Abstract
Background
This study strived to explore the role and mechanism of glucagon‐like peptide‐1 receptor (GLP1R) in endometrial carcinoma (EC).
Methods
In detail, after transfection of GLP1R overexpression vector and small interfering RNA targeting PKA, the mRNA expressions of GLP1R and PKA in EC cells (Ishikawa and RL95‐2) were quantified by quantitative reverse transcription polymerase chain reaction (qRT‐PCR). The cell biological behaviors, including proliferation, migration, invasion, and apoptosis, were detected using 5‐ethynyl‐2′‐deoxyuridine (EdU), wound healing, transwell, and flow cytometry assays, respectively. The cyclic adenosine monophosphate (cAMP) content and related protein expressions (GLP1R, p‐PKA, and PKA) were determined by enzyme‐linked immunosorbent assay (ELISA) and western blot. The effects of GLP1R and PKA on tumorigenesis were evaluated by measuring the tumor volume and weight of mice bearing EC.
Result
According to the results, GLP1R expression was downregulated in EC tissues and cells, and there was a positive correlation between GLP1R and PKA expressions. Upregulation of GLP1R promoted apoptosis and activated the cAMP/PKA signaling pathway in EC cells, while hindering the EC cell proliferation, invasion, migration, and the growth of tumor in mice. However, these effects were blunted by downregulation of PKA, which also accelerated the progression of EC in vitro and in vivo via inhibiting the activation of cAMP/PKA signaling pathway.
Conclusion
Collectively, upregulation of GLP1R impeded EC progression via inducing the activation of cAMP/PKA signaling pathway, which may be a potential treatment for EC.
Keywords: cyclic adenosine monophosphate, endometrial carcinoma, glucagon‐like peptide‐1 receptor, protein kinase A
Glucagon‐like peptide‐1 receptor (GLP1R) expression was reduced in EC cells, and its upregulation played a inhibiting role on the malignant behaviors of EC cells and in vivo tumorigenesis by activating cAMP/PKA signaling pathway, while si‐PKA could reversed its above effects.
1. INTRODUCTION
Endometrial carcinoma (EC) refers to the malignant transformation of cells derived from the endometrium. 1 It ranks second among the most common female reproductive system tumors and fourth among female malignant tumors in developed countries, threatening the health of women all over the world. 2 EC comprises about 5% of cancer cases and 2% of deaths in female cancer patients. 3 EC has a predilection for perimenopausal women, and people with obesity, diabetes, hypertension, non‐pregnancy, and long‐term non‐ovulation are all high‐risk groups. More than 90% of EC patients are over the age of 50 years, but still 4% of the patients develop the disease before the age of 40. 4 , 5 Nearly 80% of EC patients are diagnosed at an early stage and have an overall good prognosis, but about 20% will eventually die of the disease. 6 Despite that most EC cases are effectively controlled by comprehensive treatments such as surgery, radiotherapy, chemotherapy and hormones, the prognosis of EC has not been improved significantly. 7 Therefore, intensive investigations on the mechanism underlying the occurrence and development of EC are warranted, which may be instrumental for the prevention, diagnosis and treatment of the disease at an earlier stage.
Recently, glucagon‐like peptide‐1 receptor (GLP1R) agonist has been reported to induce autophagy of EC cells, and high GLP1R expression may be associated with good prognosis of EC patients, 8 suggesting that GLP1R is likely to participate in the progression of EC. GLP1R, the primary target of GLP‐1, is localized on the cell surface. 9 GLP‐1 is an incretin and implicated in the regulation of islet cell function and blood glucose homeostasis in vivo by binding to GLP1R. 10 A previous study identified the cytostatic action and anti‐tumor effect of liraglutide (an analog of GLP‐1) on EC cells. 11 GLP1R is a class of G protein‐coupled receptor involved in metabolism, which is widely distributed in pancreatic islets, muscles, gastrointestinal tract, lung, liver, pancreas and other tissues or organs. 12 The main roles of GLP1R lie in the control of islet function and the regulation of blood glucose, 13 which are achieved by activating the downstream cyclic adenosine monophosphate (cAMP)‐protein kinase A (PKA) signaling pathway, after GLP1 binds to GLP1R to promote the expressions of assorted genes related to gluconeogenesis, glycogenolysis, and fatty acid metabolism. 14 Although the role of GLP1R in promoting autophagy in EC has been demonstrated, its specific regulatory mechanism remains dim.
Some scientific literatures verified that CRH activates cAMP/PKA pathway to inhibit the growth of human EC cell and the stabilizing effects of GLP‐1 on vascular endothelial barrier and vasoconstriction in diabetes are achieved by the activation of GLP1R/cAMP/PKA pathway. 15 , 16 Also, the existing finding reveals that GLP1R overexpression notably activates cAMP/PKA pathway in EC cells. Given the above information, we hypothesized that GLP1R could promote apoptosis in EC by activating the cAMP/PKA pathway.
2. MATERIALS AND METHODS
2.1. Animals and ethics statement
A total of 40 BALB/c nude mice (6–8 weeks old) were supplied by Vital River Laboratories (Beijing, China). Before experiment, mice were reared with free access to food and water under a comfortable environment, where the room temperature was maintained at 21 ± 0.5°C and humidity was set at 45%–50%, with lights on at 7 A.M. and off at 7 P.M. About 3 days after acclimation, the experiments on mice were started. All animal experiments were performed in Zhejiang Academy of Traditional Chinese Medicine and permitted by the Laboratory Animal Welfare Ethics Committee, Zhejiang Academy of Traditional Chinese Medicine ([2020] No. 001). All the procedures were in accordance with the guidelines of the China Council on Animal Care and Use. Every effort was made to minimize the pain and discomfort to the animals.
2.2. Cell culture
Human endometrial epithelial cells (hEECs, CP‐H058), human EC cell lines (Ishikawa (CL‐0283) and RL95‐2 (CL‐0197)), and the culture media were purchased from Procell in China. HEECs were incubated in hEEC complete culture medium (CM‐H058). Ishikawa cells were cultivated in Dulbecco's Modified Eagle Medium (DMEM, 30–2007) supplemented with 10% fetal bovine serum (FBS, 164210–500, Procell) and 1% penicillin–streptomycin solution (P/S, PB180120, Procell). RL95‐2 cells were cultured in DMEM/F12 medium (PM150312) blended with 5 μg/mL insulin (I9278, Sigma‐Aldrich), 10% FBS and 1% P/S. All cells were incubated at 37°C with 5% CO2.
2.3. Gene profiling analysis
The correlation between the expressions of GLP1R and PKA was retrieved from Tumor IMmune Estimation Resource (TIMER) database (http://cistrome.dfci.harvard.edu/TIMER/).
2.4. Transfection
Glucagon‐like peptide‐1 receptor was amplified and inserted into the pCMV6 vector (PS100001, OriGene) to obtain pCMV6‐GLP1R overexpression vector. The small interference RNA targeting PKA (si‐PKA, A01002, CTCACTCACCTCTCTCACCTATG) and the negative control (si‐NC, A06001) were synthesized by GenePharma (China). The pCMV6‐GLP1R, pCMV6 empty vector (negative control), si‐PKA and si‐NC were transfected into Ishikawa and RL95‐2 cells using Lipo6000 transfection reagent (C0526, Beyotime) following the manufacturer's instructions. Finally, the transfection efficiency was analyzed by quantitative reverse transcription polymerase chain reaction (qRT‐PCR).
2.5. Experimental grouping
Ishikawa and RL95‐2 cells were divided into the following groups: control (Con) group (normal cells), vector group (pCMV6 empty vector‐transfected cells), OE‐GLP1R group (pCMV6‐GLP1R overexpression vector‐transfected cells), si‐NC group (cells transfected with si‐NC), si‐PKA group (cells transfected with si‐PKA), and si‐PKA + OE‐GLP1R group (cells transfected with si‐PKA and pCMV6‐GLP1R overexpression vector). In addition, the mice were assigned into the Con, OE‐GLP1R, si‐PKA and si‐PKA + OE‐GLP1R groups, with 5 mice in each group.
2.6. QRT‐RCR
Total RNA of cells was extracted by RNeasy Plus Mini kit (74,136, Qiagen), and reversely transcribed to cDNA using High Capacity RNA‐to‐cDNA Kit (4,387,406, Life Technologies), in light of the manufacturer's instructions. The mRNA expression of GLP1R was quantified by Fast Start Universal SYBR Green Master (4,913,850,001, Roche) using an ABI7900‐HT‐Fast device (Applied Biosystems) as per the protocols of manufacturer. The sequences of the forward (F) and reversed (R) primers from 5′ to 3′ are listed as follows: GLP1R (F: GCGCTCCCTGACTGAGGAT; R: GAAGGTCCGGTTGCAGAACA); β‐actin (F: GGGAAATCGTGCGTGACATTAAG; R: TGTGTTGGCGTACAGGTCTTTG). β‐actin was served as the endogenous control and the expression level was calculated according to the 2‐ΔΔCT method. 17
2.7. 5‐ethynyl‐2′‐deoxyuridine (EdU) assay
Proliferation of Ishikawa and RL95‐2 cells was detected by BeyoClick EdU‐594 cell proliferation detection kit (C0071S, Beyotime). After cells (1 × 105 cells/well in a 6‐well plate) were incubated overnight, the abovementioned treatment procedures were conducted. Then, the cell culture medium was replaced with EdU medium (10 μM), and the cells were continually cultivated for 2 h. Next, the cells were harvested and treated by 4% paraformaldehyde (P6148, Sigma‐Aldrich) and immunostaining strong permeabilization solution (P0097, Beyotime), followed by the staining with EdU fluorescent dye and 4′,6‐diamidino‐2‐phenylindole (DAPI; C1002, Beyotime). Finally, the cell proliferation was observed under a XSP‐63XDV fluorescence microscope (200×, Shanghai optical instrument factory).
2.8. Wound healing assay
Ishikawa and RL95‐2 cells were planted in a 6‐well plate at a density of 5 × 104 cells/well, and cultured to 80%–90% confluence. Cell monolayers were then scratched every 0.5 cm with a pipette tip, after which the cells were incubated for another 24 h. Images of wounds and migrated cells were taken by an Olympus BX53M microscope (100×, Japan).
2.9. Transwell assay
The invasion of cells was determined using a 24‐well Transwell chamber pre‐coated with Matrigel with 8 μm pores (Cornin). In brief, 3 × 104 Ishikawa and RL95‐2 cells in serum‐free medium were added to the upper chamber, while the medium with 10% FBS was put into the lower compartment. 24 h of culture later, the cells that had invaded into the lower chamber were fixed with 4% paraformaldehyde, dyed with 0.5% crystal violet solution (ab246820, Abcam) and observed under the microscope (250×).
2.10. Flow cytometry
The determination of cell apoptosis was conducted with the help of Annexin V‐FITC/propidium iodide (PI) Cell Apoptosis Detection Kit (FA101‐01, Transgen). Ishikawa and RL95‐2 cells were seeded into a 6‐well plate and cultured for 48 h. Afterwards, cells were suspended in 100 μl cell solution at a concentration of 2 × 104 cells/ml, and then added with 5 μl Annexin V‐FITC and PI, followed by the culture for 15 min at 37°C in the dark. In the end, cell apoptosis was analyzed by a flow cytometer (Beckman Coulter).
2.11. Enzyme‐linked immunosorbent assay (ELISA)
The content of cAMP in Ishikawa and RL95‐2 cells was assessed using the cAMP ELISA Kit (ab133051, Abcam) according to the specification of manufacturer. Briefly, the transfected EC cells were lysed using 0.1 M HCl lysis buffer. The 96‐well plate was coated with goat anti‐rabbit IgG antibody that could bind with anti‐cAMP antibody. Alkaline phosphatase (AP)‐labeled cAMP was then introduced to each well of the plate to induce the competition between the AP‐labeled cAMP and the free cAMP. After washing, the amount of bound AP‐labeled cAMP was detected using the colorimetric AP substrate pNpp, followed by the measurement by a microplate reader at a wavelength of 405 nm (VL0000D2, Thermo Fisher).
2.12. Western blot
The procedures of western blot were performed as described previously. 18 Briefly, the EC cells were lysed and total protein was extracted by Radio‐Immunoprecipitation Assay (RIPA) lysis buffer (P0013K, Beyotime). After quantification of protein concentration, equal amounts of marker (5 μl; PR1910, Solarbio) and protein (45 μg) were fractionated by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred onto the polyvinylidene fluoride (PVDF) membranes (IPFL00010, Millipore). Subsequently, the membranes were blocked and cultured with primary antibodies at 4°C overnight, including those against GLP1R (1:500; Rabbit; bs‐1559R, 51 kDa, Bioss), phosphorylated‐PKA (p‐PKA; 1:1000; Rabbit; ab32390, 45 kDa, Abcam), PKA (1:10000; Rabbit; ab32514, 45 kDa, Abcam), and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH; 1:1000; Mouse; ab8245, 37 kDa). After being washed by tris‐buffered saline tween (TBST) buffer, the membranes were incubated with goat anti‐rabbit (1:2000, ab6721, Abcam) and rabbit anti‐mouse (1:3000, ab6728, Abcam) secondary antibodies for 2 h. The relative intensity of the protein bands was quantified using Tanon 5200 Imaging System (Tanon), with GAPDH serving as the loading control.
2.13. Xenograft tumor growth in nude mice
For tumor studies, cells (6 × 106 cells/mouse) after assorted treatments were subcutaneously injected into the BALB/c nude mice with reference to the previous study. 19 4 weeks later, all the mice were put into death by cervical dislocation, with their tumors harvested. Finally, the tumor weight was measured and the volume was calculated with the following formula: tumor size (mm3) = length (mm) × width (mm2) / 2.
2.14. Statistical analysis
The measurement data were expressed as mean ± standard deviation. One‐way analysis of variance (ANOVA) was utilized for comparisons among multiple groups. The statistical analysis was implemented by GraphPad 8.0 software, with statistical significance established at p < 0.05.
3. RESULTS
3.1. GLP1R expression was downregulated in EC cells
The expression of GLP1R was lower in EC cells (Ishikawa and RL95‐ 2) than in hEECs (p < 0.001, Figure 1A). To investigate the roles of GLP1R and PKA in EC cells, Ishikawa and RL95‐2 cells were transfected with GLP1R overexpression vector and si‐PKA. Then, the upregulation of GLP1R and the downregulation of PKA in EC cells were observed, which were indicative of the successful transfection (p < 0.001, Figure 1B–E).
FIGURE 1.
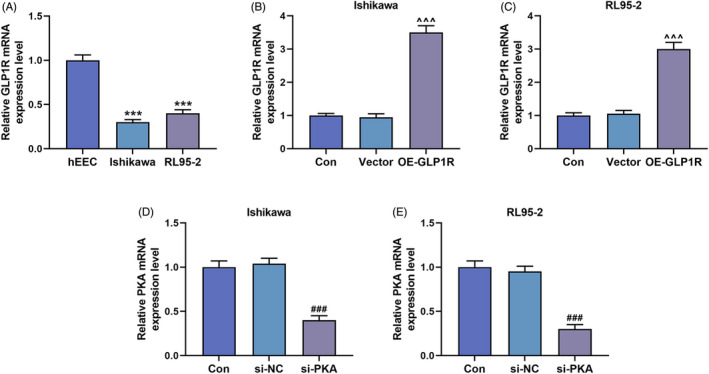
The expressions of GLP1R and PKA in EC cells. (A) The expression of GLP1R in hEEC, Ishikawa cells and RL95‐2 cells was quantified by qRT‐PCR, with β‐actin serving as the internal reference. (B and C) GLP1R expression in Ishikawa and RL95‐2 cells of the Con, Vector and OE‐GLP1R groups was also detected by qRT‐PCR, with β‐actin serving as the internal reference. (D and E) QRT‐PCR was used to determine the expression of PKA in the Con, si‐NC and si‐PKA groups, with β‐actin serving as the internal reference. All experiments were repeated three times to obtain average values. The data from three independent experiments were presented as the mean ± SD; ***p < 0.001 versus hEEC; ^^^ p < 0.001 versus Vector; ### p < 0.001 versus si‐NC. Con, control; EC, endometrial carcinoma; GLP1R, glucagon‐like peptide‐1 receptor; hEEC, human endometrial epithelial cell; OE‐GLP1R, GLP1R overexpression; PKA, protein kinase A; qRT‐PCR, quantitative reverse transcription polymerase chain reaction; SD, standard deviation; si‐NC, siRNA negative control; si‐PKA, small interference RNA targeting PKA.
3.2. Silencing of PKA reversed the inhibitory effect of overexpressed GLP1R on EC cell biological behaviors
Based on the biological behaviors of EC cells determined by EdU, wound healing, Transwell and flow cytometry assays, the proliferation, migration and invasion were observed. GLP1R overexpression inhibited the proliferation of Ishikawa and RL95‐2 cells, while silencing of PKA promoted the proliferation of Ishikawa and RL95‐2 cells, and reversed the inhibitory effect of overexpressed GLP1R on the proliferation of Ishikawa and RL95‐2 cells (Figure 2A,B). In addition, the migration (Figure 3A–D) and invasion (Figure 4A–D) were observed to be inhibited in the Ishikawa and RL95‐2 cells that were transfected with GLP1R overexpression vector (p < 0.01). Conversely, the transfection of si‐PKA resulted in a dramatic increase of cell migration and invasion abilities (p < 0.01, Figure 3A–D and Figure 4A–D). Furthermore, the cell migration and invasion abilities in the si‐PKA + OE‐GLP1R group were higher than those in the OE‐GLP1R group and lower than those in the si‐PKA group (p < 0.01, Figure 3A–D and Figure 4A–D). GLP1R overexpression promoted the apoptosis of Ishikawa and RL95‐2 cells, while silencing of PKA inhibited the apoptosis of Ishikawa and RL95‐2 cells (p < 0.01, Figure 4E–H). The apoptosis rate in the si‐PKA + OE‐GLP1R group were lower than those in the OE‐GLP1R group and higher than those in the si‐PKA group (p < 0.01, Figure 4E–H). These data indicated that silencing of PKA reversed the effects of GLP1R overexpression on biological behaviors of Ishikawa and RL95‐2 cells.
FIGURE 2.
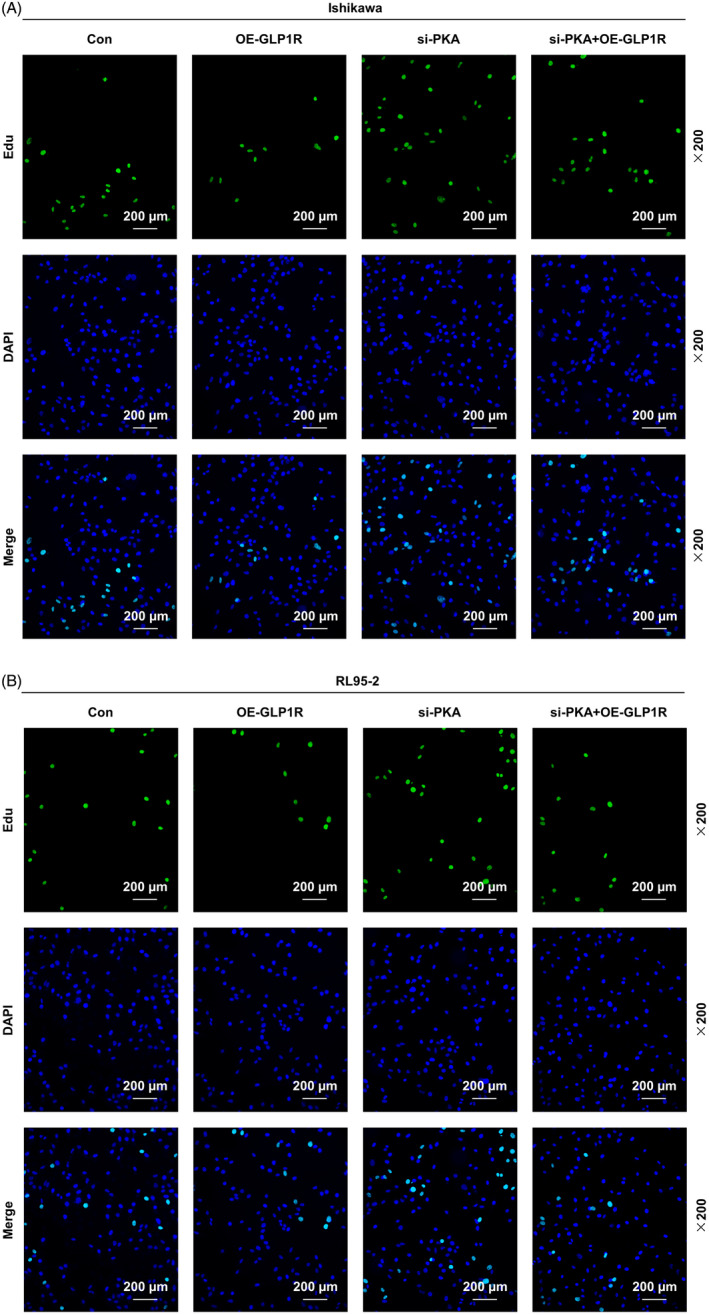
The effects of GLP1R and PKA on EC cell proliferation. (A and B) The proliferation of Ishikawa and RL95‐2 cells in the Con, OE‐GLP1R, si‐PKA and si‐PKA + OE‐GLP1R groups was measured by EdU assay (magnification 200×). Scale bar = 200 μm. Con, control; EC, endometrial carcinoma; EdU, 5‐ethynyl‐2′‐deoxyuridine; GLP1R, glucagon‐like peptide‐1 receptor; OE‐GLP1R, GLP1R overexpression; PKA, protein kinase A; si‐PKA, small interference RNA targeting PKA.
FIGURE 3.
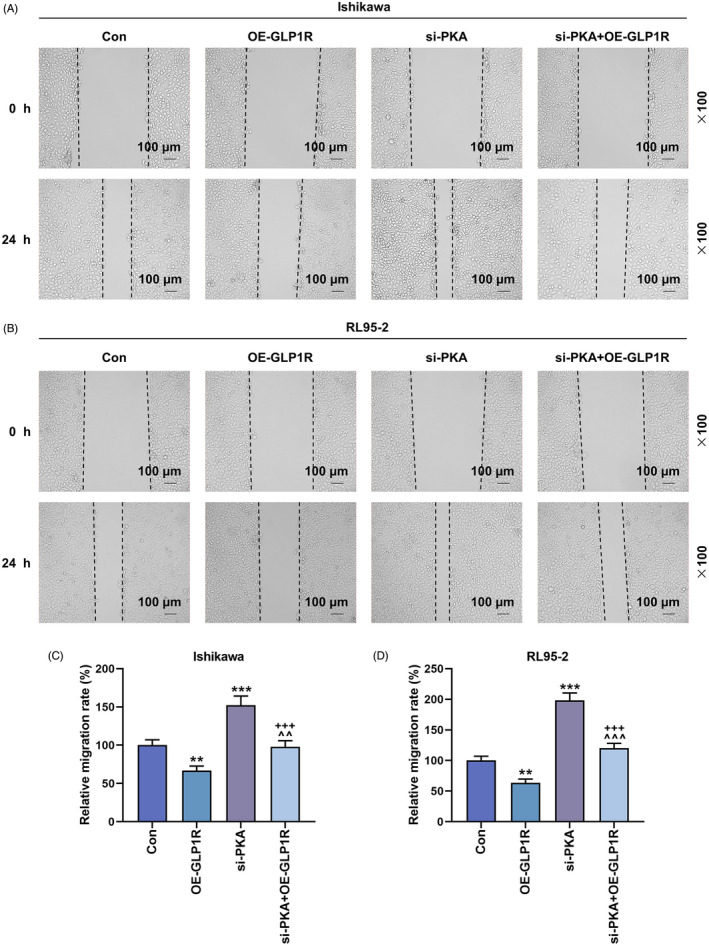
The effects of GLP1R and PKA on EC cell migration. (A–D) The migration of Ishikawa and RL95‐2 cells in the Con, OE‐GLP1R, si‐PKA and si‐PKA + OE‐GLP1R groups was evaluated by wound healing assay (magnification 100×). Scale bar = 100 μm. All experiments were repeated three times to obtain average values. The data from three independent experiments were presented as the mean ± SD; **p < 0.01; ***p < 0.001 versus Con; ^^ p < 0.01; ^^^ p < 0.001 versus OE‐GLP1R; +++ p < 0.001 versus si‐PKA. Con, control; EC, endometrial carcinoma; GLP1R, glucagon‐like peptide‐1 receptor; OE‐GLP1R, GLP1R overexpression; PKA, protein kinase A; SD, standard deviation; si‐PKA, small interference RNA targeting PKA.
FIGURE 4.
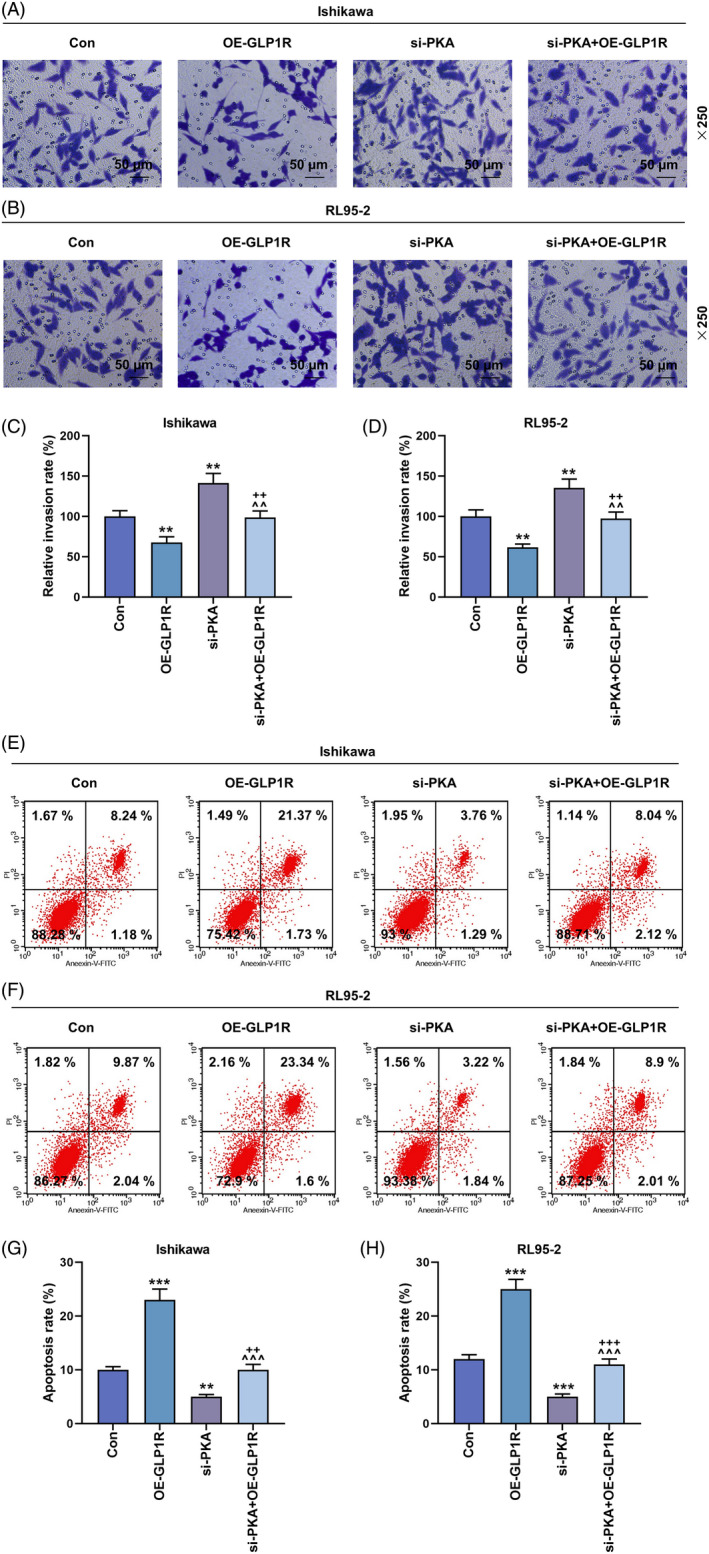
The effects of GLP1R and PKA on the invasion and apoptosis of EC cells. (A–D) Transwell assay was performed to measure the invasion of Ishikawa and RL95‐2 cells in the Con, OE‐GLP1R, si‐PKA and si‐PKA + OE‐GLP1R groups (magnification 250×). Scale bar = 50 μm. (E–H) The apoptosis of Ishikawa and RL95‐2 cells was determined by flow cytometry. All experiments were repeated three times to obtain average values. The data from three independent experiments were presented as the mean ± SD; **p < 0.01; ***p < 0.001 versus Con; ^^ p < 0.01; ^^^ p < 0.001 versus OE‐GLP1R; ++ p < 0.01, +++ p < 0.001 versus si‐PKA. Con, control; EC, endometrial carcinoma; GLP1R, glucagon‐like peptide‐1 receptor; OE‐GLP1R, GLP1R overexpression; PKA, protein kinase A; SD, standard deviation; si‐PKA, small interference RNA targeting PKA.
3.3. Overexpression of GLP1R activated the cAMP/PKA signaling pathway in vitro and reduced the tumor growth in vivo, but these effects were offset by si‐PKA
Bioinformatics analysis revealed that the expression of GLP1R was positively correlated with that of PKA (rho = 0.181, p = 2.05e‐05, Figure 5A). After overexpression of GLP1R, the concentration of cAMP in Ishikawa and RL95‐2 cells was largely increased (p < 0.001, Figure 5B,C). In contrast, silencing of PKA lessened cellular cAMP concentration (p < 0.05, Figure 5B,C), and also countervailed the upregulation of cAMP induced by GLP1R overexpression (p < 0.001, Figure 5B,C). Similarly, the rise and fall of GLP1R and p‐PKA/PKA protein expressions were separately generated by transfection of GLP1R overexpression vector and si‐PKA (p < 0.05, Figure 5D–I), and silencing of PKA neutralized the effect of GLP1R overexpression (p < 0.01, Figure 5D–I). Besides, tumor volume and weight were diminished with the transfection of GLP1R overexpression vector, but augmented post transfection of si‐PKA (p < 0.01, Figure 6A–E). Moreover, the inhibition of tumor growth by overexpressed GLP1R was reversed by silencing of PKA (p < 0.001, Figure 6A–E).
FIGURE 5.
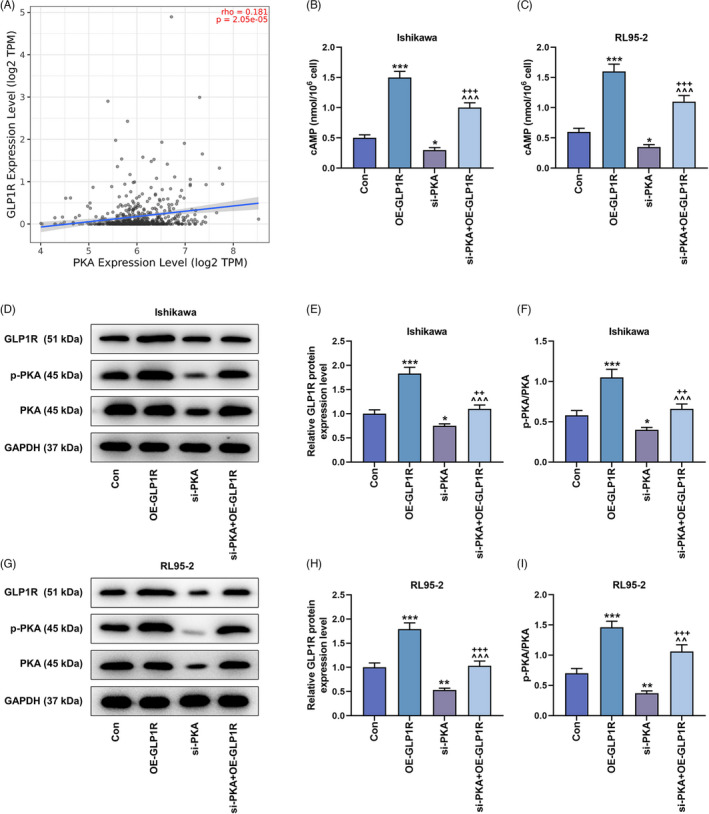
The effects of GLP1R and PKA on the cAMP/PKA signaling pathway in EC cells. (A) The correlation between the expressions of GLP1R and PKA was analyzed by TIMER database (http://cistrome.dfci.harvard.edu/TIMER/), rho = 0.181, p = 2.05e‐05. (B and C) The concentration of cAMP in Ishikawa and RL95‐2 cells was accessed by ELISA. (D–I) The detection of protein expressions (GLP1R, p‐PKA and PKA) in the Con, OE‐GLP1R, si‐PKA and si‐PKA + OE‐GLP1R groups was conducted by western blot, with GAPDH serving as the internal reference. All experiments were repeated three times to obtain average values. The data from three independent experiments were presented as the mean ± SD; *p < 0.05, **p < 0.01; ***p < 0.001 versus Con; ^^ p < 0.01; ^^^ p < 0.001 versus OE‐GLP1R; ++ p < 0.01, +++ p < 0.001 versus si‐PKA. GLP1R, glucagon‐like peptide‐1 receptor; cAMP, cyclic adenosine monophosphate; Con, control; EC, endometrial carcinoma; ELISA, enzyme‐linked immunosorbent assay; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; OE‐GLP1R, GLP1R overexpression; PKA, protein kinase A; SD, standard deviation; si‐PKA, small interference RNA targeting PKA.
FIGURE 6.
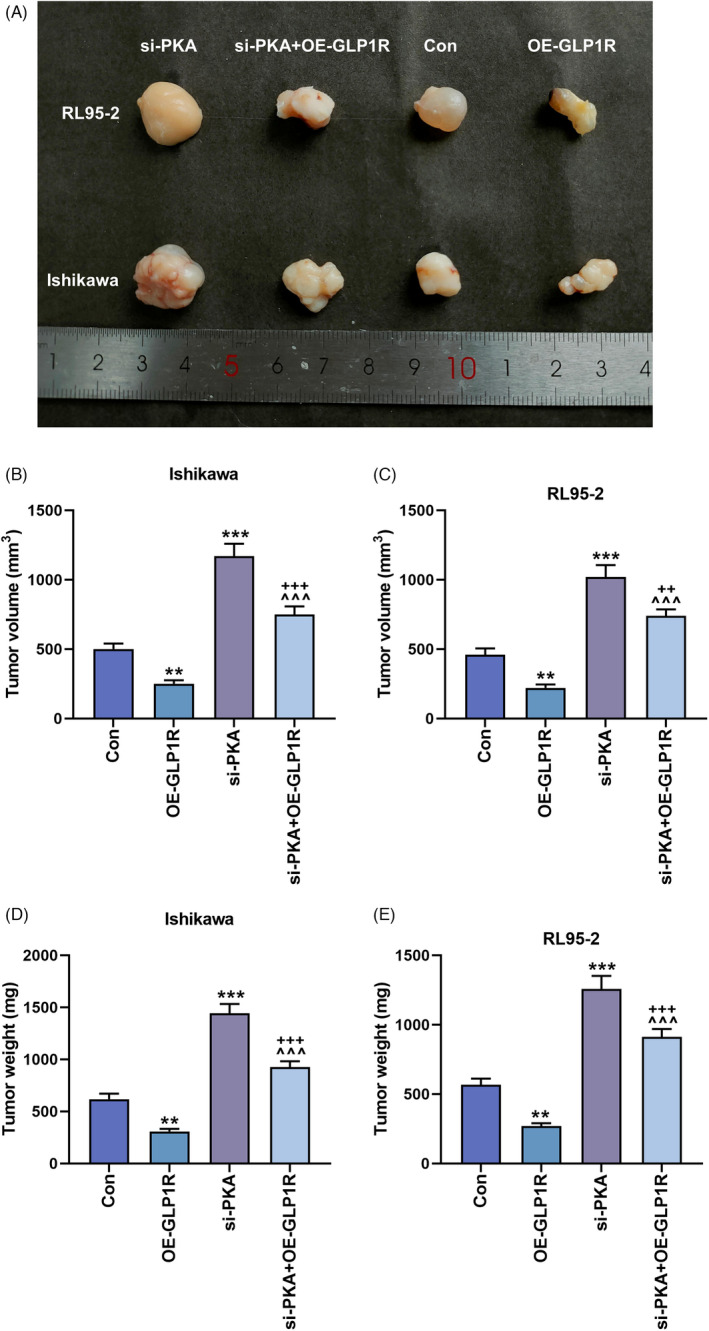
The effects of GLP1R and PKA on the tumor growth in mice. (A–E) The tumor‐initiating ability was evaluated by comparing the tumor volume and weight in control, OE‐GLP1R, si‐PKA and si‐PKA + OE‐GLP1R groups. **p < 0.01; ***p < 0.001 versus Con; ^^^ p < 0.001 versus OE‐GLP1R; ++ p < 0.01, +++ p < 0.001 versus si‐PKA. Con, control; GLP1R, glucagon‐like peptide‐1 receptor; OE‐GLP1R, GLP1R overexpression; PKA, protein kinase A; si‐PKA, small interference RNA targeting PKA.
4. DISCUSSION
Endometrial carcinoma is often associated with endocrine and metabolic diseases, such as obesity, hypertension and diabetes. 20 A meta‐analysis by Lotfolah Saed et al. 21 has demonstrated that compared with women without diabetes, the risk of EC is increased by 72% in women with diabetes mellitus, implying that diabetes may elevate the risk of EC in women. Although the underlying mechanisms for the association between diabetes and EC are not fully uncovered, a prior research has revealed that insulin resistance, hyperinsulinemia, hyperglycemia, inflammation, and disturbance of the insulin‐like growth factor 1 pathway may contribute to the development of EC in patients with diabetes. 22 In the present study, we focused on the key molecules involved in the cancer progression from the perspective of metabolic syndrome‐related EC.
A relevant study has proved that GLP‐1 can induce insulin secretion and enhance the sensitivity of insulin secretion β cells to glucose, which is mediated by GLP1R on cell membrane. 23 Besides, exenatide (a GLP1R agonist) reduces the viability and proliferation of Ishikawa cells but induces cell apoptosis, and it also inhibits the growth of EC xenografts in nude mice. 19 These literatures have illustrated the involvement of GLP1R in both diabetes and EC, signifying that GLP1R may be a potential target for EC. In this study, we observed notable downregulation of GLP1R expression in EC cells, which was in accordance with the previous study. GLP1R overexpression attenuated the proliferation, migration and invasion of EC cells, whereas promoting their apoptosis. Additionally, in vivo experiments revealed that overexpressed GLP1R suppressed the growth of transplanted tumor in nude mice and also decreased the tumor volume and weight.
On the basis of a previous literature, 16 we postulated that GLP1R may exert its tumor‐suppressive function in EC through the downstream cAMP/PKA pathway. The cAMP/PKA pathway is a classical cell signaling pathway mediated by G protein‐coupled receptors, via which the stimulation of membranous bodies induced by external signaling molecules activates the adenylyl cyclase in cells, leading to an increased intracellular cAMP level, so as to activate PKA and produce a series of biological reactions. 24 This pathway has also been confirmed to be involved in cell proliferation, differentiation and apoptosis, ion transport, regulation of metabolism and gene transcription. 25 As the downstream molecule of cAMP, PKA is composed of catalytic subunit and regulatory subunit. 26 , 27 The catalytic subunit can mediate the phosphorylation of various substrate proteins, while the two subtypes of regulatory subunit, RI and RII, are mainly implicated in cell proliferation and differentiation. 28 Once external factors lead to the dominant expression of type RI subunit in cells, cancer cells will proliferate in an unlimited manner, thus enabling tumor formation. 29 Ample evidence has highlighted that upregulation of GLP1R generates the neuroprotective effect and could inhibit the serum deprivation‐induced apoptosis of MC3T3‐E1 cells through promoting the activation of the cAMP/PKA pathway. 30 , 31 In the current study, it is worth noting that GLP1R positively regulated PKA level and activated the cAMP/PKA signaling pathway, while silenced PKA reversed the function of GLP1R overexpression in EC and thereby facilitated the cancer progression in vivo and in vitro.
In summary, upregulated GLP1R is able to block the growth of EC cells via activating the cAMP/PKA signaling pathway. This study digs into the pathogenesis of EC in a different insight, providing a new clue for the establishment of EC therapeutic regimens. However, several limitations need to be optimized in our study. For instance, since only two endometrial cancer cells are selected, the effects of GLP1R on other EC cells cannot be determined; and the animal experiments are not comprehensive enough.
AUTHOR CONTRIBUTIONS
Wu Li provided substantial contributions to conception and design and drafting the article or critically revising it for important intellectual content. Yanpin Gu, Songjun Liu, Fan Ruan, and Wen Lv involved in data acquisition, data analysis, and interpretation. Wu Li, Yanpin Gu, Songjun Liu, Fan Ruan, and Wen Lv gave the final approval of the version to be published and provided the agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
FUNDING INFORMATION
This work was supported by the Basic Public Welfare Research Project of Zhejiang Province [LGF20H160013]; the Medical and Health Science and Technology Project of Zhejiang Province [2018ZD016].
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Li W, Gu Y, Liu S, Ruan F, Lv W. GLP1R inhibits the progression of endometrial carcinoma through activation of cAMP/PKA pathway. J Clin Lab Anal. 2022;36:e24604. doi: 10.1002/jcla.24604
DATA AVAILABILITY STATEMENT
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15(7):e268‐e278. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7‐34. [DOI] [PubMed] [Google Scholar]
- 3. Clarke MA, Long BJ, Morillo AD, Arbyn M, Bakkum‐Gamez JN, Wentzensen N. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta‐analysis. JAMA Intern Med. 2018;178(9):1210‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soslow RA, Tornos C, Park KJ, et al. Endometrial carcinoma diagnosis: use of FIGO grading and genomic subcategories in clinical practice: recommendations of the International Society of Gynecological Pathologists. Int J Gynecol Pathol. 2019;38 Suppl 1(Iss 1 Suppl 1):S64‐S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sorosky JI. Endometrial cancer. Obstet Gynecol. 2012;120(2 Pt 1):383‐397. [DOI] [PubMed] [Google Scholar]
- 6. Huvila J, Pors J, Thompson EF, Gilks CB. Endometrial carcinoma: molecular subtypes, precursors and the role of pathology in early diagnosis. J Pathol. 2021;253(4):355‐365. [DOI] [PubMed] [Google Scholar]
- 7. Tran AQ, Gehrig P. Recent advances in endometrial cancer. F1000Res. 2017;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kanda R, Hiraike H, Wada‐Hiraike O, et al. Expression of the glucagon‐like peptide‐1 receptor and its role in regulating autophagy in endometrial cancer. BMC Cancer. 2018;18(1):657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Almutairi M, Al Batran R, Ussher JR. Glucagon‐like peptide‐1 receptor action in the vasculature. Peptides. 2019;111:26‐32. [DOI] [PubMed] [Google Scholar]
- 10. Wang M. GLP1 fragments protect the kidney. Nat Rev Nephrol. 2018;14(10):599. [DOI] [PubMed] [Google Scholar]
- 11. Zhu XX, Feng ZH, Liu LZ, Zhang Y. Liraglutide suppresses the proliferation of endometrial cancer cells through the adenosine 5′‐monophosphate (AMP)‐activated protein kinase signaling pathway. Chin Med J (Engl). 2021;134(5):576‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drucker DJ. Mechanisms of action and therapeutic application of glucagon‐like Peptide‐1. Cell Metab. 2018;27(4):740‐756. [DOI] [PubMed] [Google Scholar]
- 13. Lyseng‐Williamson KA. Glucagon‐like Peptide‐1 receptor analogues in type 2 diabetes: their use and differential features. Clin Drug Investig. 2019;39(8):805‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li JH, Jain S, McMillin SM, et al. A novel experimental strategy to assess the metabolic effects of selective activation of a G(q)‐coupled receptor in hepatocytes in vivo. Endocrinology. 2013;154(10):3539‐3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Graziani G, Tentori L, Portarena I, et al. CRH inhibits cell growth of human endometrial adenocarcinoma cells via CRH‐receptor 1‐mediated activation of cAMP‐PKA pathway. Endocrinology. 2002;143(3):807‐813. [DOI] [PubMed] [Google Scholar]
- 16. Tang ST, Tang HQ, Su H, et al. Glucagon‐like peptide‐1 attenuates endothelial barrier injury in diabetes via cAMP/PKA mediated down‐regulation of MLC phosphorylation. Biomed Pharmacother. 2019;113:108667. [DOI] [PubMed] [Google Scholar]
- 17. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C[T]) method. Methods. 2001;25(4):402‐408. [DOI] [PubMed] [Google Scholar]
- 18. Kaur J, Bachhawat AK. A modified Western blot protocol for enhanced sensitivity in the detection of a membrane protein. Anal Biochem. 2009;384(2):348‐349. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Xu F, Liang H, et al. Exenatide inhibits the growth of endometrial cancer Ishikawa xenografts in nude mice. Oncol Rep. 2016;35(3):1340‐1348. [DOI] [PubMed] [Google Scholar]
- 20. Bjørge T, Stocks T, Lukanova A, et al. Metabolic syndrome and endometrial carcinoma. Am J Epidemiol. 2010;171(8):892‐902. [DOI] [PubMed] [Google Scholar]
- 21. Saed L, Varse F, Baradaran HR, et al. The effect of diabetes on the risk of endometrial cancer: an updated a systematic review and meta‐analysis. BMC Cancer. 2019;19(1):527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shikata K, Ninomiya T, Kiyohara Y. Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer Sci. 2013;104(1):9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahrén B. Glucagon‐like peptide‐1 receptor agonists for type 2 diabetes: a rational drug development. J Diabetes Investig. 2019;10(2):196‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riccetti L, Sperduti S, Lazzaretti C, Casarini L, Simoni M. The cAMP/PKA pathway: steroidogenesis of the antral follicular stage. Minerva Ginecol. 2018;70(5):516‐524. [DOI] [PubMed] [Google Scholar]
- 25. Zhang H, Kong Q, Wang J, Jiang Y, Hua H. Complex roles of cAMP‐PKA‐CREB signaling in cancer. Exp Hematol Oncol. 2020;9(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gold MG. Swimming regulations for protein kinase a catalytic subunit. Biochem Soc Trans. 2019;47(5):1355‐1366. [DOI] [PubMed] [Google Scholar]
- 27. Mucignat‐Caretta C, Caretta A. Protein kinase a catalytic and regulatory subunits interact differently in various areas of mouse brain. Int J Mol Sci. 2020;21(9):3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Autenrieth K, Bendzunas NG, Bertinetti DD, Herberg FWPD, Kennedy EJPD. Defining A‐kinase anchoring protein (AKAP) specificity for the protein kinase a subunit RI (PKA‐RI). Chembiochem. 2016;17(8):693‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang JZ, Lu TW, Stolerman LM, et al. Phase separation of a PKA regulatory subunit controls cAMP compartmentation and oncogenic signaling. Cell. 2020;182(6):1531‐1544.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bao Y, Jiang L, Chen H, Zou J, Liu Z, Shi Y. The neuroprotective effect of liraglutide is mediated by glucagon‐like peptide 1 receptor‐mediated activation of cAMP/PKA/CREB pathway. Cell Physiol Biochem. 2015;36(6):2366‐2378. [DOI] [PubMed] [Google Scholar]
- 31. Wu X, Li S, Xue P, Li Y. Liraglutide inhibits the apoptosis of MC3T3‐E1 cells induced by serum deprivation through cAMP/PKA/β‐catenin and PI3K/AKT/GSK3β signaling pathways. Mol Cells. 2018;41(3):234‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.


