The presence of right ventricular (RV) injury in the context of acute respiratory distress syndrome (ARDS) is significantly associated with increased mortality posing a clinical challenge [1]. In a recent systematic review and meta-analysis of nine ARDS studies (n = 1861), RV injury defined as RV dysfunction, acute cor-pulmonale (ACP), RV dysfunction with hemodynamic compromise, or RV failure, was present in 21% of the cohort [1]. In this state-of-the-art concise article, we aim to discuss the potential mechanisms of abnormal RV biomechanics and the spectrum of RV injury phenotypes in ARDS. Understanding the pathophysiology and natural history of RV injury may inform the intensivist’s approach to diagnosis and RV monitoring, and application of personalized interventions with potential therapeutic relevance.
Right ventricular injury definition and phenotypes in ARDS
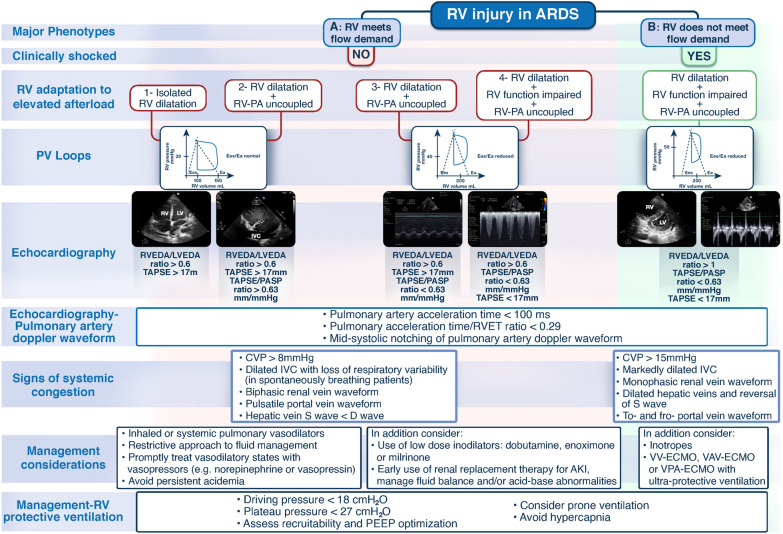
A universally accepted RV injury definition does not exist. The majority of proposed definitions focus on advanced-stage RV injury which may be refractory to treatment [2]. Three distinct RV injury phenotypes (normal RV function, RV dilatation, RV dilatation with impaired systolic function) have been identified recently in patients with coronavirus disease 2019 (COVID-19)-related ARDS and found to be associated with distinguishable clinical outcomes [3]. In a recent multi-national study which included patients with COVID-19-related ARDS, the presence of the ACP phenotype of RV injury (defined as right to left ventricular end-diastolic area ratio (RVEDA/LVEDA) > 0.6 with paradoxical septal motion) was associated with increased mortality [4]. In cases of isolated RV dilatation or RV dilatation with impaired function with or without evidence of venous congestion (assessed by surrogate static hemodynamic parameters e.g. central venous pressure ≥ 8 mmHg, right-sided venous flow patterns e.g. in hepatic, portal and intra-renal veins or inferior vena cava (IVC) size and respirophasic IVC variation assessed by echocardiography), the RV may still meet flow demands occasionally by excessively using the Frank–Starling mechanism (Fig. 1) [2, 5, 6]. In the presence of venous congestion, secondary organ injury (kidney and/or liver) may develop due to decreased perfusion pressure (mean arterial pressure minus venous pressure) [6]. Right ventricular dilatation with impaired function and the inability of the RV to meet flow demands despite excessive use of Frank–Starling mechanism leading to systemic shock could be seen as advanced-stage RV injury/RV failure [2–4]. It should be noted, however, that RV injury phenotypes in ARDS, phenotypic endpoints, and transition through phenotypes over time are not large-scale data-derived. There may be an overlap between different stages of RV injury or absence of RV pathology at initial hemodynamic evaluation and development of RV injury later in the intensive care unit episode particularly when respiratory function deteriorates [7]. This is likely related to the underlying mechanisms of RV injury in ARDS making temporal characterization of RV injury rather challenging even when serial evaluation of the RV (e.g. by echocardiography) is performed [7].
Fig. 1.
Proposed theoretical RV injury major phenotypes based on RV biomechanics as assessed by clinical examination, RV P–V relationship at different loading conditions, and echocardiography. It should be noted that the different methods and combination of parameters proposed to differentiate the different phenotypes and management strategies need to be validated in prospective studies since this is not a ‘standard’ time sequence regularly encountered on clinical grounds; in practice, the hemodynamic assessment is often performed at various timepoints in the RV injury course. The RV P–V loops show the RV pressure–volume relation at different loading conditions wherein the RV may or may not meet flow demand. Assessment of systemic venous congestion using central venous pressure and right-sided venous flow patterns in hepatic, portal and intra-renal veins, and IVC may also be part of RV injury severity evaluation (morphology of S and D venous Doppler waveforms, during RV contraction and relaxation respectively, for hepatic and intra-renal veins; pulsatility or interruption of flow giving a to-and-fro appearance, for portal vein; and size and respirophasic variation, for IVC). AKI acute kidney injury, ARDS acute respiratory distress syndrome, CVP central venous pressure, COVID-19 coronavirus disease-19, Ea pulmonary arterial elastance, ECMO extracorporeal membrane oxygenation, Ees right ventricular end-systolic elastance, IVC inferior vena cava, LV left ventricle, LVEDA left ventricular end-diastolic area, PV loops pressure–volume loops, PA pulmonary artery, PASP pulmonary artery systolic pressure, PEEP positive end-expiratory pressure, RV right ventricle, RVEDA right ventricular end-diastolic area, RVET right ventricle ejection time, TAPSE tricuspid annular plane systolic excursion, VAV-ECMO veno-arterio-venous extracorporeal membrane oxygenation, VPA- ECMO veno-pulmonary arterial extracorporeal membrane oxygenation, VV-ECMO veno-venous extracorporeal membrane oxygenation
Mechanisms of right ventricular injury in ARDS
Cardiovascular mechanisms and right ventricular-pulmonary arterial coupling
The interplay between the RV and pulmonary arterial (PA) circulation under different loading conditions, namely RV-PA coupling, determines the mechano-energetic relationship between RV contractility, (measured by end-systolic elastance (Ees)) and PA afterload (measured by pulmonary arterial elastance (Ea)) [4–6] (Fig. 1). It should be emphasized that RV afterload refers to pulmonary vascular vasomotor tone, a composite of a resistive and pulsatile component, and not pulmonary vascular resistance only [8]. The system is coupled when Ees/Ea ratio is > 1 [4, 5]. Acute pulmonary vascular dysfunction due to obstruction (driven by thrombosis and remodeling), compression (driven by pulmonary edema and invasive ventilation), and constriction (driven by hypoxemia, hypercapnia, acidemia) leads to development of acute pulmonary arterial hypertension [2, 8]. Acutely elevated PA pressure results in augmentation of the intrinsic RV contractile force to compensate for the increase in RV load (homeometric adaptation or Anrep mechanism) which in critical illness may be limited due to systemic inflammation and hypotension [2, 8]. Subsequent RV dilatation to preserve blood flow (heterometric adaptation), and reduction in Ees/Ea ratio (< 1, caused by elevated Ea) may lead to RV-PA uncoupling and negative diastolic interaction between the RV and LV with the RV end-diastolic pressure exceeding LV end-diastolic pressure, adversely affecting LV filling and cardiac output [2, 8]. Coronary ischemia ensues due to reduced cardiac output and systemic arterial pressure, reduced coronary perfusion pressure, and systemic or supra-systemic RV pressure leading to perfusion of the RV myocardium in diastole only [2]. The resultant negative systolic interaction between the RV and LV worsens RV-PA uncoupling causing spiraling systemic shock and inability of the RV to meet flow demands (advanced-stage RV injury/RV failure) (Fig. 1) [2, 8, 9]. Although the concept of RV-PA uncoupling seems physiologically sound, the point of acute transition from homeometric to heterometric RV adaptation and acute decompensation leading to advanced-stage RV injury and shock cannot be predicted. Additionally, the non-linear adaptation of the RV to altered loading conditions coupled with the limited intrinsic RV contractility reserve seen in critical illness could potentially explain why identifying a temporal sequence (chronology) of distinct RV hemodynamic /echocardiography phenotypes in ARDS is a real challenge thus making RV injury a complex clinical syndrome [2, 8, 9].
Heart–lung ‘cross talk’ and iatrogenic mechanisms
Right ventricular afterload is highest at the extremes of lung volume [2, 10]. Patients with severe ARDS have significantly reduced functional residual capacity (FRC) and the non-physiological stress (transpulmonary pressure) and strain (tidal volume to FRC ratio) induced by invasive ventilation may cause elevated RV afterload when transpulmonary pressure exceeds pulmonary venous pressure [2, 10]. Low lung volumes cause terminal airway and extra-alveolar vessel collapse whereas at high lung volumes there is an increase in alveolar wall tension and intra-alveolar vessel collapse, both resulting in elevated RV afterload [2, 10]. Driving pressure (plateau pressure minus positive-end expiratory pressure) ≥ 18 cm H2O has been identified as a risk factor for the development of RV injury (ACP) in moderate-severe ARDS [11]. Besides, intrinsic RV contractility defect in the context of critical illness or septic cardiomyopathy can worsen RV function.
A multimodal approach to diagnosis and monitoring of RV injury
The matching of RV contractility and afterload and the adaptation of the RV to acute changes in RV afterload in ARDS may be assessed using a combination of echocardiography, pulmonary hemodynamic measurements, and clinical examination (Fig. 1). RV-PA coupling may be assessed non-invasively using echocardiography markers (tricuspid annular plane systolic excursion (TAPSE)/systolic PA pressure (PASP) ratio). Although TAPSE/PASP ratio has been strongly associated with RV-PA coupling and outcomes in COVID-19-related ARDS, there is a need for multicenter prospective studies to prove this relationship [9]. Echocardiography derived diastolic and systolic parameters and RV diastolic pressure waveform morphology using a pulmonary artery catheter with RV port (Edwards Lifescience, Irvine, CA) may be utilized to evaluate RV-PA coupling, grade severity, and phenotype RV injuries [12]. This approach may enable monitoring of the progression of RV injury and pulmonary vascular dysfunction, and dynamic assessment of the response to therapeutic interventions [12]. The benefit of the proposed multimodal diagnostic approach, however, should be tested in large prospective studies.
Management strategies in RV injury
Pharmacological management of RV injury in ARDS includes: vasoactive agents which improve RV-PA coupling (e.g. inodilators), maintain coronary perfusion pressure by keeping systemic pressure above PA pressure (e.g. vasopressors), and drugs that reduce RV afterload and stroke work (e.g. pulmonary vasodilators) (Fig. 1) [2, 13]. Non-pharmacological potential therapies include: (a) RV-protective ventilation [14]; (b) prone positioning [14]; and (c) extracorporeal membrane oxygenation ((ECMO) veno-venous, veno-arterio-venous or veno-pulmonary arterial). It would make physiological sense that reversal of factors which increase RV afterload (hypoxemia, hypercapnia, acidemia) facilitated by ECMO, combined with low stress/strain (i.e. ‘RV-protective’) ventilation may aid in unloading the ‘injured’ RV (Fig. 1). [15]. Given the effect of RV injury on mortality and the physiological benefits of ECMO, future prospective research should evaluate whether the subset of patients with severe ARDS and RV injury should be targeted when selecting patients for ECMO support [16]. The aforementioned approaches and in particular the mechanical RV support options are largely based on understanding of disease mechanisms and strong physiological rationale rather than evidence-based medicine and the proposed algorithm (Fig. 1) remains to be strictly validated.
In conclusion, RV injury should be routinely sought for in ARDS since it is prognostic and may lead to altered management with potential outcome benefits. Understanding the mechanisms of RV injury in particular RV-PA uncoupling, may inform the type and timing of hemodynamic interventions and adjunctive therapies applied to protect and ‘resuscitate’ the RV (Fig. 1). A multi-modal diagnostic and monitoring approach potentially aids in RV injury phenotyping and understanding response to therapies, better personalization of care and prognostic enrichment of patient populations for future studies.
Author contributions
VZ wrote the first draft, HY and MS reviewed the manuscript and made important edits and revisions.
Funding
The authors did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflicts of interest
VZ is the chair and HY co-chair of the Protecting the Right Ventricle Network (PRORVnet), with MS as a member. MS reports lecture fees from Getinge, Drager, and Xenios outside the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sato R, Dugar S, Cheungpasitporn W, Schleicher M, Collier P, Vallabhajosyula S, Duggal A. The impact of right ventricular injury on the mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care. 2021;25:172. doi: 10.1186/s13054-021-03591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vieillard-Baron A, Naeije R, Haddad F, Bogaard HJ, Bull TM, Fletcher N, Lahm T, Magder S, Orde S, Schmidt G, Pinsky MR. Diagnostic workup, etiologies and management of acute right ventricle failure: a state-of-the-art paper. Intensive Care Med. 2018;44:774–790. doi: 10.1007/s00134-018-5172-2. [DOI] [PubMed] [Google Scholar]
- 3.Chotalia M, Ali M, Alderman JE, Patel JM, Parekh D, Bangash MN. Cardiovascular subphenotypes in patients with COVID-19 pneumonitis whose lungs are mechanically ventilated: a single-centre retrospective observational study. Anaesthesia. 2022;77:763–771. doi: 10.1111/anae.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang S, Vignon P, Mekontso-Dessap A, Tran S, Prat G, Chew M, Balik M, Sanfilippo F, Banauch G, Clau-Terre F, Morelli A, De Backer D, Cholley B, Slama M, Charron C, Goudelin M, Bagate F, Bailly P, Blixt PJ, Masi P, Evrard B, Orde S, Mayo P, McLean AS, Vieillard-Baron A, ECHO-COVID research group Echocardiography findings in COVID-19 patients admitted to intensive care units: a multi-national observational study (the ECHO-COVID study) Intensive Care Med. 2022;48:667–678. doi: 10.1007/s00134-022-06685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vieillard-Baron A, Prigent A, Repessé X, Goudelin M, Prat G, Evrard B, Charron C, Vignon P, Geri G. Right ventricular failure in septic shock: characterization, incidence and impact on fluid responsiveness. Crit Care. 2020;24:630. doi: 10.1186/s13054-020-03345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiegel R, Teeter W, Sullivan S, Tupchong K, Mohammed N, Sutherland M, Leibner E, Rola P, Galvagno SM, Jr, Murthi SB. The use of venous Doppler to predict adverse kidney events in a general ICU cohort. Crit Care. 2020;24:615. doi: 10.1186/s13054-020-03330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evrard B, Goudelin M, Giraudeau B, François B, Vignon P. Right ventricular failure is strongly associated with mortality in patients with moderate-to-severe COVID-19-related ARDS and appears related to respiratory worsening. Intensive Care Med. 2022;48:765–767. doi: 10.1007/s00134-022-06730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol. 2017;69:236–243. doi: 10.1016/j.jacc.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 9.D'Alto M, Marra AM, Severino S, Salzano A, Romeo E, De Rosa R, Stagnaro FM, Pagnano G, Verde R, Murino P, Farro A, Ciccarelli G, Vargas M, Fiorentino G, Servillo G, Gentile I, Corcione A, Cittadini A, Naeije R, Golino P. Right ventricular-arterial uncoupling independently predicts survival in COVID-19 ARDS. Crit Care. 2020;24:670. doi: 10.1186/s13054-020-03385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vieillard-Baron A, Loubieres Y, Schmitt JM, Page B, Dubourg O, Jardin F. Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol. 1999;87:1644–1650. doi: 10.1152/jappl.1999.87.5.1644. [DOI] [PubMed] [Google Scholar]
- 11.Mekontso Dessap A, Boissier F, Charron C, Bégot E, Repessé X, Legras A, Brun-Buisson C, Vignon P, Vieillard-Baron A. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med. 2016;42:862–870. doi: 10.1007/s00134-015-4141-2. [DOI] [PubMed] [Google Scholar]
- 12.Raymond M, Grønlykke L, Couture EJ, Desjardins G, Cogan J, Cloutier J, Lamarche Y, L'Allier PL, Ravn HB, Couture P, Deschamps A, Chamberland ME, Ayoub C, Lebon JS, Julien M, Taillefer J, Rochon A, Denault AY. Perioperative right ventricular pressure monitoring in cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33:1090–1104. doi: 10.1053/j.jvca.2018.08.198. [DOI] [PubMed] [Google Scholar]
- 13.Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care. 2010;14:R169. doi: 10.1186/cc9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paternot A, Repessé X, Vieillard-Baron A. Rationale and description of right ventricle-protective ventilation in ARDS. Respir Care. 2016;61:1391–1396. doi: 10.4187/respcare.04943. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M, Tachon G, Devilliers C, Muller G, Hekimian G, Bréchot N, Merceron S, Luyt C-E, Trouillet J-L, Chastre J, Leprince P, Combes A. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med. 2013;39:838–846. doi: 10.1007/s00134-012-2785-8. [DOI] [PubMed] [Google Scholar]
- 16.Petit M, Mekontso-Dessap A, Masi P, Legras A, Vignon P, Vieillard-Baron A. Evaluation of right ventricular function and driving pressure with blood gas analysis could better select patients eligible for VV ECMO in severe ARDS. Crit Care. 2021;25:220. doi: 10.1186/s13054-021-03646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]