Abstract
Transcription of the Azotobacter vinelandii algD gene, which encodes GDP-mannose dehydrogenase (the rate-limiting enzyme of alginate synthesis), starts from three sites: p1, p2, and p3. The sensor kinase GacS, a member of the two-component regulatory system, is required for transcription of algD from its three sites during the stationary phase. Here we show that algD is expressed constitutively throughout the growth cycle from the p2 and p3 sites and that transcription from p1 started at the transition between the exponential growth phase and stationary phase. We constructed A. vinelandii strains that carried mutations in gacA encoding the cognate response regulator of GacS and in rpoS coding for the stationary-phase ςS factor. The gacA mutation impaired alginate production and transcription of algD from its three promoters. Transcription of rpoS was also abolished by the gacA mutation. The rpoS mutation impaired transcription of algD from the p1 promoter and increased it from the p2 ςE promoter. The results of this study provide evidence for the predominant role of GacA in a regulatory cascade controlling alginate production and gene expression during the stationary phase in A. vinelandii.
Azotobacter vinelandii is a nitrogen-fixing soil bacterium that undergoes differentiation to form desiccation-resistant cysts and produces two polymers of industrial importance: alginate and poly-β-hydroxybutyrate (PHB).
A. vinelandii has been shown to posses an alginate biosynthetic gene cluster organized in three operons (5, 25, 29, 30, 49), one of which transcribes algD, which encodes GDP-mannose dehydrogenase, the key enzyme of the alginate biosynthetic pathway. The algUmucABCD cluster has been characterized in A. vinelandii and in Pseudomonas aeruginosa and has been shown to control alginate production (28, 34, 37, 44, 45, 53). It has been shown for P. aeruginosa that the activity of the alternative sigma factor ςE (AlgU) encoded by algU is negatively regulated by the anti-sigma factor MucA (9, 10, 16, 27, 45, 54) and in an indirect manner by MucB (27). In several bacterial species, ςE regulates expression of functions related to the extracytoplasmic compartments (32). In A. vinelandii, transcription of algD can initiate at three promoters, one of which (p2) is regulated by ςE (28, 34) but presumably in an indirect manner (37).
The global two-component GacS/GacA system is conserved in a variety of gram-negative bacteria. In Erwinia carotovora and some Pseudomonas species, it controls the expression of genes involved in secondary metabolism, phytopathogenesis, and quorum sensing (7, 8, 11, 15, 20, 24, 40, 41). In Pseudomonas syringae B728a, gacA and gacS mutations negatively affect alginate production and algD expression (52).
The GacS histidine kinase controls alginate production in A. vinelandii. In gacS mutants transcription of algD is significantly reduced during exponential growth and abolished in the stationary phase (6). Regulation of alginate synthesis by GacS during the stationary phase was shown to be exerted on algD transcription from its three promoters (6).
In Escherichia coli and other bacteria, the alternative sigma factor ςS (RpoS) functions as a global regulator and is responsible for the activation of many genes expressed mainly during the stationary phase and under various stress conditions (18). One way in which GacA regulates gene expression in Pseudomonas fluorescens is by influencing accumulation of the ςS factor (50). In P. aeruginosa, ςS controls the production of virulence factors, such as exotoxin A, pyocyanin, and alginate in an alginate-overproducing strain (48). A relationship between ςS and quorum sensing has also been reported in P. aeruginosa (22, 51).
In E. coli transcription of rpoS in exponentially growing cells is dependent on BarA (35). BarA was recently identified as the cognate sensor kinase of UvrY, the E. coli GacA homologue (39). As GacS/GacA influences the level of ςS in several bacterial species, expression of algD in A. vinelandii was proposed to be regulated by ςS (6). In agreement with this proposition, the A. vinelandii algD-p1 promoter has the −10 sequence CTATAAT and also has an intrinsic DNA curvature observed in promoters preferentially recognized by ςS (12, 13, 38).
Most of the two-component systems are composed of a transmembrane histidine phosphokinase that senses environmental signals and a cytoplasmic response regulator that activates transcription upon phosphorylation by the sensor (19, 47).
This study reports the identification and characterization of the A. vinelandii gacA gene, which encodes the GacS cognate response regulator, and rpoS, which encodes the ςS factor. Our data show that entering into the stationary phase results in expression of rpoS and of algD from its p1 promoter and that a mutation in gacA abrogates transcription of algD and rpoS, indicating the predominant role of GacA in a regulatory cascade that controls gene expression in the stationary phase and alginate production in A. vinelandii.
MATERIALS AND METHODS
Microbiological procedures.
Bacterial strains and plasmids used are listed in Table 1. Medium and growth conditions were as follows: A. vinelandii was grown at 30°C in Burk's nitrogen-free salts medium supplemented with 2% sucrose (21). E. coli strain DH5α was grown on Luria-Bertani medium (31) at 37°C. Antibiotic concentrations used (in micrograms per milliliter) for A. vinelandii and E. coli, respectively, were as follows: tetracycline, 20 and 20; kanamycin, 5 and 30; rifampin, not used and 20; ampicillin, not used and 100; nalidixic acid, 20 and 20; spectinomycin, 100 and 100; streptomycin, 2 and 20; and gentamicin, 1.5 and 10. A. vinelandii transformation was carried out as previously described (3).
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| A. vinelandii strains | ||
| ATCC 9046 | Highly mucoid, wild type | ATCC |
| JM3 | ATCC 9045 with a gacA::Gm mutation | This work |
| CNS59 | ATCC 9046 with a rpoS::Sp mutation | This work |
| E. coli strain | ||
| DH5α | supE44 ΔlacU169 hsdR17 recA1 endA1 gyrA96 hi-1 relA1 | GIBCO-BRL |
| Plasmids | ||
| pBluescript KS(+) | Stratagene | |
| pKT230 | Broad-host-range vector, Kmr Smr | 2 |
| pHP45Ω-Sp | 14 | |
| pBSL141-Gm | 1 | |
| pSMU1886 | pCP13 cosmid vector containing 25 kb of A. vinelandii DNA, including gacA | This work |
| pSAFA1 | pBluescript KS(+) carrying the gacA gene cloned by PCR | This work |
| pSAFA2 | pBluescript KS(+) carrying a 3.0-kb ClaI fragment with gacA | This work |
| pSAFA3 | pSAFA2 derivative with a gacA::Sp mutation | This work |
| pSAFA4 | pKT230 vector carrying the gacA gene cloned by PCR | This work |
| pCNS59 | pBluescript KS(+) carrying a 0.8-kb fragment with rpoS | This work |
| pSMS7 | pCNS59 derivative with an rpoS::Sp mutation | This work |
Alginate and PHB production was determined as previously described (30, 46). All measurements were done in triplicate. Protein concentration was determined by the Lowry method (26).
Nucleic acid procedures.
RNA and DNA isolation and cloning, Southern blotting, and random primer procedures were carried out as described earlier (42). Plasmids pSAFA2 and pCNS59 were used to determine the nucleotide sequences reported in this study. DNA sequencing was done with the Thermosequenase sequencing kit by the dideoxy-chain termination method of Sanger et al. (43). Primer extension of algD and algU was carried out as previously described (5, 37). Reactions were performed with a primer extension system (Amersham) as instructed by the manufacturer.
Northern blot analysis.
Total RNA was extracted from the ATCC 9046 and JM3 strains using a High-Pure RNA Isolation Kit (Roche) and was quantified spectrophotometrically by measuring optical density at 260 nm. For Northern analysis 10 μg of RNA was loaded per lane. As loading and transfer controls, all blots were reprobed with a probe specific to 16S rRNA derived from plasmid pKK3535 (4).
Cloning of A. vinelandii gacA and rpoS genes.
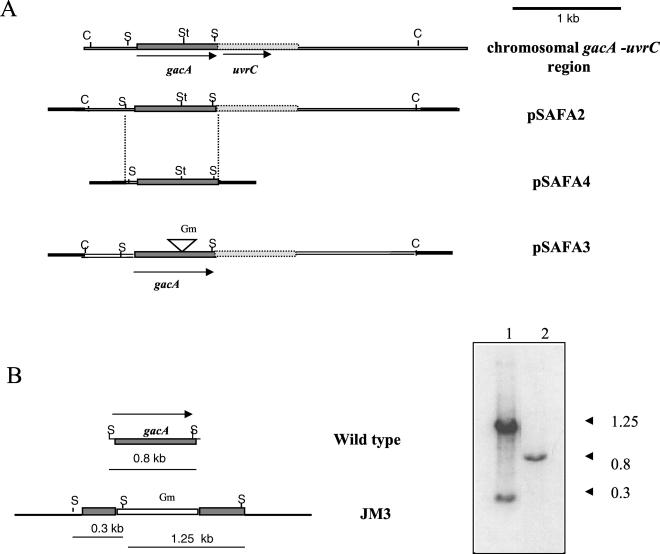
Oligonucleotides gacA1 (5′-GATTAAGGTGCTGGTGGTCGACC-3′) and gacA2 (5′-GCGGTGCCGTACCAGCTACGGCGG-3′) and total DNA from P. aeruginosa PAO1 were used to isolate by PCR a fragment containing the P. aeruginosa gacA gene (40). This fragment was used as probe to identify a cosmid clone denoted pSMU1886, which was derived from an A. vinelandii genomic library, and contained a 3-kb ClaI fragment that hybridized to the gacA probe. This 3-kb ClaI fragment was cloned into the pBluescript KS(+) vector (Stratagene) to yield plasmid pSAFA2 (Fig. 1). Oligonucleotides jsf2 (5′-TTGCCCACCTCCCGGGTGG-3′) and jsf3 (5′-GCAGGGATCCAGAAAAGCCG-3′) were used to isolate by PCR a fragment containing the gacA gene. This fragment was cloned into plasmid pKT230 (2) to produce pSAFA4 (Fig. 1).
FIG. 1.
(A) Physical map of the A. vinelandii chromosomal gacA-uvr region and plasmids constructed in this study. Arrows indicate direction of transcription. Antibiotic resistance cassette is represented by the inverted triangle. Vector sequences are represented by black bars. (B) Physical map of insertional inactivation of the gacA gene in A. vinelandii ATCC 9046. Southern blot hybridization of total genomic DNA digested with SalI endonuclease, with the 0.8-kb SalI fragment as probe. Lane 1, ATCC 9046; lane 2, JM3. Abbreviations: S, SalI; C, ClaI; St, StuI.
Oligonucleotides rpoS5 (5′-TTGGACGCAACGCAGCTGTATC-3′) and rpoS3 (5′-CTGGATCTGACGAACCCGCTC-3′) were designed based on the P. aeruginosa rpoS sequence and correspond to a ςS conserved region among various species. Total DNA from A. vinelandii ATCC 9046 and these oligonucleotides were used to clone by PCR a 756-bp fragment that was ligated into the pBluescript KS(+) vector to yield plasmid pCNS59. Sequence analysis of this fragment confirmed the presence of the A. vinelandii rpoS gene.
Construction of gacA and rpoS mutants.
Plasmid pSAFA2 (Fig. 1), which carries a 3.0-kb ClaI DNA fragment including gacA, was used to construct a gacA::Gm mutation. A 0.8-kb fragment containing a gentamicin cassette from plasmid pBSL141-Gm (1) was inserted into the unique StuI site to create a gacA::Gm mutation within the codon for amino acid residue 137 of GacA. The resultant plasmid pSAFA3 (Fig. 1), which is unable to replicate in A. vinelandii, was introduced into strain ATCC 9046. Strain JM3, a Gmr Aps transformant, was selected. Plasmid PCNS59 was used to construct an rpoS mutation. A 2-kb fragment containing a Ω-spectinomycin cassette from plasmid pHP45Ω-Sp (14) was inserted into the unique StuI site to create the rpoS::Sp mutation within the codon for amino acid residue 130 of RpoS. The resultant plasmid pSMS7, which is unable to replicate in A. vinelandii, was introduced into strain ATCC 9046. Strain CNS59, a Spr Aps transformant, was selected and confirmed by Southern blot analysis to carry the rpoS::Sp mutation (data not shown).
Nucleotide sequence accession number.
The nucleotide sequences of the gacA and rpoS genes reported here have been assigned GenBank accession numbers AF382827 and AY029155, respectively.
RESULTS AND DISCUSSION
DNA sequence of A. vinelandii gacA gene.
The A. vinelandii GacS sensor kinase was previously shown to play a role as a positive regulator of polymer synthesis, since a gacS mutation significantly reduced alginate and PHB production. To further study regulation of alginate production by the global two-component GacSA system, we cloned, as described in Materials and Methods, an A. vinelandii sequence that hybridized to P. aeruginosa gacA. DNA sequence analysis of this fragment revealed an open reading frame encoding a 214-amino-acid polypeptide (GacA). The identity of A. vinelandii GacA was 85% with GacA present in the following Pseudomonas species: P. syringae (41), Pseudomonas viridiflava (24), P. fluorescens (8), Pseudomonas aureofaciens (7), and Pseudomonas tolaasii (17). Following gacA, a partial orf gene encoding 22 amino acids sharing similarity to UvrC, an exonuclease that participates in DNA repair after UV damage (33), was found. A potential Shine-Dalgarno sequence (AGGAG) is present upstream of the gacA start codon. As in other bacteria, the uvrC start codon overlaps the gacA TGA termination codon (11, 33, 40), suggesting that these two genes form an operon. As with other response regulators, GacA contains two highly conserved aspartate residues, Asp8 and the predicted phospho-accepting aspartate Asp54.
Alginate and PHB production is under GacA control.
As the GacS cognate response regulator, GacA was expected to act as positive regulator of biosynthesis of both alginate and PHB. Strain JM3, an ATCC 9046 derivative carrying a gacA::Gm mutation, was constructed as described in Materials and Methods and was shown by Southern blot analysis to carry the gacA::Gm mutation (Fig. 1B). Strain JM3 was unable to produce alginate and PHB (Table 2), confirming that GacA is an activator of the synthesis of these polymers.
TABLE 2.
Alginate and PHB production in A. vinelandii strainsa
| Strain | Alginate concn (mg/mg of protein) | PHB concn (μg/mg of protein) |
|---|---|---|
| ATCC 9046 | 1.93 ± 0.3 | 460 ± 24 |
| JM3 | <1.0 | <10.0 |
| JM3/pSAFA3 | 1.96 ± 0.3 | 455 ± 21 |
| CNS59 | 1.70 ± 0.3 | NDb |
Alginate and PHB concentrations were determined in cells grown for 48 h in Burk's liquid medium supplemented with 2% sucrose. Values are means ± standard errors of the means.
ND, not determined.
UvrC is involved in resistance to UV in both Pseudomonas species and E. coli. Strain JM3 was more sensitive to UV light than was wild-type strain ATCC 9046 or the gacS mutant (data not shown). Plasmid pSAFA4 restored to the JM3 mutant the ability to produce alginate and PHB (Table 2) but did not restore resistance to UV, suggesting that the gacA mutation exerted polarity on uvrC transcription and that gacA and uvrC are organized as an operon. This data also confirmed that the inability to produce alginate and PHB is caused by the absence of the gacA gene product. These results provide genetic evidence supporting the conclusion that GacA is the cognate response regulator of GacS.
Growth-phase-dependent expression of algD and its control by GacA.
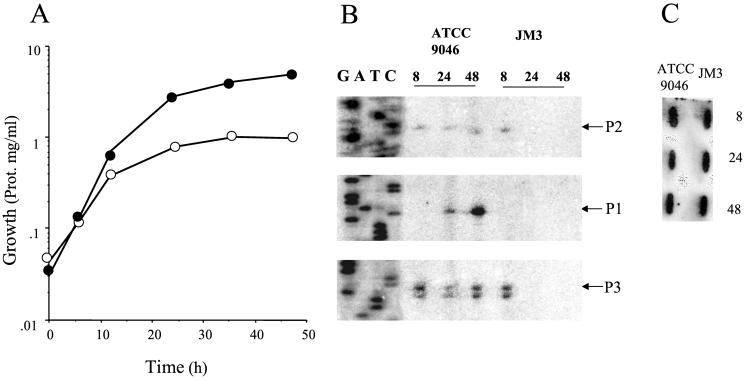
In previous studies, transcription of algD from its three promoters was documented by primer extension experiments carried out in stationary-phase cells collected after 48 h of growth in Burk's sucrose medium (6, 34, 36). We also reported that a gacS mutation abolished transcription of algD during the stationary phase; however, during exponential growth some transcription of algD (determined by β-galactosidase activity with an algD-lacZ fusion) was detected in a gacS mutant (6). To further study the control of algD expression in A. vinelandii, the transcriptional induction kinetic of algD was determined by primer extension in cells of ATCC 9046 and the gacA mutant JM3 throughout a growth cycle on liquid Burk's sucrose medium (Fig. 2A). A reduction of growth was observed in strain JM3, suggesting a GacA requirement for the control of factors contributing to optimal growth. In the exponential phase, algD transcription initiated from the p2 and p3 but not from the p1 promoter. Transcription from the p1 promoter started at the transition between exponential growth and stationary phase and increased when cells reached the stationary phase (Fig. 2). This result is in agreement with the hypothesis that p1 is a ςS-dependent promoter.
FIG. 2.
Growth and primer extension analysis of algD. (A) Growth of ATCC 9046 (solid circles) and JM3 strains (empty circles) in Burk's sucrose medium. (B) Primer extension at 8 (lane 1), 24 (lane 2), and 48 h (lane 3) incubation in Burk's sucrose medium. (C) Hybridization of a sample of the RNA (10 μg) used as template for the primer extension with a probe specific for 16S rRNA over 8, 24, and 48 h (4).
The effect of the gacA mutation on transcription of the algD throughout a growth cycle is shown in Fig. 2B. Similar to results for the wild type, primer extension products corresponding to the p2 and p3 promoter but not from p1 were detected in strain JM3 in exponentially growing cells (Fig. 2). This is an unexpected result, since in the gacS mutant, transcription of algD (measured as β-galactosidase activity with an algD-lacZ fusion) was reduced during exponential growth (6). However, similar to the result reported with the gacS mutant (6), during the stationary phase no primer extension products corresponding to the three promoters were detected in this gacA mutant. This result indicates that the GacS/GacA system is essential for activation of the three algD promoters during the stationary phase. These data also imply that control of alginate synthesis is to some extent growth phase dependent.
algD-p1 is a ςS-dependent promoter.
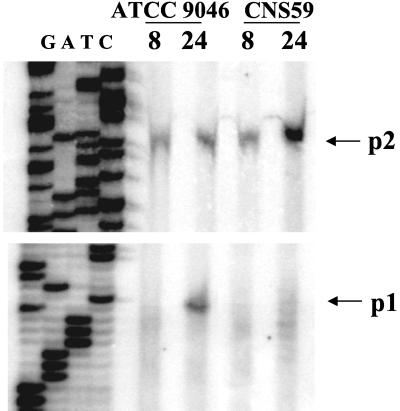
ςS is the sigma factor responsible for the activation of many genes expressed mainly during the stationary phase (18). As shown above, transcriptional activation of the p1-algD promoter specifically occurs in the stationary phase. We cloned, as described in Materials and Methods, an A. vinelandii rpoS internal fragment encoding amino acids 60 to 313 of ςS and constructed by reverse genetics strain CNS59, a derivative of ATCC 9046 carrying an rpoS::Sp mutation (see Materials and Methods). As predicted, transcription of algD from the p1 promoter in the CNS59 strain was not detected (Fig. 3), confirming that p1 is a ςS-dependent promoter. In addition transcription from p2, the ςE-dependent promoter during the stationary phase, was found to increase in the rpoS mutant (Fig. 3), suggesting that the absence of ςS results in ςE activation. Transcription from the p3 site was similar in the wild type and the rpoS mutant (data not shown). The rpoS mutation did not significantly affect the production of alginate (Table 2), suggesting that the increase in the activity of the p1 ςE promoter compensates for the negative effect on the p2 ςS promoter. These data suggest that both GacA and ςS participate in the same regulatory cascade and that GacA functions upstream of ςS.
FIG. 3.
Primer extension analysis of algD transcription from p1 and p2 in ATCC 9046 and CNS59 strains after 8 and 24 h of growth on Burk's sucrose medium.
Growth-phase-dependent expression of rpoS and its control by GacA.
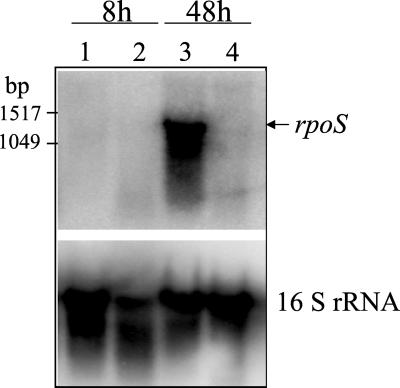
We determined the levels of rpoS mRNA by Northern analysis in cells of ATCC 9046 and the gacA mutant JM3 harvested from exponential (8 h) and stationary phase (48 h) cultures. In the wild-type strain ATCC 9046, rpoS mRNA was detected in the stationary phase but not during exponential growth (Fig. 4). Thus, as in other bacteria rpoS expression in A. vinelandii is under growth phase regulation. In E. coli, for example, the highest ςS concentration is found in early stationary phase; however, a low-level expression of rpoS as determined by Northern blot analysis is detected in exponentially growing cells in minimal or rich media (23, 35). Correspondingly some ςS-dependent genes are also expressed during exponential growth, implying a role for ςS in growing cells (18). We did not detect rpoS RNA in exponential cultures grown in Burk's minimal medium; however, as regulation of ςS is unknown in A. vinelandii, this result does not rule out a role for this factor in growing cells.
FIG. 4.
Northern analysis of rpoS RNA isolated from ATCC 9046 (lanes 1 and 3) and JM3 (lanes 2 and 4) after 8 and 48 h of incubation in Burk's sucrose medium.
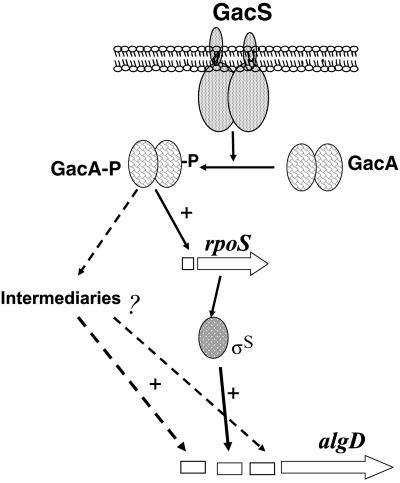
The effect of the gacA mutation on transcription of the rpoS is also shown in Fig. 4. No RNA corresponding to rpoS was detected in the gacA mutant. Together, these results indicate that GacA mediates signal transduction between GacS and the activation of the rpoS promoter; in turn, ςS mediates activation of the algDp1 promoter by GacA (Fig. 5). Whether GacA directly interacts with the rpoS promoter region remains to be determined.
FIG. 5.
Model for regulation of algD expression by the global regulators GacA and ςS.
The gacA mutation has no effect on algU transcription.
The lack of transcription from p2 in JM3 during the stationary phase suggested that transcription of algU, the gene encoding ςE, might be under GacA control. We carried out primer extension analysis of algU, with RNA isolated from strains ATCC 9046 and JM3 (data not shown). We found that the gacA mutation has no effect on transcription of algU; thus, stationary-phase induction of the algD-p2 promoter by GacA seems to be exerted via a ςE-independent intermediary (Fig. 5).
The results of this study show that the gacA gene cloned encodes the cognate response regulator of GacS which is required for polymer synthesis and which is specifically required to activate transcription of algD from its three promoters, one of which was shown to be a ςS-dependent promoter.
Activation of gene expression by the GacS/GacA system appears to use different signal pathways or cascades, one of which includes rpoS, since we showed that GacA is required for transcription of rpoS. By regulating expression of rpoS, the GacS/GacA system must play an important role in the control of stationary-phase functions. GacA was also shown to be required to activate the algD non-ςS promoters; thus, activation of alginate synthesis by GacS/GacA is also mediated by another as-yet-unidentified pathway.
ACKNOWLEDGMENTS
This work was supported by grant 27767 from CONACyT.
We acknowledge Rene Hernandez and Josefina Guzman for technical support and G. Soberón-Chávez for reviewing the manuscript.
REFERENCES
- 1.Alexeyev M F, Shokolenko I, Croughan T P. Improved antibiotic-resistance gene cassettes and omega elements for Escherichia coli vector construction and in vitro deletion/insertion mutagenesis. Gene. 1995;160:63–67. doi: 10.1016/0378-1119(95)00108-i. [DOI] [PubMed] [Google Scholar]
- 2.Bagdasarian M, Lurz R, Ruckert B, Franklin F C, Bagdasarian M M, Frey J, Timmis K N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981;16:237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- 3.Bali A, Blanco G, Hill S, Kennedy C. Excretion of ammonium by a nifL mutant of Azotobacter vinelandii fixing nitrogen. Appl Environ Microbiol. 1992;58:1711–1718. doi: 10.1128/aem.58.5.1711-1718.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brosius J, Ullrich A, Raker M A, Gray A, Dull T J, Gutell R R, Noller H F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of Escherichia coli. Plasmid. 1981;6:112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- 5.Campos M E, Martínez-Salazar J, Lloret L, Núñez C, Espín G, Soberón-Chávez G. Characterization of the gene coding for GDP-mannose dehydrogenase (algD) from Azotobacter vinelandii. J Bacteriol. 1996;178:1793–1799. doi: 10.1128/jb.178.7.1793-1799.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castañeda M, Guzmán J, Moreno S, Espín G. The GacS sensor kinase regulates alginate and poly-β-hydroxybutyrate production in Azotobacter vinelandii. J Bacteriol. 2000;182:2624–2628. doi: 10.1128/jb.182.9.2624-2628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chancey S T, Wood D W, Pierson L S., III Two-component transcriptional regulation of N-acyl-homoserine lactone production in Pseudomonas aureofaciens. Appl Environ Microbiol. 1999;65:2294–2299. doi: 10.1128/aem.65.6.2294-2299.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbell N, Loper J E. A global regulator of secondary metabolite production in Pseudomonas fluorescens Pf-5. J Bacteriol. 1995;177:6230–6236. doi: 10.1128/jb.177.21.6230-6236.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deretic V, Martin D W, Schurr M J, Mudd M H, Hibler N S, Curcic R, Boucher J C. Conversion to mucoidy in Pseudomonas aeruginosa. Bio/Technology. 1993;11:1133–1136. doi: 10.1038/nbt1093-1133. [DOI] [PubMed] [Google Scholar]
- 10.Deretic V, Schurr M J, Boucher J C, Martin D W. Conversion of Pseudomonas aeruginosa to mucoidy in cystic fibrosis: enviromental stress and regulation of bacterial virulence by alternative sigma factors. J Bacteriol. 1994;176:2773–2780. doi: 10.1128/jb.176.10.2773-2780.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriksson A R, Andersson R A, Pirhonen M, Palva E T. Two-component regulators involved in the global control of virulence in Erwinia carotovora subsp. carotovora. Mol Plant-Microbe Interact. 1998;11:743–752. doi: 10.1094/MPMI.1998.11.8.743. [DOI] [PubMed] [Google Scholar]
- 12.Espinosa-Urgel M, Chamizo C, Tormo A. A consensus structure for sigma S-dependent promoters. Mol Microbiol. 1996;21:657–659. doi: 10.1111/j.1365-2958.1996.tb02573.x. [DOI] [PubMed] [Google Scholar]
- 13.Espinosa-Urgel M, Tormo A. Sigma s-dependent promoters in Escherichia coli are located in DNA regions with intrinsic curvature. Nucleic Acids Res. 1993;21:3667–3670. doi: 10.1093/nar/21.16.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 15.Frederick R D, Chiu J, Bennetzen J L, Handa A K. Identification of a pathogenicity locus, rpfA, in Erwinia carotovora subsp. carotvora that encodes a two-component sensor-regulator protein. Mol Plant-Microbe Interact. 1997;10:407–415. doi: 10.1094/MPMI.1997.10.3.407. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg J B, Gorman W L, Flynn J L, Ohman D E. A mutation in algN permits trans activation of alginate production by algT in Pseudomonas species. J Bacteriol. 1993;175:1303–1308. doi: 10.1128/jb.175.5.1303-1308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han B, Arnab P, Johnstone K. Spontaneous duplication of a 661 bp element within a two-component sensor regulator gene causes phenotypic switching in colonies of Pseudomonas tolaasii, cause of brown blotch disease of mushrooms. Mol Microbiol. 1997;25:211–218. doi: 10.1046/j.1365-2958.1997.4411811.x. [DOI] [PubMed] [Google Scholar]
- 18.Hengge-Aronis R. Regulation of gene expression during entry into stationary phase. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1497–1512. [Google Scholar]
- 19.Hoch J A, Silhavy T J. Two-component signal transduction. Washington, D.C.: American Society for Microbiology; 1995. [Google Scholar]
- 20.Hrabak E M, Willis D K. The lemA gene required for pathogenicity of Pseudomonas syringae pv. syringae on bean is a member of a family of two-component regulators. J Bacteriol. 1992;174:3011–3020. doi: 10.1128/jb.174.9.3011-3020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy C, Gamal R, Humphrey R, Ramos J, Brigle K, Dean D. The nifH, nifM, and nifN genes of Azotobacter vinelandii: characterization by Tn5 mutagenesis and isolation from pLARF1 gene banks. Mol Gen Genet. 1986;205:318–325. [Google Scholar]
- 22.Lafiti A, Foglino M, Tanaka K, Williams P, Lazdunski A. A hierarchial quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhlR (VsmR) to expression of the stationary phase sigma factor RpoS. Mol Microbiol. 1996;21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 23.Lange R, Hengge-Aronis R. The cellular concentration of the sigma S subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes Dev. 1994;8:1600–1612. doi: 10.1101/gad.8.13.1600. [DOI] [PubMed] [Google Scholar]
- 24.Liao C H, MacCullus D E, Fett W F. Molecular characterization of two gene loci required for production of the key pathogenicity factor pectate lyase in Pseudomonas viridiflava. Mol Plant-Microbe Interact. 1994;7:391–400. doi: 10.1094/mpmi-7-0391. [DOI] [PubMed] [Google Scholar]
- 25.Lloret L, Barreto R, León R, Moreno S, Martínez-Salazar J, Espín G, Soberón-Chávez G. Genetic analysis of the transcriptional arrangement of Azotobacter vinelandii alginate biosynthetic genes: identification of two independent promoters. Mol Microbiol. 1996;21:449–457. doi: 10.1111/j.1365-2958.1996.tb02554.x. [DOI] [PubMed] [Google Scholar]
- 26.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Mathee K, McPherson C J, Ohman D E. Postranscriptional control of algT (algU)-encoded ς22 for expression of the alginate regulon in Pseudomonas aeruginosa and localization of its antagonist proteins MucA and MucB. J Bacteriol. 1997;179:3711–3720. doi: 10.1128/jb.179.11.3711-3720.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez-Salazar J, Moreno S, Nájera R, Boucher J C, Espín G, Soberón-Chávez G, Deretic V. Characterization of the genes coding for the putative sigma factor AlgU and its negative regulators MucA, MucB, MucC, and MucD in Azotobacter vinelandii and evaluation of their role in alginate biosynthesis. J Bacteriol. 1996;178:1800–1808. doi: 10.1128/jb.178.7.1800-1808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mejía-Ruíz H, Guzmán J, Moreno S, Soberón-Chávez G, Espín G. The Azotobacter vinelandii alg8 and alg44 genes are essential for alginate synthesis and can be transcribed from an algD-independent promoter. Gene. 1997;199:271–277. doi: 10.1016/s0378-1119(97)00380-6. [DOI] [PubMed] [Google Scholar]
- 30.Mejía-Ruíz H, Moreno S, Guzmán J, Nájera R, León R, Soberón-Chávez G, Espín G. Isolation and characterization of an Azotobacter vinelandii algK mutant. FEMS Microbiol Lett. 1997;156:101–106. doi: 10.1111/j.1574-6968.1997.tb12712.x. [DOI] [PubMed] [Google Scholar]
- 31.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. pp. 431–435. [Google Scholar]
- 32.Missiakas D, Raina S. The extracytoplasmic function sigma factors: role and regulation. Mol Microbiol. 1998;28:1059–1066. doi: 10.1046/j.1365-2958.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 33.Moolenaar G F, van Sluis C A, Backendorf C, van de Putte P. Regulation of the Escherichia coli excision repair gene uvrC. Overlap between the uvrC structural gene and the region coding for a 24 kD protein. Nucleic Acids Res. 1987;15:4273–4289. doi: 10.1093/nar/15.10.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreno S, Guzmán J, Nájera R, Soberón-Chávez G, Espín G. Role of the alternative ς factor AlgU in encystment of Azotobacter vinelandii. J Bacteriol. 1998;180:2766–2769. doi: 10.1128/jb.180.10.2766-2769.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukhopadhyay S, Audia J P, Roy R N, Schellhorn H E. Transcriptional induction of the conserved alternative factor RpoS in Escherichia coli is dependent on BarA, a probable two-component regulator. Mol Microbiol. 2000;37:371–381. doi: 10.1046/j.1365-2958.2000.01999.x. [DOI] [PubMed] [Google Scholar]
- 36.Núñez C, Moreno S, Soberón-Chávez G, Espín G. The Azotobacter vinelandii response regulator AlgR is essential for encystment. J Bacteriol. 1999;181:141–148. doi: 10.1128/jb.181.1.141-148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Núñez C, León R, Guzmán J, Espín G, Soberón-Chávez G. Role of Azotobacter vinelandii mucA and mucC gene products in alginate production. J Bacteriol. 2000;182:6550–6556. doi: 10.1128/jb.182.23.6550-6556.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page W J, Tindale A, Chandra M, Kwon E. Alginate formation by Azotobacter vinelandii UWD during stationary phase and the turnover of poly-β-hydroxybutyrate. Microbiology. 2001;147:483–490. doi: 10.1099/00221287-147-2-483. [DOI] [PubMed] [Google Scholar]
- 39.Pernestig A K, Melefors O, Georgellis D. Identification of UvrY as the cognate response regulator for the BarA sensor kinase in Escherichia coli. J Biol Chem. 2001;276:225–231. doi: 10.1074/jbc.M001550200. [DOI] [PubMed] [Google Scholar]
- 40.Reimmann C, Beyeler M, Latifi A, Winteler H, Foglino M, Lazdunski A, Haas D. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol Microbiol. 1997;24:309–319. doi: 10.1046/j.1365-2958.1997.3291701.x. [DOI] [PubMed] [Google Scholar]
- 41.Rich J J, Kinscherf T G, Kitten T, Willis D K. Genetic evidence that the gacA gene encodes the cognate response regulator for the lemA sensor in Pseudomonas syringae. J Bacteriol. 1994;176:7468–7475. doi: 10.1128/jb.176.24.7468-7475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 43.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schurr M J, Yu H, Boucher J C, Hibler N S, Deretic V. Multiple promoters and induction by heat shock of the gene encoding the alternative factor AlgU (ςE) which controls mucoidy in cystic fibrosis isolates of Pseudomonas aeruginosa. J Bacteriol. 1995;177:5670–5679. doi: 10.1128/jb.177.19.5670-5679.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schurr M J, Yu H, Martínez-Salazar J M, Boucher J C, Deretic V. Control of AlgU, a member of the ςE-like family of stress sigma factors, by the negative regulators MucA and MucB and Pseudomonas aeruginosa conversion to mucoidy in cystic fibrosis. J Bacteriol. 1996;178:4997–5004. doi: 10.1128/jb.178.16.4997-5004.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segura D, Espín G. Mutational inactivation of a gene homologous to Escherichia coli ptsP affects poly-β-hydroxybutyrate accumulation and nitrogen fixation in Azotobacter vinelandii. J Bacteriol. 1998;180:4790–4798. doi: 10.1128/jb.180.18.4790-4798.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stock J B, Ninfa A J, Stock A M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suh S J, Silo-Suh L, Woods D E, Hassett D J, West S E, Ohman D E. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J Bacteriol. 1999;181:3890–3897. doi: 10.1128/jb.181.13.3890-3897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vázquez A, Moreno S, Guzmán J, Alvarado A, Espín G. Transcriptional organization of the Azotobacter vinelandii algGXLIVFA genes: characterization of algF mutants. Gene. 1999;232:217–222. doi: 10.1016/s0378-1119(99)00119-5. [DOI] [PubMed] [Google Scholar]
- 50.Whistler C A, Corbell N A, Sarniguet A, Ream W, Loper J E. The two-component regulators GacS and GacA influence accumulation of the stationary-phase sigma factor ςS and the stress response in Pseudomonas fluorescens Pf-5. J Bacteriol. 1998;180:6635–6641. doi: 10.1128/jb.180.24.6635-6641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whiteley M, Parsek M R, Greenberg E P. Regulation of quorum sensing by RpoS in Pseudomonas aeruginosa. J Bacteriol. 2000;182:4356–4360. doi: 10.1128/jb.182.15.4356-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willis D K, Holmstadt J J, Kinscherf T G. Genetic evidence that loss of virulence associated with gacS or gacA mutations in Pseudomonas syringae B728a does not result from effects on alginate production. Appl Environ Microbiol. 2001;67:1400–1403. doi: 10.1128/AEM.67.3.1400-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wozniak D J, Ohman D E. Transcriptional analysis of the Pseudomonas aeruginosa genes algR, algB, and algD reveals a hierarchy of alginate gene expression which is modulated by algT. J Bacteriol. 1994;176:6007–6014. doi: 10.1128/jb.176.19.6007-6014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie Z-D, Hershberger C D, Shankar S, Ye R W, Chakrabarty A M. Sigma factor–anti-sigma factor interaction in alginate synthesis: inhibition of AlgT by MucA. J Bacteriol. 1996;178:4990–4996. doi: 10.1128/jb.178.16.4990-4996.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]