Abstract
Medicinal or herbal spices are grown in tropical moist evergreen forestland, surrounding most of the tropical and subtropical regions of Eastern Himalayas in India (Sikkim, Darjeeling regions), Bhutan, Nepal, Pakistan, Iran, Afghanistan, a few Central Asian countries, Middle East, USA, Europe, South East Asia, Japan, Malaysia, and Indonesia. According to the cultivation region surrounded, economic value, and vogue, these spices can be classified into major, minor, and colored tropical spices. In total, 24 tropical spices and herbs (cardamom, black jeera, fennel, poppy, coriander, fenugreek, bay leaves, clove, chili, cassia bark, black pepper, nutmeg, black mustard, turmeric, saffron, star anise, onion, dill, asafoetida, celery, allspice, kokum, greater galangal, and sweet flag) are described in this review. These spices show many pharmacological activities like anti-inflammatory, antimicrobial, anti-diabetic, anti-obesity, cardiovascular, gastrointestinal, central nervous system, and antioxidant activities. Numerous bioactive compounds are present in these selected spices, such as 1,8-cineole, monoterpene hydrocarbons, γ-terpinene, cuminaldehyde, trans-anethole, fenchone, estragole, benzylisoquinoline alkaloids, eugenol, cinnamaldehyde, piperine, linalool, malabaricone C, safrole, myristicin, elemicin, sinigrin, curcumin, bidemethoxycurcumin, dimethoxycurcumin, crocin, picrocrocin, quercetin, quercetin 4’-O-β-glucoside, apiol, carvone, limonene, α-phellandrene, galactomannan, rosmarinic acid, limonene, capsaicinoids, eugenol, garcinol, and α-asarone. Other than that, various spices are used to synthesize different types of metal-based and polymer-based nanoparticles like zinc oxide, gold, silver, selenium, silica, and chitosan nanoparticles which provide beneficial health effects such as antioxidant, anti-carcinogenic, anti-diabetic, enzyme retardation effect, and antimicrobial activity. The nanoparticles can also be used in environmental pollution management like dye decolorization and in chemical industries to enhance the rate of reaction by the use of catalytic activity of the nanoparticles. The nutritional value, phytochemical properties, health advantages, and both traditional and modern applications of these spices, along with their functions in food fortification, have been thoroughly discussed in this review
Keywords: Phytochemicals, Ethnobotany, Essential oil, Antimicrobial, Bio-prospecting, Nanoparticles
Introduction
Nowadays, people have been more conscious about the co-relation between the food product and healthy life to cure nutrition-related diseases and promote quality of life [178]. This thought brought many advantages to the food industry, including the provision to provide functional food products that fulfill people’s demands along with good standards
In order to meet a purchaser’s expectations in the quality and hygiene of food products, nowadays researchers are trying to apply nanotechnology in current food science [361]. Therefore, different types of nanostructures like nanoliposomes [195], nanoemulsions [514, 881], and nanoparticles [700] are used in the food industry to sustain and develop proposal features [102]. The process of better packaging methods and governance of food standards and protectiveness were a few of the very important studied areas in food nanotechnology [179, 336]. There is a debate about the application of nanotechnology in food, as this might compromise food indemnity,therefore, recent provisions of the application of nanomaterials in food and medicine are incipient [73] only through recommendations given by the US (FDA) Department of Health and Human Services 2014 and the European Food Safety Association (EFSA) [265]. The scientific community has focused on nanotechnology-based techniques in order to detect hygiene-related problems and hazards of recent foods that might develop during and after food processing [168]
Investigation into the effectiveness of nanotechnology in the formulation of food products might associate with the encapsulation of nutritious ingredients like vitamins [93], antioxidants [370, 411], and polyphenols [179]. Thus, various additives applied during food development, especially those derived from agricultural processing wastage or naturally like fruits and spices, have a significant potential for application in nanotechnology [179]. All these products are utilized for their unique properties like color, aroma, flavor, and preservation of food [278]
The technology and science in which nanoparticles have been utilized are as follows: medical [709], electronics, agricultural [772], chemical [942], and pharmaceutical [757]. Most of the research studies till now performed on the application of nanoparticles with spices mainly focus on 1) in vitro studies, 2) fortification, 3) food industry, 4) packaging, 5) aroma and drug industry and 6) textile industry
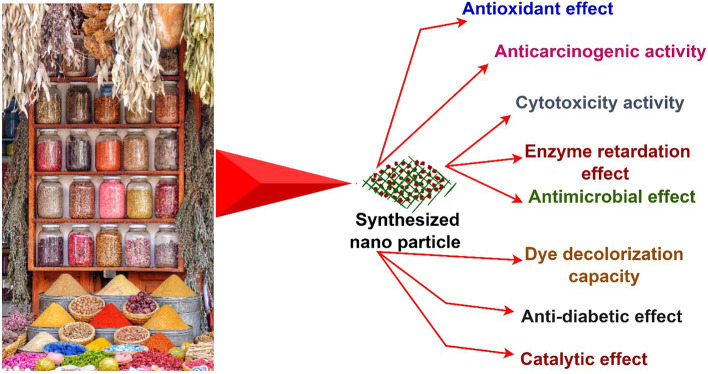
Since all spices contain an ample amount of bioactive components that are used to synthesize nanoparticles, these nanoparticles can be used in various types of food products to make them more nutritious that have enormous health-beneficial effects. The antibacterial activity of green synthesized silver nanoparticles has been evaluated against Klebsiella pneumoniae and Bacillus subtilis [836]. Soshnikova et al. [880] prepared the gold and silver nanoparticles with the water extract of dried fruits of Amomum villosum called Fructus Amomi (cardamom) to assess catalytic and antioxidant effects and prevention activity for breast cancer cells [880]. Krishnan et al. [487] synthesized silver nanoparticles with seed extracts of cardamom that showed its cytotoxic effect against Hep-2 cell line [487]. Taami et al. [906] evaluated the antioxidant efficacy of biodegradable starch film containing nanoemulsions of Bunium persicum essential oil fortified with cinnamaldehyde [906]. Arif et al. [84] investigated a substitute treatment therapy to cure rheumatoid arthritis with fennel seed selenium nanoparticles in arthritic BALB/c mice [84]
Nowadays, in human nutrition, spices provide eminent historical significance. The bioactive components present in spices make them more popular for centuries. They have been applied for their health advantages and also for coloring or flavoring food products [207]. Day by day, the application of spices in different types of food has been enhanced as they provide various pharmacological and physiological benefits. Medicinal spices have been taken great importance by the recent biomedical research, as spices have been used traditionally in producing either nutraceuticals or functional foods due to their health-beneficial properties. India possesses rank three in the world spices market with an 8.8% share. India possesses the first position in turmeric, coriander, pepper, fenugreek, and some other spices export. The USA, Germany, and Malaysia are the main trading countries. Spices provide aroma due to the presence of volatile oils and oleoresin [147]
Spices play an important role in terms of medicinal benefits. Spices are utilized as anti-inflammatory, carminative, antioxidants, and antiseptic. In the current scenario, spices are gaining interest due to the bioactive compounds and their biological effect and chemical structure. Phytochemicals like alkaloids, phenolic compounds, flavonoids, tannins, and flavones are present in spices and can be used as a powerful drug against dengue and Ebola viruses. Chikungunya virus can be cured by using ginger extract. Spices have antioxidant properties and have proof of oxidative alternation of low-density lipoprotein cholesterol in the formation of atherosclerosis. Spices containing various bioactive components have an anticancer effect as examined in model animals [147]. The active compounds of the spices are essential oils, and the spices, namely black pepper, cinnamon, cloves, coriander, chili, and cumin, are enriched with essential oils having pharmaceutically active components such as piperine, cinnamaldehyde, eugenol, allicin, curcumin, and linalool [658]. Spices are used as therapeutic agents with antimicrobial, anti-inflammatory, anticancer, and antioxidant properties. Carotenoids, eugenol, and curcumin in saffron, clove, and turmeric are the active constituents in spices evidenced by a phytochemical evaluation that exposes the effective nature of these spices. The benefits represented by these spices are immunity boosters, especially during the phase of the pandemic, and their incorporation into our regular diet could enhance disease control mechanisms [773]
In recent years, the societal needs and interest in the application of renewable, natural, and biodegradable resources have enhanced. Food consumers and producers have increased their demands for the quality of processed food products, especially in the area of enhancing shelf life while protecting nutritional and organoleptic properties. Spices have been reported to have a high potential to be applied as important, renewable, and biodegradable sources of chemicals like polyphenols having great antimicrobial/antioxidant properties [460]. Spices have been significantly utilized to increase or improve the flavor of food through their preservative properties [956]. Spices possess a significant role in food safety. The retardation effect of spices and derivatives on the propagation of fungi, bacteria, yeasts, and microbial toxins synthesis has been reported,therefore, they have been applied in food preservation. Spices are recently utilized for increasing the flavor and shelf life of food products because of their bactericidal or bacteriostatic effect [282]
In this review, the ethnobotanical aspects of 24 spices have been covered along with their bioactive and nutritional potential. The role of those spices in food fortification and their potential in nanotechnology have also been explored
Ethnobotanical Knowledge Related to Spices and Herbs
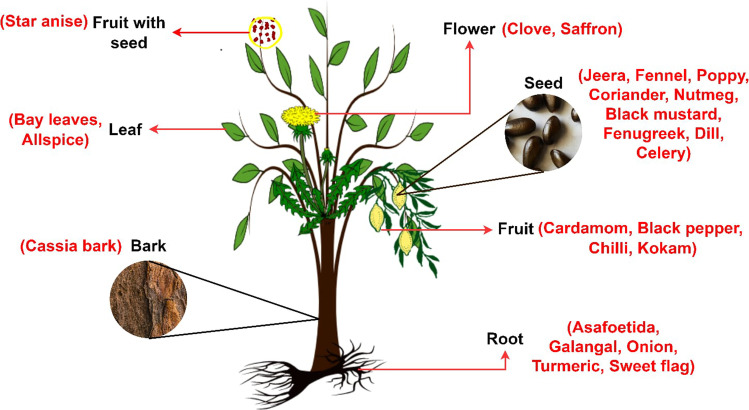
Spices and herbs are the plant parts. Figure 1 describes the botanical perspective of the spices and herbs
Fig. 1.
Spices as different plant parts
Cardamom (Elettaria cardamomum)
Small cardamom belongs to the family Zingiberaceae. Small cardamom capsules (fruits) show beneficial health effects which are related to the traditional and modern pharmaceutical aspects. It is used in traditional medicines to prevent asthma, nausea, teeth and gum infections, diarrhea, cataracts, indigestion, cardiac attack, and kidney failure. Cardamom fruits are utilized as a fragrance, spice, and flavoring ingredient in food products [89]. In Ayurveda, for the treatment of food poisoning, cardamom has been applied and at present, plant-based creams and soaps are made with cardamom oils [973]. The large cardamom is called ‘cash crops’ cultivated between altitudes of 600 and 2000 m in the tropical moist evergreen jungle of Eastern Himalayas in India (Sikkim, and Darjeeling regions), Bhutan, and Nepal. The fruit is reddish-brown to dark pink, trilocular, and many-seeded capsule. The seeds possess 2–3% essential oils that contain medicinal properties. These are utilized as a supplement to formulate different types of medicine [379, 866]. India is the highest producer of cardamom (26.51 thousand tons) [563]. The small cardamom is called ‘Queen of all Spices’ and grows in the tropical rain forest at altitudes of 762–1524 m, where it rains about 381 cm per year. It is cultivated commercially in Guatemala, India, Sri Lanka, and Tanzania. The fruits are narrow-walled, soft-skinned, and oblong. The green fruits contain 15–20 aromatic reddish-brown seeds. The seeds possess volatile oil, which is utilized for the flavoring of bread, cakes, curries, and other culinary purposes. Confectionery and coffee products are also flavored with small cardamom. It is also applied in several neural, gastrointestinal, and cardiovascular diseases [681]
Black jeera (Bunium persicum)
In traditional and folkloric medicine, black jeera has been utilized for the treatment of different types of diseases like nasopharyngeal, gastrointestinal, cardiac, respiratory, ocular, neurological, and urinary tract problems [559]. This plant is popular as a spice [352]. The roots and seeds are used as spices that provide flavor to the food [360]. About 70% of the world’s cumin is produced in India (725.42 thousand tons) [104]. Black jeera belongs to the family Apiaceae, which is a topical plant and is found in Pakistan, Iran, Afghanistan, and a few Central Asian countries. This plant is a branched and perennial herb, its glandular root is irregular and circular in shape, and the height of this herb is around 40–60 cm. The leaves are properly dissected, freely, pinnate, and capillary, and the white color flowers little in shape have symmetrical little pollen tubes, sepals, and petals and are present in compact umbels. The darkish-brown-colored fruits are round in shape and warm to the taste [617]
Fennel (Foeniculum vulgare)
Fennel belongs to the family Apiaceae and is an aromatic and medicinal plant applied for the treatment of galactagogue, carminative, digestive, diuretic, respiratory problems, and gastrointestinal diseases. The seeds are utilized as a flavoring ingredient to prepare dishes like ice cream, baked goods, meat and fish dishes, alcoholic beverages, and herb mixtures [749]. Fennel is applied as medicine with purgatives to diminish their side effects due to their carminative properties. Fennel seeds are consumed fresh as a sweetener and help in curing eyesight. Fennel contains phytoestrogens that improve the growth of breast tissue and induces an increased milk supply in breastfeeding mothers [20]. In the year 2020, 137.29 thousand tons of fennel seeds were produced in India [512]. Fennel is an upright, branching perennial herb that has tender, feathery, hair-like foliage. The height of the tree is around 6.6 ft (2 m), and it is used for its anise-flavored foliage. The seeds are utilized in cooking. It has been cultivated in almost all countries [620]
Poppy (Papaver somniferum)
Poppy is a traditional plant commonly called Khashkhash/Afyon belonging to the family Papaveraceae and is used as medicine. As per Unani literature, it has significant therapeutic values and is applied as a sedative, analgesic, narcotic, stimulant, and nutritive agent. It helps to prevent various types of diseases like insomnia, headache, cough, cardiac asthma, and biliary colic [582]. Poppy is cultivated throughout the world and is native and grown as an ornamental flower in South East Asia, Europe, South America, and North America. Poppyseed oil is a healthy edible oil and is utilized for various purposes. The purified opium (dried latex from plant fruit) is also used as a major therapeutic component. Purified poppy has been used to balance Vata and Kapha, dosha, and pitta dosha [572]. Poppy seeds are popular as a spice and are utilized to make fortified bread and confections [170]. Afghanistan possesses the highest acreage (6800 tons) for opium poppy cultivation [891]. The opium poppy grows for the whole year and is diploid (2n = 14) with a dominating self-pollinating mode of cross-breeding [926]. Plants are vertical, yearly herbs, height is about 1–1.5 m, stalk green, soft, and hairy. The roots are fine and medium and the leaves are tall, broad, and alternately arranged with serrated margins. The color of the flowers may be varied from red to black to white. The fruits have various cells and are of little size, and they get broken on their own, and the capsule is round and longitudinally grooved [492]
Clove (Syzygium aromaticum)
Cloves have many medicinal applications and are popular in treating mouth and throat inflammation and toothache. The main constituent of clove is eugenol possessing wide antibacterial properties against gram-negative, gram-positive, and acid-fast bacteria, and also fungi. Cloves are popular for their carminative, and antiemetic activities. In China, cloves have been consumed for medicinal purposes to prevent different types of diseases since 240 BC. Traditionally, cloves have been consumed for the treatment of stomach irritation, diarrhea, liver disease, bowel problems, flatulence, nausea, vomiting, and to maintain the nerve system. Cloves have been used to prevent different infections such as tuberculosis, malaria, cholera, and scabies. In America, it is traditionally utilized for preventing candida, worms, viruses, and several bacterial and protozoan infections [151]. The flower buds called clove are popular as a spice [119]. India is the leading producer of clove (1.20 thousand tons) [563]. Clove belongs to the family Myrtaceae and is a medium-sized plant (8–12 m), indigenous to the Maluku islands in East Indonesia. Currently, it is cultivated in Malaysia, Indonesia, India, Sri Lanka, Madagascar, Tanzania, and mostly the Zanzibar island [422]
Cassia bark (Cinnamomum cassia)
Cassia bark belongs to the family Lauraceae and is an evergreen, tropical aromatic plant that is utilized as a traditional spice. It is also used in traditional Chinese medicine utilized throughout the world [1012]. The leaves and bark of this plant are utilized as spices in home kitchens and their synthetic analogs or distilled essential oils are utilized as flavoring agents in the beverage and food industry [188, 230]. Cinnamon is obtained from the bark of tender branches and is utilized as a fragrance. It is used for its spicy flavor throughout the world. It is applied as a regular condiment. Cassia bark is distributed throughout Vietnam, China, India, and Indonesia. The bark of Cinnamomum cassia is Cinnamomi cortex that is popular as spices and is used as a seasoning in the western part of the world. It is also used as food supplement in some countries. It is used as a source of coumarin in America [983]. As per the Japanese, Unani, Ayurveda, and traditional Chinese medicine, the herb is utilized to prevent various diseases such as ischemic brain injury, dyspepsia, peptic ulcer, diabetes, and cancer [1005]. The major producer and exporter of cassia bark is Sri Lanka (48,002 MT) [817]. The plant is evergreen, its height is around 10 m, the branches are rigid, the bark is soft, and the color is yellowish. The leaves are leathery and grow up to 11–16 cm with dot tips,the color of the upper leaf is dark green and that of the below leaf is light green. The color of the flower is inconspicuous yellow and has a bad odor; the flowers are cylindrical in shape with 6 lobes. The size of the fruits is small with flabby berrylike structure [385]
Black pepper (Piper nigrum)
Piper nigrum belongs to the family Piperaceae Black pepper fruit is considered as “King of spices.” It is used to increase the flavor, and taste of foods. It shows biological activities due to the presence of different bioactive phytocompounds. Traditionally, the black pepper is used as veterinary drugs and in the treatment of gastrointestinal diseases and ear–nose–throat problems. Black pepper is considered as part of the kingdom of medicinal agents due to its important bioactive compounds with potential pharmacological and nutraceutical utilizations [910]. Black pepper is grown in various tropical regions such as India, Brazil, and Indonesia. Pungent and hot peppercorns are obtained from black pepper and are used as a preservative, medicinal agents, and in perfumery. The application of whole peppercorn or its active components has been used to formulate medicines, to prepare foods, sauces, and meat dishes. It is used in various traditional medicinal systems like Unani and Ayurvedic [821]. India is the greatest producer of black pepper (91.63 thousand tons) [563]. This plant is a flowering timbered perennial climbing vine and easily grows in the shadow of backing trees, maximum height is about 13 feet or 4 m and roots may emerge from leaf nodes if the vine contact with the ground. The plant leaves are heart-shaped, the length is 5–10 cm, and 3–6 cm diagonally, it has 5–7 eminent palmate veins. The flowers are little in shape, monoecious with different male and female flowers but may be polygamous. The fruits are 3–4 mm in diameter, known as a drupe. The dried fruits of black pepper are called peppercorn. The color of fully ripe fruits is dark red, and the diameter is about 5 mm. Each of the fruits consists of one seed, and a stem possesses 20–30 fruits [220]
Coriander (Coriandrum sativum)
Coriander seed is used as a spice, which possesses medicinal and nutritional properties. It helps in the treatment of nausea, bed cold, vomiting, belly diseases, and seasonal fever. It is utilized as a medicine to prevent rheumatism, worms, indigestion, and joint pain [737]Coriandrum sativum belongs to the family Umbelliferae The mature fruits contain delightful and fresh flavor due to the presence of essential oil. It is mostly utilized throughout the world in the ground or volatile isolate form to add flavor to tobacco products, sweets, beverages, and baked goods and as a basic ingredient in curry powder and also to make perfumes and soaps. It is cultivated as a household plant [175]. The largest producer of coriander seed is India (677.21 thousand tons) [701]. It is cultivated in India, especially in Karnataka, Tamil Nadu, Rajasthan, Andhra Pradesh, Madhya Pradesh, and Bihar. This plant is thin, glabrous, and branched. The fresh leaves are round and aerial leaves are extended. The flowers are white and look like an eggplant. The fruits are oval and partitioned into two parts. The flowering season of coriander is winter [611]
Nutmeg (Myristica fragrans)
Drugs are produced with the essential oil, and extracts of nutmeg. It has various pharmacological properties. In traditional medicines, nutmeg is utilized as a narcotic, carminative, stimulant, emmenagogue, abortifacient, reduced appetite, diarrhea, rheumatism, and muscle spasm. In China and India, nutmeg is used in producing medicines, and foods due to its availability and biological properties [412]Myristica fragrans is called “nutmeg.” Two spices are formed from it, namely mace and nutmeg. Nutmeg is the seed kernel beneath the fruit, and mace is the red lacy enveloping the kernelMyristica fragrans belongs to the family Myristicaceae It is indigenous to the Moluccas, native to Sri Lanka, India, and Indonesia. It is cultivated in many tropical countries like South Africa and Sri Lanka [675]. In 2021, the production of nutmeg in India is 15.24 thousand tons [563]. Nutmeg is an evergreen aromatic plant. The height of this tree is about 5–13 m, occasionally 20 m. The bark possesses watery red to pink juice. Leaves are dark green, have shiny surface, and settled upon alternately along the branches. The leaf stem is about 1 cm long. Flowers are bell-shaped, pale yellow, flabby, and fleshy. The fruits are yellow, fleshy, drooping, soft, and 6–9 cm long with a lengthwise ridge. Seeds are extremely ovoid (2–3 cm long), whitish, firm, fleshy, and lateral by red-brown veins and the mace becomes bright red and is more corneous when fresh, and when dried becomes brittle and the color becomes yellowish-brown. Nutmeg is famous as a spice and contains several therapeutic activities. It shows a characteristic pleasant fragrance and a little hot taste. It is utilized to increase the flavor of puddings, baked foods, confections, meats, sausages, saucers, vegetables, beverages, curry powder, teas, soft drinks, or added to milk, and alcohol [664]
Black mustard (Brassica nigra)
Brassica nigra belongs to the family Brassicaceae. It is popular as a spice and a cheap source of antimicrobial agents [732]. It is cultivated in the Mediterranean region and several other countries such as Europe, and India. It is the main source of the mustard seed which is utilized as a spice [22]. In 2019, Nepal is the world’s largest producer of mustard seeds accounting for more than 32% of the global production [529]. For the treatment of malaria, seeds are powdered and pasted with water and then taken with or without “ Injera” [320]. The height of this tree is about 2–7 cm, broadly branching, pubescent or glabrate. The lower leaves are thin petiolate and densely pinnatifid and have one marginal big lobe and 2–4 little adjacent ones,the upper leaves are little-petiolate or sessile, dentate. The flowers are bright yellow colored, 3–5 inches wide [939]. Black mustard seed has been utilized as a remedy for brain and lung edema, neurotic pain, rheumatoid arthritis, paralysis, migraine, and epilepsy in Iranian traditional medicine [470]
Turmeric (Curcuma longa)
Curcuma longa L belongs to the family Zingiberaceae family. It is a perennial herb. The height of this tree is approximately 3.5 ft [83]. It is indigenous to Southern Asia [345]. In 2021, the major producer of turmeric is India (1102.91 thousand tons) [563]. Curcumin plays an important role as a therapeutic agent. For different diseases, it is used in human clinical trials [496]. Indian turmeric is most famous compared to other countries due to the presence of curcuminCurcuma longa rhizome is called Haldi or turmeric. Turmeric is also known as “Indian saffron.” It is commonly used as an antiseptic and shows high medicinal and nutritional value. Rhizome of Curcuma longa is utilized as a spice for its flavoring properties. It is utilized as medicinal food due to its therapeutic properties [187]. In Ayurveda, it has been utilized as an ethnomedicine and coloring agent to dye unmordant cotton, wool, and silk. It is utilized in the Indian medicinal system to prevent stomachache, antacid, carminative, blood purifier, wound healing, and inflammation [545]. It is cultivated broadly in Asia, especially in China, and India. It is distributed throughout subtropic, and tropic areas of the world. It is also cultivated in Japan, Southern China, Taiwan, Burma, and Indonesia as well as in Africa. The color of the flower of this plant is dull yellow [967]
Bay leaves (Laurus nobilis)
Laurus nobilis belongs to the family Lauraceae It is a small evergreen plant, marketed as sweet bay leaves, and Roman or Turkish laurel [932]. It is a fragrant and aromatic plant that contains volatile components and fixed oil (non-volatile oil of plant origin) as well as camphor. It is indigenous to South Europe [123]. The dried leaves and essential oil are applied in cosmetics, in foods as a spice, in drugs, and also for industrial purposes for the seasoning of fish, meat products, and soups. Bay leaves are utilized in the food industry as food preservatives due to their antimicrobial properties. The volatile and fixed oil present in fruits is mainly required for the formation of soap [163]. It is cultivated in the tropical and subtropical regions of East Asia, South, and North America. The natural residence of this herb is found in the Mediterranean area. Traditionally, bay leaves have been utilized in Mediterranean cuisine for seasoning, as well as fruits are used to treat rheumatism, viral infections, cough, digestive problems, diarrhea, and other health conditions in the folk medicine system [256]. India is the largest producer of bay leaves (6.20 thousand tons) [563]. This plant is a shrub. The height of this plant is about 2–20 m, thin, glabrous twigs, and thin oblong-lanceolate, sturdy leaves and dioecious with the female, and male flowers on a different tree. The flower grows in couples alongside a leaf with a pale yellow-green color. The diameter of fruits is about 1 cm. The fruits ripen in the downfall, and the shape of the fruit is oval [56]
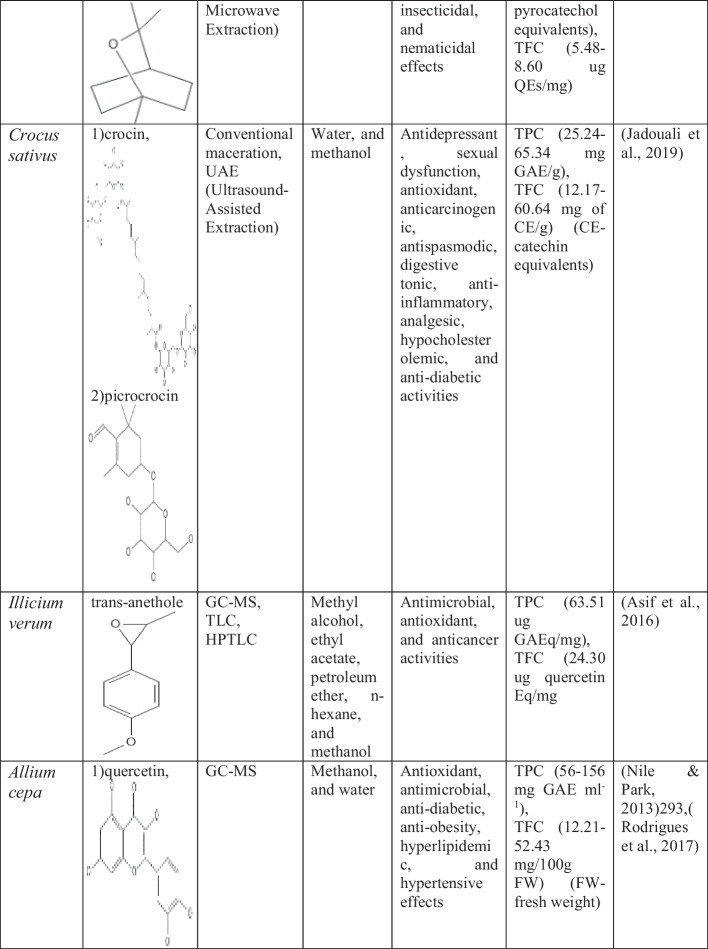
Saffron (Crocus sativus)
Saffron is the dried orifice of the Crocus sativus flower that is used as a spice. It is considered among the main terroir products and a source of income for many areas of Morocco. According to Chinese, Ayurvedic, Mongolian, Egyptian, Greek, and Arabic medicines, saffron has been taken into account as a preventive measure for various diseases. It is utilized as a source of traditional medicine from ancient times. Saffron shows many therapeutic properties like antidepressant activity, treating sexual problems, digestion problems, lowering cholesterol levels, controlling blood sugar levels, healing second-degree burns, and treating eye disability [633]. The largest producer and exporter of Saffron is Iran (430 tons) [826]. The Crocus sativus belongs to the family Iridaceae It is an herbaceous perennial plant that grows up to 10 to 25 cm long, developing from its bulbs. The bulb is of sub-ovoid shape and available in different forms and sizes [1022]
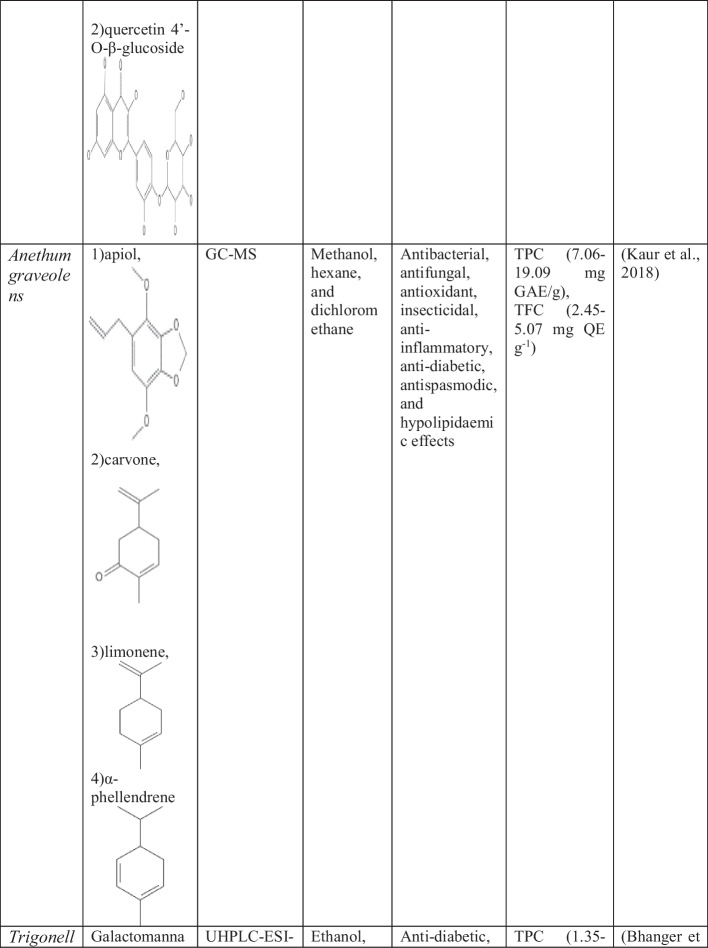
Star anise (Illicium verum)
Illicium verum belongs to the family Illicuaceae It is an evergreen aromatic plant sometimes contaminated with very poisonous Japanese star anise and toxic star anise that possess various neurotoxic sesquiterpenes [981]. Star anise is indigenous to the Southwest of China. It is cultivated in tropical, and subtropical areas of Asia [274]. The production of star anise seed in Vietnam is more than 5000 tons per year and the combined production of Vietnam, and China is more than 25,000 tons per year [832]. It is a medium-sized tree. The height of this tree is about 8–15 m and the breadth is 30 cm. The color of the bark is white to bright grey. The leaves are 6–12 cm tall, sturdy, alternate, simple, complete, glabrous, very bright, and usually packed with bundles at the end of the branches. The flower is large, diameter is 1–1.5 cm, bisexual, white–pink to red to greenish-yellow, axillary, and lonely [962]. Dry fruits and seeds of star anise are popular as a spice in Chinese cuisine. Star anise oil is applied naturally for otalgia, and rheumatism, as an antiseptic, cough, toothache, sinusitis, and also as food preservatives traditionally. The star anise oil is utilized to treat dysentery, flatulence, and spasmodic pains and relieves colic [831]
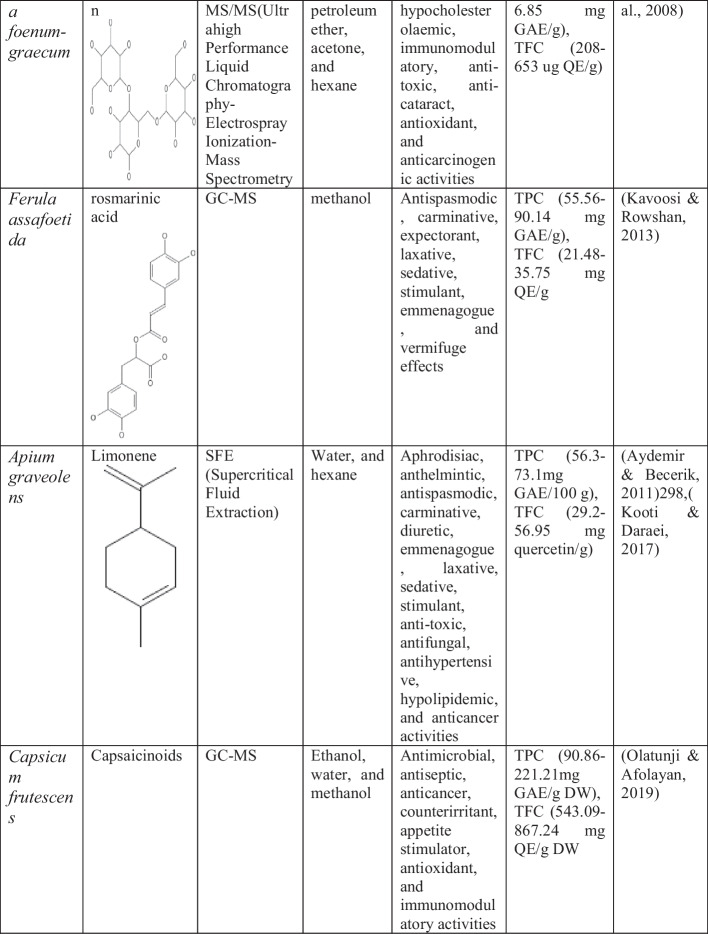
Shallot/ Onion (Allium cepa)
A highly consumable vegetable, onion, is well known for its flavor. It is the third most important horticulture spice having remarkable marketable importance [925]Allium cepa belongs to the family Amaryllidaceae. The edible portion of the onion is the root, which is also called as the bulb. The colors of onion varied from purple to yellow to red to white green and may be categorized by its rancidity [870]. According to the medicinal characteristics, traditionally onion has been utilized in the prevention of several diseases and applied as a blood purifier for athletes in ancient Greece. Onion is used to heal wound, pneumonia, and diuretic. Onion is considered as one of the essential spice or vegetables and has been considered as a medicine in India since the sixth century [416]. India is the highest onion producer (28,853.35 thousand T) in 2020–2021 [257]. Onion is distributed in temperate areas like North America, Europe, Asia, and Africa [156]. This plant is a biennial plant with fortuitous, fibrous roots, and glaucous leaves. The bulb is produced of converging, large flabby leaf bases. When the bulb matures, the outward leaf base dries, becomes slender, many-colored, and produces a defensive coat, while the inside leaf bases thicken and develop the bulb which may be globose, egg-shaped, or extended. According to the cultivar, the size of the onion varies differently [533]
Dill (Anethum graveolens)
Anethum graveolens belongs to the family Apiaceae It has been utilized since ancient times in Ayurveda. It is a famous, aromatic, and annual herb. Dill seeds are used as a spice and also produce essential oil. The dill seeds are used as a preventive agent for diuretic, carminative, and stomachache in Ayurveda [403]. The highest producer country of dill is India (30.40 thousand T) [563]. The height of this plant is about 90 cm. It has thin stems. Leaves are alternate and are finally divided three or four times into winged sections slightly wider than the same type leaf of fennel, and the yellow flower forms into umbels. The seeds are very little in size. The dry fruits are known as schizocarps [747]. It naturally grows in the Mediterranean area, Southern, and Central Asia. It is cultivated broadly throughout the world [997]
Fenugreek (Trigonella foenum-graecum)
Trigonella foenum-graecum belongs to the family Fabaceaeis. It is an ancient medicinal plant. It is widespread throughout the world. It has been utilized as medicine and traditional food. Current investigation has determined fenugreek as an important herbal tree having a powerful effect on curing diseases and as a source of bioactive compounds for the pharmaceutical industry such as steroidal hormones [871]. The leaf and the seed of fenugreek are consumed as a spice. It is also utilized as an ingredient in traditional medicine due to its strong flavor and aroma [4]. Fenugreek is recommended as a valuable medicine to prevent various diseases like mucosal problems, digestive problems, fever, sore throat, wounds, swollen glands, skin irritation, diabetes, bronchitis, and ulcer in the ancient Indian traditional medicinal system like Ayurveda [687]. The largest producer of fenugreek is India (115,929 metric tons). But a substantial amount of the production is eaten internally in the country [347]. The height of the fenugreek plant is about 1–2 feet. It has green trifoliate leaves. The flower color changes from white to yellow. The plant retains narrow pods. The length of the pods is about 15 cm, and they possess an average of 10–20 seeds [589]
Asafoetida (Ferula assafoetida)
Ferula assafoetida belongs to the family Apiaceae It is one of the more valuable plants among the 30 species of Ferula. It is distributed in Iran. It is an herbaceous, and perennial plant. It has been found in Iranian folk medicine as a carminative, antispasmodic, aromatic, digestive, expectorant, laxative, sedative, nerving, analgesic, anthelmintic, antiseptic, and aphrodisiac agent. It is a valuable plant used in veterinary, traditional medicine, and also for non-medicinal purposes. Kerman in Iran is the leading producer of this plant [157, 331]. The rhizome or tap root is used as a spice. It is popular in curing digestive disorders. It is utilized in recent herbalism in preventing bronchitis, hysteria, nervous situations, asthma, whooping cough, infantile pneumonia, reduced blood pressure, and flatulent colic. Since ancient times, in Ayurveda, and the Unani system, it is broadly utilized. Asafoetida is naturally found in a topical zone, particularly in Central Asia, Eastern Iran to Afghanistan. Afghanistan is the highest producer of asafoetida (23,021 thousand tons). At present time, it grows in Afghanistan and Iran from where it is exported to other places. It has been utilized as a cookery agent. It has large carrot-shaped roots, and when they become 4–5 years old the diameter is about 12.5–15 cm [554]
Celery (Apium graveolens)
Apiumgraveolens belongs to the family Apiaceae. The dried and ripe celery seeds are popular as a spice. It has been applied for the treatment of diuretics, spasms, stomach problems, and heart tonic to reduce blood pressure in African traditional medicine [515]. India is the highest producer of celery (30.40 thousand tons) [563]. It is also used to cure joint pain. The center portion of the root of celery is fugitive. The stem is branched, notched, juicy, and hardy. The leaves are winged and ovate. The flower size is small and the color is white to greenish-white. Petals are circular and fruits are schizocarp with two mericarps, suborbicular to ellipsoid in shape and a little bitter in taste [55]. It is mainly famous in countries like Iran, Algeria, the Caucasus, India, and America [479]
Chilli (Capsicum frutescens)
Capsicum frutescens belongs to the family Solanaceae It is a temperate plant. Cayenne pepper/chili is used traditionally in medicine and in the diet as an ingredient in warm sauces [32]. Chilli is utilized in ethnomedicinal treatments of postnatal care to cure erectile problems, nutrition therapy, pain management, as a circulatory medicine, as a tonic for arthritis, and rheumatic pains, lower the blood glucose level, for cholesterol extraction [413]. India is the largest producer of chili (3992 thousand tons) [563]. It is a perennial shrub/household annual plant. It has little vertical, wheat-shaped fruit that is spicy in flavor, and the fruit color changes from green to pale yellow that converts into red color when it ripens. The most commonly wild pepper species is found in the tropical region of China, namely Hainan and Yunnan territory [238]. This plant is a yearly growing plant or short-lived perennial herb. Its stem is striate, glabrous, 1–4 feet long according to growing conditions and weather. The leaves are oval, slightly leathery, the color is dark green, soft, height is 2.5 inches, and 1 inch broad. The flowers are naturally conifer or funnel, having five petals, combined, and white. The fruits are vertical, ellipsoid-conical to lanceoloid, with a length of about 10–20 mm and a diameter of 3–7 mm [50]
Allspice (Pimenta dioica)
Pimenta dioica belongs to the family Myrtaceae It contains an aromatic flavor and tastes similar to a mixture of nutmeg, cinnamon, and cloves; hence, it is called allspice. It is topical to the Caribbean area mainly Cuba and Jamaica. It grows naturally at an average temperature between 180 and 240. The plant is broadly cultivated in temperate areas throughout the world as a decorative plant, because of its catching appearance, and fragrance [745]. It is an evergreen plant. The height of this plant is up to 15 m with pale brown bark. The leaves are normal, opposite, oblong-elliptical, 6–20 cm long, with pellucid glands that provide an all-spice odor when squeezed. The flowers are tiny in size, whitish with a peculiar aroma, each of the flowers contains four petals, and the color of the flower is white and deciduous. The flowers are grown during March–June. The fruits mature in 3–4 months and are picked for spice collection when it is fully matured but still green. The fruits contain two kidney-shaped seeds [859, 860]. Since time immemorial, allspice is popular as a valuable spice due to its medicinal, and culinary properties. Its leaves are utilized to flavor the rice and provide a good aroma. Water extract of the berries is applied to cure diarrhea and flatulence. The powdered fruits are utilized for rheumatism, neuralgia, and aromatic provoking in digestive problems traditionally in India. It is effective in tonics, purgatives, and anodyne against neuralgia. In Turkey, it is utilized as an aphrodisiac when taken along with honey. The oil of the fruits and leaves are used in the food industry, specially in tanning, and meat as well as in cosmetic products, and perfumery compositions [745]
Kokam (Garcinia indica)
Garcinia Indica belongs to the family Clusiaceae It is a little to moderate-sized plant that is used as traditional house medicine for infections, flatulence, and heart attack. Numerous therapeutic activities of the fruit are documented in Ayurveda, like curing infusion, skin disease (rashes caused by allergies), scalds, chaffed skin, relief from sunstroke, treatment for dysentery, treatment of burns and mucous diarrhea, as an appetizer, to recover appetite, to reduce thirst, cardiotonic, piles, bleeding, and tumors. Kokam is prepared by sun-drying. The outer part of the fruit is used as a spice to add flavor and color to dishes. Kokam is a tropical evergreen tree. It is a thin plant having drooping dals height of about 15–20 m. It is native to the Western ghats area of India and distributed along North, and South Karnataka, Konkan, Goa, North Malabar, Coorg, West Bengal, Wayanad, and Assam [367, 395]
Greater galangal (Alpinia galangal)
Alpinia galangal belongs to the family Zingiberaceae It is distributed in tropical regions. It is mainly utilized in ethnomedicine and in food preparation. It is native to Indochina and Southeast China, especially Hainan, Guangdong, and Guangxi. It is cultivated throughout Eastern Himalayas, West Bengal, and Assam. It is utilized conventionally in Chinese medicine and Ayurveda. In China, rhizome from this plant has been utilized to treat bracing the circulatory system, stomach aches, colds, and to lower swelling [133]. It is commonly utilized for the treatment of bronchitis, eczema, coryza, gastritis, otitis interna, ulcers, morbilli, pityriasis Versicolor, cholera, to wash the mouth, and emaciation [279]. Indonesia is the leading producing country of galangal (303.53 million kg) [222]. It contains aromatic and tuberous rootstocks. The flowers are 30 cm long, greenish-white, and bracts ovate are lanceolate. Leaves are glabrous, oblong, lanceolate, and green, paler beneath, to some extent callus white margins. The color of the fruits varied from red cherry to orange [904, 965]
Sweet flag (Acorus calamus)
Since ancient times, the sweet flag exhibits various traditional, and ethnomedicinal applications in Siddha, Ayurveda, Unani, and Chinese medicine to treat different types of health problems such as bronchitis, nervous problem, appetite loss, chest pain, colic, cramps, diarrhea, digestive problem, flatulence, gastric problem, indigestion, rheumatism, sedative, cough, fever, inflammation, depression, tumors, hemorrhoids, skin ailment, numbness, and vascular diseases. The rhizome is used as a spice to provide flavor to food and alcoholic beverages [741]. Since ancient times, the sweet flag has been harvested. It is commonly cultivated in Asian countries. It is a very important tree in medical sciences [865]Acorus calamus belongs to the family Acoraceae It is an annual plant having weird and extensively branched, aromatic rhizome, tubular, up to 205 cm thick, the color changes from purplish-brown to lightly brown on the outside and white in the middle portion. The leaves contain one eminent midvein. This plant rarely grows flowers or produces fruits. The flowers are 3–8 cm long, tubular in shape, greenish-brown and are surrounded by a multitude of circular spikes. The fruits are little in size like berries, having some seeds [121]
Nutritional Potential of Spices
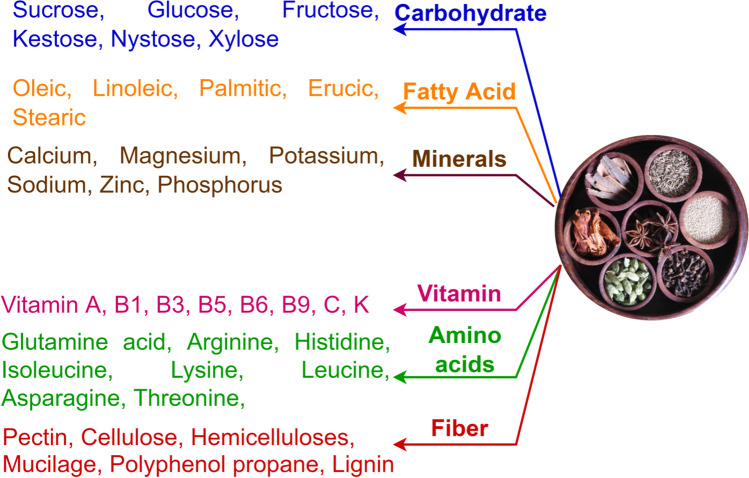
Spices help to keep the body healthy and fit [86] (Fig. 2). The spices contain carbohydrates, minerals, proteins, and vitamins. Table 1 and Table 2 portray the nutritional composition of the spices and herbs. Though spices are used in very small quantities in food, the amount of protein, fat, carbohydrate, and energy, which can be acquired from the spices is very negligible; however, the spices are rich source of various essential oils and fatty acids; thus, it is a source of essential nutrients for body development and to maintain immune system
Fig. 2.
Nutritional potential of spices and herbs
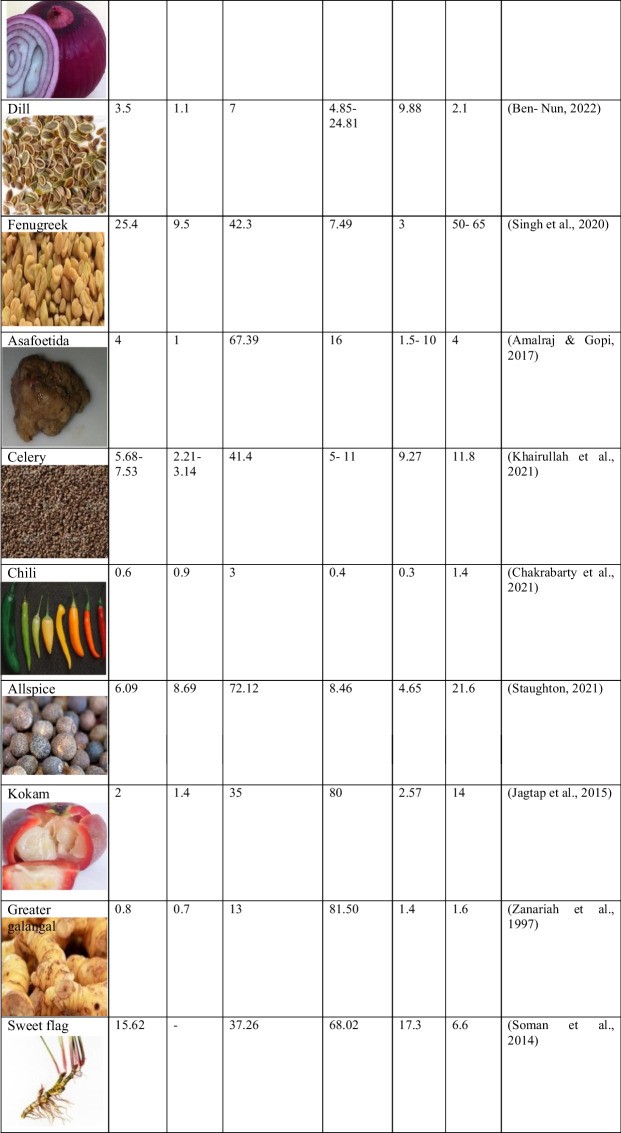
Table 1.
Proximate composition of different spices
Table 2.
Mineral composition (mg/100 g) of selective spices
| Spices | Ca | Mg | Mn | Fe | Cu | Zn | Na | Reference |
|---|---|---|---|---|---|---|---|---|
| Cardamom | 92.7 | 181.5 | 41.7 | 12.8 | 0.5 | 3.6 | 17 | [89] |
| Shah jeera | 689 | 258 | 1.3 | 16.2 | 0.91 | 5.5 | 17 | [949] |
| Fennel | 49 | 17 | - | 0.73 | - | 0.2 | 52 | [110] |
| Poppy | 690.50 | 287.20 | 3.84 | 5.47 | 2.58 | 2.57 | 81.16 | [621] |
| Clove | 0.64 | 60 | 0.25 | 8.68 | 0.23 | 2.32 | 243 | [500, 950] |
| Cassia bark | 1002 | 60 | 17.46 | 8.32 | 0.33 | 1.83 | - | [329] |
| Black pepper | 400 | 235.8–249.8 | 12.8 | 17 | 1.33 | 1.45–1.72 | 10 | [88] |
| Coriander | 709 | 330 | - | 17.9 | - | 4.70 | 35 | [145] |
| Nutmeg | 189 | 183 | 2.90 | 3.04 | 1.03 | 2.15 | 16 | [419] |
| Black mustard | 266 | 370 | 2.45 | 9.21 | 0.64 | 6.08 | 13 | [636] |
| Turmeric | 200 | 208 | 19.8 | 47.5 | 1.3 | 4.5 | 10 | [714, 951] |
| Bay leaves | 834 | 120 | 8.16 | 43 | 0.41 | 3.70 | 23 | [135] |
| Saffron | 111 | 264 | 28.40 | 11.10 | 0.32 | 1.09 | 148 | [794] |
| Star anise | 646 | 170 | 2.3 | 37 | 0.91 | 5.3 | 16 | [948] |
| Onion | 23 | 10 | 1.29 | 0.21 | 0.03 | 0.17 | 4 | [947] |
| Dill | 208 | 55 | 1.3 | 6.6 | 0.14 | 0.9 | 61 | [138] |
| Fenugreek | 176 | 191 | 1.23 | 33.53 | 1.11 | 2.5 | 67 | [560] |
| Asafoetida | 690 | 80 | 1.1 | 39 | 0.4 | 0.8 | - | [70] |
| Celery | 403–709 | 243–556 | 35.3–39.3 | 101.4–305.2 | 39.98–56.90 | 11.96–15.61 | 80 | [443] |
| Chili | 7.8 | 8 | 0.1 | 0.4 | - | 0.1 | 1.6 | [182] |
| Allspice | 661 | 135 | 2.94 | 7.06 | 0.55 | 1.01 | 77 | [892] |
| Greater galangal | 5000 | 2000 | 1000 | 1000 | - | 1000 | - | [228] |
| Sweet flag | 158.56 | 64.4 | - | - | 1.15 | 1.80 | 182.3 | [876] |
Carbohydrate
Carbohydrates are present in fennel, especially glucose, fructose, and sucrose, and are found in all the parts of fennel. The carbohydrates are composed of sugars that are naturally formed. Sucrose is the most valuable sugar in plants. Few percentages of sucrose could have been hydrolyzed to their monosaccharides to enhance the fructose, and glucose level in fennel that plays a valuable role for the contribution of carbon skeletons for the production of other compounds, and in the energetic metabolism. Mainly carbohydrates play an important role as major structural compounds and as short-term energy storage compounds [130]. Fennel and nutmeg contain an abundant amount of carbohydrates [21, 958]. Onion contains nonstructural carbohydrates (NSC) like sucrose, glucose, fructose, and fructo-oligosaccharides (FOS) such as fructo-furanosylnystose, ketose, and nystose [140]. Bay leaves are rich source of carbohydrates especially sucrose, fructose, and glucose [374]. Carbohydrates like L-arabinose, glucose, galactose, rhamnose, and polysaccharides present in asafetida [969]. Kokam contains carbohydrates, especially xylose, and glucose [1004]. The carbohydrate content of different types of spices is described in Table 1. The highest carbohydrate-rich spice is cassia bark (80.59%), and the minimum amount is observed in chili (3%)
Protein
Protein helps in building up the body tissue, distributes as fuel source. Protein helps in the survival period of humans, and animal. Protein plays an important role in diet for maintaining proper functions of the body [35]. It is observed that cassia bark contains protein and provides good nutritional quality due to the presence of many essential amino acids like glutamic acid, lysine, aspartic acid, leucine, and valine. Naturally, the protein quality is determined by the amino acid profile [47]. The major amino acids present in onion are glutamine acid, and arginine [897]. Fenugreek is rich source of free amino acids such as histidine, and isoleucine that can provoke the secretion of insulin. Fenugreek provides an adequate amount of lysine. The quality of fenugreek lysine is the same as soybean lysine. For this reason, fenugreek is taken as dietary supplement. It is noted that fenugreek seeds contain significant concentrations of leucine, glutamine, asparagine, threonine, and arginine [1023]. Asafoetida contains mainly arabinogalactan protein [444]. Chili and poppy have several amino acids like isoleucine, tryptophan, threonine, leucine, lysine, methionine, cysteine, phenylalanine, tyrosine, valine, arginine, histidine, alanine, aspartic acid, glutamine acid, glycine, proline, and serine [182, 922]. Black mustard is the rich source of protein among the spices covered in this review article (26.08 g/100 g)
Fatty Acids
Nowadays, the importance of essential fatty acids like alpha-linolenic acid, and linoleic acid, and their metabolites in animal, and human health is a vital topic in science [828]. Fatty acids help in the metabolic mechanism of living organisms. It is a source of several bioactive particles, energy, and structural elements [357]. Dietary fatty acids especially monounsaturated, and polyunsaturated fatty acids constituent the plasma lipoprotein profile and decrease the chances of heart problems [898]. Fatty acids can be divided into (1) saturated (2) monounsaturated (3) polyunsaturated. Cardamom contains fatty acids like saturated (myristic acid, palmitic acid, stearic acid, arachidic acid), mono-unsaturated (palmitoleic acid, oleic acid), polyunsaturated (linoleic acid, alpha-linolenic acid, eicosenoic acid) [89]. It is evidenced that palmitic acid is the major fatty acid present in the oil of cassia bark [47]. Fennel fixed oil contains some major fatty acids like palmitic acids, oleic acid, and linoleic acid [762]. The major fatty acid in poppy is linoleic acid covering 70.7–75.2% of the total fatty acid content [542]. During the ripening of coriander fruit, the amount of monounsaturated fatty acid has been increased, while the amount of polyunsaturated and saturated fatty acids is decreased. Coriander contains various fatty acids, namely myristic acid, stearic acid, palmitic acid, behenic acid, arachidic acid, petroselinic acid, oleic acid, linoleic acid, and linolenic acid [653]. Palmitic acid, lauric acid, myristic acid, myristoleic acid, stearic acid, oleic acid, palmitoleic acid, and linoleic acid are obtained from nutmeg [1016]. The fatty acids like stearic, palmitic, heptadecanoic, linoleic, oleic, linolenic, eicosenoic, arachidic, behanic, heneicosanoic, erucic, lignoceric, docosadienoic, and nervonic are present in Brassica nigra [435, 436]. Saturated fatty acids exist in an abundant amount in saffron [209]. Chinese star anise contains more palmitic, oleic, and linoleic acid [426]. The predominant fatty acid in Allium cepa is linoleic acid followed by oleic and palmitic acid [332]. Dill seed oil contains about 8.51% of saturated fatty acids (stearic acid, and palmitic acid) and about 91.35% of an unsaturated fatty acids [873]. Egyptian fenugreek oil contains 13.8% linolenic acid, 33.7% linoleic, and 35.1% oleic acid [898]
Minerals
Minerals are known as micronutrients that play an important role in the body's immunity system and metabolism. Spices are considered as good sources of minerals. The insufficiency of mineral content in human body is attributed due to 1) improper absorbance and 2) inadequate intake. Enzymes required several minerals as the co-factor for proper activity and function. These enzymes got deactivated due to the absence of minerals [979]. Minerals are important for the antioxidant activity. Inadequate consumption of mineral-enriched food products is observed among a vast number of economically backward people. Mustafa [631] studied that calcium (Ca), phosphorus (P), potassium (k), sodium (Na), and magnesium (Mg) are present in spices. The spices, Elattaria cardamomum, and Curcuma longa contain ample amounts of important trace elements like manganese (Mn), iron (Fe), and zinc (Zn). Derivative of minerals in spices helps in various activities of the body’s growth like Fe assists in cellular growth, oxygen transport, and oxidative metabolism. Similarly, calcium and copper are critical to maintain various physiological activities. Zn plays a valuable role in replication and cellular immune response. Consumption of spices can add minerals into the diet and contributes a nutritious effect. But excess level of minerals consumption can cause toxicity. It is observed that Ca, Na, K, and Mg were comprehensively higher in all the spices. Ca content is higher in laurus leaves and black seed. Copper is relatively very less in all spices [631]. K, Ca, Mg, P, sulfur (S), and iron (Fe) are present in Foeniculum vulgare and cardamom. Particularly, cardamom leaves and capsules possess significant levels of Mn and Zn [89, 749]. Poppy contains minerals like K, P, Ca, Mg, Na, and Fe [630]. Clove is one of the spices that is one of the highest rich sources of manganese and is important for enhancement of bone strength and metabolism and contributes in the development of enzymes. The occurrence of the strong appearance of the clove is due to the presence of K, Mg, and Ca [151]Cinnamomum cassia possesses an ample amount of Ca [47]. The electrolytes like K, and Na and minerals such as Fe, Ca, Cu, Mg, Mn, P, Zn are present in Myristica fragrans [21]. Mg, P, Ca, and K are present in adequate amount in turmeric [710]. The seeds of star anise are source of minerals like Cu, Ca, Fe, K, Mn, Mg, and Zn [831]. Calcium is present in a higher amount in the brown skin of the onion and the whole onion contains an ample amount of Zn, Mg, Fe, and Mn [140]. Dill contains micronutrients like Fe, Zn, Cu, and Mn and four macronutrients Mg, K, Ca, and P but Fe, Ca, and P [782]. Fenugreek seed and asafoetida are good sources of Fe and Ca [679, 710]. P, Zn, Mg, Mn, Ca, Se, Cu, Na, Pt, Fe, and K are some important minerals present in celery [822]. Chili contains minerals like K, Ca, Fe, P, Na, Mg, Cu, and Zn that play a valuable role in human health development [669]. kokum contains minerals like Ca, Mn, Mg, and K that protect against heart disease, control blood pressure, and heart rate [109]
Vitamins
Vitamins like vitamin A, C, riboflavin, thiamine, niacin, and pyridoxine (B6) are present at higher amounts in cardamom [281]. The coriander green leaves possess important vitamins like vitamin C, riboflavin, niacin, and vitamin A [634]. The valuable vitamins like niacin (B3), thiamin (B1), riboflavin (B2), pantothenic acid (B5), pyridoxine (B6), folic acid (B9), and vitamins E and C are present in poppy [673]. Cassia bark has important vitamins like vitamin C, and A [329]. Fennel is rich source of vitamins like B2, C, B1, B3, B6, folate, and vitamins A, E, and K [130]. Vitamins E, A, and C are the main vitamins of nutmeg [21]. Clove is one of the rich sources of vitamin K, and C [151]. Vitamins like B2, vitamin C, K, and B6 are present in abundant amounts in black pepper [853]. Turmeric is an exuberant source of vitamin C, and pyridoxine [710]. One of the main constituents of saffron is vitamins especially B1, and B2 [321]. Many essential B-complex vitamins like riboflavin, pyridoxine, niacin, thiamin, and vitamins C and A are present in adequate amounts in anise seeds [830]. Onion provides high amounts of folic acid, and vitamin B6 [897]. Dill contains mainly vitamin E, and A [782]. Fenugreek seeds contain adequate amount of vitamin C, A, B1, and nicotinic acid [1023]. Vitamin C is mainly present in celery [443]. Chili is one of the great source of vitamin C that is a potent antioxidant that develops natural immunity against diseases. Chili also contains vitamin A which is a fat-soluble vitamin that assists to reduce the health hazards caused by free radicals and assists in the formation of red blood cells and other B-complex vitamins B6, K, B3, B2, and B1 [182]. It is noted that kokam leaves are rich source of ascorbic acid and B-complex vitamins that assist to maintain blood pressure and heart rate [395]. Vitamins C and A are found in adequate amount in greater galangal [122]
Fiber
Fiber is a very important nutrient in maintaining the human health and has been found to decrease the level of cholesterol, decrease the chances of different types of cancers and bowel disorders, maintain carbohydrates and lipid metabolism, cure gut function, and assist in constipation problem and well-being of individual [35]. Fibers are composed of a group of compounds such as non-starch polysaccharides (pectin, cellulose, hemicelluloses, and mucilage), polyphenol propane, and lignin. The fibers are present in most of the plants and cannot be hydrolyzed within the human body [310]. Cassia bark contains a high amount of crude fiber [47]. Fennel, nutmeg, and clove are rich sources of dietary fiber [21, 130, 151]. It has been observed that total dietary fiber is high in brown skin of onion [140]. Black pepper, turmeric, onion, and kokam are good sources of dietary fiber [710, 756, 853, 897]. The maximum fiber content is seen in cassia bark (53.1%), and the lowest amount is reported in chili (1.4%)
Bioactive Potential of Selected Spices and Herbs
Nowadays, it has become important to search for natural bioactive components to replace synthetic compounds. The bioactive compounds from natural sources have several advantages over the synthetic drug molecules and have several side effects [809, 810]. Spices are a very necessary resource due to the presence of an ample amount of bioactive components (Table 3), for the searching of a new substitute, and bioactive molecules in drug formation studies for different types of diseases [803]. Spices possess various phytochemicals that contain antioxidative activity and lead to a decrease in inflammation, modulation of detoxification of enzymes, manifestation of the immune system, antibacterial, and antiviral activities [513]. Spices contain various active components like phenolic acids, phthalides, polyacetylenes, flavonoids, coumarins, and terpenes and are recommended to be potent antioxidants [86]
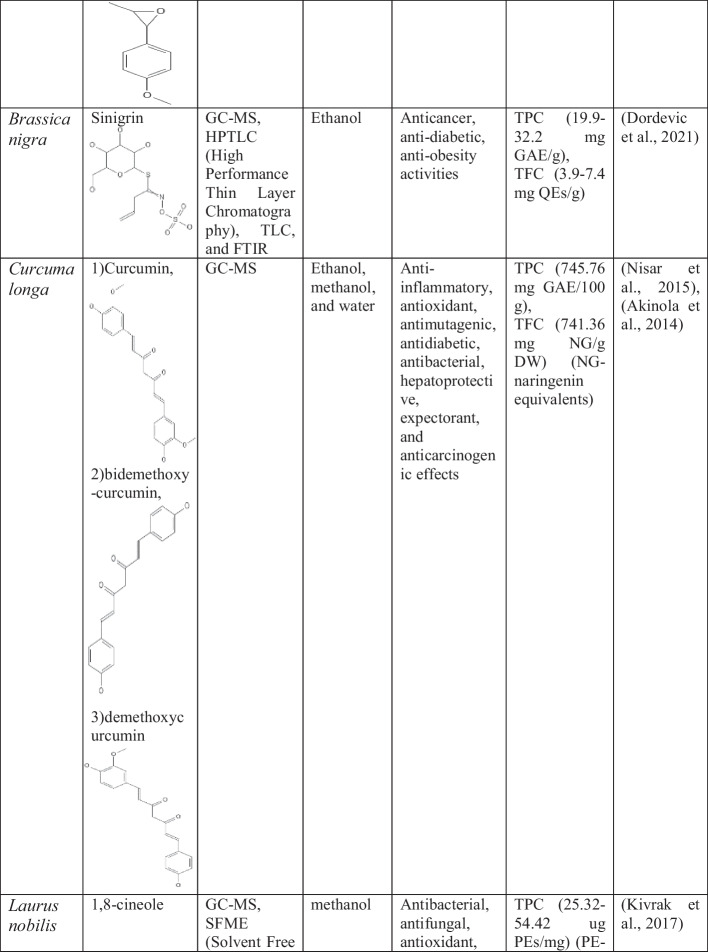
Table 3.
Bioactive potential and health-beneficial effects of spices

Elettaria cardamomum (Cardamom)
The main constituent present in cardamom is 1,8-cineole, which is responsible for its characteristic aroma. The potential antimicrobial effect is observed in cardamom essential oil (CEO) against gram-negative and gram-positive microorganisms [656]. The volatile oil is the most important component of cardamom, has its characteristic aroma. The oil contains small mono- or sesquiterpene hydrocarbons. It is mainly formed by oxygenated compounds,all of these are potential aroma compounds. The aroma variations in different sources of cardamom are due to the alteration of proportion of the 1,8-cineole and esters. The minor compounds like alcohols, methyl eugenol, and terpene hydrocarbon are present in cardamom. The characteristic aroma of cardamom is formed by the combined effects of major components like 1,8-cineole and alpha-terpinyl acetate. These compounds are found to be carminative, antiseptic, anti-inflammatory, and stimulating [481]. The cancer can be treated by various methods like chemotherapy, radiation therapy, palliative care, surgery, targeted cancer therapy, etc. However, these methods exhibited toxic side effects on the patient’s normal cell, and overall health condition. For this reason, spices have been used as an alternative way to treat the breast cancer, with bioactive components like anthocyanin, alkaloids, flavonoids, terpenes, and phenylpropanoids which can retard biological activities occurred with breast cancer cell growth. In traditional Ayurveda, cardamom is used in human diet to prevent the growth of cancerous cell. Cardamom is a profoundly used spice and shows anti-carcinogenic potential due to the presence of DCM (diindolylmethane) and IC3 (indole-3-carbinol) that can destroy breast cancer cells and retard prioliferation. These compounds also give additional anticancer benefits by developing host immune response. For this reason, it is suggested that cardamom should be intake regular basis. Phytochemicals like limenonene and cineole present in cardamom act as chemoprotective [977]
Bunium persicum (Black jeera)
The various extracts of seeds, and essential oil of Bunium persicum has been analized for antioxidant activity by three methods, namely ammonium thiocyanate, DPPH assay, and beta-carotene bleaching. It is observed that the highest antioxidant activity is found in methanolic extract of oil. The major components of Bunium persicum oil are gamma-terpinene and cuminaldehyde as evaluated by the GC/MS method. With the help of column chromatography, the methanol extract of the plant has been fractioned. In this fraction, p-coumaric acid, kaempferol, and caffeic acid have been observed in the antioxidant activity. It is confirmed that cardamom oil and methanolic extract showed radical scavenging and antioxidant activityBunium persicum is hydrodistillized to form the pale yellow-colored essential oil which contains about 24 compounds that comprise 97.20% of the oil. The oil is made of oxygenated monoterpenes, and hydrocarbons like alpha-thujene, sabinene, alpha-pinene, myrcene, beta-pinene, alpha-terpinene, p-cymene, alpha-terpinolene, p-Menthe-3-ene-7-al, cuminyl alcohol, beta-caryophyllene, gamma-eleman, beta-bisabolene, beta-selinene, myristicin, germacrene B, and dillapiol. Other main components of the oil are limonene, cuminaldehyde, and p-cymene [839]. It has been observed that various phytochemicals like terpenes (sesquiterpene, monoterpene, oxygenated monoterpenes, oxygenated sesquiterpenes), aliphatic compounds, steroids, terpenoids, campesterol, esters, stigmasterol, fatty acids, alkaloids, resins, tannins, thymoquinone, phenolics, saponins, and flavonoids are obtained in Bunium persicum. Some components such as gamma-terpinene-7-al, and beta-sinensal are found in black jeera. The pleasant aroma of the oil is mainly contributed by cumaldehyde. The oil is used to produce perfumes, and other cosmetics. The reduction in the quality of the spice is due to the presence of beta-pinene, gamma-terpinene, and p-cymeneBunium persicum showed potential antifungal effects due to the existence of p-cymene and cuminaldehyde [126]
Foeniculum vulgare (Fennel)
In the methanolic extract of fennel seed, 56 bioactive phytochemical compounds were determined. The phytochemicals detected depend on the molecular formula, peak location, retention time, molecular weight, MS-fragments, and pharmacological actions. Alcohols, alkanes, ethers, carboxylic acids, esters, nitro compounds, alkenes, hydrogen-bonded alcohols, aliphatic fluoro compounds, and phenols are identified in fennel seeds through Fourier Transform Infrared Spectroscopy (FTIR). Fennel contains ample amounts of phytochemical compounds like cyclohexene, 4-isopropenyl-1-methoxymethoxymethyl, O-alpha-D-glucopyranosyl-(1- > 3)-beta-D-fructo, L-fenchone, estragole, 2-propyl-tetrahydropyran-3-ol, benzaldehyde, 4-methoxy, anethole, 2-methoxy-4-vinylphenol, ascaridole epoxide, d-mannose, pterin-6-carboxylic acid, 4-methoxybenzoic acid, allyl ester, arisaldehyde dimethyl acetal, 1-propyl-3, 6-diazahomoadamantan-9-ol, 4-(2,5-dihydro-3-methoxyphenyl) butylamine, corymbolone, apiol, fenretinide, dihydroxanthin, 1-(4-methoxyphenyl)-1, 5-pentanediol, 1-heptatriacotanol, gibberellic acid, 2, 3-dimethoxy-5-methyl-6-decaisoprenyl-chinon, 2-[4-methyl-6-(2,6,6-trimethylcyclohex-1-enyl) hexa-1,3,5-trienyl] cyclo, cis-vaccenic acid, 6,9,12,15-docosatetraenoic acid, methyl ester, dl-3beta-hydroxyl-d-homo-18-nor-5 alpha, 8 alpha, 14 beta-androst-13 (1), 5 Ah-3a, 12-methano-1H-cyclopropa [ 5’, 6’] cyclodeca [1’, 2’: 1, 5] cyclo, (22S)-21-acetoxy-6alpha, 11beta-dihydroxy-16alpha, 17alpha-propylmethylenedioxyp, oxiraneoctanoic acid, 3-octyl-, methyl ester, ingol 12-acetate, alpha-D-glucopyranoside, and 2,24a,6a,8a,9,12b,14a-octamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,1 as identified by chromatogram GC–MS method [381]. Essential oil is present in ample amount in fennel seeds that provides the characteristic flavor. Seeds contain several lipophilic and hydrophilic compounds like carotenoids, phenols, chlorophylls, unsaturated fatty acids, and phytosterols. These compounds act as potential antioxidant agent and prevent various types of diseases [760]. The major phytoconstituents of fennel seeds are volatile aroma compounds, phenols, and phenolic glycosides like trans-anethole (81.63% and 87.85%), fenchone, and estragole. It is reported that the major acaricidal agents are fenchone and p-anisaldehyde that have proven effectiveness against dermatoghagoides pteronyssinus, and dermatophagoides farinae. In another investigation, anethole and its polymer like photo anethole, and dianethole are proved to be active oestrogenic agents. It is proved that anethole acts as protective antithrombotic agent due to its vasorelaxant action, antiplatelet activity, and clot destabilizing effect. However, estragole may cause anxiety as the structure of estragole is similar to methyleugenol. Estragole has carcinogenic property. The maximum limit of estragole in non-alcoholic beverage is 10 mg/ kg. Phenolic acids like 5-O-caffeoylquinic acid, 3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 1,3-O-di-caffeoylquinic acid, and 1,4-O-di-caffeoylquinic acid, and the flavonoids such as rosmarinic acid, eriodictyol-7-rutinoside, and quercetin-3-rutinoside are found in fennel. Phenolic compounds are considered to be a preventive measure against various diseases like inflammation, cardiovascular disease, and cancer [749]
Papaver somniferum (Poppy)
The main active secondary compound of poppy seed is benzylisoquinoline alkaloids (BIAs). Several bioactive compounds like phenolic compounds, alkaloids, flavonoids, and polyunsaturated fatty acids are present in poppy seed. It has been utilized as food ingredients. Poppy seed oil is a rich source of polyunsaturated fatty acids. Poppy possesses lactic acid, meconic acid, and alkaloids like morphine, codeine, noscapine, thebaine, papaverine, aporphines, protoberberine, rhoeadines, benzophenanthridine, and tetrahydroisoquinolines. Poppy seed oil contains tocopherol, and unsaturated fatty acids. At present, poppy seed oil is utilized in infant formulas due to the absence of narcotic properties. Due to the presence of important phytochemicals like tocopherol, the seed oil is used as a nutraceutical supplements in different types of food products. Linoleic acid (68%) is the major fatty acid found in poppy seed oil. Poppy seed oil contains unsaturated fatty acids like linolenic acid, oleic acid, linoleic acid, and palmitoleic acid, and the principal saturated fatty acids such as arachidic acid, stearic acid, and palmitic acid. The poppy seed oil is used for food processing due to the presence of a high amounts of linoleic acid. Poppy possesses neutral elements like melanoidin, opionin, and meconin. Poppy contains organic acids such as meconic, lactic, caffeic, and ferulic acids. Tocotrienols and tocopherols have made the poppy seeds valuable for preventing various diseases like cancer, autoimmune, cardiovascular, metabolic, bone, and neurological disorders. Due to the presence of excessive amounts of tocotrienols and α-tocotrienols in the plasma membrane, the poppy seed oil possessed antioxidant activity and it has the potential to retard the lipid oxidation [621]. Various secondary metabolites such as saponins, tannins, cardiac glycosides, phytosterols, and terpenoids are present in poppy seed. Volatile compounds like 9-octadecanoic acid, methylester, (E, E),9-tetradecen-1-ol, acetate, (E),9,12-octadecadienoic acid, cis-9,10-epoxyoctadecan-1-ol, and undec-10-ynoic acid as determined by GC–MS. Different types of functional compounds like carboxylic acid, alcohols, phenols, aldehydes, anhydrides, amides, esters, ketones, unsaturated aliphatics, unsaturated heterocycles, aromatics, amines, nitro compound, alkanes, and alkenes are determined by FT-IR in poppy seed. It is utilized potentially in various ways in the field of pharmacy and food technology to prevent various human diseases [632]
Syzygium aromaticum (Clove)
Phenolic compounds like hydroxycinnamic acids, flavonoids, hydroxybenzoic acids, and hydroxyphenyl propens are present in abundant amounts in clove flowers. Eugenol is the main bioactive compound of clove ranging from 9381.70 to 14,650 mg/100 g of fresh plant material. Clove contains gallic acid, phenolic acid, and other gallic acid derivatives as hydrolysable tannins at a higher level. Clove possesses phenolic acids like elagic, ferulic, caffeic, and salicylic acid and the flavonoids such as quercetin, kaempferol, and its glycosylated moiety. Eugenol is contributed 89% of the clove essential oil. Eugenol acetate and β-cariofileno contributed 5%-15% of the clove essential oil. The other valuable compound is obtained in the essential oil of clove is α-humulene at a level up to 2.1%. Clove has some volatile compounds in its essential oil like farnesol, limonene, benzaldehyde, 2-heptanone, β-pinene, and ethyl hexanoate in lower concentrations. This plant is utilized for centuries as a medicinal plant and food preservative especially as an antioxidant agent and also showed antimicrobial effects [607]. The phytochemicals like hydrocarbon, monoterpenes, and sesquiterpenes are found in ample amounts in clove. Various studies demonstrated that eugenol showed anticancer, antioxidant, antiseptic, antidepressant, antispasmodic, anti-inflammatory, antiviral, antifungal, analgesic, and antibacterial effects against various pathogenic bacteria like S. aureus and methicillin-resistant Staphylococcus epidermidis It has protective activity against CCl4-induced hepatotoxicity and it provides powerful lethal effectiveness against the propagation of several parasites such as Haemonchus contortus, Giardia lamblia, Fasciola gigantica, and Schistosoma mansoni. Eugenol is broadly applied in dentistry due to its penetrating power into dental pulp tissue. Sesquiterpenes have been found to possess anti-carcinogenic activity. It is reported that eugenol is able to donate the hydrogen atom and subsequently neutralized the phenoxyl radical that results in the occurrence of steady molecules which do not develop or enhance the rate of oxidation. Eugenol possessed carbon chain link combined with the aromatic ring that can be engaged in phenoxil radical stabilization with the help of resonance. Calamenene, calacorene, and humulenol are identified in clove by gas chromatography–mass spectroscopy (GC–MS). Biflorin, 5,7-dihydroxy-2-methylchromone-8-C-β-D-glucopyranoside, orsellinic acid glucoside, myricetin, rhamnocitrin, and oleanolic acid are found in clove and showed their efficacy in retarding oral pathogens [134]. The gas chromatography evaluation of hexane extract of clove determined the presence of compounds like chavibetol, 2,6,6,9-tetramethyl-1,4,8-cycloundecatriene, and copaene [113]
Cinnamomum cassia (Cassia bark)
The main active component of cassia bark is essential oil like cinnamaldehyde (0.003 mg/ mL). Essential oil consists of cinnamic acid, coumarin, cinnamyl alcohol, and 2-methoxycinnamaldehyde. Essential oil showed anti-platelet aggregation, antioxidant, anti-diabetic, and antifungal activities [1017]. Terpenes are found in cassia bark oil in abundant quantity. Four chemical classes are obtained in the crude extract of cassia bark like oxygenated sesquiterpenes, oxygenated monoterpenes, sesquiterpene hydrocarbon, and other oxygenated compounds. Trans-cinnamaldehyde is predominantly present in the extracted cinnamon oil. The other compounds are guaiacol, benzenepropanal, cis-cinnamaldehyde, bornyl acetate, acetophenone, geranyl acetate, tetradecanal. Oxygenated monoterpenes are eucalyptol, linalool, borneol, L-α-terpineol, benzaldehyde, anethole, and eugenol. The sesquiterpene hydrocarbons are α-cubebene, copaene, β-caryophyllene, α-muurolene, trans-α-bergamotene, α-humulene, α-amorphene, 1 s-cis-calamenene, calarene, cedrene, and β-cadinene. Oxygenated sesquiterpenes consist of caryophyllene oxide, and tau.-muurolol [410]. Glycosides, terpenoids, and phenylpropanoids are other main compounds in cassia bark. Cinnzeylanol, anhydrocinnzeylanol, 2,3-dehydroanhydrocinnzeylanine, 1-acetylcinncassiol A, 16-O-β-D-glucopyranosyl-19-deoxycinncassiol G, perseanol, and D1 glucoside are the diterpenoids isolated from cassia bark. Cinnacasside B, cinnacassoside D, cinnacasolide E, and samwiside are glycosides isolated from cassia bark. Other chemical compounds are present in cassia bark like benzyl benzoate, 2-hydroxybenzaldehyde, 3-phenylpropanol, 2,2,4,6,6-pentamethylheptane, 2,5,9-trimethyldecane, 2-ethyl-5propylphenol, 3,4-dimethoxyphenethyl alcohol, 2,5-dimethylundecane, benzaldehyde, phenylethyl alcohol, benzenepropanal, acetophenone, 1,3-dimethylbenzene, styrene, 2,2,4-trimethyl-1,3-pentanediol, decanal, 2,6,10-trimethyldodecane, rosavin, coumarin, dihydromelilotoside, evofolin B, and cinncassin C [1012]
Piper nigrum (Black pepper)
The most abundant chemical alkaloid present in black pepper is piperine. Other alkaloids are piperanine, piperylin A, piperettine, pipericine, and piperolein B. Black pepper possesses an abundant amount of polyphenols as compared to white pepper. It is recorded that alkaloids, some aromatic compounds, flavonoids, amides, and lignans are found in black pepper. Some volatile oils like γ-cadinol, γ-guanine, and (E)-β-ocimene were determined by high-resolution gas chromatography, column chromatography, and gas chromatography-mass spectrometry (GC–MS) in black pepper [5]. Piperine enhances the bioavailability of many medicines, and nutrients by retarding several metabolizing enzymes. Piperine shows various pharmacological activities like antioxidant, antihypertensive, antiplatelet, antitumor, anti-asthmatic, analgesic, anti-inflammatory, anti-diarrheal, antidepressants, antispasmodic, immunomodulatory, anti-thyroids, anticonvulsant, antibacterial, antifungal, hepato-protective, larvicidal, and insecticidal activities. Piperine is found to increase fertility, cognitive action, provoke the intestinal activity, and improve the pancreatic enzymatic activity that help to fight indigestion. Most of the researchers identified various compounds in black pepper-like phenolics, steroids, neolignans, terpenes, and chalcones. Some of the compounds are brachyamide B, dihydro-pipericide, N-trans-feruloyltyramine, N-formylpiperidine, guineensine, pentadienoyl as piperidine, isobutyl-eicosatrienamide, piperamide, sarmentine, sarmentosine, and retrofractamide. Four isomers have been isolated from piperine like chavicine, piperine, isopiperine, and isochavicine [220]. Phytochemical compounds of black pepper fruit are 3-carene, propanedioic acid, dimethyl ester, cyclohexene, 1,6-octadien-3-ol,3,7-dimethyl, 2-methyl-1-ethylpyrrolidine, 2-isopropenyl-5-methylhex-4-enal, L-α-terpineol, pyrrolizin-1,7-dione-6-carboxylic acid, methyl ester, 7-epi-cis-sesquisabinene hydrate, phenol, eugenol, α.copaene, naphthalene, epiglobulol, caryophyllene,1,4,7-cycloundecatriene, 1,5,9,9-tetramethyl-,Z,Z,Z,α-ylangene, cedran-diol, 8S,13,isocalamendiol, cinnami acid, desacetylanquidine, trans-1,2-diaminocyclohexane-N,N,N,N-tetraacetic acid, phytol, eicosanoic acid, 2,5,5,8a-tetramethyl-6,7,8,8a-tetrahydro-5H-chromen-8-ol, Z-5-methyl-6-heneicosen-11-one, 2H-1,2-benzoxazine-3-carbonitrile, fenretinide, 11-dehydrocorticosterone, ursodeoxycholic acid, 5α-cholan-24-oic acid, and stigmasterol as determined by GC/ MS [607]
Coriandrum sativum (Coriander)
Coriander seed contains geranyl acetate, linalool, and camphor as phytochemical compounds. Coriander seed possesses phenols and flavonoids. Essential oils from coriander seeds contain camphene, β-pinene, β-myrcene, and 4-carene. Monoterpene hydrocarbons like limonene, γ-terpinene, and p-cymene are the second principal chemical group found in the essential oil, the other chemical classes include the sesquiterpenes compounds [2]. Linalool is present at high concentrations in the essential oil of coriander seed. The other essential oils are triglyceride oil and petroselinic acid. The compositional evaluation of coriander seed revealed the presence of alcohols (linalool, geraniol, α-terpineol, terpinene-4-ol), hydrocarbons, ketones (camphor), and esters (linalyl acetate). Linalool provides a pleasant, and floral-like odor [570]. In an investigation, the HPLC analysis revealed the existence of hesperidin, apigenin, luteolin, hyperoside, diosmin, vicenin, orientine, dihydroquercetin, chrysoeriol, catechin, salicylic acid, ferulic acid, dicoumarin, gallic acid, esculetin, 4-hydroxycoumarin, esculin, maleic acid, tartaric acid, and arbutin in coriander [634]
Myristica fragrans (Nutmeg)
Nutmeg seeds contain malabaricone C, dehydrodiisoeugenol, and malabaricone B. Malabaricone C is the active component present in high amount in nutmeg seed which showed higher antioxidant activity than the other two compounds. Polyphenols are isolated at higher amount in the methanol extract of nutmeg [525]. Secondary metabolites like steroids, saponins, alkaloids, flavonoids, phenols, tannins, phlobatannins, anthraquinones, coumarins, cardiac glycosides, anthocyanin, emodins, chalcones, and triterpenoids are identified by qualitative evaluation of the nutmeg seed extracts. Nutmeg seed contains resin, quinines, thiols, terpenoids, gum, and mucilages [933]. Nutmeg seed contains major components like terpene hydrocarbons (camphene, sabinene, pinene, phellandrene, p-cymene, terpinene, myrcene, and limonene,all together they constitute approximately 60%-80% of the oil. Oxygenated terpenes (terpineol, linalool, and geraniol contribute at least 5%-15% of the oil. Aromatic ethers like safrole, myristicin, elemicin, eugenol derivatives, and eugenol contribute about 15–20% of the total composition. Lignans have been found in nutmeg seed and showed antimicrobial effects against Shigella dysenteriae, Bacillas subtilis, and Staphylococcus aureus [585]
Brassica nigra (Black mustard)
Black mustard contains some bioactive compounds like indoles, isothiocyanates, thiocyanates, and oxazolidine-2-thiones [663]. Various phytochemical compounds are found in Brassica plants like phenolic acid, phenolics, polyphenols, carotenoids (β-carotene, zeaxanthin, and lutein), tannins, saponins, anthocyanins, phytosteroids, aromatic, and aliphatic amines, flavonoids, phytosterols chlorophyll, alkaloids, glucosinolates, glycosides, and terpenoids. Black mustard seed contains phenolics (catechin, epicatechin, myricetin, quercetin, gallic acid, and rutin), phlobatannins, tocopherols, glutathione reducing sugar, and volatile oil and possessed antiradical and antioxidant potential [643]. The major glucosinolate present in black mustard seed is sinigrin (24.5–61.2 g/kg fresh weight) present in black mustard seed. Sinigrin can be hydrolyzed to allyl-isothiocyanate which provides the characteristics of the pungent odor. The other glucosinolate is sinalbin [529]
Curcuma longa (Turmeric)
The main active component of turmeric is curcumin (10.16 mg/g-16.48 mg/g). Turmeric is an orange-yellow-colored lipophilic polyphenol substance which is derived from the rhizomes of the plant. Curcumin has anticancer, antioxidant, anti-inflammatory, anticoagulant, antifertility, antifungal, antiprotozoal, antiviral, antifibrotic, antivenom, antiulcer, hypotensive, and anticholesteremic activities. It plays a valuable role in the treatment, and prevention of different types of diseases like neurological, cancer, autoimmune, cardiovascular disease, and diabetes [328]. Curcumin possessed antimicrobial activity against Mycobacterium tuberculosis, Staphylococcus aureus, Salmonella paratyphi, and Trichophyton gypseum Curcumin is a compound naturally considered as safe, and effective. Turmeric root possesses curcuminoid, which is comprised of bidemethoxy-curcumin, curcumin, and demethoxycurcumin. Curcumin is a valuable compound of the traditional treatment method known as Jiawei Xiaoyao in China. The compound also prevents metabolic disease, lung disease, and liver disease. Curcumin is applied to decrease postoperative inflammation and is safe to consume at a high dose without any toxicity [476]. Some bioactive compounds found in the rhizome of turmeric are α-turmerone, ar-turmerone, and β-turmerone as determined by high-performance liquid chromatography (HPLC) [189]. Turmeric contains volatile compounds like camphor, eucalyptol, and β-pinene which are considered as strong antifungal compounds. Other volatile compounds are present in ample amounts in the essential oil of turmeric like phenyl propionoids, monoterpenes, and sesquiterpenes. Some other volatile compounds are α-pinene, camphene, α-phellandrene, 3-carene, β-cymene, β-elemene, α-santalene, caryophyllene, α-farnesene, zingiberene, β-cedrene, α-bisabolol, and β-sesquiphellandrene as identified by SH-GC–MS in turmeric essential oil [375, 377]
Laurus nobilis (Bay leaves)
Bay leaf contains volatile oils and alkaloids. Current investigation on bay leaves proposed that leaves contain the major sesquiterpene lactone, costunolide, and its α-methylene-γ-butyrolactone moiety [584]. They play an important role in preventing flatulence colic and enhancing the gastric fluid secretion. Bay leaves possess isoquercitrin compound which is responsible for its alkyl radical scavenging activity. Dehydrocostus lactone, zaluzanin D, and (1 R,4S)-1-hydro-peroxy-p-menth-2-en-8-ol acetate are identified from methanol extract of bay leaves that is responsible for the trypanocidal activity. Isoquercetin (flavonoid) is an active principle of bay leaves. In another investigation, bay leaves contain guaianolides eremanthin and germacronolide costunolide [129]. 1,8-cineole is the major component of bay leaves. The other predominant components are α-terpinyl acetate, sabinene, apinene, limonene, linalool, terpinene-4-ol, β-pinene, bornyl acetate, aterpineol, myrcene, α-phellandrene, p-cymene, methyleugenol, germacrene D, α-thujene, β-elemol, camphene, and eugenol [239]. The other sesquiterpene lactones are (1 3b-chlorodehydrocostuslactone, and (2 5a,9-dimethyl-3-methylene-3,3a,4,5,5a,6,7,8-octahydro-1-oxacyclopenta [c] azulene-2-one as identified by chromatographic separation. Various secondary metabolites are isolated from bay leaves like monoterpene, germacrene alcohols, catechin, procyanidin derivatives, laurenobiolide, glycosylated flavonoids, and megastigmane glucosides [217]
Crocus sativus (Saffron)
Safranal, crocins, and picrocrocin constituents present in saffron provide flavor and color to food products and have health-promoting properties. Other phytochemical compounds are phenolic (flavonols and anthocyanins), flavonoids, degraded carotenoid compounds like crocins, and crocetin. Crocin 1 or α-crocin is the major compound of saffron flower. It exhibits antioxidant potential through neutralizing free radicals and by defending cells, and tissues from oxidation [432]. Saffron contains little amount of carotenoids like zeaxanthin, carotenes, lycopene, xanthone-carotenoid glycosidic conjugate, mangicrocin, and picrocrocin. Picrocrocin is colorless glycoside and product of zeaxanthin degeneration. It is the second most abundant constituent which is responsible for the bitter taste of saffron [528]. The odor of saffron occurs due to the presence of safranal which is a product of natural deglycosylation of picrocrocin [548]. Some investigations revealed that saffron flower extract contains an abundant amount of polyphenol components that help to decrease the free radicals activity and contributes in building the defense mechanism to the various organs like lung, kidneys, liver, and heart. It is proved that saffron and its carotenoid pigment, crocin, showed antioxidant and hypolipidemic properties. Some studies showed that crocin has neuroprotective activity [725]. Saffron flower contains other compounds like delphinidin, and kaempferol (flavonoid) that exerted antioxidant activity and these can be used in cosmetics, food products, and phytopharmaceuticals. The red color and yellowish red hues of saffron occurs due to the presence of crocetin esters. Pollen has isorhamnetin glycosides and kaempferide compounds. Malvidin glycoside, delphinidin, kaempferol aglycone, and petunidin are the phenolic compounds found in the perianth. Flavonol, quercetin, and 3-O-β-sophoroside are found in the petals of saffron flower [615]. The aroma rich active compounds of saffron like 4-ketoisophorone, isophorone, phenylethyl alcohol, 2-pentanol, α,β-ionone, isophorone oxide which are evaluated by GC–MS [211]
Illicium verum (Star anise)
Shikimic acid is the precursor molecule present in abundant amount in star anise fruit. It is applied in the preparation of oseltamivir, which acts as an antiviral drug for influenza A and influenza B. The essential oil of anise consists of sesquiterpenes, prenylated C6-C3 compounds, lignans, and flavonoids that provide various medical properties. The characteristics taste of anise is due to the presence of anethole compound. Star anise essential oil contains myrcene, α-pinene, γ-terpineol, α-phellandrene limonene, linalool, estragole, α-cubebene caryophyllene oxide, trans-anethole, and α-humulene that have various biological properties. Tannins, alkaloids, limone, safrol, and farnesol and some small amount of nitrogenous compounds, 14 hydrocarbon compounds, and 22 oxygenated hydrocarbon derivatives such as p-allylanisole, anisyl acetone, anisaldehyde, p-cumin aldehyde, p-allylpen, palmitic acid, linoleic acid, and foeniculin are isolated from the fruit of anise [201]. Other investigations suggested that anise fruit contains β-sitosterol, car-3-ene, cineol, 4 (10)-thujene, and hydroquinone ethyl ether. Sesquicitronellene, copaene, anisketone, methyl-3-methoxy-benzoate, methyl isoeugenol, p-hydroxy benzoic acid, nerolidol, and m-methoxy-a-benzyl benzene acetic acid are identified in anise [748]. Sabinene, 1,8-cineole, anisaldehyde, borneol, 4-methoxypropiophenone, bisabolene, O-nitrobenzoic acid, germacrene D, trans-nerolidol, geranyl isobutyrate, chavicol, and p-cumic aldehyde are some volatile oils which have been identified in star anise. Star anise fruit also contains toluene, octane, and undecane [693]. Trans-anethole is the main active compound present in star anise fruit which provides a characteristics aroma. Researchers observed that anethole shows a peripheral antinociceptive activity, and provides treatment for painful, and inflammatory problems [231]. Antifungal properties are found in anise fruit due to the presence of trans-anethole in the oil. Trans-ocimene, β-clemene, bergamotene, and α-cadinol are the other most valuable compounds found in star anise [831]
Allium cepa (Onion)
Onion contains several secondary metabolites like saponins, flavonoids, and phytosterols. The most active compounds of onion are quercetin, and quercetin 4’-O-β-glucoside which are responsible for the formation of brown, and yellow compounds [579]. Various phytochemicals that present in onion are polysaccharides, organosulfur compounds, and phenolic. Sulfur-containing compounds such as onionin A and cysteine sulfoxides are the major bioactive compounds of onion. Anthocyanins are also found in onion. The compound cysteine sulfoxides consists of methiin, cyclophilin, isoalliin, and propiin [1015]. Quercetin aglycon is also found in onion. Onion contains three types of alkyl cysteine sulfoxides (ACSOs,(1 trans-(+ -S-1-propenyl-L-cysteine sulfoxide (PECSO which is naturally obtained in the higher amount and is responsible for the lachrymatory activity, (2 (+ -S-methyl-L-cysteine sulfoxide (MCSO, and (3 (+ -S-propyl-L-cysteine sulfoxide (PCSO [140]
Anethum graveolens (Dill)
Some phytochemicals like polyphenols, essential oil, and furanocoumarin are present in dill. Dill seeds essential oil contains 1-methoxy-4-(2-propenyl)benzene, D-( +)-carvone, humulene, 6-methyl-2,4-di-t-butyl-phenol, 1-allyl-2,5-dimethoxy-3,4-methylenedioxybenzene(diplaniol), eicosane, heptadecane, docosane, n-heneicosane, n-pentacosane, tricosane, octacosane, dioctyl ester of 1,2-phenyldicarboxylic acid, and n-nonacosane [999]. Dill seeds possess the most valuable bioactive compounds like flavonoids, glycosides, alkaloids, tannins, saponins, steroids, terpenoids, phenols, resins, and iridoid glycosides [45]. The main active components of dill seeds are dill apiol, carvone, limonene, and α-phellandrene. Ethanol extract of dill exhibited phenolic acids like protocatechuic, vanillic, caffeic, ferulic, p-coumaric, syringic, chlorogenic, o-coumaric, rosmarinic, and trans-cinnamic acid. Dill seed essential oil consists of sabinene, α-thujene, α-pinene, p-cymene, myrcene, dill ether, γ-terpinene, trans-carveol, iso-dihydro carveol, and anethole in small amounts. Other compounds of dill seed essential oil are myristicin, trans-dihydrocarvone, E, E-2,6 dimethyl-3,5 octatetraene, linalyl acetate, piperitone, thujyl alcohol, grandisol, 2-carene, o-isopropenyltolune, 1,2-diethoxyethane, diphenol, and bis-1,2 benzene dicarboxylic acid. 3-hydroxy-α-ionol, 8-hydroxygeraniol-D-glucopyranoside, p-menth-2-ene-1,6-diol-D-glucopyranoside, 3-hydroxy-β-ionol 3-O-β-D-glucopyranoside, and quercetin 3-O-β-D-glucuronide [180]
Trigonella foenum-graecum (Fenugreek)
Fenugreek seeds contain tannins and polyphenol. Alkaloids, steroids, and saponins are present in fenugreek seed showed anti-diabetic effects. Some other bioactive compounds like trigonelline, orientin, isoorientin, isovitexin, and vitexin are isolated and quantified by HPLC system. The method UHPLC-ESI–MS/MS identify some compounds like sarsasapogenin, and pinitol [857, 861]. Fenugreek seed also contains flavonoids that provide anti-inflammatory and antinociceptive properties. Polysaccharides and volatile compounds are obtained in an abundant amount in fenugreek seed. Some volatile compounds are dextroamphetamine, 2-methyl pyrrolidine, benzene methanol,a-(chloromethyl), astaxanthin, buffa-20,22-dienolide, 4a-phorbol 12,13-decanoate, psi.,.psi.-carotene, 9,19-cyclolanost-24-en-3-ol,acetate, hydrocortisone acetate, 2-propane-1-amine,N-ethyl, methyl ester, ethyl iso-allocholate, lycoxanthin, betulin, 1,1,2,2-tetrahydro-1,1’-dimethoxy, and rhodopin [838]. The main active compound of fenugreek seed is galactomannan which is a long-chain polysaccharide. Galactomannan occupies a huge portion in the total bioactive composition of the seed extract. 4-hydroxy isoleucine and diosgenin are isolated in fenugreek seed. Fenugreek seed provides its characteristics odor due to the presence of trigonelline [888]. It is applied in the production of imitation maple syrup, rum, artificial flavoring for licorice, vanilla, and butterscotch. The other phytochemicals found in fenugreek seeds are sapogenins, diosgenin, gitogenin, yamogenin, yuccagenin, neogitogenin, sarsasapogenin, tigogenin, smilagenin, and choline. The secondary metabolites are lignins and flavonoids (flavones, flavonones, flavanols, flavonols, proanthocyanidins, isoflavones, and anthocyanins). The most common flavonoids obtained from fenugreek are kaempferol, quercetin, and luteolin. Fenugreek contains phenolic compounds such as chlorogenic, scopoletin, coumarin, caffeic, and p-coumaric acids [519]
Ferula assafoetida (Asafoetida)
Assafoetida is rich source of polysulfides, sesquiterpene, coumarins, phenolic acid, and flavonoids. The main active compound of assafoetida is rosmarinic acid (RA) (phenolic acid) which is isolated by the HPLC. RA shows anti-inflammatory properties by decreasing the manifestation of COX-2 enzymes and their activity. The strong defensive and therapeutic activities of this compound for communicable diseases are recommended because of its antioxidant, and anti-inflammatory effects. The other phenolic acids are apigenin, luteolin, rutin (asafetida), vanillic acid, and ferulic acid which are formed by SBUp1 isolate [685]. Assafoetida root contains various coumarins, and terpenoids that provide anti-HSV activity. The sesquiterpene coumarins are badrakemin acetate, Pellerin, assafoetidnol A, assafoetidnol B, gummosis, polyanthus, neveskone, galbanic acid, 5-hydroxyumbelliprenin, and 8-acetoxyumbelliprenin are identified in the gum resin of assafoetida [3]. Assafoetida possesses polyphenols like flavonoid glycosides, and tannins which have anti-HSV effect [322]. Monoterpenes, and sulfur-containing compounds are volatile compounds found in assafoetida. Three main sulfur compounds are 2-butyl 3-(methylthio)-2-propenyl disulfide, 2-butyl 1-propenyl disulfide, and 1-(methylthio) propyl 1-propenyl disulfide. The other chemical compounds present in asafetida are umbelliprenin, tadshiferin, galbanic acid, conferol, franesiferol A, assafoetidin, ferocaulicin, kamolonol, saradaferin, 10-R-acetoxy-11-hydroxyumbelliprenin, 10-R-karatavicinol, lehmferin, feselol, ligupersin A, epi-conferdione, microlobin, asadisulfide, foetisulfide C, 7-oxocallitristic acid, picealactone C, 15-hydroxy-6-en-dehydroabietic acid, vanillin, taraxacin, and fetidone A [946]
Apium graveolens (Celery)
Celery seed oil contains flavonoids, terpenoids, and lactones. Essential oil is extracted from celery seed by steam distillation. The main active constituent of celery seed is limonene (essential oil). The other oils are a-p-dimethyl styrene, caryophyllene, N-pertyl benzene, a-selinene, sedanenolide, N-butyl phthalide, b-element, sabinene, linalool, trans-1 2-epoxylimonene, isovaleric acid, cis-dihydrocarvone, terpinene-4-ol, trans-dihydrocarvone, trans-p-menth-2,8-diene-1-ol, 1-cis-p-menth-2,8-diene-1-ol, α-terpineol, trans-8-diene 1-ol, carvone, salience, several sesquiterpene, thymol, and perialdehyde. The characteristics flavor of celery is mainly due to the presence of two lactones which are 3-butyl 4,5 dihydrophthalide (sedanolide), and 3-n-butylphthalide [293]. Various phenolics like furanocoumarins, and flavones found in celery. Glycosides, and steroids are present in celery. Celery seeds contain phytochemicals like apiin, caffeic acid, chlorogenic acid, apigenin, ocimene, rutaretin, isopimpinellin, bergapten, seslin, osthenol, umbelliferone, isoimperatorin, and gravebioside A and B. Sedanolide, and sedanonic anhydride found in celery seed oil that is responsible for its aroma. The other chemical constituents of celery are 8-hydroxyl-5-methoxypsoralen, myristic acid, and octadecanoic acid [42]
Capsicum frutescens (Chili)
Chili contains valuable bioactive compounds like steroids, phenolic compound, flavonoids, saponins, coumarins, anthroquinones, proanthocyanidins, carotenoids, polyphenols, terpenoids, and alkaloids. The main active component of chili is capsaicinoids [665]. The capsaicinoid alkaloid is responsible for pungent taste of chili. The major capsaicinoids are dihydrocapsaicin (DHCap), and capsaicin (Cap). The minor capsaicinoids are homodihydrocapsaicin (HDHCap), nordihydrocapsaicin (NDHCap), and homocapsaicin (HCap) [311]
Pimenta dioica (Allspice)
Allspice leaves contain bioactive compounds like gallic acid, ferulic acid, rutin, and chlorogenic acid. Allspice is utilized in traditional medicine and provides characteristic aroma due to the presence of volatile oil. Allspice contains phenolic compounds like quercetin, ericifolin, and eugenol [924]. Some other phytochemical compounds are catechin, methyl gallate, syringic acid, caffeic acid, pyro catechol, ellagic acid, coumaric acid, vanillin, naringenin, cinnamic acid, taxifolin, and kaempferol. Polyphenols are also found in allspice [270]. The main active constituent of allspice leaves is eugenol present in essential oil. The others are β-caryophyllene, linalool, cineole, and α-humulene. Eugenol showed therapeutic activities like anti-inflammatory, analgesic, local anesthetic, and antibacterial effects. Some of the essential oils are α-pinene, β-myrcene, limonene, α-terpineol, chavicol, and germacrene [248]. Volatile compounds of allspice leaves are methyl eugenol, β-guaiene, and naphthalene. The compounds α-phellandrene and β-phellandrene are identified in allspice [923]
Garcinia indica (Kokum)
Kokam is a rich source of anthocyanin pigments like cyaniding-3-sambubioside, and cyaniding-3-glucoside. Hydroxyl citric acid that has been isolated from kokum possess anti-obesity effect. A polyisoprenylated benzophenone derivative garcinol is the main active component present in kokam fruit. It provides chelating, and antioxidant activity. The chemical constituents of kokam fruits are tannin, pectin, and organic acid [644]. Isogarcinol is also found in kokam fruit. Xanthohumol and isoxanthochymol are isolated at higher amounts in kokum. Flavonoids, benzophenones, xanthones, and lactones are identified in kokam fruit. Kokam fruit contains an abundant amount of Cambogia, besides Garcia-2, and garcim-1, which are the main oxidative products of garcinol along with mangosteen, macurin, clusianone, guttiferone (I, J, K, M, N), gambogic acid, and oblongifolin (A, B, C) [485]. Garcinol is a fat-soluble and yellow-colored pigment. It is isolated by hexane and ethanol extraction. Garcinol exhibits antimicrobial effects against Staphylococcus aureus It acts as an antitumor agent by apoptosis through the initiation of caspases and also acts as an anticancer agent. Flavonols, leucoanthocyanidins, and catechins are the bioflavonoids that alter the permeability of capillaries, accelerate the ethanol metabolism and decrease inflammation and edematic reactions [395]
Alpinia galangal (Greater galangal)
Greater galangal contains various phytochemical compounds like 1’S-1’-acetoxy eugenol, α-fenchyl acetate, β-farnesene, α-bergamotene, β-bisabolene, β-sitosterol diglucoside (AG-7), β-pinene, 1’-acetoxychavicol acetate (galangal acetate), β-sitsterylarabinoside (AG-8), 1’S-1’-acetoxychavicol acetate (ACE), and p-hydroxycinnamaldehyde. The essential oils like mono sesquiterpene, as well as (E)-methyl cinnamate, are present in greater galangal root and are responsible for the characteristic odor. These essential oils are used in food products, and medicine. Volatile oils and flavonoids are isolated from galangal. 1’S-1’-acetoxychavicol acetate (ACE) provides a pungent flavor of galangal root. Galangal also possesses p-hydroxybenzaldehyde, and phenylpropanoids. 1,8-cineol is the main active compound of galangal root. Galangal rhizome possesses P-coumaryldiacetate, 4-hydroxybenzaldehyde, trans-coniferyldiacetate, phenylpropanoids, (E)-4-acetoxy cinnamyl ethylether, (E)-4-acetoxy cinnamyl alcohol, (E)-4-hydroxycinnamaldehyde, (S)-1’-ethoxy chavicol acetate, and 4-acetoxy cinnamyl acetate. Some other compounds are diterpene ((E)-8β,17-epoxylabd-12-ene-15,16-dial), curcuminoid (1,7-bis (4-hydroxyphenyl)-1,4,6-heptatrien-3-one), and bisdemethoxycurcumin [203]. Rhizome essential oil consists of camphor and guaiol. Galangal rhizome exerted eleven flavonols (galangin, major flavonols), one flavan 3-ol, dihydroflavonols, and flavanone [203]. The non-methylated flavonols are myricetin, kaempferol, and quercetin,methoxylated flavonols are galangin 3-methyl ether, kaempferide, kumatakenin, isorhamnetin, and quercetin 3-methyl ether; polyglycosylated flavonol called as galangoflavonoside is isolated from galangal rhizome. The dihydroflavonol ((2R,3R-alpinone (sd, pinobanksin 3-acetate, flavan 3-ol (catechin, and flavanone (pinocembrin are also isolated from this spice [944]
Acorus calamus (Sweet flag)
The main active compound of sweet flag rhizome is α-asarone (essential oil) [741]. The essential oil of sweet flag rhizome consists of (E)-methyl isoeugenol, methyl eugenol, α-cedrene, β-asarone, and camphor. Other chemical constituents of essential oil extracted from sweet flag rhizome are α-pinene, camphene, (D)-limonene, 1,8-cineol, linalool, terpinene-4-ol, α-terpineol, bornyl acetate, estragole, α-funebrene, copaene, α-gurjunene, α-bulnesene, β-caryophyllene, germacrene D, cuparene, α-cadinene, β-calacorene, α-cadinol, acorenone, α-bisabolol, acorone, preisocalamenediol, shyobunone, cryptoacorone, (Z)-sesquilavandulol, dehydroxyisocalamendiol, α-selinene, cedrol, berganotene, α-palchoulene, α-guaiadiene, 4-(5-hydroxy-2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one, dehydrofukinone, elemicin, monoterpenoids, and sesquiterpenoids [538]. Sweet flag also contains some bioactive compounds like 2-allyl-5-ethoxy-4-methoxyphenol, lysidine, epieudesmin, spathulenol, furylethyl ketone, borneol, nonanoic acid, galgravin, retusin, (9E,12E,15E)-9,12,15-octadecatrien-1-ol, geranyl acetate, butyl butanoate, sakuranin, acetic acid, acetophenone, α-ursolic acid, dehydroabietic acid, methyl ether, and apigenin 4,7-dimethyl ether. Phenylpropanoids, xanthone glycosides, flavones, lignans, and steroids are found in sweet flag, which possesses several pharmacological activities such as antibacterial, insecticidal, larvicidal, mutagenic, cytotoxic, hepatoprotective, anticonvulsant, smooth muscle relaxant, neuroleptic, and smooth muscle stimulant activity. The essential oil also contains trans-β ocimene, aristolene, α-humulene, viridiflorene, kessane, asaronaldehyde, aspidinol, and phytol [684]
Health Benefit Potential
Spices and herbs provide phytochemicals that protect from various diseases, inhibit oxidation, and remove free radicals (the byproducts of biochemical methods). They also provide preventive measures against various neurological diseases, cardiac disorders, other physiological diseases and protect valuable biomolecules from oxidative damage [643] (Fig. 3). Table 3 summarizes the health benefit potential of the important spices and herbs
Fig. 3.
Health-beneficial effects of the selected spices
Elettaria cardamomum (Cardamom)
Anti-inflammatory Effects
Cardamom seed oil has been applied at a dose of 175–280 µl/kg on carrageenan-induced hind paw edema in male albino rats to analyze its anti-inflammatory effects [52]. 30 mg/kg of indomethacin has been taken as standard. 86.4% inhibition has been found with 280 µl/kg of cardamom oil,thus, the cardamom oil has a high potency with respect to the inflammatory activity of indomethacin. 69.2% inhibition has been observed with 175 µl/kg of cardamom oil [494]
Cardiovascular Health
Various bioactive compounds such as flavonoids, alkaloids, saponins, terpenes, and glycosides steroids have been extracted from cadamom, which have cardioprotective effects. It has been observed that at a high dose of 500 mg/kg of cardamom extract is able to reduce the SGPT (serum glutamic pyruvic transaminase) levels, LDH (lactate dehydrogenase) levels, SGOT (serum glutamic-oxaloacetic transaminase) levels, total protein levels, serum albumin levels, cholesterol levels, LDL cholesterol level, VLDL cholesterol level, total chlorides level, alkaline phosphatase levels, and triglycerides, and recover HDL cholesterol level. It has been observed that at a low dose of 100 mg/kg.bw the cardamom extract is able to reduce the triglyceride level in doxorubicin-induced cardiac problem in rats [829]
Gastrointestinal Activity
Cardamom extract (unfiltered methanolic extract (TM), petroleum ether solution (PS), insoluble (PI) methanolic extract, and essential oil (EO)) at a dose of 100–500, 12.5–50, 12.5–150, and 450 mg/kg has been administered to rats to analyze its gastrointestinal protective activity. The extracts of cardamom considered are able to retard the gastric damages induced by aspirin and ethanol significantly though the damage induced by pylorus ligation has not been inhibited successfully. For the EtOH-induced gastric ulcer, TM reduced lesions by 70% at the dose of 500 mg/kg of cardamom oil. At the dose of 50–100 mg/kg of cardamom oil, the PS fraction lowered the problems by 50%; the same effect has been observed for the fraction of PI at a dose of 450 mg/kg. The best gastroprotective activity has been observed in the PS fraction at the dose of 12.5 mg/kg of oil, which retarded lesions by 100% [400]
Anti-diabetic Effect
The role of cardamom has been proved and accepted by accurate scientific analysis to control diabetes. At a dose of 1 mg/mL, aqueous and methanol extracts of cardamom have been analyzed for their in vitro α-amylase and α-glucosidase inhibition activity and antioxidant properties. 10.41% and 13.73% retardation of α-glucosidase have been observed for aqueous and methanol extract of cardamom, respectively. 82.99% and 39.93% retardation for α-amylase have been observed for methanol and aqueous extract of cardamom, respectively [28]
Obesity Management
In an experiment, cardamom has been given at the dose of 3 g/day for 3 months to the non-alcoholic fatty liver patients, suffering from excess level of SIRT1 (sirtuins) [221]. Researchers have suggested that several parameters related to diabetes, and obesity like fat mass and obesity-associated (FTO), leptin receptor (LEPR), carnitine palmitoyltransferase 1A (CPT1A), lamin A/ C, and peroxisome proliferator-activated receptor gamma (PPAR-gamma) can be managed by the administration of green cardamom at a 3 g/day doses for 16 weeks in women suffering from polycystic ovary syndrome [197]. Researchers have observed that obesity can be managed with a dose of 1% of cardamom powder for 8 weeks for high-fat diet-induced obese rats [992]
Antimicrobial Effect
The growth of Salmonella typhi, Staphylococcus aureus, E. coli, Streptococcus mutans, Candida albicans, Bacillus pulmilus, and Listeria monocytogenes have been inhibited by the application of 10 mg/ml of cardamom essential oil (CEO). CEO showed a potential antifungal, and antibacterial effects [89]
Antioxidant Activity
The thiocyanate method has been used by researchers to estimate the antioxidant potential of methanolic extract of green cardamom extract. The percentage retardation of peroxidation is 84.2–90%. With varying solvent concentrations, the antioxidant activity of green cardamom extract various [148]
Central Nervous System Activity
Scholars have investigated the effectiveness of cardamom extract and its main phytoconstituents over Alzheimer's disease (AD). It has been reported that multiple drug targets have been bound with α-terpinyl acetate. Cardamom extract retarded the butyrylcholinesterase (BuChE), acetylcholinesterase (AChE), decreased Aβ-induced neurotoxicity, hydrogen peroxide-induced oxidative stress, and anti-amyloidogenic activity. The cardamom extract has improved the effects of AD and multi-targeted directed ligand (MTDL) capacity [204]
Bunium persicum (Black jeera)
Anti-inflammatory Effects
Hydroalcoholic extract and essential oil derived from Bunium persicum extracts have been tested for anti-inflammatory activity. The extracts of black jeera exhibited significant anti-inflammatory effects. Researchers have studied the anti-inflammatort activity of black jeera extract on Carrageenan-, Formalin-, and Croton oil-induced ear edema for rats [824, 825]
Anti-diabetic Effect and Anti-obesity Effect
The extract of Bunium persicum has been investigated for its anti-diabetic and anti-obesity activities. It has been evidenced that black jeera is able to retard the glycoside hydrolase activity. Hydroalcoholic extract of Bunium persicum is able to reduce the albumin glycation and aggregation because of the presence of higher concentrations of polyphenolic compounds [126]
Antimicrobial Effect
Different types of extracts of Bunium persicum essential oil (BPEO) have been studied for their antimicrobial properties against various strains of bacteria. The test revealed that essential oil possessed higher inhibition effect on gram-positive bacteria than gram-negative bacteria. Phenolic compounds like cuminaldehyde, γ-terpinene, and p-cymene are responsible for antimicrobial activity. The essential oil present in black jeera is responsible for the bacterial decay. Various food-borne pathogens like Escherichia coli, Bacillus cereus, Bacillus subtilis, Klebsiella pneumonia, Listeria monocytogenes, Proteus vulgaris, Pseudomonas aeruginosa, Salmonella enteritidis, and Staphylococcus aureus have been inhibited by BPEO. P-cymene and cuminaldehyde are responsible for the antifungal activities against various phytopathogenic fungi like Candida albicans, Aspergillus spp., Saccharomyces cerevisiae, Penicillium chrysogenum, Alternaria mali, Botrytis cinerea, Colletotrichum lindemuthianum, Fusarium oxysporum, and Verticillium dahlia [126]
Cardiovascular Health
In animal (rats) model, the cardiovascular effect of Bunium persicum extract have been analyzed. Hypercholesterolemic rats have been selected in the experiment, and Bunium persicum extract has been administered to them to measure the effectiveness of black jeera extract in terms of protecting cardiovascular health. Researchers observed that the extract is able to maintain the lipid profile; thus, the cardiorespiratory ability has also been enhanced [824, 825]
Gastrointestinal Activity
A placebo-controlled, randomized, and double-blind study have been indicated the potential of Bunium persicum as an herbal remedy for gastrointestinal problem. Anti-inflammatory, spasmolytic, antioxidant, carminative, and immunomodulatory properties have been reported for caraway [439]. No significant activity of caraway has been found on the symptoms or inflammatory markers like irritable bowel syndrome. Researchers found the neutral effect of caraway on gastrointestinal symptoms [1]
Antioxidant Activity
The essential oil derived from black jeera and methanol and aqueous extract of the same have been analyzed for its antioxidant potential. It has been reported that the methanol extract possess higher antioxidant potential in comparison with the other extracts as evidenced by DPPH assay and inhibitory effect against the lipid peroxidation and betacarotene oxidation [839]
Foeniculum vulgare (Fennel)
Anti-inflammatory Activity
Compared to the indomethacin, fennel essential oil showed significantly lesser anti-inflammatory activity. The fennel oil exhibited the similar activity at the doses of 0.050 and 0.200 mL kg−1 in comparison with etodolac. It has been reported that fennel essential oil possessed anti-inflammatory activity as observed in animal model (rats) [672]
Anti-diabetic Effect
Essential oil is derived from fennel has been administered to diabetic rats. It has been reported that the essential oil is able to reduce the hyperglycemia. The serum glutathione peroxidase can be managed successfully with the fennel essential oil. The detrimental effects on pancreas, and kidney can also be recovered. Therefore, fennel seed can be utilized as a anti-diabetic drug [269]
Anti-obesity Effect
It has been reported that ethanolic extract of fennel seed is able to decrease the body weight of rats by 12%, while the same has been reduced by 6.4% for rats fed with Orlistat enriched diet. The triglyceride level has not been influenced by Orlistat and plant extract. The ethanolic extract of fennel seed has been used as a promising anti-obesity drug as it is able to maintain the lipid profile; simultaneously, it is able to decrease the body weight. Molecular docking study revealed that stigmast-5-en-3-ol ( a bioactive compound found in fennel seed) is able to control the obesity problem [323]
Antimicrobial Effect
Essential oil of fennel seed is more effective in terms of antimicrobial activity in comparison with the alcoholic and hexane extracts of the seed. Essential oil, alcoholic, and diethyl ether extracts exhibited potential inhibition effect against Bacillus cereus, E. coli, Salmonella typhi, and Staphylococcus aureus Lower minimum inhibitory concentration (MIC) value has been found in essential oil extract for the microorganisms, namely Bacillus subtilis, Bacillis cereus, Bacillus megaterium, E. coli, Klebsiella sp., Pseudomonas aeruginosa, Salmonella typhi, Sarcina lutea, Shigella boydii, Shigella dysenteriae, Shigella shiga, Shigella sonnie, and Staphylococcus aureus. The alcoholic, diethyl ether, and hexane extracts showed lower MIC value against Candida albicans, Aspergillus flavus, Bacillus cereus, E. coli, Salmonella typhi, and Staphylococcus aureus Both the methanolic, and ethanolic extracts have showed similar antimicrobial effect. In the same experiment, acetone extract showed lower MIC value compared to aqueous extract. The higher antifungal effect has been reported in acetone and diethyl ether extracts compared to essential oil [44]
Cardiovascular Health
Methanolic, and water extracts of fennel had potential cardiovascular activity. The extract has been reported for its ability to decrease down the level of plasma lipid. The decreased triglyceride level has the ability to cure the fatty liver problem and it also helps to maintain a healthy blood flow in the coronary arteries. The fennel extract has been given intravenously to the rats, which is able to decrease the blood pressure significantly without influencing the heart rate and respiration. This may be due to its anti-atherogenic and hypolipidemic properties. The water extract possessed lower hypotensive activity [110]
Gastrointestinal Effect
Researchers have studied the effectivity of Foeniculum vulgare aqueous extract (FVE) for its effectiveness in prevention of gastrointestinal ulcer. Fennel aqueous extract has been administered at the doses of 75, 150, and 300 mg/kg to rats. It has been reported that the ethanol-induced gastric problem can be cured with the FVE. The research has been provided to evaluate the potential of heated fennel therapy in increasing the recovery of gastrointestinal function [193]. Three hundred and eighty-one patients suffering from gastric tumor, hepatobiliary, and pancreatic have been selected. The patients are partitioned into two groups: 1) group has given heated fennel therapy, while 2) the other group has taken heated rice husk therapy. Researchers have reported that time to first flatus, first defecation, and fasting time are significantly reduced for the group has been treated with heated fennel therapy [593]
Antioxidant Activity
Researchers have investigated the effectiveness of water, and methanolic extracts of ajwain, and fennel seeds for their antioxidant activity, and phenolic potential. Ascorbic acid has been considered as control. At a concentration of 240 µg/ml of methanolic extract of fennel seed possessed 71.67% greater OH-scavenging activity by in comparison with control. It has been reported by the researchers that the FRAP activity of methanol, and aqueous extracts is in the range of 7–48 µm Fe (II)/g. Polyphenolic compounds derived from fennel seed may be responsible for antioxidant capacity [887]
Central Nervous System Activity
Researchers have investigated the neuroprotective activity of fennel seed on lead (Pb)-induced brain neurotoxicity of rat model. The male BALB/c mice of same age group have fed with 75%, 100% ethanol extracts of fennel seed, simultaneously, 0.1% Pb has also been administered at various doses (20 mg/kg/day, and 200 mg/kg/day). The 75% of ethanol extract has been found with most promising effect. Researchers have concluded that neuronal toxicity can be reduced with fennel seed by controlling the oxidative stress [149]
Papaver somniferum (Poppy)
Cardiovascular Health
Poppy seed helps in increasing the oxygen flow in the blood. The seed protects the activity of the heart may be due to the presence of essential micronutrients [599]
Anti-diabetic Effect
Poppy seed is considered as an effective ant-diabetic agent. The seed helps in the treatment of diabetes may be due to the presence of manganese [599]
Antimicrobial Effect
Poppy seed has been tested for its antimicrobial potential against E.coli, and Salmonella It has been reported that the methanol extract of poppy seed successfully inhibits the growth of E.coli and Salmonella species. The aqueous extract inhibits the growth of coliform bacteria [599]
Antioxidant Activity
Researchers have considered poppy seed oil as a potential antioxidant agent. They have fed 35 Wistar rats with poppy seed oil, and sun flower oil have been administered orally at a doses of 250, and 500 mg/kg/day to 35 Wistar rats. The diesel has been dissolved in equal levels of sunflower oil for 21 days, while untreated rats have been introduced as control. The serum nitrite concentrations have been progressively increased for all of the rats included in the experiment. The blood MDA (malondialdehyde) concentrations have also increased. Though it has been reported that the rats were fed with poppy seed oil at a dose of 500 mg/kg/day, the blood MDA level has enhanced slightly for the rats those have fed with poppy seed at a dose of 500 mg/kg/day. The erythrocyte catalase activity and blood glutathione concentrations have been reduced for poppy seed oil-treated rats [41]
Central Nervous System Activity
The opium alkaloids like codeine, morphine, thebaine, and papaverine are present in small amounts in dry poppy seed. The components have a little effect on the human nervous system. In contrary, the chemicals may possess few health benefits. The poppy seed may act as a painkiller for nervous irritability [751]
Syzygium aromaticum (Clove)
Anti-inflammatory Activity
At the doses of 50 mg/kg, 100 mg/kg, and 200 mg/kg, the ethanol extract of clove showed a significant anti-inflammatory activity for formalin-induced edema in rats. The anti-inflammatory activity is exerted may be due to the presence of tannins, and flavonoids, as these compounds are able to retard the phosphodiesterases activity. The activity of the compounds rely on the biosynthesis of protein cytokines. Prostaglandin is one of the key anti-inflammatory substance. Prostaglandins can be retarded by tannins and flavonoids. The anti-inflammatory activity has been demonstrated for different types of non-steroidal anti-inflammatory agents (NSAIDS) [917]
Anti-diabetic Effect
The anti-diabetic effect of essential oil of clove has been evaluated by researchers with the help of an α-amylase enzyme assay. α-amylase is an enzyme in humans that breaks down the starch into simple sugars. Researchers have observed a decrease in carbohydrate digestion and glucose absorption value may be due to the lower enzyme activity. The postprandial rise is reduced in blood glucose. The clove essential oil showed poor anti-diabetic activity than the standard anti-diabetic compound like ascorbase. The presence of insulin-mimetic agents in clove is responsible for anti-diabetic activity [907]
Anti-obesity Effect
Researchers have evaluated the anti-obesity effect of S. aromaticum ethanol extract (SAE) both in vitro and in vivo condition. In vitro study, the effect of SAE treatment on adipocyte differentiation in 3T3-L1 cells has been evaluated for analyzing the anti-obesity activity of SAEIn vivo study, to investigate the anti-obesity activity of SAE, mice are divided into 3 groups: a control group, a group that consumed a high-fat diet (HFD group), and a group that consumed an HFD supplemented with 0.5%(w/w) SAE (HFD + SAE group). All the parameters like serum triglyceride (TG), the body weight, white adipose tissue (WAT), total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, glucose, insulin, leptin, hepatic lipid accumulation, and levels of lipid metabolism-related parameters have been assessed after 9 weeks of feedingIn vitro evaluation of the effect of SAE treatment on 3T3-L1 cells showed that it had potentially retarded the transformation of cells into adipocytes in a dose-dependent manner. SAE supplementation had significantly reduced HFD-induced enhancement in the body weight, liver weight, WAT mass, serum TG, TC, lipid, glucose, insulin, and leptin levels. In HFD-fed mice, fat deposition are retarded by SAE via the suppression of transcription factors integral to lipogenesis, and adipogenesis [414]
Antimicrobial Effect
The alcoholic extract of clove has decreased the bacterial propagation as perceived by the researchers through zone inhibition test. The extract contains quinine, which may responsible for the reduction in bacterial growth [242, 607]
Cardiovascular Health
Clove extract have a membrane-stabilizing effect on the cardiac cell membrane. Clove extract is able to reduce the enzyme salvation. The clove extract possessed free radical scavenging activity, lipid peroxidation, and antioxidant activity may be due to the presence of flavonoids, and other phenolic compounds. Researchers have investigated and showed that clove extract is able to prevent myocardium damage as evidenced by isoproterenol-induced damages in myocardium [721]
Gastrointestinal Effect
Gastric mucus is a protective factor for the gastric mucosa and contains viscous, elastic, adherent, and transparent gel, which has been made by water, and glycoproteins, which enveloped the whole gastrointestinal mucosa [797]. Eugenol, and clove oil have been examined in the rat model with gastric problem. Compared to the control, it has been found that the doses of 100 and 250 mg/kg of clove oil and eugenol have increased the mucus formation. The free radical scavenging activity of the clove extract may be the reason for a decreased mucosal injury. It is concluded that the gastroprotective mechanism of the eugenol and clove essential oil is connected to factors that enhanced the resistance of the mucus barrier and mucus production [798]
Antioxidant Activity
Researchers have investigated the antioxidant potential of clove essential oil with DPPH radical scavenging activity and ferric reducing power. They have found the DPPH radical scavenging activity and the ferric reducing power. With the help of the DPPH method, the samples have been estimated for their radical scavenging capacity, which relied on the scavenging of the stable DPPH [816]
Central Nervous System Activity
Researchers have carried out in vivo investigation to estimate the neuropharmacological effects of the ethanolic extract of clove with the help of behavioral models of mice. The anxiolytic activities of clove extract have been evaluated through plus maze (EPM), open field test (OFT), hole cross test (HCT), and hole-board test (HBT), respectively. The tail suspension test (TST), and forced swimming test (FST) have been conducted to identify the antidepressant effects. The thiopental sodium (TS)-induced sleeping time test have been conducted to analyze the sedative-hypnotic capacity of the extract. Researchers have observed that in the extract-treated group, the behavior of the mice are altered as perceived through HCT, and OFT in comparison with the control group. Thus, it is obvious that the locomotor actions of the mice have been reduced. The potential sedative response of the extract has been ensured by a TS-induced sleeping time test. The sleeping time for control group is 45.4 min, while the same for 500 mg/kg BW extract-treated group is 87 min. It can be concluded that the inlaying effect of any phytoconstituents with γ-aminobutyric acid (GABAA) or benzodiazepine (BZD) receptors is responsible for the neurological response [373]
Cinnamomum cassia (Caasia bark)
Anti-inflammatory Activity
Researchers have investigated the anti-inflammatory activity of cinnamic acid, cinnamic aldehyde, cinnamic alcohol, and coumarin, the compounds derived from cassia bark. The carrageenan (Carr)-induced mouse paw edema and lipopolysaccharide (LPS)-stimulated mouse macrophage models have been considered to analyze the anti-inflammatory activity. The nitric oxide (NO) generation and tumor necrosis factor have been retarded in cinnamic aldehyde-treated mouse. The Carr-induced paw edema of mouse has been reduced by cinnamic aldehyde. The glutathione peroxidase, catalase, and superoxide dismutase activities are enhanced in the paw tissue with cinnamic aldehyde. It is reported that cinnamic aldehyde has emaciated myeloperoxidase (MPO) and MDA activities in the paw edema after Carr administration [527]
Anti-diabetic Effect
500 mg of capsules of cassia bark is supplied twice daily to the patients. For the treatment of type 2 diabetes, 1 g of cassia bark powder is given for 12 weeks to reduce glycosylated Hb, fasting blood glucose level, and serum level of malondialdehyde, to enhance serum glutathione and superoxide dismutase level [778]. In another investigation, at various doses of cassia bark extract are given for 6 weeks. It is observed that blood glucose level is significantly reduced in a dose-dependent manner in comparison with the control. The intestinal glycosidase activity is reduced, while the serum insulin level and HDL-cholesterol level are increased after 6 weeks of administration of cassia bark extract. It can be concluded that blood glucose level has been maintained with cassia bark extract. The cassia bark extract is able to reduce the blood glucose level by decreasing the carbohydrate absorption in the small intestine [49]
Anti-obesity Effect
Researchers have demonstrated that at various doses of 50, 100, and 200 µg/mL of cassia bark extract are able to increase the lipid deposition in white adipocytes and to enhance the oxidation ability of fatty acid and are able to prevent obesity-induced type 2 diabetes. The doses of 100 and 300 mg/kg of WEBC (water extracts of barks of C. cassia) are able to reduce the total cholesterol level, serum glucose level, insulin, and lipid storage significantly in obese mice. The doses of 0.1 and 0.2 mg/mL of WEBC are capable to enhance the ATP levels [1012]
Cardiovascular Health
Scholars have reported that various doses of 10, 30, and 50 µg/mL of WEBC are able to retard the proliferation of vascular smooth muscle cells (VSMCs), which may prevent cardiovascular disorder. It is demonstrated that a dose of 750 mg/kg of WEBC is able to reduce the serum levels of LDL, TG, and BNP (brain natriuretic peptide) significantly and is able to increase the Ca2+Mg2+-ATP enzyme activity, and to incresae the contents of PCR (polymerase chain reaction), ATP, and ADP in streptozotocin (STZ)-induced myocardial damage diabetic rats [1012]
Antimicrobial Effect
Researchers have investigated about the antimicrobial activity of cassia bark extract. The extract exhibited a wide spectrum of antibacterial effects against fish pathogenic bacteria. Particularly, it showed the potential inhibitory effect against Listonella anguillarum, Streptococcus iniae, and Edwardsiella tarda In liquid medium, the Minimum Inhibitory Concentration (MIC) value of the extract is 75.8 ~ 189.6 µg/mL against gram-positive bacteria and is 75.8 ~ 113.8 µg/mL against gram-negative bacteria. It showed potential bactericidal action against gram-negative bacteria in comparison with gram-positive bacteria. The viable cell counts of L. anguillarum and S. iniae are decreased with the increase in the concentrations of the cassia bark extract [610]
Gastrointestinal Effect
Researchers have investigated about the strong co-relation between bioactive components present in cinnamon bark and its gastrointestinal (GI) actions. Trans-cinnamic acid (TrCin), an organic acid, and its derivatives have been analyzed for regulating the activity of gastrointestinal track. The gastric emptying (GE) test has been conducted to analyze the gastrointestinal activity. 6 cinnamic derivatives like 3,4,5-trimethoxycinnamic acid, cinnamic acid, p-methoxy cinnamic acid, 2-(trifluoromethyl) cinnamic acid, 3-(trifluoromethyl cinnamic acid, and trans 4-(trifluoromethyl) cinnamic acid showed better delaying effect. TrCin, rosmarinic acid, p-coumaric acid, and its derivatives have been analyzed for their α-glucosidase inhibitory action, which may be co-related with gastric emptying and leads to weight loss, and satiety. Therefore, tannins, which are present in cassia bark extract, are able to reduce the demand for food intake by delaying the digestion action and by delaying the digestive track emptying. Researchers have reported that cassia bark extract is helpful in different GI-related disorders like constipation, and diarrhea [815]
Antioxidant Activity
Researchers have investigated the antioxidant activity of cassia bark extract. 70% of ethanol and warm aqueous extracts of cassia bark have been used to evaluate its antioxidant activity by the DPPH radical scavenging activity, and ABTS radical scavenging activity. It is reported that the warm aqueous extract possess the DPPH radical scavenging activity and ABTS radical scavenging activity of 84.93% and 82.20%, respectively, while the ethanolic extract possesses the DPPH radical scavenging activity and ABTS radical scavenging activity of 90.25% and 92.21%, respectively. Researchers have analyzed the NO retardation activity for the cassia bark extract. It has been reported that the NO generation is reduced by 10.15 µM in the ethanol extract and 14.57 µM in the warm aqueous extract [523]
Central Nervous System Activity
Scholars have proposed that the ischemic injury in the animal brain is improved by the administration of 80 mg/kg of cinnamophilin (component of cassia bark). The oxygen–glucose deprivation-induced neuronal injury can be treated with cinnamophilin. The aqueous-soluble extract of cassia bark possessed a substance known as procyanidin Type-A trimer (trimer1), which is able to decrease the cell lumps through improving the movement of intracellular calcium. It is also able to reduce the oxygen–glucose deprivation-induced decreasing activities on glutamate uptake [746]
Piper nigrum (Black pepper)
Anti-inflammatory Activity
At the doses of 10 and 15 mg/kg of plethysmometer, piperine showed anti-inflammatory activity in rats. At a dose of 10 mg/kg, both ethanol and hexane extracts exhibited the anti-inflammatory effect [919]
Anti-diabetic Effect
Piperine (main component of black pepper) showed hypoglycemic effect in normal mice. In hyperglycemic rats, anti-lipid peroxidative, anti-hyperglycemic, and antioxidant activities are found through the administration black pepper orally. In acute and subacute study models, blood glucose levels are influenced with piperine in alloxan-induced diabetic rats. Piperine is given orally at doses of 10, 20, and 40 mg/kg body weight to alloxan-induced diabetic rats. It is reported that piperine is able to increase the blood glucose level at the high dose in the acute study. In the subacute study, piperine is given at a doses of 5, 10, and 20 mg/kg body weight. It is reported that piperine is able to reduce the glucose level. 5% of black pepper has been administered for 8 weeks in the diet of rats. It is reported that it is able to enhance the serum cholesterol, serum liver cholesterol concentration, and hepatic cholesterol-7a-hydroxylase level [445]
Anti-obesity Effect
Researchers investigated the effect of black pepper to control the obesity as a herbal drug [781]. Scholars have investigated the anti-obesity activity of black pepper, Terminalia arjuna, and Lagenaria siceraria. Obesity has been induced in Wistar albino rats with high-fat diet for 28 days. Group I is considered as control (1% of carboxy methyl cellulose (CMC)); group II is considered as obese control (1% of CMC) with high-fat diet; groups III, IV, V, and VI have been treated with several polyherbal tablet formulations of Lagenaria siceraria alone or in combination with other plant extracts of black pepper and Terminalia arjuna (400 mg/kg bw); group VII is taken formulation (400 mg/kg bw), and group VIII is considered as standard (Orlistat 45 mg/kg bw). In comparison with standard, the organ weight, food consumption tendency, body weight TC, TG, LDL, and locomotor activity are decreased, and HDL levels are enhanced in high-fat diet-fed rats treated with the black pepper, Terminalia arjuna, and Lagenaria siceraria [730]
Antimicrobial Effect
A dose of 40 µg/disc of black pepper extract has been selected for examining its inhibitory effect against gram-positive bacteria like S. albus, and B. megaterium and gram-negative bacteria such as E. coli, S. typhi, and P. aeruginosa, and a fungus A. niger. Carbon tetrachloride, benzene, chloroform, ethyl acetate, acetone, ethanol, and aqueous extracts of black pepper showed the inhibitory activity against S. albus. Carbon tetrachloride, benzene, chloroform, ethyl acetate, acetone, and ethanol extracts showed the inhibitory activity against P. aeruginosa and S. typhi. carbon tetrachloride, benzene, ethanol, and distilled water exhibited inhibitory activity against E. coli and B. megaterium [453]
Cardiovascular Health
The alkaloid piperine is derived from black pepper ranging from ~ 5–13%. Piperine is a compound with proven beneficial effect on cardiovascular disease (CVDs). In CVDs, the oxidation status, lipid metabolism, and inflammation have been mediated by the use of black pepper. Piperine showed favorable effects for atherosclerosis. Piperine is capable to inhibit lipid droplet development, lipid peroxidation, oxidized low-density lipoprotein uptake in macrophages, adherence of inflammatory cells to endothelial monolayer, to develop cholesterol efflux from macrophages, to recover lipid profile, to promote myocardial ischemia, cardiac damage, cardiac fibrosis, show antihypertensive, antithrombosis effect, and to protect arterial stenosis through retarding vascular smooth muscle cell proliferation [980]
Gastrointestinal Effect
Researchers have proved that the black pepper and its main constituent piperine is an effective gastrointestinal stimulant and relaxant [707]. A neurotransmitter of the parasympathetic nervous system is Ach, cause gastrointestinal stimulation by the activation of muscarinic receptors. The piperine, and pepper extract showed similar effect to that of Ach [569]. Pepper has been utilized for medicinal purpose like constipation [591]
Antioxidant Activity
Few in vitro investigations proved that reactive oxygen species are retarded by piperine and showed defensive activity against oxidative lesions [244]. In an in vivo study, it has been observed that piperine or black pepper are able to reduce lipid peroxidation and exhibited antioxidant potential. The oxidative stress is inhibited by black pepper by holding superoxide and hydroxyl free radicals, retarding lipid peroxidation and human lipoxygenase, and reducing lung carcinogenesis in rats [220]
Central Nervous System Activity
At various doses of 50, and 100 mg/kg of methanolic extract of black pepper are administered orally for 21 days for memory increasing activity in Alzheimer’s disease model in rats. In an in vivo study, the memory-increasing activities through the administration of the extract of black pepper have been estimated by radical arm-maze. In the radical arm-maze task, the reference memory is reduced and work memory is increased. The memory activity is enhanced by the use of the methanolic extract of black pepper. Researchers have proposed that amyloid β (1–42)-induced local memory diminished has been ameliorated by methanolic extract of black pepper through depletion of the oxidative stress in the hippocampus of rats [220]
Coriandrum sativum (Coriander)
Antimicrobial Activity
Researchers have reported that coriander essential oil is able to retard a wide spectrum of micro-organisms. The essential oil showed antibacterial activity against Staphylococcus aureus, gram-negative bacteria strain like E. coli, Klebsiella pneumonia, Salmonella typhimurium, and Pseudomonas aeruginosa The cell death may be due to the action of coriander essential oil. The fungicidal activity has been found in coriander essential oil against the Candida strains, which has been examined by minimal lethal concentrations (MLC). It is similar to that of MIC value extent from 0.05 to 0.4% (v/v). Coriander essential oil constitutes cyclodecane, 2-Hexen-1-ol, and 3-hexane-1-ol, which may responsible for the antimicrobial activity [87, 412]
Anti-diabetic Effect
In an obese-hyperglycemic, and hyperlipidemic rat model, a dose of 20 mg/kg of coriander extract is given orally that is able to control insulin resistance, and glycemia. It also able to reduce the increased level of insulin, total cholesterol (TC), LDL-cholesterol, triglyceride (TG), and various components of the metabolic syndrome. It can able to enhance the cardioprotective indices. It is concluded that the extract of coriander is capable to enhance insulin secretion, glucose uptake, metabolism, and to reduce hyperglycemia [87, 412]
Anti-inflammatory Activity
The injection of carrageenan is given to hind paw of rats, resulting in the development of edema, arising at its highest within the first 60 min. During the first hour, the retardation is peaked by 29% for the group of rats, which has been examined by polyphenol fraction of Coriandrum sativum seed (PCS) at the dose of 25 mg/kg body weight (BW). The retardation is peaked by 48% for the group, which has been treated with 50 mg/kg BW of coriander seed. The retardation is peaked by 34% for the group, which has been experimented with diclofenac. The retardation is peaked continuously at the 3rd hour by 39, 55, and 57% for group, which has been tested with PCS at a dose of 25 and 50 mg/kg BW and diclofenac, respectively. At the later stage of the experiment, the amounts have been fixed on an ultimate retardation, which is around 87, 92, and 95%. Carrageenan is applied as a chemical, which is able to provoke the salvation of inflammatory mediators. During the first hour after injection, the first phase is characterized by the salvation of histamine, serotonin, and kinins. The second phase is characterized by salvation of prostaglandin [586]
Cardiovascular Health
Arterial blood pressure of anesthetized rabbits is partly blocked through atropine, which is due to the use of coriander crude extract. In rabbit aorta, the development of vasodilatation for phenylephrine and K+-induced constriction are due to the utilization of coriander crude extract and caused cardio-depressant effect. In the organic, and water fractions, bioassay-directed fractionation showed the isolation of spasmolytic, and spasmogenic components. Therefore, diuresis is developed by coriander crude extract at a dose of 1–10 mg/kg. Coriander seed extract is able to retard the electrically evoked constrictions of spiral strips and tubular segments of separated central middle ear artery of rabbits [587]. The analysis of the protective effect of coriander seed on the cardiac injury has been done on isoproterenol-induced cardiotoxicity model in male rats. Rats are pre-examined with methanolic extract of coriander seed at various doses of 100, 20, or 300 mg/kg orally for 30 days. They are subsequently given with isoproterenol (85 mg/kg BW) for the last 2 days. It has been reported that in the cardiac tissue of isoproterenol-tested rabbits the levels of endogenous antioxidants, ATPases, plasma lipids, and the other markers of cardiac injury have been reduced. It is concluded that myocardial infarction are inhibited with methanolic extract of coriander seed through retarding myofibrillar injury and also postulated the oxidative damage, which is inhibited due to the presence of the ample amount of polyphenolic content in coriander seed extract. It has been reported that the coriander seed extract is able to scavenged the isoproterenol generated reactive oxygen species (ROS) efficiently [51]
Gastrointestinal Effect
In rats, the activity of coriander has been examined for gastric mucosal damage caused through ethanol, NaCl, NaOH, indomethacin, and pylorus ligation. In the treatment, the doses of 250 and 500 mg/kg of coriander have been administered orally. It is observed that it is able to prevent the gastric mucosal damage through pylorus ligation, ethanol-related reduction in nonprotein sulfhydryl groups (NP-SH), ulcerogenic activities of various necrotizing agents, and ethanol-induced histopathological wounds. The gastroprotective effect of coriander is able to prevent gastric mucus, and indomethacin-induced ulcers. The protective activity of coriander against ethanol-induced injury of gastric tissue has been connected to the free-radical scavenging property of various antioxidant constituents like coumarins, linalool, flavonoids, catechins, terpenes, and polyphenolic compounds that exist in coriander. The compounds create a protective layer through hydrophobic interactions to retard the ulcer [51]
Antioxidant Activity
Researchers have evaluated the free radical scavenging activity and antioxidant activity of coriander seed. Coriander seed is able to minimize the oxidative stress of streptozotocin-induced diabetic rats. The insulin level is enhanced and blood glucose level is reduced by the administration of seed powder in the diet of diabetic rats. The method of peroxidative destruction is retarded, antioxidant levels, and antioxidant enzymes are reactivated by the administration of coriander seed powder to diabetic rats. The scavenging effect has been observed in the seed against hydroxyl radicals, and superoxides in a concentration-related manner [234]
Central Nervous System Activity
Scholars have identified the chemical composition of coriander essential oil to analyze its cytotoxic activity in SH-SY5Y human neuroblastoma cells, to evaluate its neuronal electrophysiological effects. Apart from this the changes in the extracellular signal-regulated kinase (ERK) and adenylate cyclase1 (ADCY1) expression are also been evaluated. It has been evidenced from the Western blotting, and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide MTT assay that the coriander essential oil can influence the ERK and ADCY1 expression. The cytotoxic effects of the coriander essential oil and linalool has been reported by researchers [173]
Myristica fragrans (Nutmeg)
Anti-inflammatory Activity
Researchers have proved that nutmeg seed extract possessed anti-inflammatory activity through the retardation of TNF-α, IL-6, IL-1β, and NO formation. It is observed that the main macrophage-derived inflammatory mediators are PGE2, and NO. IL-1β, IL-6, and TNF-α are valuable inflammatory cytokines. However, it has been recommended that inhibition of IL-1β, IL-6, TNF-α, and NO is the best technique to decrease inflammation [243]
Antimicrobial Effect
Some investigations exhibited that 60% of nutmeg seed essential oil is able to inhibit the S. typhi, S. aureus, S. dysenteriae, and S. epidermidis The growth of S. aureus, and E. coli are inhibited by the use of nutmeg essential oil. 0.5% of essential oil is able to inhibit the growth of E.faecalis, which has been found in teeth canals. Nutmeg essential oil is considered as a safe protective component for oral care products [585]
Anti-diabetic Effect
Scholars have evaluated the effectiveness of nutmeg seed fot the anti-diabetic effect on alloxan-induced diabetic rats. Normal fasted, glucose fed, and alloxan-induced diabetic rats are given the petroleum ether extract of Myristica fragrans (PEMF) orally. It is observed that a dose of 200 mg/kg of PEMF is able to reduce the blood glucose level after regular treatment of PEMF for 2 weeks. The body weight, organ-like pancreas, liver weight, Hb content, and lipid profile are improved in alloxan treated rats in comparison with diabetic control rats [878]
Anti-obesity Effect
Nutmeg is utilized as tonic, stomachic, carminative, aphrodisiac, and nervous stimulant. It has been demonstrated that in the tetrahydrofuran type lignans (THF)-treated mice, the body weights, and weight gain have been reduced compared to the high-fat diet (HFD)-induced obese mice. A group treated with THF can inhibit the enhancement in the body weight, adipose tissue mass, glucose, and LDL levels than HFD treated group. The acetyl-CoA carboxylase (ACCs) and AMP-activated protein kinase (AMPK) activators are the important factors which regulate the energy storage and management within the animal body. The ACCs, and AMPK are abundant in nutmeg extract, which may responsible for the anti-obesity effect of nutmeg [652]
Cardiovascular Health
Hyalinization of muscle fibers with focal cellular infiltration or infarction of muscle fiber has been revealed by the administration of isoproterenol (ISO) in the heart tissue of rats. Deposition of inflammatory substance is found in the focal cells, which revealed that ISO may destroy the heart tissue. There are no changes in cardiac structure by the administration of nutmeg extract (NM) in heart tissue of treated rats. The effect is same as that of the control group. It is revealed that cardiac tissue is not injured by NM. It is concluded that NM showed protective activity on the myocardium against ISO [429]
Gastrointestinal Effect
Ethanol-induced ulcer models are mostly recommended for the investigation of the gastro-protective effect of nutmeg. In the ethanol-induced ulcer model, the histopathological evaluation of gastric mucosa revealed the damage in lamina propria, damage of superficial epithelium, infiltration of mononuclear cells in the mucosa, degenerative changes in gastric glands, completion of blood vessels, and gastric damage. But pretreatment with sucralfate, and nutmeg ethanolic extract significantly decreased the changes in gastric mucosa and have been reported to provide protection against alcohol-induced gastric damages. Therefore, researchers have concluded that ethanol-induced gastric ulcer is suppressed with nutmeg seed ethanolic extract [812]
Antioxidant Activity
Researchers have compared that the antioxidant activity of nutmeg seed with the synthetic antioxidants like propyl gallate butylated hydroxytoluene (BHT), and butylated hydroxyanisole (BHA). Nutmeg exhibited potential activity in the deoxyribose assay. It is able to maintain the durability of fats like margarine, and butter and oils such as olive, sunflower, and corn against oxidation at 1100 C. In comparison with the BHT, the greater antioxidant activity of nutmeg has been evidenced through Trolox equivalent antioxidant capacity. The antioxidant activity of nutmeg may be due to the presence of compounds like phenylpropanoid, lignans, monoterpenoid including 4-allyl-2, 6-dimethoxyphenol, terpinene-4-ol, and α-terpineol [399]
Central Nervous System Activity
Psychoactive properties such as anxiogenic, and hallucination can be prevented with nutmeg. The physiology of central nervous system has been modulated by the use of nutmeg. The forced swim test (FST) and the tail suspension test (TST) have been used to assess the antidepressant activity of the n-hexane extracts of nutmeg seed in mice. They are subjected to three various doses, e.g., 5, 10, and 20 mg/kg BW, orally. It has been reported that the nutmeg seed extract exhibited antidepressant activity in nutmeg extract treated mice [346]
Brassica nigra (Black mustard)
Anti-inflammatory Activity
Researchers have analyzed the anti-inflammatory effect of ethanolic and water extracts of black mustard seed. At the doses of 200 mg/kg, and 500 mg/kg the extract of black mustard seed are made. Wistar albino rats have been considered for Carrageenan-induced paw edema in vivo analysis to ascertain the anti-inflammatory activity of the black mustard seed. Aspirin-treated group has been considered as the control group. Both the ethanolic, and aqueous extracts of black mustard seed showed the significant retardation of paw edema in comparison with the control group as reported by the researchers [146]
Antimicrobial Effect
Silver nanoparticles along with antibiotics Vancomycin has been applied as a standard anti-bacterial agent against K. pneumonia, P. acnes, and P. aeruginosa The organisms cannot be inhibited by 1 mM AgNO3 But the fabricated silver nanoparticles alone, and a combination of antibiotics exerted significant inhibitory activity against the bacteria considered. The highest synergistic and antibacterial effects have been observed for P. acnes. Researchers have reported that the efficacy of silver nanoparticles are increased in conjunction with standard antibiotics. The organisms, namely P. acne, Candida albicans, Microsporum canis, and Trichophyton mentagrophytes, can be inhibited with mustard seed-silver nanoparticles. The free radicals generated from silver nanoparticles, the interaction between microbial cell membrane and the AgNPs, and the interaction between silver ion and the respiratory cycle of microbes may be the reason for which the inhibitory activities can be observed [680]
Anti-diabetic Effect
The acetone, aqueous, ethanol, and chloroform extracts of black mustard seed are selected to treat streptozotocin-induced diabetic rats. The dose of 200 mg/kg BW of aqueous extract are given to diabetic rats daily for 30 days. The mustard seed extracts are able to reduce the fasting serum glucose (FSG) levels compared to control groups. The serum lipid is lowered, and glycosylated hemoglobin (HbA1c) is enhanced in tested rats in comparison with the control group [76]
Cardiovascular Health
The black mustard seed is a rich source of antioxidant α-tocopherol. The aqueous extract of mustard seed is able to retard the lipid peroxidation induced through ferrous sulfate ascorbate in the human erythrocyte membranes. The mucilaginous fraction of mustard seed is administered into rats. It showed no differences in triglyceride levels and serum cholesterol [284]. The activities between mustard seed oil and fish oil have been evaluated in 360 patients suffering from acute myocardial infarction (MI) in a 1-year randomized placebo-controlled experiment. The treatment has been given to all patients for 18 h after symptoms of an acute MI. 1.08 g/day of fish oil are administered orally to group A patient, 2.9 g/day of mustard seed oil are given to group B orally, and 118 patients are taken as the control group. It has been reported that decrease in total angina pectoris, cardiac arrhythmias, and left ventricular is observed in mustard seed oil- and fish oil-treated patients [642]
Antioxidant Activity
Superoxide dismutase (SOD) is antioxidant enzyme. The enzyme catalyzed the transformation of superoxide to hydrogen peroxide. It defends the cell against oxidative stress. Researchers have carried out an investigation, where 150 mg/kg of Brassica nigra seed has been used to enhance the SOD level of pentylenetetrazole (PTZ)-treated mice. It has been reported that the mustard seed extract is able to remove free radicals and is a potent antioxidant [470]
Central Nervous System Activity
Researchers have evaluated the effect of B. nigra fixed oil (BNO) on the alterations in memory caused by β-amyloid. 42 Wistar rats are divided into 7 groups: 1) control, 2–3) the group is taken BNO at the doses of 462.5 and 925 mg/kg, 4) sham group, 5) Alzheimer group is taken 50 mg/µl/side β-amyloid. The regular gavage of BNO has been carried out for 2–21 days post-amyloid injection. In Morris water maze task, the spatial memory has been analyzed from 21 to 26 days. It is reported that the BNO treated rats the journey distance has been reduced in comparison with the group, which has been treated with β-amyloid alone. The brain memory improving activity of BNO may be due to the presence of 11-eicosenoic acid and erucic acid [647]
Curcuma longa (Turmeric)
Anti-inflammatory Activity
Researchers have reported about the effect of curcumin derived from turmeric on anti-inflammatory activity in rats, and human trials [583, 989]. The laboratory investigation has determined the number of various molecules like cyclooxygenase 2, phospholipase, lipoxygenase, leukotrienes, thromboxane, prostaglandins, nitric oxide, collagenase, elastase, hyaluronidase, monocyte chemoattractant protein-1 (MCP-1), interferon-inducible protein, tumor necrosis factor (TNF), and interleukin-12 (IL-12) that evolved in inflammation. These molecules can be inhibited with curcumin [181]
Antimicrobial Effect
The antimicrobial effect of various fractions of Curcuma longa rhizome has been evaluated against Staphylococcus aureas. The Staphylococcus aureas has been treated with C. longa extract. The microbial cell death may be due to the deformation of cytoplasmic membrane as reported from the scanning electron microscopic observation. It is reported that C. longa rhizome extract showed a wide spectrum of antimicrobial activity. Thus, it can be stated that the turmeric is able to control the microbial infections [345, 851]
Anti-diabetic Effect
Diabetes is treated with turmeric rhizome powder mixed with amla juice, and honey. Researchers have reported that postprandial serum insulin levels are enhanced by the administration of 6 g of turmeric. But it is not able to influence the plasma glucose concentrations or GI within healthy persons. Turmeric showed the activity on the secretion of insulin. Curcuminoids are the active component derived from turmeric rhizome. The curcuminoid is able to reduce the lipid peroxidation by controlling the effects of antioxidant enzymes such as glutathione peroxidase, superoxide dismutase, and catalase at maximum levels [290]. Curcumin and its derivatives like diacetyl curcumin, dimethoxy curcumin, and bisdemethoxycurcumin are responsible for antioxidant activities. Freeze-dried turmeric rhizome powder along with milk can be used as an efficient and safe anti-diabetic supplement as explored by the researchers [729]. Isopropanol and acetone extracts of turmeric are capable to retard the enzyme Human Pancreatic Amylase (HPA) resulting in the lowering of the starch hydrolysis, which ultimately reduce the glucose levels [961]
Gastrointestinal Effect
The curcumin functions by nuclear factor (NF)-kB inhibition. The formation of adherence molecules and inflammatory cytokines are decreased with curcumin. It is able to ameliorate the gastric damage, to recover from gastric mucosal injury, to reduce the leucocyte adherences, intracellular adherence molecule 1, and tumor necrosis factor (TNF)-α formation in nonsteroidal anti-inflammatory drugs (NSAIDs)-induced gastropathy in rats [934]. Abdominal pain or irritation, and Irritable Bowel Syndrome (IBS) are reduced by the administration of Curcuma longa extract. It is also able to enhance the IBS quality. Curcumin is able to treat the acetaminophen (APAP)-induced hepatitis through the recovery of liver histopathology through reduced restitution of GSH, oxidative stress, and inflammation of the liver [877]
Cardiovascular Health
Turmeric contains antioxidants that inhibit damage to cholesterol; therefore, has provided a protective activity against atherosclerosis. Turmeric is able to reduce free radicals as it contains vitamins C and E. It is reported that triglycerides, cholesterol, and other lipids move in the bloodstream. They are the risk factors for cardiovascular disorders which may be reduced with curcumin. A standard American diet rich in refined saturated fat, carbohydrates, and fiber has been administered to mice. The diet has been mixed with turmeric has been given to mice. It has been reported that the mice those have been fed with the turmeric added diet possessed 20% less blockage of the arteries after 4 months in comparison with that mice fed with the American diet. In another investigation, rabbits fed with turmeric enriched diet to treat the atherosclerosis disease. It has been reported that the cholesterol, and triglycerides levels have been reduced in the turmeric treated rabbits [961]
Anti-obesity Effect
Researchers have investigated about the potential anti-obesity activity of the turmeric, and curcumin extracts. A high level of turmeric extract is able to prevent the formation of cholesterol and triglycerides [743]. The anti-adipogenesis potential of turmeric extract may be due to its ability to retard the formation of lipid droplets, triglycerides, and cholesterol in HepG2 cells. The turmeric extract showed better anti-obesity activity in comparison with curcumin [165]
Antioxidant Activity
Researchers have evaluated the antioxidant activity of different fractions of methanolic extract of turmeric rhizome [761]. The ethylacetate fraction possessed DPPH free radical scavenging activity with IC50 = 9.86 µg/mL. At a similar concentration, the ethylacetate fraction showed higher DPPH radical scavenging activity, and ABTS radical scavenging activity in comparison with n-hexane, and water fractions [199]
Central Nervous System Activity
Researchers have investigated the chemical composition of the essential oil of turmeric rhizome to analyze its neuropharmacological effect. At a dose of 50–200 mg/kg of EO of turmeric has been given to albino mice to assess its sedative, behavioural, anxiolytic, and anticonvulsant activities. It is reported that the head dips are reduced, locomotor activities are retarded, and total sleeping time are extended with the application of EO. EO is able to defend the mice from pentylenetetrazol-induced convulsions. It is reported that EO of turmeric possessed anticonvulsant, anxiolytic, and sedative effects [671]
Laurus nobilis (Bay leaves)
Anti-inflammatory Activity
Researchers have considered mice for analyzing the anti-inflammatory activity of bay leaf essential oil. In the carrageenan-induced hind paw edema in mice, the water, and ethanol extracts of bay leaf have been reported for its potential of anti-inflammatory effect without causing any gastric injury [931]
Antimicrobial Effect
A wide range of microorganisms are inhibited with Laurus leave essential oil [124]. The greater antibacterial activity has been found in Laurus essential oil in comparison with tetracycline antibiotics [212]. It is reported that bay leave essential oil can alter membrane-embedded proteins, damage cellular membranes, enhance the permeability of the membrane, damage the transit system of the membrane [868]. The EO showed antibacterial properties may be due to the presence of terpenes. Researchers have revealed the gram-positive bacterial strains and the gram-negative bacteria can be inhibited with essential oil. The outside membrane of gram-negative bacteria provides protection against the EO, that is why the essential oils are more effective against the gram-positive bacteria than the gram-negative bacteria [909]. 1,8-cineole is the main active compound responsible for the antimicrobial effect of bay leave EO [172]. The antagonistic, and synergistic effects of 1,8-cineole along with the oxygenated terpenes may be the reason behind the inhibitory potential of Laurus essential oil on microbial growth. Microorganisms like Kocuria rhizophila, Staphylococcus aureus, E. coli, Pseudomonas aeruginosa, and Salmonella abony can be inhibited with bay leaf essential oil [298]
Anti-diabetic Effect
Researchers have investigated the effect of bay leave extract on biochemical, and histopathological changes in β-cells for Streptozotocin (STZ)-induced diabetic male albino rats. 30 healthy adult male albino rats have been considered. The rats have been divided equally into 5 groups: diabetic Laurus nobilis extract group (DLN), control group (C), diabetic group (D), Laurus nobilis extract group (LN), and diabetic acarbose (DA) group. It is reported that the D group rats have exerted several degenerative, and necrotic changes in their kidney, liver, and pancreas, while the normal histology is found in DLN rats. D groups showed a reduction in insulin immunostaining in the pancreatic β-cells in comparison with the C groups. The diabetic rats have been treated with Laurus nobilis, and have also been treated with acarbose, which have exhibited the reduction in glucose concentration, aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), and alanine aminotransferase (ALT) enzyme in comparison with D groups. It is reported that bay leave showed important activity on blood glucose levels. The bay leave is able to increase the regeneration of pancreatic islets. It is also able to restore the altered liver enzymes, total protein levels, urea, creatinin kinase, calcium, and ferritin to near-normal levels [609]
Gastrointestinal Effect
Scholars have considered rats to evaluate the gastroprotective effect of various extracts of bay leave (chloroform and methanol). The methanolic and chloroform crude extracts can decrease the gastric damage significantly. The extracts are able to provide more efficient protection. The extracts of bay leave is able to ensure the relationship between the antiradical activity, and pharmacological efficacy [885]
Antioxidant Activity
Various flavonols and glycosides are present in bay leave. Bay leave contains water-soluble antioxidant compounds that can be connected to the several health-beneficial effects [216]
Crocus sativus (Saffron)
Anti-inflammatory Activity
Anti-inflammatory effect has been found in water extract of saffron petals. The presence of crocin in saffron is responsible for the anti-inflammatory activity [633]
Antimicrobial Effect
Researchers have evaluated the antimicrobial activity of the non-polar extract of saffron flower stamens. It has been reported that saffron flower extract can inhibit both the growth of gram-positive, and gram-negative bacteria [903]. The diethyl ether extract of saffron stamens (DES) is able to inhibit the foodborne pathogen species like Listeria monocytogenes, Salmonella enterica subsp In. bongori, Staphylococcus aureus, and E. coli [1009]
Anti-diabetic Effect
Scholars have evaluated the anti-diabetic activity of water extract of saffron. α-Amylase and α-glucosidase enzymes are retarded with saffron water extract. Thus, researchers have concluded that saffron water extract is able to prevent diabetes [509]. It is reported that phenolic compounds derived from saffron showed hypoglycemic activity through the maintenance of insulin sensitivity, postprandial blood glucose levels, and acute insulin secretion. The extract is also able to retard the glucose absorption rate from the intestines into bloodstream [1001]
Gastrointestinal Effect
Many studies have demonstrated that the effectiveness of saffron on gastrointestinal system. It is reported that saffron is able to treat the stomach related problems, amenorrhea, menstruation problem, the problem related to anus displacement, hemorrhoids, decrease in appetite, intestinal fermentation related problems. Safranal derived from saffron is able to decrease the surface spreading of gastric ulcers, to normalize gastric volume, and to maintain the histological changes in gastrointestinal track [633]
Cardiovascular Health
Researchers have demonstrated the capability of saffron on cardiovascular activity. The saffron is able to provoke chronotropic, inotropy, and to decrease KCI induced constriction in vascular, non-vascular smooth muscle. In hypertensive and normotensive anesthetized rats, the blood pressure is decreased with water extract of saffron. This investigation recommended that smooth muscle contractility may not be affected with crocin [706]. The hydroalcoholic extract of saffron showed the hypotensive effect because of the retardation of the rennin-angiotensin system in angiotensin II hypertensive rats. The saffron extract showed hypotensive activity by decreasing the calcium salvation into the sarcoplasmic reticulum, antagonism toward adrenergic receptors, and retardation of calcium channel [1011]
Anti-obesity Effect
The body weight of rats is decreased with saffron stigma ethanolic extract. The ethanolic extract of saffron is able to reduce the appetite. It is reported that the saffron extract has possessed mood-improving activity. The saffron extract is able to reduce snacking, and appetite. It is observed that saffron extract intake in women can reduce their body weight, and snacking within 60 days. Researchers have recommended that body weight can be reduced with the consumption of saffron supplemented diet [581]
Antioxidant Activity
Researchers have identified the antioxidant potential of saffron in various extracts [725]. Saffron is able to protect the free radical-induced injured organs like lungs, kidney, liver, and heart as observed in hamsters [955]. It is reported that at a dose of 0.45 mg/ml of soaked, and decocted saffron is the most efficient one to cure the injured organs. The superoxide dismutase activity can be enhanced with saffron extract, while the lipid peroxidation can be reduced [561]
Central Nervous System Activity
Scholars have evaluated the neuropharmacological activities of crocetin, saffron, crocin, and safranal in the peripheral, and CNS [984]. Saffron and its active constituents showed protective activities like anxiolytic, antidepressant, antinociceptive, hypnotic, and cytoprotective [506]. Saffron, and its constituents are able to decrease the opioid withdrawal syndrome, and to cure neurodegenerative diseases such as Parkinson’s disease, epilepsy disease, brain ischemia, and memory impairment. It is reported that age-related macular degeneration and Alzheimer’s disorders are prevented with saffron [640]
Illicium verum (Star anise)
Antimicrobial Effect
The crude ethanol extract of star anise exhibited antimicrobial properties against S. aureus and A. baumannii. The star anise ethanol extract is also able to inhibit P. aeruginosa. The diethyl ether (EE) fraction of star anise has provided greater inhibitory activity in comparison with crude extract. The supercritical CO2 extract of star anise exhibited an antibacterial effect against A. baumannii. Though the supercritical CO2 extract did not show any antibacterial property against the other two bacteria. The EE fractions and alcohol extract showed a wide antibacterial spectrum against these three bacteria. The EE fraction of star anise has possessed MIC value of 0.25 mg/mL. It showed higher antimicrobial activity against S. aureus in comparison with the crude extract. Similar results have been reported by the researchers for gram-negative bacteria, namely A. baumannii and P. aeruginosa [994]
Anti-inflammatory Activity
Researchers have revealed the anti-inflammatory activity of star anise. The anti-inflammatory activity has been reported in water extract of star anise as per the investigation carried out on the intestinal smooth muscles of mice. The water extract of star anise is able to reduce the serum level of TNF-α, and IL-1β [94]
Anti-diabetic Effect
Scholars have investigated the anti-glycation effect of ethanolic extract of star anise. The protein glycation retardation has been evaluated by human serum albumin (HAS)-fructose glycation model. Similarly, sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis has been conducted to analyze the cross-linked advanced glycation endproducts (AGEs). The star anise extract has been given to streptozotocin-induced diabetic rats for 7 weeks. After that, the anti-glycation activity has been analyzed. Star anise extract exhibited a good inhibitory activity in comparison with the standard inhibitor (rutin). The extract of star anise is able to decrease the lipid, blood glucose level, urea, liver functions parameters, and renal AGEs levels in streptozotocin-induced diabetic rats. It is reported that star anise can prevent diabetes-associated problems [447, 448, 455]
Gastrointestinal Effect
Researchers have investigated the star anise extract as an anti-gastroulcerogenic agent in the rat model. Various extracts like aqueous–alcoholic, petroleum ether, and essential oil have been considered. The rats are fed with a dose of 1250 mg/kg bw petroleum ether extract and 2500 mg/kg bw for aqueous ethanol extract. Rats have been divided into three main groups: treated animals, negative control groups (fed with distilled water), and positive control groups (administered with various extracts). It is reported that aqueous–alcoholic extract possesses the greatest antioxidant activity. The petroleum ether extract showed the lowest effect. The higher anti-ulcerogenic activity has been found in aqueous–alcoholic extract in comparison with famotine (a reference drug). The aqueous–alcoholic extract is able to increase the superoxide dismutase activity and catalase activity and is able to induce the glutathione reductase activity [382]
Central Nervous System Activity
Scholars have investigated the action of star anise extract on the central nervous system (CNS). In this experiment, the male albino mice and rats have been examined. The parameters like locomotor activity, general behavior, sleeping pattern, anxiety, and myocoordination activity have been used to analyze the CNS activity. The rats and mice are separated into 5 groups with 6 animals in each group and have examined for 1 week. At a dose of 200 mg/kg of all extracts have been administered through intraperitoneal injection. It is reported that the extracts of star anise are able to decrease the locomotor activity, prolong the phenobarbitone induced sleeping time, form alteration in a general behavior pattern, and possess anxiolytic effects. Though, the extracts are not able to alter the muscle coordination activity. The methanol extracts are more effective in comparison with the ethyl acetate, and hexane extracts. It is reported that the most potential CNS depressant activity and anxiolytic effects are found in these three extracts without altering the motor coordination [202]
Antioxidant Activity
Researchers have evaluated the antioxidant activity of star anise constituents. The methanolic extract of star anise showed antioxidant potential (as appeared in DPPH assay) with IC50 of 61 mg/ml. Among the different types of fractions, antioxidant activity has been found in the ethyl acetate (IV-EA) with IC50 of 18 mg/ml. In the sub-fractions, the most potent antioxidant activity has been observed in the ethyl acetate soluble sub-fraction of ethyl acetate fraction (IV-EA-EA-S) with IC50 value of 42 mg/ml. The protocatechuic acid, Illicium verum extract (IV-E), and IV-EA showed antioxidant potential with IC50 of 2000, 1400, and 600 mg/ml, respectively [92]
Allium cepa (Onion)
Anti-inflammatory Activity
Researchers have evaluated the anti-inflammatory activity of ethanolic extract of onion. Sprague Dawley rats (250–300 g) have been considered to analyze the anti-inflammatory activity of onion extract. The standard drugs like tramadol and diclofenac sodium have been considered to compare the anti-inflammatory activity of onion. The carrageenan-induced paw edema of rats can be retarded with the administration of ethanolic extract of onion [415]
Antimicrobial Activity
Scholars have evaluated the antimicrobial activity of onion EO at various concentrations (50, 100, 200, 300, and 500 ml/l) against Salmonella enteritidis and Staphylococcus aureus. The EO extracts of onion are able to inhibit fungies like Aspergillus niger, Penicillium cyclopium, and Fusarium oxysporum [141]
Anti-diabetic Activity
Researchers have analyzed the anti-diabetic activity of onion in alloxan-induced diabetic rats. Thirty-six male albino rats are given with alloxan through intraperitoneal route to evaluate the anti-diabetic activity of onion. The rats have been divided into six groups (6 rats/group). The group A consists of rats those are not induced with alloxan and untreated, group B rats (induced but untreated), group C rats (administered with glibenclaimide), rats of group D, E, and F are induced, treated with 1, 2, and 3 mL/100 g BW of onion juice, respectively. It is reported that onion is able to reduce the blood glucose levels, and the lipid profile significantly in untreated rats [33]
Anti-obesity Activity
Scholars have investigated the effects of the onion extract on the expression of inflammatory mediators from the adipose tissue in a high-fat diet-induced obese rat model. Dawley rats have been separated into three groups: control, high-fat diet (HF), and high-fat diet with onion extract. The all the groups have been treated for 8 weeks. It is reported that the epididymal, and perirenal fat weights are not influenced significantly, whereas the mesenteric fat weight is reduced significantly in the groups treated by high-fat diet with onion peel extract in comparison with the HF groups. A greater adiponectin mRNA level has been identified for the group fed with high-fat diet with onion supplementation in comparison with the other groups. IL-6 mRNA level has been reduced slightly in rats fed onion extract supplementation in comparison with the HF groups [579]
Cardiovascular Activity
Flavonoids derived from onion, which are able to prevent cardiovascular disease, and heartburn. Quercetin is able to decrease the blood pressure in hypertensive persons, to activate platelets, and to treat cardiovascular disease [398]. Stroke, coronary heart disease, and hypertension have been associated with inflammation or atherosclerosis. Researchers have revealed that oxidative stress can be reduced with quercetin through mopping free radicals. Therefore, quercetin is able to decrease the chances of stroke, and heart disease. In another study, 24 healthy persons with ages between 35 and 55 years have been considered. They have been separated into two groups of 12 each having 7 females and 5 males suffering from moderate hypercholesterolemia. One of the groups has been administered with 100 mL of onion, while the other group has been considered as control. It is reported that LDL-C, waist circumference, and total cholesterol are decreased after 8 weeks substantially for the group fed with onion. It is reported that onion juice is able to enhance the LDL oxidation lag time, and antioxidant activity, which is helpful in the prevention of CVD [183]
Antioxidant Activity
Researchers have evaluated the antioxidant capacity of flavonoids, organosulfur compounds, and polyphenols are present in ample amounts in onion. Three onion cultivars, namely Borettana di Rovato, Dorata di Parma, and Rossa di Toscana, have been considered to evaluate their antioxidant potential in terms of total phenolic content, DPPH, FRAP (ferric reducing antioxidant power), and TEAC (Trolox equivalent antioxidant capacity) potential. The highest antioxidant potential has been observed in Rossa di Toscana [536]. The subcritical water, hot water, and ethanol extracts of onion have been considered to evaluate the antioxidant potential in terms of ferric thiocyanate (FTC), DPPH, and lipid peroxidation inhibitory ability [518]. Other researchers have investigated a relevance between the antioxidant activity, and organosulfur compounds of the onion by the methods like ABTS (2,2’-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid), ORAC (oxygen radical absorbance capacity), and DPPH [473]. The antioxidant activity has been reported in organosulfur compounds. Researchers have selected 400 broiler chickens to evaluate the effects of phenol-rich onion for their antioxidant capacity, production, digestion, and immune response. At various concentrations, the phenol-rich onion extract (PROE) has been given to each group (100/group) for 35 days. It is confirmed that antioxidant enzyme activities (glutathione peroxidase, catalase, and superoxide dismutase) are enhanced with PROE because of flavonoids, and phenol [667]
Central Nervous System Activity
Researchers have evaluated the neuroprotective effect of Onion in aluminium chloride-induced neurotoxicity. 50 mg/kg/day of aluminium chloride, and 50–200 mg/kg/day of onion have been administered to Swiss albino male mice. It is reported that onion is able to enhance catalase, and glutathione (GSH) activities and to lower the nitrate/ nitrite, acetylcholinesterase (AChE), and lipid peroxidation. Quercetin, kaempferol, cycloartenol, and phytosterols like lophenol, 24-ethyl cycloartenol, and 24-methyl lophenol present in onion possessed neuroprotective effect [183]
Anethum graveolens (Dill)
Anti-inflammatory Activity
Researchers have evaluated the effects of dill seed, and the extract of aerial part to determine its anti-inflammation potential. The dill extract has been evaluated for its anti-inflammatory activity against formalin-induced inflammation in rats and the diclofenac gel as a reference. The inflammation has been induced by injecting formalin in the paw of rats. After that the weight of the rats has been measured by plethysmometer and the paw volume has been assessed. The experiment has been conducted for 8 days. Researchers have divided the rats into 3 groups of 6 male rats: the diclofenac gel groups, formalin groups, and dill-oil groups. Two grams of dill oil with 100 mg dill extract has been administered to dill-oil groups. Two grams of gel with 20 mg diclofenac Na has been given to the diclofenac groups. In these groups, the mean paw amounts have been changed after Formalin-induced inflammation on the 1st day. In diclofenac gel and dill-oil-administered groups, the paw volume has been changed after injecting the formalin on daily basis for 8 days in comparison with the control groups, which may show a significant reduction. Paw volume has been more reduced in dill groups in comparison with the diclofenac groups. Plethysmometer has been conducted to evaluate the results of paw volume. It is reported that the dill-oil is capable to reduce the paw volume significantly [639]
Antimicrobial Activity
Dill extracts have been reported for its Helicobacter pylori inhibiting activity. The potential antibacterial activity has been found in organic, and water extracts, and the essential oils of dill against fungi (two molds like Aspergillus flavus, Penicillium islandicum, yeast such as Candida albicans)Candida albicans, Aspergillus niger, and Saccharomyces cerevisiae are inhibited by D-carvone and D-limonene. The antimicrobial effect may be characterized by furanocoumarin present in dill [607]
Gastrointestinal Activity
The dill extract has been reported to possess antisecretory, and mucosal protective activities against ethanol, and HCl-induced gastric mucosa damage in mice. Dill extract is capable enough to reduce the total acid content as perceived by the researchers. Rabbit intestine constrictions are decreased with dill essential oil. Ethanol extract of dill has been retarded histamine and acetylcholine-induced constrictions of guinea-pig ileum. Some digestive problems like flatulence, stomachache, and indigestion are improved with dill [354]. Dill water is reported to show a cooling effect in stomach. Dill is able to prevent gripe, to improve colic, and hiccups. It is reported that essential oil is able to decrease foaming, and also to act as a light carminative [378]
Antioxidant Activity
The antioxidant potential of the acetone, and essential oil extracts of dill has been investigated by researchers through the methods like DPPH, reducing power, and chelating effect. The ethanol, hexane, and ethyl acetate extracts of dill have been evaluated for their antioxidant potential through the methods like reducing power, DPPH radical scavenging, Trolox equivalent antioxidant capacity, chelating power, and β-carotene bleaching [854]. The carbon tetrachloride-induced hepatotoxicity in albino rats have been considered to analyze the antioxidant activity of ethanolic extract of dill [914]. It is reported that ethanolic extract of dill (100 mg/Kg wt) is able to restore the antioxidant enzymes and serum enzymes activity that are increased in treated rats. The antioxidant capacity of ethyl acetate, dichloromethane, n-hexane, and ethanol extracts of dill have been examined by nitric oxide radical scavenging, DPPH, N,N-dimethyl-p phenylendiamine, ferric ion-chelating capacity, ferric reducing antioxidant power, and phosphor molybdenum-reducing antioxidant power. It has been reported that the ethanolic extract of dill possessed the highest antioxidant power. Though, in terms of ferric ion chelation effect the dichloromethane extract possess the maximum value. The chlorogenic acid (6.04 µg/g extract) is the dominant phenolic acid as reported by the researchers. The EO fraction contains lower amounts of phenolic compounds (35.1 mg GAE g−1 extract) [180]
Anti-diabetic Activity
Various extractions of dill, and its essential oil have been given to diabetic rats. The extracts, and EO of dill are able to decrease low-density lipoprotein cholesterol, triglycerides, total cholesterol, very-low-density lipoprotein cholesterol, and glucose levels remarkably, and to enhance the HDL-C levels. The antioxidant and hypoglycemic activities have been found in dill. Flavonoids, and phenolic proanthocyanidins compounds derived from dill showed antioxidant potential. The anti-diabetic activity of dill may be due to the enhancement of fecal excrement, bile acids formation, retardation of the absorption of intestinal cholesterol, and the ability to bind the bile acids in the intestine [402]. The hypolipidemic activity of dill may be due to the presence α-phellandrene, carvone, and limonene. Histopathological tests exhibited various extracts of dill, which are used to treat diabetic rats are able to normalize the deposition of lipid in the heart, liver, and pancreas. The hypoglycemic effect of dill may be due to the presence of alkaloids, flavonoids, terpenoids, tannins, and phytosterols in dill extract. It is proposed that the dose of 300 mg/kg of hydroalcoholic extract of dill is able to reduce the fasting blood glucose level. Various extracts of dill at the concentrations of 0.032, 0.065, 0.125, 0.25, 0.5, and 1 mg/ml have been fed to the type 2 diabetic rats to decrease the fructosamine level [333]
Anti-obesity Activity
Researchers have investigated the anti-obesity activity of Anethum graveolens aqueous extract (AGAE). AGAE is able to act as a strong anti-obesity agent through the 5-hydroxytryptamine (5-HT) metabolism mediation, to enhance 5-HT turnover in the brain of rats, the concentrations of tryptophan in the brain and blood. AGAE has been administered to rats orally for 7 days. It is able to decrease the tendency in food consumption of obese rats, resulting in reduced body weight. Researchers have described important facts about the 5-HT concentration in the brain which is a neurotransmitter. It plays an important role in feeding behavior. Obesity and hyperphagia have been produced with reduced concentration of 5-HT in the brain [125]
Cardiovascular Activity
Scholars have evaluated the effectiveness of the hydroalcoholic extract of dill on the cardiovascular activity of overweighted male rats. Thirty-two overweight male rats weighing 350–400 g having aged 12 weeks have been separated into four equal groups like endurance training + dill extract (ETr + DEx), endurance training (ETr) for 10 weeks in 5 sessions/week, dill extract (DEx) at a dose of 300 mg/kg BW, and control (Ct). Eight rats weighing 240–280 g in the non-obese control (NCt) groups have been considered to analyze the cardiovascular activity. After the final intervention session, the fasting plasma lipid concentration has been assessed for 48 h. It is reported that in the Ct groups, LDL-C, TC, TG, VLDL-C, TC/HDL-C, and HDL-C levels are enhanced in comparison with the NCt groups. The plasma concentration of LDL-C, VLDL-C, TG, TC/HDL-C, and LDLC/HDL-C is reduced in the ETr + DEx groups and in the ETr groups in comparison with Ct groups. It is reported that the plasma lipid profile is maintained with endurance training. It has been confirmed by the researchers that the dill extract is more efficient to prevent cardiovascular disease [59]
Trigonella foenum-graecum (Fenugreek)
Anti-inflammatory Activity
Researchers have evaluated the anti-inflammatory effect of petroleum ether extract of fenugreek. Fenugreek petroleum ether extract (FSPEE) has been evaluated and examined on rats against formaldehyde, and carrageenan-induced paw edema. At a dose of 0.5 mL/kg of FSPEE has been given to rats to analyze the anti-inflammatory activity. It is reported that FSPEE is able to decrease 37%, and 85% inflammation of the paw in the formaldehyde and carrageenan-induced paw edema, respectively. It is reported that anti-inflammatory activity has been found in fenugreek petroleum ether extract because of the presence of linoleic, and linolenic acids [716]
Antimicrobial Activity
Scholars have evaluated the antimicrobial activity of acetone, aqueous, and methanol extracts of fenugreek against Staphylococcus and E. coli. The methanol extract has been reported as the most efficient one in terms of antimicrobial activity followed by acetone extract, and aqueous extract [840]
Anti-diabetic Activity
Researchers have evaluated the anti-diabetic effect of extract of Trigonella foenum-graecum (IND01) on the neonatal streptozotocin-induced rat (n-STZ) model with diabetes mellitus (DM). At a dose of 50 mg/kg of streptozotocin (STZ) has been injected in the rat pups. It is reported that DM has been evaluated after 8 weeks through measuring fasting serum glucose (SG) level. At a dose of 10 mg/kg of standard drug (glyburide) has been given to n-STZ rats orally. At a dose of 100 mg/kg of IND01 has been given to n-STZ rats for 28 days. In the acute study, the SG levels have been taken at periodical intervals. In the subacute study, the serum insulin, and glycosylated hemoglobin (HbA1c) levels have been recorded on day 28. The HbA1c, SG levels, and body weights are increased significantly for n-STZ induced rats. Pancreatic islet β-cells and serum insulin levels are reduced in control groups. The treatment with glyburide and IND01 exhibited significant reversal of n-STZ-induced changes. The histology sections of the pancreas showed the ability of IND01 to enhance the size and number of pancreatic islet β-cells. It is reported that IND01 is able to treat DM and also to maintain the glycemic activities in n-STZ induced diabetic rats [491]
Anti-obesity Activity
Scientists have evaluated the anti-obesity activity of Fenugreek (INDUS810). The mice aged 4 weeks have been consumed with a high-fat diet, and a normal diet together with or without of 200 mg/kg of INDUS810 by intraperitoneal injection twice/week to analyze the anti-obesity activity of fenugreek. It is reported that in high-fat diet-induced mice, the weight are decreased with INDUS810 in liver, epididymal white adipose tissue, interscapular brown adipose tissue. INDUS810 is able to reduce the serum cholesterol level, and low-density lipoprotein cholesterol, to maintain the insulin sensitivity in mice. INDUS810 is also able to retard the lipid deposition and to enhance the lipolysis activity in mature adipocytes. INDUS810 can enhance protein levels of sirtuin 1, peroxisome proliferator-activated receptor α, peroxisome-proliferator-activated receptor-gamma co-activator 1β, and sirtuin 3. It is capable to activate the adenosine monophosphate-activated protein kinase that may decrease the lipid levels within adipocytes. It is reported that INDUS810 is considered as a potential anti-obesity agent [196]
Gastrointestinal Activity
Researchers have observed that the fenugreek seed exhibited anti-ulcer activity. Omeprazole, a well known proton pump blocker has been comparable to the effect of fenugreek. The drug is utilized to treat gastrointestinal disorders like gastritis, gastroesophageal reflux disorder, and duodenum and gastric ulcer. A gel fraction, and aqueous extract of fenugreek exhibited effective ulcer defensive activities, which may be characterized by its antisecretory activity and effectiveness over mucosal glycoproteins as reported by researchers in a ethanol-induced gastric ulcer in rats. It is reported that water extract of fenugreek has possessed antioxidant activity, which may retard the ethanol-induced gastric damage in the rats. In comparison with omeprazole, a better gastroprotective activity has been reported in insoluble gel fraction of fenugreek. This may be due to the presence of flavonoids and beneficial polysaccharides in fenugreek [338]. The defensive mechanism of flavonoids in the mucosa may be the reason for the gastrointestinal protective effect of fenugreek [991]
Antioxidant Activity
Scholars have reported that the lipid peroxidation is enhanced, and the antioxidant molecules like α-tocopherol, glutathione, and β-carotene are altered with fenugreek in alloxan-diabetic rats. 2 g/kg BW of fenugreek powder supplementation has been given to alloxan-diabetic rats for 1 month to evaluate the antioxidant and lipid peroxidation capacity. The fenugreek powder is able to normalize the oxidative stress, and lipid peroxidation. It is reported that antioxidant activity has been found in insoluble part of fenugreek [888]
Cardiovascular Activity
Researchers have investigated the cardioprotective activity of fenugreek, and garlic on hypercholesterolemic rats. For 8 weeks, the rats are given a high-cholesterol diet along with garlic (2%), and fenugreek (10%), and their combination. Myocardial infarction (MI) has been induced with isoproterenol. MI has been associated with enhancement of serum iron, circulatory troponin, disarrangement of activities of cardiac ATPase, reduced ceruloplasmin. It is reported that isoproterenol-induced compromised antioxidant status is improved with garlic and fenugreek and the combination of both garlic and fenugreek [627]
Central Nervous System Activity
Researchers have investigated the neuropharmacological effect of fenugreek. The neuropharmacological activity has been observed with petroleum ether extract (PE), total alcoholic extract (TA), total aqueous extract (TQ), total alkaloidal extract (TK), total glycoside extract (TG), fenugreek oil (FO) in diosgenin (DI), and trigonelline (TR)-induced albino Wistar rats. At the dose of 100 mg/kg BW of PE, TA, TQ, and FO have been given to rats. At the dose of 50 mg/kg BW of DI, TK, TG, and TR have been given to rats intraperitoneally. At a dose of 150 ug/kg of chlorpromazine hydrochloride (CP) and at a dose of 48 mg/kg of caffeine (CF) have been utilized as standard. It is reported that except TQ, all the other extracts are able to stimulate of the activity of central nervous system (CNS). While, TQ possessed CNS depressant activity. It is reported that fenugreek extracts contain bioactive components (isovitexin, and rhaponticin) that showed CNS depressant, and stimulant potential [641]
Ferula assafoetida (Asafoetida)
Anti-inflammatory Activity
Researchers have evaluated the anti-inflammatory activity of asafoetida. Phenolic compound present in asafoetida is responsible for anti-inflammatory activity. 2.5 mg/kg of asafoetida has been administered to mouse to treat the carrageenan-induced paw edema. The bioactive compounds like sesquiterpene coumarins are present in asafoetida, which may retard the 5-lipoxygenase activity. Ferulic acid and flavonoid components derived from asafoetida possessed strong antioxidant potential and act as an anti-inflammatory agent [114]
Antimicrobial Activity
Scholars have investigated the antimicrobial effect of ethanol, ethyl acetate, methanol, chloroform, and aqueous extracts of asafoetida. The antifungal effect against Candida albicans and Aspergillus niger and the antibacterial effect of asafoetida extracts against Klebsiella pneumonia, Bacillus subtilis, Staphylococcus aureus, and E. coli have been analyzed through well diffusion process and assessment has been done with the minimum inhibitory concentration (MIC). The inhibition zone has been compared with standards such as fluconazole (0.1 mg/ml) and ciprofloxacin (0.1 mg/ml). It is reported that antimicrobial activity has been found in methanol, ethyl acetate, and ethanol extracts. The highest inhibitory activity has been observed in methanol extract. The ethyl acetate, methanol, and ethanol extracts showed the MIC values of 1 mg/ml and 2 mg/ml, respectively, for the examined organisms [690]
Anti-diabetic Activity
Scientists have analyzed the hypoglycemic effect of the asafoetida extract in streptozotocin-induced diabetic rats. Male Wistar rats have been divided into several groups like control groups, rats fed with 50, 100, and 300 mg/kg doses of asafoetida. Asafoetida extract has been administered regularly through drinking water to diabetic rats for 4 weeks. The serum glucose level is reduced in diabetic rats treated with 50 mg/kg of asafoetida extract in comparison with diabetic rats. It is reported that hypoglycemic activity has been found in streptozotocin-induced diabetic rats with asafoetida extract (50 mg/kg) after 2 weeks. Tannins and phenolic acids (ferulic acid) present in asafoetida are responsible for the anti-diabetic activity [38]
Anti-obesity Activity
Researchers have identified the effectiveness of asafoetida over liver steatosis, weight enhancement, lipid deposition, and leptin level. Aqueous solution of fructose (10%) has been administered in a regular basis to both the control and treated rats. At the doses of 25 or 50 mg/kg (PO) of asafoetida oleo-gum resin has been administered to two treated groups. Tap water, and standard chow food have been given to the control group. It is reported that asafoetida is able to reduce the size of epididymal adipocytes, body weights, abdominal fat, and serum leptin levels. Researchers have observed that fat lowering and anti-obesity activity have been found in asafoetida that may also prevent liver steatosis in type 2 diabetic rats [106]
Gastrointestinal Activity
Scholars have evaluated the effects of asafoetida oleo-gum resin on gastrointestinal diseases like digestion, parasite, bloating, and cancer [387]. Scholars have assessed the effectiveness of asafoetida in the prevention of dyspepsia. Asafoetida possessed anti-diarrhea, anti-parasite, anticancer, and liver defensive effects. Asafoetida is able to prevent gastrointestinal diseases [553]
Cardiovascular Activity
Scientists have analyzed the effect of asafoetida essential oil (AEO) on ischemia–reperfusion-induced damage in isolated rat hearts. Forty-eight male Wistar rats have been separated into 6 groups: 1–3) AEO groups, 4) control groups, 5) vehicle groups, and 6) carvedilol groups. The hearts have been subjected to ischemia treatment (for 30 min) followed by reperfusion (120 min) for the control group. In other groups, hearts have been perfused with carvedilol (10 µM), vehicle (tween 0.1%), and AEO (0.125, 0.25, or 0.50 µL/g heart) for 5 min. The myocardial dysfunction has been significantly more acute only in group 3 in comparison with the control group. In group 3, the creatine kinase and lactate dehydrogenase activities are reported as the markers of myocardial damage. It is reported that the isolated rat hearts have been perfused with AEO (0.5 µl/g heart), which may spoil the myocardial ischemic-reperfusion damage [285]
Antioxidant Activity
Researchers have reported that NO scavenging activity has been found in methanolic gum extract of asafoetida [235]. Researchers have revealed that the superoxide dismutase (SOD) enzyme activity are reduced in mouse brain tissue in the pentylenetetrazole (PTZ)-induced mouse [304]. The superoxide radical has been formed on the surface of the brain of mouse. The superoxide radical has not been scavenged with asafoetida by enhancing the superoxide dismutase enzyme. In the asafoetida treated groups, MDA (malondialdehyde) level is decreased in comparison with PTZ groups. Asafoetida gum extract is able to reduce the lipid peroxidation and oxidative destruction because of antioxidant activity [469]
Central Nervous System Activity
Some studies proposed that asafoetida acts as a neuroprotective, and nerve stimulative agent [371]. Asafoetida oleo gum resin is able to increase the re-myelination and regeneration, and to reduce the rate of lymphocyte infiltration in the neuropathic tissue in mice. It is proved that monoamine oxidase B (MAO-B) are retarded with asafoetida resin. Asafoetida resin is able to treat the neurodegenerative diseases like Alzheimer’s and Parkinson’s diseases. Furthermore, it is noted that asafoetida showed acetylcholinesterase (AChE) retarding activity in the snail nervous system. It also possessed memory-enhancing activity [459]
Apium graveolens (Celery)
Anti-inflammatory Activity
Researchers have proposed that isolated, and total fractions of celery seed may possess anti-inflammatory activity [524]. The aqueous extract of celery possessed flavonoids like apiin, apigenin, and phenolic acids are responsible for anti-inflammatory activity. It is evidenced that antioxidants like apigenin can reduce the generation of hydrogen peroxide and anti-immunoglobin E-induced histamine salvation. The flavonoids can retard the cyclooxygenase-2 (COX-2) activity. It is reported that coumarins, and phthalides are present in hexane extract of celery are responsible for the anti-inflammatory activity [742]
Antimicrobial Activity
Researchers have evaluated the antimicrobial effect of methylated spirit, methanol, ethanol, and hexane extracts of celery against different bacterial strains like Psteropseda aeruginosa, Staphylococcus aureus, E. coli, Salmonella typhi, and Bacillus subtilis and fungal strains like Candida glaberata, Trchophyton longifusus, Candida albicans, Fusarium solani, and Aspergillus flavus. It is reported that Salmonella typhi, E. coli, Staphylococcus aureus, Bacillus subtilis, and Psteropseda aeruginosa are inhibited effectively by the different celery extracts. While the B. subtilis, and S. typhi are inhibited with the methanolic fraction. The methanolic fraction showed lower inhibitory activity against E. coli. P. aeruginosa is less inhibited with methylated spiritA. flavus is strongly inhibited with the ethanolic fraction of celery [480]
Antioxidant Activity
Methanolic fractions of celery showed the highest antioxidant potential followed by methylated spirit, and ethanolic extract. It is reported that the highest ABTS radical scavenging activity has been possessed by ethanolic fraction followed by the methylated spirit and ethanolic extract [254, 480]
Anti-diabetic Activity
Researchers have analyzed the anti-diabetic activity of celery extract with a few biochemical and hematological parameters in alloxan-induced diabetic rats. 50 adults albino rats have been divided into five equal groups: group 1—control groups (administered with normal saline of 0.5 ml/kg dose), group II—administered with 1 ml of the extract (425 mg/kg BW) for 1 month, group III—2 doses of alloxan (150 mg/kg), group IV—1 ml extract (425 mg/kg BW) injected to treat the diabetic rats for 30 successive days, and group V—14.2 mg/kg of metformin-treated diabetic rats (for 30 successive days). It is reported that the blood glucose level is decreased and insulin secretion, RBC, WBC count, PVC, and the neutrophil percentage are enhanced in diabetic rats with ethanol extract of celery after 1 month of treatment. Therefore, researchers have reported that the celery extract is able to reduce the WBC count, the average value of LDL-C, serum cholesterol, triglyceride, ESR, urea, uric acid, and creatinine in diabetic groups. The anti-diabetic activity of celery extract is similar to that of metformin (a standard drug for treatment of diabetes) [576]
Anti-obesity Activity
Researchers have investigated the anti-obesity activities of celery. Celery seed ethyl acetate fraction (CSEA) (contains p-coumaric acid, and isoferulic acid) showed higher anti-adipogenesis in the 3T3-L1 cells in comparison with the ethanolic extract of celery seed. This may be due to the reduction in adipogenic hormones like adiponectin and leptin. The adipocyte-related transcription factors and gene expression levels including αp2, C/EBPα, and PPAR-gamma are reduced with CSEA [521]
Cardiovascular Activity
Scholars have evaluated the effect of alcoholic, and water extracts of celery on the blood pressure of anesthetized rabbits [12]. It is proposed that the hypotensive effect has been higher in ethanol extract in comparison with aqueous extract. At a dose of 0.3 mg/kg of Atropine can block the hypotensive effect of ethanol extract [344]
Gastrointestinal Activity
Scientists have proposed that the ethanol extract of celery is able to prevent the cytodestructive, and indomethacin agents (25% NaCl, 80% ethanol, and 0.2 M NaOH) induced gastric ulcer. Basal gastric secretion are reduced and gastric mucosa are protected with ethanol extract, which may be due to its antioxidant activity. Tannins, and flavonoids derived from celery are able to treat ulcer [42]
Central Nervous System Activity
Researchers have analyzed the neuroprotective activity of celery. The insilico study has been considered to evaluate the neuroprotective activity of celery. It is reported that Choline present in celery is able to bind the acetylcholinesterase (Ache), Slc5a7, and choline acetyltransferase (Chat). The insilico research showed the activity in the neurotransmitter secretion process, neurotransmitter biosynthesis process, and neurotransmitter metabolic process. It is reported that neuroprotective activity has been found in celery by the interaction of Ache, Slc5a7, and Chat [555]
Capsicum frutescens (Chili)
Antimicrobial Activity
Researchers have evaluated the effect of bell pepper on few foodborne bacteriaSalmonella typgimurium is inhibited with bell pepper extract [174]. It is observed that Listeria monocytogenes, Salmonella typhimurium, Staphylococcus aureus, and Bacillus cereus are inhibited with bell pepper extract. The isopropanol, and water extracts of chili showed anti-bacterial effects, which may be due to the presence of capsaicin in bell pepper. It is recommended that Pseudomonas aeruginosa, and Salmonella found in beef meat are inhibited by isopropanol and aqueous extract of pepperClostridium, Bacillus, and Salmonella sp are inhibited by an aqueous extract of pepper. The antibacterial activity has been found in isopropanol extract against Salmonella typhimurium, Listeria monocytogenes, Staphylococcus aureus, and Bacillus cereus [477]
Anti-inflammatory Activity
At a range of 0–100 µg/mL concentration, the yellow, red, and green chili extracts exhibited anti-inflammatory activity. The anti-inflammatory activity of chili may be due to its free radicals scavenging property [719]
Antioxidant Activity
Researchers have assessed the antioxidant potential of chili through different in vitro methods, namely ABTS, DPPH, and FRAP. The yellow and red chili have possessed the antioxidant potential of 5.18 µmol TE/g fw and 3.89 µmol TE/g fw, respectively, as assessed by ABTS method. The antioxidant activity of green chili (43.29 µmol TE/g fw) has been found greater than red chili (2.82 µmol TE/g fw) as assessed by DPPH method. Similar results have also been reported for green bell (46.36 µmol TE/g fw) and red chili (16.15 µmol TE/g fw) as assessed by FRAP method [719]
Anti-diabetic Activity
Scholars have evaluated the anti-glycation properties of crude methanolic extract of chili. The anti-glycation capability of the water, ethyl acetate, n-hexane, and crude methanolic fractions of chili is higher than that of chloroform fraction. Scholars have proposed that the chili extracts are able to treat complications associated with diabetes [449]
Anti-obesity Activity
Capsaicin derived from chili has been reported for its high-fat diet-induced chronic low-grade inflammation (CLGI) decreasing activity, which may be connected with anti-obesity activity. Capsaicin is able to reduce the amount of glycerol-3-phosphate dehydrogenase (GDPH) activity and intracellular triglyceride in 3T3-L1 adipocytes, to retard the leptin, PPAR-gamma, and C/EBPα expression. 0.075% capsaicin is able to reduce the lipid deposition in epididymal adipose tissue and mesenteric tissue in high-fat diet-induced obese mice. The serum level of glucose, triglycerides, and cholesterol levels are reduced with capsaicin [107]
Cardiovascular Activity
Researchers have evaluated the cardioprotective activity of capsaicin in anesthetized dogs, and rabbits at a dose of 10–300 µg/kg. The induced hypertension is not affected with phentolamine, hexamethonium, and tolazoline in dogs and rabbits. In dogs, the hypotension has been reduced with atropine. The heart rate has not been affected or slightly enhanced in atropine-treated dogs with capsaicin. The contractile force or rate of atria of rabbit, and dog is not affected with capsaicin. In the dog, the constrictive activity of capsaicin is decreased slightly in upper mesenteric arteries. The capsaicin is able to contract the cerebral arterial strips [50]
Gastrointestinal Activity
Researchers have investigated about the digestion provoking activity of chili may be due the presence of capsaicin. The activities of digestive enzymes in the small intestine and pancreas, stimulation of saliva, and secretion of bile have been connected to the digestive stimulatory activity of chili that also augmented the salivary amylase activity. Chili is able to increase the fat absorption, and digestion in high-fat-consumed rats, as reported by researchers. Capsaicin, chili extracts, and other phytochemicals are able to enhance the permeability of intestinal epithelial cells and the rate of gastric emptying. Chili can stimulate the acid secretion, and irritation character. The concentration-dependent cytopathic activity has been observed with chili extracts on oral mucosal fibroblasts. The defensive nature against gastric mucosa damages has been found with capsaicin, and chili extract. Chili is able to defend the gastric mucosa due to the activation of gastrointestinal transient receptor potential vanilloid subtype 1 (TRPV1), antioxidant enzymes, and retardation of inflammatory factors. Capsaicin is capable to treat ulcers by retarding acid secretion. The epigastric pain, fullness, dyspeptic symptoms, nausea, and heartburn are reduced by the administration of capsaicin, and red pepper in heartburn, and dyspeptic patients [784]
Central Nervous System Activity
Scholars have investigated about the neuroprotective activity of phenolic extract of chili, and as an inhibitor of monoamine oxidase and cholinesterase activities. Scholars have evaluated the OH radicals scavenging capabilities, DPPH, and membrane-stabilizing activity of the phenolic extract of chili. The activities may take place may be due to the retardation of Fe2+-induced lipid peroxidation in rat brain tissue. It is reported that the mitochondrial MAO, BChE, and AChE inhibitory activity are found in the phenolic extract of chili. Chili is able to control the neurodegenerative disease like Parkinson’s disease [661]
Pimenta dioica (Allspice)
Anti-inflammatory Activity
Researchers have evaluated the effect of allspice on anti-inflammatory activity. It is reported that the extract of Pimenta dioica (PD) is able to enhance the proinflammatory cytokines IL-6 and TNF-α by 150% and 166%, respectively. Eugenol derived from essential oil of PD is able to modulate the inflammatory response [540]
Antimicrobial Activity
Scholars have examined the essential oil of PD against pathogenic fungi, and bacteria. It is reported that the five bacterial strains, namely P. aeruginosa, E. coli, A. baumannii, and S. aureus, and the yeast such as C. albicans are inhibited with EO extract of PD. The antibacterial effect has been found in EO of allspice against P. aeruginosa and MRSA [260]
Anti-diabetic Activity
It has been reported by researchers that the protein glycation can be retarded with allspice. Therefore, allspice is utilized as a potential anti-diabetic agent [745]
Antioxidant Activity
The antioxidant capacity for the aqueous soluble fraction, dichloromethane extract, and ethyl acetate soluble fraction of allspice has been evaluated through the oil stability index process, and ferric thiocyanate method. The free radical scavenging potential of allspice has been evaluated through DPPH assay [471]. Allspice is able to retard the xanthine oxidase activity by 74.83% [745]
Central Nervous System Activity
The vaso relaxing, and hypotensive activity have been reported for water extract of allspice. The rodents have been considered to evaluate the hypotensive activity of water extract of allspice. It is reported that the aqueous extract of allspice is able to depress the CNS spontaneously in hypertensive rodents. The hypotensive action is not mediated by cholinergic or β-adrenergic ways [902]
Cardiovascular Activity
Researchers have considered the anesthetized normotensive rats to evaluate the hypotensive effect of water, ethanolic extracts of allspice, and various fractions of the aqueous extract. At various doses (30, 70, and 100 mg/kg) of aqueous extracts of allspice have been given to the anesthetized normotensive rats intravenously. It is reported that allspices extracts are able to reduce the mean arterial blood pressure. A dose of 100 mg/kg of the aqueous extract showed the highest hypotensive effect in comparison with the ethanolic extract. The highest hypotensive effect has been reported in the aqueous fraction of allspice [893]
Garcinia indica (Kokum)
Anti-inflammatory Activity
Researchers have evaluated the effect of water extract of Garcinia indica fruit rind (GIE) for anti-inflammatory activity in cotton pellet-induced granuloma, and carrageenan-induced paw edema in rats. The dose of 400 mg/kg, and 800 mg/kg of GIE have been administered to the Wistar rats orally. A dose of 10 mg/kg of diclofenac sodium has been considered as standard drug. Four sterile cotton pellets have been implanted in the ventral portion in the granuloma model in each rat. Pellet implanted rats are administered with diclofenac, and GIE orally for 8 days. The functions of the aspartate transaminase (AST), alkaline phosphatase (ALP), and alanine transaminase (ALT) have been examined from the serum. Rats, which are treated with GIE, decrease the cotton pellet granuloma, and paw edema in comparison with carrageenan treated and cotton pellet implanted rats, respectively. The ALP, AST, and ALT activities attenuated with the treatment of GIE. It has been reported that anti-inflammatory activity has been observed in GIE because of antioxidant potential, and lysosomal membrane stabilization through virtue of its phenolic compounds [677]
Antioxidant Activity
Some investigations showed the effect of ethanolic, and water extracts of kokam for its antioxidant activity in Wistar albino rats. The biochemical parameters such as lipid peroxidation (LPO), sulfoxide dismutase (SOD), glutathione (GSH), and catalase (CAT) have been considered to analyze the antioxidant activity. It has been reported that the methanolic extract of kokam possessed free radical retardation activity. The β-carotene linoleate and DPPH methods have been considered to evaluate the free radical scavenging activity of chloroform extract of kokam. A strong antioxidant property has been found in methanolic extract of kokam in comparison with the standard ascorbic acid. The superoxide anion scavenging function has been reported for garcinol derived from kokum by phenazine methosulphate/ NADH nitroblue tetrazolium method [395]
Gastrointestinal Activity
Researchers have suggested that the ethanolic, and water extracts of kokam possessed ulcer protective activity. It has been reported that the ethanolic and aqueous extracts of kokam possessed ulcer protective properties against HCl/ethanol-induced gastric damage and indomethacin-inducedulcerogenesis. At a dose of 500 mg/kg of ethanolic, aqueous extracts of kokam has been administered to rats orally. It has been reported the ethanolic, and aqueous extracts of kokum are able to decrease ulcer in the HCl/ethanol and indomethacin induced gastric damage in rats [678]
Anti-obesity Activity
The hyperlipidemic effect has been found in methanolic extract of kokam as observed by the researchers in cholesterol-induced hyperlipidemic rat model. It has been reported that the methanolic extract of kokum is able to reduce the LDL-C, total cholesterol, triglycerides, VLDL-C, and to enhance HDL-C. It has been reported that hydroxy citric derived from kokam is able to decrease the body weight, appetite, and retard lipogenesis [557]
Anti-diabetic Activity
Fasting blood glucose level is reduced with kokam extract in streptozotocin-induced hyperglycemic rats. At a dose of 400 mg/kg of water extract of kokum has been administered to rats. It has been reported that the water extract possessed anti-hyperglycemic effect and can enhance the oral glucose tolerance. Garcinol derived from Kokam possessed strong glycation retarding effect as it can reduce the protein glycation in a bovine serum albumin/fructose process [395]
Central Nervous System Activity
The neuroprotective activity has been found in methanolic extract of kokam against 6-hydroxydopamine (6-OHDA), which is an indicator for its anti-Parkinson’s activity in rats. Lipopolysaccharide (LPS) induced anti-inflammatory mediators expression is decreased with garcinol. The anticholinesterase activity has been observed in kokam. The expression of neurofilament proteins and neurite outgrowth are inhibited with cyanidin-3-glucoside [81]
Antimicrobial Activity
Researchers have evaluated the antibacterial effect of the crude methanolic extract of kokam. The doses of 500, and 1000 µg/mL of methanolic extract of kokum have been considered to analyze the antimicrobial activity. At a dose of 500 mg/mL of kokam extract exhibited the highest inhibition against S. aureus, and P. aeruginosa At a dose of 1000 mg/mL of kokam extract exhibited the highest inhibition against S. aureus, and P. aeruginosa. It is reported that the methanol extract of kokam cannot exert any inhibition against E. coli [930]
Cardiovascular Activity
Scholars have studied about the effectiveness of kokum extract on cardiovascular diseases. The values of atherogenic coefficient (AC), atherogenic index of plasma (AIP), and cardiac risk ratio (CRR) have been significantly greater in the group of rats fed with Western diet in comparison with normal groups. The AC, AIP, and CRR levels are normalized with garcinol-enriched fraction (GEF) [532]
Alpinia galangal (Greater galangal)
Anti-inflammatory Activity
Many researchers have evaluated the anti-inflammatory activity of galangal. The p-coumaryl alcohol-γ-O-methyl ether substantially, and selectively reduce the formation of interferon-gamma (IFN-γ) in CD4 + Th (T helper) cells. Hydroxychavicol acetate and acetoxychavicol derived from galangal are able to prevent the inflammatory immune diseases caused through indulgent activation. Galangal is able to reduce the granuloma weight in croton oil-induced granuloma pouch rat model and carrageenan-induced paw inflammation [205]
Anti-diabetic Activity
Researchers have reported that aqueous, and methanolic extracts of galangal have been administered to normal rabbits to evaluate the anti-diabetic activity of galangal. It has been reported that galangal is able to reduce the blood glucose level [39]
Antimicrobial Activity
Scholars have reported that the human immunodeficiency virus type-1 (HIV-1), and human cytomegalovirus (HCMV) are retarded potentially with methanolic extract of galangal. The antimicrobial activity has been observed in the essential oil of galangal. It is evidenced by scholars that ethyl acetate and ether extracts of galangal possessed antibacterial activityStaphylococcus aureus is inhibited by 1,8-cineole derived from galangal. It has been reported that the ethanol extract of galangal exhibited potential inhibitory activity as observed by the broth dilution method against Staphylococcus aureus. Pseudomonas aeruginosa, Klebsiella pneumonia, E. coli, S. aureus, and Streptococcus pyogenes are inhibited with water extract of galangalErysipelothrix rhusiopathiac, Staphylococcus aureus, Streptococcus suis, Pseudomonas aeruginosa, E. coli, Pasteurella multocida, and Arcanobacterium pyogenes are inhibited by essential oil extracted from galangal. The combined effect of α-bisabolene, 1,8-cineole, and 4-allyphenyl acetate derived from galangal are responsible for the antimicrobial activity [744]
Gastrointestinal Activity
The antiulcer activity has been found in galangal, which may be due to the action of cytoprotective, and antisecretory. At a dose of 500 mg/kg of ethanolic extract of galangal is able to decrease the gastric secretion in hypothermic restraint stressing, and pyrrolic ligation rats [53]
Antioxidant Activity
Many studies have proposed that the antioxidant potential has been found in galangal and its isolates. A potential antioxidant property with an IC50 value of 550 µg/ml has been observed in the essential oil of galangal. It has been reported that the aqueous, methanolic extracts and volatile oils exhibited free radical scavenging activity significantly as appeared through the DPPH method. At neutral pH, galangal showed higher antioxidant capacity in comparison with the acidic pH. The reducing power, superoxide anion scavenging activity, and Fe2+ chelating activity have been found in ethanolic extract of galangal in a concentration-related manner. Furthermore, lipoxygenase inhibitor activity has been found in galangal. It is reported that the methanol and dichloromethane (DCM) of galangal possessed antioxidant activity [551]
Anti-obesity Activity
Researchers have investigated the anti-obesity activity of ethanol extract of galangal in a cafeteria diet-fed obese rats. In albino rats, obesity has been induced with a cafeteria diet consumption regularly for 6 weeks. At a dose of 500 mg/kg of ethanol extract of galangal has been administered for 6 weeks regularly. It has been reported that the ethanol extract of galangal is able to reduce the parametrial adipose tissue weight, body weight, energy intake, deposition of hepatic triglycerides, liver weight, leptin, and serum lipid levels. The galangal extract is also able to retard the pancreatic lipase activity [850]
Cardiovascular Activity
Scholars have found the bioactive constituents such as 5-hydroxy-7-(4″-hydroxy-3″-methoxyphenyl)-1-phenyl-3-heptanone, and galangin present in galangal which are capable to exert antioxidant, and anti-inflammatory effect through retarding the COX-2 activity, salvation of histamine, serotonin, and kinin. The LDL level is reduced in the blood with the extracts of galangal [13]. It is reported that galangin can control the cell membrane integrity, can decrease oxidative stress, and is able to improve cardiac systolic/diastolic dysfunction in the cardiac myocytes stressed with doxorubicin induced rats [752]. The galangal juice has been administered to broiler chicken. It has been reported that the galangal juice is able to decrease the level of triglyceride in the broiler bloodstream. For improving cardiovascular problems, the galangal extract has been mixed with Phyllanthus embilica, which is able to reduce the level of circulating LDL-C [224, 227]
Central Nervous System Activity
Scientists have reported that the effectiveness of galangal to stimulate the cognitive activity and to enhance the antioxidant activity, and Na+/K+ATPase, simultaneously it can reduce acetylcholinesterase activity. Therefore, galangal is able to cure the neurodegenerative diseases such as Alzheimer’s, and Parkinson’s disorders [224, 227]
Acorus calamus (Sweet flag)
Central Nervous System Activity
Researchers have evaluated the effect of ethanol extract of Acorus calamus (AC) on monoamine levels of the brain and unconditioned electrical function. It is reported that sweet flag is able to enhance α activity, serotonin level, and the norepinephrine level within the cerebral cortex in rats. Furthermore, sweet flag is able to enhance the dopamine level in the midbrain, and caudate nucleus. Sweet flag showed anti-depressive activity through changing the brain monoamine levels in various brain areas and through altering electrical action. Researchers have conducted a clinical investigation in 50 cases of depression. It is reported that the degree of acute depression is decreased with AC [626]
Anti-inflammatory Activity
Scholars have investigated the anti-inflammatory effect of AC on severe, and chronic treated rat models. Croton oil granuloma pouch inflammatory response, carrageenan-induced paw edema, and cotton pellet granuloma production have been retarded with AC extract orally. The anti-inflammatory activity has been found with AC extract in severe, chronic, and immunologic rat models [976]
Antioxidant Activity
The ethyl acetate extract of AC has been reported to possess antioxidant activity as reported through DPPH method [14]. It is reported that 0.2 g/mL of AC extract exhibited the highest antioxidant effect (86.43%). α-asarone is an active compound of AC has been administered peritoneally before the rats are going to be expressed to noise-stress for 1 month. It has been reported that α-asarone possessed antioxidant activity against sounding-stress-induced alterations in the rat brain [573]
Cardiovascular Activity
Researchers have investigated the effectiveness of AC essential oil to reduce blood pressure. β-asarone and α-asarone derived from sweet flag possessed hypotensive effect as evaluated on the anesthetized dogs. The alcoholic extract of AC showed hypotensive activity on dog. 45 patients with ischemic heart disorders have been considered by researchers. They have been treated with AC extract. It has been reported that AC is able to decrease the body weight, serum cholesterol, SLDL (serum low-density lipoprotein), cure chest pain, improve ECG, and to enhance SHDL (serum high-density lipoproteins) [567]
Gastrointestinal Activity
Scholars have reported that ethanol extract of AC has defended gastroduodenal mucosa and retarded gastric secretion in rats against the damages caused by reserpine, pyloric ligation, indomethacin, and cysteamine administration. A dose of 500 mg/kg of AC extract showed anti-ulcerogenic and anti-secretory effects in rats subjected to reserpine, indomethacin, pyloric ligation, and cysteamine administration. The AC extract possessed protective activity against cytodestructive agents. It is reported that AC is able to treat gastropathy [131]
Antimicrobial Activity
Scientists have reported that Bacillus subtilis, Staphylococcus aureus, E. coli, Bacillus megaterium, Salmonella paratyphi A and B, Salmonella marcescens, Proteus vulgaris, Staphylococcus citreus, and Shigella dysomei have been inhibited with alcohol extract of ACStaphylococcus aureus has been inhibited with the acetate buffer, alcohol, ether, and dilute sulfuric acid extract of ACStreptococcus viridans, Streptococcus pyogenes, Diplococcus pneumoniae, Corynebacterium diphtheriae, E. coli, Salmonella typhi, Salmonella paratyphi A and B, Staphylococcus aureus, and Shigella flexneri have been inhibited with the extract of AC. Antifungal activity has been found in essential oil against A. jumigatis, A. nidulans, Penicillium aculeatum, Phomopsis destuctum, citrus decay pathogens Penicillium digitatum, P. italicum, Diplodia natalensis, Altemaria tenuis, Candida albicans, Epidermophyton cresens, Aspergillus oryzae, and Microsporum gypseumPenicillium selenium, Aspergillus niger, and Saccharomyces (yeast) have been inhibited with alcoholic extract. It is reported that antiviral activity has been observed in alcoholic extract of AC against Herpes simplex virus HSV-1, and HSV-2 [957]
Anti-diabetic Activity
The methanolic extract of sweet flag showed α-glucosidase inhibitory activity. The α-glucosidase inhibitory activity has been measured with IC50 value at 54.90 µg/ml. Standard acarbose has been showed the IC50 value of 39.12 µg/ml for α-glucosidase inhibitory activity. The flavonoids and polyphenols present in the sweet flag are responsible for the anti-diabetic effect [185]
Anti-obesity Activity
Researchers have evaluated the effectiveness of β-asarone in high-fat diet (HFD)-induced obese mice. The β-asarone is able to retard the metabolic transformations and glucose intolerance, to reduce the body weight, cholesterol level in adipose tissue in mice. The lipid-lowering effect has been observed with water extract of sweet flag [844, 845]
Food Industry Application of Selected Spices
Spices and herbs have been used in different food products either in the form of powder, extract, encapsulated material, or in raw form (Table 4). Figure 4 describes the application of spices and herbs in different food systems along with the methods of application
Table 4.
Food application of different spices
| Spices | Food system | Food product | Method of application | Tests conducted | Key results | Reference |
|---|---|---|---|---|---|---|
| cardamom | Meat product | Chicken drumsticks | Chitosan coating loaded with 1–2% essential oil | Proximate analysis, pH, peroxide value, TVN, TBARS, and sensory analysis | Shelf life increased by 2–3 days | [464] |
| Lamb meat | Chitosan coating with 1%, and 2% of ethanol extract of cardamom | Flavonol, total flavonoid, antioxidant, MIC, MBC, pH, and sensory analysis | Increased the quality, flavor, taste, and shelf life | [837] | ||
| Dairy product | Labneh | Powder | Microbiological, physiochemical, texture profile, and organoleptic properties evaluation | Increased the physicochemical, sensory properties, microbiological, and shelf life up to 30 days | [921] | |
| Jeera | Meat product | Turkey meat | Chitosan-loaded nanoemulsion enriched with essential oil of Bunium persicum, and zataria multiflora | Microbial indicators and sensory analysis | Protected microbial quality and enhanced shelf life up to 15–18 days | [441] |
| Broiler chicks | Dried jeera | Nutrient digestibility and metabolites | Enhanced the blood profile of cholesterol, and nutrient digestibility | [852] | ||
| Ground beef | Poly-lactic acid film matrix with Bunium persicum essential oil (EO), Mentha piperita EO, and nanocellulose | Antibacterial, organoleptic, and sensory evaluation | Shelf life enhanced up to 4–7 days | [911] | ||
| Broiler chicken | Jeera powder | Serum biochemical, nutrient digestibility, antibody-mediated immunity, and cell-mediated immunity estimated | Positive effects have not been found on these parameters | [823] | ||
| Hamburger | Chitosan/cellulose nanofiber film coating with nanoemulsions of Trachyspermum ammi essential oil (EO), and Bunium persicum EO | Microbial evaluation | Increased microbial protection of perishable foods | [875] | ||
| Chicken fillet | Chitosan film with 0, 1, and 2% of Bunium persicum EO | Microbial and chemical analysis | Enhanced efficient factors connected with microbial, and chemical spoilage | [425] | ||
| Dairy product | Gouda cheese | Edible coating with dried Bunium persicum EO and lactoperoxidase system | Bacteriological, chemical (pH, lipid extraction, thiobarbituric acid, free fatty acid, and peroxide value), and sensory evaluation | Reduced lipid oxidation, retarded gram-positive and gram-negative microorganisms, and enhanced shelf life | [806] | |
| Iranian white cheese | Bunium persicum EO | Phytochemical, antibacterial, antioxidant, and sensory analysis | Enhanced flavor, color, odor, and texture, | [266] | ||
| Miscellaneous product | Mushroom | 80% of ethanol extracts of aerial portions of Marrubium vulgare, Physalis alkekengi, Alcea rosea, and the seed of Bunium persicum | Enzyme kinetic parameters | Retarded mushroom tyrosinase | [638] | |
| Fruit and vegetable products | Corn starch | Dried jeera powder | Antibacterial activity analysis | Retarded food pathogens, enhanced protection of food products, and extended shelf life | [75] | |
| Fennel | Meat product | Lambs meat | Powder | Quality, digestibility, and ruminal features evaluated | Enhanced ruminal nitrogen concentration, ammonia, acetate, improved fat oxidation, quality, and composition | [351] |
| Poultry (broiler, laying hens, and Japanese quail) | Powder, EO, and extract | Body weight, carcass traits, egg quality, formation, immunity, the relative weight of lymphoid organs, antioxidant potential, and blood biochemistry analysis | Enhanced body weight, carcass traits, egg quality, formation, immunity, the relative weight of lymphoid organs, reduced antioxidant capacity, and improved blood biochemistry | [447, 448, 455] | ||
| Chicken thighs | Dried EO | Microbiological evaluation | Extended shelf life | [417] | ||
| Sausage | Powder of fennel, nitrite, and lettuce | Microbial property, physicochemical, and sensory properties assessed | Reduced bacterial growth, and aroma | [726] | ||
| Dairy product | Cottage cheese | Powder and phenolic-rich extract | Antioxidant activity, antimicrobial properties, color, and nutritional composition estimated | Enhanced antioxidant activity, and shelf life up to 14 days | [169] | |
| Yogurt | 2.5, 5, and 7.5 µl of EO | Sensory, microbiological, and physicochemical properties analysis | Prolonged shelf life up to 29 days | [139] | ||
| Processed cheese | Aqueous extract of fennel, and ajwain seed | Sensory, physicochemical (pH, free fatty acid, tyrosine value, UREA PAGA), and microbiological assessed | Enhanced microbial stability, shelf life, flavor, and acceptance | [577] | ||
| Butter | Plantago major seed gum-based nanocomposite active films with fennel EO | Moisture absorption, water vapor permeability estimated | Improved shelf life | [578] | ||
| Probiotic yogurt | Whole milk powder with water extract of dried fennel seed (2, 4, and 6%) | Physicochemical, antioxidant potential, total phenolic content, microbiological, and sensory analysis | Improved texture, flavor, enhanced protection, and bioactive components | [99] | ||
| Steamed yoghurt | Fennel, and parsley EO | Proximate biochemical, microbiological, and sensory estimated | Enhanced nutritional qualities up to 29 days, durability biochemical and physicochemical characteristics, texture, acidity, taste, reduced total fat content, and prolonged shelf life | [356] | ||
| Fruit and vegetable products | Pistachio | Potato starch-based films with 1, 3, and 5% of nano-ZnO, and 1, 2, and 3% of fennel EO | Microbiological activities, physicochemical, and sensory properties analysis | Enhanced fat, moisture, carbohydrate, and sensory properties (appearance, texture, flavor, acceptance), inhibited microbial formation, and propagation | [108] | |
| Miscellaneous product | Herbal candy | Powder | Proximate, physicochemical, and sensory analysis | Improved nutritional value, and acceptance of sensory and Physicochemical properties | [418] | |
| Poppy | Meat product | Buffalo meat cookies | Powder | Physicochemical, textural properties, color values, and sensory profile assessed | Decreased fat content, and improved sensory scores | [334] |
| Chevon nuggets | Powder | Physicochemical evaluation | Decreased fat content, microbial activity enhanced protein, emulsion stability, sensory score, and storage quality | [421] | ||
| Chevon patties | 5% of Poppy seed | Physicochemical and sensory analysis | Reduced cholesterol, fat content, increased moisture, Fe, K, Ca, Mn, Zn, and color | [645] | ||
| Beef | Seed EO | Proximate, lipid oxidation, texture profile, color, cholesterol, fatty acid, and sensory properties assessed | Reduced saturated fatty acid, cholesterol, cardiac arrhythmias, blood pressure, enhanced polyunsaturated fatty acid, and sensory acceptance | [330] | ||
| Cereal-based product | Gluten-free bread | Seed flour | Spectroscopic and physicochemical properties evaluated | Useful in diabetes | [987] | |
| White chocolates | Poppy seed | Sensory properties estimated | Improved texture, and flavor | [1010] | ||
| Clove | Meat product | Ground beef | 0.1% of clove powder, and 0.02% of BHT | pH, lipid oxidation, volatile compound, and color analysis | Decreased lipid oxidation, retarded volatile compound production, and enhanced product oxidative stability | [1003] |
| Broiler chickens | 1, 2, 3, 4, 5, and 6% of clove seed | Carcass and quality measured | Improved quality, sensory, carcass characteristic, and provided protective effect to antibiotics | [899] | ||
| Indigenous chickens | Clove and turmeric powder | Physical and chemical, WAP, WHC, lipoprotein, cholesterol, triglyceride content, sensory, and color value analyzed | Reduced moisture content, HDL, enhanced acceptance, and tenderness | [801, 802] | ||
| Hamburger | The bioactive coating based on chitosan with dried clove EO | Antimicrobial activity evaluated | Inhibited microbiological activities | [68] | ||
| Beef patties | Extract | Sensory characteristics and oxidative stability evaluated | Reduced hue angle, TBARS value, carbonyl content, lipid peroxidation, enhanced redness value, the durability of sensory characteristics, prolonged shelf life up to 10 days, and also quality | [1002] | ||
| Frozen beef patties | Clove and marjoram EOs | pH, color, thiobarbituric acid-reactive substances, microbiological, and sensory properties assessed | Enhanced oxidative stability, and quality | [6] | ||
| Chicken meat | LLDPE surface has been modified through chromic acid treatment and coated with clove EO | Antimicrobial activity analyzed | Promoted the yellow color, inhibited microbial activity, and improved packaging stability for 21 days | [628] | ||
| Buffalo patty | 0.1, and 0.2% of grape seed extract and 0.1% of clove EO | Lipid oxidation and microbial properties evaluated | Inhibited the microbial propagation, and reduced the lipid oxidation | [908] | ||
| Goat meat balls | Hydroethanol, ethanol (1:1), and aqueous extract | Antimicrobial and antioxidant capacity analyzed | Inhibited microbial proliferation, and enhanced antioxidant activity, improved free fatty acid, pH, TBARS, total mesophilic count, sensory characteristics, extended shelf life | [864] | ||
| Almond and walnut fortified chevon nuggets | EO | Sensory, oxidative stability and microbiological properties evaluated | Enhanced pH value, acceptance up to 14 days reduced TBARS, and microbial activity | [143] | ||
| Chevon cutlets | 2, 4, and 6% of sorghum flour with 100 ppm of clove EO | PH, proximate, thiobarbituric acid-reactive substances value, emulsion stability, microbiological profile, sensory properties, texture profile, and color value assessed | Reduced fat content, enhanced fiber, storage quality, acceptance, and nutritive value | [863] | ||
| Fresh beef patties | Extract powder | pH, TBARS value, carbonyl content, and color estimated | Reduced lipid oxidation, hue angle, protein oxidation, enhanced chroma value, redness, and improved quality | [90] | ||
| Chinese-style sausages | Powder clove added with 400 mL of 95% edible ethanol | Protein carbonyl, color, texture profile, and sensory properties analyzed | Retarded protein and lipid oxidation, enhanced color, texture, flavor, acceptance, and quality | [1013] | ||
| Raw chicken sausage | 500 ppm of Clove powder extract and 700 ppm of green tea extract | Chemical, physical parameters, sensory quality, and bacteriological status estimated | Reduced microbiological, chemical parameters, physical parameters, sensory attributes, increased antioxidant activity, inhibited lipid oxidation, and prolonged shelf life up to 3–6 days | [424] | ||
| Beef sucuks | Deboned chicken meat protein coating containing thyme or clove EO | Microbial, physical, and chemical parameters evaluated | Enhanced thiobarbituric acid reactive substances, weight loss, pH, reduced water activity, and prolonged shelf life | [808] | ||
| Dairy product | Soft cheese | Thyme, ginger, and clove EO | Sensory attributes and microbial protection measured | Provided protection, and enhanced sensory scores | [30] | |
| Mayonnaise | Eugenol-lean fraction of clove extract | Sensory, pH, color, non-thermal and thermal creaming value, rheological, and phytochemical properties analyzed | Reduced thermal and non-thermal creaming, and enhanced color intensity | [190] | ||
| Probiotic yoghurt | 0.1, 0.3, and 1% of clove, and 0.03% of propolis | Sensory, microbiological, and chemical parameters evaluated | Altered sensory, microbiological, and chemical properties | [343] | ||
| Fruit and vegetable | Strawberries | Mustard, and clove EO | Antifungal activity of EO vapors estimated | Retarded fungal growth | [24] | |
| Banana varieties | Cassava starch films with clove EO | Solubility, moisture, thickness, WVP, biodegradability, color, and antifungal activity assessed | Enhanced film thickness, decreased moisture content, solubility, TTA, mass loss, Improved antifungal activity, quality, and prolonged shelf life | [67] | ||
| Cassia bark | Meat product | Meat | Thermoplastic cassava starch with cinnamon, and sappan powder extract | Microbial count and appearance identified | Reduced total microbial count, extended shelf life, and formed active packaging | [467] |
| Chevon rolls | 0.25% of ethanolic cinnamon bark extract, and 0.40% of ethanolic aloe vera extract | pH, oxidative stability, microbial quality, and sensory properties evaluated | Improved acceptance, color, odor, and prolonged shelf life | [750] | ||
| Fresh beef | 0, 0.5, 1, 1.5, and 2% of EO | Shelf life estimated | Prolonged shelf life up to 15 days | [953] | ||
| Beef slices | Sodium alginate coating with cinnamon essential oil nanocapsules, and nisin | Physicochemical parameters (TVB-N, pH value, weight loss), antimicrobial activity, sensory characteristics, and color parameters estimated | Inhibited microbial growth, decreased lipid oxidation, prolonged shelf life up to 15 days, enhanced texture, color, odor, juicy characteristic, quality, antioxidant, and sensory properties | [1014] | ||
| Chicken meat | Edible coating of Rosemary, and cinnamon EO with alginate coating | Antioxidant potential, chemical quality, and sensory properties analyzed | Improved antioxidant activity, sensory characteristic, quality, decreased lipid oxidation, and extended shelf life | [724] | ||
| Chicken fillets | Edible coating of carrageenan and cinnamon EO | TBA, drip loss, pH, TV, ERV, WBSFV, color, microbiological, and sensory attributes evaluated | Increased sensory score, and prolonged shelf life | [456] | ||
| Dairy product | Butter | Extract | Sensory, proximate, chemical, phytochemical, and microbiological activity estimated | Improved physiochemical properties, inhibited microbial propagation, and spoilage | [966] | |
| Eastern European curd cheese | Liquid whey protein concentrate-based edible coating with 0.3% of Chinese cinnamon bark CO2 extract | Microbiological, physicochemical (protein, pH, lactic acid, fat, moisture, color, rheological, and sensory attributes) assessed | Inhibited microbial growth and prolonged shelf life | [597] | ||
| African yogurt | Cinnamon bark and black pepper seed powder extract | Antimicrobial potential and pH value evaluated | Inhibited bacterial propagation | [662] | ||
| Yogurt | 0, 0.1, 0.2, 0.3, and 0.4 mL of cinnamon oleoresin | Sensory, quality and shelf life analyzed | Enhanced microbial count and obtained shelf life up to 10 days | [442] | ||
| Cereal-based product | Bread | 0, 1, 2, 3, and 4% of powder | Antioxidant potential, total phenol content, sensory quality, texture, and microbial properties estimated | Enhanced loaf volume by 2%, acceptability, phenol content, antioxidant potential, inhibited mold growth, and extended shelf life up to 6 days | [251] | |
| Cake | 3% of cinnamon | Proximate, and sensory attributes analyzed | Enhanced protein, carbohydrate, crude fiber, Ca, Fe, Zn, reduced total energy, and fat content | [259] | ||
| Sponge cake | 0, 1, 2, 3, and 4% of powder | Quality and sensory attributes measured | Reduced volume, pH, moisture content, color, lightness, yellowness, enhanced stiffness, and acceptability with 1% powder | [522] | ||
| Spanish bread | Mustard, and cinnamon EO | Antifungal activity and shelf life evaluated | Control fungal growth, enhanced acceptability | [208] | ||
| Cookies | 4% of Powder | Antioxidant potential and quality analyzed | Reduced loss ratio, moisture content, spread rate, yellowness, lightness, enhanced stiffness, redness, antioxidant capacity, and acceptability | [879] | ||
| Fruit and vegetable products | Processed apple | Nutmeg and cassia bark EOs in alginate-based edible coatings | Shelf life and sensory attributes estimated | Inhibited microbial growth, reduced flavor, consistency, aroma, and extended shelf life up to 15 days | [800] | |
| “Thomson navel” orange fruit | Shellac edible coating with cinnamon EO | Ascorbic acid, fruit spoilage, weight loss, consistency, and sensory attributes assessed | Decreased weight loss, consistency loss, and improved quality | [463] | ||
| Mango | Bees wax-based edible coatings with bark EO and hexanal | Quality, physicochemical, sugar, organic acid, internal gas, volatile compound, and sensory attributes evaluated | Improved quality, physicochemical, and flavor | [342] | ||
| Fresh cut fruits (Grapes, pears, raspberries, strawberries, gala apples, Valencia, Hamlin orange, fuji apples, flor de Invierno pears, granny smith apples, golden delicious apples) | Vanilla, Cinnamomum zeylanicum, and Cinnamommum cassia | Shelf life analyzed | Inhibited pathogenic propagation, extended shelf life | [619] | ||
| Miscellaneous product | Mushrooms | cinnamon EO | Browning, weight loss, antioxidant activity, and volatile oils evaluated | Neglected browning, and weight loss | [264] | |
| Chocolates | Cinnamon, shellac, and xanthan extract | Antioxidant potential and physicochemical parameters analyzed | Prolonged bioactive profile, enhanced antioxidant capacity, phenolic content, acceptability, and flavor | [622] | ||
| Black pepper | Meat product | Buffalo meat steaks | Carrageenan edible film with 0.5% of oleoresins of black pepper | Physicochemical characteristics, microbiological properties, proximate, and sensory attributes estimated | Increased quality and extended shelf life | [575] |
| Chicken meat | Hot red pepper, garlic, and black pepper powder | Proximate composition, cholesterol level, and TBARS assessed | Enhanced composition, protein, antioxidant capacity, quality reduced lipid oxidation, and cholesterol level | [718] | ||
| Dairy product | Paneer | 0.50% of cardamom, and 0.25% of black pepper powder | Sensory, proximate, and physicochemical parameters evaluated | Enhanced phenolic compound, and extended shelf life | [112] | |
| Burfi | 0.5,1, and 1.5% of turmeric, and 0.6, 0.8, and 1% of black pepper powder | Sensory, and textural profiles analyzed | Improved texture, flavor, color, appearance, acceptance, and nutritional value | [501] | ||
| Fruit and vegetable | Pineapple | Chitosan and alginate-based edible coatings with black pepper, and Schinus terebinthifolia EO | Microbiological activity and shelf life estimated | Retarded microorganism, and extended shelf life up to 45 days | [229] | |
| Cereal-based product | Snack like pastry | Tigernut, black pepper, and plantain flour | Proximate composition, mineral, vitamin, vitamin C, and sensory characteristics evaluated | Increased protein, fat, dietary fiber, mineral, vitamin, flavor, and taste | [17] | |
| Coriander | Meat product | Chicken fillet | The bioactive edible coating is based on sodium alginate with coriander EO in 650 mL of aqueous extract | MBC, MIC, antimicrobial activity, TBARS, TVBN, peroxide value, and sensory attributes evaluated | Retarded pathogen growth, enhanced chemical quality, antioxidant capacity, and extended shelf life | [430] |
| Quails meat | Coriander, and black cumin seed | Feed conversion, BW (Body Weight) gain, carcass attributes, organ production, and fatty acid composition analyzed | Reduced BW, enhanced fatty acid composition | [428] | ||
| Lambs meat | 5% of powder | Digestibility, blood metabolites, immune system, rumen, and chemical composition estimated | Exhibited important effect on blood cells, and blood metabolite concentration | [446] | ||
| Porcine meat | Meat and bone meal protein films with coriander EO | Moisture content, mechanical parameters, water vapor permeability, and optical properties assessed | Enhanced antimicrobial food packaging capacity | [520] | ||
| Broiler chicken | Cichorium intybus, and coriander water extract | Antioxidant potential, phytochemicals, minerals, carcass traits, blood parameters, and growth examined | Enhanced total phenolic acid, flavonoid content, Na, Fe, K, lipid profile, carcass attributes, liver activity, and antioxidant capacity | [316] | ||
| Dairy product | Ghee | Coriander steam distilled to extract | Antioxidant activity evaluated | Enhanced antioxidant capacity | [688] | |
| Cereal-based product | Wheat and wheat product | Coriander, thyme, and oregano essential oils | Antifungal activity, phytotoxicity effect, antimicotoxigenic capacity, and sensory properties evaluated | Inhibited fungal growth | [160] | |
| Fruit and vegetable products | Stick carrots | Seed EO, extract, and hydrosol | MBC, MIC, inactivation, growth kinetics, chemical properties, and sensory attributes analyzed | Inhibited microorganisms, enhanced sensory characteristics, and color | [697] | |
| Nutmeg | Meat product | Beef | The edible coating is based on sage seed mucilage with nutmeg EO | Shelf life and quality estimated | Reduced microbial count, lipid oxidation, enhanced stiffness, acceptability, color stability, and sensory attributes | [468] |
| Bovine loins | Active films formulated with polyvinyl alcohol, various oleoresin concentration, and gelatin of nutmeg | Physicochemical characterization assessed | Reduced pH, humidity percentage, TVB-N, and prolonged the shelf life | [305] | ||
| Dairy product | Fresh Baladi cheese | Nutmeg and cinnamon EO | MIC and inhibition zone diameter evaluated | Provided less safety to the fresh Baladi cheese against Brucella | [46] | |
| Black mustard | Meat product | Normal ground beef, and lean ground beef | Cellulose acetate film with ground mustard seed | Moisture absorption, sinigrin content, allyl isothiocyanate, AITC, microbiological, and shelf life analyzed | Decreased moisture absorption, and extended shelf life up to 3.68 days | [118] |
| Turmeric | Meat product | Chicken breast, fresh pork loin, and beef loin | The edible alginate-based film with turmeric | Structural, physical properties, thickness, WS, MC, antioxidant activity, color, transparency, WVP, OP, mechanical properties, infrared spectroscopy, pH, and lipid oxidation evaluated | Enhanced antioxidant activity, decreased lipid oxidation, improved quality, and shelf life | [155] |
| Chicken meatballs | 0.5 and 1% of powder | Quality analyzed | Prolonged lipid oxidation | [472] | ||
| Beef burger, and minced beef | Turmeric and marjoram ethanolic extract | The bacterial pathogen, AST, molecular, and antimicrobial activity estimated | could not provide any microbial effect | [783] | ||
| Broiler chicken | Powder | Antibacterial property, body weight gain, FCR, and quality assessed | Increased palatability, flavor, crude protein, digestion absorption, decreased saturated fatty acid, and triglycerides | [508] | ||
| Sausages | Edible hydrogel coatings with turmeric residue and gelatin hydrogels or cassava starch and gelatin hydrogel | Microbial culture and antimicrobial activity measured | Retarded microbial growth | [941] | ||
| Chicken breast fillets | Carboxymethyl cellulose solution with turmeric, and black pepper seed extract | Quality, microbiological property, total lipid extraction, FFA, PV, TBA, TVB-N, and sensory attributes evaluated | Reduced lipid oxidation, proteolysis, controlled bacterial count, and extended shelf life up to 16 days | [218] | ||
| Chicken, and fish meat | Film-based curcumin with rice starch | MC, WAC, WS, biodegradability, color, pH, temperature, and storage stability analyzed | Altered color from yellow to reddish-brown, decreased water solubility, and water absorptivity | [283] | ||
| Dairy product | yogurt | Gel-like stable suspension using cellulose nanofibers with turmeric | Titratable acidity, pH, syneresis, color, rheology, and sensory attributes evaluated | Enhanced syneresis, provided yellow color, and improved sensory characteristic | [341] | |
| Whole, skim, and low-fat milk | Encapsulated curcumin | Physical stability, bioaccesability, and antioxidant activity analyzed | Enhanced chemical stability, bioaccesibility, and bioactivity | [309] | ||
| Buffalo milk ghee | 0.5, 1, and 1.5% of powder | Sensory attributes estimated | Enhanced acceptability | [72] | ||
| Stirred yoghurt | Ethanol, and aqueous extracts | Proximate composition, total carbohydrate content, moisture content, fat, crude protein, digestion, distillation, titration, micronutrients, physicochemical, microbial, and sensory attributes assessed | Enhanced nutrient composition, pH, vitamins, minerals, quality, acceptance altered color from white to yellowish-orange, reduced carbohydrate, protein content, and inhibited microbial activity | [580] | ||
| Dairy yogurt | Turmeric, and cinnamon oleoresins | Sensory attributes, proximate composition, shelf life, microbial, physicochemical parameters, and glycemic impact examined | Enhanced acceptability, quality, decreased blood glucose level, postprandial blood glucose level, and chances of type 2 diabetes | [696] | ||
| Pasteurized milk | Turmeric, ginger oleoresins, and pomegranate peel ethanolic extract | GCMS, sensory, proximate composition, color, antioxidant, TPC, microbial count, and antimicrobial potential measured | Inhibited gram-positive, gram-negative bacteria, fungi, enhanced TPC, and antioxidant capacity | [407] | ||
| Buffalo milk paneer | Raw turmeric powder aqueous extract | Sensory properties evaluated | Increased nutritional, and quality | [884] | ||
| Herbal milk | 0.1% of turmeric powder, 25% of tulsi juice, and 3% of ginger juice | Physicochemical, sensory, and microbiological properties analyzed | Enhanced nutritional value, acceptance, sensory attributes, TPC, antioxidant activity, and flavor | [314] | ||
| Custard | Paw-paw and turmeric powder | Proximate composition, functional parameter, microbiological, and sensory attributes estimated | Reduced sensory acceptance | [660] | ||
| Functional kulfi | Curcumin encapsulated in calcium alginate | Morphology, color, encapsulation efficiency, FTIR, SEM, release behavior, physical characteristic, microbiological properties, and sensory attributes assessed | Enhanced sensory characteristics (flavor, color, appearance, taste), acceptance, and lower microbial load | [841, 843] | ||
| Herbal lassi | Turmeric extract | Sensory, and TPC measured | Enhanced sensory attributes, TPC, and extended shelf life up to 9 days | [558] | ||
| Paneer | Fresh raw turmeric powder aqueous extract, and buffalo milk | Hardness, cohesiveness, elasticity, gumminess, and chewiness evaluated | Improved hardness, cohesiveness, elasticity, gumminess, and chewiness | [461] | ||
| Fresh shanklish cheese | Extract and kefir | Chemical properties analyzed | No effect on the physical–chemical composition | [856] | ||
| Manchego-type cheese | Nanoemulsified curcumin | Sensory and physicochemical properties estimated | Enhanced antioxidant potential, TPC, improved appearance, color, and odor | [807] | ||
| Cokelek cheese | The edible film with 0.5% of alginate, 1.5% of sorbitol, 5% of egg white protein powder, and 2% of turmeric EO | Volatile compounds, EOs, physical–chemical properties, and microbiological properties assessed | Enhanced EO, inhibited water vapor transmission, microbial growth, and reduced weight loss | [437] | ||
| Pineapple ice cream | Curcumin-loaded nanoemulsions | Sensory and technological parameters measured | Decreased application of artificial dyes | [158] | ||
| Dairy product | Plantain syrup and turmeric powder | Oxidation, color, water activity, titratable acidity, pH, syneresis, WRC, total soluble solids, carotenoids, antioxidant potential, TPC, mineral contents, and microbiological quality-examined | Reduced yellow color, enhanced oxidation value, syneresis ratio, WRC, TPC, antioxidant capacity, Ca content, and extended shelf life | [164] | ||
| Cereal-based product | Bread | 4% of Powder | Proximate composition, physical attributes, curcumin content, TPC, and sensory properties evaluated | Enhanced antioxidant capacity, curcumin content, and TPC | [531] | |
| Yellow layer cake | Powder | Quality, physicochemical, and sensory attributes analyzed | Enhanced crumb color, the viscosity of cake batter, cake volume, crude fiber, curcuminoid content, TPC, reduced density, water activity, stiffness, improved antioxidant potential, and physicochemical properties | [530] | ||
| Functional biscuits | Turmeric powder, wheat flour, and soya bean flour | Proximate composition, TPC, reducing power, color, and sensory attributes estimated | Enhanced nutritional quality, protein content, and antioxidant activity | [16] | ||
| Moin-Moin | 2.5 g of Powder | Proximate, mineral content, physical, microbiological, and sensory attributes assessed | Lowered microbial count | [34] | ||
| Gluten-free cracknel biscuits | Turmeric rhizome, and chia seed powder | Sensory attributes measured | Inhibited microbial growth | [507] | ||
| Wheat flour dough, and cake | 0, 2, 4, 6, and 8% of powder | Rheological, physical properties, curcumin content, and sensory attributes examined | Enhanced antioxidant capacity, and curcumin content | [683] | ||
| Breakfast cereal | Encapsulated turmeric extract (5%) | Physical attributes, TPC, curcuminoid content, antioxidant potential, and consumer acceptability evaluated | Enhanced antioxidant capacity, curcuminoid content, and TPC | [516] | ||
| Biscuits | Turmeric, carrot water extracts, and grape leaves ethanol extract | Antioxidant activity, chemical, and physical properties estimated | Improved antioxidant capacity | [362] | ||
| Cake | Enriched cornstarch with curcumin-loaded lyophilized liposomes and the enriched cornstarch | Water absorption, water activity, moisture content, bulk properties, flowability, and wetting time assessed | Reduced acute, homogeneous yellow color, stiffness, and chewiness | [296] | ||
| Snacks | Turmeric powder, and broken rice grains | Sensory, physicochemical, microbiological properties, chemical composition, phenolic compound, and antioxidant activity measured | Improved sensory and nutritional properties | [666] | ||
| Corn snacks | Turmeric, ginger, and bay leaves powder | Organoleptic, proximate, minerals, vitamins, functional properties, TPC, TFC, polyphenolic compounds, physical properties, color, texture, and microbial properties examined | Enhanced vitamins, mineral content, phytochemical content, improved rheological properties, and extended shelf life | [74] | ||
| Fruit and vegetable products | Pumpkin | EO | Microbial activity and quality parameters evaluated | Retarded microbial growth, improved quality, extended shelf life up to 15 days | [171] | |
| Bay leaves | Meat product | Meatballs | Whey protein edible films with bay leaves, and sage | Antioxidant potential, TPC, PV, CD, thiobarbituric acid value, color, and sensory attributes evaluated | Reduced CD, peroxide value, enhanced sensory characteristic, acceptance, and extended shelf life | [37] |
| Minced beef | Encapsulation of bay leaves extract (hydroalcoholic, water, and alcoholic) with nano-liposome | Antioxidant, phenolic, flavonoid content, antimicrobial activity, shelf life, and chemical properties analyzed | Enhanced antioxidant activity, inhibited microbial load, oxidation, and extended shelf life | [940] | ||
| Minced maronesa beef | Rosemary, and bay leaves EO | Volatile composition, microbiological activity, color, and pH estimated | Enhanced pH, decreased microbial load, improved color, and extended shelf life | [970] | ||
| Dry fermented game sausages | Bay leaves EO | Technological traits, sensory acceptability, TBARS, pH, and water activity assessed | Reduced pH, TBARS, water activity value, and enhanced acceptance | [482] | ||
| Dairy product | Domiati cheese | Mint, bay leaves, and dill EO extract | Sensory, and fungal evaluated | Reduced total count of fungi, and contamination | [389] | |
| Cereal-based product | Meat bread | Bay and oregano | Shelf life, storage stability, oxidation parameters, microbiological, color, and sensorial profile evaluated | Extended shelf life | [945] | |
| Cookies | 6% of powder | Palatability, postprandial glycemia, appetite, and gastrointestinal problem analyzed | Decreased blood glucose level and enhanced applicability | [450] | ||
| Rice | Clove leaf and bay leaf EO | Chemical composition and antifungal activity | Prolonged shelf life | [702] | ||
| Fruit and vegetable | Fried potatoes | Multilayer food packaging films with bay leaves, Algerian sage leaves ethanol, and aqueous extract | Bioactive molecules, packaging color, tangible migration, and lipid oxidation evaluated | Improved antioxidant activity, extended shelf life | [670] | |
| Fresh cut muskmelons | Bay leaves EO nanoemulsion | Physicochemical properties, and microbiological analyzed | Inhibited TA level, oxidative browning, improved TPC, retarded spoilage microorganisms, and improved physiological quality | [770] | ||
| Saffron | Meat product | Poultry | Saffron | Growth, and feed additive parameters evaluated | Improved antioxidant activity, protection, and quality | [159] |
| Cereal-based product | Wheat flour pasta | Powder | Characterization, moisture content, water activity, antioxidant potential, TPC, HPLC, TPA, color, and sensory attributes evaluated | Enhanced sensory properties | [85] | |
| Functional cookies | 50 mg of aqueous extract | Proximate composition, sensory, physical, texture, color, TPC, antioxidant activity, and lipid peroxidation analyzed | Improved antioxidant activity, enhanced sensory properties, increased acceptance, and shelf life | [144] | ||
| Fruit and vegetable products | Fresh cut cucumber | Edible film with saffron petal alcoholic extract | Physical parameters, moisture content, transparency, water vapor permeability, antibacterial activity, TPC, antimicrobial activity, and total soluble solids evaluated | Enhanced physical properties, quality, reduced spoilage, and extended shelf life | [359] | |
| Salad dressings | Pomegranate juice and saffron powder | Rheology, storage stability, color, and sensory attributes analyzed | Reduced viscosity, structured emulsion, improved rheological properties, enhanced color, and taste | [420] | ||
| Star anise | Meat product | Yao meat | Nanoemulsion-based active coating with star anise EO, nisin, and polylysine | Shelf life, quality, sensory, physicochemical, and microbial properties evaluated | Improved stability, inhibited microbial growth, decreased total volatile, pH, improved acceptability, color, odor, quality, and extended shelf life from 8 to 16 days | [537] |
| Dairy product | Milk | Ethanol and aqueous extracts of star anise powder | MICs inhibition evaluated | Retarded bacterial growth, delayed spoilage, improved protection level, and reduced health problems | [727] | |
| Fruit and vegetable products | Strawberry | Edible film from the base formulation with star anise EO, and cinnamon extract | Quality and shelf life evaluated | Reduced titratable acidity, pH, extended shelf life up to 41 days | [77] | |
| Onion | Meat product | Beef burger patties | Edible onion film | Shelf life, sensory, and quality parameters evaluated | Enhanced chrome, redness, yellowness, reduced pH, increased chewing property, flavor, texture, color, odor, and appearance | [872] |
| Ground beef meat | Water extract | Phenolic compounds, MIC, antibacterial activity, microbiological property, physicochemical, and sensory attributes analyzed | Enhanced meat protection, delayed protein, lipid oxidation, and inhibited pathogens propagation | [618] | ||
| Cereal-based product | Fermented millet-based porridge | Alliam cepa, and Allium parvum extracts | Antibiotic susceptibility, MIC, and FTIR evaluated | Improved food protection | [286] | |
| Wheat pasta | Powder | Chemical composition, antioxidant activity, quality, color, and sensory attributes analyzed | Enhanced nutritional value, flavonoids, total dietary fiber, TPC, and antioxidant activity | [596] | ||
| Fruit and vegetable products | Pesticide-treated vegetables (tomato, beet, cucumber, cherry tomato, lettuce, round aubergine, and pepper), and grapes | Onion | Genotoxicity and toxicity evaluated | Enhanced mutagenicity and micronucleus frequency | [294] | |
| Dill | Meat product | Beef | Plantago major seed mucilage edible coating with dill seed EO | Antioxidant activity, TPC, antimicrobial activity, sensory, and chemical properties evaluated | Prolonged shelf life up to 9 days | [137] |
| Meat | A water-soluble polysaccharide (AGP1) of dill seed | AGP1 characterization analyzed | Good thermal stability, lower lipid peroxidation, and enhanced bacterial stability | [353] | ||
| Broiler chicks meat | Dill seed and dietary hemp | Quality, physicochemical properties, lipid peroxidation, oxidative stability, and sensory attributes estimated | Decreased lipid peroxidation, enhanced sensory characteristics, lipid profile, and oxidative stability | [975] | ||
| Dairy product | Probiotic yogurt | 100 ppm of EO | Sensory and physicochemical properties evaluated | Enhanced probiotic bacteria, titratable, reduced pH, increased sensory scores, and physicochemical properties | [590] | |
| Yogurt | Aqueous extract of dill seed powder | Physicochemical, textural, colorimetric, and sensory attributes analyzed | Enhanced antioxidant capacity, TPC, acidity, lower water content, increased acceptability, nutritional value, and taste | [938] | ||
| Fruit and vegetable product | Tomato pomace | Dill seed, and Mentha piperita | Shelf life stability and physicochemical properties evaluated | Extended shelf life | [98] | |
| Dried fruit | Methanol; a fraction, EO fraction, and aqueous fraction | Bacterial cultures, antibacterial effect, antioxidant potential, and TPC analyzed | Enhance antioxidant capacity and TPC | [918] | ||
| Fenugreek | Meat product | Meat with olive oil | 2 and 4% of Powder | Emulsion stability, lipid oxidation degree, water salvation, fat released, TPA characteristic, and color evaluated | Reduced fluid salvation, fat released, gumminess, stiffness, chewiness, and increased quality | [303] |
| Ground beef patties | Extract | Storage stability analyzed | Reduced thiobarbituric acid value, delayed bringing stage of oxidative rancidity, improved oxidative stability, and antioxidant capacity | [369] | ||
| Tunisian beef sausage | Water-soluble polysaccharide of fenugreek seed | Functional parameters, and oxidative method estimated | Retarded myoglobin, lipid oxidation, and improved storage stability | [490] | ||
| Beef burger | 3, 6, 9, and 12% of seed flour | Antimicrobial and antioxidant activity assessed | Enhanced microbiological quality, essential amino acid, physicochemical quality, improved acceptance, and sensory attributes | [363] | ||
| Hen meat patties | Powder | pH, proximate composition, TBARS value, microbiological quality, and sensory attributes measured | Enhanced sensory properties | [720] | ||
| Rabbit sausage | 5, 10, and 15% of powder | Physical, color, TBARS, and sensory properties examined | Lower lipid oxidation and improved antioxidant capacity | [1006] | ||
| Dairy product | Buffalo yogurt | Moringa oleifera and fenugreek seed flours | Proximate, polyphenols, AOA, TPC, physicochemical, mineral content, microbiological properties, antibacterial activity, and sensory attributes evaluated | Enhanced viability of yogurt culture, AOA, TPC, antibacterial effect, mineral, functional properties, and nutritional value | [250] | |
| Milk | Sprouted | Dairy and growth performance analyzed | Enhanced dairy performance, improved regular weight gain, and increased growth ratio | [161] | ||
| Cereal-based product | Rice and chickpea | 15% of fenugreek polysaccharide flour | Moisture obtained, expansion rate, WAI, color, texture, sensory, and glycemic index evaluated | Reduced GI, improved sensory, and physical properties | [849] | |
| Dark wheat flour | 2, 5, and 8% of the flour | Proximate composition, baking, antioxidant activity, TPC, texture, sensory, and microbiological properties estimated | Enhanced acceptance, and improved quality | [568] | ||
| Brabari, and lavash flatbreads with wheat doughs | 4.93% of seed gum | Quality assessed | Higher water absorption, improvement, extensibility, less tendering degree, and optimum amounts of retardation to stretch | [728] | ||
| Muffins | Seed husk | Rheological, chemical properties, color, dietary fiber, tangible gravity, viscosity, sensory, physical, texture, and composition measured | Enhanced fiber content, improved quality, volume, and tender texture | [889] | ||
| Biscuits and bread | Wheat and fenugreek flour | Proximate composition, functional characteristic, physicochemical, microbiological properties, and sensory attributes examined | Improved flour quality, reduced gluten content, enhanced protein, fiber, mineral, and acceptability | [915] | ||
| Oat | Powder | Bulk density, stiffness, storage stability, and shelf life evaluated | Reduced stiffness, bulk density, and extended shelf life | [986] | ||
| Legumes, and millets (laddu, dhokla, and uppuma) | Seed | Hypoglycemic effect analyzed | Improved acceptability and maintained diabetes | [689] | ||
| Bread | 5% of the fenugreek powder | Carbohydrate metabolism, taste, acceptance, insulin, blood glucose, nutrient composition, and sensory characteristic estimated | Reduced insulin, glucose, controlled functional property, decreased insulin resistance, and type 2 diabetes | [541] | ||
| Flatbreads and buns | 10% of fenugreek powder | GI, and glycemic response assessed | Reduced glucose curve, GI, glycemic response, and postprandial glycemia | [767] | ||
| Wheat biscuits | Fenugreek powder | Thickness and sensory attributes measured | Enhanced thickness, quality, acceptability, dietary fiber, protein content, lysine, total Ca, and total Fe | [372] | ||
| Pea and oat | Seed powder and leaf powder | Barrel temperature and stiffness examined | Improved quality | [985] | ||
| Injera | Fenugreek powder | Mineral, proximate composition, total microbial load, and sensory properties evaluated | Enhanced crude fiber, crude protein, mineral, lower microbial growth, increased crude fat content, and nutritional value | [327] | ||
| Pearl millet | EO | Amylose content, solubility, moisture content, film thickness, opacity, water solubility, tensile properties, SEM, and antimicrobial activity analyzed | Enhanced tendril break, tensile power, melting enthalpy, thermal transition temperature, morphological properties, surface softness, retarded microbial growth, increased mechanical, obstacle properties, quality, and prolonged shelf life | [253] | ||
| Biscuit and wheat flour | 10% of fenugreek powder | Physical properties, sensory, chemical, and rheological properties analyzed | Enhanced sensory, chemical properties, and prevented degenerative disorders | [268] | ||
| Whole wheat flour | 5, 10, 15, and 20% of fenugreek powder | Rheological, functional, and thermal characteristics estimated | Developed yellowish color, enhanced bulk density, emulsion potentiality, water retention capacity, lower melting enthalpies, increased viscosities, consistency, power-law firmness coefficient values, and functional properties | [780] | ||
| Gluten-free fresh pasta | Fenugreek, tiger nut, and chickpea powder | Chemical properties, water activity, glycemic index, rheological, color, and sensory attributes assessed | Enhanced nutritional value, protein content, soluble, insoluble fiber, health benefits, reduced glycemic response, slow down starch enzymatic digestion, increased texture, developed more reddish color, and improved sensory acceptability | [539] | ||
| Semolina-based upma | Raw, soaked, and germinated chickpea, and fenugreek seed powder | Sensory and nutritive value examined | Enhanced acceptability, dietary fiber, protein, fat, Fe, Ca, moisture content, reduced calories, carbohydrates, GI, and improved nutritional value | [503] | ||
| Wheat flour rusk | Fenugreek powder | Mineral, proximate composition, dietary fiber, functional, physical parameters, stiffness, color, antioxidant activity, and sensory attributes evaluated | Improved antioxidant, nutritional properties, reduced sensory characteristic, enhanced mineral, fiber, phytochemical properties, stiffness, loaf weight, quality, acceptability, lower loaf volume, and developed dark color | [252] | ||
| Mungbean | Fenugreek | Soil pathogenic fungi analyzed | Lower frequency of soil pathogenic fungi, enhanced percent pollen fertility, chlorophyll content, and nitrate reductase activity | [937] | ||
| Asafoetida | Dairy product | Milk dessert | Lactobacillus reuteri encapsulated with sodium alginate with zedo, and asafoetida gum | Sensory, physicochemical, microbiological, and bacterial survival evaluated | Enhancement in MLR survival | [431] |
| Celery | Cereal-based product | Wheat flour and bread | Powder | Chemical, Physical parameters, antioxidant potential, TPC, starch digestibility, and sensory attributes evaluated | Enhanced antioxidant capacity, phenolics content, crumb stiffness, sensory characteristic reduced GI, starch digestibility, and bread volume, | [982] |
| Biscuits | Oleoresin and EO | Microstructural and physicochemical properties analyzed | Enhanced hydration properties of fiber, stiffness, and quality | [882] | ||
| Chili | Meat product | Hubbard broiler | Powder | Physicochemical, biochemical, and hematological parameters evaluated | Decreased blood glucose level | [258] |
| Vietnamese fermented pork roll (nem chua) | Powder | TBA, Enterobacteriaceae load, pH, TVB-N, sensory attributes analyzed | Reduced Enterobacteriaceae load, pH, TVB-N, TBA, enhanced sensory score, and maintained physicochemical | [598] | ||
| Broiler chickens | Chili, and turmeric powder | Proximate composition, quality, refrigeration loss, water absorptive capability, pH, lipid profile, TBARS, and sensory attributes estimated | Reduced lipid oxidation, enhanced flavor, and storage stability | [801, 802] | ||
| Dairy product | Gouda cheese | Microencapsulation of chili pepper powder extract added with olive oil | Quality, pH, amino acid content, texture, and sensory attributes assessed | Enhanced quality, reduced stiffness, and extended utilization | [474] | |
| Yogurt | Encapsulate chilli waste bioactives | Physicochemical and sensory attributes measured | Improved solubility, water activity, moisture content, flowing, color, polyphenol retention, acceptance, sensory properties, controlled lactic acid bacteria, enhanced bioactive, nutritional, and color parameters | [819] | ||
| Allspice | Fruit and vegetable product | Tomato | Oregano, allspice, and garlic EO | Antimicrobial and physical properties evaluated | Decreased viscosity, enhanced elongation, developed dark color, and improved physical parameters | [261] |
| Kokam | Dairy product | Cocoa butter | Phulwara butter and kokum fat fraction | Physical and chemical parameters evaluated | Improved triacylglycerol, fatty acids composition, solidification properties, and low melting limits | [755] |
| Cereal-based product | Rice extrudates | Encapsulated fruit powder | Proximate composition, nutritional value, starch, protein, lipids, vitamins, phytochemicals, moisture, color, relative humidity, antioxidant potential, breaking strength, texture, TPC, and bulk density analyzed | Improved color, reduced loss of antioxidant capacity, TPC, and enhanced bulk density | [366] | |
| Miscellaneous product | Chocolate | Cocoa butter, and 5% of kokam powder | Triglyceride composition, rheology, and stiffness evaluated | Enhanced stiffness, solids fat content, physical parameters, and heat resistance properties | [556] |
Fig. 4.
Food application of spices and herbs
Meat-based Food Product
Green and dried cardamom has been ground, dipped in water, and the hydrodistillation method with the help of clevenger type apparatus has been used to extract the EO. Thereafter, the EO has been applied to the chicken drumsticks to increase its shelf life by 2–3 days [464]. Cardamom seeds have been dried in shade and ground. 500 cc of 80% of ethanol has been added to 100 g of the dried powder and stored at room temperature (220 C) for 1 day. After filtration, the alcohol extract has been dried at 400 C temperature. The extract has been used on lamb meat to increase the quality in terms of flavor, taste, and enhance the shelf life of lamb meat [837]
EOs have been extracted from Zataria Multiflora and Bunium persicum The extracted EOs have been used to produce chitosan-loaded nanoemulsions. The nanoemulsion has been applied to turkey meat to enhance its shelf life up to 15–18 days and to improve its microbial quality [441]. To improve the cholesterol profile and nutrient digestibility of broiler chicks, the dried and milled seeds of Zataria Multiflora and Bunium persicum are used [852]. Nanocellulose, Mentha piperita essential oil, and Bunium persicum essential oil have been mixed at various percentages to prepare a biodegradable active poly-lactic acid composite films to enhance the shelf life of ground beef up to 4–7 days [911]. To improve the nutritional quality of boiler chicken meat, the chicks have been fed with different diet composition where corn-soybean meal-based diet has been taken as control. The other diet compositions are 10 ppm avilamycin + basal diet, 0.25% of cumin powder + basal diet, 0.75% of cumin powder + basal diet, 0.25% of black cumin powder + basal diet, and 0.75% black cumin powder + basal. It has been observed that the basal diet give satisfactory results [823]. The Trachyspermum ammi EO (NTEO), and Bunium persicum EO (NBPEO) have been used to prepare chitosan based nanoemulsions and chitosan based nanoemulsions have been applied to hamburgers to improve its microbial quality [875]. 1–2% of Bunium persicum EO along with tween 80 (emulsifier), and glycerol (plasticizer) have been used to make chitosan films and the films have been applied on chicken fillet to retard its chemical spoilage and to improve its microbial quality [425]
Lambs have been fed with 1.5% FSP (fennel seed powder) and diet without FSP (control) to improve the nutritional composition and to reduce the fat oxidation [351]. EO has been extracted from the fennel seed and applied to chicken thighs to extend its shelf life [417]. Different formulas have been considered with fennel and lettuce powders to reduce the bacterial growth, to improve the aroma and to replace the nitrite in sausage [726]
Dried and powdered poppy seeds have been used to decrease fat content and to improve the sensory scores, pungent flavor, textural attributes, and color feature of buffalo meat cookies [334]. Dried Poppy seeds have been used to prepare poppy paste, the paste has been applied to chevon nuggets to reduce the microbial activity, fat content, and to increase the protein percentage, sensory score, and shelf life [421]. Poppy seeds at different percentages (1, 3, and 5%) have been administered to chevon patties to reduce cholesterol and fat content and to increase the moisture, color, and mineral (Fe, K, Mn, Zn, Ca) profile [645]
Clove extract has been applied to cooked ground beef to decrease its lipid oxidation and to enhance the storage stability and flavor profile of the sample [1003]. The broiler chickens have been fed with diet enriched with clove to improve the physical sensory and microbial characteristics of carcass [899]. The tenderness, moisture content, water holding capacity, and sensory quality of chicken meat has been improved with the application of dried clove powder along with turmeric rhizome [801, 802]. EO derived from clove has been applied to frozen beef patties to improve its sensory quality and to improve oxidative stability [68]. Clove flower EOs and marjoram EOs have been applied to frozen beef patties to enhance oxidative stability, and quality [6]. At 1:5 proportion, clove extract has been applied to fresh beef patties to decrease the lipid, protein oxidation, and hue angle and to improve the quality attributes like elevated chroma value and redness value [90]. 400 mL of 95% of edible ethanol has been added into dried cloves. The mixture has been applied to Chinese-style sausages to retard protein and lipid oxidation, enhance the color, texture, flavor, acceptance, and quality [1013]
Cinnamon and sappan powders and thermoplastic starch have been mixed and extruded to produce active meat packaging film to extend its shelf life [467]. The shelf life of fresh beef has been extended up to15 days through the application of edible film comprised of 0.5, 1, 1.5, and 2% of cinnamon bark EO along with glycerol, and tapioca starch [953]. Nisin and cinnamon EO nanocapsules have been incorporated with sodium alginate coating. The mixture has been applied to beef slices to inhibit microbial growth, decrease lipid oxidation, prolong shelf life up to 15 days, enhance texture, color, odor, juicy characteristic, quality, antioxidant, and sensory properties [1014]. At 4:1 proportion, potassium chloride, carrageenan, and citric acid have been mixed with 0.05% cinnamon EO. The solution has been applied to chicken fillets to reduce the sensory score, and prolong shelf life [456]
0.5% of black pepper has been applied to buffalo meat steaks to increase quality and extend shelf life [575]
1.5% of EO of coriander seeds have been applied to chicken fillet to retard pathogen growth, enhance chemical quality and antioxidant capacity, and extend shelf life [430]
Cellulose acetate film with 500 mg of powdered mustard seeds has been applied to meat product to form moisture-activated antimicrobial film, decrease moisture absorption, and extend shelf life of meat up to 3.68 days [118]
Turmeric and alginate have been applied to chicken, pork, and beef to enhance its storage stability, quality, and to decrease the lipid oxidation. The mixture has improved the shelf life for chicken, and pork up to 12 days, while the shelf life of beef has been extended to 16 days [155]. Dried turmeric rhizomes powder has been applied to broiler chicks to enhance its quality, and marketability [612]. Turmeric and black pepper extracts have been applied to chicken breast fillet to reduce lipid oxidation and proteolysis, to control bacterial count, and to extend shelf life up to 16 days [218]. Acetone solution has been applied to extract curcumin. 200 mL of acetone has been added to10 g of turmeric powder. The solution has been applied to chicken and fish meat to alter color from yellow to reddish brown, to decrease water solubility, and to improve the water absorptivity [283]
Powder of sage and laurel leaves have been applied to cooked meatballs to reduce peroxide value, to enhance sensory characteristic and acceptance, and to extend shelf life [37]. Hydrodistillation by Clevenger-type apparatus has been used to extract EOs from rosemary and bay leaves. The EOs have been applied to minced maronesa beef to enhance pH, decrease microbial load, improve color, and extend the shelf life [970]
Onion peel extract has been applied to ground beef meat to delay protein and lipid oxidation and to inhibit the growth of pathogenic bacteria [618]
The fenugreek seed powder has been applied to rabbit sausage to lower lipid oxidation and to improve antioxidant capacity [1006]
Dairy Based Food Product
Raw bay leaves and cardamom powder have been applied to labneh to increase the physiochemical, sensory, and microbiological properties and shelf life up to 30 days [921]
100 g of dried Bunium persicum seed EOs has been applied to gouda cheese to reduce lipid oxidation, to retard the growth of gram-positive and gram-negative microorganisms, and to enhance shelf life [806]
Phenolic-enriched extracts of dried fennel seeds have been applied to cottage cheese to enhance antioxidant activity and shelf life up to 14 days [169]. 250 g of aqueous extract of ajwain and fennel seeds has been applied to processed cheese to enhance microbial stability, shelf life, flavor, and acceptance [577]. The water extract of dried fennel seeds has been applied to probiotic yogurt to improve texture, flavor, enhance protection, and bioactive components [99]. EOs have been extracted from Petroselinum crispum and fennel; the EOs have been applied to steamed yogurts to improve its nutritional qualities, physicochemical characteristics, texture, acidity, and taste and to extend the shelf life up to 29 days [356]
The eugenol-lean fraction of clove buds has been applied to mayonnaise to reduce thermal and non-thermal creaming, homogenous, enhance color intensity, reduce power, and enhance antioxidant potential and phenolic content [190]
The EOs of cinnamon bark and black pepper have been applied to traditional African yoghurt to inhibit bacterial propagation [662]
Cardamom and black pepper powder have been applied to paneer to enrich its phenolic profile and to extend shelf life [112]
0.9 wt.% of cellulose nanofibers (CNFs) and 30wt.% of turmeric have been mixed together. The solution has been applied to yoghurt to enhance the color quality and sensory characteristics and to reduce the syneresis [341]. Aqueous extract of curcumin has been applied to milk food product to enhance chemical stability, bioaccessibility, and bioactivity and improve human health [309]. Water extract of turmeric has been applied to yoghurt to enhance nutrient composition, pH, alter color from white to yellowish-orange, reduce carbohydrate, protein content, increase vitamins, minerals, inhibit microbial activity, and acceptance of the product [580]. Water extract of raw turmeric slices have been applied to buffalo milk paneer to increase nutritional, health properties, and quality of the product [884]. In distilled water, 0.50% of lecithin, 4% of curcumin, and an emulsifier have been dissolved to formulate thick emulsion. The emulsion has been applied to kulfi to enhance sensory characteristics (flavor, color, appearance, taste), acceptance, and lower microbial load [841, 843]. Water extract of 5% raw turmeric has been applied to paneer to improve the hardness, cohesiveness, elasticity, gumminess, and chewiness [461]. Nanoemulsified curcumin has been applied to Pelibuey sheep milk to enhance antioxidant potential, TPC, appearance, color, odor, beneficial health effect [807]. The EOs extracted from turmeric has been applied to Cokelek Cheese to inhibit microbial growth, and to reduce the weight loss [437]
Water–ethanol extract of 100 g of star anise has been applied to milk to inhibit bacterial growth, delay spoilage, and to improve the keeping quality of the final product [727]
At the 1:10 proportion, water extract of dill has been applied to yogurt to enhance antioxidant capacity, TPC, acidity, acceptability, nutritional value, and taste, and to lower the water content of the product [938]
The Moringa oleifera and fenugreek seed flours have been applied to buffalo yogurt to enhance the viability of yogurt culture, TPC, antibacterial effect, minerals, functional properties, and nutritional value [250]
At the proportion of 1:5, the olive oil has been added into chili pepper powder; the prepared extract has been applied to Gouda cheese to enhance its quality, to reduce stiffness, and to extend its shelf life [474]
Cereal-based Food Product
The hydro-distillation process with the help of Clevenger-type apparatus has been operated for 3 h at 1000 C to extract EOs of thyme flower shoots, and black cumin seeds. The extracted oils have been applied to corn starch to retard the propagation of food pathogens, and to extend its shelf life [75]
Cinnamon powder has been applied to bread to enhance loaf volume by 2%, acceptability, phenolic content, antioxidant potential, to inhibit mold growth, and to extend shelf life up to 6 days [251]
Black pepper flour has been applied to snack food product to increase the nutritional profile, flavor profile, mineral profile, and the marketability product [17]
Coriander EOs have been applied to wheat, and wheat product to inhibit fungal growth [160]
Turmeric rhizome powder has been applied to biscuits to enhance the nutritional quality, protein content, and antioxidant activity [16]. Turmeric rhizome powder has been applied to Moin-Moin to lower the microbial count [34]. Korean turmeric rhizomes powder has been applied to cake, and wheat flour dough to enhance the antioxidant capacity, and curcumin content [683]. 0.35 g of turmeric extract has been applied to breakfast cereal to enhance antioxidant capacity, curcuminoid content, and TPC [516]
Water extract of 1 g of saffron has been applied to cookies to improve antioxidant activity, enhance sensory properties, increase acceptance, and shelf life [144]
onion powder has been applied to wheat pasta to enhance nutritional value, flavonoids, total dietary fiber, TPC, and antioxidant activity [596]
Germinated fenugreek seed powder has been applied to bread, and biscuits to improve flour quality, nutritional quality, mineral content, acceptability, to reduce gluten content [915]. Water extract of fenugreek seed has been applied to Injera to enhance crude fiber, crude protein, minerals, crude fat content, and to lower the microbial growth [327]. Fenugreek EO has been applied to pearl millet to enhance tendril break, tensile power, melting enthalpy, thermal transition temperatures, morphological properties, surface softness, to retard microbial growth, to increase mechanical, obstacle properties, quality, and prolonged shelf life [253]. Water extract of germinated fenugreek seed has been applied to wheat flour, and biscuits to enhance sensory, chemical properties, and to prevent degenerative disorders [268]
Fruit and Vegetable-based Food Product
Clove EO, and mustard EO have been applied to strawberries to retard fungal growth [24]
Hexane extract of sage, and bay leaves has been applied to fried potato to improve antioxidant activity, and to extend shelf life [670]
Ethanol extract of 40 g of saffron petals has been applied to fresh-cut cucumber to enhance physical properties, quality, to reduce spoilage, and to extend the shelf life [359]
The methanol fraction of dill has been applied to dried fruit extracts to enhance antioxidant capacity, TPC [918]
Miscellaneous Food Product
80% of ethanol extracts of Marrubium vulgare, Physalis alkekengi, Alcea rosea, and Bunium persicum seeds have been applied to mushroom tyrosinase to retard mushroom tyrosinase [638]
Fennel seed powder has been applied to herbal candy to improve the nutritional value, acceptance, and other physicochemical properties [418]
Nanotechnology Application of Spices
Different types of spices like cardamom, black jeera, fennel, poppy, clove, turmeric, bay leaves, fenugreek, etc. are along with some specific solvent such as zinc acetate solution, silver nitrate solution, chitosan solution, etc. are the key elements to synthesize metal, and polymer nanoparticles, zinc oxide nanoparticles, silver nanoparticles, gold nanoparticles, selenium nanoparticles, and polymer nanoparticles which have several health benefits like antioxidant, anti-carcinogenic, anti-diabetic, cytotoxicity activity, enzyme retardation effect, antimicrobial activity, dye decolorization effect, and catalytic activity. A complete discussion on nanoparticles synthesis with the help of different types of spices using various methods and its beneficial applications are portrayed in Fig. 5 and Table 5
Fig. 5.
Nanoparticles from spices and herbs and their application
Table 5.
Nanotechnology application of spices
| Spices | NP synthesized | Characterization | NP synthesis technique used | Application | Reference |
|---|---|---|---|---|---|
| Cardamom | Zinc oxide nanoparticles (ZnONPs) | Ultraviolet–visible (UV–Vis), spectroscopy, X-ray powder diffraction (XRD), SEM, and FTIR | Zn acetate solution with green cardamom solution | Antimicrobial effects, anticancer activity | [676] |
| Gold (Au NP) | SAED pattern, PerkinElmer Lambda-35 spectrophotometer, TEM, XRD, FTIR, and IR prestige-21 Shimadzu spectrometer | 2.5 × 10–4 M Chloroauric acid with water extract of cardamom (2 g sample in 100 ml) | Anticarcinogenic effect, antioxidant, and antibacterial activities | [733] | |
| AuNP | XRD, SEM, TEM and UV–Vis spectra | Green synthesis | NA | [694] | |
| Silver NPs (AgNPs) | FTIR, UV–visible spectroscopy | Green synthesis | Cytotoxic effect, anti-carcinogenic activity | [487] | |
| AgNPs | EDAX, XRD, FTIR and SEM | Green synthesis | Antimicrobial effects | [326] | |
| AgNPs | SEM, UV–Vis absorbance spectroscopy, and XRD | Green synthesis | Antibacterial effects | [835] | |
| Gelatin NPs (GNPs) | Zeta potential, encapsulation efficacy, average particle size, DLS, UV–Vis spectrophotometry, DSC, XRD, SEM, and FE-SEM | Cardamom extract-loaded gelatin NPs with Water extract of green cardamom | Glioblastoma treatment | [649] | |
| ZnO-NPs | FTIR, UV–Vis, XRD, SEM, and TEM | Biogenic synthesis | Antibiofilm function, antibacterial, and mosquito larvicidal effect | [972] | |
| Black jeera | Ag-NPs | FE-SEM, FTIR, XRD, EDS, TEM, and TG–DTA | Green synthesis | Catalytic decreased of organic dyes | [769] |
| Ag-NPs | SEM, UV–Vis spectrometer, FTIR, EDX | Sodium chloride solution with alcoholic extract of black cumin | Pharmacological activities, antibacterial activity, sedative, analgesic, hypertensive, and bronchodilator activities | [447, 448, 455] | |
| Au-NPs | SEM, UV–Vis spectroscopy, FTIR, EDX, and XRD | 1 mM hot Au solution with methanol or alcoholic extract of black jeera seed | Urease retardation activity, enzyme retardation, antifungal, antibacterial activities, xanthine oxidase effect, and carbonic anhydrase function | [117] | |
| Encapsulation of black cumin EO | FTIR, SEM, AFM, and XRD | Chitosan nanopolymer | Free radical scavenging activity, antifungal, and aflatoxin B1 retardation activity | [990] | |
| Cellulose NPs | GC–MS | Mentha pepperita, and black cumin EO with polylactic acid solution | Antibacterial effect | [912] | |
| Fennel | Au-NPs | UV–Vis absorption spectra, TEM, XRD, and FTIR, | A biomimetic synthesis | Catalytic activity | [200] |
| Ag-NPs | XRD, UV–Vis spectrophotometer, FTIR, SEM, and EDS | Rapid green synthesis | Antibacterial effect | [409] | |
| Selenium NPs (Se-NPs) | XRD, UV–Vis, FTIR, SEM, and EDS | 173 mg of sodium selenite salt solution with fennel seed extract | Toxicity evaluation, arthritis treatment | [84] | |
| Au-NPs | UV–Vis, FTIR, TEM, and FE-SEM | Green synthesis | Antioxidant potential, anti-human breast cancer, and qualitative evaluation | [194] | |
| Chitosan nanocomposite | XRD, SEM, and FTIR | Chitosan-based nano-encapsulated fennel EO | Aflatoxin B1, antifungal retardation activities | [497] | |
| ZnO-NPs | FTIR, UV–Vis, XRD, TEM, and EDX | Green synthesis | Cytotoxicity evaluation, cell treatment, and antimicrobial activity | [61] | |
| Ag-NPs | UV–Vis spectral | Methylene blue dye solution with fennel seed extract | Phytochemical evaluation, and catalytic activity | [603] | |
| Ag-NPs | Viability | Ag-NPs dilutions with 100 g fennel seed EO | Scolicidal effect for protoscoleces of hydatid cyst | [517] | |
| Chitosan NPs | CNPs | Chitosan solution with fennel seed EO | Fish trail, peroxide value, total volatile base nitrogen evaluation, microbial activity, and sensorial attributes | [549] | |
| ZnO-NPs | DLS | ZnO dispersion with water extract of anise, and fennel seed | Liver enzyme evaluation, MDA, immunohistochemistry of interleukin 6 analysis, and histopathological assessment | [127] | |
| ZnO-NPs | EXD, FTIR, SEM, and XRD | Green synthesis | Antioxidant activity, biological, and antibacterial activity | [465] | |
| ZnO-NPs | XRD, UV–Vis spectrophotometry, and SEM | Green synthesis | Antimicrobial activity, pharmaceutical products development, agricultural area improvement | [895] | |
| Titanium dioxide NPs (TiO2-NPs) | Response surface methodology | 2% of TiO2 and 2% of fennel EO | Antibacterial activity, packaging film production | [562] | |
| Mono-disperse carbon quantum dots (C-QDs) | NMF-ARD-SO, PCA, MCR-ALS, UV–Vis absorption spectroscopy, TEM, EDS, FTIR, and TLC | Purified C-QDs with fennel seed | Photoluminescence evaluation | [214] | |
| Transdermal nanoemulsions | HPLC | 68% of Smix, 2% of fennel EO, and 5.6% of oleic acid | Anti-diabetic activity evaluated | [616] | |
| TiO2-NPs | TEM | TiO2-NPs with fennel | Chemical evaluation, heavy elements analysis | [458] | |
| Nano-chitosan | FTIR | Nano-chitosan, pectin with fennel EO, and potato peel extract | Antimicrobial activity, antioxidant activity, mechanical, thickness, optical parameter, total soluble matter, and morphological evaluation | [774] | |
| Oil in water nano-emulsions | Ultrasonic emulsification | Nano-emulsions with 0.05, and 0.01wt% of fennel seed EO | Herbicidal capacity evaluated | [435, 436] | |
| Poppy | Au-NPs | FTIR, UV–Vis spectrophotometer, and SEM | Green synthesis | NA | [624] |
| Lead oxide NPs (PbO-NPs), and Fe2O3-NPs | EDX, XRD, FTIR, and SEM | Green synthesis | Antifungal, antibacterial activity, antioxidant activity, reducing capacity, free radical scavenging activity, and anti-carcinogenic effect | [625] | |
| Mesostructured silica-coated magnetic NPs | EDS, SEM, and XRD | Fe3O4 solution with solid–liquid extract of poppy seeds | Na | [176] | |
| Clove | ZnO-NPs | TEM and SEM | Zinc nitrate solution with a clove flower solution | Anti-mycotoxin, antifungal effect, lipid peroxidation evaluation, and ergosterol content analysis | [511] |
| Ag-NPs | UV–Vis spectroscopy, and FTIR | Biomimetic synthesis | Antifungal, antibacterial effect | [36] | |
| Ag-NPs | HR-TEM, FTIR, UV–visible spectroscopy and EDAX | Green synthesis | Cytotoxic activity, and anti-carcinogenic effect | [964] | |
| FeO-NPs | UV–Visible spectral, SEM, EDAX, and FTIR | Bee venom with water extract of clove | Anti-carcinogenic effect | [116] | |
| Ag-NPs | TEM, FTIR, UV–Vis spectroscopy, and XRD | Green synthesis | Antiviral effect, micro haemagglutination evaluation, cytotoxic activity | [592] | |
| Pt and Au/Pt bimetallic NPs | FTIR, UV–visible spectra, XRD, XPS, cyclic voltammetry, zeta potential | Phyto-mediated synthesis | Antioxidant activity, antibacterial, and catalytic efficacy evaluated | [841, 843] | |
| PVP/clay nanocomposites | DSC, XRD, SAXS, SEM, and FTIR | Clove EO | Aedes aegypti larvae management | [799] | |
| Fe-NPs | XRD, TGA, and XPS | Green synthesis | NA | [497] | |
| Chitosan NPs | TEM, UV–Vis spectra, IR, and XRD | Chitosan solution with 1 ml of clove EO | Biochemical evaluation and insecticidal effect | [271] | |
| Fe2O3-NPs | Microscopic and spectroscopic | Biogenic green synthesis | Methylene blue dispelled | [397] | |
| ZnO-NPs | SEM and XRD | Green hydrothermal synthesis | Physicochemical evaluation, and bactericidal activity | [82] | |
| Ag-NPs | UV–visible spectra, SEM, and FTIR | Silver solution with methanolic extract of clove flower | Antifungal, and antibacterial effect | [547] | |
| Ag-NPs | Analytical | Ag solution with water extract of clove | Oxidative stress, biochemical, hematological evaluation, testis, liver histological analysis, antifungal effect, anti-inflammatory, and antioxidant activity | [162] | |
| Zn-NPs | UV–visible spectroscopy | Green synthesis | Antibacterial effect | [405] | |
| Carbon dots | XPS, FTIR, and UV–visible spectrometer | Hydrothermal synthesis | Antioxidant effect, catalytic, and cytotoxic effect | [495, 498] | |
| Cassia bark | Ag-NPs | FTIR, UV–Vis absorption spectroscopy, and SEM | Silver nitrate solution with water extract of cassia bark | Pathogenic avian influenza virus subtype H7N3 effect, and cytotoxicity evaluation | [292] |
| Au-NPs | TEM, and UV–Vis spectroscopy | Green synthesis | Fluorescence activity | [275] | |
| Colloidal NPs | Cryo-SEM | Shellac solution with polyphenol-rich extract of cassia bark | Encapsulation efficacy, loading potential, TPC, antioxidant activity, pH, and stability evaluation | [623] | |
| Cobalt aluminate NPs (CoAl2O4-NPs) | TEM, XRD, SEM, XPS, IR, and UV–Vis spectroscopy | Cobalt aluminate solution with cassia bark extract | NA | [325] | |
| Ag-NPs | UV–Vis spectra, FTIR, TEM, and XRD | AgNO3 with water extract of cassia bark | Blood glucose assessment, histopathological evaluation of liver, and kidney tissue | [483] | |
| Cinnamon NPs | UV–Vis spectrophotometer, FTIR, SAED, BIO-TEM, HRTEM, EDX, and DLSLC-MS | Pulse laser ablation in liquid with the cinnamon stick | Antibacterial effect | [787] | |
| Ag-NPs | FTIR, ATR, TEM, and SAED | AgNO3 water solution with water extract of cassia bark | Type 2 diabetes treatment | [484] | |
| Ag-NPs | FESEM, UV–Vis spectrophotometer, XRD, EDAX, and FTIR | Green synthesis | Antibacterial activity | [715] | |
| TiO2 /cellulose nanocomposite | EDX, FTIR, XRD, and FE-SEM | Green synthesis | Decreased toxic organic compounds | [65] | |
| Se-NPs | FTIR and SEM | Green biosynthesis | Antibacterial activity | [57] | |
| Cinnamon NPs (CN-NPs) | UV–Vis absorption, TEM, EDX, PL emission, and FTIR-ATR | Methanol media and green pulsed laser ablation in liquid | NA | [785, 787, 788] | |
| Cinnamon NPs | SAED, TEM, HRTEM, FTIR, UV–Vis, and photoluminescence | 4 ml of Citric acid, olive oil, and ethanolic extract of cinnamon | Antioxidant, and antimicrobial activities | [786] | |
| AuNPs | TEM, and UV–Vis spectrophotometer | Starch solution with alcoholic extract of cinnamon | Cytotoxicity, and prostate cancer treatment | [186] | |
| Nickel NPs (Ni-NPs) | XRD, FTIR, and SEM | Nickel solution with a methanol solution of cassia bark | Serum, blood biomarker evaluation, oxidative stress, and histological assessment | [386] | |
| Nanoemulsion | TEM | Bacterial nanocrystals with cinnamon EO | Surfactant effect of gelatin evaluation | [753] | |
| CNPs | TEM, and EDX spectra | Citric acid medium applying pulse laser ablation in liquid | NA | [785, 787, 788] | |
| AuNPs | FTIR, UV–visible spectroscopy, XRD, and TEM | Green synthesis | Antibacterial effect | [337] | |
| Amorphous cinnamon NPs | TEM, XRD, FTIR, and photoluminescence spectra | Ethanol solution with nanosecond-pulse laser ablation in liquid technique | Biomedicine utilization | [790] | |
| Mg-NPs | TEM and UV–visible spectroscopy | MgCl solution with cassia bark oleoresin | NA | [80] | |
| CNPs | XRD, UV–Vis, FTIR, TEM, HRTEM, SAED, EDX, DLS, and HPLC | Cinnamon stick with 5 mL of liquid methanol | Antibacterial effect | [789] | |
| Core/shell NPs | ZP, DLS, and PDI | 1% of acetic acid solution with ethanol extract of oregano leaves, and cassia bark | Cytotoxic effect, and mitochondrial transmembrane capacity evaluation | [423] | |
| TiO2-NPs | ZP | Green synthesis | Antioxidant activity, oxidative stress, and histological evaluation | [791] | |
| Ag-NPs | DLS, UV spectroscopy, STEM, and ZP | Pectin films with cinnamon bark, and garlic extract | Antibacterial activity | [340] | |
| Cinnamon-loaded poly-NPs | TEM, FTIR, and XRD | 50 mg of PLGA with 10 mg of methanolic extract of dried cinnamon bark | Antimicrobial effect | [971] | |
| Edible coatings of nanostructures chitosan | ZP, FTIR, DLS, and TEM | Chitosan solution with cinnamon EO | Quality of cucumber fruits, total chlorophylls content evaluation, and microbiological analysis | [390] | |
| Black pepper | Au-NPs | UV–visible spectroscopy, EDX, SEM, and FTIR | Green synthesis | Catalytic effect, antibacterial, antifungal effect, urease inhibitory, xanthine oxidase effect, carbonic anhydrase-II effect, sedative, oedema evaluation, and severe toxicity analysis | [136] |
| Ag-NPs | UV–visible spectroscopy, FTIR, HR-TEM, | Green synthesis | Anti-carcinogenic effect | [486] | |
| Ag-NPs | SEM, UV–Vis spectroscopy, XRD, TEM, and FTIR | Green synthesis | Phytochemical constituents, antibacterial effect, cytotoxic activity | [427] | |
| Ag-NPs | XRD, UV–Vis spectrophotometer, TEM, and FTIR | Rapid green biogenic synthesis | NA | [312] | |
| Chitosan NP | XRD, UV–Vis spectrophotometry, and ZP | Chitosan solution with black pepper EO | Insecticidal effect, toxicity, neurotransmitter evaluation, and pest control | [739] | |
| Copper NPs | XRD, UV–visible spectroscopy, FTIR, FEG-SEM, EDS, and HR-TEM | Green synthesis | Antibacterial effect | [824, 825] | |
| Ag-NPs | UV–Vis spectra, XRD, SEM, EDX, and FTIR | Phyto-synthesis | Antibacterial effect | [406] | |
| Undoped cobalt oxide, and cerium ion-doped cobalt oxide NPs | AFM, UV–Vis, and FTIR | Green synthesis | Photocatalytic effect | [805] | |
| Coriander | Ag-NPs | TEM, UV spectroscopy, XRD, SEM | Situ green synthesis | Antimicrobial effect | [648] |
| Ag-NPs | FTIR, UV–visible spectroscopy, PXRD, DLS, ZP, XRD, FESEM, and EDAX | Green synthesis | Antibacterial effect | [818] | |
| Ag-NPs | UV spectral, SEM, and FTIR | Biogenic synthesis | Antimicrobial effect | [574] | |
| ZnO-NPs | SEM and PSA | Zn acetate solution with coriander seed, and leaves extract | Free radical scavenging activity, TPC, TFC evaluation | [867] | |
| Cu-NPs | XRF | Cu solution with coriander | Phytotoxic, and genotoxic activities | [60] | |
| ZnO-NPs | SAED, TEM, and HRTEM | Zinc-based solution, ZnSO4 with coriander seeds | Carotenoid, chlorophyll pigment content evaluation, and antioxidant enzyme function | [771] | |
| ZnO-NPs | TEM | Photo-synthesis | Carotenoid content, malondialdehyde content evaluation | [758] | |
| ZnO-NPs | FTIR, UV–Vis spectroscopy, XRD, SEM, and EDS | Green synthesis | Proline, chlorophyll content evaluation, and lipid peroxidation analysis | [451, 454] | |
| CdS-NPs, and CuO-NPs | XRD, UV–Vis, FTIR, DLS, and FESEM | Ammonium hydroxide solution mixed with copper chloride solution, and coriander | Endogenous H2O2 evaluation, antioxidant enzyme activity, and genotoxicity analysis | [713] | |
| CdS-NPs and CuO-NPs | FESEM, XRD, and DLS | Ammonium hydroxide solution into copper chloride and sodium dodecyl sulfate solution with coriander seed | Mitotic evaluation | [712] | |
| Ag-based hybrid nanostructures | FTIR | Bio-synthesis | Antibacterial activity | [543] | |
| Si-NPs | UV–Vis spectrophotometer | Silicon dioxide solution and salicylic acid with coriander | TPC and TFC evaluation | [19] | |
| Ag-NPs | EDX, UV–visible spectrophotometry, SEM, XRD, and FTIR | AgNO3 solution with coriander seed extract | Antibacterial activity | [813] | |
| ZnO-NPs | UV–visible spectroscopy | Green synthesis | Antibacterial effect, cytotoxic assay | [695] | |
| Ag-NPs | TEM and UV–visible spectroscopy | Green synthesis | Antioxidant potential | [319] | |
| Nutmeg | Ag-NPs | TEM, atomic absorption spectroscopy, XRD, FTIR and UV–visible | Green synthesis | Antimicrobial effect | [842] |
| TiO2-NPs | UV–Vis spectroscopy, XRD, FTIR, FESEM, and EDX | Green synthesis | Photocatalytic effect, morphological, physicochemical parameters, and antibacterial effect | [776] | |
| Ag-NPs | UV–Vis absorption spectrum, FTIR, XRD, TEM, and SEM–EDS | Bio-synthesis | Antibacterial effect, and cytotoxic activity | [120] | |
| Ag-NPs | UV–visible spectroscopy, SEM, EDX, XRD, FTIR, and ZP | AgNO3 solution with hydroethanolic extract of nutmeg seed | Anti-diabetic activity | [698] | |
| Chitosan nano-matrix | XRD, SEM, and FTIR | Bio-synthesis | Antimicrobial effect, aflatoxin inhibitory capacity, and lipid peroxidation | [224, 227] | |
| Ag-NPs | UV spectrophotometer, FTIR, and AFM | AgNO3 solution with nutmeg seed powder | Antimicrobial effect, biofilm activity, and plasmid curing evaluation | [349] | |
| Ag-NPs | FTIR, SEM, and EDX | Green synthesis | Antibacterial effect | [935] | |
| Ag-NPs | SEM, UV–Vis spectrophotometer, XRD, EDAX, and FTIR | Green synthesis | Antibacterial activity | [408] | |
| Ag-NPs | XRD, UV–visible, FTIR, SEM, TEM, and AFM | Green synthesis | Antibacterial effect | [564] | |
| Ag-NPs | UV–Vis spectrophotometry, DLS, TEM, FTIR, and EDX | 450 ml of 1 mM AgNO3 solution with water extract of nutmeg seed | Antibacterial, antifungal, and cytotoxic activity | [766] | |
| Black mustard | Ag-NPs | FTIR and ZP | Green synthesis | Antimicrobial effect | [680] |
| Turmeric | Ag-NPs | XRD, UV–visible spectroscopy, FTIR, TEM, and SEM | Green synthesis | Anti-carcinogenic effect | [963] |
| Cu-NPs | TEM, UV–visible spectroscopy, and FTIR | Biochemical synthesis | Antibacterial effect | [959] | |
| Ag-NPs | UV–visible spectra, TEM, and FTIR | Bio-synthesis | Antibacterial effect | [505] | |
| Magnetic NPs | TEM | NaBH4 solution in ammonia with rhizome of turmeric | Curcuminoids and free phenolic acids evaluation | [550] | |
| Nickel oxide NPs, CuO-NPs, and Cu-Ni biometallic hybrid NPs | EDX, UV spectroscopy, FTIR, XRD, SEM, TEM, and TGA | Biosynthesis | Antibacterial activity, anti-leishmanial evaluation, protein kinase retardation, cytotoxic activity, anti-diabetic activity, anti-Alzheimer’s activity, and antioxidant potential | [289] | |
| Curcumin NPs | XRD, FTIR, TEM, and SEM | Crude curcuminoid powder with ethanol extract of turmeric powder | NA | [368] | |
| Ag-NPs, and Au-NPs | TEM and UV–Vis spectroscopy | Au, Ag solution with turmeric rhizome extract | Antimicrobial, and anticancer activities | [844, 845] | |
| Fe-NPs, Ag-NPs, and Cu-NPs | EDAX spectroscopy, UV–visible spectrophotometry, SEM, DLS, FTIR, and XRD | Facile-synthesis | Physicochemical parameter | [795] | |
| Ag-NPs | FTIR, UV–vis spectroscopy, XRD, TEM, EDX, DLS, and fluorescence spectroscopy | Ag solution with extract of turmeric rhizome | Antibacterial effect | [318] | |
| Curcumin NPs | SEM, and XRD | Green synthesis | Antibacterial effect and antifungal effect | [451, 454] | |
| Ag-NPs | XRD, UV–visible spectroscopy, TEM, and FTIR | Bio-synthesis | Antibacterial effect | [295] | |
| Nano-ZnO, and nano-Curcuma longa | SEM, and FTIR | In deionized water, 200 ml solution of each 0.25 M Zn (CH3COO)22H2O and 0.5 M NaOH have been added with turmeric rhizome | Third-degree burn treatment | [152] | |
| Au-NPs | TEM, FTIR, and UV–Visible spectroscopy | Green synthesis | Antioxidant activity | [650] | |
| Ag/CS nanocomposite | UV–visible spectrum, TEM, SAED, SEM, FTIR, and XRD | 100 ml of 1 mM AgNO3 solution with turmeric extract | COVID-19 treatment | [263] | |
| Ag-NPs | XRD, UV–Vis spectroscopy, FTIR, EDX, and TEM | AgNO3 solution with turmeric powder | Antibacterial effect | [943] | |
| Ag-NPs | AFM, and UV–Vis spectrophotometer | 90 ml of 5 mM aqueous of Ag(NO3)2 with turmeric rhizome extract | Antimicrobial effect | [894] | |
| Ag-NPs | FTIR, UV–visible spectroscopy, XRD, and ZP | Green synthesis | Antimicrobial effect, anti-carcinogenic, and cytotoxic activity | [308] | |
| Ag-NPs | DSC, ZP, AFM, XRD, and FTIR | 100 ml of 1 mM of silver nitrate solution with ethanol extract of turmeric rhizome | Cytotoxic activity | [313] | |
| Ag-NPs | TEM, UV–visible spectrophotometer, and SEM | 10 µl of AgNO3 solution with turmeric extract | Cytotoxic activity | [847] | |
| Ag-NPs | XRD, SEM, UV–visible spectroscopy, FTIR, and HPLC | Green synthesis | Antimicrobial effect | [315] | |
| Silver-curcumin nanoconjugates (Ag-CurNCs) | UV irradiation | Green synthesis | Cytotoxic activity, antibacterial, and photostability evaluation | [7] | |
| Curcumin NPs | TEM, UV spectra, SEM, and DLS | 1 mL of dichloromethane and curcumin solution | MIC value, zone of inhibition evaluation | [150] | |
| Au-NPs | FTIR, UV–Vis spectroscopy, and TEM | Green synthesis | Cytotoxic activity, and stability evaluation | [886] | |
| Magnetic NPs | HRTEM, Mossbauer spectroscopy, FTIR spectroscopy, XRD, and magnetization measurement | NaBH4 solution in ammonia with turmeric rhizomes | NA | [297] | |
| Polymeric NPs | DLS, FTIR, and TEM | Curcumin–nanocurcumin with micellar aggregates of cross-linked and random copolymers of N-isopropyl acrylamide and N-vinyl-2-pyrrolidone and poly-monoacrylate | Cancer treatment | [154] | |
| Silk fibroin NPs (SFNs) | ZP, DLS, FESEM, TEM, ATR-FTIR, fluorescence spectroscopy, and UV–Vis spectrophotometric | SF-ionic liquid solution with curcumin | Free radical scavenging activity, and cytotoxic activity | [613] | |
| Nano silver | XRD, DLS, SEM, EDX, and TEM | Green synthesis | Antibacterial activity | [272] | |
| InP/ZnS quantum dots embedded mesoporous NPs | GC–MS, and CLSM | InP/ZnS quantum dots with turmeric crude extract | NA | [376] | |
| Nanocurcumin | TEM, DLS, and SLS | Ethanolic extract of curcumin powder solution | Antimicrobial effect | [833] | |
| Ag-NPs | TEM, UV–vis spectroscopy, FTIR, SEM, and EDS | Green synthesis | Antibacterial effect | [62] | |
| Biopolymer core–shell NPs | DSC, and FTIR | Curcumin with hydrophobic protein like zein and hydrophilic polysaccharide like pectin | NA | [375, 377] | |
| Curcumin NPs | PL, IR, FL, FTIR and FE-SEM | Polymer solution with ethanol solution of curcumin | Anti-carcinogenic effect | [348] | |
| Ag-NPs | UV–Vis spectroscopy | Extracellular biosynthesis | Antimicrobial effect | [608] | |
| Nano-encapsulated curcuminoids | Photoacoustic spectroscopy (PAS) | Polyvinyl pyrrolidone with curcuminoids | Anti-inflammatory activity, oxidative stress evaluation | [534] | |
| Goldmag NPs (CD-GMNs) | XRD, FTIR, TGA, DLS, TEM, and VSM | Hydroxypropyl-β-cyclodextrin with turmeric | NA | [526] | |
| Polymeric NPs | Liquid chromatography-tandem mass spectrometry (LC–MS/MS) | N-isopropyl acrylamide, acrylic acid, vinylpyrrolidone with curcumin solution | Tumor, and metastases treatment | [153] | |
| Garlic, turmeric, and zedoary NPs | SEM | Garlic, turmeric, and zedoary added to the water | Antibiotic, and antibacterial activities | [355] | |
| PLA-TPGS NPs | DLS, FTIR, UV–vis spectrophotometer and FESEM | 100 ml water solution of copolymer PLA-TPGS with 10 mg of curcumin | NA | [651] | |
| Cu-NPs | TEM, FTIR, SEM, and XRD | Copper solution with curcumin | Antifungal activity | [811] | |
| Ag-NPs | UV–visible spectroscopy | 90 mL of 1 mM silver nitrate distilled water with turmeric EO | Anti-inflammatory effect | [223] | |
| Poly-l-lysine mediated NP | Fluorescence microscope | Silica, trisodium citrate solution with curcumin | NA | [692] | |
| Au-NPs | FTIR, UV–Visible spectroscopy, and TEM | Rapid bio-synthesis | Radical scavenging activity, TPC, and TFC | [246] | |
| Chitosan phosphate NPs | TEM, and DLS | 6 g of orthophosphoric acid, 2 g of chitosan powder, 100 mL of 2% of acetic acid solution with curcumin solution | Antimicrobial effect, and cytotoxic activity | [236] | |
| ZnO-NPs | UV–visible spectroscopy, FTIR, XRD, and electron microscopy | Green synthesis | Antioxidant potential, anti-carcinogenic effect, trypan blue dye exclusion evaluation, MTT, LDH leakage analysis, colony developing, nitrite assessment, and caspase effects | [393] | |
| Ag-NPs | Fluorescence life time, UV–Visible absorption spectroscopy, and fluorescence emission spectroscopy | Ag solution with curcumin dye solution | NA | [291] | |
| ZnO-NPs | TEM, and XRD | Zinc solution with turmeric rhizome | Antibacterial effect, antifungal effect, antioxidant potential, and hydrogen peroxide evaluation | [392] | |
| Curcuma longa NPs | FTIR, XRD, SEM, and PL | Turmeric powder | Physicochemical, morphological, and optical parameters | [71] | |
| Nanocurcumin | TEM, FTIR, and SEM | Myristica acid, chitosan solution with curcumin | Cell viability | [466] | |
| Ag-NPs | FESEM, UV–Vis spectroscopy, PXRD, HRTEM, SAED, and ZP | Rapid green synthesis | NA | [452] | |
| Curcumin PLGA-encapsulated NPs | ZP, SEM, XRD, and DSC | 50 mg of PLGA with 5 mg of curcumin | Anti-plasmodial, toxicity evaluation, and cytotoxic activity | [167] | |
| Ag-NPs | UV–vis absorption spectrum, SEM, TEM, FTIR, X-ray photoelectron spectrometer, and DLS | 10 mM AgNO3 solution with 250 µl of 20 mM curcumin | Antiviral effect | [996] | |
| Apotransferrin NPs | Electron and atomic force microscopy, SEM, and TEM | 10 mg of apotransferrin in 100 µl of phosphate-buffered saline with 3.6 mg of curcumin | HIV-1 neutralization | [306] | |
| Au-NPs | UV–Vis spectroscopy, TEM, and DLS | Green synthesis | Anti-carcinogenic activity, cytotoxic activity, and anti-tumor effect | [273] | |
| Alginate-chitosan-pluronic composite NPs | SEM, AFM, and FTIR | Pluronic, alginate, chitosan with curcumin | Cytotoxic activity | [225] | |
| Niosomes NPs | TEM, and DLS | Niosomes solution with curcumin | Physical attributes, encapsulation efficiency, and drug release | [659] | |
| Ag-NPs | TLC, and HPLC | Silver salts with ethanol extract of turmeric rhizome | Anti-carcinogenic effect | [365] | |
| Ag-NPs | UV–Vis spectroscopy | Bio-synthesis | Antifungal activity | [1019] | |
| Curcumin niosomal NPs | ZP, PCS, and FTIR | Cholesterol, tween 60, span 60 with 50 mg of curcumin | NA | [1020] | |
| Au-NPs | UV–visible spectrophotometer | Bio-synthesis | TPC | [247] | |
| Curcumin loaded chitosan-alginate-sTPP NPs | AFM, and FESEM | Ultrasonic-mediated synthesis | Adsorption isotherm evaluation, cell culture analysis | [26] | |
| Chitosan NPs | PCS, and DLS | Chitosan solution with curcumin | Stability, and hemocompatibility evaluation | [960] | |
| Ag-NPs | TEM, and DLS | Green synthesis | Human pterygium-derived keratinocytes treatment | [890] | |
| Silica NPs | N2adsorption-desorption measurement, TEM, SEM, TGA, XRD,IR, FTIR, ZP, and DLS | PEGylated KIT-6 suspension with curcumin solution | Cytotoxic activity, and apoptosis evaluation | [546] | |
| PEG-albumin-curcumin NPs | FTIR, SEM, ZP, and DSC | Polymeric solution with curcumin | Breast cancer treatment | [927] | |
| Cassava starch NPs | DSC, TEM, FTIR, SEM, XRD, and fluorescence spectra | 5 g of cassava starch solution with curcumin | DPPH, cytotoxic activity, and cellular absorption evaluation | [96] | |
| Au-nonaspheres | FTIR, UV–VIS spectrophotometer, and TEM | 500 µl of 10 mM of Au solution with 46 mg of curcumin | Cytotoxic activity, superoxide anion development evaluation, nitric oxide release, MPO release analysis, SOD, GSH, and catalase assessment | [860, 862] | |
| ZnO-NPs, and Ag-NPs | UV–VIS spectrophotometry, FTIR, XRD, SEM, TEM, fluorescence spectra, DLS, ZP, EDX, and HRTEM | Zinc chloride solution, AgNO3 solution with curcumin solution | Antibacterial activity | [913] | |
| Ag-NPs | EDS, UV–visible spectrum, SEM, zeta sizer, FTIR, and TEM | Green synthesis | Antibacterial activity | [48] | |
| Ag-NPs | TEM, UV–visible spectroscopy, DLS, EDX, XRD, ZP, FESEM,EDAX, and FTIR | Green synthesis | Cytotoxic activity | [629] | |
| Poly-magnetic FeO-NPs | TEM | Polymer templated iron oxide solution with curcumin | Morphogenesis, and synergistic free radical scavenging activity | [478] | |
| Polycaprolactone (PCL) NPs | DSC, HPLC, and ZP | PCL solution with curcumin | NA | [936] | |
| Curcumin NPs | ESEM | Curcumin | Anticarcinogenic effect, and antibacterial activity | [15] | |
| Silica NPs | FTIR, XRD, UV–visible, HPLC, TEM, XRF, XRD, and AFM | 40 g of rice husk with turmeric powder | Anti-carcinogenic effect | [763] | |
| Ag-NPs | HPLC, tandem mass spectra | Pulse with dried rhizome of turmeric | Anti-carcinogenic activity | [602] | |
| Ag-NPs | UV absorption spectrometry, SEM, TEM, spectrofluorimetry, and Z scan | AgNO3 solution with curcumin | NA | [245] | |
| Ag-NPs | TEM, and UV–visible absorption spectroscopy | 75 ml of 1 mM of silver nitrate solution with 10 mM of curcumin | Antibacterial effect | [249] | |
| Au-NPs | Surface plasmon resonance spectrum, and SEM | Green synthesis | Nucleic acid evaluation | [691] | |
| Au-NPs | FTIR, UV–visible spectroscopy, and TEM | PVP with curcumin | Cytotoxic activity | [929] | |
| Starch NPs | TEM, SEM, UV–Vis spectrophotometer, and LSM | Starch solution with curcumin | Swelling evaluation | [198] | |
| Silver/silver chloride NPs | XRD, UV–vis, FTIR, SANS, SAXS, AFM, SEM, ZP, SAS, and EDS | Green synthesis | Antioxidant potential, antibacterial activity, cell viability, and hemocompatibility | [128] | |
| Curcumin-PBCANPs | TEM, DLS, ZP, fluorescence spectra, and HPLC | Chitosan solution, PBCA solution with curcumin | Anti-tumor activity | [262] | |
| PLGA-PEG-Fe3O4 NPs | VSM, SEM, and FTIR | Fe3O4 and curcumin encapsulated in PLGA-PEG co-polymer | Cytotoxic activity | [775] | |
| Au-NPs | TEM, and UV–Vis | 100 mL of miliq water solution with curcumin | Antimicrobial effect | [210] | |
| Polymeric NPs | SEM, UV–vis, and ZP | PVA solution with curcumin | Cellular toxicity evaluation | [779] | |
| Ag-NPs | FTIR, XRD, and SEM | Silver nitrate solution with curcumin | NA | [18] | |
| Chitosan tripolyphosphate NPs | ZP, PCS, AFM, TEM, FTIR, and UV spectrophotometer | TPP salt with curcumin | Antibiotic activity | [396] | |
| Fluorescent carbon dots | Fluorescence spectra, UV–spectrophotometer, TEM, and FTIR | Facile synthesis | NA | [848] | |
| Nitrogen-doped fluorescence carbon dots | Fluorescence spectra, TEM, XPS, XRD, UV–vis absorption spectra, and FTIR | Nitrogen-doped carbon dots powder with curcumin | NA | [916] | |
| Carbon nanodots | TEM, FTIR, EDAX and FESEM | Carbon soot with turmeric rhizome | Antibacterial activity, cell compatibility, and anti-carcinogenic effect | [206] | |
| Curcumin quantum dots | CLSM, TEM, SEM, UV–VIS, fluorescence, Raman spectroscopy, and ZP | 60 g of zirconia beads with curcumin solution | Antibiofilm effect | [860, 862] | |
| Curcumin quantum dots | TEM, and DLS | Curcumin quantum dots solution | Antimicrobial activity | [764] | |
| Bay leaves | FeO-NPs | XRD, UV–Visible, FTIR, SEM, TEM, and EDS | Green synthesis | Antimicrobial effect | [401] |
| ZnO-NPs | XRD, UV–Vis spectroscopy, FTIR, TEM, SEM, and EDX | Green synthesis | Antibacterial effect, cytotoxic activity | [968] | |
| TiO2-NPs | FTIR, UV–visible absorption spectroscopy, XRD, SEM, particle size, and ZP | Green synthesis | Antimicrobial effect, and antioxidant activity | [738] | |
| Zirconia NPs | XRD, UV–visible absorption spectrophotometer, FTIR, SEM, and DLS | Green biogenic synthesis | Antimicrobial activity | [191] | |
| Ag-NPs | XRD, UV–Visible spectroscopy, FTIR, SEM, and TEM | Green synthesis | TPC, TFC, and radical scavenging activity | [433] | |
| CuO-NPs | UV–vis spectroscopy, FTIR, SEM, and TEM | Green synthesis | Antioxidant activity, antibacterial effect, and dye decolorization capacity | [166] | |
| Ag-NPs | XRD, UV–visible spectroscopy, FTIR, SEM–EDX, and TEM | Green synthesis | Antimicrobial effect, and catalytic effect | [43] | |
| PLGA-NPs | SEM, UV–Vis spectrometry, DLS, and ZP | Polylactic-co-glycolic acid solution with bay leaves EO | Cancer treatment | [280] | |
| Ag-NPs | UV–Visible spectroscopy, SEM, ZSP, and FTIR | Green synthesis | Antimicrobial activity | [103] | |
| Saffron | Au-NPs | UV–vis spectroscopy, FTIR, SEM, EDX, AFM, and XRD | Green synthesis | Catalytic effect, antibacterial effect, enzyme retardation effect, sedative effect, carrageenan-induced paw edema evaluation, and severe toxicity analysis | [58] |
| ZnO-NPs, and CuO-NPs | UV–visible spectroscopy, SEM, XRD, EDS, and FTIR | Green synthesis | NA | [846] | |
| Ag-NPs | XRD, UV–vis spectrum, HRTEM, and FTIR | Green synthesis | Antibacterial activity | [115] | |
| Exogenous calcium NP | HPLC | Copper sulfate fungicide + acaricide with saffron | TFC, and antioxidant enzyme activity | [111] | |
| Ag-NPs | FESEM, UV–Vis, and FTIR | Green synthesis | Antibacterial effect | [1021] | |
| Ag-NPs | XRD, FESEM, EDX, and FTIR | Green synthesis | Antimicrobial effect | [462] | |
| Ag-NPs, and Au-NPs | XRD, UV–Vis spectroscopy, and TEM | Phyto-synthesis | NA | [105] | |
| Nano-bimetallic Ag/Pt alloy | Electron microscopy, UV–visible, infrared spectroscopy, FTIR, and XRD | Bio-synthesis | Antioxidant activity, antimicrobial, cytotoxic activity, and catalytic effect | [995] | |
| FeO-NPs | EDX, UV–visible spectroscopy, FTIR, XRD, and SEM | Biogenic synthesis | Antifungal activity | [54] | |
| Chitosan NPs | SEM, FTIR, XRD, WAXD, and DLS | Chitosan solution with water extract of saffron | Cytotoxic activity | [657] | |
| Nano-silica | HPLC | 2 mM of silica solution with corms of saffron | Antioxidant enzymes evaluation | [920] | |
| Chitosan-gum Arabic complex nanocarriers | FTIR, XRD, TEM, and ZP | Chitosan, gum Arabic solution with saffron extract | NA | [731] | |
| Cu-NPs | ZP, UV–visible absorption spectroscopy, particle size, FTIR, and electron microscope | Cu solution with ethanolic extract of saffron | Oxidative stress, and antioxidant activity | [97] | |
| Star anise | Ag-NPs | XRD, UV–Vis, FTIR, SEM, TEM, AFM, and particle size distribution | Green synthesis | Anti-diatom effect | [510] |
| Ag-NPs | XRD, TEM, SAED, EDS, UV–visible absorption spectra, FTIR, and Raman spectroscopy | Silver nitrate solution with star anise seed extract | NA | [544] | |
| PLGA-NPs | TEM, and SEM | Polylactic-co-glycolic acid with star anise | NA | [504] | |
| Onion | Ag-NPs | TEM, UV–vis spectroscopy, and SEM | Green synthesis | NA | [364] |
| Ag-NPs | UV–Vis spectral, EDS, and FTIR | Green synthesis | Cytotoxic activity | [646] | |
| ZnO-NPs | TEM, and SEM | ZnO solution with onion root | NA | [502] | |
| Au-NPs, and Ag-NPs | TEM, UV–Vis spectrophotometer, SEM–EDX, and XRD | AgNO3, and Au solution with dried onion root | NA | [233] | |
| Ag-NPs | ZP, TEM, DLS, LIBS, and ICP/OES | Silver solution with onion root | Cytotoxic, and genotoxic activities | [814] | |
| Ag-NPs | TEM, AFM, XRD, and FTIR | Biogenic synthesis | NA | [777] | |
| Cu-NPs | UV–Visible spectrum, ZP, TEM, and SAED | Bioinspired synthesis | NA | [635] | |
| Ag-NPs | UV–visible spectrophotometer, FTIR, TEM, and EDX | Green synthesis | NA | [998] | |
| Al2O3-NPs | FTIR, and CLSM | Al2O3 solution with onion root | Oxidative stress evaluation | [734] | |
| Cr2O3-NPs | CLSM | The water solution of Cr2O3 with onion root | Cytogenetic evaluation | [499] | |
| WO3-NPs | EDX, and TEM | Tungsten solution with onion | Cytotoxic activity, and genotoxic evaluation | [535] | |
| Ag-NPs | TEM | Silver ions, PVP with onion root | Cytological evaluation | [300] | |
| Ag-NPs | TEM, ZP, UV–vis | 200 mg/mL of the silver ion with onion root | Antioxidant activity, and LPO evaluation | [177] | |
| MgO-NPs | TEM, FESEM, DLS, and LDV | MgO solution with onion bulbs | ROS, H2O2 evaluation, hydroxyl radical, and lipid peroxidation analysis | [571] | |
| Au-NPs | TEM, UV–Vis spectroscopy, XRD, and SEM | Green synthesis | NA | [682] | |
| Au-nanorods | ZP, and electron microscopic | Au capped with CTAB or PEG, and onion | Cytogenetic analysis | ||
| Zn-NPs, and Ag-NPs | EDX, UV–vis spectra, TEM, particle size, PDI, and ZP | Silver ion, Zn solution with onion root tip | Genotoxicity evaluation | [9] | |
| Au-NPs | DLS, and TEM | Au solution with onion root | Toxicity analysis | [735, 736] | |
| TiO2-NPs | Optical, fluorescence, and confocal laser scanning microscopy | 12.5, 25, 50, and 100 µg/mL of TiO2 solution with onion root tip | Genotoxicity evaluation | [674] | |
| ZnO-NPs | UV–visible, XRD, FTIR, SEM–EDX and TEM | ZnO solution, ZnO bulk, zinc ions with onion root | NA | [28] | |
| TiO2-NPs, and ZnO-NPs | TEM, DLS, and LDV | TiO2, ZnO solution with onion root | Cell viability evaluation | [237] | |
| Chitosan capped Ag-NPs | AFM, and SEM | Chitosan coated AgNO3 solution with onion bulbs | NA | [699] | |
| ZnO-NPs | TEM, and ZP | Stock solution with onion bulbs | Cytotoxic activity, ROS generation, and genotoxicity evaluation | [900] | |
| Ag-NPs | FTIR, SEM/EDS, AFM, and ZP | Green synthesis | Anti-corrosion activity | [391] | |
| ZnO-NPs | FTIR, DLS, and FESEM | Green synthesis | Phytotoxicity evaluation | [604] | |
| TiO2-NPs, Al2O3-NPs, and CuO-NPs | UV–visible spectrophotometer, FTIR, XRD, SEM, EDX, and TEM | CuO, Al2O3, TiO2 solution with onion bulbs | Superoxide radicals, ROS formation, and SOD, CAT activities evaluation | [29] | |
| Ag-NPs | TEM | Ag stock solution with onion bulbs | Cytological, comet evaluation, lipid peroxidation, and hydrogen peroxide analysis | [350] | |
| Ag-NPs | SEM | Green synthesis | NA | [740] | |
| ZnO-NPs | UV–visible spectroscopy, XRD, FTIR, TEM, SEM, and EDAX | 90 ml of 1 mM zinc nitrate solution with onion bulbs extract | Cell viability, antioxidant activity, and TBARS evaluation | [988] | |
| Ag-NPs | UV–Visible spectra, FTIR, and SEM | Rapid green synthesis | Antioxidant machinery evaluation | [874] | |
| Ag-NPs | UV–Vis spectroscopy, and XRD | Green synthesis | NA | [796] | |
| Ag-NPs | FTIR, DLS, XRD, TEM, SAED, XPS, and UV–VIS | Green synthesis | Antibacterial, and cytotoxic activity | [834] | |
| Ag-NPs | UV–Visible spectroscopy, FTIR, and SEM | Green synthesis | Antioxidant potential, cell viability, and physical stability evaluation | [8] | |
| Dill | Chitosan nanomatrix | SEM, XRD, and FTIR | 1.5 g of chitosan solution with dill seed EO | TPC, and phytotoxicity evaluation | [226] |
| Nanosilver | UV–visible spectroscopy, XRD, FTIR, and SEM | Green synthesis | NA | [595] | |
| Fenugreek | Au-NPs | UV–Visible spectroscopy, TEM, XRD, FTIR, and SAED | Green synthesis | Catalytic effect | [95] |
| Ag-NPs | UV–vis spectroscopy, and SEM | Biogenic synthesis | Photocatalytic, and antibacterial effect | [100] | |
| Fe-NPs | UV–visible spectrometry, XRD, TGA/DTG, FTIR, and TEM | Bio-synthesis | Photocatalytic methyl orange dye degradation, and antibacterial activity | [722] | |
| TiO2-NPs | FTIR, UV, XRD, HRTEM, XRD, and HRSEM | Green synthesis | Antimicrobial activity | [896] | |
| ZnO-NPs | UV–Vis spectroscopy, UV–Visible diffuse reflectance spectroscopy, FTIR, XRD, SEM, PL, and EDX | Bio-synthesis | Photocatalytic activity | [63] | |
| Ag-NPs | UV–Vis spectroscopy, FTIR, SEM, HRTEM, and EDS | Bio-synthesis | NA | [404] | |
| Au-NPs | UV–Vis spectra, fluorescence, DLS, and TEM | 2.5 mg of HAuCl4 with water extract of fenugreek seed | Antioxidant activity | [324] | |
| Lanthanum NPs | SEM, and FTIR | Green synthesis | NA | [184] | |
| Ag-NPs | UV–VIS spectra, SEM, and XRD | 1 mM AgNO3 solution with aqueous extract of fenugreek seed | Antimicrobial activity | [708] | |
| Ag-NPs, and Au-NPs | TEM, and SEM | Green synthesis | Anti-diabetic effect | [974] | |
| Au-NPs | UV–Vis spectra, fluorescence, DLS, and TEM | Green synthesis | NA | [1000] | |
| ZnO-NPs | DLS, XRD, FTIR, SEM, and TEM | 50 mL of NH4OH solution with fenugreek seed | NA | [192] | |
| SnO2-NPs | XRD, SEM, TEM, FTIR, and UV–Visible spectrometer | Biogenic synthesis | Photocatalytic, and antimicrobial effect | [339] | |
| MnO-NPs | SEM, XRD, TEM, FTIR, EDX, and UV–Vis spectroscopy | Green synthesis | NA | [765] | |
| Organic–inorganic hybrid nanoflower | SEM, XRD, EDX, and FTIR | Green synthesis | Antimicrobial potential | [66] | |
| Fe-NPs, and Ag-NPs | TEM, XRD, EDX, and FTIR | Green synthesis | Antibacterial, and antioxidant potential | [241] | |
| Ag-NPs | TLC, and HPLC | Aqueous AgNO3 solution with methanolic extract of fenugreek seed | TFC, metal chelating evaluation, FRAP, α-amylase inhibition analysis, anti-inflammatory effect, antimicrobial activity assessment | [255] | |
| Au-NPs | UV–Vis spectrophotometry, and SEM | Bio-synthesis | NA | [302] | |
| Fe-NPs | XRD, TEM, UV–Visible spectroscopy, and FTIR | Green synthesis | NA | [78] | |
| ZnO-NPs | XRD, and TEM | Zinc acetate, citric acid with fenugreek seed | Seed germination, and seedling growth evaluation | [855] | |
| Ag-NPs | XRD, UV–Vis spectra, FTIR, and SEM | AgNO3 solution with water extract of fenugreek seed | Optical parameters evaluation | [588] | |
| Ag-NPs | XRD, and SEM | Green synthesis | Antibacterial activity | [31] | |
| Magnetic Fe-NPs | XRD, FTIR, SEM, and EDX | Green synthesis | NA | [883] | |
| Au-NPs | UV–Vis spectrophotometry, EDX, XRD, and FTIR | Bio-synthesis | NA | [301] | |
| Nano-hydroxyapatite chitosan | SEM | Chitosan solution with fenugreek seed polysaccharide | Bone tissue engineering evaluation | [1018] | |
| Lanthanum NPs | FTIR and SEM | 100 mg/ml of lanthanum solution with fenugreek seed extract | Osteosarcoma treatment | [219] | |
| Pd-NPs | SAED, UV–Visible spectroscopy, SEM, FTIR, and XRD | Green synthesis | Catalytic activity | [565] | |
| Ag-NPs | Particle size analyzer, UV–Visible spectroscopy, and XRD | Green synthesis | NA | [215] | |
| TiO2-NPs | XRD | TiO2 solution with alcoholic extract of fenugreek seed | NA | [358] | |
| Ag-NPs | SEM | Green synthesis | Antifungal activity | [277] | |
| Au-NPs | XRD, fluorescence spectra, SEM, TEM, EDAX, and FTIR | Facile green synthesis | NA | [79] | |
| Ag-NPs | FTIR, UV–Visible spectroscopy, and TEM | Green synthesis | Antibacterial, and antifungal effects | [69] | |
| ZnO-NPs | XRD, SEM, EDX, UV–V spectroscopy, and TEM | Biogenic synthesis | Anti-tumor capacity evaluation | [723] | |
| Nanosized TiO2 | DLS, FTIR, and infrared spectra | 6 µL of TiO2 stock suspension with fenugreek seed powder | NA | [600] | |
| Ag-NPs | XRD, and HRTEM | Green synthesis | NA | [380] | |
| Se-NPs | TEM, DLS, and FTIR | 1 mM of selenium dioxide with water extract of fenugreek seed | Tumoricidal activity evaluation | [267] | |
| Au–Pd bimetallic NPs | UV–Vis, SEM, TEM, XRD, XPS, and FTIR | Green synthesis | Catalytic evaluation | [566] | |
| ZnO-NPs, Pd-NPs, Cd-NPs, Ni-NPs, and Ag-NPs | EDS, UV, SEM, and FTIR | Green synthesis | Antimicrobial activity | [1007] | |
| Au-NPs | UV–Vis absorption spectroscopy, FTIR, and SEM | Green synthesis | NA | [276] | |
| Carbon quantum dots | SEM, UV–Vis absorption spectroscopy, TEM, TLC, Raman spectroscopy, EDS, FTIR, and XRP | Ultrafast synthesis | NA | [213] | |
| Nanosized TiO2 | XRD, FTIR, and SEM | Photo-synthesis | Seedling growth, biomass, TFC evaluation, TPC, total soluble protein content analysis, antioxidant enzyme activity, and lipid peroxidation assessment | [601] | |
| Carbon nanodots | Fluorescence spectroscopy, and CFM | Carbon synthesis | NA | [978] | |
| CeO2-NPs | SEM, XRD, FTIR, TEM, Raman spectroscopy, and XRP | Sol–gel solution with fenugreek extract | Fluorescence sensing of picric acid evaluation | [759] | |
| Au-NPs | TEM | Au solution with fenugreek hydrogel-agarose matrix | Carbamates evaluation | [440] | |
| Graphene quantum dots | FTIR, SEM, AFM, and fluorescence microscopy | Fenugreek β-amylase with aminopropyltriethoxysilane, and glutaraldehyde | Biochemical, thermodynamic, and kinetic evaluation | [23] | |
| Asafoetida | Ag-NPs | FTIR, and TEM | Bio-synthesis | NA | [793] |
| Ag-NPs | SEM, FTIR, and XRD | Green synthesis | Larvicidal effect | [804] | |
| Ag-NPs | EDX, FESEM, XRD, UV–visible spectroscopy, and FTIR | Green synthesis | Antibacterial activity | [11] | |
| Chili | Ag-NPs | TEM, UV–Vis spectroscopy, SEM, EDX, XRD, and FTIR | 0.1 M silver nitrate solution with water extract of chili, garlic, and ginger | Antimicrobial activity, and antioxidant activity | [668] |
| Allspice | Au-NPs | TEM, UV–Vis absorption spectroscopy, FTIR, and XRD | Green synthesis | Antibacterial activity, photocatalyst, and antioxidant activity evaluation | [288] |
| Ag-NPs | AFM, UV–Vis spectroscopy | Green synthesis | NA | [317] | |
| CuO-NPs | XRD, UV–Visible, FTIR, SEM–EDS, TEM, and TGA-DTA | Green synthesis | Cytotoxicity, anti-carcinogenic activity, antioxidant potential, and anti-diabetic effect evaluation | [703] | |
| Au-NPs | FTIR, XRD, UV–Vis absorption spectroscopy, Raman spectroscopy, and electron microscopy | Green synthesis | NA | [457] | |
| Ag-NPs | UV–visible spectroscopy, XRD, SEM, TEM, EDX, GC–MS, FTIR and LC–MS | Green synthesis | Larvicidal activity evaluation | [495, 498] | |
| Kokam | Ag-NPs | FESEM, UV–vis absorption spectroscopy, FTIR, EDS, XRD, TEM, and SAED | Biogenic synthesis | Antioxidant, and antibacterial effect | [792] |
| Au-NPs | UV–Vis spectroscopy, XRD, FESEM, and FTIR | Bio-synthesis | NA | [488] | |
| Au-NPs | TEM, UV–spectroscopy, and SEM | Biogenic synthesis | Anti-carcinogenic effect | [132] | |
| Au-NPs | FTIR, UV–visible spectroscopy, EDS, XRD, SAED, and TEM | Green synthesis | Antioxidant potential, photoluminescent, and photocatalytic effect evaluation | [240] | |
| Ag-NPs | UV–visible spectroscopy, FTIR, XRD, and TEM | Phytobiological synthesis | Antibacterial activity | [905] | |
| Au-NPs | UV–Vis spectroscopy, and FESEM | Au solution with kokum fruit extract | NA | [489] | |
| Greater galangal | Ag-NPs | XRD, FTIR, UV–vis spectroscopy, SEM, TEM, SAED, and EDX | Bio-synthesis | Antibacterial effect | [384] |
| Sweet flag | BFNPs | XRD, SEM, Raman spectroscopy, and IR | Green synthesis | Antifungal activity | [928] |
| Au-NPs | UV–Visible spectral, XRD, FTIR, SEM, HRTEM, and EDAX | Green synthesis | UV-blocking and antibacterial activity | [307] | |
| ZnONPs | XRD, SEM, and FTIR | Green synthesis | Antioxidant activity | [717] | |
| CeO2-NPs | Confocal, light, and electron microscopy | Green synthesis | Antibiofilm effect | [64] | |
| ZnONPs | EDX, UV spectrophotometer, SEM, FTIR, and TEM | 1 mM of zinc acetate solution with aqueous extract of sweet flag | Antimicrobial effect, and cytotoxic activity | [954] | |
| Ag-NPs | UV–Visible spectra | 1 mm of AgNO3 solution with methanolic extract of Agaricus bisporus, and sweet flag rhizome | Antibacterial activity | [704] | |
| Cu-NPs | FTIR, UV–Visible absorption spectrometer, SEM, and TEM | Green synthesis | NA | [869] | |
| Ag-NPs | TEM, FESEM, EDAX, and FTIR | Green synthesis | Antibacterial activity, and cytotoxic activity | [10] | |
| Ag-NPs | TEM, UV spectrophotometer, and SEM | Green synthesis | Antioxidant potential | [705] | |
| Au-NPs | UV spectroscopic, FTIR, SEM, and TEM | 3 mM of gold chloride solution with water extract of sweet flag | Antimicrobial activity | [820] | |
| Ag-NPs | DLS, UV–visible spectroscopy, SEM, EDX, and FTIR | Green synthesis | Antioxidant activity, antibacterial effect, and cytotoxic activity | [637] | |
| ZnO-NPs | UV–visible spectrophotometric | Biogenical synthesis | Sun protection factor evaluation | [299] | |
| Hydroxyapatite NPs | TEM, XRD, SEM, and HRTEM | 0.6 M Na2HPO4, 1 M CaCl2 with water extract of sweet flag rhizome | Anti-acetylcholinesterase retardation, computational pharmacokinetics evaluation | [711] |
Zinc Oxide Nanoparticles
The pure green cardamoms have been cleaned many times and air dried. To produce ZnONPs, 0.2 M zinc acetate solution has been added to 2.2 g of cardamom powder dissolved in 50 ml deionized water and stirred on a magnetic stirrer till dissolve. At 800 C, 5 g of green cardamoms have been boiled in deionized water for 20 min to obtain the purified extract. The produced nanoparticle has been analyzed for its antimicrobial, and anticancer activity [676]
10 mg of fennel seed powder has been soaked in 50 mL of deionized water for 24 h. To the extract 250 mL of deionized water, 41.3 mg of ZnO has been added, the mixture has been placed in an incubator at 990 C. Dark yellow-colored ZnONPs has been formed. The purified ZnONPs has been analyzed for its cytotoxic activity and antimicrobial activity [61]P. anisum and fennel seeds powdered have been dissolved in 800 mL of distilled water and stored in a refrigerator for 1 day. The extract has been used to assess the liver functionality test [127]
5 g of coriander seeds or leaves have been mixed with zinc acetate solution to synthesize ZnO-NPs. The ZnO-NPs have been evaluated for its TFC and TPC content [867]. 90 mL, 1 mM zinc oxide solution has been added into 10 mL of Coriander oleoresin extract to produce ZnO-NPs. The ZnO-NPs have been analyzed for its antibacterial effect, and cytotoxic activity [695]. Water extract of turmeric rhizome has been mixed with Zn acetate solution to prepare ZnONPs. The ZnONPs have been assessed for its antioxidant potential, anti-carcinogenic effect, trypan blue dye exclusion, colony developing, nitrite, and caspase effects [393]
To synthesize ZnONPs, NH4OH, and zinc acetate dehydrates have been added to fenugreek seeds. The prepared ZnONPs have been measured for its capability of seedling growth [192]
The Pure sweet flag rhizome has been mixed with 0.1% of mercuric chloride, and 1 mM of zinc acetate solution to form ZnONPs. The ZnONPs have been estimated for its antimicrobial effect, and cytotoxicity activity [954]
Gold Nanoparticles
To prepare AuNPs, 30 mL of 2.5 × 10–4 M boiling HAuCl4 solution was mixed with 1 mL cardamom seed extract. The synthesized AuNPs have been evaluated for their anticarcinogenic effect, antioxidant, and antibacterial activities [733]
To synthesize Au-NPs, methanol, and alcoholic extracts of black cumin have been mixed with various proportions of 1 mM HAuCl4 The prepared Au-NPs have been analyzed for its urease retardation activity, enzyme retardation, antifungal, antibacterial activities, xanthine oxidase effect, and carbonic anhydrase function [117]
Water extract of 25 mg of cinnamon has been added to 100 µL of 0.1 M NaAuCl4 solution to synthesize AuNPs. The AuNPs have been assessed for their cytotoxicity activity and prostate cancer diminishing activity [186]
30 mg of turmeric rhizome has been added with 2 mL of 10 mM NaOH solution and 1 mL of 1 mM HAuCl4 solution to produce AuNPs. The AuNPs have been measured for their cytotoxic activity [886]
The water extract of fenugreek seeds has been mixed with 2.5 mg of HAuCl4 solution to form AuNPs. The AuNPs have been evaluated for their antioxidant activity [324]
The water extract of sweet flag rhizomes has been mixed with gold chloride solution to synthesize the greenish-yellow to pale pink Acorus calamus gold nanoparticles. The Acorus calamus gold nanoparticles have been evaluated for its antimicrobial activity [820]
Silver Nanoparticles
40 mL of aqueous silver nitrate solution (0.14, 0.16, 0.18, 0.20, and 0.22 g) have been mixed with 10 ml of cardamom seed extract to synthesize the AgNPs. The AgNPs have been evaluated for their antibacterial effects [835]
90 ml of silver nitrate has been mixed with 10 ml of fennel seed extract to produce AgNPs, which have been analyzed for its antibacterial effect [409]
Clove extract- AgNPs have been synthesized with clove bud powder and aqueous 1 mM AgNO3 solution. The Clove extract- AgNPs have been assessed for its antiviral effect, micro hemagglutination, and cytotoxicity activity [592]
10 ml of the fennel seed extract has been mixed with 90 ml of 1 mM silver nitrate solution to prepare AgNPs. The AgNPs have been measured for its anti-viral activity against influenza virus subtype H7N3 and cytotoxicity [292]
To synthesize of AgNPs, AgNO3 has been mixed with 100 mL cassia bark aqueous extract. The AgNPs have been estimated for its capacity to reduce blood glucose level, improve the histopathology of the liver, and kidney tissue conditions [483]
10 ml of black pepper has been mixed with 90 ml of 1 mM silver nitrate solution to form AgNPs. The AgNPs have been evaluated for its anti-carcinogenic effect [486]
To synthesize silver nanoparticles, 100 ml of 0.1 N AgNO3 solution has been mixed with 50 ml of coriander seed extract to evaluate the antimicrobial effects of AgNPs [648]. 10 mL of 0.5 M of aqueous AgNO3 solution has been added into coriander extract to synthesize AgNPs to observe its antibacterial activity [543]. 100 ml of 1 mM AgNO3 solution has been mixed with 20 ml of coriander seed extract to synthesize AgNPs for its antibacterial activity [813]. 99.5 ml of water and 0.5 ml of Coriander oleoresin have been mixed with 1 M of silver nitrate solution to synthesize AgNPs, which have been evaluated for its antioxidant potential [319]. 10 g of coriander seed powder has been mixed with 45 mL of 1 × 10–3 M of AgNO3 solution to synthesize AgNPs to observe its antimicrobial effect, anti-biofilm activity and plasmid curing effect [349]
Nutmeg seed and bark were cleaned continuously with deionized water, dried, and powdered. For biosynthesis of silver nanoparticles, 1 mM of silver nitrate solution has been mixed with 10 g of nutmeg seed powder, which has been done to evaluate the antibacterial activity of AgNPs [408]
Water extract obtained from 5 g of black mustard seed has been mixed with 1 mM of silver nitrate solution. The AgNPs have been evaluated for its antimicrobial effect [680]
AgNO3 solution has been added to turmeric powder to prepare AgNPs and then the AgNPs have been evaluated for its antibacterial effect [943]. 10 ml of curcumin extract has been added to 100 ml of 1 mM silver nitrate to synthesize yellow to yellowish-brown color silver nanoparticles. The nanoparticles have been evaluated for its cytotoxic activity [313]. 6.8 g of curcumin powder or organic turmeric powder has been added to 100 mL of Milli-Q water to make water extract of turmeric powder. To synthesize AgNPs, 2 mL of aqueous extract of turmeric powder has been added to 8 mL of 1 mM AgNO3 solution. The AgNPs have been evaluated for its antibacterial effect [62]. To prepare AgNPs, 1 mM of silver nitrate has been mixed with turmeric rhizome extract. The nanoparticles have been evaluated for its antimicrobial effects [608]. 250 µL of 20 mM curcumin has been dissolved in DMSO, and 2.5 mL AgNO3 to synthesize cAgNPs. The cAgNPs have been evaluated for its antiviral activities [996]. To decrease the curcumin particle size, the filtrate was sonicated in ultrasonicator at 20 kHz frequency for around 20 min. To prepare silver nanoparticles, 20 g of the turmeric powder has been mixed with silver salts. The synthesized AgNPs has been estimated for its anti-carcinogenic effect of AgNPs [365]. Curcumin solution has been added to 75 ml of 1 mM of silver nitrate solution to synthesize AgNPs to observe its antibacterial effect [249]. 1 mL of turmeric extract has been added to 100 mL of 1 mM AgNO3 water solution to produce AgNPs, which have been evaluated for their antioxidant potential, antibacterial activity, cell viability, and hemocompatibility [128]
10 mL of the water extract of bay leaves has been mixed with 50 mL solution of AgNO3 to prepare AgNPs. The AgNPs have been analyzed for its radical scavenging activity [433]
1 mM AgNO3 has been added to 10 ml of the water extract of fenugreek seed to prepare silver nanoparticles, and its antimicrobial activity has been measured [708]
Selenium Nanoparticles
173 mg of sodium selenite salt has been homogenized in 90 mL of deionized water. The mixture has been mixed with 10 mL of fennel seed extract to synthesize 100 mL of selenium nanoparticles to observe its toxicity, and the capability to prevent arthritis [84]
Water extract of fenugreek seed has been added with 1 mM selenium dioxide solution to synthesize SeNPs. The SeNPs have been evaluated for their tumoricidal activity [267]
Carbon Dots Nanoparticles
0.5 g of fine clove powder has been dissolved in 30 mL of water (hydrothermal synthesis) to synthesize carbon dot nanoparticles. The nanoparticles have been evaluated for its antioxidant effect, catalytic, and cytotoxicity effect [495, 498]
Quantum Dot Nanoparticles
600 mg of curcumin has been added to 10 g of zirconia beads in 15 ml of ethanol to synthesis curcumin quantum dots and then tested it for its antimicrobial activity [764]
Fennel seed has been passed through single-step thermal decomposition method to prepare mono-disperse carbon quantum dots. The quantum dots have been analyzed for its photoluminescence [214]
To synthesize graphene quantum dots, fenugreek β-amylase, glutaraldehyde, and aminopropyltriethoxysilane have been used. The graphene quantum dots have been assessed for its biochemical, thermodynamic, and kinetic properties [23]
Polymeric Nanoparticles
To synthesize gelatin nanoparticles, 200–500 mg of gelatin has been mixed with 150 g of cardamom powder. The gelatin nanoparticles have been evaluated to observe its capacity to prevent Glioblastoma disease [649]
The equal amounts of aqueous tripolyphosphate solution and chitosan solution have been added together to ethanol extract of 50 g of fennel seed to synthesize chitosan nanoparticles and then the nanoparticles are tested for its peroxide value, total volatile nitrogen, microbial activity, and sensory attributes [549]
To synthesize chitosan nanoparticles, the water extract of 30 g of clove has been mixed with 1 gm of chitosan solution. After that, 0.5 g of sodium tripolyphosphate has been poured into the solution. The chitosan nanoparticles have been analyzed for its biochemical, and insecticidal effect [271]
2% of shellac powder has been mixed with ethanol extract of 10 g of cassia bark to synthesize colloidal nanoparticles and then the nanoparticles have been tested for its encapsulation efficiency, loading potential, TPC, antioxidant activity [623]. Ethanol extract of 100 g of oregano and cassia bark have been mixed with chitosan solution to synthesize core/shell nanoparticles and then the nanoparticles have been examined for its cytotoxic effect, and mitochondrial transmembrane capacity [423]
NaBH4 solution has been mixed with turmeric powder to synthesize magnetic nanoparticles and then the nanoparticles have been tested for its capacity to obtain curcuminoids, and free phenolic acids [550]. To synthesize curcumin silk fibroin nanoparticles, 0.5 g of silk fibroin (SF) has been mixed with an SF-ionic liquid solution. The curcumin silk fibroin nanoparticles have been evaluated for its free radical scavenging activity and cytotoxic activity [613]. To synthesize nanocurcumin, ethanol extract of curcumin powder has been dissolved into water. The nanocurcumin has been assessed for its antimicrobial effect [833]. To prepare curcumin-loaded chitosan phosphate nanoparticles, the curcumin has been mixed with chitosan solution. The antimicrobial effect, and cytotoxic activity of curcumin-loaded chitosan phosphate nanoparticles have been analyzed [236]. To synthesize curcumin-encapsulated nanoparticles, chitosan solution, curcumin, and TPP have been mixed together. The curcumin-encapsulated nanoparticles have been measured for their stability and hemocompatibility [960]. 5 g of cassava starch has been mixed with 50 mL of 3.16 M sulphuric acid solution to produce starch nanoparticles. The cytotoxic activity, antioxidant activity, and cellular absorption of the synthesized nanoparticles have been observed [96]. To synthesize PBCANPs, 0.1% of chitosan has been dissolved in HCl,0.01% of curcumin powder and 100 µL of BCA monomer have been added to the solution. The PBCANPs have been evaluated for their anti-tumor activity [262]
Conclusion
Spices are gaining interest in terms of functional food formulation, as a source of new drug molecule, in biotechnological industries as a source of novel bioactive components and effective source of functional nanoparticles. Phenolic acids, flavonoids, organic acids, alkaloids, tannins, and health-beneficial pigments are found in spices abundantly. Synthetic antioxidants may possess detrimental health effect, which can be replaced with natural antioxidants that are present in different spices. The application of synthetic and costly antioxidants is reduced which provides health problems and toxicity. Spices are produced mostly in the economically backward countries or in the developing countries; therefore, it is obvious that spices are not only significant in terms of human health development, or in the field of biotechnology, it is also an aid to improve the economy of the countries. The 24 spices expressed in this article are achieving worth financially and improving the lifestyle of the stakeholders associated with the harvesting and post-harvest administration of the spices. In this article, 24 spices have been covered; it has been found that most of the spices have been explored for their bioactive, health-beneficial effects, and functional food development potential, whereas some of them are yet to be searched for their food fortification potential, e.g., sweet flag and galangal have not been explored for their food fortification potential. Most of the spices considered in this article are seasonal in nature. Therefore, adequate preservation methods need to be searched to overcome the issue of seasonal availability of spices described. Spices may be used for a source of natural food pigment. The inedible portions of the spices may be utilized as a source of natural colorant and novel bioactive compounds. Therefore, the wastage parts or inedible portions of the spices can be utilized as a source of natural pigment, and bioactive extraction. Carbon dot-, quantum dot-, metal-, and polymer-based nanoparticles are prepared with several spices like asafoetida, cardamom, black jeera, fennel, fenugreek, poppy, clove, turmeric, bay leaves, sweet flag, etc., using different methods such as green or biogenic synthesis. These nanoparticles possess different health-beneficial potentials like antioxidant, anti-carcinogenic, anti-diabetic, enzyme retardation effect, and antimicrobial activity. The nanoparticles have also been analyzed for their cytotoxic effect. The synthesized nanoparticles possess a significant role in environmental pollution management as some of the nanoparticles hold dye decolorization activity
Author Contribution
D.M, T.S., and R.C. contributed to conceptualization; D.M, T.S., and R.C. helped in writing, reviewing, and editing; R.C. provided resources. All the authors read and approved the final version of the manuscript
Data Availability
All relevant data are within the paper
Code Availability
Not applicable
Declarations
Ethical Approval
Not applicable
Consent to Participate
The authors have agreed to participate in the publication of the paper
Consent to Publish
All authors have agreed to publish the paper
Conflicts of Interest
The authors declare no conflict of interest
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Contributor Information
Tanmay Sarkar, Email: tanmays468@gmail.com.
Runu Chakraborty, Email: crunu@hotmail.com.
References
- 1.Abbasi B, Daniali M, Ramezani H, Derakhshande M, Ghiasvand R. The effect of Bunium persicum on gastrointestinal symptoms and inflammatory mediators in irritable bowel syndrome patients. Islamic Azad University Science and Research Branch. 2018;1(1):43–46. [Google Scholar]
- 2.Abbassi A, Mahmoudi H, Zaouali W, M’Rabet Y, Casabianca H, Hosni K. Enzyme-aided release of bioactive compounds from coriander (Coriandrum sativum L.) seeds and their residue by-products and evaluation of their antioxidant activity. Journal of Food Science and Technology. 2018;55(8):3065–3076. doi: 10.1007/s13197-018-3229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abd El-Razek MH, Ohta S, Ahmed AA, Hirata T. Sesquiterpene coumarins from the roots of Ferula assa-foetida. Phytochemistry. 2001;58(8):1289–1295. doi: 10.1016/S0031-9422(01)00324-7. [DOI] [PubMed] [Google Scholar]
- 4.Abd Elhamid EM, Sadak MS, Tawfik MM. Physiological response of Fenugreek plant to the application of proline under different water regimes. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2016;7(3):580–594. [Google Scholar]
- 5.Abdallah, E. M. (2018). Black pepper fruit (Piper nigrum L.) as antibacterial agent: A mini-reviewJournal of Bacteriology & Mycology: Open Access, 6(2)10.15406/jbmoa.2018.06.00192
- 6.Abdel-Aziz ME, Morsy NFS. Keeping quality of frozen beef patties by marjoram and clove essential oils. Journal of Food Processing and Preservation. 2015;39(6):956–965. doi: 10.1111/jfpp.12309. [DOI] [Google Scholar]
- 7.Abdellah AM, Sliem MA, Bakr M, Amin RM. Green synthesis and biological activity of silver-curcumin nanoconjugates. Future Medicinal Chemistry. 2018;10(22):2577–2588. doi: 10.4155/fmc-2018-0152. [DOI] [PubMed] [Google Scholar]
- 8.Abdellatif, A. A. H., Mahmood, A., Alsharidah, M., Mohammed, H. A., Alenize, S. K., Bouazzaoui, A., Al Rugaie, O., Alnuqaydan, A. M., Ahmad, R., Vaali-Mohammad, M. A., Alfayez, M., Traiki, T. B., Al-Regaiey, K. A., Ali, A. T., Hassan, Y. A. H., & Abdulla, M. H. (2022). Bioactivities of the green synthesized silver nanoparticles reduced using Allium cepa L aqueous extracts induced apoptosis in colorectal cancer cell linesJournal of Nanomaterials,202210.1155/2022/1746817
- 9.Abdelsalam NR, Fouda MMG, Abdel-Megeed A, Ajarem J, Allam AA, El-Naggar ME. Assessment of silver nanoparticles decorated starch and commercial zinc nanoparticles with respect to their genotoxicity on onion. International Journal of Biological Macromolecules. 2019;133:1008–1018. doi: 10.1016/j.ijbiomac.2019.04.134. [DOI] [PubMed] [Google Scholar]
- 10.Abirami, B., Silva, D., Abinaya, D., Praveenkumar, D., Vinothkumar, A., Saravanan, G., & Achiraman, S. (2017). Biosynthesis of silver nanoparticles derived Acorus calamus rhizome extract and their biomedical applicationJournal of Advanced Applied Scientific Research
- 11.Abootalebi, S. N., Mousavi, S. M., Hashemi, S. A., Shorafa, E., Omidifar, N., & Gholami, A. (2021). Antibacterial effects of green-synthesized silver nanoparticles using Ferula asafoetida against acinetobacter baumannii isolated from the hospital environment and assessment of their cytotoxicity on the human cell linesJournal of Nanomaterials,202110.1155/2021/6676555
- 12.Aboud, N. S., Hussein, N. J., & Al-Musawi, H. K. (2014). Studying the therapeutic effect of watery &alcoholic extracts extract of Apium graveolens leaves on urinary tract infections caused by Staphylococcus aureus in rabbitsJournal of Babylon University, 22(22), 779–788https://www.researchgate.net/publication/334657403
- 13.Achuthan CR, Padikkala J. Hypolipidemic effect of Alpinia Galanga (Rasna) and Kaempferia Galanga (Kachoori) Indian Journal of Clinical Biochemistry. 1997;12(1):55–58. doi: 10.1007/BF02867956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acuña UM, Atha DE, Ma J, Nee MH, Kennelly EJ. Antioxidant capacities of ten edible North American plants. Phytotherapy Research. 2002;16(1):63–65. doi: 10.1002/ptr.1031. [DOI] [PubMed] [Google Scholar]
- 15.Adahoun MA, Al-Akhras MAH, Jaafar MS, Bououdina M. Enhanced anti-cancer and antimicrobial activities of curcumin nanoparticles. Artificial Cells, Nanomedicine and Biotechnology. 2017;45(1):98–107. doi: 10.3109/21691401.2015.1129628. [DOI] [PubMed] [Google Scholar]
- 16.Adegoke GO, Makinde O, Falade KO, Uzo-Peters PI. Extraction and characterization of antioxidants from framomum melegueta and Xylopia aethiopica. European Food Research and Technology. 2017;216(6):526–528. doi: 10.1007/s00217-003-0683-6. [DOI] [Google Scholar]
- 17.Adelekan, E. O., Adegunwa, M. O., Adebowale, A. A., Bakare, H. A., & Alamu, E. O. (2019). Quality evaluation of snack produced from black pepper (Piper nigrum L.), plantain (Musa paradisiaca L.), and tigernut (Cyperus esculentus L.) flour blendsCogent Food and Agriculture, 5(1)10.1080/23311932.2019.1614285
- 18.Adibzadeh P, Motakef-kazemi N. Preparation and characterization of curcumin-silver nanoparticle and evaluation of the effect of poly ethylene glycol and temperature. Journal of Nanoanalysis. 2018;5(3):156–162. [Google Scholar]
- 19.Afshari, M., Pazoki, A., & Sadeghipour, O. (2022). Biochemical changes of coriander ( Coriandrum sativum L. ) plants under drought stress and foliar application of salicylic acid and silicon nanoparticlesJournal of Medicinal Plants and By-Products
- 20.Agarwal R, Gupta SK, Agrawal SS, Srivastava S, Saxena R. Oculohypotensive effects of Foeniculum vulgare in experimental models of glaucoma. Indian Journal of Physiology and Pharmacology. 2008;52(1):77–83. [PubMed] [Google Scholar]
- 21.Agbogidi, O. M., & Azagbaekwe, O. P. (2013). Health and Nutritional Benefits of Nut Meg (Mystica Fragrans Houtt.)Scientia Agriculturae, 1(2), 40–44http://pscipub.com/Journals/Data/JList/ScientiaAgriculturae/2013/Volume1/Issue2/2.pdf
- 22.Agrawal S, Yallatikar T, Gurjar P. Brassica nigra: Ethnopharmacological review of a routinely used condiment. Current Drug Discovery Technologies. 2019;16:40–47. doi: 10.2174/1570163815666180308143400. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal DC, Yadav A, Kesarwani R, Srivastava ON, Kayastha AM. Immobilization of fenugreek β-amylase onto functionalized graphene quantum dots (GQDs) using Box-Behnken design: Its biochemical, thermodynamic and kinetic studies. International Journal of Biological Macromolecules. 2020;144:170–182. doi: 10.1016/j.ijbiomac.2019.12.033. [DOI] [PubMed] [Google Scholar]
- 24.Aguilar-González AE, Palou E, López-Malo A. Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innovative Food Science and Emerging Technologies. 2015;32(September):181–185. doi: 10.1016/j.ifset.2015.09.003. [DOI] [Google Scholar]
- 25.Ahmad A, Husain A, Mujeeb M, Khan SA, Alhadrami HAA, Bhandari A. Quantification of total phenol, flavonoid content and pharmacognostical evaluation including HPTLC fingerprinting for the standardization of Piper nigrum Linn fruits. Asian Pacific Journal of Tropical Biomedicine. 2015;5(2):101–107. doi: 10.1016/S2221-1691(15)30152-0. [DOI] [Google Scholar]
- 26.Ahmadi F, Ghasemi-Kasman M, Ghasemi S, Tabari MG, Pourbagher R, Kazemi S, Alinejad-Mir A. Induction of apoptosis in HeLa cancer cells by an ultrasonic-mediated synthesis of curcumin-loaded chitosan-alginate-STPP nanoparticles. International Journal of Nanomedicine. 2017;12:8545–8556. doi: 10.2147/IJN.S146516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed ASh, Uddin AQ, Kumar SA, Parveen J. Evaluation of in vitro antidiabetic and antioxidant characterizations of Elettaria cardamomum (L.) Maton (Zingiberaceae), Piper cubeba L. f. (Piperaceae), and Plumeria rubra L. (Apocynaceae) Pakistan Journal of Pharmaceutical Sciences. 2017;30(1):113–126. [PubMed] [Google Scholar]
- 28.Ahmed B, Dwivedi S, Abdin MZ, Azam A, Al-Shaeri M, Khan MS, Saquib Q, Al-Khedhairy AA, Musarrat J. Mitochondrial and chromosomal damage induced by oxidative stress in Zn2+ ions, ZnO-bulk and ZnO-NPs treated Allium cepa roots. Scientific Reports. 2017;7(January):1–14. doi: 10.1038/srep40685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed B, Shahid M, Khan MS, Musarrat J. Chromosomal aberrations, cell suppression and oxidative stress generation induced by metal oxide nanoparticles in onion (Allium cepa) bulb. Metallomics. 2018;10(9):1315–1327. doi: 10.1039/c8mt00093j. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed LI, Ibrahim N, Abdel-Salam AB, Fahim KM. Potential application of ginger, clove and thyme essential oils to improve soft cheese microbial safety and sensory characteristics. Food Bioscience. 2021;42:101177. doi: 10.1016/j.fbio.2021.101177. [DOI] [Google Scholar]
- 31.Ahmed RM, Alshafei N, Mohamed HA. Green method of synthesis silver nanoparticles as using fenugreek seeds extract ( Trigonella foenum-graecum ) and its application as antibacterial agent. University of Bahri Journal of Veterinary Sciences. 2022;1(1):8–14. [Google Scholar]
- 32.Ahuja, K, D, K., Robertson, I, K., & Geraghty, D, P., Ball, M, J. (2006)Effects of chili consumption on postprandial glucose, insulin, and energy metabolism10.1093/ajcn/84.1.63 [DOI] [PubMed]
- 33.Airaodion AI, Akaninyene IU, Ngwogu KO, Ekenjoku JA, Ngwogu AC. Hypolipidaemic and antidiabetic potency of Allium cepa (onions) bulb in alloxan-induced diabetic rats. Acta Scientifci Nutritional Health. 2020;4(3):01–08. doi: 10.31080/asnh.2020.04.0648. [DOI] [Google Scholar]
- 34.Ajayi OA, Segun-Taiwo BA. Effect of turmeric (Curcuma longa) spice on nutrititional, microbial content of moin-moin and efficacy of spice against selected foodborne pathogens. African Journal of Agriculture and Food Science. 2021;4(3):28–40. doi: 10.52589/ajafs-8vinvy88. [DOI] [Google Scholar]
- 35.Ajenu, C., & Imhontu, E. (2021). Comparative evaluation of the proximate and micro-nutritional benefits of pawpaw, carrots, turmeric and coconutJournal of Food Technology & Nutrition Sciences, 1–510.47363/jftns/2021(3)124
- 36.Ajitha, B., Reddy, Y. A. K., Lee, Y., Kim, M. J., & Ahn, C. W. (2019). Biomimetic synthesis of silver nanoparticles using Syzygium aromaticum (clove) extract: Catalytic and antimicrobial effectsApplied Organometallic Chemistry, 33(5)10.1002/aoc.4867
- 37.Akcan T, Estévez M, Serdaroğlu M. Antioxidant protection of cooked meatballs during frozen storage by whey protein edible films with phytochemicals from Laurus nobilis L. and Salvia officinalis. LWT - Food Science and Technology. 2017;77:323–331. doi: 10.1016/j.lwt.2016.11.051. [DOI] [Google Scholar]
- 38.Akhlaghi F, Rajaei Z, Hadjzadeh MAR, Iranshahi M, Alizadeh M. Antihyperglycemic effect of asafoetida (Ferula assafoetida oleo-gum-resin) in streptozotocin-induced diabetic rats. World Applied Sciences Journal. 2012;17(2):157–162. [Google Scholar]
- 39.Akhtar MS, Khan MA, Malik MT. Hypoglycaemic activity of Alpinia galanga rhizome and its extracts in rabbits. Fitoterapia. 2002;73(7–8):623–628. doi: 10.1016/S0367-326X(02)00235-6. [DOI] [PubMed] [Google Scholar]
- 40.Akinola AA, Ahmad S, Maziah M. Total anti-oxidant capacity, flavonoid, phenolic acid and polyphenol content in ten selected species of Zingiberaceae rhizomes. African Journal of Traditional Complementary Alternative Medicine. 2014;11(3):7–13. doi: 10.4314/ajtcam.v11i3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aksoy L. Oxidant/antioxidant equilibrium in rats supplemented with diesel fuel or with opium poppy (Papaver somniferum L.) seed oil biodiesel. Revue de Medecine Veterinaire. 2013;164(1):34–38. [Google Scholar]
- 42.Al-Asmari AK, Athar MT, Kadasah SG. An updated phytopharmacological review on medicinal plant of Arab region: Apium graveolens Linn. Pharmacognosy Reviews. 2017;11(21):13–18. doi: 10.4103/phrev.phrev_35_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Ghamdi AY. Antimicrobial and catalytic activities of green synthesized silver nanoparticles using bay laurel (Laurus nobilis) leaves extract. Journal of Biomaterials and Nanobiotechnology. 2019;10(01):26–39. doi: 10.4236/jbnb.2019.101003. [DOI] [Google Scholar]
- 44.Al-Hadid KJ. Quantitative analysis of antimicrobial activity of Foeniculum vulgare. A review. Plant OMICS. 2017;10(1):28–36. doi: 10.21475/poj.10.01.17.322. [DOI] [Google Scholar]
- 45.Al-Ma’adhedi SHF. Phytochemical screening, estimation of some heavy metals concentrations, and specific extraction of bioactive components from Iraqi Anethum graveolens l. seeds and studying their Antibacterial. Al-Anbar Journal of Veterinary Sciences. 2012;5(2):28–36. [Google Scholar]
- 46.Al-Mariri A, Ismail R, Allaham A, Alobeid B, Alhallab L. Inhibitory effects of essential oils of Cinnamon zeylanicum and Myristica fragrans against Brucella abortus 544 inoculated in fresh baladi cheese. Journal of Food Quality and Hazards Control. 2021;8(1):34–40. doi: 10.18502/JFQHC.8.1.5461. [DOI] [Google Scholar]
- 47.Al-Numair KS, Ahmad D, Ahmed SB, Al-Assaf AH. Nutritive value, levels of polyphenols and anti-nutritional factors in Sri Lankan cinnamon (Cinnamomum Zeyalnicum) and chinese cinnamon (Cinnamomum cassia) Food Science & Agriculture Research Center. 2007;154(154):5–21. [Google Scholar]
- 48.Al-Saif SSAL, Awad MA, Siddiqui MI. Formation, characterization and pathogen activities of green synthesis of curcuma silver nanoparticles. Journal of Computational and Theoretical Nanoscience. 2018;15(4):1300–1306. doi: 10.1166/jctn.2018.7306. [DOI] [Google Scholar]
- 49.Al-Samydai A, Al-Mamoori F, Shehadeh M, Hudaib M. Anti-diabetic activity of cinnamon : A review. International Research Journal of Pharmacy and Medical Sciences. 2018;1(5):43–45. [Google Scholar]
- 50.Al-Snafi, A. E. (2015). Therapeutic properties of medicinal plants: a review of their immunological effects (part 1)Asian Journal of Pharmaceutical Research, 5(3), 208–216
- 51.Al-Snafi PDAE. A review on chemical constituents and pharmacological activities of Coriandrum sativum. IOSR Journal of Pharmacy (IOSRPHR) 2016;06(07):17–42. doi: 10.9790/3013-067031742. [DOI] [Google Scholar]
- 52.Al-Zuhair H, El-Sayeh B, Ameen HA, Al-Shoora H. Pharmacological studies of cardamom oil in animals. Pharmacological Research. 1996;34(1–2):79–82. doi: 10.1006/phrs.1996.0067. [DOI] [PubMed] [Google Scholar]
- 53.Al-Yahya MA, Rafatullah S, Mossa JS, Ageel AM, Al-Said MS, Tariq M. Gastric antisecretory, antiulcer and cytoprotective properties of ethanolic extract of Alpinia galanga willd in rats. Phytotherapy Research. 1990;4(3):112–114. doi: 10.1002/ptr.2650040308. [DOI] [Google Scholar]
- 54.Alam, T., Akbar, F., Ali, M., Munis, M. F. H., & Khan, J. (2019). Biosynthesis of iron oxide nanoparticles via Crocus sativus and their antifungal efficacy against verticillium wilt pathogen verticillium dahliaeBioRxiv, 861401
- 55.Alejandro Celis Toledo, O., Sharifi-Rad, J., Sufiyan Fazal, S., & Singla, R. K. (2012). Review on the pharmacognostical & pharmacological characterization of Apium graveolens linn related papers Apium graveoleons a healt h boon Ravi kant Apium plant s: beyond simple food and phyt opharmacological applicat ions review on the pharmacognostical. In Indo Global Journal of Pharmaceutical Sciences (Vol. 2, Issue 1)
- 56.Alejo-Armijo A, Altarejos J, Salido S. Phytochemicals and biological activities of laurel tree (Laurus nobilis) Natural Product Communications. 2017;12(5):743–757. doi: 10.1177/1934578x1701200519. [DOI] [PubMed] [Google Scholar]
- 57.Alghuthaymi, M. A., Diab, A. M., Elzahy, A. F., Mazrou, K. E., Tayel, A. A., & Moussa, S. H. (2021). Green biosynthesized selenium nanoparticles by cinnamon extract and their antimicrobial activity and application as edible coatings with nano-chitosanJournal of Food Quality,202110.1155/2021/6670709
- 58.Alhumaydhi FA, Khan I, Rauf A, Qureshi MN, Aljohani ASM, Khan SA, Khalil AA, El-Esawi MA, Muhammad N. Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles. Green Processing and Synthesis. 2021;10(1):230–245. doi: 10.1515/gps-2021-0024. [DOI] [Google Scholar]
- 59.Aliakbari-Baydokhty M, Marziyeh Saghebjoo HS, Hedayati M. The effect of endurance training and hydroalcoholic extract of Anethum Graveolens L. (dill) on biochemical cardiovascular risk factors in obese male rats. Journal of Basic Research in Medical Science. 2019;6(4):1–11. [Google Scholar]
- 60.Alquraidi AO, Mosa KA, Ramamoorthy K. Phytotoxic and genotoxic effects of copper nanoparticles in coriander (Coriandrum sativum—apiaceae) Plants. 2019;8(1):19. doi: 10.3390/plants8010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alsalhi MS, Devanesan S, Atif M, Alqahtani WS, Nicoletti M, Del Serrone P. Therapeutic potential assessment of green synthesized zinc oxide nanoparticles derived from fennel seeds extract. International Journal of Nanomedicine. 2020;15:8045–8057. doi: 10.2147/IJN.S272734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alsammarraie FK, Wang W, Zhou P, Mustapha A, Lin M. Green synthesis of silver nanoparticles using turmeric extracts and investigation of their antibacterial activities. Colloids and Surfaces B: Biointerfaces. 2018;171:398–405. doi: 10.1016/j.colsurfb.2018.07.059. [DOI] [PubMed] [Google Scholar]
- 63.Alshehri AA, Malik MA. Biogenic fabrication of znonps using trigonella foenum-graecum for proficient photocatalytic degredation of methylene blue under uv irradiation. Journal of Materials Science Materials in Electronics. 2019;30:16156–16173. doi: 10.1007/s10854-019-01985-8. [DOI] [Google Scholar]
- 64.Altaf M, Manoharadas S, Zeyad MT. Green synthesis of cerium oxide nanoparticles using Acorus calamus extract and their antibiofilm activity against bacterial pathogens. Microscopy Research and Technique. 2021;84(8):1638–1648. doi: 10.1002/jemt.23724. [DOI] [PubMed] [Google Scholar]
- 65.Althomali RH, Alamry KA, Hussein MA, Khan A, Alosaimi AM. A green nanocomposite based modified cellulose/TiO2/Cinnamon bark for the reduction of toxic organic compounds using spectrophotometric technique. Journal of Materials Research and Technology. 2021;12:947–966. doi: 10.1016/j.jmrt.2021.03.002. [DOI] [Google Scholar]
- 66.Altinkaynak C, Ildiz N, Baldemir A, Ozdemir N, Yilmaz V, Ocsoy I. Synthesis of organic-inorganic hybrid nanoflowers using Trigonella foenum-graecum seed extract and investigation of their anti-microbial activity. Derim. 2019;36(2):159–167. [Google Scholar]
- 67.Alves de Figueiredo Sousa H, Gonçalves de Oliveira Filho J, Egea MB, Rosada Silva E, Macagnan D, Pires M, Peixoto J. Active film incorporated with clove essential oil on storage of banana varieties. Nutrition and Food Science. 2019;49(5):911–924. doi: 10.1108/NFS-09-2018-0262. [DOI] [Google Scholar]
- 68.Alves PIC, Radünz M, Borges CD, Bastos CP, Timm CD, Gandra EA. Antimicrobial potential of a bioactive coating based on chitosan incorporated with clove essential oil in hamburger-like meat product. Research, Society and Development. 2021;10(11):e73101119373. doi: 10.33448/rsd-v10i11.19373. [DOI] [Google Scholar]
- 69.Alwhibi MS, Soliman DA, Awad MA, Rizwana H, Marraiki NA. Biosynthesis of silver nanoparticles using fenugreek seed extract and evaluation of their antifungal and antibacterial activities. Journal of Computational and Theoretical Nanoscience. 2018;15(4):1255–1260. doi: 10.1166/jctn.2018.7301. [DOI] [Google Scholar]
- 70.Amalraj A, Gopi S. Biological activities and medicinal properties of Asafoetida: A review. Journal of Traditional and Complementary Medicine. 2017;7(3):347–359. doi: 10.1016/j.jtcme.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aman AK, Singh RK, Kumar R, Ghosh AK. Effect of high energy ball milling grinding on physico-chemical, morphological and optical properties of Curcuma longa nanoparticles powders. International Journal of Pharmaceutical Sciences and Research. 2018;9(2):672–677. doi: 10.13040/IJPSR.0975-8232.9(2).672-77. [DOI] [Google Scholar]
- 72.Ambhore SS, Padghan PV, Thombre BM, Jamadar KS. Studies on turmeric powder ( Curcuma Longa L. ) added ghee. The Pharma Innovation Journal. 2020;9(12):9–14. [Google Scholar]
- 73.Amenta V, Aschberger K, Arena M, Bouwmeester H, Botelho Moniz F, Brandhoff P, Gottardo S, Marvin HJP, Mech A, Quiros Pesudo L, Rauscher H, Schoonjans R, Vettori MV, Weigel S, Peters RJ. Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regulatory Toxicology and Pharmacology. 2015;73(1):463–476. doi: 10.1016/j.yrtph.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 74.Amer, S. A., & Rizk, A. E. (2022). Production and evaluation of novel functional extruded corn snacks fortified with ginger, bay leaves and turmeric powderFood Production, Processing and Nutrition, 4(1)10.1186/s43014-022-00083-3
- 75.Aminzare, M., Hashemi, M., Hassanzadazar, H., Amiri, E., & Abbasi, Z. (2017). Antibacterial activity of corn starch films incorporated with Zataria multiflora and Bonium persicum essential oilsAnnual Research and Review in Biology, 19(1)10.9734/ARRB/2017/37103
- 76.Anand P, Murali KY, Tandon V, Chandra R, Murthy PS. Preliminary studies on antihyperglycemic effect of aqueous extract of Brassica nigra (L.) Koch in streptozotocin induced diabetic rats. Indian Journal of Experimental Biology. 2007;45(8):696–701. [PubMed] [Google Scholar]
- 77.Angelita, A. (2016). Characterization of edible films prepared from base formulations and cinnamon extract on anise oil as natural antimicrobial and application as edible coating to strawberryUniversitas Pelita Harapan
- 78.Anitha, M. L., Jose, R., Rinita, J., Jothi, N. S. N. (2020). Green synthesis of trigonella foenum graecum mediated iron nanoparticles by co precipitation methodThe Journal of Chemical Physics
- 79.Anitha, M. L., Riya, J., Rinita, J., Eunice, P. C., & Jothi, N. S. N. (2021). Facile green synthesis and characterisation of gold nanoparticles using fenugreek seeds and honeyJournal of Physics: Conference Series, 2070(1)10.1088/1742-6596/2070/1/012048
- 80.Anjana, G., Roy, A., Rajeshkumar, S., & Ezhilarasan, D. (2020). Cassia oleoresin mediated synthesis of magnesium oxide nanoparticles and brine shrimp lethality assayJournal of Pharmaceutical Research International, August, 75–8210.9734/jpri/2020/v32i1530628
- 81.Antala BV, Patel MS, Bhuva SV, Gupta S, Rabadiya S, Lahkar M. Protective effect of methanolic extract of Garcinia indica fruits in 6-OHDA rat model of Parkinson’s disease. Indian Journal of Pharmacology. 2012;44(6):683–687. doi: 10.4103/0253-7613.103242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anvarinezhad M, Javadi A, Jafarizadeh-Malmiri H. Green approach in fabrication of photocatalytic, antimicrobial, and antioxidant zinc oxide nanoparticles - Hydrothermal synthesis using clove hydroalcoholic extract and optimization of the process. Green Processing and Synthesis. 2020;9(1):375–385. doi: 10.1515/gps-2020-0040. [DOI] [Google Scholar]
- 83.Araújo, C., & Leon, L. L. (2001). Biological Activities of Curcuma longa L. In Mem Inst Oswaldo Cruz (Vol. 96, Issue 5) [DOI] [PubMed]
- 84.Arif A, Bhatti A, John P. Therapeutic potential of Foeniculum vulgare mill. Derived selenium nanoparticles in arthritic Balb/c mice. International Journal of Nanomedicine. 2019;14:8561–8572. doi: 10.2147/IJN.S226674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Armellini R, Peinado I, Pittia P, Scampicchio M, Heredia A, Andres A. Effect of saffron (Crocus sativus L.) enrichment on antioxidant and sensorial properties of wheat flour pasta. Food Chemistry. 2018;254:55–63. doi: 10.1016/j.foodchem.2018.01.174. [DOI] [PubMed] [Google Scholar]
- 86.Aruna G, Baskaran V. Comparative study on the levels of carotenoids lutein, zeaxanthin and β-carotene in Indian spices of nutritional and medicinal importance. Food Chemistry. 2010;123(2):404–409. doi: 10.1016/j.foodchem.2010.04.056. [DOI] [Google Scholar]
- 87.Asgarpanah J. Phytochemistry, pharmacology and medicinal properties of Coriandrum sativum L. African Journal of Pharmacy and Pharmacology. 2012;6(31):2340–2345. doi: 10.5897/ajpp12.901. [DOI] [Google Scholar]
- 88.Ashokkumar, K., Murugan, M., Dhanya, M. K., Pandian, A., & Warkentin, T. D. (2021). Phytochemistry and therapeutic potential of black pepper [Piper nigrum (L.)] essential oil and piperine: a reviewClinical Phytoscience, 7(1)10.1186/s40816-021-00292-2
- 89.Ashokkumar, K., Murugan, M., Dhanya, M. K., & Warkentin, T. D. (2020). Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton] – A critical review. In Journal of Ethnopharmacology (Vol. 246). Elsevier Ireland Ltd10.1016/j.jep.2019.112244 [DOI] [PubMed]
- 90.Ashrafuzzaman Zahid M, Seo JK, Parvin R, Ko J, Yang HS. Comparison of butylated hydroxytoluene, ascorbic acid, and clove extract as antioxidants in fresh beef patties at refrigerated storage. Food Science of Animal Resources. 2019;39(5):768–779. doi: 10.5851/kosfa.2019.e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Asif M, Yehya AHS, Al-Mansoub MA, Revadigar V, Ezzat MO, Khadeer Ahamed MB, Oon CE, Murugaiyah V, Abdul Majid AS, Abdul Majid AMS. Anticancer attributes of Illicium verum essential oils against colon cancer. South African Journal of Botany. 2016;103:156–161. doi: 10.1016/j.sajb.2015.08.017. [DOI] [Google Scholar]
- 92.Aslam Bhatti, H., Khan, S., Faizi, S., Abbas, G., Ali, I., Jawaid, S., Naushir Akbar Ali, A., Jamy, R., Shahid, F., Ali Versiani, M., & Dar, A. (2017). Protocatecheuic acid underlies the antioxidant activity exhibited by Illicium verum fruitJournal of Analytical & Pharmaceutical Research, 6(3)10.15406/japlr.2017.06.00177
- 93.Assadpour E, Jafari SM, Maghsoudlou Y. Evaluation of folic acid release from spray dried powder particles of pectin-whey protein nano-capsules. International Journal of Biological Macromolecules. 2017;95:238–247. doi: 10.1016/j.ijbiomac.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 94.Assiry AA, Karobari MI, Bhavikatti SK, Marya A. Crossover analysis of the astringent, antimicrobial, and anti-inflammatory effects of Illicium verum/star anise in the oral cavity. BioMed Research International. 2021;2021:3–8. doi: 10.1155/2021/5510174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aswathy Aromal S, Philip D. Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy. 2012;97:1–5. doi: 10.1016/j.saa.2012.05.083. [DOI] [PubMed] [Google Scholar]
- 96.Athira GK, Jyothi AN. Preparation and characterization of curcumin loaded cassava starch nanoparticles with improved cellular absorption. International Journal of Pharmacy and Pharmaceutical Sciences. 2014;6(10):171–176. [Google Scholar]
- 97.Attia, A. A., Ramdan, H. S., Al-Eisa, R. A., Adle Fadle, B. O. A., & El-Shenawy, N. S. (2021). Effect of saffron extract on the hepatotoxicity induced by copper nanoparticles in male miceMolecules, 26(10)10.3390/molecules26103045 [DOI] [PMC free article] [PubMed]
- 98.Attila B, Muste S, Pop A, Ucean APĂ. Valorization Of tomato pomace in innovative food products and evaluation of synergetic effect with Mentha piperita l. and Anethum graveolens l -a comprehensive review. Hop and Medicinal Plants. 2021;1:164–172. [Google Scholar]
- 99.Atwaa ESH, Shahein MR, El-Sattar ESA, Hijazy HHA, Albrakati A, Elmahallawy EK. Bioactivity, physicochemical and sensory properties of probiotic yoghurt made from whole milk powder reconstituted in aqueous fennel extract. Fermentation. 2022;8(2):52. doi: 10.3390/fermentation8020052. [DOI] [Google Scholar]
- 100.Awad MAA, Hendi A, Ortashi KM, Alzahrani B, Soliman D, Alanazi A, Alenazi W, Taha RM, Ramadan R, El-Tohamy M, AlMasoud N, Alomar TS. Biogenic synthesis of silver nanoparticles using Trigonella foenum-graecum seed extract: Characterization, photocatalytic and antibacterial activities. Sensors and Actuators A: Physical. 2021;323:112670. doi: 10.1016/j.sna.2021.112670. [DOI] [Google Scholar]
- 101.Aydemir T, Becerik S. Phenolic content and antioxidant activity of different extracts from ocimum basilicum, apium graveolens and lepidium sativum seeds. Journal of Food Biochemistry. 2011;35(1):62–79. doi: 10.1111/j.1745-4514.2010.00366.x. [DOI] [Google Scholar]
- 102.Ayelén Vélez, M., Cristina Perotti, M., Santiago, L., María Gennaro, A., & Hynes, E. (2017). Bioactive compounds delivery using nanotechnology: Design and applications in dairy foodNutrient Delivery,221–25010.1016/b978-0-12-804304-2.00006-8
- 103.Ayışığı M, Yalçın T, Yıldız Aktaş L. Antimicrobial potentials of phyto-synthesized silver nanoparticles from Laurus nobilis L. Celal Bayar Üniversitesi Fen Bilimleri Dergisi. 2019;15(3):317–321. doi: 10.18466/cbayarfbe.582161. [DOI] [Google Scholar]
- 104.Azad, R., Delwar, A., Surya, B., Michael, T., Tieneke, T., & Sanjaya, T. (2019)Market analysis of cumin seed. 1–14https://crcna.com.au/resources/publications/market-analysis-cumin-seed
- 105.Azizian-Shermeh O, Valizadeh M, Taherizadeh M, Beigomi M. Phytochemical investigation and phytosynthesis of eco-friendly stable bioactive gold and silver nanoparticles using petal extract of saffron (Crocus sativus L.) and study of their antimicrobial activities. Applied Nanoscience (Switzerland) 2020;10(8):2907–2920. doi: 10.1007/s13204-019-01059-5. [DOI] [Google Scholar]
- 106.Azizian H, Rezvani ME, Esmaeili DM, Bagheri SM. Anti-obesity, fat lowering and liver steatosis protective effects of ferula asafoetida gum in type 2 diabetic rats posiible involvement of leptin. Iranian Journal of Diabetes and Obesity. 2012;4(3):120–126. [Google Scholar]
- 107.Azlan A, Sultana S, Huei CS, Razman MR. Antioxidant, anti-obesity, nutritional and other beneficial effects of different chili pepper: A review. Molecules. 2022;27(3):1–11. doi: 10.3390/molecules27030898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Babapour, H., Jalali, H., Nafchi, A. M., & Jokar, M. (2022). Effects of active packaging based on potato starch / nano zinc oxide / fennel ( Foeniculum vulgare miller ) essential oil on fresh pistachio during cold storageJournal of Nuts, 13(February)10.22034/jon.2022.1941353.1129
- 109.Badami, R. C., Razdan, M. K. (1972). Isolation and identification of L- leucine as DNP-L- leucine hydrochloride in the leaves of garcinia indicaIndian Chem Soc Journal
- 110.Badgujar SB, Patel VV, Bandivdekar AH. Foeniculum vulgare mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. BioMed Research International. 2014 doi: 10.1155/2014/842674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Badihi L, Gerami M, Akbarinodeh D, Shokrzadeh M, Ramezani M. Physio-chemical responses of exogenous calcium nanoparticle and putrescine polyamine in Saffron (Crocus sativus L.) Physiology and Molecular Biology of Plants. 2021;27(1):119–133. doi: 10.1007/s12298-020-00923-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Badola R, Danish M, Kumar S, Fahad M, Kanade PP, Upadhayay S, Kohli D, Rautela I. Effect of incorporation of black pepper and cardamom on quality characteristics of paneer. International Journal of Applied Science and Engineering. 2018;6(2):121–127. doi: 10.30954/2322-0465.2.2018.4. [DOI] [Google Scholar]
- 113.Bagavan A, Rahuman AA, Kamaraj C, Elango G, Zahir AA, Jayaseelan C, Santhoshkumar T, Marimuthu S. Contact and fumigant toxicity of hexane flower bud extract of Syzygium aromaticum and its compounds against Pediculus humanus capitis (Phthiraptera: Pediculidae) Parasitology Research. 2011;109(5):1329–1340. doi: 10.1007/s00436-011-2425-1. [DOI] [PubMed] [Google Scholar]
- 114.Bagheri SM, Hedesh ST, Mirjalili A, Dashti-R MH. Evaluation of anti-inflammatory and some possible mechanisms of antinociceptive effect of Ferula assa foetida oleo gum resin. Journal of Evidence-Based Complementary and Alternative Medicine. 2016;21(4):271–276. doi: 10.1177/2156587215605903. [DOI] [PubMed] [Google Scholar]
- 115.Bagherzade G, Tavakoli MM, Namaei MH. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pacific Journal of Tropical Biomedicine. 2017;7(3):227–233. doi: 10.1016/j.apjtb.2016.12.014. [DOI] [Google Scholar]
- 116.Bagyalakshmi B, Priyadarshini SL, Balamurugan A. Anticancer activity of bee venom against lung cancer cell line ( A549 Cells ) enhanced by iron oxide nanoparticles synthesized from syzygium aromaticum. Journal of Drug Delivery and Therapeutics Open. 2019;9:248–254. [Google Scholar]
- 117.Bahattab O, Khan I, Bawazeer S, Rauf A, Qureshi MN, Al-Awthan YS, Muhammad N, Khan A, Akram M, Islam MN, Bin Emran T. Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications. Green Processing and Synthesis. 2021;10(1):440–445. doi: 10.1515/gps-2021-0041. [DOI] [Google Scholar]
- 118.Bahmid NA, Dekker M, Fogliano V, Heising J. Development of a moisture-activated antimicrobial film containing ground mustard seeds and its application on meat in active packaging system. Food Packaging and Shelf Life. 2021;30(August):100753. doi: 10.1016/j.fpsl.2021.100753. [DOI] [Google Scholar]
- 119.Bährle-Rapp, M. (2007). Syzygium aromaticumSpringer Lexikon Kosmetik Und Körperpflege, 541–54110.1007/978-3-540-71095-0_10277
- 120.Balakrishnan S, Sivaji I, Kandasamy S, Duraisamy S, Kumar NS, Gurusubramanian G. Biosynthesis of silver nanoparticles using myristica fragrans seed extract and its antibacterial activity against multidrug resistant salmonella enterica serovar. Environmental Science and Pollution Research. 2017;24:14758–14769. doi: 10.1007/s11356-017-9065-7. [DOI] [PubMed] [Google Scholar]
- 121.Balakumbahan, R., Rajamani, K., & Kumanan, K. (2010). Acorus calamus: an overviewJournal of Medicinal Plants Research, 4(25), 2740–2745http://www.academicjournals.org/JMPR
- 122.Baldo DEB, Serrano JE, Diomerl C, Baldo EB. Screening for intestinal anti-inflammatory activity of Alpinia galanga against acetic acid-induced colitis in Mice (Mus musculus). ~ 72 ~. Journal of Medicinal Plants Studies. 2016;4(1):72–77. [Google Scholar]
- 123.Balfour, H. (1857)The Plants of the Bible. Trees and Shrubs
- 124.Mounyt,B., Moulay, S., & Saad, I. (2016). Methods for in vitro evaluating antimicrobial activity: A reviewJournal of Pharmaceutical Analysis, 6(6), 17–79https://ac.els-cdn.com/S2095177915300150/1-s2.0-S2095177915300150-main.pdf?_tid=98fc0310-a3c9-45ad-a705-f4789eae18fd&acdnat=1552489821_3bebd92b334682aa98528a548985b3fd [DOI] [PMC free article] [PubMed]
- 125.Bano F, Ahmed A, Ahmed M, Parveen T. Anethum graveolens seeds aqueous extract stimulates whole brain 5-hydroxytryptamine metabolism and reduces feeding behavior and body weight in obese rats. Pakistan Journal of Pharmaceutical Sciences. 2015;28(1):221–225. [PubMed] [Google Scholar]
- 126.Bansal S, Sharma K, Gautam V, Lone AA, Malhotra EV, Kumar S, Singh R. A comprehensive review of Bunium persicum: A valuable medicinal spice. Food Reviews International. 2021;00(00):1–20. doi: 10.1080/87559129.2021.1929305. [DOI] [Google Scholar]
- 127.Barakat A. Ameliorating role of Foeniculum vulgare (fennel) and Pimpinella anisum (anise) against Zinc oxide nanoparticles induced hepatotoxicity in male albino rats. Journal of Bioscience and Applied Research. 2019;5(3):262–277. doi: 10.21608/jbaar.2019.146878. [DOI] [Google Scholar]
- 128.Barbinta-Patrascu ME, Gorshkova Y, Ungureanu C, Badea N, Bokuchava G, Lazea-Stoyanova A, Bacalum M, Zhigunov A, Petrovič S. Characterization and antitumoral activity of biohybrids based on turmeric and silver/silver chloride nanoparticles. Materials. 2021;14(16):4726. doi: 10.3390/ma14164726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barla A, Topçu G, Öksüz S, Tümen G, Kingston DGI. Identification of cytotoxic sesquiterpenes from Laurus nobilis L. Food Chemistry. 2007;104(4):1478–1484. doi: 10.1016/j.foodchem.2007.02.019. [DOI] [Google Scholar]
- 130.Barros L, Carvalho AM, Ferreira ICFR. The nutritional composition of fennel (Foeniculum vulgare): Shoots, leaves, stems and inflorescences. LWT - Food Science and Technology. 2010;43(5):814–818. doi: 10.1016/j.lwt.2010.01.010. [DOI] [Google Scholar]
- 131.Barua, C. C., Haloi, A., Sen, S., & Barua, I. (2015). Evaluation of gastric ulcer protective activity of Acorus calamus linn. in laboratory animalsMedicinal Plants: Phytochemistry, Pharmacology and Therapeutics, May
- 132.Baskararaj, S., & Kunjiappan, S. (2019). Biogenic synthesis of Garcinia indica mediated gold nanoparticles for enhanced anticancer activity on human hepatoma cell lines Biogenic synthesis of Garcinia indica mediated gold nanoparticles for enhanced anticancer activity on human hepatoma cell linesIPMN, February
- 133.Basri AM, Taha H, Ahmad N. A review on the pharmacological activities and phytochemicals of Alpinia officinarum (Galangal) extracts derived from bioassay-guided fractionation and isolation. Pharmacognosy Reviews. 2017;11(21):43–56. doi: 10.4103/phrev.phrev_55_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Batiha GES, Alkazmi LM, Wasef LG, Beshbishy AM, Nadwa EH, Rashwan EK. Syzygium aromaticum l. (myrtaceae): traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules. 2020;10(2):202. doi: 10.3390/biom10020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Batool, S., Khera, R. A., Hanif, M. A., & Ayub, M. A. (2020). Bay leafMedicinal Plants of South Asia, January
- 136.Bawazeer S, Khan I, Rauf A, Aljohani ASM, Alhumaydhi FA, Khalil AA, Qureshi MN, Ahmad L, Khan SA. Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications - A green approach. Green Processing and Synthesis. 2022;11(1):11–28. doi: 10.1515/gps-2022-0002. [DOI] [Google Scholar]
- 137.Behbahani BA, Shahidi F, Yazdi FT, Mortazavi SA, Mohebbi M. Use of Plantago major seed mucilage as a novel edible coating incorporated with Anethum graveolens essential oil on shelf life extension of beef in refrigerated storage. International Journal of Biological Macromolecules. 2017;94:515–526. doi: 10.1016/j.ijbiomac.2016.10.055. [DOI] [PubMed] [Google Scholar]
- 138.Ben- Nun, L. (2022). Health effects of dillMedical Research in Biblical Times
- 139.Ben Abdesslem S, Ben Moussa O, Boulares M, Elbaz M, Chouaibi M, Ayachi S, Hassouna M. Evaluation of the effect of fennel (Foeniculum vulgare Mill) essential oil addition on the quality parameters and shelf-life prediction of yoghurt. International Journal of Dairy Technology. 2020;73(2):403–410. doi: 10.1111/1471-0307.12667. [DOI] [Google Scholar]
- 140.Benítez V, Mollá E, Martín-Cabrejas MA, Aguilera Y, López-Andréu FJ, Cools K, Terry LA, Esteban RM. Characterization of industrial onion wastes (Allium cepa L.): dietary fibre and bioactive compounds. Plant Foods for Human Nutrition. 2011;66(1):48–57. doi: 10.1007/s11130-011-0212-x. [DOI] [PubMed] [Google Scholar]
- 141.Benkeblia N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum) LWT - Food Science and Technology. 2004;37(2):263–268. doi: 10.1016/j.lwt.2003.09.001. [DOI] [Google Scholar]
- 142.Bhanger MI, Bukhari SB, Memon S. Antioxidative activity of extracts from a fenugreek seeds. Pakistan Journal of Analytical and Environmental Chemistry. 2008;9(2):6. [Google Scholar]
- 143.Bhat, A. A., Kumar, A., & Kaur, M. (2016). Effect of clove oil on the oxidative stability and microbial quality of almond and walnut enriched chevon nuggetsJournal of Pure and Applied Microbiology, 10, 1341+https://link.gale.com/apps/doc/A481650224/AONE?u=anon~11b2e98a&sid=googleScholar&xid=feea037b
- 144.Bhat NA, Hamdani AM, Masoodi FA. Development of functional cookies using saffron extract. Journal of Food Science and Technology. 2018;55(12):4918–4927. doi: 10.1007/s13197-018-3426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bhat, S. (2013). Nutrient composition of coriander leaf and seeds as per usdaNational Nutrition Data Base
- 146.Bhat, S. P., Rizvi, W., & Kumar, P. A. (2021). Analgesic and anti-inflammatory activity of Brassica nigra l. seed analgesic and anti-inflammatory activity of Brassica nigra l. seedInternational Journal of Drug Formulation and Research, 5(5)
- 147.Bhattacharya E, Pal U, Dutta R, Bhowmik PC, Mandal Biswas S. Antioxidant, antimicrobial and DNA damage protecting potential of hot taste spices: A comparative approach to validate their utilization as functional foods. Journal of Food Science and Technology. 2022;59(3):1173–1184. doi: 10.1007/s13197-021-05122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bhatti HN, Zafar F, Jamal MA. Evaluation of phenolic contents and antioxidant potential of methanolic extracts of green cardamom (Elettaria cardamomum) Asian Journal of Chemistry. 2010;22(6):4787–4794. [Google Scholar]
- 149.Bhatti S, Ali Shah SA, Ahmed T, Zahid S. Neuroprotective effects of Foeniculum vulgare seeds extract on lead-induced neurotoxicity in mice brain. Drug and Chemical Toxicology. 2018;41(4):399–407. doi: 10.1080/01480545.2018.1459669. [DOI] [PubMed] [Google Scholar]
- 150.Bhawana, Basniwal RK, Buttar HS, Jain VK, Jain N. Curcumin nanoparticles: preparation, characterization, and antimicrobial study. Journal of Agricultural and Food Chemistry. 2011;59(5):2056–2061. doi: 10.1021/jf104402t. [DOI] [PubMed] [Google Scholar]
- 151.Bhowmik, D., Sampath Kumar, K. P., Yadav, A., Srivastava, S., Paswan, S., & Dutta, A. S. (2012). Recent trends in Indian traditional herbs Syzygium aromaticum and its health benefitsJournal of Pharmacognosy and Phytochemistry, 1(1)www.phytojournal.comwww.phytojournal.com
- 152.Bhutta ZA, Ashar A, Mahfooz A, Khan JA, Saleem MI, Rashid A, Aqib AI, Kulyar MFeA, Sarwar I, Shoaib M, Nawaz S, Yao W. Enhanced wound healing activity of nano ZnO and nano Curcuma longa in third-degree burn. Applied Nanoscience (Switzerland) 2021;11(4):1267–1278. doi: 10.1007/s13204-020-01661-y. [DOI] [Google Scholar]
- 153.Bisht S, Mizuma M, Feldmann G, Ottenhof N, Hong S-M, Pramanik D, Chenna V, Karikari C, Sharma R, Goggins MG, Rudek MA, Ravi R, Maitra A, Maitra A. Systemic administration of polymeric nanoparticles encapsulated curcumin blocks tumor growth and metastases in preclinical models of pancreatic cancer. Molecular Cancer Therapeutics. 2010;9(8):2255–2264. doi: 10.1158/1535-7163.MCT-10-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, Maitra A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): A novel strategy for human cancer therapy. Journal of Nanobiotechnology. 2007;5:1–18. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bojorges H, Ríos-Corripio MA, Hernández-Cázares AS, Hidalgo-Contreras JV, Contreras-Oliva A. Effect of the application of an edible film with turmeric (Curcuma longa L.) on the oxidative stability of meat. Food Science and Nutrition. 2020;8(8):4308–4319. doi: 10.1002/fsn3.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bora K, Sharma A. Phytoconstituents and therapeutic potential of allium cepa linn.- A Review. Pharmacognosy Reviews. 2009;3(5):170–180. [Google Scholar]
- 157.Borjoeifar M, Nabieyan S, Saadatfar A, Mehrjerdi MRZ. Development of Operational Strategies for Branding Ferula assa-foetida L. Medicinal Plant (Case study: Rangelands of Kerman Province, Iran) Journal of Rangeland Science. 2021;11(2):224–240. [Google Scholar]
- 158.Borrin TR, Georges EL, Brito-Oliveira TC, Moraes ICF, Pinho SC. Technological and sensory evaluation of pineapple ice creams incorporating curcumin-loaded nanoemulsions obtained by the emulsion inversion point method. International Journal of Dairy Technology. 2018;71(2):491–500. doi: 10.1111/1471-0307.12451. [DOI] [Google Scholar]
- 159.Bostoglou E, Govaris A, Giannenas I, Botsoglou N. Use of saffron (Crocus sativus L.) as a feed additive for improving growth and meat or egg quality in poultry. Functionnal Plant Science and Biotechnology. 2010;4(2):98–107. [Google Scholar]
- 160.Bota V, Sumalan RM, Obistioiu D, Negrea M, Cocan I, Popescu I, Alexa E. Study on the sustainability potential of thyme, oregano, and coriander essential oils used as vapours for antifungal protection of wheat and wheat products. Sustainability. 2022;14(7):4298. doi: 10.3390/su14074298. [DOI] [Google Scholar]
- 161.Boukhechem S, Bougherara H, Mimoune N, Redouane R, Nia N, Kaidi R. Effect of sprouted Trigonella foenum graecum L. incorporation into the diet on milk production of rabbit does and growth of young rabbits in the Northeast of Algeria. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Veterinary Medicine. 2021;78(1):48. doi: 10.15835/buasvmcn-vm:2020.0047. [DOI] [Google Scholar]
- 162.Bouras., Mesbahi, Y., Amine, A. (2021)Invitro and invivo antifungal effect of silver oxide and syzygium aromaticum in wistars ratsApplied Biochemistry
- 163.Bozan B, Karakaplan U. Antioxidants from laurel (Laurus nobilis L.) berries: Influence of extraction procedure on yield and antioxidant activity of extracts. Acta Alimentaria. 2007;36(3):321–328. doi: 10.1556/AAlim.36.2007.3.4. [DOI] [Google Scholar]
- 164.Britto, G. C. S. de, Bécker, G., Soares, W. P., Nascimento, E., Rodrigues, E. C., Picanço, N. F. M., Faria, R. A. P. G. de, & Scabora, M. H. (2020). Bioactive compounds and physicochemical properties of dairy products supplemented with plantain and turmericJournal of Food Processing and Preservation, 44(9)10.1111/jfpp.14720
- 165.Budiman I, Tjokropranoto R, Widowati W, Fauziah N, Erawijantari P. Potency of tumeric (Curcuma longa L.) extract and curcumin as anti-obesity by inhibiting the cholesterol and triglycerides synthesis in HepG2 cells. International Journal of Research in Medical Sciences. 2015;3(5):1165. doi: 10.5455/2320-6012.ijrms20150525. [DOI] [Google Scholar]
- 166.Bulut Kocabas B, Attar A, Peksel A, Altikatoglu Yapaoz M. Phytosynthesis of CuONPs via Laurus nobilis: Determination of antioxidant content, antibacterial activity, and dye decolorization potential. Biotechnology and Applied Biochemistry. 2021;68(4):889–895. doi: 10.1002/bab.2010. [DOI] [PubMed] [Google Scholar]
- 167.Busari, Z. A., Dauda, K. A., Morenikeji, O. A., Afolayan, F., Oyeyemi, O. T., Meena, J., Sahu, D., & Panda, A. K. (2017). Antiplasmodial activity and toxicological assessment of curcumin PLGA-encapsulated nanoparticlesFrontiers in Pharmacology, 8(SEP)10.3389/fphar.2017.00622 [DOI] [PMC free article] [PubMed]
- 168.Busquets, R. (2017). Emerging nanotechnologies in food scienceEmerging Nanotechnologies in Food Science, 1–222
- 169.Caleja C, Barros L, Antonio AL, Ciric A, Soković M, Oliveira MBPP, Santos-Buelga C, Ferreira ICFR. Foeniculum vulgare mill. as natural conservation enhancer and health promoter by incorporation in cottage cheese. Journal of Functional Foods. 2015;12:428–438. doi: 10.1016/j.jff.2014.12.016. [DOI] [Google Scholar]
- 170.Calixto JB, Beirith A, Ferreira J, Santos ARS, Filho VC, Yunes RA. Naturally occurring antinociceptive substances from plants. Phytotherapy Research. 2000;14(6):401–418. doi: 10.1002/1099-1573(200009)14:6<401::AID-PTR762>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 171.Campo Velasco JA, Vanegas Mahecha P, Andrade-Mahecha MM. Essential oil turmeric (Curcuma longa) antifungal agent as in edible coatings applied to pumpkin minimally processed. Revista de Ciências Agrárias. 2017;40(3):641–654. doi: 10.19084/RCA16130. [DOI] [Google Scholar]
- 172.Caputo, L., Nazzaro, F., Souza, L. F., Aliberti, L., De Martino, L., Fratianni, F., Coppola, R., & De Feo, V. (2017). Laurus nobilis: composition of essential oil and its biological activitiesMolecules, 22(6)10.3390/molecules22060930 [DOI] [PMC free article] [PubMed]
- 173.Caputo L, Souza LF, Alloisio S, Cornara L, De Feo V. Coriandrum sativum and Lavandula angustifolia essential oils: Chemical composition and activity on central nervous system. International Journal of Molecular Sciences. 2016;17(12):1999. doi: 10.3390/ijms17121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Careaga M, Fernández E, Dorantes L, Mota L, Jaramillo ME, Hernandez-Sanchez H. Antibacterial activity of capsicum extract against Salmonella typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. International Journal of Food Microbiology. 2003;83(3):331–335. doi: 10.1016/S0168-1605(02)00382-3. [DOI] [PubMed] [Google Scholar]
- 175.Carrubba, A., Torre, R, L., Prima, A, D., Saiano, F., Alonzo, G. (2002)Statistical analyses on the essential oil of Italian coriander (Coriandrum sativum l.) fruits of different ages
- 176.Casado-Hidalgo G, Pérez-Quintanilla D, Morante-Zarcero S, Sierra I. Mesostructured silica-coated magnetic nanoparticles to extract six opium alkaloids in poppy seeds prior to ultra-high-performance liquid chromatography-tandem mass spectrometry analysis. Foods. 2021;10(7):1587. doi: 10.3390/foods10071587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Casillas-figueroa F, Arellano-garcía ME, Leyva-aguilera C, Ruíz-ruíz B, Vázquez-gómez RL, Radilla-chávez P, Chávez-santoscoy RA, Pestryakov A, Toledano-magaña Y, García-ramos JC, Bogdanchikova N. Argovit™ silver nanoparticles effects on allium cepa: Plant growth promotion without cyto genotoxic damage. Nanomaterials. 2020;10(7):1–20. doi: 10.3390/nano10071386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cavaliere A, De Marchi E, Banterle A. Investigation on the role of consumer health orientation in the use of food labels. Public Health. 2017;147:119–127. doi: 10.1016/j.puhe.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 179.Cerqueira, M. Â., Pinheiro, A. C., Ramos, O. L., Silva, H., Bourbon, A. I., & Vicente, A. A. (2017). Advances in food nanotechnologyEmerging Nanotechnologies in Food Science,11–3810.1016/B978-0-323-42980-1.00002-9
- 180.Chahal, K., Kaur, R., Kumar, A., & Bhardwaj, U. (2017). Chemistry and biological activities of Anethum graveolens L. (dill) essential oil: A reviewJournal of Pharmacognosy and Phytochemistry, 6(2), 295–306https://www.phytojournal.com/archives/2017/vol6issue2/PartF/6-2-67-817.pdf
- 181.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa) Journal of Alternative and Complementary Medicine. 2003;9(1):161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 182.Chakrabarty S, Islam AKMM, Islam AKMA. Nutritional benefits and pharmaceutical potentialities of chili : A review. Fundamental and Applied Agriculture. 2021;2:227–232. [Google Scholar]
- 183.Chakraborty, A. J., Uddin, T. M., Matin Zidan, B. M. R., Mitra, S., Das, R., Nainu, F., Dhama, K., Roy, A., Hossain, M. J., Khusro, A., & Emran, T. B. (2022). Allium cepa: A treasure of bioactive phytochemicals with prospective health benefitsEvidence-Based Complementary and Alternative Medicine,202210.1155/2022/4586318 [DOI] [PMC free article] [PubMed]
- 184.Chakraborty P, Dam D, Abraham J. Bioactivity of lanthanum nanoparticle synthesized using Trigonella foenum-graecum seed extract. Journal of Pharmaceutical Sciences and Research. 2016;8(11):1253–1257. [Google Scholar]
- 185.Chakraborty R, Sen S, Ali Ahmed F, Barakoti H. Evaluation of in vitro anti-diabetic and anti-oxidant potential of Acorus calamus rhizome. Indo Global Journal of Pharmaceutical Sciences. 2020;10(03):35–40. doi: 10.35652/igjps.2020.10304. [DOI] [Google Scholar]
- 186.Chanda N, Shukla R, Zambre A, Mekapothula S, Kulkarni RR, Katti K, Bhattacharyya K, Fent GM, Casteel SW, Boote EJ, Viator JA, Upendran A, Kannan R, Katti KV. An effective strategy for the synthesis of biocompatible gold nanoparticles using cinnamon phytochemicals for phantom CT imaging and photoacoustic detection of cancerous cells. Pharmaceutical Research. 2011;28(2):279–291. doi: 10.1007/s11095-010-0276-6. [DOI] [PubMed] [Google Scholar]
- 187.Chanda, S., & Ramachandra, T. V. (2019). Phytochemical and pharmacological importance of turmeric (Curcuma longa): a reviewResearch & Reviews: A Journal of Pharmacology, 16–23www.stmjournals.com
- 188.Chang ST, Chen PF, Chang SC. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. Journal of Ethnopharmacology. 2001;77(1):123–127. doi: 10.1016/S0378-8741(01)00273-2. [DOI] [PubMed] [Google Scholar]
- 189.Chao IC, Wang CM, Li SP, Lin LG, Ye WC, Zhang QW. Simultaneous quantification of three curcuminoids and three volatile components of curcuma longa using pressurized liquid extraction and high-performance liquid chromatography. Molecules. 2018;23(7):1568. doi: 10.3390/molecules23071568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chatterjee D, Bhattacharjee P. Use of eugenol-lean clove extract as a flavoring agent and natural antioxidant in mayonnaise: Product characterization and storage study. Journal of Food Science and Technology. 2015;52(8):4945–4954. doi: 10.1007/s13197-014-1573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chau TP, Kandasamy S, Chinnathambi A, Alahmadi TA, Brindhadevi K. Synthesis of zirconia nanoparticles using Laurus nobilis for use as an antimicrobial agent. Applied Nanoscience (Switzerland) 2021 doi: 10.1007/s13204-021-02041-w. [DOI] [Google Scholar]
- 192.Chemingui H, Smiri M, Missaoui T, Hafiane A. Zinc oxide nanoparticles induced oxidative stress and changes in the photosynthetic apparatus in fenugreek (Trigonella foenum graecum L.) Bulletin of Environmental Contamination and Toxicology. 2019;102(4):477–485. doi: 10.1007/s00128-019-02590-5. [DOI] [PubMed] [Google Scholar]
- 193.Chen B, He Y, Xiao Y, Guo D, Liu P, He Y, Sun Q, Jiang P, Liu Z, Liu Q. Heated fennel therapy promotes the recovery of gastrointestinal function in patients after complex abdominal surgery: A single-center prospective randomized controlled trial in China. Surgery (United States) 2020;168(5):793–799. doi: 10.1016/j.surg.2020.05.040. [DOI] [PubMed] [Google Scholar]
- 194.Chen J, Ding J, Li D, Wang Y, Wu Y, Yang X, Chinnathambi A, Salmen SH, Ali Alharbi S. Facile preparation of Au nanoparticles mediated by Foeniculum Vulgare aqueous extract and investigation of the anti-human breast carcinoma effects. Arabian Journal of Chemistry. 2022;15(1):103479. doi: 10.1016/j.arabjc.2021.103479. [DOI] [Google Scholar]
- 195.Chen X, Zou LQ, Niu J, Liu W, Peng SF, Liu CM. The stability, sustained release and cellular antioxidant activity of curcumin nanoliposomes. Molecules. 2015;20(8):14293–14311. doi: 10.3390/molecules200814293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cheng C-Y, Yang A-J, Ekambaranellore P, Huang K-C, Lin W-W. Antiobesity action of INDUS810, a natural compound from trigonella foenum graecum AMPK dependent lipolysis effect in adipocytes. Obesity Research & Clinical Practice. 2018;12(6):562–569. doi: 10.1016/j.orcp.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 197.Cheshmeh, S., Elahi, N., Ghayyem, M., Moradi, S., Pasdar, Y., & Tahmasebi, S. (2021). Effects of green cardamom supplementation on obesity and diabetes gene expression among obese women with polycystic ovary syndrome; a double blind randomized controlled trialResearch Square, PREPRINT(version 1), 1–12
- 198.Chin, S. F., Mohd Yazid, S. N. A., & Pang, S. C. (2014). Preparation and characterization of starch nanoparticles for controlled release of curcuminInternational Journal of Polymer Science,201410.1155/2014/340121
- 199.Choi, H.-Y. (2009). Antioxidant activity of Curcuma longa l., novel foodstuff. In Molecular and Cellular Toxicology (Vol. 5, Issue 3, pp. 237–242)
- 200.Choudhary MK, Kataria J, Sharma S. A biomimetic synthesis of stable gold nanoparticles derived from aqueous extract of Foeniculum vulgare seeds and evaluation of their catalytic activity. Applied Nanoscience (Switzerland) 2017;7(7):439–447. doi: 10.1007/s13204-017-0589-4. [DOI] [Google Scholar]
- 201.Chouksey, D., Sharma, P., & Pawar, R. S. (2010). Biological activities and chemical constituents of Illicium verum hook fruits (chinese star anise)Der Pharmacia Sinica, 1(3), 1–10http://www.imedpub.com/articles/biological-activities-and-chemical-constituents-of-illicium-verum-hook-fruits-chinese-star-anise.pdf
- 202.Chouksey D, Upmanyu N, Pawar RS. Central nervous system activity of Illicium verum fruit extracts. Asian Pacific Journal of Tropical Medicine. 2013;6(11):869–875. doi: 10.1016/S1995-7645(13)60155-8. [DOI] [PubMed] [Google Scholar]
- 203.Chouni A, Paul S. A review on phytochemical and pharmacological potential of Alpinia galanga. Pharmacognosy Journal. 2018;10(1):9–15. doi: 10.5530/pj.2018.1.2. [DOI] [Google Scholar]
- 204.Chowdhury S, Kumar S. Alpha-terpinyl acetate: A natural monoterpenoid from Elettaria cardamomum as multi-target directed ligand in Alzheimer’s disease. Journal of Functional Foods. 2020;68(February):103892. doi: 10.1016/j.jff.2020.103892. [DOI] [Google Scholar]
- 205.Chudiwal AK, Jain DP, Somani RS. Alpinia galanga willd.- an overview on phyto-pharmacological properties. Indian Journal of Natural Products and Resources. 2010;1(2):143–149. [Google Scholar]
- 206.Chun S, Muthu M, Gansukh E, Thalappil P, Gopal J. The ethanopharmacological aspect of carbon nanodots in turmeric smoke. Scientific Reports. 2016;6(November):1–12. doi: 10.1038/srep35586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cicero N, Gervasi T, Durazzo A, Lucarini M, Macrì A, Nava V, Giarratana F, Tardugno R, Vadalà R, Santini A. Mineral and microbiological analysis of spices and aromatic herbs. Foods. 2022;11(4):1–12. doi: 10.3390/foods11040548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Clemente I, Aznar M, Nerín C. Synergistic properties of mustard and cinnamon essential oils for the inactivation of foodborne moulds in vitro and on Spanish bread. International Journal of Food Microbiology. 2019;298:44–50. doi: 10.1016/j.ijfoodmicro.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 209.Cortez MV, Perovic NR, Soria EA, Defagó MD. Effect of heat and microwave treatments on phenolic compounds and fatty acids of turmeric (curcuma longa l.) and saffron (crocus sativus l.) Brazilian Journal of Food Technology. 2020;23:1–7. doi: 10.1590/1981-6723.20519. [DOI] [Google Scholar]
- 210.Courrol, D. dos S., Teixeira, B. H., & Pereira, C. B. P. (2016). Pegylated curcumin with gold nanoparticles: antimicrobial agent evaluationJournal of Biomedical Engineering and Biosciences, 310.11159/jbeb.2016.008
- 211.Cusano E, Consonni R, Petrakis EA, Astraka K, Cagliani LR, Polissiou MG. Integrated analytical methodology to investigate bioactive compounds in Crocus sativus L. flowers. Phytochemical Analysis. 2018;29(5):476–486. doi: 10.1002/pca.2753. [DOI] [PubMed] [Google Scholar]
- 212.Da Silveira SM, Luciano FB, Fronza N, Cunha A, Scheuermann GN, Vieira CRW. Chemical composition and antibacterial activity of Laurus nobilis essential oil towards foodborne pathogens and its application in fresh Tuscan sausage stored at 7°C. LWT - Food Science and Technology. 2014;59(1):86–93. doi: 10.1016/j.lwt.2014.05.032. [DOI] [Google Scholar]
- 213.Dager A, Baliyan A, Kurosu S, Maekawa T, Tachibana M. Ultrafast synthesis of carbon quantum dots from fenugreek seeds using microwave plasma enhanced decomposition: Application of C-QDs to grow fluorescent protein crystals. Scientific Reports. 2020;10(1):1–15. doi: 10.1038/s41598-020-69264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dager A, Uchida T, Maekawa T, Tachibana M. Synthesis and characterization of Mono-disperse Carbon Quantum Dots from Fennel Seeds: Photoluminescence analysis using Machine Learning. Scientific Reports. 2019;9(1):1–10. doi: 10.1038/s41598-019-50397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Daisy, E., Angelina, R., Bavyaa, R., & Rajagopal, R. (2013). Green synthesis and characterization of silver nanoparticles using fenugreek seed extractInternational Journal of Scientific and Research Publications, 3(7), 2250–3153www.ijsrp.org
- 216.Dall’Acqua S, Cervellati R, Speroni E, Costa S, Guerra MC, Stella L, Greco E, Innocenti G. Phytochemical composition and antioxidant activity of Laurus nobilis L. leaf infusion. Journal of Medicinal Food. 2009;12(4):869–876. doi: 10.1089/jmf.2008.0119. [DOI] [PubMed] [Google Scholar]
- 217.Dall’Acqua S, Viola G, Giorgetti M, Loi MC, Innocenti G. Two new sesquiterpene lactones from the leaves of Laurus nobilis. Chemical and Pharmaceutical Bulletin. 2006;54(8):1187–1189. doi: 10.1248/cpb.54.1187. [DOI] [PubMed] [Google Scholar]
- 218.Dalvandi F, Almasi H, Ghanbarzadeh B, Hosseini H, Karimian N. Effect of vacuum packaging and edible coating containing black pepper seeds and turmeric extracts on shelf life extension of chicken breast fillets. Journal of Food and Bioprocess Engineering. 2020;3(1):69–78. [Google Scholar]
- 219.Dam D, Banerjee K, Mukhopadhyay A. 519P Lanthanum nanoparticles synthesized from fenugreek seed extract as targeted therapy for osteosarcoma. Annals of Oncology. 2016;27:ix168. doi: 10.1016/s0923-7534(21)00677-3. [DOI] [Google Scholar]
- 220.Damanhouri, Z. A. (2014). A review on therapeutic potential of Piper nigrum l. (black pepper): the king of spicesMedicinal & Aromatic Plants, 03(03)10.4172/2167-0412.1000161
- 221.Daneshi-Maskooni, M., Keshavarz, S. A., Qorbani, M., Mansouri, S., Alavian, S. M., Badri-Fariman, M., Jazayeri-Tehrani, S. A., & Sotoudeh, G. (2018). Green cardamom increases Sirtuin-1 and reduces inflammation in overweight or obese patients with non-alcoholic fatty liver disease: A double-blind randomized placebo-controlled clinical trialNutrition and Metabolism, 15(1)10.1186/s12986-018-0297-4 [DOI] [PMC free article] [PubMed]
- 222.Daru M. Partnership expansion between farmers and the herbal medicine industry for community economic development. E3S Web of Conferences. 2021;306:02006. doi: 10.1051/e3sconf/202130602006. [DOI] [Google Scholar]
- 223.Das A, Roy A, Rajeshkumar S, Lakshmi T. Anti-inflammatory activity of turmeric oil mediated silver nanoparticles. Research Journal of Pharmacy and Technology. 2019;12(7):3507–3510. doi: 10.5958/0974-360X.2019.00596.1. [DOI] [Google Scholar]
- 224.Das G, Patra JK, Gonçalves S, Romano A, Gutiérrez-Grijalva EP, Heredia JB, Talukdar AD, Shome S, Shin HS. Galangal, the multipotent super spices: A comprehensive review. Trends in Food Science and Technology. 2020;101(May):50–62. doi: 10.1016/j.tifs.2020.04.032. [DOI] [Google Scholar]
- 225.Das RK, Kasoju N, Bora U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine: Nanotechnology, Biology and Medicine. 2010;6(1):153–160. doi: 10.1016/j.nano.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 226.Das S, Singh VK, Dwivedy AK, Chaudhari AK, Dubey NK. Anethum graveolens essential oil encapsulation in chitosan nanomatrix: investigations on in vitro release behavior, organoleptic attributes, and efficacy as potential delivery vehicles against biodeterioration of rice (Oryza sativa l.) Food and Bioprocess Technology. 2021;14(5):831–853. doi: 10.1007/s11947-021-02589-z. [DOI] [Google Scholar]
- 227.Das S, Singh VK, Dwivedy AK, Chaudhari AK, Upadhyay N, Singh A, Deepika, Dubey NK. Fabrication, characterization and practical efficacy of Myristica fragrans essential oil nanoemulsion delivery system against postharvest biodeterioration. Ecotoxicology and Environmental Safety. 2020;189:110000. doi: 10.1016/j.ecoenv.2019.110000. [DOI] [PubMed] [Google Scholar]
- 228.Database, U. S. nutrition. (2021)Galangal: health benefits, nutrition, uses in ayurveda, recipes, side effects
- 229.de Araujo CIM, Bonato LB, Mangucci CB, Malpass GRP, Okura MH, Granato AC. Comparison of biopolymer-based edible coatings incorporating Piper nigrum and Schinus terebinthifolia applied on minimally processed pineapple. British Food Journal. 2022;124(4):1274–1284. doi: 10.1108/BFJ-04-2021-0453. [DOI] [Google Scholar]
- 230.De M, De AK, Banerjee AB. Antimicrobial screening of some Indian spices. Phytotherapy Research. 1999;13(7):616–618. doi: 10.1002/(SICI)1099-1573(199911)13:7<616::AID-PTR475>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 231.De M, De AK, Sen P, Banerjee AB. Antimicrobial properties of Star anise (Illicium verum Hook f) Phytotherapy Research. 2002;16(1):94–95. doi: 10.1002/ptr.989. [DOI] [PubMed] [Google Scholar]
- 232.De Sousa Leite, A., Dantas, A. F., Da Silva Oliveira, G. L., Gomes Júnior, A. L., De Lima, S. G., Das Graças Lopes Citó, A. M., De Freitas, R. M., Melo-Cavalcante, A. A. D. C., & Lopes, J. A. D. (2015). Evaluation of toxic, cytotoxic, mutagenic, and antimutagenic activities of natural and technical cashew nut shell liquids using the allium cepa and artemia salina bioassaysBioMed Research International,201510.1155/2015/626835 [DOI] [PMC free article] [PubMed]
- 233.Debnath P, Mondal A, Hajra A, Das C, Mondal NK. Cytogenetic effects of silver and gold nanoparticles on Allium cepa roots. Journal of Genetic Engineering and Biotechnology. 2018;16(2):519–526. doi: 10.1016/j.jgeb.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Deepa B, Anuradha CV. Antioxidant potential of Coriandrum sativum l. seed extract. Indian Journal of Experimental Biology. 2011;49(1):30–38. [PubMed] [Google Scholar]
- 235.Dehpour AA, Ebrahimzadeh MA, Fazel NS, Mohammad NS. Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas y Aceites. 2009;60(4):405–412. doi: 10.3989/gya.010109. [DOI] [Google Scholar]
- 236.Deka C, Aidew L, Devi N, Buragohain AK, Kakati DK. Synthesis of curcumin-loaded chitosan phosphate nanoparticle and study of its cytotoxicity and antimicrobial activity. Journal of Biomaterials Science, Polymer Edition. 2016;27(16):1659–1673. doi: 10.1080/09205063.2016.1226051. [DOI] [PubMed] [Google Scholar]
- 237.Demir E, Kaya N, Kaya B. Genotoxic effects of zinc oxide and titanium dioxide nanoparticles on root meristem cells of allium cepa by comet assay. Turkish Journal of Biology. 2014;38(1):31–39. doi: 10.3906/biy-1306-11. [DOI] [Google Scholar]
- 238.Deng M, Wen J, Zhu H, Zou X. The hottest pepper variety in China. Genetic Resources and Crop Evolution. 2009;56(5):605–608. doi: 10.1007/s10722-009-9445-z. [DOI] [Google Scholar]
- 239.Derwich E, Benziane Z, Boukir A. Chemical composition and antibacterial activity of Leaves essential oil of laurus nobilis from Morocco. Australian Journal of Basic and Applied Sciences. 2009;3(4):3818–3824. [Google Scholar]
- 240.Desai MP, Sangaokar GM, Pawar KD. Kokum fruit mediated biogenic gold nanoparticles with photoluminescent, photocatalytic and antioxidant activities. Process Biochemistry. 2018;70:188–197. doi: 10.1016/j.procbio.2018.03.027. [DOI] [Google Scholar]
- 241.Deshmukh, A. R., Gupta, A., & Kim, B. S. (2019). Ultrasound assisted green synthesis of silver and iron oxide nanoparticles using fenugreek seed extract and their enhanced antibacterial and antioxidant activitiesBioMed Research International,201910.1155/2019/1714358 [DOI] [PMC free article] [PubMed]
- 242.Dev M, Ghosh M, Bhattacharyya Dkumar. Physico-chemical, antimicrobial, and organoleptic properties of roasted aromatic spice (clove bud) in baked product. Applied Biochemistry and Biotechnology. 2021;193(6):1813–1835. doi: 10.1007/s12010-021-03504-0. [DOI] [PubMed] [Google Scholar]
- 243.Dewi, K., Widyarto, B., Erawijantari, P., & Widowati, W. (2015)In vitro study of Myristica fragrans seed (Nutmeg) ethanolic extract and quercetin compound as anti-inflammatory agentInternational Journal of Research in Medical Sciences, 2303–231010.18203/2320-6012.ijrms20150621
- 244.Dey, T., Ghosh, A., Mishra, S., Pal, P. K., Chattopadhyay, A., Pattari, S. K., & Bandyopadhyay, D. (2020). Attenuation of arsenic induced high fat diet exacerbated oxidative stress mediated hepatic and cardiac injuries in male Wistar rats by piperine involved antioxidative mechanismsFood and Chemical Toxicology,14210.1016/j.fct.2020.111477 [DOI] [PubMed]
- 245.Dhanya NP. Non linear optical investigations of silver nanoparticles synthesised by curcumin reduction. Optical Materials. 2017;73:384–387. doi: 10.1016/j.optmat.2017.08.026. [DOI] [Google Scholar]
- 246.Dhanyaraj D, Thomas A. Phyto-assisted synthesis of gold nanoparticles by aqueous extract of curcuma longa and the evaluation of total phenolic and flavonoid contents. Uttar Pradesh Journal of Zoology. 2021;42(18):82–88. [Google Scholar]
- 247.Dhanyaraj, D., Shine, F., Shibu, J, S, T., Akhila, T, A. (2019). Gold nanoparticles synthesis in extract of curcuma longa, evaluation of its total phenolic contentBISFAA
- 248.Dharmadasa RM, Abeysinghe DC, Abeywardhane KW, Dissanayake MN, Fernando NS. Leaf essential oil composition, antioxidant activity, total phenolic content and total flavonoid content of Pimenta dioica (l.)merr (myrtaceae): a superior quality spice grown in Sri Lanka. Universal Journal of Agricultural Research. 2015;3(2):49–52. doi: 10.13189/ujar.2015.030203. [DOI] [Google Scholar]
- 249.Dharman S, Maragathavalli, Rajeshkumar, Shanmugasundaram K. Ecofriendly synthesis, characterisation and antibacterial activity of curcumin mediated silver nanoparticles. International Journal of Dentistry and Oral Science. 2021;8(4):2314–2318. doi: 10.19070/2377-8075-21000457. [DOI] [Google Scholar]
- 250.Dhawi F, El-Beltagi HS, Aly E, Hamed AM. Antioxidant, antibacterial activities and mineral content of buffalo yoghurt fortified with fenugreek and Moringa oleifera seed flours. Foods. 2020;9(9):1157. doi: 10.3390/foods9091157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Dhillon GK, Amarjeet K. Quality evaluation of bread incorporated with different levels cinnamon powder. International Journal of Food Science, Nutrition and Dietetics. 2013;2(7):70–74. doi: 10.19070/2326-3350-1300013. [DOI] [Google Scholar]
- 252.Dhull, S. B., Punia, S., Sandhu, K. S., Chawla, P., Kaur, R., Singh, A. (2020). Effect of debittered fenugreek flour addition on physical, nutritional, antioxidant and sensory properties of wheat flour ruskLegume Science, 2(1)
- 253.Dhull SB, Bangar SP, Deswal R, Dhandhi P, Kumar M, Trif M, Rusu A. Development and characterization of active native and cross-linked pearl millet starch-based film loaded with fenugreek oil. Foods. 2021;10(12):3097. doi: 10.3390/foods10123097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Din ZU, Shad AA, Bakht J, Ullah I, Jan S. In vitro antimicrobial, antioxidant activity and phytochemical screening of Apium graveolens. Pakistan Journal of Pharmaceutical Sciences. 2015;28(5):1699–1704. [PubMed] [Google Scholar]
- 255.Divya KS, Harshitha BS, Kumar RD, Bala CJ. In vitro investigation of antioxidant potentiality of methanol and silver nanoparticles extract from Trigonella foenum-graecum. Journal of Pharmacognosy and Phytochemistry. 2019;8(3):2213–2221. [Google Scholar]
- 256.Dobroslavic E, Repajic M, Dragovic- Uzelac V, Garofulic IE. Isolation of laurus nobilis leaf polyphenols: A review on current techniques and future perspectives. Foods. 2022;11:235. doi: 10.3390/foods11020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Dossa, F. (2018). Onion (Allium Cepa) production in urban and peri-urban areas: financial performance and importance of this activity for market gardeners in southern beninCurrent Investigations in Agriculture and Current Research, 3(2)10.32474/ciacr.2018.03.000159
- 258.Dougnon TJ, Kiki P, Dougnon TV, Youssao I. Evaluation of Capsicum frutescens powder effects on the growth performances, biochemical and hematological parameters in Hubbard broiler. Journal of Applied Pharmaceutical Science. 2014;4(10):38–43. doi: 10.7324/JAPS.2014.40107. [DOI] [Google Scholar]
- 259.Doweidar MM, Amer AM, Tawfek A. Preparation and evaluation of healthy cinnamon cake. Egyptian Journal of Nutrition. 2016;XXXI(4):157–195. [Google Scholar]
- 260.Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs. 2007;67(3):351–368. doi: 10.2165/00003495-200767030-00003. [DOI] [PubMed] [Google Scholar]
- 261.Du WX, Olsen CW, Avena-Bustillos RJ, McHugh TH, Levin CE, Mandrell R, Friedman M. Antibacterial effects of allspice, garlic, and oregano essential oils in tomato films determined by overlay and vapor-phase methods. Journal of Food Science. 2009;74(7):390–397. doi: 10.1111/j.1750-3841.2009.01289.x. [DOI] [PubMed] [Google Scholar]
- 262.Duan J, Zhang Y, Han S, Chen Y, Li B, Liao M, Chen W, Deng X, Zhao J, Huang B. Synthesis and in vitro/in vivo anti-cancer evaluation of curcumin-loaded chitosan/poly(butyl cyanoacrylate) nanoparticles. International Journal of Pharmaceutics. 2010;400(1–2):211–220. doi: 10.1016/j.ijpharm.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 263.Dwivedi P. Curcuma longa aided Ag/CS nanocomposite coating of surfaces as SARS-CoV-2 contamination minimizing measure towards containment of COVID-19: a perspective. Letters in Applied NanoBioScience. 2020;9(4):1485–1493. doi: 10.33263/lianbs94.14851493. [DOI] [Google Scholar]
- 264.Echegoyen Y, Nerín C. Performance of an active paper based on cinnamon essential oil in mushrooms quality. Food Chemistry. 2015;170:30–36. doi: 10.1016/j.foodchem.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 265.EFSA Scientific Commitee Guidance for risk assessment of engineered nanomaterials. EFSA Journal. 2011;9(5):2–36. [Google Scholar]
- 266.Ehsani A, Hashemi M, Naghibi SS, Mohammadi S, Khalili Sadaghiani S. Properties of Bunium persicum essential oil and its application in Iranian white cheese against Listeria monocytogenes and Escherichia coli O157:H7. Journal of Food Safety. 2016;36(4):563–570. doi: 10.1111/jfs.12277. [DOI] [Google Scholar]
- 267.El-Batal, A. I., Ahmed, N. H., Barakat, L. A. A., & Khirallah, S. M. (2019). Tumoricidal effect of Trigonella foenum-graceum extract and selenium nanoparticles on ehrlich carcinoma bearing miceAsian Journal of Research in Biochemistry, March, 1–1610.9734/ajrb/2019/v4i130059
- 268.El-Naggar EA. Influence of fenugreek seeds flour on the rheological characteristics of wheat flour and biscuit quality. Zagazig Journal of Agricultural Research. 2019;46(3):721–738. doi: 10.21608/zjar.2019.40961. [DOI] [Google Scholar]
- 269.El-Soud NA, El-Laithy N, El-Saeed G, Wahby MS, Khalil M, Morsy F, Shaffie N. Antidiabetic activities of foeniculum vulgare mill. essential oil in streptozotocin-induced diabetic rats. Macedonian Journal of Medical Sciences. 2011;4(2):139–146. doi: 10.3889/MJMS.1857-5773.2011.0173. [DOI] [Google Scholar]
- 270.El Gizawy, H. A., Boshra, S. A., Mostafa, A., Mahmoud, S. H., Ismail, M. I., Alsfouk, A. A., Taher, A. T., & Al-Karmalawy, A. A. (2021). Pimenta dioica (L.) merr. bioactive constituents exert anti-sars-cov-2 and anti-inflammatory activities: Molecular docking and dynamics, in vitro, and in vivo studiesMolecules, 26(19)10.3390/molecules26195844 [DOI] [PMC free article] [PubMed]
- 271.El Gohary EGE, Farag SM, El-Sayed AA, Khattab RR, Mahmoud DM. Insecticidal activity and biochemical study of the clove oil (Syzygium aromaticum) nano-formulation on culex pipiens l. (diptera: Culicidae) Egyptian Journal of Aquatic Biology and Fisheries. 2021;25(1):227–239. doi: 10.21608/ejabf.2021.137233. [DOI] [Google Scholar]
- 272.El Khoury E, Abiad M, Kassaify ZG, Patra D. Green synthesis of curcumin conjugated nanosilver for the applications in nucleic acid sensing and anti-bacterial activity. Colloids and Surfaces B: Biointerfaces. 2015;127:274–280. doi: 10.1016/j.colsurfb.2015.01.050. [DOI] [PubMed] [Google Scholar]
- 273.Elbialy NS, Abdelfatah EA, Khalil WA. Antitumor activity of curcumin-green synthesized gold nanoparticles: In vitro study. BioNanoScience. 2019;9(4):813–820. doi: 10.1007/s12668-019-00660-w. [DOI] [Google Scholar]
- 274.Elmasry, T. A., Al-Shaalan, N. H., Tousson, E., El-Morshedy, K., & Al-Ghadeer, A. (2018). Star anise extracts modulation of reproductive parameters, fertility potential and DNA fragmentation induced by growth promoter equigan in rat testesBrazilian Journal of Pharmaceutical Sciences, 54(1)10.1590/s2175-97902018000117261
- 275.ElMitwalli, O. S., Barakat, O. A., Daoud, R. M., Akhtar, S., & Henari, F. Z. (2020). Green synthesis of gold nanoparticles using cinnamon bark extract, characterization, and fluorescence activity in Au/eosin Y assembliesJournal of Nanoparticle Research, 22(10)10.1007/s11051-020-04983-8
- 276.Elsayid MA, Mohamed HA, Hassan SA, Khidi AM, Shaddad SA. Antifungal effect of gold as nanoparticle synthesis by fenugreek seed extract. World Journal of Pharmaceutical Research. 2018;8(1):1–9. [Google Scholar]
- 277.Elsiddig, E. S. E. H. (2022). Green synthesis of silver nano-particles by using fenugreek seeds extract and application as antifungal agentSUST Repository
- 278.Embuscado, M. E. (2015). Spices and herbs: natural sources of antioxidants - a mini review. In Journal of Functional Foods (Vol. 18, pp. 811–819). Elsevier Ltd10.1016/j.jff.2015.03.005
- 279.Eram, S., Mujahid, M., Bagga, P., Ansari, V. A., Ahmad, M. A., Kumar, A., Ahsan, F., & Akhter, M. S. (2019). A review on phytopharmacological activity of Alpinia galangaInternational Journal of Pharmacy and Pharmaceutical Sciences, 6–1110.22159/ijpps.2019v11i3.31352
- 280.Ercin E, Kecel-Gunduz S, Gok B, Aydin T, Budama-Kilinc Y, Kartal M. Laurus nobilis L. essential oil-loaded PLGA as a nanoformulation candidate for cancer treatment. Molecules. 2022;27(6):1–20. doi: 10.3390/molecules27061899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ereifej KI, Feng H, Rababah TM, Tashtoush SH, Al-U’datt MH, Al-Rabadi GJ, Torley P, Alkasrawi M. Microbiological status and nutritional composition of spices used in food preparation. Food and Nutrition Sciences. 2015;06(12):1134–1140. doi: 10.4236/fns.2015.612118. [DOI] [Google Scholar]
- 282.Eresam EKK, Pinto S, Aparnathi KD. Concise and informative title: Evaluation of selected spices in extending shelf life of paneer. Journal of Food Science and Technology. 2015;52(4):2043–2052. doi: 10.1007/s13197-013-1226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Erna KH, Felicia WXL, Rovina K, Vonnie JM, Huda N. Development of curcumin/rice starch films for sensitive detection of hypoxanthine in chicken and fish meat. Carbohydrate Polymer Technologies and Applications. 2022;3:100189. doi: 10.1016/j.carpta.2022.100189. [DOI] [Google Scholar]
- 284.Eskin NAM, Raju J, Bird RP. Novel mucilage fraction of Sinapis alba L. (mustard) reduces azoxymethane-induced colonic aberrant crypt foci formation in F344 and Zucker obese rats. Phytomedicine. 2007;14(7–8):479–485. doi: 10.1016/j.phymed.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 285.Esmaeili H, Hafezimoghadam Z, Esmailidehaj M, Rezvani ME, Hafizibarjin Z. The effect of asafoetida essential oil on myocardial ischemic-reperfusion injury in isolated rat hearts. Avicenna Journal of Phytomedicine (Ajp) 2018;8(4):338–349. doi: 10.22038/ajp.2018.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Etikala A, Thamburaj S, Johnson AM, Sarma C, Mummaleti G, Kalakandan SK. Incidence, toxin gene profile, antibiotic resistance and antibacterial activity of Allium parvum and Allium cepa extracts on Bacillus cereus isolated from fermented millet-based food. Lwt. 2022;160(August 2021):113314. doi: 10.1016/j.lwt.2022.113314. [DOI] [Google Scholar]
- 287.Đorđević, B. S., Todorović, Z. B., Troter, D. Z., Stanojević, L, P., Stojanović, G. S., Đalović, I. G., Mitrović, P. M., & Veljković V. B. (2021). Extraction of phenolic compounds from black mustard (Brassica nigra L.) seed by deep eutectic solventsJournal of Food Measurement and Characterization, 15(2), 1931–1938
- 288.Fadaka A, Aluko O, Awawu S, Theledi K. Green synthesis of gold nanoparticles using Pimenta dioica leaves aqueous extract and their application as photocatalyst, antioxidant, and antibacterial agents. Journal of Multidisciplinary Applied Natural Science. 2021;1(2):78–88. doi: 10.47352/jmans.v1i2.81. [DOI] [Google Scholar]
- 289.Faisal S, Al-Radadi NS, Jan H, Abdullah, Shah SA, Shah S, Rizwan M, Afsheen Z, Hussain Z, Uddin MN, Idrees M, Bibi N. Curcuma longa mediated synthesis of copper oxide, nickel oxide and Cu-Ni bimetallic hybrid nanoparticles: Characterization and evaluation for antimicrobial, anti-parasitic and cytotoxic potentials. Coatings. 2021;11(7):1–22. doi: 10.3390/coatings11070849. [DOI] [Google Scholar]
- 290.Faizal P, Suresh S, Satheesh Kumar R, Augusti KT. A study on the hypoglycemic and hypolipidemic effects of an ayurvedic drug Rajanyamalakadi in diabetic patients. Indian Journal of Clinical Biochemistry. 2009;24(1):82–87. doi: 10.1007/s12291-009-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Fathima R, Mujeeb A. Plasmon enhanced linear and nonlinear optical properties of natural curcumin dye with silver nanoparticles. Dyes and Pigments. 2021;189:109256. doi: 10.1016/j.dyepig.2021.109256. [DOI] [Google Scholar]
- 292.Fatima M, Zaidi NSS, Amraiz D, Afzal F. In vitro antiviral activity of Cinnamomum cassia and its nanoparticles against H7N3 influenza a virus. Journal of Microbiology and Biotechnology. 2015;26(1):151–159. doi: 10.4014/jmb.1508.08024. [DOI] [PubMed] [Google Scholar]
- 293.Fazal, S., & Singla, R. (2012). Review on the pharmacognostical & pharmacological characterization of Apium graveolens linnIndo Global Journal of Pharmaceutical Sciences, 2(1), 36–42http://iglobaljournal.com/wp-content/uploads/2012/05/3.-Fazal-Singla-2012.pdf
- 294.Feretti D, Zerbini I, Zani C, Ceretti E, Moretti M, Monarca S. Allium cepa chromosome aberration and micronucleus tests applied to study genotoxicity of extracts from pesticide-treated vegetables and grapes. Food Additives and Contaminants. 2007;24(6):561–572. doi: 10.1080/02652030601113602. [DOI] [PubMed] [Google Scholar]
- 295.Ferfera-Harrar H, Berdous D, Benhalima T. Hydrogel nanocomposites based on chitosan-g-polyacrylamide and silver nanoparticles synthesized using Curcuma longa for antibacterial applications. Polymer Bulletin. 2018;75(7):2819–2846. doi: 10.1007/s00289-017-2183-z. [DOI] [Google Scholar]
- 296.Ferreira LS, Chaves MA, Dacanal GC, Pinho SC. Wet agglomeration by high shear of binary mixtures of curcumin-loaded lyophilized liposomes and cornstarch: Powder characterization and incorporation in cakes. Food Bioscience. 2018;25:74–82. doi: 10.1016/j.fbio.2018.08.003. [DOI] [Google Scholar]
- 297.Ferreira MI, Magro M, Ming LC, da Silva MB, Ormond Sobreira Rodrigues LF, Zanoni do Prado D, Bonaiuto E, Baratella D, De Almeida Roger J, Pace Pereira Lima G, Rossetto M, Zennaro L, Vianello F. Sustainable production of high purity curcuminoids from Curcuma longa by magnetic nanoparticles: A case study in Brazil. Journal of Cleaner Production. 2017;154:233–241. doi: 10.1016/j.jclepro.2017.03.218. [DOI] [Google Scholar]
- 298.Fidan H, Stefanova G, Kostova I, Stankov S, Damyanova S, Stoyanova A, Zheljazkov VD. Chemical composition and antimicrobial activity of Laurus nobilis l essential oils from bulgaria. Molecules. 2019;24(4):1–10. doi: 10.3390/molecules24040804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Firdhouse MJ, Lalitha P. Formulations of sunscreen lotions using Acorus calamus and zinc oxide nanoparticles and their in vitro evaluation of sun protection factor ( Spf ) World Journal of Pharmaceutical Research. 2018;7(9):510–515. doi: 10.20959/wjpr20189-11562. [DOI] [Google Scholar]
- 300.Fouad AS, Hafez RM. The effects of silver ions and silver nanoparticles on cell division and expression of cdc2 gene in Allium cepa root tips. Biologia Plantarum. 2018;62(1):166–172. doi: 10.1007/s10535-017-0751-6. [DOI] [Google Scholar]
- 301.Fragoon A, Frah L, Mamoun A. Biosynthesis of gold nanoparticles by fenugreek (Trigonella foenum-graecum) extract. Advances in Science, Technology and Engineering Systems. 2016;1(5):50–55. doi: 10.25046/aj010509. [DOI] [Google Scholar]
- 302.Fragoon, A., Mamoun, A., Frah, L., & Abd Alwahab, S. (2016). Biosynthesis of gold nanoparticle by fenugreek seed (trigonella foenum) extractProceedings - 2015 International Conference on Computing, Control, Networking, Electronics and Embedded Systems Engineering, ICCNEEE 2015, 388–39110.1109/ICCNEEE.2015.7381397
- 303.Frangopoulos T. Incorporation of Trigonella Foenum-Graecum seed powder in meat emulsion systems with olive oil: Effects on physicochemical, texture, and color characteristics. Journal of Food Science and Technology. 2021 doi: 10.1007/s13197-021-05220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Freitas RM. Investigation of oxidative stress involvement in hippocampus in epilepsy model induced by pilocarpine. Neuroscience Letters. 2009;462(3):225–229. doi: 10.1016/j.neulet.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 305.Galeano YL, Torres OV, Garcia AS. Evaluation of nutmeg as active component during storage of bovine loins. Revista de Ciencias Agricolas. 2018;35(1):48–57. doi: 10.22267/rcia.183501.82. [DOI] [Google Scholar]
- 306.Gandapu, U., Chaitanya, R. K., Kishore, G., Reddy, R. C., & Kondapi, A. K. (2011). Curcumin-loaded apotransferrin nanoparticles provide efficient cellular uptake and effectively inhibit HIV-1 replication In vitroPLoS ONE, 6(8)10.1371/journal.pone.0023388 [DOI] [PMC free article] [PubMed]
- 307.Ganesan RM, Gurumallesh Prabu H. Synthesis of gold nanoparticles using herbal Acorus calamus rhizome extract and coating on cotton fabric for antibacterial and UV blocking applications. Arabian Journal of Chemistry. 2019;12(8):2166–2174. doi: 10.1016/j.arabjc.2014.12.017. [DOI] [Google Scholar]
- 308.Ganesan, S., Mehalingam, P., & Selvam, G. S. (2019). Green synthesis of silver nanoparticles from de-oiled rhizomes of Curcuma longa L. and its biomedical potentialSpringer Proceedings in Materials, 94–10610.1007/978-3-030-25135-2_10
- 309.Gao, H., Cheng, C., Fang, S., McClements, D. J., Ma, L., Chen, X., Zou, L., Liang, R., & Liu, W. (2022). Study on curcumin encapsulated in whole nutritional food model milk: Effect of fat content, and partitioning situationJournal of Functional Foods,9010.1016/j.jff.2022.104990
- 310.Garcia-Amezquita LE, Tejada-Ortigoza V, Campanella OH, Welti-Chanes J. Influence of drying method on the composition, physicochemical properties, and prebiotic potential of dietary fibre concentrates from fruit peels. Journal of Food Quality. 2018 doi: 10.1155/2018/9105237. [DOI] [Google Scholar]
- 311.García-González CA, Silvar C. Phytochemical assessment of native ecuadorian peppers (Capsicum spp.) and correlation analysis to fruit phenomics. Plants. 2020;9(8):1–25. doi: 10.3390/plants9080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Garg, S. (2012). Rapid biogenic synthesis of silver nanoparticles using black pepper (Piper nigrum) corn extract Indo-Hungarian project View project Nanoparticle, wound healing, anti-cancer studies View projectInternational Journal of Innovations in Biological and Chemical Sciences, 3(July), 5–10https://www.researchgate.net/publication/280007266
- 313.Garg S, Garg A. Encapsulation of curcumin in silver nanoparticle for enhancement of anticancer drug delivery. International Journal of Pharmaceutical Sciences and Research. 2018;9(3):1160. doi: 10.13040/IJPSR.0975-8232.9(3).1160-66. [DOI] [Google Scholar]
- 314.Gaur GK, Rani R, Dharaiya CN. Development of herbal milk using tulsi juice, ginger juice and turmeric powder. International Journal of Chemical Studies. 2019;7(2):1150–1157. [Google Scholar]
- 315.Gaurav I, Tanuja Green synthesis and characterization of silver nanoparticles with Rhizome extract of Curcuma longa (AgNPs-RECL) for Antimicrobial activity towards Xanthomonas and Erwinia species. Research Journal of Pharmacy and Technology. 2021;14(1):325–330. doi: 10.5958/0974-360x.2021.00060.3. [DOI] [Google Scholar]
- 316.Gazwi HSS, Mahmoud ME, Toson EMA. Analysis of the phytochemicals of Coriandrum sativum and Cichorium intybus aqueous extracts and their biological effects on broiler chickens. Scientific Reports. 2022;12(1):1–11. doi: 10.1038/s41598-022-10329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Geetha, A. R., George, E., Srinivasan, A., & Shaik, J. (2013). Optimization of green synthesis of silver nanoparticles from leaf extracts of pimenta dioica (Allspice)The Scientific World Journal, 2013(i)10.1155/2013/362890 [DOI] [PMC free article] [PubMed]
- 318.George JM, Mathew B. Curcuma longa rhizome extract mediated unmodified silver nanoparticles as multisensing probe for Hg(II) ions. Materials Research Express. 2019;6(11):1150h5. doi: 10.1088/2053-1591/ab5240. [DOI] [Google Scholar]
- 319.George R, Roy A, Rajeshkumar S, Lakshmi T. Coriander oleoresin assisted synthesis and characterization of silver nanoparticles and its antioxidant activity. Biomedicine (India) 2020;40(3):309–312. doi: 10.51248/.v40i3.15. [DOI] [Google Scholar]
- 320.Ghafari, S., Esmaeili, S., Naghibi, F., & Mosaddegh, M. (2013). Plants used to treat “tabe rebá” (malaria like fever) in Iranian Traditional MedicineInternational Journal of Traditional and Herbal Medicine, 1(6), 168–176http://www.ijthmjournal.com
- 321.Ghaffari S, Roshanravan N. Saffron; an updated review on biological properties with special focus on cardiovascular effects. Biomedicine and Pharmacotherapy. 2019;109(May 2018):21–27. doi: 10.1016/j.biopha.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 322.Ghannadi A, Fattahian K, Shokoohinia Y, Behbahani M, Shahnoush A. Anti-viral evaluation of sesquiterpene coumarins from ferula assa-foetida against HSV-1. Iranian Journal of Pharmaceutical Research. 2014;13(2):523–530. [PMC free article] [PubMed] [Google Scholar]
- 323.Ghinny DA, Alsafi MY, Al-Bashir RM, Elsammani TO, Alshreef AM, El-hadiyah TMH, Saeed AA. Investigation of anti-obesity activity of ethanolic extract of Foeniculum vulgare seeds, in vivo and in silico models. World Journal of Pharmacy and Pharmaceutical Sciences. 2019;8(11):111–124. doi: 10.20959/wjpps201911-14880. [DOI] [Google Scholar]
- 324.Ghosh S, Sengupta J, Datta P, Gomes A. Hematopoietic and antioxidant activities of gold nanoparticles synthesized by aqueous extract of fenugreek ( Trigonella foenum-graecum ) seed. Advanced Science, Engineering and Medicine. 2014;6(5):546–552. doi: 10.1166/asem.2014.1511. [DOI] [Google Scholar]
- 325.Gingasu D, MÎNdru I, Patron L, Marinescu G, Ianculescu A, Surdu VA, Somacescu S, Preda S, Oprea O. Synthesis of cobalt aluminate nanoparticles by combustion methods using cinnamon bark extract. Revue Roumaine de Chimie. 2018;63(5–6):459–466. [Google Scholar]
- 326.Gnanajobitha, G., Annadurai, G., & Kannan, C. (2012). Green synthesis of silver nanoparticle using Elettaria cardamomom and assesment of its antimicrobial activityInternational Journal of Pharmaceutical Sciences and Research (IJPSR), 3(3), 323–330http://www.ijpsr.info/docs/IJPSR12-03-03-011.pdf
- 327.Godebo DD, Dessalegn E, Niguse G. Nutritional composition, microbial load and sensory properties of fenugreek (Trigonella foenum-graecum l.) flour substituted Injera. Journal of Food Processing & Technology. 2019;10(7):1–7. doi: 10.4172/2157-7110.1000799. [DOI] [Google Scholar]
- 328.Goel A, Ajaikumar B, Kunnumakkara B, Bharat B, Aggarwal B. Curcumin as ‘“curecumin”’: From kitchen to clinic. Biochemical Pharmacology. 2007;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 329.Goel, B., & Mishra, S. (2020). Medicinal and nutritional perspective of cinnamon: a mini-reviewEuropean Journal of Medicinal Plants, March, 10–1610.9734/ejmp/2020/v31i330218
- 330.Gök V. Effect of replacing beef fat with poppy seed oil on quality of Turkish sucuk. Korean Journal for Food Science of Animal Resources. 2015;35(2):240–247. doi: 10.5851/kosfa.2015.35.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Golmohammadi, F. (2013). Medical plant of Ferula assa-foetida and its cultivating , main characteristics and economical importance in South khorasan province - east of IranGlobal Health, 2334–2346
- 332.Golubkina NA, Nadezhkin SM, Agafonov AF, Kosheleva OV, Molchanova AV, Russo G, Cuciniello A, Caruso G. Seed oil content, fatty acids composition and antioxidant properties as affected by genotype in Allium cepa L. and perennial onion species. Advances in Horticultural Science. 2015;29(4):199–206. doi: 10.13128/ahs-22738. [DOI] [Google Scholar]
- 333.Goodarzi, M. T., Khodadadi, I., Tavilani, H., & Abbasi Oshaghi, E. (2016). The role of Anethum graveolens l. (dill) in the management of diabetesJournal of Tropical Medicine, 201610.1155/2016/1098916 [DOI] [PMC free article] [PubMed]
- 334.Goswami M, Sharma BD, Mendiratta SK, Pathak V. Evaluation of quality characteristics low fat buffalo meat cookies incorporated with poppy seeds (Papaver somniferum) Buffalo Bulletin. 2018;37(4):535–544. [Google Scholar]
- 335.Goswami, N., & Chatterjee, S. (2014). Assessment of free radical scavenging potential and oxidative DNA damage preventive activity of Trachyspermum ammi l. (carom) and Foeniculum vulgare mill. (fennel) seed extractsBioMed Research International, 201410.1155/2014/582767 [DOI] [PMC free article] [PubMed]
- 336.Gottesman R, Shukla S, Perkas N, Solovyov LA, Nitzan Y, Gedanken A. Sonochemical coating of paper by microbiocidal silver nanoparticles. Langmuir. 2011;27(2):720–726. doi: 10.1021/la103401z. [DOI] [PubMed] [Google Scholar]
- 337.Goyal, D., Saini, A., Saini, G. S. S., & Kumar, R. (2019). Green synthesis of anisotropic gold nanoparticles using cinnamon with superior antibacterial activityMaterials Research Express, 6(7)10.1088/2053-1591/ab15a6
- 338.Goyal, S., Gupta, N., & Chatterjee, S. (2016). Investigating therapeutic potential of trigonella foenum-graecum L. As our defense mechanism against several human diseasesJournal of Toxicology, 201610.1155/2016/1250387 [DOI] [PMC free article] [PubMed]
- 339.Goyal V, Singh A, Singh J, Kaur H, Kumar S, Rawat M. Biogenically structural and morphological engineering of Trigonella foenum-graecum mediated SnO2 nanoparticles with enhanced photocatalytic and antimicrobial activities. Materials Chemistry and Physics. 2022;282:125946. doi: 10.1016/j.matchemphys.2022.125946. [DOI] [Google Scholar]
- 340.Granja Alvear, L. A. (2019). Evaluation of the reducing and capping agent of garlic and cinnamon bark extracts In the synthesis of silver nanoparticlesQuimica
- 341.Guerra, A. S., Hoyos, C. G., Velásquez-Cock, J., Vélez, L., Gañán, P., & Zuluaga, R. (2022). The effects of adding a gel-alike Curcuma longa l. suspension as color agent on some quality and sensory properties of yogurtMolecules, 27(3)10.3390/molecules27030946 [DOI] [PMC free article] [PubMed]
- 342.Gunasekera N, Wijeratnam SW, Perera S, Hewajulige I, Gunathilaka S, Paliyath G, Subramanian J. Extending storage life of mango (Mangifera indica L.) using a new edible wax formulation incorporated with hexanal and cinnamon bark oil. Tropical Agriculture. 2018;95(1):97–110. [Google Scholar]
- 343.Gunes-Bayir A, Bilgin MG, Kutlu SS, Demirci D, Gölgeci FN. Microbiological, chemical and sensory analyzes of produced probiotic yoghurts added clove and propolis. Icontech International Journal. 2020;4(2):1–14. doi: 10.46291/icontechvol4iss2pp1-14. [DOI] [Google Scholar]
- 344.Gupta J, Gupta R, Mathur K. Pharmacognostical, pharmacological and traditional perspectives of apium graveolens an ethnomedicinal plant. International Journal of Life Science and Pharma Research. 2019;9(3):38–47. [Google Scholar]
- 345.Gupta A, Mahajan S, Sharma R. Evaluation of antimicrobial activity of Curcuma longa rhizome extract against Staphylococcus aureus. Biotechnology Reports. 2015;6:51–55. doi: 10.1016/j.btre.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Gupta, E. (2020). Elucidating the phytochemical and pharmacological potential of Myristica fragrans (nutmeg)Ethnopharmacological Investigation of Indian Spices, January, 52–6110.4018/978-1-7998-2524-1.ch004
- 347.Güzel, Y., & Özyazıcı, G. (2021). Adoption of promising fenugreek (Trigonella foenum-graceum L.) genotypes for yield and quality characteristics in the semiarid climate of TurkeyAtmosphere, 12(9)10.3390/atmos12091199
- 348.Ha, P. T., Le, M. H., Hoang, T. M. N., Le, T. T. H., Duong, T. Q., Tran, T. H. H., Tran, D. L., & Nguyen, X. P. (2012). Preparation and anti-cancer activity of polymer-encapsulated curcumin nanoparticlesAdvances in Natural Sciences: Nanoscience and Nanotechnology, 3(3)10.1088/2043-6262/3/3/035002
- 349.Hadi SM, Etheb AM, Omran AH, Hassan SH. Characterization, biofilm and plasmid curing effect of silver nanoparticles synthesis by aqueous extract of Myristica fragrans seeds. Jordan Journal of Biological Sciences. 2022;15(1):149–158. doi: 10.54319/jjbs/150120. [DOI] [Google Scholar]
- 350.Hafez RM, Fouad AS. Mitigation of genotoxic and cytotoxic effects of silver nanoparticles on onion root tips using some antioxidant scavengers. Egyptian Journal of Botany. 2020;60(1):133–145. doi: 10.21608/ejbo.2019.13390.1321. [DOI] [Google Scholar]
- 351.Hajalizadeh Z, Dayani O, Khezri A, Tahmasbi R. Digestibility, ruminal characteristics, and meat quality of fattening lambs fed different levels of fennel (Foeniculum vulgare) seed powder. Journal of Livestock Science and Technologies. 2020;8(1):37–46. doi: 10.22103/jlst.2020.15855.1319. [DOI] [Google Scholar]
- 352.Hajhashemi V, Sajjadi SE, Zomorodkia M. Antinociceptive and anti-inflammatory activities of Bunium persicum essential oil, hydroalcoholic and polyphenolic extracts in animal models. Pharmaceutical Biology. 2011;49(2):146–151. doi: 10.3109/13880209.2010.504966. [DOI] [PubMed] [Google Scholar]
- 353.Hajji M, Falcimaigne-Gordin A, Ksouda G, Merlier F, Thomasset B, Nasri M. A water soluble polysaccharide from anethum graveolens seeds structural characterization, antioxidant activity and potential use as meat preservative. International Journal of Biological Macromolecules. 2021;167(15):516–527. doi: 10.1016/j.ijbiomac.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 354.Hameed IH, Hamza LF, Kamal SA. Analysis of bioactive chemical compounds of Aspergillus niger by using gas chromatography-mass spectrometry and fourier-transform infrared spectroscopy. Journal of Pharmacognosy and Phytotherapy. 2015;7(8):132–163. doi: 10.5897/JPP2015.0354. [DOI] [Google Scholar]
- 355.Handharyani E, Sutardi LN, Mustika AA, Andriani A, Yuliani S. Antibacterial activity of Curcuma longa (turmeric), Curcuma zedoaria (zedoary), and Allium sativum (garlic) nanoparticle extract on chicken with chronic respiratory disease complex: in vivo study. E3S Web of Conferences. 2020;151:1–5. doi: 10.1051/e3sconf/202015101054. [DOI] [Google Scholar]
- 356.Hariri A, Ouis N. Effect of volatile oils from Petroselinum crispum and Foeniculum vulgare on the quality and shelf-life of steamed yoghurts. Algerian Journal of Natural Products. 2020;1:723–731. [Google Scholar]
- 357.Harlina PW, Ma M, Shahzad R, Gouda MM, Qiu N. Effect of clove extract on lipid oxidation, antioxidant activity, volatile compounds and fatty acid composition of salted duck eggs. Journal of Food Science and Technology. 2018;55(12):4719–4734. doi: 10.1007/s13197-018-3367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Hasan, A. A., Abdullah, R. M., & Hameed, H. Q. (2020). Effect of trigonellafoenum extract and TiO2 nanoparticles against acinetobacter baumanniiAnnals of Tropical Medicine and Public Health, 23(18)10.36295/ASRO.2020.231814
- 359.Hashemi SMB, Jafarpour D. The efficacy of edible film from Konjac glucomannan and saffron petal extract to improve shelf life of fresh-cut cucumber. Food Science and Nutrition. 2020;8(7):3128–3137. doi: 10.1002/fsn3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Hassanzadazar H, Taami B, Aminzare M, Daneshamooz S. Bunium persicum (boiss.) b. fedtsch: an overview on phytochemistry, therapeutic uses and its application in the food industry. Journal of Applied Pharmaceutical Science. 2018;8(10):150–158. doi: 10.7324/JAPS.2018.81019. [DOI] [Google Scholar]
- 361.He, X., & Hwang, H. M. (2016). Nanotechnology in food science: Functionality, applicability, and safety assessment. In Journal of Food and Drug Analysis (Vol. 24, Issue 4, pp. 671–681). Elsevier Taiwan LLC10.1016/j.jfda.2016.06.001 [DOI] [PMC free article] [PubMed]
- 362.Hefnawy HT, El-Shourbagy GA, Ramadan MF. Phenolic extracts of carrot, grape leaf and turmeric powder antioxidant potential and application in biscuits. Journal of Food Measurement and Characterization. 2016;10:576–583. doi: 10.1007/s11694-016-9339-7. [DOI] [Google Scholar]
- 363.Hegazy A, Hegazy AI. Influence of using fenugreek seed flour as antioxidant and antimicrobial agent in the manufacturing of beef burger with emphasis on frozen storage stability. World Journal of Agricultural Sciences. 2011;7(4):391–399. [Google Scholar]
- 364.Heikal YM, Şuţan NA, Rizwan M, Elsayed A. Green synthesized silver nanoparticles induced cytogenotoxic and genotoxic changes in Allium cepa L. varies with nanoparticles doses and duration of exposure. Chemosphere. 2020;243:125430. doi: 10.1016/j.chemosphere.2019.125430. [DOI] [PubMed] [Google Scholar]
- 365.Hemalatha, P., & Premnath, A. (2016). Study on silver nanoparticle encapsulated curcumin for anticancer activityHemalatha et Al World Journal of Pharmaceutical Research, 5(October1), 95810.20959/wjpr201610-7170
- 366.Hendrayady, A., & Audi Ghaffari, M. (2011). Application of Garcinia indica as a colorant and antioxidant in rice extrudatesJurnal Fisip Umrah, Vol 1 No(1), 287–295http://e-journal.usd.ac.id/index.php/LLT; http://jurnal.untan.ac.id/index.php/jpdpb/article/viewFile/11345/10753; 10.1016/j.sbspro.2015.04.758;www.iosrjournals.org
- 367.Herbst, R., & Herbst, S. T. (2015)The deluxe food lover’s companion. 854https://books.google.com/books?id=e8BoCwAAQBAJ&pg=PT901
- 368.Hettiarachchi SS, Dunuweera SP, Dunuweera AN, Rajapakse RMG. Synthesis of curcumin nanoparticles from raw turmeric rhizome. ACS Omega. 2021;6(12):8246–8252. doi: 10.1021/acsomega.0c06314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Hettiarachchy NS, Glenn KC, Gnanasambandam R, Johnson MG. Natural antioxidant extract from Fenugreek (Trigonella foenumgraecum) for ground beef patties. Journal of Food Science. 1996;61(3):516–519. doi: 10.1111/j.1365-2621.1996.tb13146.x. [DOI] [Google Scholar]
- 370.Ho KKHY, Schroën K, San Martín-González MF, Berton-Carabin CC. Physicochemical stability of lycopene-loaded emulsions stabilized by plant or dairy proteins. Food Structure. 2017;12:34–42. doi: 10.1016/j.foostr.2016.12.001. [DOI] [Google Scholar]
- 371.Homayouni Moghadam F, Dehghan M, Zarepur E, Dehlavi R, Ghaseminia F, Ehsani S, Mohammadzadeh G, Barzegar K. Oleo gum resin of Ferula assa-foetida L. ameliorates peripheral neuropathy in mice. Journal of Ethnopharmacology. 2014;154(1):183–189. doi: 10.1016/j.jep.2014.03.069. [DOI] [PubMed] [Google Scholar]
- 372.Hooda S, Jood S. Organoleptic and nutritional evaluation of wheat biscuits supplemented with untreated and treated fenugreek flour. Food Chemistry. 2005;90(3):427–435. doi: 10.1016/j.foodchem.2004.05.006. [DOI] [Google Scholar]
- 373.Hossain, M. M., Das Aka, T., Rahman, M. S., Uddin, A. H. M. M., Rahman, N., & Rashid, M. M. O. (2019). Neuropharmacological activity of the crude ethanolic extract of Syzygium aromaticum flowering budDiscovery Phytomedicine, 6(4)10.15562/phytomedicine.2019.109
- 374.Howard LA, Wong AD, Perry AK, Klein BP. β-Carotene and ascorbic acid retention in fresh and processed vegetables. Journal of Food Science. 1999;64(5):929–936. doi: 10.1111/j.1365-2621.1999.tb15943.x. [DOI] [Google Scholar]
- 375.Hu K, Huang X, Gao Y, Huang X, Xiao H, McClements DJ. Core–shell biopolymer nanoparticle delivery systems: Synthesis and characterization of curcumin fortified zein–pectin nanoparticles. Food Chemistry. 2015;182:275–281. doi: 10.1016/j.foodchem.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 376.Hu Y, Fu A, Miao Z, Zhang X, Wang T, Kang A, Shan J, Zhu D, Li W. Fluorescent ligand fishing combination with in-situ imaging and characterizing to screen Hsp 90 inhibitors from Curcuma longa L. based on InP/ZnS quantum dots embedded mesoporous nanoparticles. Talanta. 2018;178:258–267. doi: 10.1016/j.talanta.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 377.Hu Y, Luo J, Kong W, Zhang J, Logrieco AF, Wang X, Yang M. Uncovering the antifungal components from turmeric (Curcuma longa L.) essential oil as Aspergillus flavus fumigants by partial least squares. RSC Advances. 2015;5(52):41967–41976. doi: 10.1039/c5ra01725d. [DOI] [Google Scholar]
- 378.Huda JA, Hameed IH, Hamza LF. Anethum graveolens: physicochemical properties, medicinal uses, antimicrobial effects, antioxidant effect, anti-inflammatory and analgesic effects: a review. International Journal of Pharmaceutical Quality Assurance. 2017;8(3):88–91. doi: 10.25258/ijpqa.v8i03.9569. [DOI] [Google Scholar]
- 379.Hussain J, Khan AL, ur Rehman N, Zainullah, Khan F, Hussain ST, Shinwari ZK. Proximate and nutrient investigations of selected medicinal plants species of Pakistan. Pakistan Journal of Nutrition. 2009;8(5):620–624. doi: 10.3923/pjn.2009.620.624. [DOI] [Google Scholar]
- 380.Hussein NH, Shaarawy HH, Hawash SI, Abdel-Kader AE. Green synthesis of silver nanoparticle using fenugreek seeds extract. ARPN Journal of Engineering and Applied Sciences. 2018;13(2):1–5. [Google Scholar]
- 381.Hussein HJ, Hadi MY, Hameed IH. Study of chemical composition of Foeniculum vulgare using Fourier transform infrared spectrophotometer and gas chromatography - mass spectrometry. Journal of Pharmacognosy and Phytotherapy. 2016;8(3):60–89. doi: 10.5897/JPP2015.0372. [DOI] [Google Scholar]
- 382.Ibrahim FM, Ibrahim AY, El Gohary AE, Hussein MS, Ahmed KA. Illicium verum extracts anti-gastro ulcerogenic potential on experimentally rat models. International Journal of PharmTech Research. 2016;9(5):65–80. [Google Scholar]
- 383.Ikpeama A, Onwuka GI, Nwankwo C. Nutritional composition of tumeric (Curcuma longa) and its antimicrobial properties. International Journal of Scientific & Engineering Research. 2014;5(10):1085–1089. [Google Scholar]
- 384.Imchen P, Ziekhrü M, Zhimomi BK, Phucho T. Biosynthesis of silver nanoparticles using the extract of Alpinia galanga rhizome and Rhus semialata fruit and their antibacterial activity. Inorganic Chemistry Communications. 2022;142:109599. doi: 10.1016/j.inoche.2022.109599. [DOI] [Google Scholar]
- 385.Indu Rawat, N. V., & K. J. (2020). Cinnamon (Cinnamomum zeylanicum)Medicinal Plants in India: Importance and Cultivation, 164–177
- 386.Iqbal S, Jabeen F, Peng C, Ijaz MU, Chaudhry AS. Cinnamomum cassia ameliorates Ni-NPs-induced liver and kidney damage in male Sprague Dawley rats. Human and Experimental Toxicology. 2020;39(11):1565–1581. doi: 10.1177/0960327120930125. [DOI] [PubMed] [Google Scholar]
- 387.Iranshahy M, Iranshahi M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin) - A review. Journal of Ethnopharmacology. 2011;134(1):1–10. doi: 10.1016/j.jep.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 388.Ishtiaque S, Naz S, Siddiqi R, Jabeen S, Ahmed J. Antioxidant activity and phenolic contents of ajwain, mustard, fenugreek and poppy seed. Recent Innovations in Chemical Engineering (Formerly Recent Patents on Chemical Engineering) 2015;7(2):119–127. doi: 10.2174/2405520407666150425004125. [DOI] [Google Scholar]
- 389.Ismail MA, Darwish AZM, Hussein NA, Darwish SM. In vivo effect of essential oils from Laurus nobilis, Anethum graveolens and Mentha piperita on mycobiota associated with domiati cheese during storage. Food and Public Health. 2014;4(3):110–122. doi: 10.5923/j.fph.20140403.07. [DOI] [Google Scholar]
- 390.Istúriz-Zapata MA, Hernández-López M, Correa-Pacheco ZN, Barrera-Necha LL. Quality of cold-stored cucumber as affected by nanostructured coatings of chitosan with cinnamon essential oil and cinnamaldehyde. Lwt. 2020;123(September 2019):109089. doi: 10.1016/j.lwt.2020.109089. [DOI] [Google Scholar]
- 391.Ituen, E., Singh, A., Yuanhua, L., & Akaranta, O. (2021). Green synthesis and anticorrosion effect of Allium cepa peels extract-silver nanoparticles composite in simulated oilfield pickling solutionSN Applied Sciences, 3(6)10.1007/s42452-021-04670-w
- 392.Jacob V, P R. In vitro analysis: the antimicrobial and antioxidant activity of zinc oxide nanoparticles from Curcuma longa. Asian Journal of Pharmaceutical and Clinical Research. 2019;12(1):200. doi: 10.22159/ajpcr.2018.v12i1.28808. [DOI] [Google Scholar]
- 393.Jacob V, Rajiv P, Dhanasekaran S. Antioxidant and anticancerous activities of biologically synthesized zinc oxide nanoparticles (Zno nps) from the rhizomes of curcuma longa. International Journal of Pharmaceutical Research. 2020;12(September):2304–2313. doi: 10.31838/ijpr/2020.SP1.283. [DOI] [Google Scholar]
- 394.Jadouali SM, Atifi H, Mamouni R, Majourhat K, Bouzoubaâ Z, Laknifli A, Faouzi A. Chemical characterization and antioxidant compounds of flower parts of Moroccan crocus sativus L. Journal of the Saudi Society of Agricultural Sciences. 2019;18(4):476–480. doi: 10.1016/j.jssas.2018.03.007. [DOI] [Google Scholar]
- 395.Jagtap, P., Bhise, K., & Prakya, V. (2015). A Phytopharmacological Review on Garcinia indica~ 2 ~ International Journal of Herbal Medicine, 3(4)
- 396.Jahromi, M. A. M., Al-Musawi, S., Pirestani, M., Ramandi, M. F., Ahmadi, K., Rajayi, H., Hassan, Z. M., Kamali, M., & Mirnejad, R. (2014). Curcumin-loaded chitosan tripolyphosphate nanoparticles as a safe, natural and effective antibiotic inhibits the infection of staphylococcus aureus and pseudomonas aeruginosa in vivoIranian Journal of Biotechnology, 12(3)10.15171/ijb.1012
- 397.Jain A, Wadhawan S, Mehta SK. Biogenic synthesis of non-toxic iron oxide NPs via Syzygium aromaticum for the removal of methylene blue. Environmental Nanotechnology, Monitoring & Management. 2021;16:100464. doi: 10.1016/j.enmm.2021.100464. [DOI] [Google Scholar]
- 398.Jain S, Buttar HS, Chintameneni M, Kaur G. Prevention of cardiovascular diseases with anti-inflammatory and anti- oxidant nutraceuticals and herbal products: An overview of pre-clinical and clinical studies. Recent Patents on Inflammation & Allergy Drug Discovery. 2018;12(2):145–157. doi: 10.2174/1872213x12666180815144803. [DOI] [PubMed] [Google Scholar]
- 399.Jaiswal P, Kumar P, Singh VK, Singh DK. Biological effects of Myristica fragrans. Annual Review of Biomedical Sciences. 2009;11:21–29. doi: 10.5016/1806-8774.2009v11p21. [DOI] [Google Scholar]
- 400.Jamal A, Javed K, Aslam M, Jafri MA. Gastroprotective effect of cardamom, Elettaria cardamomum Maton. fruits in rats. Journal of Ethnopharmacology. 2006;103(2):149–153. doi: 10.1016/j.jep.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 401.Jamzad M, Kamari Bidkorpeh M. Green synthesis of iron oxide nanoparticles by the aqueous extract of Laurus nobilis L. leaves and evaluation of the antimicrobial activity. Journal of Nanostructure in Chemistry. 2020;10(3):193–201. doi: 10.1007/s40097-020-00341-1. [DOI] [Google Scholar]
- 402.Jana S, Shekhawat G. Anethum graveolens: An Indian traditional medicinal herb and spice. Pharmacognosy Reviews. 2010;4(8):179–184. doi: 10.4103/0973-7847.70915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Jana S, Shekhawat G. Anethum graveolens: An Indian traditional medicinal herb and spice. Pharmacognosy Reviews. 2010;4(8):179–184. doi: 10.4103/0973-7847.70915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Jasim B, Thomas R, Mathew J, Radhakrishnan EK. Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.) Saudi Pharmaceutical Journal. 2017;25(3):443–447. doi: 10.1016/j.jsps.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Javad S. Antibacterial activity of plant extract and zinc nanoparticles obtained from Syzigium aromaticum L. Pure and Applied Biology. 2017;6(4):1079–1087. doi: 10.19045/bspab.2017.600115. [DOI] [Google Scholar]
- 406.Jayaprakash N, Judith Vijaya J, John Kennedy L, Priadharsini K, Palani P. One step phytosynthesis of highly stabilized silver nanoparticles using Piper nigrum extract and their antibacterial activity. Materials Letters. 2014;137:358–361. doi: 10.1016/j.matlet.2014.09.027. [DOI] [Google Scholar]
- 407.Jayathilake AL, Jayasinghe MA, Walpita J. Development of ginger, turmeric oleoresins and pomegranate peel extracts incorporated pasteurized milk with pharmacologically important active compounds. Applied Food Research. 2022;2(1):100063. doi: 10.1016/j.afres.2022.100063. [DOI] [Google Scholar]
- 408.Jelin FJ, Kumar SS, Malini M, Vanaja M, Annadurai G. Environment -assisted green approach agnps by nutmeg inhibition potential accustomed to pharmaceuticals. European Journal of Biomedical AND Pharmaceutical Sciences. 2015;2(3):258–274. [Google Scholar]
- 409.Jerlin Showmya J, Harini K, Pradeepa M, Thiyagarajan M, Manikandan R, Venkatachalam P, Geetha N. Rapid green synthesis of silver nanoparticles using seed extracts of Foeniculum vulgare and screening of its antibacterial activity. Plant Cell Biotechnology and Molecular Biology. 2012;13(1–2):29–34. [Google Scholar]
- 410.Jeyaratnam N, Nour AH, Kanthasamy R, Nour AH, Yuvaraj AR, Akindoyo JO. Essential oil from Cinnamomum cassia bark through hydrodistillation and advanced microwave assisted hydrodistillation. Industrial Crops and Products. 2016;92:57–66. doi: 10.1016/j.indcrop.2016.07.049. [DOI] [Google Scholar]
- 411.Jin H, Xia F, Jiang C, Zhao Y, He L. Nanoencapsulation of lutein with hydroxypropylmethyl cellulose phthalate by supercritical antisolvent. Chinese Journal of Chemical Engineering. 2009;17(4):672–677. doi: 10.1016/S1004-9541(08)60262-1. [DOI] [Google Scholar]
- 412.Asgarpanah,J. (2012). Phytochemistry and pharmacologic properties of Myristica fragrans Hoyutt.: A reviewAfrican Journal of Biotechnology, 11(65)10.5897/ajb12.1043
- 413.Jolayemi AT, Ojewole JAO. Comparative anti-inflammatory properties of Capsaicin and ethyl-aAcetate extract of Capsicum frutescens linn [Solanaceae] in rats. African Health Sciences. 2013;13(2):357–361. doi: 10.4314/ahs.v13i2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Jung CH, Ahn J, Jeon TI, Kim TW, Ha TY. Syzygium aromaticum ethanol extract reduces high-fat diet-induced obesity in mice through downregulation of adipogenic and lipogenic gene expression. Experimental and Therapeutic Medicine. 2012;4(3):409–414. doi: 10.3892/etm.2012.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Kaashmeera NK, Krupavaram B, Singh JCH, Anandarajagopal K, Aasminerjiit K, Shopana P. Evaluation of ethanolic root extract of Allium cepa. l for analgesic and anti-inflammatory activities in animal models. World Journal of Current Medical and Pharmaceutical Research. 2020;02(01):67–73. doi: 10.37022/wjcmpr.2020.02017. [DOI] [Google Scholar]
- 416.Kabrah, A. (2015). The Antibacterial Activity of Onion on MSSA and MRSA Isolates of Staphylococcus aureusProQuest Dissertations and Theses, 198https://search.proquest.com/docview/1718551102?accountid=26642;http://link.periodicos.capes.gov.br/sfxlcl41?url_ver=Z39.88-2004&rft_val_fmt=info:ofi/fmt:kev:mtx:dissertation&genre=dissertations+%26+theses&sid=ProQ:ProQuest+Dissertations+%26+Theses+Globa
- 417.Kačániová M, Mellen M, Vukovic NL, Kluz M, Puchalski C, Haščík P, Kunová S. Combined effect of vacuum packaging, fennel and savory essential oil treatment on the quality of chicken thighs. Microorganisms. 2019;7(5):2–11. doi: 10.3390/microorganisms7050134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Kailey R, Dhawan K, Rasane P, Singh J, Kaur S, Singh BP, Kaur N, Kaur D. Utilization of Foeniculum vulgare in herbal candy preparation and analysing its effect on the physico-chemical and sensory properties. Current Science. 2019;116(12):2013–2019. doi: 10.18520/cs/v116/i12/2013-2019. [DOI] [Google Scholar]
- 419.Kalam MA. An Overview of Myristica fragrans (Nutmeg) -Its benefits and adverse effects to Humans. Indian Journal of Integrated Medicine. 2020;2:45–50. [Google Scholar]
- 420.Kaltsa O, Yanniotis S, Polissiou M, Mandala I. Stability, physical properties and acceptance of salad dressings containing saffron (Crocus sativus) or pomegranate juice powder as affected by high shear (HS) and ultrasonication (US) process. Lwt. 2018;97(January):404–413. doi: 10.1016/j.lwt.2018.07.015. [DOI] [Google Scholar]
- 421.Kamal SB, Kumar A, Tanwar T. Physico-chemical, proximate, sensory and storage quality attributes analysis of Papaver somniferum (poppy) fortified chevon nuggets. Journal of Applied and Natural Science. 2017;9(1):114–120. doi: 10.31018/jans.v9i1.1158. [DOI] [Google Scholar]
- 422.Kamatou GP, Vermaak I, Viljoen AM. Eugenol - from the remote Maluku islands to the international market place: a review of a remarkable and versatile molecule. Molecules. 2012;17(6):6953–6981. doi: 10.3390/molecules17066953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Kamel KM, Khalil IA, Rateb ME, Elgendy H, Elhawary S. Chitosan-coated cinnamon/oregano-loaded solid lipid nanoparticles to augment 5-fluorouracil cytotoxicity for colorectal cancer: Extract standardization, nanoparticle optimization, and cytotoxicity evaluation. Journal of Agricultural and Food Chemistry. 2017;65(36):7966–7981. doi: 10.1021/acs.jafc.7b03093. [DOI] [PubMed] [Google Scholar]
- 424.Kamel Moawad R, Saleh O, Mohamed S, Abdelmaguid NM. Shelf-life evaluation of raw chicken sausage incorporated with green tea and clove powder extracts at refrigerated storage. Plant Archives. 2020;20(2):8821–8830. [Google Scholar]
- 425.Kamkar, A., Khanjari, A. O. M., & Aghaee, E, M. (2017). Effect of packaging with chitosan film containing Bunium persicum L. essential oil on chemical and microbial properties of chicken filletJournal of Fasa University of Medical Science, 7(1)
- 426.Kamoun J, Krichen F, Koubaa I, Zouari N, Bougatef A, Abousalham A, Aloulou A. In vitro lipolysis and physicochemical characterization of unconventional star anise oil towards the development of new lipid-based drug delivery systems. Heliyon. 2021;7(4):e06717. doi: 10.1016/j.heliyon.2021.e06717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Kanniah P, Chelliah P, Thangapandi JR, Gnanadhas G, Mahendran V, Robert M. Green synthesis of antibacterial and cytotoxic silver nanoparticles by Piper nigrum seed extract and development of antibacterial silver based chitosan nanocomposite. International Journal of Biological Macromolecules. 2021;189:18–33. doi: 10.1016/j.ijbiomac.2021.08.056. [DOI] [PubMed] [Google Scholar]
- 428.Karadağoğlu Ö, Şahin T, Ölmez M, Ahsan U, Özsoy B, Önk K. Fatty acid composition of liver and breast meat of quails fed diets containing black cumin (Nigella sativa L.) and/or coriander (Coriandrum sativum L.) seeds as unsaturated fatty acid sources. Livestock Science. 2019;223:164–171. doi: 10.1016/j.livsci.2019.03.015. [DOI] [Google Scholar]
- 429.Kareem MA, Krushna GS, Hussain SA, Devi KL. Effect of aqueous extract of nutmeg on hyperglycaemia, hyperlipidaemia and cardiac histology associated with isoproterenol-induced myocardial infarction in rats. Tropical Journal of Pharmaceutical Research. 2009;8(4):337–344. doi: 10.4314/tjpr.v8i4.45227. [DOI] [Google Scholar]
- 430.Kargozari M, Hamedi H, Amirnia SA, Montazeri A, Abbaszadeh S. Effect of bioactive edible coating based on sodium alginate and coriander ( Coriandrum sativum L. ) essential oil on the quality of refrigerated chicken fillet. Food & Health. 2018;1(6):30–38. [Google Scholar]
- 431.Karimi M, Sekhavatizadeh SS, Hosseinzadeh S. milk dessert containing lactobacillus reuteri encapsulated with sodium alginate, ferula asafoetida and zedo gum as three layers of wall materials. Food and Bioproducts Processing. 2021;127:244–254. doi: 10.1016/j.fbp.2021.03.003. [DOI] [Google Scholar]
- 432.Karimi E, Oskoueian E, Hendra R, Jaafar HZE. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules. 2010;15(9):6244–6256. doi: 10.3390/molecules15096244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Kashkouli, S., Jamzad, M., & Nouri, A. (2018). Total phenolic and flavonoids contents, radical scavenging activity and green synthesis of silver nanoparticles by Laurus nobilis L. leaves aqueous extractJournal of Medicinal Plants and By-Products, 1, 25–32. moz-extension://0074b4dd-988d-4d53–9b16-bec6dea4bf70/enhanced-reader.html?openApp&pdf https://jmpb.areeo.ac.ir/article_116725_dac1a5ad6a5cc68538510268c5c720dc.pdf
- 434.Kaur N, Kaur CK, Singh RU. Phytochemical screening and antioxidant activity of Anethum graveolens L. seed extracts. ~ 324 ~. The Pharma Innovation Journal. 2018;7(6):324–329. [Google Scholar]
- 435.Kaur G, Kaur R, Kaur S. Studies on physiochemical properties of oil extracted from Brassica nigra and Brassica rapa toria. Materials Today: Proceedings. 2021;48(October):1645–1651. doi: 10.1016/j.matpr.2021.09.527. [DOI] [Google Scholar]
- 436.Kaur P, Gupta S, Kaur K, Kaur N, Kumar R, Bhullar MS. Nanoemulsion of Foeniculum vulgare essential oil: A propitious striver against weeds of Triticum aestivum. Industrial Crops and Products. 2021;168:113601. doi: 10.1016/j.indcrop.2021.113601. [DOI] [Google Scholar]
- 437.Kavas N, Kavas G. Use of turmeric (Curcuma longa l.) essential oil added to an egg white protein powder-based film in the storage of Çökelek cheese. Journal of Food Chemistry and Nanotechnology. 2017;3(3):105–110. doi: 10.17756/jfcn.2017-045. [DOI] [Google Scholar]
- 438.Kavoosi G, Rowshan V. Chemical composition, antioxidant and antimicrobial activities of essential oil obtained from Ferula assa-foetida oleo-gum-resin: Effect of collection time. Food Chemistry. 2013;138(4):2180–2187. doi: 10.1016/j.foodchem.2012.11.131. [DOI] [PubMed] [Google Scholar]
- 439.Keshavarz A, Minaiyan M, Ghannadi A, Mahzouni P. Effects of carum carvi L. (Caraway) extract and essential oil on TNBS-induced colitis in rats. Research in Pharmaceutical Sciences. 2013;8(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 440.Kestwal RM, Bagal-Kestwal D, Chiang B-H. Fenugreek hydrogel–agarose composite entrapped gold nanoparticles for acetylcholinesterase based biosensor for carbamates detection. Analytica Chimica Acta. 2015;886:143–150. doi: 10.1016/j.aca.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 441.Keykhosravy K, Khanzadi S, Hashemi M, Azizzadeh M. Chitosan-loaded nanoemulsion containing Zataria Multiflora Boiss and Bunium persicum Boiss essential oils as edible coatings: Its impact on microbial quality of turkey meat and fate of inoculated pathogens. International Journal of Biological Macromolecules. 2020;150:904–913. doi: 10.1016/j.ijbiomac.2020.02.092. [DOI] [PubMed] [Google Scholar]
- 442.Khadka, G. (2018). Preparation and shelf life study of cinnamon oleoresin incorporated yoghurtFood Technology Thesishttp://202.45.146.37:8080/jspui/handle/123456789/89
- 443.Khairullah AR, Solikhah TI, Ansori ANM, Hidayatullah AR, Hartadi EB, Ramandinianto SC, Fadholly A. Review on the pharmacological and health aspects of apium graveolens or celery: An update. Systematic Reviews in Pharmacy. 2021;12(1):606–612. doi: 10.31838/srp.2021.1.87. [DOI] [Google Scholar]
- 444.Khajeh M, Yamini Y, Bahramifar N, Sefidkon F, Reza Pirmoradei M. Comparison of essential oils compositions of Ferula assa-foetida obtained by supercritical carbon dioxide extraction and hydrodistillation methods. Food Chemistry. 2005;91(4):639–644. doi: 10.1016/j.foodchem.2004.06.033. [DOI] [Google Scholar]
- 445.Khaliq T, Sarfraz M, Ashraf MA. Recent progress for the utilization of Curcuma longa, Piper nigrum and Phoenix dactylifera Seeds against type 2 diabetes. West Indian Medical Journal. 2015;64(5):527–532. doi: 10.7727/wimj.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Khamisabadi H, Ahmadpanah J. The effect of diets supplemented with coriandrum sativum seeds on carcass performance, immune system, blood metabolites, rumen parameters and meat quality of lambs. Acta Scientiarum - Animal Sciences. 2020;43(1):1–13. doi: 10.4025/actascianimsci.v43i1.52048. [DOI] [Google Scholar]
- 447.Khan I, Bawazeer S, Rauf A, Qureshi MN, Muhammad N, Al-Awthan Y, Bahattab O, Maalik A, Rengasamy KRR. synthesis, biological investigation and catalytic application using the alcoholic extract of black cumin seeds based silver nanoparticles. Journal of Nanostructure in Chemistry. 2022;12:59–77. doi: 10.1007/s40097-021-00402-z. [DOI] [Google Scholar]
- 448.Khan HN, Rasheed S, Choudhary MI, Ahmed N, Adem A. Anti-glycation properties of Illicium verum Hook. f. fruit in-vitro and in a diabetic rat model. BMC Complementary Medicine and Therapies. 2022;22(1):1–15. doi: 10.1186/s12906-022-03550-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Khan I, Ahmad H, Ahmad B. Anti-glycationand anti-oxidation properties of Capsicum frutescens and Curcuma longa fruits: Possible role in prevention of diabetic complication. Pakistan Journal of Pharmaceutical Sciences. 2014;27(5):1359–1362. [PubMed] [Google Scholar]
- 450.Khan I, Shah S, Ahmad J, Abdullah A, Johnson SK. Effect of incorporating bay leaves in cookies on postprandial glycemia, appetite, palatability, and gastrointestinal well-Being. Journal of the American College of Nutrition. 2017;36(7):514–519. doi: 10.1080/07315724.2017.1326324. [DOI] [PubMed] [Google Scholar]
- 451.Khan MA, Moghul NB, Butt MA, Kiyani MM, Zafar I, Bukhari AI. Assessment of antibacterial and antifungal potential of Curcuma longa and synthesized nanoparticles: A comparative study. Journal of Basic Microbiology. 2021;61(7):603–611. doi: 10.1002/jobm.202100010. [DOI] [PubMed] [Google Scholar]
- 452.Khan, M. J., Shameli, K., Sazili, A. Q., Selamat, J., & Kumari, S. (2019). Rapid green synthesis and characterization of silver nanoparticles arbitrated by curcumin in an alkaline mediumMolecules, 24(4)10.3390/molecules24040719 [DOI] [PMC free article] [PubMed]
- 453.Khan M, Siddiqui M. Antimicrobial activity of piper fruits. Indian Journal of Natural Products and Resources. 2007;6(2):111–113. [Google Scholar]
- 454.Khan, M. T., Ahmed, S., Shah, A. A., Shah, A. N., Tanveer, M., El-Sheikh, M. A., & Siddiqui, M. H. (2021). Influence of zinc oxide nanoparticles to regulate the antioxidants enzymes, some osmolytes and agronomic attributes in coriandrum sativum l. Grown under water stressAgronomy, 11(10)10.3390/agronomy11102004
- 455.Khan, R. U., Fatima, A., Naz, S., Ragni, M., Tarricone, S., & Tufarelli, V. (2022). Perspective, opportunities and challenges in using fennel (Foeniculum vulgare) in poultry health and production as an eco-friendly alternative to antibiotics: a reviewAntibiotics, 11(2)10.3390/antibiotics11020278 [DOI] [PMC free article] [PubMed]
- 456.Khare AK, Abraham RJJ, Rao VA, Babu RN. Utilization of carrageenan, citric acid and cinnamon oil as an edible coating of chicken fillets to prolong its shelf life under refrigeration conditions. Veterinary World. 2016;9(2):166–175. doi: 10.14202/vetworld.2016.166-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Kharey P, Dutta SB, Gorey A, Manikandan M, Kumari A, Vasudevan S, Palani IA, Majumder SK, Gupta S. Pimenta dioica mediated biosynthesis of gold nanoparticles and evaluation of its potential for theranostic applications. ChemistrySelect. 2020;5(26):7901–7908. doi: 10.1002/slct.202001230. [DOI] [Google Scholar]
- 458.Khater MS, Osman YAH. Influence of TiO2 nanoparticles on growth, chemical constituents and toxicity of fennel plant. Arab Journal of Nuclear Sciences and Applications. 2015;48(4):178–186. [Google Scholar]
- 459.Khazdair MR, Anaeigoudari A, Hashemzehi M, Mohebbati R. Neuroprotective potency of some spice herbs, a literature review. Journal of Traditional and Complementary Medicine. 2019;9(2):98–105. doi: 10.1016/j.jtcme.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Kheeree N, Sangvanich P, Puthong S, Karnchanatat A. Antifungal and antiproliferative activities of lectin from the rhizomes of Curcuma amarissima Roscoe. Applied Biochemistry and Biotechnology. 2010;162(3):912–925. doi: 10.1007/s12010-009-8804-8. [DOI] [PubMed] [Google Scholar]
- 461.Khobragade SP, Padghan PV, Deshmukh AP. textural properties of paneer prepared from blends of raw turmeric extract and buffalo milk. Journal of Pharmacognosy and Phytochemistry. 2021;10(1):149–152. [Google Scholar]
- 462.Khorasani S, Ghandehari Yazdi AP, Saadatfar A, Kamali Rousta L, Nejatian M, Abarian M, Jafari SM. Valorization of saffron tepals for the green synthesis of silver nanoparticles and evaluation of their efficiency against foodborne pathogens. Waste and Biomass Valorization. 2022 doi: 10.1007/s12649-022-01791-0. [DOI] [Google Scholar]
- 463.Khorram F, Ramezanian A. Cinnamon essential oil incorporated in shellac, a novel bio-product to maintain quality of ‘Thomson navel’ orange fruit. Journal of Food Science and Technology. 2020 doi: 10.1007/s13197-020-04798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Khorshidi S, Mehdizadeh T, Ghorbani M. The effect of chitosan coatings enriched with the extracts and essential oils of Elettaria Cardamomum on the shelf-life of chicken drumsticks vacuum-packaged at 4 °C. Journal of Food Science and Technology. 2021;58(8):2924–2935. doi: 10.1007/s13197-020-04794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Khoso S, Mehar S, Anam I, Naheed N, Saeed F, Khan N, Abbasi BH. Green synthesis of ZnO nanoparticles from Foeniculum vulgare, its characterization and biological potential against bacteria. Journal of Animal and Plant Sciences. 2022;32(1):229–237. doi: 10.36899/JAPS.2022.1.0418. [DOI] [Google Scholar]
- 466.Khosropanah MH, Dinarvand A, Nezhadhosseini A, Haghighi A, Hashemi S, Nirouzad F, Khatamsaz S, Entezari M, Hashemi M, Dehghani H. Analysis of the antiproliferative effects of curcumin and nanocurcumin in MDA-MB231 as a breast cancer cell line. Iranian Journal of Pharmaceutical Research. 2016;15(1):231–239. [PMC free article] [PubMed] [Google Scholar]
- 467.Khumkomgool A, Saneluksana T, Harnkarnsujarit N. Active meat packaging from thermoplastic cassava starch containing sappan and cinnamon herbal extracts via LLDPE blown-film extrusion. Food Packaging and Shelf Life. 2020;26:100557. doi: 10.1016/j.fpsl.2020.100557. [DOI] [Google Scholar]
- 468.Kiarsi, Z., Hojjati, M., Behbahani, B. A., & Noshad, M. (2020)In vitro antimicrobial effects of Myristica fragrans essential oil on foodborne pathogens and its influence on beef quality during refrigerated storageJournal of Food Safety, 40(3)10.1111/jfs.12782
- 469.Kiasalari Z, Khalili M, Roghani M, Heidari H, Azizi Y. Antiepileptic and antioxidant effect of hydroalcoholic extract of ferula assa foetida gum on pentylentetrazoleinduced kindling in male mice. Basic and Clinical Neuroscience. 2013;4(4):21–28. [PMC free article] [PubMed] [Google Scholar]
- 470.Kiasalari Z, Khalili M, Roghani M, Sadeghian A. Antiepileptic and antioxidant effect of Brassica nigra on pentylenetetrazol-induced kindling in mice. Iranian Journal of Pharmaceutical Research. 2012;11(4):1209–1217. doi: 10.22037/ijpr.2012.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Kikuzaki H, Sato A, Mayahara Y, Nakatani N. Galloylglucosides from berries of Pimenta dioica. Journal of Natural Products. 2000;63(6):749–752. doi: 10.1021/np9906121. [DOI] [PubMed] [Google Scholar]
- 472.Kilic, S., Oz, E., & Oz, F. (2021). Effect of turmeric on the reduction of heterocyclic aromatic amines and quality of chicken meatballsFood Control,12810.1016/j.foodcont.2021.108189
- 473.Kim S, Kim DB, Jin W, Park J, Yoon W, Lee Y, Kim S, Lee S, Kim S, Lee OH, Shin D, Yoo M. Comparative studies of bioactive organosulphur compounds and antioxidant activities in garlic (Allium sativum L.), elephant garlic (Allium ampeloprasum L.) and onion (Allium cepa L.) Natural Product Research. 2018;32(10):1193–1197. doi: 10.1080/14786419.2017.1323211. [DOI] [PubMed] [Google Scholar]
- 474.Kim YK, Nam MS, Bae HC. Characteristics of gouda cheese supplemented with chili pepper extract microcapsules. Korean Journal for Food Science of Animal Resources. 2017;37(6):833–839. doi: 10.5851/kosfa.2017.37.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Kivrak Ş, Gokturk T, Kivrak İ. Assessment of volatile oil composition, phenolics and antioxidant activity of bay (Laurus nobilis) leaf and usage in cosmetic applications. International Journal of Secondary Metabolite. 2017;4(2):148–148. doi: 10.21448/ijsm.323800. [DOI] [Google Scholar]
- 476.Kocaadam B, Şanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Critical Reviews in Food Science and Nutrition. 2017;57(13):2889–2895. doi: 10.1080/10408398.2015.1077195. [DOI] [PubMed] [Google Scholar]
- 477.Koffi-Nevry R, Kouassi KC, Nanga ZY, Koussémon M, Loukou GY. Antibacterial activity of two bell pepper extracts: Capsicum annuum L. and Capsicum frutescens. International Journal of Food Properties. 2012;15(5):961–971. doi: 10.1080/10942912.2010.509896. [DOI] [Google Scholar]
- 478.Konwarh R, Saikia JP, Karak N, Konwar BK. ‘Poly(ethylene glycol)-magnetic nanoparticles-curcumin’ trio: Directed morphogenesis and synergistic free-radical scavenging. Colloids and Surfaces B: Biointerfaces. 2010;81(2):578–586. doi: 10.1016/j.colsurfb.2010.07.062. [DOI] [PubMed] [Google Scholar]
- 479.Kooti W, Ali-Akbari S, Asadi-Samani M, Ghadery H, Ashtary-Larky D. A review on medicinal plant of Apium graveolens. Advanced Herbal Medicine. 2015;1(1):48–59. [Google Scholar]
- 480.Kooti W, Daraei N. A review of the antioxidant activity of celery (Apium graveolens l) Journal of Evidence-Based Complementary and Alternative Medicine. 2017;22(4):1029–1034. doi: 10.1177/2156587217717415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Korikanthimath, V. S., Prasath, D., Govardhan, R. (2001). Medicinal properties of cardamom elettaria cardamomumICAR IISR
- 482.Kos I, Bedekovic D, Siric I, Vnucec I, Pecina M, Glumpak A, Stanko KC. Technological characterization and consumer perception of dry fermented game sausages with bay leaf (Laurus nobilis L.) essential oil. Journal of Central European Agriculture. 2017;18(4):794–805. doi: 10.5513/jcea01/18.4.1961. [DOI] [Google Scholar]
- 483.Kouame K, Peter AI, Akang EN, Adana M, Moodley R, Naidu EC, Azu OO. Effect of long-term administration of cinnamomum cassia silver nanoparticles on organs (Kidneys and liver) of sprague-dawley rats. Turkish Journal of Biology. 2018;42(6):498–505. doi: 10.3906/biy-1805-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Kouame K, Peter AI, Akang EN, Moodley R, Naidu EC, Azu OO. Histological and biochemical effects of Cinnamomum cassia nanoparticles in kidneys of diabetic Sprague-Dawley rats. Bosnian Journal of Basic Medical Sciences. 2019;19(2):138–145. doi: 10.17305/bjbms.2019.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Krishnamurthy, N., Lewis, Y. S., & Ravindranath, B. (1982). Chemical constituents of kokam fruit rindJournal of Food Science and Technology, 19(3), 97–100http://ir.cftri.com/id/eprint/8578
- 486.Krishnan, V., Bupesh, G., Manikandan, E., Thanigai, A. K., Magesh, S., Kalyanaraman, R., Maaza, M. (2016). Green synthesis of silver nanoparticles using Piper nigrum concoction and its anticancer activity against MCF-7 and Hep-2 cell linesJournal of Antimicrobial Agents, 2(3)10.4172/2472-1212.1000123
- 487.Krishnan V, Kalyanaraman R, Fathima G. Green biosynthesis of silver nanoparticles from Elettaria cardamomum ( Seed ) and its in vitro cytotoxic activity. World Journal of Pharmacy and Pharmaceutical Sciences. 2015;4(04):723–733. [Google Scholar]
- 488.Krishnaprabha, M., & Pattabi, M. (2016a). Synthesis of gold nanoparticles using garcinia indica fruit rind extractInternational Journal of Nanoscience, 15(5–6)10.1142/S0219581X16600152
- 489.Krishnaprabha, M., & Pattabi, M. (2016b). Synthesis of gold nanostructures using fruit extract of Garcinia IndicaAIP Conference Proceedings,173110.1063/1.4947776
- 490.Ktari N, Feki A, Trabelsi I, Triki M, Maalej H, Slima SB, Nasri M, Amara IB, Salah RB. Structure, functional and antioxidant properties in tunisian beef sausage of a novel polysaccharide from trigonella foenum-graecum seeds. International Journal of Biological Macromolecules. 2017;98:169–181. doi: 10.1016/j.ijbiomac.2017.01.113. [DOI] [PubMed] [Google Scholar]
- 491.Kulkarni CP, Bodhankar SL, Ghule AE, Mohan V, Thakurdesai PA. Antidiabetic activity of Trigonella foenumgraecum L. seeds extract (IND01) in neonatal streptozotocin-induced (n-STZ) rats. Diabetologia Croatica. 2012;41(1):29–40. [Google Scholar]
- 492.Kulkarni, P. (2004). The ayurvedic plantsSri Satguru Publications, 98
- 493.Kumar SJT, Akshay VR, Vasundhara M, Arumugam M. Biosynthesis of multiphase iron nanoparticles using Syzygium aromaticum and their magnetic properties. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2020;603:125241. doi: 10.1016/j.colsurfa.2020.125241. [DOI] [Google Scholar]
- 494.Kumar S, Kumari R. Traditional, phytochemical and biological activities of Elettaria cardamomum (L.) maton-a review. International Journal of Pharmaceutical Sciences and Research. 2021;12(8):4122. doi: 10.13040/IJPSR.0975-8232.12(8).4122-31. [DOI] [Google Scholar]
- 495.Kumar, A., Bankoti, K., Kumar, T., & Dhara, S. (2022)Hydrothermal synthesis of clove bud-derived multifunctional carbon dots passivated with PVP- antioxidant , catalysis , and bioimaging applications [DOI] [PubMed]
- 496.Kumar, A., Dora, J., & Singh, A. (2011). A review on spice of life Curcuma longa (Turmeric)International Journal Applied Biological Pharmaceutical Technology, 2, 371–379www.ijabpt.com
- 497.Kumar A, Pratap Singh P, Prakash B. Unravelling the antifungal and anti-aflatoxin B1 mechanism of chitosan nanocomposite incorporated with Foeniculum vulgare essential oil. Carbohydrate Polymers. 2020;236:116050. doi: 10.1016/j.carbpol.2020.116050. [DOI] [PubMed] [Google Scholar]
- 498.Kumar D, Kumar P, Vikram K, Singh H. Fabrication and characterization of noble crystalline silver nanoparticles from Pimenta dioica leave extract and analysis of chemical constituents for larvicidal applications. Saudi Journal of Biological Sciences. 2022;29(2):1134–1146. doi: 10.1016/j.sjbs.2021.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Kumar D, Rajeshwari A, Jadon PS, Chaudhuri G, Mukherjee A, Chandrasekaran N, Mukherjee A. Cytogenetic studies of chromium (III) oxide nanoparticles on Allium cepa root tip cells. Journal of Environmental Sciences. 2015;38:150–157. doi: 10.1016/j.jes.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 500.Kumar Dey, B., Sanyal Mukherjee Professor, S., Author, C., & Sanyal Mukherjee, S. (2021). Potential of clove and its nutritional benefits in physiological perspective: A review~ 103 ~ International Journal of Physiology, 6(1), 103–106www.journalofsports.com
- 501.Kumare KD, Londhe GK, Anarthe NK. Effect of black pepper ( Piper nigrum ) and turmeric ( Curcuma longa ) powder on sensory and rheological quality of burfi. The Pharma Innovation Journal. 2022;11(1):539–543. [Google Scholar]
- 502.Kumari M, Khan SS, Pakrashi S, Mukherjee A, Chandrasekaran N. Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. Journal of Hazardous Materials. 2011;190(1):613–621. doi: 10.1016/j.jhazmat.2011.03.095. [DOI] [PubMed] [Google Scholar]
- 503.Kumari, P. (2013). Development of semolina based upma incorporated with fenugreek and chickenpea seeds flour and evaluation of its sensory and nutritional attributes along with its glycemic indexTrends in Biosciences Journal
- 504.Kumari V, Tyagi P, Sangal A. In-vitro kinetic release study of illicium verum (Chakraphool) polymeric nanoparticles. Materials Today: Proceedings. 2022;60:14–20. doi: 10.1016/j.matpr.2021.11.014. [DOI] [Google Scholar]
- 505.Kurian M, Varghese B, Athira TS, Krishna S. Novel and efficient synthesis of silver nanoparticles using Curcuma longa and Zingiber officinale rhizome extracts. International Journal of Nanoscience and Nanotechnology. 2016;12(3):175–181. [Google Scholar]
- 506.Kyriaki, H., Eleni, K., George, L. I., Kostas, B., Petros, T. A. (2019). Antioxidant properties of crocus Sativus L. and its constituents and relevance to neurodegenerative diseases; Focus on Alzheimer’s and Parkinson’s DiseaseCurrent Neuropharmacology, 17(4), 377–402http://www.eurekaselect.com/603/journal/current-neuropharmacology [DOI] [PMC free article] [PubMed]
- 507.Laczkowski MS, Baqueta MR, de Oliveira VMAT, Gonçalves TR, Gomes STM, Março PH, Matsushita M, Valderrama P. Application of chemometric tools in the development and sensory evaluation of gluten-free cracknel biscuits with the addition of chia seeds and turmeric powder. Journal of Food Science and Technology. 2021;58(11):4118–4126. doi: 10.1007/s13197-020-04874-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Lagua EB, Ampode KMB. Turmeric powder: potential alternative to antibiotics in broiler chicken diets. Journal of Animal Health and Production. 2021;9(3):243–253. doi: 10.17582/journal.jahp/2021/9.3.243.253. [DOI] [Google Scholar]
- 509.Lahmass I, Sabouni A, Elyoubi M, Benabbes R, Mokhtari S, Saalaoui E. Anti-diabetic effect of aqueous extract Crocus sativus L. in tartrazine induced diabetic male rats. Physiology and Pharmacology (Iran) 2017;21(4):312–321. [Google Scholar]
- 510.Lakhan MN, Chen R, Shar AH, Chandio MB, Shah AH, Ahmed R, Wang J. Illicium verum as a green source for the synthesis of silver nanoparticles and investigation of antidiatom activity against Paeodactylum Tricornutum. Sukkur IBA Journal of Emerging Technologies. 2020;3(1):23–30. doi: 10.30537/sjet.v3i1.495. [DOI] [Google Scholar]
- 511.Lakshmeesha, T. R., Kalagatur, N. K., Mudili, V., Mohan, C. D., Rangappa, S., Prasad, B. D., Ashwini, B. S., Hashem, A., Alqarawi, A. A., Malik, J. A., Abd-Allah, E. F., Gupta, V. K., Siddaiah, C. N., & Niranjana, S. R. (2019). Biofabrication of zinc oxide nanoparticles with Syzygium aromaticum flower buds extract and finding its novel application in controlling the growth and mycotoxins of Fusarium graminearumFrontiers in Microbiology, 10(JUN)10.3389/fmicb.2019.01244 [DOI] [PMC free article] [PubMed]
- 512.Lal, G., Saran, P. L., Devi, G., & Bijarniya, D. (2020). Production technology of fennel (Foeniculum vulgare Mill)Advances in Vegetable Agronomy, 232–243
- 513.Lampe, J. W. (2003). Spicing up a vegetarian diet: Chemopreventive effects of phytochemicalsAmerican Journal of Clinical Nutrition, 78(3 SUPPL.)10.1093/ajcn/78.3.579s [DOI] [PubMed]
- 514.Lane KE, Li W, Smith CJ, Derbyshire EJ. The development of vegetarian omega-3 oil in water nanoemulsions suitable for integration into functional food products. Journal of Functional Foods. 2016;23:306–314. doi: 10.1016/j.jff.2016.02.043. [DOI] [Google Scholar]
- 515.Lans, C. A. (2006). Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitusJournal of Ethnobiology and Ethnomedicine,210.1186/1746-4269-2-45 [DOI] [PMC free article] [PubMed]
- 516.Laokuldilok N, Thakeow P, Kopermsub P, Utama-ang N. Quality and antioxidant properties of extruded breakfast cereal containing encapsulated turmeric extract. Chiang Mai Journal of Science. 2017;44(3):946–955. [Google Scholar]
- 517.Lashkarizadeh MR, Asgaripour K, Saedi Dezaki E, Fasihi Harandi M. Comparison of scolicidal effects of amphotricin B, silver nanoparticles, and Foeniculum vulgare mill on hydatid cysts protoscoleces. Iranian Journal of Parasitology. 2015;10(2):206–212. [PMC free article] [PubMed] [Google Scholar]
- 518.Lee KA, Kim K-T, Kim HJ, Chung MS, Chang PS, Park H, Pai HD. Antioxidant activities of onion (Allium cepa L.) peel extracts produced by ethanol, hot water, and subcritical water extraction. Food Science and Biotechnology. 2014;23(2):615–621. doi: 10.1007/s10068-014-0084-6. [DOI] [Google Scholar]
- 519.Lee, E. L. (2009). Genotype X environment impact on selected bioactive compound content of fenugreek (Trigonella foenum-graecum L.)ProQuest Dissertations and Theses, 166https://search.proquest.com/docview/193686920?accountid=27575
- 520.Lee JH, Won M, Song KB. Physical properties and antimicrobial activities of porcine meat and bone meal protein films containing coriander oil. Lwt. 2015;63(1):700–705. doi: 10.1016/j.lwt.2015.03.043. [DOI] [Google Scholar]
- 521.Lee MKH, Yang HBS, Jae H, Kim LM. Metabolite analysis and anti-obesity effects of celery seed in 3T3- L1 adipocytes. Food Science and Biotechnology. 2021;30(2):277–286. doi: 10.1007/s10068-020-00866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Lee S, Lee JH. Quality of sponge cakes supplemented with cinnamon. Journal of the Korean Society of Food Science and Nutrition. 2013;42(4):650–654. doi: 10.3746/jkfn.2013.42.4.650. [DOI] [Google Scholar]
- 523.Lee YS, Ryu MJ. Antioxidant effects of Cinnamomum cassia bark extract and its effectiveness as a cosmetics ingredient. Asian Journal of Beauty and Cosmetology. 2019;17(1):69–80. doi: 10.20402/ajbc.2018.0262. [DOI] [Google Scholar]
- 524.Lewis DA, Tharib SM, Veitch GBA. The anti-inflammatory activity of celery apium graveolens L. (Fam. Umbelliferae) Pharmaceutical Biology. 1985;23(1):27–32. doi: 10.3109/13880208509070685. [DOI] [Google Scholar]
- 525.Li, C. W., Chu, Y. C., Huang, C. Y., Fu, S. L., & Chen, J. J. (2020). Evaluation of antioxidant and anti-α-glucosidase activities of various solvent extracts and major bioactive components from the seeds of Myristica fragransMolecules (Basel, Switzerland), 25(21)10.3390/molecules25215198 [DOI] [PMC free article] [PubMed]
- 526.Lian T, Peng M, Vermorken AJM, Jin Y, Luo Z, Van De Ven WJM, Wan Y, Hou P, Cui Y. Synthesis and characterization of curcumin-functionalized hp-β-cd-modified goldmag nanoparticles as drug delivery agents. Journal of Nanoscience and Nanotechnology. 2016;16(6):6258–6264. doi: 10.1166/jnn.2016.11370. [DOI] [PubMed] [Google Scholar]
- 527.Liao, J. C., Deng, J. S., Chiu, C. S., Hou, W. C., Huang, S. S., Shie, P. H., & Huang, G. J. (2012). Anti-inflammatory activities of Cinnamomum cassia constituents in vitro and in vivoEvidence-Based Complementary and Alternative Medicine,201210.1155/2012/429320 [DOI] [PMC free article] [PubMed]
- 528.Licón CC, Carmona M, Rubio R, Molina A, Berruga MI. Preliminary study of saffron (Crocus sativus L. stigmas) color extraction in a dairy matrix. Dyes and Pigments. 2012;92(3):1355–1360. doi: 10.1016/j.dyepig.2011.09.022. [DOI] [Google Scholar]
- 529.Lietzow J. Biologically active compounds in mustard seeds: A toxicological perspective. Foods. 2021;10(9):2089. doi: 10.3390/foods10092089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Lim HS, Kashif G, Park SH, Hwang SY, Park J. quality and antioxidant properties of yellow layer cack containing korean turmeric powder. Journal of Food and Nutrition Research. 2010;49(3):123–133. [Google Scholar]
- 531.Lim HS, Park SH, Ghafoor K, Hwang SY, Park J. Quality and antioxidant properties of bread containing turmeric (Curcuma longa L.) cultivated in South Korea. Food Chemistry. 2011;124(4):1577–1582. doi: 10.1016/j.foodchem.2010.08.016. [DOI] [Google Scholar]
- 532.Lim SH, Lee HS, Lee CH, Choi CI. Pharmacological Activity of Garcinia indica (Kokum): An Updated Review. Pharmaceuticals. 2021;14(12):1–13. doi: 10.3390/ph14121338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Lim, T. K. (2016). Edible medicinal and non-medicinal plants. In Edible Medicinal and Non-Medicinal Plants (Vol. 10)10.1007/978-94-017-7276-1
- 534.Lima EP, Gonçalves OH, Ames FQ, Castro-Hoshino LV, Leimann FV, Cuman RKN, Comar JF, Bersani-Amado CA. Anti-inflammatory and antioxidant activity of nanoencapsulated curcuminoids extracted from Curcuma longa L. in a model of cutaneous inflammation. Inflammation. 2021;44(2):604–616. doi: 10.1007/s10753-020-01360-4. [DOI] [PubMed] [Google Scholar]
- 535.Liman R, Başbuğ B, Ali MM, Acikbas Y, Ciğerci İH. Cytotoxic and genotoxic assessment of tungsten oxide nanoparticles in Allium cepa cells by Allium ana-telophase and comet assays. Journal of Applied Genetics. 2021;62(1):85–92. doi: 10.1007/s13353-020-00608-x. [DOI] [PubMed] [Google Scholar]
- 536.Lisanti A, Formica V, Ianni F, Albertini B, Marinozzi M, Sardella R, Natalini B. Antioxidant activity of phenolic extracts from different cultivars of Italian onion (Allium cepa) and relative human immune cell proliferative induction. Pharmaceutical Biology. 2016;54(5):799–806. doi: 10.3109/13880209.2015.1080733. [DOI] [PubMed] [Google Scholar]
- 537.Liu, Q., Zhang, M., Bhandari, B., Xu, J., & Yang, C. (2020). Effects of nanoemulsion-based active coatings with composite mixture of star anise essential oil, polylysine, and nisin on the quality and shelf life of ready-to-eat Yao meat productsFood Control,10710.1016/j.foodcont.2019.106771
- 538.Liu XC, Zhou LG, Liu ZL, Du SS. Identification of insecticidal constituents of the essential oil of Acorus calamus rhizomes against Liposcelis bostrychophila badonnel. Molecules. 2013;18(5):5684–5696. doi: 10.3390/molecules18055684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Llavata B, Albors A, Martin-Esparza ME. High fibre gluten-free fresh pasta with tiger nut, chickpea and fenugreek: Technofunctional, sensory and nutritional properties. Foods. 2020;9(1):11. doi: 10.3390/foods9010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Lorenzo-Leal, A. C., Palou, E., López-Malo, A., & Bach, H. (2019). Antimicrobial, cytotoxic, and anti-inflammatory activities of Pimenta dioica and Rosmarinus officinalis essential oilsBioMed Research International,201910.1155/2019/1639726 [DOI] [PMC free article] [PubMed]
- 541.Losso JN, Holliday DL, Finley JW, Martin RJ, Rood JC, Yu Y, Greenway FL. Fenugreek bread: a treatment for diabetes mellitus. Journal of Medicinal Food. 2009;12(5):1046–1049. doi: 10.1089/jmf.2008.0199. [DOI] [PubMed] [Google Scholar]
- 542.Luhmer K, Schulze-Kaysers N, Feuereisen M, Wirth L, Maretzky F, Wüst M, Blum H, Dörr E, Pude R. Fatty acid composition, tocopherols, volatile compounds, and sensory evaluation of low morphine yielding varieties of poppy (Papaver somniferum l.) seeds and oils. Journal of Agricultural and Food Chemistry. 2021;69(11):3439–3451. doi: 10.1021/acs.jafc.0c07183. [DOI] [PubMed] [Google Scholar]
- 543.Luna C, Barriga-Castro ED, Gómez-Treviño A, Núñez NO, Mendoza-Reséndez R. Microstructural, spectroscopic, and antibacterial properties of silver-based hybrid nanostructures biosynthesized using extracts of coriander leaves and seeds. International Journal of Nanomedicine. 2016;11:4787–4798. doi: 10.2147/IJN.S105166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Luna C, Chávez VHG, Barriga-Castro ED, Núñez NO, Mendoza-Reséndez R. Biosynthesis of silver fine particles and particles decorated with nanoparticles using the extract of Illicium verum (star anise) seeds. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy. 2015;141:43–50. doi: 10.1016/j.saa.2014.12.076. [DOI] [PubMed] [Google Scholar]
- 545.Luthra PM, Singh R, Chandra R. Therapeutic uses of curcuma longa (Turmeric) Indian Journal of Clinical Biochemistry. 2001;16(2):153–160. doi: 10.1007/BF02864854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Ma’Mani L, Nikzad S, Kheiri-Manjili H, Al-Musawi S, Saeedi M, Askarlou S, Foroumadi A, Shafiee A. Curcumin-loaded guanidine functionalized PEGylated I3ad mesoporous silica nanoparticles KIT-6: Practical strategy for the breast cancer therapy. European Journal of Medicinal Chemistry. 2014;83(March 2018):646–654. doi: 10.1016/j.ejmech.2014.06.069. [DOI] [PubMed] [Google Scholar]
- 547.Mafakheri, S., Niri Dehghan, F., & Mirhosseini, M. (2017). Study the biological production and antibacterial and antifungal effects of silver nanoparticles synthesized by the methanolic extract of clove (Syzygium aromaticum)Journal of Biotechnology, 8(2), 11http://biot.modares.ac.ir/article-22-3170-en.html
- 548.Maggi L, Carmona M, Kelly SD, Marigheto N, Alonso GL. Geographical origin differentiation of saffron spice (Crocus sativus L. stigmas) - Preliminary investigation using chemical and multi-element (H, C, N) stable isotope analysis. Food Chemistry. 2011;128(2):543–548. doi: 10.1016/j.foodchem.2011.03.063. [DOI] [PubMed] [Google Scholar]
- 549.Maghami M, Motalebi AA, Anvar SAA. Influence of chitosan nanoparticles and fennel essential oils (Foeniculum vulgare) on the shelf life of Huso huso fish fillets during the storage. Food Science and Nutrition. 2019;7(9):3030–3041. doi: 10.1002/fsn3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Magro M, Campos R, Baratella D, Ferreira MI, Bonaiuto E, Corraducci V, Uliana MR, Lima GPP, Santagata S, Sambo P, Vianello F. Magnetic purification of curcumin from Curcuma longa rhizome by novel naked maghemite nanoparticles. Journal of Agricultural and Food Chemistry. 2015;63(3):912–920. doi: 10.1021/jf504624u. [DOI] [PubMed] [Google Scholar]
- 551.Mahae N, Chaiseri S. Antioxidant activities and antioxidative components in extracts of Alpinia galanga (l.) sw. Kasetsart Journal - Natural Science. 2009;43(2):358–369. [Google Scholar]
- 552.Mahae N, Chaiseri S. Antioxidant activities and antioxidative components in extracts of Alpinia galanga (L.) Sw. Kasetsart Journal - Natural Science. 2009;43(2):358–369. [Google Scholar]
- 553.Mahboubi M. The beneficial effects of Ferula asafoetida oleo-gum resin in gastrointestinal disorders. Bulletin of Faculty of Pharmacy Cairo University. 2022;59(1):50–63. doi: 10.54634/2090-9101.1025. [DOI] [Google Scholar]
- 554.Mahendra P, Bisht S. Ferula asafoetida : Traditional uses and pharmacological activity. Pharmacognosy Reviews. 2012;6(12):141–146. doi: 10.4103/0973-7847.99948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Mahendra R, Rofik A, Salim HM, Ulfa M, Awwaliyah ES. Neuroprotective activity of extract of celery (Apium graveolens) in insilico study. Medical and Health Science Journal. 2021;5(2):27–31. doi: 10.33086/mhsj.v5i2.2362. [DOI] [Google Scholar]
- 556.Maheshwari B, Yella Reddy S. Application of kokum (Garcinia indica) fat as cocoa butter improver in chocolate. Journal of the Science of Food and Agriculture. 2005;85(1):135–140. doi: 10.1002/jsfa.1967. [DOI] [Google Scholar]
- 557.Majeed, M., Majeed, S., Nagabhushanam, K., Lawrence, L., & Mundkur, L. (2020). Garcinia indica extract standardized for 20% Garcinol reduces adipogenesis and high fat diet-induced obesity in mice by alleviating endoplasmic reticulum stressJournal of Functional Foods,6710.1016/j.jff.2020.103863
- 558.Maji S, Ray PR, Ghatak PK, Chakraborty C. Total phenolic content (TPC) and quality of herbal lassi fortified with Turmeric (Curcuma longa) extract. Asian Journal of Dairy and Food Research. 2018;37(of):273–277. doi: 10.18805/ajdfr.dr-1391. [DOI] [Google Scholar]
- 559.Majidi, Z., Bina, F., Kahkeshani, N., & Rahimi, R. (2020). Bunium persicum: A Review of Ethnopharmacology, Phy-tochemistry, and Biological Activities. In Traditional & Integrative Medicine (Vol. 5, Issue 3)http://jtim.tums.ac.irhttp//jtim.tums.ac.ir
- 560.Majumdar, M. (2012)Fenugreek - health benefits, uses, and side effectshttp://www.medindia.net/patients/lifestyleandwellness/fenugreek-reference.htm
- 561.Makhlouf H, Saksouk M, Habib J, Chahine R. Determination of antioxidant activity of saffron taken from the flower of Crocus sativus grown in Lebanon. African Journal of Biotechnology. 2011;10(41):8093–8100. doi: 10.5897/ajb11.406. [DOI] [Google Scholar]
- 562.Maleki, M., & Mohsenzadeh, M. (2022). Optimization of a biodegradable packaging film based on carboxymethyl cellulose and Persian gum containing titanium dioxide nanoparticles and Foeniculum vulgare essential oil using response surface methodologyJournal of Food Processing and Preservation, 46(4)10.1111/jfpp.16424
- 563.Malhotra, S. K., & Welfare, F. (2021)Spices statistics at a glance 2021 (Issue December)
- 564.Malini PSG, Premalatha V, Rani S. Green synthesis and characterization of silver nanoparticles using ethanol extract of Myristica fragrans (Nutmeg) and its biological applications. Journal of Nanoscience and Technology. 2019;5(3):738–740. doi: 10.30799/jnst.s02.19050309. [DOI] [Google Scholar]
- 565.Mallikarjuna K, Bathula C, Buruga K, Shrestha NK, Noh Y-Y, Kim H. Green synthesis of palladium nanoparticles using fenugreek tea and their catalytic applications in organic reactions. Materials Letters. 2017;205:138–141. doi: 10.1016/j.matlet.2017.06.081. [DOI] [Google Scholar]
- 566.Mallikarjuna K, Bathula C, Dinneswara Reddy G, Shrestha NK, Kim H, Noh Y-Y. Au-Pd bimetallic nanoparticles embedded highly porous Fenugreek polysaccharide based micro networks for catalytic applications. International Journal of Biological Macromolecules. 2019;126:352–358. doi: 10.1016/j.ijbiomac.2018.12.137. [DOI] [PubMed] [Google Scholar]
- 567.Mamgain P, Singh RH. Controlled clinical trial of the lekhaniya drugvaca (Acorus calamus) in cases of Ischaemic heart diseases. Journal of Research in Ayurveda and Siddha. 1994;15(1):35–51. [Google Scholar]
- 568.Man, S. M., Pǎucean, A., Cǎlian, I. D., Murean, V., Chi, M. S., Pop, A., Murean, A. E., Bota, M., & Muste, S. (2019). Influence of fenugreek flour (Trigonella foenum-graecum l.) addition on the technofunctional properties of dark wheat flourJournal of Food Quality, 201910.1155/2019/8635806
- 569.Manap, A. S. A., Tan, A. C. W., Leong, W. H., Chia, A. Y. Y., Vijayabalan, S., Arya, A., Wong, E. H., Rizwan, F., Bindal, U., Koshy, S., & Madhavan, P. (2019). Synergistic effects of curcumin and piperine as potent acetylcholine and amyloidogenic inhibitors with significant neuroprotective activity in sh-sy5y cells via computational molecular modeling and in vitro assayFrontiers in Aging Neuroscience, 10(JUL)10.3389/fnagi.2019.00206 [DOI] [PMC free article] [PubMed]
- 570.Mandal S, Mandal M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pacific Journal of Tropical Biomedicine. 2015;5(6):421–428. doi: 10.1016/j.apjtb.2015.04.001. [DOI] [Google Scholar]
- 571.Mangalampalli B, Dumala N, Grover P. Allium cepa root tip assay in assessment of toxicity of magnesium oxide nanoparticles and microparticles. Journal of Environmental Sciences (China) 2018;66(May):125–137. doi: 10.1016/j.jes.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 572.Mani D, Dhawan SS. Scientific basis of therapeutic uses of opium poppy (Papaver somniferum) in Ayurveda. Acta Horticulturae. 2014;1036:175–180. doi: 10.17660/ActaHortic.2014.1036.20. [DOI] [Google Scholar]
- 573.Manikandan S, Devi RS. Antioxidant property of α-asarone against noise-stress-induced changes in different regions of rat brain. Pharmacological Research. 2005;52(6):467–474. doi: 10.1016/j.phrs.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 574.Manisha DR, Merugu R, Vijay Babu AR, Pratap Rudra MP. Microwave assisted biogenic synthesis of silver nanoparticles using dried seed extract of Coriandrum sativum, Characterization and antimicrobial activity. International Journal of ChemTech Research. 2014;6(7):3957–3961. [Google Scholar]
- 575.Manjunath N, Nayar R, Akkara SS, Sathu T, Vasudevan VN, Rajkumar SR. Effect of carrageenan edible film with oleoresins of Piper nigrum ( black pepper ) on quality of buffalo meat steaks Effect of carrageenan edible film with oleoresins of Piper nigrum ( black pepper ) on quality of buffalo meat steaks. The Pharma Innovation Journal. 2019;8(9):397–400. [Google Scholar]
- 576.Mans, K., & Aburjai, T. (2019). Accessing the hypoglycemic effects of seed extract from celery (Apium graveolens) in alloxan-induced diabetic ratsJournal of Pharmaceutical Research International, April, 1–1010.9734/jpri/2019/v26i630152
- 577.Mansha Rafiq S, Ghosh CB. Fennel (Foeniculum vulgare) and Ajwain (Trachyspermum ammi) extracts as potential preservatives in processed cheese foods. Indian Journal of Dairy Science. 2021;74(3):199–207. doi: 10.33785/ijds.2021.v74i03.002. [DOI] [Google Scholar]
- 578.Marand SA, Alizadeh Khaledabad M, Almasi H. Optimization and characterization of plantago major seed gum/nanoclay/Foeniculum vulgare essential oil active nanocomposite films and their application in preservation of local butter. Food and Bioprocess Technology. 2021;14(12):2302–2322. doi: 10.1007/s11947-021-02724-w. [DOI] [Google Scholar]
- 579.Marrelli, M., Amodeo, V., Statti, G., & Conforti, F. (2019). Biological properties and bioactive components of allium cepa L.: Focus on potential benefits in the treatment of obesity and related comorbiditiesMolecules, 24(1)10.3390/molecules24010119 [DOI] [PMC free article] [PubMed]
- 580.Martina EC, Oludayo AK, Linda NC, Chinasa OP, Ambrose OC, Muoneme OT. Effect of the incorporation of graded levels of turmeric ( Curcuma longa ) on different qualities of stirred yoghurt. African Journal of Food Science. 2020;14(April):71–85. doi: 10.5897/AJFS2020.1903. [DOI] [Google Scholar]
- 581.Mashmoul M, Azlan A, Khaza’Ai H, Yusof BNM, Noor SM. Saffron: a natural potent antioxidant as a promising anti-obesity drug. Antioxidants. 2013;2(4):293–308. doi: 10.3390/antiox2040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Masihuddin M, Jafri M, Siddiqui A, Chaudhary S. Traditional uses, phytochemistry and pharmacological activities of papaver somniferum with special reference of unani medicine an updated review. Journal of Drug Delivery and Therapeutics. 2018;8(5-s):110–114. doi: 10.22270/jddt.v8i5-s.2069. [DOI] [Google Scholar]
- 583.Matacchione G, Gurău F, Silvestrini A, Tiboni M, Mancini L, Valli D, Rippo MR, Recchioni R, Marcheselli F, Carnevali O, Procopio AD, Casettari L, Olivieri F. Anti-SASP and anti-inflammatory activity of resveratrol, curcumin and β-caryophyllene association on human endothelial and monocytic cells. Biogerontology. 2021;22(3):297–313. doi: 10.1007/s10522-021-09915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Matsuda H, Shimoda H, Ninomiya K, Yoshikawa M. Inhibitory mechanism of costunolide, a sesquiterpene lactone isolated from Laurus nobilis, on blood-ethanol elevation in rats: Involvement of inhibition of gastric emptying and increase in gastric juice secretion. Alcohol and Alcoholism. 2002;37(2):121–127. doi: 10.1093/alcalc/37.2.121. [DOI] [PubMed] [Google Scholar]
- 585.Matulyte I, Jekabsone A, Jankauskaite L, Zavistanaviciute P, Sakiene V, Bartkiene E, Ruzauskas M, Kopustinskiene DM, Santini A, Bernatoniene J. The essential oil and hydrolats from myristica fragrans seeds with magnesium aluminometasilicate. Foods. 2020;9(37):1–12. doi: 10.3390/foods9010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Mechchate, H., Es-Safi, I., Amaghnouje, A., Boukhira, S., Alotaibi, A. A., Al-Zharani, M., Nasr, F. A., Noman, O. M., Conte, R., Amal, E. H. E. Y., Bekkari, H., & Bousta, D. (2021). Antioxidant, anti-inflammatory and antidiabetic proprieties of LC-MS/MS identified polyphenols from coriander seedsMolecules, 26(2)10.3390/molecules26020487 [DOI] [PMC free article] [PubMed]
- 587.Medhin, D. G., Bakos, P., & Hadhazy, P. (1986). Inhibitory effects of extracts of Lupinus termis and Coriandrum sativum on electrically induced contraction of the rabbit ear artery. In Acta Pharmaceutica Hungarica (Vol. 56, Issue 3, pp. 109–113) [PubMed]
- 588.Meena, R. K., & Chouhan, N. (2015). Biosynthesis of silver nanoparticles from plant ( Fenugreek Seeds ) reducing method and their optical propertiesResearch Journal of Recent Sciences Res. J. Recent. Sci, 4(January), 47–52http://www.isca.in/rjrs/archive/v4/iIVC-2015/10.ISCA-IVC-2015-04CS-004.pdf
- 589.Meghwal, M. (2012). A review on the functional properties, nutritional content, medicinal utilization and potential application of fenugreekJournal of Food Processing & Technology, 03(09)10.4172/2157-7110.1000181
- 590.Mehdizadeh, T., Langroodi, A, M., Shakouri, R., Khorshidi, S. (2019). physicochemical,microbial and sensory characteristics of probiotic yoghurt enhanced with anethum graveolens essential oilJournal of Food Safety, 39(5)
- 591.Mehmood MH, Gilani AH. Pharmacological basis for the medicinal use of black pepper and piperine in gastrointestinal disorders. Journal of Medicinal Food. 2010;13(5):1086–1096. doi: 10.1089/jmf.2010.1065. [DOI] [PubMed] [Google Scholar]
- 592.Mehmood Y, Farooq U, Yousaf H, Riaz H, Mahmood RK, Nawaz A, Abid Z, Gondal M, Malik NS, Barkat K, Khalid I. Antiviral activity of green silver nanoparticles produced using aqueous buds extract of Syzygium aromaticum. Pakistan Journal of Pharmaceutical Sciences. 2020;33(2):839–845. doi: 10.36721/PJPS.2020.33.2.SUP.839-845.1. [DOI] [PubMed] [Google Scholar]
- 593.Mehra N, Tamta G, Nand V. A review on nutritional value, phytochemical and pharmacological attributes of Foeniculum vulgare Mill. Journal of Pharmacognosy and Phytochemistry. 2021;10(2):1255–1263. doi: 10.22271/phyto.2021.v10.i2q.13983. [DOI] [Google Scholar]
- 594.Melnyk JP, Wang S, Marcone MF. Chemical and biological properties of the world’s most expensive spice: Saffron. Food Research International. 2010;43(8):1981–1989. doi: 10.1016/j.foodres.2010.07.033. [DOI] [Google Scholar]
- 595.Menaga S, Kripa K, Sangeetha R. Evaluation of in vitro inhibition of proton-pump by nanosilver particles synthesized using seeds of anethum graveolens. Asian Journal of Pharmaceutical and Clinical Research. 2017;10(6):363–366. doi: 10.22159/ajpcr.2017.v10i6.16896. [DOI] [Google Scholar]
- 596.Michalak-Majewska M, Teterycz D, Muszyński S, Radzki W, Sykut-Domańska E. Influence of onion skin powder on nutritional and quality attributes of wheat pasta. PLoS ONE. 2020;15(1):1–15. doi: 10.1371/journal.pone.0227942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Mileriene J, Serniene L, Henriques M, Gomes D, Pereira C, Kondrotiene K, Kasetiene N, Lauciene L, Sekmokiene D, Malakauskas M. Effect of liquid whey protein concentrate–based edible coating enriched with cinnamon carbon dioxide extract on the quality and shelf life of Eastern European curd cheese. Journal of Dairy Science. 2021;104(2):1504–1517. doi: 10.3168/jds.2020-18732. [DOI] [PubMed] [Google Scholar]
- 598.Minh, N. P. (2021). effectiveness of chili powder incorporation to microbial loads,physicochemical and sensory characteristics of vietnamese fermented pork rollJournal of Pure and Applied Microbiology, 15(1)
- 599.Mishra S, Pathak V. Phytochemical study and antimicrobial activity of khus khus seeds. World Journal of Pharmaceutical Research. 2021;10(11):1663–1681. [Google Scholar]
- 600.Missaoui T, Smiri M, Chemingui H, Jbira E, Hafiane A. Regulation of mitochondrial and cytosol antioxidant systems of fenugreek (Trigonella foenum graecum L.) exposed to nanosized titanium dioxide. Bulletin of Environmental Contamination and Toxicology. 2018;101(3):326–337. doi: 10.1007/s00128-018-2414-5. [DOI] [PubMed] [Google Scholar]
- 601.Missaoui T, Smiri M, Chmingui H, Hafiane A. Effects of nanosized titanium dioxide on the photosynthetic metabolism of fenugreek (Trigonella foenum-graecum L.) Comptes Rendus - Biologies. 2017;340(11–12):499–511. doi: 10.1016/j.crvi.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 602.Mittal L, Camarillo IG, Varadarajan GS, Srinivasan H, Aryal UK, Sundararajan R. High-throughput, label-free quantitative proteomic studies of the anticancer effects of electrical pulses with turmeric silver nanoparticles: An in vitro model study. Scientific Reports. 2020;10(1):1–18. doi: 10.1038/s41598-020-64128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Mobeen, S. A., & Riazunnisa, K. (2019). Methylene blue dye degradation by silver nanoparticles biosynthesized using seed extracts of Foeniculum vulgareZero Waste,219–22510.1201/9780429059247-14
- 604.Modi S, Yadav VK, Choudhary N, Alswieleh AM, Sharma AK, Bhardwaj AK, Khan SH, Yadav KK, Cheon J-K, Jeon BH. Onion peel waste mediated-green synthesis of zinc oxide nanoparticles and their phytotoxicity on mung bean and wheat plant growth. Materials. 2022;15:2393. doi: 10.3390/ma15072393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Mohammed GJ, Omran AM, Hussein HM. Antibacterial and phytochemical analysis of piper nigrum using gas chromatography – mass spectrum and fourier-transform infrared spectroscopy. International Journal of Pharmacognosy and Phytochemical Research. 2016;8(6):977–996. [Google Scholar]
- 606.Mohammed KAK, Abdulkadhim HM, Noori SI. Chemical composition and anti-bacterial effects of clove (Syzygium aromaticum) flowers. International Journal of Current Microbiology and Applied Sciences. 2016;5(2):483–489. doi: 10.20546/ijcmas.2016.502.054. [DOI] [Google Scholar]
- 607.Mohammed GJ, Omran AM, Hussein HM. Antibacterial and phytochemical analysis of piper nigrum using gas chromatography – mass spectrum and fourier-transform infrared spectroscopy. International Journal of Pharmacognosy and Phytochemical Research. 2016;8(6):977–996. [Google Scholar]
- 608.Mohammed NA. In vitro study of antimicrobial activity of silver nanoparticles usage (Curcuma longa L.) rhizomes against Helicobacterpylori. Plant Archives. 2019;19(1):1102–1106. [Google Scholar]
- 609.Mohammed RR, Omer AK, Yener Z, Uyar A, Ahmed AK. Biomedical effects of Laurus nobilis L. leaf extract on vital organs in streptozotocin-induced diabetic rats: Experimental research. Annals of Medicine and Surgery. 2021;61(October 2020):188–197. doi: 10.1016/j.amsu.2020.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Mok J-S, Song K-C, Chol N-J, Yang H-S. antibacterial effect of cinnamon bark extract against fish pathogenic bacteria. Korean Journal of Fisheries and Aquatic Sciences. 2001;34(5):545–549. [Google Scholar]
- 611.Momin AH, Acharya SS, Gajjar AV. coriandrum sativum- review of advances in phytopharmacology. International Journal of Pharmaceutical Sciences and Research. 2012;3(5):1233–1239. [Google Scholar]
- 612.Mondal, M. A., Yeasmin, T., Karim, R., Siddiqui, M. N., Nabi, S. R., Sayed, M. A., & Siddiky, M. N. A. (2015). Effect of dietary supplementation of turmeric (Curcuma longa) powder on the growth performance and carcass traits of broiler chicks. SAARC Journal of Agriculture, 13(1), 188–199
- 613.Montalbán MG, Coburn JM, Lozano-Pérez AA, Cenis JL, Víllora G, Kaplan DL. Production of curcumin-loaded silk fibroin nanoparticles for cancer therapy. Nanomaterials. 2018;8(2):7–13. doi: 10.3390/nano8020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Moradi MT, Karimi A, Alidadi S, Hashemi L. Anti-adenovirus activity, antioxidant potential, and phenolic content of dried flower buds of Syzygium aromaticum extract in HEp2 cell line. Marmara Pharmaceutical Journal. 2017;21(4):852–859. doi: 10.12991/mpj.2017.4. [DOI] [Google Scholar]
- 615.Moratalla-López, N., Bagur, M. J., Lorenzo, C., Martínez-Navarro, M. E., Rosario Salinas, M., & Alonso, G. L. (2019). Bioactivity and bioavailability of the major metabolites of Crocus sativus l. flowerMolecules, 24(15)10.3390/molecules24152827 [DOI] [PMC free article] [PubMed]
- 616.Mostafa DM, Abd El-Alim SH, Asfour MH, Al-Okbi SY, Mohamed DA, Awad G. Transdermal nanoemulsions of Foeniculum vulgare Mill. essential oil: Preparation, characterization and evaluation of antidiabetic potential. Journal of Drug Delivery Science and Technology. 2015;29:99–106. doi: 10.1016/j.jddst.2015.06.021. [DOI] [Google Scholar]
- 617.Mozafarian, V. (2018)Identification of medicinal and aromatic plants of Iran. 1350
- 618.Mtibaa, A. C., Smaoui, S., Ben Hlima, H., Sellem, I., Ennouri, K., & Mellouli, L. (2019). Enterocin BacFL31 from a Safety Enterococcus faecium FL31: Natural Preservative Agent Used Alone and in Combination with Aqueous Peel Onion (Allium cepa) Extract in Ground Beef Meat StorageBioMed Research International,201910.1155/2019/4094890 [DOI] [PMC free article] [PubMed]
- 619.Muche BM, Rupasinghe HPV. Natural antimicrobial agents of cinnamon ( Cinnamomum zeylanicum L. and C. cassia ) and vanilla ( Vanilla planifola, V. fresh-cut fruits. Ethiop. Journal of Applied Science and Technology. 2011;2(1):1–13. [Google Scholar]
- 620.Muckensturm B, Foechterlen D, Reduron JP, Danton P, Hildenbrand M. Phytochemical and chemotaxonomic studies of Foeniculum vulgare. Biochemical Systematics and Ecology. 1997;25(4):353–358. doi: 10.1016/S0305-1978(96)00106-8. [DOI] [Google Scholar]
- 621.Muhammad A, Akhtar A, Aslam S, Khan RS, Ahmed Z, Khalid N. Review on physicochemical, medicinal and nutraceutical properties of poppy seeds: a potential functional food ingredient. Functional Foods in Health and Disease. 2021;11(10):522–547. doi: 10.31989/ffhd.v11i10.836. [DOI] [Google Scholar]
- 622.Muhammad DRA, Saputro AD, Rottiers H, Van de Walle D, Dewettinck K. Physicochemical properties and antioxidant activities of chocolates enriched with engineered cinnamon nanoparticles. European Food Research and Technology. 2018;244(7):1185–1202. doi: 10.1007/s00217-018-3035-2. [DOI] [Google Scholar]
- 623.Muhammad DRA, Sedaghat Doost A, Gupta V, bin Sintang MD, Van de Walle D, Van der Meeren P, Dewettinck K. Stability and functionality of xanthan gum–shellac nanoparticles for the encapsulation of cinnamon bark extract. Food Hydrocolloids. 2020;100(August 2019):105377. doi: 10.1016/j.foodhyd.2019.105377. [DOI] [Google Scholar]
- 624.Muhammad, W., As, S., Sumaira, S., Muhammad, N., Safia, H., & Muhammad, J. (2017). Green synthesis of gold nanoparticles and their characterizations using plant extract of Papaver somniferumNano Science & Nano Technology: An Indian Journal, 11(2), 1–8https://www.tsijournals.com/articles/green-synthesis-of-gold-nanoparticles-and-their-characterizations-using-plant-extract-of-papaver-somniferum-13484.html%0Awww.tsijournals.com
- 625.Muhammad W, Khan MA, Nazir M, Siddiquah A, Mushtaq S, Hashmi SS, Abbasi BH. Papaver somniferum L. mediated novel bioinspired lead oxide (PbO) and iron oxide (Fe2O3) nanoparticles: In-vitro biological applications, biocompatibility and their potential towards HepG2 cell line. Materials Science and Engineering C. 2019;103(April):109740. doi: 10.1016/j.msec.2019.109740. [DOI] [PubMed] [Google Scholar]
- 626.Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acorus calamus: Scientific validation of ayurvedic tradition from natural resources. Pharmaceutical Biology. 2007;45(8):651–666. doi: 10.1080/13880200701538724. [DOI] [Google Scholar]
- 627.Mukthamba P, Srinivasan K. Dietary fenugreek (Trigonella foenum-graecum) seeds and garlic (Allium sativum) alleviates oxidative stress in experimental myocardial infarction. Food Science and Human Wellness. 2017;6(2):77–87. doi: 10.1016/j.fshw.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 628.Mulla M, Ahmed J, Al-Attar H, Castro-Aguirre E, Arfat YA, Auras R. Antimicrobial efficacy of clove essential oil infused into chemically modified LLDPE film for chicken meat packaging. Food Control. 2017;73:663–671. doi: 10.1016/j.foodcont.2016.09.018. [DOI] [Google Scholar]
- 629.Murugesan K, Koroth J, Srinivasan PP, Singh A, Mukundan S, Karki SS, Choudhary B, Gupta CM. Effects of green synthesised silver nanoparticles (ST06-AgNPs) using curcumin derivative (ST06) on human cervical cancer cells (HeLa) in vitro and EAC tumor bearing mice models. International Journal of Nanomedicine. 2019;14:5257–5270. doi: 10.2147/IJN.S202404. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 630.Musa Özcan M, Atalay Ç. Determination of seed and oil properties of some poppy (Papaver somniferum L.) varieties. Grasas y Aceites. 2006;57(2):169–174. doi: 10.3989/gya.2006.v57.i2.33. [DOI] [Google Scholar]
- 631.Mustafa RA. Phytochemicals analysis and minerals present in the dried used as spices: health risk implication in Northern of Iraq. Pakistan Journal of Medical and Health Sciences. 2021;15(7):2006–2013. doi: 10.53350/pjmhs211572006. [DOI] [Google Scholar]
- 632.Muthukumaran P, Kumaravel S, Nimia N. Phytochemical, GC-MS and FT-IR Analysis of Papaver somniferum L. Journal of Pharmaceutical and Biological Sciences. 2019;7(1):1–8. doi: 10.18231/j.jpbs.2019.001. [DOI] [Google Scholar]
- 633.Mzabri, I., Addi, M., & Berrichi, A. (2019). Traditional and modern uses of saffron (Crocus sativus). In Cosmetics (Vol. 6, Issue 4, pp. 1–11). MDPI AG10.3390/COSMETICS6040063
- 634.Nadeem M, Anjum FM, Khan MI, Tehseen S, El-Ghorab A, Sultan JI. Nutritional and medicinal aspects of coriander (Coriandrum sativum L.): A review. British Food Journal. 2013;115(5):743–755. doi: 10.1108/00070701311331526. [DOI] [Google Scholar]
- 635.Nagaonkar D, Shende S, Rai M. Biosynthesis of copper nanoparticles and its effect on actively dividing cells of mitosis in Allium cepa. Biotechnology Progress. 2015;31(2):557–565. doi: 10.1002/btpr.2040. [DOI] [PubMed] [Google Scholar]
- 636.Nagdeve, M. (2021). 11 benefits that prove why mustard is good for healthOrganic Facts
- 637.Nakkala JR, Mata R, Gupta AK, Sadras SR. Biological activities of green silver nanoparticles synthesized with Acorous calamus rhizome extract. European Journal of Medicinal Chemistry. 2014;85:784–794. doi: 10.1016/j.ejmech.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 638.Namjoyan F, Jahangiri A, Azemi ME, Arkian E, Mousavi H. Inhibitory effects of physalis alkekengi l., Alcea rosea l., Bunium persicum b. fedtsch. and Marrubium vulgare l. on mushroom tyrosinase. Jundishapur Journal of Natural Pharmaceutical Products. 2015;10(1):1–6. doi: 10.17795/jjnpp-23356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Naseri M, Mojab F, Khodadoost M, Kamalinejad M, Davati A, Choopani R, Hasheminejad A, Bararpoor Z, Shariatpanahi S, Emtiazy M. The study of anti-infammatory activity of oil-based dill (Anethum graveolens L.) extract used topically in formalin-induced infammation male rat paw. Iranian Journal of Pharmaceutical Research. 2012;11(4):1169–1174. [PMC free article] [PubMed] [Google Scholar]
- 640.Nassiri-Asl, M., & Hosseinzadeh, H. (2015). chapter 3-neuropharmacology effects of saffron and its active constituentsBioactive Neutraceuticals and Dietary Supplements in Neurological and Brain Disease, 29–39
- 641.Natarajan B, Muralidharan A, Satish R, Dhananjayan R. Neuropharmacological activity of Trigonella foenum graecum Linn. seeds. Journal of Natural Remedies. 2007;7(1):160–165. [Google Scholar]
- 642.Nauman SM, Mohammad I. Role of khardal (Brassica Nigra) in non-communicable diseases: An overview. International Journal of Drug Development and Research. 2015;7(1):137–144. [Google Scholar]
- 643.Nawaz H, Shad MA, Muzaffar S. Phytochemical composition and antioxidant potential of Brassica. Brassica Germplasm - Characterization, Breeding and Utilization. 2018;1:7–26. doi: 10.5772/intechopen.76120. [DOI] [Google Scholar]
- 644.Nayak CA, Rastogi NK, Raghavarao KSMS. Bioactive constituents present in garcinia indica choisy and its potential food applications: A review. International Journal of Food Properties. 2010;13(3):441–453. doi: 10.1080/10942910802626754. [DOI] [Google Scholar]
- 645.Nayak NK, Pathak V. Development of low fat chevon patties using poppy seed as fat replacer. Indian Journal of Small Ruminants (The) 2017;23(2):236. doi: 10.5958/0973-9718.2017.00038.1. [DOI] [Google Scholar]
- 646.Nayaka, S., Chakraborty, B., Bhat, M. P., Nagaraja, S. K., Airodagi, D., Swamy, P. S., Rudrappa, M., Hiremath, H., Basavarajappa, D. S., & Kanakannanavar, B. (2020). Biosynthesis, characterization, and in vitro assessment on cytotoxicity of actinomycete-synthesized silver nanoparticles on Allium cepa root tip cellsBeni-Suef University Journal of Basic and Applied Sciences, 9(1)10.1186/s43088-020-00074-8
- 647.Nazari E, Khanavi M, Amani L, Sharifzadeh M, Vazirian M, Saeedi M, Sanati M, Lamardi SNS. Beneficial effect of Brassica nigra fixed oil on the changes in memory caused by Β-amyloid in an animal model. Pharmaceutical Sciences. 2020;26(3):261–269. doi: 10.34172/PS.2020.19. [DOI] [Google Scholar]
- 648.Nazeruddin GM, Prasad NR, Prasad SR, Shaikh YI, Waghmare SR, Adhyapak P. Coriandrum sativum seed extract assisted in situ green synthesis of silver nanoparticle and its anti-microbial activity. Industrial Crops and Products. 2014;60:212–216. doi: 10.1016/j.indcrop.2014.05.040. [DOI] [Google Scholar]
- 649.Nejat H, Rabiee M, Varshochian R, Tahriri M, Jazayeri HE, Rajadas J, Ye H, Cui Z, Tayebi L. Preparation and characterization of cardamom extract-loaded gelatin nanoparticles as effective targeted drug delivery system to treat glioblastoma. Reactive and Functional Polymers. 2017;120:46–56. doi: 10.1016/j.reactfunctpolym.2017.09.008. [DOI] [Google Scholar]
- 650.Nellore J, Pauline C, Amarnath K, Shilpa PN. Antioxidant effect of gold nanoparticles synthesised from Curcuma Longa restrains 1-methyl-2-phenyl pyridinium ion induced stress in PC12 cells. Journal of Nanoneuroscience. 2012;2(1):63–74. doi: 10.1166/jns.2012.1018. [DOI] [Google Scholar]
- 651.Nguyen, H. N., Ha, P. T., Nguyen, A. S., Nguyen, D. T., Do, H. D., Thi, Q. N., & Hoang, T. M. N. (2016). Curcumin as fluorescent probe for directly monitoring in vitro uptake of curcumin combined paclitaxel loaded PLA-TPGS nanoparticlesAdvances in Natural Sciences: Nanoscience and Nanotechnology, 7(2)10.1088/2043-6262/7/2/025001
- 652.Nguyen PH, Le TVT, Kang HW, Chae J, Kim SK, Kwon KiI, Seo DB, Lee SJ, Oh WK. AMP-activated protein kinase (AMPK) activators from Myristica fragrans (nutmeg) and their anti-obesity effect. Bioorganic and Medicinal Chemistry Letters. 2010;20(14):4128–4131. doi: 10.1016/j.bmcl.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 653.Nguyen QH, Talou T, Cerny M, Evon P, Merah O. Oil and fatty acid accumulation during coriander (Coriandrum sativum L.) fruit ripening under organic cultivation. Crop Journal. 2015;3(4):366–369. doi: 10.1016/j.cj.2015.05.002. [DOI] [Google Scholar]
- 654.Nile SH, Park SW. Total phenolics, antioxidant and xanthine oxidase inhibitory activity of three colored onions (Allium cepa L.) Frontiers in Life Science. 2013;7(3–4):224–228. doi: 10.1080/21553769.2014.901926. [DOI] [Google Scholar]
- 655.Nisar T, Iqbal M, Raza A, Iftikhar F, Safdar M, Waheed M. Estimation of total phenolics and free radical scavenging of turmeric (Curcuma longa) Journal of Agricultural & Environmental Sciences. 2015;15(7):1272–1277. doi: 10.5829/idosi.aejaes.2015.15.7.9527. [DOI] [Google Scholar]
- 656.Noumi, E., Snoussi, M., Alreshidi, M. M., Rekha, P. D., Saptami, K., Caputo, L., De Martino, L., Souza, L. F., Msaada, K., Mancini, E., Flamini, G., Al-Sieni, A., & De Feo, V. (2018). Chemical and biological evaluation of essential oils from cardamom speciesMolecules, 23(11)10.3390/molecules23112818 [DOI] [PMC free article] [PubMed]
- 657.Ntohogian, S., Gavriliadou, V., Christodoulou, E., Nanaki, S., Lykidou, S., Naidis, P., Mischopoulou, L., Barmpalexis, P., Nikolaidis, N., & Bikiaris, D. N. (2018). Chitosan nanoparticles with encapsulated natural and Uf-purified annatto and saffron for the preparation of UV protective cosmetic emulsionsMolecules, 23(9)10.3390/molecules23092107 [DOI] [PMC free article] [PubMed]
- 658.Nur Manik MI, Ali MH, Nath Ray M, Islam MM, Zobayed A, Hossain MT, Md A, Fahad TM, Khan A. A comprehensive review on medicinal values and health benefits of spices and condiments commonly used in the Indian sub-continent. Research Journal of Pharmacognosy and Phytochemistry. 2022;14(01):11–18. doi: 10.5271/0975-4385.2022.00003. [DOI] [Google Scholar]
- 659.Obeid MA, Khadra I, Albaloushi A, Mullin M, Alyamani H, Ferro VA. Microfluidic manufacturing of different niosomes nanoparticles for curcumin encapsulation: Physical characteristics, encapsulation efficacy, and drug release. Beilstein Journal of Nanotechnology. 2019;10:1826–1832. doi: 10.3762/bjnano.10.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Odimegwu NE, Ubbaonu CN, Ofoedu CE, Akajiaku LO, Njoku NE, Agunwah LM, Alagbaoso SO, Iwuh GE. comparative study on the proximate composition, functional and sensory properties of turmeric and pawpaw custard products. Current Journal of Applied Science and Technology. 2019;33(4):1–11. doi: 10.9734/cjast/2019/v33i430090. [DOI] [Google Scholar]
- 661.Ogunruku OO, Oboh G, Ogunsuyi OB. Neuroprotective potential of phenolic extracts of Capsicum frutescens as an inhibitor of monoamine oxidase and cholinesterase activities. Ife Journal of Science. 2018;20(2):403. doi: 10.4314/ijs.v20i2.20. [DOI] [Google Scholar]
- 662.Ogwaro BA, O’gara EA, Hill DJ, Gibson H. A study of the antimicrobial activity of combined black pepper and cinnamon essential oils against escherichia fergusonii in traditional african yoghurt. Foods. 2021;10(11):2847. doi: 10.3390/foods10112847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Okunade OA, Ghawi SK, Methven L, Niranjan K. Thermal and pressure stability of myrosinase enzymes from black mustard, brown mustard and yellow mustard seeds. Food Chemistry. 2015;187:485–490. doi: 10.1016/j.foodchem.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 664.Olaleye, M. T., Akinmoladun, A. C., & Akindahunsi, A. A. (2006). Antioxidant properties of Myristica fragrans (Houtt) and its effect on selected organs of albino ratsAfrican Journal of Biotechnology, 5(13), 1274–1278http://www.academicjournals.org/AJB
- 665.Olatunji, T. L., & Afolayan, A. J. (2019). Comparative quantitative study on phytochemical contents and antioxidant activities of capsicum annuum L. and Capsicum frutescens LScientific World Journal, 201910.1155/2019/4705140 [DOI] [PMC free article] [PubMed]
- 666.Oliveira AR, Ribeiro AEC, Oliveira ER, Ribeiro KO, Garcia MC, Careli-Gondim I, Junior MSS, Caliari M. physicochemical, microbiological and sensory characteristics of snacks developed from broken rice grains and turmeric powder. International Journal of Food Science + Technology. 2020;55(7):2719–2729. doi: 10.1111/ijfs.14525. [DOI] [Google Scholar]
- 667.Omar, A. E., Al-Khalaifah, H. S., Mohamed, W. A. M., Gharib, H. S. A., Osman, A., Al-Gabri, N. A., & Amer, S. A. (2020). Effects of phenolic-rich onion (Allium cepa L.) extract on the growth performance, behavior, intestinal histology, amino acid digestibility, antioxidant activity, and the immune status of broiler chickensFrontiers in Veterinary Science, 710.3389/fvets.2020.582612 [DOI] [PMC free article] [PubMed]
- 668.Otunola, G, A., Afolayan, A, J., Ajayi, E, O., Odeyemi, S, W. (2017). Characterization, antibacterial and antioxidant properties of silver nanoparticles synthesized from aqueous extracts of allium sativum,zingiber officinale and capsicum frutescensA Multifaceted Peer Reviewed Journal in the Field of Pharmacognosy and Natural Products [DOI] [PMC free article] [PubMed]
- 669.Otunola GA, Oloyede OB, Oladiji AT, Afolayan AJ. Comparative analysis of the chemical composition of three spices - Allium sativum L. Zingiber officinale Rosc. and Capsicum frutescens L. commonly consumed in Nigeria. African Journal of Biotechnology. 2010;9(41):6927–6931. doi: 10.5897/ajb10.183. [DOI] [Google Scholar]
- 670.Oudjedi K, Manso S, Nerin C, Hassissen N, Zaidi F. New active antioxidant multilayer food packaging films containing Algerian Sage and Bay leaves extracts and their application for oxidative stability of fried potatoes. Food Control. 2019;98:216–226. doi: 10.1016/j.foodcont.2018.11.018. [DOI] [Google Scholar]
- 671.Oyemitan IA, Elusiyan CA, Onifade AO, Akanmu MA, Oyedeji AO, McDonald AG. Neuropharmacological profile and chemical analysis of fresh rhizome essential oil of Curcuma longa (turmeric) cultivated in Southwest Nigeria. Toxicology Reports. 2017;4(February):391–398. doi: 10.1016/j.toxrep.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Ozbek H. The anti-inflammatory activity of the Foeniculum vulgare l. essential oil and investigation of its median lethal dose in rats and mice. International Journal of Pharmacology. 2005;1(4):329–331. doi: 10.3923/ijp.2005.329.331. [DOI] [Google Scholar]
- 673.Özkutlu F, Kara SM, Sekeroglu N. Determination of mineral and trace elements in some spices cultivated in Turkey. Acta Horticulturae. 2007;756:321–327. doi: 10.17660/actahortic.2007.756.34. [DOI] [Google Scholar]
- 674.Pakrashi, S., Jain, N., Dalai, S., Jayakumar, J., Chandrasekaran, P. T., Raichur, A. M., Chandrasekaran, N., & Mukherjee, A. (2014)In vivo genotoxicity assessment of titanium dioxide nanoparticles by Allium cepa root tip assay at high exposure concentrationsPLoS ONE, 9(2)10.1371/journal.pone.0087789 [DOI] [PMC free article] [PubMed]
- 675.Pal M, Srivastava M, Soni DK, Kumar A, Tewari SK. Composition and anti-microbial activity of essential oil of Myristica fragrans from Andaman Nicobar Island. Inernational Journal of Pharmacy & L Ife Sciences. 2011;2(10):1115–1117. [Google Scholar]
- 676.Pal, S., Pal, K., Mukherjee, S., Bera, D., Karmakar, P., & Sukhen, D. (2020). Green cardamom mediated phytosynthesis of ZnONPs and validation of its antibacterial and anticancerous potentialMaterials Research Express, 7(1)10.1088/2053-1591/ab69c8
- 677.Panda VS, Islam A. In vivo anti-inflammatory activity of Garcinia indica fruit rind (Kokum) in rats. The Journal of Phytopharmacology. 2013;2(5):8–14. doi: 10.31254/phyto.2013.2502. [DOI] [Google Scholar]
- 678.Panda VS, Khambat PD. Antiulcer activity of Garcinia indica fruit rind (kokum berry) in rats. Biomedicine and Aging Pathology. 2014;4(4):309–316. doi: 10.1016/j.biomag.2014.07.008. [DOI] [Google Scholar]
- 679.Pandey H, Awasthi P. Effect of processing techniques on nutritional composition and antioxidant activity of fenugreek (Trigonella foenum-graecum) seed flour. Journal of Food Science and Technology. 2015;52(2):1054–1060. doi: 10.1007/s13197-013-1057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Pandit R. Green synthesis of silver nanoparticles from seed extract of Brassica nigra and its antibacterial activity. Nusantara Bioscience. 1970;7(1):15–19. doi: 10.13057/nusbiosci/n070103. [DOI] [Google Scholar]
- 681.Pandya KK, Patel RB, Chakravarthy BK. Antibacterial activity of some Indian medicinal plants. Indian Drugs. 1990;27(8):415–417. [Google Scholar]
- 682.Parida UK, Bindhani BK, Nayak P. Green synthesis and characterization of gold nanoparticles using onion (Allium cepa) extract. World Journal of Nano Science and Engineering. 2011;01(04):93–98. doi: 10.4236/wjnse.2011.14015. [DOI] [Google Scholar]
- 683.Park SH, Lim HS, Hwang SY. Evaluation of antioxidant, rheological, physical and sensorial properties of wheat flour dough and cake containing turmeric powder. Food Science and Technology International. 2012;18(5):435–443. doi: 10.1177/1082013211428220. [DOI] [PubMed] [Google Scholar]
- 684.Parki A, Chaubey P, Prakash O, Kumar R, Pant AK. Seasonal variation in essential oil compositions and antioxidant properties of Acorus calamus l. accessions. Medicines. 2017;4(4):81. doi: 10.3390/medicines4040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Parvandi M, Rezadoost H, Farzaneh M. Introducing Alternaria tenuissima SBUp1, as an endophytic fungus of Ferula assa-foetida from Iran, which is a rich source of rosmarinic acid. Letters in Applied Microbiology. 2021;73(5):569–578. doi: 10.1111/lam.13542. [DOI] [PubMed] [Google Scholar]
- 686.Pashapoor A, Mashhadyrafie S, Mortazavi P. The antioxidant potential and antihyperlipidemic activity of Myristica fragrans seed (nutmeg) extract in diabetic rats. Journal of Human, Environment, and Health Promotion. 2020;6(2):91–96. doi: 10.29252/jhehp.6.2.7. [DOI] [Google Scholar]
- 687.Passano P. The many uses of methi. Manushi. 1995;2:31–34. [Google Scholar]
- 688.Patel S, Shende S, Arora S, Singh AK. An assessment of the antioxidant potential of coriander extracts in ghee when stored at high temperature and during deep fat frying. International Journal of Dairy Technology. 2013;66(2):207–213. doi: 10.1111/1471-0307.12023. [DOI] [Google Scholar]
- 689.Pathak P, Srivastava S, Grover S. Development of food products based on millets, legumes and fenugreek seeds and their suitability in the diabetic diet. International Journal of Food Sciences and Nutrition. 2000;51(5):409–414. doi: 10.1080/096374800427019. [DOI] [PubMed] [Google Scholar]
- 690.Patil SD, Shinde S, Kandpile P, Jain AS. Evaluation of antimicrobial activity of asafoetida. International Journal of Pharmaceutical Science and Research. 2015;6(2):722–727. doi: 10.13040/IJPSR.0975-8232.6(2).722-27. [DOI] [Google Scholar]
- 691.Patra, D., & Moussawi, R. N. (2015). Curcumin conjugated gold nanoparticles for nucleic acid sensingIEEE-NANO 2015 - 15th International Conference on Nanotechnology, 401–40410.1109/NANO.2015.7388621
- 692.Patra D, Sleem F. A new method for pH triggered curcumin release by applying poly(l-lysine) mediated nanoparticle-congregation. Analytica Chimica Acta. 2013;795:60–68. doi: 10.1016/j.aca.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 693.Patra JK, Das G, Bose S, Banerjee S, Vishnuprasad CN, del Pilar Rodriguez-Torres M, Shin HS. Star anise (Illicium verum): Chemical compounds, antiviral properties, and clinical relevance. Phytotherapy Research. 2020;34(6):1248–1267. doi: 10.1002/ptr.6614. [DOI] [PubMed] [Google Scholar]
- 694.Pattanayak M, Muralikrishnan T, Nayak PL. Green synthesis of gold nanoparticles using Elettaria cardamomum ( ELAICHI ) aqueous extract. World Journal of Nano Science & Technology. 2013;2(2):52–58. doi: 10.5829/idosi.wjnst.2013.2.1.21131. [DOI] [Google Scholar]
- 695.Paul R, Roy A, Rajeshkumar S, Thangavelu L. Cytotoxic effect and antibacterial activity of coriander oleoresin mediated zinc oxide nanoparticles. International Journal of Pharmaceutical Research. 2020;12(October):3057–3062. doi: 10.31838/ijpr/2020.SP1.411. [DOI] [Google Scholar]
- 696.Pavalakumar D, Jayasinghe M, Edirisinghe M, Wijesekara I, Senadheera S. Cinnamomum zeylanicum and Curcuma longa incorporated dairy yoghurts with hindered glycaemic properties for healthy people. Journal of Future Foods. 2021;1(1):104–112. doi: 10.1016/j.jfutfo.2021.09.006. [DOI] [Google Scholar]
- 697.Pellegrini, M., Rossi, C., Palmieri, S., Maggio, F., Chaves-López, C., Lo Sterzo, C., Paparella, A., De Medici, D., Ricci, A., & Serio, A. (2020). Salmonella enterica control in stick carrots through incorporation of coriander seeds essential oil in sustainable washing treatmentsFrontiers in Sustainable Food Systems,410.3389/fsufs.2020.00014
- 698.Perumalsamy R, Krishnadhas L. Anti-diabetic activity of silver nanoparticles synthesized from the hydroethanolic extract of Myristica fragrans seeds. Applied Biochemistry and Biotechnology. 2022;194(3):1136–1148. doi: 10.1007/s12010-022-03825-8. [DOI] [PubMed] [Google Scholar]
- 699.Pesnya DS. Cytogenetic effects of chitosan-capped silver nanoparticles in the Allium cepa test. Caryologia. 2013;66(3):275–281. doi: 10.1080/00087114.2013.852342. [DOI] [Google Scholar]
- 700.Peters R, Kramer E, Oomen AG, Herrera Rivera ZE, Oegema G, Tromp PC, Fokkink R, Rietveld A, Marvin HJP, Weigel S, Peijnenburg AACM, Bouwmeester H. Presence of nano-sized silica during in vitro digestion of foods containing silica as a food additive. ACS Nano. 2012;6(3):2441–2451. doi: 10.1021/nn204728k. [DOI] [PubMed] [Google Scholar]
- 701.Pgs, M. (2020). Seed production technology of coriander (Coriandrum sativum)In Advances in Vegetable Agronomy, 214–222
- 702.Pilar Santamarina M, Roselló J, Giménez S, Amparo Blázquez M. Commercial Laurus nobilis L. and Syzygium aromaticum L. Merr. & Perry essential oils against post-harvest phytopathogenic fungi on rice. LWT - Food Science and Technology. 2016;65:325–332. doi: 10.1016/j.lwt.2015.08.040. [DOI] [Google Scholar]
- 703.Pillai RR, Sreelekshmi PB, Meera AP. Enhanced biological performance of green sythesized copper oxide nanoparticles using Pimenta dioica leaf extract. Materials Today: Proceedings. 2022;50:163–172. doi: 10.1016/j.matpr.2021.11.547. [DOI] [Google Scholar]
- 704.Pipriya S. Evaluation of antibacterial potential & phytochemical screening by the medicinal plant of Acorus calamus & Agaricus bisporus & their synthesis of herbal silver nanoparticles with different solvents. International Journal of Engineering Research and Technology (IJERT) 2019;8(05):158–169. [Google Scholar]
- 705.Pipriya S, Kundu N, Tiwari U. Green synthesis, characterization and antioxidant activity of silver nanoparticles in extracts of Acorus calamus and Agaricus bisporus. International Journal of Biochemistry Research & Review. 2018;21(4):1–15. doi: 10.9734/ijbcrr/2018/41615. [DOI] [Google Scholar]
- 706.Plangar AF, Anaeigoudari A, Khajavirad A, Shafei MN. Beneficial cardiovascular effects of hydroalcoholic extract from crocus sativus in hypertension induced by angiotensin II. Journal of Pharmacopuncture. 2019;22(2):95–101. doi: 10.3831/KPI.2019.22.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Pongkorpsakol P, Wongkrasant P, Kumpun S, Chatsudthipong V, Muanprasat C. Inhibition of intestinal chloride secretion by piperine as a cellular basis for the anti-secretory effect of black peppers. Pharmacological Research. 2015;100:271–280. doi: 10.1016/j.phrs.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 708.Pooloth, J. (2013). Biosynthesis of silver nanoparticles using Trigonella foenum graecum and the determination of their antimicrobial activityInternational Journal of Science and Research (IJSR), 2(5), 287–290https://www.ijsr.net/archive/v2i5/IJSRON2013955.pdf
- 709.Prabhu, S., & Poulose, E. K. (2012). Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effectsInternational Nano Letters, 2(1)10.1186/2228-5326-2-32
- 710.Pradeep KU, Geervani P, Eggum BO. common indian spices:nutrient composition, consumption and contribution to dietary value. Plant Foods for Human Nutrition. 1993;44(2):137–148. doi: 10.1007/BF01088378. [DOI] [PubMed] [Google Scholar]
- 711.Pradeep, S., Jain, A. S., Dharmashekara, C., Prasad, S. K., Akshatha, N., Pruthvish, R., Amachawadi, R. G., Srinivasa, C., Syed, A., Elgorban, A. M., Kheraif, A. A. A., Ortega-Castro, J., Frau, J., Flores-Holguin, N., Shivamallu, C., Kollur, S. P., Glossm, D. (2021). Synthesis, computational pharmacokinetics report, conceptual DFT-based calculations and anti-acetylcholinesterase activity of hydroxyapatite nanoparticles derived from Acorus calamus plant extractMedicinal and Pharmaceutical Chemistry [DOI] [PMC free article] [PubMed]
- 712.Pramanik A, Datta AK, Das D, Kumbhakar DV, Ghosh B, Mandal A, Gupta S, Saha A, Sengupta S. Assessment of nanotoxicity (Cadmium Sulphide and Copper Oxide) using cytogenetical parameters in Coriandrum sativum L. (Apiaceae) Cytology and Genetics. 2018;52(4):299–308. doi: 10.3103/S0095452718040084. [DOI] [Google Scholar]
- 713.Pramanik A, Datta AK, Gupta S, Ghosh B, Das D, Kumbhakar DV. Assessment of genotoxicity of engineered nanoparticles (cadmium sulphide – CdS and copper oxide - CuO) using plant model (Coriandrum sativum L.) International Journal of Research in Pharmaceutical Sciences. 2017;8(4):741–753. [Google Scholar]
- 714.Prasad, S., & Aggarwal , B, B. (2011). chapter 13: turmeric, the golden spiceHerbal Medicine: Biomolecular and Clinical Aspects
- 715.Premkumar J, Sudhakar T, Dhakal A, Shrestha JB, Krishnakumar S, Balashanmugam P. Synthesis of silver nanoparticles (AgNPs) from cinnamon against bacterial pathogens. Biocatalysis and Agricultural Biotechnology. 2018;15:311–316. doi: 10.1016/j.bcab.2018.06.005. [DOI] [Google Scholar]
- 716.Pundarikakshudu K, Shah DH, Panchal AH, Bhavsar GC. Anti-inflammatory activity of fenugreek (Trigonella foenum-graecum Linn) seed petroleum ether extract. Indian Journal of Pharmacology. 2016;48(4):441–444. doi: 10.4103/0253-7613.186195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Puthur S, Raj KK, Anoopkumar AN, Rebello S, Aneesh EM. Acorus calamus mediated green synthesis of ZnONPs: A novel nano antioxidant to future perspective. Advanced Powder Technology. 2020;31(12):4679–4682. doi: 10.1016/j.apt.2020.10.016. [DOI] [Google Scholar]
- 718.Puvača N, Kostadinović L, Popović S, Lević J, Ljubojević D, Tufarelli V, Jovanović R, Tasić T, Ikonić P, Lukač D. Proximate composition, cholesterol concentration and lipid oxidation of meat from chickens fed dietary spice addition (Allium sativum, Piper nigrum, Capsicum annuum) Animal Production Science. 2016;56(11):1920–1927. doi: 10.1071/AN15115. [DOI] [Google Scholar]
- 719.Qiao GH, Wexxin D, Zhigang X, Sami R, Khojah E, Amanullah S. Antioxidant and anti-inflammatory capacities of pepper tissues. Italian Journal of Food Science. 2020;32(2):265–274. doi: 10.14674/IJFS-1700. [DOI] [Google Scholar]
- 720.Qureshi AI, Sheikh RA, Tahir N, Pal MA, Asif HS, Mir R, Heena J. Efficacy of fenugreek seed powder for the development of functional spent hen meat patties. Journal of Entomology and Zoology Studies. 2018;6(5):353–356. [Google Scholar]
- 721.Rabadia J, Hirani U, Kardani D, Kaneria A, Rabadia J. Cardioprotective effect of methanolic extract of Syzygium aromaticum on isoproterenol induced myocardial infarction in rat. Asian Journal of Phamacology and Toxicology. 2014;02(04):1–6. [Google Scholar]
- 722.Radini IA, Hasan N, Malik MA, Khan Z. Biosynthesis of iron nanoparticles using Trigonella foenum-graecum seed extract for photocatalytic methyl orange dye degradation and antibacterial applications. Journal of Photochemistry and Photobiology B: Biology. 2018;183:154–163. doi: 10.1016/j.jphotobiol.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 723.Radwan AM, Aboelfetoh EF, Kimura T, Mohamed TM, El-Keiy MM. Fenugreek-mediated synthesis of zinc oxide nanoparticles and evaluation of its in vitro and in vivo antitumor potency. Biomedical Research and Therapy. 2021;8(8):4483–4496. doi: 10.15419/bmrat.v8i8.687. [DOI] [Google Scholar]
- 724.Raeisi M, Hashemi M, Afshari A, Tabarraei A, Aminzare M, Jannat B. Cinnamon and rosemary essential oils incorporated into alginate coating improve chemical and sensorial quality of chicken meat. Iranian Journal of Chemistry and Chemical Engineering. 2019;38(5):293–304. doi: 10.2139/ssrn.3627606. [DOI] [Google Scholar]
- 725.Rahaiee S, Moini S, Hashemi M, Shojaosadati SA. Evaluation of antioxidant activities of bioactive compounds and various extracts obtained from saffron (Crocus sativus L.): a review. Journal of Food Science and Technology. 2015;52(4):1881–1888. doi: 10.1007/s13197-013-1238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726.Rahman, A. (2021). Effects of lettuce and foeniculum vulgare powder as a natural source of nitrate on the physical, chemical and microbiological properties and sensory evaluation of vacuum packed sausageIranian Food Science and Technology Research Journal
- 727.Rahman RT, Lou Z, Zhang J, Yu F, Timilsena YP, Zhang C, Zhang Y, Bakry AM. Star anise (Illicium verum hook. f.) as quorum sensing and biofilm formation inhibitor on foodborne bacteria: study in milk. Journal of Food Protection. 2017;80(4):645–653. doi: 10.4315/0362-028X.JFP-16-294. [DOI] [PubMed] [Google Scholar]
- 728.Rahnama, F., Mohammadzadeh Milani, J., & Gohari Ardabili, A. (2017). Improved quality attributes of brabari and lavash flat breads with wheat doughs incorporated with fenugreek seed (Trigonella foenum graecum) gumJournal of Food Processing and Preservation, 41(1)10.1111/jfpp.12741
- 729.Rai PK, Jaiswal D, Mehta S, Rai DK, Sharma B, Watal G. Effect of Curcuma longa freeze dried rhizome powder with milk in STZ induced diabetic rats. Indian Journal of Clinical Biochemistry. 2010;25(2):175–181. doi: 10.1007/s12291-010-0032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Rai PD, Dambal AA, Khatri S, Khadabadi SS. Development of an anti-obesity polyherbal formulation containing Terminalia arjuna, Lagenaria siceraria and Piper nigrum. The Pharma Innovation. 2015;3(11):33–37. [Google Scholar]
- 731.Rajabi H, Jafari SM, Rajabzadeh G, Sarfarazi M, Sedaghati S. Chitosan-gum arabic complex nanocarriers for encapsulation of saffron bioactive components. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2019;578:123644. doi: 10.1016/j.colsurfa.2019.123644. [DOI] [Google Scholar]
- 732.Rajamurugan R, Suyavaran A, Selvaganabathy N, Ramamurthy CH, Reddy GP, Sujatha V, Thirunavukkarasu C. Brassica nigra plays a remedy role in hepatic and renal damage. Pharmaceutical Biology. 2012;50(12):1488–1497. doi: 10.3109/13880209.2012.685129. [DOI] [PubMed] [Google Scholar]
- 733.Rajan A, Rajan AR, Philip D. Elettaria cardamomum seed mediated rapid synthesis of gold nanoparticles and its biological activities. OpenNano. 2017;2(November 2016):1–8. doi: 10.1016/j.onano.2016.11.002. [DOI] [Google Scholar]
- 734.Rajeshwari A, Kavitha S, Alex SA, Kumar D, Mukherjee A, Chandrasekaran N, Mukherjee A. Cytotoxicity of aluminum oxide nanoparticles on Allium cepa root tip—effects of oxidative stress generation and biouptake. Environmental Science and Pollution Research. 2015;22(14):11057–11066. doi: 10.1007/s11356-015-4355-4. [DOI] [PubMed] [Google Scholar]
- 735.Rajeshwari A, Roy B, Chandrasekaran N, Mukherjee A. Cytogenetic evaluation of gold nanorods using Allium cepa test. Plant Physiology and Biochemistry. 2016;109:209–219. doi: 10.1016/j.plaphy.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 736.Rajeshwari A, Suresh S, Chandrasekaran N, Mukherjee A. Toxicity evaluation of gold nanoparticles using an Allium cepa bioassay. RSC Advances. 2016;6(29):24000–24009. doi: 10.1039/c6ra04712b. [DOI] [Google Scholar]
- 737.Rajeshwari U, Andallu B. Medicinal benefits of coriander(Coriandrum Sativum L) Spatula DD - Peer Reviewed Journal on Complementary Medicine and Drug Discovery. 2011;1(1):51. doi: 10.5455/spatula.20110106123153. [DOI] [Google Scholar]
- 738.Rajeswari VD, Eed EM, Elfasakhany A, Badruddin IA, Kamangar S, Brindhadevi K. Green synthesis of titanium dioxide nanoparticles using Laurus nobilis (bay leaf): Antioxidant and antimicrobial activities. Applied Nanoscience (Switzerland) 2021 doi: 10.1007/s13204-021-02065-2. [DOI] [Google Scholar]
- 739.Rajkumar V, Gunasekaran C, Dharmaraj J, Chinnaraj P, Paul CA, Kanithachristy I. Structural characterization of chitosan nanoparticle loaded with Piper nigrum essential oil for biological efficacy against the stored grain pest control. Pesticide Biochemistry and Physiology. 2020;166:104566. doi: 10.1016/j.pestbp.2020.104566. [DOI] [PubMed] [Google Scholar]
- 740.Rajoriya P, Misra P, Shukla PK, Ramteke PW. Light-regulatory effect on the phytosynthesis of silver nanoparticles using aqueous extract of garlic (Allium sativum) and onion (Allium cepa) bulb. Current Science. 2016;111(8):1364–1368. doi: 10.18520/cs/v111/i8/1364-1368. [DOI] [Google Scholar]
- 741.Rajput, S. B., Tonge, M. B., & Karuppayil, S. M. (2014). An overview on traditional uses and pharmacological profile of Acorus calamus Linn. (Sweet flag) and other Acorus species. In Phytomedicine (Vol. 21, Issue 3, pp. 268–276). Urban und Fischer Verlag Jena10.1016/j.phymed.2013.09.020 [DOI] [PubMed]
- 742.Ramezani M, Nasri S, Yassa N. Antinociceptive and anti-inflammatory effects of isolated fractions from Apium graveolens seeds in mice. Pharmaceutical Biology. 2009;47(8):740–743. doi: 10.1080/13880200902939283. [DOI] [Google Scholar]
- 743.Ramírez-Tortosa MC, Mesa MD, Aguilera MC, Quiles JL, Baró L, Ramirez-Tortosa CL, Martinez-Victoria E, Gil A. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 1999;147(2):371–378. doi: 10.1016/S0021-9150(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 744.Rao K, Ch B, Narasu LM, Giri A. Antibacterial activity of Alpinia galanga (L) willd crude extracts. Applied Biochemistry and Biotechnology. 2010;162(3):871–884. doi: 10.1007/s12010-009-8900-9. [DOI] [PubMed] [Google Scholar]
- 745.Rao PS, Navinchandra S, Jayaveera K. An important spice, Pimenta dioica (Linn.) Merill: A Review. International Current Pharmaceutical Journal. 2012;1(8):221–225. doi: 10.3329/icpj.v1i8.112555. [DOI] [Google Scholar]
- 746.Rao, P. V., & Gan, S. H. (2014). Cinnamon: A multifaceted medicinal plantEvidence-Based Complementary and Alternative Medicine,201410.1155/2014/642942 [DOI] [PMC free article] [PubMed]
- 747.Rao S, Ramakrishna A. Indian medicinal plants. Indian Medicinal Plants. 2020 doi: 10.1201/9781003001898. [DOI] [Google Scholar]
- 748.Rashid MA, Zuberi RH. Pharmacognostical studies for standardisation of a medicinal spice, the fruit of Illicium Verum hook. f. PharmaTutor. 2016;4(8):36–41. [Google Scholar]
- 749.Rather, M. A., Dar, B. A., Sofi, S. N., Bhat, B. A., & Qurishi, M. A. (2016). Foeniculum vulgare: a comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. In Arabian Journal of Chemistry (Vol. 9, pp. S1574–S1583). Elsevier B.V10.1016/j.arabjc.2012.04.011
- 750.Rathour M, Malav OP, Kumar P, Chatli MK, Mehta N. Storage stability of chevon rolls incorporated with ethanolic extracts of Aloe vera and Cinnamon bark at refrigeration temperature (4±1°C) Journal of Animal Research. 2017;7(1):183. doi: 10.5958/2277-940x.2017.00025.0. [DOI] [Google Scholar]
- 751.Raut VRA, Ghotankar AM. Review of khuskhus ( khaskhas ) ( seeds of Papaver somniferum linn. ) with special reference of ayurveda medicine. Journal of Ayurveda and Integrated Medical Sciences. 2019;4(06):228–231. [Google Scholar]
- 752.Ravichandra V, Hanumantharayappa B, Madhava Reddy Papasani V. Evaluation of cardio protective activity of Galangin against doxorubicin induced cardiomyopathy. International Journal of Pharmacy and Pharmaceutical Sciences. 2014;6(9):86–90. [Google Scholar]
- 753.Razavi MS, Golmohammadi A, Nematollahzadeh A, Fiori F, Rovera C, Farris S. Preparation of cinnamon essential oil emulsion by bacterial cellulose nanocrystals and fish gelatin. Food Hydrocolloids. 2020;109:106111. doi: 10.1016/j.foodhyd.2020.106111. [DOI] [Google Scholar]
- 754.Ražná K, Khasanova N, Ivanišová E, Qahramon D, Habán M. Antioxidant properties of cumin (Bunium persicum Boiss.) extract and its protective role against ultrasound-induced oxidative stress tested by microrna based markers. Potravinarstvo Slovak Journal of Food Sciences. 2018;12(1):11–19. doi: 10.5219/838. [DOI] [Google Scholar]
- 755.Reddy SY, Prabhakar JV. cocoa butter extenders from kokum and phulwara butter. Journal of the American Oil Chemists’ Society. 1994;71(2):217–219. doi: 10.1007/BF02541559. [DOI] [Google Scholar]
- 756.Reddy, G. C., & Raghuveer, S. (2018)Non-traditional products from kokum: Inland and global opportunities5(6), 102–104
- 757.Reddy LH, Arias JL, Nicolas J, Couvreur P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chemical Reviews. 2012;112(11):5818–5878. doi: 10.1021/cr300068p. [DOI] [PubMed] [Google Scholar]
- 758.Reddy Pullagurala VL, Adisa IO, Rawat S, Kalagara S, Hernandez-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL. ZnO nanoparticles increase photosynthetic pigments and decrease lipid peroxidation in soil grown cilantro (Coriandrum sativum) Plant Physiology and Biochemistry. 2018;132:120–127. doi: 10.1016/j.plaphy.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 759.Remani KC, Binitha NN. Fluorescence sensing of picric acid by ceria nanostructures prepared using fenugreek extract. Journal of the Iranian Chemical Society. 2022;19(2):619–633. doi: 10.1007/s13738-021-02327-4. [DOI] [Google Scholar]
- 760.Repajić M, Ekić S, Kruk V, Dragović Uzelac V. Effect of accelerated solvent extraction conditions on the isolation of bioactive compounds from fennel (Foeniculum vulgare Mill.) seeds. Hrvatski Časopis Za Prehrambenu Tehnologiju, Biotehnologiju i Nutricionizam. 2021;15(3–4):102–106. doi: 10.31895/hcptbn.15.3-4.7. [DOI] [Google Scholar]
- 761.Restrepo-Osorio J, Nobile-Correa DP, Zuñiga O, Sánchez-Andica RA. Determination of nutritional value of turmeric flour and the antioxidant activity of Curcuma longa rhizome extracts from agroecological and conventional crops of Valle del Cauca-Colombia. Revista Colombiana de Quimica. 2020;49(1):26–32. doi: 10.15446/rev.colomb.quim.v1n49.79334. [DOI] [Google Scholar]
- 762.Rezaei-Chiyaneh E, Amirnia R, Amani Machiani M, Javanmard A, Maggi F, Morshedloo MR. Intercropping fennel (Foeniculum vulgare L.) with common bean (Phaseolus vulgaris L.) as affected by PGPR inoculation: A strategy for improving yield, essential oil and fatty acid composition. Scientia Horticulturae. 2020;261(September):108951. doi: 10.1016/j.scienta.2019.108951. [DOI] [Google Scholar]
- 763.Rezaei S, Mohammadi M, Najafpour GD, Moghadamnia A, Kazemi S, Nikzad M. Separation of curcumin from Curcuma longa L. and its conjugation with silica nanoparticles for anti-cancer activities. International Journal of Engineering, Transactions B: Applications. 2018;31(11):1803–1809. doi: 10.5829/ije.2018.31.11b.01. [DOI] [Google Scholar]
- 764.Ring LC, Yenn TW, Wen-Nee T, Tumin ND, Yusof FAM, Yacob LS, Rosli MIHB, Taher MA. Synthesis of curcumin quantum dots and their antimicrobial activity on necrotizing fasciitis causing bacteria. Materials Today: Proceedings. 2020;31(January):31–35. doi: 10.1016/j.matpr.2020.01.082. [DOI] [Google Scholar]
- 765.Rinita J, Jose R, Jothi NSN. Acclimated growth and characterization of manganese oxide nanospheres using natural extract from trigonella foenum-graecum. Materials Technology. 2021;36(7):412–419. doi: 10.1080/10667857.2020.1761660. [DOI] [Google Scholar]
- 766.Rizwana, H., Bokahri, N. A., Alkhattaf, F. S., Albasher, G., & Aldehaish, H. A. (2021). Antifungal, antibacterial, and cytotoxic activities of silver nanoparticles synthesized from aqueous extracts of mace-arils of myristica fragransMolecules, 26(24)10.3390/molecules26247709 [DOI] [PMC free article] [PubMed]
- 767.Robert SD, Ismail AAsafi, Rosli WIW. Reduction of postprandial blood glucose in healthy subjects by buns and flatbreads incorporated with fenugreek seed powder. European Journal of Nutrition. 2016;55(7):2275–2280. doi: 10.1007/s00394-015-1037-4. [DOI] [PubMed] [Google Scholar]
- 768.Rodrigues AS, Almeida DP, Gandara JS, Gregorio MRP. Onions : a source of flavonoids world ’ s largest science, technology & medicine open access book publisher. Biosynthesis Hum Health. 2017;23(September):439. [Google Scholar]
- 769.Rostami-Vartooni A, Nasrollahzadeh M, Alizadeh M. Green synthesis of seashell supported silver nanoparticles using Bunium persicum seeds extract: Application of the particles for catalytic reduction of organic dyes. Journal of Colloid and Interface Science. 2016;470:268–275. doi: 10.1016/j.jcis.2016.02.060. [DOI] [PubMed] [Google Scholar]
- 770.Ru, Q., Hu, Q., Dai, C., Zhang, X., & Wang, Y. (2022). Formulation of Laurus nobilis essential oil nanoemulsion system and its application in fresh-cut muskmelonsCoatings, 12(2)10.3390/coatings12020159
- 771.Ruiz-Torres N, Flores-Naveda A, Barriga-Castro ED, Camposeco-Montejo N, Ramírez-Barrón S, Borrego-Escalante F, Niño-Medina G, Hernández-Juárez A, Garza-Alonso C, Rodríguez-Salinas P, García-López JI. Zinc oxide nanoparticles and zinc sulfate impact physiological parameters and boosts lipid peroxidation in soil grown coriander plants (Coriandrum sativum) Molecules. 2021;26(7):1–14. doi: 10.3390/molecules26071998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 772.Sabir, S., Arshad, M., & Chaudhari, S. K. (2014). Zinc oxide nanoparticles for revolutionizing agriculture: Synthesis and applications. In Scientific World Journal (Vol. 2014). Hindawi Limited10.1155/2014/925494 [DOI] [PMC free article] [PubMed]
- 773.Sachdeva, C., & Kaushik, N, K. (2021). Spices reservoir of health benefitsNatural Medicinal Plants
- 774.Sadadekar AS, Shruthy R, Preetha R, Kumar N, Pande KR, Nagamaniammai G. Enhanced antimicrobial and antioxidant properties of Nano chitosan and pectin based biodegradable active packaging films incorporated with fennel (Foeniculum vulgare) essential oil and potato (Solanum tuberosum) peel extracts. Journal of Food Science and Technology. 2022 doi: 10.1007/s13197-021-05333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 775.Sadeghzadeh, H., Pilehvar-Soltanahmadi, Y., Akbarzadeh, A., Dariushnejad, H., Sanjarian, F., & Zarghami, N. (2017). The effects of nanoencapsulated curcumin-Fe3O4 on proliferation and hTERT gene expression in lung cancer cellsAnti-Cancer Agents in Medicinal Chemistry, 17(10)10.2174/1871520617666170213115756 [DOI] [PubMed]
- 776.Sagadevan S, Anita Lett J, Vennila S, Varun Prasath P, Saravanan Kaliaraj G, Fatimah I, Léonard E, Mohammad F, Al-Lohedan HA, Alshahateet SF, Lee CT. Photocatalytic activity and antibacterial efficacy of titanium dioxide nanoparticles mediated by Myristica fragrans seed extract. Chemical Physics Letters. 2021;771:138527. doi: 10.1016/j.cplett.2021.138527. [DOI] [Google Scholar]
- 777.Saha N, Dutta Gupta S. Low-dose toxicity of biogenic silver nanoparticles fabricated by Swertia chirata on root tips and flower buds of Allium cepa. Journal of Hazardous Materials. 2017;330:18–28. doi: 10.1016/j.jhazmat.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 778.Sahib AS. Anti-diabetic and antioxidant effect of cinnamon in poorly controlled type-2 diabetic Iraqi patients: A randomized, placebo-controlled clinical trial. Journal of Intercultural Ethnopharmacology. 2016;5(2):108–113. doi: 10.5455/jice.20160217044511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 779.Saikia T, Lalhlenmawia H, Laldinchhana, Roy PK, Thanzami K. Effect of polymeric nanoparticles of curcumin on a549 cell line. International Journal of Current Pharmaceutical Research. 2020;12(5):50–53. doi: 10.22159/ijcpr.2020v12i5.39765. [DOI] [Google Scholar]
- 780.Sakhare SD, Prabhasankar P. Effect of roller milled fenugreek fiber incorporation on functional, thermal and rheological characteristics of whole wheat flour. Journal of Food Measurement and Characterization. 2017;11(3):1315–1325. doi: 10.1007/s11694-017-9509-2. [DOI] [Google Scholar]
- 781.Saleem A, Naureen I, Naeem M, Tasleem G, Ahmed H, Farooq U. Therapeutic role of piper nigrum L (Black Pepper) and pharmacological activities. Scholars International Journal of Biochemistry. 2022;5(1):15–21. doi: 10.36348/sijb.2022.v05i01.003. [DOI] [Google Scholar]
- 782.Saleh, A. M., Selim, S., Jaouni, S. Al, & AbdElgawad, H. (2018). CO 2 enrichment can enhance the nutritional and health benefits of parsley (Petroselinum crispum L.) and dill (Anethum graveolens L.). In Food Chemistry (Vol. 269)10.1016/j.foodchem.2018.07.046 [DOI] [PubMed]
- 783.Saleh SR, Salam HSH, Hassan AHA. Antibacterial activity of origanum majorana and Curcuma longa extracts against multiple drug-resistant pathogenic E. coli and methicillin-resistant Staphylococcus aureus isolates recovered from meat products. Journal of Advanced Veterinary Research. 2021;11(4):221–229. [Google Scholar]
- 784.Salehi B, Hernández-Álvarez AJ, Contreras MDM, Martorell M, Ramírez-Alarcón K, Melgar-Lalanne G, Matthews KR, Sharifi-Rad M, Setzer WN, Nadeem M, Yousaf Z, Sharifi-Rad J. Potential phytopharmacy and food applications of capsicum spp.: A comprehensive review. Natural Product Communications. 2018;13(11):1543–1556. doi: 10.1177/1934578x1801301133. [DOI] [Google Scholar]
- 785.Salim AA, Bakhtiar H, Bidin N, Ghoshal SK. Unique attributes of spherical cinnamon nanoparticles produced via PLAL technique: Synergy between methanol media and ablating laser wavelength. Optical Materials. 2018;85:100–105. doi: 10.1016/j.optmat.2018.08.054. [DOI] [Google Scholar]
- 786.Salim AA, Bidin N. Pulse Q-switched Nd:YAG laser ablation grown cinnamon nanomorphologies: Influence of different liquid medium. Journal of Molecular Structure. 2017;1149:694–700. doi: 10.1016/j.molstruc.2017.08.055. [DOI] [Google Scholar]
- 787.Salim AA, Bidin N, Ghoshal SK. Growth and characterization of spherical cinnamon nanoparticles: Evaluation of antibacterial efficacy. LWT - Food Science and Technology. 2018;90(September 2017):346–353. doi: 10.1016/j.lwt.2017.12.047. [DOI] [Google Scholar]
- 788.Salim AA, Bidin N, Ghoshal SK, Islam S, Bakhtiar H. Synthesis of truncated tetrahedral cinnamon nanoparticles in citric acid media via PLAL technique. Materials Letters. 2018;217:267–270. doi: 10.1016/j.matlet.2018.01.103. [DOI] [Google Scholar]
- 789.Salim AA, Bidin N, Lafi AS, Huyop FZ. Antibacterial activity of PLAL synthesized nanocinnamon. Materials and Design. 2017;132:486–495. doi: 10.1016/j.matdes.2017.07.014. [DOI] [Google Scholar]
- 790.Salim AA, Ghoshal SK, Bakhtiar H. Growth mechanism and optical characteristics of Nd:YAG laser ablated amorphous cinnamon nanoparticles produced in ethanol: Influence of accumulative pulse irradiation time variation. Photonics and Nanostructures - Fundamentals and Applications. 2021;43:100889. doi: 10.1016/j.photonics.2020.100889. [DOI] [Google Scholar]
- 791.Salman AS, Al-Shaikh TM, Hamza ZK, El-Nekeety A, Bawazir SS, Hassan NS, Abdel-Wahhab MA. maltodextrin-cinnamon essential oil nanoformulation as a potent protective against titanium nanoparticles induced oxidative stress, genotoxicity, and reproductive disturbances. Environmental Science and Pollution Research. 2021;28:39035–39051. doi: 10.1007/s11356-021-13518-0. [DOI] [PubMed] [Google Scholar]
- 792.Sangaonkar GM, Pawar KD. Garcinia indica mediated biogenic synthesis of silver nanoparticles with antibacterial and antioxidant activities. Colloids and Surfaces B: Biointerfaces. 2018;164:210–217. doi: 10.1016/j.colsurfb.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 793.Sangeetha J, Sandhya J, Philip J. Biosynthesis and functionalization of silver nanoparticles using Nigella sativa, Dioscorea alata and Ferula asafoetida. Science of Advanced Materials. 2014;6(8):1681–1690. doi: 10.1166/sam.2014.1991. [DOI] [Google Scholar]
- 794.Sani AM, Kakhki AH, Moradi E. Chemical composition and nutritional value of saffron’s pollen (Crocus sativus L.) Nutrition and Food Science. 2013;43(5):490–495. doi: 10.1108/NFS-04-2012-0040. [DOI] [Google Scholar]
- 795.Sankar R, Rahman PKSM, Varunkumar K, Anusha C, Kalaiarasi A, Shivashangari KS, Ravikumar V. Facile synthesis of Curcuma longa tuber powder engineered metal nanoparticles for bioimaging applications. Journal of Molecular Structure. 2017;1129:8–16. doi: 10.1016/j.molstruc.2016.09.054. [DOI] [Google Scholar]
- 796.Santhosh A, Theertha V, Prakash P, Smitha Chandran S. From waste to a value added product: Green synthesis of silver nanoparticles from onion peels together with its diverse applications. Materials Today: Proceedings. 2019;46:4460–4463. doi: 10.1016/j.matpr.2020.09.680. [DOI] [Google Scholar]
- 797.Santin JR, Lemos M, Júnior LCK, Niero R, de Andrade SF. Antiulcer effects of Achyrocline satureoides (Lam) DC (Asteraceae) (Marcela), a folk medicine plant, in different experimental models. Journal of Ethnopharmacology. 2010;130(2):334–339. doi: 10.1016/j.jep.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 798.Santin JR, Lemos M, Klein-Júnior LC, Machado ID, Costa P, De Oliveira AP, Tilia C, De Souza JP, De Sousa JPB, Bastos JK, De Andrade SF. Gastroprotective activity of essential oil of the Syzygium aromaticum and its major component eugenol in different animal models. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2011;383(2):149–158. doi: 10.1007/s00210-010-0582-x. [DOI] [PubMed] [Google Scholar]
- 799.Santos AJ, Pina LTS, Galvão JG, Trindade GGG, Nunes RKV, Santos JS, Santos CP, Gonsalves JKMC, Lira AAM, Cavalcanti SCH, Santos RLC, Sarmento VHV, Nunes RS. Clay/PVP nanocomposites enriched with Syzygium aromaticum essential oil as a safe formulation against Aedes aegypti larvae. Applied Clay Science. 2020;185:105394. doi: 10.1016/j.clay.2019.105394. [DOI] [Google Scholar]
- 800.DosSantos, S. M., Malpass, G. R. P., Okura, M. H., & Granato, A. C. (2018). Edible active coatings incorporated with cinnamomum cassia and myristica fragrans essential oils to improve shelf-life of minimally processed applesCiencia Rural, 48(12)10.1590/0103-8478cr20180447
- 801.Sanwo KA, Adegoke AV, Akinola OS, Njoku CP, Okolo SO, Oladipo NA, Oladejo AS. Meat quality characteristics Of improved indigenous chickens (funaab-alpha) fed turmeric (Curcuma longa) or clove (Syzygium aromaticum) as feed additives. Journal of Agricultural Science and Environment. 2020;19(1):102–112. doi: 10.51406/jagse.v19i1.2018. [DOI] [Google Scholar]
- 802.Sanwo KA, Adegoke AV, Egbeyale LT, Abiona JA, Sobayo RA, Obajuluwa OV, Abdulazeez AO. Meat quality and lipid profile of broiler chickens fed diets containing turmeric (Curcuma Longa) powder and cayenne pepper (Capsicum Frutescens) powder as antioxidants. Journal of Agricultural Science and Environment. 2020;19(1):73–91. doi: 10.51406/jagse.v19i1.2016. [DOI] [Google Scholar]
- 803.Sarac, H. (2021). Bioactive components and biological activities of the cardamom ( Elettaria cardamomum L. ) plantResearch and Reviews in Science and Mathematics, 91
- 804.Saranyadevi S, Suresh K, Mathiyazhagan N, Muthusamy R, Thirumalaisamy R. Silver nanoparticles synthesized using asafoetida resin, characterization of their broad spectrum and larvicidal activity. Annals of the Romanian Society for Cell Biology. 2021;25(4):15035–15049. [Google Scholar]
- 805.Saravanakumar, P., Muthukumar, M., Muthuchudarkodi, R. R., & Ramkumar, P. (2018). Charecterization of undoped cobalt oxide and cerium ion doped cobalt oxide nanoparticlesInternational Journal of Recent Research Aspects, August, 918–923
- 806.Saravani M, Ehsani A, Aliakbarlu J, Ghasempour Z. Gouda cheese spoilage prevention: Biodegradable coating induced by Bunium persicum essential oil and lactoperoxidase system. Food Science and Nutrition. 2019;7(3):959–968. doi: 10.1002/fsn3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Sardiñas-Valdés M, Hernández-Becerra JA, García HS, Chay-Canul AJ, Velázquez-Martínez JR, Ochoa-Flores AA. Physicochemical and sensory properties of manchego-type cheese fortified with nanoemulsified curcumin. International Food Research Journal. 2021;28(2):326–336. doi: 10.47836/ifrj.28.2.13. [DOI] [Google Scholar]
- 808.Saricaoglu FT, Turhan S. Performance of mechanically deboned chicken meat protein coatings containing thyme or clove essential oil for storage quality improvement of beef sucuks. Meat Science. 2019;158:107912. doi: 10.1016/j.meatsci.2019.107912. [DOI] [PubMed] [Google Scholar]
- 809.Sarkar T, Salauddin M, Chakraborty R. In-depth pharmacological and nutritional properties of bael (Aegle marmelos): A critical review. Journal of Agriculture and Food Research. 2020;2(October):100081. doi: 10.1016/j.jafr.2020.100081. [DOI] [Google Scholar]
- 810.Sarkar T, Salauddin M, Roy A, Sharma N, Sharma A, Yadav S, Jha V, Rebezov M, Khayrullin M, Thiruvengadam M, Chung IM, Shariati MA, Simal-Gandara J. Minor tropical fruits as a potential source of bioactive and functional foods. Critical Reviews in Food Science and Nutrition. 2022 doi: 10.1080/10408398.2022.2033953. [DOI] [PubMed] [Google Scholar]
- 811.Sathiyabama M, Indhumathi M, Amutha T. Preparation and characterization of curcumin functionalized copper nanoparticles and their application enhances disease resistance in chickpea against wilt pathogen. Biocatalysis and Agricultural Biotechnology. 2020;29:101823. doi: 10.1016/j.bcab.2020.101823. [DOI] [Google Scholar]
- 812.Sattar A, Abdo A, Mushtaq MN, Anjum I, Anjum A. Evaluation of gastro-protective activity of myristica fragrans on ethanol-induced ulcer in albino rats. Anais Da Academia Brasileira de Ciencias. 2019;91(2):1–8. doi: 10.1590/0001-3765201920181044. [DOI] [PubMed] [Google Scholar]
- 813.Saygi, K. O., Kacmaz, B., & Gul, S. (2021). Antimicrobial activities of coriander seed essential oil and silver nanoparticlesResearch Squar10.21203/rs.3.rs-526332/v1
- 814.Scherer MD, Sposito JCV, Falco WF, Grisolia AB, Andrade LHC, Lima SM, Machado G, Nascimento VA, Gonçalves DA, Wender H, Oliveira SL, Caires ARL. Cytotoxic and genotoxic effects of silver nanoparticles on meristematic cells of Allium cepa roots: A close analysis of particle size dependence. Science of The Total Environment. 2019;660:459–467. doi: 10.1016/j.scitotenv.2018.12.444. [DOI] [PubMed] [Google Scholar]
- 815.Sebai H, Rtibi K, Selmi S, Jridi M, Balti R, Marzouki L. Modulating and opposite actions of two aqueous extracts prepared from: Cinnamomum cassia L. bark and Quercus ilex L. on the gastrointestinal tract in rats. RSC Advances. 2019;9(38):21695–21706. doi: 10.1039/c9ra02429h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 816.Selles SMA, Kouidri M, Belhamiti BT, Ait Amrane A. Chemical composition, in-vitro antibacterial and antioxidant activities of Syzygium aromaticum essential oil. Journal of Food Measurement and Characterization. 2020;14(4):2352–2358. doi: 10.1007/s11694-020-00482-5. [DOI] [Google Scholar]
- 817.Senaratne, R., & Pathirana, R. (2020)Cinnamon - botany, agronomy, chemistry and industrial applicationshttps://www.springer.com/gp/book/9783030544256
- 818.Senthilkumar N, Aravindhan V, Ruckmani K, Potheher IV. Coriandrum sativum mediated synthesis of silver nanoparticles and evaluation of their biological characteristics. Materials Research Express. 2018;5(5):55032. doi: 10.1088/2053-1591/aac312. [DOI] [Google Scholar]
- 819.Šeregelj V, Tumbas Šaponjac V, Lević S, Kalušević A, Ćetković G, Čanadanović-Brunet J, Nedović V, Stajčić S, Vulić J, Vidaković A. Application of encapsulated natural bioactive compounds from red pepper waste in yogurt. Journal of Microencapsulation. 2019;36(8):704–714. doi: 10.1080/02652048.2019.1668488. [DOI] [PubMed] [Google Scholar]
- 820.Sethumadhavan SC, Pottail L. Acorus calamus gold nanoparticles as enhanced anti-microbial agents for transdermal patches. Micro and Nano Letters. 2019;14(4):367–372. doi: 10.1049/mnl.2018.5512. [DOI] [Google Scholar]
- 821.Sg A, Ah M, Av G. Review of piperine as a bio-enhancer. American Journal of PharmTech Research. 2012;2(February):32–44. [Google Scholar]
- 822.Shad AA, Shah HU, Bakht J, Choudhary MI, Ullah J. Nutraceutical potential and bioassay of Apium graveolens L. grown in Khyber Pakhtunkhwa-Pakistan. Journal of Medicinal Plant Research. 2011;5(20):5160–5166. [Google Scholar]
- 823.Shafiee M, Akbari MR, Asadi-Khoshoei E, Bahadoran S, Hassanpour H. Growth performance, nutrients digestibility, immune system, and blood parameters in broiler chickens fed on diets supplemented with cumin (Cuminum cyminum) or black cumin (bunium persicum) seed powders. Poultry Science Journal. 2020;8(2):223–231. doi: 10.22069/psj.2020.18155.1601. [DOI] [Google Scholar]
- 824.Shah Z, Ali T, Shafi S. Phytopharmacological review of Bunium persicum (Boiss)B. fedtsch. Journal of Drug Delivery and Therapeutics. 2019;9(2):458–460. doi: 10.22270/jddt.v9i2.2509. [DOI] [Google Scholar]
- 825.Shah R, Pathan A, Vaghela H, Ameta SC, Parmar K. Green synthesis and characterization of copper nanoparticles using mixture (Zingiber officinale, Piper nigrum and Piper longum) extract and its antimicrobial activity. Chemical Science Transactions. 2019;8(1):63–69. doi: 10.7598/cst2019.1517. [DOI] [Google Scholar]
- 826.Shahbandeh, M. (2019). Saffron: leading producers worldwide 2019Statistahttps://www.statista.com/statistics/1135621/leading-saffron-producers-worldwide/
- 827.Shahi, Z., Sayyed-Alangi, S. Z., & Najafian, L. (2020). Effects of enzyme type and process time on hydrolysis degree, electrophoresis bands and antioxidant properties of hydrolyzed proteins derived from defatted Bunium persicum Bioss. press cakeHeliyon, 6(2)10.1016/j.heliyon.2020.e03365 [DOI] [PMC free article] [PubMed]
- 828.Shahid, S., Chand, N., Khan, R. U., Suhail, S. M., & Khan, N. A. (2015). Alternations in cholesterol and fatty acids composition in egg yolk of Rhode island red x fyoumi hens fed with hemp seeds (Cannabis sativa l.)Journal of Chemistry10.1155/2015/362936
- 829.Shahidullah, M., Janarthan, M., & Khan, S. (2017). Evaluation of cardioprotective activity of maceration extract of Elettaria cardamomum in doxorubicin induced cardiotoxicity in ratsIndian Journal of Research in Pharmacy and Biotechnology, 5(6), 366–370www.ijrpb.comjournalhomepage: http://www.ijrpb.com
- 830.Shahrajabian MH, Sun W, Cheng Q. Chinese star anise and anise, magic herbs in traditional Chinese medicine and modern pharmaceutical science. Asian Journal of Medical and Biological Research. 2019;5(3):162–179. doi: 10.3329/ajmbr.v5i3.43584. [DOI] [Google Scholar]
- 831.Shahrajabian MH, Sun W, Cheng Q. Chinese star anise (Illicium verum) and pyrethrum (Chrysanthemum cinerariifolium) as natural alternatives for organic farming and health care-A review. Australian Journal of Crop Science. 2020;14(3):517–523. doi: 10.21475/ajcs.20.14.03.p2209. [DOI] [Google Scholar]
- 832.Shahrajabian MH, Sun W, Cheng Q. Survey on chemical constituent, traditional and modern pharmaceutical and health benefits of chinese star anise, a treasure from the East. Pharmacognosy Communications. 2021;11(1):31–35. doi: 10.5530/pc.2021.1.7. [DOI] [Google Scholar]
- 833.Shailendiran, D., Pawar, N., Chanchal, A., Pandey, R. P., Bohidar, H. B., & Verma, A. K. (2011). Characterization and antimicrobial activity of nanocurcumin and curcumin2011 International Conference on Nanoscience, Technology and Societal Implications, NSTSI11, February 202110.1109/NSTSI.2011.6111984
- 834.Shanmugam J, Dhayalan M, Savaas Umar MR, Gopal M, AliKhan M, Simal-Gandara J, Cid-Samamed A. Green synthesis of silver nanoparticles using Allium cepa var aggregatum natural extract: antibacterial and cytotoxic properties. Nanomaterials. 2022;12(10):1725. doi: 10.3390/nano12101725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835.Sharada SOV. Green synthesis and characterization of silver nanoparticles and evaluation of their antibacterial activity using Elettaria cardamom seeds. Journal of Nanomedicine & Nanotechnology. 2015;06(02):2–5. doi: 10.4172/2157-7439.1000266. [DOI] [Google Scholar]
- 836.Sharada, S. O. V. (2015b). Green Synthesis and Characterization of Silver Nanoparticles and Evaluation of their Antibacterial Activity using Elettaria Cardamom SeedsJournal of Nanomedicine & Nanotechnology, 06(02)10.4172/2157-7439.1000266
- 837.Sharafati-Chaleshtori F, Sharafati-Chaleshtori R. In vitro antibacterial and antioxidant properties of elettaria cardamomum maton extract and its effects, incorporated with chitosan, on storage time of lamb meat. Veterinarski Arhiv. 2017;87(3):301–315. doi: 10.24099/vet.arhiv.160117. [DOI] [Google Scholar]
- 838.Sharara MS. Effect of germination and heat treatment on chemical composition and bioactive components of fenugreek seeds. World Journal of Diary and Food Sciences. 2017;12(1):33–41. doi: 10.5829/idosi.wjdfs.2017.33.41. [DOI] [Google Scholar]
- 839.Sharififar F, Yassa N, Mozaffarian V. Bioactivity of major components from the seeds of Bunium persicum (Boiss.) FEDTCH. Pakistan Journal of Pharmaceutical Sciences. 2010;23(3):300–304. [PubMed] [Google Scholar]
- 840.Sharma, V., Singh, P., Rani, A. (2017). Antimicrobial activity of Trigonella foenum-graecum l. (fenugreek)European Journal of Experimental Biology, 07(01)10.21767/2248-9215.100004
- 841.Sharma C, Ansari S, Ansari MS, Satsangee SP. Phyto-mediated synthesis of Pt and Au/Pt bimetallic nanoparticles using Syzygium aromaticum bud-extract: Study of their catalytic, antibacterial, and antioxidant activities. Journal of Industrial and Engineering Chemistry. 2022 doi: 10.1016/j.jiec.2022.04.031. [DOI] [Google Scholar]
- 842.Sharma G, Sharma AR, Kurian M, Bhavesh R, Nam JS, Lee SS. Green synthesis of silver nanoparticle using Myristica fragrans (nutmeg) seed extract and its biological activity. Digest Journal of Nanomaterials and Biostructures. 2014;9(1):325–332. [Google Scholar]
- 843.Sharma M, Inbaraj BS, Dikkala PK, Sridhar K, Mude AN, Narsaiah K. Preparation of curcumin hydrogel beads for the development of functional kulfi, a tailoring delivery system. Foods. 2022;11(2):182. doi: 10.3390/foods11020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 844.Sharma M, Monika, Thakur P, Saini RV, Kumar R, Torino E. Unveiling antimicrobial and anticancerous behavior of AuNPs and AgNPs moderated by rhizome extracts of Curcuma longa from diverse altitudes of Himalaya. Scientific Reports. 2020;10(1):1–11. doi: 10.1038/s41598-020-67673-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 845.Sharma, V., Sharma, R., Gautam, D. N. S., Kuca, K., Nepovimova, E., & Martins, N. (2020). Role of vacha (Acorus calamus Linn.) in neurological and metabolic disorders: Evidence from ethnopharmacology, phytochemistry, pharmacology and clinical studyJournal of Clinical Medicine, 9(4)10.3390/jcm9041176 [DOI] [PMC free article] [PubMed]
- 846.Shashanka R. Investigation of optical and thermal properties of CuO and ZnO nanoparticles prepared by Crocus Sativus (Saffron) flower extract. Journal of the Iranian Chemical Society. 2021;18(2):415–427. doi: 10.1007/s13738-020-02037-3. [DOI] [Google Scholar]
- 847.Sheikh E, Bhatt MLB, Tripathi M. Bio-based synthesised and characterized monodispersed Curcuma longa silver nanoparticles induces targeted anticancer activity in breast cancer cells. Pharmacognosy Magazine. 2018;14(57):S340–S345. doi: 10.4103/pm.pm_71_18. [DOI] [Google Scholar]
- 848.Shi Y, Li C, Liu S, Liu Z, Zhu J, Yang J, Hu X. Facile synthesis of fluorescent carbon dots for determination of curcumin based on fluorescence resonance energy transfer. RSC Advances. 2015;5(79):64790–64796. doi: 10.1039/c5ra13404h. [DOI] [Google Scholar]
- 849.Shirani G, Ganesharanee R. Extruded products with Fenugreek (Trigonella foenum-graecium) chickpea and rice: Physical properties, sensory acceptability and glycaemic index. Journal of Food Engineering. 2009;90(1):44–52. doi: 10.1016/j.jfoodeng.2008.06.004. [DOI] [Google Scholar]
- 850.Kumar S, Alagawadi KR. Influence Of Alpinia galanga rhizomes on cafeteria diet induced obesity in rats. Pharmacologyonline. 2010;3:84–91. [Google Scholar]
- 851.Shome, S., Talukdar, A. Das, & Upadhyaya, H. (2021). Antibacterial activity of curcumin and its essential nanoformulations against some clinically important bacterial pathogens: A comprehensive reviewBiotechnology and Applied Biochemistry, August, 1–3010.1002/bab.2289 [DOI] [PubMed]
- 852.Shuaib, M., Ullah, N., Hafeez, A., Alhidary, I. A., Abdelrahman, M. M., & Khan, R. U. (2020). Effect of dietary supplementation of wildCumin (Bunium persicum) seeds on performance, nutrient digestibility and circulating metabolites in broiler chicks during the finisher phaseAnimal Biotechnology, 1–510.1080/10495398.2020.1844222 [DOI] [PubMed]
- 853.Shukla R, Rai N, Singhai M, Singhai AK. a magical medicinal fruit of piper nigrum. World Journal of Pharmaceutical Research. 2018;7(8):418–425. [Google Scholar]
- 854.Shyu YS, Lin JT, Chang YT, Chiang CJ, Yang DJ. Evaluation of antioxidant ability of ethanolic extract from dill (Anethum graveolens L.) flower. Food Chemistry. 2009;115(2):515–521. doi: 10.1016/j.foodchem.2008.12.039. [DOI] [Google Scholar]
- 855.Siani NG, Rostamnejadi A. Evaluation of inhibition effect of ZnO nanoparticles concentration regarding seed germination and seedling growth of fenugreek ( Trigonella foenum-graecum L.) Journal of Medicinal Plants and By-Products. 2016;2:235–243. [Google Scholar]
- 856.da Silva BM, Vieira LGdeF, Beltrami JM, Serenini GDF, dos Santos NS, Soares AA, Alves G. Physical-Chemical characterization of fresh shanklish cheese with kefir and turmeric extract (Curcuma longa l.) Arquivos de Ciências Veterinárias e Zoologia Da UNIPAR. 2020;23(2cont):23–27. doi: 10.25110/arqvet.v23i2cont.2020.8265. [DOI] [Google Scholar]
- 857.Singh, P., Bajpai, V., Gond, V., Kumar, A., Tadigoppula, N., Kumar, B. (2020). determination of bioactive compound of fenugreek seeds using lc-ms techniquesLegume Genomics, 377–393 [DOI] [PubMed]
- 858.Singh TP, Chauhan G, Mendiratta SK, Agarwal RK, Arora S, Verma AK, Rajkumar V. In vitro antioxidant and antimicrobial activities of clove extract and its effectiveness in bio-composite film on storage stability of goat meat balls. Journal of Food Science. 2022;87(5):2083–2095. doi: 10.1111/1750-3841.16135. [DOI] [PubMed] [Google Scholar]
- 859.Singh, A. K. (2017). Spices and condimentsWild Relatives of Cultivated Plants in India,137–15410.1007/978-981-10-5116-6_11
- 860.Singh, A. K., Prakash, P., Singh, R., Nandy, N., Firdaus, Z., Bansal, M., Singh, R. K., Srivastava, A., Roy, J. K., Mishra, B., & Singh, R. K. (2017). Curcumin quantum dots mediated degradation of bacterial biofilmsFrontiers in Microbiology, 8(AUG)10.3389/fmicb.2017.01517 [DOI] [PMC free article] [PubMed]
- 861.Singh A, Singh S, Sharma R. Nutritional potentials and nutrient profile of fenugreek (Trigonella foenum-graecum l.): review article. International Journal of Current Microbiology and Applied Sciences. 2020;9(10):3606–3615. doi: 10.20546/ijcmas.2020.910.417. [DOI] [Google Scholar]
- 862.Singh LM, Chakraborty B, Pal R, Nath A, Pal S, Rahman DS, Ghosh SK, Sengupta M. A comparative study on the antioxidant and immunomodulatory properties of curcumin conjugated gold nanospheres and free curcumin. Journal of Applied Pharmaceutical Science. 2017;7(11):56–63. doi: 10.7324/JAPS.2017.71108. [DOI] [Google Scholar]
- 863.Singh PK, Kumar S, Bhat ZF, Kumar P. Effect of sorghum bicolour and clove oil on the quality characteristics and storage quality of aerobically packaged chevon cutlets. Nutrition and Food Science. 2015;45(1):145–163. doi: 10.1108/NFS-02-2014-0017. [DOI] [Google Scholar]
- 864.Singh, P., Roy, T. K., Kanupriya, C., Tripathi, P. C., Kumar, P., & Shivashankara, K. S. (2022). Evaluation of bioactive constituents of Garcinia indica (kokum) as a potential source of hydroxycitric acid, anthocyanin, and phenolic compoundsLwt,15610.1016/j.lwt.2021.112999
- 865.Singh R, Kumar Sharma P, Malviya R. Pharmacological properties and ayurvedic value of Indian buch plant (Acorus calamus): A short review. Advances in Biological Research. 2011;5(3):145–154. [Google Scholar]
- 866.Sinu PA, Shivanna KR. Pollination biology of large cardamom (Amomum subulatum) Current Science. 2007;93(4):548–552. [Google Scholar]
- 867.Siregar TM, Cahyana AH, Gunawan RJ. Characteristics and free radical scavenging activity of zinc oxide (ZnO) nanoparticles derived from extract of coriander (Coriandrum sativum L.) Reaktor. 2017;17(3):145. doi: 10.14710/reaktor.17.3.145-151. [DOI] [Google Scholar]
- 868.Siriken, B., Yavuz, C., & Guler, A. (2018). Antibacterial activity of Laurus nobilis: a review of literatureMedical Science and Discovery, 374–37910.17546/msd.482929
- 869.Sivakumar C, Rajan AP, Kousalya GN. Roots extract mediated green synthesis of copper nano particles using Acorus calamus and its characterization. World Journal of Pharmaceutical Research. 2019;8(9):975–983. doi: 10.20959/wjpr20199-15432. [DOI] [Google Scholar]
- 870.Slimestad R, Fossen T, Vågen IM. Onions: A source of unique dietary flavonoids. Journal of Agricultural and Food Chemistry. 2007;55(25):10067–10080. doi: 10.1021/jf0712503. [DOI] [PubMed] [Google Scholar]
- 871.Snehlata, H. S., & Payal, D. R. (2012). Fenugreek (Trigonella foenum-graecum l.): an overview. In Available online on www.ijcpr.com International Journal of Current Pharmaceutical Review and Research (Vol. 2, Issue 4)www.ijcpr.com
- 872.Soares KS, Souza MP, Silva-Filho EC, Barud HS, Ribeiro CA, Santos DD, Rocha KNS, de Moura JFP, Oliveira RL, Bezerra LR. Effect of edible onion (Allium cepa L.) film on quality, sensory properties and shelf life of beef burger patties. Molecules. 2021;26(23):1–13. doi: 10.3390/molecules26237202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 873.Soleimani Far, M., Niyazmand, R., & Shahdi Noghabi, M. (2017). Evaluation and comparison of physicochemical properties, fatty acid, oxidative stability of coriander and dill seeds TT - ارزیابی و مقایسه خصوصیات فیزیکوشیمیایی، ساختار اسید چرب و پایداری اکسایشی روغن بذر گشنیز و شویدMdrsjrns, 14(62), 123–133http://fsct.modares.ac.ir/article-7-8010-en.html
- 874.Soliman, M., Qari, S. H., Abu-Elsaoud, A., El-Esawi, M., Alhaithloul, H., & Elkelish, A. (2020). Rapid green synthesis of silver nanoparticles from blue gum augment growth and performance of maize, fenugreek, and onion by modulating plants cellular antioxidant machinery and genes expressionActa Physiologiae Plantarum, 42(9)10.1007/s11738-020-03131-y
- 875.Soltaninezhad B, Khanzadi S, Hashemi M, Azizzadeh M. The Inhibition of Escherichia coli O157:H7 Inoculated in Hamburger Using a Chitosan/Cellulose Nanofiber Film Containing the Nanoemulsion of Trachyspermum ammi and Bunium persicum Essential Oils. Journal of Human, Environment, and Health Promotion. 2020;6(1):30–34. doi: 10.29252/jhehp.6.1.6. [DOI] [Google Scholar]
- 876.Soman, M., Ramachandran Nair, P. C., Vinod, V. M., & Kesavamongalam, S. P. (2014). Nutritional and anti nutritional status of Acorus calamus l. rhizomeAnnals: Food Science & Technology, 15(1), 51–59http://search.ebscohost.com/login.aspx?direct=true&db=fsr&AN=101022536&site=ehost-live
- 877.Somanawat K, Thong-Ngam D, Klaikeaw N. Curcumin attenuated paracetamol overdose induced hepatitis. World Journal of Gastroenterology. 2013;19(12):1962–1967. doi: 10.3748/wjg.v19.i12.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 878.Somani, R. S., & Singhai, A K. (2008). Hypoglycaemic and antidiabetic activities of seeds of Myristica fragrans in normoglycaemic and alloxan-induced diabetic ratsAsian Journal of Experimental Sciences, 22(1), 95–102http://www.ajes.in/PDFs/08-1/11. Hypoglycaemic and Antidiabetic Activity.pdf
- 879.Song JH, Lim JA, Lee JH. Quality and antioxidant properties of cookies supplemented with cinnamon powder. Journal of the Korean Society of Food Science and Nutrition. 2014;43(9):1457–1461. doi: 10.3746/jkfn.2014.43.9.1457. [DOI] [Google Scholar]
- 880.Soshnikova V, Kim YJ, Singh P, Huo Y, Markus J, Ahn S, Castro-Aceituno V, Kang J, Chokkalingam M, Mathiyalagan R, Yang DC. Cardamom fruits as a green resource for facile synthesis of gold and silver nanoparticles and their biological applications. Artificial Cells, Nanomedicine and Biotechnology. 2018;46(1):108–117. doi: 10.1080/21691401.2017.1296849. [DOI] [PubMed] [Google Scholar]
- 881.Sotomayor-Gerding D, Oomah BD, Acevedo F, Morales E, Bustamante M, Shene C, Rubilar M. High carotenoid bioaccessibility through linseed oil nanoemulsions with enhanced physical and oxidative stability. Food Chemistry. 2016;199:463–470. doi: 10.1016/j.foodchem.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 882.Sowbhagya HB, Mahadevamma S, Indrani D, Srinivas P. Physicochemical and microstructural characteristics of celery seed spent residue and influence of its addition on quality of biscuits. Journal of Texture Studies. 2011;42(5):369–376. doi: 10.1111/j.1745-4603.2011.00294.x. [DOI] [Google Scholar]
- 883.Sowmiya Rajalakshmi, B., Ancy Jenifer, A., Ahila, K. G., Vasanthy, M., & Thamaraiselvi, C. (2019). Effective removal of TDS and COD from sugar effluent using green synthesized magnetic iron nanoparticle with Trigonella foenum-graecum seed mucilageWaste Management and Resource Efficiency,961–97310.1007/978-981-10-7290-1_80
- 884.Sp K, Pv P, At S, Ambhore S. Studies on process standardization and sensory properties of buffalo milk paneer blended with raw turmeric extract ( Curcuma longa L. ) The Pharma Innovation Journal. 2020;9(11):34–40. [Google Scholar]
- 885.Speroni E, Cervellati R, Dall’Acqua S, Guerra MC, Greco E, Govoni P, Innocenti G. Gastroprotective effect and antioxidant properties of different laurus nobilis L. Leaf extracts. Journal of Medicinal Food. 2011;14(5):499–504. doi: 10.1089/jmf.2010.0084. [DOI] [PubMed] [Google Scholar]
- 886.Sreelakshmi C, Goel N, Datta KKR, Addlagatta A, Ummanni R, Reddy BVS. Green synthesis of curcumin capped gold nanoparticles and evaluation of their cytotoxicity. Nanoscience and Nanotechnology Letters. 2013;5(12):1258–1265. doi: 10.1166/nnl.2013.1678. [DOI] [Google Scholar]
- 887.Chatterjee S, Nandini Goswami PB. Estimation of phenolic components and in vitro antioxidant activity of fennel (Foeniculum vulgare) and ajwain (Trachyspermum ammi) seeds. Advances in Bioresearch. 2012;3(June):109–118. [Google Scholar]
- 888.Srinivasan K. Fenugreek (Trigonella foenum-graecum): A review of health beneficial physiological effects. Food Reviews International. 2006;22(2):203–224. doi: 10.1080/87559120600586315. [DOI] [Google Scholar]
- 889.Srivastava D, Rajiv JM, Naidu MM, Puranaik J, Srinivas P. Effect of fenugreek seed husk on the rheology and quality characteristics of muffins. Food and Nutrition Sciences. 2012;03(11):1473–1479. doi: 10.4236/fns.2012.311191. [DOI] [Google Scholar]
- 890.Stati G, Rossi F, Trakoolwilaiwan T, Tung LD, Mourdikoudis S, Thanh NTK, Di Pietro R. Development and characterization of curcumin-silver nanoparticles as a promising formulation to test on human pterygium-derived keratinocytes. Molecules. 2022;27(1):1–11. doi: 10.3390/molecules27010282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 891.Statistica. (2020)Main countries for opium poppy cultivation based on acreage in 2019https://www.statista.com/statistics/264744/top-countries-for-oppium-cultivation-based-on-acreage/
- 892.Staughton, J. (2021). 7 impressive benefits of allspiceOrganic Facts
- 893.Suárez A, Ulate G, Ciccio JF. Cardiovascular effects of ethanolic and aqueous extracts of Pimenta dioica in Sprague-Dawley rats. Journal of Ethnopharmacology. 1997;55(2):107–111. doi: 10.1016/S0378-8741(96)01485-7. [DOI] [PubMed] [Google Scholar]
- 894.Subbaiah KPV, Ramanjaneyulu G, Savithramma N. Synthesis of silver nanoparticles and validation from rhizome powder of Curcuma longa L. – an ethnobotanical plant for skin disease. Indian Streams Research Journal. 2013;3(May):1–7. [Google Scholar]
- 895.Subham, K., Kaur, J., Chakroborty, M., Manuel, S. G. A., & Pradeep, N. (2020). Green synthesis of zinc oxide nanoparticles using the extracts of Calotropis gigantea, Foeniculum vulgare and Murraya koenigii and their antimicrobial propertiesJournal of Advanced Scientific Research, 11(3), 183–188https://ezproxy.lib.swin.edu.au/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=a9h&AN=145639198&site=ehost-live&scope=site
- 896.Subhapriya S, Gomathipriya P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microbial Pathogenesis. 2018;116:215–220. doi: 10.1016/j.micpath.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 897.Suleria HAR, Butt MS, Anjum FM, Saeed F, Khalid N. Onion: Nature protection against physiological threats. Critical Reviews in Food Science and Nutrition. 2015;55(1):50–66. doi: 10.1080/10408398.2011.646364. [DOI] [PubMed] [Google Scholar]
- 898.Sulieman AME, Ali AO, Hemavathy J. Lipid content and fatty acid composition of fenugreek (Trigonellafoenum- graecum L.) seeds grown in Sudan. International Journal of Food Science and Technology. 2008;43(2):380–382. doi: 10.1111/j.1365-2621.2006.01446.x. [DOI] [Google Scholar]
- 899.Suliman GM, Alowaimer AN, Al-Mufarrej SI, Hussein EOS, Fazea EH, Naiel MAE, Alhotan RA, Swelum AA. The effects of clove seed (Syzygium aromaticum) dietary administration on carcass characteristics, meat quality, and sensory attributes of broiler chickens. Poultry Science. 2021;100(3):100904. doi: 10.1016/j.psj.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 900.Sun Z, Xiong T, Zhang T, Wang N, Chen D, Li S. Influences of zinc oxide nanoparticles on Allium cepa root cells and the primary cause of phytotoxicity. Ecotoxicology. 2019;28(2):175–188. doi: 10.1007/s10646-018-2010-9. [DOI] [PubMed] [Google Scholar]
- 901.Susanah RW, Retno K, Made Dira SI. Total phenolic and flavonoid contents and antimicrobial activity of acorus calamus L Rhizome ethanol extract. Research Journal of Chemistry and Environment. 2018;22(Special issue II):65–70. [Google Scholar]
- 902.Swamibhut. (2017). allspice/pimenta dioicaNturalherbssite
- 903.Swamy, M. K., Akhtar, M. S., & Sinniah, U. R. (2016). Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated reviewEvidence-Based Complementary and Alternative Medicine,201610.1155/2016/3012462 [DOI] [PMC free article] [PubMed]
- 904.Swapna TS, Binitha M, Manju TS. In vitro multiplication in Kaempferia galanga Linn. Applied Biochemistry and Biotechnology - Part A Enzyme Engineering and Biotechnology. 2004;118(1–3):233–241. doi: 10.1385/ABAB:118:1-3:233. [DOI] [PubMed] [Google Scholar]
- 905.Syed B, Bhat PS, Bisht N, Karthik RN, Farangis-Rakhimova D, Devi AT, Prasad A, Satish S, Nanjundaswamy S, Nagendra Prasad MN. Phytobiological mediated production of silver nanoparticles from Garcinia indica and their bactericidal potential. Journal of Biologically Active Products from Nature. 2018;8(3):154–161. doi: 10.1080/22311866.2018.1486227. [DOI] [Google Scholar]
- 906.Taami B, Rostami Zadeh K, Aminzare M, Hassanzad Azar H. Antioxidant efficacy of biodegradable starch film containing of bunium persicum essential oil nanoemulsion fortified with cinnamaldehyde. SSRN Electronic Journal. 2021 doi: 10.2139/ssrn.3972558. [DOI] [Google Scholar]
- 907.Tahir HU, Sarfraz RA, Ashraf A, Adil S. Chemical composition and antidiabetic activity of essential oils obtained from two spices (Syzygium aromaticum and Cuminum cyminum) International Journal of Food Properties. 2016;19(10):2156–2164. doi: 10.1080/10942912.2015.1110166. [DOI] [Google Scholar]
- 908.Tajik H, Farhangfar A, Moradi M, Razavi Rohani SM. Effectiveness of clove essential oil and grape seed extract combination on microbial and lipid oxidation characteristics of raw buffalo patty during storage at abuse refrigeration temperature. Journal of Food Processing and Preservation. 2014;38(1):31–38. doi: 10.1111/j.1745-4549.2012.00736.x. [DOI] [Google Scholar]
- 909.Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Control. 2010;21(9):1199–1218. doi: 10.1016/j.foodcont.2010.02.003. [DOI] [Google Scholar]
- 910.Takooree H, Aumeeruddy MZ, Rengasamy KR, Venugopala KN, Jeewon R, Zengin G, Mahomoodally MF. a systematic review on black pepper (piper nigrum L) from folk uses to pharmacological applications. Critical Reviews in Food Science and Nutrition. 2019;59:S210–S243. doi: 10.1080/10408398.2019.1565489. [DOI] [PubMed] [Google Scholar]
- 911.Talebi F, Misaghi A, Khanjari A, Kamkar A, Gandomi H, Rezaeigolestani M. Incorporation of spice essential oils into poly-lactic acid film matrix with the aim of extending microbiological and sensorial shelf life of ground beef. LWT. 2018;96:482–490. doi: 10.1016/j.lwt.2018.05.067. [DOI] [Google Scholar]
- 912.Talebi F, MIsaghi A, Khanjari A, Kamkar A, Gandomi H, Saeedi M. Evaluation of antimicrobial activity of Poly Lactic Acid (PLA) films containing cellulose nanoparticle and Bunium persicum and Mentha pepperita essential oils (EOs) Iranian Journal of Veterinary Medicine. 2017;11(4):289–298. doi: 10.22059/ijvm.2017.223435.1004783. [DOI] [Google Scholar]
- 913.Talodthaisong C, Plaeyao K, Mongseetong C, Boonta W, Srichaiyapol O, Patramanon R, Kayunkid N, Kulchat S. The decoration of ZnO nanoparticles by gamma aminobutyric acid, curcumin derivative and silver nanoparticles: Synthesis, characterization and antibacterial evaluation. Nanomaterials. 2021;11(2):1–19. doi: 10.3390/nano11020442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 914.Tamilarasi R, Sivanesan D, Kanimozhi P, College STETW. Hepatoprotective and antioxidant efficacy of Anethum graveolens linn in carbon tetrachloride induced hepatotoxicity in albino rats. Journal of Chemical and Pharmaceutical Research. 2012;4(4):1885–1888. [Google Scholar]
- 915.Tamiru Kasaye A. Evaluation of composite blends of fermented fenugreek and wheat flour to assess its suitability for bread and biscuit. International Journal of Nutrition and Food Sciences. 2015;4(1):29. doi: 10.11648/j.ijnfs.20150401.15. [DOI] [Google Scholar]
- 916.Tang X, Yu H, Bui B, Wang L, Xing C, Wang S, Chen M, Hu Z, Chen W. Nitrogen-doped fluorescence carbon dots as multi-mechanism detection for iodide and curcumin in biological and food samples. Bioactive Materials. 2021;6(6):1541–1554. doi: 10.1016/j.bioactmat.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 917.Tanko Y, Mohammed A, Okasha MA, Umar AH, Magaji RA. Anti-nociceptive and anti-inflammatory activities of ethanol extract of Syzygium aromaticum flower bud in Wistar rats and mice. African Journal of Traditional, Complementary and Alternative Medicines. 2008;5(2):209–212. doi: 10.4314/ajtcam.v5i2.31275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 918.Tanruean K, Kaewnarin K, Rakariyatham N. Antibacterial and antioxidant activities of Anethum graveolens L. dried fruit extracts. Chiang Mai Journal of Science. 2014;41(3):649–660. [Google Scholar]
- 919.Tasleem F, Azhar I, Ali SN, Perveen S, Mahmood ZA. Analgesic and anti-inflammatory activities of Piper nigrum L. Asian Pacific Journal of Tropical Medicine. 2014;7(S1):S461–S468. doi: 10.1016/S1995-7645(14)60275-3. [DOI] [PubMed] [Google Scholar]
- 920.Tavakoli F, Rafieiolhossaini M, Ravash R. Effects of PEG and nano-silica elicitors on secondary metabolites production in Crocus sativus L. Russian Journal of Plant Physiology. 2021;68(5):931–940. doi: 10.1134/S1021443721050216. [DOI] [Google Scholar]
- 921.Tawfek MAEM, Ali ARM. Effectiveness of cardamom or bay leaf powder in improving the quality of labneh. ACTA. 2022;21(1):39–52. doi: 10.17306/J.AFS.0984. [DOI] [PubMed] [Google Scholar]
- 922.Tekeli, A., Kop-Bozbay, C., Atan, H., Karamik, S. (2020). Nutritional values of cold pres poppy ( Papaver somniferum L. ) and sesame ( Sesamum indicum L. ) meal as alternative protein sourceInternational Agriculture Congress, January
- 923.Tenne PCRK, Karunaratne MMSC. Phytochemical profile and bioactivity of essential oil from Pimenta dioica leaves on Cowpea beetle, Callosobruchus maculatus (f.) (coleoptera: bruchidae): a farmer friendly solution for postharvest pest management. Open Agriculture. 2018;3(1):301–309. doi: 10.1515/opag-2018-0033. [DOI] [Google Scholar]
- 924.Tenne PCRK, Karunaratne MMSC. Phytochemical profile and bioactivity of essential oil from Pimenta dioica leaves on Cowpea beetle, Callosobruchus maculatus (F) (Coleoptera: Bruchidae): a farmer friendly solution for postharvest pest management. Open Agriculture. 2018;3(1):301–309. doi: 10.1515/opag-2018-0033. [DOI] [Google Scholar]
- 925.Teshika JD, Zakariyyah AM, Zaynab T, Zengin G, Rengasamy KR, Pandian SK, Fawzi MM. Traditional and modern uses of onion bulb (Allium cepa L.): A systematic review. Critical Reviews in Food Science and Nutrition. 2019;59:S39–S70. doi: 10.1080/10408398.2018.1499074. [DOI] [PubMed] [Google Scholar]
- 926.Tétényi, P. (2010). Opium poppy ( Papaver somniferum ): Botany and horticultureHorticultural Reviews,373–40810.1002/9780470650622.ch7
- 927.Thadakapally R, Aafreen A, Aukunuru J, Habibuddin M, Jogala S. Preparation and characterization of PEG-albumin-curcumin nanoparticles intended to treat breast cancer. Indian Journal of Pharmaceutical Sciences. 2016;78(1):65–72. doi: 10.4103/0250-474X.180250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 928.Thakur A, Sharma N, Bhatti M, Sharma M, Trukhanov AV, Trukhanov SV, Panina LV, Astapovich KA, Thakur P. Synthesis of barium ferrite nanoparticles using rhizome extract of Acorus Calamus: Characterization and its efficacy against different plant phytopathogenic fungi. Nano-Structures and Nano-Objects. 2020;24:100599. doi: 10.1016/j.nanoso.2020.100599. [DOI] [Google Scholar]
- 929.Thanh, V, N, T. (2014). Synthesis and evaluation of gold nanoparticle conjugated with curcuminBachelor Thesis - Biotechnology & Related Fields
- 930.Tharachand C, Immanuel Selvaraj C, Abraham Z. Comparative evaluation of anthelmintic and antibacterial activities in leaves and fruits of Garcinia cambogia (gaertn.) desr. and Garcinia indica (dupetit-thouars) choisy. Brazilian Archives of Biology and Technology. 2015;58(3):379–386. doi: 10.1590/S1516-8913201500062. [DOI] [Google Scholar]
- 931.Tharun, G., Ramana, G., Sandhya, R., & Shravani, M. (2017a). Phytochemical and pharmacological review on Laurus nobilisJournal of Pharmacy Research, 11(3), 249–256http://jprsolutions.info
- 932.Tharun, G., Ramana, G., Sandhya, R., & Shravani, M. (2017b). Phytochemical and Pharmacological Review on Laurus NobilisJournal of Pharmacy Research, 11(3), 249–256http://jprsolutions.info
- 933.Thomas RA, Krishnakumari S. Phytochemical profiling of Myristica fragrans seed extract with different organic solvents. Asian Journal of Pharmaceutical and Clinical Research. 2015;8(1):303–307. [Google Scholar]
- 934.Thong-Ngam D, Choochuai S, Patumraj S, Chayanupatkul M, Klaikeaw N. Curcumin prevents indomethacin-induced gastropathy in rats. World Journal of Gastroenterology. 2012;18(13):1479–1484. doi: 10.3748/wjg.v18.i13.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 935.Thummaneni, C., Surya Prakash, D. V, Golli, R., & Vangalapati, M. (2022). Green synthesis of silver nanoparticles and characterization of caffeic acid from Myristica fragrans (Nutmeg) against antibacterial activityMaterials Today: Proceedings10.1016/j.matpr.2022.04.586
- 936.Tinca AL, Iurian S, Rus LM, Porfire A, Tefas LR. Development and characterization of curcumin-loaded polycaprolactone nanoparticles by Design of Experiments. Romanian Journal of Pharmaceutical Practice. 2020;13(3):127–138. doi: 10.37897/rjphp.2020.3.3. [DOI] [Google Scholar]
- 937.Tiyagi SA, Mahmood I, Rizvi R. application of organic additives for the management of plant parasitic nematodes and soil pathogenic fungi on fenugreek and mungbean. Journal of Medicinal and Aromatic Plant Sciences. 2010;32(4):420–431. [Google Scholar]
- 938.Tizghadam P, Roufegari-nejad L, Asefi N, Jafarian Asl P. Physicochemical characteristics and antioxidant capacity of set yogurt fortified with dill (Anethume graveolens) extract. Journal of Food Measurement and Characterization. 2021;15(4):3088–3095. doi: 10.1007/s11694-021-00881-2. [DOI] [Google Scholar]
- 939.Tomar RS, Shrivastava V. Efficacy evaluation of ethanolic extract of Brassica nigra as potential anitmicrobial agent against selcted microorganisms. International Journal of Pahramaceutical Acience and Health Care. 2014;3(4):117–123. [Google Scholar]
- 940.Tometri SS, Ahmady M, Ariaii P, Soltani MS. Extraction and encapsulation of Laurus nobilis leaf extract with nano-liposome and its effect on oxidative, microbial, bacterial and sensory properties of minced beef. Journal of Food Measurement and Characterization. 2020;14(6):3333–3344. doi: 10.1007/s11694-020-00578-y. [DOI] [Google Scholar]
- 941.Tosati JV, de Oliveira EF, Oliveira JV, Nitin N, Monteiro AR. Light-activated antimicrobial activity of turmeric residue edible coatings against cross-contamination of Listeria innocua on sausages. Food Control. 2018;84:177–185. doi: 10.1016/j.foodcont.2017.07.026. [DOI] [Google Scholar]
- 942.Toshima N, Yonezawa T. Bimetallic nanoparticles - novel materials for chemical and physical applications. New Journal of Chemistry. 1998;22(11):1179–1201. doi: 10.1039/a805753b. [DOI] [Google Scholar]
- 943.Trung D, Nhuan T, Van TC, Minh V, Anh P. Biofabrication of silver nanoparticles using Curcuma longa extract : Effects of extraction and synthesis conditions, characteristics, and its antibacterial activity. J Biochem Tech. 2020;11(1):57–66. [Google Scholar]
- 944.Tungmunnithum D, Tanaka N, Uehara A, Iwashina T. Flavonoids profile, taxonomic data, history of cosmetic uses, anti-oxidant and anti-aging potential of alpinia galanga (L.) willd. Cosmetics. 2020;7(4):1–8. doi: 10.3390/cosmetics7040089. [DOI] [Google Scholar]
- 945.Umaraw, P., Chauhan, G., Mendiratta, S. K., Verma, A. K., & Arya, A. (2020). Effect of oregano and bay as natural preservatives in meat bread for extension of storage stability at ambient temperatureJournal of Food Processing and Preservation, 44(4)10.1111/jfpp.14375
- 946.Upadhyay PK, Singh S, Agrawal G, Vishwakarma VK. Pharmacological activities and therapeutic uses of resins obtained from Ferula asafoetida Linn.: A Review. International Journal of Green Pharmacy. 2017;11(2):S240–S247. [Google Scholar]
- 947.Upadhyay RK. Nutraceutical, pharmaceutical and therapeutic uses of Allium cepa: A review. International Journal of Green Pharmacy. 2016;10(1):S46–S64. [Google Scholar]
- 948.USDA. (2019a). spices, anise seedFood Data Central
- 949.USDA. (2019b). spices, caraway seedFood Data Central
- 950.USDA. (2019c). spices, cloves, groundFood Data Central
- 951.USDA. (2019d). spices, turmeric, groundFood Data Central
- 952.USDA. (2019e)Spices , caraway seed02005
- 953.Utami R, Khasanah LU, Manuhara GJ, Ayuningrum ZK. Effects of cinnamon bark essential oil (cinnamomum burmannii) on characteristics of edible film and quality of fresh beef. Pertanika Journal of Tropical Agricultural Science. 2019;42(4):1173–1184. [Google Scholar]
- 954.Vakayil R, Muruganantham S, Kabeerdass N, Rajendran M, Mahadeo Palve A, Ramasamy S, Awad Alahmadi TS, Almoallim H, Manikandan V, Mathanmohun M. Acorus calamus-zinc oxide nanoparticle coated cotton fabrics shows antimicrobial and cytotoxic activities against skin cancer cells. Process Biochemistry. 2021;111(P1):1–8. doi: 10.1016/j.procbio.2021.08.024. [DOI] [Google Scholar]
- 955.Vakili S, Savardashtaki A, Moghaddam MAM, Nowrouzi P, Shirazi MK, Ebrahimi G. The effects of saffron consumption on lipid profile, liver enzymes, and oxidative stress in male hamsters with high fat diet. Trends in Pharmaceutical Sciences. 2017;3(3):201–208. [Google Scholar]
- 956.Valdés A, Mellinas AC, Ramos M, Burgos N, Jiménez A, Garrigós MC. Use of herbs, spices and their bioactive compounds in active food packaging. RSC Advances. 2015;5(50):40324–40335. doi: 10.1039/c4ra17286h. [DOI] [Google Scholar]
- 957.Valsaraj R, Pushpangadan P, Smitt UW, Adsersen A, Nyman U. Antimicrobial screening of selected medicinal plants from India. Journal of Ethnopharmacology. 1997;58(2):75–83. doi: 10.1016/S0378-8741(97)00085-8. [DOI] [PubMed] [Google Scholar]
- 958.Vardavas CI, Majchrzak D, Wagner KH, Elmadfa I, Kafatos A. Lipid concentrations of wild edible greens in Crete. Food Chemistry. 2006;99(4):822–834. doi: 10.1016/j.foodchem.2005.08.058. [DOI] [Google Scholar]
- 959.Varghese B, Kurian M, Krishna S, Athira TS. Biochemical synthesis of copper nanoparticles using Zingiber officinalis and Curcuma longa: Characterization and antibacterial activity study. Materials Today: Proceedings. 2020;25:302–306. doi: 10.1016/j.matpr.2020.01.476. [DOI] [Google Scholar]
- 960.Varuna KJB, Madhusudhan B. Synthesis, characterization and hemocompatibility evaluation of curcumin encapsulated chitosan nanoparticles for oral delivery. International Journal of Advanced Research. 2015;3(4):604–611. [Google Scholar]
- 961.Vasavda K, Hegde P, Harini A. Pharmacological activities of turmeric: A review. Journal of Traditional Medicinal and Clinical Nuropathy. 2013;2:133. [Google Scholar]
- 962.Vecchio MG, Gulati A, Minto C, Lorenzoni G. Pimpinella anisum and Illicium verum: The multifaceted role of anise plants. The Open Agriculture Journal. 2016;10(1):81–86. doi: 10.2174/1874331501610010084. [DOI] [Google Scholar]
- 963.Venkatadri B, Shanparvish E, Rameshkumar MR, Arasu MV, Al-Dhabi NA, Ponnusamy VK, Agastian P. Green synthesis of silver nanoparticles using aqueous rhizome extract of Zingiber officinale and Curcuma longa: In-vitro anti-cancer potential on human colon carcinoma HT-29 cells. Saudi Journal of Biological Sciences. 2020;27(11):2980–2986. doi: 10.1016/j.sjbs.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 964.Venugopal K, Rather HA, Rajagopal K, Shanthi MP, Sheriff K, Illiyas M, Rather RA, Manikandan E, Uvarajan S, Bhaskar M, Maaza M. Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. Journal of Photochemistry and Photobiology B: Biology. 2017;167:282–289. doi: 10.1016/j.jphotobiol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 965.Verma RK, Mishra G, Singh P, Jha KK, Khosa RL. Alpinia galanga – an important medicinal plant : A review. Der Pharmacia Sinica. 2011;2(1):142–154. [Google Scholar]
- 966.Vidanagamage SA, Pathiraje PMHD, Perera ODAN. Effects of Cinnamon (Cinnamomum Verum) Extract on Functional Properties of Butter. Procedia Food Science. 2016;6(Icsusl 2015):136–142. doi: 10.1016/j.profoo.2016.02.033. [DOI] [Google Scholar]
- 967.Vigyan Kendra K, Preeti Kumari I, Kumar Maurya Scholar R, Kumar V, Kumar Verma R, Kumari P, Kumar Maurya R, Verma R, Kumar Singh R. Medicinal properties of turmeric (Curcuma longa L.): A review. ~ 1354 ~. International Journal of Chemical Studies. 2018;6(4):1354–1357. [Google Scholar]
- 968.Vijayakumar S, Vaseeharan B, Malaikozhundan B, Shobiya M. Laurus nobilis leaf extract mediated green synthesis of ZnO nanoparticles: Characterization and biomedical applications. Biomedicine & Pharmacotherapy. 2016;84:1213–1222. doi: 10.1016/j.biopha.2016.10.038. [DOI] [PubMed] [Google Scholar]
- 969.Vijayasteltar L, Jismy IJ, Joseph A, Maliakel B, Kuttan R, Krishnakumar IM. Beyond the flavor: A green formulation of Ferula asafoetida oleo-gum-resin with fenugreek dietary fibre and its gut health potential. Toxicology Reports. 2017;4(June):382–390. doi: 10.1016/j.toxrep.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 970.Vilela J, Martins D, Monteiro-Silva F, González-Aguilar G, de Almeida JMMM, Saraiva C. Antimicrobial effect of essential oils of Laurus nobilis L. and Rosmarinus officinallis L. on shelf-life of minced “Maronesa” beef stored under different packaging conditions. Food Packaging and Shelf Life. 2016;8:71–80. doi: 10.1016/j.fpsl.2016.04.002. [DOI] [Google Scholar]
- 971.Vinod K, Aditi S. Synthesis, characterization, antimicrobial activity and release study of cinnamon loaded poly nanoparticles. Research Journal of Pharmacy and Technology. 2019;12(4):1529–1535. doi: 10.5958/0974-360X.2019.00253.1. [DOI] [Google Scholar]
- 972.Vinotha V, Yazhiniprabha M, Raj DS, Mahboob S, Al-Ghanim KA, Al-Misned F, Govindarajan M, Vaseeharan B. Biogenic synthesis of aromatic cardamom-wrapped zinc oxide nanoparticles and their potential antibacterial and mosquito larvicidal activity: An effective eco-friendly approach. Journal of Environmental Chemical Engineering. 2020;8(6):104466. doi: 10.1016/j.jece.2020.104466. [DOI] [Google Scholar]
- 973.Vinothini P, Suthacini V. Cardamom crop production and harvesting: A review. Shanlax International Journal of Economics. 2021;9(3):31–37. [Google Scholar]
- 974.Virk P. Antidiabetic activity of green gold-silver nanocomposite with trigonella foenum graecum L. seeds extract on streptozotocin- induced diabetic rats. Pakistan Journal of Zoology. 2018;50(2):711–718. doi: 10.17582/JOURNAL.PJZ/2018.2.711.718. [DOI] [Google Scholar]
- 975.Vispute MM, Sharma D, Biswas AK, Rokade JJ, Chaple AR, Biswas A, Gopi M, Kapgate MG. Dietary hemp ( Cannabis sativa l.) and dill seed ( Anethum graveolens ) improve physicochemical properties, oxidative stability, and sensory attributes of broiler meat. ACS Food Science & Technology. 2021;1(3):453–461. doi: 10.1021/acsfoodscitech.0c00049. [DOI] [Google Scholar]
- 976.Vohra SB, Shah SA, Sharma K, Naqvi SAH, Dandiya PC. Antibacterial, antipyretic, analgesic and anti-inflammatory studies on Acorus calamus Linn. Annals of National Academy of Medical Sciences. 1989;25:13–20. [Google Scholar]
- 977.Vutakuri N, Somara S. Natural and herbal medicine for breast cancer using Elettaria cardamomum (L.) Maton. International Journal of Herbal Medicine. 2018;6(2):91–96. [Google Scholar]
- 978.Waghmare RD, Gunjal DB, Naik VM, Gore AH, Nimbalkar MS, Anbhule PV, Bhosale SV, Sohn D, Kolekar GB. Carbon nanodots derived from kitchen waste biomass as a growth accelerator for fenugreek plant. Journal of Nanoscience and Nanotechnology. 2021;21(4):2234–2245. doi: 10.1166/jnn.2021.19028. [DOI] [PubMed] [Google Scholar]
- 979.Wagner K-H, Elmadfa I. Biological relevance of terpenoids. Annals of Nutrition and Metabolism. 2003;47(3–4):95–106. doi: 10.1159/000070030. [DOI] [PubMed] [Google Scholar]
- 980.Wang, D., Zhang, L., Huang, J., Himabindu, K., Tewari, D., Horbanczuk, J. O., Xu, S., Chen, Z., Atanasov, A. G. (2021). Cardiovascular protective effect of black pepper (Piper nigrum L.) and its major bioactive constituent piperineTrends in Food Science and Technology, 117, 34–45https://inpst.net/cardiovascular-protective-effect-of-black-pepper-piper-nigrum-l-and-its-major-bioactive-constituent-piperine/
- 981.Wang GW, Hu WT, Huang BK, Qin LP. Illicium verum: A review on its botany, traditional use, chemistry and pharmacology. Journal of Ethnopharmacology. 2011;136(1):10–20. doi: 10.1016/j.jep.2011.04.051. [DOI] [PubMed] [Google Scholar]
- 982.Wang N, Xu Y, Chao H, Zhang M, Zhou Y, Wang M. Effects of celery powder on wheat dough properties and textural, antioxidant and starch digestibility properties of bread. Journal of Food Science and Technology. 2020;57(5):1710–1718. doi: 10.1007/s13197-019-04204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 983.Wang YH, Avula B, Nanayakkara NPD, Zhao J, Khan IA. Cassia cinnamon as a source of coumarin in cinnamon-flavored food and food SUPPL.ements in the United States. Journal of Agricultural and Food Chemistry. 2013;61(18):4470–4476. doi: 10.1021/jf4005862. [DOI] [PubMed] [Google Scholar]
- 984.Wang Y, Han T, Zhu Y, Zheng CJ, Ming QL, Rahman K, Qin LP. Antidepressant properties of bioactive fractions from the extract of crocus sativus L. Journal of Natural Medicines. 2010;64(1):24–30. doi: 10.1007/s11418-009-0360-6. [DOI] [PubMed] [Google Scholar]
- 985.Wani SA, Kumar P. Development and parameter optimization of health promising extrudate based on fenugreek oat and pea. Food Bioscience. 2016;14:34–40. doi: 10.1016/j.fbio.2016.02.002. [DOI] [Google Scholar]
- 986.Wani S, Solanke N, Kumar P. Extruded product based on oat and fenugreek and their storage stability. Current Nutrition & Food Science. 2015;11(1):78–84. doi: 10.2174/1573401311666150304235616. [DOI] [Google Scholar]
- 987.Wójcik, M., Różyło, R., Schönlechner, R., Matwijczuk, A., & Dziki, D. (2022). Low-Carbohydrate, High-Protein, and Gluten-Free Bread Supplemented with Poppy Seed Flour: Physicochemical, Sensory, and Spectroscopic PropertiesMolecules, 27(5)10.3390/molecules27051574 [DOI] [PMC free article] [PubMed]
- 988.Wu F, Chen Y, Li G, Zhu D, Wang L, Wang J. Zinc oxide nanoparticles synthesized from Allium cepa prevents UVB radiation mediated inflammation in human epidermal keratinocytes (HaCaT cells) Artificial Cells, Nanomedicine and Biotechnology. 2019;47(1):3548–3558. doi: 10.1080/21691401.2019.1642905. [DOI] [PubMed] [Google Scholar]
- 989.Yadav VR, Suresh S, Devi K, Yadav S. Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPS PharmSciTech. 2009;10(3):752–762. doi: 10.1208/s12249-009-9264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 990.Yadav A, Kujur A, Kumar A, Singh PP, Gupta V, Prakash B. Encapsulation of Bunium persicum essential oil using chitosan nanopolymer: Preparation, characterization, antifungal assessment, and thermal stability. International Journal of Biological Macromolecules. 2020;142:172–180. doi: 10.1016/j.ijbiomac.2019.09.089. [DOI] [PubMed] [Google Scholar]
- 991.Yadav UCS, Baquer NZ. Pharmacological effects of Trigonella foenum-graecum L. in health and disease. Pharmaceutical Biology. 2014;52(2):243–254. doi: 10.3109/13880209.2013.826247. [DOI] [PubMed] [Google Scholar]
- 992.Yahyazadeh R, Rahbardar MG, Razavi BM, Karimi G, Hosseinzadeh H. The effect of elettaria cardamomum (cardamom) on the metabolic syndrome: narrative review. Iranian Journal of Basic Medical Sciences. 2021;24(11):1462–1469. doi: 10.22038/IJBMS.2021.54417.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 993.Yang CH, Li RX, Chuang LY. Antioxidant activity of various parts of Cinnamomum cassia extracted with different extraction methods. Molecules. 2012;17(6):7294–7304. doi: 10.3390/molecules17067294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 994.Yang JF, Yang CH, Chang HW, Yang CS, Wang SM, Hsieh MC, Chuang LY. Chemical composition and antibacterial activities of Illicium verum against antibiotic-resistant pathogens. Journal of Medicinal Food. 2010;13(5):1254–1262. doi: 10.1089/jmf.2010.1086. [DOI] [PubMed] [Google Scholar]
- 995.Yang M, Lu F, Zhou T, Zhao J, Ding C, Fakhri A, Gupta VK. Biosynthesis of nano bimetallic Ag/Pt alloy from Crocus sativus L. extract: Biological efficacy and catalytic activity. Journal of Photochemistry and Photobiology B: Biology. 2020;212:112025. doi: 10.1016/j.jphotobiol.2020.112025. [DOI] [PubMed] [Google Scholar]
- 996.Yang XX, Li CM, Huang CZ. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale. 2016;8(5):3040–3048. doi: 10.1039/c5nr07918g. [DOI] [PubMed] [Google Scholar]
- 997.Yazdanparast R, Bahramikia S. Evaluation of the effect of Anethum graveolens L. crude extracts on serum lipids and lipoproteins profiles in hypercholesterolaemic rats. Daru. 2008;16(2):88–94. [Google Scholar]
- 998.Yekeen TA, Azeez MA, Lateef A, Asafa TB, Oladipo IC, Badmus JA, Adejumo SA, Ajibola AA. Cytogenotoxicity potentials of cocoa pod and bean-mediated green synthesized silver nanoparticles on Allium cepa cells. Caryologia. 2017;70(4):366–377. doi: 10.1080/00087114.2017.1370260. [DOI] [Google Scholar]
- 999.Yili A, Yimamu H, Maksimov VV, Aisa HA, Veshkurova ON, Salikhov SI. Chemical composition of essential oil from seeds of Anethum graveolens cultivated in China. Chemistry of Natural Compounds. 2006;42(4):491–492. doi: 10.1007/s10600-006-0190-7. [DOI] [Google Scholar]
- 1000.Younis FAI, Abdelbagi H, Ahmed M, Ahmed F, Mohmmed R. Green synthesis and characterization of gold nanoparticles (AuNPs) using fenugreek seeds extract (Trigonella foenum - gracum) European Journal of Biomedical and Pharmaceutical Sciences. 2018;5(4):100–107. [Google Scholar]
- 1001.Zaazaa, L., Naceiri Mrabti, H., Ed-Dra, A., Bendahbia, K., Hami, H., Soulaymani, A., & Ibriz, M. (2021). Determination of mineral composition and phenolic content and investigation of antioxidant, antidiabetic, and antibacterial activities of Crocus sativus l. aqueous stigmas extractsAdvances in Pharmacological and Pharmaceutical Sciences, 202110.1155/2021/7533938 [DOI] [PMC free article] [PubMed]
- 1002.Zahid MA, Choi JY, Seo J-K, Parvin R, Ko J, Yang H-S. Effects of clove extract on oxidative stability and sensory attributes in cooked beef patties at refrigerated storage. Meat Science. 2020;161:107972. doi: 10.1016/j.meatsci.2019.107972. [DOI] [PubMed] [Google Scholar]
- 1003.Zahid, M. A., Eom, J. U., Parvin, R., Seo, J. K., & Yang, H. S. (2022). Changes in quality traits and oxidation stability of Syzygium aromaticum extract-added cooked ground beef during frozen storageAntioxidants, 11(3)10.3390/antiox11030534 [DOI] [PMC free article] [PubMed]
- 1004.Zahid, Z., Rezgui, M., Nisar, S., & Azeem, M. W. (2016). Phytochemistry and medicinal uses of underutilized tree Garcinia indica: A detailed reviewIjcbs, 10(September), 40–45www.iscientific.org/Journal.html
- 1005.Zaidi SF, Aziz M, Muhammad JS, Kadowaki M. Diverse pharmacological properties of Cinnamomum cassia: A review. Pakistan Journal of Pharmaceutical Sciences. 2015;28(4):1433–1438. [PubMed] [Google Scholar]
- 1006.Zaki FE. Incorporation of fenugreek seed powder in the manufacturing of rabbit sausage and its effects on the quality properties during frozen storage. Journal of Advances in Food Science & Technology. 2018;5(1):8–14. [Google Scholar]
- 1007.Zambre D, Raut N, Raut S, Zode R, Umekar M. Effect of green synthesized fenugreek metal-ion nanoparticles on resistant microorganism. Multidisciplinary International Research Journal of Gujarat Technological University. 2022;4(1):113–119. [Google Scholar]
- 1008.Zanariah J, Rehan AN, Rosnah O. Nutritional composition of common Zingiberaceae species used in traditional medicines and cooking. J. Trop. Agric. and Fd. Sc. 1997;25(2):225–229. [Google Scholar]
- 1009.Zara, S., Petretto, G. L., Mannu, A., Zara, G., Budroni, M., Mannazzu, I., Multineddu, C., Pintore, G., & Fancello, F. (2021). Antimicrobial activity and chemical characterization of a non-polar extract of saffron stamens in food matrixFoods, 10(4)10.3390/foods10040703 [DOI] [PMC free article] [PubMed]
- 1010.Zay K, Gere A. Sensory acceptance of poppy seed-flavored white chocolates using just-about-right method. LWT. 2019;103:162–168. doi: 10.1016/j.lwt.2018.12.069. [DOI] [Google Scholar]
- 1011.Zeka K, Marrazzo P, Micucci M, Ruparelia KC, Arroo RRJ, Macchiarelli G, Nottola SA, Continenza MA, Chiarini A, Angeloni C, Hrelia S, Budriesi R. Activity of antioxidants from crocus sativus l. Petals: Potential preventive effects towards cardiovascular system. Antioxidants. 2020;9(11):1–18. doi: 10.3390/antiox9111102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1012.Zhang, C., Fan, L., Fan, S., Wang, J., Luo, T., Tang, Y., Chen, Z., & Yu, L. (2019). Cinnamomum cassia presl: a review of its traditional uses, phytochemistry, pharmacology and toxicologyMolecules, 24(19)10.3390/molecules24193473 [DOI] [PMC free article] [PubMed]
- 1013.Zhang H, Peng X, Li X, Wu J, Guo X. The application of clove extract protects chinesestyle sausages against oxidation and quality deterioration. Korean Journal for Food Science of Animal Resources. 2017;37(1):114–122. doi: 10.5851/kosfa.2017.37.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1014.Zhang M, Luo W, Yang K, Li C. Effects of sodium alginate edible coating with cinnamon essential oil nanocapsules and Nisin on quality and shelf life of beef slices during refrigeration. Journal of Food Protection. 2022 doi: 10.4315/jfp-21-380. [DOI] [PubMed] [Google Scholar]
- 1015.Zhao, X. X., Lin, F. J., Li, H., Li, H. Bin, Wu, D. T., Geng, F., Ma, W., Wang, Y., Miao, B. H., & Gan, R. Y. (2021). Recent advances in bioactive compounds, health functions, and safety concerns of onion (Allium cepa l.)Frontiers in Nutrition, 8(July)10.3389/fnut.2021.669805 [DOI] [PMC free article] [PubMed]
- 1016.Zheng CJ, Yoo JS, Lee TG, Cho HY, Kim YH, Kim WG. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Letters. 2005;579(23):5157–5162. doi: 10.1016/j.febslet.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 1017.Zhou W, Liang Z, Li P, Zhao Z, Chen J. Tissue-specific chemical profiling and quantitative analysis of bioactive components of Cinnamomum cassia by combining laser-microdissection with UPLC-Q/TOF–MS. Chemistry Central Journal. 2018;12(1):1–9. doi: 10.1186/s13065-018-0438-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1018.Zia I, Mirza S, Jolly R, Rehman A, Ullah R, Shakir M. Trigonella foenum graecum seed polysaccharide coupled nano hydroxyapatite-chitosan: A ternary nanocomposite for bone tissue engineering. International Journal of Biological Macromolecules. 2019;124:88–101. doi: 10.1016/j.ijbiomac.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 1019.Ziadi SA, Mahmood HR. The effect of silver bio-nanoparticles synthesized by Curcuma longa L. on pathogenic fungi. International Journal of ChemTech Research. 2017;10(3):508–514. [Google Scholar]
- 1020.Zinatloo-Ajabshir Z, Zinatloo-Ajabshir S. Preparation and characterization of curcumin niosomal nanoparticles via a simple and eco-friendly route. Journal of Nanostructures. 2019;9(4):784–790. doi: 10.22052/JNS.2019.04.020. [DOI] [Google Scholar]
- 1021.Zivyar, N., Bagherzade, G., … M. M.-J. of H., & 2021, undefined. (2021). Evaluation of the green synthesis, characterization and antibacterial activity of silver nanoparticles from corm extract of Crocus sativus var. HaussknechtiiJhpr.Birjand.Ac.Ir, 4, 19–3210.22077/jhpr.2021.3812.1177
- 1022.Zubor, Á. A., Surányi, G., Győri, Z., Borbély, G., & Prokisch, J. (2003). Molecular biological approach of the systematics of Crocus sativus l. and its allies. In I International Symposium on Saffron Biology and Biotechnology (Vol. 650, pp. 85–93)
- 1023.Żuk-Gołaszewska K, Wierzbowska J. Fenugreek: Productivity, nutritional value and uses. Journal of Elementology. 2017;22(3):1067–1080. doi: 10.5601/jelem.2017.22.1.1396. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper
Not applicable