Key Points
Question
Does the efficacy of endovascular therapy (EVT) in patients with acute large ischemic stroke differ between Alberta Stroke Program Early Computed Tomography Scores (ASPECTS) 3 or less vs 4 to 5?
Findings
In this secondary analysis of a randomized clinical trial, EVT was not associated with improvement in functional outcome at 90 days in patients with ASPECTS 3 or less and was associated with a significantly higher incidence of any intracranial hemorrhage within 48 hours. EVT was associated with safe and effective outcomes in patients with ASPECTS 4 to 5.
Meaning
Considering the lower likelihood of returning to functional independence and the higher incidence of symptomatic intracranial hemorrhage in patients with ASPECTS 3 or less, the findings in this study indicate that EVT for this subgroup should be carefully considered.
This secondary analysis of the Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism—Japan Large Ischemic Core Trial (RESCUE-Japan LIMIT) evaluates the safety and efficacy of endovascular therapy in patients with acute large ischemic stroke by Alberta Stroke Program Early Computed Tomography Score.
Abstract
Importance
Endovascular therapy (EVT) has been found to reduce functional disability in patients with acute stroke due to large-vessel occlusion. However, the extent of the ischemic region, measured using Alberta Stroke Program Early Computed Tomography Scores, may limit the efficacy of EVT.
Objective
To compare the efficacy and safety of EVT according to ASPECTS 3 or less vs 4 to 5.
Design, Setting, and Participants
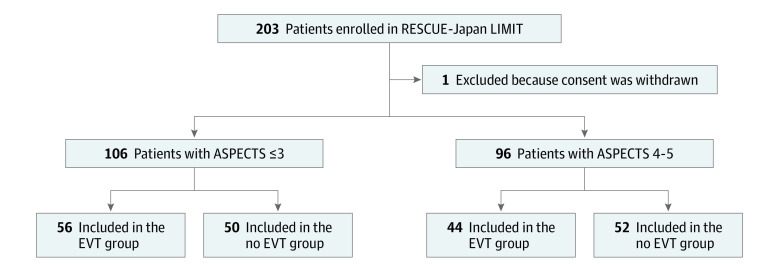
The Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism—Japan Large Ischemic Core Trial (RESCUE-Japan LIMIT) was an open-label randomized clinical trial conducted from November 2018 to December 2021 at 45 stroke centers across Japan. The trial enrolled adult patients with acute ischemic stroke with a large ischemic region, defined as ASPECTS 3 to 5 primarily determined by magnetic resonance imaging, with occlusion site at the internal carotid artery or middle cerebral artery segment 1. Among 203 enrolled patients, 1 withdrew consent and 202 were included in the original trial and secondary analysis. This secondary analysis was conducted in April 2022.
Interventions
Patients were randomly assigned to EVT with medical therapy or medical therapy alone.
Main Outcomes and Measures
Modified Rankin Scale (mRS) score at 90 days and symptomatic and any intracranial hemorrhage within 48 hours.
Results
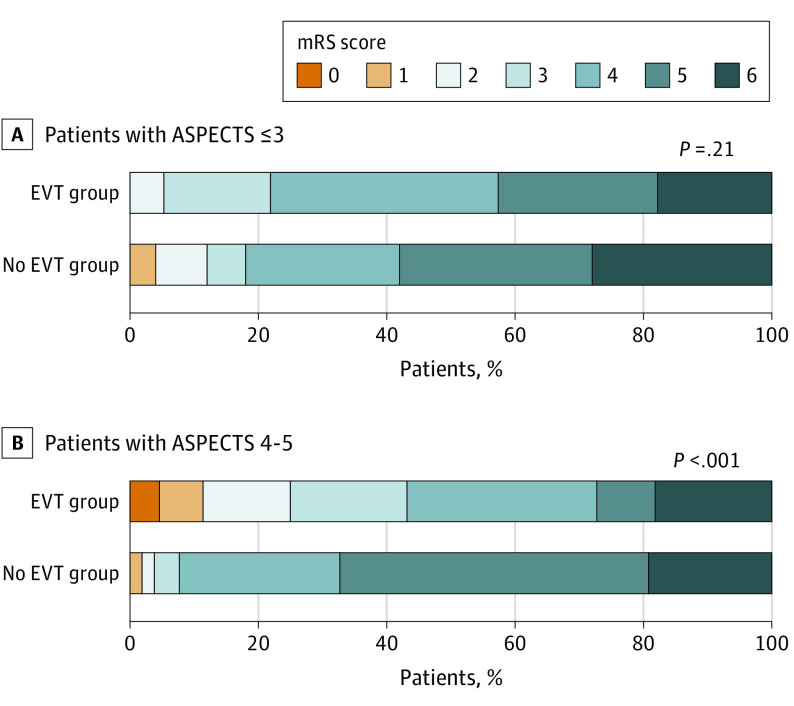
Among 202 patients, 106 (52%) had ASPECTS 3 or less (mean [SD] age, 76.7 [9.6] years; 54 female individuals [50.9%]) and 96 had ASPECTS 4 to 5 (mean [SD] age, 75.6 [10.6] years; 36 female individuals [37.5%]). Of patients with ASPECTS 3 or less, 12 (21.4%) in the EVT group and 9 (18.0%) in the no EVT group had an mRS score of 0 to 3 (odds ratio [OR], 1.24; 95% CI, 0.47-3.26). Of patients with ASPECTS 4 to 5, 19 patients (43.2%) in the EVT group and 4 (7.7%) in the no EVT group had an mRS score of 0 to 3 at 90 days (OR, 9.12; 95% CI, 2.80-29.70; interaction P = .01). The ordinal shift across the range of mRS scores toward a better outcome was not significant in those with ASPECTS or 3 or less (common OR, 1.56; 95% CI, 0.79-3.10) but was significant in those with ASPECTS 4 to 5 (common OR, 4.48; 95% CI, 2.07-9.71; interaction P = .046). The risk of intracranial hemorrhage was significantly increased in patients with ASPECTS 3 or less when EVT was conducted (OR, 4.14; 95% CI, 1.84-9.32) and nonsignificantly increased in those with ASPECTS 4 to 5 (OR, 2.05; 95% CI, 0.89-4.73; interaction P = .24).
Conclusions and Relevance
In this study, EVT was associated with improved 90-day functional outcomes in patients with acute large vessel occlusive stroke and ASPECTS was 4 to 5 but not in those with ASPECTS 3 or less.
Trial Registration
ClinicalTrials.gov Identifier: NCT03702413
Introduction
Endovascular therapy (EVT) has become a standard treatment for acute stroke caused by large vessel occlusion. Current guidelines recommend EVT for patients with an occlusion site in the middle cerebral artery segment 1 or internal carotid artery, for which imaging findings indicate that the size of the infarct area is not large, as defined by an Alberta Stroke Program Early Computed Tomographic Score (ASPECTS) of at least 6,1 if EVT can be performed within 6 hours after onset. If EVT can be performed at 6 to 24 hours after onset, an additional condition of mismatch between infarction volume and penumbra is recommended.2,3,4,5 ASPECTS measures the number of infarct regions by dividing the hemisphere fed by the middle cerebral artery into 10 regions. The ASPECTS value ranges from 0 to 10, with lower values indicating a greater infarct area.6,7 Patients with large infarctions (ASPECTS 5 or less) have generally been excluded from clinical trials investigating EVT or are represented in only small numbers, partly because of concerns that bleeding might occur in the area of infarction after reperfusion and patients might be unable to regain functional independence.8,9,10,11
Our randomized clinical trial12 suggested efficacy of EVT in patients with a large ischemic core (ASPECTS 3 to 5).13 However, there was still a difference in disease severity in patients with ASPECTS 3 to 5, and thus we do not know at what ASPECTS threshold EVT stops being beneficial and becomes unhelpful and potentially harmful. Thus, we conducted a secondary subanalysis using data from the Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism—Japan Large Ischemic Core Trial (RESCUE-Japan LIMIT)12 to investigate possible differences in efficacy and safety of EVT in patients with large ischemic stroke and ASPECTS 3 or less vs 4 to 5.
Methods
Study Design
RESCUE-Japan LIMIT was a prospective multicenter non–industry-supported open-label randomized clinical trial aimed at evaluating the efficacy and safety of standard medical care with EVT compared to standard medical care without EVT in adult patients with acute large vessel occlusion with a large ischemic core at 45 stroke centers across Japan.12,13 This trial exclusively enrolled patients with ASPECTS 3 to 5 on computed tomography or diffusion-weighted imaging–magnetic resonance imaging (DWI-MRI). Additional eligibility criteria were acute ischemic stroke, age 18 years and older, National Institutes of Health Stroke Scale (NIHSS) score 6 or higher on admission,14 modified Rankin scale (mRS) score of 0 to 1 before onset, occlusion site at the internal carotid artery or middle cerebral artery segment 1 on computed tomography angiography or magnetic resonance angiography, randomization could be completed within 6 hours from the time the patient was last known to be well or 6 to 24 hours from the time the patient was last known to be well if there were no ischemic changes on fluid-attenuated inversion recovery imaging,15 EVT could be initiated within 60 minutes of randomization, and the patient or a legally authorized representative signed the informed consent form. Patients were excluded if they had a significant mass effect with midline shift on computed tomography or MRI, evidence of acute intracranial hemorrhage (ICH) on computed tomography or MRI, or a high risk of hemorrhage or other conditions.12 This study was overseen by independent steering, monitoring, imaging evaluation, and event-adjudication committees and was performed in compliance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan and the Declaration of Helsinki. The institutional review boards of Hyogo College of Medicine (approval number 3015) and all participating hospitals approved this study and written informed consent was obtained from all patients or their legal representatives.
RESCUE-Japan LIMIT enrolled 203 patients from November 2018 to September 2021, with follow-up ending in December 2021. This article reports associations with efficacy and safety outcomes in the EVT group compared to the no EVT group stratified by ASPECTS 3 or less vs 4 to 5.
Measurements
Baseline characteristics and treatment details were obtained from the neurologists at the study hospitals. Baseline characteristics included premorbid mRS score, NIHSS score, occlusion site, ASPECTS, type of stroke, and laboratory tests.
Imaging studies were conducted using either noncontrast computed tomography (NCCT) or DWI-MRI. The selection of imaging modalities was determined by the treating neurologists. Computed tomography angiography was performed to determine the occlusion site when acute ischemic stroke due to large vessel occlusion with NCCT was suspected. If MRI was used, magnetic resonance angiography was simultaneously performed to determine the occlusion site. The treating neurologists were trained and certified in advance to assess ASPECTS using either NCCT or DWI-MRI.6,7 All imaging studies were sent to the core laboratory and independently evaluated by the imaging evaluation committee. Eligibility was based on the initial evaluation by the treating neurologists but the imaging evaluation committee made the final judgments. We analyzed all patients enrolled in the trial, including those who were ultimately found to have ASPECTS outside the 3 to 5 range. When both NCCT and DWI-MRI were obtained, DWI-MRI was used to determine the ASPECTS.
Outcomes
Consistent with the main report,12 the primary outcome in this study13 was an mRS score of 0 to 3 at 90 days after onset. The secondary outcomes were mRS score of 0 to 2, mRS score of 0 to 1, an ordinal shift across the range of mRS scores toward a better outcome at 90 days, and an improvement of at least 8 points on the NIHSS at 48 hours after randomization. Safety outcomes included symptomatic ICH, defined as any ICH type with the NIHSS score worsening by 4 or more points within 48 hours, and any ICH within 48 hours.16 The mRS score at 90 days was assessed by a neurologist or physical therapist who was unaware of the treatment assignment.
Statistical Analysis
We recorded patient characteristics by group (ASPECTS 3 or less vs 4 to 5). Continuous variables were expressed as either mean and standard deviation or median and interquartile range and were compared using the t test or Wilcoxon rank sum test based on their distributions. Categorical variables were presented as numbers and percentages and were compared using the χ2 test.
The distributions of ordinal mRS scores were presented according to whether the ASPECTS was 3 or less or 4 to 5, and the differences in distribution between the EVT and no EVT groups were assessed using the Wilcoxon rank sum test. We calculated the proportion of primary, secondary, and safety outcomes in the stratum of ASPECTS 3 or less and 4 to 5 and separately compared differences in the studied outcomes. Comparisons between the EVT group and no EVT group were presented as odds ratios (ORs) with 95% CIs, except for the ordinal shift of mRS score. We subsequently constructed an ordinal logistic model to estimate the common ORs with 95% CIs for 1 scale lower of mRS score at 90 days after the assessment of the proportional odds assumption. We constructed a logistic regression model using the treatment group as a variable, ASPECTS 3 or less vs 4 to 5, and an interaction term to estimate the interaction P values. Because the treatment group was randomly assigned, we did not adjust the estimates for all the analyses.
All statistical analyses were performed using JMP version 15.1 (SAS Institute) and SAS version 9.4 (SAS Institute). Missing data on baseline characteristics were not imputed and were eliminated from the corresponding analyses. All reported P values were 2-tailed, and P values less than .05 were considered statistically significant. Because of the exploratory nature of the subanalysis, we did not adjust P values considering multiplicity.
Results
Patient Characteristics
Of the 203 enrolled patients, 202 were included in this subanalysis, after excluding 1 patient who withdrew consent (Figure 1). Of these, 106 (52%) had ASPECTS 3 or less (mean [SD] age, 76.7 [9.6] years; 54 female individuals [50.9%]) and 96 had ASPECTS 4 to 5 (mean [SD] age, 75.6 [10.6] years; 36 female individuals [37.5%]) (Figure 1), evaluated with DWI-MRI in 174 (86.1%) and NCCT in 28 (13.9%). Eight patients were initially considered to have an ASPECTS of 3 but were ascertained as having 0 to 2 by the imaging evaluation committee, including 1 patient in the no EVT group with an ASPECTS of 0, 2 in the EVT group with an ASPECTS of 1, and 3 in the EVT group and 2 in the no EVT group with an ASPECTS of 2. The characteristics were numerically similar between those with ASPECTS 3 or less and 4 to 5, except that patients with ASPECTS 3 or less had larger infarction volume (126 mL vs 89 mL; P < .001) and higher median C-reactive protein values (0.25 mg/dL vs 0.13 mg/dL; P = .008) (Table 1).
Figure 1. Study Flow Diagram.
ASPECTS indicates Alberta Stroke Program Early Computed Tomography Score; EVT, endovascular therapy; RESCUE-Japan LIMIT, Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism—Japan Large Ischemic Core Trial.
Table 1. Patient Characteristics.
| Variable | No. (%) | P value | |
|---|---|---|---|
| ASPECTS ≤3 (n = 106) | ASPECTS 4-5 (n = 96) | ||
| Age, mean (SD), y | 76.7 (9.6) | 75.6 (10.6) | .46 |
| Female | 54 (50.9) | 36 (37.5) | .055 |
| Male | 52 (49.1) | 60 (62.5) | |
| Modified Rankin Scale score before onset, median (IQR) | 0 (0-1) | 0 (0-1) | .38 |
| Baseline NIHSS score, median (IQR) | 23 (17-26) | 21 (18-25) | .14 |
| Medical history | |||
| Hypertension | 74 (69.8) | 67 (69.8) | >.99 |
| Diabetes | 24 (22.6) | 19 (19.8) | .62 |
| Hyperlipidemia | 30 (28.3) | 19 (19.8) | .16 |
| Atrial fibrillation | 64 (60.4) | 55 (57.3) | .66 |
| Current smoking | 18 (17.0) | 21 (21.9) | .38 |
| Blood pressure, mean (SD), mm Hga | |||
| Systolic | 159.5 (30.3) | 159.6 (27.3) | .99 |
| Diastolic | 86.4 (21.0) | 89.6 (18.2) | .25 |
| Laboratory tests | |||
| Cholesterol, mean (SD), mg/dLb | |||
| LDL | 110.8 (32.2) | 106.5 (32.7) | .39 |
| HDL | 56.7 (16.6) | 56.2 (16.9) | .86 |
| Blood glucose, median (IQR), mg/Lc | 138 (116-163) | 129 (116-147) | .16 |
| Creatinine, median (IQR), mg/dLd | 0.80 (0.66-1.08) | 0.83 (0.70-1.05) | .45 |
| CRP, median (IQR), mg/dLe | 0.25 (0.10-0.92) | 0.13 (0.06-0.37) | <.001 |
| Internal carotid artery occlusion | 55 (51.9) | 40 (41.7) | .15 |
| MRI | 95 (89.6) | 79 (82.3) | .13 |
| ASPECTS | NA | ||
| 0-2 | 8 (7.5) | 0 | |
| 3 | 98 (92.5) | 0 | |
| 4 | 0 | 56 (58.3) | |
| 5 | 0 | 40 (41.7) | |
| Infarction volume, median (IQR), mLf | 126 (83-168) | 89 (60-122) | <.001 |
| Intravenous rt-PA use | 28 (26.4) | 27 (28.1) | .79 |
| Time from onset to image, median (IQR), min | 198 (103-376) | 159 (101-400) | .46 |
| Time from onset to randomization, median (IQR), min | 224 (140-405) | 207 (145-427) | .62 |
| Time from onset to puncture, median (IQR), min | 263 (170-523) | 237 (165-388) | .38 |
| Time from onset to reperfusion, median (IQR), min | 320 (223-559) | 292 (207-479) | .33 |
| Reperfusion degree with TICI ≥2b | 47/56 (83.9) | 39/44 (88.6) | .50 |
| Stroke classification | |||
| Cardioembolic | 84 (79.2) | 75 (78.1) | .87 |
| Atherothrombotic | 5 (4.7) | 7 (7.3) | |
| Cryptogenic | 14 (13.2) | 11 (11.5) | |
| Other | 3 (2.8) | 3 (3.1) | |
Abbreviations: ASPECTS, Alberta Stroke Program Early Compute Tomography Score; CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MRI, magnetic resonance imaging; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; rt-PA, recombinant tissue plasminogen activator; TICI, thrombolysis in the cerebral infarction.
Data missing for 2 participants for systolic and diastolic blood pressure.
To convert to mmol/L, multiply by 0.0259. Data missing for 33 participants for LDL cholesterol and 34 for HDL cholesterol.
To convert to mmol/L, multiply by 0.0555. Data missing for 2 participants for blood glucose.
To convert to μmol/L, multiply by 76.25.
To convert to mg/L, multiply by 10.
Data missing for 20 participants for infarction volume.
Outcomes
Outcomes are summarized in Table 2. Among patients with ASPECTS 3 or less, mRS score at 90 days was similar between the EVT and no EVT groups (Figure 2A). Of patients with ASPECTS 3 or less, 12 (21.4%) in the EVT group and 9 (18.0%) in the no EVT group had an mRS score of 0 to 3 (odds ratio [OR], 1.24; 95% CI, 0.47-3.26). Of patients with ASPECTS 4 to 5, 19 patients (43.2%) in EVT group and 4 (7.7%) in the no EVT group had an mRS score of 0 to 3 at 90 days (OR, 9.12; 95% CI, 2.80-29.70; interaction P = .01) (Figure 2B).
Table 2. Outcomes.
| Outcome | No. (%) | OR (95% CI) | P value | P value for interaction | |
|---|---|---|---|---|---|
| EVT group | No-EVT group | ||||
| ASPECTS ≤3 (n = 106), No. | 56 | 50 | NA | NA | NA |
| Primary outcome | |||||
| mRS score of 0-3 at 90 d | 12 (21.4) | 9 (18.0) | 1.24 (0.47-3.26) | .66 | .01 |
| Secondary outcomes | |||||
| mRS score of 0-2 at 90 d | 3 (5.4) | 6 (12.0) | 0.42 (0.10-1.77) | .23 | .003 |
| mRS score of 0-1 at 90 d | 0 | 2 (4.0) | NA | NA | NA |
| Ordinal shift across the range of mRS toward a better outcome | NA | NA | 1.56 (0.79-3.10) | .20 | .046 |
| Improvement of ≥8 NIHSS points at 48 h | 14 (25.0) | 4 (8.0) | 3.83 (1.17-12.6) | .03 | .60 |
| Safety outcomes | |||||
| Symptomatic intracranial hemorrhage within 48 h | 6 (10.7) | 3 (6.0) | 1.88 (0.44-7.95) | .39 | .98 |
| Any intracranial hemorrhage within 48 h | 37(66.1) | 16 (32.0) | 4.14 (1.84-9.32) | <.001 | .24 |
| Death within 90 d | 10 (17.9) | 14 (28.0) | 0.56 (0.22-1.40) | .22 | .47 |
| Recurrence of cerebral infarction within 90 d | 4 (7.1) | 1 (2.0) | 3.77 (0.41-34.9) | .24 | .054 |
| Decompressive craniectomy within 7 d | 7 (12.5) | 7 (14.0) | 0.88 (0.28-2.70) | .82 | .50 |
| ASPECTS 4-5 (n = 96), No. | 44 | 52 | NA | NA | NA |
| Primary outcome | |||||
| mRS score of 0-3 at 90 d | 19 (43.2) | 4 (7.7) | 9.12 (2.80-29.7) | <.001 | NA |
| Secondary outcomes | |||||
| mRS score of 0-2 at 90 d | 11 (25.0) | 2 (3.8) | 8.33 (1.73-40.0) | .008 | NA |
| mRS score of 0-1 at 90 d | 5 (11.4) | 1 (1.9) | 6.54 (0.73-58.3) | .09 | NA |
| Ordinal shift across the range of mRS toward a better outcome | NA | NA | 4.48 (2.07-9.71) | <.001 | NA |
| Improvement of 8 points or greater on the NIHSS at 48 h | 17 (38.6) | 5 (9.6) | 5.92 (1.96-17.8) | .002 | NA |
| Safety outcomes | |||||
| Symptomatic intracranial hemorrhage within 48 h | 3 (6.8) | 2 (3.8) | 1.83 (0.29-11.5) | .52 | NA |
| Any intracranial hemorrhage within 48 h | 21 (47.7) | 16 (30.8) | 2.05 (0.89-4.73) | .09 | NA |
| Death within 90 d | 8 (18.2) | 10 (19.2) | 0.93 (0.33-2.62) | .90 | NA |
| Recurrence of cerebral infarction within 90 d | 1 (2.3) | 6 (11.5) | 0.18 (0.02-1.54) | .12 | NA |
| Decompressive craniectomy within 7 d | 3 (6.8) | 7 (13.5) | 0.47 (0.11-1.94) | .30 | NA |
Abbreviations: ASPECTS, Alberta Stroke Program Early Computed Tomography Score; EVT, endovascular therapy; mRS, modified Rankin Scale; NA, not applicable; NIHSS, National Institutes of Health stroke scale; OR, odds ratio.
Figure 2. Distribution of Modified Rankin Scale (mRS) Score at 90 Days.
ASPECTS indicates Alberta Stroke Program Early Computed Tomography Score; EVT, endovascular therapy.
An mRS score of 0 to 2 was also significant in participants with ASPECTS 4 to 5 (OR, 8.33; 95% CI, 1.73-40.0) but not in those with ASPECTS 3 or less (OR, 0.42; 95% CI, 0.10-1.77). This interaction was also significant (P = .003). The ordinal shift across the range of mRS scores toward a better outcome was also significant in those with ASPECTS 4 to 5 (common OR, 4.48; 95% CI, 2.07-9.71) but not in those with ASPECTS 3 or less (common OR, 1.56; 95% CI, 0.79-3.10), and the differences in the effect were significant (interaction P = .046). Improvement in NIHSS score at 48 hours with EVT was similar in patients with ASPECTS 3 or less and 4 to 5 (OR, 3.83; 95% CI, 1.17-12.6 vs OR, 5.92; 95% CI, 1.96-17.8, respectively; interaction P = .60).
Among participants with ASPECTS 3 or less, the incidences of symptomatic ICH were 10.7% in the EVT group (n = 6) and 6.0% in the no EVT group (n = 3) (OR, 1.88; 95% CI, 0.44-7.95) and among those with ASPECTS 4 to 5, 6.8% in the EVT group (n = 3) and 3.8% in the no EVT group (n = 2) (OR, 1.83, 95% CI, 0.29-11.5) (interaction P = .98). Conversely, the risk of any ICH was more prominent in patients with ASPECTS 3 or less when EVT was conducted (OR, 4.14; 95% CI, 1.84-9.32) than in those with ASPECTS 4 to 5 (OR, 2.05; 95% CI, 0.89-4.73), but the interaction was not significant (P = .24).
Discussion
In this study, we report the results of additional analyses of a randomized clinical trial of EVT for patients with acute large stroke defined by ASPECTS of 3 to 5. An association of efficacy outcomes with EVT was prominent in patients with ASPECTS 4 to 5 in terms of mRS scores of 0 to 3, 0 to 2, and a shift of mRS score toward lower, with significant interaction between groups with ASPECTS 3 or less and 4 to 5. An association was observed between EVT and improved NIHSS score at 48 hours in both ASPECTS groups. Although the incidence of symptomatic ICH was not statistically different between the EVT group and the no EVT group in patients with ASPECTS 3 or less and 4 to 5, symptomatic ICH occurred in 1 of 10 patients with ASPECTS 3 or less when EVT was administered. In addition, the incidence of any ICH within 48 hours was significantly higher in the EVT group than in the no EVT group in patients with ASPECTS 3 or less.
Previous patient-level meta-analyses of randomized clinical trials have reported the efficacy of EVT in terms of shift of mRS at 90 days by each value of ASPECTS, indicating that EVT is associated with beneficial outcomes in patients with ASPECTS of 3 but not in those with ASPECTS of 0 to 2 or 4 or 5.11 We suspected that the beneficial associations observed only in those with ASPECTS 3 were due to the small sample size. Considering the consistent association between ASPECTS and efficacy with EVT, the inflection point of efficacy with EVT should be between ASPECTS 0 to 2 and 4. Our study supports the hypothesis that EVT would have neutral efficacy in patients with large ischemic stroke and ASPECTS of 3.
This analysis also demonstrated that the risk of ICH within 48 hours was higher in patients with ASPECTS 3 or less than in those with ASPECTS 4 to 5. The OR for any ICH of EVT was twice as high in patients with ASPECTS 3 or less than in those with ASPECTS 4 to 5. In addition, the incidence of symptomatic ICH in both the EVT and no EVT groups among those with ASPECTS 4 to 5 in our study was similar to the approximately 5% rate observed in previous studies with higher ASPECTS.9 However, the incidence of symptomatic ICH increased to 10.7% when EVT was administered to patients with ASPECTS 3 or less. From the perspective of the risk of ICH, EVT should be carefully considered in patients with ASPECTS 3 or less.
Limitations
Our study has several limitations that should be acknowledged. The first and most important issue was that DWI-MRI was the predominant imaging modality used to evaluate ASPECTS and 86.1% of individuals underwent DWI-MRI to measure ASPECTS. Although DWI-MRI is prioritized to assess suspected acute ischemic stroke in Japan, many Western countries use NCCT followed by computed tomography angiography or angiography.17 Because DWI-MRI has a higher sensitivity to detect ischemic regions, discrepancies in the assessment of ASPECTS between DWI-MRI and NCCT have been reported. Although not limited in large vessel occlusions, the ASPECTS measured by DWI-MRI in patients with acute ischemic stroke was reported 1 scale lower than that measured by NCCT.18 If such discrepancy is common, the classification of ASPECTS in this study may change. Therefore, our findings should be tested in other settings where NCCT is dominant. Second, we used ASPECTS to measure the extent of the ischemic regions in this study. Recent advancements in Rapid Processing of Perfusion and Diffusion (RAPID) software or other tools to measure the volume of ischemic regions more precisely could replace the ASPECTS on NCCT or DWI-MRI.19,20 However, the wide use of such advanced and expensive modalities may take a long time, and the current practice with ASPECTS should be used to select candidates with large ischemic stroke who might benefit from EVT. Third, the sample size for subgroups of ASPECTS 3 or less and 4 to 5 was not large enough to confirm the efficacy and safety of EVT as the current subanalysis was not predetermined. Indeed, the achievement of mRS score 0 to 3 was inversely higher in the ASPECTS 3 or less group than in the ASPECTS 4 to 5 group if they did not receive EVT. Therefore, the findings of this study should be considered exploratory and will need to be verified by an independent powered study focusing on the subgroups of patients with ASPECTS 3 or less and 4 to 5.
Conclusions
The findings in this study indicate that EVT was not associated with improved functional outcomes at 90 days in patients with acute large ischemic stroke and ASPECTS 3 or less. Considering the lower likelihood of regaining functional independence and the higher incidence of symptomatic ICH in patients with ASPECTS 3 or less, ASPECTS 3 should be further investigated as the threshold of indication for EVT. Because those with ASPECTS 4 to 5 had similar efficacy and safety outcomes with EVT compared with those with ASPECTS 6 or higher, they may be able to return to functional independence with EVT.
The RESCUE-Japan LIMIT investigators
References
- 1.Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001;22(8):1534-1542. [PMC free article] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 3.Yamagami H, Hayakawa M, Inoue M, et al. ; JSS/JNS/JSNET Joint Guideline Authoring Committee . Guidelines for mechanical thrombectomy in Japan, the fourth edition, march 2020: a guideline from the Japan Stroke Society, the Japan Neurosurgical Society, and the Japanese Society for Neuroendovascular Therapy. Neurol Med Chir (Tokyo). 2021;61(3):163-192. doi: 10.2176/nmc.nmc.st.2020-0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 5.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber PA, Demchuk AM, Zhang J, Buchan AM; ASPECTS study group . Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355(9216):1670-1674. doi: 10.1016/S0140-6736(00)02237-6 [DOI] [PubMed] [Google Scholar]
- 7.Singer OC, Kurre W, Humpich MC, et al. ; MR Stroke Study Group Investigators . Risk assessment of symptomatic intracerebral hemorrhage after thrombolysis using DWI-ASPECTS. Stroke. 2009;40(8):2743-2748. doi: 10.1161/STROKEAHA.109.550111 [DOI] [PubMed] [Google Scholar]
- 8.Cucchiara B, Kasner SE, Tanne D, et al. ; SAINT Investigators . Factors associated with intracerebral hemorrhage after thrombolytic therapy for ischemic stroke: pooled analysis of placebo data from the Stroke-Acute Ischemic NXY Treatment (SAINT) I and SAINT II trials. Stroke. 2009;40(9):3067-3072. doi: 10.1161/STROKEAHA.109.554386 [DOI] [PubMed] [Google Scholar]
- 9.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 10.Bracard S, Ducrocq X, Mas JL, et al. ; THRACE investigators . Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138-1147. doi: 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 11.Román LS, Menon BK, Blasco J, et al. ; HERMES collaborators . Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018;17(10):895-904. doi: 10.1016/S1474-4422(18)30242-4 [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura S, Uchida K, Sakai N, et al. Randomized clinical trial of endovascular therapy for acute large vessel occlusion with large ischemic core (RESCUE-Japan LIMIT): rationale and study protocol. Neurol Med Chir (Tokyo). 2022;62(3):156-164. doi: 10.2176/nmc.rc.2021-0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimura S, Sakai N, Yamagami H, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2022;386(14):1303-1313. doi: 10.1056/NEJMoa2118191 [DOI] [PubMed] [Google Scholar]
- 14.Lyden P, Brott T, Tilley B, et al. ; NINDS TPA Stroke Study Group . Improved reliability of the NIH Stroke Scale using video training. Stroke. 1994;25(11):2220-2226. doi: 10.1161/01.STR.25.11.2220 [DOI] [PubMed] [Google Scholar]
- 15.Sakakibara F, Yoshimura S, Numa S, Uchida K, Kinjo N, Morimoto T. Diffusion-weighted imaging-fluid-attenuated inversion recovery mismatch is associated with 90-day functional outcomes in patients undergoing mechanical thrombectomy. Cerebrovasc Dis. 2020;49(3):292-300. doi: 10.1159/000508369 [DOI] [PubMed] [Google Scholar]
- 16.Wahlgren N, Ahmed N, Dávalos A, et al. ; SITS-MOST investigators . Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275-282. doi: 10.1016/S0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 17.Yoshimura S, Uchida K, Morimoto T. Endovascular therapy for large acute strokes. reply. N Engl J Med. 2022;386(25):2440-2441. doi: 10.1056/NEJMc2205925 [DOI] [PubMed] [Google Scholar]
- 18.Nezu T, Koga M, Nakagawara J, et al. Early ischemic change on CT versus diffusion-weighted imaging for patients with stroke receiving intravenous recombinant tissue-type plasminogen activator therapy: stroke acute management with urgent risk-factor assessment and improvement (SAMURAI) rt-PA registry. Stroke. 2011;42(8):2196-2200. doi: 10.1161/STROKEAHA.111.614404 [DOI] [PubMed] [Google Scholar]
- 19.Vagal A, Wintermark M, Nael K, et al. Automated CT perfusion imaging for acute ischemic stroke: Pearls and pitfalls for real-world use. Neurology. 2019;93(20):888-898. doi: 10.1212/WNL.0000000000008481 [DOI] [PubMed] [Google Scholar]
- 20.Hotter B, Pittl S, Ebinger M, et al. Prospective study on the mismatch concept in acute stroke patients within the first 24 h after symptom onset—1000Plus study. BMC Neurol. 2009;9:60. doi: 10.1186/1471-2377-9-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The RESCUE-Japan LIMIT investigators