Abstract
Purpose
Porous orbital implants are commonly used materials following enucleation or evisceration. Implant-associated inflammation is a rare but serious complication which may necessitate explantation.
Observations
We report a case of a patient who developed extensive orbital inflammation six months after implantation of a vicryl (polyglactin 910) mesh-wrapped Bioceramic (aluminum oxide) spherical implant. An orbital biopsy demonstrated an extensive fibroinflammatory reaction with multinucleated giant cells. Removal of the implant resulted in complete resolution of symptoms.
Conclusions and importance
We surmise that the Bioceramic implant played a significant contributory role in this patient's orbital inflammation, a complication which has not been described previously.
Keywords: Bioceramic implant, Aluminum oxide, Orbital inflammation
1. Introduction
Orbital implants are commonly used to restore lost volume to the anophthalmic cavity following enucleation or evisceration. Porous implant materials, such as aluminum oxide, hydroxyapatite and porous polyethylene, are generally favored for their low side-effect profile, biocompatibility and reduced risk of extrusion due to improved integration with orbital tissue.1,2 Non-porous alternatives, such as silicone and polymethylmethacrylate, also demonstrate high biocompatibility and similar rates of complications when compared with porous materials.1 Complications associated with orbital implants include conjunctival thinning, implant exposure, discharge and infection.1 Implant-associated inflammation is a particularly rare complication and only a few cases have been reported in patients with hydroxyapatite implants.3,4 Furthermore, details of the radiological findings associated with this rare complication are scarce.
We report a case of extensive orbital inflammation which developed six months after primary implantation of a vicryl mesh-wrapped Bioceramic (aluminum oxide) implant. Explantation resulted in complete resolution of inflammation. Bioceramic implant-associated orbital inflammation has not been previously reported.
2. Case report
A 60-year-old woman presented with a two-month history of progressively worsening left retro-orbital pain and an ill-fitting ocular prosthesis. She remained systemically well. She had undergone a left enucleation for stage T4b choroidal melanoma six months earlier, with primary implantation of a 22mm porous Bioceramic (aluminum oxide) orbital implant (Spectrum Surgical, Australia). The implant was wrapped in vicryl mesh, which was secured with 5/0 vicryl sutures. An external prosthesis was fitted following surgery and initially fit well. The patient had no history of radiotherapy, immunosuppressive treatment or any other known risk factors for incomplete fibrovascular growth.
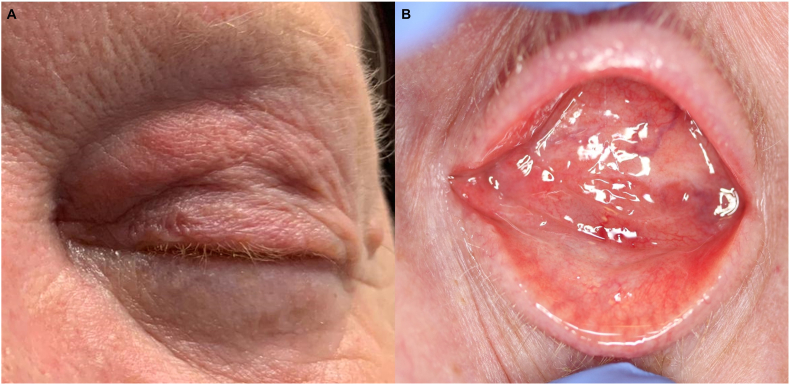
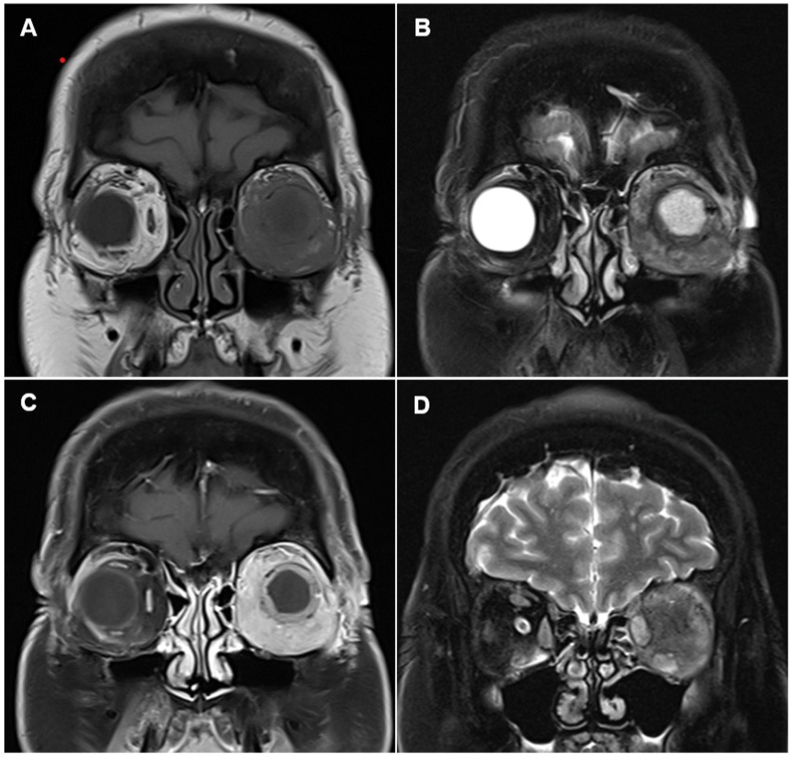
On examination, the prosthesis was inferiorly displaced >1cm with limited motility. There was mild, non-tender oedema and erythema of the left upper lid (Fig. 1A). Two firm, mobile nodules were found overlying the inferior orbital rim. The conjunctiva was hyperemic but there was no sign of melanomatous deposit, implant exposure or discharge (Fig. 1B). Magnetic resonance imaging (MRI) of the orbits revealed diffuse soft tissue infiltration of the left orbit and circumferential changes surrounding the implant periphery; the latter suggestive of fibrovascular ingrowth. The infiltrate demonstrated an isointense T1 signal, intermediate T2 signal and intense post-contrast enhancement (Fig. 2). Diffusion-weighted imaging revealed an intermediate apparent diffusion coefficient (ADC) signal intensity overlying the infiltrate, indicating less water content. The absence of oedema signal on T2 and ADC sequences made it difficult to determine if the infiltrate was secondary to a diffuse sclerosing inflammation or extensive local tumor recurrence. Of note, however, the left medial and inferior recti muscles were swollen and hyperintense on T2, indicating muscle oedema which was more consistent with an inflammatory process. A systemic screen for metastatic melanoma two weeks prior to her presentation, including liver ultrasound and chest X-ray, was unremarkable.
Fig. 1.
Clinical appearance. There is oedema and erythema of the left upper lid (A) with conjunctival hyperemia and thickening, but no evidence of implant exposure (B).
Fig. 2.
MRI coronal T1 (A), fat suppressed T2 (B) and fat suppressed T1, post contrast (C) images demonstrating diffuse infiltration of the left orbit. The peripheral rim of soft tissue within the orbital implant proved to be fibrovascular ingrowth. The changes are homogenous and isointense on T1, intermediate on T2, with intense enhancement. The fat suppressed T2 coronal image (D) reveals enlarged, somewhat edematous medial and inferior recti with preserved architecture and no evidence of intramuscular infiltration. The oedema signal on T2 is supportive of peri-implant inflammation as opposed to tumor recurrence.
As the orbital infiltrate had an equivocal radiological appearance, we proceeded with an orbital biopsy with a view to explantation. The patient declined a dermis fat graft. The orbital biopsy was performed under general anesthesia with intraoperative frozen sections of the tissue surrounding the implant and the infraorbital nodules. There was no evidence of malignant cells on frozen section analysis. The orbital sphere was explanted. Intraoperatively, there was no evidence of residual vicryl mesh.
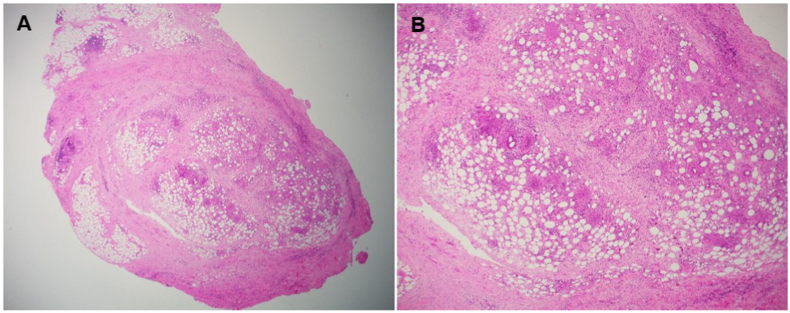
Histopathological analysis of tissue obtained from the primary orbital mass showed a florid fibro-inflammatory response to the prosthetic implant material. The fat surrounding the orbital implant showed extensive fibrosis and inflammation including foreign body-type multinucleate giant cell granulomas (Fig. 3). There was no evidence of recurrent melanoma. An inflammatory screen, which included angiotensin-converting enzyme, antineutrophil cytoplasmic antibodies (ANCA), extractable nuclear antigen (ANA) panel, anti-dsDNA, IgG subclasses, rheumatoid factor, anti-cyclic citrullinated peptide (anti-CCP) antibody, immunophenotyping, and lymphoid screens were negative. There were no fungal elements or residual intracellular vicryl seen on histopathology. One microbiological culture grew Staphylococcus epidermidis, though the chronicity of the clinical presentation and florid granulomatous foreign body reaction on histology were not compatible with a standard staphylococcus infection. A previous trial of oral amoxicillin/clavulanic acid had no effect and thus staphylococcus was thought to be a contaminant. The implant was also negative for atypical mycobacteria or any other growth on culture. Six months after the implant was removed, her inflammation had resolved without any additional treatment. The patient declined vicryl and aluminum oxide patch testing.
Fig. 3.
(A) Peri-orbital implant fat showing extensive fibrosis and inflammation including foreign body-type multinucleate giant cell granulomas (H&E stain, 2x); (B) Fibrosis and mixed inflammation including foreign body-type granulomas (H&E stain, 4x).
3. Discussion
Bioceramic spheres, composed of aluminum oxide, are a widely used form of biocompatible, integrative implant with a comparable safety profile to commonly used alternatives such as hydroxyapatite and porous polyethylene.5,6 Aluminum oxide has been readily used for orthopedic and dental prostheses due to its properties as a durable and bioinert material.6 Its surface promotes proliferation of fibroblasts and, upon insertion into the body, a protein coating acts as an “immune camouflage”, thereby promoting its integration with orbital tissue.6
Aluminum oxide is a highly biocompatible material and therefore secondary orbital inflammation should be rare. However, our patient had a negative serological and histological screen for alternate causes of orbital inflammation, and experienced a complete resolution of symptoms upon explantation, leading to the conclusion that this was most likely a rare case of Bioceramic implant associated orbital inflammation. Although patch testing for hypersensitivity reactions to aluminum oxide and vicryl would have been necessary to determine a definitive cause, unfortunately the patient declined these investigations.
Orbital inflammation secondary to porous orbital implants is rare. A review of the literature found only five previous cases of spherical orbital implant-associated inflammation with radiological findings reported, all of which had occurred with hydroxyapatite implants.3,4 Presenting symptoms included inflammation, discharge, pain and prosthesis intolerance. The delay from implantation to onset of symptoms ranged from 3 weeks to 24 years. Examination features consistent with chronic inflammation included forniceal shortening, fibrosis, symblepharon and socket contraction.3,4 Our patient's clinical presentation of pain, ill-fitting prosthesis and conjunctival inflammation mirror the inflammatory changes previously reported. Similar to the histological features in our case, Chee et al. and Galindo-Ferreiro et al.’s cases of hydroxyapatite-induced inflammation had microscopic features of chronic inflammatory infiltrate, giant cells, multiple small foreign bodies, eosinophils and dense fibrosis.3,4 It is unlikely that these peri-implant histological findings represented idiopathic sclerosing orbital inflammation given the complete quiescence achieved following implant removal. Four patients showed some improvement with topical antibiotics and steroids, however their symptoms recurred upon cessation of treatment. None were treated with intraorbital or systemic steroids. All patients ultimately underwent explantation and two required a dermis fat graft for orbital volume augmentation. In all except one case, symptoms resolved following removal of the implant and all patients were able to hold an external prosthesis at last follow-up. The remaining case did not comment on the patient's outcome. None of these cases reported their final follow up period.
Due to the rarity of the condition, the radiological characteristics of orbital implant-associated inflammation are not well elucidated. In Galindo-Ferreiro et al.’s case series, computed tomography scan and histopathological studies were conducted in all except one case, and none had an MRI performed.4 The radiological features varied between patients, with two cases describing inflammation surrounding the implant whilst two others found only preseptal inflammation. Our case was further complicated by the patient's recent history of choroidal melanoma, which, together with an ambiguous radiological appearance, raised the possibility of tumour recurrence. A surgical biopsy was necessary to confirm the diagnosis and rule out other causes of inflammatory response, such as a fungal infection.
Vicryl (polyglactin 910) is a highly effective wrap for orbital implants as it averts the need for donor sclera and promotes more rapid fibrovascular ingrowth.7 Vicryl is well-tolerated in most cases and is generally absorbed in the first 4–8 weeks, though it may persist for up to 6 months and can incite a mild foreign body reaction in some patients.8,9 Several reports have also described significant hypersensitivity reactions triggered by vicryl sutures, characterized clinically by oedema, erythema and pruritis.8,10 The onset of a vicryl hypersensitivity reaction can be immediate or delayed up to three months after implantation, despite no visible residual material.10 Histologically, vicryl-associated inflammation is characterized by a significant infiltrate of giant cell histiocytes, granulocytes and eosinophils.9 Limited studies suggest vicryl may appear completely digested on histopathology despite an active foreign body-type inflammatory response.10 However, given that there was no macroscopic or microscopic evidence of residual vicryl, and there was a prolonged time course between implantation to symptoms, we surmise that it was less likely that vicryl contributed to the extensive inflammatory response seen in this patient. Instead, we postulate that the surface of the Bioceramic implant became increasingly exposed to orbital tissue as the vicryl was absorbed, which in turn incited the inflammatory reaction.
4. Conclusion
Although significant foreign body reactions requiring explantation have been associated with orbital hydroxyapatite implants in rare instances, the present case describes a severe inflammatory response to a Bioceramic implant, a complication which, to the authors’ knowledge, has not previously been reported.
5. Patient consent
The patient provided written consent to publication of their information in the form of a journal article.
Funding
No funding or grant support
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The authors have no financial disclosures or conflicts of interest.
Acknowledgements
None.
References
- 1.Jordan D.R. Porous versus nonporous orbital implants: a 25-year retrospective. Ophthalmology. 2018;125(9):1317–1319. doi: 10.1016/j.ophtha.2018.03.055. [DOI] [PubMed] [Google Scholar]
- 2.Quaranta-Leoni F.M., Moretti C., Sposato S., Nardoni S., Lambiase A., Bonini S. Management of porous orbital implants requiring explantation: a clinical and histopathological study. Ophthalmic Plast Reconstr Surg. 2014;30(2):132–136. doi: 10.1097/IOP.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 3.Chee E., Kim Y.-D., Woo K.I., Lee J.H., Kim J.H., Suh Y.-L. Inflammatory mass formation secondary to hydroxyapatite orbital implant leakage. Ophthalmic Plast Reconstr Surg. 2013;29(2):e40–e42. doi: 10.1097/IOP.0b013e3182696577. [DOI] [PubMed] [Google Scholar]
- 4.Galindo-Ferreiro A., Elkhamary S.M., Maktabi A., Galvez-Ruiz A., Schellini S.A. Chronic orbital inflammation associated to hydroxyapatite implants in anophthalmic sockets. Case Rep Ophthalmol. 2017;8(3):574–580. doi: 10.1159/000485498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schellini S., Jorge E., Sousa R., Burroughs J., El-Dib R. Porous and nonporous orbital implants for treating the anophthalmic socket: a meta-analysis of case series studies. Orbit. 2016;35(2):78–86. doi: 10.3109/01676830.2016.1139591. [DOI] [PubMed] [Google Scholar]
- 6.Jordan D.R., Gilberg S., Mawn L.A. The bioceramic orbital implant: experience with 107 implants. Ophthalmic Plast Reconstr Surg. 2003;19(2):128–135. doi: 10.1097/01.IOP.0000056027.63698.FE. [DOI] [PubMed] [Google Scholar]
- 7.Jordan D.R., Klapper S.R., Gilberg S. The use of vicryl mesh in 200 porous orbital implants: a technique with few exposures. Ophthalmic Plast Reconstr Surg. 2003;19(1):53–61. doi: 10.1097/00002341-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Ogbechie O.A., Paul S., Schalock P.C. A technique for identifying vicryl suture hypersensitivity. Dermatitis. 2014;25(6):370–371. doi: 10.1097/DER.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 9.FitzGerald J.F., Kumar A.S. Biologic versus synthetic mesh reinforcement: what are the pros and cons? Clin Colon Rectal Surg. 2014;27(4):140–148. doi: 10.1055/s-0034-1394155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stocco C., Berton F., Papa G., Bussani R., Arnež Z.M. Vicryl hypersensitivity test with histological response. Dermatitis. 2016;27(3):145–146. doi: 10.1097/DER.0000000000000182. [DOI] [PubMed] [Google Scholar]