Abstract
Background: Preoperative anemia is an independent risk factor for higher rates of blood transfusion in cardiac surgery. This study aimed to evaluate the effects of intravenous iron sucrose and erythropoietin on transfusion requirements in patients with preoperative iron deficiency anemia (IDA) undergoing on-pump coronary artery bypass graft (CABG) surgery.
Methods: In this open-label, randomized clinical trial, patients with preoperative IDA who were candidates for on-pump CABG were randomized into intervention (iron plus erythropoietin) or control groups. Iron sucrose was administered as a 200 mg intravenous dose and erythropoietin as a 100 IU/kg bolus 1 to 2 days before surgery. The primary outcome was the amount of blood transfusion during the first 4 postoperative days.
Results: The study population consisted of 114 patients. The mean age was 64.11±8.18 years in the intervention group and 63.35±8.70 years in the control group. Twenty-seven patients (47.4%) in the intervention group and 25 (43.9%) in the control group were males. The number of red blood cell units transfused per patient exhibited a significant fall in the intervention group compared with the control group (P˂0.001). The ferritin level showed a significant rise in the intervention group on postoperative day 7 (P=0.027). The length of stay in the intensive care unit and the hospital was significantly lower in the intervention arm (P=0.041 and P=0.006, respectively). No adverse events were reported in both groups.
Conclusion: The use of erythropoietin and iron sucrose 1 to 2 days before surgery significantly decreased the need for blood transfusion in patients with IDA undergoing CABG without any significant adverse events.
Key Words: Anemia, iron-deficiency; Blood transfusion; Coronary artery bypass; Erythropoietin
Introduction
Each year, more than 800 000 patients undergo coronary artery bypass graft (CABG) surgery.1 Preoperative anemia, mainly caused by iron deficiency, is a common condition among candidates for CABG.2 The prevalence of preoperative anemia in cardiac surgery is between 10.0% and 50.0%.3 Preoperative anemia is a modifiable risk factor that can increase mortality4, morbidity, and transfusion requirements.5-8 Furthermore, cardiac surgeries are at high risk for bleeding and transfusion requirements due to the complexity of the procedures, contact with cardiopulmonary bypass pumps, and the pre- or intraoperative use of antithrombotic agents.6
Blood transfusion is associated with adverse outcomes such as acute renal failure, acute lung injury,7 and infection; additionally, it is an independent risk factor for prolonged lengths of stay in the intensive care unit (ICU) and the hospital as well as increased short- and long-term mortality rates. Thus, using strategies for blood preservation aimed at reducing transfusion requirements is an important step to prevent complications.8
According to the international consensus statement on the perioperative management of anemia and iron deficiency, in all surgical procedures with expected blood loss exceeding 500 mL, it is essential to diagnose and treat preoperative anemia. In the time available before most cardiac surgeries, however, effective treatment with oral iron is not feasible. Indeed, preoperative treatment with intravenous iron is more effective and faster than oral iron when the time for surgery is within 6 weeks.9
Recently, Spahn et al10 showed the beneficial efficacy of an ultra-short-term combination treatment of intravenous iron and erythropoietin in lessening blood transfusion requirements in patients with preoperative anemia undergoing elective cardiac surgery. The safety and efficacy of erythropoietin in conjunction with iron have been demonstrated in anemic patients undergoing valvular heart surgery.11
In the present study, we sought to evaluate the effects of preoperative intravenous iron and intravenous bolus erythropoietin on transfusion requirements in patients with preoperative iron deficiency anemia (IDA) undergoing on-pump CABG.
Methods
This prospective, randomized, single-center trial was performed in Tehran Heart Center, Tehran, Iran, from May 2019 through March 2020, on patients with IDA undergoing on-pump CABG. The trial protocol was approved by the Ethics Committee of Tehran University of Medical Sciences (approval code: IR.TUMS.tips.REC.1398.027). The trial was registered at the Iranian Registry of Clinical Trials (IRCT20190121042447N1) in November 2019. Written informed consent was obtained from the study participants after they had received verbal explanations about the study.
The study enrolled 114 adult patients with preoperative IDA who were candidates for on-pump CABG. According to the World Health Organization, the criterion for the diagnosis of anemia is a hemoglobin concentration level of less than 12 g/dL in women and less than 13 g/dL in men.11 In the current investigation, iron deficiency was defined as a ferritin level of less than 30 μg. In addition, if the ferritin level was between 30 μg and 100 μg, the transferrin iron-binding capacity had to be less than 20.0%, and/or C-reactive protein had to be more than 0.5 mg/dL.9 Eligible patients were randomized to either the intervention or the control group via permuted block randomization.
The exclusion criteria consisted of preexisting uncontrolled hypertension (systolic blood pressure>180 mmHg), a platelet count of more than 450000/mm3, a history of thromboembolism, a history of seizure, malignancies, liver dysfunction (liver function test≥3 times the upper limit of normal), renal impairment (serum creatinine≥2 mg/dL), hypersensitivity to iron, and ongoing bleeding.
The intervention group received 200 mg of iron sucrose (Venofer, Vifor Pharma, Ltd, Switzerland) in 200 mL of a normal saline infusion in 30 minutes plus 100 IU/kg of erythropoietin (Pooyesh Darou, Iran) via intravenous bolus administration at 1 to 2 days before surgery. The control group received no additional intervention apart from the standard treatment.
The medications were administered by nurses who were not involved in the study, and the surgery team was blinded to the patients’ groups until the end of the study.
The primary outcome was a comparison in terms of the mean number of units of packed red blood cells (RBCs), fresh frozen plasma (FFP), and platelets transfused per patient between the surgical time and the fourth postoperative day. The secondary outcomes were composed of the adverse events associated with injection, the patterns of hemoglobin concentration changes between the surgical time and postoperative day 7, the pattern of changes in the iron profile, and the incidence of postoperative complications.
The transfusion threshold was a hemoglobin concentration of less than 8 mg/dL after CABG and less than 7 mg/dL during surgery. Additionally, liberal transfusions were considered regardless of the hemoglobin concentration in patients with hemodynamic instability.
Preoperative data encompassed demographic characteristics, past medical history (including diabetes mellitus, hypertension, dyslipidemia, chronic renal failure, cerebrovascular accidents, congestive heart failure, chronic obstructive pulmonary disease, and myocardial infarction), and medications. Intraoperative data comprised the duration of aortic cross-clamping and cardiopulmonary bypass. In addition, the amounts of heparin, protamine, and tranexamic acid administered during surgery were recorded for evaluation. Postoperative data were comprised of the amounts of packed-RBCs, FFP, and platelets transfused until postoperative day 4 and the hemoglobin concentration until postoperative day 7, as well as the iron profile (ie, the serum iron concentration, the total iron-binding capacity, the ferritin concentration, and transferrin saturation) measured preoperatively and on postoperative day 7.
Other parameters evaluated included the incidence of acute kidney injury in the first 48 postoperative hours; atrial fibrillation, infection, liver impairment, and coagulopathy in the first 7 postoperative days; the length of stay in the ICU and the hospital; and the rate of in-hospital mortality.
Acute kidney injury was defined according to The Kidney Disease: Improving Global Outcomes (KDIGO) guideline as an elevation in serum creatinine by at least 0.3 mg/dL or a rise in serum creatinine to at least 1.5 times the baseline value.12 Surgical mortality was defined as all deaths occurring during the hospital stay. Liver impairment was considered an increase in the liver function test (ALT and AST) exceeding 3 times the upper normal limit. Postoperative infection was defined as any kind of infection occurring within 30 days of surgery, likely related to the operation itself or the postoperative course. Coagulopathy was considered a platelet count below 50000/m3 or an international normalized ratio above 1.5 or a partial thromboplastin time at least twice the control value.
Continuous variables were presented as the mean with the standard deviation (SD) for normally distributed data. The Student t test was used for between-group comparisons. Nonparametric variables were reported as the median with 25th and 75th percentiles. The Mann–Whitney U test was employed to compare the intervention and control groups. In addition, the χ2 test was applied to compare categorical variables, which were expressed as frequencies with percentages. All the statistical analyses were conducted using IBM SPSS Statistics for Windows, version 23.0 (Armonk, NY: IBM Corp).
Results
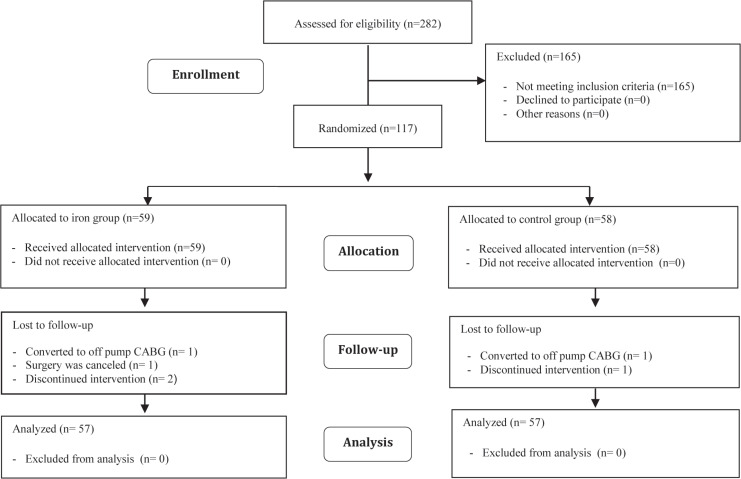
In this study, 282 patients were assessed for eligibility, and 165 patients were excluded: 127 patients did not have IDA, 20 patients with serum creatinine levels exceeding 2 mg/dL, 3 patients with liver function test values exceeding 3 times the upper normal limit, 5 patients with a history of malignancy, and 10 patients with ongoing bleeding. Of the remaining 117 patients, 59 were allocated to the iron group and 58 were allocated to the control group. One patient from each group was converted to off-pump revascularization. Surgery was canceled for 1 patient in the intervention group (Figure 1).
Figure 1.
The image depicts the CONSORT flow chart of patient enrollment in the present study.
CABG, Coronary artery bypass graft surgery
Of 114 patients, 62 (54.4%) were females. As is shown in Table 1, both groups had similar age, sex, history of diabetes mellitus, hypertension, and dyslipidemia. Laboratory data before surgery were similar in both groups (Table 2). The mean ejection fraction was 42.81±10.44% in the intervention group and 41.49±8.96% in the control group (P=0.472). Surgical data, including the duration of aortic cross-clamping and cardiopulmonary bypass, were similar in the intervention and control groups (Table 3).
Table 1.
Comparison of the baseline characteristics and drug history between the iron and control groups*
| Control Group (n=57) | Iron Group (n=57) | P | |
|---|---|---|---|
| Age (y) | 64.11±8.18 | 63.35±8.70 | 0.634 |
| Sex (male) | 27 (47.4) | 25 (43.9) | 0.707 |
| Dyslipidemia | 33 (57.9) | 28 (49.1) | 0.348 |
| MI | 11 (19.3) | 5 (8.8) | 0.106 |
| Hypertension | 35 (61.4) | 34 (59.6) | 0.848 |
| Diabetes mellitus | 36 (63.2) | 34 (59.6) | 0.700 |
| Heart failure | 25 (43.9) | 17 (29.8) | 0.120 |
| CKD | 8 (14.0) | 10 (17.5) | 0.607 |
| COPD | 3 (5.3) | 0 | 0.243 |
| CVA | 3 (5.3) | 5 (8.8) | 0.716 |
| ACEI/ARB | 39 (68.4) | 38 (66.7) | 0.841 |
| β-blocker | 49 (86.0) | 48 (84.2) | 0.793 |
| CCB | 9 (15.8) | 16 (28.1) | 0.113 |
MI, Myocardial infarction; CKD, Chronic kidney disease; COPD, Chronic obstructive pulmonary disease; CVA, Cerebrovascular accidents; ACEI, Angiotensin-converting enzyme inhibitor; ARB, Angiotensin receptor block; CCB, Calcium channel blocker
Data are presented as mean±SD or n (%).
Table 2.
Comparison of preoperative laboratory and echocardiography data between the iron and control groups*
| Control Group (n=57) | Iron Group (n=57) | P | |
|---|---|---|---|
| Serum creatinine (mg/dL) | 1 (0.9-1.2) | 1.08 (0.9-1.3) | 0.230 |
| BUN (mg/dL) | 40.0 (29.0-48.0) | 37.0 (30.0-53.0) | 0.616 |
| MCV (fl) | 82.73±6.61 | 81.88±6.15 | 0.476 |
| Platelet ×)1000/cumm) | 243.25±67.04 | 243.95±61.39 | 0.954 |
| LFT (normal) | 55 (96.5) | 55 (96.5) | 0.999 |
| Hemoglobin (g/dL) | 11.64±1.08 | 11.72±1.03 | 0.673 |
| Iron (µg/dL) | 45.04±17.03 | 46.96±15.65 | 0.530 |
| TIBC (µg/dL) | 306.11±59.51 | 300.11±52.26 | 0.569 |
| TSAT (%) | 14.48±4.82 | 15.24±4.45 | 0.386 |
| Ferritin (µg/L) | 73 (38.0-148.0) | 86 (30.0-187.0) | 0.762 |
| CRP (mg/dL) | 0.8 (0.3-1.4) | 0.36 (0.2-2.4) | 0.116 |
| Ejection fraction (%) | 41.49±8.96 | 42.81±10.44 | 0.472 |
BUN, Blood urea nitrogen; MCV, Mean corpuscular volume; LFT, Liver function test; TIBC, Total iron-binding capacity; TSAT, Transferrin saturation; CRP, C-Reactive protein
Data are presented as mean±SD, n (%), or median (IQR25-75%).
Table 3.
Comparison of intraoperative data between the iron and control groups*
| Control Group (n=57) | Iron Group (n=57) | P | |
|---|---|---|---|
| CCT (min) | 42 (30.0-51.0) | 44 (33.0-57.0) | 0.200 |
| CPB (min) | 70 (55.0-90.0) | 70 (57.0-100.0) | 0.642 |
| Heparin (number of ampules) | 8 (7.0-10.0) | 8 (6.0-9.0) | 0.135 |
| Protamine (number of ampules) | 8 (7.0-8.0) | 8 (6.0-8.0) | 0.056 |
| Tranexamic Acid (number of ampules) | 7.09±3.40 | 5.81±1.82 | 0.014 |
CCT, Cross-clamp time; CPB, Cardiopulmonary bypass
Data are presented as mean±SD or median (IQR25-75%).
The amounts of heparin and protamine administered during surgery were similar in both groups, but the amount of tranexamic acid administered was significantly lower in the intervention group (P=0.014; 95% CI: 0.51–1.28).
The mean number of units of RBCs transfused per patient showed a significant drop in the intervention group compared with the control group (2.56±1.35 vs 1.53±1.04; P˂0.001; 95% CI: 0.58–1.48).
Postoperative serum iron concentrations, total iron-binding capacity, and transferrin saturation were similar in both groups, but the ferritin level was significantly higher in the intervention group (P=0.027; 95% CI, 0.28–0.48) (Table 4).
Table 4.
Comparison of the postoperative iron profile between the iron and control groups*
| Control Group (n=57) | Iron Group (n=57) | P | |
|---|---|---|---|
| Iron (μg /dL) | 26 (22.0-40.0) | 25 (21.0-43.0) | 0.977 |
| TIBC (μg/dL) | 231.39±54.60 | 226.79±55.40 | 0.656 |
| TSAT (%) | 12.7 (10.0-15.7) | 12 (9.0-18.2) | 0.901 |
| Ferritin (μg/L) | 226 (145.0-302.0) | 251 (174.0-468.0) | 0.027 |
TIBC, Total iron-binding capacity; TSAT, transferrin saturation
Data are presented as mean±SD or median (IQR25-75%).
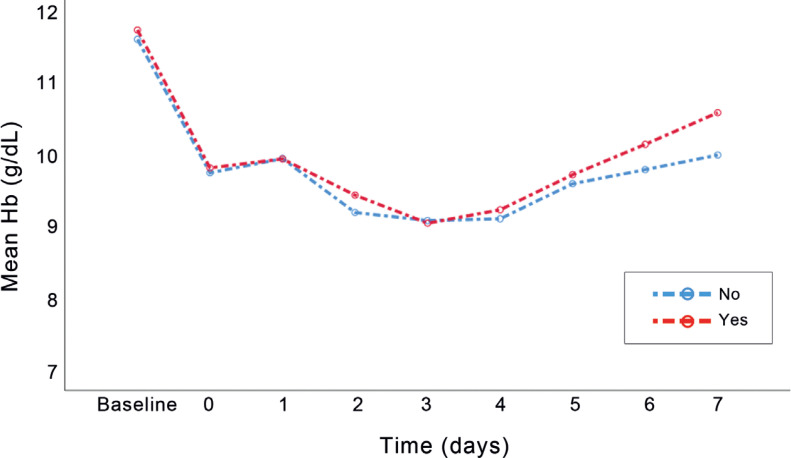
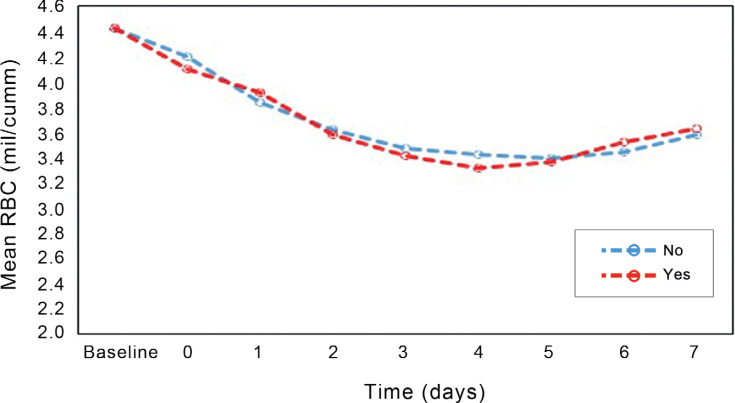
The effect of time on the increase in the hemoglobin and RBC concentration was significant; still, the average change in the concentration of hemoglobin in the iron group exhibited a slight rise, which was not significantly different (Figures 2 & 3). Changes in hemoglobin concentrations and RBCs were consistent between the groups (Table 5).
Figure 2.
The figure presents changes in the mean hemoglobin concentration over time in the iron and control groups.
Figure 3.
The diagram illustrates changes in the mean red blood cells over time in the iron and control groups.
Table 5.
Comparison of changes in the mean hemoglobin concentration and the mean number of red blood cells between the iron and control groups in POD days*
| Control Group (n=57) | Iron Group (n=57) | P | |
|---|---|---|---|
| Hemoglobin (g/dL) (base) | 11.62±1.08 | 11.75±1.01 | 0.673 |
| Hemoglobin (g/dL) (POD0) | 9.75±1.39 | 9.82±1.43 | 0.435 |
| Hemoglobin (g/dL) (POD1) | 9.95±1.23 | 9.95±1.05 | 0.852 |
| Hemoglobin (g/dL) (POD2) | 9.20±1.08 | 9.44±1.25 | 0.276 |
| Hemoglobin (g/dL) (POD3) | 9.09±1.08 | 9.05±1.19 | 0.744 |
| Hemoglobin (g/dL) (POD4) | 9.11±0.89 | 9.24±1.07 | 0.273 |
| Hemoglobin (g/dL) (POD5) | 9.60±0.92 | 9.73±0.88 | 0.467 |
| Hemoglobin (g/dL) (POD6) | 9.80±0.93 | 10.15±1.40 | 0.323 |
| Hemoglobin (g/dL) (POD7) | 10.00±1.00 | 10.59±1.41 | 0.008 |
| RBC (mil/cumm) (base) | 4.42±0.52 | 4.42±0.42 | 0.932 |
| RBC (mil/cumm) (POD0) | 4.21±0.51 | 4.10±0.41 | 0.715 |
| RBC (mil/cumm) (POD1) | 3.83±0.54 | 3.91±0.38 | 0.089 |
| RBC (mil/cumm) (POD2) | 3.61±0.55 | 3.57±0.47 | 0.952 |
| RBC (mil/cumm) (POD3) | 3.46±0.47 | 3.40±0.47 | 0.519 |
| RBC (mil/cumm) (POD4) | 3.42±0.49 | 3.31±0.52 | 0.180 |
| RBC (mil/cumm) (POD5) | 3.39±0.46 | 3.36±0.50 | 0.812 |
| RBC (mil/cumm) (POD6) | 3.44±0.36 | 3.51±0.49 | 0.407 |
| RBC (mil/cumm) (POD7) | 3.57±0.32 | 3.62±0.42 | 0.553 |
POD, Postoperative day; RBC, Red blood cell
Data are presented as mean±SD.
The incidence of acute kidney injury, atrial fibrillation, liver impairment, and coagulopathy was not significantly different between the groups; the incidence of postoperative infection was, however, significantly lower in the intervention group (P=0.018; 95% CI: 0.34–0.48) (Table 6).
Table 6.
Comparison of the postoperative outcome between the intervention and control groups*
| Control Group (n=57) | Iron Group (n=57) | P | |
|---|---|---|---|
| AKI | 13 (22.8) | 9 (15.8) | 0.342 |
| Liver impairment | 3 (5.3) | 1 (1.8) | 0.618 |
| Infection | 16 (28.1) | 6 (10.5) | 0.018 |
| Coagulopathy | 11 (19.3) | 6 (10.5) | 0.189 |
| AF | 9 (15.8) | 8 (14.0) | 0.793 |
| ICU duration | 3 (2.0-5.0) | 2 (2.0-4.0) | 0.041 |
| Hospital | 12 (10.0-16.0) | 10 (9.0-12.0) | 0.006 |
| Death (%) | 5 (8.8) | 5 (8.8) | 0.999 |
AKI, Acute kidney injury; AF, Atrial fibrillation; ICU, Intensive care unit
Data are presented as n (%) or median (IQR25-75%).
The median (IQR25–75%) length of hospital stay was 10 (IQR25–75%: 9.00–12.00) days in the intervention group and 12 (IQR25–75%: 10.00–16.00) days in the control group (P=0.006; 95% CI, 0.26–0.45). The median (IQR25–75%) length of stay in the ICU was 2 (IQR25–75%: 2.00–4.00) days in the intervention group and 3 (IQR25–75%: 2.00–5.00) days in the control group (P=0.041, 95% CI: 0.30–0.49). The mortality rate was similar in both groups. There were no reports of hypersensitivity reactions to iron or erythropoietin in the intervention group.
Discussion
Cardiac surgery is associated with blood loss, decreased hematopoiesis, and postoperative anemia. Blood transfusion is recognized as a primary solution for patient blood management. About 20% of transfusions are for cardiac surgery.13 Nonetheless, not only is blood transfusion associated with adverse outcomes such as acute renal failure, acute lung injury,7 and infection but also it is an independent risk factor for prolonged lengths of stay in the ICU and the hospital, as well as short- and long-term mortality rates.8
Preoperative anemia is common in patients undergoing cardiac surgery.2 Multiple studies have shown that preoperative anemia is associated with more blood transfusion and adverse clinical outcomes such as increased ICU and hospital lengths of stay and high mortality.8
Iron plays an important role in erythropoiesis and hemoglobin synthesis, and erythropoietin requires adequate iron stores for the production of new RBCs.14 On the other hand, the absorption of iron is decreased by inflammation, and 150 mg of stored iron is required to increase hemoglobin levels by 1 g/dL. Accordingly, in the present study, we used 200 mg of iron intravenously, similar to the study by Yoo et al.11
A salient difference between our study and previous investigations is the type of surgery. Several studies have shown that the amount of bleeding after off-pump surgery is low by comparison with on-pump surgery.6 We excluded both off-pump CABG and CABG with concomitant valve surgery because the amount of bleeding differs based on the type of surgery and we sought a lesser heterogeneity. Thus, to the best of our knowledge, we are the first to evaluate the efficiency of iron and erythropoietin in reducing blood transfusion in on-pump CABG.
In this clinical trial, we observed a statistically significant reduction in the mean number of RBC units transfused per patient between the surgical time and the fourth postoperative day in the intervention group. In line with our study, a meta-analysis of 11 randomized controlled trials showed that the rate of allogeneic blood transfusion was decreased with the administration of erythropoietin before cardiac surgery.15
Weltert et al14 assessed 320 patients undergoing off-pump CABG and compared the effects of a preoperative high-dose erythropoietin treatment protocol (cumulative dose=52000 IU) and a placebo starting 2 days before and ending 2 days after surgery. Postoperative transfusion requirements were lower in the erythropoietin group than in the placebo group (0.33 vs 0.76 units per patient; P=0.008).
Yoo et al11 evaluated 74 patients with heart valve replacement and reported that a single administration of 500 IU/kg of erythropoietin and 200 mg of iron sucrose 1 day before surgery reduced the RBC transfusion rate (from 86% to 59%) and the number of transfused RBCs (3±2.2 to 1.0±1.1 RBC units per patient).
Spahn et al10 demonstrated that ultra-short-term treatment with a combination of iron, erythropoietin, vitamin B12, and folic acid in 505 patients with iron deficiency or anemia undergoing cardiac surgery significantly lessened RBC transfusions and augmented hemoglobin concentrations in the first 7 days.
There are some concerns regarding the safety of erythropoietin. Recent studies have mentioned that the long-term, high dose, and repeated use of erythropoietin leads to thromboembolism events, hypertension, and other complications,16 which prompted us to prescribe 100 IU/kg of erythropoietin 1 to 2 days before surgery in the current investigation. We also administered erythropoietin via intravenous route due to microcirculation and generalized atherosclerotic condition in cardiac surgery, causing the subcutaneous absorption of erythropoietin.11
Chiming in with a previous study,17 we showed that lower doses of erythropoietin exerted a significant effect on reducing blood transfusion requirements, with no thromboembolic events.
Ferritin levels tend to reach the peak level between 7 and 9 days after iron administration. We also demonstrated a statistically significantly higher elevation in ferritin concentrations on postoperative day 7 in the iron group compared with the control group, suggesting that perioperative intravenous iron supplementation maintains iron stores after cardiac surgery.
In agreement with previous studies,18 our results showed that cardiac surgery caused changes in iron metabolism, including increased serum ferritin concentrations and decreased serum iron concentrations and transferrin saturation. Indeed, despite intravenous iron and erythropoietin administration 1 to 2 days before surgery, the serum iron concentration and transferrin saturation decreased in both intervention and control groups, which may have been a consequence of the rapid clearance of iron during increased erythropoiesis. Notably, a single RBC unit contains approximately 200 to 250 mg of iron.19 According to our results, the amount of RBC transfusion was significantly higher in the control group, which is concordant with a previous study reporting that transfusion significantly increased serum iron concentrations.20
According to Waltert et al,13 the administration of erythropoietin in cardiac patients increased hemoglobin concentrations 4 days after surgery. We observed a rise in the concentration of hemoglobin in the treatment group 5 days after surgery, although the change was not significant compared with the control group. The discrepancy in the results could be because of the lower dose of erythropoietin and the type of surgery (on-pump) in our investigation, as well as the fact that the patients in their study had higher baseline hemoglobin concentrations than did our patients.
Tranexamic acid is widely used to reduce bleeding and blood transfusion in cardiac surgery.21 In our study, intraoperative tranexamic acid administration was significantly lower in the iron group, which may indicate less bleeding in the intervention group.
Earlier studies have reported that erythropoietin has protective properties in the kidney against ischemia/reperfusion injury and, thus, reduces postoperative acute kidney injury.11-22 We had fewer patients who developed acute kidney injury, but the difference from previous investigations is not statistically significant. A reason for the lower number of patients developing acute kidney injury in the current study could be our use of a low erythropoietin dose by comparison with the aforementioned studies.
Various studies have concluded that preoperative anemia, as well as blood transfusion, is a risk factor for postoperative infection.5 There is also concern over intravenous iron insofar as it may increase the amount of free iron in the body, which plays a role in the growth of microorganisms and may lead to increased infection.4 In contrast to a similar study,14 our results showed that iron therapy not only did not increase the rate of infection but also, by effectively reducing blood transfusion, led to a significant decline in infection compared with the control group.
Similar to a study by Cladellas et al,14 the length of stay in the hospital and the ICU in our study was significantly shorter in the patients who received iron and erythropoietin than in the control group. This is a valuable finding because although these drugs are expensive, shortening the length of hospital stay may make the combination cost-effective.
The rate of mortality was similar in both of our studied groups. Nonetheless, this rate is higher than that reported by Yoo et al.11 This dissimilarity is justifiable because whereas Yoo and colleagues evaluated 30-day mortality, we assessed mortality until hospital discharge, which is known to be higher.
Short follow-up periods and the non-measurement of inflammatory factors after surgery could be mentioned as the limitations of our study.
Conclusion
Our result demonstrated that the administration of a single dose of erythropoietin and intravenous iron sucrose 1 to 2 days before surgery significantly decreased the need for blood transfusion and the length of stay in the ICU and the hospital stay in patients with iron deficiency anemia undergoing on-pump CABG. We, therefore, conclude that this protocol could be a safe and effective intervention for patient blood management in cardiac surgery.
Acknowledgments
We hereby appreciate the assistance of all the participants in this study and all the staff members of Tehran Heart Center. This study was approved and supported by Tehran University of Medical Sciences, Tehran, Iran.
Notes:
This paper should be cited as: Jafari S, Talasaz AH, Salehiomran A, Ariannejad H, Jalali A. Effects of Iron Sucrose and Erythropoietin on Transfusion Requirements in Patients with Preoperative Iron Deficiency Anemia Undergoing on-Pump Coronary Artery Bypass Graft. J Teh Univ Heart Ctr 2022;17(1):7-14.
References
- 1.Johansson PI, Rasmussen AS, Thomsen LL. Intravenous iron isomaltoside 1000 (Monofer®) reduces postoperative anaemia in preoperatively non-anaemic patients undergoing elective or subacute coronary artery bypass graft, valve replacement or a combination thereof: a randomized double-blind placebo-controlled clinical trial (the PROTECT trial) Vox Sang. 2015;109:257–266. doi: 10.1111/vox.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein AA, Chau M, Yeates JA, Collier T, Evans C, Agarwal S, Richards T. UK Cardiac and Vascular Surgery Interventional Anaemia Response (CAVIAR) study team. Preoperative intravenous iron before cardiac surgery: a prospective multicentre feasibility study. Br J Anaesth. 2020;124:243–250. doi: 10.1016/j.bja.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Meybohm P, Westphal S, Ravn HB, Ranucci M, Agarwal S, Choorapoikayil S, Spahn DR, Ahmed AB, Froessler B, Zacharowski K. Perioperative anemia management as part of PBM in cardiac surgery - a narrative updated review. J Cardiothorac Vasc Anesth. 2020;34:1060–1073. doi: 10.1053/j.jvca.2019.06.047. [DOI] [PubMed] [Google Scholar]
- 4.Ranucci M, Di Dedda U, Castelvecchio S, Menicanti L, Frigiola A, Pelissero G, Surgical Clinical Outcome Research (SCORE) Group. Impact of preoperative anemia on outcome in adult cardiac surgery: a propensity-matched analysis. Ann Thorac Surg. 2012;94:1134–1141. doi: 10.1016/j.athoracsur.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 5.Padmanabhan H, Siau K, Curtis J, Ng A, Menon S, Luckraz H, Brookes MJ. Preoperative anemia and outcomes in cardiovascular surgery: systematic review and meta-analysis. Ann Thorac Surg. 2019;108:1840–1848. doi: 10.1016/j.athoracsur.2019.04.108. [DOI] [PubMed] [Google Scholar]
- 6.Pagano D, Milojevic M, Meesters MI, Benedetto U, Bolliger D, von Heymann C, Jeppsson A, Koster A, Osnabrugge RL, Ranucci M, Ravn HB, Vonk ABA, Wahba A, Boer C. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53:79–111. doi: 10.1093/ejcts/ezx325. [DOI] [PubMed] [Google Scholar]
- 7.Bux J. Transfusion-Related Acute Lung Injury (TRALI): a serious adverse event of blood transfusion. Vox Sang. 2005;89:1–10. doi: 10.1111/j.1423-0410.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 8.Lim J, Miles L, Litton E. Intravenous iron therapy in patients undergoing cardiovascular surgery: a narrative review. J Cardiothorac Vasc Anesth. 2018;32:1439–1451. doi: 10.1053/j.jvca.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz M, Acheson AG, Auerbach M, Besser M, Habler O, Kehlet H, Liumbruno GM, Lasocki S, Meybohm P, Rao Baikady R, Richards T, Shander A, So-Osman C, Spahn DR, Klein AA. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia. 2017;72:233–247. doi: 10.1111/anae.13773. [DOI] [PubMed] [Google Scholar]
- 10.Spahn DR, Schoenrath F, Spahn GH, Seifert B, Stein P, Theusinger OM, Kaserer A, Hegemann I, Hofmann A, Maisano F, Falk V. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet. 2019;393:2201–2212. doi: 10.1016/S0140-6736(18)32555-8. [DOI] [PubMed] [Google Scholar]
- 11.Yoo YC, Shim JK, Kim JC, Jo YY, Lee JH, Kwak YL. Effect of single recombinant human erythropoietin injection on transfusion requirements in preoperatively anemic patients undergoing valvular heart surgery. Anesthesiology. 2011;115:929–937. doi: 10.1097/ALN.0b013e318232004b. [DOI] [PubMed] [Google Scholar]
- 12.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 13.Weltert L, D'Alessandro S, Nardella S, Girola F, Bellisario A, Maselli D, De Paulis R. Preoperative very short-term, high-dose erythropoietin administration diminishes blood transfusion rate in off-pump coronary artery bypass: a randomized blind controlled study. J Thorac Cardiovasc Surg. 2010;139:621–626. doi: 10.1016/j.jtcvs.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Cladellas M, Farré N, Comín-Colet J, Gómez M, Meroño O, Bosch MA, Vila J, Molera R, Segovia A, Bruguera J. Effects of preoperative intravenous erythropoietin plus iron on outcome in anemic patients after cardiac valve replacement. Am J Cardiol. 2012;110:1021–1026. doi: 10.1016/j.amjcard.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 15.Alghamdi AA, Albanna MJ, Guru V, Brister SJ. Does the use of erythropoietin reduce the risk of exposure to allogeneic blood transfusion in cardiac surgery? A systematic review and meta-analysis. J Card Surg. 2006;21:320–326. doi: 10.1111/j.1540-8191.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- 16.Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, Song HK, Clough ER, Shore-Lesserson LJ, Goodnough LT, Mazer CD, Shander A, Stafford-Smith M, Waters J, Baker RA, Dickinson TA, FitzGerald DJ, Likosky DS, Shann KG Society of Thoracic Surgeons Blood Conservation Guideline Task Force; Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion; International Consortium for Evidence Based Perfusion. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–982. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 17.Yazicioğlu L, Eryilmaz S, Sirlak M, Inan MB, Aral A, Taşöz R, Eren NT, Kaya B, Akalin H. Recombinant human erythropoietin administration in cardiac surgery. J Thorac Cardiovasc Surg. 2001;122:741–745. doi: 10.1067/mtc.2001.115426. [DOI] [PubMed] [Google Scholar]
- 18.van Iperen CE, Kraaijenhagen RJ, Biesma DH, Beguin Y, Marx JJ, van de Wiel A. Iron metabolism and erythropoiesis after surgery. Br J Surg. 1998;85:41–45. doi: 10.1046/j.1365-2168.1998.00571.x. [DOI] [PubMed] [Google Scholar]
- 19.Remacha A, Sanz C, Contreras E, De Heredia CD, Grifols JR, Lozano M, Nuñez GM, Salinas R, Corral M, Villegas A Spanish Society of Blood Transfusion; Spanish Society of Haematology and Haemotherapy. Guidelines on haemovigilance of post-transfusional iron overload. Blood Transfus. 2013;11:128–139. doi: 10.2450/2012.0114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boshuizen M, van Hezel ME, van Manen L, Straat M, Somsen YBO, Spoelstra-de Man AME, Blumberg N, van Bruggen R, Juffermans NP. The effect of red blood cell transfusion on iron metabolism in critically ill patients. Transfusion. 2019;59:1196–1201. doi: 10.1111/trf.15127. [DOI] [PubMed] [Google Scholar]
- 21.Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T, Cooper DJ, Marasco S, McNeil J, Bussières JS, McGuinness S, Byrne K, Chan MT, Landoni G, Wallace S ATACAS Investigators of the ANZCA Clinical Trials Network. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2017;376:136–148. doi: 10.1056/NEJMoa1606424. [DOI] [PubMed] [Google Scholar]
- 22.Paschos N, Lykissas MG, Beris AE. The role of erythropoietin as an inhibitor of tissue ischemia. Int J Biol Sci. 2008;4:161–168. doi: 10.7150/ijbs.4.161. [DOI] [PMC free article] [PubMed] [Google Scholar]