Abstract
Background
Interleukin (IL)‐41, also known as Metrnl, is a novel immunomodulatory cytokine, which is involved in the pathogenesis of many inflammatory and metabolic diseases, but its role in thyroid autoimmune diseases is not clear. The aim of this study was to evaluate the serum IL‐41 levels in patients with Graves' disease (GD) and its relationship with GD.
Methods
This study included a total of 49 GD patients and 47 age‐ and sex‐matched healthy individuals. All baseline data were obtained by physical examination. Free triiodothyronine 3 (FT3), free triiodothyronine 4 (FT4), thyroid‐stimulating hormone (TSH), anti‐thyroglobulin antibodies (TgAb), thyroid peroxidase antibody (TPOAb), and thyrotropin receptor antibody (TRAb) levels in plasma of GD patients were measured by chemiluminescence. The high‐sensitivity C‐reactive protein (CRP) and white blood cell count (WBC) were detected using automated biochemical analyzer. Serum IL‐41 levels were measured by enzyme‐linked immunosorbent assay.
Results
Serum IL‐41 levels in patients with GD were significantly lower than those in healthy controls (201.0 vs. 260.8 pg/mL, p < 0.05). There was a significant positive correlation between IL‐41 level and CRP (r = 0.2947, p = 0.0385) and WBC (r = 0.4104, p = 0.0034) in GD patients. CRP was positively correlated with TRAb (r = 0.2874, p = 0.0452) and TSH (r = 0.3651, p = 0.0099) levels in GD patients.
Conclusions
This study demonstrates that GD patients have decreased serum IL‐41 levels, and IL‐41 plays a potential role in abnormal immune response of GD patients.
Keywords: autoimmune thyroid disease, Graves' disease, interleukin‐41, Metrnl
Serum IL‐41 levels were significantly lower in GD patients compared to HC (A). We used correlation heat maps to show the correlation between IL‐41 and inflammatory indicators or thyroid markers, and then analyzed those with significant correlations using Pearson analysis (B). In patients with GD, serum IL‐41 level was positively correlated with CRP (C) and WBC (D).

1. INTRODUCTION
It is well known that Graves' disease (GD) is recognized as a representative disease of clinical hyperthyroidism. 1 The peak incidence of GD is between the ages of 30 and 50 and six times more common in women than in men. The immune disorder of GD leads to thyroid‐stimulating hormone (TSH) receptor antibody (TRAb) to stimulate thyroid, which makes thyroid follicular cells proliferate and increases the secretion of thyroid hormone. 2 Excessive thyroid hormone production can lead to abnormal metabolism of the body. 3 The occurrence of GD is mainly attributed to genetic influences and environmental triggers, as well as the resulting abnormal immune function. However, none of these theories can fully explain the pathogenesis of GD, which has also led to limited therapeutic modalities for GD. 4 Although the pathogenesis of GD remains elusive, it is well accepted that the immune response plays a key role in the development of GD, involving a variety of cytokines. 5
Cytokines participate in immune response and the pathogenesis of autoimmune diseases. Serum cytokines, including interleukin (IL)‐6, IL‐10, IL‐27, IL‐29, IL‐35, IL‐36, IL‐37, and tumor necrosis factor (TNF)‐α, have been reported to be abnormally expressed in GD and can influence its severity. 6 , 7 , 8 , 9 IL‐41, 10 , 11 also named Metrnl, Cometin, Subfatin, Meteorin (Metrn)‐like, or Meteorin‐β, is expressed in many tissues, with the most notable expression in subcutaneous white adipose tissue, nervous system, and barrier tissues, such as skin, intestinal, and respiratory epithelium. In addition, IL‐41 can also be expressed in immune cells, including activated macrophages. IL‐41 was discovered as a neurotrophic factor in 2004. 12 As a newly discovered adipokine, IL‐41 contains 311 amino acids, and the N‐terminal signal peptide is 45 amino acids. Previous studies have confirmed that IL‐41 is involved in the development of metabolic and inflammatory diseases, such as type 2 diabetes (T2D), 13 , 14 coronary heart disease, 15 colitis, 16 , 17 psoriasis, and arthritis. 11 In these diseases, IL‐41 has shown to inhibit inflammation, improve metabolism, regulate adipose function, and reduce obesity‐induced insulin resistance. In addition to acting as an anti‐inflammatory cytokine, IL‐41 also plays a role in acquired immunity, IL‐41 involved in Th1, Th2, and Th17 immune responses. 18 However, the role of IL‐41 in GD is unknown.
In the present study, we compared serum levels of IL‐41 in Chinese GD patients and healthy controls. Moreover, we analyzed the relationship between serum IL‐41 and inflammatory indicators in these GD patients, which may provide new insights into the pathogenesis of GD.
2. MATERIALS AND METHODS
2.1. Participants' selection
Serum samples from 49 patients with GD were collected in The Affiliated Lihuili Hospital of Ningbo University from February 2020 to February 2021. GD was defined by: (1) diffuse goiter with soft or tough texture; (2) hyperthyroidism defined as: serum total triiodothyronine (TT3) > 2.45 nmol/L and/or free triiodothyronine 3 (FT3) > 5.70 pmol/L; total thyroxine (TT4) > 150.84 nmol/L and/or free thyroxine 4 (FT4) > 19.05 pmol/L; TSH <0.35 mIU/L. The healthy control group (HC) was healthy individuals recruited from the Medical Examination Department of the same hospital and ensured that they were matched for sex and age with GD patients and have no history of GD or other autoimmune diseases. Informed consent from each subject and Ethics Committee approval were obtained from The Affiliated Lihuili Hospital of Ningbo University. All baseline data were obtained by physical examination.
2.2. Measurement of thyroid indicators
Determination of TRAb level in human plasma was detected via Staphylococcal Protein A antigen sandwich method using the full‐automatic chemiluminescence immunoassay analyzer (MAGLUMI4000plus; Shenzhen New Industries Biomedical Engineering Co., Ltd, Shenzhen, China). Thyroid hormones (FT3, FT4), TSH, anti‐thyroglobulin antibodies (TgAb), thyroid peroxidase antibody (TPOAb) were measured using automated chemiluminescent immunoassays (i‐2000; Abbott, IL, USA).
2.3. Enzyme‐linked immunosorbent assay (ELISA)
Blood samples were drawn from all recruited patients and control persons, centrifuged at 3500 g for 10 min to get serum, and frozen at −80°C. Human IL‐41 in serum samples was quantified by ELISA kits (Jianglai Biotechnology Co., Ltd, Shanghai, China) according to the manufacturer's instructions. The procedure is as follows: 50 μl of sample and standard were added sequentially to wells pre‐coated with IL‐41 antibody, while blank wells were added with sample dilution, then 100 μl of HRP‐labeled detection antibody was added to each well. The plates were covered with the adhesive strip provided, incubated for 60 min, and washed five times. Substrates A and B were then added at 50 μl each, incubated at 37°C for 15 min, and protected from light. Finally, 50 μl of stop solution was added, and the absorbance was measured at 450 nm within 15 min. The concentration of IL‐41 was calculated from the standard curve. The intra‐ or the inter‐assay coefficients of variation was <9 and <11%, respectively.
2.4. Statistical analysis
All statistical analyses were performed using GraphPad Prism 9.0 software (La Jolla, CA, USA). Continuous data were presented as mean ± SD. Count data were expressed as percentages. Differences between the two groups were compared using the t test, and the chi‐squared test was used to compare count data. Pearson correlation test was used to assess the association between IL‐41 levels and inflammatory indicators or thyroid indicators. A value of p < 0.05 was regarded as denoting statistical significance in all the analyses.
3. RESULTS
3.1. Characteristics of the GD and control groups
As shown in Table 1, a total of 96 subjects were enrolled in this study: 49 GD patients and 47 HC persons. There was no statistically significant difference between the two groups in terms of age, sex ratio, and also their inflammatory parameters such as WBC, CRP (p > 0.05). Compared with HC, GD patients had significantly higher levels of FT3, FT4, TgAb, TPOAb, and TRAb, and significantly lower levels of TSH (p < 0.001).
TABLE 1.
Demographic and clinical characteristics of the participants
| Parameter | HC (n = 47) | GD (n = 49) | t/χ 2 | p |
|---|---|---|---|---|
| Female (n, %) | 31, 65.9% | 29, 59.1% | 0.4696 | 0.4931 |
| Age (years) | 36.34 ± 11.85 | 36.84 ± 14.54 | 0.1829 | 0.8553 |
| TSH (mIU/L) | 1.79 ± 0.86 | 0.003 ± 0.007 | 14.56 | <0.0001**** |
| FT3 (pmol/L) | 4.43 ± 0.44 | 12.59 ± 8.58 | 6.509 | <0.0001**** |
| FT4 (pmol/L) | 12.83 ± 1.01 | 24.72 ± 7.75 | 10.43 | <0.0001**** |
| TgAb (IU/mL) | 1.089 ± 0.71 | 328.6 ± 602.3 | 3.727 | 0.0003*** |
| TPOAb (IU/mL) | 0.33 ± 0.25 | 863.5 ± 973.8 | 6.075 | <0.0001**** |
| TRAb (IU/mL) | 0.36 ± 0.20 | 15.43 ± 16.41 | 6.294 | <0.0001**** |
| WBC (109/L) | 5.88 ± 1.36 | 5.59 ± 1.92 | 0.8283 | 0.4096 |
| CRP (mg/L) | 0.85 ± 0.54 | 1.28 ± 2.28 | 1.266 | 0.2086 |
Note: Compared with controls. ***p < 0.001; ****p < 0.0001, p values were calculated by t test, and chi‐squared test.
Abbreviations: CRP, C‐reactive protein; GD, Graves' disease group; HC, healthy control group; FT3, free triiodothyronine 3; FT4, free triiodothyronine 4; TgAb, anti‐thyroglobulin antibody; TPOAb, thyroid peroxidase antibody; TRAb, TSH‐receptor antibody; TSH, thyroid stimulating hormone; WBC, white blood cell count.
3.2. The serum concentration of IL‐41
As shown in Figure 1, serum IL‐41 levels were significantly lower in GD patients (260.8 ± 183.9) compared with HC (201.0 ± 97.33, p < 0.05). The standard curve for IL‐41 measured by ELISA is shown in Figure S1.
FIGURE 1.
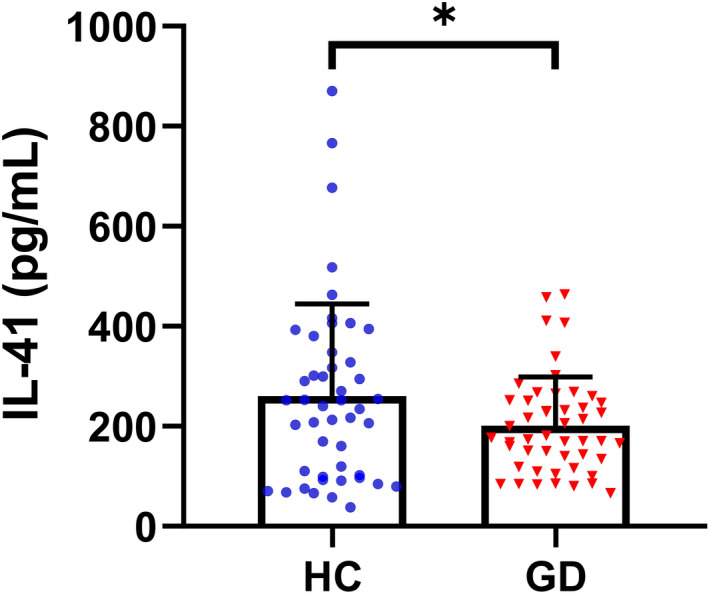
Serum IL‐41 levels of GD and HC. Serum IL‐41 was significantly lower in GD than in HC. *p < 0.05. HC, healthy control group; GD, Graves' disease group
3.3. Correlation of serum IL‐41 with inflammatory and thyroid markers
We used correlation heat maps to show the correlation between IL‐41 and inflammatory indicators or thyroid markers and then analyzed those with significant correlations using Pearson analysis (Figure 2A). The results showed that IL‐41 was positively correlated with CRP (r = 0.2947, p = 0.0385, Figure 2B) and WBC (r = 0.4104, p = 0.0034, Figure 2C). However, there was no significant correlation between IL‐41 levels and serum TRAb, FT3, or FT4 levels (p > 0.05, Figure 2D–F). Additionally, CRP, an indicator of inflammation, was positively correlated with the thyroid markers TSH (r = 0.3651, p = 0.0099, Figure 2G) and TRAb (r = 0.2874, p = 0.0452, Figure 2H).
FIGURE 2.
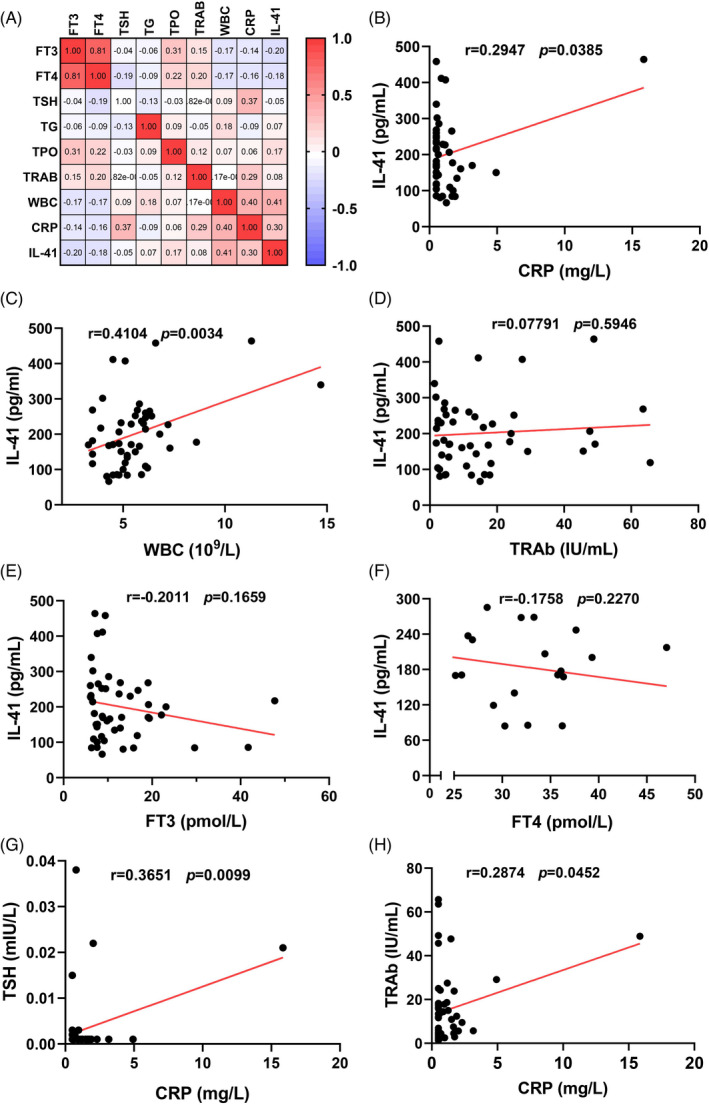
Correlation analysis of serum IL‐41 levels, thyroid indicators, and inflammatory indicators in patients with GD. Correlations were determined by Pearson correlation test. p < 0.05 means statistically significant. CRP, C‐reactive protein; GD, Graves' disease group; FT3, free triiodothyronine 3; FT4, free triiodothyronine 4; TSH, thyroid stimulating hormone; TgAb, anti‐thyroglobulin antibody; TPOAb, thyroid peroxidase antibody; TRAb, TSH‐receptor antibody; WBC, white blood cell count
4. DISCUSSION
In the present study, we found that serum levels of IL‐41 were significantly lower in patients with GD than in HC. Correlation analysis showed that IL‐41 level in GD patients was positively correlated with both inflammatory indicators CRP and WBC, while there was no correlation with thyroid hormones (FT3, FT4) and thyroid autoantibody TRAb. Meanwhile, both thyroid indicators TRAb and TSH in GD patients were positively correlated with CRP.
IL‐41 is a novel immunomodulatory cytokine produced by activated macrophages that plays an important role in the inflammatory system. Rao et al. 19 reported that IL‐41 exerts anti‐inflammatory effects via IL‐4 released by eosinophils. Recent studies showed that serum IL‐41 levels were reduced in many diseases, especially in patients with inflammation‐related diseases. Cai et al. 20 found that serum IL‐41 levels were lower in chronic heart failure patients when compared to control subjects. Several studies demonstrated that serum IL‐41 levels were decreased in T2D patients compared with the healthy controls. 21 , 22 , 23 Moreover, serum IL‐41 concentrations were considerably lower in patients with coronary artery disease 15 and ulcerative colitis and Crohn's disease 24 in comparison with healthy controls. In this study, we also observed a decrease in serum IL‐41 levels in GD patients. However, other studies showed that serum IL‐41/Metrnl levels were higher in T2D patients compared with healthy controls. 13 , 25 , 26 Bridgewood et al. 11 examined IL‐41 level in the synovial tissue of rheumatoid arthritis and psoriatic arthritis and found that IL‐41 was compensatory upregulated in both diseases. These results suggest that the expression level of IL‐41 in serum varies with different diseases, and even there are contradictory results of the expression level of IL‐41 in serum of the same disease (such as T2D), which needs further study.
Several reports have confirmed that GD is a T‐cell‐dominated autoimmune disease characterized by increased thyroid hormone (FT3, FT4), which inhibit the synthesis and release of TSH. 27 FT4 combined with TSH can be used to detect thyroid dysfunction. 4 Furthermore, GD diagnosis is based on the presence of hyperthyroidism and positive TRAb title. IL‐41 plays an important role in inflammation and various autoimmune diseases, but its role in thyroid diseases is not clear. In the present study, we found that IL‐41 were decreased significantly in GD patients (p < 0.05), while there was no significant correlation between IL‐41 and thyroid hormone (p > 0.05). We hypothesized that IL‐41 is involved in the development of GD and may be related to macrophage polarization. Macrophages can be divided into two phenotypes: classically activated macrophages (M1), which promotes inflammation, and the other alternatively activated macrophages (M2), which inhibits inflammation. 28 Th1 cytokine, which is closely related to GD, also induces macrophages to polarize towards M1. Imam et al. 29 found that in patients with GD, NK cells are activated, and macrophages exhibit an M1‐like phenotype. The upregulation of the M1 phenotype is accompanied by a decrease in the M2 phenotype. IL‐41 has been shown to be produced by M2‐like macrophages. 30 Therefore, we hypothesize that the decrease in serum IL‐41 in GD patients may be related to M1/M2 imbalance.
In order to explore the potential relationship between GD and inflammation, we further analyzed the relationship between TSH, TRAb, and inflammatory marker (CRP). Interestingly, we observed that the inflammatory CRP was positively correlated with TSH and TRAb. Our results also demonstrated a positive correlation between serum IL‐41 concentrations and inflammatory indices (CRP, WBC) in patients with GD.
In conclusion, our study shows that IL‐41 is downregulated in GD and serum IL‐41 is correlated with GD, suggesting that IL‐41 may play a potential role in abnormal immune response of GD patients. Further studies are needed to expand the current findings, especially to determine IL‐41 expression in thyroid tissue and explore the exact mechanism of IL‐41 in the pathogenesis of GD.
FUNDING INFORMATION
National Natural Science Foundation of China, Grant/Award Number: 81970735; Clinical Research and Application Project of Zhejiang Health Science and Technology Program, Grant/Award Number: 2022KY1148; Natural Science Foundation of Ningbo, Grant/Award Number: 2021 J080, 2021 J246, and 2021 J280.
CONFLICT OF INTEREST
None declared.
Supporting information
Figure S1
Gong L, Huang G, Weng L, et al. Decreased serum interleukin‐41/Metrnl levels in patients with Graves' disease. J Clin Lab Anal. 2022;36:e24676. doi: 10.1002/jcla.24676
Contributor Information
Wugeng Cui, Email: cuiwugeng@nbu.edu.cn.
Mingcai Li, Email: limingcai@nbu.edu.cn.
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study can be obtained from the corresponding author upon reasonable request.
REFERENCES
- 1. Smith TJ, Hegedus L. Graves' disease. N Engl J Med. 2016;375(16):1552‐1565. [DOI] [PubMed] [Google Scholar]
- 2. Nojiri T, Kurano M, Araki O, et al. Serum autotaxin levels are associated with Graves' disease. Endocr J. 2019;66(5):409‐422. [DOI] [PubMed] [Google Scholar]
- 3. Ji X, Wan J, Chen R, et al. Low frequency of IL‐10‐producing B cells and high density of ILC2s contribute to the pathological process in Graves' disease, which may be related to elevated‐TRAb levels. Autoimmunity. 2020;53(2):78‐85. [DOI] [PubMed] [Google Scholar]
- 4. Antonelli A, Ferrari SM, Ragusa F, et al. Graves' disease: epidemiology, genetic and environmental risk factors and viruses. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101387. [DOI] [PubMed] [Google Scholar]
- 5. Zhang J, Zeng H, Ren M, et al. Interleukin‐21 is associated with disease activity in patients with Graves' disease. Endocrine. 2014;46(3):539‐548. [DOI] [PubMed] [Google Scholar]
- 6. Yao QM, Li L, Song ZY, et al. Elevated interleukin‐36alpha and CD4(+)IL‐36alpha(+)T cells are involved in the pathogenesis of Graves' disease. Front Endocrinol (Lausanne). 2018;9:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saeed MH, Kurosh K, Zahra A, Hossein DM, Davood R, Ataollahi MR. Decreased serum levels of IL‐27and IL‐35 in patients with Graves disease. Arch Endocrinol Metab. 2021;64(5):521‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Falkowski B, Szczepanek‐Parulska E, Sawicka‐Gutaj N, Krygier A, Ruchala M. Evaluation of IL‐29 in euthyroid patients with Graves' orbitopathy: a preliminary study. Mediators Inflamm. 2020;2020:4748612‐4748616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Y, Wang Z, Yu T, et al. Increased expression of IL‐37 in patients with Graves' disease and its contribution to suppression of proinflammatory cytokines production in peripheral blood mononuclear cells. PLoS One. 2014;9(9):e107183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miao ZW, Hu WJ, Li ZY, Miao CY. Involvement of the secreted protein Metrnl in human diseases. Acta Pharmacol Sin. 2020;41(12):1525‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bridgewood C, Russell T, Weedon H, et al. The novel cytokine Metrnl/IL‐41 is elevated in psoriatic arthritis synovium and inducible from both entheseal and synovial fibroblasts. Clin Immunol. 2019;208:108253. [DOI] [PubMed] [Google Scholar]
- 12. Jorgensen JR, Thompson L, Fjord‐Larsen L, et al. Characterization of Meteorin‐‐an evolutionary conserved neurotrophic factor. J Mol Neurosci. 2009;39(1–2):104‐116. [DOI] [PubMed] [Google Scholar]
- 13. Wang K, Li F, Wang C, et al. Serum levels of Meteorin‐like (Metrnl) are increased in patients with newly diagnosed type 2 diabetes mellitus and are associated with insulin resistance. Med Sci Monit. 2019;25:2337‐2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lappas M. Maternal obesity and gestational diabetes decrease Metrnl concentrations in cord plasma. J Matern Fetal Neonatal Med. 2021;34(18):2991‐2995. [DOI] [PubMed] [Google Scholar]
- 15. Liu ZX, Ji HH, Yao MP, et al. Serum Metrnl is associated with the presence and severity of coronary artery disease. J Cell Mol Med. 2019;23(1):271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zuo L, Ge S, Ge Y, et al. The adipokine Metrnl ameliorates chronic colitis in Il‐10−/− mice by attenuating mesenteric adipose tissue lesions during spontaneous colitis. J Crohns Colitis. 2019;13(7):931‐941. [DOI] [PubMed] [Google Scholar]
- 17. Zhang SL, Li ZY, Wang DS, et al. Aggravated ulcerative colitis caused by intestinal Metrnl deficiency is associated with reduced autophagy in epithelial cells. Acta Pharmacol Sin. 2020;41(6):763‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ushach I, Arrevillaga‐Boni G, Heller GN, et al. Meteorin‐like/Meteorin‐beta is a novel immunoregulatory cytokine associated with inflammation. J Immunol. 2018;201(12):3669‐3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rao RR, Long JZ, White JP, et al. Meteorin‐like is a hormone that regulates immune‐adipose interactions to increase beige fat thermogenesis. Cell. 2014;157(6):1279‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cai J, Wang QM, Li JW, et al. Serum Meteorin‐like is associated with weight loss in the elderly patients with chronic heart failure. J Cachexia Sarcopenia Muscle. 2022;13(1):409‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmid A, Karrasch T, Schaffler A. Meteorin‐like protein (Metrnl) in obesity, during weight loss and in adipocyte differentiation. J Clin Med. 2021;10(19):4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. El‐Ashmawy HM, Selim FO, Hosny TAM, Almassry HN. Association of low serum Meteorin like (Metrnl) concentrations with worsening of glucose tolerance, impaired endothelial function and atherosclerosis. Diabetes Res Clin Pract. 2019;150:57‐63. [DOI] [PubMed] [Google Scholar]
- 23. Fadaei R, Dadmanesh M, Moradi N, et al. Serum levels of subfatin in patients with type 2 diabetes mellitus and its association with vascular adhesion molecules. Arch Physiol Biochem. 2020;126(4):335‐340. [DOI] [PubMed] [Google Scholar]
- 24. Gholamrezayi A, Mohamadinarab M, Rahbarinejad P, et al. Characterization of the serum levels of Meteorin‐like in patients with inflammatory bowel disease and its association with inflammatory cytokines. Lipids Health Dis. 2020;19(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. AlKhairi I, Cherian P, Abu‐Farha M, et al. Increased expression of Meteorin‐like hormone in type 2 diabetes and obesity and its association with irisin. Cells. 2019;8(10):1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chung HS, Hwang SY, Choi JH, et al. Implications of circulating Meteorin‐like (Metrnl) level in human subjects with type 2 diabetes. Diabetes Res Clin Pract. 2018;136:100‐107. [DOI] [PubMed] [Google Scholar]
- 27. Antonelli A, Fallahi P, Elia G, et al. Graves' disease: clinical manifestations, immune pathogenesis (cytokines and chemokines) and therapy. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101388. [DOI] [PubMed] [Google Scholar]
- 28. Shapouri‐Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425‐6440. [DOI] [PubMed] [Google Scholar]
- 29. Imam S, Dar P, Paparodis R, et al. Nature of coexisting thyroid autoimmune disease determines success or failure of tumor immunity in thyroid cancer. J Immunother Cancer. 2019;7(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ushach I, Burkhardt AM, Martinez C, et al. METEORIN‐LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clin Immunol. 2015;156(2):119‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Data Availability Statement
The data supporting the findings of this study can be obtained from the corresponding author upon reasonable request.


