Version Changes
Revised. Amendments from Version 1
We implemented the changes suggested by the reviewers. -Added a paragraph on current treatment guidelines for RA -Elaborated on the selection/inclusion criteria for articles included in the systematic review and discussion -Edited the conclusion to make it more concise -Corrected errors in dates and used full-forms for various acronyms
Abstract
Background: Rheumatoid arthritis (RA) is a highly prevalent, chronic inflammatory condition of the synovial joints that affects approximately 1% of the global population. The pathogenesis of RA is predominantly inflammatory in nature, thereby accelerating the co-occurrence of other immunoinflammatory conditions such as atherosclerosis. Apart from traditional cardiovascular risk factors, RA patients possess a multitude of other factors that predispose them to early atherosclerotic disease. The aim of this systematic review is to assess the prevalence of premature atherosclerosis in RA patients and elucidate the role that proinflammatory cytokines, RA-related autoantibodies, and endothelial dysfunction play in the pathophysiology of RA-mediated atherosclerosis. We also discussed novel biomarkers that can be used to predict early atherosclerosis in RA and current guidelines used to treat RA.
Methods: This review followed the PRISMA guidelines to select and analyze relevant articles. A literature search for articles was performed on February 25, 2022, through three research databases including PubMed, ProQuest, and ScienceDirect. The query used to identify relevant publications was “Rheumatoid arthritis and atherosclerosis” and the search duration was set from 2012-2022. Relevant articles were selected based on the inclusion and exclusion criteria.
Results: Our initial search generated 21,235 articles. We narrowed our search according to the inclusion and exclusion criteria. After assessing eligibility based on the full content of the articles, 73 articles were ultimately chosen for this review.
Conclusion: There is an increased prevalence of accelerated atherosclerosis among RA patients. We found evidence to explain the role of proinflammatory cytokines, RA-related autoantibodies, and endothelial dysfunction in the pathophysiology RA-mediated atherosclerosis. Therapies targeting either the inflammatory load or traditional CV risk-factors seem to improve vascular outcomes in RA patients. Novel markers of atherosclerosis in RA may be useful in predicting premature atherosclerosis and serve as new targets for therapeutic intervention.
Keywords: Rheumatoid arthritis, atherosclerosis, atherogenesis, premature, pathophysiology, inflammation, cytokines, endothelial dysfunction, autoantibodies
Introduction and background
Rheumatoid arthritis (RA) is an autoimmune disorder, often described as a debilitating condition, that severely impairs quality of life by causing extra-articular manifestations. 1 – 3 RA affects approximately 1% of the global population and poses a significant societal and economic burden in terms of cost and disability. 4 , 5 The incidence of comorbidities such as atherosclerosis-related cardiovascular diseases, lung cancer, osteoporosis, and depression are higher among individuals with RA, making it a multisystem disease. 6 RA is described as a chronically progressive inflammatory condition that affects the synovial lining of joints in the fingers, wrists, feet, and ankles. 1 Since the pathological mechanism that lead to RA is predominantly inflammatory in nature, it facilitates the co-occurrence of other immunoinflammatory conditions such as atherosclerosis. 7 , 8 Atherosclerosis refers to the hardening of an artery due to the buildup of fatty, cholesterol-rich plaque within the intimal lining of the vessel wall. 8 , 9 The pathophysiological link between RA and atherosclerosis has its roots in complex inflammatory pathways that interconnect the two conditions and serve as an explanation for the increased cardiovascular morbidity in RA patients. 10 , 11 Tumor necrosis factor alpha (TNF-α), is a proinflammatory cytokine that is highly elevated in the synovial fluid of individuals with RA. 12 , 13 TNF-α, along with interleukin-6 (IL-6), promotes the accumulation of oxidized low density lipoprotein (oxLDL) within the vessel wall, which directly contributes to the formation of lipid-laden macrophages, also known as foam cells. 13 – 15 Macrophagic foam cells are considered to be the prototypical cells involved in the development of atherosclerotic plaques. 15 , 16 Interleukin-1 (IL-1) is another cytokine associated with RA that shares its proinflammatory properties with TNF-α as they both upregulate the expression of adhesion molecules on vascular endothelial surfaces, stimulate cytokine production, and induce the expression of proinflammatory genes, all of which favor the initiation of atherogenesis. 17 – 20
The role of neutrophil extracellular traps (NETs) in the pathogenesis of RA has been increasingly gaining attention. 21 NETs are a complex network of granular proteins, nuclear chromatin, and extracellular fibers that eliminate pathogens through the activation of the ROS-mediated suicidal NETosis pathway. 21 – 23 The role of neutrophils in atherogenesis has been historically denied, but recent evidence shows that neutrophils are involved in progressive endothelial damage, recruitment of proinflammatory monocytes, and foam cell formation, thus implicating them in the process of atherosclerosis. 24 – 26 The presence of citrullinated proteins within the synovia of patients with RA has been recognized as a target for anti-citrullinated peptide antibodies (ACPAs). 27 , 28 Citrullinated fibrinogen within atherosclerotic plaques have also shown to be targeted by RA-derived ACPAs, contributing to the development of atherosclerosis in the setting of RA. 27 , 29 It is well known that atherosclerosis is a consequence of progressive endothelial damage and dysfunction. 30
There is ample evidence to support the presence of both micro- and macrovascular endothelial dysfunction in RA which helps to strengthen the pathophysiological link between the two entities. 31 Our systematic review will assess the prevalence of premature atherosclerosis in RA patients and elucidate the role that proinflammatory cytokines, RA-related autoantibodies, and endothelial dysfunction play in the pathophysiology of RA-mediated atherosclerosis through analysis of available literature. We will also discuss carotid intima media thickness, flow mediated dilation, lipoprotein-associated phospholipase A2 enzyme activity, osteocalcin and osteoprotegerin levels as markers of predicting atherosclerosis in RA patients.
Methods
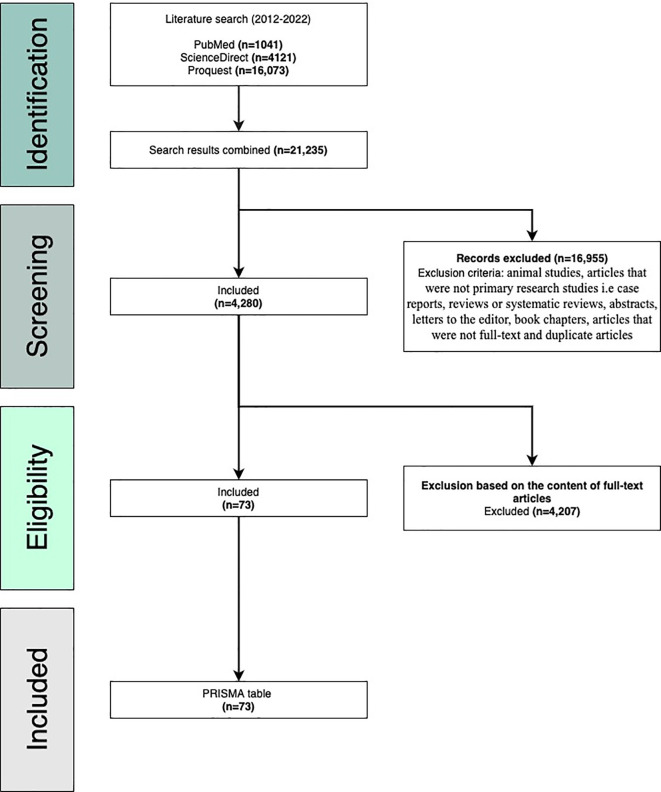
This review strictly follows the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines as this is a widely accepted and validated methodology for choosing relevant publications to be included in systematic reviews. 32 A literature search for articles was performed on February 25, 2022, through three research databases: PubMed, ProQuest, and ScienceDirect. The query used to identify relevant publications was “Rheumatoid arthritis and atherosclerosis” and the search duration was set from 2012–2022. We included primary case–control studies, cohort studies, cross-sectional studies, observational studies, comparative studies, and meta-analyses. Once the search was complete, three co-authors worked independently to screen the results and extract data from each article. We acknowledge that despite our maximum efforts, some relevant articles may have been left off accidently. Our initial search generated 21,235 articles. Using manual screening, we narrowed the search according to the inclusion and exclusion criteria and a total of 73 articles were ultimately included in this systematic review ( Figure 1).
Figure 1. Preferred Reporting Items for Systematic Reviews (PRISMA) flow diagram.
Inclusion criteria
The following inclusion criteria were used: research studies conducted on humans and written in English, studies published in or after 2011, studies relevant to our topic of interest (accelerated atherosclerosis in rheumatoid arthritis), and articles that were full text, peer-reviewed, and primary or original research publications. The articles that were ultimately chosen were manually screened and read before being considered for this review.
Exclusion criteria
The following criteria were used for exclusion: animal studies, articles that were not primary research studies i.e. case reports, reviews or systematic reviews, abstracts, letters to the editor, book chapters, articles published outside of range (2011–2022), and articles not relevant to our review. All duplicates and non-full-text articles were also excluded. This information is visually presented in the PRISMA flow diagram ( Figure 1).
Data items
The information collected from each study included the name of the first author, year of publication, study design, study population, study aim, findings, and conclusion.
Risk of bias in selected studies
Once studies were selected, three co-authors were to grade the risk of bias in individual studies using using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) system. GRADE evaluates study flaws such as bias risk, indirectness, imprecision, and publication bias. Two reviewers would have used these criteria on a study-by-study basis, with a third reviewer assessing and providing an outcome if there was any inconsistency.
Results
Our literature search yielded 21,235 articles: 1,041 from PubMed, 4121 from ScienceDirect, and 16,073 from ProQuest. 16,955 articles were excluded based on the exclusion criteria (animal studies, articles that were not primary research studies i.e. case reports, reviews or systematic reviews, abstracts, letters to the editor, book chapters, articles published outside of range, and articles not relevant to our review). All duplicates and non-full-text articles were also excluded. This resulted in 4,280 articles to be checked for eligibility. After manual screening of full-text articles, 73 articles were considered relevant and included in this review. We acknowledge that some relevant articles may have been accidently left off. Ultimately, there were 37 prospective studies, 10 case-control studies, eight cross-sectional studies, five comparative studies, three observational studies, three prospective observational studies, three meta-analyses, three retrospective studies, and one cohort analysis included in this review. Study characteristics are tabulated in Table 1.
Table 1. Baseline characteristics of the included studies.
| Author | Country | Design & study population | Aim | Findings | Conclusion | |
|---|---|---|---|---|---|---|
| 1 | Ruscitti et al., 2017 33 | Italy | Prospective observational study (n=347) | To investigate the existence of atherosclerosis in RA. | There was a significant increase in the development of subclinical atherosclerosis in RA patients during the follow-up (p<0.00001). | Both chronic inflammation and traditional cardiovascular risk factors are responsible for the occurrence of premature atherosclerosis in RA. |
| 2 | Ambrosino et al., 2015 34 | Italy | Meta-analysis (n=7923) | To assess the impact of RA on cIMT and carotid plaques. | RA patients had a higher common carotid artery intima media thickness (CCA-IMT) (p<0.00001) and a higher prevalence of plaques (p<0.00001) compared with the control group. | RA is associated with subclinical atherosclerosis and regular CVD screening and prevention strategies would benefit RA patients. |
| 3 | Bes et al., 2018 35 | Turkey | Case–control study (n=65) | To evaluate whether Lp-PLA2 activity was associated with atherosclerosis in RA. | Lipoprotein-associated phospholipase A2 (Lp-PLA2) enzyme activity was found to be similar in RA patients and healthy control subjects but lower than in patients with diabetes mellitus (DM) (p=0.006). | There was no association between Lp-PLA2 atherogenic activity and RA when compared to the control group and DM group, possibly because the RA patients were undergoing therapy and had low disease activity scores. |
| 4 | Ruscitti et al., 2019 36 | Italy | Prospective observational study (n=841) | To determine occurrence of atherosclerosis in RA. | RA patients had a significantly increased rate of subclinical and clinical atherosclerosis (p<0.0001). | The long-term vascular outcomes of RA patients can be improved by recognizing the role of inflammation and traditional CVD risk factors in order to develop treatment regimens accordingly. |
| 5 | Verma et al., 2017 37 | India | Prospective study (n=60) | To assess endothelial function in patients with RA. | In RA patients, flow mediated dilatation (FMD) was found to be significantly lower than the control group (p<0.001) whereas cIMT was found to be higher in RA patients compared with controls (p=0.003). | These findings were consistent with endothelial dysfunction and accelerated atherosclerosis in patients with RA. |
| 6 | Hannawi et al., 2020 38 | UAE | Cross-sectional study (n=250) | To assess the occurrence of subclinical atherosclerosis in RA. | Patients with RA had a significantly higher cIMT compared with the control group (p=0.03). | This study concluded that subclinical atherosclerosis is more prevalent in RA patients compared with healthy controls. |
| 7 | Krajnc et al., 2021 39 | Slovenia | Prospective observational study (n=110) | To assess the importance of traditional CV factors and inflammation in RA. | Both plaque formation (p=0.005) and cIMT (p=0.001) was greater in patients with RA compared with the matched control group. | Inflammatory mediators seem to be more significant than traditional CVD risk factors as indicators of the atherosclerotic burden in premenopausal women with RA. |
| 8 | Burggraaf et al., 2018 40 | The Netherlands | Cohort analysis (n=212) | To assess cIMT in RA patients following a treat-to-target intervention and in patients with concomitant MetS. | Treat-to-target intervention resulted in lower progression of cIMT. In RA patients with metabolic syndrome (MetS), carotid intima media thickness (cIMT) was higher than in patients without MetS (p<0.001). | In RA patients without MetS, implementing CVD therapeutic regimens can help curb the progression of atherosclerotic plaques. |
| 9 | Suarez et al., 2019 41 | Mexico | Case–control study (n=209) | To evaluate carotid ultrasound findings in RA patients. | The incidence of bilateral carotid plaque (CP) was more than double in RA patients compared with the control group (15.5% versus 6.6%). | This study confirmed the increased prevalence of RA-related subclinical atherosclerosis. |
| 10 | Ursini et al., 2017 42 | Italy | Meta-analysis (n=346) | To assess whether anti-TNF-α therapy improves endothelial function. | RA patients receiving anti-TNF-α therapy showed a statistically significant improvement of endothelial function (p<0.0001). | Endothelial dysfunction is one of the mechanisms implicated in the increased cardiovascular morbidity in individuals with RA. |
| 11 | Végh et al., 2019 43 | Hungary | Prospective cohort study (n=53) | To assess whether anti-TNF-α therapy improves vascular function in RA patients. | Anti-TNFα treatment showed an improvement in FMD and pulse wave velocity (PWV) in patients with RA during the course of the study (p=0.004 and (p=0.034 respectively). | This shows that TNFα inhibition improved and stabilized vascular-related outcomes in patients with RA. |
| 12 | Pérez-Sánchez et al., 2017 44 | Spain | Case–control study (n=146) | To evaluate the role of NETosis in the development of CVD in RA. | Patients with RA demonstrated enhanced (neutrophil extracellular trap) NET formation (p=0.02). | NETosis-derived products have a role in atherosclerosis and these products could be used to assess therapeutic response in RA patients. |
| 13 | Anghel et al., 2021 45 | Romania | Retrospective observational study (n=115) | To assess the role of anti-TNFα drugs on cIMT. | After 1 year of anti-TNFα drugs, patients with RA showed a significant decrease in cIMT (p<0.001) and homocysteine levels. | This study concluded that biological treatments such as anti-TNF-α drugs are effective in reducing the cardiovascular risk in RA. |
| 14 | Majka et al., 2017 46 | USA | Prospective cohort study (n=6532) | To assess whether RA-related antibodies are independent risk factors for atherosclerosis in RA. | Rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibodies (anti-CCP) were increased in women with RA with coronary artery calcium (CAC) ≥300 (odds ratio 2.4 [95% CI 1.2–5.1] and odds ratio 4.1 [95% CI 1.3–12.7] respectively). | RA-autoantibodies are an independent risk factor for the development and propagation of subclinical and clinical atherosclerosis, indicating their role in atherogenesis in RA patients. |
| 15 | Rodríguez-Carrio et al., 2014 47 | Spain | Prospective study (n=255) | To assess the role of EPC populations in RA. | Long-standing RA was correlated with a reduction in endothelial progenitor cell (EPC) population (p<0.001). | EPC imbalance is associated with an increased risk of CVD in RA and this association may be due to defective endothelial repair. |
| 16 | Nowak et al., 2016 48 | Poland | Observational study (n=61) | To investigate the role of non-traditional risk factors in mediating atherosclerosis in RA. | RA patients had a higher level of oxidized low-density lipoprotein (oxLDL) compared to controls (p=0.04) and the presence of anti-CCP antibodies were associated with a greater cIMT (p=0.04). | The presence of anti-CCP antibodies and the oxLDL fraction can be determinants of CVD risk in RA. |
| 17 | Adawi et al., 2018 49 | Israel | Prospective cohort study (n=57) | To assess endothelial function in patients with RA. | Out of 44 patients with RA, only 6 (13.6%) had normal endothelial function. RF was positive in 26 subjects (59.1%), whereas anti-CCP antibodies were found in 25 (56.8%) patients. | This study acknowledges the presence of accelerated atherosclerosis in RA and proposes the clinical use of FMD% as a measure of endothelial function to predict subsequent atherosclerosis. |
| 18 | Dimitroulas et al., 2017 50 | UK | Prospective study (n=197) | To evaluate whether ADMA and ADMA levels are associated with atherosclerosis in RA. | There was a significant association between acetylcholine (Ach) and both SDMA (p=0.014) and ADMA:SDMA ratio (p=0.027), especially in the group with a higher inflammatory status. | Along with being novel biomarkers of endothelial dysfunction, SDMA and ADMA may also cause endothelial injury in patients with RA due to being mediators of inflammation. |
| 19 | Spinelli et al., 2017 51 | Italy | Case–control study (n=80) | To assess whether anti-CarP antibodies are associated with atherosclerosis in RA. | There was a statistically significant association of anti-carbamylated protein antibodies (anti-CarP) with FMD in RA patients (p=0.04). | This study concluded that patients with RA experience endothelial dysfunction without the presence of traditional CVD risk factors. |
| 20 | González-Juanatey et al., 2011 52 | Spain | Prospective study (n=118) | To assess endothelial function and cIMT in RA patients. | Patients with longer RA disease duration had higher cIMT values (p<0.001) and lower FMD (p<0.001), indicating increased cardiovascular risk. | Irrespective of CVD, endothelial dysfunction and cIMT were shown to increase as the time course of RA progressed. |
| 21 | Rodríguez-Carrio et al., 2015 53 | Spain | Comparative study (n=194) | To assess RDW in relation to endothelial repair failure in RA patients. | An increase in red cell distribution width (RDW) caused a decrease in EPC counts in patients with established RA after adjusting for disease duration and traditional CVD risk factors (p<0.009). | RDW is associated with lower EPC populations and endothelial dysfunction and repair failure, thus contributing to the pathophysiology of CVD in RA. |
| 22 | Karpouzas et al., 2020 54 | USA | Prospective cohort study (n=101) | To evaluate the role of inflammation in mediating atherosclerosis in RA. | Plaque burden was increased in 48% of patients and was associated with age, higher inflammation, and prednisone dose (p<0.05). | This study concluded that inflammation is an independent risk factor for subsequent premature atherosclerosis in RA. |
| 23 | Huaranga et al., 2018 55 | Spain | Cross-sectional study (n=119) | To investigate the role of RA disease activity in the development of atherosclerosis. | High-risk patients, as defined by age, gender, blood pressure, presence of anti-cyclic citrullinated peptide antibodies and high disease activity, had a 3× increased risk of developing atherosclerotic plaques (p=0.037). | This study found that moderate or high disease activity is significantly associated with an increased risk of RA-related subclinical atherosclerosis. |
| 24 | Dehghan et al., 2015 56 | Iran | Case–control study (n=70) | To investigate the prevalence of atherosclerosis in patients with inactive RA. | RA patients presented with more carotid plaques and a higher cIMT compared with the control group (48.6 versus 14.3% and 0.705±0.140 versus 0.580±0.125 respectively). | In patients with inactive RA, chronic inflammation is predictive of atherosclerosis. |
| 25 | Lo Gullo et al., 2014 57 | Italy | Comparative study (n=66) | To assess the endothelial function and CPC populations in RA. | RA patients were found to have lower circulating progenitor cells (CPCs) compared to controls (p<0.001) and higher levels of ROS compared with controls (p<0.001). | Accelerated atherosclerosis and endothelial dysfunction in RA may be due to inflammation and oxidative stress caused by a reduction in CPCs. |
| 26 | Sahari et al., 2014 58 | Malaysia | Case–control study (n=80) | To assess cIMT in RA patients without traditional CV risk factors. | RF antibodies and an active disease status were significantly correlated with higher cIMT values as compared with healthy controls (p=0.03 and p=0.02, respectively). | RA patients have a higher co-occurrence of premature atherosclerosis despite the absence of traditional CVD risk factors. |
| 27 | del Rincón et al., 2015 59 | USA | Comparative study (n=487) | To investigate factors that mediate accelerated atherosclerosis in RA. | Increase in cIMT was significantly associated with ESR (Odds ratio 1.12 per 10 mm/h, [95% CI 1.02–1.23]) and the presence of CVD risk factors (Odds ratio 1.27 per risk factor, [95% CI 1.01–1.61]). | This study concluded that abnormal thickening of the cIMT in patients with RA was associated with chronic inflammation and the presence of traditional CVD risk factors. |
| 28 | Södergren et al., 2019 60 | Sweden | Prospective cohort study (n=111) | To assess whether inflammatory biomarkers are associated with atherosclerosis in RA. | RA patients had significantly elevated levels of inflammatory biomarkers and pro-inflammatory cytokines such as MIC-1, TNF-R2, ICAM-1, VCAM-1 and endostatin compared with controls. | Inflammatory biomarkers may be involved in the development of premature subclinical atherosclerosis in patients with RA. |
| 29 | Södergren et al., 2015 61 | Sweden | Prospective cohort study (n=111) | To assess the role of traditional CV risk factors and inflammation in mediating atherosclerosis in RA. | Patients had higher cIMT and poorer FMD values five years after being diagnosed with RA (p<0.01). | Both traditional CVD risk factors and inflammatory load are predictive of accelerated atherosclerosis in patients with RA. |
| 30 | Mohan et al., 2014 62 | India | Case–control study (n=64) | To assess RA-related atherosclerosis in adult south Indian patients. | RA patients had a higher mean cIMT compared to healthy age and gender-matched controls (p=0.001). | cIMT is a useful marker to assess progression of atherosclerosis in patients with RA. |
| 31 | Agca et al., 2021 63 | The Netherlands | Prospective study (n=89) | To assess the existence of arterial wall inflammation in RA patients. | A higher 18F-FDG uptake was detected in the wall of the carotid arteries (p<0.01) and the aorta (p<0.01) in patients with RA, even after adjusting for CVD risk factors. | This study concluded that arterial wall inflammation is implicated in the pathophysiology of RA-related vascular complications. |
| 32 | Yamamoto et al., 2019 64 | Japan | Prospective study (n=454) | To determine risk factors for the occurrence of atherosclerosis in RA patients. | 238 (52%) RA-patients had atherosclerosis. Traditional CVD risk factors including age, hypertension, and total/HDL cholesterol ratio were significantly associated with cIMT (p<0.01). | Inflammatory mediators involved in the RA disease process influence the development of atherosclerotic plaques so the management of RA should involve controlling CVD risk factors and disease activity. |
| 33 | Guin et al., 2013 65 | India | Prospective study (n=35) | To assess whether DMARDs improve vascular function in RA patients. | cIMT value decreased after 1 year of DMARD treatment (p=0.002). | DMARDs are useful in the management of RA and halting the progression of RA-related atherosclerosis. |
| 34 | Dalbeni et al., 2017 66 | Italy | Prospective study (n=256) | To assess risk-factors that accelerate atherosclerosis in RA. | Among non-modifiable CVD risk-factors, age was significantly associated with carotid segmental distensibility, cIMT, and atherosclerotic plaques in RA patients (p<0.001). | Age is implicated as the major determinant of premature atherosclerosis in arthritis. |
| 35 | Ajeganova et al., 2012 67 | Sweden | Prospective study (n=105) | To assess the influence of atherosclerosis and disease activity in development of CVD. | Improvement in DAS28 and usage of methotrexate (MTX) reduced the risk of a CVD event [hazard ratio 0.68 (95% CI 0.5–0.97) and 0.34 (95% CI 0.12–0.91), respectively]. | Elevated levels of oxLDL may contribute to the pathogenesis of chronic inflammation. Controlling disease activity and early treatment of RA is pivotal in improving vascular-related outcomes in RA patients. |
| 36 | Liu et al., 2017 68 | China | Prospective study (n=85) | To investigate the progression of vascular calcification in RA patients. | Coronary calcium score (CS) was found to be significantly increased in the coronary artery (p<0.01), carotid artery (p<0.01), and aorta (p<0.01). | This study concluded that there is a significant increase in vascular calcification over 10 years following RA diagnosis. |
| 37 | Udachkina et al., 2018 69 | Russia | Prospective study (n=74) | To assess CAC in patients with RA. | Coronary artery calcification (CAC) was detected in 34 (46%) RA patients. In RA patients who had ischemic heart disease, occurrence of CAC was 100%. | This study found that CAC was highly prevalent in patients with early RA and this association was positively correlated with age. |
| 38 | Tuzcu et al., 2019 70 | Turkey | Case–control study (n=69) | To evaluate whether endocan can be used as a marker for endothelial dysfunction in RA patients. | RA patients had significantly higher values of human endothelial cell-specific molecule-1 (endocan) compared to age and sex-matched controls (p=0.009). | Endocan can be used as a biomarker to assess endothelial dysfunction and atherosclerotic plaques in patients with RA. |
| 39 | Fan et al., 2012 71 | China | Case–control study (n=148) | To assess the presence of atherosclerosis in RA patients using cIMT and FMD%. | Patients with RA had a significantly higher cIMT compared to the control group (p<0.001) and significantly lower brachial artery FMD% than controls (p<0.001). | This study concluded that RA patients had subclinical atherosclerosis as assessed by cIMT and FMD%. |
| 40 | Yiu et al., 2011 72 | Hong Kong | Comparative study (n=170) | To assess the prevalence of vascular calcification in patients with RA. | Aortic valve calcification (AVC) and mitral valve calcification (MVC) was highly prevalent in patients with RA or systemic lupus erythematosus (SLE) (p<0.01). | MVC was an independent risk factor for premature atherosclerosis in patients with RA or SLE after adjusting for clinical parameters. |
| 41 | Lo Gullo et al., 2014 73 | Italy | Comparative study (n=50) | To assess the impact of inflammation in mediating atherosclerosis in RA. | RA patients had a lower count of CD34+ cells, but higher expressions of ROS, TLR3 and IL-1β compared to the control group. RA patients also had elevated levels of CRP, suggesting a proinflammatory milieu. | Accelerated atherosclerosis in RA is mediated by RA-related inflammatory changes in the vasculature. |
| 42 | Chung et al., 2013 74 | USA | Prospective study (n=990) | To assess risk-factors associated with CAC in RA. | There was no statistical significance in the incidence of coronary artery calcium (CAC) in RA patients compared to controls (p=0.68). | Inflammatory markers were not associated with development of CAC in RA patients. Traditional CVD risk factors, rather than inflammation, are associated with CAC in RA. |
| 43 | Vázquez-Del Mercado et al., 2015 75 | Mexico | Cross-sectional study (n=82) | To assess the impact of anti-CCP antibodies on cIMT in RA patients. | RA patients with anti-CCP positive antibodies had a significantly increased cIMT compared to the control group and anti-CCP negative group (p<0.001). | Increased incidence of atherosclerosis and CVD events in RA patients are associated with anti-CCP and CRP levels. |
| 44 | Di Minno et al., 2015 76 | Italy | Meta-analysis (n=1688) | To assess endothelial function in RA patients using FMD. | Patients with RA had a lower FMD compared to controls (p=0.0003), but no significant difference was found in nitrate-mediated dilation (NMD) (p=0.49). | This meta-analysis concluded that patients with RA should be monitored for endothelial dysfunction and therapeutic strategies must be planned to prevent vascular damage in these individuals. |
| 45 | Di Franco et al., 2012 77 | Italy | Prospective study (n=40) | To assess the role of SDMA and ADMA in mediating endothelial dysfunction in RA patients. | In patients with early RA, ADMA levels were higher than in the control group (p=0.007). After treatment with DMARDs, ADMA levels were significantly decreased in this group (p=0.012). | This study concluded that asymmetric-dimethylarginine and apelin can be used as markers of endothelial impairment in RA patients. |
| 46 | Kerekes et al., 2011 78 | Hungary | Prospective study (n=8) | To assess the effect of TNF-α inhibitors on vascular function in RA patients. | In patients treated with adalimumab, there was a significant decrease in CRP levels (p=0.04) and DAS28 (p<0.0001). Endothelial function, as measured by FMD also improved (p<0.05). | Treatment with TNF-α inhibitors improved endothelial function and ameliorated atherosclerosis in patients with early RA, suggesting that TNFα is a key mediator of premature atherosclerosis in RA. |
| 47 | Wahlin et al., 2016 79 | Sweden | Prospective study (n=22) | To evaluate factors that are associated with CAC in RA patients. | Patients with RA who had coronary artery calcification (CAC) were found to have increased values of DAS28 and ESR (p<0.01 and p<0.01, respectively). | In patients with long-standing RA, inflammation is a key mediator of CAC. |
| 48 | Ahmed et al., 2016 80 | Norway | Prospective study (n=39) | To assess the role of inflammatory molecules in mediating atherosclerosis in RA. | In patients with RA, TNF was found in 63% of biopsy specimens whereas only 30% of specimens from the control group expressed TNF (p=0.04). | Proinflammatory cytokines are involved in the pathophysiology of mediating accelerated atherosclerosis in RA. |
| 49 | Im et al., 2015 81 | Korea | Prospective study (n=615) | To assess inflammatory burden in RA patients. | RA patients had a higher mean cIMT compared to controls (p<0.001). Plaques were more common RA patients (74%) than in controls (26%) (p=0.004). | Inflammatory mediators and traditional CVD risk factors act synergistically to contribute to the development of premature atherosclerotic disease in RA. |
| 50 | Profumo et al., 2012 82 | Italy | Prospective study (n=62) | To assess biomarkers that can be used to predict atherosclerosis in RA. | RA patients had higher levels of ox-LDL compared to controls. RA patients also had lower levels of NO compared to healthy subjects (p<0.0001). | This study concluded that ox-LDL and NO are biomarkers of premature atherosclerotic disease in RA. |
| 51 | Chandrasekharan et al., 2018 83 | USA | Cross-sectional study (n=357) | To assess the role of SDMA and ADMA in mediating CVD in RA patients. | Patients with RA had elevated levels of ADMA (p<0.001) and SMDA (p<0.001) and lower levels of nitric oxide synthase (NOS) (p<0.001) compared with healthy age- and sex-matched controls. | This study concluded that dysregulated L-arginine metabolism may contribute to the pathophysiology of developing CVD in RA. |
| 52 | Okano et al., 2017 84 | Japan | Prospective cohort study (n=413) | To assess whether anti-CCP antibodies contribute to atherosclerosis in RA. | Carotid plaque was found more commonly in RA patients than control subjects (p=0.027). RA patients with plaques had higher levels of anti-CCP antibodies compared to RA patients without plaques (p=0.005). | High serum levels of anti-CCP antibodies are associated with causing severe atherosclerotic plaque in RA. |
| 53 | Geraldino-Pardilla et al., 2017 85 | USA | Prospective study (n=195) | To assess the role of ACPAs in mediating CAC in RA. | In patients with RA, high levels of ACPAs were positively correlated with higher CAC scores (p=0.001) and this association was observed even after adjusting for traditional CVD and RA risk factors (p=0.03). | ACPAs play a significant role in the pathophysiology of atherosclerosis in RA. |
| 54 | Marder et al., 2011 86 | USA | Prospective study (n=51) | To evaluate whether IL-17 is involved in mediating endothelial dysfunction in RA patients. | Patients with RA who had anti-CCP antibodies in the serum had higher levels of IL-17 compared to patients who were anti-CCP negative (p=0.01). | IL-17 participates in the development and propagating of endothelial impairment and CVD in patients with RA. |
| 55 | Barbarroja et al., 2014 87 | Italy | Prospective study (n=106) | To assess the role of RA-derived antibodies in mediating atherosclerosis in RA. | In RA patients, anti-CCPs antibody levels were significantly associated with age and DAS28 (p=0.024 and p=0.001, respectively). RA patients who were anti-CCP positive were found to have a strong association with cIMT (p<0.01). | This study concluded that autoantibodies such as anti-CCPs are significantly associated with the proatherogenic milieu seen in RA patients. |
| 56 | Davies et al., 2021 88 | UK | Cross-sectional study (n=182) | To assess the role of IL-6 in mediating atherosclerosis in RA. | In patients with established RA, a positive correlation was found between the DAS28 index and sVCAM-1 (p = 0.017). | This study concluded that sVCAM-1 is a risk-factor for the progression of atherosclerosis in patients with RA and that IL-6 plays a role in mediating this. |
| 57 | van Breukelen-van der et al., 2015 89 | The Netherlands | Cross-sectional study (n=360) | To assess cIMT values in RA patients and determine the clinical implications of this. | There was no association between RA and cIMT. In RA patients, cIMT was associated with age and systolic blood pressure (p<0.001) and (p=0.003). | This study concluded that in RA patients with low disease activity, there is no significant association with cIMT, suggesting that therapeutic strategies that target CVD risk factors seem to improve cardiovascular risk in RA. |
| 58 | Karpouzas et al., 2014 90 | USA | Prospective study (n=300) | To assess the prevalence of CAC in RA patients. | RA patients had higher CAC compared with controls (p < 0.0001). Atheroscletic plaques were more frequently found in patients with RA compared to the control cohort (71% versus 45%, p<0.0001). | This study concluded that RA patients without any known CVD had a higher occurrence and severity of coronary atherosclerotic plaque. |
| 59 | Kassem et al., 2011 91 | Egypt | Prospective study (n=30) | To assess the importance of non-traditional risk factors in mediating atherosclerosis in RA. | There was an association between RA patients who harbored carotid atherosclerosis and inflammatory markers such as CRP*, ESR* and IL-6** and VCAM-1*, a marker of endothelial impairment (*p<0.001 and **p<0.05). | This study found a significant correlation between atherosclerosis and inflammatory markers, endothelial dysfunction, and rheumatoid arthritis related antibodies. |
| 60 | Wahab et al., 2015 92 | Egypt | Prospective study (n=75) | To assess whether anti-CCP antibodies are involved in mediating atherosclerosis in RA. | RA patients also had higher levels of anti-CCP antibodies compared to controls (p=0.001). | Anti-CCP antibodies are a useful indicator of subclinical atherosclerosis in patients with RA. |
| 61 | Mondal et al., 2011 93 | India | Prospective study (n=146) | To assess endothelial function in RA patients. | RA patients had a lower FMD value than controls (p<0.001). There was a negative association between FMD and CRP levels (p<0.01). | RA patients without any known CVD risk factors have altered endothelial function and an increased susceptibility to premature atherosclerosis. |
| 62 | Arida et al., 2021 94 | Greece | Prospective study (n=85) | To investigate whether PCSK9 or LDLR levels are associated with subclinical CVD in RA patients. | PCSK9 was significantly associated with atheromatous plaques (p=0.033) and LDLR concentration was also correlated with plaque presence (p=0.005) in RA patients. | The PCSK9/LDLR system was found to be associated with subclinical atherosclerosis in RA. |
| 63 | Mena-Vázquez et al., 2022 95 | Spain | Observational study (n=160) | To analyze association of postprandial lipidemia with subclinical atherosclerosis in RA. | Postprandial hyperlipidemia (PPHL) in RA patients was associated with subclinical atherosclerosis (p=0.037), TNF-α (p=0.048), and high-sensitivity C-reactive protein (p=0.027). | PPHL in RA is associated with inflammation and subclinical atherosclerosis. |
| 64 | Wahlin et al., 2021 96 | Sweden | Prospective study (n=79) | To examine whether regulators of bone formation or turnover are associated with RA-related atherosclerosis. | Osteocalcin (OCN) and osteoprotegerin (OPG) were significantly associated with IMT after 11 years (p=0.03). | Markers of bone turnover were associated with IMT in RA patients. |
| 65 | Beyazal et al., 2016 97 | Turkey | Retrospective cohort study (n=116) | To assess whether serum OPG levels are associated with arterial stiffness and cIMT in RA patients. | OPG levels were higher in RA patients than controls (p < 0.001) and were significantly associated with cIMT. | OPG may be a useful marker to assess CV risk in RA patients. |
| 66 | Esaily et al., 2021 98 | Egypt | Case–control study (n=200) | To examine the relationship between the cellular communication network factor 1 (CCN1) and RA-induced atherosclerosis. | CCN1 was positively correlated with cIMT in RA patients (p<0.001). | Disruptions in serum CCN1 levels are associated with subclinical atherosclerosis in RA patients. |
| 67 | Mulumba et al., 2019 99 | Democratic Republic of Congo | Cross-sectional study (n=75) | To describe the prevalence of subclinical atherosclerosis in RA patients. | Risk factors associated with subclinical atherosclerosis in RA patients included being a woman aged >55 years (p=0.028), DAS28-ESR >2.6 (p=0.044), severe RA (p=0.035) and obesity (p=0.026). | Subclinical atherosclerosis is highly prevalent in RA patients. |
| 68 | Elshereef et al., 2015 100 | Egypt | Retrospective study (n=152) | To evaluate prevalence of atherosclerosis in RA patients and correlate it with disease activity. | 31.3% of asymptomatic RA patients had atherosclerosis compared with 5% control subjects (p=0.003). | Long-term RA patients have a high frequency of subclinical atherosclerosis. |
| 69 | Ristić et al., 2015 101 | Serbia | Cross-sectional study (n=74) | To determine whether von Willebrand factor (vWF) activity is associated with subclinical atherosclerosis in RA patients. | vWF activity was higher in RA patients with subclinical atherosclerosis (p<0.05) or atherosclerotic plaques (p<0.05). | vWF is a valuable biomarker to determine premature atherosclerosis in RA patients. |
| 70 | Södergren et al., 2015 102 | Sweden | Prospective study (n=71) | To assess whether the level of Lp-PLA2 is associated with subclinical atherosclerosis. | Lp-PLA2 was significantly associated with IMT at T0 and T5 and flow mediated dilation (FMD) (p<0.05). | Lp-PLA2 is associated with both subclinical atherosclerosis and disease severity in RA patients. |
| 71 | Mena-Vázquez et al., 2020 103 | Spain | Observational study (n=80) | To analyze association of postprandial lipidemia with subclinical atherosclerosis in RA. | In RA patients, cIMT was associated with postprandial ApoB48 (OR (95% CI), 1.15 (1.0-1.3)) and total ApoB (OR [95% CI], 1.12 [1.1–1.2]). | Atherogenic particles such as ApoB48 are associated with endothelial dysfunction in RA patients. |
| 72 | Södergren et al., 2010 104 | Sweden | Prospective study (n=123) | To assess the presence of premature atherosclerosis in patients with very early RA. | RA patients had a significantly increased IMT and higher levels of VWF, sICAM-1 (p<0.05), and MCP-1 (p=0.001) compared with controls. | IMT and FMD are related to biomarkers associated with endothelial dysfunction and atherosclerosis in RA patients. |
| 73 | Barbara et al., 2018 105 | Brazil | Prospective study (n=180) | To investigate the association of serum MBL levels and its association with IMT in RA patients. | RA patients had a significantly lower MBL serum concentration in relation to controls (528 ng/mL versus 937.5 ng/mL, p= 0.05, respectively). | RA patients had lower MBL serum levels than controls. MBL was not associated with disease activity, ESR, autoantibodies, or IMT. |
Discussion
The role of inflammatory cytokines
The pathophysiology of accelerated atherosclerosis in RA is due to a complex interplay between various proinflammatory mediators. Tumor necrosis factor-alpha (TNF-α) is an inflammatory cytokine that is highly elevated in the synovial fluid of individuals with RA and is involved in mediating premature atherogenesis by complex signaling pathways involving the p38 mitogen-activated protein kinase (MAPK) and the transcription factor nuclear factor-κB (NF-κB). 11 , 106 As described earlier, TNF-α acts synergistically with interleukin-6 (IL-6) to form lipid-laden macrophages (foam cells), which are prototypic in the development of atherosclerotic plaques. 13 – 16 The mechanistic pathway that contributes to this process involves the upregulation of the human scavenger receptor-A (SR-A) and lectin-like oxidized Low density lipoprotein receptor-1 (LOX1) on macrophages. 15 Anti-TNF-α therapy has become a mainstay for treatment of RA. 106 , 107 Some studies reported that TNF-α inhibition was found to significantly improve vascular function in RA patients. 42 , 43 , 45 , 78
Davies et al. found a positive correlation between RA disease activity and soluble vascular adhesion molecule-1 (sVCAM-1), and associated it with the role of IL-6 in mediating atherosclerosis in RA. 88 They went on to conclude that IL-6 trans-signaling plays an important role in vascular dysfunction in RA and blocking this pathway may be useful for RA patients. IL-1 is considered to be a proatherogenic cytokine as it stimulates smooth muscle cell (SMC) proliferation by autocrine induction of the platelet-derived growth factor (PDGF). 20 , 108 SMCs contribute to the initiation of atherosclerosis by producing an extracellular matrix (ECM), precipitating lipid uptake, and inducing foam cell formation to ultimately form a fibrous cap within the vessel wall. 109 IL-1 also induces its own gene expression, thus creating a powerful positive feedback loop to maintain a proatherogenic milieu. 20 Lo Gullo et al. reported that RA patients had a higher expression of IL-1β and elevated levels of C-reactive protein (CRP) compared with the control group, suggesting a proinflammatory milieu mediated by this cytokine 73 Kassem et al. assessed the role of non-traditional risk factors and found that RA patients with carotid atherosclerosis harbored inflammatory markers such as CRP, erythrocyte sedimentation rate (ESR), IL-6, and vascular cell adhesion molecule-1 (VCAM-1). 91 Similar findings were reported by Karpouzas et al. and Agca et al. who concluded that inflammation is implicated in the pathophysiology of RA-related vascular complications. 54 , 63 Other studies also found significantly elevated levels of inflammatory biomarkers and pro-inflammatory cytokines in RA patients, further supporting the role of cytokines in premature atherosclerosis in RA. 60 , 80 Individuals with RA have elevated circulating levels of IL-17 as a result of T-helper 17 (Th17) cell activation and differentiation. 10 , 11 , 110 Although the pathomechanisms that implicate IL-17 as a mediator of atherogenesis in the setting of RA remain unclear, Marder et al. proposed that IL-17 may disrupt normal endothelial function, stimulate myocardial fibrosis, and lower arterial compliance, thus promoting atherosclerosis. 86 Yamamoto et al. reported that both traditional cardiovascular disease (CVD) risk-factors and inflammatory mediators influence the development of atherosclerosis in RA patients and concluded that the management of RA should involve controlling CVD risk factors and RA disease activity. 64 A wealth of data shows that the role of inflammatory cytokines is pivotal when considering the pathomechanisms that explain the increased incidence of premature atherosclerosis in RA.
The role of neutrophil extracellular traps
The role of neutrophil extracellular traps (NETs) in autoimmune disorders is explained by the process of NETosis, which is described as an imbalance between NET formation and NET breakdown. 10 In patients with RA, NET formation is thought to be triggered by autoantibodies and immunostimulatory molecules. 21 NETs contain citrullinated vimentin (cVim), which is involved in secreting proinflammatory cytokines such as TNF-α and IL-1, thus continually maintaining a state of inflammation in RA. 21 , 111 Even though the role of neutrophils in mediating atherosclerosis has been historically denied, recent evidence shows that neutrophils are involved in progressive endothelial damage, recruitment of proinflammatory monocytes, and foam cell formation. 24 – 26 Researchers have detected the presence of NETs in atherosclerotic plaques of both humans and mice, which may contribute to the pathophysiology of RA-related atherosclerosis. 23 , 112 , 113 Pérez-Sánchez et al. found that RA patients had enhanced NETosis, which correlated with disease activity and inflammatory and oxidative profiles in these patients. They concluded that NETosis-derived products play a role in mediating atherosclerosis in RA and may be used as a diagnostic tool. 44 The lack of primary research studies on this topic area make it difficult to draw definitive conclusions about the role NETosis in RA and atherosclerosis. More research in this particular field would be beneficial in evaluating the diagnostic potential of NETosis-derived products and assessing whether the inhibition of NETs would hold therapeutic value in RA.
The role of RA-related autoantibodies
Citrullinated proteins within the synovia of patients with RA are a target for anti-citrullinated peptide antibodies (ACPAs). 27 , 28 Sokolove et al. found that citrullinated fibrinogen within atherosclerotic plaques are also targeted by RA-derived ACPAs. 28 Clavel et al. found that RA-specific ACPA immune complexes have the potential of inducing macrophage-driven TNF-α secretion via the Fc-gamma receptor IIa (FcγRIIa). 114 As detailed earlier, the contribution of TNF-α as an inflammatory mediator of atherosclerosis is remarkable, which provides evidence to support the pathophysiology of RA autoantibody-derived atherosclerosis. RA-derived autoantibodies like ACPAs were found in the serum of patients with RA and were considered an independent risk factor for the development of subclinical and clinical atherosclerosis. 51 Nowak et al. found that the presence of anti-CCP antibodies were associated with greater cIMT values, as also confirmed by Vázquez-Del Mercado et al. 48 , 75 Wahab et al. also found higher levels of anti-CCP antibodies in RA patients compared to controls and suggested its use as a useful indicator of subclinical atherosclerosis. 92 A number of studies included in this review found similar results concerning RA-derived autoantibodies and atherosclerosis. 51 , 55 , 84 , 85 , 87 An interesting phenomenon was reported by Jacobsen et al., who found polymorphic variations in the mannose-binding lectin gene (MBL) were associated with high scores of RA disease activity, C-reactive protein-based DAS28, and physical disability in anti-CCP-positive RA patients. 115 MBL, a serum protein, plays an instrumental role in regulating innate immunity by binding to repeating sugar motifs to activate the complement system via MBL-associated serum proteases. 116 Barbara et al. found that RA patients had a significantly lower MBL serum concentration in relation to controls and found no statistically significant association between MBL and disease activity, ESR, autoantibodies, or IMT. 105 A cross-sectional study found that both high and low levels of MBL in RA patients were associated with an increased common carotid artery intima-media thickness (cc-IMT), indicating a quadratic U-shaped relation between serum MBL and ccIMT. 117 More studies would be valuable to assess whether MBL truly plays a role in mediating atherosclerosis in RA patients.
The role of endothelial dysfunction
Atherosclerosis is a consequence of progressive endothelial damage and dysfunction. 30 The endothelium, as an organ, functions as an intimate vascular barrier that is involved with maintaining vascular tone, blood hemostasis, leukocyte migration, and antigen presentation, among other physiological processes. 118 , 119 Endothelial dysfunction is heavily involved in the pathogenesis of atherosclerosis by inducing cell adhesion molecules, facilitating leukocyte emigration, promoting cytokine production, platelet activation, and SMC proliferation 120 A number of studies have found elevated serum levels of asymmetric dimethylarginine (ADMA) in RA patients. 121 – 124 ADMA is a potent inhibitor of endothelial nitric oxide synthase (eNOS), an enzyme responsible for the synthesis of vasoprotective nitric oxide (NO) 125 NO serves as a cardioprotective molecule due to its vasodilatory properties and its ability to inhibit platelet aggregation, suppress adhesion molecules, maintain endothelial barrier integrity, and regulate SMC proliferation. 126
Di Franco et al. found that RA patients had elevated levels of ADMA and proposed its use as a biomarker of vascular endothelial dysfunction, as also concluded by Dimitroulas et al. and Chandrasekharan et al. 50 , 77 , 83 Endothelial progenitor cells (EPCs) have also been implicated in RA-related atherogenesis. 10 An inverse relationship between circulating EPCs and endothelial function has been established by numerous studies. 127 – 129 When considering RA, a reduction in EPCs has been reported, possibly due to C-reactive protein-mediated apoptosis of EPCs 130 Rodríguez-Carrio et al. reported long-standing RA was correlated with a reduction in EPCs and concluded that EPC imbalance is associated with endothelial dysfunction and subsequent CVD in RA. 47 Similar findings were also reported by other studies included in this review. 53 , 57 Progressive endothelial dysfunction along with a reduction in EPCs in RA results in the creation of a proatherogenic environment, thus facilitating atherosclerosis and CVD in affected individuals.
Prevalence of accelerated atherosclerosis in RA
Carotid intima media thickness (cIMT) has popularly been used as a marker for subclinical atherosclerosis. In our review, a number of studies utilized cIMT to assess the prevalence of subclinical atherosclerosis in RA. In a cross-sectional study by Hannawi et al., patients with RA had significantly higher cIMT values and a higher carotid plaque burden compared to healthy controls. 38 Krajnc et al. also found that patients with RA had a higher cIMT compared with controls, suggesting atherosclerotic plaque build-up and increased risk for subsequent CVD. 39 A plethora of other studies included in this review reported similar findings and concluded that there is a high prevalence of premature atherosclerosis in patients with RA. 33 , 34 , 36 , 40 , 41 , 52 , 56 , 58 – 62 , 65 , 66 , 71 , 81 , 99 , 100 , 104 However, in a cross-sectional study by van Breukelen-van der et al., no significant association was found between RA and cIMT. These conflicting findings were attributed to the patients having low disease activity and well-controlled RA, suggesting therapeutic strategies that target CVD risk factors seem to improve overall cardiovascular risk in RA. 89
Flow-mediated dilation (FMD) is a non-invasive method to assess endothelial dysfunction and has been used as a predictor of early atherosclerosis. Verma et al. found that RA patients had lower FMD and higher cIMT compared to controls, consistent with endothelial dysfunction and accelerated atherosclerosis in patients with RA. 37 Adawi et al. also acknowledged the high prevalence of accelerated atherosclerosis in RA patients and proposed the clinical use of FMD as a measure of endothelial function to predict subsequent atherosclerosis. 49 Two other studies also reported similar findings regarding endothelial dysfunction in RA patients and the usefulness of FMD as an early marker of atherosclerosis. 76 , 93
Coronary calcium score (CS) utilizes computed tomography to detect the presence of vascular calcification. In a prospective study by Liu et al., the CS was found to be significantly increased in the coronary artery, carotid artery, and aorta of RA patients. 68 In another prospective study, coronary artery calcification (CAC) was detected in 46% of RA patients. 69 Other studies also reported similar findings. 72 , 90 In a prospective study by Wahlin et al., patients with RA who had CAC were found to have increased values of DAS28 and ESR, implying that inflammation plays a role in mediating RA-induced CAC. 79 Contrastingly, Chung et al. found no statistical significance in the incidence of CAC in RA patients compared to controls and concluded that traditional risk-factors for CVD, rather than inflammatory markers are responsible for CAC and atherosclerosis in RA. 74 This study also reported that once RA patients developed CAC, the rate of progression was found to be similar to the progression seen in control participants. This study acknowledges that their results were not concordant with their primary hypothesis, and one explanation offered was that the study focused on subclinical atherosclerosis, and RA patients with prior events, who may have contributed to a greater progression of CAC had been excluded, resulting in differential bias. Overall, there is abundant evidence to conclude that premature atherosclerosis in RA is highly prevalent, and that both traditional CVD risk-factors and inflammatory mediators play a role in mediating this process.
Novel markers associated with accelerated atherosclerosis in RA
Postprandial hyperlipidemia (PPHL) has been shown to be an independent predictor of CVD. 131 Mena-Vázquez et al. found that PPHL in RA patients was significantly associated with subclinical atherosclerosis, TNF-α, and high-sensitivity C-reactive protein, suggesting that PPHL in RA is associated with inflammation and subclinical atherosclerosis. 95 Other studies have also found that cIMT is associated with postprandial ApoB48 and total ApoB, providing evidence that atherogenic chylomicron remnants contribute to atherosclerosis in RA. 103 , 132
Biomarkers associated with bone turnover such as osteoprotegerin (OPG) have been associated with CAC, carotid plaque, and IMT. 133 – 135 Wahlin et al. examined the role of bone turnover markers in mediating atherosclerosis in RA and found that Osteocalcin (OCN) and OPG were significantly associated with IMT after 11 years, especially in patients with joint erosions. However, there was no significant association between collagen markers of ongoing bone turnover and IMT, suggesting that bone turnover and atherosclerosis may have different pathogenic mechanisms in the setting of RA. 96 Beyazal et al. also found higher OPG levels in RA patients than controls and concluded that OPG may be a useful marker to assess CV risk in RA patients. 97 More studies investigating this topic area may be beneficial to clarify whether bone turnover truly plays a role in premature atherosclerosis in RA.
Other novel markers found to be associated with accelerated atherosclerosis in RA patients were von Willebrand factor (vWF), 101 cellular communication network factor 1 (CCN1), 98 human endothelial cell-specific molecule-1 (endocan), 70 PCSK9/LDLR system, 94 and oxLDL. 67 , 82 Lipoprotein-associated phospholipase A 2 (Lp-PLA 2) is a biomarker used to assess vascular inflammation. 136 Södergren et al. found that Lp-PLA2 is associated with both subclinical atherosclerosis and disease severity in RA patients, 102 however, Bes et al. found no significant difference between Lp-PLA2 enzyme in RA patients and healthy controls, possibly because RA patients were undergoing treatment and had low disease activity scores. 35 Similarly, there are other novel biomarkers being investigated in their role in developing accelerated atherosclerosis in the setting of RA. More studies in this topic area may be beneficial to predict premature atherosclerosis in RA and identify new therapeutic targets.
Current therapies to treat RA
The treatment of RA is individually tailored to each patient to optimize patient care. The goal of treating RA involves reducing joint inflammation and preventing progressive joint damage. The target is to achieve a state of low disease activity within 6 months of RA diagnosis. 137 Non-pharmacologic therapies that can be used to control symptoms and manage RA include physical activity, occupational therapy, lifestyle changes such as quitting smoking and reducing alcohol consumption, and dietary therapies such as implementing a Mediterranean diet. 138 It is recommended that pharmacologic drugs should be started as soon as RA is diagnosed. 139 The mainstay drugs in the treatment of RA are disease-modifying antirheumatic drugs (DMARDs). These are further subdivided into synthetic and biological forms. Methotrexate is a conventional synthetic DMARD (csDMARD) and is considered first-line in treatment of RA and is typically prescribed at a weekly dose of 25 mg in combination with short-term glucocorticoids. 140 , 141 If first-line treatment fails, patients are given a biological DMARD (bDMARD) in addition to the csDMARD. If remission is not achieved with second-line therapy, third-line treatment involves continuing the csDMARD along with a bDMARD and adding a targeted synthetic DMARD such as tofacitinib which acts on enzymes such as janus kinases to interfere with intracellular cytokine signalling. 137 75% to 80% of patients achieve a state of remission or low disease activity with these therapies and have normal life expectancies. Early diagnosis and intervention is key in preventing disease progression.
Limitations
Due to the rigorous inclusion and exclusion criteria, studies that may contain supportive evidence according to their results were not included because their aim was not in line with our search criteria. Moreover, some studies did not explicitly state information regarding the study setting and type of study. Based on the current literature and evidence, there are various pathophysiological processes involved in accelerating atherosclerosis in RA which made it difficult to draw definitive conclusions on whether inflammation or traditional cardiovascular risk-factors work together or independently to contribute to these findings.
Conclusion
In conclusion, abundant evidence exists to support an increased prevalence of accelerated atherosclerosis among RA patients. Since cardiovascular morbidity and mortality in RA is strikingly high, it is important to understand the mechanisms that initiate and govern atherosclerosis in RA so tailored therapeutic regimens can be developed to reduce the CV burden that RA patients carry. Proinflammatory cytokines such as IL-6 and TNF-α are involved in the formation of atherogenic foam cells. RA-derived autoantibodies are involved in exacerbating the inflammatory potential of macrophages and endothelial dysfunction is involved in disrupting the integrity of the vascular barrier which create a proatherogenic milieu and favour subsequent atherosclerotic disease. The question of whether inflammation and traditional CV risk factors work synergistically to produce atherosclerosis in RA or if one is more significant than the other remains. Nevertheless, therapies targeting both the inflammatory load or traditional CV risk-factors seem to improve vascular outcomes in RA patients. Lastly, novel markers of atherosclerosis in RA may be useful in predicting early atherosclerosis and serve as new targets for pharmacological intervention.
Data availability
No data are associated with this article.
Reporting guidelines
Raj, Rhea; Gorantla, Vasavi; Thomas, Sneha (2022): Accelerated atherosclerosis in rheumatoid arthritis: a systematic review. figshare. Dataset. https://doi.org/10.6084/m9.figshare.19618947.v1
Ethical statement
Not applicable. No patient data or animal studies were used in this review.
Consent from patients
In this review, no patient information was used. Consent is not applicable.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; peer review: 3 approved]
References
- 1. Guo Q, Wang Y, Xu D, et al. : Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6(1):15. 10.1038/s41413-018-0016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khanna S, Jaiswal KS, Gupta B: Managing Rheumatoid Arthritis with Dietary Interventions. Front. Nutr. 2017;4. 10.3389/fnut.2017.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turesson C: Comorbidity in rheumatoid arthritis. Swiss Med. Wkly. 2016 Apr 5;146:w14290. 10.4414/smw.2016.14290 [DOI] [PubMed] [Google Scholar]
- 4. Woude D, Helm-van Mil AHM: Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2018 Apr;32(2):174–187. 10.1016/j.berh.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 5. Lee DM, Weinblatt ME: Rheumatoid arthritis. Lancet. 2001 Sep 15;358(9285):903–911. 10.1016/S0140-6736(01)06075-5 [DOI] [PubMed] [Google Scholar]
- 6. Dougados M: Comorbidities in rheumatoid arthritis. Curr. Opin. Rheumatol. 2016 May;28(3):282–288. 10.1097/BOR.0000000000000267 [DOI] [PubMed] [Google Scholar]
- 7. Smolen JS, Aletaha D, McInnes IB: Rheumatoid arthritis. Lancet. 2016 Oct 22;388(10055):2023–2038.Epub 2016 May 3. Erratum in: Lancet.2016 Oct 22; 388(10055): 1984. 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 8. Falk E: Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006 Apr 18;47(8 Suppl):C7–C12. 10.1016/j.jacc.2005.09.068 [DOI] [PubMed] [Google Scholar]
- 9. Schaftenaar F, Frodermann V, Kuiper J, et al. : Atherosclerosis: the interplay between lipids and immune cells. Curr. Opin. Lipidol. 2016 Jun;27(3):209–215. 10.1097/MOL.0000000000000302 [DOI] [PubMed] [Google Scholar]
- 10. Adawi M, Firas S, Blum A: Rheumatoid Arthritis and Atherosclerosis. Isr. Med. Assoc. J. 2019 Jul;21(7):460–463. [PubMed] [Google Scholar]
- 11. Kahlenberg JM, Kaplan MJ: Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu. Rev. Med. 2013;64:249–263. 10.1146/annurev-med-060911-090007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rho YH, Chung CP, Oeser A, et al. : Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009 Nov 15;61(11):1580–1585. 10.1002/art.25009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adawi M, Firas S, Blum A: Rheumatoid Arthritis and Atherosclerosis. Isr. Med. Assoc. J. 2019 Jul;21(7):460–463. [PubMed] [Google Scholar]
- 14. Zelová H, Hošek J: TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm. Res. 2013 Jul;62(7):641–651. 10.1007/s00011-013-0633-0 [DOI] [PubMed] [Google Scholar]
- 15. Hashizume M, Mihara M: Atherogenic effects of TNF-α and IL-6 via up-regulation of scavenger receptors. Cytokine. 2012 Jun;58(3):424–430. 10.1016/j.cyto.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 16. Yu XH, Fu YC, Zhang DW, et al. : Foam cells in atherosclerosis. Clin. Chim. Acta. 2013 Sep 23;424:245–252. 10.1016/j.cca.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 17. Kay J, Calabrese L: The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford). 2004 Jun;43 Suppl 3:iii2–iii9. 10.1093/rheumatology/keh201 [DOI] [PubMed] [Google Scholar]
- 18. Proudman SM, Cleland LG, Mayrhofer G: Effects of tumor necrosis factor-alpha, interleukin 1beta, and activated peripheral blood mononuclear cells on the expression of adhesion molecules and recruitment of leukocytes in rheumatoid synovial xenografts in SCID mice. J. Rheumatol. 1999 Sep;26(9):1877–1889. [PubMed] [Google Scholar]
- 19. Abbot SE, Kaul A, Stevens CR, et al. : Isolation and culture of synovial microvascular endothelial cells. Characterization and assessment of adhesion molecule expression. Arthritis Rheum. 1992 Apr;35(4):401–406. 10.1002/art.1780350407 [DOI] [PubMed] [Google Scholar]
- 20. Libby P: Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J. Am. Coll. Cardiol. 2017 Oct 31;70(18):2278–2289. 10.1016/j.jacc.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song W, Ye J, Pan N, et al. : Neutrophil Extracellular Traps Tied to Rheumatoid Arthritis: Points to Ponder. Front. Immunol. 2021 Jan 29;11:578129. 10.3389/fimmu.2020.578129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Papayannopoulos V: Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018 Feb;18(2):134–147. 10.1038/nri.2017.105 [DOI] [PubMed] [Google Scholar]
- 23. Döring Y, Soehnlein O, Weber C: Neutrophil Extracellular Traps in Atherosclerosis and Atherothrombosis. Circ. Res. 2017 Feb 17;120(4):736–743. 10.1161/CIRCRESAHA.116.309692 [DOI] [PubMed] [Google Scholar]
- 24. Soehnlein O: Multiple roles for neutrophils in atherosclerosis. Circ. Res. 2012 Mar 16;110(6):875–888. 10.1161/CIRCRESAHA.111.257535 [DOI] [PubMed] [Google Scholar]
- 25. Drechsler M, Megens RT, Zandvoort M, et al. : Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010 Nov 2;122(18):1837–1845. 10.1161/CIRCULATIONAHA.110.961714 [DOI] [PubMed] [Google Scholar]
- 26. Zernecke A, Bot I, Djalali-Talab Y, et al. : Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 2008 Feb 1;102(2):209–217. 10.1161/CIRCRESAHA.107.160697 [DOI] [PubMed] [Google Scholar]
- 27. DeMizio DJ, Geraldino-Pardilla LB: Autoimmunity and Inflammation Link to Cardiovascular Disease Risk in Rheumatoid Arthritis. Rheumatol Ther. 2020 Mar;7(1):19–33. 10.1007/s40744-019-00189-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sokolove J, Bromberg R, Deane KD, et al. : Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7(5):e35296. 10.1371/journal.pone.0035296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sokolove J, Brennan MJ, Sharpe O, et al. : Brief report: citrullination within the atherosclerotic plaque: a potential target for the anti-citrullinated protein antibody response in rheumatoid arthritis. Arthritis Rheum. 2013 Jul;65(7):1719–1724. 10.1002/art.37961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Falk E: Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006 Apr 18;47(8 Suppl):C7–C12. 10.1016/j.jacc.2005.09.068 [DOI] [PubMed] [Google Scholar]
- 31. Bordy R, Totoson P, Prati C, et al. : Microvascular endothelial dysfunction in rheumatoid arthritis. Nat. Rev. Rheumatol. 2018 Jul;14(7):404–420. 10.1038/s41584-018-0022-8 [DOI] [PubMed] [Google Scholar]
- 32. Liberati A: The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. Ann. Intern. Med. 2009 Aug 18;151(4):W–W94. 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 33. Ruscitti P, Cipriani P, Masedu F, et al. : Increased Cardiovascular Events and Subclinical Atherosclerosis in Rheumatoid Arthritis Patients: 1 Year Prospective Single Centre Study. PLoS One. 2017 Jan 19;12(1):e0170108. 10.1371/journal.pone.0170108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ambrosino P, Lupoli R, Di Minno A, et al. : Subclinical atherosclerosis in patients with rheumatoid arthritis. A meta-analysis of literature studies. Thromb. Haemost. 2015 May;113(5):916–930. 10.1160/TH14-11-0921 [DOI] [PubMed] [Google Scholar]
- 35. Bes C, Gürel S, Buğdaycı G, et al. : Atherosclerosis assessment and rheumatoid arthritis. Z. Rheumatol. 2018 May;77(4):330–334. 10.1007/s00393-016-0239-3 [DOI] [PubMed] [Google Scholar]
- 36. Ruscitti P, Cipriani P, Liakouli V, et al. : Subclinical and clinical atherosclerosis in rheumatoid arthritis: results from the 3-year, multicentre, prospective, observational GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Arthritis Res. Ther. 2019 Sep 3;21(1):204. 10.1186/s13075-019-1975-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verma I, Syngle A, Krishan P: Predictors of endothelial dysfunction and atherosclerosis in rheumatoid arthritis in Indian population. Indian Heart J. 2017 Mar-Apr;69(2):200–206. 10.1016/j.ihj.2016.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hannawi SM, Hannawi H, Alokaily F, et al. : Subclinical atherosclerosis in rheumatoid arthritis patients of the Gulf Cooperated Council. Saudi Med. J. 2020 Sep;41(9):1022–1025. 10.15537/smj.2020.9.25319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koren Krajnc M, Hojs R, Holc I, et al. : Accelerated atherosclerosis in premenopausal women with rheumatoid arthritis - 15-year follow-up. Bosn. J. Basic Med. Sci. 2021 Aug 1;21(4):477–483. 10.17305/bjbms.2020.5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burggraaf B, Stoep DF, Vries MA, et al. : Progression of subclinical atherosclerosis in subjects with rheumatoid arthritis and the metabolic syndrome. Atherosclerosis. 2018 Apr;271:84–91. 10.1016/j.atherosclerosis.2018.02.019 [DOI] [PubMed] [Google Scholar]
- 41. Wah-Suarez MI, Galarza-Delgado DA, Azpiri-Lopez JR, et al. : Carotid ultrasound findings in rheumatoid arthritis and control subjects: A case-control study. Int. J. Rheum. Dis. 2019 Jan;22(1):25–31. 10.1111/1756-185X.13377 [DOI] [PubMed] [Google Scholar]
- 42. Ursini F, Leporini C, Bene F, et al. : Anti-TNF-alpha agents and endothelial function in rheumatoid arthritis: a systematic review and meta-analysis. Sci. Rep. 2017 Jul 13;7(1):5346. 10.1038/s41598-017-05759-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Végh E, Kerekes G, Pusztai A, et al. : Effects of 1-year anti-TNF-α therapy on vascular function in rheumatoid arthritis and ankylosing spondylitis. Rheumatol. Int. 2020 Mar;40(3):427–436. 10.1007/s00296-019-04497-0 [DOI] [PubMed] [Google Scholar]
- 44. Pérez-Sánchez C, Ruiz-Limón P, Aguirre MA, et al. : Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in Rheumatoid Arthritis patients. J. Autoimmun. 2017 Aug;82:31–40. 10.1016/j.jaut.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 45. Anghel D, Sîrbu CA, Hoinoiu EM, et al. : Influence of anti-TNF therapy and homocysteine level on carotid intima-media thickness in rheumatoid arthritis patients. Exp. Ther. Med. 2022 Jan;23(1):59. 10.3892/etm.2021.10981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Majka DS, Vu TT, Pope RM, et al. : Association of Rheumatoid Factors With Subclinical and Clinical Atherosclerosis in African American Women: The Multiethnic Study of Atherosclerosis. Arthritis Care Res (Hoboken). 2017 Feb;69(2):166–174. 10.1002/acr.22930 [DOI] [PubMed] [Google Scholar]
- 47. Rodríguez-Carrio J, Paz B, López P, et al. : IFNα serum levels are associated with endothelial progenitor cells imbalance and disease features in rheumatoid arthritis patients. PLoS One. 2014 Jan 21;9(1):e86069. 10.1371/journal.pone.0086069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nowak B, Madej M, Łuczak A, et al. : Disease Activity, Oxidized-LDL Fraction and Anti-Oxidized LDL Antibodies Influence Cardiovascular Risk in Rheumatoid Arthritis. Adv. Clin. Exp. Med. 2016 Jan-Feb;25(1):43–50. 10.17219/acem/29847 [DOI] [PubMed] [Google Scholar]
- 49. Adawi M, Watad A, Bragazzi NL, et al. : Endothelial function in rheumatoid arthritis. QJM. 2018 Apr 1;111(4):243–247. 10.1093/qjmed/hcy010 [DOI] [PubMed] [Google Scholar]
- 50. Dimitroulas T, Hodson J, Sandoo A, et al. : Endothelial injury in rheumatoid arthritis: a crosstalk between dimethylarginines and systemic inflammation. Arthritis Res. Ther. 2017 Feb 10;19(1):32. 10.1186/s13075-017-1232-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Spinelli FR, Pecani A, Ciciarello F, et al. : Association between antibodies to carbamylated proteins and subclinical atherosclerosis in rheumatoid arthritis patients. BMC Musculoskelet. Disord. 2017 May 25;18(1):214. 10.1186/s12891-017-1563-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. González-Juanatey C, Llorca J, González-Gay MA: Correlation between endothelial function and carotid atherosclerosis in rheumatoid arthritis patients with long-standing disease. Arthritis Res. Ther. 2011 Jun 22;13(3):R101. 10.1186/ar3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rodríguez-Carrio J, Alperi-López M, López P, et al. : Red cell distribution width is associated with endothelial progenitor cell depletion and vascular-related mediators in rheumatoid arthritis. Atherosclerosis. 2015 May;240(1):131–136. 10.1016/j.atherosclerosis.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 54. Karpouzas GA, Ormseth SR, Hernandez E, et al. : Impact of Cumulative Inflammation, Cardiac Risk Factors, and Medication Exposure on Coronary Atherosclerosis Progression in Rheumatoid Arthritis. Arthritis Rheumatol. 2020 Mar;72(3):400–408. 10.1002/art.41122 [DOI] [PubMed] [Google Scholar]
- 55. Ramírez Huaranga MA, Mínguez Sánchez MD, Díaz Z, et al. : What role does rheumatoid arthritis disease activity have in cardiovascular risk?. Reumatol. Clin. (Engl Ed). 2018 Nov-Dec;14(6):339–345. 10.1016/j.reuma.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 56. Dehghan P, Rajaei A, Moeineddin R, et al. : Prevalence of atherosclerosis in patients with inactive rheumatoid arthritis. Clin. Rheumatol. 2015 Aug;34(8):1363–1366. 10.1007/s10067-015-2996-9 [DOI] [PubMed] [Google Scholar]
- 57. Lo A, Mandraffino G, Sardo MA, et al. : Circulating progenitor cells in rheumatoid arthritis: association with inflammation and oxidative stress. Scand. J. Rheumatol. 2014;43(3):184–193. [DOI] [PubMed] [Google Scholar]
- 58. Sahari NS, Shaharir SS, Ismail MR, et al. : Subclinical atherosclerosis among rheumatoid arthritis patients without overt cardiovascular risk factors. Mod. Rheumatol. 2014 Nov;24(6):920–925. 10.3109/14397595.2014.891497 [DOI] [PubMed] [Google Scholar]
- 59. Rincón I, Polak JF, O'Leary DH, et al. : Systemic inflammation and cardiovascular risk factors predict rapid progression of atherosclerosis in rheumatoid arthritis. Ann. Rheum. Dis. 2015 Jun;74(6):1118–1123. 10.1136/annrheumdis-2013-205058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Södergren A, Karp K, Bengtsson C, et al. : Biomarkers associated with cardiovascular disease in patients with early rheumatoid arthritis. PLoS One. 2019 Aug 5;14(8):e0220531. 10.1371/journal.pone.0220531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Södergren A, Karp K, Bengtsson C, et al. : The Extent of Subclinical Atherosclerosis Is Partially Predicted by the Inflammatory Load: A Prospective Study over 5 Years in Patients with Rheumatoid Arthritis and Matched Controls. J. Rheumatol. 2015 Jun;42(6):935–942. 10.3899/jrheum.140694 [DOI] [PubMed] [Google Scholar]
- 62. Mohan A, Sada S, Kumar BS, et al. : Subclinical atherosclerosis in patients with rheumatoid arthritis by utilizing carotid intima-media thickness as a surrogate marker. Indian J. Med. Res. 2014 Sep;140(3):379–386. [PMC free article] [PubMed] [Google Scholar]
- 63. Agca R, Blanken AB, Sijl AM, et al. : Arterial wall inflammation is increased in rheumatoid arthritis compared with osteoarthritis, as a marker of early atherosclerosis. Rheumatology (Oxford). 2021 Jul 1;60(7):3360–3368. 10.1093/rheumatology/keaa789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yamamoto H, Nakajima T, Kawahara R, et al. : Evaluation of risk factors for atherosclerosis using carotid ultrasonography in Japanese patients with rheumatoid arthritis. Int. J. Rheum. Dis. 2019 Jul;22(7):1312–1318. 10.1111/1756-185X.13591 [DOI] [PubMed] [Google Scholar]
- 65. Guin A, Chatterjee Adhikari M, Chakraborty S, et al. : Effects of disease modifying anti-rheumatic drugs on subclinical atherosclerosis and endothelial dysfunction which has been detected in early rheumatoid arthritis: 1-year follow-up study. Semin. Arthritis Rheum. 2013 Aug;43(1):48–54. 10.1016/j.semarthrit.2012.12.027 [DOI] [PubMed] [Google Scholar]
- 66. Dalbeni A, Giollo A, Tagetti A, et al. : Traditional cardiovascular risk factors or inflammation: Which factors accelerate atherosclerosis in arthritis patients?. Int. J. Cardiol. 2017 Jun 1;236:488–492. 10.1016/j.ijcard.2017.01.072 [DOI] [PubMed] [Google Scholar]
- 67. Ajeganova S, Faire U, Jogestrand T, et al. : Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis–an inception cohort study. J. Rheumatol. 2012 Jun;39(6):1146–1154. 10.3899/jrheum.111334 [DOI] [PubMed] [Google Scholar]
- 68. Liu JH, Ng MY, Cheung T, et al. : Ten-year progression of coronary artery, carotid artery, and aortic calcification in patients with rheumatoid arthritis. Clin. Rheumatol. 2017 Apr;36(4):807–816. 10.1007/s10067-016-3536-y [DOI] [PubMed] [Google Scholar]
- 69. Udachkina HV, Novikova DS, Popkova TV, et al. : Calcification of coronary arteries in early rheumatoid arthritis prior to anti-rheumatic therapy. Rheumatol. Int. 2018 Feb;38(2):211–217. 10.1007/s00296-017-3860-9 [DOI] [PubMed] [Google Scholar]
- 70. Tuzcu G, Uslu AU, Tuzcu A, et al. : A novel marker relationship between carotid intima media thickness and disease activity score-28 in patients with rheumatoid arthritis: Human endothelial cell-specific molecule-1. Turk. J. Med. Sci. 2019 Dec 16;49(6):1599–1605. 10.3906/sag-1806-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fan CY, Zhang ZY, Mei YF, et al. : Impaired brachial artery flow-mediated dilation and increased carotid intima-media thickness in rheumatoid arthritis patients. Chin. Med. J. 2012 Mar;125(5):832–837. [PubMed] [Google Scholar]
- 72. Yiu KH, Wang S, Mok MY, et al. : Relationship between cardiac valvular and arterial calcification in patients with rheumatoid arthritis and systemic lupus erythematosus. J. Rheumatol. 2011 Apr;38(4):621–627. 10.3899/jrheum.100844 [DOI] [PubMed] [Google Scholar]
- 73. Lo Gullo A, Mandraffino G, Imbalzano E, et al. : Toll-like receptor 3 and interleukin 1β expression in CD34+ cells from patients with rheumatoid arthritis: association with inflammation and vascular involvement. Clin. Exp. Rheumatol. 2014 Nov-Dec;32(6):922–929. [PubMed] [Google Scholar]
- 74. Chung CP, Giles JT, Kronmal RA, et al. : Progression of coronary artery atherosclerosis in rheumatoid arthritis: comparison with participants from the Multi-Ethnic Study of Atherosclerosis. Arthritis Res. Ther. 2013 Sep 25;15(5):R134. 10.1186/ar4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vázquez-Del Mercado M, Nuñez-Atahualpa L, Figueroa-Sánchez M, et al. : Serum levels of anticyclic citrullinated peptide antibodies, interleukin-6, tumor necrosis factor-α, and C-reactive protein are associated with increased carotid intima-media thickness: a cross-sectional analysis of a cohort of rheumatoid arthritis patients without cardiovascular risk factors. Biomed. Res. Int. 2015;2015:342649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mondal M, Sarkar RN, Chakroborty A, et al. : Atherosclerosis in an Indian cohort of rheumatoid arthritis with low disease activity and its correlation with inflammatory markers. Indian J. Rheumatol. 2011;6(2):61–67. 10.1016/S0973-3698(11)60059-9 [DOI] [Google Scholar]
- 77. Di Franco M, Spinelli FR, Metere A, et al. : Serum levels of asymmetric dimethylarginine and apelin as potential markers of vascular endothelial dysfunction in early rheumatoid arthritis. Mediat. Inflamm. 2012;2012:347268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kerekes G, Soltész P, Szucs G, et al. : Effects of adalimumab treatment on vascular disease associated with early rheumatoid arthritis. Isr. Med. Assoc. J. 2011 Mar;13(3):147–152. [PubMed] [Google Scholar]
- 79. Wahlin B, Meedt T, Jonsson F, et al. : Coronary Artery Calcification Is Related to Inflammation in Rheumatoid Arthritis: A Long-Term Follow-Up Study. Biomed. Res. Int. 2016;2016:1261582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ahmed A, Hollan I, Curran SA, et al. : Brief Report: Proatherogenic Cytokine Microenvironment in the Aortic Adventitia of Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2016 Jun;68(6):1361–1366. 10.1002/art.39574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Im CH, Kim NR, Kang JW, et al. : Inflammatory burden interacts with conventional cardiovascular risk factors for carotid plaque formation in rheumatoid arthritis. Rheumatology (Oxford). 2015 May;54(5):808–815. 10.1093/rheumatology/keu376 [DOI] [PubMed] [Google Scholar]
- 82. Profumo E, Di Franco M, Buttari B, et al. : Biomarkers of subclinical atherosclerosis in patients with autoimmune disorders. Mediat. Inflamm. 2012;2012:503942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chandrasekharan UM, Wang Z, Wu Y, et al. : Elevated levels of plasma symmetric dimethylarginine and increased arginase activity as potential indicators of cardiovascular comorbidity in rheumatoid arthritis. Arthritis Res. Ther. 2018 Jun 8;20(1):123. 10.1186/s13075-018-1616-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Okano T, Inui K, Sugioka Y, et al. : High titer of anti-citrullinated peptide antibody is a risk factor for severe carotid atherosclerotic plaque in patients with rheumatoid arthritis: the TOMORROW study. Int. J. Rheum. Dis. 2017 Aug;20(8):949–959. 10.1111/1756-185X.13106 [DOI] [PubMed] [Google Scholar]
- 85. Geraldino-Pardilla L, Giles JT, Sokolove J, et al. : Association of Anti-Citrullinated Peptide Antibodies With Coronary Artery Calcification in Rheumatoid Arthritis. Arthritis Care Res. (Hoboken). 2017 Aug;69(8):1276–1281. 10.1002/acr.23106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Marder W, Khalatbari S, Myles JD, et al. : Interleukin 17 as a novel predictor of vascular function in rheumatoid arthritis. Ann. Rheum. Dis. 2011 Sep;70(9):1550–1555. 10.1136/ard.2010.148031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Barbarroja N, Pérez-Sanchez C, Ruiz-Limon P, et al. : Anticyclic citrullinated protein antibodies are implicated in the development of cardiovascular disease in rheumatoid arthritis. Arterioscler. Thromb. Vasc. Biol. 2014 Dec;34(12):2706–2716. 10.1161/ATVBAHA.114.304475 [DOI] [PubMed] [Google Scholar]
- 88. Davies R, Williams J, Sime K, et al. : The role of interleukin-6 transsignalling on cardiovascular dysfunction in inflammatory arthritis. Rheumatology (Oxford). 2021 Jun 18;60(6):2852–2861. 10.1093/rheumatology/keaa725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. van Breukelen-van der Stoep DF, Zeben D, Klop B, et al. : Association of Cardiovascular Risk Factors with Carotid Intima Media Thickness in Patients with Rheumatoid Arthritis with Low Disease Activity Compared to Controls: A Cross-Sectional Study. PLoS One. 2015 Oct 20;10(10):e0140844. 10.1371/journal.pone.0140844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Karpouzas GA, Malpeso J, Choi TY, et al. : Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann. Rheum. Dis. 2014 Oct;73(10):1797–1804. 10.1136/annrheumdis-2013-203617 [DOI] [PubMed] [Google Scholar]
- 91. Kassem E, Ghonimy R, Adel M, El-Sharnoby G: Non traditional risk factors of carotid atherosclerosis in rheumatoid arthritis. The Egyptian Rheumatologist. 2011;33(3):113–119. 10.1016/j.ejr.2011.03.005 [DOI] [Google Scholar]
- 92. Wahab MAKA, El Laban A, Hasan AA, et al. : The clinical value of anti-cyclic citrullinated peptide (anti-ccp) antibodies and insulin resistance (IR) in detection of early and subclinical atherosclerosis in rheumatoid arthritis (RA). The Egyptian Heart Journal. 2016;68(2):109–116. 10.1016/j.ehj.2015.01.001 [DOI] [Google Scholar]
- 93. Di Minno MN, Ambrosino P, Lupoli R, et al. : Clinical assessment of endothelial function in patients with rheumatoid arthritis: A meta-analysis of literature studies. Eur. J. Intern. Med. 2015 Dec;26(10):835–842. 10.1016/j.ejim.2015.10.016 [DOI] [PubMed] [Google Scholar]
- 94. Arida A, Legaki AI, Kravvariti E, et al. : PCSK9/LDLR System and Rheumatoid Arthritis-Related Atherosclerosis. Front. Cardiovasc. Med. 2021 Oct 8;8:738764. 10.3389/fcvm.2021.738764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mena-Vázquez N, Redondo-Rodríguez R, Rioja J, et al. : Postprandial Hyperlipidemia: Association with Inflammation and Subclinical Atherosclerosis in Patients with Rheumatoid Arthritis. Biomedicines. 2022 Jan 8;10(1):133. 10.3390/biomedicines10010133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wahlin B, Ramnemark A, Rantapää-Dahlqvist S, et al. : Osteoprotegerin and osteocalcin are associated with atherosclerosis in patients with rheumatoid arthritis: a prospective cohort study. Clin. Exp. Rheumatol. 2021 Nov-Dec;39(6):1402–1409. Epub 2021 Feb 25. [DOI] [PubMed] [Google Scholar]
- 97. Beyazal MS, Erdoğan T, Devrimsel G, et al. : Relationship of osteoprotegerin to pulse wave velocity and carotid intima-media thickness in rheumatoid arthritis patients. Z. Rheumatol. 2016 Sep;75(7):723–8. English. 10.1007/s00393-015-1675-1 [DOI] [PubMed] [Google Scholar]
- 98. Esaily HA, Serag DM, Rizk MS, et al. : Relationship between cellular communication network factor 1 (CCN1) and carotid atherosclerosis in patients with rheumatoid arthritis. Med. J. Malaysia. 2021 May;76(3):311–317. [PubMed] [Google Scholar]
- 99. Mulumba C, Lebughe P, Mbuyi-Muamba JM, et al. : Prevalence and associated factors of subclinical atherosclerosis in rheumatoid arthritis at the university hospital of Kinshasa. BMC Rheumatol. 2019 Sep 9;3:37. 10.1186/s41927-019-0085-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Elshereef RR, Darwish A, Ali A, et al. : Asymptomatic atherosclerosis in egyptian rheumatoid arthritis patients and its relation to disease activity. Int. J. Rheumatol. 2015;2015:381931. Epub 2015 Feb 8. 10.1155/2015/381931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ristić GG, Subota V, Lepić T, et al. : Subclinical Atherosclerosis in Patients with Rheumatoid Arthritis and Low Cardiovascular Risk: The Role of von Willebrand Factor Activity. PLoS One. 2015 Aug 6;10(8):e0130462. 10.1371/journal.pone.0130462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Södergren A, Karp K, Bengtsson C, et al. : Is Lipoprotein-Associated Phospholipase A2 a Link between Inflammation and Subclinical Atherosclerosis in Rheumatoid Arthritis?. Biomed. Res. Int. 2015;2015:673018. Epub 2015 Oct 4. 10.1155/2015/673018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mena-Vázquez N, Rojas-Gimenez M, Jimenez Nuñez FG, et al. : Postprandial Apolipoprotein B48 is Associated with Subclinical Atherosclerosis in Patients with Rheumatoid Arthritis. J. Clin. Med. 2020 Aug 2;9(8):2483. 10.3390/jcm9082483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Södergren A, Karp K, Boman K, et al. : Atherosclerosis in early rheumatoid arthritis: very early endothelial activation and rapid progression of intima media thickness. Arthritis Res. Ther. 2010;12(4):R158. Epub 2010 Aug 16. 10.1186/ar3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kahlow BS, Nisihara R, Petisco R, et al. : Cardiovascular risk and mannose binding lectin in patients with rheumatoid arthritis from southern Brazil. IJC Heart Vasc. 2018;20:27–31. 10.1016/j.ijcha.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tousoulis D, Oikonomou E, Economou EK, et al. : Inflammatory cytokines in atherosclerosis: current therapeutic approaches. Eur. Heart J. 2016 Jun 7;37(22):1723–1732. 10.1093/eurheartj/ehv759 [DOI] [PubMed] [Google Scholar]
- 107. McKellar GE, McCarey DW, Sattar N, et al. : Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat. Rev. Cardiol. 2009 Jun;6(6):410–417. 10.1038/nrcardio.2009.57 [DOI] [PubMed] [Google Scholar]
- 108. Libby P, Warner SJ, Friedman GB: Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J. Clin. Invest. 1988 Feb;81(2):487–498. 10.1172/JCI113346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Doran AC, Meller N, McNamara CA: Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008 May;28(5):812–819. 10.1161/ATVBAHA.107.159327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Carbone F, Bonaventura A, Liberale L, et al. : Atherosclerosis in Rheumatoid Arthritis: Promoters and Opponents. Clin. Rev. Allergy Immunol. 2020 Feb;58(1):1–14. 10.1007/s12016-018-8714-z [DOI] [PubMed] [Google Scholar]
- 111. Fan LY, He DY, Wang Q, et al. : Citrullinated vimentin stimulates proliferation, pro-inflammatory cytokine secretion, and PADI4 and RANKL expression of fibroblast-like synoviocytes in rheumatoid arthritis. Scand. J. Rheumatol. 2012 Oct;41(5):354–358. 10.3109/03009742.2012.670263 [DOI] [PubMed] [Google Scholar]
- 112. Moschonas IC, Tselepis AD: The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis. 2019 Sep;288:9–16. 10.1016/j.atherosclerosis.2019.06.919 [DOI] [PubMed] [Google Scholar]
- 113. Döring Y, Libby P, Soehnlein O: Neutrophil Extracellular Traps Participate in Cardiovascular Diseases: Recent Experimental and Clinical Insights. Circ. Res. 2020 Apr 24;126(9):1228–1241. 10.1161/CIRCRESAHA.120.315931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Clavel C, Nogueira L, Laurent L, et al. : Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 2008 Mar;58(3):678–688. 10.1002/art.23284 [DOI] [PubMed] [Google Scholar]
- 115. Jacobsen S, Garred P, Madsen HO, et al. : Mannose-binding lectin gene polymorphisms are associated with disease activity and physical disability in untreated, anti-cyclic citrullinated peptide-positive patients with early rheumatoid arthritis. J. Rheumatol. 2009 Apr;36(4):731–735. 10.3899/jrheum.080846 [DOI] [PubMed] [Google Scholar]
- 116. Turner MW, Hamvas RM: Mannose-binding lectin: structure, function, genetics and disease associations. Rev. Immunogenet. 2000;2(3):305–322. [PubMed] [Google Scholar]
- 117. Troelsen LN, Garred P, Christiansen B, et al. : Double role of mannose-binding lectin in relation to carotid intima-media thickness in patients with rheumatoid arthritis. Mol. Immunol. 2010 Jan;47(4):713–8. Epub 2009 Nov 24. 10.1016/j.molimm.2009.10.021 [DOI] [PubMed] [Google Scholar]
- 118. Bordy R, Totoson P, Prati C, et al. : Microvascular endothelial dysfunction in rheumatoid arthritis. Nat. Rev. Rheumatol. 2018 Jul;14(7):404–420. 10.1038/s41584-018-0022-8 [DOI] [PubMed] [Google Scholar]
- 119. Haller H: Endothelial function. General considerations. Drugs. 1997;53(Suppl 1):1–10. 10.2165/00003495-199700531-00003 [DOI] [PubMed] [Google Scholar]
- 120. Hadi HA, Carr CS, Al Suwaidi J: Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005;1(3):183–198. [PMC free article] [PubMed] [Google Scholar]
- 121. Şentürk T, Yılmaz N, Sargın G, et al. : Relationship between asymmetric dimethylarginine and endothelial dysfunction in patients with rheumatoid arthritis. Eur. J. Rheumatol. 2016 Sep;3(3):106–108. 10.5152/eurjrheum.2016.15096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Naghashian F, Hosseinzadeh-Attar MJ, Akhlaghi M, et al. : The relationship between anthropometric status and rheumatoid arthritis. Exploring the role of nesfatin and asymmetric dimethylarginine. Acta Reumatol. Port. 2019 Apr-Jun;44(2):126–131. [PubMed] [Google Scholar]
- 123. Kwaśny-Krochin B, Głuszko P, Undas A: Plasma asymmetric dimethylarginine in active rheumatoid arthritis: links with oxidative stress and inflammation. Pol. Arch. Med. Wewn. 2012;122(6):270–276. [DOI] [PubMed] [Google Scholar]
- 124. Zafari P, Zarifian A, Alizadeh-Navaei R, et al. : Asymmetric and symmetric dimethylarginine concentration as an indicator of cardiovascular diseases in rheumatoid arthritis patients: a systematic review and meta-analysis of case-control studies. Clin. Rheumatol. 2020 Jan;39(1):127–134. 10.1007/s10067-019-04713-z [DOI] [PubMed] [Google Scholar]
- 125. Förstermann U, Münzel T: Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006 Apr 4;113(13):1708–1714. 10.1161/CIRCULATIONAHA.105.602532 [DOI] [PubMed] [Google Scholar]
- 126. Rosselli M, Keller PJ, Dubey RK: Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum. Reprod. Update. 1998 Jan-Feb;4(1):3–24. 10.1093/humupd/4.1.3 [DOI] [PubMed] [Google Scholar]
- 127. Hill JM, Zalos G, Halcox JP, et al. : Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003 Feb 13;348(7):593–600. 10.1056/NEJMoa022287 [DOI] [PubMed] [Google Scholar]
- 128. Lee ST, Chu K, Jung KH, et al. : Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology. 2008 Apr 22;70(17):1510–1517. 10.1212/01.wnl.0000294329.93565.94 [DOI] [PubMed] [Google Scholar]
- 129. Werner N, Kosiol S, Schiegl T, et al. : Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 2005 Sep 8;353(10):999–1007. 10.1056/NEJMoa043814 [DOI] [PubMed] [Google Scholar]
- 130. Fujii H, Li SH, Szmitko PE, et al. : C-reactive protein alters antioxidant defenses and promotes apoptosis in endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2006 Nov;26(11):2476–2482. 10.1161/01.ATV.0000242794.65541.02 [DOI] [PubMed] [Google Scholar]
- 131. Nordestgaard BG, Benn M, Schnohr P, et al. : Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007 Jul 18;298(3):299–308. 10.1001/jama.298.3.299 [DOI] [PubMed] [Google Scholar]
- 132. Burggraaf B, Breukelen-van der Stoep DF, Zeben J, et al. : Evidence for increased chylomicron remnants in rheumatoid arthritis. Eur. J. Clin. Investig. 2018 Feb;48(2). Epub 2018 Jan 8. 10.1111/eci.12873 [DOI] [PubMed] [Google Scholar]
- 133. Asanuma Y, Chung CP, Oeser A, et al. : Serum osteoprotegerin is increased and independently associated with coronary-artery atherosclerosis in patients with rheumatoid arthritis. Atherosclerosis. 2007;195:e135–e141. 10.1016/j.atherosclerosis.2007.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Asanuma YF, Shimada Y, Kouzu N, et al. : Serum osteoprotegerin concentration is associated with carotid atherosclerotic plaque in patients with rheumatoid arthritis. Mod. Rheumatol. Jpn. Rheum. Assoc. 2013;23:269–275. 10.3109/s10165-012-0654-5 [DOI] [PubMed] [Google Scholar]
- 135. Dessein PH, López-Mejias R, Gonzálezjuanatey C, et al. : Independent relationship of osteoprotegerin concentrations with endothelial activation and carotid atherosclerosis in patients with severe rheumatoid arthritis. J. Rheumatol. 2014;41:429–436. 10.3899/jrheum.131037 [DOI] [PubMed] [Google Scholar]
- 136. De Stefano A, Mannucci L, Tamburi F, et al. : Lp-PLA2, a new biomarker of vascular disorders in metabolic diseases. Int. J. Immunopathol. Pharmacol. 2019 Jan-Dec;33:2058738419827154. 10.1177/2058738419827154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Aletaha D, Smolen JS: Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA. 2018 Oct 2;320(13):1360–1372. 10.1001/jama.2018.13103 [DOI] [PubMed] [Google Scholar]
- 138. Küçükdeveci AA: Nonpharmacological treatment in established rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2019 Oct;33(5):101482. Epub 2020 Jan 25. 10.1016/j.berh.2019.101482 [DOI] [PubMed] [Google Scholar]
- 139. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. : EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020 Jun;79(6):685–699. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 140. Hoffmeister RT: Methotrexate therapy in rheumatoid arthritis: 15 years experience. Am. J. Med. 1983;75(6A):69–73. 10.1016/0002-9343(83)90477-1 [DOI] [PubMed] [Google Scholar]
- 141. Aga AB, Lie E, Uhlig T, et al. : Time trends in disease activity, response and remission rates in rheumatoid arthritis during the past decade: results from the NOR-DMARD study 2000-2010. Ann. Rheum. Dis. 2015;74(2):381–388. 10.1136/annrheumdis-2013-204020 [DOI] [PubMed] [Google Scholar]