Abstract
Immune checkpoint inhibitors (ICPis) are a novel class of immunotherapeutic agents that have revolutionized the treatment of cancer; however, these drugs can also cause a unique spectrum of autoimmune toxicity. Autoimmune hemolytic anemia (AIHA) is a rare but often severe complication of ICPis. We identified 14 patients from 9 institutions across the US who developed ICPi-AIHA. The median interval from ICPi initiation to development of AIHA was 55 days (interquartile range [IQR], 22–110 days). Direct antiglobulin test (DAT) results were available for 13 of 14 patients: eight patients (62%) had a positive DAT and five (38%) had a negative DAT. The median pre-treatment and nadir hemoglobin concentrations were 11.8 g/dL (IQR, 10.2–12.9 g/dL) and 6.3 g/dL (IQR, 6.1–8.0 g/dL), respectively. Four patients (29%) had a pre-existing lymphoproliferative disorder, and two (14%) had a positive DAT prior to initiation of ICPi therapy. All patients were treated with glucocorticoids, with 3 requiring additional immunosuppressive therapy. Complete and partial recoveries of hemoglobin were achieved in 12 (86%) and 2 (14%) patients, respectively. Seven patients (50%) were re-challenged with ICPis, and one (14%) developed recurrent AIHA. Clinical and laboratory features of ICPi-AIHA were similar in DAT positive and negative patients. ICPi-AIHA shares many clinical features with primary AIHA; however, a unique aspect of ICPi-AIHA is a high incidence of DAT negativity. Glucocorticoids are an effective first-line treatment in the majority of patients with ICPi-AIHA, and most patients who are re-challenged with an ICPi do not appear to develop recurrence of AIHA.
Keywords: Immune checkpoint inhibitors, ipilimumab, nivolumab, pembrolizumab, autoimmune hemolytic anemia, direct antiglobulin test
INTRODUCTION
Immune checkpoint inhibitors (ICPis) have dramatically altered the landscape of cancer immunotherapy, providing a novel strategy to inhibit tumor growth and improve long-term outcomes across a wide spectrum of malignancies.1 The ICPi monoclonal antibodies target suppressor receptors located on the surface of immune cells, including anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed cell death protein 1 (PD-1), as well as receptors expressed by tumor cells, such as programmed cell death ligand 1 (PD-L1). ICPis thereby down-regulate native “breaks” on the immune system and promote an adaptive immune response. However, as predicted by mouse models,2,3 the activated T cells are not antigen-specific, and immune checkpoint blockade may result in the unwanted development of autoimmune disease, collectively referred to as immune related adverse events (IRAEs). Common IRAEs include rash, colitis, and endocrinopathies;4 however, other organ systems can be affected as well, including the liver,5 lungs,6 kidneys,7 nervous system,8 and heart.9 Less commonly, hematologic IRAEs have been described, and include immune thrombocytopenia,10 autoimmune neutropenia,11 pure red cell aplasia,12 and autoimmune hemolytic anemia (AIHA).13,14
AIHA is defined as antibody-mediated destruction of red blood cells (RBCs), with or without complement activation, leading to decreased RBC survival.15 The direct antiglobulin test (DAT), or Coombs test, confirms the presence of immunoglobulin and/or complement on the red cell surface and is central in the evaluation of patients with AIHA, though DAT negative AIHA is known to occur.16 Approximately 50% of AIHA cases develop secondary to an underlying condition or exposure such as autoimmune disease, lymphoproliferative disorders, or drugs. The other half are categorized as idiopathic, or primary AIHA.17,18
AIHA has been reported as a potential complication of ICPis, but existing data are limited to isolated case reports, small case series, and two recent database reviews that included only limited individual patient-level data.13,14 In addition to the paucity of reported cases, heterogeneity of clinical and laboratory data reported across studies has further hampered progress in understanding the clinical features of ICPi-AIHA. Further, no formal definition exists for ICPi-AIHA, and other than first-line treatment with glucocorticoids, there are no definitive recommendations for second-line therapy.19 Finally, the risk of recurrence of AIHA with ICPi re-challenge is unknown.
Here we present the largest series to date of ICPi-AIHA, with a focus on clinical characteristics, laboratory features, response to treatment, and AIHA recurrence rates with ICPi re-challenge.
MATERIALS AND METHODS
Overview
We contacted hematology and oncology departments at 18 major academic medical centers across the United States to inquire about potential cases of ICPi-AIHA. We identified 14 patients from 9 institutions with ICPi-AIHA. A list of institutions that provided cases is shown in Supplemental Table 1. All protocols were approved by Massachusetts General Hospital’s Institutional Review Board.
Definition of ICPi-AIHA
We defined ICPi-AIHA according the following criteria: 1) an abrupt decrease in hemoglobin ≥2g/dL; 2) at least two laboratory features of hemolysis (serum lactate dehydrogenase [LDH] above the upper limit of normal without an alternative explanation; elevated reticulocyte percentage or absolute count; low or undetectable serum haptoglobin; and presence of spherocytes on peripheral blood smear); 3) AIHA occurrence after initiation of an ICPi; 4) exclusion of other causes of anemia; and 5) ICPi therapy was considered the most likely etiology of AIHA by the treating hematologist or oncologist. We included both DAT positive and negative cases. Our proposed definition of ICPi-AIHA, and the definition used to identify patients for this study, is summarized in Table 1.
Table 1.
ICPi-AIHA Definitions and Outcomes.
| Definition of ICPi-AIHA (must include each of the following): | |
|---|---|
| 1. Abrupt decrease in Hgb ≥2g/dL | |
| 2. At least 2 of the following features of hemolysis: | |
| • LDH >ULN (without other explanation) | |
| • Elevated reticulocyte percentage or absolute count | |
| • Low or undetectable serum haptoglobin | |
| • Presence of spherocytes on peripheral blood smear | |
| 3. Development of AIHA after initiation of ICPi | |
| 4. Exclusion of other causes of anemia | |
| 5. ICPi considered by treating physician to be the most likely etiology of AIHA | |
|
| |
| Outcomes | |
|
| |
| Complete recovery of Hgb: | Increase in Hgb to within 0–1.0 g/dL of the pre-ICPi treatment value* |
| Partial recovery of Hgb: | Increase in Hgb to within 1.1–2.0 g/dL of the pre-ICPi treatment value* |
| Complete remission from AIHA: | Increase in Hgb to within 0–1.0 g/dL of the pre-ICPi treatment value* in the absence of immunosuppression or ongoing hemolysis |
Without pRBC transfusion in the preceding 2 weeks. Abbreviations: AIHA, autoimmune hemolytic anemia; Hgb, hemoglobin; ICPi, immune checkpoint inhibitor; pRBC, packed red blood cell; ULN, upper limit of normal.
Data Collection
We collected the following patient data: age; gender; race; past medical history, including history of autoimmune disease; type of malignancy; ICPi(s) used and dosing regimen; prior chemotherapeutic regimens; other IRAEs; baseline hemoglobin prior to ICPi treatment (pre-treatment hemoglobin), nadir, and time to recovery; reticulocyte baseline and peak; LDH baseline and peak; presence of spherocytes on peripheral blood smear; DAT results with type of antibody detected and/or complement protein and strength; requirement for packed red blood cell (pRBC) transfusion and erythropoiesis-stimulating agents; treatment for AIHA, including discontinuation of ICPis, initiation, dosing, and duration of glucocorticoids, and requirement for other immunosuppressive agents; response of malignancy to ICPi; and recurrence of AIHA or other IRAEs if the patient was re-challenged with an ICPi. LDH values were normalized to the reference range at Massachusetts General Hospital (110–210 U/L) in order to standardize data across institutions.
Definitions of hemoglobin recovery and remission from AIHA
Consensus-based definitions for hemoglobin recovery following an episode of AIHA do not exist. We defined complete and partial hemoglobin recovery as an increase in the hemoglobin concentration to within 0–1.0 and 1.1–2.0 g/dL of the pre-treatment value, respectively, and without any pRBC transfusions during the preceding 2 weeks. Patients on any amount of immunosuppression could achieve a complete or partial hemoglobin recovery. We also defined a third outcome variable, complete remission from AIHA, as an increase in the hemoglobin concentration to within 0–1.0 g/dL of the pre-treatment value in the absence of immunosuppression, ongoing hemolysis, or requirement for pRBC transfusion during the preceding 2 weeks (Table 1).
Reported cases of ICPi-AIHA
We searched Pubmed and Google Scholar (with the last search performed on December 14, 2018) for reported cases of ICPi-AIHA using the following terms: 1) autoimmune hemolytic (haemolytic) anemia; AIHA; Evans syndrome; cytopenias; immunohematological (immunohaematological); 2) immune checkpoint inhibitor; immunotherapy; immune related adverse events; cytotoxic T-lymphocyte associated protein 4 (CTLA-4); programmed cell death protein 1 (PD-1); programmed cell death ligand 1 (PDL-1); 3) ipilimumab; nivolumab; pembrolizumab; atezolizumab; avelumab; durvalumab.
Statistical analyses
We performed the statistical analyses with SAS Version 9.4 (Cary, NC). Summary data are presented as median and 25th–75th interquartile range (IQR). We compared clinical and laboratory characteristics between DAT positive versus DAT negative patients using the Wilcoxon rank-sum and Fisher’s exact tests for continuous and categoric variables, respectively. All comparisons are two-tailed, with P<0.05 considered significant.
RESULTS
Baseline characteristics
Baseline characteristics of the 14 patients with ICPi-AIHA are summarized in Table 2. The median age was 65 years (IQR, 50–69 years). Seven patients (50%) were male. Melanoma was the most common malignancy (n = 9). Other malignancies included non-small cell lung cancer (n = 3), colorectal cancer (n = 1), and acute myelogenous leukemia (AML, n = 1). All patients had metastatic disease except for patient 9, who was treated with ICPi therapy after standard chemotherapy for AML as part of a clinical trial.
Table 2.
Baseline Characteristics.
| Pt | Age/Sex | Malignancy | Prior therapy | Comorbidities | ICPi regimen (and # of cycles) prior to AIHA |
|---|---|---|---|---|---|
| 1 | 85M | Melanoma | None | MZL (not treated), DM II | Pembro 2mg/kg Q3 weeks (2) |
| 2 | 48M | Melanoma | None | None | Ipi 3mg/kg + nivo 1mg/kg both Q3 weeks (3) |
| 3 | 67F | Melanoma | None | None | Ipi 3mg/kg + nivo 1mg/kg both Q3 weeks (4) |
| 4 | 68M | Melanoma | None | Leukopeniaa | Pembro 2mg/kg/day Q3 weeks (12)b |
| 5 | 18M | Melanoma | None | None | Ipi 3mg/kg +nivo 1mg/kg Q3 weeks (4) |
| 6 | 47M | Melanoma | pINF, IL-2 with TILs and TBI | HTN, HLD | Ipi 3mg/kg Q3 weeks (1) |
| 7 | 59F | NSCLC | Multiple agentsc | MZL (in remission) | Nivo 3mg/kg Q2 weeks (12) |
| 8 | 63F | NSCLC | Carboplatin/paclitaxel with XRT | CLL (not treated), DM II | Nivo 3mg/kg Q2 weeks (4) |
| 9 | 33F | AMLd | FLAG-IDA | None | Nivo 3mg/kg Q2 weeks (1) |
| 10 | 85F | Melanoma | None | DM II, breast cancer | Pembro 2mg/kg Q3 weeks (10) |
| 11 | 69F | Colorectal | Multiple agentse | HTN | Pembro 200mg Q3 weeks (1)f |
| 12 | 67M | Melanoma | None | DVT | Ipi 2mg/kg + nivo 1mg/kg both Q3 weeks (2) |
| 13 | 55M | Melanoma | Multiple agentsg | CLL, ITP | Pembro 2mg/kg Q3 weeks (1) |
| 14 | 71F | NSCLC | None | None | Pembro 200mg Q3 weeks (4) |
Long-standing history of leukopenia of unclear etiology, bone marrow biopsy negative for malignancy and leukopenia believed to be autoimmune in nature.
Received indoximod in combination with ICPi therapy as part of a clinical trial.
R-CHOP, fludarabine (received >3 years prior to development of AIHA), XRT, Auto-SCT for treatment of MZL, cisplatin and etoposide for treatment of NSCLC.
Received nivolumab on a clinical trial after conventional chemotherapy.
5-FU, oxaliplatin, irinotecan, bevacizumab, panitumumab, regorafenib, trifluridine/tipiracil.
Received GVAX and cyclophosphamide in combination with ICPi therapy as part of a clinical trial
Dabrafenib, trametinib for treatment of melanoma, fludarabine (received >3 years prior to development of AIHA), cyclophosphamide, bendamustine, rituximab, obinutuzumab, and ibrutinib for treatment of CLL.
Abbreviations: 5-FU, 5-fluorouracil; AML, acute myelogenous leukemia; Auto-SCT, autologous stem cell transplant; CLL, chronic lymphocytic leukemia; DM II, type II diabetes mellitus; DVT, deep venous thrombosis; FLAG-IDA, fludarabine, cytarabine, granulocyte colony stimulating factor, and idarubicin; GVAX, granulocyte-macrophage colony-stimulating factor allogeneic cancer vaccine; HLD, hyperlipidemia; HTN, hypertension; ICPi, immune checkpoint inhibitor; IL-2, interleukin 2; Ipi, ipilimumab; ITP, immune thrombocytopenia; MZL, marginal zone lymphoma; nivo, nivolumab; NSCLC, non-small cell lung cancer; Pembro, pembrolizumab; pIFN, pegylated interferon; R-CHOP, rituximab, cyclophosphamide, doxorubicin (Hydroxydaunomycin), vincristine (Oncovin), prednisone; TBI, total body irradiation; TILs, tumor infiltrating lymphocytes; XRT, radiation therapy.
Four patients (29%) had a pre-existing diagnosis of a lymphoproliferative disorder (patients 8 and 13 had chronic lymphocytic leukemia [CLL] and patients 1 and 7 had marginal zone lymphoma). Both patients with CLL were known to have a positive DAT prior to initiation of ICPi therapy. Patient 4 had a long-standing history of leukopenia that was considered to be autoimmune in nature. This patient also received indoximod, an investigational agent that maintains tryptophan levels and enhances T cell activity, in conjunction with ICPi therapy as part of a clinical trial. Patient 6 received immune-based therapy with pegylated interferon, interleukin 2, and tumor infiltrating lymphocytes three months prior to receipt of ICPi therapy. Patients 7 and 13 had previously received fludarabine, a well-described cause of drug-induced AIHA, for marginal zone lymphoma and chronic lymphocytic leukemia, respectively. In both patients, fludarabine therapy was completed more than 3 years prior to initiation of ICPi therapy. Importantly, none of the 14 patients, including those with known positive DATs, had evidence of active hemolysis at the time of ICPi initiation.
ICPi regimens consisted of pembrolizumab alone (n = 6), ipilimumab in combination with nivolumab (n = 4), nivolumab alone (n = 3), and ipilimumab alone (n = 1). Dosing regimens are shown in Table 2. Concomitant medications administered at the time that ICPi-AIHA was diagnosed are listed in Supplemental Table 2. None of these medications were deemed to be the cause of AIHA by the patient’s primary hematologist/oncologist.
Clinical and laboratory features of ICPi-AIHA
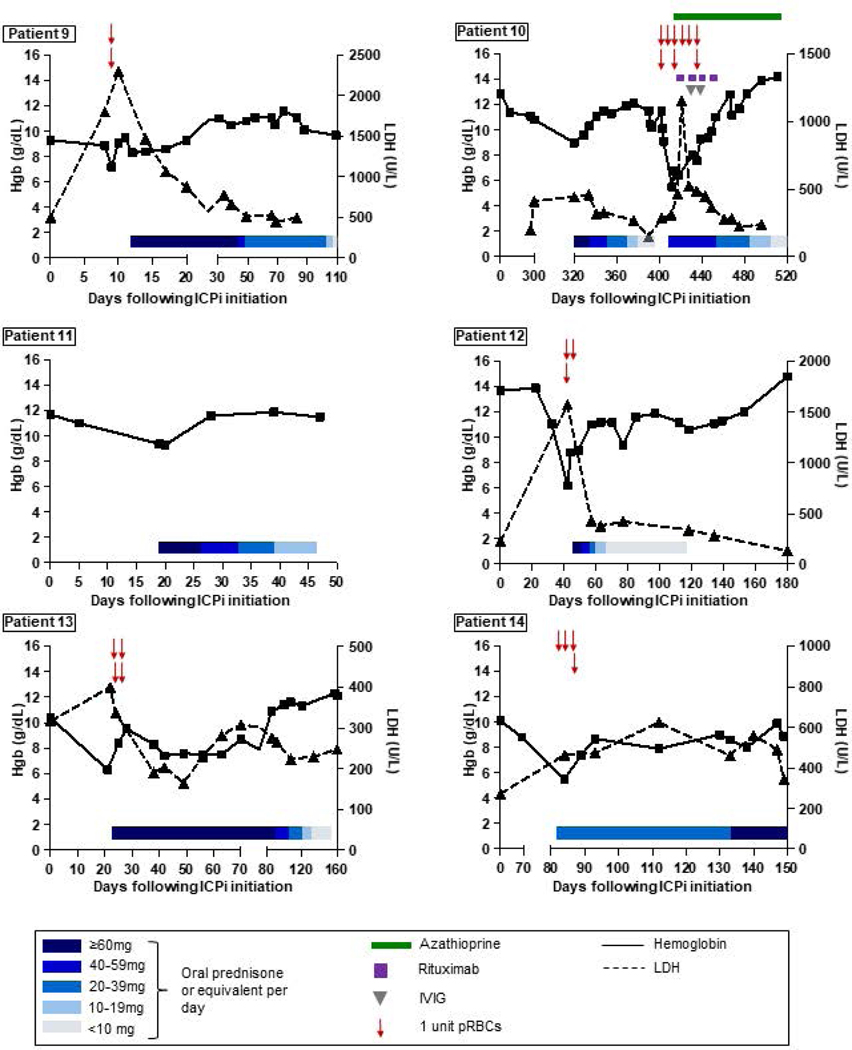
Clinical and laboratory features of ICPi-AIHA are shown in Table 3. The median interval from initiation of ICPi therapy to AIHA was 55 days (IQR, 22–100 days; overall range, 9–377 days). The median interval from the most recent dose of ICPi to AIHA was 21 days (IQR, 15–24 days; overall range, 5–70 days). The median pre-treatment and nadir hemoglobin concentrations were 11.8 g/dL (IQR, 10.2–12.9 g/dL) and 6.3 g/dL (IQR, 6.1–8.0 g/dL), respectively, and the median peak LDH was 743 U/L (IQR, 524–862 U/L). Hemoglobin and LDH trends for each patient are shown in Figure 1.
Table 3.
Clinical Features of ICPi-AIHA
| Pt | Day of AIHAa | Days since last dose of ICPi | Hgb (g/dL) | LDH peakb (U/L) | Peak retic (%) | Hapto (mg/dL) | Sphero | WBC (x109) | Plt (x109) | DAT | Transfusion requirement | Other IRAEs | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| PT | Nadir | Δ | ||||||||||||
| 1 | 30 | 9 | 12.8 | 8.2 | 4.6 | 885 | 3.3 | UD | Yes | 4.4 | 69c | Neg | pRBCs x4 | ITP |
| 2 | 84 | 42 | 7.2d | 3.5 | 3.7 | 770 | 11.1 | UD | Yes | 4.4 | 279 | IgG (1+), C3 (1+) | pRBCs x4 | None |
| 3 | 119 | 70 | 13.2 | 8.7 | 4.5 | 426 | 8.8 | UD | Yes | 10.6 | 202 | IgG (1+), C3 neg | None | Hepatitis |
| 4 | 265 | 22 | 13.6 | 8.5 | 5.1 | 530 | 4.7 | UD | No | 4.4 | 286 | IgG (2+), C3 neg | None | None |
| 5 | 25 | 25 | 12.4 | 6.2 | 6.2 | 784 | <0.5 | UD | Yese | 4.6 | 344 | Neg | pRBCs x8 | Pyrexia, PRCAf |
| 6 | 13 | 13 | 9.5 | 6.2 | 3.3 | 522g | 3.2 | UD | NA | 3.5 | 71h | NA | pRBCs x2 | None |
| 7 | 377 | 48 | 11.9 | 6 | 5.9 | 505 | 16.8 | UD | Yes | 10.7 | 211 | IgG weak, C3 (2+)i | pRBCs x6 | None |
| 8 | 68 | 5 | 11.7 | 4 | 7.7 | 976 | 14.8 | UD | NA | 38.3 | 127 | IgG3(+), C3 neg | pRBCs x10 | None |
| 9 | 9 | 9 | 9.3 | 7.2 | 2.1 | 791 | 4.9 | UD | Yes | 2.1 | 30 | Neg | pRBCs x2 | ITP |
| 10 | 320 | 21 | 12.9 | 6.4 | 6.5 | 1151 | 6.4 | UD | NA | 13.2 | 347 | IgG weak+, C3 neg; Recurrence: IgG (3+), C3 (1+) | pRBCs x9 | Rash, hypothyroidism |
| 11 | 19 | 19 | 11.7 | 9.4 | 2.3 | 716 | 4.3 | 7 | Yes | 12.3 | 265 | Polyspecific weak | None | None |
| 12 | 42 | 20 | 13.7 | 6.2 | 7.5 | 1574 | 8.5 | UD | No | 16.2 | 200 | Neg | pRBCs x3 | Colitis |
| 13 | 21 | 21 | 10.4 | 6.3 | 4.1 | 399 | 0.3 | 2 | No | 14.5 | 4j | IgG weak+, C3 (2+) | pRBCs x4 | None |
| 14 | 84 | 22 | 10.1 | 5.5 | 4.6 | 623 | 0.3 | UD | Yes | 5.6 | 422k | Neg | pRBCs x4* | ITP; AKI; hepatitis |
| M | 55 | 21 | 11.8 | 6.3 | 4.6 | 743 | 5 | 8.1 | 207 | 4 | ||||
| IQR | 22–110 | 15–24 | 10–13 | 6–8 | 4–6 | 524–862 | 3.3–8.8 | 4.4–13.0 | 85–284 | 2–6 | ||||
Number of days between the first ICPi dose and AIHA onset.
LDH values were normalized to the reference range at Massachusetts General Hospital (110–210 U/L).
BL platelet count 125×109.
BL anemia reported after hemorrhage during recent orthopedic surgery.
Patient had two episodes of AIHA, spherocytes were only reported on the second AIHA episode.
Based on an undetectable reticulocyte count, this patient was suspected of having a concurrent PRCA; however, due to a decline in clinical status, a BMBx was not performed to confirm the diagnosis.
Only one LDH value for this patient.
BL platelet count 70–100 ×109.
Cold agglutinin titer and thermal amplitude negative; 14 of the DATs were positive for C3 (2+) and negative for IgG, and one was weakly positive for IgG and negative for C3.
Patient had a known diagnosis of ITP related to CLL with BL platelet count 4–30×109; platelet count improved to 170 ×109 with glucocorticoids given for ICPi-AIHA.
Within 4 weeks of AIHA, platelet count dropped to 7×109.
Abbreviations: AIHA, autoimmune hemolytic anemia; AKI, acute kidney injury; BMBx, bone marrow biopsy; CLL, chronic lymphocytic leukemia; DAT, direct antiglobulin test; Hapto, haptoglobin; Hgb, hemoglobin; IQR, interquartile range; IRAE, immune related adverse events; ITP, immune thrombocytopenia; LDH, lactate dehydrogenase; M, median; NA, not available; Neg, negative; pembro, pembrolizumab; Plt, platelet count; pRBCs, packed red blood cells; PRCA, pure red cell aplasia; PT, pre-treatment; retic, reticulocyte percentage; RP, retroperitoneal; Sphero, spherocytes; UD, undetectable; WBC, white blood cell count.
Figure 1. Hemoglobin and LDH trends, and response to treatment.
The X axes represent the number of days following initiation of ICPi therapy (with day 0 indicating the first day of ICPi administration). The Y axes on each graph represent hemoglobin (left) and LDH concentrations (right). Hemoglobin and LDH trends are depicted with solid and dashed lines, respectively. Blue rectangles indicate steroid tapers. Red arrows represent pRBC transfusions. Green rectangles represent azathioprine. Purple squares represent rituximab infusions. Gray triangles represent IVIG infusions. Abbreviations: ICPi, immune checkpoint inhibitor; IVIG, intravenous immune globulin; LDH, lactate dehydrogenase; pRBCs, packed red blood cells.
Thirteen patients had a DAT assessed: eight (62%) were positive and five (38%) were negative. All patients had a low (n = 2) or undetectable (n = 12) serum haptoglobin. The peak reticulocyte count was variable, with some patients mounting an appropriate increase in reticulocytes and others presenting with reticulocytopenia.20 Hematinic deficiencies (e.g., iron, vitamin B12, and folate) as a cause of anemia were excluded in all patients. Transfusion of pRBCs was required in eleven (79%) patients, and the median number of units transfused was 4 (IQR, 2–6). Patient 7 received a single dose of darbepoetin 300mcg subcutaneously while hospitalized. No other patient received erythropoiesis-stimulating agents.
Treatment
The regimens used to treat ICPi-AIHA are shown in Table 4. ICPi therapy was discontinued or held in 11 (79%) patients. All patients were initially treated with glucocorticoids. Five patients (36%) were given intravenous glucocorticoids and then transitioned to oral therapy. Three patients (1, 7, and 10) required additional immunosuppressive treatment for AIHA relapse upon glucocorticoid withdrawal: rituximab (patient 1); rituximab and intravenous immune globulin (IVIG; patient 7); and rituximab, IVIG, and azathioprine (patient 10).
Table 4. Treatment and Outcomes.
Complete hemoglobin recovery (cHR) and partial hemoglobin recovery (pHR) were defined as hemoglobin within 1g/dL and 2g/dL of the pre-treatment value, respectively, and without RBC transfusion within the preceding 2 weeks. Patients on any amount of immunosuppression could still achieve a cHR or pHR. Complete remission from AIHA (CR-AIHA) was defined as hemoglobin within 1g/dL of the pre-treatment value in the absence of immunosuppression and without features of ongoing hemolysis or red blood cell transfusion within the preceding 2 weeks.
| Pt | Treatment | Hgb recovery | CR-AIHA | Time to Hgb recovery (days) | ICPi initially held? | Re-challenged with ICPi? | IRAE with retreatment? | Response of malignancy to initial ICPia |
|---|---|---|---|---|---|---|---|---|
| 1 | Pred 1mg/kg PO QD tapered over 2 months, ritux 375mg/m2/week IV x4 | cHR | Y | 122 | Y | N | N/A | Partial response, progression in months |
| 2 | MP 100–200mg IV x4 doses, Pred 60mg PO QD tapered over 25 days | cHR | Y | 21 | Y | N | N/A | Partial response |
| 3 | Pred 60mg PO QD tapered over 5 weeks | cHR | Y | 32 | Y | Nivo | N | Complete response |
| 4 | Pred 70mg PO QD tapered over 8 weeks | cHR | Y | 47 | Y | Nivo | Nb | Complete response, recurrence in months |
| 5 | MP 100mg IV x1, Pred 60mg PO QD tapered over 2 weeks then restarted at 60mg PO QD due to pyrexia | cHR | N | 56 | N | Continued on Ipi/Nivo x4 cycles, then Nivo maintenance | AIHA, pyrexia | Stable disease, progression in months |
| 6 | Dex 10mg IV BID tapered over 7 weeks, continued on dex 6mg PO QD for brain metastases | pHR | N | 29 | N | Continued on Ipi | AIHA | Progression |
| 7 | 1. Pred 100mg PO QD tapered over 6 months and IVIG 0.5g/kg QD x2 days 2. Relapse: Pred 60mg PO QD tapered over 4 months, ritux 375mg/m2/week IV x4, IVIG 1g/kg/day x2 days |
cHR | N | 44 | Y | N | N/A | Partial response |
| 8 | MP 60mg IV x1, prednisone 60mg PO QD tapered over 2.5 months | pHR | N | 160 | Y | N | N/A | Stable disease |
| 9 | MP 1.25mg/kg IV QD x3, pred 1.5mg/kg PO QD tapered over 3.5 months | cHR | Y | 18 | Y | N | N/A | Not applicable (ICPi given as adjuvant treatment) |
| 10 | 1. Pred 60mg PO QD tapered over 2.5 months 2. Relapse: Pred 50mg PO QD tapered over 3.5 months, IVIG 1g/kg x2, ritux 375mg/m2 IV x4, azathioprine 150mg PO QD |
cHR | N | 49 | Y | N | N/A | Partial response |
| 11 | Pred 60mg PO QD tapered over 4 weeks | cHR | Y | 9 | Y | N | N/A | Partial response, progression in weeks |
| 12 | Dex 12mg IV x1, pred 1mg/kg PO QD tapered over 10 weeks | cHR | Y | 138 | Y | Ipi/Nivo held x2 cycles then continued | N | Progression |
| 13 | Pred 1mg/kg PO QD tapered over 4.5 months | cHR | Y | 64 | Y | Pembro | N | Progression |
| 14 | Dex 4mg PO BID tapered over 1 month | cHR | N | 46 | N | Continued on Pembro | ITP, AKI, hepatitis | Partial response |
| M (IQR) | 47 (30–62) |
As defined by the treating clinician.
This patient did not have a recurrence of ICPi-AIHA when treated re-challenged with nivolumab alone. However, after 8 months of single-agent nivolumab, the patient’s underlying malignancy recurred. The patient was subsequently treated with combination ipilimumab/nivolumab and experienced recurrent ICPi-AIHA within one week of treatment. ICPi-AIHA was refractory to prednisone and IVIG, though did respond to rituximab. As a result, ICPis were permanently discontinued.
Abbreviations: AKI, acute kidney injury; BID, twice per day; Dex, dexamethasone; Hgb, hemoglobin; ICPi, immune checkpoint inhibitor; Ipi, ipilimumab; IQR, intraquartile range; IRAEs, immune related adverse events; ITP, immune thrombocytopenia; IV, intravenous; IVIG, intravenous immune globulin; M; median; MP, methylprednisolone; N, no; N/a, not applicable; Nivo, nivolumab; Pembro, pembrolizumab; PO, by mouth; Pred, prednisone; QD, once/day; Ritux, rituximab; Y, yes.
Response to treatment
Response to treatment is shown in Table 4 and Figure 1. A complete and partial hemoglobin recovery was achieved in 12 (86%) and 2 (14%) patients, respectively. Among the 12 patients who achieved a complete hemoglobin recovery, the median interval from hemoglobin nadir to recovery was 47 days (IQR, 29–58 days). A complete remission of AIHA was achieved in eight (57%) patients, and the median interval from hemoglobin nadir to complete remission of AIHA was 89 days (IQR, 34–130 days). Seven patients (50%) experienced at least one other IRAE, including 3 patients (1, 9, and 14) with ICPi-associated immune thrombocytopenia (ITP), and one patient (patient 5) with suspected ICPi-associated pure red cell aplasia (PRCA). In patient 9, ITP occurred simultaneously with ICPi-AIHA. In patients 1 and 14, ITP occurred within 6 weeks following the diagnosis of ICPi-AIHA. In patient 5, PRCA occurred concurrently with ICPi-AIHA.
AIHA recurrence with re-challenge
Seven patients (50%) either continued on or were re-challenged with ICPi therapy after the diagnosis of AIHA. Patients 3 and 4 were re-challenged with nivolumab after complete hemoglobin recovery, and neither had a recurrence of AIHA. Patient 5, who initially received ipilimumab and nivolumab, resumed nivolumab alone as maintenance therapy with concurrent glucocorticoids for nausea and brain lesions; although the patient’s hemoglobin initially stabilized, AIHA recurred within four months and ICPi therapy was permanently discontinued. Patient 6 continued to receive ipilimumab, however, this patient was also maintained on glucocorticoids for brain lesions. In patient 12, ipilimumab and nivolumab were held for two cycles, and were then resumed while the patient remained on a steroid taper; AIHA did not recur. In patient 13, pembrolizumab was held for five months and was subsequently resumed without recurrence of AIHA. Patient 14 continued on pembrolizumab for 2 additional cycles after the diagnosis of AIHA, but ICPi was permanently discontinued after the development of ITP, nephritis, and hepatitis. Altogether, six of seven (86%) patients who were re-challenged with ICPi did not develop recurrent AIHA, and five of seven (71%) patients who were re-challenged with ICPi did not develop any IRAE.
Clinical and laboratory characteristics of published cases of ICPi-AIHA
We identified 17 published cases of ICPi-AIHA.12,14,21–33 The clinical and laboratory features of ICPi-AIHA in these 17 cases are summarized in Supplemental Table 3. Notably, 14 (82%) patients were DAT positive and 3 (18%) were DAT negative. All 17 patients received glucocorticoids, and 15 (88%) responded. Two (12%) patients required additional immunosuppressive treatment with rituximab, IVIG, and other agents (Supplemental Table 3).12,28 Five (29%) patients were re-challenged with an ICPi, and only one had recurrence of ICPi-AIHA.21 Two patients died due to complications of AIHA.14,24 We also identified one case of ICPi-associated cold agglutinin disease,34 but did not include this patient in our analysis.
Summary of current and published cases of ICPi-AIHA analyzed in aggregate
Finally, we examined the characteristics of all 31 cases of ICPi-AIHA (14 patients from the current series and 17 previously reported patients) analyzed in aggregate (Supplemental Figure 1). The number of cycles of ICPis administered prior to development of AIHA ranged from 1 to 39, with 21 (68%) patients developing ICPi-AIHA after administration of 1 to 4 cycles (Supplemental Figure 1A). Overall, 8 of 30 patients (27%) tested were DAT negative. DAT positive patients had a similar median hemoglobin nadir (Supplemental Figure 1B), LDH peak (Supplemental Figure 1C), and requirement for additional immunosuppression beyond glucocorticoids (Supplemental Figure 1D) compared to DAT negative patients.
DISCUSSION
We present the detailed clinical and laboratory characteristics of ICPi-AIHA that occurred in 14 patients from 9 institutions across the US. The median interval from ICPi initiation to development of AIHA was 55 days (IQR, 22–110 days). DAT results were positive in eight (62%) and negative in five (38%) patients; one patient was not tested. The median pre-treatment and nadir hemoglobin concentrations were 11.8 g/dL (IQR, 10.2–12.9 g/dL) and 6.3 g/dL (IQR, 6.1–8.0 g/dL), respectively. All patients were treated with glucocorticoids, and all had a complete or partial hemoglobin recovery, with eight (57%) patients achieving a complete remission from AIHA. Six patients remained on immunosuppression, either for treatment of ICPi-AIHA or for other co-morbid conditions. Seven patients were re-challenged with an ICPi, and only one developed recurrent hemolysis. We also identified 17 previously published cases of ICPi-AIHA: we summarized the key findings from each case, and we performed analyses in aggregate with the 14 patients in the current study to identify clinical and laboratory patterns. We found that all patients received glucocorticoids as first-line treatment, that the severity of hemolysis observed is similar in DAT positive and negative patients, and that ICPi-AIHA can be a fatal condition, with two of the 17 previously published cases ending in death. Finally, we developed a standardized set of definitions for ICPi-AIHA and related outcomes, which we propose could be used in future studies of ICPi-AIHA.
The current study is consistent with and expands upon prior descriptions of ICPi-AIHA, which are mainly limited to isolated case reports and two database reviews. A recent publication identified 68 cases of AIHA associated with ICPis that were reported in the FDA Adverse Events Reporting System database.14 The authors concluded that the incidence of ICPi-AIHA is low, affecting fewer than 1% of all patients treated with ICPis. However, this report did not include detailed data on the time course of AIHA in relation to ICPis, serologic work-up of AIHA, including DAT status, treatment regimens, response to treatment, or recurrence rates of AIHA upon re-challenge with ICPis. A separate recent report that queried three French pharmacovigilance databases identified 35 patients with hematologic IRAEs, including 9 patients with ICPi-AIHA.13 All 9 patients were DAT positive, 2 had a previous diagnosis of CLL, 4 responded to glucocorticoids alone, and 5 required second-line treatment with rituximab. However, no patients with DAT-negative AIHA were included. Further, longitudinal hemoglobin and LDH trends were not provided, and only one patient was re-challenged with an ICPi (this patient did not have AIHA recurrence). These database reports are useful in illuminating this new phenomenon, but supporting details are lacking. Our study complements and expands on these previous studies by providing a nuanced description of the clinical and laboratory features of ICPi-AIHA, and by including DAT negative cases and a larger number of patients who were re-challenged with ICPis.
This observational study was not designed to elucidate the mechanisms of ICPi-AIHA, for which detailed laboratory correlates are required. However, we may speculate on potential mechanisms of ICPi-AIHA stemming from our understanding of the pathophysiology of primary AIHA. Pathways involved in primary AIHA include production of abnormal T and B cell clones, aberrant cytokine expression, lack of effective self-antigen presentation, molecular mimicry, and altered levels of complement proteins.35,36 This failure of immune surveillance leads to identification of RBCs as foreign, with subsequent Fc receptor-mediated phagocytosis by splenic and hepatic macrophages, or direct lysis via the complement cascade.36 ICPis, which lead to “reprogramming” of the immune system, may result in a similar loss of tolerance against endogenous RBC antigens, and thus the mechanism of AIHA could be similar to other forms of drug-induced AIHA.37,38
Interestingly, we observed that 4 of the 14 patients in our series experienced other hematologic IRAEs in addition to AIHA. This finding is reminiscent of observations demonstrating the co-occurrence of ICPi-associated myocarditis and myositis, which are speculated to involve shared, muscle-specific antigens.39,40 Notably, outside of these two examples (hematologic and muscle-specific), no obvious patterns involving other organ or tissue-specific concurrent IRAEs have been reported, even in large pharmacovigilance studies.41 It is also noteworthy that four (29%) of the patients in the current series and 2 of the 9 patients (22%) in the French pharmacovigilance database study13 had an underlying lymphoproliferative disorder, suggesting that patients with baseline immune dysfunction may be predisposed to developing ICPi-AIHA.
Although only 3–11% of primary AIHA cases are DAT negative,42 we found that 38% of the patients in our series were DAT negative. Four of the five patients in our series who had a negative DAT had spherocytes on peripheral blood smear, which is highly suggestive of splenic immune-mediated RBC destruction, and all responded to treatment with glucocorticoids. What accounts for the high proportion of DAT-negativity in our series? One possibility is that the negative DATs were actually false negatives due to technical reasons. This can occur due to a low concentration of IgG molecules deposited on the RBC surface, which may be below the detection threshold of a standard DAT; removal of low-affinity IgG molecules by washing during DAT processing; failure of the Coombs reagent to crosslink IgG on the RBC surface; or, rarely, AIHA that is due to IgA or IgM (without complement fixation), since standard DAT techniques only detect IgG and complement.42 Advanced techniques, also known as “Super-Coombs” testing, include flow cytometry, use of 4⁰C low-ionic-strength saline wash, and use of anti-IgA and IgM reagents.17,43 These techniques should be performed by specialized laboratories when the clinical suspicion for AIHA is high despite a negative DAT. No patients in our series underwent “Super-Coombs” testing.
It is also plausible that DAT negative and DAT positive ICPi-AIHA represent two distinct pathological entities. DAT-negative cases may reflect direct macrophage phagocytosis of the RBCs without need for antibody, akin to macrophage-mediated clearance of RBCs that have sustained irreparable damage.44 The engulfment of RBCs by macrophages, which themselves express PD-1,45 could be a consequence of a pro-inflammatory state induced by ICPis, akin to hemophagocytic lymphohistiocytosis in which high circulating levels of interferon gamma and other activating cytokines result in macrophage engulfment of RBCs.46 Laboratory studies are clearly needed to characterize the distinctions between DAT positive and negative cases of ICPi-AIHA. Importantly, all of the patients in our study, regardless of DAT status, had at least an initial improvement in hemoglobin with administration of glucocorticoids, and the severity of hemolysis did not appear to differ on the basis of DAT status.
Given the relative rarity of ICPi-AIHA, treatment guidelines for this condition are still evolving. The American Society of Oncology recommends prednisone (or its equivalent) at a dose of 0.5–1 mg/kg per day as first-line treatment for ICPi-AIHA, with permanent discontinuation of ICPi therapy if the hemoglobin falls below 8.0 g/dL.19 However, these guidelines fail to take into account the patient’s pre-treatment hemoglobin concentration, which may be low as a consequence of their underlying malignancy and previous treatment. Accordingly, we defined ICPi-AIHA according to relative declines in hemoglobin concentration from the pre-treatment value, along with other criteria consistent with hemolysis (Table 1). For patients who fail glucocorticoids, second-line therapies include rituximab, IVIG, cyclosporin A, azathioprine, and mycophenolate mofetil; however, the ideal second-line agent is unknown.19 In our series, three patients were treated with rituximab, IVIG, and/or azathioprine, and all three had a complete hemoglobin recovery. Finally, glucocorticoid-sparing agents may be a particularly attractive option given the concern that glucocorticoids may mitigate the anti-tumor effect of ICPis.47 Two non-glucocorticoid investigational agents are in clinical trials for treatment of primary AIHA: fostamatinib, a spleen tyrosine kinase inhibitor (NCT02612558), and an antibody to the neonatal Fc Receptor (NCT03075878). These agents may prove useful in ICPi-AIHA as well.
Our study has several strengths. We conducted the largest study of ICPi-AIHA to date that includes highly granular patient-level data, including detailed clinical, laboratory, treatment, and outcomes data. By collecting cases from large academic medical centers across the US, we were able to showcase various management strategies of ICPi-AIHA by a wide range of clinicians. We did not limit our inclusion criteria to patients with DAT positive AIHA, thus allowing for an unbiased description of this newly-identified phenomenon. We speculate that the larger percentage of DAT negative cases of ICPi-AIHA in the current study (38%) compared to published cases (18%) could reflect publication bias. Finally, we developed a standardized set of definitions for ICPi-AIHA and related outcomes, which we propose could be used in future studies of ICPi-AIHA.
We also acknowledge several limitations, including observational design, retrospective collection of data, and absence of advanced techniques such as Super-Coombs testing in the DAT-negative cases. Further, the number of patients with ICPi-AIHA that we identified was modest, and we were therefore unable to perform multivariable-adjusted analyses to determine prognostic factors for hemoglobin recovery in patients with ICPi-AIHA. We were also unable to determine the precise incidence of ICPi-AIHA, although we estimate it to be less than 0.1% based on the 14 cases we identified from 20 large academic cancer centers that have cumulatively treated tens of thousands of patients with ICPis. Finally, we did not include a control group of patients treated with ICPis who did not develop AIHA, and thus we were unable to determine risk factors for ICPi-AIHA.
In conclusion, ICPi-AIHA is a rare but often severe complication of ICPi therapy. As the use of ICPis becomes more widespread, clinicians will likely encounter ICPi-AIHA with increasing frequency. ICPi-AIHA shares many clinical features with primary AIHA. However, a unique aspect of ICPi-AIHA includes a high incidence of DAT negativity. Thus, clinicians should maintain a high index of suspicion for AIHA in patients being treated with ICPis, even in the absence of a positive DAT. Glucocorticoids are an effective first-line treatment strategy for most patients with ICPi-AIHA, though 3 of the patients in our series required additional immunosuppressive therapy. Finally, the majority of patients in our series who were re-challenged with ICPis did not develop recurrence of AIHA. Thus, development of ICPi-AIHA should not necessarily prompt permanent discontinuation of ICPis, particularly in patients who have limited alternative treatment options. Future studies should be conducted to explore the risk factors, pathophysiology, and optimal treatment strategies for ICPi-AIHA.
Supplementary Material
Acknowledgements
The authors thank Reed Drews, M.D. (Beth Israel Deaconess Medical Center) and George Karp, M.D. (Regional Cancer Care Associates) for their critical review of the manuscript.
Disclosures
R.K.L. has received consulting fees from GLG Consulting. T.A. reports active funding from the ASH Research Training Award for Fellows, the Mayo Clinic SPORE, the Mayo Clinic Immune Monitoring Core, and the Mayo Clinic Hematology Research Division. A.K.S. receives research funding (paid to institution) from BMS, Celldex, Dynavax, Genentech, Immunocore, Merck, Reata; she is a consultant for Merck, BMS, Array; she is a speaker (unbranded) for BMS. D.B.J. serves on advisory boards of Array Biopharma, BMS, Genoptix, Incyte, Merck, and Novartis; he receives research funding from BMS and Incyte. O.R. receives research support from Merck; he is a speaker for activities supported by educational grants from BMS and Merck; he is a consultant for Merck, Celgene, Five Prime, GFK, Defined Health INC, Roche/Genentech, Puretech, Leerink and PRMA Consulting; he has a patent pending for methods of using pembrolizumab and trebananib. D.E.L. is supported by grant K23DK106448 from the National Institute of Diabetes and Digestive and Kidney Diseases. The remaining authors declare no competing financial interests.
REFERENCES
- 1.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015;33:1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 1995;270:985–8. [DOI] [PubMed] [Google Scholar]
- 3.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995;3:541–7. [DOI] [PubMed] [Google Scholar]
- 4.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378:158–68. [DOI] [PubMed] [Google Scholar]
- 5.De Martin E, Michot JM, Papouin B, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol 2018;68:1181–90. [DOI] [PubMed] [Google Scholar]
- 6.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2016;2:1607–16. [DOI] [PubMed] [Google Scholar]
- 7.Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mancone S, Lycan T, Ahmed T, et al. Severe neurologic complications of immune checkpoint inhibitors: a single-center review. J Neurol 2018. [DOI] [PubMed] [Google Scholar]
- 9.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol 2018;71:1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiuan E, Beckermann KE, Ozgun A, et al. Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy. J Immunother Cancer 2017;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbacki A, Maliha PG, Hudson M, Small D. A case of severe Pembrolizumab-induced neutropenia. Anticancer Drugs 2018;29:817–9. [DOI] [PubMed] [Google Scholar]
- 12.Nair R, Gheith S, Nair SG. Immunotherapy-Associated Hemolytic Anemia with Pure Red-Cell Aplasia. N Engl J Med 2016;374:1096–7. [DOI] [PubMed] [Google Scholar]
- 13.Delanoy N, Michot JM, Comont T, et al. Haematological immune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: a descriptive observational study. Lancet Haematol 2018. [DOI] [PubMed] [Google Scholar]
- 14.Tanios GE, Doley PB, Munker R. Autoimmune hemolytic anemia associated with the use of immune checkpoint inhibitors for cancer: 68 cases from the Food and Drug Administration database and review. Eur J Haematol 2018. [DOI] [PubMed] [Google Scholar]
- 15.Barcellini W. New Insights in the Pathogenesis of Autoimmune Hemolytic Anemia. Transfus Med Hemother 2015;42:287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill QA, Stamps R, Massey E, et al. The diagnosis and management of primary autoimmune haemolytic anaemia. Br J Haematol 2017;176:395–411. [DOI] [PubMed] [Google Scholar]
- 17.Go RS, Winters JL, Kay NE. How I treat autoimmune hemolytic anemia. Blood 2017;129:2971–9. [DOI] [PubMed] [Google Scholar]
- 18.Hill AH QA Autoimmune hemolytic anemia. American Society of Hematology Education Book; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crosby WH, Rappaport H. Reticulocytopenia in autoimmune hemolytic anemia. Blood 1956;11:929–36. [PubMed] [Google Scholar]
- 21.Khan U, Ali F, Khurram MS, Zaka A, Hadid T. Immunotherapy-associated autoimmune hemolytic anemia. J Immunother Cancer 2017;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tardy MP, Gastaud L, Boscagli A, Peyrade F, Gallamini A, Thyss A. Autoimmune hemolytic anemia after nivolumab treatment in Hodgkin lymphoma responsive to immunosuppressive treatment. A case report. Hematol Oncol 2017;35:875–7. [DOI] [PubMed] [Google Scholar]
- 23.Simeone E, Grimaldi AM, Esposito A, et al. Serious haematological toxicity during and after ipilimumab treatment: a case series. J Med Case Rep 2014;8:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palla AR, Kennedy D, Mosharraf H, Doll D. Autoimmune Hemolytic Anemia as a Complication of Nivolumab Therapy. Case Rep Oncol 2016;9:691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong BY, Micklethwaite KP, Swaminathan S, Kefford RF, Carlino MS. Autoimmune hemolytic anemia induced by anti-PD-1 therapy in metastatic melanoma. Melanoma Res 2016;26:202–4. [DOI] [PubMed] [Google Scholar]
- 26.Schwab KS, Heine A, Weimann T, Kristiansen G, Brossart P. Development of Hemolytic Anemia in a Nivolumab-Treated Patient with Refractory Metastatic Squamous Cell Skin Cancer and Chronic Lymphatic Leukemia. Case Rep Oncol 2016;9:373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Algaze SD, Park W, Harrington TJ, Mudad R. Autoimmune haemolytic anaemia in a patient with advanced lung adenocarcinoma and chronic lymphocytic leukaemia receiving nivolumab and intravenous immunoglobulin. BMJ Case Rep 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lott A BM, Leighl N, Cserti-Gazdewich CM. Evan’s Syndrome Associated with Pembrolizumab Therapy in Metastatic Non-Small Cell Lung Cancer. Blood 2015. [Google Scholar]
- 29.Le Burel S, Champiat S, Mateus C, et al. Prevalence of immune-related systemic adverse events in patients treated with anti-Programmed cell Death 1/anti-Programmed cell Death-Ligand 1 agents: A single-centre pharmacovigilance database analysis. Eur J Cancer 2017;82:34–44. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Lee SK, Oo TH, Rojas-Hernandez CM. Management of Immune-mediated Cytopenias in the Era of Cancer Immunotherapy: A Report of 4 Cases. J Immunother 2018;41:32–4. [DOI] [PubMed] [Google Scholar]
- 31.Ramos B, Gastal G, Rovere RK. An Autoimmune Haemolytic Anaemia Secondary to Ipilimumab Treatment. Klin Onkol;30:128–30. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa K, Ito J, Fujimoto D, et al. Exacerbation of autoimmune hemolytic anemia induced by the first dose of programmed death-1 inhibitor pembrolizumab: a case report. Invest New Drugs 2018;36:509–12. [DOI] [PubMed] [Google Scholar]
- 33.Shaikh H, Daboul N, Albrethsen M, Fazal S. A case of autoimmune haemolytic anaemia after 39 cycles of nivolumab. BMJ Case Rep 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasanov M, Konoplev SN, Hernandez CMR. Nivolumab-induced cold agglutinin syndrome successfully treated with rituximab. Blood Adv 2018;2:1865–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev 2010;24:195–210. [DOI] [PubMed] [Google Scholar]
- 36.Semple JW, Freedman J. Autoimmune pathogenesis and autoimmune hemolytic anemia. Semin Hematol 2005;42:122–30. [DOI] [PubMed] [Google Scholar]
- 37.Garratty G, Arndt P, Prince HE, Shulman IA. The effect of methyldopa and procainamide on suppressor cell activity in relation to red cell autoantibody production. Br J Haematol 1993;84:310–5. [DOI] [PubMed] [Google Scholar]
- 38.Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program 2009:73–9. [DOI] [PubMed] [Google Scholar]
- 39.Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018;391:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 2016;375:1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang DY, Salem JE, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:1721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segel GB, Lichtman MA. Direct antiglobulin (“Coombs”) test-negative autoimmune hemolytic anemia: a review. Blood Cells Mol Dis 2014;52:152–60. [DOI] [PubMed] [Google Scholar]
- 43.Leaf RK, O’Brien KL, Leaf DE, Drews RE. Autoimmune hemolytic anemia in a young man with acute hepatitis E infection. Am J Hematol 2017;92:E77–E9. [DOI] [PubMed] [Google Scholar]
- 44.de Back DZ, Kostova EB, van Kraaij M, van den Berg TK, van Bruggen R. Of macrophages and red blood cells; a complex love story. Front Physiol 2014;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang X, Venet F, Wang YL, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A 2009;106:6303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zoller EE, Lykens JE, Terrell CE, et al. Hemophagocytosis causes a consumptive anemia of inflammation. J Exp Med 2011;208:1203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.