Abstract
Background:
Evidence on mortality risks associated with MS-immunotherapies during the SARS-CoV2 pandemic derived thus far mainly from single country experiences.
Objective:
In this analysis, we aim to determine the frequency of COVID-19 associated fatality reports of patients receiving an MS-immunotherapy as reported to the international Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) from February 2020 to March 2021.
Methods:
In all, 1071 cases for this cross-sectional analysis were retrieved from FAERS and a multivariable logistic regression was performed. We adjusted for sex, age, region, month of report to FDA, immunotherapy-class and additionally for healthcare-system and pandemic-related metrics.
Result:
Anti-CD20 therapies (60%) followed by sphingosine-1 phosphate modulators (12%) and dimethylfumarat (10%) were reported most frequently. In 50% of the cases, MS-phenotype is not reported, relapsing MS in 35% and progressive MS in 15%. Besides older age (odds ratio [OR]: 1.1; 95% confidence interval [CI]: 1.07–1.13; p < 0.01), anti-CD20 therapies were significantly associated with a higher risk of death (OR: 4.1; 95% CI: 1.17–14.46; p = 0.03), whereas female sex was associated with a reduced mortality risk (OR: 0.4, 95% CI: 0.22–0.72; p < 0.01).
Conclusion:
Using international open access data and a multidisciplinary approach for risk prediction, we identified an increased mortality risk associated with anti-CD20 therapies, which is in line with national and multi-national cohort studies.
Keywords: disease-modifying therapy, immunosuppression, multiple sclerosis, pharmacovigilance, SARS-CoV2
Introduction
The COVID-19 pandemic raises questions about the adequate medical treatment of people with multiple sclerosis (pwMS). Multiple sclerosis (MS) is a chronic inflammatory autoimmune disease of the central nervous system (CNS) that is treated with immunotherapies in most patients.1 Many MS-immunotherapies, dependent on their mechanism of action, possess specific risks of infections.2 A growing number of reports and cohort studies have emerged over the past months reporting cases of pwMS with COVID-19. A systematic review reports highest rates of mortality and hospitalization in the non-treated pwMS, followed by those treated with B-cell depleting (anti-CD20) therapies.3 The latter has been reported in other indications of anti-CD20 therapies like rheumatology.4 Most of these studies focused on national or monocentric cohorts and did not include the dynamic spread of the pandemic and the capacities of the respective healthcare systems and problems of, for example, patient triage.5,6
Methods
The US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) is a post-marketing pharmacovigilance platform that contains voluntary reports from healthcare professionals and consumers and is coded following the MedDRA (Medical Dictionary for Regulatory Activities) terminology.7 The data presented are derived from FAERS ‘public dashboard’8 (as of 31 March 2020, downloaded on 18 May, 2021). Additional epidemiological data is derived from ‘Our World in Data’ run by Oxford University (downloaded on 21 May 2021)9 and from the World Bank (downloaded 21 May, 2021)10,11 (Supplemental eTable 1). In preparation of the datasets, duplicates, incomplete and implausible observations (e.g. reports before February 2020) were removed (Supplemental eFigure 1). A part of the dataset presented here (474/1071 cases) has already been included in a prior analysis of our group.12 For this cross-sectional study, we performed a multivariable logistic regression analysis (MLR) with the dependent variable ‘death’ and age, sex, region, MS-phenotype, month of the filed report (‘initial FDA received date’) and the respective immunotherapy as independent variables. We controlled for local epidemiological factors by adjusting the MLR for a) potential capacities of the healthcare system of each country and b) temporal and spatial infection dynamics. For a), “hospital beds per 1000 persons per country” and “health expenditure per person per country (USD)” retrieved from the World Bank,10,11 served as proxies. For b), “monthly COVID-19 case fatality rate of each country,” “new cases per population, month and country” and “new deaths per population, month and country” computed from “Our World in Data”9 were used. To control for a COVID-19-related change in reporting behavior to FAERS–resembling notoriety bias–we included the variable “ratio of total reports to FAERS to the number of reported deaths to FAERS for each drug in 2018-2020 excluding COVID-19 cases,” retrieved from FAERS.8 A two-sided hypothesis test with a level of significance of p < 0.05 was used. Nagelkerke’s R2 is indicated for each MLR. Analyses were conducted with IBM© SPSS© Statistics 25 (2017, USA). Our study did not require an ethical board approval because it solely contains open access data of anonymized patient reports. The information within these analyses does not allow patient’s identification.
Results
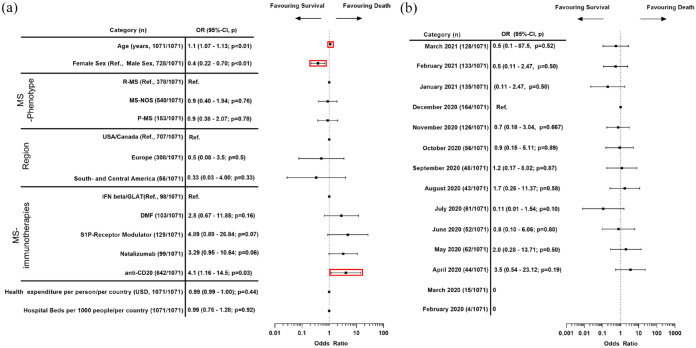
In our final cohort (n = 1071), the mean age of the pwMS was 48.5 (95% confidence interval [CI]: 47.69–49.25) with a female predominance (68%). Two thirds of reports originated from the United States and Canada (66%). The MS-phenotype was not specified in 50% of cases, relapsing MS was reported in 35% and progressive MS in 15%. Anti-CD20 therapies were the most frequently reported class of MS-immunotherapy within our cohort (59.5%), followed by sphingosine-1 phosphate modulators (S1PRM) (12.4%) and dimethyl fumarate (10.2%). Other immunotherapy classes, the respective medication therein and additional cohort characteristics are shown in Table 1. Monthly distribution of COVID-19 cases and death rates were different between regions (Supplemental eFigure 2A and B). The regional distribution of the cases per country is shown on a world map (Supplemental Figure 2C). 36% of the reported pwMS were hospitalized (386/1071) and the overall reported mortality rate in our cohort is 6.5% (70/1071; other outcomes: see Supplemental eTable 3). In the MLR, age (odds ratio [OR]: 1.1; 95% CI: 1.07–1.3; p < 0.01), treatment with anti-CD20 therapies (4.1; 1.16–14.5; p = 0.03), and with a borderline significance also treatment with natalizumab (3.3, 0.99–10.95, p = 0.053) were significant predictors of COVID-19-related death, whereas female sex was found to be protective (0.4; 0.22–0.72; p < 0.01) (Figure 1).
Table 1.
Cohort characteristics.
| Overall n = 1071 No. (%) |
Dead n = 70 No. (%) |
Survived n = 1001 No. (%) |
||
|---|---|---|---|---|
| Age in years [mean (SD)] | 48.5 (SD = 13.0) | 58.3 (SD = 10.0) | 47.8 (SD = 12.9) | |
| Sex | Male | 343 (32) | 34 (48.6) | 309 (30.9) |
| Female | 728 (68) | 36 (51.4) | 692 (69.1) | |
| Region | USA/Canada | 707 (66) | 43 (61.4) | 664 (66.3) |
| Europe | 308 (28.8) | 24 (34.3) | 284 (28.4) | |
| South- and Central America | 56 (5.2) | 3 (4.3) | 53 (5.3) | |
| MS-phenotype | Relapsing multiple sclerosis (R-MS) | 378 (35.3) | 17 (24.3) | 361 (36.1) |
| Progressive multiple sclerosis (P-MS) | 153 (15.3) | 16 (52.9) | 137 (13.7) | |
| Multiple Sclerosis—phenotype not specified | 540 (50.4) | 37 (22.9) | 503 (50.2) | |
| IFN Beta/GLAT | 98 (9.2) | 8 (11.4) | 90 (9) | |
| Interferon beta | 48 (49) | 8 (100) | 40 (44.4) | |
| Glatiramer acetate | 50 (51) | 0 (0) | 50 (55.6) | |
| Dimethyl fumarate | 103 (10.2) | 7 (10) | 96 (9.6) | |
| S1 P-receptor modulators | 129 (12.4) | 5 (7.1) | 124 (12.4) | |
| MS-immunotherapies | Fingolimod | 101 (78.3) | 3 (60) | 98 (79) |
| Siponimod | 20 (15.5) | 1 (20) | 19 (15.3) | |
| Ozanimod | 8 (6.2) | 1 (20) | 7 (5.6) | |
| Natalizumab | 99 (9.2) | 10 (14.3) | 89 (8.9) | |
| Anti-CD20 | 642 (59.9) | 40 (57.7) | 602 (60.1) | |
| Ocrelizumab | 597 (93) | 33 (82.5) | 564 (93.7) | |
| Rituximab | 35 (5.5) | 7 (17.5) | 28 (4.7) | |
| Ofatumumab | 10 (1.5) | 0 (0) | 10 (1.7) |
GLAT, glatiramer acetate; IFNeta, interferon beta; MS, multiple sclerosis; n, sample size; nos, not otherwise specified; P-MS, progressive MS; R-MS, relapsing MS; S1P-receptor modulator, sphingosine -1 phosphate modulator; USA, United States of America.
Absolute numbers of reported deaths and survived cases are displayed. Percentages relate to the dead or survived population. Percentage of the drugs listed below the medication groups (e.g. Ocrelizumab) in respect to their proportion within their medication group (e.g. anti-CD20).
Figure 1.
Multivariable logistic regression analysis of COVID-19 mortality risk factor. (a) ORs for age, sex, hospital beds per 1000 persons/per country, health expenditure per person/per country (in USD), region and immunotherapy. (b) ORs for each month of “initial FDA received date.”
CI, confidence interval; DMF, dimethyl fumarate; FDA, U.S. Food and Drug Administration; GLAT, glatiramer acetate; IFN beta, interferon beta; MLR, multivariable logistic regression; MS, multiple sclerosis; n, sample size; OR, Odds Ratio; p, p-value; Ref./ref., Reference; R-MS, relapsing MS; P-MS, progressive MS; S1 P-Receptor Modulator, Sphingosine-1 phosphate receptor modulator; USA, United States of America.
Multivariable-adjusted OR for “death”: 95%-CIs and p-values of the independent variables on a 10-log scale. Not displayed: “case fatality rate per month of initial FDA received date/country,” the “ratio of total reports to FAERS to the number of reported deaths to FAERS for each drug in 2018-2020 excluding COVID-19 cases,” “new cases per population per month and country,” and “new deaths per population per month and country”—OR, 95%-CI and p-value reported in the online-only supplement. Nagelkerke’s R2 is 0.33. For February and March 2020, no deaths have been reported to FAERS, therefore, the OR for February and March 2020 are 0.
Adjustment of the MLR by “month of event” instead of “month of initial FDA received date” (month of report)12 reduced the sample size (892 vs 1071); however, the significance levels and directions of the ORs of age (1.09; 1.06–1.13; p < 0.01), female sex (0.3; 0.15 - 0.60; p < 0.01), anti-CD20 (6.5, 1.3–32d; p = 0.02) and natalizumab (4.2, 1.04–16.88, p = 0.04) remained unchanged (see Supplemental eFigure 3).
Discussion
Within this retrospective observational study based on FAERS and the inclusion of not only patient-specific factors, but also metrics for the regional capacities of the healthcare system and the local dynamics of the SARS-CoV2 pandemic, we could confirm age and male sex as relevant predictors of COVID-19 mortality in pwMS. Concerning MS-immunotherapies, anti-CD20 therapies increased the mortality risk. Our results for natalizumab were inconclusive and need validation from other studies. It is though worth mentioning, that one report demonstrated a higher risk of contracting COVID-19 of natalizumab-treated MS-patients, the reason for this is still unclear.13
Using the FAERS database has several methodological limitations. It has a self-reporting structure thus favoring reporting- and notoriety bias and some reports might be incomplete, incorrect or duplicated, despite our measures. FAERS is not designed to reflect real estimations of incidences of an adverse event in the pwMS population and is not intended to prove causalities. In addition, it has a focus on the indication for the drug (“reason for use”) and is not reporting co-morbidities, MS-disability, the specific MS-phenotype and the concomitant use of steroids to a large extent. We therefore could not control for these factors, although their importance is well-established in COVID-19. Since FAERS is a pharmacovigilance database, a non-DMT treated control cohort is not available and we observe a higher proportion of more recently approved DMT compared to older ones, which is a known phenomenon in FAERS.14 During the observation period, COVID-19 mRNA vaccines were approved by emergency authorizations in late 2020 in the United States.15,16 To assess potential vaccination-associated effects, the time span covered by our analysis is small as vaccination started in the United States on 14 December 202017 and our observation period ended with March 2021 when only 13% of US population had been fully vaccinated (30% once).17 Likewise, this time interval (January to March 2021) for potentially vaccinated MS patients between vaccination and infection might be too short to allow for protection by prior vaccination. Our data are thus not suited to thoroughly address shifts in DMD-related COVID-19 outcome of MS patients with the introduction of vaccinations. Nonetheless, our findings are valid since they reflect known characteristics of COVID-19 mortality (age and male sex as risk factors).18 The observed risk increase linked to anti-CD20 therapies is additionally in line with national and multi-national cohort studies.5,19–22
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864221129383 for Multiple Sclerosis immunotherapies and COVID-19 mortality: an analysis of the FDA Adverse Event Reporting System by Maximilian Pistor, Robert Hoepner, Andreas G.F. Hoepner, Yanan Lin, Simon Jung, Claudio L. Bassetti, Andrew Chan and Anke Salmen in Therapeutic Advances in Neurological Disorders
Acknowledgments
None
Footnotes
ORCID iDs: Maximilian Pistor  https://orcid.org/0000-0002-0703-9974
https://orcid.org/0000-0002-0703-9974
Robert Hoepner  https://orcid.org/0000-0002-0115-7021
https://orcid.org/0000-0002-0115-7021
Anke Salmen  https://orcid.org/0000-0002-4751-299X
https://orcid.org/0000-0002-4751-299X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Maximilian Pistor, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse, 3010 Bern, Switzerland.
Robert Hoepner, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Andreas G.F. Hoepner, Department of Banking & Finance, Michael Smurfit Graduate Business School, University College Dublin, Dublin, Republic of Ireland Platform for Sustainable Finance, Department for Financial Stability and Capital Markets (DG FISMA), European Commission, European Union (EU), Brussels, Belgium.
Yanan Lin, Department of Banking & Finance, Michael Smurfit Graduate Business School, University College Dublin, Dublin, Republic of Ireland.
Simon Jung, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Claudio L. Bassetti, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
Andrew Chan, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Anke Salmen, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Declarations
Ethics approval and consent to participate: This study did not require an ethical board approval because it soley contains open access data of anonymized patient reports. The information within these analyses does not allow patient’s identification.
Consent for publication: No consent for publication is necessary.
Author contributions: Maximilian Pistor: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Visualization; Writing—original draft.
Robert Hoepner: Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Writing—review & editing.
Andreas G.F. Hoepner: Formal analysis; Methodology; Writing—review & editing.
Yanan Lin: Data curation; Methodology; Writing—review & editing.
Simon Jung: Formal analysis; Methodology; Writing—review & editing.
Claudio L. Bassetti: Methodology; Resources; Writing—review & editing.
Andrew Chan: Conceptualization; Methodology; Resources; Supervision; Writing—review & editing.
Anke Salmen: Conceptualization; Formal analysis; Supervision; Writing—review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Pistor M declares that he has no competing interests in relation to this study. Pistor M was funded by a translational research grant by the Department of Neurology, University Hospital Bern and received a research grant from the Swiss MS Society.
Hoepner R received speaker/advisor honorary from Merck, Novartis, Roche, Biogen, Alexion, Sanofi, Bristol-Myers Squibb, and Almirall. He received research support within the last 5 years from Roche, Merck, Sanofi, Biogen, and Bristol-Myers Squibb. He also received research grants from the Swiss MS Society.
Hoepner AGF and Lin Y declare that they have no conflict of interest. They have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. Hoepner AGF and Lin Y acknowledge funding from the European Union’s Horizon 2020 research and innovation program for research on Fintech (Grant No. H2020-ICT-825215), and Science Foundation Ireland (Award 19/FIP/AI/7539). The views expressed in this paper are not necessarily shared by other members of the Platform for Sustainable Finance or DG FISMA.
Jung S declares that he has no competing interests in relation to this study.
Bassetti CL declares that he has no competing interests in relation to this study.
Chan A has served on advisory boards for, and received funding for travel or speaker honoraria from, Actelion-Janssen, Almirall, Bayer, Biogen, Celgene, Sanofi-Genzyme, Merck, Novartis, Roche, and Teva, all for hospital research funds; and research support from Biogen, Genzyme and UCB. Chan A is associate editor of the European Journal of Neurology and serves on the editorial board for Clinical and Translational Neuroscience and as topic editor for the Journal of International Medical Research.
Salmen A received speaker honoraria and/or travel compensation for activities with Almirall Hermal GmbH, Biogen, Merck, Novartis, Roche, and Sanofi Genzyme and research support by the Swiss MS Society.
Availability of data and materials: Data are available on reasonable request via the corresponding author.
References
- 1. Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler J 2018; 24: 96–120. [DOI] [PubMed] [Google Scholar]
- 2. Winkelmann A, Loebermann M, Reisinger EC, et al. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol 2016; 12: 217–233. [DOI] [PubMed] [Google Scholar]
- 3. Barzegar M, Mirmosayyeb O, Gajarzadeh M, et al. COVID-19 among patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2021; 8: e1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2021; 80: 930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol 2021; 89: 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenbaum L. Facing Covid-19 in Italy—ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med 2020; 382: 1873–1875. [DOI] [PubMed] [Google Scholar]
- 7. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Medical dictionary for regulatory activities, 2021, https://www.meddra.org/ (accessed 5 July 5 2021).
- 8. U.S. Food and Drug Administration. FAERS—FDA adverse event reporting system, 2021, https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard (accessed 22 February 2021).
- 9. Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus—our world data, 2021, https://ourworldindata.org/coronavirus (accessed 14 March 2021).
- 10. The World Bank. Current health expenditure per capita (current US$), n.d., https://data.worldbank.org/indicator/SH.XPD.CHEX.PC.CD (accessed 13 March 2021).
- 11. The World Bank. Hospital beds (per 1,000 people), 2021, https://data.worldbank.org/indicator/SH.MED.BEDS.ZS (accessed 13 March 2021).
- 12. Pistor M, Hoepner A, Lin Y, et al. Immunotherapies and COVID-19 mortality: a multidisciplinary open data analysis based on FDA’s Adverse Event Reporting System. Ann Rheum Dis 2021; 80: 1633–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iaffaldano P, Lucisano G, Manni A, et al. Risk of getting COVID-19 in people with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2022; 9: e1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weiss-Smith S, Deshpande G, Chung S, et al. The FDA drug safety surveillance program: adverse event reporting trends. Arch Intern Med 2011; 171: 591. [DOI] [PubMed] [Google Scholar]
- 15. U.S. Food and Drug Administration. Moderna COVID-19 vaccine emergency authorization, n.d., https://www.fda.gov/media/144636/download
- 16. U.S. Food and Drug Administration. Biontech/Pfizer COVID-19 vaccine emergency authorization, n.d., https://www.fda.gov/media/144416/download
- 17. Roser M, Ortiz-Ospina E. COVID-19 vaccinations. Our World in Data, 2022, https://ourworldindata.org/covid-vaccinations
- 18. Goodman KE, Magder LS, Baghdadi JD, et al. Impact of sex and metabolic comorbidities on coronavirus disease 2019 (COVID-19) mortality risk across age groups: 66 646 inpatients across 613 U.S. hospitals. Clin Infect Dis 2021; 73: e4113–e4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American Registry of patients with multiple sclerosis. JAMA Neurol 2021; 78: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oksbjerg NR, Nielsen SD, Blinkenberg M, et al. Anti-CD20 antibody therapy and risk of infection in patients with demyelinating diseases. Mult Scler Relat Disord 2021; 52: 102988. [DOI] [PubMed] [Google Scholar]
- 21. Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology 2021; 97: e1870–e1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prosperini L, Tortorella C, Haggiag S, et al. Determinants of COVID-19-related lethality in multiple sclerosis: a meta-regression of observational studies. J Neurol 2022; 269: 2275–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864221129383 for Multiple Sclerosis immunotherapies and COVID-19 mortality: an analysis of the FDA Adverse Event Reporting System by Maximilian Pistor, Robert Hoepner, Andreas G.F. Hoepner, Yanan Lin, Simon Jung, Claudio L. Bassetti, Andrew Chan and Anke Salmen in Therapeutic Advances in Neurological Disorders