Abstract
The ability to attach to host ligands is a well-established pathogenic factor in invasive Staphylococcus aureus disease. In addition to the family of adhesive proteins bound to the cell wall via the sortase A (srtA) mechanism, secreted proteins such as the fibrinogen-binding protein Efb, the extracellular adhesion protein Eap, or coagulase have been found to interact with various extracellular host molecules. Here we describe a novel protein, the extracellular matrix protein-binding protein (Emp) initially identified in Western ligand blots as a 40-kDa protein due to its broad-spectrum recognition of fibronectin, fibrinogen, collagen, and vitronectin. Emp is expressed in the stationary growth phase and is closely associated with the cell surface and yet is extractable by sodium dodecyl sulfate. The conferring gene emp (1,023 nucleotides) encodes a signal peptide of 26 amino acids and a mature protein of a calculated molecular mass of 35.5 kDa. Using PCR, emp was demonstrated in all 240 S. aureus isolates of a defined clinical strain collection as well as in 6 S. aureus laboratory strains, whereas it is lacking in all 10 S. epidermidis strains tested. Construction of an allelic replacement mutant (mEmp50) revealed the absence of Emp in mEmp50, a significantly decreased adhesion of mEmp50 to immobilized fibronectin and fibrinogen, and restoration of these characteristics upon complementation of mEmp50. Emp expression was also demonstrable upon heterologous complementation of S. carnosus. rEmp expressed in Escherichia coli interacted with fibronectin, fibrinogen, and vitronectin in surface plasmon resonance experiments at a Kd of 21 nM, 91 nM, and 122 pM, respectively. In conclusion, the biologic characterization of Emp suggests that it is a member of the group of secreted S. aureus molecules that interact with an extended spectrum of host ligands and thereby contribute to S. aureus pathogenicity.
To initiate invasive infection, Staphylococcus aureus must adhere to extracellular matrix substrates and eukaryotic cells by virtue of different surface proteins (“adhesins”) (23). Biologic substrates such as a tissue wound or the injured vessel wall expose a panoply of adhesive glycoproteins possessing strong attachment-promoting activities for eukaryotic cells. Many of these molecules have also been shown to be of major importance in the initial adherence phase of pathogens (37). In fact, specific interaction of S. aureus has previously been described for fibronectin (Fn), fibrinogen (Fg), vitronectin (Vn), thrombospondin, bone sialoprotein, glycosaminoglycans, elastin, collagens, and other adhesive host factors (11, 19, 39). Typically, bacterial interaction with these adhesive proteins occurs independently of the RGD epitope and is mediated by bacterial surface molecules specifically recognizing the eukaryotic ligands.
Recent evidence clearly indicates that the specific bacterial interaction with these adhesive proteins not only allows for adhesion and colonization of tissues but is also pivotal in uptake of S. aureus by nonprofessional phagocytic cells such as epithelial or endothelial cells (5, 43, 44, 45). Consequently, the bacterial adhesins binding to extracellular matrix molecules can be used as candidates for prophylaxis or therapeutics, e.g., in anti-adhesin strategies (4, 7). These strategies, however, are complicated by the fact that staphylococci may use multiple mechanisms of adhesion, mutually complementing the loss-of-function of a given adhesin (32). Therefore, a comprehensive identification of all adhesins recognizing putative host ligands is warranted. In this respect, we sought to identify previously unknown staphylococcal adhesins, to characterize these molecules genetically, and to generate isogenic deletion mutants for analysis of their role in the biology of staphylococcal cells. Here we report the identification of a novel S. aureus surface molecule with an extended binding spectrum for adhesive glycoproteins.
MATERIALS AND METHODS
Bacterial strains and media.
Six laboratory strains of S. aureus—Newman (ATCC 25905), Cowan 1 (ATCC 12598), SA113 (ATCC 35556), 8325-4 (22), 6850 (1), and Wood 46 (ATCC 10832)—as well as a collection of 240 methicillin-susceptible S. aureus isolates obtained either from blood (n = 100) or from the anterior nares of patients (n = 140) (49) were investigated. Only one isolate per patient was tested. In addition, two reference strains of Staphylococcus epidermidis (ATCC 35984 and DSM 20044) and eight clinical S. epidermidis isolates were included. Clinical isolates were from patients in the University Hospital of Muenster, Muenster, Germany, and identified at the species level as S. aureus or S. epidermidis by standard microbiological methods, including commercial phenotype testing kits (Api Staph ID 32; BioMérieux, Marcy l'Etoile, France).
S. aureus Newman was used to generate the Δemp mutant. Recombinant plasmids cloned in Escherichia coli were passaged in the restriction-negative S. aureus strain SA113 before electroporation to Staphylococcus aureus Newman. S. carnosus TM300 (13) was used for complementation studies. The following strains of E. coli were used as cloning hosts: TG1, DH5α, and INVαF′ (Invitrogen, Gronigen, The Netherlands) and M-15 (Qiagen, Hilden, Germany).
For cultivation of staphylococci, chemically defined medium HHW (20), medium B (14), tryptic soy broth or agar (Difco, Detroit, Mich.), brain heart infusion (BHI) broth or agar (Merck, Darmstadt, Germany), Mueller-Hinton broth or agar (Mast, Merseyside, United Kingdom), and Luria-Bertani (LB) broth or agar (Difco) was used. For cultivation of E. coli LB broth or agar was used.
Preparation of cell surface proteins, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis.
To prepare cell surface proteins, staphylococci were grown in 5 ml of BHI broth (Merck) at 37°C for 18 h and then centrifuged at 6,000 × g for 5 min. The pellet was resuspended in extraction buffer (125 mM Tris-HCl [pH 7.0] plus 2% SDS; Sigma-Aldrich Chemie GmbH, Deisenhofen, Germany) at 10 μl/mg (wet weight) of pellet to adjust for differences in cell number, heated at 95°C for 3 min, and then centrifuged at 10,000 × g for 3 min. The supernatant was dialyzed against distilled water to remove SDS. To 20 μl of liquid supernatant, 5 μl of 5× sample buffer (60 mM Tris-HCl, pH 6.8; 25% glycerol; 2% SDS; 14.4 mM 2-mercaptoethanol; 0.1% bromophenol blue [Merck]) was added, and the mixture was then heated at 95°C for 3 min and separated in a SDS–12% PAGE minigel. For Western ligand blot analysis, either Fn (Chemicon, Temecula, Calif.), Fg (Calbiochem, San Diego, Calif.), collagen type I (Cn; Sigma product 7774), or Vn purified by the method of Yatohgo et al. (51) were used. Fn, Fg, Cn, and Vn were labeled with biotin according to instructions of the supplier (Roche, Mannheim, Germany). For Western ligand blot analysis, proteins separated by SDS-PAGE were electrophoretically transferred (Trans-Blot SD; Bio-Rad, Munich, Germany) onto a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany), and then the membrane was blocked with 3% bovine serum albumin (BSA; fraction V; Sigma). Blotted proteins on nitrocellulose were probed with biotinylated ligands and subsequently detected by using an avidin alkaline phosphatase color reaction (Bio-Rad). Alternatively, Fn, Vn, and Fg were labeled with DIG (digoxigenin-3-O-methylcarbonyl-ɛ-aminocaproic acid-N-hydroxysuccinimide ester; Roche), and blotted S. aureus surface proteins were incubated with DIG-labeled ligands; blots were subsequently exposed to anti-DIG-antibodies (Roche) and developed with a color reaction (Roche).
Detection of the surface location of Emp by indirect immunofluorescence.
Newman cells were grown overnight, centrifuged, and suspended in phosphate-buffered saline (PBS) to an optical density at 578 nm (OD578) of 1.0. Next, 5 μl of the bacterial suspension was dried on an immunofluorescence slide (BioMérieux) and then fixed with cold acetone. Fixed cells were exposed to a 1:1,000 dilution of polyclonal antibodies raised in rabbits immunized with recombinant Emp expressed in E. coli (prepared as described below) and preadsorbed with SDS extract from mutant mEmp50 (characterized below). After three washes with PBS, a 1:100 dilution of the fluorescein isothiocyanate (FITC)-conjugated anti rabbit immunoglobulin G (Sigma) was added. Subsequently, slides were washed three times with PBS, dried and, after the addition of mounting medium Fluoprep (BioMérieux), examined by fluorescence microscopy.
Determination of time course of Emp production.
A 500-ml flask containing 200 ml of BHI was inoculated with 2 ml of an overnight culture of S. aureus Newman in the same medium. The flask was incubated at 37°C with shaking, and the first sample was collected after 2 h and subsequently after each hour. The OD650 of each sample was measured to establish a growth curve, and cell surface proteins from S. aureus cells were extracted with 2% SDS as described earlier.
N-terminal sequence determination.
Cell surface proteins were separated by SDS-PAGE and blotted electrophoretically onto Immobilon-P (polyvinylidene difluoride membrane; Millipore, Bedford, Mass.). After being stained with Coomassie blue R-250 (0.01%; Serva, Heidelberg, Germany), protein bands of interest were cut out, and the N-terminal amino acid sequence was determined with an automated 473A sequencer (Perkin-Elmer, Weiterstadt, Germany).
DNA manipulations and transformations.
Manipulations were performed according to standard procedures (40). Genomic DNA was isolated by using the QIAamp DNA Mini Kit (Qiagen) with the modification that lysostaphin (20 U/ml) for the S. aureus strains or lysostaphin (20 U/ml) plus lysozyme (1 mg/ml) for the S. epidermidis strains was used for cell lysis. Plasmid DNA was prepared by using the Qiagen plasmid kit. DNA fragments were isolated from agarose gels by using the QIAquick gel extraction kit (Qiagen). Selection for resistance to antibiotics in E. coli or S. aureus was performed with ampicillin (100 μg/ml; Sigma), erythromycin (10 μg/ml; Serva), and chloramphenicol (10 μg/ml; Serva).
Cloning, DNA sequencing, and computer analysis of emp
In order to amplify emp from genomic DNA of S. aureus Newman, primer sequences were designed as follows: upper primer P1, 5′-CTC GGA TCC ATG CTG TTA GTG AAT ATA ACA GGG-3′ (encoding nucleotides 1259 to 1236 of the sequence of contig >4366 of the S. aureus strain COL sequence [TIGR Microbial Databases]; the BamHI restriction site is underlined); and lower primer P2, 5′-CTC GGTACC ACA ACC ACA TAG ATT GTA GCC TAT ATT TA-3′ (including nucleotides 92 to 120 of the contig >4366; the KpnI restriction site is underlined). The PCR mixture included 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl, a 200 μM concentration of each deoxynucleoside triphosphate, 100 pmol of each primer, 2.5 U of Ampli Taq DNA polymerase (Perkin-Elmer), and template DNA. The PCR was carried out in a PE 2400 thermocycler (Perkin-Elmer) and, after initial denaturation at 96°C for 4 min, consisted of 30 cycles of consecutive denaturation, annealing, and extension (30 s at 96°C, 1 min at 55°C, and 3 min at 72°C, respectively). Then, 10 μl of the product was analyzed on a 1% agarose gel. The PCR product was ligated into a pCR2.1 vector (Invitrogen), and the ligation mixture was transformed in E. coli INVαF′ (Invitrogen). A plasmid containing the emp gene from S. aureus strain Newman was designated pEmpN. Plasmid DNA, prepared by using the Qiagen Plasmid Midi Kit, was used as a template for sequencing on the ABI 310 Genetic Analyzer (Perkin-Elmer) with the dRhodamine terminator cycle sequencing ready reaction kit according to the instructions of the supplier (Perkin-Elmer). Sequencing of both strands was started with M13 reverse and T7 standard primers of the vector upstream and downstream of inserted DNA, and sequencing was completed by primer walking. The obtained nucleotide sequence and deduced protein sequence were analyzed by using the database available at the European Molecular Biology Laboratory outstation European Bioinformatic Institute (EBI; Cambridge, United Kingdom). The emp genes from the chromosomal DNA of two additional S. aureus strains, SA113 and 6850, were amplified by PCR with primers P1 and P2. The obtained PCR products were ligated in cloning vector pCR2.1 as described above. The resultant plasmids containing emp from strains SA113 and 6850 were designated pEmpS and pEmp6, respectively. The DNA sequences of emp cloned in these two plasmids were determined.
Expression of recombinant Emp in E. coli
The gene emp (lacking the signal peptide) of S. aureus Newman was amplified by PCR with upper primer P3 (5′-CTC GGA TCC GCA TCA GTG ACA GAG AGT GTT GAC-3′; including nucleotides 148 to 171 of the sequence of emp of S. aureus Newman; the BamHI restriction site is underlined) and lower primer P4 (5′-CTC GGT ACC TTA TAC TCG TGG TGC TGG TAA GC-3′; including nucleotides 1073 to 1095 of the sequence of the emp gene of S. aureus Newman; the KpnI restriction site is underlined). The PCR product was ligated into the QIAexpress pQE30 vector (Qiagen). The ligation mixture was transformed in freshly prepared competent cells of E. coli M-15. A representative plasmid containing the emp gene was designated pQEmpN. His6 tag recombinant Emp was expressed, and purification was achieved in a single step on Ni-nitrilotriacetic acid (NTA) resin (Qiagen) according to the protocol provided by the manufacturer.
Screening for prevalence of emp among clinical isolates.
To determine the prevalence of emp in various S. aureus and S. epidermidis clinical isolates, emp was amplified from genomic DNA by using primers P3 and P4.
Construction of an Emp-deficient mutant. (i) Insertion of ermB
The plasmid pEC4 [pBluescript KS(+) containing the 1.45-kb ermB fragment of the staphylococcal transposon Tn551 in a ClaI restriction site] (27) was digested with HpaI and SmaI, and the ermB cassette was isolated by using the QIAquick gel extraction kit (Qiagen). The ermB cassette was ligated into the EcoRV-restricted emp in pQEmpN. The ligation mixture was transformed into freshly prepared competent cells of E. coli DH5α (40), and the transformation mixture was plated on LB plates containing ampicillin and erythromycin. One representative plasmid containing the emp::ermB fragment was designated pMH5.
(ii) Construction of an emp::ermB shuttle vector.
The emp::ermB fragment was isolated from pMH5 as a 2.4-kb fragment by restriction with KpnI and BamHI and ligated with plasmid pBT2. pBT2, composed of parts of pBR322 and pTV1ts (52), is a shuttle vector able to replicate in E. coli and staphylococci and contains a temperature-sensitive replicon for staphylococci. This vector was used to inactivate the emp gene in the genome of S. aureus Newman. The ligation mixture was transformed in E. coli TG1 cells and subsequently plated on LB plates containing ampicillin, erythromycin, and chloramphenicol. One representative plasmid conferring resistance to all three antibiotics was designated pMH6.
(iii) Inactivation of emp.
pMH6 was first propagated in restriction-deficient S. aureus SA113, and one representative plasmid was isolated and designated pMH7. Plasmid pMH7 was transformed into S. aureus strain Newman by electroporation (29) and a transformant clone containing pMH7 was selected. For construction of an emp allelic replacement mutant, the method described by Palma et al. (34) was used. S. aureus Newman containing pMH7 was cultivated overnight in LB medium in the presence of erythromycin (10 μg/ml) and chloramphenicol (10 μg/ml) with shaking at 32°C. The overnight culture was reinoculated (1:20) into LB medium with erythromycin and grown at the nonpermissive temperature (43°C) overnight. This selects for clones with the plasmid integrated by single recombination into the chromosomal DNA. The culture was reinoculated (at 1:20) and grown in LB medium at 43°C for 24 h without antibiotics selecting for stable chromosomal integration (emp::ermB) and concomitant loss of chloramphenicol resistance. Various dilutions of this culture were plated on LB plates containing erythromycin (10 μg/ml) and then incubated overnight at 43°C. Chloramphenicol-sensitive and erythromycin-resistant colonies were detected by replica plating onto plates containing chloramphenicol or erythromycin at 37°C overnight. Clone number 50, sensitive for chloramphenicol and resistant against erythromycin was designated mEmp50 and selected for further analysis. Correct insertion of the ermB fragment was confirmed by using primers Eryfor (5′-ATG AAC AAA AAT ATA AAA TAT TCT CAA AAC-3′) and Eryrev (5′-TTA TTT CCT CCC GTT AAA TAA TAG ATA AC-3′). These primers are designed from sequences of Tn551 (NCBI accession no. Y13600) and ermB of S. intermedius (NCBI accession no. AF239773).
Complementation of S. carnosus TM300 and mEmp50.
A PCR product of emp of genomic DNA of S. aureus Newman, including the ribosomal binding site, was prepared by using primers P5 (5′-CTC GGA TCC AAG GAG AAA TAA CAG ATG AAA AAG AAA TTA GTT TTA 3-′; including the nucleotide sequence from position 58 to 93 of emp of S. aureus Newman; the BamHI restriction site is underlined) and P4 and ligated into pCX19. The ligation mixture was transformed into S. carnosus cells. A representative plasmid containing emp as an insert was designated pCXEmp and, accordingly, the transformed S. carnosus strain was designated TM300(pCXEmp). To express recombinant Emp, S. carnosus containing pCXEmp was grown in medium B to an OD650 of 0.5 and induced by xylose (final concentration of 0.25%), and the culture was grown for an additional 5 h. Thereafter, cells were pelleted by centrifugation, washed once with 10 mM Tris-buffered saline (pH 8.0), and suspended in the same buffer. Lysostaphin (20 U/ml) was added to the cell suspension and incubated for 1 h at 37°C. The recombinant Emp in the lysate was determined by SDS-PAGE and Western ligand blot analysis.
The plasmid pCXEmp was isolated from S. carnosus and transformed by electroporation into mEmp50. Transformants were grown on tryptic soy agar plates (containing 10 μg of chloramphenicol and 10 μg of erythromycin per ml), and one representative clone expressing Emp upon xylose induction (as detailed above for the expression of recombinant Emp) was designated mEmp50 (pCXEmp).
Adherence to Fn- and Fg-coated PMMA coverslips.
A radiometric assay described earlier (48) was used to determine the binding of bacteria to coated surfaces. Briefly, an inoculum containing 4 × 106 CFU (40 μl) of [3H]thymidine-labeled staphylococci was incubated with a polymethylmethacrylate (PMMA) coverslip preadsorbed with human plasma Fn or Fg in a tube containing 960 μl of PBS with Ca2+ and Mg2+ (Gibco/Life Technologies, Paisley, United Kingdom) supplemented with 0.5% human serum albumin and incubated at 37°C for 1 h in a shaking water bath. Thereafter, the PMMA coverslip was removed, washed with PBS three times, and the adherent counts per minute were determined.
Biologic interaction analysis (BIA).
Surface plasmon resonance measurements were performed by using the Biacore2000 instrument from Biacore AB (Uppsala, Sweden). Sensor chip C1 (research grade), amine coupling kit, surfactant P20, and sample tubes and caps were also obtained from Biacore AB. Immobilization of the proteins and analysis of the interaction was carried out by an automatic method in a Biacore2000. The protein Emp was covalently coupled to sensor chip C1 via primary amine groups. After activation of the carboxylated matrix of C1 sensor chip with a single injection of 50 μl of 0.1 M N-hydroxysuccinimide–0.4 M N-ethyl-N′-(3-dimethylaminopropyl)-carbodiimide (NHS-EDC), 100 μl of Emp (Emp was diluted 1:10 in 10 mM sodium acetate [pH 4.5]) was injected over the activated surface. Excess activated esters were blocked by injection of 55 μl of 1 M ethanolamine (pH 8.5). The immobilized amount of Emp was in the range of 92 to 166 pg. Binding experiments were performed at 25°C in a pH 7.4 buffer containing 10 mM HEPES, 150 mM NaCl, 3.4 mM EDTA, and 0.005% surfactant P-20. Sequential injections of Fn, Fg, Vn, and Cn allowed determination of the respective binding kinetics to Emp. The sensor chip was regenerated between each run with a pulse of 100 mM NaOH. The association and dissociation rate constants, kon and koff, were analyzed by using BIA evaluation 3.1 software from Biacore AB as described previously (26).
RESULTS
Interaction of cell surface proteins with eukaryotic ligands.
For identification of hitherto-unrecognized surface proteins, 2% SDS extracts of S. aureus cells were separated in Coomassie blue-stained gels. Two proteins (70 and 40 kDa) were found in larger quantity compared to other proteins (Fig. 1A, lane 1). Putative cell surface adhesins were subsequently identified by Western ligand blotting of SDS-extracted proteins onto nitrocellulose membranes and then probing with labeled ligands. Several proteins were found to bind biotinylated Fn, Fg, Vn, and Cn in separate Western ligand blots. Three proteins (70, 40, and 32 kDa) recognized Fn and Fg (Fig. 1A, lanes 2 and 3) as well as Vn and Cn albeit to a more variable extent (not shown). Control Western ligand blots prepared with SDS- extracted surface proteins and exposed with avidin alkaline phosphatase did not reveal any recognition (not shown). The 70-kDa protein has already been characterized as Map or Eap (25, 33) as determined in our laboratory by the N-terminal sequence of the protein (M. Hussain et al., unpublished data). Further, an ∼40-kDa protein avidly bound labeled Fn, Fg, Vn, and Cn (Fig. 1A, arrow). Despite binding of the 40-kDa protein to these various extracellular matrix proteins, this interaction appears to be targeted, since Western blots exposed with biotinylated lysozyme, lysostaphin, and BSA did not show reaction with the 40-kDa protein. Finally, a 32-kDa protein was recognizable. In addition to recognizing Fn, Fg, Vn, and Cn, this molecule also reacted with the lysozyme, lysostaphin, and BSA. Currently, the nature of this 32-kDa protein is undetermined; however, size comparisons suggest it is the recently determined neutral phosphatase of S. aureus (9) or an analogous molecule. The 40-kDa protein has not yet been described; therefore, we proceeded with its further characterization.
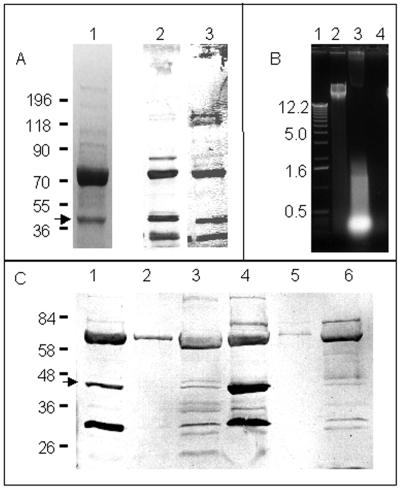
FIG. 1.
Expression of a 40-kDa protein recognized by Fn and Fg. (A) S. aureus Newman cell surface proteins detected with Coomassie blue-stained SDS-PAGE (lane 1) and Western ligand blot analysis with biotinylated Fn (lane 2) and Fg (lane 3). Cell surface proteins were extracted as described in Materials and Methods and then probed with biotinylated ligands. Molecular mass standards are expressed in kilodaltons. (B) Ethidium bromide-stained 1% agarose gel showing, from left to right: lane 1, 1-kbp DNA ladder (Gibco); lane 2, genomic DNA isolated by using the QIAamp DNA Mini Kit from strain Newman; lane 3, LiCl extract of strain Newman prepared by shaking bacteria in 1 M LiCl at 42°C for 2 h; and lane 4, 2% SDS extract of strain Newman. (C) Western ligand blots of cell surface and secreted proteins of strain Newman developed with DIG-labeled Fn. Cells of strain Newman were grown in HHW (lanes 1 to 3) and BHI (lanes 4 to 5). Lanes 1 and 4, cell surface proteins extracted with 2% SDS extract; lanes 2 and 5, cell-secreted proteins in liquid supernatant; lanes 4 and 6, cell-secreted proteins in 40× concentrated liquid supernatant. Arrows indicate the ∼40-kDa protein further characterized in this study.
The 40-kDa protein is a cell surface molecule.
The SDS extraction method used in this study to prepare cell surface proteins is less prone to release intracellular molecules than other cell surface protein extraction methods such as with 1 M LiCl (21). The 40-kDa protein appears to be localized extracellularly, since we have previously shown that the 2% SDS extraction method does not release intracellular molecules. We confirmed these findings for strain Newman used in this study, and we have found that nucleic acids as markers for release of intracellular molecules were detected in the extracts of cells treated with 1 M LiCl but not in cells extracted with 2% SDS (Fig. 1B). To determine whether the 40-kDa protein is cell surface associated or released into the medium, 2% SDS cell surface extracts and native or 40× concentrated supernatants were examined by SDS-PAGE, followed by Western ligand reactions by using DIG-labeled Fn (Fig. 1C). Control blots exposed with anti-DIG antibodies did not reveal any band recognition. While the 40-kDa protein was detectable only in SDS extracts (Fig. 1C, lanes 1 and 4, arrow), Eap but not the 40-kDa protein was also detected in unconcentrated supernatants (Fig. 1C, lanes 2 and 5). Traces of the 40-kDa protein, together with other secreted proteins, were only detected upon concentration of the supernatant (Fig. 1C, lanes 3 and 6). This finding suggests tight association of the 40-kDa protein with the bacterial cell surface.
By indirect immunofluorescence with polyclonal antibodies directed against the 40-kDa protein, recognition of cell S. aureus Newman cells could be observed (Fig. 2, lower panel), while preimmune antibodies failed to elicit a positive signal (Fig. 2, upper panel). Together with the observation that the 40-kDa protein is retained on the cell surface but released upon SDS extraction, this observation strongly suggests surface localization of the molecule.
FIG. 2.
Detection of the 40-kDa protein (Emp) on the surface of S. aureus Newman by immunofluorescence microscopy. S. aureus cells were incubated with preimmune serum (top panel) or with antiserum directed against recombinant Emp preadsorbed with surface molecules from mutant mEmp50. Thereafter, bound immunoglobulins were detected by using FITC-labeled anti-IgG antibodies and examined by fluorescence microscopy. Magnification, ×200.
The 40-kDa protein is expressed in stationary phase.
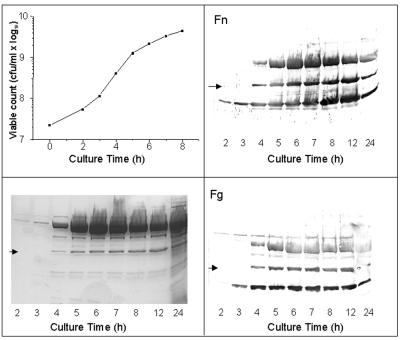
To determine the expression kinetics of the 40-kDa molecule, aliquots of different time points of growth were taken from a culture medium, adjusted for the wet weight of the pellet, and cell surface molecules were extracted with SDS. The 40-kDa protein was not detected in 2- and 3-h cultures but did appear in 4-h cultures and gradually increased in concentration as detected by SDS-PAGE and on Western blots probed with biotinylated Fn and Fg. This time course of the 40-kDa protein expression is similar to Eap, which was also not seen in early-log-phase cultures but started appearing in late-log-phase cultures (Fig. 3).
FIG. 3.
Time course of production of the 40-kDa protein of S. aureus Newman in BHI. A total of 200 ml of BHI was inoculated with 2 ml of an overnight culture in the same medium and incubated at 37°C with shaking. Samples were taken after 2 h and subsequently each hour to monitor the OD650 nm of growing culture and to extract cell surface proteins with 2% SDS. Panels display the growth curve (upper left), SDS-PAGE gels of surface protein extracts (lower left), and Western ligand blots of surface protein extracts incubated with biotinylated Fn (upper right) and Fg (lower right). Arrows indicate the 40-kDa protein.
The 40-kDa protein is a newly described protein.
For determination of the nature of the 40-kDa protein, samples purified by gel chromatography were subjected to N-terminal sequence analysis. The obtained N-terminal sequence of 40-kDa protein of S. aureus Newman was SVTES VDKKF VVPES GINKI IPAYD EFKNS PKVNV SN. The 37-amino-acid unambiguous N-terminal sequence was evaluated for homology with the Swissprot, TrEMBL, and Swall databases (EBI). The search results did not reveal any considerable homology (≤36.4%) to deposited protein sequences. Accordingly, the 40-kDa protein was considered a novel adhesin recognizing various extracellular matrix molecules, and we propose to designate it “extracellular matrix protein-binding protein” (Emp). Subsequently, the N-terminal sequence was evaluated for homology to the S. aureus strain COL sequence in the TIGR Microbial Genomic Database. A 100% identity was found in contig >4366, and an open reading frame (ORF) encoding a protein of 38.5 kDa was revealed from this particular contig. This information was used for further molecular characterization of Emp.
Analysis of emp sequence of S. aureus Newman.
The DNA sequence of the gene encoding Emp revealed an ORF of 1,023 nucleotides encoding a protein of 38.5 kDa; the gene was subsequently designated emp. The emp start codon ATG is preceded by a sequence typical for a ribosomal binding site and the ORF is terminated by a stop codon. The sequence has a GC content of 31.1% corresponding to the ∼30% GC content for the staphylococcal genome. The first 26 amino acids have features characteristic of a bacterial signal peptide. On the basis of the demonstrated cleavage site between positions 26 and 27, the mature peptide has a calculated molecular mass of 35.5 kDa. Statistical analysis of the deduced amino acid sequence was evaluated by the program SAPS available at EBI and by PC/Gene (IntelliGenetics, Inc., Mountain View, Calif.). The mature Emp protein carries a net positive charge, and the isoelectric point is 10.44. Two aligned matching blocks of four amino acids (NHAK at positions 22 to 25 and 175 to 178, and KNFV at positions 68 to 71 and 92 to 95, respectively) and one matching block of 10 amino acids (VDKKFVVPES at positions 32 to 41 and 90 to 99) were observed. The deduced amino acid sequence of Emp did not show any significant homologies to known proteins deposited in the EBI data bank, and the emp nucleotide sequence was deposited in the EMBL database under accession number AJ271347.
Alignment of Emp from four strains.
The deduced amino acid sequences of the Emp of three test strains (Newman, SA113, and 6850) and of the ORF derived from the sequence of strain COL (contig >4366) were aligned. When we compared their respective amino acid sequences, the Emps of strains Newman, SA113, and COL showed 100% identity, whereas they displayed 95% identity compared to strain 6850. The EMBL accession numbers for emp of strains SA113 and 6850 are AJ272083 and AJ272084, respectively.
Prevalence of emp
In all 240 S. aureus clinical isolates and in 6 reference strains which were examined by PCR, an amplicon of ∼1 kbp was detected. In contrast, upon amplification of DNA extracted from S. epidermidis, an amplicon was not found in any of the 10 strains tested (eight clinical isolates and two reference strains).
Genetic characterization of the Δemp mutant, mEmp50.
For further evaluation of the putative function of Emp, an allelic replacement mutant of wild-type strain Newman was generated by insertion of an ermB cassette into the emp gene in E. coli, cloning the construct in a temperature-sensitive shuttle vector, and transfer into S. aureus 113 and subsequently into the wild-type strain Newman. For allelic replacement of emp, after temperature shift, clones with stable expression of erythromycin resistance and loss of chloramphenicol resistance were selected. One clone identified was designated mEmp50. The double-crossover emp::ermB allelic replacement in mEmp50 was confirmed by PCR. The expected PCR products of 1 and 2.4 kbp from the wild type and mEmp50, respectively, were obtained by PCR with primers P3 and P4 (used for emp amplification and subsequent preparation of the emp::ermB construct) (either of the primers was also used in combination with primers specific for the ermB resistance cassette), as well as with primers P5 and P2 (located externally of the construct) (not shown). The observed size increases for the PCR product generated from mEmp50 template DNA, as well as amplicons generated by using internal ermB primers upon amplification of mEmp50 DNA but not of wild-type DNA, confirmed the allelic replacement of the wild-type emp gene by emp::ermB.
Phenotypic analysis of mEmp50.
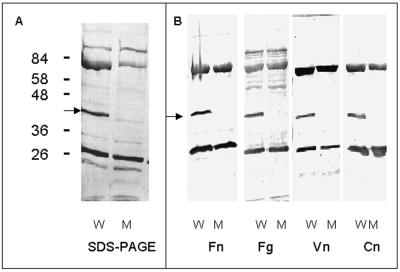
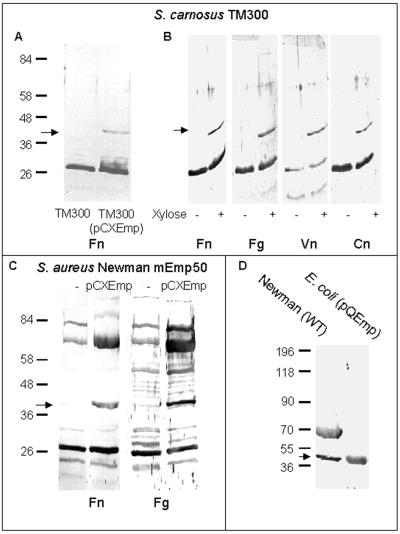
Extensive phenotypic comparison of strain Newman and mEmp50 by conventional microbiological tests and three commercial identification systems did not reveal any difference. For determination of cell surface protein expression patterns, proteins of the wild-type strain and mEmp50 were extracted with 2% SDS and analyzed by SDS-PAGE and Western ligand blot analysis. In Coomassie blue-stained gels, the band patterns of proteins obtained from the mutant were similar to those of the wild type, except that the band corresponding to Emp was missing in the SDS-extract of the mEmp50 (Fig. 4A, lane 2). In Western ligand assays, surface protein extracts of mEmp50 lacked recognition of Fn, Fg, Vn, and Cn at the position of Emp (Fig. 4B). Subsequently, both S. carnosus TM300 and mEmp50, complemented with emp, were examined for expression of functional Emp. While Emp was not demonstrable in 2% SDS surface protein extracts of wild-type TM300 analyzed either by SDS-PAGE (not shown) or by Western ligand assay (Fig. 5A), Fn-recognizing Emp was detected in 2% SDS surface protein extracts of xylose-induced TM300(pCXEmp) cultures (Fig. 5A). Upon xylose induction, recognition of Fg, Vn, and Cn by recombinant Emp could also be demonstrated (Fig. 5B). Furthermore, functional Emp recognizing Fn and Fg was found to be present in SDS extracts of the complemented mutant mEmp50(pCXEmp) (Fig. 5C). Finally, Emp was successfully cloned and expressed in E. coli. Figure 5D showing Western ligand analyses with labeled Fn demonstrates recognition of His-tagged recombinant Emp purified on an Ni-NTA resin (lane 2), while in cell surface extracts of wild-type Newman both Emp and Eap were recognized (lane 1). In immunofluorescence experiments, anti-Emp antibodies failed to recognize Emp on the surface of mEmp50 (not shown).
FIG. 4.
Western ligand blot analysis of cell surface proteins of S. aureus strains Newman (W) and mEmp50 (M). (A) SDS-PAGE of surface proteins extracted with SDS as described in Materials and Methods. (B) Western ligand blots of SDS-extracted surface proteins. Blots were prepared and incubated with biotinylated Fn, Fg, Vn, or Cn as described in Materials and Methods.
FIG. 5.
Western ligand blot analyses of cell surface proteins extracted from emp complemented strains. Arrows indicate Emp. (A) SDS extracts from xylose-induced cultures of S. carnosus TM300 (lane 1) or TM300(pCXEmp) (lane 2). (B) SDS extracts of S. carnosus TM300(pCXEmp) without or with xylose induction incubated with Fn, Fg, Vn, and Cn. (C) SDS extracts of S. aureus mEmp50 without or with complementation with emp and incubated either with Fn or Fg. (D) SDS extracts from S. aureus Newman (lane 1) and rEmp from E. coli (lane 2) incubated with Fn. Molecular mass standards are expressed in kilodaltons.
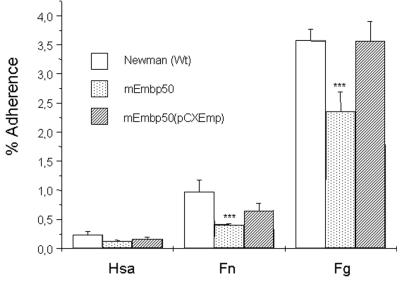
Adherence of S. aureus Newman, mEmp50, and mEmp50(pCXEmp).
In a solid-phase bacterial adhesion assay, strain Newman was found to bind weakly to Fn-coated surfaces and, to a comparably greater extent, to Fg-coated surfaces. Compared to the wild type, however, there was significantly less adhesion of mEmp50 to Fn-coated or Fg-coated coverslips. Upon complementation of the mEmp50 with pCXEmp, the adhesive function was restored (Fig. 6).
FIG. 6.
Adhesion of strains Newman, mEmp50, and mEmp50(pCXEmp) on immobilized Fg and Fn adsorbed to PMMA coverslips. PMMA coverslips were coated with either human serum albumin, Fg, or Fn. A radiolabeled bacterial suspension of strains Newman, mEmp50, or mEmp50(pCXEmp) was added to coverslips and incubated at 37°C with shaking. After 1 h the bacterial suspension was removed, the coverslips were washed with PBS, and the adherent radioactivity was measured. Experiments were performed twice in triplicates. Shown are means ± the standard deviation. ∗∗∗, P < 0.0001 (unpaired t test).
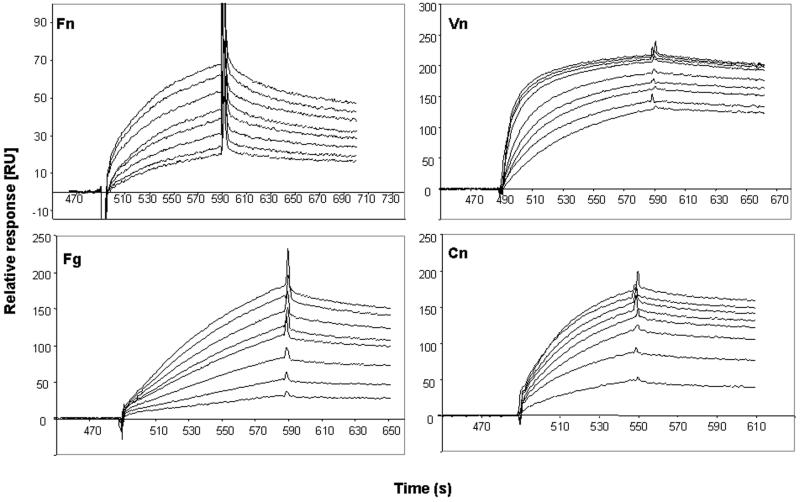
Interaction of recombinant Emp with extracellular matrix ligands.
The specific interaction between Emp and either Fn, Fg, Vn, or Cn was evaluated by using surface plasmon resonance. For this purpose, recombinant Emp from E. coli was attached to a C1 sensor chip, a solution containing either Fn, Fg, or Vn was perfused over the surface, and the interaction was analyzed (Fig. 7). All three ligands interacted with immobilized recombinant Emp (rEmp); however, the affinity of ligand interaction was found to vary greatly in extent. For Fn, mean values of the rate constants and of the equilibrium dissociation constant in eight determinations with various ligand concentrations were as follows: kon = 2.154/mol/liter · s (Ms), koff = 4.41−3/s, and Kd = 20.5 nM. For Fg, the respective values were kon = 2.513/Ms, koff = 2.26−3/s, and Kd = 91.3 nM. The values for Vn (assuming a mean molecular mass of the native [i.e., polymeric] Vn molecule of 1.200 kDa (17) were kon = 7.566/Ms, koff = 9.29−4/s, and Kd = 122 pM. For Cn, the rate constant and equilibrium dissociation constant determinations were not achievable since a molecular mass determination of the commercial Cn preparation was not available.
FIG. 7.
Sensorgrams showing binding of Fg, Fn, Cn, and Vn to and dissociation from Emp. Emp (158 pg) was immobilized on sensor chip C1 as described in Materials and Methods. Sensor chip surfaces were subsequently exposed with different concentrations of purified extracellular matrix proteins, and surface plasmon resonance was determined as described in the text. Mean values of the rate constants and of the equilibrium dissociation constant are given below the respective sensorgrams. The following conditions and concentrations were used (bottom to top sensorgrams of each panel, respectively: Fn at 495, 630, 720, 810, 900, 990, 1,080, and 1,170 nM; Fg at 590, 1,180, 1,770, 2,360, 2,950, 3,540, 4,130, and 5,020 nM; Vn at 1.33, 2, 2.66, 3.33, 5, 8.3, 10, 13.3, and 16.6 μg/ml; Cn at 40, 80, 120, 160, 200, 240, 280, and 320 μg/ml.
DISCUSSION
In this study, we characterized a hitherto-undescribed adhesin of S. aureus recognizing numerous extracellular matrix molecules. The protein was initially identified in Western ligand blot analyses. The N-terminal sequence analysis suggested that this protein was different from other staphylococcal binding proteins interacting with adhesive host molecules. The nucleotide sequence of the intact emp gene of S. aureus Newman revealed an ORF of 1,023 nucleotides conferring a protein of a calculated molecular mass of 38.5 kDa. The sequence encodes a signal peptide of 26 amino acids typical for gram-positive bacteria, and the mature protein of calculated molecular mass of 35.5 kDa closely matches the molecular mass (∼40 kDa) apparent in SDS-PAGE determinations. The overall homology of the mature protein was found to be of <25% amino acid identity with any other proteins deposited in the EBI databases. Subdomain homology searches did not reveal any additional significant homologies. Thus, molecular analysis of emp conferring expression of Emp clearly demonstrated this protein to be novel.
In gram-positive bacteria, a large number of sequenced surface proteins (6), many of whom display binding functions for eukaryotic host proteins and are involved in phagocytosis resistance, tissue colonization, or bacterial invasion, are covalently bound to peptidoglycan via the LPXTG consensus sequence in a reaction catalyzed by the sortase mechanism (42). In S. aureus, five of these surface proteins, i.e., the Fn-binding proteins A and B (FnbpA and FnbpB), the Fg-binding proteins (ClfA and ClfB), and the collagen binding protein (Cna), have been described (10). The interaction of these proteins with their ligands, previously perceived as monofunctional, more recently has been understood as multifunctional (50), encompassing adhesive ligands as well as other host molecules (15). Emp does not contain an LPXTG consensus sequence; however, it is tightly associated with the bacterial cell surfaces, since we failed to demonstrate the molecule in concentrated liquid supernatants but were able to extract it from the cell surface by using SDS.
How does Emp bind to the staphylococcal cell surface? Several mechanisms have been implicated in protein association with the gram-positive cell surface. In addition to the described sortase-mediated mechanism conserved in many gram-positive species, in S. pneumoniae a repeat of 20 amino acids binds LytA to choline-substituted teichoic acids or lipoteichoic acids (18). In Listeria monocytogenes, InlB binds via carboxy-terminal tandem repeats, including dipeptide repeats of GW, directly to lipoteichoic acids (3, 24), while other surface proteins, such as ActA, are anchored to the membrane via a hydrophobic domain of 20 amino acids and a positively charged amino acid tail (28). The binding of Emp to the cell surface may be mediated through hydrophobic interaction since the mature molecule contains 79 (25.2%) major hydrophobic residues (leucine, valine, isoleucine, phenylalanine, and methionine), as well as 35 alanine and tyrosine residues carrying hydrophobic R groups, resulting in 114 (36.3%) hydrophobic amino acids; this hydrophobicity may allow for the interaction of Emp with lipoteichoic acids or other lipid moieties on the cell surface. Alternatively or additionally, Emp contains 42 (13.4%) positively and 19 (6.1%) negatively charged residues contributing to a net positive charge of 23 (7.3%) residues, which may allow electrostatic interaction with negatively charged surface molecules such as teichoic acids. Finally, Emp may be recognized by receptor molecules on S. aureus.
In contrast to the increasingly well-established role of the wall-associated, surface-located proteins of the MSCRAMM family as virulence factors (32, 35, 46; Y. A. Que, P. Francois, J. A. Haefliger, J. M. Entenza, J. Vouillamoz, P. Vaudaux, P. Francioli, and P. Moreillon, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. S713, 2000), much less is known about the role of the secreted S. aureus proteins. While pathogenicity could be attributed to the monofunctional proteins, i.e., the 19-kDa Fb-binding protein Efb in a wound infection model (34) and the staphylococcal coagulase in a pulmonary infection model (41), the biologic role of the Map (major histocompatibility complex type II analog protein)/Eap (extracellular adhesive protein) homologues (25, 31, 33) is less clear. Map/Eap homologues are prominent molecules in extracellular S. aureus extracts (31) and possess extended-spectrum binding functions for eukaryotic ligands displaying binding activities with bone sialoprotein, Fn, Fg, Vn, thrombospondin (31), and osteopontin (25). For Eap, it has been shown that this protein binds back to the staphylococcal cell surface and may thus contribute to bridging of the staphylococcal cell with eukaryotic ligands (33). Similarly to Map/Eap, the extended-spectrum binding Emp was found in SDS extracts of strain Newman in large quantities, and the conferring gene emp was demonstrated by PCR in all tested S. aureus isolates.
The difficulties in evaluating a role of staphylococcal surface-located adhesins have to be viewed with respect to the complex staphylococcal background with one or several surface molecules potentially compensating for loss-of-function in deletion mutants. In experimental infections, functional deletion of single adhesins does not completely prevent infection (32) or may even have no effect at all (8). Accordingly, we could not demonstrate a lack of binding of mEmpb50 to Fn or Fg; however, the significant reduction of adhesion to Fn and Fg suggests an important contribution of Emp to the overall adhesion of strain Newman. As previously described (47), we found here the adhesion of Newman (wild type) to Fn to be low compared to attachment to Fg. With respect of adhesion to surface Fg, loss of Emp may be partially compensated for by the Clfs; however, our studies indicating a significantly reduced adhesion of mEmp50 to surface Fg may suggest an independent role of Emp in interactions with these ligands.
In contrast to the low-affinity interaction of recombinant Emp with Fn and Fg, rEmp did interact with soluble Vn in a high-affinity reaction with an apparent Kd of ca. 10−10 M. By using fluorescence resonance energy transfer, we have previously shown that cascade-blue heparin is displaced by S. aureus proteins (but not by S. epidermidis proteins) from Vn or from Vn347-361 peptides (containing the heparin-binding region in Vn) (12). These findings confirmed the interaction of S. aureus molecules with Vn through its heparin-binding site. Yet it remains unresolved whether Eap, another 60-kDa Vn-binding protein (30), the here-described Emp, or other S. aureus molecules confer this binding. Furthermore, despite the high affinity of the binding of soluble Vn to S. aureus, in a previous study we found only a modest increase in adhesion of S. aureus Cowan 1 to solid-phase Vn (16) and obtained similar results by using different (native and multimeric) Vn preparations on both glass and polymer surfaces (unpublished data). Irrespective of its impact as an adhesive molecule for S. aureus, it may be speculated that an important biologic role of Vn binding by Emp may consist in localizing Vn functions such as complement activation, hemostasis, or tissue remodeling (38) on the staphylococcal cell surface.
In conclusion, Emp is a hitherto-undescribed S. aureus surface protein different from previously described secreted adhesive molecules such as coagulase (36), the Fg-binding protein Efb (2), Map/Eap (25), or a novel 30-kDa S. aureus phosphatase (9). The observation that emp is present in all S. aureus isolates but not in S. epidermidis, taken together with the high-affinity binding of rEmp to Vn and the decreased adhesion of an emp::ermB mutant to adsorbed Fg and Fn, underlines its putative importance in staphylococcal pathogenesis. For full apprehension of the contribution of Emp to pathogenicity, further evaluation of the role of Emp, for example, in heterologous in vitro or in appropriate infection models, is warranted.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft, by the Collaborative Research Center 492 (Project B9), and by the German Ministry for Education, Science, Research, and Technology (grant 01 KI 9750/9).
We thank G. S. Chhatwal, Braunschweig, Germany, for amino-terminal sequence determination; R. Brueckner and B. Krismer, Tubingen, Germany, for providing the pEC4, pBT2, and the pCX19 vectors; and B. Specht, Muenster, Germany, for surface plasmon resonance measurements.
REFERENCES
- 1.Balwit J M, van Langevelde P, Vann J M, Proctor R A. Gentamicin-resistant, menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J Infect Dis. 1994;170:1033–1036. doi: 10.1093/infdis/170.4.1033. [DOI] [PubMed] [Google Scholar]
- 2.Bodén M K, Flock J-I. Evidence for three different fibrinogen-binding proteins with unique properties from Staphylococcus aureus strain Newman. Microb Pathog. 1992;12:289–298. doi: 10.1016/0882-4010(92)90047-r. [DOI] [PubMed] [Google Scholar]
- 3.Braun L, Dramsi S, Dehoux P, Bierne H, Lindahl G, Cossart P. InlB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol Microbiol. 2001;25:285–294. doi: 10.1046/j.1365-2958.1997.4621825.x. [DOI] [PubMed] [Google Scholar]
- 4.Bryskier A. Novelties in the field of anti-infective compounds in 1999. Clin Infect Dis. 2000;31:1423–1466. doi: 10.1086/317490. [DOI] [PubMed] [Google Scholar]
- 5.Dziewanowska K, Patti J M, Deobald C F, Bayles K W, Trumble W R, Bohach G A. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect Immun. 1999;67:4673–4678. doi: 10.1128/iai.67.9.4673-4678.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischetti V A. Surface proteins of gram-positive bacteria. In: Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Gram-positive pathogens. Washington, D.C.: ASM Press; 2000. pp. 11–24. [Google Scholar]
- 7.Flock J I. Extracellular-matrix-binding proteins as targets for the prevention of Staphylococcus aureus infections. Mol Med Today. 1999;5:532–537. doi: 10.1016/s1357-4310(99)01597-x. [DOI] [PubMed] [Google Scholar]
- 8.Flock J I, Hienz S A, Heimdahl A, Schennings T. Reconsideration of the role of fibronectin binding in endocarditis caused by Staphylococcus aureus. Infect Immun. 1996;64:1876–1878. doi: 10.1128/iai.64.5.1876-1878.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flock M, Flock J I. Rebinding of extracellular adherence protein Eap to Staphylococcus aureus can occur through a surface-bound neutral phosphatase. J Bacteriol. 2001;183:3999–4003. doi: 10.1128/JB.183.13.3999-4003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster T J, Höök M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–488. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 11.Francois P, Vaudaux P, Foster T J, Lew D P. Host-bacteria interactions in foreign body infections. Infect Control Hosp Epidemiol. 1996;17:514–520. doi: 10.1086/647358. [DOI] [PubMed] [Google Scholar]
- 12.Francois P P, Preissner K T, Herrmann M, Haugland R P, Vaudaux P, Lew D P, Krause K H. Vitronectin interaction with glycosaminoglycans. Kinetics, structural determinants, and role in binding to endothelial cells. J Biol Chem. 1999;274:37611–37619. doi: 10.1074/jbc.274.53.37611. [DOI] [PubMed] [Google Scholar]
- 13.Götz F, Kreutz B, Schleifer K H. Protoplast transformation of Staphylococcus carnosus by plasmid DNA. Mol Gen Genet. 1983;189:340–342. [Google Scholar]
- 14.Götz F, Schumacher B. Improvement of protoplast transformation in Staphylococcus carnosus. FEMS Microbiol Lett. 1987;40:285–288. [Google Scholar]
- 15.Hartleib J, Köhler N, Dickinson R B, Chhatwal G S, Sixma J J, Hartford O M, Foster T J, Peters G, Kehrel B E, Herrmann M. Protein A is the von Willebrand factor binding protein on Staphylococcus aureus. Blood. 2000;96:2149–2156. [PubMed] [Google Scholar]
- 16.Herrmann M, Suchard S J, Boxer L A, Waldvogel F A, Lew P D. Thrombospondin binds to Staphylococcus aureus and promotes staphylococcal adherence to surfaces. Infect Immun. 1991;59:279–288. doi: 10.1128/iai.59.1.279-288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess S, Stockmann A, Völker W, Preissner K T. Multimeric vitronectin: structure and function. In: Preissner K T, Rosenblatt S, Kost C, Wegerhoff J, Mosher D, editors. Biology of the vitronectins and their receptors. Amsterdam, The Netherlands: Elsevier Science Publishers; 1993. pp. 21–29. [Google Scholar]
- 18.Holtje J V, Tomasz A. Specific recognition of choline residues in the cell wall teichoic acid by the N-acetylmuramyl-l-alanine amidase of Pneumococcus. J Biol Chem. 1975;250:6072–6076. [PubMed] [Google Scholar]
- 19.Hudson M C, Ramp W K, Frankenburg K P. Staphylococcus aureus adhesion to bone matrix and bone-associated biomaterials. FEMS Microbiol Lett. 1999;173:279–284. doi: 10.1111/j.1574-6968.1999.tb13514.x. [DOI] [PubMed] [Google Scholar]
- 20.Hussain M, Hastings J G M, White P J. A chemically defined medium for slime production by coagulase-negative staphylococci. J Med Microbiol. 1991;34:143–147. doi: 10.1099/00222615-34-3-143. [DOI] [PubMed] [Google Scholar]
- 21.Hussain M, Peters G, Chhatwal G S, Herrmann M. A lithium chloride-extracted, broad-spectrum-adhesive 42-kilodalton protein of Staphylococcus epidermidis is ornithine carbamoyltransferase. Infect Immun. 1999;67:6688–6690. doi: 10.1128/iai.67.12.6688-6690.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iordanescu S, Surdeanu M. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J Gen Microbiol. 1976;96:277–281. doi: 10.1099/00221287-96-2-277. [DOI] [PubMed] [Google Scholar]
- 23.Joh D, Wann E R, Kreikemeyer B, Speziale P, Hook M. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 1999;18:211–223. doi: 10.1016/s0945-053x(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 24.Jonquieres R, Bierne H, Fiedler F, Gounon P, Cossart P. Interaction between the protein InlB of Listeria monocytogenes and lipoteichoic acid: a novel mechanism of protein association at the surface of gram-positive bacteria. Mol Microbiol. 1999;34:902–914. doi: 10.1046/j.1365-2958.1999.01652.x. [DOI] [PubMed] [Google Scholar]
- 25.Jönsson K, McDevitt D, McGavin M H, Patti J M, Höök M. Staphylococcus aureus expresses a major histocompatibility complex class II analog. J Biol Chem. 1995;270:21457–21460. doi: 10.1074/jbc.270.37.21457. [DOI] [PubMed] [Google Scholar]
- 26.Karlsson R, Michaelsson A, Mattsson L. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J Immunol Methods. 1991;145:229–240. doi: 10.1016/0022-1759(91)90331-9. [DOI] [PubMed] [Google Scholar]
- 27.Khan S A, Novick R P. Terminal nucleotide sequences of Tn551, a transposon specifying erythromycin resistance in Staphylococcus aureus: homology with Tn3. Plasmid. 1980;4:148–154. doi: 10.1016/0147-619x(80)90004-9. [DOI] [PubMed] [Google Scholar]
- 28.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. Listeria monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 29.Lee J C. Electroporation protocols for microorganisms: electrotransformation of staphylococci. Methods Mol Biol. 1993;47:209–216. doi: 10.1385/0-89603-310-4:209. [DOI] [PubMed] [Google Scholar]
- 30.Liang O D, Flock J-I, Wadström T. Isolation and characterisation of a vitronectin-binding surface protein from Staphylococcus aureus. Biochim Biophys Acta. 1995;1250:110–116. doi: 10.1016/0167-4838(95)00076-7. [DOI] [PubMed] [Google Scholar]
- 31.McGavin M H, Krajewska Pietrasik D, Rydén C, Höök M. Identification of a Staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infect Immun. 1993;61:2479–2485. doi: 10.1128/iai.61.6.2479-2485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreillon P, Entenza J M, Francioli P, McDevitt D, Foster T J, Francois P, Vaudaux P. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun. 1995;63:4738–4743. doi: 10.1128/iai.63.12.4738-4743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palma M, Haggar A, Flock J I. Adherence of Staphylococcus aureus is enhanced by an endogenous secreted protein with broad binding activity. J Bacteriol. 1999;181:2840–2845. doi: 10.1128/jb.181.9.2840-2845.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palma M, Nozohoor S, Schennings T, Heimdahl A, Flock J I. Lack of the extracellular 19-kilodalton fibrinogen-binding protein from Staphylococcus aureus decreases virulence in experimental wound infection. Infect Immun. 1996;64:5284–5289. doi: 10.1128/iai.64.12.5284-5289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patti J M, Bremell T, Krajewska Pietrasik D, Abdelnour A, Tarkowski A, Rydén C, Höök M. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect Immun. 1994;62:152–161. doi: 10.1128/iai.62.1.152-161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phonimdaeng P, O'Reilly M, Nowlan P, Bramley A J, Foster T J. The coagulase of Staphylococcus aureus 8325-4. Sequence analysis and virulence of site-specific coagulase-deficient mutants. Mol Microbiol. 1990;4:393–404. doi: 10.1111/j.1365-2958.1990.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 37.Preissner K T, Chatwal G S. Extracellular matrix and host cell surfaces: potential sites of pathogen interaction. In: Cossart P, Boquet P, Normark S, Rappuoli R, editors. Cellular microbiology. Washington, D.C.: ASM Press; 2000. pp. 49–80. [Google Scholar]
- 38.Preissner K T, Seiffert D. Role of vitronectin and its receptors in haemostasis and vascular remodeling. Thromb Res. 1998;89:1–21. doi: 10.1016/s0049-3848(97)00298-3. [DOI] [PubMed] [Google Scholar]
- 39.Proctor R A. Toward an understanding of biomaterial infections: a complex interplay between the host and bacteria. J Lab Clin Med. 2000;135:14–15. doi: 10.1016/s0022-2143(00)70015-1. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 41.Sawai T, Tomono K, Yanagihara K, Yamamoto Y, Kaku M, Hirakata Y, Koga H, Tashiro T, Kohno S. Role of coagulase in a murine model of hematogenous pulmonary infection induced by intravenous injection of Staphylococcus aureus enmeshed in agar beads. Infect Immun. 1997;65:466–471. doi: 10.1128/iai.65.2.466-471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneewind O, Fowler A, Faull K F. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science. 1995;268:103–106. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- 43.Sinha B, Francois P, Que Y A, Hussain M, Heilmann C, Moreillon P, Lew D, Krause K H, Peters G, Herrmann M. Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect Immun. 2000;68:6871–6878. doi: 10.1128/iai.68.12.6871-6878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinha B, Francois P P, Nüsse O, Foti M, Hartford O M, Vaudaux P, Foster T J, Lew D P, Herrmann M, Krause K H. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell Microbiol. 1999;1:101–117. doi: 10.1046/j.1462-5822.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 45.Sinha B, Herrmann M, Krause K H. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 2000;8:343–344. doi: 10.1016/s0966-842x(00)01813-8. [DOI] [PubMed] [Google Scholar]
- 46.Stutzmann Meier P, Entenza J M, Vaudaux P, Francioli P, Glauser M P, Moreillon P. Study of Staphylococcus aureus pathogenic genes by transfer and expression in the less virulent organism Streptococcus gordonii. Infect Immun. 2001;69:657–664. doi: 10.1128/IAI.69.2.657-664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaudaux P E, Francois P, Proctor R A, McDevitt D, Foster T J, Albrecht R M, Lew P D, Wabers H, Cooper S L. Use of adhesion-defective mutants of Staphylococcus aureus to define the role of specific plasma proteins in promoting bacterial adhesion to canine arteriovenous shunts. Infect Immun. 1995;63:585–590. doi: 10.1128/iai.63.2.585-590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaudaux P E, Waldvogel F A, Morgenthaler J J, Nydegger U E. Adsorption of fibronectin onto polymethylmethacrylate and promotion of Staphylococcus aureus adherence. Infect Immun. 1984;45:768–774. doi: 10.1128/iai.45.3.768-774.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 50.Wann E R, Gurusiddappa S, Hook M. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J Biol Chem. 2000;275:13863–13871. doi: 10.1074/jbc.275.18.13863. [DOI] [PubMed] [Google Scholar]
- 51.Yatohgo T, Izumi M, Kashiwagi H, Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988;13:281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- 52.Youngman P, Poth H, Green K, York K, Olmedo G, Smith K. Methods for genetic manipulation, cloning, and functional analysis of sporulation in genes in Bacillus subtilis. In: Smith I, Slepecky R A, Setlow P, editors. Regulation of procaryotic development. Washington, D.C.: American Society of Microbiology; 1989. pp. 65–87. [Google Scholar]