Abstract
Background:
Previous research suggests that testosterone (T) plays a key role in shaping competitive and aggressive behavior in humans, possibly by modulating threat-related neural circuitry. However, this research has been limited by the use of T augmentation that fails to account for baseline differences and has been conducted exclusively in women. Thus, the extent to which normal physiologic concentrations of T affect threat-related brain function in men remains unknown.
Methods:
In the current study, we use a novel two-step pharmacologic challenge protocol to overcome these limitations and to evaluate causal modulation of threat- and aggression-related neural circuits by T in healthy young men (n = 16). First, we controlled for baseline differences in T through administration of a gonadotropin releasing hormone antagonist. Once a common baseline was established across participants, we then administered T to within the normal physiologic range. During this second step of the protocol we acquired functional neuroimaging data to examine the impact of T augmentation on neural circuitry supporting threat and aggression.
Results:
Gonadotropin releasing hormone antagonism successfully reduced circulating concentrations of T and brought subjects to a common baseline. Administration of T rapidly increased circulating T concentrations and was associated with heightened reactivity of the amygdala, hypothalamus, and periaqueductal grey to angry facial expressions.
Conclusions:
These findings provide novel causal evidence that T rapidly potentiates the response of neural circuits mediating threat processing and aggressive behavior in men.
Keywords: Aggression, amygdala, androgens, anger, emotion, fMRI, testosterone
Testosterone (T) has been clearly associated with aggression across numerous species (1). However, evidence in humans has revealed a relatively weak and inconsistent association between baseline T concentrations and aggression (2). These inconsistencies might arise from an exclusive focus on baseline T rather than experimentally evoked T responses, which are recognized as highly variable and rapidly fluctuating (3,4). Indeed, current theory holds that changes in T during competitive interactions are key for modulating ongoing and/or future aggression and dominance-related behaviors (5,6). In support of this model, studies have found that changes in T during competition are positively correlated with subsequent competitive motivation (7,8) and reactive aggression (9–11). Although acute changes in T have proven useful in predicting aggression (12), the extent to which T plays a causal role in shaping variability in human aggression is not clear. Explication of possible causal pathways between T and aggression is particularly timely and relevant, because T augmentation is increasingly being promoted as a pharmacologic approach to recovering and maintaining physical and reproductive vitality in aging men with “low T” (13).
Of particular value in this context is identifying the effects of T augmentation on neural circuitry—including the amygdala, hypothalamus, and periaqueductal gray (PAG)—that mediate threat processing and aggressive behavior (14–16). Notably, individual differences in T concentrations are associated with variability in the function of these neural structures in humans. Specifically, functional neuroimaging studies in men and women indicate that endogenous T concentrations are positively correlated with the reactivity of the amygdala (17–20) and hypothalamus (18) to facial threat displays (e.g., angry and fearful faces). Going beyond correlational work, evidence indicates that a single administration of T increases amygdala, hypothalamic, and midbrain reactivity to facial signals of threat (18,20–22). Although these studies provide support for a causal role of T in potentiating threat-related neural function, they are limited in certain ways. Most notably, these pharmacologic challenge studies have been performed exclusively in women, for whom there is lacking evidence for a relationship between acute endogenous changes in T and aggressive behavior (9,11). Also, the standard sublingual T protocols employed in women fail to account for baseline differences and increase T concentrations above the normal physiologic range (mean = 18.41 nmol/L vs. .6–7.2 nmol/L) (23,24). Finally, most previous work in women has used a significant time lag (4–4.5 hours) between drug administration and assessment of physiological and behavioral processes, and there is some evidence that T might have much more rapid, perhaps non-genomic effects on neural responses to social threat (18). Thus, the extent to which raising T concentration to within the normal physiologic range in healthy young men would rapidly potentiate threat-related neural function remains unclear.
Understanding the modulatory role of T on heightened threat-related amygdala reactivity in men is particularly important, because aggression is generally more common in men than women (25), and pathological extremes of aggression are also more often exhibited in men than women (26). Psychopathology characterized by heightened reactive aggression (e.g., intermittent explosive disorder, borderline personality disorder) is associated with heightened amygdala reactivity to the presentation of angry facial expressions (27,28). Also, functional genetic polymorphisms in pathways linked to aggression, such as the androgen receptor (29) and monoamine oxidase A (30), are associated with increased amygdala reactivity to fearful (19) and angry (31) facial expressions. On the basis of this evidence and work in animal models (1), we have proposed that acute changes in T within the context of competitive interactions might modulate aggressive behavior through its effects on amygdala, hypothalamic, and PAG function (12).
To begin to address this hypothesis, the current study developed a novel human pharmacologic challenge protocol similar to that used in California mice (32). In this animal model, male mice are physically castrated before engaging in a resident-intruder paradigm, a manipulation that serves to clamp the hypothalamic-pituitary-gonadal (HPG) axis, thus ensuring that endogenous changes in T do not occur in response to competitive interactions. After winning a competitive interaction, mice are given an acute dose of T or placebo. This manipulation reveals that mice are more aggressive in subsequent competitive interactions but only if they received T after winning an initial interaction (32). A remarkably similar effect has been observed in healthy young men: winning is associated with increased aggressive behavior, an effect mediated by T reactivity (11).
We adapted the T suppression/replacement animal model to experimentally modulate T in healthy young men with a dual-stage, placebo-controlled, double-blind, within-subject design. Specifically, we first used a gonadotropin releasing hormone (GnRH) antagonist to acutely suppress the HPG axis. By antagonizing GnRH receptors on the anterior pituitary, this drug effectively inhibits the release of luteinizing hormone, ultimately suppressing endogenous T concentrations to within the hypogonadal range (33). Critically, this first challenge simultaneously reduces variability in baseline T concentrations. After clamping the HPG axis and achieving T suppression, participants then received T or placebo and performed a challenge paradigm involving perceptual processing of emotional facial expressions during blood oxygen level–dependent (BOLD) functional magnetic resonance imaging (fMRI).
The primary goal of this study was to examine whether acute manipulation of T affects threat-related neural function in men. On the basis of recent pharmacologic challenge work in women (18,20) and neurobiological models of aggression in animal models (1), we hypothesized that T administration would increase the reactivity of the amygdala, hypothalamus, and periaqueductal gray (PAG) to angry facial expressions. Testosterone administration has also been shown to decrease fear-potentiated startle (34), suggesting that T administration might decrease amygdala reactivity to fearful facial expressions. However, recent evidence indicates that T administration increases amygdala reactivity to fearful expressions in women (22); and correlational studies, which have combined angry and fearful facial expressions in their analyses, have found positive correlations between T and amygdala reactivity to both types of threat-related expressions (17,19). Given inconclusive evidence with regard to T and neural responses to fearful expressions, we made no directional hypotheses concerning the role of T in modulating neural reactivity to these stimuli. Finally, we also examined the effect of T administration on neural responses to surprise facial expressions.
Methods and Materials
Participants
Participants were 16 healthy adult male volunteers (18–44 years of age; mean age 26.81) who self-identified as Caucasian (75%), Hispanic (12.5%), African-American (6.25%), and Asian (6.25%). Exclusion criteria were history of endocrine or psychiatric disorder, current prescription medication use, left-hand dominance, history of closed-head injury, and presence of ferromagnetic foreign bodies or medical devices. Participants were given an honorarium of $125 per visit. The protocol was approved by the Wayne State University Institutional Review Board.
Procedure
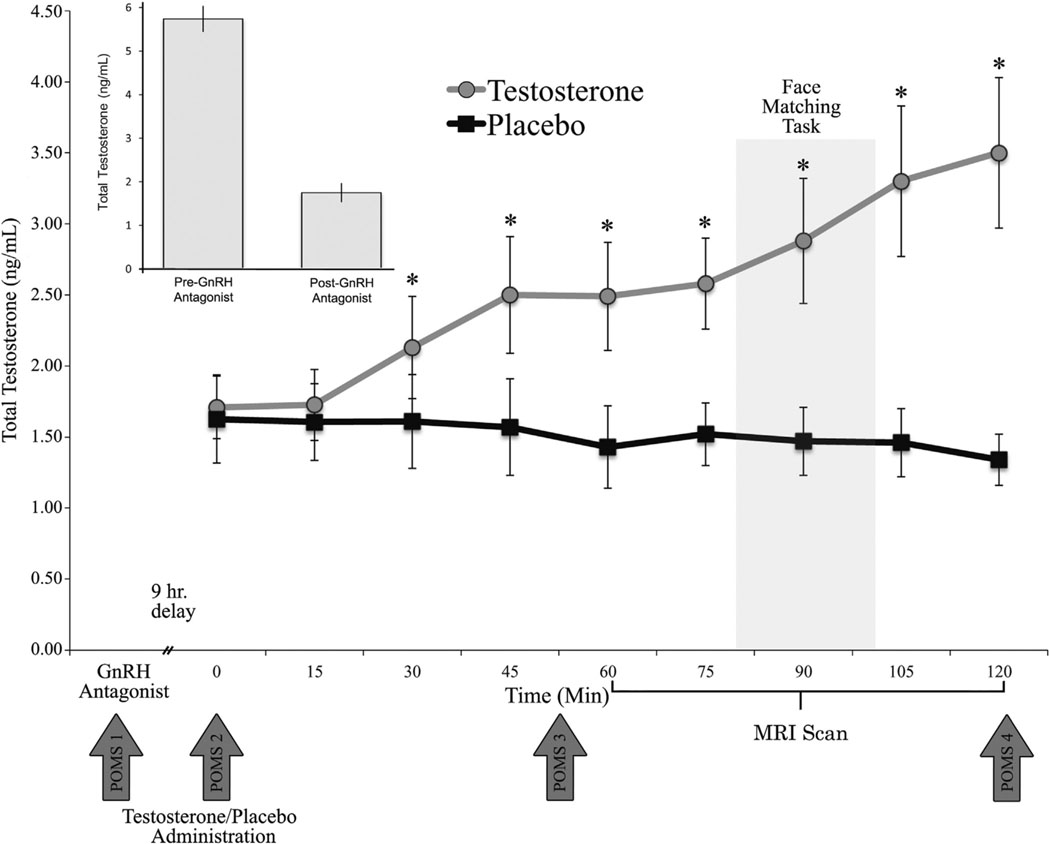
The current study employed a placebo-controlled, double-blinded, within-subject, crossover design. The procedure entailed 2 testing days spaced at least 3 weeks apart. On both visits, participants came to the clinic between 8:00 AM and 9:00 AM, completed a Profile of Mood States questionnaire (POMS) (35) and provided demographic information. Blood (10 mL) was then collected from the antecubital vein. Next, participants received a subcutaneous injection of a GnRH antagonist (cetrorelix acetate, 3 mg). After receiving the GnRH antagonist, participants were free to go about their normal daily activities before returning between 5:00 PM and 6:00 PM for the fMRI portion of the study. Upon arrival, participants had an indwelling catheter inserted into their antecubital vein, and blood (10 mL) was drawn every 15 min for the 2-hour visit. We were unable to obtain blood samples from two participants. After their first blood draw, participants were randomly assigned to receive either 100 mg T gel (Androgel; Abbvie, North Chicago, Illinois) or placebo gel (order of gel administration was fully counterbalanced) and then completed a second POMS questionnaire. Approximately 50 min after gel application, participants completed a third POMS questionnaire before entering the scanning suite. The MRI scan lasted approximately 60 min. After the scan, participants completed their fourth and final POMS questionnaire. See Figure 1 for experimental protocol. After the final visit, participants were paid, debriefed, and asked whether they believed they received the T gel on the first or second visit. A binomial test indicated that participants were no better than chance at guessing when they received T (p = .607). None of the research staff conducting the questionnaire or fMRI portion of the study had knowledge of assignment to Androgel (Abbvie) or placebo until completion of the entire study.
Figure 1.
Experimental design and serum testosterone concentrations. Androgel (Abbvie, North Chicago, Illinois) administration increased serum testosterone concentrations above the placebo condition within 30 min of drug application. Error bars depict SEM. *p < .05. GnRH, gonadotropin releasing hormone; MRI, magnetic resonance imaging; POMS, Profile of Mood States.
Hormone Assessment
To assess the efficacy of our T manipulation protocol, total T concentrations were assayed with commercially available enzyme linked immunoassay kits (DRG International, Spring-field, New Jersey). Moreover, we also assayed for estradiol and serum hormone binding globulin (SHBG) to assess the specificity of the T suppression/replacement manipulation. All samples were spun in a refrigerated centrifuge (4°C) for 15 min at 3000 rpm. The plasma supernatant was aliquoted and stored at −80°C. For T, the intra- and inter-assay coefficients (CVs) of variation were 6.72% and 8.29%, respectively. For SHBG, the intra- and inter-assay CVs were 7.20% and 6.57%, respectively. For estradiol, the intra- and inter-assay CVs were 11.05% and 7.50%, respectively.
fMRI Task
The fMRI challenge paradigm used in the current study has been used extensively to elicit a robust and replicable amygdala response across an array of experimental protocols and sample populations (36–40). In the paradigm, there are four blocks of a perceptual face-matching task interleaved with five blocks of a sensorimotor control. During task blocks, participants view a trio of faces and select one of two faces (on the bottom) identical to a target face (on the top). Each task block consists of six different trios, balanced for gender, all of which were derived from a standard set of pictures of facial affect (41). Thus, in each block, participants see 18 faces (6 trials × 3 faces of the same expression). We used the Duke Neurogenetics Study version of the task (42–44) consisting of one block each of fearful, angry, surprised, and neutral facial expressions presented in a pseudorandom order across participants. These four task blocks are interleaved with five control blocks, in which subjects match simple geometric shapes (circles and vertical and horizontal ellipses). Each control block consists of six different shape trios. All blocks are preceded by a brief instruction (“Match Faces” or “Match Shapes”) that lasts 2 sec. In the task blocks, each of the six face trios is presented for 4 sec with a variable interstimulus interval of 2–6 sec (mean = 4 sec), for a total block length of 48 sec. A variable interstimulus interval is used to minimize expectancy effects and resulting habituation and maximize amygdala reactivity throughout the paradigm. In the control blocks, each of the six shape trios is presented for 4 sec with a fixed interstimulus interval of 2 sec, for a total block length of 36 sec. Total task time is 390 sec. The stimuli were presented with E-prime software (version 2.0; Psychology Software Tools, Pittsburgh, Pennsylvania).
Neural Regions of Interest
Because of our strong a priori hypotheses, we focused our analyses primarily on the amygdala, hypothalamus, and PAG. Functional reactivity of the amygdala was assessed within anatomical regions of interest (ROIs) on the basis of cytoarchitectonic probability maps as implemented in the anatomy toolbox for SPM8 (Wellcome Department of Imaging Neuroscience, London, United Kingdom) (45,46). In keeping with recent imaging work (47–49) and in recognition of the functional and anatomical heterogeneity of the amygdala (50), we specifically considered the corticomedial (centromedial and superficial) and basolateral subregions in our analyses. The PAG ROI was a 6-mm sphere centered on Montreal Neurological Institute (MNI) coordinates x = 1, y = −29, z = −11 (51). The hypothalamic ROI was constructed on the basis of the PickAtlas toolbox of SPM8 (http://fmri.wfubmc.edu/software/PickAtlas) and was dilated (×1) to accommodate between-subject variability in this small structure.
To correct for multiple comparisons within our ROIs and to guard against Type I error, we used the Alphasim function in AFNI. Monte Carlo simulations (10,000 iterations) were performed with the smoothness values estimated from the residuals obtained from the GLM. At a per-voxel p value of .05 (two-tailed), the following cluster sizes provided for a corrected family-wise error rate of α < .05: 38 voxels in corticomedial amygdala (CMA), 43 voxels basolateral amygdala (BLA), 14 voxels in the hypothalamus, and 28 voxels in the PAG. Unless stated otherwise, all results reported in this article survive correction for multiple comparisons.
Behavioral Data Analysis
The response time (RT) and accuracy data for each participant during the imaging task were obtained. A total of 17 of 1728 trials (16 trials of shapes, 1 trial of neutral faces) with RTs more than 3 SDs from the mean were excluded. The RT and accuracy data were averaged for participants by different stimulus type and drug condition and reconstructed for paired comparisons. As expected, participant accuracy was very high (M = 97.7%, SD = 5.9). There were no effects of drug or drug × expression interaction on either accuracy (p values > .71) or RT (p values > .69).
POMS Questionnaires
The POMS questionnaire consisted of 37 items. Six subscales (tension, depression, anger, vigor, fatigue, and confusion) were aggregated from individual items. To account for missing items, the average score was used instead of the sum. A Total Mood Disturbance score was calculated as the average of the six subscales. The six subscales of POMS (tension, depression, anger, vigor, fatigue, and confusion) achieved high reliability (average Cronbach’s α = .81). There were no effects of drug condition on any of the subscales (p values > .05), and thus we do not report further on this measure.
BOLD fMRI Data Acquisition
Each participant was scanned with a research-dedicated Siemens Vario 3T scanner at Wayne State University. T2*-weighted BOLD images were acquired with echo-planar imaging (EPI) (repetition time/echo time/flip angle = 2000 msec/25 msec/90; field-of-view = 220; voxel size = 3.44 × 3.44 × 4 mm; interslice skip = 0). Siemens MRI motion correction software was used to retroactively reduce the relative motion across the dataset by applying post-processing interpolation of frame-to-frame movement. After this, mean movement for each of six translational (x, y, z) and rotational (pitch, roll, yaw) movement directions were calculated. Average participant movement was <.5 mm across three translational directions and <.3° across three rotational directions. No significant differences in movement were found between the two drug conditions for any of the six movement parameters (p values > .05).
BOLD fMRI Data Preprocessing
Preprocessing steps were performed with SPM8 software (Wellcome Department of Imaging Neuroscience). The first six EPI volumes were discarded to allow for signal stabilization. Images were then realigned and spatially normalized to the MNI template with the participant-specific transformation parameters created by fitting mean functional images to the single reference EPI standard SPM template (final resolution of functional images = 2 mm isotropic voxels). After normalization, images were spatially smoothed with a relatively small Gaussian kernel of 4 mm full width at half maximum as used in recent work (52–54). Finally, low-frequency BOLD signal drift was removed by applying a standard high-pass filter (128-sec cutoff).
After preprocessing, linear contrasts with canonical hemodynamic response functions convolved with the block duration were used to estimate expression-specific (anger > neutral; fear > neutral; surprise > neutral) BOLD responses for each individual. These individual contrast images (i.e., weighted sum of the beta images) were then used in second-level random-effects models to determine mean expression-specific neural reactivity with one-sample t tests and simple main effects of drug challenge (i.e., T > placebo and placebo > T) with paired t tests. To examine the specificity of the effects of drug on threat-related neural activation within our a priori ROIs, we performed a 2 × 3 flexible factorial analysis with drug condition (T vs. placebo) and expression (anger > neutral vs. fear > neutral vs. surprise > neutral) as within-subject factors. Statistical parametric maps for the effect of drug were superimposed onto a high-resolution T1-weighted image of a single individual transformed into MNI space.
Results
Hormone Responses to Drug Challenge
Total T.
A repeated-measure analysis of variance (ANOVA) revealed main effects of drug and time on total T concentrations (p values < .001). Testosterone concentrations were reduced to within the hypogonadal range after GnRH antagonist administration (MpreGnRHantagonist = 5.73 ng/mL vs. MpostGnRHantagonist = 1.74 ng/mL; t13 = 12.185, p < .001). The main effects of drug and time were qualified by a significant drug × time interaction (F9,99 = 10.075, p < .001). Post hoc analyses indicated that total T concentrations were significantly higher in the T condition compared with the placebo condition within 30 min of gel application (M = 2.13 ng/mL vs. M = 1.64 ng/mL, respectively; t13 = 5.819, p < .001) (Figure 1) and continued to increase throughout the session (maximum at 2 hours, T: M = 3.48 ng/mL; placebo: M = 1.34 ng/mL).
SHBG.
Repeated measures ANOVA revealed no main effects or drug × time interaction (all p values > .128).
T Effects on Neural Responses to Angry vs. Neutral Expressions
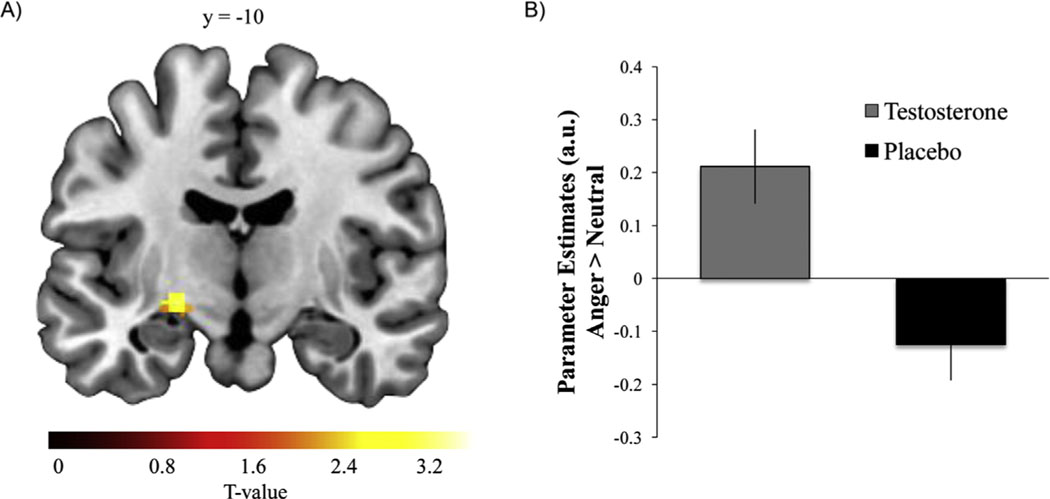
Results revealed significant amygdala (CMA and BLA) reactivity to angry expressions in comparison with neutral expressions across placebo and T conditions (Figure S1A in Supplement 1). Direct comparisons between T and placebo conditions showed the T condition was associated with increased left CMA reactivity (Figure 2) and heightened reactivity within the PAG and hypothalamus (Table 1).
Figure 2.
Testosterone (T) administration increased corticomedial amygdala (CMA) reactivity to angry compared with neutral faces. (A) Statistical parametric map illustrating relatively increased left CMA reactivity to angry compared with neutral expressions (p < .05, corrected for multiple comparisons within the CMA) after T administration. (B) Parameter estimates obtained from peak voxel in CMA demonstrating effect of drug (T > placebo) for the contrast of angry versus neutral faces. Error bars depict SEM. a.u., arbitrary units.
Table 1.
Effects of Testosterone on Amygdala, Hypothalamus, and Periaqueductal Gray Function
| Contrast | ROI | X | Y | Z | Peak Z Value | Cluster Size |
|---|---|---|---|---|---|---|
| Angry > Neutral | ||||||
| Testosterone | Left CMA | −26 | −12 | −6 | 2.78 | 71 |
| Right CMA | 28 | −10 | −10 | 2.31 | 89 | |
| Hypothalamus | −6 | −6 | −8 | 3.12 | 20 | |
| PAG | 2 | −22 | −12 | 2.22 | 48 | |
| Placebo | Right BLA | 34 | −6 | −16 | 2.24 | 43 |
| Testosterone > | Left CMA | −24 | −12 | −6 | 2.71 | 44 |
| placebo | Hypothalamus | −8 | −6 | −8 | 2.67 | 17 |
| PAG | 0 | −32 | −6 | 2.85 | 110 | |
| Fearful > Neutral | ||||||
| Testosterone | Left CMA | −16 | −6 | −20 | 2.13 | 105 |
| Left BLA | −20 | −6 | −20 | 2.03 | 68 | |
| Placebo | Left CMA | −16 | 2 | −20 | 3.22 | 71 |
| Left BLA | −20 | 2 | −24 | 3.09 | 119 | |
| Right BLA | 32 | −4 | −16 | 2.88 | 76 | |
| PAG | 2 | −28 | −18 | 2.28 | 28 | |
| Surprise > Neutral | ||||||
| Testosterone | Left CMA | −26 | −2 | −10 | 2.09 | 169 |
| Right CMA | 28 | −6 | −10 | 2.30 | 198 | |
| Left BLA | −28 | −12 | −12 | 2.43 | 120 | |
| Right BLA | 26 | −6 | −18 | 2.45 | 197 | |
| PAG | 0 | −32 | −14 | 2.19 | 113 | |
| Placebo | Left CMA | −28 | −2 | −16 | 2.02 | 44 |
| Right CMA | 32 | −2 | −16 | 2.07 | 47 | |
| Right BLA | 34 | −6 | −16 | 2.21 | 70 | |
| PAG | 0 | −24 | −14 | 2.27 | 29 |
Activations are reported at p < .05, corrected for multiple comparisons within the regions of interest (ROIs). Peak coordinates of each cluster are reported in Montreal Neurological Institute space. There were no effects of testosterone > placebo or placebo > testosterone for the fearful > neutral or surprise > neutral contrasts.
BLA, basolateral amygdala; CMA, corticomedial amygdala; PAG, periaqueductal gray.
T Effects on Neural Responses to Fearful vs. Neutral Expressions
There was significant amygdala (CMA and BLA) reactivity to fearful expressions in comparison with neutral expressions across placebo and T conditions (Figure S1B in Supplement 1). Also, we observed significant PAG reactivity across placebo and T conditions (Z = 2.26, x = 0, y = −24, z = −14, cluster size = 42 voxels, p < .05, corrected). Direct comparisons between T and placebo conditions showed no significant effects of drug condition (T > placebo or placebo > T) on amygdala, hypothalamus, or PAG reactivity.
T Effects on Neural Responses to Surprise vs. Neutral Expressions
Results revealed significant amygdala (CMA and BLA) reactivity to surprise expressions in comparison with neutral expressions across placebo and T conditions (Figure S1C in Supplement 1). Also, we observed significant PAG reactivity across placebo and T conditions (Z = 2.82, x = 0, y = −24, z = −14, cluster size = 104 voxels, p < .05, corrected). Direct comparisons between T and placebo conditions showed no significant effects of drug condition (T > placebo or placebo > T) on amygdala, hypothalamus, or PAG reactivity (Table 1).
Flexible Factorial Analysis
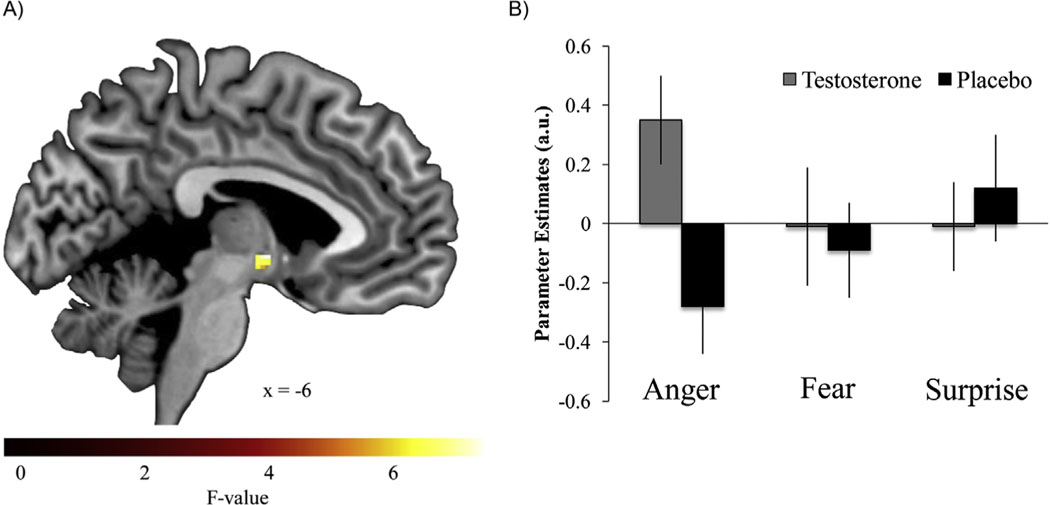
To formally investigate the extent to which the effect of T on threat-related neural function was specific to the processing of angry or fearful expressions (i.e., drug × emotional expression interaction), a 2 × 3 flexible factorial analysis was performed with drug condition (T vs. placebo) and expression (angry, fearful, and surprise vs. neutral) as within-subject factors. Results revealed a significant drug × emotional expression interaction in the hypothalamus (F = 7.13, x = −6, y = −2, z = −4, cluster size = 16 voxels, p < .05, corrected) but not the amygdala or PAG (Figure 3). Also, there was a significant main effect of drug condition (T > placebo) within the PAG (F = 7.41, x = 4, y = −32, z = −6, cluster size = 46 voxels, p < .05, corrected).
Figure 3.
Testosterone administration increased hypothalamus reactivity to angry but not fearful or surprise compared with neutral expressions. (A) Statistical parametric map illustrating a significant drug × emotion interaction in the hypothalamus (p < .05, corrected for multiple comparisons within the hypothalamus). (B) Parameter estimates obtained from peak voxel in the hypothalamus demonstrating drug × emotion interaction. Error bars depict SEM. a.u., arbitrary units.
Discussion
With a novel pharmacologic challenge protocol that effectively controls for variability in baseline concentrations of T and uniformly raises these concentrations to a normal physiologic range, we provide causal evidence that T rapidly increases threat-related reactivity of core neural structures mediating aggression. Importantly, the current study is the first to examine the causal role of T in mediating neural responses to ecologically valid facial threat cues in healthy young men and thus represents a significant extension of the existing literature on the neuroendocrine modulation of threat- and aggression-related neural function.
The effects of T on amygdala reactivity were found within the centromedial subregion, which encompasses the central and medial nuclei. Critically, the central nucleus of the amygdala can mediate physiologic arousal and threat vigilance through projections to the hypothalamus, brain stem, and basal forebrain cholinergic cell populations (55). Terburg and van Honk (56) have argued that T increases social aggression by modulating neural function within the medial amygdala. Consistent with this idea, stimulation of the medial amygdala increases rage behavior in cats (57,58), mainly through its downstream effects on the hypothalamus and PAG. Notably, we also observed increased hypothalamic and PAG reactivity to angry facial expressions after T administration. Collectively, these neural structures are rich in both androgen and estrogen receptors (59–63) and form part of the neural circuitry underlying reactive aggression (1).
Most previous pharmacologic challenge work in humans has assessed the effects of T on brain and behavior 4–4.5 hours after T administration (21). This is a legacy effect from the initial landmark study demonstrating that effects of sublingual T administration on vaginal pulse amplitude in response to sexual stimuli emerged only 4 hours after drug administration (64). This relatively long temporal interval between drug administration and behavioral testing is consistent with a genomic mechanism wherein binding of T to androgen receptors (or estrogen receptors after aromatization) in the cytosol initiates their translocation to the nucleus where they act as transcription factors directly regulating gene expression (65).
In contrast, the effects observed in our study were prominent within 90 min after T administration, which is consistent with a rapid non-genomic mechanism (66). Moreover, work by van Wingen et al. (20) indicates that T administration in women increases threat-related amygdala function within 45 min. Research in animal models has identified extranuclear androgen and estrogen receptors in the hippocampus, amygdala, hypothalamus, and cortex (67–70). Such extranuclear sex steroid receptors are positioned to regulate rapid membrane and cytoplasmic signaling in axons and dendrites (71), thus facilitating the modulation of brain function and social behavior through non-genomic mechanisms (72–74). These findings converge to suggest that T can have both rapid and sustained effects on threat-related neural processes.
Although the present findings provide novel causal evidence for the importance of T in modulating threat-related neural processing, some study limitations should be noted. First, our T administration protocol only raised T concentrations to within the low normal range (75). Other pharmacologic challenge work conducted in healthy young women increased T concentrations to a much higher degree (64). Thus, perhaps the effects observed in the current study would have been more robust (similar to that observed in young women) had we used a larger dose of Androgel (Abbvie) (76). Despite differences in absolute T concentrations achieved after drug administration, both our study and the aforementioned work in young women converge on the finding that T modulates the neural circuitry underpinning threat processing and aggressive behavior in a similar fashion. Nevertheless, we believe that it will be critical to determine the extent to which T has dose-dependent effects on threat-related neural function in both men and women.
Another limitation of our work is that, although the effect of T on threat-related neural processing seemed to be specific to angry facial expressions, the factorial analysis failed to show significant drug × expression interactions for the amygdala and PAG. Thus, we cannot make strong claims concerning the specificity of the effect of T on the processing of angry facial expressions within these two regions. In contrast, the factorial analysis revealed that the effect of T on hypothalamic reactivity was emotion-specific. T was associated with increased hypothalamic reactivity to angry but not for fearful or surprise expressions, consistent with our hypothesis and with previous work (18,20).
Currently it is unclear whether the few behavioral studies in humans reporting associations between changes in T and subsequent aggressive behavior are caused by these neuroendocrine responses or are related only indirectly through a third variable (8,9,11). Our findings indicate that acutely raising T concentrations (similar to the changes in T observed during competitive interactions) can rapidly increase threat-related neural processing. A next critical step will be to implement our dual-stage pharmacologic challenge during protocols assessing aggressive behavior explicitly (e.g., Point Subtraction Aggression Paradigm, Taylor Aggression Paradigm). This will extend our current work to testing the role of increased amygdala, hypothalamus, and PAG reactivity in mediating the effects of T on aggressive behavior. Furthermore, such application of our challenge design could be used more broadly to examine the effects of T on other behavioral processes previously linked to T (e.g., risk-taking, cooperation).
In summary, we provide novel, causal evidence that exogenously administered T potentiates threat-related neural function in healthy young men. These effects were observed shortly after T administration, which is consistent with a rapid non-genomic mechanism of action. Adopting the current approach in behavioral studies will be an important next step in establishing the role that hormone dynamics, particularly those of T, play in modulating competitive and aggressive behavior.
Supplementary Material
Acknowledgments
This research was supported in part by the Wayne State University Department of Psychology (to JMC), the Merrill Palmer Skillman Institute for Child and Family Development, Wayne State University School of Medicine and by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (to MET). ARH receives support through the National Institute on Drug Abuse grants R01DA033369 and R01DA031579.
Footnotes
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2014.01.016.
All authors declare no biomedical financial interests or potential conflicts of interests.
References
- 1.Nelson RJ, Trainor BC (2007): Neural mechanisms of aggression. Nat Rev Neurosci 8:536–546. [DOI] [PubMed] [Google Scholar]
- 2.Archer J, Graham-Kevan N, Davies M (2005): Testosterone and aggression: A reanalysis of Book, Starzyk, and Quinsey’s (2001) study. Aggress Violent Behav 10:241–261. [Google Scholar]
- 3.Wingfield JC, Hegner RE, Dufty AM Jr, Ball GF (1990): The “challenge hypothesis”: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat 829–846. [Google Scholar]
- 4.Mazur A (2005): Biosociology of Dominance and Deference. Oxford, UK: Rowman and Littlefield. [Google Scholar]
- 5.Mazur A (1985): A biosocial model of status in face-to-face primate groups. Soc Forces 64:377–402. [Google Scholar]
- 6.Archer J (2006): Testosterone and human aggression: An evaluation of the challenge hypothesis. Neurosci Biobehav Rev 30:319–345. [DOI] [PubMed] [Google Scholar]
- 7.Mehta PH, Josephs RA (2006): Testosterone change after losing predicts the decision to compete again. Horm Behav 50:684–692. [DOI] [PubMed] [Google Scholar]
- 8.Carré JM, McCormick CM (2008): Aggressive behavior and change in salivary testosterone concentrations predict willingness to engage in a competitive task. Horm Behav 54:403–409. [DOI] [PubMed] [Google Scholar]
- 9.Carré JM, Putnam SK, McCormick CM (2009): Testosterone responses to competition predict future aggressive behaviour at a cost to reward in men. Psychoneuroendocrinology 34:561–570. [DOI] [PubMed] [Google Scholar]
- 10.Geniole SN, Carré JM, McCormick CM (2011): State, not trait, neuro-endocrine function predicts costly reactive aggression in men after social exclusion and inclusion. Biol Psychol 87:137–145. [DOI] [PubMed] [Google Scholar]
- 11.Carré JM, Campbell JA, Lozoya E, Goetz SM, Welker KM (2013): Changes in testosterone mediate the effect of winning on subsequent aggressive behaviour. Psychoneuroendocrinology 38:2034–2041. [DOI] [PubMed] [Google Scholar]
- 12.Carré JM, McCormick CM, Hariri AR (2011): The social neuroendocrinology of human aggression. Psychoneuroendocrinology 36:935–944. [DOI] [PubMed] [Google Scholar]
- 13.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS (2013): Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med 173:1465–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegel A, Bhatt S, Bhatt R, Zalcman SS (2007): The neurobiological bases for development of pharmacological treatments of aggressive disorders. Curr Neuropharm 5:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair R (2010): Psychopathy, frustration, and reactive aggression: The role of ventromedial prefrontal cortex. Br J Psychol 101:383–399. [DOI] [PubMed] [Google Scholar]
- 16.Blair R (2010): Neuroimaging of psychopathy and antisocial behavior: A targeted review. Curr Psychiatry Rep 12:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derntl B, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, Moser E, et al. (2009): Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology 34: 687–693. [DOI] [PubMed] [Google Scholar]
- 18.Hermans EJ, Ramsey NF, van Honk J (2008): Exogenous testosterone enhances responsiveness to social threat in the neural circuitry of social aggression in humans. Biol Psychiatry 63:263–270. [DOI] [PubMed] [Google Scholar]
- 19.Manuck SB, Marsland AL, Flory JD, Gorka A, Ferrell RE, Hariri AR (2010): Salivary testosterone and a trinucleotide (CAG) length polymorphism in the androgen receptor gene predict amygdala reactivity in men. Psychoneuroendocrinology 35:94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Wingen GA, Zylicz A, Pieters S, Mattern C, Verkes RJ, Buitelaar JK, et al. (2008): Testosterone increases amygdala reactivity in middle-aged women to a young adulthood level. Neuropsychopharmacology 34:539–547. [DOI] [PubMed] [Google Scholar]
- 21.Bos PA, Panksepp J, Bluthé RM, van Honk J (2012): Acute effects of steroid hormones and neuropeptides on human social–emotional behavior: A review of single administration studies. Front Neuro-endocrinol 33:17–35. [DOI] [PubMed] [Google Scholar]
- 22.Bos PA, van Honk J, Ramsey NF, Stein DJ, Hermans EJ (2012): Testosterone administration in women increases amygdala responses to fearful and happy faces. Psychoneuroendocrinology 38:808–817. [DOI] [PubMed] [Google Scholar]
- 23.van Rooij K, Bloemers J, de Leede L, Goldstein I, Lentjes E, Koppeschaar H, et al. (2012): Pharmacokinetics of three doses of sublingual testosterone in healthy premenopausal women. Psychoneuroendocrinology 37:773–781. [DOI] [PubMed] [Google Scholar]
- 24.Taieb J, Mathian B, Millot F, Patricot M-C, Mathieu E, Queyrel N, et al. (2003): Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography–mass spectrometry in sera from 116 men, women, and children. Clin Chem 49:1381–1395. [DOI] [PubMed] [Google Scholar]
- 25.Archer J (2004): Sex differences in aggression in real-world settings: A meta-analytic review. Rev Gen Psychol 8:291–322. [Google Scholar]
- 26.Kessler RC, Coccaro EF, Fava M, Jaeger S, Jin R, Walters E (2006): The prevalence and correlates of DSM-IV intermittent explosive disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry 63: 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL (2007): Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry 62:168–178. [DOI] [PubMed] [Google Scholar]
- 28.New AS, Hazlett EA, Buchsbaum MS, Goodman M, Mitelman SA, Newmark R, et al. (2007): Amygdala–prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology 32: 1629–1640. [DOI] [PubMed] [Google Scholar]
- 29.Rajender S, Pandu G, Sharma J, Gandhi K, Singh L, Thangaraj K (2008): Reduced CAG repeats length in androgen receptor gene is associated with violent criminal behavior. Int J Legal Med 122:367–372. [DOI] [PubMed] [Google Scholar]
- 30.Brunner HG, Nelen M, Breakefield X, Ropers H, Van Oost B (1993): Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 262:578–578. [DOI] [PubMed] [Google Scholar]
- 31.Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, et al. (2006): Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A 103:6269–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trainor BC, Bird IM, Marler CA (2004): Opposing hormonal mechanisms of aggression revealed through short-lived testosterone manipulations and multiple winning experiences. Horm Behav 45:115–121. [DOI] [PubMed] [Google Scholar]
- 33.Behre HM, Klein B, Steinmeyer E, McGregor G, Voigt K, Nieschlag E (1992): Effective suppression of luteinizing hormone and testosterone by single doses of the new gonadotropin-releasing hormone antagonist cetrorelix (SB-75) in normal men. J Clin Endocrinol Metab 75: 393–398. [DOI] [PubMed] [Google Scholar]
- 34.Hermans EJ, Putman P, Baas JM, Koppeschaar HP, van Honk J (2006): A single administration of testosterone reduces fear-potentiated startle in humans. Biol Psychiatry 59:872–874. [DOI] [PubMed] [Google Scholar]
- 35.Curran SL, Andrykowski MA, Studts JL (1995): Short Form of the Profile of Mood States (POMS-SF): Psychometric information. Psychological Assessment 7:80–83. [Google Scholar]
- 36.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. (2002): Serotonin transporter genetic variation and the response of the human amygdala. Sci Signal 297:400–403. [DOI] [PubMed] [Google Scholar]
- 37.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. (2005): A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry 62:146–152. [DOI] [PubMed] [Google Scholar]
- 38.Fisher P, Meltzer C, Ziolko S, Price J, Hariri A (2006): Capacity for 5-HT1A–mediated autoregulation predicts amygdala reactivity. Nat Neurosci 9:1362–1363. [DOI] [PubMed] [Google Scholar]
- 39.Fisher PM, Meltzer CC, Price JC, Coleman RL, Ziolko SK, Becker C, et al. (2009): Medial prefrontal cortex 5-HT2A density is correlated with amygdala reactivity, response habituation, and functional coupling. Cereb Cortex 19:2499–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Z, Zhu G, Hariri AR, Enoch M-A, Scott D, Sinha R, et al. (2008): Genetic variation in human NPY expression affects stress response and emotion. Nature 452:997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ekman P, Friesen WV (1975): Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- 42.Prather AA, Bogdan R, Hariri AR (2013): Impact of sleep quality on amygdala reactivity, negative affect, and perceived stress. Psychosom Med 75:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carré JM, Hyde LW, Neumann CS, Viding E, Hariri AR (2012): The neural signatures of distinct psychopathic traits. Soc Neurosci 8:122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carré JM, Murphy KR, Hariri AR (2013): What lies beneath the face of aggression? Soc Cogn Affect Neurosci 8:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah N, et al. (2005): Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl) 210:343–352. [DOI] [PubMed] [Google Scholar]
- 46.Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. (2005): A new SPM toolbox for combining probabilistic cytoarch-itectonic maps and functional imaging data. Neuroimage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- 47.Boll S, Gamer M, Gluth S, Finsterbusch J, Büchel C (2012): Separate amygdala subregions signal surprise and predictiveness during associative fear learning in humans. Eur J Neurosci 37:758–767. [DOI] [PubMed] [Google Scholar]
- 48.Morgan B, Terburg D, Thornton HB, Stein DJ, van Honk J (2012): Paradoxical facilitation of working memory after basolateral amygdala damage. PLoS One 7:e38116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terburg D, Morgan B, Montoya E, Hooge I, Thornton H, Hariri A, et al. (2012): Hypervigilance for fear after basolateral amygdala damage in humans. Transl Psychiatry 2:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis M, Whalen PJ (2001): The amygdala: Vigilance and emotion. Mol Psychiatry 6:13–34. [DOI] [PubMed] [Google Scholar]
- 51.Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D (2012): Neuroimaging of the periaqueductal gray: State of the field. Neuro-image 60:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knutson B, Adams CM, Fong GW, Hommer D (2001): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21:RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH (2008): Neural responses to monetary incentives in major depression. Biol Psychiatry 63:686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sacchet MD, Knutson B (2012): Spatial smoothing systematically biases the localization of reward-related brain activity. Neuroimage 66C: 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LeDoux JE (2000): Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184. [DOI] [PubMed] [Google Scholar]
- 56.Terburg D, van Honk J (2013): Approach–avoidance versus dominance–submissiveness: A multilevel neural framework on how testosterone promotes social status. Emotion Review 5:296–302. [Google Scholar]
- 57.Brutus M, Shaikh MB, Edinger H, Siegel A (1986): Effects of experimental temporal lobe seizures upon hypothalamically elicited aggressive behavior in the cat. Brain Res 366:53–63. [DOI] [PubMed] [Google Scholar]
- 58.Stoddard-Apter SL, MacDonnell MF (1980): Septal and amygdalar efferents to the hypothalamus which facilitate hypothalamically elicited intraspecific aggression and associated hissing in the cat. An autoradiographic study. Brain Res 193:19–32. [DOI] [PubMed] [Google Scholar]
- 59.Wood R, Newman SW (1999): Androgen receptor immunoreactivity in the male and female Syrian hamster brain. J Neurobiol 39:359–370. [DOI] [PubMed] [Google Scholar]
- 60.Fernández-Guasti A, Kruijver FP, Fodor M, Swaab DF (2000): Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol 425:422–435. [DOI] [PubMed] [Google Scholar]
- 61.Roselli CE, Klosterman S, Resko JA (2001): Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J Comp Neurol 439:208–223. [DOI] [PubMed] [Google Scholar]
- 62.Donahue JE, Stopa EG, Chorsky RL, King JC, Schipper HM, Tobet SA, et al. (2000): Cells containing immunoreactive estrogen receptor-α in the human basal forebrain. Brain Res 856:142–151. [DOI] [PubMed] [Google Scholar]
- 63.Murphy AZ, Shupnik MA, Hoffman GE (1999): Androgen and estrogen ( α ) receptor distribution in the periaqueductal gray of the male rat. Horm Behav 36:98–108. [DOI] [PubMed] [Google Scholar]
- 64.Tuiten A, Van Honk J, Koppeschaar H, Bernaards C, Thijssen J, Verbaten R (2000): Time course of effects of testosterone administration on sexual arousal in women. Arch Gen Psychiatry 57:149–153. [DOI] [PubMed] [Google Scholar]
- 65.Aranda A, Pascual A (2001): Nuclear hormone receptors and gene expression. Physiol Rev 81:1269–1304. [DOI] [PubMed] [Google Scholar]
- 66.Foradori C, Weiser M, Handa R (2008): Non-genomic actions of androgens. Front Neuroendocrinol 29:169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DonCarlos LL, Garcia-Ovejero D, Sarkey S, Garcia-Segura LM, Azcoitia I (2003): Androgen receptor immunoreactivity in forebrain axons and dendrites in the rat. Endocrinology 144:3632–3638. [DOI] [PubMed] [Google Scholar]
- 68.Tabori N, Stewart L, Znamensky V, Romeo R, Alves S, McEwen B, et al. (2005): Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience 130: 151–163. [DOI] [PubMed] [Google Scholar]
- 69.Blaustein J, Lehman M, Turcotte J, Greene G (1992): Estrogen receptors in dendrites and axon terminals in the guinea pig hypothalamus. Endocrinology 131:281–290. [DOI] [PubMed] [Google Scholar]
- 70.McEwen BS (2001): Invited review: Estrogens effects on the brain: Multiple sites and molecular mechanisms. J Appl Physiol 91: 2785–2801. [DOI] [PubMed] [Google Scholar]
- 71.Sarkey S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D, DonCarlos LL (2008): Classical androgen receptors in non-classical sites in the brain. Horm Behav 53:753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trainor BC, Lin S, Finy MS, Rowland MR, Nelson RJ (2007): Photoperiod reverses the effects of estrogens on male aggression via genomic and non-genomic pathways. Proc Natl Acad Sci U S A 104:9840–9845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trainor BC, Sima Finy M, Nelson RJ (2008): Rapid effects of estradiol on male aggression depend on photoperiod in reproductively nonresponsive mice. Horm Behav 53:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oliveira RF, Silva A, Canário AV (2009): Why do winners keep winning? Androgen mediation of winner but not loser effects in cichlid fish. Proc Biol Sci 276:2249–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang C (2007): Challenges in the diagnosis of the right patient for testosterone replacement therapy. European Urology Supplements 6: 862–867. [Google Scholar]
- 76.Eisenegger C, von Eckardstein A, Fehr E, von Eckardstein S (2013): Pharmacokinetics of testosterone and estradiol gel preparations in healthy young men. Psychoneuroendocrinology 38:171–178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.