Abstract
The development of the vertebrate vascular system is an extremely important and complex process. The circulatory system is the first organ system to develop during embryogenesis. The development of the vasculature into highly branched canals must occur clearly in many places in order to supply oxygen and nutrients to the rapidly developing embryo. This process is mediated by a coordinated response of vascular endothelial and parietal cells to heterogeneous angiogenic signals provided by tissues and organs. MicroRNAs regulate gene expression at the transcriptional and post-transcriptional levels and participate in many important physiological and pathological processes. MicroRNAs mainly play an important role in the developmental regulation of vascular smooth muscle cells and vascular endothelial cells. This article summarizes the research progress of microRNAs in vascular development in recent years, focusing on the regulatory mechanism of miR-126 and miR-17/92 families in vascular endothelial cells, as well as the miR-143/145 family, miR-21 in vascular smooth muscle cell's regulation. The research prospects of the role of microRNAs in vascular development are also presented in this article.
Keywords: Vascular development, Regulation, Endothelial cells, Noncoding RNAs, microRNAs
1. Introduction
In the 1950s, scientists used light microscopy to discover that invertebrates lack endothelial cells in the blood circulation system, so invertebrates cannot undergo a series of evolutions like vertebrates to form a closed vascular system [1]. The appearance of closed vascular system in vertebrates contributes to the growth and development of vertebrates, and is also an important link in the process of biological evolution. The vascular system provides oxygen transport and various nutrient supply for the growth, development and survival of individual organisms. During embryonic development, disturbances in the development of the vascular system may lead to embryonic death or individual developmental disability. In higher vertebrates, vascular development is a very complex process. There are two vascular systems in higher vertebrates, the blood vascular system and the lymphatic vascular system [2]. There are certain differences between the lymphatic vascular system and the blood vascular system: blood vessels are a ring-shaped system, while the lymphatic vascular system is a linear system; capillaries are surrounded by vascular endothelial cells, and the endothelial cells that make up capillary lymph have connections between them similar to imbricate, where one cell edge overlaps the other [3]. The two are related to each other in the functions of tissue fluid reabsorption [2]. The existence of the blood vascular system and the lymphatic vascular system and the close connection between the two are essential in higher vertebrates [4]. MicroRNAs are a class of single-stranded non-coding small RNAs with a length of 21–23 nt, which function by recognizing the 3′ untranslated region sequence of the target mRNA and binding to its target, and inhibiting the translation level of its target after transcription [[5], [6], [7], [8], [9], [10]]. At present, there have been many reports on the regulation of microRNAs in vascular development. This article mainly reviews the research progress of microRNAs in the regulation of vascular development.
2. microRNAs in vascular development
MicroRNAs widely exist in various animal and plant genomes, maintain high homology and conservation during species evolution, and have strict expression specificity and timing in species [11]. In 1993, Lee et al. discovered the first microRNA in Caenorhabditis elegans, which is antisense complementary to the temporal gene lin-4 that regulates larval embryo development [12]. So far, thousands of microRNAs have been found in Drosophila, C. elegans, mice, and humans. Studies have shown that microRNAs can not only play a regulatory role at the post-transcriptional mRNA level, but also mediate many key physiological and pathological processes at the transcriptional level, including cell proliferation, cell fate determination, cell differentiation, cell metabolism, cell apoptosis, etc., so it's an important class of non-coding RNAs [13].
3. microRNAs affecting vascular endothelial cells
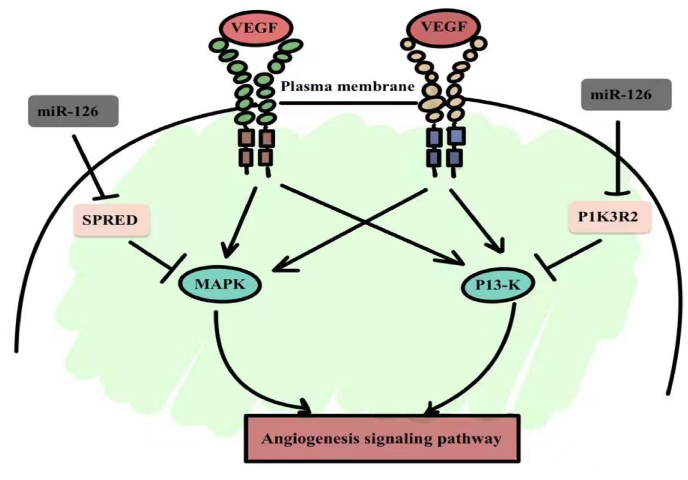
The miR-17/92 family includes six microRNAs: 17, 18a, 19a, 20a, 19b-1 and 92a-1 [14]. Studies have shown that lack of this microRNA family leads to postnatal death in mice due to cardiac septal defect [15,73]. This family is highly expressed in endothelial cells and inhibits angiogenesis by hindering endothelial cell motility [16]. The miR-17/92 family regulates the expression of proto-oncogenes, and this family of microRNAs downregulates the expression of anti-angiogenic molecules, thrombospondin and connective tissue growth factor [17]. There are many studies on individual microRNAs in this family, for example, inhibiting the expression of miR-92a can enhance the growth of blood vessels during ischemic injury or myocardial infarction [18]. In vitro experiments, overexpression of miR-92a can inhibit the formation of vascular sprouting and vascular network; in animal experiments, overexpression of miR-92a inhibits the expression of angiogenic growth factors [19]. Other members of the miRNA-17/92 family, including miR-17 and miR-20a, also inhibit angiogenesis [20]. Studies have shown that miR-126 is highly expressed in endothelial cells [21]. miR-126 inhibits PI3K and MAPK signaling pathways to promote angiogenesis and downregulate the expression of inflammatory adhesion molecules and vascular cell adhesion molecule 1 (VCAM1) (Fig. 1) [22]. miR-126 can inhibit the formation of atherosclerosis and increase the stability of platelets, can regulate the angiogenic signaling cascade, and can act as an anti-inflammatory mediator in endothelial cells to inhibit the inflammatory response [23]. These studies suggest that miR-126 can regulate angiogenesis in a range of pathological conditions, suggesting that it may play a role in future cancer therapy. When human umbilical vein endothelial cells are injured, overexpression of miR-126 significantly enhances the PI3K/Akt signaling pathway [24]. In mice, knockout of miR-126 resulted in rupture of blood vessels during embryonic stage, and it can increase the activity of pro-angiogenic factors in vascular injury in adult tissues, and promote the formation of new blood vessels [25]. Recent studies have shown that miR-221 can down-regulate vascular endothelial growth factor (VEGF) receptor signaling by regulating PI3K regulatory subunits [26]. The miR-221/222 family is the target gene of c-Kit and let-7f, which can promote angiogenesis through thrombospondin 1 [66]. Deep sequencing showed that miR-221 increased endothelial cells in zebrafish embryos [26]. Knockdown of miR-221 did not affect embryonic vascular development, but resulted in defects in angiogenesis and lymphatic vasculature, similar to the absence of vascular endothelial growth factor receptor 3 (VEGFR-3) [26]. Overexpression of miR-221 can cause changes in apical cell behavior, such as increased proliferation and migration [27].
Fig. 1.
The regulation of the angiogenesis of the miR-126 through the signaling pathways. VEGF, vascular endothelial growth factor; PI3K, hosphoinositide 3-kinase pathway; MAPK, mitogen-activated protein kinase pathway; PI3KR2, phosphoinositide 3-kinase, regulatory subunit 2; SPRED1, Sprouty-related, EVH1 domain-containing 1.
MiR-378 promotes VEGF expression by competing with miR-125a for the same region of the VEGF 3′-UTR [28]. miR-378 promotes cell survival by targeting the genes Sufu and Fus-1, and regulates tumor angiogenesis by indirectly upregulating VEGF. Studies have shown that injection of miR-378-transfected cancer cells in mice produces larger blood vessels than injection of cancer cells alone; overexpression of miR-378 in tumor cells increases cell viability and reduces cell death and promotes tumor growth and angiogenesis [29]. The miR-23/27/24 families is widespread in vascularized tissues and endothelial cells. Silencing of miR-23 and miR-27 can inhibit the activation of VEGF on MAPK and PI3K/PKB signaling pathways, thereby inhibiting angiogenesis, and inhibiting choroidal angiogenesis after laser injury [30]. In the absence of intersegmental vessels in zebrafish embryos, downregulation of miR-27 induces venous remodeling and angiogenesis [31]. The loss-of-function phenotype of miR-27 can be compensated by repressing one of Sprouty or DLL4 genes, therefore, these two genes may be the main target genes of miR-27 in zebrafish vascular development [31].
In vascular endothelial cells, hypoxia can induce the expression of miR-210 [32]. miR-210 can promote the formation of capillary sprouting and reduce apoptosis by inhibiting Ephrin-A3 under hypoxic conditions [32]. Downregulation of miR-200b promotes angiogenesis in endothelial cells when the skin is damaged [33]. In endothelial cells, overexpression of miR-181b can inhibit the expression of nuclear factor-κB (NF-κB) responsive genes; in the stimulatory response of mouse vascular endothelial cells to pro-inflammatory factors, miR-181b decreased expression [34]. In addition to the microRNAs mentioned above, many microRNAs also affect the development of blood vessels. Table 1 lists some of the microRNAs that affect the development of blood vasculature, and lists their target genes and functions.
Table 1.
Some microRNAs involved in the vascular development.
| MiRNA | Subject | Method | Target gene | Function |
|---|---|---|---|---|
| Mir-126 | Mouse | Knockout | F4C | Plays a role in the formation of new blood vessels [35] |
| Mouse | Knockout | Egfl7 | Plays an important role in embryonic blood vessel formation development [35] | |
| Mouse | Injection of miR-126 | Cxcl12 | Regulation of apoptotic body makes it have the function of anti-atherosclerosis [23] | |
| Human umbilical vein | Transfection of miR-126 endothelial cells | VCAM-1 | Inhibits VCAM-1 and regulates vascular inflammation [36] | |
| Zebrafish | Knockdown | Flt4 | Inhibits the development of lymphatic vessels in the face and torso [37] | |
| Mouse | Knockout | Flt4 | Regulates the development of the lymphatic network [38] | |
| Zebrafish | Knockdown | Spred1 | Enhances Spred1 activity [37] | |
| Human coronary endothelial cells | Knockdown | Spred1 | Regulates Spredl expression [38] | |
| Endothelial progenitor cell | Overexpression | P13KR2 | Regulates angiogenesis via targeting PI3KR2 [39] | |
| Zebrafish | Knockdown | Pak1 | Regulates the expression of Pakl in endothelial cells and causing head hemorrhage in zebrafish [40] | |
| Mir-126a | Zebrafish embryos | Knockdown | Cxcl12a | Regulates the formation of lymphatic vascular cavity [41] |
| Mir-92a | Vascular smooth muscle cells | Overexpression | MKK4, JNK1 | Down-regulates MKK4 and JNK1 [42] |
| Mouse | Knockout | Itga5 | Damages the development of the neointima [43] | |
| Mir-19a | Endothelial cell | Overexpression | Cyclin Dl | Inhibits endothelial cell proliferation via negatively regulating Cyclin Dl [44] |
| Mir-146a, mir-21 | Human coronary smooth muscle cells | Overexpression | Notch2 | Inhibits expression of Notch2 to regulate proliferation of smooth muscle cells [45] |
| Mir-146a | Human umbilical vein Endothelial cell | Overexpression | IRAKI | Down-regulates IRAKI [46] |
| Vascular smooth muscle cells | Knockdown | NF-kB, KLF4 | Regulates the proliferation and migration of vascular smooth muscle cells via targeting NF-kB and KLF4 [47,48] | |
| Mir-155 | Mouse | Knockout | MST2 | Regulates vascular smooth muscle cells by down-regulating MST2 [49] |
| Mouse | Knockout | TNF-a | Regulates vascular inflammatory response and proliferation of neointima [50] | |
| Mouse | Knockout | CCN1 | Promotes angiogenesis [51] | |
| Mir-10a | Mouse umbilical vein endothelial cells | Overexpression | BMP2 | Reduces proliferation and migration of umbilical vein endothelial cells and the formation of lumen [52] |
| Mouse smooth muscle cells | Transfection of miR-10a | HDAC4 | Reduces smooth muscle cell differentiation [53] | |
| Human arterial endothelial cells | Knockdown | HOXAl | Inhibits the expression of HOXAl [54] | |
| Mir-10a, mir-22 | Endothelial progenitor cell | Overexpression | Hmga2 | Inhibits Hmga2 expression [55] |
| Mir-100 | Mouse | Silent expression | mTOR | Inhibits the formation of blood vessels [56] |
| Mir-296 | Human umbilical vein endothelial cells | Overexpression | HGS | Regulates HGS and promotes angiogenesis [57] |
| Mir-378 | NCI–H292 cells | Overexpression | HMOX1 | Regulates HMOX1 and affects angiogenesis and growth of non-small cell lung cancer [58] |
| Mouse | Injection of miR-378-transfected cancer cells | VEGF | Affects angiogenesis [28] | |
| Mir-23/27 | Endothelial cells | Overexpression | SEMA6A, SPROUTY | Inhibits the expression of SEMA6A and SPROUTY and promotes angiogenesis [30] |
| Mir-96 | Vascular smooth muscle cells | Injection of anti-miR-96 | BMP4 | Regulates vascular smooth muscle cells via targeting BMP4 [59] |
| Mir-34a | Vascular smooth muscle cells | Overexpression | SIRTl | Down-regulates SIRTl and promotes senescence of vascular smooth muscle cells [60] |
| Mir-217 | Vascular smooth muscle cells | Transfection of mimics | NMDAR | Inhibits proliferation of vascular smooth muscle cells [61] |
| Human umbilical vein endothelial cells | Transfection of mimics | SIRTl | Inhibits SIRTl and regulates FoxOl resulting in angiogenesis damage and promotes endothelial cell senescence [62] | |
| Mir-182 | Zebrafish | Knockout | FoxOl | Regulates angiogenesis via targeting FoxOl [63] |
4. microRNAs affecting vascular smooth muscle cells
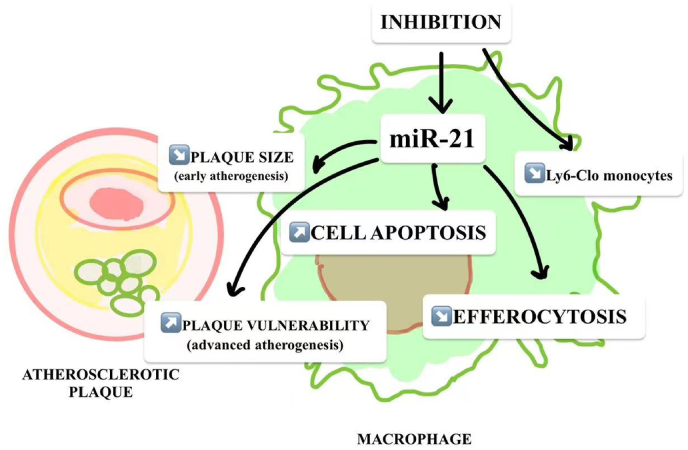
So far, many microRNAs related to vascular development have been reported, and many are related to vascular smooth muscle cells. For example, miR-21 has an important regulatory role in the proliferation and migration of vascular smooth muscle cells [64], and There are also higher levels of expression in endothelial cells [65]. Down-regulation of miR-21 expression increases apoptosis and inhibits the proliferation of adventitial fibroblasts and myofibroblasts [66]. miR-21 regulates vascular smooth muscle cell differentiation by affecting bone morphogenetic protein 4 (BMP4) and transforming growth factor β (TGF-β) signaling pathways [67]. miR-21 also regulates smooth muscle cells and endothelial cells, affecting vascular remodeling [15]. Studies have shown that the expression of miR-21 is increased in human atherosclerotic lesions (Fig. 2) [68].
Fig. 2.
MiR-21 in regulating lipid metabolism, apoptosis, macrophage inflammation and efferocytosis during atherogenesis.
In vitro experiments in serum-depleted conditions, human or mouse aortic smooth muscle cell differentiation reduces miR-21 expression [65]. In vitro experiments in mouse aortic smooth muscle cells and injured mouse carotid arteries, silencing of miR-21 will inhibit cell proliferation and increase apoptosis [65]. In mice, knockdown of miR-21 inhibits vascular remodeling in carotid injury [65]. Studies have shown that miR-21 has a higher expression level in various solid tumors than normal cells [69], so miR-21 can both promote cell proliferation and inhibit cell proliferation. Studies have shown that miR-146a can promote the proliferation of vascular smooth muscle cells in vitro and the intimal proliferation of angiogenesis in vivo [70]. Transfection of antisense miR-146a oligonucleotides into carotid balloon-injured mice significantly reduced neointimal hyperplasia [70], suggesting that miR-146a can promote vascular smooth muscle proliferation. At the same time, miR-146 can form a negative feedback loop to inhibit Toll-like receptor (TLR) signaling pathway, this negative feedback leads to endotoxin-induced tolerance to a certain extent, and can inhibit the production of inflammatory factors [71]. Studies have also shown that miR-147 and miR-155 also have similar functions [72]. Numerous studies have shown that vascular cell motility plays a crucial role in the development of various cancers and cardiovascular diseases, and the miR-143/145 family can regulate vascular cell motility [73]. In mice, knockdown of both miR-143 and miR-145 resulted in abnormal vascular tone and down-regulation of vascular smooth muscle cell-specific genes [74]. It has been shown that deletion of this family results in a reduced intimal proliferative response in vascular injury [74]. The miR-143/145 family is highly expressed in normal vascular smooth muscle cells, but the family is less expressed in acute and chronic vascular stress and human aortic aneurysms [75], and decreased expression in proliferating vascular smooth muscle cells [75,76]. In this family, miR-145 interacts with miR-143 to upregulate the expression of numerous target genes, including Kruppellike factor 4 (KLF4), Elk-1 (member of the ETS oncogene family) [76], vascular Angiotensin-converting enzyme (ACE) [77], serum response factor (SRF), and its co-activator, myocardin [74], It is shown that miR-145 can regulate the differentiation of human embryonic stem cells and the self-renewal of bone marrow stem cells. miR-143 can inhibit the migration of vascular smooth muscle cells through versican [78]. Platelet-derived growth factor induces miR-24 transcription, which induces a synthetic phenotype of vascular smooth muscle cells [79]. miR-24 is highly expressed in endothelial cells under stress conditions, such as oxidative stress. In mice, antisense expression of miR-24 enhanced angiogenesis and cardiac function [80].
Transfection of antisense miR-155 inhibitor in vascular smooth muscle cells can up-regulate the expression of endogenous angiotensin II type 1 receptor (AGTR1) [81]. miR-155 is expressed in smooth muscle cells, and its absence in atherogenesis reduces fat accumulation in macrophages [82]. The expression of miR-155 is up-regulated in mice and humans with atherosclerotic injury. Although up-regulation of miR-155 expression can promote atherosclerotic injury in humans, the circulating levels of miR-155 in patients with coronary artery disease are decreased [68]. Studies have shown that implantation of the bone marrow of LDLR-deficient mice with a high-fat diet into the bone marrow of miR-155-deficient mice will aggravate atherosclerotic lesions and inflammatory responses [82]. Down-regulation of miR-30b and miR-30c expression can lead to increased calcification of vascular smooth muscle cells [83]. Inhibition of miR-26a can accelerate the differentiation of vascular smooth muscle cells, and regulation of TGF-β signaling pathway by miR-26a may alter the phenotype of vascular smooth muscle [84]. During the proliferation of vascular smooth muscle and the growth of vascular wall neointima, the expression of miR-31 is significantly increased [85]; knockout of miR-31 can down-regulate serum and platelet-derived growth factors, thereby inducing vascular smooth muscle cell proliferation [85]. Overexpression of miR-208 can promote the proliferation of vascular smooth muscle cells, and can increase the regulatory effect of insulin on the proliferation of vascular smooth muscle cells [86]. In vascular smooth muscle cells, overexpression of miR-181a down-regulates the expression of angiotensin II (Ang II), up-regulates the expression of osteopontin (OPN), and enhances the adhesion of vascular smooth muscle cells to collagen [87]. In human aortic smooth muscle cells, overexpression of myocardin upregulates the expression of miR-1 and inhibits the proliferation of vascular smooth muscle cells [88].
5. Research on micrornas related to the development of lymphatic vasculature
MicroRNAs regulate not only the development of blood vasculature, but also the development of lymphatic vasculature. MiR-31 functions in early Xenopus embryonic lymphatic vasculature development [89]. Studies have shown that in lymphatic endothelial cells, knockdown of FAT4, which is a target gene of miR-31, enhances cell migration [90]. Studies have shown that both miR-31 and miR-181a are expressed in vascular endothelial cells during zebrafish embryonic lymphangiogenesis, and miR-31 or miR-181a regulates lymphangiogenesis by regulating the BMP2b/BMP2 signaling pathway [91]. In vitro experiments demonstrated that miR-184 inhibits corneal lymphangiogenesis, and overexpression of miR-184 reduces the migration of lymphatic endothelial cells and inhibits the formation of lymphatic endothelial cells [92]. In human lymphatic endothelial cells, overexpression of miR-27a reduces the formation and migration of lymphatic vessels, and the target gene of miR-27a is SMAD4, which negatively regulates the length of lymphatic vessels in the formation and migration of human lymphatic endothelial cells [93]. miR-206 inhibits tumor lymphangiogenesis in pancreatic ductal adenocarcinoma cells, thereby delaying tumor growth, which has certain significance in cancer therapy research [94]. Studies on the morphogenesis of lymphatic vessels in zebrafish have shown that miR-182 knockdown in zebrafish has defects in parachordal lymphatic vessels [63].
6. Conclusion
Vascular development is an extremely important and complex process, including the differentiation of endothelial cells, angiogenesis and angiogenesis, and the formation of lymphatic vasculature. It also involves many signaling pathways and transcription factors such as Notch and BMP. These regulatory factors control the differentiation and movement of endothelial cells, thereby regulating the development of blood vessels. Therefore, studying the mechanism of vascular development and its related signaling pathways has a certain role in promoting the evolution, growth and reproduction of animals. Many studies related to vascular development have shown that microRNAs play an important role in vascular development. MicroRNAs mainly inhibit vascular development or make vascular proliferation by regulating their target genes, and have vascular smooth muscle cells and vascular endothelial cells in the blood vascular system. Although many studies have revealed the regulatory mechanism of microRNAs in vascular development, many microRNAs with important functions in vascular development have not been discovered. Further exploration of the regulation of microRNAs on vascular development will help us to deeply understand the process of life, which is of great significance for the study of biological evolution.
Author statement
Albert Sufianov and Sema Begliarzade: conceptualized and designed the study. All authors participated in the acquisition, analysis and interpretation of the data. Valentin Kudriashov, Radmila Nafikova: drafted the manuscript. Tatiana Ilyasova, Yanchao Liang: contributed to critical revisions of the manuscript. All authors agreed on the journal to which the article would be submitted, gave final approval for the version to be published, and agreed to be accountable for all aspects of the work.
Funding
This study was supported by the Bashkir State Medical University Strategic Academic Leadership program (PRIORITY-2030).
Declaration of competing interest
The authors declare that no conflicts of interest exist.
Contributor Information
Albert Sufianov, Email: Federation.sufianov@gmail.com.
Sema Begliarzade, Email: semanagiyeva@yandex.ru.
Valentin Kudriashov, Email: vkudryashov.uro@gmail.com.
Radmila Nafikova, Email: nafikova.radmila@mail.ru.
Tatiana Ilyasova, Email: Iltanya67@yandex.ru.
Yanchao Liang, Email: liangyanchao@hrbmu.edu.cn.
References
- 1.Choi I., Lee S., Hong Y.K. The new era of the lymphatic system: no longer secondary to the blood vascular system. Cold Spring Harb Perspect Med. 2012;2(4):a006445. doi: 10.1101/cshperspect.a006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong Y.K., Shin J.W., Detmar M. Development of the lymphatic vascular system: a mystery unravels. Dev Dyn. 2004;231(3):462–473. doi: 10.1002/dvdy.20179. [DOI] [PubMed] [Google Scholar]
- 3.Shigei T., Tsuru H., Ishikawa N., Yoshioka K. Absence of endothelium in invertebrate blood vessels: significance of endothelium and sympathetic nerve/medial smooth muscle in the vertebrate vascular system. Jpn J Pharmacol. 2001;87(4):253–260. doi: 10.1254/jjp.87.253. [DOI] [PubMed] [Google Scholar]
- 4.Alitalo K., Tammela T., Petrova T.V. Lymphangiogenesis in development and human disease. Nature. 2005;438(7070):946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 5.Sufianov A., Begliarzade S., Ilyasova T., Liang Y., Beylerli O. MicroRNAs as prognostic markers and therapeutic targets in gliomas. Noncoding RNA Res. 2022 Jul 6;7(3):171–177. doi: 10.1016/j.ncrna.2022.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beylerli O., Gareev I., Pavlov V., Chen X., Zhao S. The role of long noncoding RNAs in the biology of pituitary adenomas. World Neurosurg. 2020 May;137:252–256. doi: 10.1016/j.wneu.2019.10.137. [DOI] [PubMed] [Google Scholar]
- 7.Beylerli O., Gareev I., Sufianov A., Ilyasova T., Guang Y. Long noncoding RNAs as promising biomarkers in cancer. Noncoding RNA Res. 2022 Feb 25;7(2):66–70. doi: 10.1016/j.ncrna.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gareev I., Gileva Y., Dzidzaria A., Beylerli O., Pavlov V., Agaverdiev M., Mazorov B., Biganyakov I., Vardikyan A., Jin M., Ahmad A. Long non-coding RNAs in oncourology. Noncoding RNA Res. 2021 Aug 26;6(3):139–145. doi: 10.1016/j.ncrna.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gareev I., Beylerli O., Aliev G., Pavlov V., Izmailov A., Zhang Y., Liang Y., Yang G. The role of long non-coding RNAs in intracranial aneurysms and subarachnoid hemorrhage. Life (Basel) 2020 Aug 20;10(9):155. doi: 10.3390/life10090155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beylerli O., Khasanov D., Gareev I., Valitov E., Sokhatskii A., Wang C., Pavlov V., Khasanova G., Ahmad A. Differential non-coding RNAs expression profiles of invasive and non-invasive pituitary adenomas. Noncoding RNA Res. 2021 Jun 30;6(3):115–122. doi: 10.1016/j.ncrna.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 12.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 13.Engels B.M., Hutvagner G. Principles and effects of microRNAmediated post-transcriptional gene regulation. Oncogene. 2006;25(46):6163–6169. doi: 10.1038/sj.onc.1209909. [DOI] [PubMed] [Google Scholar]
- 14.Concepcion C.P., Bonetti C., Ventura A. The microRNA-17-92 family of microRNA clusters in development and disease. Cancer J. 2012;18(3):262–267. doi: 10.1097/PPO.0b013e318258b60a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendell J.T. miRiad roles for the miR-17-92 cluster in development and disease. Cell. 2008;133(2):217–222. doi: 10.1016/j.cell.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ventura A., Young A.G., Winslow M.M., Lintault L., Erkeland A.M., Newman J., Bronson R.T., Crowley D., Stone J.R., Sharp F.A., Jack T. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132(5):875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonauer A., Carmona G., Iwasaki M., Mione M., Koyanaqi M., Fischer A., Burchfield J., Fox H., Doebele C., Ohtani K., Chavakis E., Potente M., Tjwa M., Urbich C., Zeiher A.M., Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324(5935):1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 18.Dews M., Homayouni A., Yu D., Murphy D., Sevignani C., Wentzel E., Furth E.E., Lee W.M., Enders G.H., Mendell J.T., Tikhonenko A.T. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38(9):1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang L.T., Lawson N.D., Fish J.E. MicroRNA control of vascular endothelial growth factor signaling output during vascular development. Arterioscler Thromb Vasc Biol. 2013;33(2):193–200. doi: 10.1161/ATVBAHA.112.300142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doebele C., Bonauer A., Fischer A., Scholz A., Reiss Y., Urbich C., Hofmann W.K., Zeiher A.M., Dimmeler S. Members of the microRNA-17-92 cluster exhibit a cell intrinsic anti-angiogenic function in endothelial cells. Blood. 2010;115(23):4944–4950. doi: 10.1182/blood-2010-01-264812. [DOI] [PubMed] [Google Scholar]
- 21.Suarez Y., Wang C., Manes T.D., Pober J.S. TNF-induced miRNAs regulate TNF-induced expression of E-Selectin and ICAM-1 on human endothelial cells: feedback control of inflammation. J Immunol. 2010;184(1):21–25. doi: 10.4049/jimmunol.0902369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fish J.E., Santoro M.M., Morton S.U., Yu S., Yeh R.F., Wythe J.D., Ivey K.N., Bruneau B.G., Stainier D.Y., Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zernecke A., Bidzhekov K., Noels H., Shaqdarsuren E., Gan L., Deneche B., Hristov M., Kopple T., Jahantiqh M.N., Lutqens E., Wang S., Olson E.N., Schober A., Weber C. Delivery of microRNA-126 by apoptotic bodies induces CXCL12- dependent vascular protection. Sci Signal. 2009;2(100):81–92. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 24.Chen L., Wang J., Wang B., Yang J., Gong Z., Zhao X., Zhang C., Du K. MiR-126 inhibits vascular endothelial cell apoptosis through targeting PI3K/Akt signaling. Ann Hematol. 2016;95(3):365–374. doi: 10.1007/s00277-015-2567-9. [DOI] [PubMed] [Google Scholar]
- 25.Wang S., Aurora A.B., Johnson B.A., Qi X., McAnally J., Hill J.A., Richardson J.A., Bassel-Duby R., Olson E.N. The endothelial- specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicoli S., Knyphausen C.P., Zhu L.J., Lakshmanan A., Lawson N.D. miR-221 is required for endothelial tip cell behaviors during vascular development. Dev Cell. 2012;22(2):418–429. doi: 10.1016/j.devcel.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J.F., Zhang X., Groopman J.E. Activation of vascular endothelial growth factor receptor-3 and its downstream signaling promote cell survival under oxidative stress. J BiolChem. 2004;279(26):27088–27097. doi: 10.1074/jbc.M314015200. [DOI] [PubMed] [Google Scholar]
- 28.Hua Z., Lv Q., Ye W., Wong C.K., Cai G., Gu D., Ji Y., Zhao C., Wang J., Yang B.B., Zhang Y. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One. 2006;1(1):116–129. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee D.Y., Deng Z., Wang C.H., Yang B.B. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A. 2007;104(51):20350–20355. doi: 10.1073/pnas.0706901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Q., Gallagher R., Ufret-Vincenty R., Li X., Olson E.N., Wang S. Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23∼27∼24 clusters. Proc Natl Acad Sci U S A. 2011;108(20):8287–8292. doi: 10.1073/pnas.1105254108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biyashev D., Veliceasa D., Topczewski J., Topczewska J.M., Mizgirev I., Vinokour E., Reddi A.L., Licht J.D., Revskoy S.Y., Volpert O.V. miR-27b controls venous specification and tip cell fate. Blood. 2012;119(11):2679–2687. doi: 10.1182/blood-2011-07-370635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fasanaro P., D'Alessandra Y., Di Stefano V., Melchionna R., Romani S., Pompilio G., Capogrossi M.C., Martelli F. MicroRNA- 210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283(23):15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan Y.C., Roy S., Khanna S., Sen C.K. Downregulation of endothelial MicroRNA-200b supports cutaneous wound angiogenesis by desilencing GATA binding protein 2 and vascular endothelial growth factor receptor 2. Arterioscler Thromb Vasc Biol. 2012;32(6):1372–1382. doi: 10.1161/ATVBAHA.112.248583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X.H., Sit A., Feinberg M.W. Role for miR-181 family in regulating vascular inflammation and immunity. Trends Cardiovasc Med. 2014;24(3):105–112. doi: 10.1016/j.tcm.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S., Aurora A.B., Johnson B.A., Qi X., McAnally J., Hill J.A., Richardson J.A., Bassel-Duby R., Olson E.N. An endothelialspecific microRNA governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris T.A., Yamakuchi M., Ferlito M., Mendell J.T., Lowenstein C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105(5):1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kontarakis Z., Rossi A., Ramas S., Dellinger M.T., Stainier D.Y.R. Mir-126 is a conserved modulator of lymphatic development. Dev Biol. 2018;437(2):120–130. doi: 10.1016/j.ydbio.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Jansen F., Yang X., Hoelscher M., Cattelan A., Schmitz T., Proebsting S., Wenzel D., Vosen S., Franklin B.S., Fleischmann B.K., Nickenig G., Werner N. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 2013;128(18):2026–2038. doi: 10.1161/CIRCULATIONAHA.113.001720. [DOI] [PubMed] [Google Scholar]
- 39.Meng Q., Wang W., Yu X., Li W., Kong L., Qian A., Li C., Li X. Upregulation of microRNA-126 contributes to endothelial progenitor cell function in deep vein thrombosis via its target PIK3R2. J Cell Biochem. 2015;116(8):1613–1623. doi: 10.1002/jcb.25115. [DOI] [PubMed] [Google Scholar]
- 40.Zou J., Li W.Q., Li Q., Li X.Q., Zhang J.T., Liu G.Q., Chen J., Qiu X.X., Tian F.J., Wang Z.Z., Zhu N., Qin Y.W., Shen B., Liu T.X., Jing Q. Two functional microRNA-126s repress a novel target gene p21-activated kinase 1 to regulate vascular integrity in zebrafish. Circ Res. 2011;108(2):201–209. doi: 10.1161/CIRCRESAHA.110.225045. [DOI] [PubMed] [Google Scholar]
- 41.Chen J., Zhu R.F., Li F.F., Liang Y.L., Wang C., Qin Y.W., Huang S., Zhao X.X., Jing Q. MicroRNA-126a directs lymphangiogenesis through interacting with chemokine and Flt4 signaling in zebrafish. Arterioscler Thromb Vasc Biol. 2016;36(12):2381–2393. doi: 10.1161/ATVBAHA.116.308120. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L., Zhou M., Wang Y., Huang W., Qin G., Weintraub N.L., Tang Y. miR-92a inhibits vascular smooth muscle cell apoptosis: role of the MKK4-JNK pathway. Apoptosis. 2014;19(6):975–983. doi: 10.1007/s10495-014-0987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daniel J.M., Penzkofer D., Teske R., Dutzmann J., Koch A., Bielenberg W., Bonauer A., Boon R.A., Fischer A., Bauersachs J., van Rooij E., Dimmeler S., Sedding D.G. Inhibition of miR-92a improves re-endothelialization and prevents neointima formation following vascular injury. Cardiovasc Res. 2014;103(4):564–572. doi: 10.1093/cvr/cvu162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin X., Wang X., Wang Y., Tang Z., Cui Q., Xi J., Li Y., Chien S., Wang N. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2010;107(7):3240–3244. doi: 10.1073/pnas.0914882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao J., Zhang K., Zheng J., Dong R. MicroRNA-146a and -21 cooperate to regulate vascular smooth muscle cell proliferation via modulation of the Notch signaling pathway. Mol Med Rep. 2015;11(4):2889–2895. doi: 10.3892/mmr.2014.3107. [DOI] [PubMed] [Google Scholar]
- 46.Olivieri F., Lazzarini R., Recchioni R., Marcheselli F., Rippo M.R., Di Nuzzo S., Albertini M.C., Graciotti L., Babini L., Mariotti S., Spada G., Abbatecola A.M., Antonicelli R., Franceschi C., Procopio A.D. MiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling. Age (Dordr) 2013;35(4):1157–1172. doi: 10.1007/s11357-012-9440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong S., Xiong W., Yuan J., Li J., Liu J., Xu X. MiRNA-146a regulates the maturation and differentiation of vascular smooth muscle cells by targeting NF-κB expression. Mol Med Rep. 2013;8(2):407–412. doi: 10.3892/mmr.2013.1538. [DOI] [PubMed] [Google Scholar]
- 48.Kang H., Hata A. MicroRNA regulation of smooth muscle gene expression and phenotype. Curr Opin Hematol. 2012;19(3):224–231. doi: 10.1097/MOH.0b013e3283523e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Z., Zheng B., Zhang Y., He M., Zhang X.H., Ma D., Zhang R.N., Wu X.L., Wen J.K. miR-155-dependent regulation of mammalian sterile 20-like kinase 2 (MST2) coordinates inflammation, oxidative stress and proliferation in vascular smooth muscle cells. Biochim Biophys Acta. 2015;1852(7):1477–1489. doi: 10.1016/j.bbadis.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Zhang R.N., Zheng B., Li L.M., Zhang J., Zhang X.H., Wen J.K. Tongxinluo inhibits vascular inflammation and neointimal hyperplasia through blockade of the positive feedback loop between miR-155 and TNF-α. Am J Physiol Heart Circ Physiol. 2014;307(4):552–562. doi: 10.1152/ajpheart.00936.2013. [DOI] [PubMed] [Google Scholar]
- 51.Yan L., Lee S., Lazzaro D.R., Aranda J., Grant M.B., Chaqour B. Single and compound knock-outs of microRNA (miRNA)- 155 and its angiogenic gene target CCN1 in mice alter vascular and neovascular growth in the retina via resident microglia. J Biol Chem. 2015;290(38):23264–23281. doi: 10.1074/jbc.M115.646950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J., Zhang Y., Zhao Q., Wang J., He X. MicroRNA-10a influences osteoblast differentiation and angiogenesis by regulating β-catenin expression. Cell Physiol Biochem. 2015;37(6):2194–2208. doi: 10.1159/000438576. [DOI] [PubMed] [Google Scholar]
- 53.Huang H., Xie C., Sun X., Ritchie R.P., Zhang J., Chen Y.E. miR-10a contributes to retinoid acid-induced smooth muscle cell differentiation. J Biol Chem. 2010;285(13):9383–9389. doi: 10.1074/jbc.M109.095612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang Y., Shi C., Manduchi E., Civelek M., Davies P.F. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci U S A. 2010;107(30):13450–13455. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu S., Deng S., Ma Q., Zhang T., Jia C., Zhuo D., Yang F., Wei J., Wang L., Dykxhoorn D.M., Hare J.M., Goldschmidt-Clermont P.J., Dong C. MicroRNA-10A* and MicroRNA-21 modulate endothelial progenitor cell senescence via suppressing high-mobility group A2. Circ Res. 2013;112(1):152–164. doi: 10.1161/CIRCRESAHA.112.280016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grundmann S., Hans F.P., Kinniry S., Heinke J., Helbing T., Bluhm F., Sluijter J.P., Hoefer I., Pasterkamp G., Bode C., Moser M. MicroRNA-100 regulates neovascularization by suppression of mammalian target of rapamycin in endothelial and vascular smooth muscle cells. Circulation. 2011;123(9):999–1009. doi: 10.1161/CIRCULATIONAHA.110.000323. [DOI] [PubMed] [Google Scholar]
- 57.Feng J., Huang T., Huang Q., Chen H., Li Y., He W., Wang G.B., Zhang L., Xia J., Zhang N., Liu Y. Pro-angiogenic microRNA-296 upregulates vascular endothelial growth factor and downregulates Notch1 following cerebral ischemic injury. Mol Med Rep. 2015;12(6):8141–8147. doi: 10.3892/mmr.2015.4436. [DOI] [PubMed] [Google Scholar]
- 58.Skrzypek K., Tertil M., Golda S., Ciesla M., Weglarczyk K., Collet G., Guichard A., Kozakowska M., Boczkowski J., Was H., Gil T., Kuzdzal J., Muchova L., Vitek L., Loboda A., Jozkowicz A., Kieda C., Dulak J. Interplay between heme oxygenase-1 and miR-378 affects non-small cell lung carcinoma growth, vascularization, and metastasis. Antioxid Redox Signal. 2013;19(7):644–660. doi: 10.1089/ars.2013.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim S., Hata A., Kang H. Down-regulation of miR-96 by bone morphogenetic protein signaling is critical for vascular smooth muscle cell phenotype modulation. J Cell Biochem. 2014;115(5):889–895. doi: 10.1002/jcb.24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Badi I., Burba I., Ruggeri C., Zeni F., Bertolotti M., Scopece A., Pompilio G., Raucci A. MicroRNA-34a induces vascular smooth muscle cells senescence by SIRT1 downregulation and promotes the expression of age-associated pro-inflammatory secretory factors. J Gerontol A Biol Sci Med Sci. 2015;70(11):1304–1311. doi: 10.1093/gerona/glu180. [DOI] [PubMed] [Google Scholar]
- 61.Duan H., Li Y., Yan L., Yang H., Wu J., Qian P., Li B., Wang S. MicroRNA-217 suppresses homocysteine-induced proliferation and migration of vascular smooth muscle cells via N-methyl-D-aspartic acid receptor inhibition. Clin Exp PharmacolPhysiol. 2016;43(10):967–975. doi: 10.1111/1440-1681.12611. [DOI] [PubMed] [Google Scholar]
- 62.Menghini R., Casagrande V., Cardellini M., Martelli E., Terrinoni A., Amati F., Vasa-Nicotera M., Ippoliti A., Novelli G., Melino G., Lauro R., Federici M. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120(15):1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- 63.Kiesow K., Bennewitz K., Miranda L.G., Stoll S.J., Hartenstein B., Angel P., Kroll J., Schorpp-Kistner M. Junb controls lymphatic vascular development in zebrafish via miR-182. SciRep. 2015;5(3):15007–15013. doi: 10.1038/srep15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ji R., Cheng Y., Yue J., Liu X., Chen H., Dean D.B., Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100(11):1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 65.Wang F., Zhao X.Q., Liu J.N., Wang Z.H., Wang X.L., Hou X.Y., Liu R., Gao F., Zhang M.X., Zhang Y., Bu P.L. Antagonist of microRNA-21 improves balloon injury-induced rat iliac artery remodeling by regulating proliferation and apoptosis of adventitial fibroblasts and myofibroblasts. J Cell Biochem. 2012;113:2989–3001. doi: 10.1002/jcb.24176. [DOI] [PubMed] [Google Scholar]
- 66.Kuehbacher A., Urbich C., Zeiher A.M., Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101(1):59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 67.Davis B.N., Hilyard A.C., Lagna G., Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454(2000):56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raitoharju E., Lyytikainen L.P., Levula M., Oksala N., Mennander A., Tarkka M., Klopp N., Illig T., Kahonen M., Karhunen P.J., Laaksonen R., Lehtimaki T. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis. 2011;219(1):211–217. doi: 10.1016/j.atherosclerosis.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 69.Krichevsky A.M., Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13(1):39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun S.G., Zheng B., Han M., Fang X.M., Li H.X., Miao S.B., Su M., Han Y., Shi H.J., Wen J.K. miR-146a and Kruppel- like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Reports. 2011;12(1):56–62. doi: 10.1038/embor.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taganov K.D., Boldin M.P., Chang K.J., Baltimore D. NF-κB dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu G., Friggeri A., Yang Y., Park Y.J., Tsuruta Y., Abraham E. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci U S A. 2009;106(37):15819–15824. doi: 10.1073/pnas.0901216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yue J. miRNA and vascular cell movement. Adv Drug Deliv Rev. 2011;63(8):616–622. doi: 10.1016/j.addr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xin M., Small E.M., Sutherland L.B., Qi X., McAnally J., Plato C.F., Richardson J.A., Bassel-Duby R., Olson E.N. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23(18):2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng Y., Liu X., Yang J., Lin Y., Xu D.Z., Lu Q., Deitch E.A., Huo Y., Delphin E.S., Zhang C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105(2):155–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cordes K.R., Sheehy N.T., White M.P., Berry E.C., Morton S.U., Muth A.N., Lee T.H., Miano J.M., Ivey K.N., Srivastava D. miR- 145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460(7256):705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boettger T., Beetz N., Kostin S., Schneider J., Krüger M., Hein L., Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119(9):2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang X., Hu G., Zhou J. Repression of versican expression by microRNA-143. J Biol Chem. 2010;285:23241–23250. doi: 10.1074/jbc.M109.084673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan M.C., Hilyard A.C., Wu C., Davis B.N., Hill N.S., Lal A., Lieberman J., Lagna G., Hata A. Molecular basis for antagonism between PDGF and the TGFβ family of signaling pathways by control of miR-24 expression. EMBO J. 2010;29(3):559–573. doi: 10.1038/emboj.2009.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fiedler J., Jazbutyte V., Kirchmaier B.C., Gupta S.K., Lorenzen J., Hartmann D., Galuppo P., Kneitz S., Pena J.T., Sohn-Lee C., Loyer X., Soutschek J., Brand T., Tuschl T., Heineke J., Martin U., Schulte-Merker S., Ertl G., Engelhardt S., Bauersachs J., Thum T. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124:720–730. doi: 10.1161/CIRCULATIONAHA.111.039008. [DOI] [PubMed] [Google Scholar]
- 81.Zhu N., Zhang D., Chen S., Liu X., Lin L., Huang X., Guo Z., Liu J., Wang Y., Yuan W., Qin Y. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation andmigration. Atherosclerosis. 2011;215(2):286–293. doi: 10.1016/j.atherosclerosis.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 82.Nazari-Jahantigh M., Wei Y., Noels H., Akhtar S., Zhou Z., Koenen R.R., Heyll K., Gremse F., Kiessling F., Grommes J., Weber C., Schober A. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. 2012;122(11):4190–4202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Balderman J.A., Lee H.Y., Mahoney C.E., Handy D.E., White K., Annis S., Lebeche D., Hajjar R.J., Loscalzo J., Leopold J.A. Bone morphogenetic protein-2 decreases microRNA-30b and microRNA-30c to promote vascular smooth muscle cell calcification. J Am Heart Assoc. 2012;1(6):1225–1236. doi: 10.1161/JAHA.112.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leeper N.J., Raiesdana A., Kojima Y., Chun H.J., Azuma J., Maegdefessel L., Kundu R.K., Quertermous T., Tsao P.S., Spin J.M. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol. 2011;226(4):1035–1043. doi: 10.1002/jcp.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu X., Cheng Y., Chen X., Yang J., Xu L., Zhang C. MicroRNA-31 regulated by the extracellular regulated kinase is involved in vascular smooth muscle cell growth via large tumor suppressor homolog 2. J Biol Chem. 2011;286(49):42371–42380. doi: 10.1074/jbc.M111.261065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y., Wang Y., Wang X., Zhang Y., Eisner G.M., Asico L.D., Jose P.A., Zeng C. Insulin promotes vascular smooth muscle cell proliferation via microRNA-208-mediated downregulation of p21. J Hypertens. 2011;29(8):1560–1568. doi: 10.1097/HJH.0b013e328348ef8e. [DOI] [PubMed] [Google Scholar]
- 87.Ew1 Remus, Lyle A.N., Weiss D., Landàzuri N., Weber M., Searles C., Taylor W.R. miR181a protects against angiotensin II-induced osteopontin expression in vascular smooth muscle cells. Atherosclerosis. 2013;228(1):168–174. doi: 10.1016/j.atherosclerosis.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen J., Yin H., Jiang Y., Radhakrishnan S.K., Huang Z.P., Li J., Shi Z., Kilsdonk E.P., Gui Y., Wang D.Z., Zheng X.L. Induction of microRNA-1 by myocardin in smooth muscle cells inhibits cell proliferation. Arterioscler Thromb Vasc Biol. 2011;31(2):368–375. doi: 10.1161/ATVBAHA.110.218149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pedrioli D.M., Karpanen T., Dabouras V., Jurisic G., van de Hoek G., Shin J.W., Marino D., Kälin R.E., Leidel S., Cinelli P., Schulte-Merker S., Brändli A.W., Detmar M. miR-31 functions as a negative regulator of lymphatic vascular lineage-specific differentiation in vitro and vascular development in vivo. Mol Cell Biol. 2010;30(14):3620–3634. doi: 10.1128/MCB.00185-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu Y.H., Hu T.F., Chen Y.C., Tsai Y.N., Tsai Y.H., Cheng C.C., Wang H.W. The manipulation of miRNA-gene regulatory networks by KSHV induces endothelial cell motility. Blood. 2011;118(10):2896–2905. doi: 10.1182/blood-2011-01-330589. [DOI] [PubMed] [Google Scholar]
- 91.Dunworth W.P., Cardona-Costa J., Bozkulak E.C., Kim J.D., Meadows S., Fischer J.C., Wang Y., Cleaver O., Qyang Y., Ober E.A., Jin S.W. Bone morphogenetic protein 2 signaling negatively modulates lymphatic development in vertebrate embryos. Circ Res. 2014;114(1):56–66. doi: 10.1161/CIRCRESAHA.114.302452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grimaldo S., Yuen D., Theis J., Ng M., Ecoiffier T., Chen L. MicroRNA-184 regulates corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2015;56(12):7209–7213. doi: 10.1167/iovs.15-17733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu Q., Tong J.L., Zhang C.P., Xiao Q., Lin X.L., Xiao X.Y. miR-27a induced by colon cancer cells in HLECs promotes lymphangiogenesis by targeting SMAD4. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0186718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Keklikoglou I., Hosaka K., Bender C., Bott A., Koerner C., Mitra D., Will R., Woerner A., Muenstermann E., Wilhelm H., Cao Y., Wiemann S. MicroRNA-206 functions as a pleiotropic modulator of cell proliferation, invasion and lymphangiogenesis in pancreatic adenocarcinoma by targeting ANXA2 Aand KRAS genes. Oncogene. 2015;34(37):4867–4878. doi: 10.1038/onc.2014.408. [DOI] [PMC free article] [PubMed] [Google Scholar]