Abstract
Purpose:
The purpose of this study was to systematically analyze the presence of secondary angular deformities after percutaneous epiphysiodesis based on long-standing radiographs, and to see if the occurrence and magnitude of angular deformities after percutaneous epiphysiodesis correlated with the amount of remaining growth at the time of surgery.
Methods:
From a local Health Register consisting of patients investigated using the Moseley Straight-Line Graph, we identified 269 patients who had undergone percutaneous epiphysiodesis from 2002 until 2020. Radiographic analysis included the measurement of mechanical axis and joint orientation angles on long-standing anterior–posterior radiographs. Remaining growth was analyzed based on the Menelaus method.
Results:
One hundred and forty epiphysiodeses (71 femurs and 69 tibiae) in 88 patients (39 girls and 49 boys) could be included in the study. Mean age at surgery was 13.2 (10–16.8) years, and mean skeletal age at surgery was 13.0 (9.8–15.7) years. A change of the MA (Mechanical axis) ≥10 mm was found in eight patients (9%). Secondary frontal plane deformities after percutaneous epiphysiodesis correlated significantly with the remaining growth at the time of surgery (p = 0.003).
Conclusion:
We found a high rate of secondary angular deformities after percutaneous epiphysiodesis, and the magnitude of the deformities correlated with the amount of remaining growth at the time of surgery. A modification of the original surgical method for percutaneous epiphysiodesis to also include ablation of central parts of the growth plate might be considered. Patients should be enrolled in a systematic follow-up scheme which allows for the early detection of possible angular deformities.
Level of evidence:
level III study.
Keywords: Percutaneous epiphysiodesis, limb alignment, angular deformity, complication, leg length discrepancy
Introduction
Percutaneous epiphysiodesis (PE) as originally described by Bowen and Johnson 1 is a well-established method for the treatment of leg length discrepancy (LLD). Other surgical techniques to achieve growth arrest include the Phemister technique, 2 the White and Stubbins technique, 3 epiphyseal stapling, 4 and epiphysiodesis by percutaneous screws. 5
The open methods like the Phemister and White techniques, as well as stapling, require a certain surgical approach to expose the physis, whereas only very small incisions are needed for the percutaneous technique. According to the originally described method of PE, 1 the peripheral one-third of the plate is ablated by curettage. The ablation is done from the medial and the lateral side of the physis. The method has been modified by Canale and Christian 6 to include drilling in combination with curettage. Percutaneous techniques for epiphysiodesis are most widely used today due to less surgical approach and less postoperative pain compared to open techniques.7,8 The method is considered to be safe with a low complication rate.1,7,9–11 However, failure of the procedure with continued growth either over the whole or parts of the physis might occur. Secondary angular deformities are described to a varying degree, and both valgus and varus malalignment of the knee are known risk factors for the development of osteoarthritis.12–14 Makarov et al. 15 found angular deformities in 3.6% of the patients in a large series, but only few (41 out of 863) operated with the percutaneous technique were included in their study. Craviari et al. 16 found angular deformities in 4 (7%) out of 60 patients. In a study by Blair et al., 17 all patients were operated with a modified Phemister technique, 10 (15%) out 67 patients did not show fusion of the physis, which resulted in either no slowing of growth or angular deformity. Other authors report none or only rare cases of secondary angular deformities.1,6,7,9,10,16,18–20 However, it applies to all of these studies that it is either not explained how eventual angular deformities have been assessed, or they were analyzed with adequate radiographs only in selected cases (Table 1). To date, there is no study which systematically has analyzed the presence of secondary angular deformities after PE by long-standing radiographs. Most studies report the success of the procedure in terms of correction of LLD, and there is reason to believe that the presence of secondary angular deformities might be under-reported. The aim of this study was therefore to systematically analyze the presence of secondary angular deformities after PE based on long-standing radiographs, and to see if the occurrence of angular deformities after PE correlates with the amount of remaining growth at the time of surgery.
Table 1.
Review of the literature.
| Authors | Patients (n) | Patients with PE (n) and adequate follow-up | Angular deformities (n) | Persistent growth | Evaluation based on | |||
|---|---|---|---|---|---|---|---|---|
| Clinical examination | Knee radiographs | Long-standing radiographs | Not specified | |||||
| Inan et al. 7 | 97 | 97 | 1 | 3 | + | |||
| Goedegebuure et al. 21 | 77 | 77 | 1 | + | (+) only selected patients | |||
| Edmonds and Stasikelis 19 | 63 | 63 | 1 | 7 | + | + | (+) only selected patients | |
| Kemnitz et al. 11 | 57 | 57 | 2 | + | ||||
| Surdam et al. 8 | 96 | 56 | 1 | 3 | (+) | “serial radiographs” | ||
| Makarov et al. 15 | 863 | 41 | 28 a | 3 | (+) only selected patients | + | ||
| Timperlake et al. 22 | 50 | 35 | 0 | + | ||||
| Campens et al. 23 | 92 | 27 | 0 | + | ||||
| Horton and Olney 10 | 26 | 26 | 0 | “postsurgical radiographs” | ||||
| Scott et al. 20 | 24 | 20 | 0 | 3 | + | |||
| Canale and Christian 6 | 22 | 22 | 0 | + | ||||
| Bowen and Johnson 1 | 12 | 12 | 0 | + | + | |||
PE: percutaneous epiphysiodesis.
Sorted according to the number of patients with adequate follow-up included.
Makarov et al. (2018) found 28 angular deformities after epiphysiodesis in 863 patients, including the Phemister technique and PE. Authors have not specified how many of these angular deformities occurred after PE.
Patients and methods
From an institutional Health Register consisting of patients investigated using the Moseley Straight-Line Graph, we identified 269 patients who had undergone PE between 2002 and 2020. Inclusion criteria were as follows: PE, follow-up of at least 12 months after the procedure, no underlying pathology in the operated leg which could cause angular deformity, long-standing radiographs after the procedure and if available before PE, and skeletal age (SA) determination based on hand radiographs no longer then 6 months before surgery.
The surgical technique included a 1-cm skin incision medially and laterally over the distal femoral physis and/or the proximal tibial physis. Under image intensification, an awl was advanced 1.5 cm from medial and lateral and centrally into the physeal plate followed by a 6-mm drill-bit. The peripheral 2–2.5 cm part of the physes were first ablated by fan-shaped oscillating drilling, and then further ablated by a 3-mm curved curette. Epiphysiodesis of the proximal fibula was not performed when the estimated remaining growth in the proximal tibia was ≤2 cm.
Radiographic analysis included long-standing anterior–posterior (AP) radiographs from the pelvis to the feet. These radiographs were obtained in a standard way with the patella pointing straight forward and any LLD corrected with standing blocks under the short extremity to level the pelvis. Deformity analysis was done based on the malalignment test and malorientation test and included measurement of: mechanical axis deviation (MAD), mechanical lateral distal femoral angle (mLDFA), and medial proximal tibial angle (MPTA).24,25 Sagittal plane parameters were not assessed since lateral long-standing radiographs were only obtained in very few patients. Analysis of MAD was done by experienced radiologists not involved in the study. Analysis of mLDFA and MPTA was done by the senior author of the study (J.H.).
Remaining growth at the time of surgery was calculated according to the Menelaus 26 method and based on skeletal age (SA). Radiological assessment of maturation by SA was done by experienced radiologists using hand and wrist radiographs and the Greulich and Pyle atlas. 27 Hand radiographs for analysis of SA were obtained prior to surgery in all cases with a mean time interval of 1 (0–6) months. In all but eight patients, SA was determined within 3 months before surgery. SA at surgery was extrapolated by adding the chronological time difference between the latest hand radiographs and the date of surgery to the latest calculation of SA before surgery.
For statistical evaluation, we applied the paired samples T-test for differences between paired measurements and the Pearson correlation coefficient to measure the linear correlation between two sets of data, in particular the correlation between the occurrence of angular deformity after PE and remaining growth at the time of surgery. The study was approved by the institutional review board (case nr. 18/04927) and the research committee at the department of radiology and nuclear medicine (KRNnr. 1985).
Results
Patients
Two-hundred sixty nine patients who had undergone PE could be identified, of these 140 epiphysiodeses (71 femurs and 69 tibiae) in 88 patients (39 girls and 49 boys) could be included in the study. Fifty-four of the epiphysiodeses were combined in the femur and tibia, and 32 were solely in the femur (n = 17) or tibia (n = 15). Mean age at surgery was 13.9 (10.8–16.8) years for boys and 12.4 (10.0–15.4) years in girls. Mean SA at surgery was 13.6 (10.2–15.7) years for boys and 12.3 (9.8–13.8) years for girls. The most common diagnoses were idiopathic LLD and hemihypertrophy (Table 2). Remaining growth of the physes, according to the Menelaus 26 method and based on SA at surgery, was 1.6 (0.24–5.19) cm. Follow-up for the condition (LLD) was 53 (12–181) months, whereas all but six patients were followed until skeletal maturity. Mean time from surgery to long-standing radiographs (latest follow-up for radiological analysis of alignment) was 48 (12–181) months.
Table 2.
Diagnoses in 140 epiphysiodeses (88 patients).
| Diagnosis | n |
|---|---|
| Idiopathic LLD | 25 |
| Hemihypertrophy | 25 |
| PEV sequela | 18 |
| CFD, fibular hemimelia | 13 |
| Fracture sequela | 11 |
| DDH sequela | 10 |
| Perthes’ sequela | 8 |
| Others | 30 |
LLD: limb length discrepancy; PEV: Pes equino varus; CFD: congenital femoral deficiency; DDH: developmental dislocation of the hip.
Alignment
Long-standing radiographs after surgery were available in all patients included in the study, whereas these radiographs were available in 51 patients before and after surgery. In 37 patients, postoperative mechanical axis (MA) and joint orientation angles were compared with the unoperated healthy side. We found a statistically significant change of the MA, but no significant change of joint orientation angles (Table 3).
Table 3.
Frontal plane limb alignment parameters (mean and range values).
| Patients/segments (n) | Before surgery | At latest follow-up | Change | p-value | |
|---|---|---|---|---|---|
| MAD (mm) | 88 patients | 3.9 (0–21) | 7.2 (0–71) | 5 (0–71) | 0.003 |
| mLDFA (°) | 71 segments | 88.4 (84–93) | 88.2 (72–97) | 0.2 (0–17) | 0.67 |
| MPTA (°) | 69 segments | 88.7 (85–92) | 88.3 (80–92) | 0.4 (0–7) | 0.051 |
MAD: mechanical axis deviation; mLDFA: mechanical lateral distal femoral angle; MPTA: medial proximal tibial angle.
At the latest follow-up at least 12 months after surgery, a deviation of the MA of >7 mm was found in 13 out of 88 patients (15%). Of these 13 patients, 8 (9%) showed a change of MAD ≥10 mm at the latest follow-up. All cases of asymmetric growth which resulted in ≥10 mm MAD occurred after distal femoral epiphysiodesis (5 varus and 3 valgus) (Table 4). No total failures occurred. The epiphysiodeses included in this study were performed by four different surgeons, and the occurrence of secondary angular deformities after the procedure was evenly distributed among the surgeons.
Table 4.
Cases with MAD ≥10 mm.
| Patient | Age | Bone age | Sex | Diagnosis | Physis operated (F = femur, T = tibia) | Physis failed | Remaining growth at time of surgery in failed physis (cm) | Time interval until failure was noticed (months) | Immediate action | MAD (mm) + (valgus) − (varus) |
Solution |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14.7 | 14.4 | M | CFD/fibular hemimelia | F | F | 1.53 | 38 | None | −10 | Angular deformity accepted |
| 2 | 15.9 | 12.8 | M | CFD | F + T | F | 3.07 | 9 | Re-epiphysiodesis | −42 | Osteotomy |
| 3 | 13.1 | 13.0 | M | Neurofibromatosis | F + T | F | 2.83 | 7.5 | Re-epiphysiodesis | +35 | Angular deformity accepted |
| 4 | 16.8 | 14.8 | F | Hemihypertrophia | F + T | F | 1.17 | 36 | None | −21 | Angular deformity accepted |
| 5 | 11.9 | 10.5 | M | CFD/fibular hemimelia | F | F | 5.19 | 41 | None | −16 | Angular deformity accepted |
| 6 | 12.6 | 12.2 | F | DDH sequela | F | F | 1.70 | 42 | None | −14 | Angular deformity accepted |
| 7 | 13.9 | 12.8 | M | Femoral fracture sequela | F + T | F | 3.09 | 16.5 | Re-epiphysiodesis | +71 | Osteotomy |
| 8 | 12.2 | 12.0 | F | Hypoplasia | F + T | F | 1.89 | 13 | None | +12 | Angular deformity accepted |
MAD: mechanical axis deviation; CFD: congenital femoral deficiency; DDH: developmental dislocation of the hip.
The development of secondary frontal plane deformities after PE correlated significantly with the remaining growth at time of surgery (p = 0.003). Remaining growth in those patients who developed angular deformity of more than ≥10 mm MAD was 2.6 (1.2–5.2) cm (Table 4).
Discussion
We found a high rate of secondary angular deformities after PE. The occurrence of deformities correlated with the amount of remaining growth at the time of surgery. Thus, significant remaining growth when PE is performed is associated with a certain risk of asymmetric growth after the procedure resulting in angular deformities. In this study, eight patients (9%) showed a secondary MAD of ≥10 mm. In a former study, it could be shown that the precision of repeated radiographic measurements of the MAD was ±3 mm with a mean change of MAD of 3 (0–7) mm between two measurements of an untreated healthy extremity. 25 Any change of MAD >3 mm might, therefore, be considered a real change. However, since the range in this study was from 0 to 7 mm, we consider any change of the MAD >10 mm, a significant change which without doubt can be attributed to asymmetrical growth. Furthermore, a change of 10 mm in MA might be considered significant with a risk for having long-term clinical consequences.12,13
Our finding that the occurrence of secondary angular deformities correlated with the amount of remaining growth at the time of surgery is in accordance with findings by other authors,15,20 who found higher failure rates of PE when the operation was performed at a younger age. However, we found a higher rate of secondary deformities than earlier studies. Makarov et al. 15 found angular deformities in 28 (3.2%) of 863 children after epiphysiodesis. However, only 41 of these children were operated with PE. In this study, LLD was assessed on scanograms, and if these were not available, standing AP radiographs were used. Authors have not specified if long-standing radiographs were available in all patients and how MAD and joint orientation angles have been assessed. Inan et al. 7 found one case with >5° of angular deformity, and failure of the PE in three patients in a series of 97 who were followed until skeletal maturity. Timperlake et al. 22 did not recognize any angular deformities in a group of 35 patients. However, none of these authors did specify how limb alignment was assessed. Table 1 gives an overview, although not complete literature review, over studies reporting results after PE. None of the studies have systematically assessed limb alignment with long-standing radiographs, and in most studies, it is not specified how limb alignment was examined.
The relatively high rate of secondary angular deformities might therefore to a large extent be contributed to the fact that this study is the first to present a systematic analysis of lower limb alignment after PE with long-standing radiographs. Thus, we assume that the incidence of secondary angular deformities after PE is under-reported in former studies which report the results of PE. However, several studies have investigated the occurrence of secondary angular deformities based on adequate radiographic examinations after temporary epiphysiodesis for LLD with different techniques.28,29
It is likely to assume that the partial failures of the physes to unite are due to inadequate surgical technique. However, in this study, the occurrence of secondary angular deformities was not surgeon dependent, but did correlate with the amount of remaining growth at the time of surgery. Computed tomography (CT) scans of the knee in failed epiphysiodeses indicate that percutaneous drilling was done at the correct level both in the AP and the sagittal plane (Figures 1–3). Nevertheless, the physes did not fuse and growth could be maintained, which might be attributed to the ability of the physes to repair the traumatic partial destruction of the growth plate by the PE procedure. According to the original method for PE as described by Bowen and Johnson, 1 “the peripheral one-third of the plate is ablated, leaving the middle and central one-third of the plate intact.” Some authors propose that secondary angular deformities after PE might be avoided by creating a central bone bridge in the growth plate.6,7,9,30 However, Canale and colleagues are somewhat unclear in their two papers6,9 about their technique of PE if the central portion should be ablated. In the paper from 1990, Canale and Christian 6 state that “often, the medial and lateral defects can be connected,” and Figure 3A in their paper illustrates ablation only of the peripheral parts of the physeal plate. Ogilvie and King 30 describe a single portal approach for tibial epiphysiodesis, an approach which necessarily would include central parts of the growth plate. For the femur, they do not report if central parts of the growth plate were ablated.
Figure 1.

Boy with CFD and fibular hemimelia in the right lower extremity (patient no. 2 in Table 4). PE left distal femur and left proximal tibia at chronological age 15.9 years and skeletal age 12.8 years. Within 9 months after surgery, the patient developed significant varus deformity in the femur. Remaining growth in the distal femur was 3.1 cm at the time of surgery.
Figure 2.
Same boy as in Figure 1. CT scan show apparent successful epiphysiodesis medially in the distal femur. CT scan to the left: frontal plan; CT scan to right: sagittal cut at the medial aspect of the distal femoral physis.
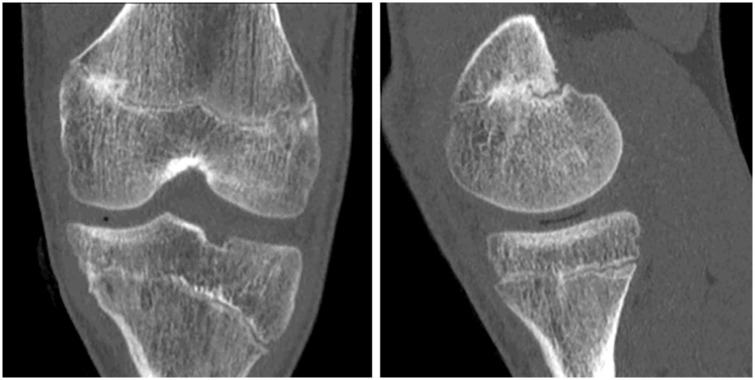
Figure 3.
Same boy as in Figures 1 and 2. Sagittal CT cuts through the lateral aspect of the distal femoral physis. Irregularity of bone marrow and the physis indicates the area which was ablated by drilling and curettage (red pricked line). Although irregular in its course, the growth plate was open laterally, resulting in asymmetric growth and varus deformity.
To our knowledge, there is no obvious evidence in the literature supporting the assumption that by ablation of the central parts of the growth plate, secondary angular deformities could be avoided. However, this theory is interesting and worthwhile to consider. In this study, we observed a high rate of secondary angular deformities following a modified technique of the one described by Canale and Christian6,9 and Bowen and Johnson. 1 We did not ablate central parts of the growth plate, but just the peripheral one-third. In a former study, a single portal approach for tibial epiphysiodesis was used and growth was monitored by radio-stereo-metric analysis (RSA), 31 a method which allows very accurate analysis of micro-movements such as growth. 32 The single portal technique includes ablation of the central parts of the growth plate, and based on RSA, no asymmetric growth after the procedure was observed. A clinical study reporting the results of single portal approach for PE found no angular deformities, 33 whereas one angular deformity for this approach was found in another study. 19 Gunderson et al. 34 found in an RSA and CT study on 27 patients (37 physes) on PE that “continuous confluencing bone-bridging formed in all patients centrally in the physis, except for 8 who had 2 separate small areas of bony healing laterally and medially in the femur.”
The original technique as described by Bowen and Johnson 1 and Canale6,9 was not safe in our patients, resulting in MAD of ≥10 mm in 9% of the patients. A modification of the surgical technique to include central parts of the growth plate should at least be considered, especially when significant growth is remaining at the time of epiphysiodesis. There is no standard method to monitor the effect of PE. Physeal arrest might be documented by conventional radiographs. 9 However, conventional radiographs of the knee are not considered sufficient to analyze the success of the procedure in terms of closure of the growth plate both medially and laterally. 32 Therefore, long-standing radiographs should be obtained at between 3 and 6 months after the procedure to detect any asymmetric growth. Alternatively, if available, tantalum beads might be implanted on each side of the operated physis to allow early detection of asymmetric growth by RSA.31,32,35
This study has certain limitations. First, preoperative long-standing radiographs were not available in all patients, and in these cases, limb alignment at latest follow-up of the operated side was compared with the untreated side. However, inclusion criteria were strict, and the untreated limb was only used for comparison when normal alignment was present. Another limitation is that we did not analyze the sagittal plane of the distal femur and the proximal tibia after PE, because sagittal long-standing radiographs were not routinely obtained in these patients. However, there is reason to believe that angular deformities in the sagittal plane also might occur with a similar frequency as in the AP plane.
Conclusion
We found a high rate of secondary angular deformities after PE, and the occurrence and magnitude of deformities correlated with the amount of remaining growth at the time of surgery. A modification of the original method for PE to include ablation of central parts of the growth plate might be considered. Patients who are operated with PE should be enrolled in a systematic follow-up which allows for early detection of possible angular deformities, that is, long-standing radiographs before surgery and at 3–6 months after surgery or monitoring of growth by RSA.
Footnotes
Author contributions: J.H., A.B.B., and H.S. initiated the study. A.B.B. and H.S. operated the database on all epiphysiodeses. H.W. and J.H. selected the patients from the database according to inclusion and exclusion criteria. J.H. and A.B. analyzed the radiographs. J.H. and H.W. analyzed the data and wrote the manuscript, and A.B.B. and H.S. revised the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The study was approved by the Institutional Review Board of the author’s hospital (case no. 18/04927). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: This is a retrospective study based on an anonymous institutional quality registry without any identifiable data. According to the evaluation by the Institutional Review Board, informed consent by the patients was not required.
References
- 1. Bowen JR, Johnson WJ. Percutaneous epiphysiodesis. Clin Orthop Relat Res 1984(190): 170–173. [PubMed] [Google Scholar]
- 2. Phemister DB. Epiphysiodesis for equalizing the length of the lower extremities and for correcting other deformities of the skeleton. Mem Acad Chir 1950; 76(26–27): 758–763. [PubMed] [Google Scholar]
- 3. White JW, Stubbins SG. Growth arrest for equalizing leg lengths. J Am Med Assoc 1944; 124: 1146–1149. [Google Scholar]
- 4. Blount WP, Clarke GR. Control of bone growth by epiphyseal stapling; a preliminary report. J Bone Joint Surg Am 1949; 31a(3): 464–478. [PubMed] [Google Scholar]
- 5. Métaizeau JP, Wong-Chung J, Bertrand H, et al. Percutaneous epiphysiodesis using transphyseal screws (PETS). J Pediatr Orthop 1998; 18(3): 363–369. [PubMed] [Google Scholar]
- 6. Canale ST, Christian CA. Techniques for epiphysiodesis about the knee. Clin Orthop Relat Res 1990(255): 81–85. [PubMed] [Google Scholar]
- 7. Inan M, Chan G, Littleton AG, et al. Efficacy and safety of percutaneous epiphysiodesis. J Pediatr Orthop 2008; 28(6): 648–651. [DOI] [PubMed] [Google Scholar]
- 8. Surdam JW, Morris CD, DeWeese JD, et al. Leg length inequality and epiphysiodesis: review of 96 cases. J Pediatr Orthop 2003; 23(3): 381–384. [PubMed] [Google Scholar]
- 9. Canale ST, Russell TA, Holcomb RL. Percutaneous epiphysiodesis: experimental study and preliminary clinical results. J Pediatr Orthop 1986; 6(2): 150–156. [PubMed] [Google Scholar]
- 10. Horton GA, Olney BW. Epiphysiodesis of the lower extremity: results of the percutaneous technique. J Pediatr Orthop 1996; 16(2): 180–182. [DOI] [PubMed] [Google Scholar]
- 11. Kemnitz S, Moens P, Fabry G. Percutaneous epiphysiodesis for leg length discrepancy. J Pediatr Orthop B 2003; 12(1): 69–71. [DOI] [PubMed] [Google Scholar]
- 12. Brouwer GM, van Tol AW, Bergink AP, et al. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum 2007; 56(4): 1204–1211. [DOI] [PubMed] [Google Scholar]
- 13. Felson DT, Niu J, Gross KD, et al. Valgus malalignment is a risk factor for lateral knee osteoarthritis incidence and progression: findings from the multicenter osteoarthritis study and the osteoarthritis initiative. Arthritis Rheum 2013; 65(2): 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Janakiramanan N, Teichtahl AJ, Wluka AE, et al. Static knee alignment is associated with the risk of unicompartmental knee cartilage defects. J Orthop Res 2008; 26(2): 225–230. [DOI] [PubMed] [Google Scholar]
- 15. Makarov MR, Dunn SH, Singer DE, et al. Complications associated with epiphysiodesis for management of leg length discrepancy. J Pediatr Orthop 2018; 38(7): 370–374. [DOI] [PubMed] [Google Scholar]
- 16. Craviari T, Bérard J, Willemen L, et al. Percutaneous epiphysiodesis. Analysis of a series of 60 full-grown patients]. Rev Chir Orthop Reparatrice Appar Mot 1998; 84(2): 172–179. [PubMed] [Google Scholar]
- 17. Blair VP, 3rd, Walker SJ, Sheridan JJ, et al. Epiphysiodesis: a problem of timing. J Pediatr Orthop 1982; 2(3): 281–284. [PubMed] [Google Scholar]
- 18. Benyi E, Berner M, Bjernekull I, et al. Efficacy and safety of percutaneous epiphysiodesis operation around the knee to reduce adult height in extremely tall adolescent girls and boys. Int J Pediatr Endocrinol 2010; 2010: 740629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edmonds EW, Stasikelis PJ. Percutaneous epiphysiodesis of the lower extremity: a comparison of single- versus double-portal techniques. J Pediatr Orthop 2007; 27(6): 618–622. [DOI] [PubMed] [Google Scholar]
- 20. Scott AC, Urquhart BA, Cain TE. Percutaneous vs modified Phemister epiphysiodesis of the lower extremity. Orthopedics 1996; 19(10): 857–861. [DOI] [PubMed] [Google Scholar]
- 21. Goedegebuure WJ, Jonkers F, Boot AM, et al. Long-term follow-up after bilateral percutaneous epiphysiodesis around the knee to reduce excessive predicted final height. Arch Dis Child 2018; 103(3): 219–223. [DOI] [PubMed] [Google Scholar]
- 22. Timperlake RW, Bowen JR, Guille JT, et al. Prospective evaluation of fifty-three consecutive percutaneous epiphysiodeses of the distal femur and proximal tibia and fibula. J Pediatr Orthop 1991; 11(3): 350–357. [PubMed] [Google Scholar]
- 23. Campens C, Mousny M, Docquier PL. Comparison of three surgical epiphysiodesis techniques for the treatment of lower limb length discrepancy. Acta Orthop Belg 2010; 76(2): 226–232. [PubMed] [Google Scholar]
- 24. Paley D. Principles of deformity correction. Berlin: Springer, 2005. [Google Scholar]
- 25. Horn J, Hvid I, Huhnstock S, et al. Limb lengthening and deformity correction with externally controlled motorized intramedullary nails: evaluation of 50 consecutive lengthenings. Acta Orthop 2019; 90(1): 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Menelaus MB. Correction of leg length discrepancy by epiphyseal arrest. J Bone Joint Surg Br 1966; 48(2): 336–339. [PubMed] [Google Scholar]
- 27. Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and the wrist. Stanford, CA: Stanford University Press, 1959. [Google Scholar]
- 28. Gorman TM, Vanderwerff R, Pond M, et al. Mechanical axis following staple epiphysiodesis for limb-length inequality. J Bone Joint Surg Am 2009; 91(10): 2430–2439. [DOI] [PubMed] [Google Scholar]
- 29. Dodwell ER, Garner MR, Bixby E, et al. Percutaneous epiphysiodesis using transphyseal screws: a case series demonstrating high efficacy. HSS J 2017; 13(3): 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ogilvie JW, King K. Epiphysiodesis: two-year clinical results using a new technique. J Pediatr Orthop 1990; 10(6): 809–811. [PubMed] [Google Scholar]
- 31. Horn J, Gunderson RB, Wensaas A, et al. Percutaneous epiphysiodesis in the proximal tibia by a single-portal approach: evaluation by radiostereometric analysis. J Child Orthop 2013; 7(4): 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lauge-Pedersen H, Hägglund G, Johnsson R. Radiostereometric analysis for monitoring percutaneous physiodesis. J Bone Joint Surg Br 2006; 88(11): 1502–1507. [DOI] [PubMed] [Google Scholar]
- 33. Elizondo T, Tompkins B, Bronson W, et al. The single portal percutaneous epiphysiodesis technique for treatment of leg length inequality stops growth as expected. J Pediatr Orthop B 2020; 31(1): e37–e43. [DOI] [PubMed] [Google Scholar]
- 34. Gunderson RB, Horn J, Kibsgård T, et al. Negative correlation between extent of physeal ablation after percutaneous permanent physiodesis and postoperative growth. Acta Orthopaedica 2013; 84(4): 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garner MR, Dow M, Bixby E, et al. Evaluating length: the use of low-dose biplanar radiography (EOS) and tantalum bead implantation. J Pediatr Orthop 2016; 36(1): e6–e9. [DOI] [PubMed] [Google Scholar]