Abstract
Extracellular vesicles (EVs) are generated by cells in the form of exosomes, microvesicles, and apoptotic bodies. They can be taken up by neighboring cells, and their contents can have functional impact on the cells that engulf them. As the mediators of intercellular communication, EVs can play important roles in both physiological and pathologic contexts. In addition, early detection of EVs in different body fluids may offer a sensitive diagnostic tool for certain diseases, such as cancer. Furthermore, targeting specific EVs may also become a promising therapeutic approach. This review summarizes the latest findings of EVs in the field of liver research, with a focus on the different contents of the EVs and their impact on liver function and on the development of inflammation, fibrosis, and tumor in the liver. The goal of this review is to provide a succinct account of the various molecules that can mediate the function of EVs so the readers may apply this knowledge to their own research.
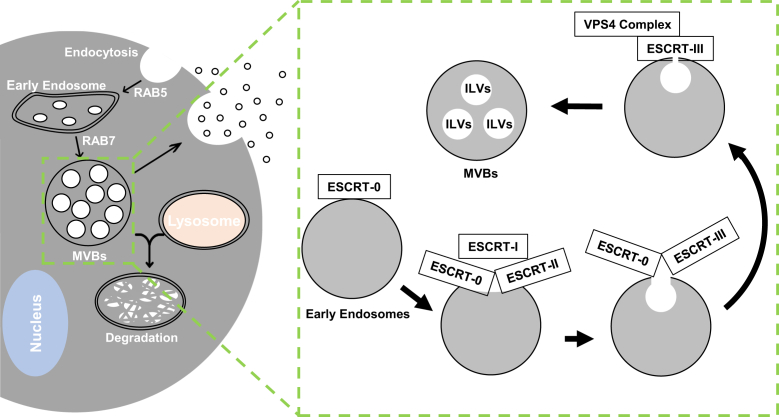
Extracellular vesicles (EVs) have attracted tremendous attention in research because they can act as critical mediators of cell-to-cell communication in both health and disease states.1,2 EVs are defined as membrane-bound, nanometer-sized vesicles released by cells into the extracellular space in a highly regulated manner. EVs are classified according to the size as exosomes (approximately 40 to 160 nm; approximately 100 nm on average), microvesicles (150 to 1000 nm), and apoptotic bodies (1000 to 4000 nm).3 Membrane invagination of vesicles allows the formation of multivesicular bodies. Some multivesicular bodies can fuse with the plasma membrane to release the internal vesicles as exosomes via exocytosis, whereas other multivesicular bodies are degraded in lysosomes (Figure 1).4 Microvesicles are derived directly from cell membrane through budding, whereas apoptotic bodies are remnants of cells following apoptotic death. EVs can be found in all major bodily fluids, including blood, breast milk, urine, bile, saliva, semen, and cerebrospinal fluid.5 EVs deliver various cargos from donor cells in the form of proteins, lipids, and nucleic acids, and they exert a wide range of effects on target cells. Some cargos are specifically found in certain disease status, which may be used as diagnosis markers, whereas other cargos can regulate physiological activity in target cells. Despite methods of EV isolation that are constantly improving, current studies may only recognize a small subpopulation of EVs with specific cargos.6,7
Figure 1.
Generation of exosomes from multivesicular bodies (MVBs). MVBs are important for the intracellular trafficking, connecting early endosomes to lysosomes. MVBs are characterized by the presence of multiple intraluminal vesicles (ILVs). The formation of ILVs requires the endosomal sorting complexes required for transport (ESCRT) machinery, which is made up of cytosolic protein complexes, known as ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III. They are recruited to the precursors of MVBs sequentially as indicated and promote membrane invagination and vesicle formation. A variety of protein and RNA molecules can be incorporated into the ILVs. Exosomes are a type of extracellular vesicles (EVs) with the size around 100 nm in average. They are derived from the ILVs of MVBs when the later fuses with the cytoplasmic membranes. Thus, EVs derived from MVBs can contain cargos contained in the ILVs, which may carry unique signatures reflecting the functional or structural features of the cells. These cargo molecules can have functional impact on cells that engulf the exosomes. RAB, member RAS oncogene family; VPS4, vacuolar protein sorting 4 homolog.
The liver is the largest internal organ of the human body with multiple functions in hematopoiesis, protein synthesis, production of bile, macromolecule metabolism, and detoxification.8, 9, 10 The liver is a multicellular organ, and virtually all types of liver cells can release EVs. Hepatocytes are the main resource of EVs from the liver. EVs from hepatocyte participate in liver fibrosis, repair, and regeneration.11, 12, 13, 14 EVs from nonhepatocytes,15 such as cholangiocytes, hepatic stellate cells (HSCs), resident hepatic macrophages (Kupffer cells), and liver sinusoidal endothelial cells are involved in liver injury, fibrosis, inflammation, ductular reaction, and cancer development.16, 17, 18, 19 A series of markers have been identified to distinguish the exosomes released by different kinds of liver cells. The exosomes released from hepatocytes have high expression of cytochrome P450 (CYP) family 2 subfamily E member 1 and asialoglycoprotein receptor 1.20 Cholangiocyte-derived exosomes express the specific marker cytokeratin 19.19 Sphingosine kinase 1 is found in exosomes isolated from liver sinusoidal endothelial cell line, SK-HEP-1,21 whereas connective tissue growth factor (CTGF/CCN2) is found in exosomes from highly activated mouse HSCs.22 Liver cells can be both the donor and the recipient of EVs, resulting in a multiway communication network among the various cells in the liver.23,24 Intercellular communications can be mediated by proteins, and perhaps more importantly, by mRNAs and miRNAs, with functional consequence in target cells.25 This review summarizes recent findings on the role of EVs in liver pathology, and their potential clinical applications.
EVs in Liver Physiology
Although most studies investigate the role of EVs in the disease state, EVs appear to participate in the physiological function of the liver with some of the well-characterized molecules found in the EVs as well (Figure 2 and Table 126, 27, 28, 29, 30, 31, 32).
Figure 2.
Extracellular vesicles (EVs) can affect both physiological and pathologic processes of liver diseases. EVs can carry molecules related to normal liver physiology. The cargos are responsible for its regulatory functions (Table 1). EVs are also important in injury, inflammation, fibrosis, and tumorigenesis related to the development of various liver diseases. EVs may be produced, secreted, and/or taken up by hepatocytes and nonhepatocytes. EVs function by delivering cargo from the producing cells (Table 2, Table 3, Table 4) to the target cells and thus modify the function of the latter in the pathogenesis of alcoholic liver disease, nonalcoholic fatty liver disease, biliary diseases, liver cancer, and viral hepatitis.
Table 1.
EV-Derived Molecules Involved in Liver Physiology
| Function | Molecules |
|---|---|
| Drug metabolism | |
| Regeneration |
Listed molecules are enriched in EVs.
CYP2E1, cytochrome P450 family 2 subfamily E member 1; CYP3A, cytochrome P450 family 3 subfamily A; ERK1/2, extracellular-regulated protein kinase 1/2; EV, extracellular vesicle; SK2, sphingosine kinase 2; UGT, uridine 5′-diphospho-glucuronosyltransferase.
Drug Metabolism
In the late 1980s, Johnstone et al26,33 reported the shedding of plasma membrane in the process of maturation of reticulocytes to erythrocytes. In addition, they showed that some superfluous proteins were eliminated through released membrane vesicles. These vesicles were found with acetylcholinesterase activities associated with the metabolism of certain drugs.26,33 This was the first report implicating a role of EVs in drug metabolism–related function. The CYP superfamily is primarily responsible for drug metabolism in the liver, kidney, and small intestine. Functional CYP isoforms can be sorted into EVs found in plasma of healthy persons.27 In particular, CYP family 2 subfamily E member 1 is expressed at a much higher level than other CYPs, such as 1B1, 2A6, 2E1, and 3A4, in EVs isolated from healthy subjects,27 and thus becomes a well-recognized marker for hepatocyte-derived EVs. In addition, exosome-derived CYP3A and uridine 5′-diphospho-glucuronosyltransferase proteins have also been found to be metabolically active under ex vivo conditions and exhibit kinetics comparable to subcellular fractions prepared from human liver tissue.28 CYP and uridine 5′-diphospho-glucuronosyltransferase families of drug-metabolizing enzymes account for the metabolic clearance of >90% of drugs.29,34 EVs may play a critical role in drug metabolism. It is possible that the liver releases CYP-containing EVs to promote drug metabolism in other cells that internalize these vesicles. However, the mechanism of CYP/uridine 5′-diphospho-glucuronosyltransferase sorting into EVs and target cell selection are largely unknown.
Regeneration
Liver is the main organ for detoxification of noxious endobiotics and xenobiotics, and is susceptible to various harmful conditions and injuries. This makes the ability of regeneration important to the liver. Interestingly, exosomes isolated from human liver stem cells are able to induce proliferation and prevent apoptosis of hepatocytes.12 The proliferation is abolished in isolated EVs treated with anti–α4-integrin–blocking antibody and RNase, indicating that α4-integrin and RNAs play a critical role in the internalization of EVs and regulation of regeneration, respectively.30 In addition, Nojima et al12 reported that hepatocyte-derived EVs increased hepatocyte proliferation and liver regeneration. This effect was abolished in EVs isolated from sphingosine kinase 2–deficient mice or when the function of sphingosine kinase 2 was blocked, highlighting the importance of sphingosine kinase 2, an enzyme that catalyzes the formation of sphingosine-1-phosphate. Sphingosine kinase 2/sphingosine-1-phosphate may play a positive role in cell growth.
Other than stimulating hepatocyte growth, EVs isolated from bile may influence cholangiocyte proliferation. Bile may contain exosomes that decrease the phosphorylation level of extracellular-regulated protein kinase 1/2, and subsequently increase the level of miR-15A, causing decreased proliferation of cholangiocytes.32,35
EVs in Liver Pathology
EVs can be released by various liver cells under pathologic conditions, which, in turn, contribute to the occurrence and development of inflammation, fibrosis, and cancer.36 This important role of EVs has now been well recognized. Table 217,37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 summarizes some of the molecules found in EVs that are involved in liver pathology.
Table 2.
EV-Derived Molecules Involved in Liver Inflammation and Fibrosis
| Disease status | Molecules |
|---|---|
| Inflammation (alcoholic liver disease) | |
| Inflammation (nonalcoholic fatty liver disease) | |
| Liver fibrosis |
Listed molecules are increased in EVs under the disease status.
CCN2, connective tissue growth factor; CD40L, CD40 ligand; EV, extracellular vesicle; HSP90, heat shock protein 90; ITGβ1, integrin β1; S1P, sphingosine-1-phosphate; SK, sphingosine kinase; TLR3, toll-like receptor 3.
Liver Inflammation
The key cellular events of liver inflammatory responses are the activation of liver-resident macrophages, also known as Kupffer cells, and the recruitment and activation of inflammatory monocytes originated from the extraliver space.
Excessive alcohol drinking, one of the most common causes of liver disease, can cause multiple pathologic effects. The number of exosomes is significantly increased in mice after binge or chronic alcohol consumption, and in the serum of healthy individuals after alcohol binge drinking.37 Early studies showed that alcohol treatment in vitro induced EV release from primary hepatocytes and HepG2 cells. The released EVs could stimulate cytokine production from THP-1 macrophage cells.38 EV release requires caspase activation, and EVs are enriched with the molecule CD40 ligand. Mice receiving a pan-caspase or a Rho kinase inhibitor and mice with genetic deletion of CD40 or the caspase-activating tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) receptor are protected from alcohol-induced injury and associated macrophage infiltration, suggesting that these EVs contribute to pathogenesis. Saha et al39 showed that the total number of circulating EVs increased in mice with chronic ethanol feeding, compared with pair-fed controls, and the mRNA and protein level of C-C motif chemokine ligand 2 increased in hepatocytes isolated from mice treated with these EVs. Furthermore, the transcriptional levels of tumor necrosis factor-α and IL-1β increased in macrophages treated with these EVs derived from mice with chronic ethanol exposure or alcoholic liver disease. The EVs thus play a proinflammation role by affecting both inflammatory cells and hepatocytes.
This proinflammatory effect of alcoholic liver disease–derived EVs is determined by their cargo, particularly miRNAs. Studies have found the significance of different cargo in alcoholic liver disease, such as heat shock protein 90, miR-192,39 and miR-122.37 Exosome-derived miR-122 inhibits heme oxygenase-1 pathway in the target THP-1 monocytes so that the cells become sensitized to stimulation of lipopolysaccharide, leading to increased production of proinflammatory cytokines. Similarly, miR-27a, a cargo of exosomes derived from alcohol-treated monocytes, activates native monocytes and promotes inflammatory response by IL-10 and transforming growth factor-β.40
Nonalcoholic fatty liver disease (NAFLD) is a common disorder with accumulation of excess fat in the liver of people who drink little or no alcohol. Recent studies have found that EVs can play a considerable role in NAFLD. Lipid accumulation in the liver under NAFLD can lead to lipotoxicity in hepatocytes, which can stimulate the release of EVs. EVs from hypoxic fat-laden hepatic cells can induce proinflammatory responses in Kupffer cells,49 which may be mediated by C-X-C motif chemokine ligand 10 contained in the EVs.41 Hepatocyte-derived EVs have a higher level of proinflammatory lipids, such as sphingosine-1-phosphate, which can also induce macrophage chemotaxis.42 Adipocytes are also a major source of EVs in NAFLD. Co-culturing adipocyte-derived EVs, isolated from high-fat–fed obese mice, with primary macrophages, activates the latter and promoted the production of macrophage colony-stimulating factor, IL-6, and tumor necrosis factor-α.50
Nonalcoholic steatohepatitis is one form of NAFLD in which the liver develops inflammation and cell damage in addition to fat accumulation. As in alcoholic liver disease, miRNAs play a role in the inflammation of NAFLD and can be a major force driving the development of nonalcoholic steatohepatitis. Hepatocyte-derived exosomes isolated from high-fat diet mice have an increased level of miR-130a,43 which promotes proinflammatory phenotype and suppresses anti-inflammatory phenotype of THP-1 cells via directly inhibiting peroxisome proliferator-activated receptor-γ (PPAR-γ).51 In rats fed with a high-fat, high-cholesterol diet, exosomes derived from hepatocyte are enriched with miR-192-5p that plays a critical role in the activation of proinflammatory macrophages and disease progression by targeting the regulatory associated protein of MTOR complex 1 (RPTOR)–independent companion of mechanistic target of rapamycin kinase (MTOR) complex 2/AKT serine/threonine kinase/forkhead box O1 signaling pathway.20 Interestingly, miR-192-5p level in serum collected from patients with NAFLD positively correlates with hepatic inflammation activity score and disease progression.20
Other factors associated with EVs can be also important for nonalcoholic steatohepatitis development. Dasgupta et al44 reported that activation of inositol-requiring transmembrane kinase endoribonuclease-1α stimulated X-box–binding protein 1 to increase transcription of serine palmitoyltransferase gene, resulting in ceramide biosynthesis and production of hepatocyte-derived EVs in a mouse model of nonalcoholic steatohepatitis. Consistently, mice given i.v. injections of inositol-requiring transmembrane kinase endoribonuclease-1α–stimulated, hepatocyte-derived, ceramide-enriched EVs recruited monocytes into the liver, resulting in inflammation and injury. Another mechanism related to EVs involves integrin β1 (ITGβ1). Guo et al17 reported that lysophosphatidylcholine treatment in hepatocytes activates ITGβ1 and mediates its endocytic trafficking and sorting into EVs. The ITGβ1-enriched EVs can then mediate monocyte adhesion to liver sinusoidal cells through an ITGβ1-dependent manner. The resulting inflammation is attenuated by anti-ITGβ1 antibody.
Liver Fibrosis
Liver fibrosis results from excessive accumulation of extracellular matrix proteins, such as collagens. Fibrosis can diminish blood flow throughout the liver. It can progress to cirrhosis, leading to liver failure and even liver cancer.52 HSCs are one of the major types of cells driving fibrosis, and their functions can be regulated by EVs as they internalize these vesicles coming from different types of cells.
Hepatocytes are the major source of EVs that can activate HSCs.45,53 Increased fat deposit in hepatocytes is an early event of NAFLD. EVs derived from fat-laden hepatocytes contain various miRNAs, some of which are known repressors of PPAR-γ, and HSC activators, such as miR-128-3p.45 Thus, EVs may promote progression of liver pathology from simple steatosis to fibrosis. An in vitro study also found that cobalt chloride, a chemical hypoxia inducer, caused the release of EVs from fat-laden HepG2 cells.53 These EVs, in turn, enhance the expression of profibrotic markers in LX-2 cells, an immortalized HSC cell line. This in vitro finding was confirmed by an in vivo model in which C57BL/6 mice given choline-deficient, L-amino acid defined (CDAA) diet and intermittent hypoxia exposure led to an increased production of exosomes to promote fibrosis.53 In yet another example of carbon tetrachloride–induced liver injury, exosomes isolated from damaged hepatocytes contain toll-like receptor 3 ligands, which induce activation of toll-like receptor 3 in HSCs to exacerbate liver fibrosis.46
Endothelial cells are another source of EVs that may affect HSCs in livers. Exosomes derived from SK1-overexpressing endothelial cells promote the migration of HSCs via AKT signaling, which were blocked by disruption of fibronectin-integrin interaction or by inhibition of dynamin. The findings suggest that the effect of endothelial cell–derived SK1-containing EVs on HSC migration relies on fibronectin-integrin–dependent EV adherence and dynamin-dependent EV internalization.47 Another study found that small EVs (exosomes) isolated from the serum of patients with systemic mastocytosis carry a mast cell signature.48 These EVs were enriched with KIT proto-oncogene, receptor tyrosine kinase (KIT), and were internalized by HSCs, leading to their proliferation, differentiation, and cytokine production. These effects were reduced by KIT inhibition and recapitulated by enforced expression of KIT. In addition to in vitro studies, HSCs purified from mice injected with systemic mastocytosis exosomes had increased expressions of α-smooth muscle actin and KIT. Their findings demonstrate that systemic mastocytosis exosomes can initiate activation of HSCs in both in vitro and in vivo models.
Interestingly, EVs can be transferred between HSCs. In a well-studied case, EVs contained CTGF/CCN2, which is a regulator of activated HSCs.54 CTGF/CCN2 can promote adhesion, activation, chemotaxis, and migration of HSCs. CTGF/CCN2 protein or Ctgf mRNA can shuttle between activated and quiescent HSCs via EVs, thus activating or amplifying fibrotic progress.22 In another interesting case, quiescent HSCs became cancer-associated fibroblasts after taking up exosomes derived from hepatocellular carcinoma cells.55 These EVs contain miR-21, which may promote pyruvate dehydrogenase kinase 1/AKT signaling. The activated cancer-associated fibroblasts can release transforming growth factor-β, matrix metallopeptidases, and fibroblast growth factor 2, to promote fibrosis and other effects.
Liver Cancer
Liver cancers can be derived from different cell types and include hepatocellular carcinoma (HCC), cholangiocarcinoma, angiosarcoma, hemangiosarcoma, and hepatoblastoma. Hepatocellular carcinoma is the most common form of liver cancer, accounting for approximately 90% of cases, and it is the second leading cause of cancer-related death globally.56 Exosomes can act as a regulatory means of transmitting signaling cargos between neighboring cells and heterogeneous populations of tumor cells to affect the nature and microenvironment of tumor cells (Table 357, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73).
Table 3.
EV-Derived Molecules Involved in Liver Tumorigenesis
| Pathology changes | Molecules |
|---|---|
| EMT | |
| Angiogenesis | |
| Proliferation, invasion, and metastasis |
Listed molecules are increased in EVs under the disease status.
ANGPT2, angiopoietin 2; Circ-CCAC1, circular RNA 1; CircRNA, circular RNA; EMT, epithelial-mesenchymal transition; EV, extracellular vesicle; HGF, hepatocyte growth factor; ITG-αMβ2, integrin αMβ2; linc-ROR, long intergenic non–protein-coding RNA, regulator of reprogramming; lncRNA, long ncRNA; ncRNA, noncoding RNA; OVOL1, ovo like transcriptional repressor 1; α-SMA, α-smooth muscle actin; ZEB, zinc finger E-box binding homeobox.
Effect of Exosomes in EMT
Epithelial-mesenchymal transition (EMT) is a pathophysiological process in which epithelial cells lose polarity and cell-cell adhesion, and acquire motile and invasive characteristics to become mesenchymal stem cells. EMT is the critical step for the invasion and metastasis of cancers.74 Emerging evidence shows that EVs can promote EMT.
Most of these studies treated in vitro one type of cells with exosomes derived from another type of cells. The expression of EMT markers, such as E-cadherin and vimentin, increased in the recipient cells.57,58,75,76 Notably, exosome-treated cells became more proliferative through increased mitogen-activated protein kinase/extracellular-regulated protein kinase signaling.75 Similarly, HCC cell–derived exosomes play a regulatory role on hepatocyte growth factor/hepatocyte growth factor receptor/AKT signaling.58
Effect of Exosomes in Angiogenesis
Angiogenesis is critical for cancer growth through increased supply of oxygen, nutrients, and growth factors. Expanded vascular network, along with altered permeability, can also promote metastasis. Liver cancer–derived EVs may be involved in the promotion of angiogenesis when they are taken up by vascular endothelial cells. A variety of molecules (such as proteins and small RNAs) contained in the EVs have been linked to this process. For example, liver cancer cells can produce angiopoietin 2–containing exosomes, which can be internalized by vascular endothelial cells.59 In another study, HCC cells produced vasorin-containing exosomes that can stimulate human umbilical vein endothelial cells for angiogenesis.60
miRNAs often transmit the angiogenesis effect after delivery to the target cells via EVs. miR-155–containing exosomes, produced by HCC cells under hypoxic condition, elevate the level of vascular endothelial growth factor and hypoxia-inducible factor-1α in target human umbilical vein endothelial cells to promote angiogenesis.61 On the other hand, miR-210–containing exosomes, secreted by HCC cells, induce angiogenesis via SMAD family member 4 and STAT6 signaling pathway.62 Cholangiocarcinoma can also release EVs that are taken up by endothelial cells. Circular RNA 1 contained within the EVs may promote angiogenesis.63 The effect of miRNA in angiogenesis can also be indirect. Quiescent HSCs can take up miR-21–containing exosomes derived from HCC cell lines to become cancer-associated fibroblasts, which can then stimulate angiogenesis in addition to other effects.55
Other small RNAs can also serve as signaling messengers. In one study, exosomes isolated from the medium of cultured cancer stem cell–like CD90+ liver cells contained long noncoding RNA (lncRNA) H19, which increased the transcripts of vascular endothelial growth factor in human umbilical vein endothelial cells to induce the formation of tube-like structures.64 In another study, high-metastatic HCC cells produced more exosomes containing circular RNA-100338, which promoted the proliferation, angiogenesis, and permeability of human umbilical vein endothelial cells.65
Effect of Exosomes in Proliferation, Invasion, and Metastasis
Most of the studies focus on the exosomes derived from tumor cells,63,66,67,77, 78, 79 although EVs from nontumor cells, such as immature myeloid cells, macrophages, mast cells, and T cells, have been examined as well.68 Although the target cells are mainly studied for hepatic tumor cells,67, 68, 69, 70, 71, 72,80 vascular endothelial cells63,72 and other nonparenchymal cells66,78,79 have also been reported. The overall effects in cancer cell proliferation, invasion, and metastasis can be direct or indirect through distinct mechanisms mediated by the content in the exosomes, which may include protein molecules, miRNA, lncRNAs, and circular RNA. The following are a few examples illustrating the heterogeneity in the way of action.
One study showed that exosomes derived from HCC cells can facilitate tumorigenesis in recipient normal hepatocytes by activating phosphoinositide 3-kinase/AKT/mitogen-activated protein kinase pathway and promote production of metalloprotease matrix metallopeptidases 2 and 9.77 Another study reported that exosomes isolated from the culture medium of HCC cells contained lncRNA TUC339, which was transferred to perhaps other cells within the same microenvironment, and consequently promoted HCC proliferation and invasion.67 Exosomes may communicate between tumor cells to enhance viability and survival of target cells through a series of regulations involving multiple small RNAs, including long intergenic non–protein-coding RNA, regulator of reprogramming, which eventually lead to the activation of hypoxia-inducible factor-1α and/or hypoxia-inducible factor-2α pathway.73 The miRNAs may be regulated by other factors under tumor microenvironment. For example, acidic microenvironment triggered the activation of hypoxia-inducible factor-1α/2α, which enhanced the level of miR-21 and miR-10b in exosomes substantially, thus promoting Hep3B cell proliferation, migration, and invasion in vivo and in vitro70,71 Unfortunately, how deep within the tumor EVs are able to reach is unclear.
Interestingly, many of the studies found that tumor-derived exosomes target to a variety of nontumor cells, which then, in turn, promote proliferation, invasion, and metastasis. For example, HCC cells, such as HepG2, MHCC97H, or QGY-7703 cells, can release exosomes that are internalized by mesenchymal stem cells. miR-181d-5p released from these exosomes targets suppressor of cytokine signaling 3 to activate focal adhesion kinase/SRC proto-oncogene, non-receptor tyrosine kinase (SRC), nonreceptor tyrosine kinase signaling pathway, which promotes the differentiation of mesenchymal stem cells into fibroblasts and accelerates EMT, invasion, and migration of tumor cells.65 Another study showed that such effects can cause the differentiation of mesenchymal stem cells to fibroblasts, which regulate local microenvironment to enhance tumor cell growth.78 In addition, adipocytes have the capacity to internalize exosomes isolated from HepG2 cell culture medium. When co-injected with HepG2 cells into mice, these exosome-treated adipocytes release inflammatory cytokine through the activation of NF-κB pathway, which recruits macrophages to the tumor region, leading to enhanced cell proliferation and tumor growth.79 Finally, another cell population that can be modified by tumor-derived exosomes is endothelial cells, which leads to increased vascular permeability via the down-regulation of tight junction protein 1, cadherin 5, and p120-catenin by miR-103, thus facilitating tumor metastasis.72
Potential Values of EVs in Clinical Applications
Considering the many effects of EVs in cell-cell communication, it is tempting to consider the possibility of administrating EVs with appropriate cargo to treat pathologic conditions. There are some advantages of using EVs for this purpose, including that i) EVs do not replicate after administration; ii) EVs may have a lower immunogenicity; iii) EVs possess a high ability to cross tissue and cellular barrier; and iv) EVs can escape from the degradation of proteases in circulation, and are stable under freeze/thaw cycles during long-term storage. However, EVs are unlikely to be used directly isolated in vivo because of limitations in achieving uniform purification and variations in the nature of cargo and tissue source. Use of engineered EVs with only intended cargo and an enhanced delivery ability is more likely. Thus, the knowledge of the specific cargo and the membrane components of the EVs may be translated to a better engineered form of artificial EVs for clinical applications.
EVs may also be readily analyzed for diagnostic purposes. The content of EVs could serve as biomarkers for a given pathologic condition. There are several advantages of EVs as diagnostic biomarkers, including that EVs can be secreted by almost all types of cells and found in all major bodily fluids. This may make the detection easy for most diseases for early diagnosis and follow-up, and because the cargo varies from cell types or situations, specific cargo may be linked to specific pathology. In case of liver diseases, the focus has been on liver cancer, in which several EV cargo molecules have been examined for their differential expression in the normal versus in cancer conditions (Table 481, 82, 83, 84, 85, 86, 87, 88, 89, 90).
Table 4.
EV-Derived Molecules with Potential Diagnostic Values for Liver Cancers
| Molecules | Examples |
|---|---|
| Proteins | |
| miRNAs | |
| lncRNAs and lincRNAs |
Listed molecules are increased in EVs under the disease status, except those marked with an asterisk.
CAP1, cytoskeleton regulatory protein 1; EV, extracellular vesicle; GAL-3BP, galectin-3–binding protein; lincRNA, long intergenic noncoding RNAs; lncRNA, long ncRNA; lnc-ROR, long intergenic non-protein coding RNA, regulator of reprogramming; ncRNA, noncoding RNA; PIGR, polymeric immune receptor; SMAD3, SMAD family member 3; VLDLR-AS1: VLDLR antisense RNA 1.
Molecules that are reduced under the disease status.
Early diagnosis of HCC has always been a critical issue in its prompt management. There are no specific clinical symptoms for the early-stage HCC, and later-stage HCC is difficult to treat. Detection of serum α-fetoprotein and ultrasonography have been the two widely used methods, but both have limitations in the early detection of HCC.91,92 An increased number of studies have discovered that tumors can secrete unique exosomes, with the number and content distinctly different from the healthy population.93,94 For example, levels of galectin-3–binding protein and polymeric immune receptor are significantly higher in exosomes isolated from the serum of HCC patients, compared with those in healthy subjects.81 SMAD family member 3 and cyclase-associated actin cytoskeleton regulatory protein 1 are also rich in exosomes isolated from the peripheral blood of HCC patients and are closely associated with HCC stage and metastasis, respectively.82,83
However, small RNAs are perhaps the most promising biomarkers for HCC. Recent studies have shown that the levels of miR-21, miR-210, miR-224, and miR-93 in exosomes isolated from HCC patients' serum are significantly higher than those in EVs from healthy donors.62,71,84,85 A recent study reported that HCC patients, particularly those with low α-fetoprotein levels, had much higher levels of miR-21-5p, miR-10b-5p, miR-221-3p, and miR-223-3p in exosomes, compared with those in chronic hepatitis/non-HCC patients.86 Similarly, exosomes from HCC patients are enriched with miR-18a, miR-221, miR-222, and miR-224, compared with the patients with liver cirrhosis or chronic hepatitis B. In contrast, miR-101, miR-106b, miR-122, miR-125b, and miR-195 are found to be significantly lower in exosomes from HCC patients.87,88 Other kinds of noncoding RNAs, including lncRNAs and long intergenic noncoding RNAs, are also associated with HCC, even though lncRNAs account for only 3% of total RNA in EVs.95 lncRNA-TUC339, lncRNA H19, lnc-ROR, VLDLR-AS1, and Linc00161 are significantly up-regulated in HCC cells.64,67,80,89,90 Nevertheless, there is still a lot to be done to develop a clinically proven diagnostic test to detect EVs and/or the specific content for a particular disease.
Conclusion
Exosome research has made great progress in recent years. Novel mechanisms related to cell-to-cell communication have been discovered. The physiological and pathologic roles of exosomes have been explored in various liver conditions. EVs contain cargo that can be important to the pathogenesis of liver diseases, including metabolic disturbance, inflammation, fibrosis, and tumorigenesis. However, many questions remain to be addressed, particularly about the in vivo role and dynamics of EVs, considering that most of the work was done in vitro using cell lines. How EVs interact with the extracellular matrix and other microenvironment elements to gain access to cells that uptake them is unclear. Toward that end, an efficient and practical way to label these EVs may be necessary, trackable by methods for in vivo imaging.
Inhibition of the biogenesis or release of EVs, or blockage of EV uptake, may evolve into potential therapies to arrest or even prevent the development of the diseases. In addition, the structural features of EVs, which help them against lysosomal degradation, can help design new methods to deliver RNAs or drugs for therapeutic purpose. However, this approach is far from ready for clinical application. Better understanding of EVs is likely to result in diagnostic approaches based on specific EV cargo, which should provide the necessary sensitivity and specificity for early detection, thus facilitating the clinical management of diseases.
Footnotes
Supported in part by NIH–National Institute of Diabetes and Digestive and Kidney Diseases grant DK116605 (X.-M.Y.).
Disclosures: None declared.
References
- 1.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng F., Magee N., Zhang Y. Decoding the role of extracellular vesicles in liver diseases. Liver Res. 2017;1:147–155. doi: 10.1016/j.livres.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsova P., Ibrahim S.H., Verma V.K., Morton L.A., Shah V.H., LaRusso N.F., Gores G.J., Malhi H. Extracellular vesicles in liver pathobiology: small particles with big impact. Hepatology. 2016;64:2219–2233. doi: 10.1002/hep.28814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowal J., Tkach M., Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Colombo M., Raposo G., Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 6.Thery C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willms E., Cabanas C., Mager I., Wood M.J.A., Vader P. Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol. 2018;9:738. doi: 10.3389/fimmu.2018.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marin J.J., Macias R.I., Briz O., Banales J.M., Monte M.J. Bile acids in physiology, pathology and pharmacology. Curr Drug Metab. 2015;17:4–29. doi: 10.2174/1389200216666151103115454. [DOI] [PubMed] [Google Scholar]
- 9.Grant D.M. Detoxification pathways in the liver. J Inherit Metab Dis. 1991;14:421–430. doi: 10.1007/BF01797915. [DOI] [PubMed] [Google Scholar]
- 10.Reinke H., Asher G. Circadian clock control of liver metabolic functions. Gastroenterology. 2016;150:574–580. doi: 10.1053/j.gastro.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 11.Chen L., Chen R., Kemper S., Brigstock D.R. Pathways of production and delivery of hepatocyte exosomes. J Cell Commun Signal. 2018;12:343–357. doi: 10.1007/s12079-017-0421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nojima H., Freeman C.M., Schuster R.M., Japtok L., Kleuser B., Edwards M.J., Gulbins E., Lentsch A.B. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol. 2016;64:60–68. doi: 10.1016/j.jhep.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsova P., Ibrahim S.H., Krishnan A., Verma V.K., Bronk S.F., Werneburg N.W., Charlton M.R., Shah V.H., Malhi H., Gores G.J. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology. 2016;150:956–967. doi: 10.1053/j.gastro.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y.S., Kim S.Y., Ko E., Lee J.H., Yi H.S., Yoo Y.J., Je J., Suh S.J., Jung Y.K., Kim J.H., Seo Y.S., Yim H.J., Jeong W.I., Yeon J.E., Um S.H., Byun K.S. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci Rep. 2017;7:3710. doi: 10.1038/s41598-017-03389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kai O.L., Bengtsson G., Salaspuro M., Väänänen H. Springer; Boston, MA: 1986. Separation of Functionally Different Liver Cell Types. [Google Scholar]
- 16.Nishio T., Hu R., Koyama Y., Liang S., Rosenthal S.B., Yamamoto G., Karin D., Baglieri J., Ma H.Y., Xu J., Liu X., Dhar D., Iwaisako K., Taura K., Brenner D.A., Kisseleva T. Activated hepatic stellate cells and portal fibroblasts contribute to cholestatic liver fibrosis in MDR2 knockout mice. J Hepatol. 2019;71:573–585. doi: 10.1016/j.jhep.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Guo Q., Furuta K., Lucien F., Sanchez L.H.G., Hirsova P., Krishnan A., Kabashima A., Pavelko K.D., Madden B., Alhuwaish H., Gao Y., Revzin A., Ibrahim S.H. Integrin beta1-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J Hepatol. 2019;71:1193–1205. doi: 10.1016/j.jhep.2019.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greuter T., Shah V.H. Hepatic sinusoids in liver injury, inflammation, and fibrosis: new pathophysiological insights. J Gastroenterol. 2016;51:511–519. doi: 10.1007/s00535-016-1190-4. [DOI] [PubMed] [Google Scholar]
- 19.Li X., Liu R., Huang Z., Gurley E.C., Wang X., Wang J., He H., Yang H., Lai G., Zhang L., Bajaj J.S., White M., Pandak W.M., Hylemon P.B., Zhou H. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes cholestatic liver injury in mouse and humans. Hepatology. 2018;68:599–615. doi: 10.1002/hep.29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X.L., Pan Q., Cao H.X., Xin F.Z., Zhao Z.H., Yang R.X., Zeng J., Zhou H., Fan J.G. Lipotoxic hepatocyte-derived exosomal microRNA 192-5p activates macrophages through Rictor/Akt/forkhead box transcription factor O1 signaling in nonalcoholic fatty liver disease. Hepatology. 2020;72:454–469. doi: 10.1002/hep.31050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye Q., Zhou Y., Zhao C., Xu L., Ping J. Salidroside inhibits CCl4-induced liver fibrosis in mice by reducing activation and migration of HSC induced by liver sinusoidal endothelial cell-derived exosomal SphK1. Front Pharmacol. 2021;12:677810. doi: 10.3389/fphar.2021.677810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charrier A., Chen R., Chen L., Kemper S., Hattori T., Takigawa M., Brigstock D.R. Exosomes mediate intercellular transfer of pro-fibrogenic connective tissue growth factor (CCN2) between hepatic stellate cells, the principal fibrotic cells in the liver. Surgery. 2014;156:548–555. doi: 10.1016/j.surg.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conde-Vancells J., Rodriguez-Suarez E., Embade N., Gil D., Matthiesen R., Valle M., Elortza F., Lu S.C., Mato J.M., Falcon-Perez J.M. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res. 2008;7:5157–5166. doi: 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fonsato V., Collino F., Herrera M.B., Cavallari C., Deregibus M.C., Cisterna B., Bruno S., Romagnoli R., Salizzoni M., Tetta C., Camussi G. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells. 2012;30:1985–1998. doi: 10.1002/stem.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 26.Johnstone R.M., Adam M., Hammond J.R., Orr L., Turbide C. Vesicle formation during reticulocyte maturation: association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 27.Gerth K., Kodidela S., Mahon M., Haque S., Verma N., Kumar S. Circulating extracellular vesicles containing xenobiotic metabolizing CYP enzymes and their potential roles in extrahepatic cells via cell-cell interactions. Int J Mol Sci. 2019;20:6178. doi: 10.3390/ijms20246178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowland A., Ruanglertboon W., van Dyk M., Wijayakumara D., Wood L.S., Meech R., Mackenzie P.I., Rodrigues A.D., Marshall J.C., Sorich M.J. Plasma extracellular nanovesicle (exosome)-derived biomarkers for drug metabolism pathways: a novel approach to characterize variability in drug exposure. Br J Clin Pharmacol. 2019;85:216–226. doi: 10.1111/bcp.13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowland A., Miners J.O., Mackenzie P.I. The UDP-glucuronosyltransferases: their role in drug metabolism and detoxification. Int J Biochem Cell Biol. 2013;45:1121–1132. doi: 10.1016/j.biocel.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Herrera M.B., Fonsato V., Gatti S., Deregibus M.C., Sordi A., Cantarella D., Calogero R., Bussolati B., Tetta C., Camussi G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14:1605–1618. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balaphas A., Meyer J., Sadoul R., Morel P., Gonelle-Gispert C., Buhler L.H. Extracellular vesicles: future diagnostic and therapeutic tools for liver disease and regeneration. Liver Int. 2019;39:1801–1817. doi: 10.1111/liv.14189. [DOI] [PubMed] [Google Scholar]
- 32.Masyuk A.I., Huang B.Q., Ward C.J., Gradilone S.A., Banales J.M., Masyuk T.V., Radtke B., Splinter P.L., LaRusso N.F. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol. 2010;299:G990–G999. doi: 10.1152/ajpgi.00093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnstone R.M., Bianchini A., Teng K. Reticulocyte maturation and exosome release - transferrin receptor containing exosomes shows multiple plasma-membrane functions. Blood. 1989;74:1844–1851. [PubMed] [Google Scholar]
- 34.Nebert D.W., Russell D.W. Clinical importance of the cytochromes P450. Lancet. 2002;360:1155–1162. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- 35.Wortzel I., Seger R. The ERK cascade: distinct functions within various subcellular organelles. Genes Cancer. 2011;2:195–209. doi: 10.1177/1947601911407328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernandez A., Arab J.P., Reyes D., Lapitz A., Moshage H., Banales J.M., Arrese M. Extracellular vesicles in NAFLD/ALD: from pathobiology to therapy. Cells. 2020;9:817. doi: 10.3390/cells9040817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Momen-Heravi F., Bala S., Kodys K., Szabo G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci Rep. 2015;5:9991. doi: 10.1038/srep09991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma V.K., Li H.Y., Wang R.S., Hirsova P., Mushref M., Liu Y.M., Cao S., Contreras P.C., Malhi H., Kamath P.S., Gores G.J., Shah V.H. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol. 2016;64:651–660. doi: 10.1016/j.jhep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saha B., Momen-Heravi F., Furi I., Kodys K., Catalano D., Gangopadhyay A., Haraszti R., Satishchandran A., Iracheta-Vellve A., Adejumo A., Shaffer S.A., Szabo G. Extracellular vesicles from mice with alcoholic liver disease carry a distinct protein cargo and induce macrophage activation through heat shock protein 90. Hepatology. 2018;67:1986–2000. doi: 10.1002/hep.29732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saha B., Momen-Heravi F., Kodys K., Szabo G. MicroRNA cargo of extracellular vesicles from alcohol-exposed monocytes signals naive monocytes to differentiate into M2 macrophages. J Biol Chem. 2016;291:149–159. doi: 10.1074/jbc.M115.694133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ibrahim S.H., Hirsova P., Tomita K., Bronk S.F., Werneburg N.W., Harrison S.A., Goodfellow V.S., Malhi H., Gores G.J. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2016;63:731–744. doi: 10.1002/hep.28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao C.Y., Song M.J., Gao Y., Mauer A.S., Revzin A., Malhi H. Hepatocyte-derived lipotoxic extracellular vesicle sphingosine 1-phosphate induces macrophage chemotaxis. Front Immunol. 2018;9:2980. doi: 10.3389/fimmu.2018.02980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J., Dong T., Chen T., Sun J., Luo J., He J., Wei L., Zeng B., Zhang H., Li W., Liu J., Chen X., Su M., Ni Y., Jiang Q., Zhang Y., Xi Q. Hepatic exosome-derived miR-130a-3p attenuates glucose intolerance via suppressing PHLPP2 gene in adipocyte. Metabolism. 2020;103:154006. doi: 10.1016/j.metabol.2019.154006. [DOI] [PubMed] [Google Scholar]
- 44.Dasgupta D., Nakao Y., Mauer A.S., Thompson J.M., Sehrawat T.S., Liao C.Y., Krishnan A., Lucien F., Guo Q.Q., Liu M.F., Xue F., Fukushima M., Katsumi T., Bansal A., Pandey M.K., Maiers J.L., DeGrado T., Ibrahim S.H., Revzin A., Pavelko K.D., Barry M.A., Kaufman R.J., Malhi H. IRE1A stimulates hepatocyte-derived extracellular vesicles that promote inflammation in mice with steatohepatitis. Gastroenterology. 2020;159:1487–1503.e17. doi: 10.1053/j.gastro.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Povero D., Panera N., Eguchi A., Johnson C.D., Papouchado B.G., de Araujo Horcel L., Pinatel E.M., Alisi A., Nobili V., Feldstein A.E. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-gamma. Cell Mol Gastroenterol Hepatol. 2015;1:646–663.e4. doi: 10.1016/j.jcmgh.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seo W., Eun H.S., Kim S.Y., Yi H.S., Lee Y.S., Park S.H., Jang M.J., Jo E., Kim S.C., Han Y.M., Park K.G., Jeong W.I. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by gammadelta T cells in liver fibrosis. Hepatology. 2016;64:616–631. doi: 10.1002/hep.28644. [DOI] [PubMed] [Google Scholar]
- 47.Wang R., Ding Q., Yaqoob U., de Assuncao T.M., Verma V.K., Hirsova P., Cao S., Mukhopadhyay D., Huebert R.C., Shah V.H. Exosome adherence and internalization by hepatic stellate cells triggers sphingosine 1-phosphate-dependent migration. J Biol Chem. 2015;290:30684–30696. doi: 10.1074/jbc.M115.671735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim D.K., Cho Y.E., Komarow H.D., Bandara G., Song B.J., Olivera A., Metcalfe D.D. Mastocytosis-derived extracellular vesicles exhibit a mast cell signature, transfer KIT to stellate cells, and promote their activation. Proc Natl Acad Sci U S A. 2018;115:E10692–E10701. doi: 10.1073/pnas.1809938115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hernandez A., Geng Y., Sepulveda R., Solis N., Torres J., Arab J.P., Barrera F., Cabrera D., Moshage H., Arrese M. Chemical hypoxia induces pro-inflammatory signals in fat-laden hepatocytes and contributes to cellular crosstalk with Kupffer cells through extracellular vesicles. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165753. doi: 10.1016/j.bbadis.2020.165753. [DOI] [PubMed] [Google Scholar]
- 50.Deng Z.B., Poliakov A., Hardy R.W., Clements R., Liu C., Liu Y., Wang J., Xiang X., Zhang S., Zhuang X., Shah S.V., Sun D., Michalek S., Grizzle W.E., Garvey T., Mobley J., Zhang H.G. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58:2498–2505. doi: 10.2337/db09-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin L., Lin H., Wang L., Wang B., Hao X., Shi Y. miR-130a regulates macrophage polarization and is associated with non-small cell lung cancer. Oncol Rep. 2015;34:3088–3096. doi: 10.3892/or.2015.4301. [DOI] [PubMed] [Google Scholar]
- 52.Bataller R., Brenner D.A. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hernandez A., Reyes D., Geng Y., Arab J.P., Cabrera D., Sepulveda R., Solis N., Buist-Homan M., Arrese M., Moshage H. Extracellular vesicles derived from fat-laden hepatocytes undergoing chemical hypoxia promote a pro-fibrotic phenotype in hepatic stellate cells. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165857. doi: 10.1016/j.bbadis.2020.165857. [DOI] [PubMed] [Google Scholar]
- 54.Huang G., Brigstock D.R. Regulation of hepatic stellate cells by connective tissue growth factor. Front Biosci (Landmark Ed) 2012;17:2495–2507. doi: 10.2741/4067. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Y., Ren H., Dai B., Li J., Shang L., Huang J., Shi X. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J Exp Clin Cancer Res. 2018;37:324. doi: 10.1186/s13046-018-0965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Llovet J.M., Zucman-Rossi J., Pikarsky E., Sangro B., Schwartz M., Sherman M., Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 57.Qu Z., Feng J.W., Pan H., Jiang Y., Duan Y.F., Fa Z.Z. Exosomes derived from HCC cells with different invasion characteristics mediated EMT through TGF-beta/Smad signaling pathway. Oncotargets Ther. 2019;12:6897–6905. doi: 10.2147/OTT.S209413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qu Z., Wu J.H., Wu J.Y., Luo D.J., Jiang C.P., Ding Y.T. Exosomes derived from HCC cells induce sorafenib resistance in hepatocellular carcinoma both in vivo and in vitro. J Exp Clin Cancer Res. 2016;35:159. doi: 10.1186/s13046-016-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie J.Y., Wei J.X., Lv L.H., Han Q.F., Yang W.B., Li G.L., Wang P.X., Wu S.B., Duan J.X., Zhuo W.F., Liu P.Q., Min J. Angiopoietin-2 induces angiogenesis via exosomes in human hepatocellular carcinoma. Cell Commun Signal. 2020;18:46. doi: 10.1186/s12964-020-00535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang A., Dong J., Li S., Wang C., Ding H., Li H., Su X., Ge X., Sun L., Bai C., Shen X., Fang T., Li J., Shao N. Exosomal transfer of vasorin expressed in hepatocellular carcinoma cells promotes migration of human umbilical vein endothelial cells. Int J Biol Sci. 2015;11:961–969. doi: 10.7150/ijbs.11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuura Y., Wada H., Eguchi H., Gotoh K., Kobayashi S., Kinoshita M., Kubo M., Hayashi K., Iwagami Y., Yamada D., Asaoka T., Noda T., Kawamoto K., Takeda Y., Tanemura M., Umeshita K., Doki Y., Mori M. Exosomal miR-155 derived from hepatocellular carcinoma cells under hypoxia promotes angiogenesis in endothelial cells. Dig Dis Sci. 2019;64:792–802. doi: 10.1007/s10620-018-5380-1. [DOI] [PubMed] [Google Scholar]
- 62.Lin X.J., Fang J.H., Yang X.J., Zhang C., Yuan Y., Zheng L., Zhuang S.M. Hepatocellular carcinoma cell-secreted exosomal microRNA-210 promotes angiogenesis in vitro and in vivo. Mol Ther Nucleic Acids. 2018;11:243–252. doi: 10.1016/j.omtn.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu Y., Leng K., Yao Y., Kang P., Liao G., Han Y., Shi G., Ji D., Huang P., Zheng W., Li Z., Li J., Huang L., Yu L., Zhou Y., Jiang X., Wang H., Li C., Su Z., Tai S., Zhong X., Wang Z., Cui Y. A circular RNA, cholangiocarcinoma-associated circular RNA 1, contributes to cholangiocarcinoma progression, induces angiogenesis, and disrupts vascular endothelial barriers. Hepatology. 2021;73:1419–1435. doi: 10.1002/hep.31493. [DOI] [PubMed] [Google Scholar]
- 64.Conigliaro A., Costa V., Lo Dico A., Saieva L., Buccheri S., Dieli F., Manno M., Raccosta S., Mancone C., Tripodi M., De Leo G., Alessandro R. CD90+liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer. 2015;14:155. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang X.Y., Huang Z.L., Huang J., Xu B., Huang X.Y., Xu Y.H., Zhou J., Tang Z.Y. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res. 2020;39:20. doi: 10.1186/s13046-020-1529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei H.M., Wang J.C., Xu Z.M., Li W.C., Wu X.J., Zhuo C.Y., Lu Y., Long X.D., Tang Q.L., Pu J. Hepatoma cell-derived extracellular vesicles promote liver cancer metastasis by inducing the differentiation of bone marrow stem cells through microRNA-181d-5p and the FAK/Src pathway. Front Cell Dev Biol. 2021;9:607001. doi: 10.3389/fcell.2021.607001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Kogure T., Yan I.K., Lin W.L., Patel T. Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Genes Cancer. 2013;4:261–272. doi: 10.1177/1947601913499020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang N., Li S., Li G., Zhang S., Tang X., Ni S., Jian X., Xu C., Zhu J., Lu M. The role of extracellular vesicles in mediating progression, metastasis and potential treatment of hepatocellular carcinoma. Oncotarget. 2017;8:3683–3695. doi: 10.18632/oncotarget.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li B.G., Mao R., Liu C.F., Zhang W.H., Tang Y., Guo Z. LncRNA FAL1 promotes cell proliferation and migration by acting as a CeRNA of miR-1236 in hepatocellular carcinoma cells. Life Sci. 2018;197:122–129. doi: 10.1016/j.lfs.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 70.Ban J.J., Lee M., Im W., Kim M. Low pH increases the yield of exosome isolation. Biochem Biophys Res Commun. 2015;461:76–79. doi: 10.1016/j.bbrc.2015.03.172. [DOI] [PubMed] [Google Scholar]
- 71.Tian X.P., Wang C.Y., Jin X.H., Li M., Wang F.W., Huang W.J., Yun J.P., Xu R.H., Cai Q.Q., Xie D. Acidic microenvironment up-regulates exosomal miR-21 and miR-10b in early-stage hepatocellular carcinoma to promote cancer cell proliferation and metastasis. Theranostics. 2019;9:1965–1979. doi: 10.7150/thno.30958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fang J.H., Zhang Z.J., Shang L.R., Luo Y.W., Lin Y.F., Yuan Y., Zhuang S.M. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology. 2018;68:1459–1475. doi: 10.1002/hep.29920. [DOI] [PubMed] [Google Scholar]
- 73.Takahashi K., Yan I.K., Haga H., Patel T. Modulation of hypoxia-signaling pathways by extracellular linc-RoR. J Cell Sci. 2014;127:1585–1594. doi: 10.1242/jcs.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen L., Guo P., He Y., Chen Z., Chen L., Luo Y., Qi L., Liu Y., Wu Q., Cui Y., Fang F., Zhang X., Song T., Guo H. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. 2018;9:513. doi: 10.1038/s41419-018-0534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dutta S., Reamtong O., Panvongsa W., Kitdumrongthum S., Janpipatkul K., Sangvanich P., Piyachaturawat P., Chairoungdua A. Proteomics profiling of cholangiocarcinoma exosomes: a potential role of oncogenic protein transferring in cancer progression. Biochim Biophys Acta. 2015;1852:1989–1999. doi: 10.1016/j.bbadis.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 77.He M., Qin H., Poon T.C.W., Sze S.C., Ding X.F., Co N.N., Ngai S.M., Chan T.F., Wong N. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis. 2015;36:1008–1018. doi: 10.1093/carcin/bgv081. [DOI] [PubMed] [Google Scholar]
- 78.Haga H., Yan I.K., Takahashi K., Wood J., Zubair A., Patel T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J Extracell Vesicles. 2015;4:24900. doi: 10.3402/jev.v4.24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang S., Xu M., Li X., Su X., Xiao X., Keating A., Zhao R.C. Exosomes released by hepatocarcinoma cells endow adipocytes with tumor-promoting properties. J Hematol Oncol. 2018;11:82. doi: 10.1186/s13045-018-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takahashi K., Yan I.K., Kogure T., Haga H., Patel T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio. 2014;4:458–467. doi: 10.1016/j.fob.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meng X.D., Pan J.C., Sun S.F., Gong Z.H. Circulating exosomes and their cargos in blood as novel biomarkers for cancer. Transl Cancer Res. 2018;7:S226–S242. [Google Scholar]
- 82.Fu Q., Zhang Q., Lou Y., Yang J., Nie G., Chen Q., Chen Y., Zhang J., Wang J., Wei T., Qin H., Dang X., Bai X., Liang T. Primary tumor-derived exosomes facilitate metastasis by regulating adhesion of circulating tumor cells via SMAD3 in liver cancer. Oncogene. 2018;37:6105–6118. doi: 10.1038/s41388-018-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang S., Chen G., Lin X., Xing X., Cai Z., Liu X., Liu J. Role of exosomes in hepatocellular carcinoma cell mobility alteration. Oncol Lett. 2017;14:8122–8131. doi: 10.3892/ol.2017.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cui Y., Xu H.F., Liu M.Y., Xu Y.J., He J.C., Zhou Y., Cang S.D. Mechanism of exosomal microRNA-224 in development of hepatocellular carcinoma and its diagnostic and prognostic value. World J Gastroenterol. 2019;25:1890–1898. doi: 10.3748/wjg.v25.i15.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xue X., Wang X., Zhao Y., Hu R., Qin L. Exosomal miR-93 promotes proliferation and invasion in hepatocellular carcinoma by directly inhibiting TIMP2/TP53INP1/CDKN1A. Biochem Biophys Res Commun. 2018;502:515–521. doi: 10.1016/j.bbrc.2018.05.208. [DOI] [PubMed] [Google Scholar]
- 86.Ghosh S., Bhowmik S., Majumdar S., Goswami A., Chakraborty J., Gupta S., Aggarwal S., Ray S., Chatterjee R., Bhattacharyya S., Dutta M., Datta S., Chowdhury A., Dhali G.K., Banerjee S. The exosome encapsulated microRNAs as circulating diagnostic marker for hepatocellular carcinoma with low alpha-fetoprotein. Int J Cancer. 2020;147:2934–2947. doi: 10.1002/ijc.33111. [DOI] [PubMed] [Google Scholar]
- 87.Liu W.F., Hu J., Zhou K.Q., Chen F.Y., Wang Z., Liao B.Y., Dai Z., Cao Y., Fan J., Zhou J. Serum exosomal miR-125b is a novel prognostic marker for hepatocellular carcinoma. Oncotargets Ther. 2017;10:3843–3851. doi: 10.2147/OTT.S140062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sohn W., Kim J., Kang S.H., Yang S.R., Cho J.Y., Cho H.C., Shim S.G., Paik Y.H. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med. 2015;47:e184. doi: 10.1038/emm.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takahashi K., Yan I.K., Wood J., Haga H., Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer Res. 2014;12:1377–1387. doi: 10.1158/1541-7786.MCR-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun L., Su Y.Y., Liu X.X., Xu M., Chen X.X., Zhu Y.F., Guo Z.R., Bai T.T., Dong L., Wei C.C., Cai X.X., He B.S., Pan Y.Q., Sun H.L., Wang S.K. Serum and exosome long non coding RNAs as potential biomarkers for hepatocellular carcinoma. J Cancer. 2018;9:2631–2639. doi: 10.7150/jca.24978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choi J., Kim G.A., Han S., Lee W., Chun S., Lim Y.S. Longitudinal assessment of three serum biomarkers to detect very early-stage hepatocellular carcinoma. Hepatology. 2019;69:1983–1994. doi: 10.1002/hep.30233. [DOI] [PubMed] [Google Scholar]
- 92.Galle P.R., Foerster F., Kudo M., Chan S.L., Llovet J.M., Qin S., Schelman W.R., Chintharlapalli S., Abada P.B., Sherman M., Zhu A.X. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39:2214–2229. doi: 10.1111/liv.14223. [DOI] [PubMed] [Google Scholar]
- 93.Abudoureyimu M., Zhou H., Zhi Y., Wang T., Feng B., Wang R., Chu X. Recent progress in the emerging role of exosome in hepatocellular carcinoma. Cell Prolif. 2019;52:e12541. doi: 10.1111/cpr.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li L.M., Liu Z.X., Cheng Q.Y. Exosome plays an important role in the development of hepatocellular carcinoma. Pathol Res Pract. 2019;215:152468. doi: 10.1016/j.prp.2019.152468. [DOI] [PubMed] [Google Scholar]
- 95.Gezer U., Ozgur E., Cetinkaya M., Isin M., Dalay N. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol Int. 2014;38:1076–1079. doi: 10.1002/cbin.10301. [DOI] [PubMed] [Google Scholar]