Abstract
Background
Stroke has a deleterious impact on human health due to its high incidence, degree of disabling sequelae and mortality, constituting one of the main causes of death and disability worldwide.
Objectives
This study aimed to assess the efficacy and safety of very early mobilization (VEMG) after thrombolysis in functional recovery in patients with acute ischemic stroke.
Methods
The present study was an open, prospective, randomized study, with no blinded outcome, carried out in the stroke unit of a tertiary referral hospital located in Salvador-Bahia, Brazil. The primary outcome was the level of functional independence. Secondary outcomes were functional mobility, balance, complications within 7 days of hospitalization and 90 days after hospital discharge, and length of stay.
Outcomes
A total of 104 patients with ischemic stroke who received thrombolytic treatment between August 2020 and July 2021 were prospectively recruited to the study. Of these, 51 patients received VEMG within 24 h of the ictus and another 53 patients receiving usual care (UCG) with mobilization 24 h after the ictus. When compared to the usual care, the VEMG group was not associated with a significant reduction in the risk of the primary outcome (relative risk [95% confidence intervals]: 0.74 [0.339–1.607]) or any of the secondary outcomes.
Conclusion
In this study, the strategy of early mobilization after thrombolysis in ischemic stroke was safe, but without evidence of short-term benefit. Brazilian Registry of Clinical Trials under the registry (registry number: RBR-8bgcs3).
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-022-11411-5.
Keywords: Stroke, Early mobilization, Stroke rehabilitation, Motor recovery
Introduction
Practical clinical guidelines recommend two key treatments for acute ischemic stroke: care in units specializing in stroke management and thrombolysis with recombinant tissue plasminogen activator (rtPA). Thrombolysis is a specific treatment for acute ischemic stroke when given within the first 4.5 h of ischemic stroke onset [1].
It may be administered to patients who wake up with stroke symptoms (wake-up stroke) or have an uncertain onset time > 4.5 h since last seen asymptomatic, when administered within 4.5 h of recognition of stroke symptoms, if NIHSS < 25 [2]. Or after 4.5 h if a mismatch is found on MRI between flair and diffusion [3].
The benefits of are well defined, however, thrombolysis has side effects and patients are carefully selected due to the risk of secondary intracranial hemorrhage, which is one of the main limiting factors for the use of this therapy in a broader spectrum of patients. Smoking, atrial fibrillation, National Institutes of Health Stroke Scale (NIHSS) score before thrombolysis ≥ 17, and high systolic blood pressure 2 h after thrombolysis are amongst the risk factors for bleeding after intravenous thrombolysis. Age is not considered a contraindication, and the use of thrombolysis is recommended regardless of age [4–6].
Starting rehabilitation in the acute phase is important both to prevent and treat complications and to improve functional independence. However, several components of rehabilitation are still poorly defined, with early mobilization being among these and further, with regard to mobilization of thrombolyzed patients, some protocols include 24 h of bed rest after treatment [1].
In theory, the best period for brain repair may be in a very narrow window after the onset of the stroke, as brain function is plastic and reorganizable and may change in structure and function to adapt to post-injury needs. early mobilization is an important factor in the functional recovery of the central nervous system [7–9].
The largest study carried out on very early mobilization in stroke found negative results at 3 months, but in the subgroup of patients who underwent thrombolytic treatment, it was not possible to say that early mobilization is harmful [10].
The main objective of mobilization is the prevention of complications and, therefore, our hypothesis is that very early mobilization in stroke patients undergoing thrombolytic treatment may associate the benefits of recanalization promoted by thrombolysis with the benefits of mobilization in a small time window after stroke, reducing the occurrence of complications and accelerating functional recovery.
Therefore, this study aimed to assess the efficacy and safety of very early mobilization (VEMG) after thrombolysis in functional recovery in patients with acute ischemic stroke.
Materials and methods
Study design
The present study was an open-label, prospective randomized trial without blinded outcome, performed in the stroke unit (SU) of a tertiary referral hospital located in Salvador, Bahia, Brazil. The report follows the CONSORT instruction (Supplementary Fig. 1).
Participants
The inclusion criteria include patients older than 18 years of age, diagnosed with acute ischemic stroke who had undergone intravenous thrombolytic therapy, hemodynamically stable, oxygen saturation > 92%, cognitively preserved (being able to respond to the examiner’s command), and who had previously reported a modified Rankin Score (mRS) (mRS ≤ 2) [11].
Exclusion criteria were any of the following: if your condition deteriorated within the first hour of hospital admission, verified by values greater than or equal to 1 on the National Institutes of Health Stroke Scale level 1c (NIHSS 1c ≥ 1), transient ischemic attacks, concomitant progressive neurological disorder, and condition unstable coronary. e.g., acute myocardial infarction) or other medical conditions that could pose a danger to the patient or if their physiological variables (blood pressure, oxygen saturation, heart rate, temperature) are beyond established safety limits, heart failure severe, lower limb fracture, mobility impairment, as well as those patients with terminal cancer.
Interventions
The intervention group (very early mobilization group; VEMG) received treatment as soon as possible after recruitment, with the aim of mobilizing within 12 h after thrombolysis. The time spent in early mobilization activities was 15 min, and mobilization was performed twice a day for 7 days or until discharge (whichever comes first), with the following activities performed: three sets of ten repetitions of bridging exercises (in the supine position on the bed, with knees bent and hands at the side, the patient is asked to lift the hip, using the hands and feet for support, until the trunk is more or less in a straight line with legs, then slowly lower), sitting in bed with lower limbs hanging, standing, ambulation and functional activities for the upper limbs.
Patients in the usual care group (UCG) group were mobilized 24 h after thrombolysis and received routine care in the stroke unit, including active mobilization (if possible), correct bed positioning, bed mobilization, sitting balance training, limb and trunk control activities, and ambulation for 45 min a day, for 7 days or until discharge.
Outcomes
The primary outcome was defined as a favorable outcome in the degree of disability and dependence on activities of daily living at 7 days or at discharge (whichever occurred before) and at 90 days after hospital discharge, measured using mRS, which is an ordinal scale ranging from 0 (no disability) to 5 (severe disability), with a score of 6 allocated to those who died [11].
The secondary outcomes evaluated were functional mobility, balance, complications (decreased level of consciousness, pulmonary embolism, delirium, hemodynamic instability, renal dysfunction, respiratory tract infection, deep vein thrombosis, headache, and cardiorespiratory arrest), and length of hospital stay.
To assess neurological severity, the NIHSS was used, which is a systematic instrument used to describe stroke-related deficits, monitor the patient's neurological status, and analyze the severity of the injury. The scale is composed of 11 categories, with 15 items of neurological examination, scored from zero to four depending on the item. The total score ranges from 0 to 42 points; the higher the score, the worse the severity of the disease [12].
The Berg Balance Scale (BBS) and Timed Up and Go test (TUG) were used to assess balance and functional mobility, respectively. The BBS is used to assess the elderly and individuals with balance deficits, for example, after stroke, regardless of their age. The cutoff point adopted was a score of 45 points, and lower values were suggestive of a high risk of falls [13].
The TUG assesses an individual’s mobility level by measuring the time taken to get up from the chair, walk a distance of three meters, turn around, and return to the place and position of origin. The cutoff point used for this test was 14 s, which was also mentioned in another study carried out with older individuals hospitalized in an SU, thus indicating a greater risk of falls [14].
The scales were applied on three occasions: admission to the unit, if they were able to do so, after completing 7 days of hospitalization in the unit or at the time of hospital discharge (whichever occurred before) and 90 days after hospital discharge.
Baseline patient characteristics were collected, including age, sex, and stroke territory, in addition to risk factors (hypertension, diabetes, cardiovascular disease, and smoking) and premorbid disability (as per the mRS).
Sample size
To define the sample size, an internal pilot study was carried out with 70 participants to calculate 02 proportions based on the Modified Rankin Score transformed into a dichotomous variable, where mRS values ≤ 2 were defined as a favorable outcome in up to 07 days of hospitalization, with favorable outcome proportions of 39.4% in the very early mobilization group and 67.6% in the usual care group. The sample size was calculated, along with these proportions. Adopting a 1:1 sample ratio, a total of 48 patients were required for each group with a two-sided significance of 0.05 (alpha 5%) and a power of 0.8 (80%).To perform the calculation, the Bioestat 5.3 Software was used [15].
Randomization
After agreeing to participate in the study, patients were randomly allocated by a block randomization process, where the randomization list was a random sequence of blocks of participants, with blocks of predetermined size with four or six participants, ensuring that the group intervention and control groups were balanced in terms of the number of participants, using a randomized sequence generated by free online software (Random.org), using a hidden opaque envelope method.
The randomization sequence was generated by a researcher who was not involved in the enrolment of the participants in the study.
Statistical analysis
Descriptive statistics were used to analyze demographic and clinical characteristics. Continuous data were presented as mean and standard deviation or median and IQI (25–75%), and categorical data were presented as numbers and percentages. Differences in severity scores, degree of disability and dependence for activities of daily living, balance and functional mobility, respectively, obtained on the 7th day or hospital discharge and 90 days after hospital discharge were compared between groups using the Mann–Whitney test. To assess the results of the study, the relative risk between the two groups was used. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS), version 16.0 (SPSS Inc., Chicago, IL, USA). The significance level was set at 5%.
Ethical aspects
The study was approved by the Research Ethics Committee of Roberto Santos General Hospital under the number CAAE: 87,271,218.0.0000.5028 and Opinion No. 3.447.930. Informed consent was obtained from all the patients or their representatives at baseline. No adverse events were associated with study participation. The present study is registered in the Brazilian Registry of Clinical Trials under the registry (registry number: RBR-8bgcs3).
Results
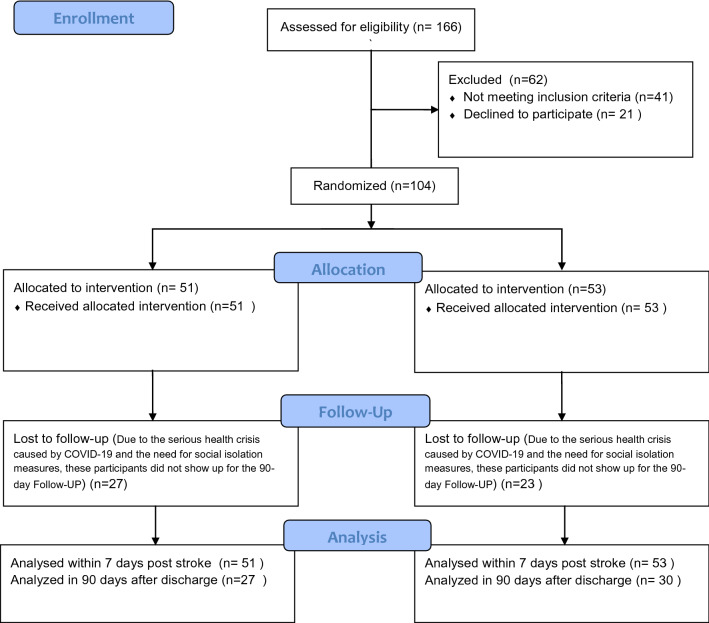
Prospectively, from August 2020 to July 2021, a total of 166 patients were evaluated for eligibility, where 104 patients with ischemic stroke who received thrombolytic treatment were recruited to this study, including 51 patients who received very early mobilization within 24 h after the ictus and another 53 patients who received care usual with mobilization 24 h after the ictus (Fig. 1).
Fig. 1.
Flow of participants in the study
Among the recruited patients, nine were excluded by the established exclusion criteria (one from the VEMG, five from the UCG, and one and two deaths in the VEMG and UCG, respectively). There were no statistical differences in age between the two groups, and the main risk factor for stroke in both groups was systemic arterial hypertension (SAH), followed by smoking and diabetes mellitus. The level of severity on admission, as assessed using the NIHSS, was predominantly mild-to-moderate in both groups (Table 1).
Table 1.
Baseline characteristics of the groups
| Very early mobilization group (N = 51) | Usual care group (N = 53) | |
|---|---|---|
| Age | ||
| Mean (SD) | 61.80 (13.03) | 58.89 (12.16) |
| Sex | ||
| Male, no. (%) | 27 (52.9) | 28 (52.8) |
| Affected side | ||
| Right, no. (%) | 30 (58.8) | 27 (50.9) |
| Stroke risk factors, no. (%) | ||
| Hypertension | 38 (74.5) | 43 (81.1) |
| Diabetes mellitus | 14 (27.5) | 15 (28.3) |
| HFrEF | 3 (5.9) | 3 (5.7) |
| Atrial fibrillation | 6 (11.8) | 5 (9.4) |
| Coronary disease | 3 (5.9) | 3 (5.7) |
| Dyslipidemia | 4 (7.8) | 4 (7.5) |
| Smoking | 22 (43.1) | 20 (37.7) |
| Severity (NIHSS), no. (%) | ||
| Light (0–7) | 36 (70.6) | 39 (73.6) |
| Moderate (8–16) | 14 (27.5) | 13 (24.5) |
| Severe (> 16) | 1 (2.0) | 1 (1.9) |
| Pre-morbidity score (mRS), no. (%) | ||
| 0 | 49 (96.1) | 51 (96.2) |
| 1 | 1 (2.0) | 1 (1.9) |
| 2 | 1 (2.0) | 1 (1.9) |
| Systolic pressure at admission (mm Hg) | ||
| Mean (SD) | 157.30 (23.74) | 158.80 (28.25) |
| Diastolic pressure at admission (mm Hg) | ||
| Mean (SD) | 88.18 (14.11) | 91.53 (18.25) |
| Time to thrombolysis (hours)* | ||
| Median (IQR) | 3.10 (2.26–4.00) | 3.08 (2.50–3.50) |
| Time for first mobilization after stroke (hours) | ||
| Mean (SD) | 12.24(5.00) | 29.14(5.82) |
HFrEF heart failure with reduced ejection fraction, SD standard deviation, IQR interquartile range, mRS modified rankin scale, NIHSS national institutes of health stroke scale
Another important aspect was the pre-morbidity score using mRS, where most patients in both groups claimed to have a score of 0 (zero), that is, without previous disabilities. In addition, there were no significant differences between blood pressure levels at admission, in the window for thrombolysis, or in the length of hospital stay.
When comparing the severity between the two groups, the difference between the level of functional independence, and balance at admission and discharge were used. It was noted that there were differences in the severity assessed through the NIHSS between the groups, with the UCG presenting a lower level of severity on admission. However, in both groups, the medians were within the range of classification of mild stroke. Moreover, in both groups, there were gains in terms of severity (NIHSS), level of functional independence (mRS), and balance (BBS) at discharge when compared to admission scores, with no differences between groups (Table 2).
Table 2.
Comparison of the scores of the assessment instruments
| Very early mobilization group (N = 51) | Usual care group (N = 53) | P* | |
|---|---|---|---|
| NIHSS | |||
| Admission | 5 (3–9) | 3 (1–8) | 0.01 |
| Hospital discharge | 2 (1–4) | 1 (0–4) | 0.91 |
| Difference (discharge–admission) | 2 (0–3) | 1 (0–2) | 0.09 |
| Difference (discharge-90 days) (N = 54) | 3 (1–5) | 2 (0–4) | 0.69 |
| mRS | |||
| Admission | 4 (1–4) | 2 (1–4) | 0.24 |
| Hospital discharge | 2 (0–5) | 2 (0–6) | 0.57 |
| Difference (discharge–admission) | 0 (0–1) | 0 (0–1) | 0.73 |
| Difference (discharge-90 days) (N = 54) | 1 (1–2) | 1 (0–1) | 0.15 |
| BBS | |||
| Admission | 38 (5–50) | 46 (17–54) | 0.47 |
| Hospital discharge | 44 (14–54) | 53 (28–55) | 0.11 |
| Difference (discharge–admission) | 3 (0–6) | 1 (0–5) | 0.14 |
| Difference (discharge-90 days) (N = 54) | 14 (4–26) | 3 (1–8) | 0.40 |
BBS berg balance scale, mRS modified rankin scale, NIHSS national institutes of health stroke scale
*Mann–Whitney U test
Owing to the serious COVID-19 pandemic that began in Brazil in March 2020, which brought about restrictive measures and social distancing to minimize the risks of spreading this disease, only 54 patients selected in the study returned for follow-up after 90 days.
For the primary endpoint assessed, a favorable outcome was defined as an mRS score of ≤ 2 (no disability or minimal disability) and an unfavorable outcome score of 3–6 (moderate to severe disability or death). It was observed that most participants (56.7% within 7 days after the stroke and 75.92% within 90 days after discharge, respectively) had no or minimal disability at discharge and, when compared between in both groups, no increased risk was observed between the groups within seven days and after 90 days of discharge (p = 0.44 and p = 0.15, respectively) (Table 3).
Table 3.
Outcome in up to 7 days of hospitalization and in 90 days
| Very early mobilization group (N=51) | Usual care group (N=53) | Relative risk (95% confidence interval) | P* | |
|---|---|---|---|---|
|
Primary outcome 7 days or discharge (N=104) mRS ≤ 2 |
27 (52.9%) | 32 (60.4%) | 0.74 (0.339–1.607) | 0.44 |
|
90 days (N=54) mRS ≤ 2 |
16 (66.7%) | 25 (83.3%) | 0.400 (0.111–0.441) | 0.15 |
|
Favorable secondary outcomes 7 days or discharge (N=104) TUG ≤14 seconds(N=61) BBS ≥45 (N-89) No complications (N-104) |
22 (81.5%) 21 (47.7%) 41 (80.4%) |
24 (70.6%) 28 (62.2%) 40 (75.5%) |
1.833 (0.542–6.207) 1.804 (0.775–4.197) 1.230 (0.478–3.166) |
0.33 0.17 0.55 |
|
90 days TUG ≤14 seconds (N=52) BBS ≥45 (N-54) No complications (N-54) |
17 (73.9%) 16 (66.7%) 22 (91.7%) |
23 (79.3%) 24 (80.0%) 26 (86.7%) |
0.739 (0.203–2.695) 2.00 (0.583–6.864) 0.59 (0.099–3.539) |
0.65 0.27 0.56 |
|
Length of hospital stay (days) Median (IQR)** |
6.0 (4.0–7.0) | 5.0 (4.0–8.0) | 0.69 |
BBS berg balance scale, mRS modified rankin scale, NIHSS national institutes of health stroke scale, TUG timed up and go scale
*Chi-squared test
**Mann–Whitney test
As secondary outcomes, favorable results for functional mobility, assessed using the TUG and balance assessed using the BBS, were considered together with complications and length of hospital stay, with no significant differences being found between groups within 7 days after stroke and 90 days after hospital discharge (Table 3).
Discussion
Exercise protocols are already used in other cardiovascular pathologies and present good results in several components of rehabilitation [16].
In this study, we observed that the post-thrombolysis early mobilization protocol was safe and effective to get the patient out of bed and get the patient to perform sitting, standing, and walking activities earlier than with usual care, but it was not capable of showing superiority up to 7 days after the stroke and 90 days after hospital discharge when compared to usual care regarding the degree of disability and dependence on activities of daily living, functional mobility, balance and complications.
The results of this study are in agreement with a systematic review that found no evidence of superiority of mobilization before 24 h compared to mobilization above 24 h in acute stroke [17]. Although there are no studies regarding very early mobilization with exclusive populations of patients diagnosed with stroke undergoing thrombolytic treatment, there is a study that evaluated very early mobilization of patients with stroke, regardless of whether the patient had been treated with thrombolysis. In that study, the authors further analyzed if there were differences between patients who were included in the study undergoing thrombolytic treatment compared to patients undergoing thrombolysis who were not included in the study. They concluded that there were no differences, indicating the possibility of performing mobilization very early in this patient profile [1].
The overall prognosis was favorable for the main outcome of the study (mRS ≤ 2), where 59 of the 104 participants had a favorable outcome within 7 days after the stroke and 41 of the 54 participants who returned for follow-up 90 days after the stroke had a favorable outcome. Hospital discharge favorable results for this outcome.
Of the 61 participants who were able to perform the TUG test within 7 days after the stroke and of the 52 participants who performed the test 90 days after discharge, 46 participants and 40 participants, respectively, showed good functional mobility. Moreover, of the 89 patients that were evaluated using the BBS up to 7 days and of the 54 patients evaluated after 90 days of hospital discharge, 49 participants and 40 participants, respectively, showed no change in balance and most participants did not present complications during hospital stay.
Regarding the main and secondary outcomes evaluated in this study, no differences were found in the degree of disability and dependence on activities of daily living, balance, and functional mobility within 7 days of hospitalization and 90 days after discharge; however, other studies have found favorable results [18] or unfavorable results for early mobilization [10], which is in agreement with three studies that found no benefits in early mobilization over usual care [19–21].
The time to first mobilization after the onset of stroke symptoms was significantly shorter in VEMG than in GCU (p < 0.001), similar to that found in the study by Sundseth and colleagues, which reported no differences between early mobilization and usual care [19]. However, the timings reported by this study are lower than what other two studies have reported [10, 18].
Regarding length of stay and complications, the results are in agreement with the study by Herisson et al. which did not observe significant differences between the groups in both cases [21].
Another study had already verified that very early mobilization was not associated with higher risks of mortality, progression of stroke or falls with injury, compared to usual care, and therefore could be considered safe, but found no evidence of benefits in use of very early mobilization, which is in agreement with the results of this study [22].
Limitations
The limitations of this study are the small sample size, slow recruitment, and especially the rate of loss of participants to follow-up, driven by the COVID-19 pandemic.
It can also be considered as a limitation of this study the non-assessment of other outcomes, such as the impact of early mobilization on the workload of the multiprofessional team, since the faster exit from bed can favor the performance of activities outside the bed, such as performing personal hygiene care, which could lead to a reduction in the workload of the nursing team.
Future directions
As a future direction, it is necessary to assess the economic impacts, cost-effectiveness and quality of life related to performing very early mobilization in patients undergoing thrombolysis after acute ischemic stroke.
Clinical messages
In this study, the strategy of early mobilization after thrombolysis in ischemic stroke was safe, but without evidence of short-term benefit.
Furthermore, very early mobilization after thrombolysis has not been shown to be superior to usual care and therefore does not provide additional recovery benefits for patients with ischemic stroke.
Larger RCTs related to very early mobilization after thrombolysis in acute stroke are needed to clarify potential benefits and provide better evidence of health guidance for patients and therapists.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to welcome the residents of the Physiotherapy in Neurofunctional Rehabilitation residency program at the Roberto Santos General Hospital in Salvador, Bahia, Brazil. Thanks also to Editage (www.editage.com) for the English edition.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
Giuseppe Biondi-Zoccai has consulted for Cardionovum, Crannmedical, Innovheart, Guidotti, Meditrial, Opsens Medical, Replycare, Teleflex, and Terumo. All other authors declare that they have no competing interest.
Ethical approval
The study complies with the ethical standards set out in the 1964 Declaration of Helsinki and its subsequent amendments. Research Ethics Committee of Roberto Santos General Hospital under the number 3.447.930.
References
- 1.Muhl L, Kulin J, Dagonnier M, et al. Mobilization after thrombolysis (rtPA) within 24 hours of acute stroke: what factors influence inclusion of patients in a very early rehabilitation trial (AVERT)? BMC Neurology. 2014 doi: 10.1186/s12883-014-0163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2019 doi: 10.1161/str.0000000000000211. [DOI] [Google Scholar]
- 3.Bai QK, Zhao ZG, Lu LJ, Shen J, Zhang JY, Sui HJ, Xie XH, Chen J, Yang J, Chen CR. Treating ischaemic stroke with intravenous tPA beyond 4.5 hours under the guidance of a MRI DWI/T2WI mismatch was safe and effective. Stroke Vasc Neurol. 2019;4(1):8–13. doi: 10.1136/svn-2018-000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li KH, Jesuthasan A, Kui C, Davies R, Tse G, Lip GY. Acute ischemic stroke management: concepts and controversies. A narrative review. Expert Rev Neurother. 2020;21(1):65–79. doi: 10.1080/14737175.2021.1836963. [DOI] [PubMed] [Google Scholar]
- 5.Sun F, Liu H, Fu HX, et al. Predictive factors of hemorrhage after thrombolysis in patients with acute ischemic stroke. Front Neurol. 2020 doi: 10.3389/fneur.2020.551157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg M, Lindley RI, Murray G, Olivot JM, Parsons M, Tilley B, Toni D, Toyoda K, Wahlgren N, Wardlaw J, Whiteley W, del Zoppo GJ, Baigent C, Sandercock P, Hacke W. Stroke thrombolysis Trialists' collaborative group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929–35. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding R, Zhang H. Efficacy of very early mobilization in patients with acute stroke: a systematic review and meta-analysis. Ann Palliat Med. 2021;10(11):11776–11784. doi: 10.21037/apm-21-2997. [DOI] [PubMed] [Google Scholar]
- 8.Langhorne P, Collier JM, Bate PJ, Thuy MN, Bernhardt J. Very early versus delayed mobilisation after stroke. Cochrane Database Syst Rev. 2018;10(10):CD006187. doi: 10.1002/14651858.CD006187.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Zhang X, Wang K, Wen J. Effects of early mobilization after acute stroke: a meta-analysis of randomized control trials. J Stroke Cerebrovasc Dis. 2018;27(5):1326–1337. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Efficacy and safety of very early mobilisation within 24 h of stroke onset AVERT A randomised controlled trial. The Lancet. 2015;386(9988):46–55. doi: 10.1016/s0140-6736(15)60690-0. [DOI] [PubMed] [Google Scholar]
- 11.Banks JL, Marotta CA. Outcomes validity and reliability of the modified rankin scale: implications for stroke clinical trials. Stroke. 2007;38(3):1091–1096. doi: 10.1161/01.str.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 12.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto ST, Lombardi Junior I, Berg KO, Ramos LR, Natour J. Brazilian version of the Berg balance scale. Braz J Med Biol Res. 2004;37(9):1411–1421. doi: 10.1590/s0100-879x2004000900017. [DOI] [PubMed] [Google Scholar]
- 14.Andersson Å, Kamwendo K, Seiger Å, Appelros P. How to identify potential fallers in a stroke unit: validity indexes of 4 test methods. J Rehabil Med. 2006;38(3):186–191. doi: 10.1080/16501970500478023. [DOI] [PubMed] [Google Scholar]
- 15.Mamirauá Institute (2013) Bioestat 5.3 [Internet]. Brasil. https://www.mamiraua.org.br/downloads/programas/. Accessed 28 Jun 2022
- 16.Kitzman DW, Whellan DJ, Duncan P, et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. 2021;385(3):203–216. doi: 10.1056/nejmoa2026141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fure B, Holte HH, Hov L, Vist GE, Kateraas LH, Indredavik B. Veldig tidlig mobilisering ved akutt hjerneslag. Tidsskrift for Den norske legeforening. 2018 doi: 10.4045/tidsskr.17.0924. [DOI] [PubMed] [Google Scholar]
- 18.Chippala P, Sharma R. Effect of very early mobilisation on functional status in patients with acute stroke: a single-blind, randomized controlled trail. Clin Rehabil. 2015;30(7):669–675. doi: 10.1177/0269215515596054. [DOI] [PubMed] [Google Scholar]
- 19.Sundseth A, Thommessen B, Rønning OM. Outcome after mobilization within 24 hours of acute stroke. Stroke. 2012;43(9):2389–2394. doi: 10.1161/strokeaha.111.646687. [DOI] [PubMed] [Google Scholar]
- 20.Yelnik AP, Quintaine V, Andriantsifanetra C, et al. AMOBES (Active Mobility Very Early After Stroke) Stroke. 2017;48(2):400–405. doi: 10.1161/strokeaha.116.014803. [DOI] [PubMed] [Google Scholar]
- 21.Herisson F, Godard S, Volteau C, Le Blanc E, Guillon B, Gaudron M. Early sitting in ischemic stroke patients (SEVEL): a randomized controlled trial. PLoS ONE. 2016;11(3):e0149466. doi: 10.1371/journal.pone.0149466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu T, Yu X, Ou S, Liu X, Yuan J, Chen Y. Efficacy and safety of very early mobilization in patients with acute stroke: a systematic review and meta-analysis. Sci Rep. 2017 doi: 10.1038/s41598-017-06871-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.