Abstract
Ubiquitin-specific peptidase 25 (USP25) is a key deubiquitylase belonging to the USP superfamily that is primarily involved in inflammation and the immune response. Thymic stromal lymphopoietin (TSLP) is an epithelial-derived cytokine that is regarded as the master switch that initiates and maintains the type 2 immune response in allergic rhinitis (AR). However, the molecular mechanisms by which USP25 regulates TSLP signaling in the nasal epithelium in AR remain unclear. The present study assessed the protein expression levels of USP25 in the nasal epithelium of patients with AR. Moreover, USP25 knockout (KO) and wild-type (WT) mice were treated with ovalbumin (OVA) to establish a model of AR. The results of western blotting and immunohistochemistry in the present study demonstrated that the protein expression levels of USP25 were significantly decreased in the nasal mucosa of patients with AR and AR mice, whereas the protein expression levels of TSLP were significantly increased. Allergic inflammation was more severe in USP25 KO mice compared with WT mice exposed to OVA, as demonstrated by increased nose scratching and sneezing, increased eosinophil infiltration, goblet cell hyperplasia and enhanced T helper type 2 (Th2) cytokine production. The results of in vitro experiments demonstrated that silencing or overexpression of USP25 decreased or increased TNF receptor-associated factor 3 (TRAF3) protein expression levels, respectively, in human nasal epithelial cells, whereas TSLP protein expression levels were negatively associated with the expression of USP25 and TRAF3. In summary, USP25 downregulation enhanced TSLP signaling in the nasal mucosal epithelium via decreased TRAF3 expression, thereby exacerbating inflammation in AR. Therefore, USP25 may act as a novel therapeutic target in AR.
Keywords: allergic rhinitis, epithelial-derived cytokine, deubiquitylase, thymic stromal lymphopoietin, ubiquitin-specific peptidase 25
Introduction
Allergic rhinitis (AR) is a common disease of the upper airway that typically manifests as sneezing, nasal congestion, rhinorrhea and pruritus and affects the quality of life of patients (1). It is estimated that 10–40% of the population is affected by AR worldwide (2,3). Moreover, the incidence of AR is increasing globally every year (4). In Europe, for example, an analysis of Polish data showed a rise in the prevalence of AR from 4.8% in 2003 to 7.7% in 2012 (5). In Asia, a survey of self-reported AR in the general Chinese adult population demonstrated that the prevalence of AR increased between 2005 and 2011 from 11.1 to 17.6% (6). In addition, the Korean National Health and Nutrition Examination Survey reported an increase of the prevalence of AR from 1.0% in 1998 to 17.1% in 2017 (7,8). Despite increasing research, the specific pathogenesis of AR remains to be fully elucidated.
Increasing evidence indicates that nasal epithelial cell dysfunction may be the major cause of nasal allergic inflammation (9,10). The nasal epithelium is the first barrier against allergen infiltration and epithelial cell-derived cytokine milieu activates different types of immune cell, thereby acting as a master immune regulator in allergic inflammation (11–13). Thymic stromal lymphopoietin (TSLP) is one of the primary epithelial-derived cytokines in T helper type 2 (Th2) reactions (14). TSLP programs dendritic cells to activate the Th2 inflammatory response and is therefore considered a key epithelial-derived molecule in the sensitization/priming phase of allergic airway disease (15,16). Moreover, TSLP activates immune cells, such as type 2 innate lymphoid cells and basophils, to sustain Th2 polarized immunity (17). Numerous studies have reported that NF-κB is a key transcription factor in the regulation of TSLP expression (18–20). However, the reasons for the aberrant activation of NF-κB and the ensuing increase in TSLP expression in AR are still poorly understood.
Ubiquitination is a common post-translational modification involved in numerous aspects of protein function, such as degradation, localization and protein-protein interactions (21). Deubiquitination is the reverse process mediated by deubiquitinating enzymes (DUBs), that serve a key role in biological activities, such as inflammation, signal transduction and the immune response (22,23). Ubiquitin-specific peptidase (USP)25 is a key deubiquitylase belonging to the USP superfamily that is primarily involved in inflammation and the immune response (24,25). TNF receptor-associated factor 3 (TRAF3) is one of the key signal transduction molecules that regulates activation of the NF-κB signaling pathway. Furthermore, USP25 serves a key role in regulating NF-κB activation associated with inflammation or the immune response by altering the ubiquitination of TRAF3. Zhong et al (26) reported that USP25 may inhibit the degradation of TRAF3 during activation of the toll-like receptor (TLR)4 signaling pathway, thereby achieving balance of innate immune response. Moreover, Lin et al (27) demonstrated that USP25 promotes antiviral responses via stabilization of TRAF3 expression. Therefore, the present study evaluated the role of USP25 in AR and whether USP25 regulates TSLP expression in nasal epithelial cells.
Materials and methods
Subjects
A total of 25 patients with AR and 15 healthy volunteers were recruited for the present study at the Renmin Hospital of Wuhan University (Wuhan, China) between June 2020 and August 2020. Characteristics of the subjects are presented in Tables SI and SII. Nasal mucosal samples were obtained from the inferior turbinate tissue of patients with AR and healthy volunteers. The diagnosis of AR was based on The Chinese Society of Allergy Guidelines for Diagnosis and Treatment of Allergic Rhinitis (28). Inclusion criteria included being diagnosed with AR, being between the ages of 18–60 years, and having the ability to provide informed consent. The exclusion criteria were: respiratory infection, uncontrolled asthma, autoimmune diseases, and receipt of immunotherapy or corticosteroids within the past month. All participants were requested to rate the severity of their rhinitis symptoms, including sneezing, nasal congestion, rhinorrhea and nasal itching, using the visual analog scale (score, 0–10) (29). The present study was approved by the Ethics Committee of Renmin Hospital of Wuhan University (approval no. WDRY2018-K014). All participants provided written informed consent.
Animals
A total of 30 female wild-type (WT) C57BL/6 mice (age, 6–8 weeks; weight, 20–25 g) were purchased from Shulaibao Biotechnology Co., Ltd (http://shulb.com/). A total of 15 female USP25 knockout (KO) C57BL/6 mice (age, 6–8 weeks; weight, 20–25 g) were donated by Dr Bo Zhong at the State Key Laboratory of Virology, College of Life Sciences, Wuhan University (Wuhan, China). Mice were housed in a specific-pathogen-free biohazard containment facility with a 12/12-h dark/light cycle and moderate humidity (45–55%) at room temperature (22±1°C) in the Animal Experiment Center of The Renmin Hospital of Wuhan University. Food and water were freely available to the mice. All experimental procedures involving animals were approved by the Animal Care and Use Committee of The Renmin Hospital of Wuhan University (approval no. WDRM-20211005).
Ovalbumin (OVA)-induced AR mouse model
USP25 KO and WT mice were randomly assigned into control and AR groups (n=6 mice/group). AR mouse model induced by OVA was established as previously described (30,31). Briefly, mice were treated with intraperitoneal injection of 300 µl phosphate-buffered saline (PBS) containing 100 µg OVA (Sigma-Aldrich; Merck KGaA) and 1 mg aluminum hydroxide on days 0, 7 and 14. On days 21–34, mice were treated intranasally with 100 mg/ml OVA solution (40 µl/mouse) daily. The control group was injected with the same dose of PBS and intranasally treated with 40 µl PBS solution on days 21–34. Nasal symptoms evaluated in mice included the frequency of sneezes and nasal rubbing. For 10 min following the last nasal treatment with OVA, nasal rubbing and sneezing frequency were assessed in each mouse by two independent, blinded investigators. The mean of all observations per group was used as the final result. At 24 h following the last nasal treatment, all mice were anesthetized using an intraperitoneal injection of sodium pentobarbital (50 mg/kg). Blood samples (1 ml/mouse) were collected from mice under anesthesia, as well as nasal lavage fluid (NLF) samples (1 ml/mouse). Following blood and NLF sample collection, the mice were sacrificed using cervical dislocation and the noses were collected for further analysis.
Histological analysis
Nasal mucosal tissue was fixed using 4% paraformaldehyde at room temperature for 24 h and embedded in paraffin. Subsequently, paraffin-embedded tissue was sectioned (5 µm). Sections were stained using hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) to evaluate eosinophil infiltration and goblet cell hyperplasia, respectively. The number of eosinophils and goblet cells in four randomly selected fields of view under a light microscope at ×400 magnification was quantified by two independent investigators and the mean value was determined.
Immunohistochemistry
Nasal mucosal tissue was fixed with 4% paraformaldehyde for 24 h at room temperature and embedded in paraffin for sectioning into 5 µm. The paraffin-embedded sections were deparaffinized with xylene, rehydrated with graded alcohol and heated with citrate buffer solution (10 mmol/l, pH 6.0) for 10 min for antigen retrieval. Subsequently, the slides were soaked in 3% hydrogen peroxide solution for 15 min at room temperature to quench the endogenous peroxidase activity. The slides were blocked with 10% normal goat serum (Wuhan Servicebio Technology, Co., Ltd.) for 15 min at room temperature. Then, the slides were incubated with primary antibodies targeting USP25 (1:200; Santa Cruz Biotechnology, Inc.) or TSLP (1:500; cat. no. ab188766; Abcam) at 4°C overnight. Following primary incubation, sections were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:100; cat. no. sc-2357; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. Immunoreactivity was visualized using 3,3-diaminobenzidine (DAB) and hematoxylin was used for counterstaining at 37°C for 25 sec. Subsequently, slides were dehydrated, cleaned and sealed. A total of three slides from each mouse were selected for evaluation. The expression and localization of USP25 and TSLP in the nasal septum were assessed in four randomly selected fields of view under a light microscope at ×400 magnification by two independent investigators who were blinded to sample identities.
ELISA
The protein expression levels of IL-4 (cat. no. M4000B; R&D Systems, Inc.), IL-5 (cat. no. M5000; R&D Systems, Inc.), IL-10 (cat. no. M1000B; R&D Systems, Inc.), IL-13 (cat. no. DY413; R&D Systems, Inc.) and IFN-γ (cat. no. MIF00; R&D Systems, Inc.) in serum and NLF were assessed using specific ELISA kits (R&D Systems, Inc.), according to the manufacturer's protocols. The levels of TSLP in cell culture supernatant were also assessed using an ELISA kit (cat. no. MTLP00; R&D Systems, Inc.).
Cell culture and treatment
Human nasal epithelial cells (HNEpCs; RPMI 2650) were purchased from BeNa Culture Collection (cat. no. BNCC356247; Beijing Beina Chunglian Institute of Biotechnology). HNEpCs were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin (Sigma-Aldrich; Merck KGaA) and incubated at 37°C in a humidified chamber containing 5% CO2. Cells were either unstimulated or stimulated with 50 µg/ml house dust mite (HDM) extract (Greer Laboratories, Inc.) at 37°C for 24 h as previously described (12).
Cell transfection
Small interfering RNA (siRNA) targeting USP25 (si-USP25; antisense, 5′-UAAUUCAGAACUAAUCUUCUA-3′) and TRAF3 (si-TRAF3; antisense, 5′-UAUCCUUAAACACCUUGUCUU-3′) were purchased from Shanghai GenePharma Co., Ltd. Scrambled sequence siRNA (Shanghai GenePharma Co., Ltd.) was used as a negative control (NC; antisense, 5′-GGTACGTCGAGTGAGTTAA-3′). The pcDNA 3.1 plasmids containing the coding sequences of USP25 (pcDNA-USP25) or TRAF3 (pcDNA-TRAF3) were constructed and purchased from BT Lab. Empty vectors (Bio-transduction Lab) were used as negative controls. HNEpCs were seeded into a 6-well plate at a density of 3×105 cells/well. When 70–80% confluence was reached, siRNAs (10 nM) and plasmids (4 g) were transfected into HNEpCs alone or in combination using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocols. Cells were collected for subsequent experiments 48 h at 37°C following transfection.
Western blotting
Total protein was extracted from cells and nasal mucosal tissue using RIPA lysis buffer (Wuhan Servicebio Technology, Co., Ltd.). Protein concentrations were determined using a bicinchoninic acid kit (Wuhan Servicebio Technology, Co., Ltd.). The sample proteins (40 µg/lane) were subjected to electrophoresis using 10% SDS-PAGE gels. Subsequently, the proteins were transferred to PVDF membranes (MilliporeSigma) and membranes were incubated with primary antibodies against USP25 (1:1,000; cat. no. ab187156; Abcam), TRAF3 (1:1,000; cat. no. ab36988; Abcam), TSLP (1:1,000; cat. no. ab188766; Abcam) and GAPDH (1:500; cat. no. sc-47724; Santa Cruz Biotechnology, Inc.) at 4°C overnight. Following three washes with tris-buffered saline with 0.1% Tween-20, membranes were incubated with HRP goat anti-rabbit secondary antibodies (1:10,000; cat. no. GB23303; Wuhan Servicebio Technology, Co., Ltd.) at room temperature for 1 h. A Servicebio® super-sensitive enhanced chemiluminescence (ECL) substrate kit (cat. no. G2020; Wuhan Servicebio Technology, Co., Ltd.) was used to visualize protein bands and GAPDH was used as an internal control. Relative protein expression levels were semi-quantified using ImageJ 1.48v software (National Institutes of Health).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.0 (GraphPad Software, Inc.). All data are presented as the mean ± standard deviation of a minimum of three independent experiments. Differences between two groups were assessed using unpaired Student's t-test. One- or two-way ANOVA followed by Tukey's post hoc test was used for comparisons between multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
USP25 expression is decreased in the nasal mucosa of patients with AR and expression of TSLP is increased
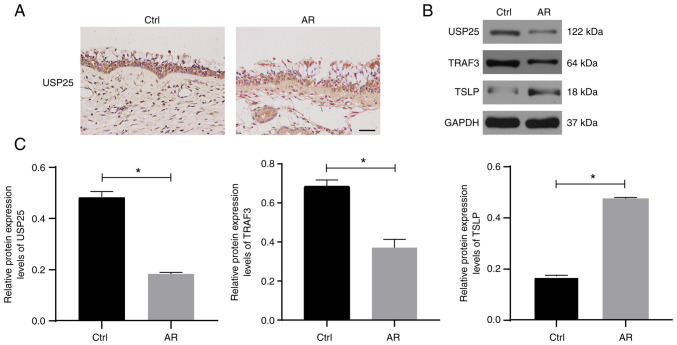
The protein expression of USP25 was analyzed using paraffinized sections of nasal biopsies obtained from patients with AR and healthy controls subjected to immunohistochemical staining. The results demonstrated that USP25 protein was primarily expressed in epithelial cells of the nasal mucosa. Moreover, markedly weaker immunostaining of USP25 was observed in nasal mucosal sections of patients with AR compared with control sections (Fig. 1A). The protein expression levels of USP25, TRAF3 and TSLP were semi-quantified in the nasal mucosal tissue of patients with AR and healthy subjects using western blotting. The results demonstrated that the protein expression levels of USP25 and TRAF3 were significantly lower in the nasal mucosal tissue of patients of AR than those of the control group, whereas protein expression levels of TSLP were significantly higher than those of the control group (Fig. 1B and C).
Figure 1.
USP25 protein expression levels are decreased in the nasal mucosa of patients with AR. (A) Representative immunohistochemical images of USP25 (magnification, ×400). (B) Protein expression of USP25, TRAF3 and TSLP in nasal mucosal tissue was assessed using western blotting. (C) Semi-quantitative analysis of western blot band intensity. Data are presented as the mean ± standard deviation (n=6). Each experiment was repeated three times. Scale bar, 50 µm. Statistical analysis was performed using independent Student's t-test. *P<0.05. USP25, ubiquitin-specific peptidase 25; AR, allergic rhinitis; TRAF3, TNF receptor-associated factor 3; TSLP, thymic stromal lymphopoietin; Ctrl, control.
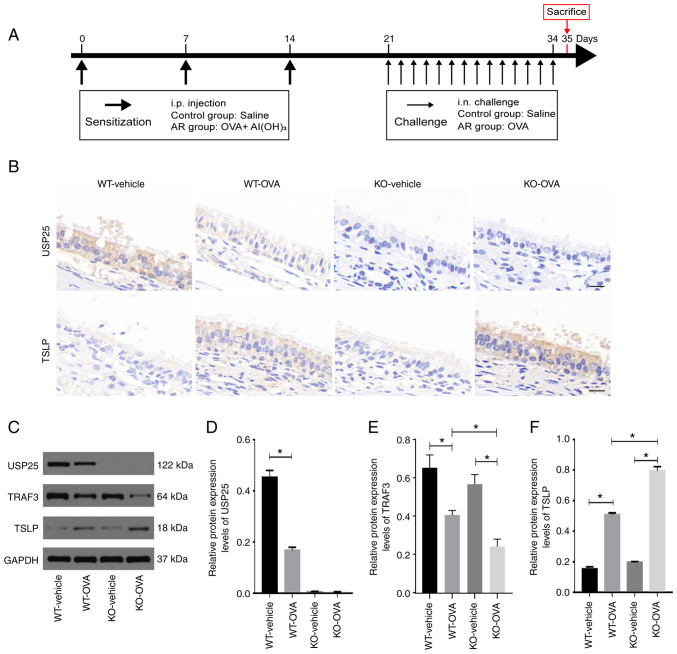
USP25 knockdown increases TSLP expression in the nasal mucosa of OVA-induced mice
To assess whether USP25 knockdown was associated with elevated TSLP protein expression levels, an AR animal model was constructed using USP25 KO and WT mice. A summary of the animal experimental procedures is presented in Fig. 2A. Consistent with the results observed in patients with AR, both USP25 and TSLP were localized in mouse nasal mucosal epithelial cells. Immunostaining of TSLP in the nasal mucosal tissue of WT mice with AR was significantly enhanced compared with the control group. Moreover, immunostaining of TSLP in nasal mucosa sections was markedly increased in OVA-induced USP25 KO mice (Fig. 2B). Furthermore, western blotting demonstrated that TSLP protein expression levels were significantly higher in nasal mucosa of OVA-induced WT mice compared with control mice and protein expression levels of TSLP and TRAF3 in the nasal mucosa were further increased in OVA-induced USP25 KO mice compared with the vehicle control (Fig. 2C-F). Collectively, these results suggested that USP25 may negatively regulate protein expression levels of TSLP.
Figure 2.
USP25 knockdown increases protein expression levels of TSLP in the nasal mucosa of OVA-induced mice. (A) Schematic experimental protocol for OVA-induced AR in USP25 KO and WT mice. (B) Representative immunohistochemical images of USP25 and TSLP staining. (C) Protein expression levels of (D) USP25, (E) TRAF3 and (F) TSLP in nasal mucosal tissue were assessed using western blotting. Semi-quantitative analysis of band intensity. Data are presented as the mean ± standard deviation (n=6). Each experiment was repeated three times. Scale bar, 50 µm. Statistical analysis was performed using two-way ANOVA followed by Tukey's post hoc test. *P<0.05. USP25, ubiquitin-specific peptidase 25; TSLP, thymic stromal lymphopoietin; OVA, ovalbumin; AR, allergic rhinitis; TRAF3, TNF receptor-associated factor 3; i.p., intraperitoneal; i.n., intranasal; KO, knockout; WT, wild-type.
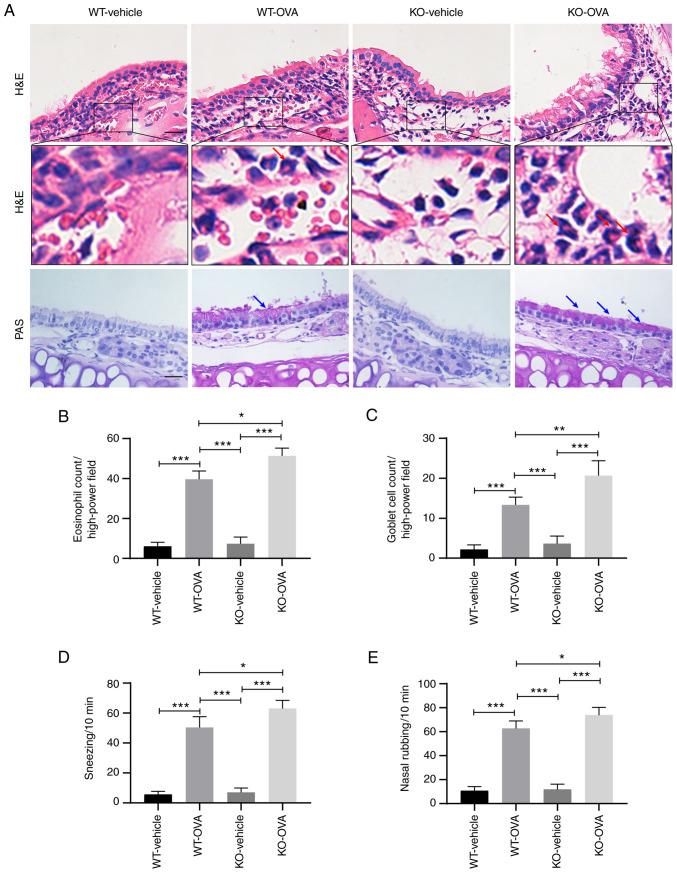
USP25 knockdown exacerbates nasal mucosal inflammation and nasal symptoms in OVA-induced mice
The primary pathological features of AR include a large infiltration of eosinophils under the stroma of the nasal mucosa and increased mucus secretion from goblet cells (2). H&E staining demonstrated that OVA induction led to significantly increased infiltration of eosinophils in the nasal mucosa of WT mice compared with WT-vehicle group and eosinophil infiltration was significantly increased in OVA-induced USP25 KO mice compared with OVA-induced WT mice (Fig. 3A and B). Furthermore, histological analysis of nasal mucosa sections using PAS staining demonstrated notable goblet cell hyperplasia in OVA-induced WT mice compared with the WT-vehicle mice and the number of goblet cells was significantly increased in OVA-induced USP25 KO mice compared with OVA-induced WT mice (Fig. 3A and C).
Figure 3.
USP25 knockdown exacerbates OVA-induced inflammatory responses in nasal mucosa and nasal symptoms. (A) H&E and PAS staining were used to assess eosinophil infiltration and goblet cell metaplasia, respectively. Red arrows indicate eosinophils. Blue arrows indicate PAS-positive goblet cells. Quantitative analysis of (B) eosinophils and (C) goblet cells. Frequency of (D) sneezing and (E) nose scratching was counted for 10 min following final treatment with OVA. Data are presented as the mean ± standard deviation (n=6). Each experiment was repeated three times. Scale bar, 50 µm. Statistical analysis was performed using two-way ANOVA followed by Tukey's post hoc test. *P<0.05, **P<0.01 and ***P<0.001. USP25, ubiquitin-specific peptidase 25; OVA, ovalbumin; H&E, hematoxylin and eosin; PAS, periodic acid-Schiff; KO, knockout; WT, wild-type.
OVA-induced mice exhibited typical nasal allergy symptoms, including nasal rubbing and sneezing. Compared with OVA-induced WT mice, OVA-induced USP25 KO mice exhibited significantly increased levels of both nose rubbing and sneezing (Fig. 3D and E).
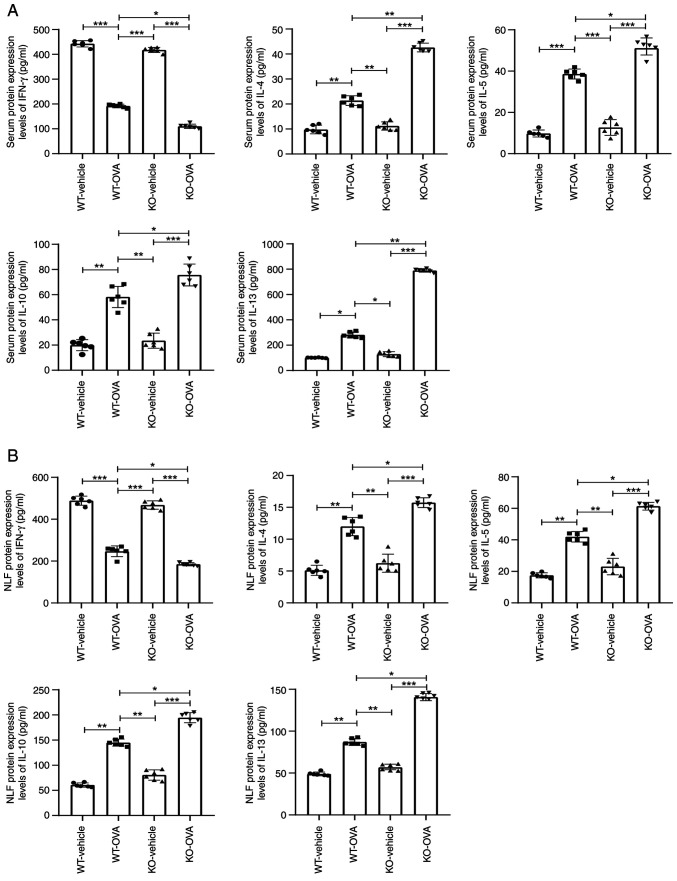
USP25 knockdown exacerbates the imbalance of Th1/Th2-associated cytokines in mice
TSLP promotes synthesis and release of Th2-type cytokines, such as IL-4, IL-5, IL-10 and IL-13, and inhibits production of Th1-type cytokines (17). Therefore, the effect of USP25 knockdown on the production of Th2 cytokines was evaluated. The protein expression levels of IL-4, IL-5, IL-10 and IL-13 were significantly increased in both serum and NLF of OVA-induced WT mice compared with WT-vehicle mice. These protein expression levels were further elevated in OVA-induced USP25 KO mice compared with OVA-induced WT mice (Fig. 4A and B). However, protein expression levels of the Th1 cytokine IFN-γ were significantly decreased in both the serum and NLF of OVA-induced WT mice compared with the WT-vehicle mice and further significantly decreased in USP25 KO mice compared with OVA- induced WT mice.
Figure 4.
USP25 knockdown promotes expression of pro-inflammatory cytokines in NLF and serum of OVA-induced mice. IFN-γ, IL-4, IL-5, IL-10 and IL-13 protein expression levels were assessed in (A) serum and (B) NLF using ELISA. Data are presented as the mean ± standard deviation (n=6). Each experiment was repeated three times. Statistical analysis was performed using two-way ANOVA followed by Tukey's post hoc test. *P<0.05, **P<0.01 and ***P<0.001. USP25, ubiquitin-specific peptidase 25; NLF, nasal lavage fluid; OVA, ovalbumin; KO, knockout; WT, wild-type.
USP25 affects TSLP expression via regulation of TRAF3 protein in human mucosal epithelial cells
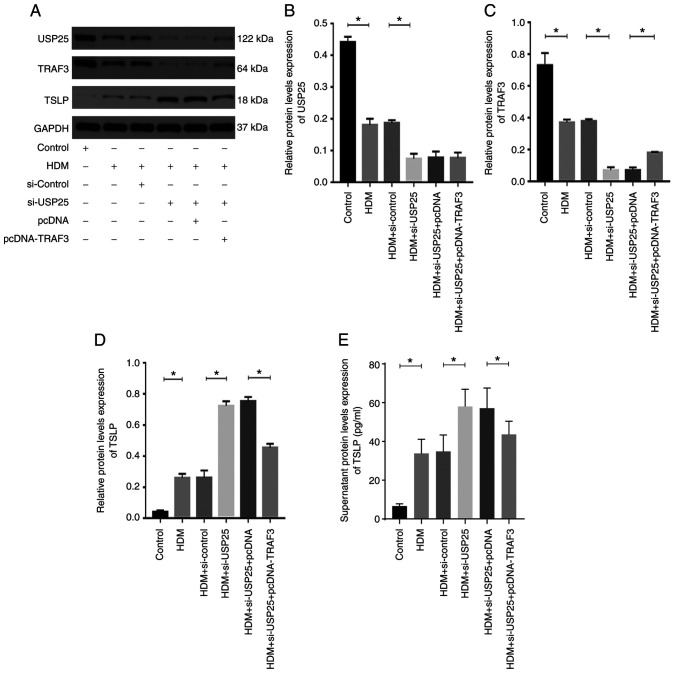
HDMs are one of the most common inhaled allergens (2). Therefore, HDM extract was used to stimulate HNEpCs to evaluate the regulatory association between USP25 and TSLP. Successful transfection of HNEpCs with the siRNAs or the plasmids was verified by western blotting experiments (Fig. S1). HDM triggered a significant increase in TSLP protein expression levels in HNEpCs and significantly decreased protein expression levels of USP25 and TRAF3 compared with the control (Fig. 5). Moreover, the protein expression levels of USP25 and TRAF3 were further significantly decreased and protein expression levels of TSLP were further increased in the HDM + si-USP25 group compared with HDM + si-control group. The TSLP protein expression levels were significantly reduced in the HDM + si-USP25 + pcDNA-TRAF3 group compared with the HDM + si-USP25 + pcDNA group.
Figure 5.
USP25 knockdown increases TSLP protein expression levels via inhibition of TRAF3 in vitro. (A) Protein expression levels of (B) USP25, (C) TRAF3 and (D) TSLP were assessed using western blotting in HDM-stimulated nasal epithelial cells following transfection with si-USP25 and pcDNA-TRAF3 alone or in combination. Semi-quantitative analysis of band intensity. (E) TSLP protein expression levels in cell culture supernatant were assessed using ELISA. Data are presented as the mean ± standard deviation (n=3). Each experiment was repeated three times. Statistical analysis was performed using one-way ANOVA followed by Tukey's post hoc test. *P<0.05. USP25, ubiquitin-specific peptidase 25; TSLP, thymic stromal lymphopoietin; TRAF3, TNF receptor-associated factor 3; HDM, house dust mite; si, small interfering.
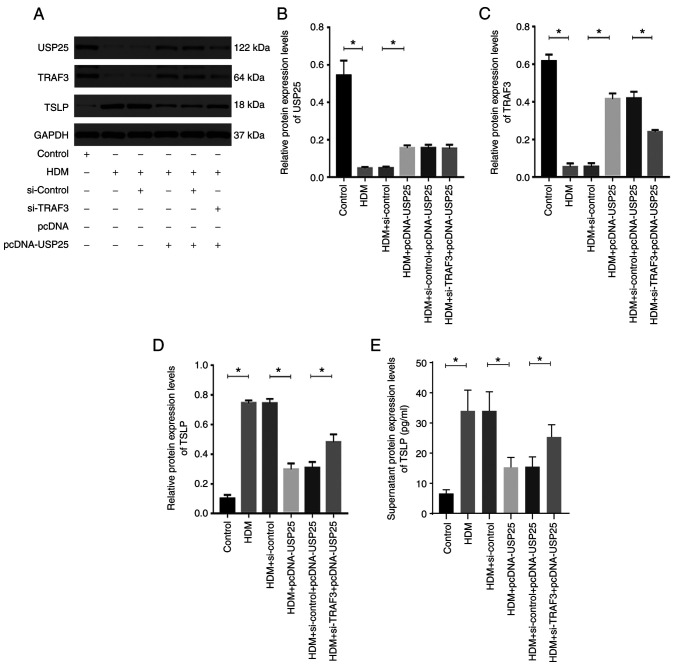
USP25 overexpression significantly increased protein expression levels of USP25 and TRAF3 and significantly decreased protein expression levels of TSLP compared with the HDM + pcDNA-USP25 group, whereas simultaneous TRAF3 knockdown and USP25 overexpression significantly reversed the effect on protein expression levels of TSLP (Fig. 6). Furthermore, protein expression levels of TSLP in cell culture supernatant were evaluated. The results demonstrated a significant increase in TSLP in supernatant following stimulation with HDM compared with the control. USP25 knockdown significantly increased protein expression levels of TSLP in supernatant compared with HDM + si-control group, whereas USP25 overexpression exerted the opposite effect. Notably, no significant changes in USP25 protein expression levels were observed following TRAF3 overexpression or knockdown, which further indicated that TRAF3 acts downstream of USP25. In conclusion, these results suggested that USP25 may regulate TSLP expression via TRAF3 protein in nasal mucosal epithelial cells.
Figure 6.
USP25 overexpression decreases TSLP protein expression levels by increasing TRAF3 protein expression levels in vitro. (A) Protein expression levels of (B) USP25, (C) TRAF3 and (D) TSLP were assessed using western blotting in HDM-stimulated nasal epithelial cells following transfection with pcDNA-USP25 and si-TRAF3 alone or in combination. Semi-quantitative analysis of band intensity. (E) TSLP levels in cell culture supernatant were assessed using ELISA. Data are presented as the mean ± standard deviation (n=3). Each experiment was repeated three times. Statistical analysis was performed using one-way ANOVA followed by Tukey's post hoc test. *P<0.05. USP25, ubiquitin-specific peptidase 25; TSLP, thymic stromal lymphopoietin; TRAF3, TNF receptor-associated factor 3; HDM, house dust mite; si, small interfering.
Discussion
Epithelial-derived cytokines, such as TSLP, IL-25 and IL-33, have been previously reported to serve a key role in allergic disease (32,33). Among these cytokines, TSLP is regarded as the master switch that initiates and maintains the type 2 immune response in AR. Moreover, TSLP activates multiple types of immune cell, thereby driving the allergic inflammatory response (14,15,34). However, it is unclear how TSLP is regulated and produced in nasal mucosal epithelial cells. To the best of our knowledge, the present study is the first to demonstrate that USP25 stabilized TRAF3 protein in the nasal mucosal epithelium, thereby inhibiting TSLP expression.
Numerous studies have reported the critical role of NF-κB in TSLP modulation (35–37). Activation of NF-κB in epithelial cells, including airway epithelial cells and keratinocytes, stimulates TSLP gene transcription and exacerbates allergic inflammation (35,36). Lee and Ziegler (37) reported that activated NF-κB binds to a site −3.8 kb of the TSLP promoter, which promotes TSLP gene expression in both humans and mice airway epithelial cells. Furthermore, Cultrone et al (38) reported a novel binding site for NF-κB located at position −0.37 kb of the TSLP promoter, a key region for NF-κB-dependent regulation of TSLP in human intestinal epithelial cells.
It has been reported that NF-κB activation is under strict regulation of DUBs (39). Abnormalities in the function or quantity of deubiquitinases has been reported to lead to development of excessive NF-κB-dependent inflammatory responses (40). The present study demonstrated that downregulation of the deubiquitylase USP25 occurred in nasal mucosal epithelium of both patients with AR and AR model mice. These results suggested that USP25 may serve an important regulatory role in AR. To evaluate the role of USP25 in AR, an AR model was established using USP25 knockout mice. The experimental results demonstrated that USP25 knockdown aggravated nasal symptoms and pathological manifestations, including markedly increased eosinophil infiltration and goblet cell hyperplasia in mice. AR is an allergic disease characterized by Th1/Th2 immune imbalance (2). Therefore, the protein expression levels of Th1/Th2 cytokines were examined in the serum and NLF in mice. USP25 knockdown exacerbated the immune imbalance of Th1/Th2 proteins, as demonstrated by the significantly higher levels of Th2 cytokines (IL-4, IL-5, IL-10 and IL-13) and significantly decreased levels of Th1 cytokines (IFN-γ) in USP25 KO mice. The present study demonstrated that USP25 knockdown significantly increased TSLP protein expression levels in nasal mucosal epithelium. It has been well documented that TSLP directly promotes Th2 cell proliferation and activation and triggers imbalance of Th1/Th2 cells by affecting other immune cells such as dendritic cells and type 2 innate lymphoid cells (17,18). Therefore, flow cytometry experiments should be performed in future experiments to verify the Th1 and Th2 cellular distribution. In summary, the results of the present study suggested that USP25 may serve a role in AR via regulation of TSLP.
Previous studies reported that USP25 serves as a signaling switch to regulate the activation of TRAF proteins during the inflammatory response, which in turn inhibits NF-κB activation (35,36). TRAF proteins are considered to serve as signaling adaptor molecules in the NF-κB signaling pathway, including both canonical and non-canonical NF-κB signaling pathways (41). TRAF3 is an E3 ubiquitin ligase that transfers activated ubiquitin molecules from E2 ubiquitin-conjugating enzymes to both itself and downstream target proteins for ubiquitin-dependent protein degradation (42). Therefore, TRAF3 serves a pivotal negative regulatory role in the non-canonical NF-κB signaling pathway (43). Accumulation of NF-κB-inducing kinase (NIK) is a key step in activation of the non-canonical NF-κB signaling pathway (44). Under normal conditions, TRAF3 ubiquitinates NIK, which leads to continuous NIK degradation. However, under certain physiological and pathophysiological situations, NIK becomes stable due to TRAF3 degradation, which results in activation of non-canonical NF-κB signaling. Furthermore, TRAF3 inhibits activation of IκB kinase by decreasing NIK protein accumulation, which in turn suppresses canonical NF-κB signaling activity (45). NIK is associated with local eosinophil infiltration and enhanced Th2 response in eosinophilic esophagitis (EoE) (46). EoE is a chronic mucosal inflammatory disease of the esophagus driven by an allergic inflammatory response to dietary allergens (46). The present study demonstrated TRAF3 protein expression levels were significantly decreased in the nasal mucosal epithelium of AR mice and USP25 knockdown further significantly decreased TRAF3 protein expression levels. Therefore, it could be hypothesized that NIK may regulate the epithelium-driven Th2 microenvironment through alterations in TSLP expression.
To evaluate the direct regulatory association between USP25 and TRAF3 in AR, in vitro experiments were performed using HNEpCs. The protein expression levels of USP25 and TRAF3 were significantly decreased in HDM-stimulated HNEpCs, whereas TSLP protein expression levels were significantly increased. Knockdown or overexpression of USP25 significantly decreased or increased TRAF3 protein expression levels, respectively, in HNEpCs, whereas TSLP protein expression levels were negatively associated with expression of USP25 and TRAF3. These results supported the hypothesis that USP25 relied on TRAF3 to negatively regulate TSLP production in nasal mucosal epithelial cells. However, the present study did not evaluate the direct interaction between USP25 and TRAF3. Several studies have reported the mechanisms underlying USP25-TRAF3 interactions (26,27,47). Notably, USP25 has been reported to specifically remove the K48-linked ubiquitin chain from TRAF3, which increases cellular abundance of TRAF3 and balances production of type I IFNs and inflammatory cytokines following TLR4 activation (26). Based on domain mapping analysis, it was reported that USP25 interacted with TRAF3 via its ubiquitin carboxyl-terminal hydrolase (UCH) domains, thereby reversing self-ubiquitination of TRAF3 (26). A further study reported that USP25 serves a key role in the antiviral immune response via stabilization of TRAF3 and TRAF6 proteins during DUB activity (27). Moreover, Wen et al (47) reported that USP25 specifically removes K48-linked polyubiquitin chains in TRAF3, which enhances Kupfer cell-mediated endotoxin tolerance. USP25 also interacts with other members of the TRAF family. For example, USP25 negatively regulates ubiquitination of TRAF5 and TRAF6 by cleaving their K63-linked polyubiquitin chains, which inhibits IL-17-induced activation of NF-κB and inflammatory responses (48). Therefore, it could be hypothesized that USP25 may negatively regulate production of TSLP protein by protecting TRAF3 from degradation and inhibiting NF-κB activation. However, this hypothesis requires further investigation.
There are some limitations in this study. Firstly, we used USP25 knockout mice in this study. However, USP25 is widely expressed as a deubiquitinating enzyme in various cell types throughout the body. Therefore, it is recommended that nasal mucosa-specific USP25 knockout mice be used for further studies. Secondly, the present study also did not explore the molecular mechanisms underlying the USP25 downregulation of nasal epithelium in AR. These limitations are to be addressed by future research.
To the best of our knowledge, the present study is the first to demonstrate that AR is associated with downregulation of USP25 in nasal mucosal epithelium. USP25 inhibited TSLP signaling in nasal mucosal epithelial cells by increasing the cellular abundance of TRAF3, thereby decreasing inflammation in AR. Therefore, regulation of USP25 expression may be key in controlling TSLP-mediated type 2 inflammatory responses. Furthermore, USP25 may serve as a potential therapeutic target for AR.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Bo Zhong (State Key Laboratory of Virology, College of Life Sciences, Wuhan University) for generously donating the USP25 KO mice.
Funding Statement
The present study was supported by the National Natural Science Foundation of China (grant no. 82071017), the National Natural Science Foundation of Hubei Province (grant no. 2021CFB125) and the Fundamental Research Funds for the Central Universities (grant no. 2042021kf0093).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
WC, HL and YX designed the study. WC and HL wrote the manuscript. WC, HL, and LT performed all experiments. LT and ZG contributed to the conception of the paper. PL, DQ, and WZ performed the data analysis. WZ recruited patients. YX critically revised the manuscript. All authors confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Written informed consent was obtained from all patients and research protocols were approved by the Ethics Committee of Renmin Hospital of Wuhan University (approval no. WDRY2018-K014). All experimental procedures involving animals were approved by The Animal Care and Use Committee of Renmin Hospital of Wuhan University (approval no. WDRM-20211005).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Vandenplas O, Vinnikov D, Blanc PD, Agache I, Bachert C, Bewick M, Cardell LO, Cullinan P, Demoly P, Descatha A, et al. Impact of rhinitis on work productivity: A systematic review. J Allergy Clin Immunol Pract. 2018;6:1274–1286.e9. doi: 10.1016/j.jaip.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, Brignardello-Petersen R, Canonica GW, Casale T, Chavannes NH, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140:950–958. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Zhang L. Increasing prevalence of allergic rhinitis in China, allergy. Asthma Immunol Res. 2019;11:156–169. doi: 10.4168/aair.2019.11.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savouré M, Bousquet J, Jaakkola JJK, Jaakkola MS, Jacquemin B, Nadif R. Worldwide prevalence of rhinitis in adults: A review of definitions and temporal evolution. Clin Transl Allergy. 2022;12:e12130. doi: 10.1002/clt2.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sozańska B, Błaszczyk M, Pearce N, Cullinan P. Atopy and allergic respiratory disease in rural Poland before and after accession to the European union. J Allergy Clin Immunol. 2014;133:1347–1353. doi: 10.1016/j.jaci.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 6.Wang XD, Zheng M, Lou HF, Wang CS, Zhang Y, Bo MY, Ge SQ, Zhang N, Zhang L, Bachert C. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71:1170–1180. doi: 10.1111/all.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myong JP, Kim H, Lee K, Chang S. Time trends of allergic rhinitis and effects of residence on allergic rhinitis in Korea from 1998 through 2007–2009. Asian Nurs Res (Korean Soc Nurs Sci) 2012;6:102–106. doi: 10.1016/j.anr.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Ha J, Lee SW, Yon DK. Ten-year trends and prevalence of asthma, allergic rhinitis, and atopic dermatitis among the Korean population, 2008–2017. Clin Exp Pediatr. 2020;63:278–283. doi: 10.3345/cep.2019.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ordovas-Montanes J, Dwyer DF, Nyquist SK, Buchheit KM, Vukovic M, Deb C, Wadsworth MH, II, Hughes TK, Kazer SW, Yoshimoto E, et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature. 2018;560:649–654. doi: 10.1038/s41586-018-0449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krohn IK, Seys SF, Lund G, Jonckheere AC, de Casterlé ID, Ceuppens JL, Steelant B, Hellings PW. Nasal epithelial barrier dysfunction increases sensitization and mast cell degranulation in the absence of allergic inflammation. Allergy. 2020;75:1155–1164. doi: 10.1111/all.14132. [DOI] [PubMed] [Google Scholar]
- 11.Bergougnan C, Dittlein DC, Hümmer E, Riepl R, Eisenbart S, Böck D, Griesbaum L, Weigl A, Damialis A, Hartwig A, et al. Physical and immunological barrier of human primary nasal epithelial cells from non-allergic and allergic donors. World Allergy Organ J. 2020;13:100109. doi: 10.1016/j.waojou.2020.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JY, Choi JH, Lee SN, Cho HJ, Ahn JS, Kim YB, Park DY, Park SC, Kim SI, Kang MJ, et al. Protein arginine methyltransferase 1 contributes to the development of allergic rhinitis by promoting the production of epithelial-derived cytokines. J Allergy Clin Immunol. 2021;147:1720–1731. doi: 10.1016/j.jaci.2020.12.646. [DOI] [PubMed] [Google Scholar]
- 13.Wang WW, Zhu K, Yu HW, Pan YL. Interleukin-17A potentiates interleukin-13-induced eotaxin-3 production by human nasal epithelial cells from patients with allergic rhinitis. Int Forum Allergy Rhinol. 2019;9:1327–1333. doi: 10.1002/alr.22382. [DOI] [PubMed] [Google Scholar]
- 14.Marković I, Savvides SN. Modulation of signaling mediated by TSLP and IL-7 in inflammation, autoimmune diseases, and cancer. Front Immunol. 2020;11:1557. doi: 10.3389/fimmu.2020.01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 16.Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Toki S, Goleniewska K, Zhang J, Zhou W, Newcomb DC, Zhou B, Kita H, Boyd KL, Peebles RS., Jr TSLP and IL-33 reciprocally promote each other's lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy. 2020;75:1606–1617. doi: 10.1111/all.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takai T. TSLP expression: Cellular sources, triggers, and regulatory mechanisms. Allergol Int. 2012;61:3–17. doi: 10.2332/allergolint.11-RAI-0395. [DOI] [PubMed] [Google Scholar]
- 19.Meng P, Chen ZG, Zhang TT, Liang ZZ, Zou XL, Yang HL, Li HT. IL-37 alleviates house dust mite-induced chronic allergic asthma by targeting TSLP through the NF-κB and ERK1/2 signaling pathways. Immunol Cell Biol. 2019;97:403–415. doi: 10.1111/imcb.12223. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda Y, Yuki T, Takahashi Y, Sakaguchi H, Matsunaga K, Itagaki H. An acid-hydrolyzed wheat protein activates the inflammatory and NF-κB pathways leading to long TSLP transcription in human keratinocytes. J Toxicol Sci. 2020;45:327–337. doi: 10.2131/jts.45.327. [DOI] [PubMed] [Google Scholar]
- 21.Song L, Luo ZQ. Post-translational regulation of ubiquitin signaling. J Cell Biol. 2019;218:1776–1786. doi: 10.1083/jcb.201902074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budroni V, Versteeg GA. Negative regulation of the innate immune response through proteasomal degradation and deubiquitination. Viruses. 2021;13:584. doi: 10.3390/v13040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georges A, Gros P, Fodil N. USP15: A review of its implication in immune and inflammatory processes and tumor progression. Genes Immun. 2021;22:12–23. doi: 10.1038/s41435-021-00125-9. [DOI] [PubMed] [Google Scholar]
- 24.Ren Y, Zhao Y, Lin D, Xu X, Zhu Q, Yao J, Shu HB, Zhong B. The type I interferon-IRF7 axis mediates transcriptional expression of Usp25 gene. J Biol Chem. 2016;291:13206–13215. doi: 10.1074/jbc.M116.718080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long C, Lai Y, Li J, Huang J, Zou C. LPS promotes HBO1 stability via USP25 to modulate inflammatory gene transcription in THP-1 cells. Biochim Biophys Acta Gene Regul Mech. 2018;1861:773–782. doi: 10.1016/j.bbagrm.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong B, Liu X, Wang X, Liu X, Li H, Darnay BG, Lin X, Sun SC, Dong C. Ubiquitin-specific protease 25 regulates TLR4-dependent innate immune responses through deubiquitination of the adaptor protein TRAF3. Sci Signal. 2013;6:ra35. doi: 10.1126/scisignal.2003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin D, Zhang M, Zhang MX, Ren Y, Jin J, Zhao Q, Pan Z, Wu M, Shu HB, Dong C, Zhong B. BInduction of USP25 by viral infection promotes innate antiviral responses by mediating the stabilization of TRAF3 and TRAF6. Proc Natl Acad Sci USA. 2015;112:11324–11329. doi: 10.1073/pnas.1509968112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng L, Chen J, Fu Q, He S, Li H, Liu Z, Tan G, Tao Z, Wang D, Wen W, et al. Chinese society of allergy guidelines for diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res. 2018;10:300–353. doi: 10.4168/aair.2018.10.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen M, Wu Y, Yuan S, Tang M, Zhang L, Chen J, Li L, Wu J, Zhang J, Yin Y. Allergic rhinitis improvement in asthmatic children after using acaricidal bait: A randomized, double-blind, cross-placebo study. Front Pediatr. 2021;9:709139. doi: 10.3389/fped.2021.709139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng YQ, Yang YQ, Wang SB, Li F, Liu MZ, Hua QQ, Tao ZZ. Intranasal administration of lentiviral miR-135a regulates mast cell and allergen-induced inflammation by targeting GATA-3. PLoS One. 2015;10:e0139322. doi: 10.1371/journal.pone.0139322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang R, Xu Y, Zhang W, Kong YG, Tan L, Chen SM, Deng YQ, Tao ZZ. Semaphorin 3A inhibits allergic inflammation by regulating immune responses in a mouse model of allergic rhinitis. Int Forum Allergy Rhinol. 2019;9:528–537. doi: 10.1002/alr.22274. [DOI] [PubMed] [Google Scholar]
- 32.Patel NN, Kohanski MA, Maina IW, Workman AD, Herbert DBR, Cohen NA. Sentinels at the wall: Epithelial-derived cytokines serve as triggers of upper airway type 2 inflammation. Int Forum Allergy Rhinol. 2019;9:93–99. doi: 10.1002/alr.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papazian D, Hansen S, Würtzen PA. Airway responses towards allergens-from the airway epithelium to T cells. Clin Exp Allergy. 2015;45:1268–1287. doi: 10.1111/cea.12451. [DOI] [PubMed] [Google Scholar]
- 34.Cianferoni A, Spergel J. The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev Clin Immunol. 2014;10:1463–1474. doi: 10.1586/1744666X.2014.967684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan Z, Zhou Y, Luo X, Ruan Y, Zhou L, Wang Q, Yan YJ, Liu Q, Chen J. Against NF-κB/thymic stromal lymphopoietin signaling pathway, catechin alleviates the inflammation in allergic rhinitis. Int Immunopharmacol. 2018;61:241–248. doi: 10.1016/j.intimp.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Kumagai A, Kubo T, Kawata K, Kamekura R, Yamashita K, Jitsukawa S, Nagaya T, Sumikawa Y, Himi T, Yamashita T, Ichimiya S. Keratinocytes in atopic dermatitis express abundant ΔNp73 regulating thymic stromal lymphopoietin production via NF-Κb. J Dermatol Sci. 2017;88:175–183. doi: 10.1016/j.jdermsci.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci USA. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cultrone A, de Wouters T, Lakhdari O, Kelly D, Mulder I, Logan E, Lapaque N, Doré J, Blottière HM. The NF-κB binding site located in the proximal region of the TSLP promoter is critical for TSLP modulation in human intestinal epithelial cells. Eur J Immunol. 2013;43:1053–1062. doi: 10.1002/eji.201142340. [DOI] [PubMed] [Google Scholar]
- 39.Harhaj EW, Dixit VM. Regulation of NF-κB by deubiquitinases. Immunol Rev. 2012;246:107–124. doi: 10.1111/j.1600-065X.2012.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aksentijevich I, Zhou Q. NF-κB pathway in autoinflammatory diseases: Dysregulation of protein modifications by ubiquitin defines a new category of autoinflammatory diseases. Front Immunol. 2017;8:399. doi: 10.3389/fimmu.2017.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi JH, Sun SC. Tumor necrosis factor receptor-associated factor regulation of nuclear factor κB and mitogen-activated protein kinase pathways. Front Immunol. 2018;9:1849. doi: 10.3389/fimmu.2018.01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Häcker H, Tseng PH, Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat Rev Immunol. 2011;11l:457–468. doi: 10.1038/nri2998. [DOI] [PubMed] [Google Scholar]
- 43.He JQ, Saha SK, Kang JR, Zarnegar B, Cheng G. Specificity of TRAF3 in its negative regulation of the noncanonical NF-kappa B pathway. J Biol Chem. 2007;282:3688–3694. doi: 10.1074/jbc.M610271200. [DOI] [PubMed] [Google Scholar]
- 44.Cildir G, Low KC, Tergaonkar V. Noncanonical NF-κB signaling in health and disease. Trends Mol Med. 2016;22:414–429. doi: 10.1016/j.molmed.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Zarnegar B, Yamazaki S, He JQ, Cheng G. Control of canonical NF-kappaB activation through the NIK-IKK complex pathway. Proc Natl Acad Sci USA. 2008;105:3503–3508. doi: 10.1073/pnas.0707959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eden K, Rothschild DE, McDaniel DK, Heid B, Allen IC. Noncanonical NF-κB signaling and the essential kinase NIK modulate crucial features associated with eosinophilic esophagitis pathogenesis. Dis Model Mech. 2017;10:1517–1527. doi: 10.1242/dmm.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen J, Bai H, Chen N, Zhang W, Zhu X, Li P, Gong J. USP25 promotes endotoxin tolerance via suppressing K48-linked ubiquitination and degradation of TRAF3 in Kupffer cells. Mol Immunol. 2019;106:53–62. doi: 10.1016/j.molimm.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Zhong B, Liu X, Wang X, Chang SH, Liu X, Wang A, Reynolds JM, Dong C. Negative regulation of IL-17-mediated signaling and inflammation by the ubiquitin-specific protease USP25. Nat Immunol. 2012;13:1110–1117. doi: 10.1038/ni.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.