‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J, Wang Q, Zhang Q, et al. Higher blood vascular cell adhesion Molecule‐1 is related to the increased risk of cardiovascular events in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2020;15:2289‐2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peres BU, Hirsch Allen AJ, Daniele P, et al. Circulating levels of cell adhesion molecules and risk of cardiovascular events in obstructive sleep apnea. PLoS One. 2021;16(7):e0255306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schunk SJ, Triem S, Schmit D, et al. Interleukin‐1alpha is a central regulator of leukocyte‐endothelial adhesion in myocardial infarction and in chronic kidney disease. Circulation. 2021;144(11):893‐908. [DOI] [PubMed] [Google Scholar]
- 24. Yin L, Bai J, Yu WJ, Liu Y, Li HH, Lin QY. Blocking VCAM‐1 prevents angiotensin II‐induced hypertension and vascular remodeling in mice. Front Pharmacol. 2022;13:825459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lang PP, Bai J, Zhang YL, et al. Blockade of intercellular adhesion molecule‐1 prevents angiotensin II‐induced hypertension and vascular dysfunction. Lab Invest. 2020;100(3):378‐386. [DOI] [PubMed] [Google Scholar]
- 26. Park IH, Cho HK, Oh JH, et al. Old age and myocardial injury in ST‐segment elevation myocardial infarction. Am J Med Sci. 2021;362(6):592‐600. [DOI] [PubMed] [Google Scholar]
- 27. Rossello X, Ferreira JP, Caimari F, et al. Influence of sex, age and race on coronary and heart failure events in patients with diabetes and post‐acute coronary syndrome. Clin Res Cardiol. 2021;110(10):1612‐1624. [DOI] [PubMed] [Google Scholar]
- 28. Fam JM, Khoo CY, Lau YH, et al. Age and diabetes mellitus associated with worse outcomes after percutaneous coronary intervention in a multi‐ethnic Asian dialysis patient population. Singapore Med J. 2021;62(6):300‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kibel A, Selthofer‐Relatic K, Drenjancevic I, et al. Coronary microvascular dysfunction in diabetes mellitus. J Int Med Res. 2017;45(6):1901‐1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghali R, Habeichi NJ, Kaplan A, et al. IL‐33 induces type‐2‐cytokine phenotype but exacerbates cardiac remodeling post‐myocardial infarction with eosinophil recruitment, worsened systolic dysfunction, and ventricular wall rupture. Clin Sci (Lond). 2020;134(11):1191‐1218. [DOI] [PubMed] [Google Scholar]
- 31. Wei Y, Lan Y, Zhong Y, et al. Interleukin‐38 alleviates cardiac remodelling after myocardial infarction. J Cell Mol Med. 2020;24(1):371‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burgess S, Juergens CP, Yang W, et al. Cardiac mortality, diabetes mellitus, and multivessel disease in ST elevation myocardial infarction. Int J Cardiol. 2021;323:13‐18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


