Author's summary
Catheter ablation using the cryoballoon is a well-established treatment strategy for patients with atrial fibrillation (AF). To our knowledge, the current sub-analysis of the Cryo Global Registry is the first multicenter study describing the outcomes of cryoballoon ablation (CBA) in real-world Korea clinical practice. Overall, 299 patients were enrolled in 3 Korean centers. Safety, efficacy, healthcare utilization, and arrhythmia disease burden outcomes were assessed. This study demonstrated that CBA according to standard-of-care usage in Korea is safe and effective in preventing AF recurrence, repeat ablation, cardiovascular-related hospitalization, and AF-related symptoms at 12 months after the index ablation.
Keywords: Atrial fibrillation, Catheter ablation, Registries, Korea
Abstract
Background and Objectives
Cryoballoon catheter ablation for the treatment of patients with symptomatic atrial fibrillation (AF) has been adopted globally, but there are limited multicenter reports of 12-month outcomes in the Korean patient population. This analysis evaluated the clinical performance and safety of cryoballoon ablation (CBA) according to standard-of-care practices in Korea.
Methods
This evaluation of Korean patients with AF was conducted within the larger Cryo Global Registry, which is a prospective, multicenter, post-market registry. Freedom from a ≥30-second recurrence of atrial arrhythmias (after a 90-day blanking period until 12 months) and procedural safety were examined in subjects treated with CBA at 3 Korean centers.
Results
Overall, 299 patients with AF (60±11 years old, 24.7% female, 50.5% paroxysmal AF) underwent CBA using the Arctic Front Advance cryoballoon. Of those, 298 were followed-up for at least 12 months. Mean procedure-, left atrial dwell- and fluoroscopy time was 76±21 minutes, 56±23 minutes, and 27±23 minutes, respectively. Freedom from AF recurrence at 12 months was 83.9% (95% confidence interval [CI], 76.9–88.9%) in the paroxysmal and 61.6% (95% CI, 53.1–69.0%) in the persistent AF cohort. Rhythm monitoring was performed on average 4.7±1.4 times during the follow-up period. Serious device- or procedure-related adverse events occurred in 2 patients (0.7%). The 12-month Kaplan-Meier estimate of freedom from repeat ablation and cardiovascular-related hospitalization was 93.8% (95% CI, 90.4–96.1%) and 89.7% (95% CI, 85.6–92.7%), respectively.
Conclusions
CBA is an efficient, effective, and safe procedure for the treatment of AF patients when used according to real-world practices in Korea.
Trial Registration
ClinicalTrials.gov Identifier: NCT02752737
INTRODUCTION
Catheter ablation (CA) is a well-established therapy for the treatment of atrial fibrillation (AF), and CA is superior to anti-arrhythmic drugs (AADs) in preventing AF recurrences and alleviating AF-related symptoms.1) Pulmonary vein isolation (PVI) is the cornerstone strategy of CA in the management of patients with either paroxysmal AF (PAF) or persistent AF (PsAF).2) In addition to radiofrequency ablation (RFA), cryoballoon ablation (CBA) with the Arctic Front Advance catheter has become a “gold standard” for AF ablation since 2012 and has more recently gained global acceptance.3) CBA-PVI has several distinct advantages over RFA, including shorter procedures, less operator-dependent experience for usage, and a shorter learning curve to establish mastery.4),5),6) Recently, 2 (single-center) trials have shown the efficacy and safety of Arctic Front Advance CBA for the treatment of patients with PAF and PsAF in Korea7),8); however, to our knowledge, the current analysis is the first multicenter study describing the outcomes of CBA in real-world Korea clinical practice.
METHODS
Ethical statement
Data collection adhered to the principles outlined in the Declaration of Helsinki (2013) and Good Clinical Practices. The study was approved by local institutional review boards (IRBs) and ethics committees at each participating center, and patients provided written informed consent prior to participation in the registry (Samsung Medical Center IRB 2019-02-061, Seoul National University Bundang Hospital B-1903/562-303 and Hallym University Sacred Heart Hospital 2019-04-025). The data, analytic methods, and study materials will not be available to other researchers. Specifically, patient data privacy within the Cryo Global Registry does not allow nor consent to data sharing with outside parties.
Study design
The Cryo Global Registry (NCT02752737) is an ongoing, prospective, multicenter post-market registry to evaluate outcomes on AF ablation procedures performed with the Arctic Front Advance Family of cryoablation catheters (Medtronic, Inc., Minneapolis, MN, USA). The current analyses aimed to describe patient baseline characteristics, procedural characteristics, and outcomes of CBA in the Korean sub-population within the Cryo Global Registry. Data regarding the ablation procedures were collected from 299 patients enrolled in 3 hospitals in Korea.
The registry has been previously described in detail by Chun et al.9) In brief, a global steering committee of international physicians oversees data quality, analyses, and publication milestones. Procedures performed in this study were performed according to standard-of-care at the time of data collection.
Patient population
Patients in this analysis were consecutively enrolled between April 2019 to May 2020 at 3 hospitals in Korea. All patients ≥18 years old with a planned CBA procedure were eligible for inclusion in the registry and were not excluded based on pre-existing characteristics nor medical conditions. All patients received PVI ablation for the first time, and patients were classified by AF disease status, including: PAF (AF that terminates spontaneously or with intervention within 7 days of onset), PsAF (continuous AF that is sustained beyond 7 days and ≤12 months), or long-standing PsAF (LsPsAF, continuous AF >12 months).3) LsPsAF patients were pooled with PsAF patients in this analysis of 3 centers for statistical considerations because of sample size considerations.
Cryoballoon ablation procedure
CBA-PVI was performed according to local standard-of-care in Korea and in accordance with typical procedural techniques.7),8),9) In brief, a dedicated 15-F OD steerable sheath (FlexCath Advance Steerable Sheath; Medtronic, Inc.) was used to introduce a 23- or 28-mm CBA catheter (Arctic Front Advance; Medtronic, Inc.) into the left atrium (LA). The CBA catheter was maneuvered in the LA either over a J-tip guidewire or a dedicated inner-lumen (octopolar or decapolar) circular mapping catheter (Achieve or Achieve Advance; Medtronic, Inc.). The CBA catheter was inflated and advanced towards the antral surface of the pulmonary vein (PV). Freezing was initiated upon antral occlusion of the targeted PV. The number of cryoapplications and freeze duration were operator determined, and PVI was confirmed by entrance and/or exit block after CA.
Phrenic nerve monitoring (by diagnostic catheter pacing at the subclavian vein and diaphragmatic movement monitoring by manual palpitation and/or adjunctive diaphragmatic movement detection methods) was recommended during all right-sided PVI. Freezes were stopped upon detection of an attenuated diaphragmatic response. Pre- and intra-procedural imaging, esophageal temperature monitoring, and post-ablation chemical testing were determined by the operator. Additional ablation tools used (e.g., focal cryoablation and radiofrequency CA), as well as lesions adjunctive to PVI, were left to the discretion of the operator and were documented. Periprocedural anti-coagulation and AAD initiation/continuation were determined by the operator, and patients were discharged according to the hospital’s standard-of-care practice.
Patient follow-up and endpoints
Patients were followed via telephone and/or in-office visits according to their hospital’s standard-of-care method. Patients were protocol-required to have at least one status visit at 12 months after the index CBA. The primary objective of the registry was to report efficacy and safety after the index CBA procedure throughout 12 months. The Korean patients that are represented in this analysis are a subset population from the larger Cryo Global AF Registry. Procedural efficacy was assessed by time-to-first ≥30-second recurrence of AF and atrial arrhythmias (defined as AF/atrial flutter [AFL]/atrial tachycardia [AT]) between a 90-day blanking period and 12-month follow-up. The method of rhythm monitoring was not dictated by the study protocol and could be conducted by any of the following methods, including: 12-lead electrocardiogram (ECG), Holter monitor, trans-telephonic monitor, insertable cardiac monitor, pacemaker, and/or implantable cardioverter defibrillator. The safety endpoint was the serious device and/or procedure-related adverse event rate as classified by the physician. Non-serious procedure-related adverse events and non-procedure related serious adverse events were also reported. Serious adverse events were defined according to international standards (ISO 14 155:2001) and included all events that led to death, or to a serious deterioration in health that resulted in either (a) a life-threatening illness or injury, (b) a permanent impairment in body structure or function, (c) in-patient or prolonged hospitalization, or (d) medical intervention to prevent life-threatening illness or injury.
Ancillary objectives were examined in this analysis including baseline characteristics, procedural characteristics, quality of life (QoL), arrhythmia monitoring, post-ablation medication management, and hospitalization rates. QoL (measured by 3-level EuroQol 5-dimensional questionnaire [EQ-5D-3L]) and predefined arrhythmia symptoms were monitored at baseline and 12-month follow-up. The EQ-5D-3L questionnaire measures 5 dimensions of health, including: 1) mobility, 2) selfcare, 3) physical activities, 4) pain and discomfort, and 5) anxiety and depression. Each question has 3 levels of response indicating no problem, some problem, or extreme problem. The resulting index score is on a scale ranging from 0 (least healthy) to 1 (most healthy). Class I/III AAD utilization was assessed at baseline, after hospital discharge for post-ablation care, and at the 12-month visit. The method and frequency of rhythm monitoring was documented throughout 12 months of follow-up, as well as the all-cause and cardiovascular (CV)-related hospitalization rates, and the repeat ablation rate.
Statistical analysis
Baseline characteristics and clinical data were summarized using the appropriate summary statistics. Continuous variables were summarized as mean and standard deviation, and categorical variables were summarized as counts and percentages. The Kaplan-Meier method was used to estimate the 12-month freedom from AF recurrence, all atrial arrhythmia recurrence, repeat ablation, and rehospitalization. Standard error was calculated with Greenwood’s formula. Separate log-rank tests were performed to assess the rate of AF recurrence and all atrial arrhythmia recurrence between PAF and PsAF subjects. Changes in QoL was assessed with a one-sample t-test. Values of p<0.05 were considered statistically significant. Statistical analyses were performed with SAS software version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Baseline characteristics
A total of 300 patients with AF were enrolled in 3 Korean hospitals. One patient was withdrawn by the investigator and exited the study prior to the index CBA; while 299 patients were treated according to standard practice. Table 1 describes baseline characteristics of the study population. Patients were on average 60±11 years of age, 75.3% male, 50.5% PAF, and diagnosed with AF for a mean of 2.3±2.8 years. PAF patients were diagnosed with AF on average 2.3±2.8 years, and PsAF patients were diagnosed for 2.4±2.8 years. The mean LA diameter was 43±7 mm (range 24–69 mm), and the left ventricular ejection fraction was 59±8%. A total of 62 (20.7%) patients had a history of prior AFL with 34 (22.5%) PAF patients and 28 (18.9%) PsAF patients. Seven patients (2.3%) had a history of prior AT with 6 (4%) in the PAF and 1 (0.7%) in the PsAF cohort. In addition, 3 (2.0%) patients in the PAF cohort had a previous AFL ablation vs. no patients in the PsAF cohort. Patients failed a mean of 1.2±0.6 AADs before the index-PVI ablation, and 4 patients (1.3%) were treated with first-line CBA.
Table 1. Patient baseline characteristics.
| Subject characteristics | Korea cohort (n = 299) | |
|---|---|---|
| Female sex | 74 (24.7) | |
| Age (year) | 60±11 | |
| Body mass index (kg/m2) | 25±3 | |
| CHA2DS2-VASc score | 1.8±1.5 | |
| PAF | 151 (50.5) | |
| Years diagnosed with AF | 2.3±2.8 | |
| Prior AFL ablation | 3 (1.0) | |
| History of AFL | 62 (20.7) | |
| History of atrial tachycardia | 7 (2.3) | |
| Left atrial diameter (mm) | 43±7 | |
| Left ventricular ejection fraction (%) | 59±8 | |
| On AAD at enrollment | 259 (86.6) | |
| Number of failed AADs | 1.2±0.6 | |
| 0 previously failed AADs | 13 (4.3) | |
| 0, on AAD at baseline | 9 (3.0) | |
| 0, not on AAD at baseline (first-line CBA) | 4 (1.3) | |
| Hypertension | 151 (50.5) | |
| Prior myocardial infarction | 4 (1.3) | |
| Prior stroke/Transient ischemic attack | 30 (10.0) | |
| Coronary artery disease | 28 (9.4) | |
| Diabetes | 53 (17.7) | |
| Sleep apnea | 9 (3.0) | |
Values are presented as number (%) or mean±standard deviation.
AAD = antiarrhythmic drugs; AF = atrial fibrillation; AFL = atrial flutter; CBA = cryoballoon ablation; PAF = paroxysmal atrial fibrillation.
Procedural characteristics
All patients underwent index CBA using the 28-mm Arctic Front Advance cryoballoon. In 1 patient, a 23-mm cryoballoon was used in addition to a 28-mm balloon. Procedural characteristics are presented in Table 2. Mean total procedure time, LA dwell time, and fluoroscopy time were 76±21 minutes, 56±23 minutes, and 27±23 minutes, respectively. Preprocedural mapping with computed tomography or magnetic resonance imaging was performed in 191 (63.9%) patients. During the procedure, intracardiac echocardiography was performed in 52.5% of cases, and a PV venogram was done in 84.3% of cases. Intra-procedural 3D electroanatomical mapping was not used, and esophageal temperature was monitored in 195 (65.2%) patients. Phrenic nerve function was monitored in all patients by pacing and palpating the phrenic nerve in conjunction with additional monitoring methods such as Compound Motor Action Potential monitoring during right-sided PV CBA.
Table 2. Procedural characteristics.
| Procedure characteristics | Korea cohort (n = 299) | |
|---|---|---|
| Total lab occupancy time (minute) | 114±24 | |
| Total procedure time (minute) | 76±21 | |
| Left atrial dwell time (minute) | 56±23 | |
| Total fluoroscopy time (minute) | 27±23 | |
| Total cryo fluoroscopy time (minute) | 18±19 | |
| Sedation method | ||
| General | 195 (65.2) | |
| Conscious | 104 (34.8) | |
| Pre-procedural mapping CT or MRI | 191 (63.9) | |
| ICE | 157 (52.5) | |
| PV venography | 252 (84.3) | |
| CTI ablation | 66 (22.1) | |
| Other non-PV ablations | 8 (2.7) | |
| Acute PVI success | 293 (98.0) | |
| Esophageal monitored at least one vein | 195 (65.2) | |
| Phrenic nerve monitoring | 299 (100.0) | |
| Cryoballoon applications | ||
| Total applications performed | 1,799 | |
| Total veins treated | 1,209 | |
| Number of applications per vein | 1.5±1.0 | |
| Duration of cryoapplication (second) | 167±54 | |
| Cryoballoon nadir temperature (°C) | −49.9±6.8 | |
Values are presented as number (%) or mean±standard deviation.
CT = computed tomography; CTI = cavotricuspid isthmus; ICE = intracardiac echocardiography; MRI = magnetic resonance imaging; PV = pulmonary vein; PVI = pulmonary vein isolation.
A total of 1,209 veins were treated with a mean of 1.5±1.0 freeze applications per vein. The average freeze duration was 167±54 seconds, and a mean nadir balloon temperature of −49.9±6.8°C was reported. There were no left common PV ostia treated with cryoballoon in this population. Acute PVI success was achieved in 98.0% of patients using only the Arctic Front Advance cryoballoon. No focal RFA or focal cryo touch-up applications were performed to achieve PVI. Overall, 72 (24.1%) patients underwent adjunctive non-PVI ablation during the index procedure. Of those, 66 patients underwent cavotricuspid isthmus (CTI) ablation, 29 (19.2%) PAF patients and 37 (25.0%) PsAF patients. In a total of 8 cases, other non-PVI and non-CTI ablations were performed, including 4 (2.6%) PAF patients and 4 (2.7%) PsAF patients. The non-PVI, non-CTI targets were LA AF triggers (1.3%, CBA), superior vena cava vein triggers (0.7%, CBA), left atrial anterior wall (0.3%, RFA), and a paroxysmal supraventricular tachycardia (0.3%, RFA).
Efficacy
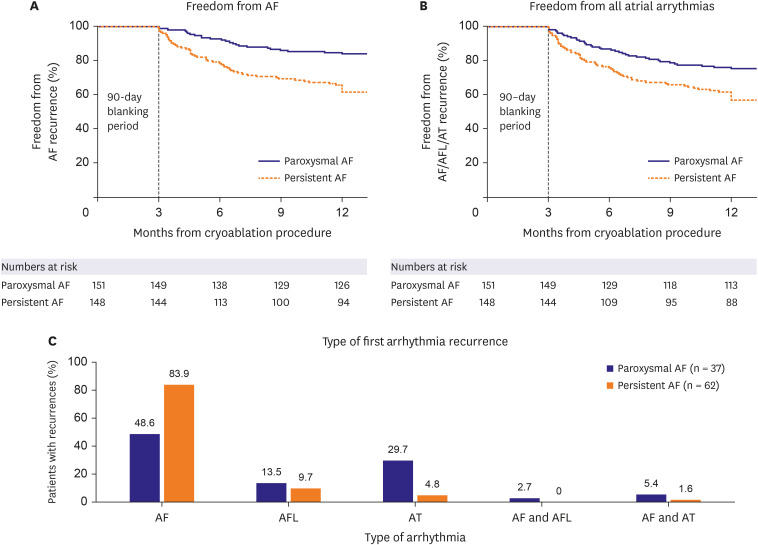
For the effectiveness analysis, PAF patients were compared to PsAF patients. Arrhythmia recurrence is shown in Figure 1. The Kaplan-Meier estimate for freedom from a ≥30-second recurrence of AF at 12 months was 83.9% (95% confidence interval [CI], 76.9–88.9%) in PAF and 61.6% (95% CI, 53.1–69.0%) in PsAF (Figure 1A). Freedom from ≥30 secones AF/AFL/AT at 12 months was lower, with 75.2% (95% CI, 67.4–81.3%) in the PAF patient cohort and 56.7% (95% CI, 48.2–64.4%) in the PsAF patient cohort (Figure 1B). AF-free survival and atrial arrhythmia-free survival were significantly lower for the PsAF patients when compared to PAF patients (p<0.01 for both). The type of arrhythmia recurrence was AF in most of the PsAF patients (83.0%). In PAF patients, 18 patients (48.6%) had recurrent AF, while 29.7% experienced AT and 13.5% AFL (Figure 1C).
Figure 1. Atrial arrhythmia recurrence. (A) Kaplan-Meier estimate of 12-month freedom from ≥30-second recurrence of AF in PAF (navy lines) and persistent AF (orange lines) after a 90-day blanking period. (B) Kaplan-Meier estimate of freedom from ≥30-second recurrence of AF/AFL/AT at 12 months after a 90-day blanking period in patients with PAF (navy lines) and persistent AF (orange lines). (C) Type of first arrhythmia recurrence in patients with PAF (navy bars) and persistent AF (orange bars).
AF = atrial fibrillation; AFL = atrial flutter; AT = atrial tachycardia.
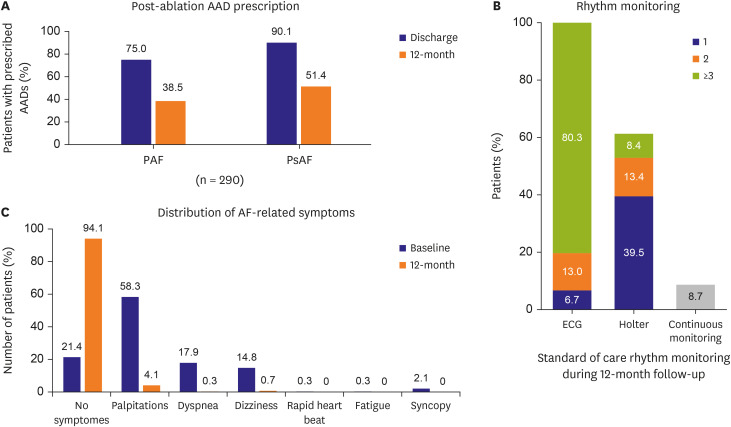
AAD continuation or prescription for post-ablation care was left to the discretion of the investigator. Of the 299 patients, 290 had AAD status reported at the index procedure discharge and 12 months. Overall AAD usage decreased from 82.4% at index procedure discharge (PAF: 75.0%, PsAF: 90.1%) to 44.8% at 12-month follow-up (PAF:38.5%, PsAF 51.4%; Figure 2A). In total, 98 patients had an atrial arrhythmia recurrence, and of those 29 (29.6%) patients were off AAD at 12 months (Table 3). During follow-up, 18 (18.4%) patients received a repeat ablation (6 patients off ADD and 12 patients on AAD at 12 months). Of the 192 patients that did not have an atrial arrhythmia recurrence, 131 (68.2%) were off AAD at 12 months, and none of those patients received a repeat ablation.
Figure 2. Post-ablation standard-of-care and distribution of AF-related symptoms. (A) AAD prescription in 290 patients with information available at discharge (navy bar) and 12 months (orange bar). (B) Proportion of patients monitored for atrial arrhythmia recurrences during the 12-month follow-up period with one (navy), 2 (orange) or 3 or more (yellow-green) 12-lead ECGs, Holter monitors, and all combined methods for arrhythmia monitoring. Patients with continuous monitoring methods (pacemaker/implantable cardiac monitor) are also depicted in red. All patients, including patients with continuous monitoring devices, received ECG monitoring at least once during the follow-up period. (C) Distribution of patients reporting on the presence of pre-specified AF-related symptoms at baseline (navy bars) and at the 12-month (orange bars) follow-up visit.
AAD = anti-arrhythmic drug; AF = atrial fibrillation; ECG = electrocardiogram.
Table 3. AAD discontinuation and repeat ablation.
| Characteristics (n = 290)* | Number of patients with AF/AFL/AT recurrence at 12M | Number of patients without AF/AFL/AT recurrence at 12M |
|---|---|---|
| Total | 98 | 192 |
| Off AAD at 12M | 29 (29.6) | 131 (68.2) |
| Repeat ablation at 12M | 18 (18.4) | 0 (0) |
Values are presented as number (%).
AAD = antiarrhythmic drugs; AF = atrial fibrillation; AFL = atrial flutter; AT = atrial tachycardia; 12M = 12 months.
*The 290 of 299 subjects completed a 12 months visit.
The method of rhythm monitoring was not dictated by the protocol. During the 12-month follow-up period, patients were seen in the clinic an average of 4.7±1.1 visits, including a study required 12-month visit, and the remaining visits were completed via standard of care follow-up. All patients were monitored for atrial arrhythmia recurrence at least once (Figure 2B). All patients received a 12-lead ECG during follow-up, and 80.3% patients had 3 or more 12-lead ECGs performed during follow-up. Holter monitoring was used at least once during the follow-up period in 61.2% of patients. In 26 patients (8.7%) with continuous monitoring devices, 74 data reviews were performed, and all these patients received an additional 12-lead ECG recordings according to standard-of-care.
Safety
A total of 62 total adverse events were reported in 51 patients (Table 4). Two serious device- and/or procedure-related events were reported in 2 patients (0.7%) within one day after the index procedure. In one patient, a vascular pseudoaneurysm was reported, and one incision site hematoma was observed in a second subject. Additionally, 5 non-serious procedure-related events (1.7%) were reported in 5 patients. Three of these events were phrenic nerve palsies, but none were classified as serious by the investigators. All phrenic nerve palsies were resolved without further clinical sequelae. In one patient, the phrenic nerve palsy was resolved at hospital discharge and the 2 others resolved at days 16 and 175 post- procedure (with a mean time to resolution of 64.0±96.4 days). Lastly, the investigators reported 55 other serious non-procedure related events (in 45 patients, 15.1%). Of those 55 serious events, 43 were related to atrial arrhythmia recurrence. Importantly, there were no atrioesophageal fistula, pericardial tamponade, or PV stenosis reported.
Table 4. Safety events.
| Adverse events | Korea cohort (n = 299) | |
|---|---|---|
| Serious* device- or procedure-related adverse events | 2 (2, 0.7) | |
| Incision site hematoma | 1 (1, 0.3) | |
| Vascular pseudoaneurysm | 1 (1, 0.3) | |
| Non-serious procedure-related adverse events | 5 (5, 1.7) | |
| Phrenic nerve injury | 3 (3, 1.0) | |
| Urinary retention | 1 (1, 0.3) | |
| Haemoptysis | 1 (1, 0.3) | |
| Non-procedure-related serious* adverse events | 55 (45, 15.1) | |
| Arrhythmia supraventricular | 6 (6, 2.0) | |
| Atrial fibrillation | 32 (29, 9.7) | |
| Atrial flutter | 5 (5, 1.7) | |
| Cholecystitis acute | 1 (1, 0.3) | |
| Coronary artery disease | 1 (1, 0.3) | |
| Gastritis | 1 (1, 0.3) | |
| Hashimoto’s encephalopathy | 1 (1, 0.3) | |
| Hypertrophic cardiomyopathy | 1 (1, 0.3) | |
| Inguinal hernia | 1 (1, 0.3) | |
| Pneumonia | 2 (2, 0.7) | |
| Sinus node dysfunction | 3 (3, 1.0) | |
| Thermal burn | 1 (1, 0.3) | |
Numbers are presented as events (subjects with events, % subjects).
*Serious adverse events were defined according to international standards (ISO 14 155:2001) and included all events that led to death, or to a serious deterioration in health that resulted in either (a) a life-threatening illness or injury, (b) a permanent impairment in body structure or function, (c) in-patient or prolonged hospitalization, or (d) medical intervention to prevent life-threatening illness or injury.
Quality of life and arrhythmia symptoms
QoL, measured by the EQ-5D index score, improved significantly in the Korean patient cohort from 0.82±0.24 at baseline to 0.90±0.20 at 12-month follow-up (p<0.001). At baseline, 78.6% of patients reported to have experienced at least one symptom related to AF, and a distribution of symptoms is presented in Figure 2C. Patients most often experienced palpitations (58.3%) followed by dyspnea (17.9%) and dizziness (14.8%) at baseline. Symptom burden improved significantly after CBA, with 94.1% of patients being free of symptoms at 12-month follow-up.
Hospitalization and repeat ablation rate
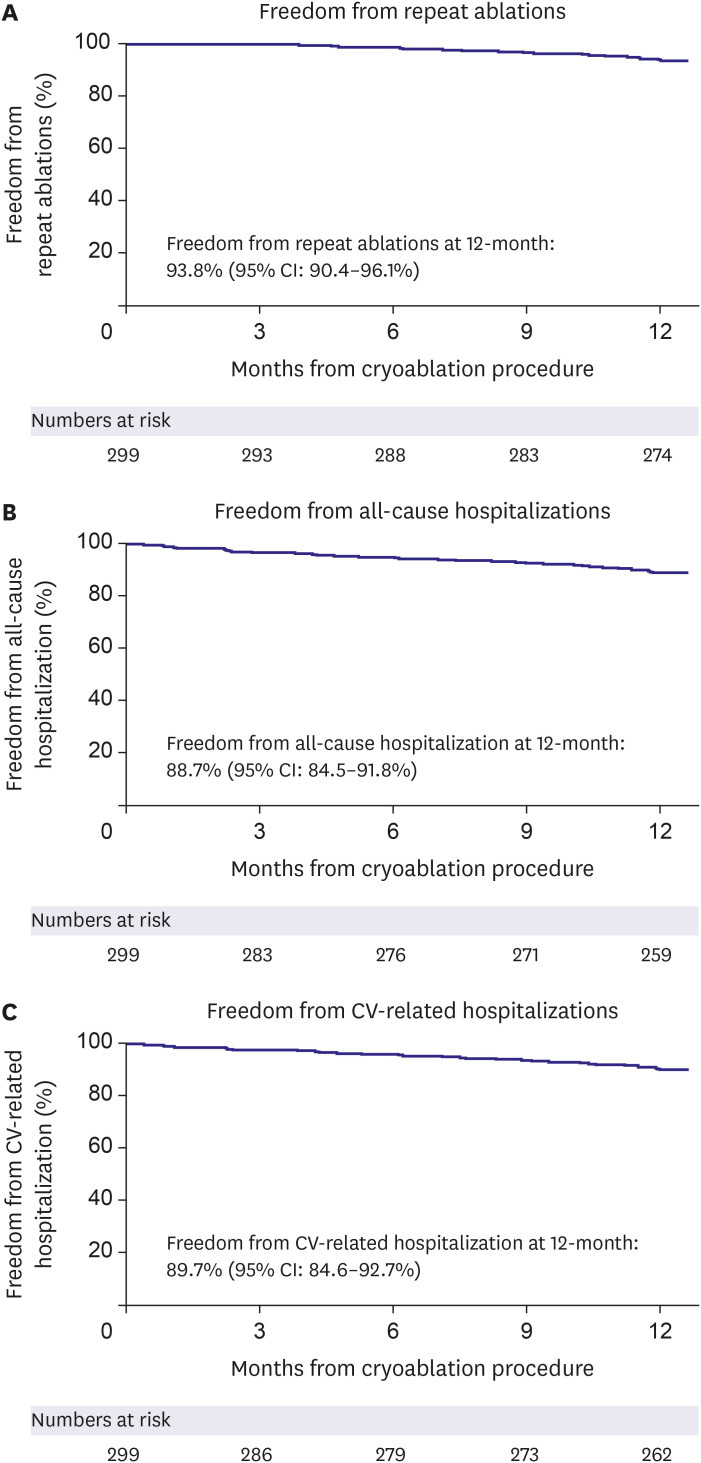
Healthcare utilization after the index ablation was demonstrated using the Kaplan-Meier method for reporting the number of repeat ablations, all-cause hospitalizations, and CV-related hospitalizations throughout 12 months (Figure 3). CBA was effective in preventing repeat ablations, with 93.8% of patients being free from repeat ablations at 12 months. In total, 18 patients had a repeat ablation within the first year, and there were no patients that underwent a second repeat ablation. The estimate for freedom from first all-cause hospitalization post-ablation was 88.7% in this Korean cohort. Most hospitalizations were due to supraventricular arrhythmias (29/33), and freedom from the first hospitalization post-ablation due to CV-related causes was 89.7%.
Figure 3. Healthcare utilization. Kaplan-Meier estimate of 12-month freedom from (A) repeat ablations, (B) all-cause hospitalizations, and (C) CV-related hospitalizations in 299 patients.
CI = confidence interval; CV = cardiovascular.
DISCUSSION
This sub-analysis of the Cryo Global Registry assessed the efficacy and safety of CBA using the Arctic Front Advance balloon for the treatment of patients with AF in Korea. In patients with PAF, freedom from a ≥30-second recurrence of AF was 83.9% at 12 months, and freedom from AF/AFL/AT was 75.2% at 12 months. Freedom from AF and AF/AFL/AT at 12 months was 61.6% and 56.7%, respectively, in the PsAF patient cohort. The rate of serious index procedure-related adverse events was 0.7%. Also, QoL significantly improved at 12-month post-ablation, and 94.1% of patients were free of AF-related symptoms at 12-month follow-up. Repeat ablations were performed in 6.2% of patients during the follow-up period, and 88.7% of patients were free from all-cause hospitalization at 12-month post-ablation.
Cappato et al.7) was the first to report on real-world evidence of outcome of CA in AF on a global scale in 2005. While the novel real-world evidence was unique and useful, the method of collection was a survey with inherent limitations. More recently, several modern registries have followed and are still ongoing. These have proven to be useful by collecting global information on patient demographics and routine clinical practice (e.g., timing and type of treatment), analyzing trends in procedural techniques, monitoring overall safety, and identifying risk factors and predictors of outcomes in large populations with different co-morbidities.8) The present analysis provides insight into the real-world use, safety and efficacy of CBA across multiple Korean centers within the larger Cryo Global Registry.9) The outcome of this Korean real-world evidence is compared below to registries around the globe.10),11),12),13) In addition, there are currently 2 publications on CBA-PVI in the Korean population for comparison of procedural outcome, and both are single-center experiences.14),15)
Patient demographics of Korean patients in this sub-analysis of the Cryo Global Registry were very comparable to what was previously reported for age, echocardiogram parameters, co-morbidities, number of failed AADs, and time to ablation.9),10),11),12),13) The participating Korean centers enrolled a slightly higher number of males (75.3%) compared to other registries. Half of the Korean patients had non-PAF, which was also seen in the GWTG-AFIB registry,10) but is higher than reported in the global cohort of the Cryo Global Registry.9) LAD was slightly larger than expected in the Korean sub-population (43±7 mm), when comparing to other registry populations. Also, 20.7% of Korean patients had a history of AFL, which is observed in some11),12) but not all registry cohorts. 9),10),13)
Cryoballoon procedures were performed following current trends. Adjunctive pre- and peri-procedural imaging and monitoring were frequently used. CBA procedure duration in this multicenter analysis (76±21 minutes) was relatively short when compared to the 2 published studies from Korea and other real-world evidence (ranging from 73±26 minutes to 136.0±46.5 minutes)9),10),11),12),13),14),15) especially when considering the number of ablations beyond PVI in this sub-analysis. A history of AFL at baseline was documented in 20.7% of patients, which explains the relatively high number of CTI ablations (22.1%) performed at the index procedure. Non-PVI ablation added on average 9 minutes to the total fluoroscopy time. The mean number of freeze applications per vein was 1.5±1.0, with a mean duration of 167±54 seconds per application, in line with contemporary dosing strategies for the Arctic Front Advance cryoballoon.9),12),13)
Freedom from AF in paroxysmal patients was within range of what was previously reported.9),12) Freedom from AF in the persistent population was significantly lower with 61.6% in the Korean sub-population vs. 73% reported by Chun et al.9) and Ferrero-De-Loma-Osorio et al.12) in the Cryo Global Registry and RECABA registry, respectively. In addition, the effectiveness of CBA for the prevention of all arrythmia recurrences (AF/AFL/AT) in the Korean sub-population was lower than expected based on outcome of the global and Japan cohorts of the Cryo Global Registry.9),13) However, it should be noted that monitoring was more rigorously performed in the Korean sub-population. The main type of arrhythmia recurrence in the present analysis was AF in 83.0% of PsAF patients vs. 48.6% in PAF patients. There was a relatively high number of patients with an AT (29.7%) and AFL (13.5%) type of recurrence in the PAF cohort compared to the other 2 Korean trials and the overall Cryo Global Registry population.9),14),15) This may be explained by the relatively high number of PAF patients enrolled in the registry with a history of AFL (22.5%) and AT (4.0%) at baseline.
AAD use was not dictated by the study protocol. According to standard of care in the participating centers, AAD were prescribed for post-ablation care in 82.4% of patients. A significant reduction in AAD use from baseline (86.6%) to 12 months (44.8%) was observed in the current analysis. Prescription of AAD at discharge and 12 months follow-up was notably higher in the 3 participating centers then in the global cohort and the Korean patients in the single-center trial described by Pak et al.14)
The total rate of procedure-related events during standard-of-care in this multicenter registry was low overall (0.7% serious and 1.7% of non-serious events). These findings on low rates of complications following a CBA procedure are consistent with previously published literature, which demonstrates the consistent safety profile across real-world usage for CBA-PVI.4),5),6),9),10),11),12),13),14) Importantly, in this analysis of 299 patients, no atrioesophageal fistula, pericardial tamponade, or PV stenosis were reported, while 12-month QoL improved significantly for the cohort.
There is limitation to our study. This sub-analysis of the Cryo Global Registry describes the clinical performance and safety of CBA with Arctic Front Advance according to real-world practice in Korea. Procedure, cardiac monitoring and AAD prescription post-ablation were not prescribed. Non-standardized monitoring could have led to under-detection of AF, especially asymptomatic AF recurrence.15) Continuous monitoring was not available in most patients, and therefore, episodes of asymptomatic AF might not have been uniformly identified. However, the current analysis describes standard of care in the participating centers. Most patients (93.3%) had a monitoring event at least 3 times during the follow-up period, mainly 12-lead ECG and Holter monitoring. The current monitoring protocol was comparable to other real-world evidence publications.9),11),12)
In conclusions, CBA according to standard-of-care usage in Korea is safe and effective in preventing AF recurrence, repeat ablation, CV-related hospitalization, and AF-related symptoms at 12 months after the index procedure.
ACKNOWLEDGMENTS
The authors sincerely thank the Cryo Global Registry Korea site staff for their commitment and contributions to the study. The authors also thank Yewon Lee, Troy Penz, Jada Selma, Hae Lim, Bob Hokanson, and Valentine Obidigbo from Medtronic for their support of the trial and generation of this manuscript.
Footnotes
Funding: The registry was sponsored by Medtronic, Inc., Minneapolis, MN, USA.
Conflict of Interest: Kueffer FJ and van Bragt KA are employees of Medtronic, Inc. The remaining authors have no conflicts of interest pertaining to this manuscript to declare.
- Conceptualization: On YK.
- Formal analysis: Kueffer FJ.
- Investigation: Lim HE, Oh IY, On YK.
- Validation: Kueffer FJ.
- Writing - original draft: Van Bragt KA, On YK.
- Writing - review & editing: Lim HE, Oh IY, Kueffer FJ, Van Bragt KA, On YK.
References
- 1.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 2.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1–e160. doi: 10.1093/europace/eux274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann E, Straube F, Wegscheider K, et al. Outcomes of cryoballoon or radiofrequency ablation in symptomatic paroxysmal or persistent atrial fibrillation. Europace. 2019;21:1313–1324. doi: 10.1093/europace/euz155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Providencia R, Defaye P, Lambiase PD, et al. Results from a multicentre comparison of cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: is cryoablation more reproducible? Europace. 2017;19:48–57. doi: 10.1093/europace/euw080. [DOI] [PubMed] [Google Scholar]
- 6.Velagić V, de Asmundis C, Mugnai G, et al. Learning curve using the second-generation cryoballoon ablation. J Cardiovasc Med (Hagerstown) 2017;18:518–527. doi: 10.2459/JCM.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 7.Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 8.Cappato R, Ali H. Surveys and registries on catheter ablation of atrial fibrillation: fifteen years of history. Circ Arrhythm Electrophysiol. 2021;14:e008073. doi: 10.1161/CIRCEP.120.008073. [DOI] [PubMed] [Google Scholar]
- 9.Chun KR, Okumura K, Scazzuso F, et al. Safety and efficacy of cryoballoon ablation for the treatment of paroxysmal and persistent AF in a real-world global setting: results from the Cryo AF Global Registry. J Arrhythm. 2021;37:356–367. doi: 10.1002/joa3.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman DJ, Holmes D, Curtis AB, et al. Procedure characteristics and outcomes of atrial fibrillation ablation procedures using cryoballoon versus radiofrequency ablation: a report from the GWTG-AFIB registry. J Cardiovasc Electrophysiol. 2021;32:248–259. doi: 10.1111/jce.14858. [DOI] [PubMed] [Google Scholar]
- 11.Padeletti L, Curnis A, Tondo C, et al. Pulmonary vein isolation with the cryoballoon technique: feasibility, procedural outcomes, and adoption in the real world: data from One Shot Technologies TO Pulmonary Vein Isolation (1STOP) project. Pacing Clin Electrophysiol. 2017;40:46–56. doi: 10.1111/pace.12975. [DOI] [PubMed] [Google Scholar]
- 12.Ferrero-De-Loma-Osorio Á, Cózar R, García-Alberola A, et al. Primary results of the Spanish Cryoballoon Ablation Registry: acute and long-term outcomes of the RECABA study. Sci Rep. 2021;11:17268. doi: 10.1038/s41598-021-96655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon HJ, Choi JH, Kim HR, et al. Radiofrequency vs. cryoballoon vs. thoracoscopic surgical ablation for atrial fibrillation: a single-center experience. Medicina (Kaunas) 2021;57:1023. doi: 10.3390/medicina57101023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pak HN, Park JW, Yang SY, et al. Cryoballoon versus high-power, short-duration radiofrequency ablation for pulmonary vein isolation in patients with paroxysmal atrial fibrillation: a single-center, prospective, randomized study. Circ Arrhythm Electrophysiol. 2021;14:e010040. doi: 10.1161/CIRCEP.121.010040. [DOI] [PubMed] [Google Scholar]
- 15.Balabanski T, Brugada J, Arbelo E, et al. Impact of monitoring on detection of arrhythmia recurrences in the ESC-EHRA EORP atrial fibrillation ablation long-term registry. Europace. 2019;21:1802–1808. doi: 10.1093/europace/euz216. [DOI] [PubMed] [Google Scholar]