This individual patient data meta-analysis evaluates the association of patent foramen ovale closure on stroke recurrence according to shunt size and/or the presence of an atrial septal aneurysm.
Key Points
Question
What association do high-risk anatomic and physiologic patent foramen ovale features have with the benefits of closure?
Findings
In this individual patient data meta-analysis, the association of closure with recurrent stroke was larger for patients with both a large shunt and an atrial septal aneurysm (ASA) than for large shunt without ASA, small shunt with ASA, and small shunt without ASA.
Meaning
Patients with both large shunt and ASA benefit substantially more from closure than patients with large shunt alone, small shunt and ASA, or neither large shunt or ASA.
Abstract
Importance
The Patent Foramen Ovale (PFO)–Associated Stroke Causal Likelihood classification system combines information regarding noncardiac patient features (vascular risk factors, infarct topography) and PFO features (shunt size and presence of atrial septal aneurysm [ASA]) to classify patients into 3 validated categories of responsiveness to treatment with PFO closure. However, the distinctive associations of shunt size and ASA, alone and in combination, have not been completely delineated.
Objective
To evaluate the association of PFO closure with stroke recurrence according to shunt size and/or the presence of an ASA.
Design, Setting, and Participants
Pooled individual patient data from 6 randomized clinical trials conducted from February 2000 to October 2017 that compared PFO closure with medical therapy. Patients in North America, Europe, Australia, Brazil, and South Korea with PFO-associated stroke were included. Analysis was completed in January 2022.
Exposures
Transcatheter PFO closure plus antithrombotic therapy vs antithrombotic therapy alone, stratified into 4 groups based on the combination of 2 features: small vs large PFO shunt size and the presence or absence of an ASA.
Main Outcomes and Measures
Recurrent ischemic stroke.
Results
A total of 121 recurrent ischemic strokes occurred in the pooled 3740 patients (mean [SD] age, 45 [10] years; 1682 [45%] female) during a median (IQR) follow-up of 57 (23.7-63.8) months. Treatment with PFO closure was associated with reduced risk for recurrent ischemic stroke (adjusted hazard ratio [aHR], 0.41 [95% CI, 0.28-0.60]; P < .001). The reduction in hazard for recurrent stroke was greater for patients with both a large shunt and an ASA (aHR, 0.15 [95% CI, 0.06-0.35]) than for large shunt without ASA (aHR, 0.27 [95% CI, 0.14-0.56]), small shunt with ASA (aHR, 0.36 [95% CI, 0.17-0.78]), and small shunt without ASA (aHR, 0.68 [95% CI, 0.41-1.13]) (interaction P = .02). At 2 years, the absolute risk reduction of recurrent stroke was greater (5.5% [95% CI, 2.7-8.3]) in patients with large shunt and ASA than for patients in the other 3 categories (1.0% for all).
Conclusions and Relevance
Patients with both a large shunt and an ASA showed a substantially greater beneficial association with PFO closure than patients with large shunt alone, patients with small shunt and ASA, and patients with neither large shunt nor ASA. These findings, combined with other patient features, may inform shared patient-clinician decision-making.
Introduction
Patent foramen ovale (PFO) is associated with cryptogenic ischemic stroke, particularly in young and middle-aged adults.1,2 However, due to its high prevalence in the general population, a discovered PFO may be incidental even in patients with cryptogenic stroke. Noncardiac and cardiac patient features contribute to causal relatedness and are combined in the recently validated PFO-Associated Stroke Causal Likelihood (PASCAL) classification system (eAppendix 1 in the Supplement).3 Noncardiac features, including the absence of vascular risk factors and presence of embolic infarct topography, are encapsulated in the Risk of Paradoxical Embolism (RoPE) score (eAppendix 2 in the Supplement).4 Cardiac features include the presence of a large shunt or an atrial septal aneurysm (ASA) (high-risk PFO).2,5 A recent individual patient data meta-analysis of 6 randomized clinical trials showed that patients classified by PASCAL as probably or possibly PFO-related benefitted from PFO closure, whereas patients classified as unlikely did not.6
However, PASCAL does not distinguish between patients with only 1 or both high-risk PFO features. Here, we assess the presence of an interaction between the stroke reduction association of PFO closure and patient subgroups defined by shunt size and ASA status.
Methods
This study is a secondary analysis of a previously reported individual patient data meta-analysis,6 which followed the PRISMA for Individual Patient Data systematic reviews reporting guideline. This collaborative individual patient data meta-analysis included all randomized phase 3 trials of transcatheter PFO closure plus antithrombotic therapy vs antithrombotic therapy alone for secondary stroke prevention in hospitals in North America, Europe, Australia, Brazil, and South Korea. All randomized clinical trials were conducted from 2000 to 2017. Trial identification, component study descriptions, data harmonization, and other details are summarized in the original Systematic, Collaborative, PFO Closure Evaluation consortium report.6
The primary end point of this study was recurrent ischemic stroke. The main exposures were treatment assignment, shunt size through the PFO (with large shunt defined as ≥20 bubbles on transesophageal echocardiography in 4 trials, ≥25 bubbles in 1 trial, and >30 in 1 trial), and the presence of an ASA (defined as septal mobility of ≥10 mm from the midline in 4 trials, ≥10-15 mm in 1 trial, and ≥15 mm in 1 trial).
Kaplan-Meier estimates were used to estimate the absolute effect sizes in each of the 4 strata defined by shunt size and ASA status at 24 and 60 months. We used Cox proportional hazards regression to estimate the hazard ratio (HR) associated with device closure with a study-specific random association. In this 1-stage individual patient data meta-analysis analysis, a study-specific random association was used to account for within-study homogeneity in outcomes and a fixed treatment association.7 To avoid distortion in estimation of interaction terms by across-study information, we used a within-study covariate centering approach to specify the treatment interaction terms.8
Patients who exited the trials early were censored at last follow-up. Multiple imputation was used to impute missing covariates as needed. We tested for heterogeneous treatment effects across the 4-level variable defined by shunt size and ASA status. P values less than .05 were considered nominally significant. As this study was a secondary analysis of the pooled data set, all findings are considered exploratory. Analysis was completed in January 2022.
Results
Of 3740 total included participants, the mean (SD) age was 45 (10) years and 1682 (45%) were female. Baseline characteristics of the subgroups are in Table 1. Patients with both PFO and ASA were 3 years older than those with PFO alone and more likely to receive oral anticoagulants alone as initial therapy. Patients with large shunts (±ASA) were more often male, had less hypertension and hyperlipidemia, and more often had ischemic stroke rather than transient ischemic attack as the index event.
Table 1. Baseline Characteristics of the 4 Groups of Patients Defined by Shunt Size and Presence or Absence of an ASAa.
| Characteristic | Missing, No. | Small shunt | Large shunt | P value | ||
|---|---|---|---|---|---|---|
| Without ASA (n = 1317) | With ASA (n = 541) | Without ASA (n = 955) | With ASA (n = 573) | |||
| Age, median (IQR), y | 12 | 45.1 (37.9-52) | 48.5 (41.7-53.8) | 45.3 (37.4-52) | 49 (41.7-55) | <.001 |
| Male | 0 | 687 (52.2) | 288 (53.2) | 561 (58.7) | 333 (58.1) | .006 |
| Female | 0 | 630 (47.8) | 253 (46.8) | 394 (41.3) | 240 (41.9) | |
| Prior MI | 0 | 12 (0.9) | 6 (1.1) | 3 (0.3) | 1 (0.2) | .08 |
| Diabetes | 0 | 92 (7.0) | 28 (5.2) | 38 (4.0) | 33 (5.8) | .02 |
| Hypertension | 0 | 357 (27.1) | 170 (31.4) | 202 (21.2) | 132 (23.0) | <.001 |
| Hyperlipidemia | 0 | 530 (40.2) | 222 (41.0) | 264 (27.6) | 198 (34.6) | <.001 |
| Prior stroke or TIA | 0 | 253 (19.2) | 93 (17.2) | 117 (12.3) | 75 (13.1) | <.001 |
| Smoking status | 1 | 248 (18.8) | 99 (18.3) | 224 (23.5) | 103 (18.0) | .01 |
| Index event (stroke vs TIA) | 2 | 1203 (91.4) | 469 (86.9) | 940 (98.4) | 557 (97.2) | <.001 |
| Superficial infarction on neuroimaging (present vs absent) | 771 | 594 (67.1) | 263 (70.7) | 591 (69.2) | 378 (75.0) | .02 |
| Initial class of antithrombotic therapy (antiplatelet vs anticoagulant) | ||||||
| Antiplatelet alone | 51 | 1060 (81.5) | 420 (78.9) | 762 (81.2) | 415 (73.6) | <.001 |
| Anticoagulant alone | 128 (9.8) | 67 (12.6) | 155 (16.5) | 120 (21.3) | ||
| Antiplatelet + anticoagulant | 99 (7.6) | 38 (7.1) | 18 (1.9) | 25 (4.4) | ||
| None | 13 (1) | 7 (1.3) | 4 (0.4) | 4 (0.7) | ||
| RoPE score (9-point nonradiology) | ||||||
| Median (IQR) | 13 | 6 (5-7) | 6 (5-7) | 6 (6-7) | 6 (5-7) | <.001 |
| Mean (SD) | 6.3 (1.5) | 6.1 (1.3) | 6.4 (1.4) | 6.1 (1.3) | ||
Abbreviations: ASA, atrial septal aneurysm; MI, myocardial infarction; RoPE, Risk of Paradoxical Embolism; TIA, transient ischemic attack.
Missing (shunt size or ASA) n = 354.
A total of 121 recurrent ischemic strokes occurred during a median (IQR) follow-up of 57 (23.7-63.8) months. The annualized incidence of stroke was 1.09% (95% CI, 0.88%-1.36%) with medical therapy and 0.47% (95% CI, 0.35%-0.65%) with device closure (adjusted HR [aHR], 0.41 (95% CI, 0.28-0.60); P < .001).6
We found a significant interaction between the reduction of recurrent stroke with PFO closure and the 4 subgroups defined by shunt size and ASA (Table 2 and Figure). The reduction was greater with large shunt and ASA (aHR, 0.15 [95% CI, 0.06-0.35]), than large shunt without ASA (aHR, 0.27 [95% CI, 0.14-0.56]), small shunt with ASA (aHR, 0.36 [95% CI, 0.17-0.78]), and small shunt without ASA (aHR, 0.68 [95% CI, 0.41-1.13]) (interaction P = .02).
Table 2. Risk of Recurrent Stroke According to the Type of Septal Abnormalities.
| Factor | No. (imputed) | HR (95% CI) | 2-y | 5-y | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Medical therapy % | PFO closure % | ARR % (95% CI)a | NNT | Medical therapy % | PFO closure % | ARR (95% CI)a | NNT | ||
| Small shunt without ASA | 1501 | 0.74 (0.45 to 1.22) | 0.68 (0.41 to 1.13) | 3.1 | 2.1 | 1.0 (–0.7 to 2.7) | 100 | 4.2 | 3.1 | 1.0 (–1.2 to 3.2) | 98 |
| Small shunt with ASA | 622 | 0.33 (0.16 to 0.71) | 0.36 (0.17 to 0.78) | 3.2 | 2.2 | 1.0 (–1.7 to 3.7) | 96 | 4.6 | 2.2 | 2.4 (–0.9 to 5.6) | 42 |
| Large shunt without ASA | 1012 | 0.32 (0.16 to 0.63) | 0.27 (0.14 to 0.56) | 1.6 | 0.7 | 1.0 (–0.4 to 2.3) | 102 | 3.0 | 0.9 | 2.0 (0.2 to 3.9) | 49 |
| Large shunt with ASA | 605 | 0.14 (0.06 to 0.34) | 0.15 (0.06 to 0.35) | 5.9 | 0.4 | 5.5 (2.7 to 8.3) | 18 | 8.0 | 0.9 | 7.1 (3.7 to 10.6) | 14 |
| Interaction P value | NA | .01 | .02 | NA | NA | NA | NA | NA | NA | NA | NA |
Abbreviations: ARR, absolute risk reduction; ASA, atrial septal aneurysm, HR, hazard ratio; NA, not applicable; NNT, number needed to treat; PFO, patent foramen ovale.
Accounting for age, sex, prior myocardial infarction, diabetes, hypertension, hyperlipidemia, prior stroke or transient ischemic attack, smoking status, index event (stroke vs transient ischemic attack), and superficial infarction on neuroimaging (present vs absent).
Figure. Kaplan-Meier Curve of Recurrent Ischemic Stroke in the 4 Subgroups of Patients Defined by Shunt Size and Atrial Septal Aneurysm (ASA).
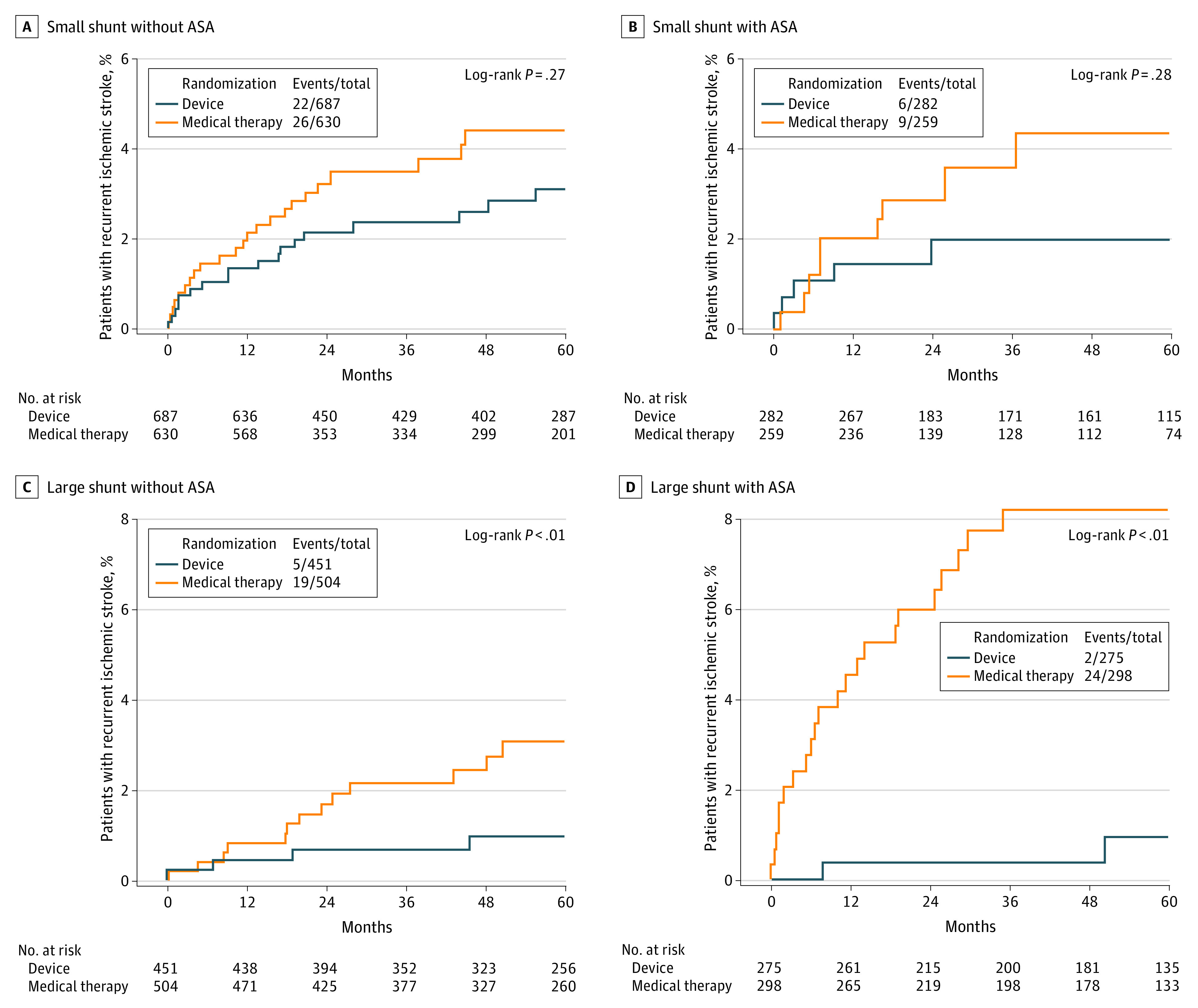
The 5-year absolute risk reduction of recurrent stroke was much higher in patients with large shunt and ASA (7%) than in other categories (1.0% to 2.4%) with a number of patients needed to treat of 14 compared with 42 to 98 in the other categories.
Discussion
This individual patient data meta-analysis indicates that the reduction in recurrent stroke risk with PFO closure varies depending on the PFO’s characteristics. Patients with both a large shunt and an ASA benefited more from PFO closure than patients with 1 or neither of these features. Patients with both a large shunt and an ASA had nominally both the highest risk of stroke recurrence when receiving medical treatment and lowest risk of stroke recurrence with PFO closure (Table 2).
Previous studies have shown that medically treated patients with both PFO and ASA have a higher risk of stroke recurrence compared with patients with PFO alone (irrespective of shunt size).9,10,11 Our exploratory results suggest that having both a large PFO and an ASA represents an especially high-benefit group, conferring a 2-year number needed to treat of 18 compared with 47 in possible and probable PASCAL categories.6 This finding may be useful in decision-making for otherwise procedure-averse patients.
It is important to note that findings from this study reinforce the appropriateness of using the PASCAL classification system to select patients for PFO closure. The PASCAL system already places all patients with both a large PFO and an ASA in categories appropriate for closure. The PASCAL system also provides guidance for management of the substantial population of patients who do not have any high-risk cardiac features, where the benefit of closure appears more uncertain using cardiac features alone. These patients are categorized as possible (and device treatment favorable) if the RoPE score is 7 or higher or unlikely (and device treatment unfavorable) if RoPE score below 7 (eTable in the Supplement).
As the presence of 1 or both high-risk traits may affect discussions with patients regarding treatment options, echocardiographic reports should routinely include the presence or absence of large-size shunt and ASA. Development and refinement of consensus definitions of shunt size and ASA by professional echocardiographic societies is desirable.
It is not yet clear how the presence of ASA and large shunt size interact to increase the risk of PFO-associated stroke. ASA may facilitate embolization through the large shunt by leading to more frequent and wider opening of the PFO channel or by directing flow from the inferior vena cava toward the foramen ovale. This action may be facilitated by a prominent Eustachian valve and Chiari network.12 Another potential mechanism is thrombus formation on the surface of the ASA13 or in the left atrium possibly mediated by ASA-related atrial dysfunction.14
Limitations
This study has limitations. First, definitions of some variables, such as large shunt and ASA, varied to a modest degree across trials. Nevertheless, this variation did not preclude robust results after pooling data across studies. Second, in several studies, transesophageal echo results were not reviewed by an independent core laboratory. Third, data regarding PFO and ASA were missing for 354 patients (9.5%), but trial procedures suggest these data are likely to be missing at random and unlikely to substantially modify the study results.
Conclusions
Among patients aged 18 to 60 years with PFO-associated stroke, those with both a large shunt and an ASA benefit more from device closure than patients with only 1 or neither of these features. This finding may inform shared patient-clinician decision-making and future trials testing closure in patients older than 60 years.
eAppendix 1. The Extended PFO-Associated Stroke Causal Likelihood (PASCAL) Classification System
eAppendix 2. The Risk of Paradoxical Embolism (RoPE) Score
eTable. PFO-Attributable Fraction by RoPE Score
eReferences.
References
- 1.Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318(18):1148-1152. doi: 10.1056/NEJM198805053181802 [DOI] [PubMed] [Google Scholar]
- 2.Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke. 2009;40(7):2349-2355. doi: 10.1161/STROKEAHA.109.547828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elgendy AY, Saver JL, Amin Z, et al. Proposal for updated nomenclature and classification of potential causative mechanism in patent foramen ovale-associated stroke. JAMA Neurol. 2020;77(7):878-886. doi: 10.1001/jamaneurol.2020.0458 [DOI] [PubMed] [Google Scholar]
- 4.Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013;81(7):619-625. doi: 10.1212/WNL.0b013e3182a08d59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabanes L, Mas JL, Cohen A, et al. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age: a study using transesophageal echocardiography. Stroke. 1993;24(12):1865-1873. doi: 10.1161/01.STR.24.12.1865 [DOI] [PubMed] [Google Scholar]
- 6.Kent DM, Saver JL, Kasner SE, et al. Heterogeneity of treatment effects in an analysis of pooled individual patient data from randomized trials of device closure of patent foramen ovale after stroke. JAMA. 2021;326(22):2277-2286. doi: 10.1001/jama.2021.20956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart GB, Altman DG, Askie LM, Duley L, Simmonds MC, Stewart LA. Statistical analysis of individual participant data meta-analyses: a comparison of methods and recommendations for practice. PLoS One. 2012;7(10):e46042. doi: 10.1371/journal.pone.0046042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riley RD, Legha A, Jackson D, et al. One-stage individual participant data meta-analysis models for continuous and binary outcomes: comparison of treatment coding options and estimation methods. Stat Med. 2020;39(19):2536-2555. doi: 10.1002/sim.8555 [DOI] [PubMed] [Google Scholar]
- 9.Mas JL, Arquizan C, Lamy C, et al. ; Patent Foramen Ovale and Atrial Septal Aneurysm Study Group . Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345(24):1740-1746. doi: 10.1056/NEJMoa011503 [DOI] [PubMed] [Google Scholar]
- 10.Turc G, Lee JY, Brochet E, Kim JS, Song JK, Mas JL; CLOSE and DEFENSE-PFO Trial Investigators . Atrial septal aneurysm, shunt size, and recurrent stroke risk in patients with patent foramen ovale. J Am Coll Cardiol. 2020;75(18):2312-2320. doi: 10.1016/j.jacc.2020.02.068 [DOI] [PubMed] [Google Scholar]
- 11.Lefebvre B, Naidu S, Nathan AS, et al. Impact of echocardiographic parameters on recurrent stroke in the randomized REDUCE PFO Cryptogenic Stroke Trial. Struct Heart. 2021;5(4):367-375. doi: 10.1080/24748706.2021.1907639 [DOI] [Google Scholar]
- 12.Schuchlenz HW, Saurer G, Weihs W, Rehak P. Persisting eustachian valve in adults: relation to patent foramen ovale and cerebrovascular events. J Am Soc Echocardiogr. 2004;17(3):231-233. doi: 10.1016/j.echo.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 13.Silver MD, Dorsey JS. Aneurysms of the septum primum in adults. Arch Pathol Lab Med. 1978;102(2):62-65. [PubMed] [Google Scholar]
- 14.Rigatelli G, Aggio S, Cardaioli P, et al. Left atrial dysfunction in patients with patent foramen ovale and atrial septal aneurysm: an alternative concurrent mechanism for arterial embolism? JACC Cardiovasc Interv. 2009;2(7):655-662. doi: 10.1016/j.jcin.2009.05.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. The Extended PFO-Associated Stroke Causal Likelihood (PASCAL) Classification System
eAppendix 2. The Risk of Paradoxical Embolism (RoPE) Score
eTable. PFO-Attributable Fraction by RoPE Score
eReferences.


