Abstract
Background
In the USA, chronic kidney disease (CKD) affects 1 in 7 adults and costs $100 billion annually. The DAPA-CKD trial found dapagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor, to be effective in reducing CKD progression and mortality in patients with diabetic and non-diabetic CKD. Currently, SGLT2 inhibitors are not considered standard of care for patients with non-diabetic CKD.
Objective
Determine the cost-effectiveness of adding dapagliflozin to standard management of patients with non-diabetic CKD.
Design
Markov model with lifetime time horizon and US healthcare sector perspective.
Patients
Patients with non-diabetic CKD
Intervention
Dapagliflozin plus standard care versus standard care only.
Main Measures
Quality-adjusted life years (QALYs), costs, and incremental cost-effectiveness ratios (ICERs), all discounted at 3% annually; total incidence of kidney failure on kidney replacement therapy; average years on kidney replacement therapy.
Key Results
Adding dapagliflozin to standard care improved life expectancy by 2 years, increased discounted QALYS (from 6.75 to 8.06), and reduced the total incidence of kidney failure on kidney replacement therapy (KRT) (from 17.4 to 11.0%) and average years on KRT (from 0.77 to 0.43) over the lifetime of the cohort. Dapagliflozin plus standard care was more effective than standard care alone while increasing lifetime costs (from $245,900 to $324,8900, or $60,000 per QALY gained). Results were robust to variations in assumptions about dapagliflozin’s efficacy over time and by CKD stage, added costs of kidney replacement therapy, and expected population annual CKD progression rates and sensitive to the cost of dapagliflozin. The net 1-year budgetary implication of treating all US patients with non-diabetic CKD could be up to $21 billion.
Conclusions
Dapagliflozin improved life expectancy and reduced progression of CKD, the proportion of patients requiring kidney replacement therapy, and time on kidney replacement therapy in patients with non-diabetic CKD. Use of dapagliflozin meets conventional criteria for cost-effectiveness.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-021-07311-5.
KEY WORDS: chronic kidney disease, cost-effectiveness analysis, health economics
INTRODUCTION
Chronic kidney disease (CKD) is the ninth leading cause of death in the USA, affecting 1 in 7 American adults as commonly defined.1 In 2019, 3.4 million people were documented to be living with CKD in the USA, but actual CKD prevalence may be as high as 37 million.1,2 While CKD affects approximately 12.5% of Medicare beneficiaries, it is responsible for a disproportionate 23% of expenditures even before accounting for enrollees with end-stage kidney disease (kidney failure requiring kidney replacement therapy).2 In addition to higher healthcare costs, CKD is associated with reduced life expectancy, increased risk of cardiovascular disease, impaired physical and cognitive function, and poorer health-related quality of life.3
Although CKD is a widespread problem, treatment options are limited. Until very recently, the only treatments with consistent evidence for slowing disease progression were angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs)4,5; hence, new evidence that sodium glucose co-transporter 2 (SGLT2) inhibitors may modify the disease course of CKD has raised significant interest.
SGLT2 inhibitors decrease renal glucose reabsorption and were initially used to improve glycemic control in patients with type 2 diabetes. The first randomized cardiovascular outcome trials using SGLT2 inhibitors were primarily designed to meet regulatory requirements for ensuring cardiovascular safety.6–9 However, large-scale cardiovascular outcomes trials have also shown various cardiovascular and renal benefits in patients with type 2 diabetes, well beyond effects that would be expected due to improvement in glycemic control,10–12 prompting further exploration of these benefits.
The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial was a large, randomized, double-blind, placebo-controlled, multicenter clinical trial of dapagliflozin in adults with CKD and albuminuria, with and without type 2 diabetes (see the Supplement for a brief description of the trial, including inclusion and exclusion criteria).13 The primary endpoint was a composite of sustained decline in eGFR of ≥ 50%, end-stage kidney disease, or kidney or cardiovascular death. Dapagliflozin reduced the kidney-specific composite outcome by 49% and all-cause mortality by 48% in patients with non-diabetic CKD.14
The DAPA-CKD trial’s promising results raise the question of whether the benefits of treatment outweigh the costs of adding dapagliflozin to the current standard of care for patients with non-diabetic CKD. In this study, we assessed the cost-effectiveness of dapagliflozin added to standard management versus standard management in patients with non-diabetic CKD from a US healthcare sector perspective.
METHODS
Decision Model
We developed a deterministic Markov model of CKD to evaluate dapagliflozin’s effect on CKD progression and all-cause mortality in US patients with non-diabetic CKD. For our base case, we report a weighted average of these outcomes estimated for each of five age-specific CKD cohorts (40-year-old men and women, 50-year-old men and women, 60-year-old men and women, 70-year-old men and women, and 80-year-old men and women)15; cohorts were defined based on the age- and sex-specific prevalence of CKD in the USA.16,17 We also report outcomes by sex and CKD stage at initiation of dapagliflozin for each age cohort (Supplement).
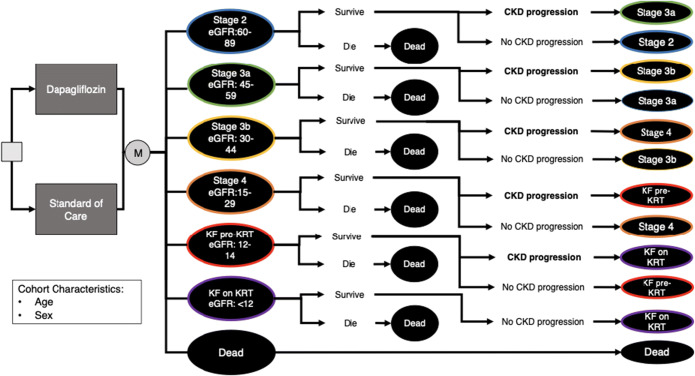
Our CKD progression model (Fig. 1) simulates patient progression through CKD stages at 3-month intervals over their lifetime. We classified CKD stage by the following eGFR levels: CKD stage 2 (60–75 mL/min/1.73 m2), CKD stage 3a (45–59 mL/min/1.73 m2), CKD stage 3b (30–44 mL/min/1.73 m2), CKD stage 4 (15–29 mL/min/1.73 m2), kidney failure not necessarily requiring kidney replacement therapy (12–14 mL/min/1.73 m2), and kidney failure generally requiring kidney replacement therapy (< 12 mL/min/1.73 m2). We henceforth refer to kidney failure not necessarily requiring kidney replacement therapy as “KF pre-KRT” and kidney failure requiring kidney replacement therapy as “KF on KRT.” While there is no specific eGFR for initiation of dialysis, more than 75% of US patients with kidney failure initiate kidney replacement therapy at an eGFR between 5 and 15.18,19 Given that CKD progression is typically relatively slow (< 5 mL/min/1.73 m2 per year), we did not allow patients to progress through more than one stage during any given 3-month time step. Consistent with prior CKD models,20,21 we did not allow substantial improvements in eGFR that would result in a shift to a lower CKD stage.
Figure 1.
Schematic diagram of the CKD Markov Model.
Model parameters are shown in Table 1. Costs, health-related quality of life, and mortality rates differ by CKD stage, age, and sex. Mortality rates specific to CKD stages were derived from published literature on patient populations with non-diabetic CKD. Mortality risk for “KF on KRT” was calculated based on age- and sex-specific life expectancy of non-diabetic patients treated with dialysis or transplant, weighted by incident counts of each treatment modality as reported by the United States Renal Data System (USRDS) (Supplement Tables 2 and 3).19 All mortality rates account for differences among men and women by using sex-specific mortality rates.
Table 1.
Inputs for Lifetime Model of Patients with Chronic Kidney Disease
| Parameters | Value | Range | |
|---|---|---|---|
| Dapagliflozin parameters | CKD progression reduction − hazard ratio14 | 0.51 | (0.34, 0.75) |
| All-cause mortality reduction − hazard ratio14 | 0.52 | (0.29, 0.93) | |
| Monthly cost of dapagliflozin23,35 | $368 | ($74, $736) | |
| CKD mortality parameters | Age- and sex-specific mortality rate36 | US life tables | (± 10%) |
| Mortality risk − CKD stage 2 − hazard ratio37 | 1.01 | (0.95, 1.07) | |
| Mortality risk − CKD stage 3a − hazard ratio37 | 1.19 | (1.1, 1.29) | |
| Mortality risk − CKD stage 3b − hazard ratio37 | 1.53 | (1.36, 1.73) | |
| Mortality risk − CKD stage 4 − hazard ratio37 | 2.27 | (1.87, 2.77) | |
| Mortality risk − kidney failure not requiring KRT − hazard ratio37 | 3.18 | (2.45, 4.07) | |
| Mortality risk − kidney failure requiring KRT19 | USRDS data | (± 10%) | |
| CKD quality-of-life adjustments for health states parameters | Quality-of-life adjustment − CKD stage 2 (30) | 0.85 | (0.7, 1.0) |
| Quality-of-life adjustment − CKD stage 3a30 | 0.80 | (0.69, 1.0) | |
| Quality-of-life adjustment − CKD stage 3b30 | 0.80 | (0.68, 1.0) | |
| Quality-of-life adjustment − CKD stage 4 (30) | 0.74 | (0.62, 1.0) | |
| Quality-of-life adjustment − kidney failure not requiring KRT30 | 0.73 | (0.62, 1.0) | |
| Quality-of-life adjustment − kidney failure requiring KRT30 | 0.60 | (0.55, 1.0) | |
| CKD cost parameters | Monthly added cost of CKD stage 3a20,23 | $196 | ($39, $392) |
| Monthly added cost of CKD stage 3b20,23 | $482 | ($96, $963) | |
| Monthly added cost of CKD stage 4 (20,23) | $625 | ($124, $1250) | |
| Monthly added cost of kidney failure not requiring KRT20,23 | $625 | ($124, $1250) | |
| Monthly added cost of kidney failure requiring KRT19,23 | $6234 | ($623, $12,468) | |
| Baseline costs23,28 | AHRQ US expenditure table | (50%, 200%) | |
| CKD cohort parameters | Initial CKD stage distribution1 | NHANES data | (± 10%) |
| Sex distribution (% female)16 | 55% | (0, 100%) | |
USRDS United States Renal Data System, AHRQ Agency for Healthcare Research and Quality, NHANES National Health and Nutrition Examination Survey
We conducted our analysis in accordance with recommendations from the Second Panel on Cost-Effectiveness in Health and Medicine.22 Our main outcomes include life years, quality-adjusted life years, healthcare sector costs computed over a lifetime horizon, and incremental cost-effectiveness ratios (ICERs), all discounted at 3% annually.22 Costs were expressed in 2019 USD, with cost estimates from other years inflation-adjusted using Consumer Price Index deflators.22,23 We incorporated all formal healthcare-related costs, including costs paid by third-party payers and out-of-pocket costs borne by patients. We included all current and future costs related to CKD, though did not explicitly incorporate costs related to heart failure hospitalizations or other non-fatal cardiovascular events.
Model inputs were derived from published literature. While results of the DAPA-CKD trial published to date provided the annual mean estimated eGFR decline for the placebo arm overall (− 3.59 mL/min/1.73 m2/year), the study group has yet to report eGFR decline separately for non-diabetic participants. To approximate long-term non-diabetic CKD progression, we calibrated the annual mean eGFR decline in this population and variability of eGFR decline to data from three longitudinal studies with long-term outcomes for patients with non-diabetic CKD (Supplement Table 4).24–26 We employed a microsimulation to convert annual rates of eGFR decline to probabilities of transition from one CKD stage to the next for our Markov model (Supplement). We compared our model’s event rates for all-cause mortality, kidney failure, and maintenance dialysis reported for the non-diabetic cohort on placebo in the DAPA-CKD trial to assess its external validity (Supplement Figures 3–6).27
Benefits of Dapagliflozin
In the base case, dapagliflozin benefited patients by slowing CKD progression and reducing mortality risk. Hence, we used the hazard ratio for the kidney-specific composite outcome among the non-diabetic cohort of the DAPA-CKD trial (0.51, 95% CI: 0.34, 0.75) to reflect the relative risk of CKD progression for patients with non-diabetic CKD treated with dapagliflozin compared to those in the placebo arm of the trial.13 To estimate reductions in all-cause mortality rates from dapagliflozin, we used the trial hazard ratio for all-cause mortality for non-diabetic patients (0.52, 95% CI: 0.29, 0.93).13 As in the DAPA-CKD trial, our model stipulates that all patients discontinue use of dapagliflozin once they require kidney replacement therapy (i.e., enter the “KF on KRT” stage).
We report our main results under the assumption that patients experience reduced effectiveness of dapagliflozin over their lifetime, simulating both reduced adherence and/or a decrease in long-term effectiveness (Supplement).
Costs and Quality of Life
Costs and quality of life vary across CKD stages. Based on patients’ age and sex, we determined a baseline level of cost using the AHRQ US expenditure table.28 While patients with CKD stage 2 were assumed to incur the same costs as the general population (a conservative assumption, given risks associated with albuminuria even among patients with normal or near-normal eGFR), patients in subsequent CKD stages accrued additional costs.29 We assumed patients in KF pre-KRT incurred the same costs as those in CKD stage 4. Monthly costs for patients in KF on KRT were estimated based on the per-person-per-year(PPY) spending of the non-diabetic ESRD population, accounting for treatment by both dialysis and transplant (Supplement).19 Dapagliflozin costs only included the medication cost, as other related expenditures, such as clinical follow-up costs, were already included in the annual costs of CKD care. With respect to health-related quality of life, patients generally experience declining health-related quality of life as they progress through CKD stages, per patient surveys.30,31
Sensitivity Analyses
All sensitivity analyses are reported as a weighted average based on the patient mix used in the base case. We conducted one-way sensitivity analyses on all model inputs, using wider uncertainty intervals than those derived from published literature on the general population of patients with CKD for all inputs. We used probabilistic sensitivity analysis to evaluate how simultaneous uncertainties in model parameters could influence outcomes.
To assess our model’s robustness to the uncertainty of long-term effectiveness of dapagliflozin, we tested several scenarios: (1) discontinuation of dapagliflozin after 2.4 years, the length of the DAPA-CKD trial, with the assumption that patients previously on dapagliflozin resume their hypothetical placebo eGFR progression trajectory at that time; (2) CKD stage–dependent effectiveness of dapagliflozin such that effectiveness decreases in later stages; and (3) continual, constant effectiveness of dapagliflozin. We also tested our model’s results with kidney replacement therapy initiation at an eGFR < 9 mL/min/1.73 m2 and an eGFR < 15 mL/min/1.73 m2.
RESULTS
Our model estimated life expectancies by age and sex for patients receiving KRT that closely matched those in the literature (Supplement Tables 2 and 3). Model event rates for all-cause mortality, kidney failure (eGFR < 15), and maintenance dialysis matched those in the placebo arm of the DAPA-CKD trial,14 further attesting to the model’s validity (Supplement Figures 3–6).
In the 40- to 80-year-old population of patients with non-diabetic CKD (base-case cohort), adding dapagliflozin yielded an increase in life expectancy of 2.0 years relative to standard of care alone (Supplement Table 10), as well as an additional 1.3 discounted QALYs. Over their lifetime, 17.4% of patients on standard of care treatment in the base-case cohort reached KF on KRT and spent an average of 0.77 life years in this stage; adding dapagliflozin to standard of care treatment reduced total incidence and time spent in KF on KRT to 11.0% and an average of 0.43 life years, respectively (Fig. 2). Dapagliflozin also increased total discounted lifetime healthcare costs by $79,000, translating to $60,000 per QALY gained compared to standard of care cost. This result indicates that adding dapagliflozin to standard of care is cost-effective relative to a willingness-to-pay threshold of $100,000 per QALY gained.
Figure 2.
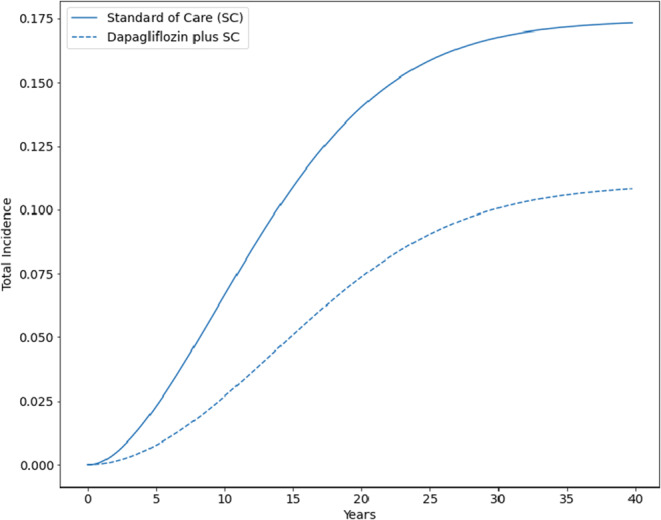
Total incidence of kidney failure requiring kidney replacement therapy (KRT), standard of care versus dapagliflozin plus standard of care.
Given that non-diabetic CKD is prevalent in the USA, we estimated the one-year net budgetary implications of treating the non-diabetic CKD population in the USA (our base-case cohort) could be as high as an increase of $21 billion, with further details provided in the supplemental material.
Age- and Stage-Specific Outcomes
Across all age cohorts and CKD stages, dapagliflozin added to standard of care provided incremental health benefits costing less than the $100,000 per QALY willingness-to-pay threshold (discounted costs, life years, and QALYs for each age-specific cohort provided in the Supplement). Cost-effectiveness ranged from being cost-saving for 40-year-old patients with stage 4 CKD to $68,400 per QALY gained for 70-year-old patients with stage 2 CKD. For cohorts aged 70 and younger, cost-effectiveness was more favorable for patients in more advanced CKD stages. In the 80-year-old cohort, cost-effectiveness remained relatively stable over the CKD stages, as shorter life expectancy in this cohort meant less potential cost savings from averted KRT for those in later stages of CKD (Fig. 3). In younger-age cohorts, there were even larger decreases in the total incidence of KF requiring KRT than in the base case (Supplement Figure 7).
Figure 3.
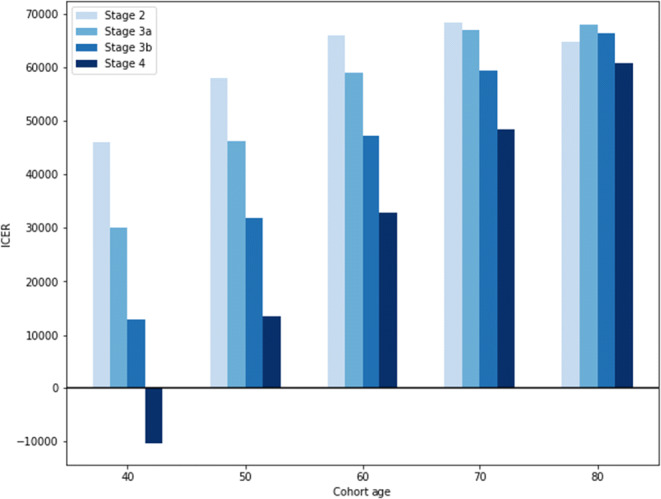
Dapagliflozin cost-effectiveness by age and CKD stage. *ICER = incremental cost-effectiveness ratio; negative ICERs indicate cost-saving results.
Sensitivity Analysis
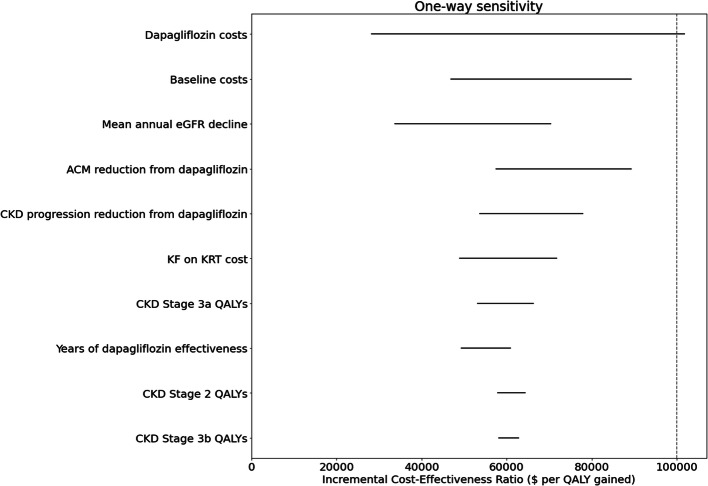
Clinical effectiveness and cost-effectiveness were robust to all conditions tested in sensitivity analyses, with the single exception of the cost of dapagliflozin as described in more detail below.
In a threshold analysis, dapagliflozin resulted in costs per QALY gained greater than $100,000 at a monthly added cost of dapagliflozin of $645, 175% of the base-case value (Fig. 4) (Supplement Figures 8–12). As above, results were robust to variation in all other one-way sensitivity analysis inputs.
Figure 4.
One-way sensitivity analysis tornado plot (base case). *Results with all tested parameters are included in the Supplement.
Probabilistic sensitivity analyses (PSA) assessed the likelihood that dapagliflozin added to standard care would be cost-effective when accounting for the simultaneous uncertainty of all model parameters (Supplement Figure 13). At a cost-effectiveness threshold of $100,000, adding dapagliflozin to standard of care was preferred over standard of care alone in 88.8% of the 1000 PSA samples, including 2.2% of samples in which dapagliflozin was cost-saving.
In additional sensitivity analyses, dapagliflozin was cost-effective at $100,000 per QALY gained across a range of alternative assumptions regarding long-term effectiveness and eGFR targets for KRT initiation (Supplement Tables 12 and 13).
DISCUSSION
Based on results from the DAPA-CKD trial, we have analyzed the cost-effectiveness of adding dapagliflozin to standard of care management of patients with non-diabetic CKD from a US healthcare sector perspective. Dapagliflozin provided substantial health benefits over the lifetime of our analyzed cohorts, including a reduction in the number of patients requiring KRT from 17.4 to 11.0%, a reduction in the time spent on KRT, increased total life years, and increased QALYs. Use of dapagliflozin increased costs but was cost-effective by currently accepted criteria in all age cohorts, and with initiation at all stages of CKD. Cost-effectiveness was more favorable in younger cohorts and in those with more advanced kidney disease (Fig. 2).
Our calculation of the long-term health and economic outcomes associated with use of dapagliflozin required us to estimate the natural history of non-diabetic CKD and the effectiveness of dapagliflozin over longer periods than were observed in the DAPA-CKD trial. To do so, we calibrated our model to observational studies of patients with non-diabetic CKD, and our calibration results provide confidence that our estimates are consistent with the available empiric evidence. Remaining questions about the long-term effectiveness of dapagliflozin include whether the effectiveness may wane over time, or whether effectiveness may differ by stage of CKD. We examined these questions in sensitivity analyses evaluating waning efficacy and differing efficacy by disease stage, respectively. For example, a 20% reduction in effectiveness of dapagliflozin for disease stage 3b and 4 has virtually no impact on cost-effectiveness (Supplement Table 12). Overall, these analyses indicated that dapagliflozin continues to provide substantial health benefits and remains cost-effective even with substantially more pessimistic assumptions about effectiveness.
The cost-effectiveness of dapagliflozin does, however, depend on its price. Dapagliflozin would not be considered cost-effective if its cost were to increase to more than 175% of the base-case price (approximately $370 per month). We also note that some large healthcare systems may be able to obtain dapagliflozin for less than our base-case estimate, which would result in more favorable cost-effectiveness.
While adding dapagliflozin to standard of care treatment was deemed cost-effective in this analysis, affordability must also be considered, given one-year net budgetary implications could be as high as a $21 billion increase.32 However, this short-term budgetary impact analysis does not reflect large and ongoing cost savings and QALY gains that are realized over the next 5 to 10 years from reducing reliance on kidney replacement therapy and estimating the long-term budgetary impact of dapagliflozin will require further analysis.
We limited our modeling of dapagliflozin’s benefits for the population of patients with non-diabetic CKD to the slowing of CKD progression and all-cause mortality; we have not separately modeled cardiovascular outcomes, including heart failure, beyond their inclusion in all-cause mortality. While cardiovascular morbidity and mortality are likely somewhat lower at baseline in patients with non-diabetic CKD as compared to patients with diabetic CKD, as indeed was demonstrated in a prespecified subgroup analysis from the DAPA-CKD trial,33 a dapagliflozin-related reduction in heart failure and other cardiovascular morbidity and mortality could nonetheless increase health benefits and result in cost savings (for example, by prevention of hospitalization for heart failure) that are not included in our analysis. If cardiovascular outcomes were added to the model, the health and economic outcomes, and thus cost-effectiveness, would likely be more favorable that we estimated.
The DAPA-CKD trial included only patients who had non-diabetic CKD with albuminuria. Patients with some conditions (polycystic kidney disease, lupus nephritis, and anti-nuclear cytoplasmic antibody (ANCA)–associated vasculitis) were excluded, as were patients with urine albumin-to-creatinine ratio < 200 or ≥ 5000 mg/g. The trial results, and therefore the results of our analysis, may not be applicable to these sub-populations.
Limitations
Our analysis was based on a single trial. The striking benefit from dapagliflozin in the DAPA-CKD, including an almost 50% reduction in all-cause mortality among patients who had non-diabetic CKD, was such that the trial was stopped early. It is possible that the early termination of the trial could have resulted in an overestimate of effectiveness; however, we assessed such a possibility in sensitivity analyses of decreased effectiveness and found our results to be robust. We note that dapagliflozin is one of several SGLT2 inhibitors, and trials are underway to assess the effects of other agents in patients with non-diabetic CKD (e.g., empagliflozin in the EMPA-KIDNEY trial)34; should these trials produce results consistent with those of DAPA-CKD, this would mitigate any potential concern around limited follow-up in DAPA-CKD.
The majority of effectiveness estimates and parameters used in our analysis were specific to non-diabetic patients, yet DAPA-CKD data on eGFR decline stratified by diabetes status have not yet been published. However, our sensitivity analyses with reduced effectiveness consistently support the cost-effectiveness of dapagliflozin for this population, indicating that modest changes in eGFR decline would not affect our conclusions.
Finally, our model has the potential to overestimate treatment benefits, as we jointly considered benefits from slowing CKD progression and reducing all-cause mortality, which may overlap. However, our model closely matched observed event rates for all-cause mortality and end-stage kidney disease as estimated for the DAPA-CKD non-diabetic placebo cohort, and results proved robust in sensitivity analyses examining the underlying rate of CKD progression. Moreover, due to our inability to disaggregate the composite cardiovascular endpoint (cardiovascular death or hospitalized heart failure), we ignored the benefits of dapagliflozin on heart failure hospitalization, thereby potentially underestimating the cardiovascular treatment benefit.
CONCLUSION
Dapagliflozin is highly effective clinically in patients with non-diabetic CKD with albuminuria, producing substantial benefits by any of several metrics including life expectancy, quality-adjusted life years, and both probability of requiring kidney replacement therapy and years spent receiving kidney replacement therapy. Short-term affordability remains a concern, as many of the accompanying cost savings and QALY gains are realized only over a 5- to 10-year time horizon. Nevertheless, dapagliflozin generates good long-term value in patients with non-diabetic CKD, with cost-effectiveness consistently within currently accepted ranges for health interventions.
Supplementary Information
(DOCX 1035 kb)
Contributors
N/A
Funding
Dr. Tisdale is supported by the Veterans Administration (VA) Office of Academic Affairs Advanced Fellowship in Health Services Research. Ms. Cusick is supported by the T32HS026128 grant from the Agency for Healthcare Research and Quality (AHRQ). Dr. Handley is supported by a graduate fellowship award from Knight-Hennessy Scholars at Stanford University. Dr. Chertow is supported by NIDDK K24 DK085446.
Declarations
Conflict of Interest
Dr. Chertow served on the Steering Committee of the DAPA-CKD trial. All other authors have nothing to disclose.
Footnotes
Prior Presentations: Oral presentation at 2021 Society for Medical Decision Making Virtual Annual Meeting, October 2021
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rebecca L. Tisdale and Marika M. Cusick contributed equally to the manuscript and are co-first authors.
References
- 1.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data [Internet]. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2019. Accessed 30 Dec 2021 from: https://www.cdc.gov/nchs/nhanes/index.htm
- 2.United States Renal Data System. 2019 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2019.
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N Engl J Med. 2004;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. The Lancet. 1999;354(9176):359–64. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- 5.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving H-H, et al. Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy. N Engl J Med. 2001;345(12):861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry: Diabetes Mellitus--Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. 2008 Dec. Accessed 30 Dec 2021 from https://www.fda.gov/media/71297/download.
- 7.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Stein P, et al. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS)—A randomized placebo-controlled trial. Am Heart J. 2013;166(2):217–223.e11. doi: 10.1016/j.ahj.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 9.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. The design and rationale for the Dapagliflozin Effect on Cardiovascular Events (DECLARE)–TIMI 58 Trial. Am Heart J. 2018;200:83–9. doi: 10.1016/j.ahj.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375(4):323–34. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 11.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–57. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 12.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380(24):2295–306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 13.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler DC, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(1):22–31. doi: 10.1016/S2213-8587(20)30369-7. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC). Chronic Kidney Disease Surveillance System [Internet]. Accessed 30 Dec 2021 from: http://www.cdc.gov/ckd/default.aspx.
- 16.Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2020;395(10225):709–33. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoerger TJ, Simpson SA, Yarnoff BO, Pavkov ME, Ríos Burrows N, Saydah SH, et al. The Future Burden of CKD in the United States: A Simulation Model for the CDC CKD Initiative. Am J Kidney Dis. 2015;65(3):403–11. doi: 10.1053/j.ajkd.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–50. [Google Scholar]
- 19.United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. [Internet]. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Accessed 30 Dec 2021 from: https://adr.usrds.org/
- 20.Erickson KF, Chertow GM, Goldhaber-Fiebert JD. Cost-Effectiveness of Tolvaptan in Autosomal Dominant Polycystic Kidney Disease. Ann Intern Med. 2013;159(6):382. doi: 10.7326/0003-4819-159-6-201309170-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin E, Chertow GM, Yan B, Malcolm E, Goldhaber-Fiebert JD. Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: A modeling study. PLoS Med [Internet]. 2018 Mar 27 [cited 2020 Oct 5];15(3). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5870947/ [DOI] [PMC free article] [PubMed]
- 22.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 23.US Bureau of Labor Statistics. Consumer Price Index [Internet]. Washington DC: 2020. Accessed 30 Dec 2021 from: https://www.bls.gov/cpi/data.htm
- 24.Perkins RM, Bucaloiu ID, Kirchner HL, Ashouian N, Hartle JE, Yahya T. GFR decline and mortality risk among patients with chronic kidney disease. Clin J Am Soc Nephrol CJASN. 2011;6(8):1879–86. doi: 10.2215/CJN.00470111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659–63. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 26.Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol JASN. 2006;17(8):2275–84. doi: 10.1681/ASN.2005121273. [DOI] [PubMed] [Google Scholar]
- 27.Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model Transparency and Validation: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force–7. Med Decis Making. 2012;32(5):733–43. doi: 10.1177/0272989X12454579. [DOI] [PubMed] [Google Scholar]
- 28.Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey: Number of people in thousands, United States, 1996-2018. [Internet]. Accessed 30 Dec 2021 from: https://meps.ahrq.gov/mepstrends/hc_use/
- 29.Rizk D, Jurkovitz C, Veledar E, Bagby S, Baumgarten DA, Rahbari-Oskoui F, et al. Quality of Life in Autosomal Dominant Polycystic Kidney Disease Patients not yet on Dialysis. Clin J Am Soc Nephrol. 2009;4(3):560–6. doi: 10.2215/CJN.02410508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper JT, Lloyd A, Sanchez JJG, Sörstadius E, Briggs A, McFarlane P. Health related quality of life utility weights for economic evaluation through different stages of chronic kidney disease: a systematic literature review. Health Qual Life Outcomes. 2020;18(1):310. doi: 10.1186/s12955-020-01559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A Systematic Review and Meta-Analysis of Utility-Based Quality of Life in Chronic Kidney Disease Treatments. Turner N, editor. PLoS Med. 2012;9(9):e1001307. doi: 10.1371/journal.pmed.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lomas J, Claxton K, Martin S, Soares M. Resolving the “Cost-Effective but Unaffordable” Paradox: Estimating the Health Opportunity Costs of Nonmarginal Budget Impacts. Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2018;21(3):266–75. doi: 10.1016/j.jval.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Heerspink HJL, Sjöström CD, Jongs N, Chertow GM, Kosiborod M, Hou FF, et al. Effects of dapagliflozin on mortality in patients with chronic kidney disease: a pre-specified analysis from the DAPA-CKD randomized controlled trial. Eur Heart J. 2021;42(13):1216–27. doi: 10.1093/eurheartj/ehab094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrington WG, Preiss D, Haynes R, von Eynatten M, Staplin N, Hauske SJ, et al. The potential for improving cardio-renal outcomes by sodium-glucoseco-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J. 2018;11(6):749–61. doi: 10.1093/ckj/sfy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pong CK, Maxted GE. Dapagliflozin (Farxiga) for Type 2 Diabetes Mellitus. Am Fam Physician. 2015;91(12):828–33. [Google Scholar]
- 36.Arias E, Xu J. National Vital Statistics Report. Natl Vital Stat Rep. 2010;58(21):66. [PubMed] [Google Scholar]
- 37.Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJL, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. The Lancet. 2012;380(9854):1662–73. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erickson KF, Japa S, Owens DK, Chertow GM, Garber AM, Goldhaber-Fiebert JD. Cost-Effectiveness of Statins for Primary Cardiovascular Prevention in Chronic Kidney Disease. J Am Coll Cardiol. 2013;61(12):1250–8. doi: 10.1016/j.jacc.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and Albuminuria to Classify CKD Improves Prediction of ESRD. J Am Soc Nephrol. 2009;20(5):1069–77. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S, Barnett PG, Holodniy M, Lo J, Joyce VR, Gidwani R, et al. Cost-Effectiveness of Treatments for Genotype 1 Hepatitis C Virus Infection in Non-VA and VA Populations. MDM Policy Pract [Internet]. 2016 Oct 3 [cited 2021 Mar 18];1(1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5942888/ [DOI] [PMC free article] [PubMed]
- 41.Smith DH, Nichols GA, Gullion CM, Johnson ES, Keith D. Predicting costs of care in chronic kidney disease: the role of comorbid conditions. Internet J Nephrol. 2007;4(1):10-5580.
- 42.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of Nationally Representative Values for the Noninstitutionalized US Adult Population for 7 Health-RelatedQuality-of-Life Scores. Med Decis Making. 2006;26(4):391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- 43.US Census Buraeu. U.S. Adult Population Grew Faster Than Nation’s Total Population From 2010 to 2020 [Internet]. Accessed 30 Dec 2021 from: https://www.census.gov/library/stories/2021/08/united-states-adult-population-grew-faster-than-nations-total-population-from-2010-to-2020.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1035 kb)