Abstract
Nitrogen-based heterocycles have aroused widespread interest due to their reoccurrence in many pharmaceuticals. Amongst these motifs, the enantioenriched lactams are the ubiquitous scaffolds found in myriad biologically active natural products and drugs. Recently, the transition metal-catalyzed asymmetric carbamoylation has been widely employed as a straightforward arsenal for chiral lactam architecture synthesis, including β-lactam and γ-lactam. However, despite the extensive efforts, there still remains no protocol to accomplish the related δ-lactam synthesis. In this manuscript, the Ni-catalyzed enantioselective carbamoylation of unactivated alkenes by the leverage of reductive dicarbofunctionalization strategy allows for the expedient access to two types of mostly common six-membered lactams: 3,4-dihydroquinolinones and 2-piperidinone in high yield and enantioselectivity. This protocol features with good functional group tolerance, as well as broad substrate scope. The newly developed chiral 8-Quinox skeleton ligand is the key parameter for this transformation, which significantly enhances the reactivity and enantioselectivity.
Subject terms: Asymmetric catalysis, Synthetic chemistry methodology
Six-membered chiral lactams are common structural motifs of pharmaceuticals. Here, the authors describe a nickel-catalyzed reductive carbamoylation of alkenes to form enantioenriched six-membered lactams.
Introduction
Nitrogen-containing heterocycles constitute the versatile structure motifs in organic and medicinal chemistry1,2. Amongst these, chiral lactam is recognized as one of the most privileged skeletons3–6, which also serves as a valuable subunit for complex molecular synthesis and drug discovery. Owing to these unique pharmacological properties and synthetic utilities, tremendous efforts have been devoted to the development of facile and robust methodologies for stereoselective synthesis of this architecture7–15. However, a particular synthetic challenge in this scenario is the enantioselective synthesis of α-alkylated lactam enabled by asymmetric catalysis, likely due to the lack of general asymmetric α-alkylation of simple lactam16–19. Organocatalyst-promoted alkylation and Michael addition were developed to tackle the aforementioned challenge. Nevertheless, this strategy is largely limited to the lactam scope wherein an electron-withdrawing group including ester or aryl group was required at the adjacent position of amide functionality to generate the reactive chiral enolate intermediate20–23.
A strategically distinct approach to forge chiral lactam is the cyclization from the acyclic fragment by the synergistic formation of amide functionality and set-up of a new stereogenic center in the formed lactam ring, namely as asymmetric carbamoylation. The representative precursor of carbamoylation is formamide and carbamoyl halide, which possess several advantages, including the one-step synthesis from the prevalent secondary amine and exceptional chemo-stable property24. Recently, several transition metal-catalyzed asymmetric transformations involving carbamoyl electrophiles have emerged as a fascinating tool box for synthesis of chiral lactam moiety25–40. Baudoin group accomplished the only example to construct the β-lactam by palladium-catalyzed desymmetric C(sp3)‒H carbamoylation (Fig. 1a)25. The most reliable approaches are the transition metal-catalyzed enantioselective cyclization of carbamoyl electrophile to the pendent alkenes in which the stereogenic center was simultaneously constructed at the α-position of amide functionality with the cyclization to afford the lactam ring. This approach allows for the facile synthesis of five-membered lactam, including oxindole and γ-lactam (Fig. 1b)27–40. The seminal Pd-catalyzed asymmetric cyanocarbamoylation of alkenes was developed by Takemoto to access the oxindole27. The hydrocarbamoylation of alkene was accomplished by Cramer to allow the expedient synthesis of α-methyl pyrrolidinone28. Very recently, a series of asymmetric carbamoylation-initiated difunctionalization of alkenes including borocarbamoylation29,30, iodocarbamoylation31, acylcarbamoylation32,33, alkylcarbamoylation34–38 and arylcarbamoylation39,40 were independently developed by Lautens, Wang, Lin, Ye and our group. All the above methods could only provide the synthesis of α-alkylated five-membered lactam by the asymmetric 5-exo-trig cyclization. Despite the apparent similarities to γ-lactam, there is no example of asymmetric α-alkylated six-membered lactams synthesis by enantioselective carbamoylation (Fig. 1c).
Fig. 1. Asymmetric carbamoylation for chiral lactam synthesis.
a β-Lactam synthesis enabled by the C(sp3)-H carbamoylation. b γ-Lactam synthesis enabled by the carbamoylation of alkenes. c Asymmetric carbamoylation for six-membered-lactam synthesis (this work). d Representative six-membered lactams skeleton in myriad biologically active natural products and drugs.
Six-membered lactams, including δ-lactam and dihydroquinolinone, are ubiquitous motif found across biologically active molecules (Fig. 1d)41,42 We envisaged that the aforesaid synthetic difficulty lies in the lack of rigidity of the longer chain and the larger spatial distances, as supported by theoretical calculations that 6-exo-trig cyclization of d10 transition metal-controlled intramolecular migratory insertion of unsaturated bond was disfavored compared with the 5-exo-trig cyclization in Baldwin-type rules43,44. Recently, the nickel-catalyzed asymmetric reductive cyclized difunctionalization of alkenes strategy has received the considerable attention, which allows the rapid stereoselective formation of five-membered carbo-skeleton or heterocycles45–66.
Herein, we report the unprecedented 6-exo-trig cyclized carbamoylation by the utilization of the nickel-catalyzed asymmetric reductive difunctionalization of alkenes strategy. It should be noted this also represents the only example for the construction of six-membered nitrogen-containing heterocycles in asymmetric reductive cross-coupling reactions64. The utilization of newly modified 8-Quinox ligand enables the synthesis of α-alkylated six-membered lactam with satisfied yields and enantioselectivities.
Results and Discussion
Reaction optimization for dihydroquinolinone synthesis
The initial investigation of the carbamoyl-alkylation was commenced with carbamoyl chloride 1a and iodoheptane 2a as electrophiles, Ni(ClO4)2•6H2O as catalyst, Mn as reductant, LiBr as additive and DMA as solvent (Fig. 2). The Quinim ligands (L1 and L2) exploited in our lab34,35, were first examined, but only trace of target product 3a was obtained with less than 30% ee. To our delight, the corrected GC yield of 3a significantly increased to 76% with the utilization of Quinox L336,37,67 instead of Quinim, though the er value was only 58.5:41.5. Encouraged by this result, a series of structurally defined Quinox ligands (L4‒L6) were examined in this transformation. Quinox L6 provided the desired 3,4-dihydroquinolinone 3a in 82:18 er with moderate yield, demonstrating that the benzyl group in oxazoline was crucial for this reductive cross-coupling reaction. It was worth noting that the benzyl group substituted Pyrox L7, which was widely used in asymmetric reductive reaction, exhibited poor reactivity. With the employment of Quinox L8 by incorporating dimethyl group at the C5-position of the oxazoline ring, the enantioselectivity of 3a was significantly increased to 93:7 er. However, replacing the substituent group of oxazoline ring from dimethyl group to diphenyl group (L9) decreased the enantioselectivity (44% yield, 86.5:13.5 er). With the optimal ligand (L8), reaction efficiency could be improved by treatment of LiI as additive. Finally, the mixed solvent system of DMA/MeCN was found to be beneficial for reactivity without dropping the enantioselectivity, and the product 3a could be obtained in 85% isolated yield and 96.5:3.5 er.
Fig. 2. Optimization of the reaction conditions.
The reaction was performed with carbamoyl chloride 1a (1.0 eq.), iodoheptane 2a (3.0 eq.), Ni(ClO4)2•6H2O (15 mol%), Ligand (18 mol%), Mn (4.0 eq.), LiBr (1.0 eq.), DMA (0.2 M), stirred for 24 h under 30 °C. The yields were reported as corrected GC yield and the er values were determined by chiral HPLC analysis on a chiral stationary phase. a1.0 eq. LiI. bThe reaction was performed in DMA/MeCN (v/v = 4/1), isolated yield on 0.2 mmol scale for 48 h was reported in parentheses.
Substrate scope of carbamoyl chloride
With optimized conditions in hand, we next investigated the scope of the carbamoyl chloride (Fig. 3). Substrates with electron-donating (-OMe) or electron-withdrawing (–F, –Cl, –CF3) on the aromatic rings were tolerated, affording corresponding products in excellent reactivity and enantioselectivity (3b‒3e). Notably, the current procedure could proceed with the carbamoyl chloride averting the direct coupling with aryl chloride, leaving a hand to further transformation. In addition, the carbamoyl chlorides with diverse substituents on N-protecting group could react smoothly to provide 3 f‒3k with up to 94% yield and up to 98:2 er. Substrates containing heteroaromatic substituents such as furan (3 h), thiophene (3i) did not impede the catalytic cycle, which were accommodated with high yield and er. Remarkably, the alkene baring ethyl group delivered the dihydroquinolinone containing all-carbon quaternary center 3 l in 60% yield and 98:2 er. Unfortunately, when we switched the aromatic ring from aryl to heteroaryl group such as pyridine, the corresponding products could not be observed during the reaction (3 m). As for thiophene group, we could not obtain the carbamoyl chloride substrate due to its instability.
Fig. 3. Substrate scope of carbamoyl chloride.
The reaction was performed with carbamoyl chloride 1 (1.0 eq.), iodoheptane 2a (3.0 eq.), Ni(ClO4)2•6H2O (15 mol%), L8 (18 mol%), Mn (4.0 eq.), LiI (1.0 eq.), DMA/MeCN (v/v = 4/1, 0.2 M), stirred for 48 h under 30 °C. Yields were for isolated products. The er values were determined by chiral HPLC analysis on a chiral stationary phase.
Substrate scope of alkyl iodide
Inspired by the high functional group tolerance of carbamoyl chloride, we next turned our attention to variations of the alkyl iodide (Fig. 4). Simple alkyl iodide such as ethyl iodide (3n) and isoamyl iodide (3o) could be compatible in this methodology, which delivered the corresponding 3,4-dihydroquinolinones in high yield and enantioselectivity. Gratifyingly, alkyl iodides bearing –Cl (3p), –F (3q), –CN (3 s) groups could also be tolerant to give the desired products with up to 96:4 er. However, CF3 substituents resulted in a sharp decline in enantioselectivity, albeit with almost quantitative yield (3r). Additionally, perfluoride-substituted alkyl electrophile had a slightly negative effect on the reaction, giving product 3 y in 59% yield and 95.5:4.5 er, and the absolute configuration was assigned unambiguously by X-ray diffraction. In addition, various functional groups, including esters (3 v), thioether (3w), cyano (3x) were also harmonious, delivering the corresponding products in moderate to excellent yields, highlighting the synthetic potential of this methodology. The alkyl iodide converted from citronellol could also be tolerant in this reaction, produced 3z in 71% yield and 97:3 dr. It should be noted that the primary 1-bromoheptane delivered the product 3a in 83% yield with 90.5:9.5 er. However, the secondary and tertiary alkyl iodides failed to deliver the corresponding products at the standard condition.
Fig. 4. Substrate scope of alkyl iodide.
The reaction was performed with carbamoyl chloride 1a (1.0 eq.), iodide 2 (3.0 eq.), Ni(ClO4)2•6H2O (15 mol%), L8 (18 mol%), Mn (4.0 eq.), LiI (1.0 eq.), DMA/MeCN (v/v = 4/1, 0.2 M), stirred for 48 h under 30 °C. Yields were for isolated products. The er values were determined by chiral HPLC analysis on a chiral stationary phase.
Study on the none aromatic ring tethered carbamoyl chloride
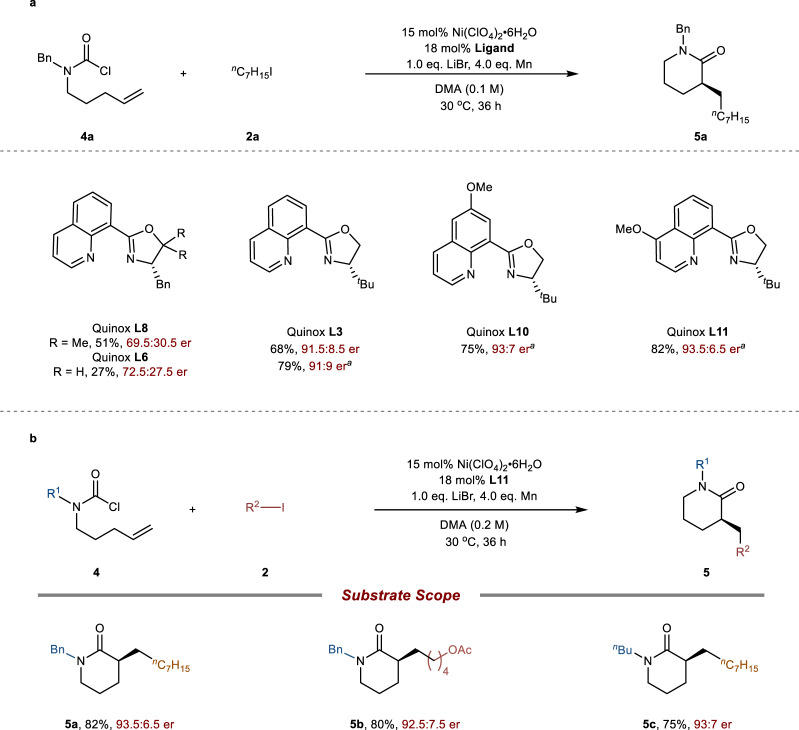
To further investigate this enantioselective tandem cyclization/reductive cross-coupling protocol, we turned our attention to the none aromatic ring tethered carbamoyl chloride which is more flexible, thus causing more challenge for the cyclized carbamoylation (Fig. 5). When readily available carbamoyl chloride 4a as starting material, Ni(ClO4)2•6H2O as catalyst, Mn as reductant, LiBr as additive and DMA as solvent, we evaluated the ligand effect in the asymmetric reductive carbamoyl-alkylation reaction (Fig. 5a). Unfortunately, the reaction performed with ligand Quinox L8 used in the above reactions provided moderate yield (51%) but with low enantioselectivity (39% ee). In contrast, Quinox L6 afford the 5a in better enantioselectivity (72.5:27.5 er). Then, it was found that Quinox L3 deliver 5a in 79% yield and 91:9 er. The modification of the electronics of quinolone revealed that the 6-OMe-Quinox L10 would further elevate the enantioselectivity into 93:7 er. Finally, switch the electronic rich methoxy group at the C-4 position of quinolone ring, namely Quinox L11 provided the optimal results, the δ-lactam 5a was obtained in 82% isolated yield and 93.5:6.5 er. Further screening of additives and solvents did not yield better results (See Supplementary Fig. 6). The exploration of alkyl iodide coupling component and substitution effect on the carbamoyl chloride precursor has little effect on both reaction efficiency and ee (5b, 5c). At current stage, this protocol was not suitable for the construction of β-lactam or seven-membered lactam skeleton.
Fig. 5. Investigation of none aromatic ring tethered carbamoyl chloride.
a Optimization of the reaction conditions of none aromatic ring tethered carbamoyl chloride. The reaction was performed with carbamoyl chloride 4a (1.0 eq.), 2a (3.0 eq.), Ni(ClO4)2•6H2O (15 mol%), Ligand (18 mol%), Mn (4.0 eq.), LiBr (1.0 eq.), DMA (0.1 M), stirred for 36 h under 30 °C. The yields were reported as corrected GC yield and the er values were determined by HPLC analysis; a0.2 M. b Substrate scope for δ-lactam synthesis.
Synthetic utility
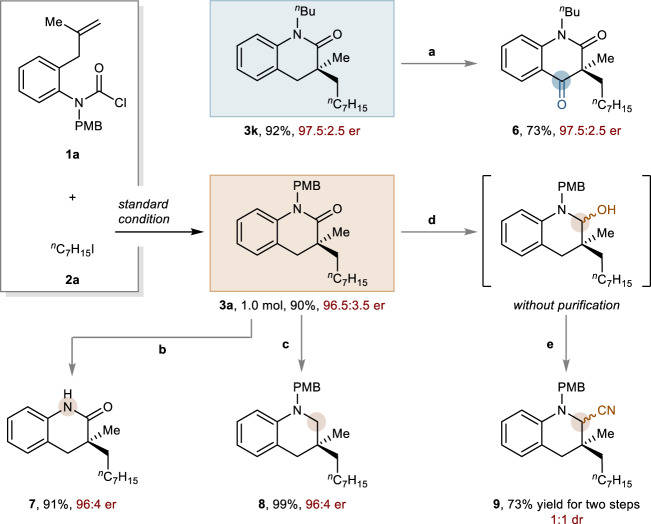
To demonstrate the synthetic utility of six-membered lactam, products 3a and 3k were employed as the building blocks for synthesis of various valuable products (Fig. 6). Firstly, direct oxidation of 3k by using ceric ammonium nitrate (CAN) delivered 6 with high yield and retention of enantioselectivity. With regards to 3a, the PMB group of quinolinone 3a could be removed by treatment with trifluoroacetic acid (TFA) to afford 7 in 91% isolated yield and 96:4 er. Subsequently, the reduction operation of 3a was carried out using diisobutylaluminum hydride (DIBAL-H) to afford chiral tetrahydroquinoline 8 in almost quantitative yield with enantiopurity maintained. Furthermore, the sequential reduction using sodium bis(2-methoxyethoxy)-aluminiumhydride (Red-Al) and cyanidation by trimethylsilyl cyanide could provide chiral α-cyano tetrahydroquinoline 9 in 73% yield.
Fig. 6. Synthetic transformations of quinolinone products.
Reaction conditions: a4.0 eq. CAN, MeCN/H2O (0.03 M), r.t.; bTFA (0.1 M), DCM (0.1 M), reflux; c2.0 eq. DIBAL-H, DCM, −78 °C.; d1.0 eq. Red-Al, THF, −2 ~ −5 °C; e1.5 eq. BF3•Et2O, 1.5 eq. TMSCN.
Mechanistic investigation
Several preliminary studies have been carried out to elucidate the plausible mechanism of this transformation (Fig. 7). Firstly, a radical ring-opening experiment of (iodomethyl)cyclopropane as electrophile has been performed. The mixture of ring-opening products 10 and 11 were obtained (Fig. 7a). Additionally, the addition of TEMPO (1.0 equiv) completely inhibited the reductive cross-couplings, and only led to decarbonylation by-product (Fig. 7b). Combined with the above experiments and our previous results, a plausible catalytic cycle could be speculated (Fig. 7c):35 Firstly, the low-valent nickel species A undergoes oxidative addition of carbamoyl chloride to form carbamoyl-Ni(II) species B. Then the carbamoyl-Ni(I) C is formed by the reduction of Mn, which then proceeds with an enantiodetermining migratory insertion into alkene to forge the intermediate D. Next, D undergoes the coupling with the alkyl iodide via a SET process to generate the alkyl-Ni(III) species F. The six-membered lactams 3 or 5 could obtain by reductive elimination of F.
Fig. 7. Mechanistic experiment.
a Radical ring-opening experiment. The yield was reported as isolated yield. b Radical trap reaction using TEMPO as radical scavenger. The yields were reported as corrected GC yield with dodecane as an internal standard. c Plausible mechanism.
In conclusion, the first enantioselective carbamoylation to access the six-membered lactam by the nickel-catalyzed asymmetric reductive cyclization was reported. The protocol using newly developed Quniox ligand allows the formation of dihydroquinolinones and δ-lactam with good yield and enantioselectivity, which can be further derived to other valuable nitrogen-containing heterocycles. The asymmetric carbamoylation that enables other chiral lactam synthesis is currently underway in our group.
Methods
The reaction to produce dihydroquinolinone 3
To a dried 8-mL vial were added Ni(ClO4)2·6H2O (15 mol%), L8 (18 mol%), Mn (4.0 equiv) and carbamoyl chloride 1 (1.0 equiv) (if solid). Then the vial was transferred into glovebox. LiI (1.0 equiv), DMA, MeCN, carbamoyl chloride 1 (1.0 equiv) (if liquid) and alkyl halide 2 (3.0 equiv) were added in sequence inside the glovebox. The vial was then taken out from the glovebox, sealed with parafilm, put into oil bath (30 °C) and stirred for 48 h. After completion, the reaction mixture was quenched with H2O, filtered through a pad of Celite and extracted with EA for three times. The combined organic phase was washed with brine and concentrated under reduced pressure to yield the crude product, which was purified by silica gel flash column chromatography to afford products 3.
The reaction to produce δ-lactam 5
To a dried 8-mL vial were added Ni(ClO4)2·6H2O (15 mol%), L11 (18 mol%), Mn (4.0 equiv). Then the vial was transferred into glovebox. LiBr (1.0 equiv), DMA, carbamoyl chloride 4 (1.0 equiv) and alkyl halide 2 (3.0 equiv) were added in sequence inside the glovebox. The vial was then taken out from the glovebox, sealed with parafilm, put into oil bath (30 °C) and stirred for 36 h. After completion, the reaction mixture was quenched with H2O, filtered through a pad of Celite and extracted with EA for three times. The combined organic phase was washed with brine and concentrated under reduced pressure to yield the crude product, which was purified by silica gel flash column chromatography to afford products 5.
Supplementary information
Acknowledgements
This work was sponsored by NSFC/China (22171079), Natural Science Foundation of Shanghai (21ZR1480400), Shanghai Rising-Star Program (20QA1402300), Shanghai Municipal Science and Technology Major Project (Grant No.2018SHZDZX03) and the Program of Introducing Talents of Discipline to Universities (B16017), and the Fundamental Research Funds for the Central Universities (222201717003) and the China Postdoctoral Science Foundation (2021M701197). The authors thank Research Center of Analysis and Test of East China University of Science and Technology for the help on NMR analysis.
Author contributions
Y.C. conceived the projects. C.Z., X.W., T.X. performed the experiments under the supervision of J.Q. and Y.C. C.Z and Y.C. wrote the manuscript with the feedbacks of all other authors
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Data availability
The crystallographic data for compound 3 y is available from the Cambridge Crystallographic Data Center under deposition numbers CCDC 2158471 respectively (https://www.ccdc.cam.ac.uk/structures/). All other data to support the conclusions are available in the main text or the Supplementary Information. (See Supplementary Method).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-33425-3.
References
- 1.Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 2.Taylor AP, et al. Modern advances in heterocyclic chemistry in drug discovery. Org. Biomol. Chem. 2016;14:6611–6637. doi: 10.1039/C6OB00936K. [DOI] [PubMed] [Google Scholar]
- 3.Pitts CR, Lectka T. Chemical synthesis of β-lactams: asymmetric catalysis and other recent advances. Chem. Rev. 2014;114:7930–7953. doi: 10.1021/cr4005549. [DOI] [PubMed] [Google Scholar]
- 4.Wang P, Yang D, Liu H. Recent advances on the synthesis of β-lactams by involving carbon monoxide. Chin. J. Org. Chem. 2021;41:3448–3458. doi: 10.6023/cjoc202104060. [DOI] [Google Scholar]
- 5.Ye L-W, Shu C, Gagosz F. Recent progress towards transition metal-catalyzed synthesis of γ-lactams. Org. Biomol. Chem. 2014;12:1833–1845. doi: 10.1039/C3OB42181C. [DOI] [PubMed] [Google Scholar]
- 6.Pandey G, Mishra A, Khamrai J. Generation of all carbon quaternary stereocenters at the C-3 carbon of piperidinones and pyrrolidinones and its application in natural product total synthesis. Tetrahedron. 2018;74:4903–4915. doi: 10.1016/j.tet.2018.05.004. [DOI] [Google Scholar]
- 7.Pedroni J, Boghi M, Saget T, Cramer N. Access to β-lactams by enantioselective palladium(0)-catalyzed C(sp3)–H alkylation. Angew. Chem. Int. Ed. 2014;53:9064–9067. doi: 10.1002/anie.201405508. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi M, Bachman S, Hashimoto S, Eichman CC, Stoltz BM. Ni-catalyzed enantioselective C-acylation of α-substituted lactams. J. Am. Chem. Soc. 2016;138:8997–9000. doi: 10.1021/jacs.6b02120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jette CI, et al. Palladium-catalyzed construction of quaternary stereocenters by enantioselective arylation of γ-lactams with aryl chlorides and bromides. Angew. Chem. Int. Ed. 2019;58:4297–4301. doi: 10.1002/anie.201814475. [DOI] [PubMed] [Google Scholar]
- 10.Park Y, Chang S. Asymmetric formation of γ-lactams via C–H amidation enabled by chiral hydrogen-bond-donor catalysts. Nat. Catal. 2019;2:219–227. doi: 10.1038/s41929-019-0230-x. [DOI] [Google Scholar]
- 11.Xing Q, Chan C-M, Yeung Y-W, Yu W-Y. Ruthenium(II)-catalyzed enantioselective γ-lactams formation by intramolecular C–H amidation of 1,4,2-dioxazol-5-ones. J. Am. Chem. Soc. 2019;141:3849–3853. doi: 10.1021/jacs.9b00535. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, et al. Iridium-catalyzed enantioselective C(sp3)–H amidation controlled by attractive noncovalent interactions. J. Am. Chem. Soc. 2019;141:7194–7201. doi: 10.1021/jacs.9b02811. [DOI] [PubMed] [Google Scholar]
- 13.Tong H-R, et al. Asymmetric synthesis of β-lactam via palladium-catalyzed enantioselective intramolecular C(sp3)–H amidation. ACS Catal. 2020;10:114–120. doi: 10.1021/acscatal.9b04768. [DOI] [Google Scholar]
- 14.Liu Y, Han S-J, Liu W-B, Stoltz B-M. Catalytic enantioselective construction of quaternary stereocenters: assembly of key building blocks for the synthesis of biologically active molecules. Acc. Chem. Res. 2015;48:740–751. doi: 10.1021/ar5004658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Yin H, Fu GC. Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins. Nature. 2018;563:379–383. doi: 10.1038/s41586-018-0669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuo J-I, Kobayashi S, Koga K. Enantioselective alkylation of lactams and lactones via lithium enolate formation using a chiral tetradentate lithium amide in the presence of lithium bromide. Tetrahedron Lett. 1998;39:9723–9726. doi: 10.1016/S0040-4039(98)02235-7. [DOI] [Google Scholar]
- 17.Groaning MD, Meyers AI. Chiral non-racemic bicyclic lactams. Auxiliary-based asymmetric reactions. Tetrahedron. 2000;56:9843–9873. doi: 10.1016/S0040-4020(00)00926-1. [DOI] [Google Scholar]
- 18.Enders D, Teschner P, Raabe G, Runsink J. Asymmetric electrophilic substitutions at the α-position of γ- and δ-lactams. Eur. J. Org. Chem. 2001;2001:4463–4477. doi: 10.1002/1099-0690(200112)2001:23<4463::AID-EJOC4463>3.0.CO;2-1. [DOI] [Google Scholar]
- 19.Amat M, Lozano O, Escolano C, Molins E, Bosch J. Enantioselective synthesis of 3,3-disubstituted piperidine derivatives by enolate dialkylation of phenylglycinol-derived oxazolopiperidone lactams. J. Org. Chem. 2007;72:4431–4439. doi: 10.1021/jo070397q. [DOI] [PubMed] [Google Scholar]
- 20.Dalpozzo R, Bartoli G, Bencivenni G. Recent advances in organocatalytic methods for the synthesis of disubstituted 2- and 3-indolinones. Chem. Soc. Rev. 2012;41:7247–7290. doi: 10.1039/c2cs35100e. [DOI] [PubMed] [Google Scholar]
- 21.Nunokawa S, Minamisawa M, Nakano K, Ichikawa Y, Kotsuki H. Asymmetric Michael addition reaction of α-aryl-substituted lactams catalyzed by chiral quaternary ammonium salts derived from cinchona alkaloids: a new short synthesis of (+)-Mesembrine. Synlett. 2015;26:2301–2305. doi: 10.1055/s-0035-1560090. [DOI] [Google Scholar]
- 22.Mukhopadhyay S, Nath U, Pan SC. Organocatalytic asymmetric synthesis of 3,3-disubstituted 3,4-dihydro-2-quinolones. Adv. Synth. Catal. 2017;359:3911–3916. doi: 10.1002/adsc.201700655. [DOI] [Google Scholar]
- 23.Inukai T, Kano T, Maruoka K. Construction of quaternary carbon center by catalytic asymmetric alkylation of 3-arylpiperidin-2-ones under phase-transfer conditions. Angew. Chem. Int. Ed. 2020;59:2211–2214. doi: 10.1002/anie.201913518. [DOI] [PubMed] [Google Scholar]
- 24.Shrestha M, Wu X, Huang W, Qu J, Chen Y. Recent advances in transition metal-catalyzed reactions of carbamoyl chlorides. Org. Chem. Front. 2021;8:4024–4045. doi: 10.1039/D0QO01648A. [DOI] [Google Scholar]
- 25.Dailler D, Rocaboy R, Baudoin O. Synthesis of β-lactams by palladium(0)-catalyzed C(sp3)−H Carbamoylation. Angew. Chem. Int. Ed. 2017;56:7218–7222. doi: 10.1002/anie.201703109. [DOI] [PubMed] [Google Scholar]
- 26.Dong W, Xu G, Tang W. Enantioselective palladium-catalyzed C(sp2)–H carbamoylation. Tetrahedron. 2019;75:3239–3247. doi: 10.1016/j.tet.2019.03.038. [DOI] [Google Scholar]
- 27.Yasui Y, Kamisaki H, Takemoto Y. Enantioselective synthesis of 3,3-disubstituted oxindoles through Pd-catalyzed cyanoamidation. Org. Lett. 2008;10:3303–3306. doi: 10.1021/ol801168j. [DOI] [PubMed] [Google Scholar]
- 28.Donets PA, Cramer N. Diaminophosphine oxide ligand enabled asymmetric nickel-catalyzed hydrocarbamoylations of alkenes. J. Am. Chem. Soc. 2013;135:11772–11775. doi: 10.1021/ja406730t. [DOI] [PubMed] [Google Scholar]
- 29.Whyte A, Burton KI, Zhang J, Lautens M. Enantioselective intramolecular copper-catalyzed borylacylation. Angew. Chem. Int. Ed. 2018;57:13927–13930. doi: 10.1002/anie.201808460. [DOI] [PubMed] [Google Scholar]
- 30.Torelli A, Whyte A, Polishchuk I, Bajohr J, Lautens M. Stereoselective construction of γ-lactams via copper-catalyzed borylacylation. Org. Lett. 2020;22:7915–7919. doi: 10.1021/acs.orglett.0c02837. [DOI] [PubMed] [Google Scholar]
- 31.Marchese AD, et al. Nickel-catalyzed enantioselective carbamoyl iodination: a surrogate for carbamoyl iodides. ACS Catal. 2020;10:4780–4785. doi: 10.1021/acscatal.0c00841. [DOI] [Google Scholar]
- 32.Fan P, Lan Y, Zhang C, Wang C. Nickel/photo-cocatalyzed asymmetric acyl-carbamoylation of alkenes. J. Am. Chem. Soc. 2020;142:2180–2186. doi: 10.1021/jacs.9b12554. [DOI] [PubMed] [Google Scholar]
- 33.Feng Z, Li Q, Chen L, Yao H, Lin A. Palladium-catalyzed asymmetric carbamoyl-carbonylation of alkenes. Sci. China Chem. 2021;64:1367–1371. doi: 10.1007/s11426-021-9992-2. [DOI] [Google Scholar]
- 34.Wu X, Qu J, Chen Y. Quinim: a new ligand scaffold enables nickel-catalyzed enantioselective synthesis of α-alkylated γ-lactam. J. Am. Chem. Soc. 2020;142:15654–15660. doi: 10.1021/jacs.0c07126. [DOI] [PubMed] [Google Scholar]
- 35.Wu X, et al. Nickel-catalyzed enantioselective reductive alkyl-carbamoylation of internal alkenes. Angew. Chem. Int. Ed. 2022;61:e202207536. doi: 10.1002/anie.202207536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu X, et al. Catalytic Desymmetric dicarbofunctionalization of unactivated alkenes. Angew. Chem. Int. Ed. 2022;61:e202111598. doi: 10.1002/anie.202111598. [DOI] [PubMed] [Google Scholar]
- 37.Luan, B., Tang, Z., Wu, X. & Chen, Y. 8-Quinolinyl oxaoline: ligand exploration in enantioselective Ni-catalyzed reductive carbamoyl-alkylation of alkene to access the chiral oxindoles. Synlett. 2022, 10.1055/a-1863-8957.
- 38.Lan Y, Wang C. Nickel-catalyzed enantioselective reductive carbo-acylation of alkenes. Commun. Chem. 2020;3:45. doi: 10.1038/s42004-020-0292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He, F., et al. Enantioselective synthesis of α-alkenylated γ-lactam enabled by Ni-catalyzed 1,4-arylcarbamoylationof 1,3-dienes. CCS Chem. 2022, 10.31635/ccschem.022.202202010.
- 40.Li Y, et al. Carbamoyl fluoride-enabled enantioselective Ni-catalyzed carbocarbamoylation of unactivated alkenes. J. Am. Chem. Soc. 2020;142:19844–19849. doi: 10.1021/jacs.0c09949. [DOI] [PubMed] [Google Scholar]
- 41.Sridharan V, Suryavanshi PA, Menéndez JC. Advances in the chemistry of tetrahydroquinolines. Chem. Rev. 2011;111:7157–7259. doi: 10.1021/cr100307m. [DOI] [PubMed] [Google Scholar]
- 42.Weintraub PM, Sabol JS, Kane JM, Borcherding DR. Recent advances in the synthesis of piperidones and piperidines. Tetrahedron. 2003;59:2953–2989. doi: 10.1016/S0040-4020(03)00295-3. [DOI] [Google Scholar]
- 43.Baldwin JE. Rules for ring closure. J. Chem. Soc. Chem. Commun. 1976;1976:734–736. doi: 10.1039/c39760000734. [DOI] [Google Scholar]
- 44.Fiser B, Cuerva JM, Gómez-Bengoa E. Baldwin-type rules for metal-controlled intramolecular migratory insertions. A computational study of Ni, Pd, and Pt case. Organometallics. 2018;37:390–395. doi: 10.1021/acs.organomet.7b00812. [DOI] [Google Scholar]
- 45.Everson DA, Weix DJ. Cross-electrophile coupling: principles of reactivity and selectivity. J. Org. Chem. 2014;79:4793–4798. doi: 10.1021/jo500507s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moragas T, Correa A, Martin R. Metal-catalyzed reductive coupling reactions of organic halides with carbonyl-type compounds. Chem. Eur. J. 2014;20:8242–8258. doi: 10.1002/chem.201402509. [DOI] [PubMed] [Google Scholar]
- 47.Gu J, Wang X, Xue W, Gong H. Nickel-catalyzed reductive coupling of alkyl halides with other electrophiles: concept and mechanistic considerations. Org. Chem. Front. 2015;2:1411–1421. doi: 10.1039/C5QO00224A. [DOI] [Google Scholar]
- 48.Diccianni JB, Diao T. Mechanisms of nickel-catalyzed cross-coupling reactions. Trends Chem. 2019;1:830–844. doi: 10.1016/j.trechm.2019.08.004. [DOI] [Google Scholar]
- 49.Poremba KE, Dibrell SE, Reisman SE. Nickel-catalyzed enantioselective reductive cross-coupling reactions. ACS Catal. 2020;10:8237–8246. doi: 10.1021/acscatal.0c01842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ping Y, Kong W. Ni-catalyzed reductive difunctionalization of alkenes. Synthesis. 2020;52:979–992. doi: 10.1055/s-0039-1690807. [DOI] [Google Scholar]
- 51.Jin Y, Wang C. Nickel-catalyzed asymmetric cross-electrophile coupling reactions. Synlett. 2020;31:1843–1850. doi: 10.1055/s-0040-1707216. [DOI] [Google Scholar]
- 52.Cherney AH, Kadunce NT, Reisman SE. Catalytic asymmetric reductive acyl cross-coupling: synthesis of enantioenriched acyclic α,α-disubstituted ketones. J. Am. Chem. Soc. 2013;135:7442–7445. doi: 10.1021/ja402922w. [DOI] [PubMed] [Google Scholar]
- 53.Qi X, Diao T. Nickel-catalyzed dicarbofunctionalization of alkenes. ACS Catal. 2020;10:8542–8556. doi: 10.1021/acscatal.0c02115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luo Y-C, Xu C, Zhang X. Nickel-catalyzed dicarbofunctionalization of alkenes. Chin. J. Chem. 2020;38:1371–1394. doi: 10.1002/cjoc.202000224. [DOI] [Google Scholar]
- 55.Wang K, Ding Z, Zhou Z, Kong W. Ni-catalyzed enantioselective reductive diarylation of activated alkenes by domino cyclization/cross-coupling. J. Am. Chem. Soc. 2018;140:12364–12368. doi: 10.1021/jacs.8b08190. [DOI] [PubMed] [Google Scholar]
- 56.Jin Y, Wang C. Nickel-catalyzed asymmetric reductive arylalkylation of unactivated alkenes. Angew. Chem. Int. Ed. 2019;58:6722–6726. doi: 10.1002/anie.201901067. [DOI] [PubMed] [Google Scholar]
- 57.Tian Z-X, et al. Highly enantioselective cross-electrophile aryl-alkenylation of unactivated alkenes. J. Am. Chem. Soc. 2019;141:7637–7643. doi: 10.1021/jacs.9b03863. [DOI] [PubMed] [Google Scholar]
- 58.Ping Y, et al. Ni-catalyzed regio- and enantioselective domino reductive cyclization: one-pot synthesis of 2,3-fused cyclopentannulated indolines. ACS Catal. 2019;9:7335–7342. doi: 10.1021/acscatal.9b02081. [DOI] [Google Scholar]
- 59.He J, et al. Nickel-catalyzed asymmetric reductive 1,2-carboamination of unactivated alkenes. Angew. Chem. Int. Ed. 2020;59:2328–2332. doi: 10.1002/anie.201913743. [DOI] [PubMed] [Google Scholar]
- 60.He Y, Zhu S. Quinim ligand-enabled Ni-catalyzed asymmetric 1,2-carbamoyl-alkylation of unactivated alkenes. Chin. J. Org. Chem. 2020;40:4377–4379. doi: 10.6023/cjoc202000088. [DOI] [Google Scholar]
- 61.Lin Z, Jin Y, Hu W, Wang C. Nickel-catalyzed asymmetric reductive aryl-allylation of unactivated alkenes. Chem. Sci. 2021;12:6712–6718. doi: 10.1039/D1SC01115D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen X-W, et al. Nickel-catalyzed asymmetric reductive carbo-carboxylation of alkenes with CO2. Angew. Chem. Int. Ed. 2021;60:14068–14075. doi: 10.1002/anie.202102769. [DOI] [PubMed] [Google Scholar]
- 63.Fang K, Huang W, Shan C, Qu J, Chen Y. Synthesis of 3,3-dialkyl-Substituted isoindolinones enabled by nickel-catalyzed reductive dicarbofunctionalization of enamides. Org. Lett. 2021;23:5523–5527. doi: 10.1021/acs.orglett.1c01871. [DOI] [PubMed] [Google Scholar]
- 64.Qiao J-B, et al. Enantioselective reductive divinylation of unactivated alkenes by nickel-catalyzed cyclization coupling reaction. J. Am. Chem. Soc. 2021;143:12961–12967. doi: 10.1021/jacs.1c05670. [DOI] [PubMed] [Google Scholar]
- 65.Sun Y, et al. Facile preparation of aryl C-glycosides by nickel-catalyzed reductive coupling of glycosyl halides with aryl halides. Chin. J. Org. Chem. 2021;41:1551–1562. doi: 10.6023/cjoc202010027. [DOI] [Google Scholar]
- 66.Zhao T-Y, Xiao L-J, Zhou Q-L. Nickel-catalyzed desymmetric reductive cyclization/coupling of 1,6-dienes: an enantioselective approach to chiral tertiary alcohol. Angew. Chem. Int. Ed. 2022;61:e202115702. doi: 10.1002/anie.202115702. [DOI] [PubMed] [Google Scholar]
- 67.Wu X-Y, Li X-H, Zhou Q-L. Chiral quinolinyl-oxazolines as ligands for copper(I)-catalyzed asymmetric cyclopropanation. Tetrahedron.: Asymmetry. 1998;9:4143–4150. doi: 10.1016/S0957-4166(98)00455-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The crystallographic data for compound 3 y is available from the Cambridge Crystallographic Data Center under deposition numbers CCDC 2158471 respectively (https://www.ccdc.cam.ac.uk/structures/). All other data to support the conclusions are available in the main text or the Supplementary Information. (See Supplementary Method).