Abstract
Biochemical studies in Azoarcus sp. strain T have demonstrated that anaerobic oxidation of both toluene and m-xylene is initiated by addition of the aromatic hydrocarbon to fumarate, forming benzylsuccinate and 3-methyl benzylsuccinate, respectively. Partially purified benzylsuccinate synthase was previously shown to catalyze both of these addition reactions. In this study, we identified and sequenced the genes encoding benzylsuccinate synthase from Azoarcus sp. strain T and examined the role of this enzyme in both anaerobic toluene and m-xylene mineralization. Based on reverse transcription-PCR experiments and transcriptional start site mapping, we found that the structural genes encoding benzylsuccinate synthase, bssCAB, together with two additional genes, bssD and bssE, were organized in an operon in the order bssDCABE. bssD is believed to encode an activating enzyme, similar in function to pyruvate formate-lyase activase. bssE shows homology to tutH from Thauera aromatica strain T1, whose function is currently unknown. A second operon that is upstream of bssDCABE and divergently transcribed contains two genes, tdiS and tdiR. The predicted amino acid sequences show similarity to sensor kinase and response regulator proteins of prokaryotic two-component regulatory systems. A chromosomal null bssA mutant was constructed (the bssA gene encodes the α-subunit of benzylsuccinate synthase). This bssA null mutant strain was unable to grow under denitrifying conditions on either toluene or m-xylene, while growth on benzoate was unaffected. The growth phenotype of the ΔbssA mutant could be rescued by reintroducing bssA in trans. These results demonstrate that benzylsuccinate synthase catalyzes the first step in anaerobic mineralization of both toluene and m-xylene.
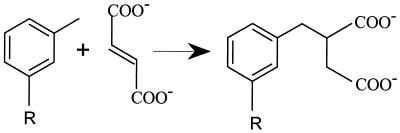
Azoarcus sp. strain T is a facultative microorganism capable of mineralizing both toluene and m-xylene anaerobically with nitrate as the electron acceptor. Based on biochemical studies, a pathway for anaerobic oxidation of toluene has been proposed (2, 6). In this pathway, a novel enzyme, benzylsuccinate synthase, catalyzes the addition of the methyl group of toluene to fumarate to form benzylsuccinate (Fig. 1). Benzylsuccinate is then oxidized to benzoyl-coenzyme A (CoA), a central intermediate in anaerobic aromatic hydrocarbon metabolism. Other studies have shown that this fumarate addition reaction is found in a wide range of microorganisms capable of anaerobic toluene mineralization, including other denitrifying bacteria, Thauera aromatica strain K172 and strain EbN1 (6, 27), several sulfate-reducing bacteria (3, 27), an anoxygenic phototrophic bacterium (35), and a methanogenic mixed culture (1). Detection of benzylsuccinate in cultures of the toluene-degrading Azoarcus tolulyticus Tol-4 and Thauera aromatica strain T1 suggests that a fumarate addition reaction may also be involved in anaerobic toluene mineralization in these microorganisms (7, 13). In addition, recent work has suggested that m-xylene (16), m-cresol (24), p-cresol (25), and the aliphatic hydrocarbons n-hexane (28) and n-dodecane (18) are also activated anaerobically by a fumarate addition reaction. Thus, it appears that the formation of benzylsuccinate, or a corresponding succinate derivative, is a common mode for initiating anaerobic mineralization of methylbenzenes, methylphenols, and long-chain n-alkanes.
FIG. 1.
Addition of toluene and m-xylene to fumarate catalyzed by benzylsuccinate synthase. R = H for toluene and R = CH3 for m-xylene.
Benzylsuccinate synthase has been purified from Thauera aromatica strain K172 and Azoarcus sp. strain T and shown to have an α2β2γ2 composition (5, 22). The enzyme was shown to be irreversibly inactivated in the presence of molecular oxygen by oxygenolytic cleavage of the α subunit of benzylsuccinate synthase (22). Furthermore, toluene addition to fumarate is believed to occur by a radical mechanism because the H atom abstracted from the methyl group of toluene during addition to fumarate is retained in the succinyl moiety of benzylsuccinate (2). These observations, in conjunction with the sequence similarity of the α subunit of benzylsuccinate synthase to glycyl radical proteins, suggested that benzylsuccinate synthase may be a glycyl radical enzyme. In addition, recent electron paramagnetic resonance studies have shown the presence of a glycyl radical in samples of active benzylsuccinate synthase purified from Azoarcus sp. strain T, demonstrating experimentally that benzylsuccinate synthase is a glycyl radical enzyme (17).
The genes encoding benzylsuccinate synthase have been independently identified in two microorganisms, T. aromatica strains T1 and K172, by a genetic and a reverse genetic approach, respectively. In T. aromatica strain T1, mutants defective in toluene utilization and benzylsuccinate formation were isolated (10). Complementation studies with these mutants led to the identification of several open reading frames, including tutE, tutFDGH, tutCB, and tutC1B1 (9, 10, 21). Based on N-terminal amino acid sequences of benzylsuccinate synthase purified from T. aromatica strain K172, bssDCAB and tdiSR were cloned and sequenced in this microorganism (22). The bssCAB genes show similarity to the tutFDG genes, which encode the γ, α, and β subunits of benzylsuccinate synthase, respectively. The predicted amino acid sequence of bssA shows similarity to the anaerobic glycyl radical enzymes pyruvate formate-lyase (PFL) and anaerobic ribonucleotide reductase (ARNR) (9, 22). The glycyl radical in PFL and ARNR is posttranslationally generated by PFL activase and ARNR activase, respectively. The predicted translation products of bssD and tutE show homology to these activases and have been proposed to perform a similar function. tutCB, tutC1B1, and tdiSR encode proteins with homology to sensor kinase and response regulator proteins of bacterial two-component regulatory systems.
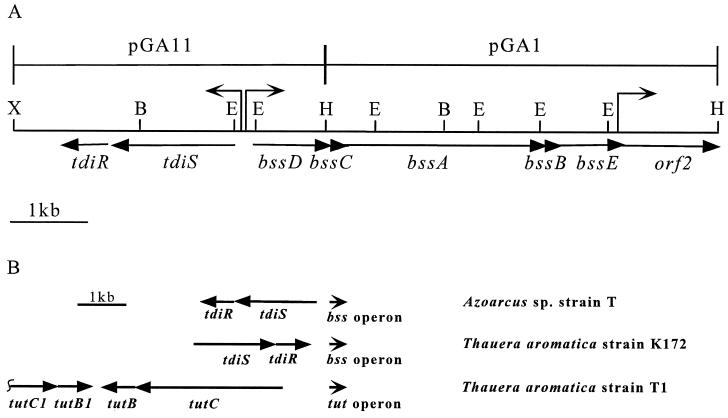
Although the predicted amino acid sequences of the benzylsuccinate synthases from T. aromatica strains K172 and T1 are almost identical, several differences exist in the organization of the genes in the bss/tut regions in these two strains (Fig. 2B). In T. aromatica strain T1, the tdiSR homologs tutC1B1 are separated from the bssDCAB homologs, tutE and tutFDG, by genes encoding another sensor kinase/response regulator pair, called tutCB (21). tutCB is transcribed divergently from both tutC1B1 and tutE. The gene products of tutCB more closely resemble the sensor kinases/response regulators believed to control aerobic toluene oxidation, including TodST, than the gene products of tdiSR (21). Since T. aromatica strain T1 is capable of both aerobic and anaerobic toluene oxidation, Leuthner et al. have proposed that TutC1B1 may be responsible for control of anaerobic toluene oxidation, while TutCB controls aerobic toluene oxidation (21). Notably, tutCB homologs are not observed in the vicinity of the bss operon in T. aromatica strain K172. Instead, tdiSR and bssDCAB are transcribed in the same direction and are not separated by any additional open reading frames (21).
FIG. 2.
(A) Restriction map of the tdiSR/bssDCABE chromosomal region of Azoarcus sp. strain T. The eight identified open reading frames are indicated with arrows below the map. Arrows above the map indicate the major transcriptional start sites identified. Restriction site abbreviations: B, BamHI; E, EcoRI; H, HindIII; X, XbaI. (B) Gene organization of tut and tdi/bss operons in Azoarcus sp. strain T, T. aromatica strain K172, and T. aromatica strain T1. Accession numbers are U57900 and AF036765 for T. aromatica T1 and AJ001848 for T. aromatica K172.
The transcriptional organization of the bss/tut genes also appears to be different in T. aromatica strains K172 and T1. In T. aromatica strain K172, Northern blot studies of toluene-grown cells showed that bssDCAB are cotranscribed. No bssDCAB mRNA was observed when the cells were grown on benzoate (22). In contrast, in T. aromatica strain T1, Northern blot and primer extension studies suggested that the bssD homolog tutE is transcribed independently from the bssCAB homologs tutFDG. tutFDG are cotranscribed with tutH, a gene downstream of tutG whose predicted amino acid sequence is similar to that of the NorQ/NirQ family of proteins. No tutFDGH mRNA was observed when cells were grown on pyruvate (8). No sequence data downstream of bssDCAB have been reported for T. aromatica strain K172, and it is unknown if a tutH homolog exists in T. aromatica strain K172.
In contrast to T. aromatica strains K172 and T1, Azoarcus sp. strain T is able to mineralize both toluene and m-xylene anaerobically. Furthermore, the specific activity of purified benzylsuccinate synthase from Azoarcus sp. strain T is relatively high compared to the enzyme purified from T. aromatica strain K172 (5). As a result, several studies of the benzylsuccinate synthase from Azoarcus sp. strain T have led to insights into this enzyme's reaction mechanism, including H atom retention in the benzylsuccinate product (2), stereoselectivity and substrate specificity of the benzylsuccinate synthase reaction (4, 5), and demonstration of a glycyl radical signal (17). However, the genes encoding benzylsuccinate synthase, their genetic organization, regulation, and function in anaerobic toluene and m-xylene mineralization have not been studied in Azoarcus sp. strain T.
In this study, we report the cloning and sequencing of the bssDCAB and tdiSR homologs from Azoarcus sp. strain T. Operon structures were determined by reverse transcription-PCR (RT-PCR) and primer extension studies. Transcriptional start sites were used to determine putative promoter regions. Analysis of the growth phenotype of a ΔbssA mutant demonstrated that BssA is essential for both toluene and m-xylene mineralization in Azoarcus sp. strain T.
MATERIALS AND METHODS
Growth conditions.
The bacterial strains, plasmids, and phages used in this study are described in Table 1. Azoarcus sp. strain T (DSM 9506) was grown at 30°C aerobically with benzoate (12) or at room temperature under denitrifying conditions with benzoate, toluene, or m-xylene, as described before (16). Escherichia coli was grown at 37°C in Luria-Bertani (LB) medium. Growth was monitored as absorbance at 600 nm. Antibiotics were used at the following concentrations: E. coli, oxytetracycline, 25 μg/ml; carbenicillin, 100 μg/ml; and kanamycin, 50 μg/ml; Azoarcus sp. strain T, kanamycin, 50 μg/ml; and oxytetracycline, 10 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Azoarcus sp. strain T | Wild type | 12 |
| AST2 | ΔbssA | This study |
| AST3 | AST2 containing pGA14 | This study |
| E. coli | ||
| DH10B | F−mcrAΔ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 endA1 recA1 deoR Δ(ara leu)7697 araD139 galU galK nupG rpsL λ− | Bethesda Research Laboratories |
| TG1recO 1504::Tn5 | K-12 supE thi Δ(lac-proAB) hsdΔ5 F′ [traD36 proA+B+ lacIqlacZΔM15] | Novagen |
| Plasmids | ||
| pBluescript II KS | ColE1 ori, lacZ, Apr | Stratagene |
| pLAFR5-SK1 | oriV, cos, Tetr | 15 |
| pBJ113 | pUC118 containing Kmr and galK; plasmid used for generating gene replacements, derived from pKG2 | 14, 32 |
| pRK415 | oriV, oriT, Tetr | 15 |
| p810F | 25-kb Sau3A fragment of partially digested chromosomal DNA of Azoarcus sp. strain T in pLAFR5-SK1 | This study |
| pGA1 | 5.3-kb HindIII fragment of 25-kb insert of p810F in pBluescript-II KS | This study |
| pGA6 | 2.6-kb PCR deletion of pGA1 in pBJ113; ΔbssA | This study |
| pGA11 | 4.2-kb XbaI/HindIII fragment of 25-kb insert of p810F in pBluescript-II KS | This study |
| pGA14 | 5.3-kb insert of pGA1 in pRK415 | This study |
Cloning and DNA manipulations.
Standard protocols were used for cloning and transformations of E. coli (29). The DNA packaging kit from Boehringer-Mannheim, Indianapolis, Ind., was used to prepare the cosmid library. Plasmids were introduced into Azoarcus sp. strain T by electroporation. Briefly, cells of Azoarcus sp. strain T were grown aerobically to an optical density at 600 nm (OD600) of ≈0.3, centrifuged at 4°C, washed twice with cold distilled water, and then resuspended to an OD600 of >200. Cells were mixed with DNA in a cold cuvette and exposed to 2 μF, 1.5 kV, and 200 Ω. Time constants were about 4.5 ms. Cells were outgrown at 30°C for 10 h in aerobic growth medium (12) augmented with 0.2% yeast extract and 0.5% Casamino Acids (see reference 11) before plating on selective medium.
Construction of a DNA library.
Chromosomal DNA from Azoarcus sp. strain T was partially digested with Sau3A and size fractionated by ultracentrifugation in a linear 10 to 40% sucrose gradient. Fractions containing DNA fragments in the size range of 18 to 28 kb were pooled and dephosphorylated. DNA fragments were then ligated to pLAFR5-SK1 digested with BamHI. The ligated DNA was packaged into λ bacteriophage with the DNA packaging kit obtained from Boehringer Mannheim Biochemicals (Indianapolis, Ind.). The phage particles were transduced into E. coli DH10B, and colonies were grown on LB agar containing oxytetracycline (25 μg/ml). Microtiter wells were filled with LB and inoculated with single colonies, and after an overnight incubation, they were augmented with 20% glycerol and stored at −80°C.
Plasmid constructions.
p810F is a cosmid clone containing an approximately 25-kb insert of Azoarcus sp. strain T chromosomal DNA, of which 9.5 kb was sequenced in this study. Subclones of this cosmid were constructed in pBluescript II KS+. pGA1 contains a 5.3-kb HindIII insert encoding the 3′ end of bssD, bssCABE, and the 5′ end of orf2. pGA11 contains a 4.2-kb HindIII/XbaI fragment encoding tdiSR and the 5′ end of bssD. pGA5 is a deletion subclone of pGA1 (see below). pGA6 contains the insert of pGA5 in pBJ113. pGA14 contains the insert of pGA1 in pRK415 (15).
DNA sequencing and sequence analysis.
Plasmid DNA for sequencing was purified using Qiagen Plasmid Kits (Qiagen Inc., Chatsworth, Calif.). The insert of pGA1 was sequenced by Bio 101, La Jolla, Calif. All other DNA sequencing was performed by the Stanford University Protein and Nucleic Acid (PAN) facility using Big Dye terminator cycle sequencing (Perkin Elmer, Foster City, Calif.). DNA sequences were analyzed with the University of Wisconsin Genetics Computer Group software package, version 10.0. Similar sequences were identified using the Blast network service at the National Center for Biotechnology Information (NCBI; version 2.1.2, 13 November 2000) (Bethesda, Md.).
Construction of a bssA null mutant.
An in-frame deletion of bssA was constructed by the method of Link et al. (23). PCR deletion products were constructed in two steps. In the first step, two different PCRs generated fragments to the left and right of the bssA sequence targeted for deletion. In the second step, the left and right fragments were annealed at the overlapping region included in the internal primers and amplified by PCR as a single fragment using external primers. Internal primers were bssNi, 5′CCCATCCACTAAACTTAAACACARCAYTTNCCYTTRTARTC3′, and bssCi, 5′TGTTTAAGTTTAGTGGATGGGAAYACNAUHAUHGCNCG3′ (H is A, C, or T; N is A, C, G, or T; R is A or G; and Y is C or T). Universal primers were used as external primers. The internal primers were designed so that only 116 bp of the 2.6-kb bssA gene remain in the final ΔbssA mutant. The final PCR product was digested with HindIII and cloned into pBluescript KS+ to form pGA5. The disrupted region was sequenced to confirm the in-frame deletion and then cloned into pBJ113. pBJ113 contains a positive-negative KG (Kmr/galK) cassette for creating the two-step integration-excision events during gene replacement (32).
pGA6 was introduced into Azoarcus sp. strain T by electroporation, and cells were plated on kanamycin selective plates. One of the Kmr colonies determined by Southern blot and PCR analysis to contain both bssA and ΔbssA was grown in nonselective liquid medium and plated on 1% galactose basal medium. Several segregants were isolated. PCR and Southern blot analyses of these segregant strains were used to distinguish between the wild-type and deletion allele by testing for the presence of ΔbssA and the absence of bssA. One ΔbssA strain was selected for further studies and designated AST2. The ΔbssA mutant was complemented with pGA14, which was introduced by electroporation.
RT-PCR.
Total RNA was prepared from cells in mid-log phase by the hot phenol method of von Gabain et al. (33). DNA was removed from the RNA by two treatments with RNase-free DNase (Boehringer-Mannheim, Indianapolis, Ind.). RT-PCRs were performed using the Access RT-PCR kit from Promega Corp. (Madison, Wis.). Primers were chosen to produce fragments less than 500 bp in length. Negative control reactions in which reverse transcriptase was omitted from the reaction mixture ensured that DNA products resulted from amplification of cDNA rather than of chromosomal DNA contamination.
Primer extension of mRNA transcripts.
The avian myeloblastosis virus reverse transcriptase primer extension system was used to determine the transcriptional start sites of tdiSR, bssDCABE, and orf2 (Promega Corp., Madison, Wis.). Primer extension products were resolved on a 6% polyacrylamide gel containing 7 M urea next to a DNA sequence generated with the same primer (fmol DNA sequencing system; Promega Corp., Madison, Wis.). The oligonucleotides used in these studies included bssDpe (5′TGAACCTCTGTATTTCGGTAACAACA3′), orf2pe2 (5′ACCTTAGGCGGCAATGTACTGAACGT3′), tdiSpe (5′TCCACCGCGACCACGTCATTCTTCAT3′), tdiRpe (5′TCATCGACGACGAACACGGTCGGCGA3′), orf1pe (5′ACAAGCCATTTGTGCTAGGAGAGGT3′), orf1pe2 (5′ATATCTTGGCGAATTTATCGAGAAGCT3′), orf1pe3 (5′ATGATCTATCGTCAACGCGGT3′), bssbpe (5′CCGCTTCCATGTTATGG3′), and bssCpe (5′CCAAAGGTATCTACTCAG3′).
Northern blotting.
Hybridization was performed in RapidHyb buffer at 70°C according to the manufacturer's instructions (Amersham Pharmacia Biotech, Piscataway, N.J.). An RNA probe for the 3′ end of bssD, approximately 800 bp in size and called bssD3, was produced using the Riboprobe T3 system from Promega Corp. (Madison, Wis.). pGA10 digested with SmaI was the template.
Nucleotide sequence accession number.
All sequence data have been assigned GenBank accession number AY032676.
RESULTS AND DISCUSSION
Cloning of benzylsuccinate synthase and neighboring genes.
In a genetic approach to identifying genes involved in anaerobic toluene utilization in T. aromatica strain T1, several mutants defective in anaerobic toluene mineralization and benzylsuccinate formation were identified (P. W. Coschigano, Abstr. 97th Annu. Meet. Am. Soc. Microbiol. 1997, p. 493). One of the mutations mapped to a gene, tutD, whose predicted amino acid sequence showed homology to pyruvate formate-lyase. Based on two regions of amino acid identity in TutD and pyruvate formate-lyase, GNDDD (Gly568 in E. coli pyruvate formate-lyase) and RVSGY (Gly734 in E. coli pyruvate formate-lyase), we designed degenerate primers, and a 162-bp fragment, called bss1, was amplified from Azoarcus sp. strain T chromosomal DNA.
The bss1 fragment was cloned and sequenced. The predicted amino acid sequence of bss1 showed homology to both pyruvate formate-lyase and TutD (data not shown). Using the bss1 fragment to probe a colony blot containing approximately 1,050 clones of a cosmid library of chromosomal DNA from Azoarcus sp. strain T identified a single hybridizing cosmid clone, p810F. A 5.3-kb HindIII fragment and a 4.2-kb XbaI/HindIII fragment from p810F were cloned into pBluescript KS+ and sequenced. Eight open reading frames were identified and, based on similarity to genes identified in T. aromatica strain K172 (22), were named tdiR, tdiS, bssD, bssC, bssA, bssB, bssE, and orf2 (Fig. 2A).
The open reading frames designated bssC, bssA, and bssB from Azoarcus sp. strain T, encoding the γ, α, and β subunits of benzylsuccinate synthase, respectively, have predicted translational start codons at A4344TG, A4550TG, and A7221TG, respectively. The start codons of bssC, bssA, and bssB are preceded by likely ribosome-binding sites, A4331GGAG, A4539GGAG, and T7209GGAG, respectively. bssC, bssA, and bssB have predicted stop codons at T4524AA, T7142GA, and T7449GA, respectively. The encoded proteins BssA, BssB, and BssC have calculated molecular masses of 97,483 Da, 8,808 Da, and 6,964 Da, respectively. These masses are similar to published data on the benzylsuccinate synthase subunits from Azoarcus sp. strain T and T. aromatica K172 (5, 22). The predicted translation products of bssC, bssA, and bssB show about 90% identity to TutF, TutD, and TutG, respectively, from T. aromatica strain T1 and between 60 and 80% identity to BssC, BssA, and BssB, respectively, from T. aromatica strain K172. Sequence analysis using Terminator (University of Wisconsin Genetics Computer Group software package, version 10.0) shows a potential weak rho-independent terminator 22 bp downstream of the predicted translational stop site for bssB.
The bssD gene, predicted to encode the activating enzyme for benzylsuccinate synthase, begins at G3327TG and ends at T4323AA. BssD has a predicted molecular mass of 37,764 Da and shows approximately 70% identity to BssD and TutE from T. aromatica strains K172 and T1, respectively. The motif CX3CX2C, proposed to coordinate a [4Fe-4S] cluster at the N termini of pyruvate formate-lyase-activating enzymes (19) and anaerobic ribonucleotide reductase activating-enzymes (31), is present, with sequence CPLRCPWC, at the N termini of all three benzylsuccinate synthase-activating proteins, beginning at Cys29 in Azoarcus sp. strain T. These proteins also contain two additional cysteine clusters of the form CX2CX2CX3C, C55VGCGRCMAVC, and C89QRCMRCVAAC in the predicted amino acid sequence of BssD in Azoarcus sp. strain T, a motif conserved in ferredoxins with two [4Fe-4S] clusters (34). This ferredoxin motif is not found in either the pyruvate formate-lyase activating enzyme or the anaerobic ribonucleotide reductase activating enzyme.
The bssE gene of Azoarcus sp. T is predicted to start at A7499TG and end at T8357AA. BssE has a predicted molecular mass of 31,818 Da and is 97% identical to the gene product of tutH from T. aromatica strain T1. Currently, the function of TutH is unknown, although it shows homology to the NorQ/NirQ family of proteins. No obvious ribosome-binding site is observed upstream of the predicted translational start of bssE. orf2 is predicted to start at A8368TG. There is a potential ribosome-binding site before the translational start site of orf2, T8357AAGG. No translational stop for orf2 was identified in the present nucleotide sequence, but the predicted gene product would be larger than 59,578 Da. An NCBI Blast search in the nonredundant GenBank, PDB, SwissProt, PIR, and PRF databases revealed no significant similarity between the deduced incomplete amino acid sequence of orf2 and any other known protein.
The predicted gene products of tdiSR show homology to sensor kinase and response regulator proteins of bacterial two-component regulatory systems. tdiS and tdiR are predicted to start at A3021TG and A1314TG and end at T1386AG and T660GA, respectively. TdiS and TdiR have predicted molecular masses of 61,694 Da and 24,220 Da, respectively, and show approximately 95% identity to TdiS and TdiR of T. aromatica strain K172 and about 80% identity to TutC1 and TutB1 from T. aromatica strain T1, respectively. The tdiSR genes are oriented in the direction opposite that of the bssDCABE and orf2 genes. The predicted ATG start codon of tdiS is 305 bp upstream of the predicted GTG start codon of bssD. tdiS and tdiR are preceded by excellent ribosome-binding sites, A3036GGAGG and A1325GGAGG, respectively. There is a potential strong rho-independent terminator 22 bp downstream of the predicted translational stop site of tdiR.
Since benzylsuccinate synthase is a glycyl radical-containing enzyme which is irreversibly inactivated in the presence of molecular oxygen, we considered that its expression might be controlled by regulatory elements similar to those that control expression of pyruvate formate-lyase. Expression of pfl is controlled by several regulators, including FNR (fumarate-nitrate reduction regulator), ArcA, NarL, and IHF (integration host factor) in response to oxygen, nitrate, and pyruvate (see review in reference 30). We searched the sequenced region for consensus binding sites for these regulatory molecules, but no perfect matches were found. Further studies will be necessary to determine if any of these regulatory proteins play a role in the control of expression of the bss operon.
The organization of the bss and tdi genes in Azoarcus sp. strain T is quite different from the organization of homologous genes in Thauera aromatica strains. In T. aromatica strains K172 and T1, tdiSR and tutB1C1 are transcribed in the same direction as bssDCAB and tutEFGH, respectively (21) (Fig. 2B). In contrast, in Azoarcus sp. strain T, a putative sensor kinase/response regulator system, closely related to tdiSR and tutB1C1, is encoded upstream of the bssDCABE genes, but is transcribed divergently to bssDCABE.
Transcriptional organization of tdiSR and bssDCABE operons.
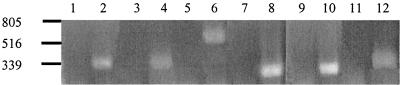
RT-PCR experiments using total RNA harvested from toluene-grown cells of Azoarcus sp. strain T were used to determine which open reading frames are cotranscribed. Amplification products were obtained using primers complementary to neighboring open reading frames to amplify the intergenic regions between tdiS and tdiR (expected size, 320 bp), bssD and bssC (expected size, 340 bp), bssC and bssA (expected size, 553 bp), bssA and bssB (expected size, 240 bp), bssB and bssE (expected size, 265 bp), and bssE and orf2 (expected size, 310 bp). Controls without reverse transcriptase were negative, indicating the absence of chromosomal DNA (Fig. 3). The RT-PCR fragments obtained were of the expected sizes. The presence of these RT-PCR products suggests that the tdiS and tdiR genes, as well as the bssDCABE genes, are each cotranscribed as an operon.
FIG. 3.
Agarose gel electrophoresis of RT-PCR products. RT-PCR products observed using primers to amplify intergenic regions between (lanes 1 and 2) tdiS and tdiR, (3 and 4) bssD and bssC, (5 and 6) bssC and bssA, (7 and 8) bssA and bssB, (9 and 10) bssB and bssE, and (11 and 12) bssE and orf2. Odd-numbered lanes are controls without reverse transcriptase. Numbers on the left represent sizes of markers (in base pairs).
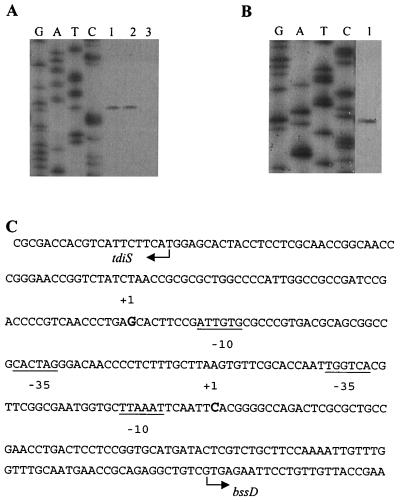
Primer extension studies were performed to determine the transcriptional start sites of the two operons. The transcriptional start site of bssDCABE was mapped to 98 bp upstream of the putative GTG intiation codon of bssD at a cytosine base (Fig. 4). The bssDCABE promoter region contains elements of a likely ς70 promoter, including two sequences, TTAAAT and TAAATT, which lie 5 and 6 bp upstream of the +1 transcriptional start site, respectively, and match the E. coli ς70 −10 consensus sequence (TATAAT) at four of the six positions. A sequence (TGGTCA) starting at −29 matches the E. coli ς70 −35 consensus sequence (TTGACA) at four of the six positions. The same transcriptional start site was observed when total RNA from m-xylene-grown cells was used. No primer extension product was observed when total RNA from benzoate-grown cells was used as the template for reverse transcription reactions. Therefore, bssDCABE is probably controlled in the same way when Azoarcus sp. strain T is growing in the presence of either toluene or m-xylene. No extension product was observed when primers (names in parentheses) were used from bssC (bssCpe), bssB (bssBpe), or bssE (orf1pe, orf1pe2, and orf1pe3).
FIG. 4.
Mapping of transcriptional start sites of bssDCABE and tdiSR by primer extension. (A) Sequencing and primer extension reactions with bssDpe primer. (B) Sequencing and primer extension reactions with tdiSpe primer. RNA was isolated from toluene-grown (lane 1), m-xylene-grown (lane 2), and benzoate-grown (lane 3) cells. (C) Nucleotide sequence of the tdiSR/bssDCABE promoter regions. Transcription start sites are indicated by +1, and putative −10 and −35 regions are underlined. Note that only one of the potential −10 regions of the bssDCABE promoter is underlined.
The transcriptional start site of the tdiSR operon was mapped 95 bp upstream of the proposed ATG initiation codon of tdiS at a cytosine base (Fig. 4). The tdiSR promoter region contained elements of a ς70 promoter, including a sequence (TAACAC) 8 bp upstream of the +1 transcriptional start site that matched the E. coli ς70 −10 consensus sequence (TATAAT) at three of the six positions. However, there is no obvious −35 sequence. No extension product was observed when a primer (tdiRpe) from within the tdiR gene was used.
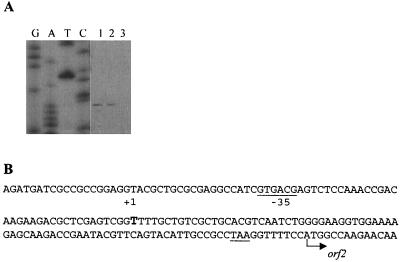
Although RT-PCR experiments are consistent with orf2 being cotranscribed with bssDCABE, a transcriptional start site of orf2 was mapped 84 bp upstream of the putative ATG initiation codon of orf2 at a thymine residue (Fig. 5). Further inspection of this region revealed that the putative orf2 promoter region contains a sequence (AAGAAG) 13 bp upstream of the +1 transcriptional start site that matches the E. coli ς70 −10 consensus sequence (TATAAT) at three of the six positions. A sequence (GTGACG) starting at −34 matches the E. coli ς70 −35 consensus sequence (TTGACA) at four of the six positions. While there is some sequence similarity, the spacing of these potential promoter sequences is not ideal. Typically, the canonical −10 sequence begins 5 to 9 bp before the transcriptional start, and the −35 sequence begins 17 bp after the −10 region. The same transcriptional start site was observed when total RNA from m-xylene-grown cells was used. No primer extension product was observed when total RNA from benzoate-grown cells was used.
FIG. 5.
Mapping of transcriptional start site of orf2 by primer extension. (A) RNA was isolated from toluene-grown cells (lane 1), m-xylene-grown cells (lane 2), and benzoate-grown cells (lane 3). A sequence ladder generated with the same primer is shown. (B) Nucleotide sequence of the orf2 promoter region, showing the start site of orf2 transcription (+1) and a potential −35 region. The stop codon for bssE is underlined.
The discovery of a primer extension product with orf2pe suggests that orf2 may be transcribed independently of bssDCABE, while RT-PCR results suggest that orf2, or its 5′ region, is cotranscribed with bssDCABE. In the former case, the RT-PCR result can be explained by a transcriptional readthrough of the bssDCABE transcript and termination of this transcript more than 202 bp downstream of the bssE stop codon. The RT-PCR product could not arise from the independent orf2 transcript because the PCR primer used to amplify the reverse-transcribed intergenic region between bssE and orf2 is complementary to the DNA sequence upstream of the start of the orf2 transcript. No factor-independent termination sites were found in this area.
Northern blot analysis using total RNA obtained from toluene- and m-xylene-grown cells of Azoarcus sp. strain T were conducted (data not shown). A 5.2-kb band was observed when total RNA from cells grown on toluene or m-xylene was probed with an RNA probe, bssD3, derived from the 3′ end of bssD. A minimum mRNA length of 5.1 kb is necessary to accommodate the bssDCABE genes. No hybridization was observed with RNA harvested from benzoate-grown cells, indicating that the presence of toluene or m-xylene is necessary to induce transcription of the bssDCABE operon under anaerobic conditions.
The data presented suggest that the bssDCABE genes are cotranscribed in Azoarcus sp. strain T. The downstream orf2 gene is transcribed independently of the bssDCABE genes, although it is also transcribed in the presence of toluene and m-xylene, but not in the presence of benzoate. In T. aromatica strain K172, bssC and bssB were reported to be located on a transcript of 4.6 kb, just 0.1 kb longer than the minimum size necessary to accommodate the bssDCAB genes (22). No sequence data downstream of bssDCAB have been reported for T. aromatica strain K172. In T. aromatica strain T1, tutE, a bssD homolog, is transcribed as a single gene, while tutFDGH are transcribed as an operon. In contrast, in Azoarcus sp. strain T, bssD and bssE are cotranscribed on the bssDCABE operon.
Phenotype of a ΔbssA mutant.
Azoarcus sp. strain T is able to grow anaerobically with either toluene or m-xylene as the sole carbon source and electron donor. Biochemical studies have established that the conversion of toluene and m-xylene to benzoyl-CoA and 3-methylbenzoyl-CoA, respectively, occurs by similar pathways (16). Notably, the addition of both toluene and m-xylene to fumarate has been observed at almost equal rates in studies with permeabilized cells of Azoarcus sp. strain T, regardless of whether the cells were grown on toluene or m-xylene (16). Furthermore, only a single copy of a bssA-like gene was observed in Southern blot studies of the chromosomal DNA of Azoarcus sp. strain T (data not shown). These observations suggested that the benzylsuccinate synthase and 3-methylbenzylsuccinate synthase reactions may be catalyzed by the same enzyme in vivo. In order to test this hypothesis, we constructed a ΔbssA mutant and analyzed its growth phenotype.
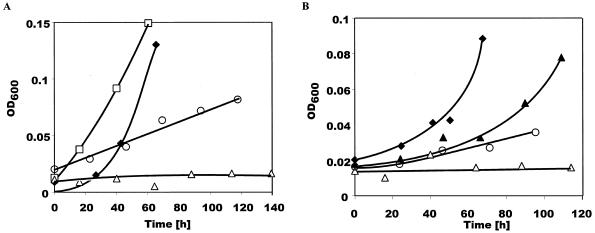
We constructed an in-frame deletion in the chromosomal copy of bssA in Azoarcus sp. strain T, generating strain AST2, as described in Materials and Methods. In AST2, 96% of the original 2.6-kb bssA gene was removed. AST2 was tested for anaerobic growth with toluene and m-xylene and found to be defective in growth on either methylbenzene (Fig. 6), while growth on benzoate was unaffected. This phenotype demonstrates that bssA is required for growth on both toluene and m-xylene. In Northern blot studies, total RNA harvested from AST2 cells grown with benzoate in the presence of toluene was probed with bssD3, a probe derived from bssD. A hybridizing band approximately 2.2 kb in size was observed, indicating that transcription of the bssDCABE operon can be induced by toluene in the presence of benzoate (data not shown). The observed size difference from the previously observed band is consistent with a 2.5-kb deletion of the 5.2-kb wild-type bssDCABE mRNA.
FIG. 6.
Role of bssA in anaerobic growth on (A) toluene and (B) m-xylene in Azoarcus sp. strain T. Growth of the wild-type strain on toluene or m-xylene (solid diamonds); AST2 (ΔbssA) on benzoate (open squares) and toluene or m-xylene (open triangles); and AST3 (AST2 + pGA14) on benzoate (solid triangles) and toluene or m-xylene (open circles). Note that wild-type Azoarcus sp. strain T has been observed to grow with a range of doubling times: 16 to 25 h on benzoate, 16 to 27 h on m-xylene, and 14 to 20 h on toluene (C. J. Krieger, unpublished data).
The mutant phenotype of AST2 could be rescued by introduction of the bssA gene in trans. A 5.6-kb HindIII fragment containing bssA, which was the insert of pGA1, was cloned into pRK415 to create pGA14. pGA14 was introduced into AST2 by electroporation to form AST3. AST3 cells were able to grow on both toluene and m-xylene, although the growth rates did not reach wild-type levels (Fig. 6). The slower growth on toluene and m-xylene may be caused by lower expression levels of bssA from the pRK415 lac promoter. The reduced growth rate observed on benzoate may be a result of the metabolic stress of tetracycline resistance, an effect that has been observed in E. coli (20, 26).
In summary, the transcriptional organization of the region encoding the genes for benzylsuccinate synthase in Azoarcus sp. strain T was determined. The genes bssDCABE and tdiSR form operons, for which transcriptional start sites were identified. The promoter regions of both of these operons show similarity to the E. coli ς70 consensus sequence. Primer extension studies and Northern blots show that the expression pattern of the bssDCABE operon is the same under toluene and m-xylene growth conditions. The growth phenotype of a ΔbssA mutant, AST2, provided the first genetic evidence in Azoarcus sp. strain T that benzylsuccinate synthase is required for growth on both toluene and m-xylene, but not on benzoate.
ACKNOWLEDGMENTS
This work was supported by NSF Career Award 9733535 and NSF MCB grant 9723312 to A.M.S. G.R.A. was the recipient of a Stanford Graduate Fellowship, and A.M.R. was supported by a postdoctoral fellowship (EX9411417811) from the Ministerio de Educación y Ciencia, Spain.
We thank Dale Kaiser and Bryan Julien for providing strains and plasmids. We also thank Jimmy Jakobsen, Cynthia Krieger, Dale Pelletier, and Mandy Ward for technical advice and useful discussions.
REFERENCES
- 1.Beller H R, Edwards E A. Anaerobic toluene activation by benzylsuccinate synthase in a highly enriched methanogenic culture. Appl Environ Microbiol. 2000;66:5503–5505. doi: 10.1128/aem.66.12.5503-5505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beller H R, Spormann A M. Anaerobic activation of toluene and o-xylene by addition to fumarate in denitrifying strain T. J Bacteriol. 1997;179:670–676. doi: 10.1128/jb.179.3.670-676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beller H R, Spormann A M. Benzylsuccinate formation as a means of anaerobic toluene activation by sulfate-reducing strain PRTOL1. Appl Environ Microbiol. 1997;63:3729–3731. doi: 10.1128/aem.63.9.3729-3731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beller H R, Spormann A M. Analysis of the novel benzylsuccinate synthase reaction for anaerobic toluene activation based on structural studies of the product. J Bacteriol. 1998;180:5454–5457. doi: 10.1128/jb.180.20.5454-5457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beller H R, Spormann A M. Substrate range of benzylsuccinate synthase from Azoarcus sp. strain T. FEMS Microbiol Lett. 1999;178:147–153. doi: 10.1111/j.1574-6968.1999.tb13771.x. [DOI] [PubMed] [Google Scholar]
- 6.Biegert T, Fuchs G, Heider J. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur J Biochem. 1996;238:661–668. doi: 10.1111/j.1432-1033.1996.0661w.x. [DOI] [PubMed] [Google Scholar]
- 7.Chee-Sanford J C, Frost J W, Fries M R, Zhou J, Tiedje J M. Evidence for acetyl coenzyme A and cinnamoyl coenzyme A in the anaerobic toluene mineralization pathway in Azoarcus tolulyticus Tol-4. Appl Environ Microbiol. 1996;62:964–973. doi: 10.1128/aem.62.3.964-973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coschigano P W. Transcriptional analysis of the tutE tutFDGH gene cluster from Thauera aromatica strain T1. Appl Environ Microbiol. 2000;66:1147–1151. doi: 10.1128/aem.66.3.1147-1151.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coschigano P W, Wehrman T S, Young L Y. Identification and analysis of genes involved in anaerobic toluene metabolism by strain T1: putative role of a glycine free radical. Appl Environ Microbiol. 1998;64:1650–1656. doi: 10.1128/aem.64.5.1650-1656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coschigano P W, Young L Y. Identification and sequence analysis of two regulatory genes involved in anaerobic toluene metabolism by strain T1. Appl Environ Microbiol. 1997;63:652–660. doi: 10.1128/aem.63.2.652-660.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dispensa M, Thomas C T, Kim M-K, Perrotta J A, Gibson J, Harwood C S. Anaerobic growth of Rhodopseudomonas palustris on 4-hydroxybenzoate is dependent on AadR, a member of the cyclic AMP receptor protein family of transcriptional regulators. J Bacteriol. 1992;174:5803–5813. doi: 10.1128/jb.174.18.5803-5813.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolfing J, Zeyer J, Binder E P, Schwarzenbach R P. Isolation and characterization of a bacterium that mineralizes toluene in the absence of molecular oxygen. Arch Microbiol. 1990;154:336–341. doi: 10.1007/BF00276528. [DOI] [PubMed] [Google Scholar]
- 13.Evans P J, Ling W, Goldschmidt B, Ritter E R, Young L Y. Metabolites formed during anaerobic transformation of toluene and o-xylene and their proposed relationship to the initial steps of toluene mineralization. Appl Environ Microbiol. 1992;58:496–501. doi: 10.1128/aem.58.2.496-501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Julien B, Kaiser A D, Garza A. Spatial control of cell differentiation in Myxococcus xanthus. Proc Natl Acad Sci USA. 2000;97:9098–9103. doi: 10.1073/pnas.97.16.9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–198. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 16.Krieger C J, Beller H R, Reinhard M, Spormann A M. Initial reactions in anaerobic oxidation of m-xylene by the denitrifying bacterium Azoarcus sp. strain T. J Bacteriol. 1999;181:6403–6410. doi: 10.1128/jb.181.20.6403-6410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieger C J, Roseboom W, Albracht S P J, Spormann A M. A stable organic free radical in anaerobic benzylsuccinate synthase of Azoarcus sp. strain T. J Biol Chem. 2001;276:12924–12927. doi: 10.1074/jbc.M009453200. [DOI] [PubMed] [Google Scholar]
- 18.Kropp K G, Davidova I A, Suflita J M. Anaerobic oxidation of n-dodecane by an addition reaction in a sulfate-reducing bacterial enrichment culture. Appl Environ Microbiol. 2000;66:5393–5398. doi: 10.1128/aem.66.12.5393-5398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulzer R, Pils T, Kappl R, Hutterman J, Knappe J. Reconstitution and characterization of the polynuclear iron-sulfur cluster in pyruvate formate-lyase-activating enzyme. J Biol Chem. 1998;273:4897–4903. doi: 10.1074/jbc.273.9.4897. [DOI] [PubMed] [Google Scholar]
- 20.Lee S W, Edlin G. Expression of tetracycline resistance in pBR322 derivatives reduces the reproductive fitness of plasmid-containing Escherichia coli. Gene. 1985;39:173–180. doi: 10.1016/0378-1119(85)90311-7. [DOI] [PubMed] [Google Scholar]
- 21.Leuthner B, Heider J. A two-component system involved in regulation of anaerobic toluene metabolism in Thauera aromatica. FEMS Microbiol Lett. 1998;166:35–41. doi: 10.1111/j.1574-6968.1998.tb13180.x. [DOI] [PubMed] [Google Scholar]
- 22.Leuthner B, Leutwein C, Schulz H, Hörth P, Haehnel W, Schiltz E, Schägger H, Heider J. Biochemical and genetic characterization of benzylsuccinate synthase from Thauera aromatica: a new glycyl radical enzyme catalysing the first step in anaerobic toluene metabolism. Mol Microbiol. 1998;28:615–628. doi: 10.1046/j.1365-2958.1998.00826.x. [DOI] [PubMed] [Google Scholar]
- 23.Link A J, Phillips D, Church G M. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol. 1997;179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller J A, Galushko A S, Kappler A, Schink B. Anaerobic degradation of m-cresol by Desulfobacterium cetonicum is initiated by formation of 3-hydroxybenzylsuccinate. Arch Microbiol. 1999;172:287–294. doi: 10.1007/s002030050782. [DOI] [PubMed] [Google Scholar]
- 25.Muller J A, Galushko A S, Kappler A, Schink B. Initiation of anaerobic degradation of p-cresol by formation of 4-hydroxybenzylsuccinate in Desulfobacterium cetonicum. J Bacteriol. 2001;183:752–757. doi: 10.1128/JB.183.2.752-757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen T N M, Phan Q G, Duong L P, Bertrand K P, Lenski R E. Effects of carriage and expression of the Tn10 tetracycline-resistance operon on the fitness of Escherichia coli K12. Mol Biol Evol. 1989;6:213–225. doi: 10.1093/oxfordjournals.molbev.a040545. [DOI] [PubMed] [Google Scholar]
- 27.Rabus R, Heider J. Initial reactions of anaerobic metabolism of alkylbenzenes in denitrifying and sulfate-reducing bacteria. Arch Microbiol. 1998;170:377–384. [Google Scholar]
- 28.Rabus R, Wilkes H, Behrends A, Armstroff A, Fischer T, Pierik A J, Widdel F. Anaerobic initial reaction of n-alkanes in a denitrifying bacterium: evidence for (1-methylpentyl)succinate as initial product and for involvement of an organic radical in n-hexane metabolism. J Bacteriol. 2001;183:1707–1715. doi: 10.1128/JB.183.5.1707-1715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 30.Sawers G. Biochemistry, physiology and molecular biology of glycyl radical enzymes. FEMS Microbiol Rev. 1999;22:543–551. [Google Scholar]
- 31.Tamarit J, Gerez C, Meier C, Mulliez E, Trautwein A, Fontecave M. The activating component of the anaerobic ribonucleotide reductase from Escherichia coli. J Biol Chem. 2000;275:15669–15675. doi: 10.1074/jbc.275.21.15669. [DOI] [PubMed] [Google Scholar]
- 32.Ueki T, Inouye S, Inouye M. Positive-negative KG cassettes for construction of multigene deletions using a single drug marker. Gene. 1996;183:153–157. doi: 10.1016/s0378-1119(96)00546-x. [DOI] [PubMed] [Google Scholar]
- 33.von Gabain A, Belasco J G, Schottel J L, Chang A C Y, Cohen S N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci USA. 1983;80:653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasonobu K T, Tanaka M. The types, distribution in nature, structure-function, and evolutionary data of the iron-sulfur proteins. In: Lovenberg W, editor. Iron-sulfur proteins. New York, N.Y: Academic Press; 1973. pp. 27–130. [Google Scholar]
- 35.Zengler K, Heider J, Rossello-Mora R, Widdel F. Phototrophic utilization of toluene under anoxic conditions by a new strain of Blastochloris sulfoviridis. Arch Microbiol. 1999;172:204–212. doi: 10.1007/s002030050761. [DOI] [PubMed] [Google Scholar]