Abstract
The rapid synthesis of diverse substituted polycyclic quinazolinones was achieved by two orthogonal Ugi four-component reaction (Ugi-4CR)-based protocols: the first two-step approach via an ammonia-Ugi-4CR followed by palladium-catalyzed annulation; in the second approach, cyanamide was used unprecedently as an amine component in Ugi-4CR followed by an AIBN/tributyltin hydride-induced radical reaction. Like no other method, MCR and cyclization could efficiently construct many biologically interesting compounds with tailored properties in very few steps.
Introduction
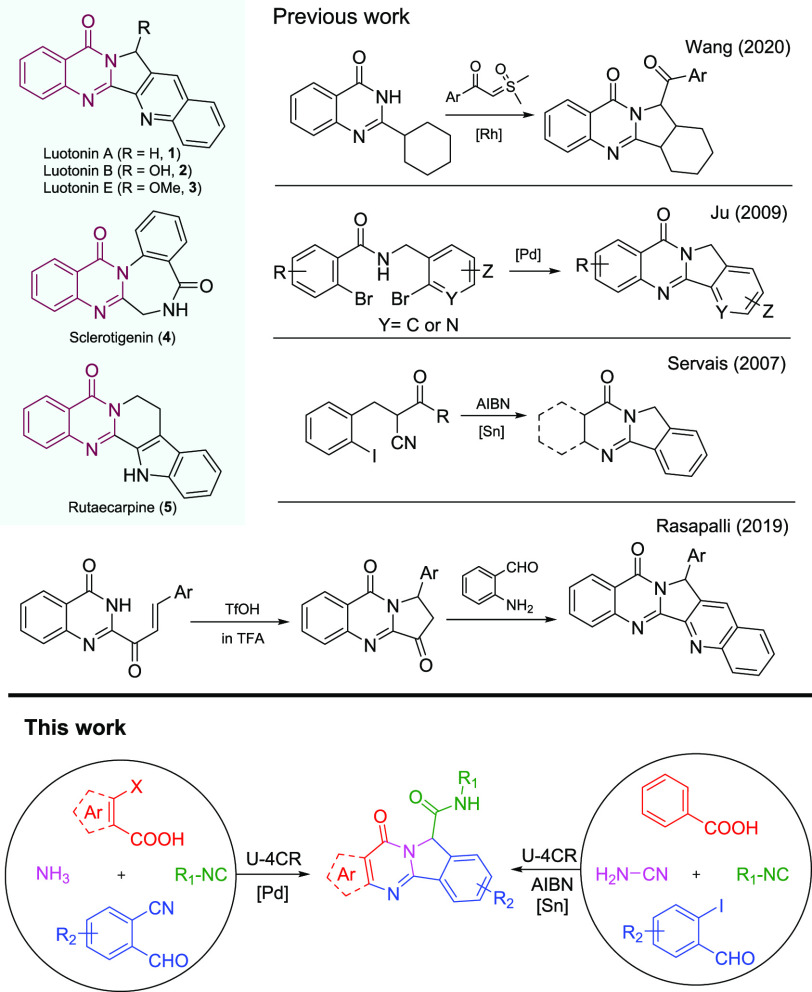
Quinazolinones are among the very important class of biologically active N-fused heterocyclic scaffolds that have been involved in many marketed drugs or potential candidates.1 Notably, polycyclic quinazolinones, such as luotonins (1–3), sclerotigenin (4), and rutaecarpine (5), have been reported to exhibit a broad spectrum of biological activities.2 Thus, the large structural diversity and promising therapeutic potential of polycyclic quinazolinone analogs have inspired chemists to further explore novel variants of quinazolinone structures or develop more efficient synthetic methodologies.
Multicomponent reactions (MCRs) are reactions that employ at least three starting materials to form one single product, where the majority of the atoms from starting materials are incorporated into final products.3 Due to their high efficiency, mild conditions, and large scaffolds diversity, the MCRs have become a unique tool for the rapid generation of a great variety of natural products and pharmaceuticals.4 For example, β-amino amides have been successfully synthesized in a one-pot manner by applying triazenyl alkynes, carboxylic acids, aldehydes, and anilines as the starting materials.5 The Ugi reaction is one of the most well-known and broadly used MCRs.6 It has been involved in the synthesis of diverse scaffolds as a key step. For instance, the synthesis of a potent amino acid antibiotic furanomycin and a naturally cyclic peptide ustiloxin D used the Ugi reaction as the critical step.7 In our previous studies, we have introduced Ugi reactions for constructing important bioactive scaffolds, such as isoquinolone-4-carboxylic acid and isoquinoline scaffolds.8
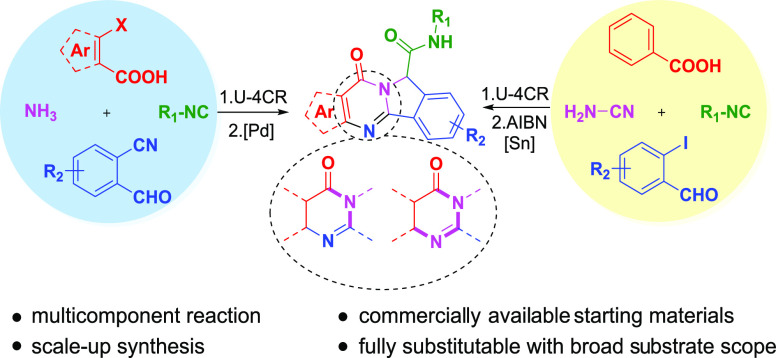
The rapid construction of polycyclic quinazolinones is an extensively studied topic. Several metal catalysts, such as copper, rhodium, and palladium, have been used for the synthesis of the corresponding compounds.9 The application of radical cyclization as well as self-catalyzed phototandem perfluoroalkylation/cyclization of unactivated alkenes also has been reported for the synthesis of the same scaffolds.10 As shown in Scheme 1, these approaches suffer from one or many issues, such as lengthy sequential synthesis, limited scope, and generality.9−11 Inspired by previous syntheses and based on our deep interest in MCR chemistry, we envisioned that isoindolo[1,2-b]quinazolinone derivatives could be synthesized in a concise manner by an Ugi-4CR reaction of o-bromobenzoic acids, o-cyanobenzaldehydes, isocyanides, and ammonia followed by a metal-catalyzed intramolecular N-arylation to form the desired products.12 Alternatively, we figured, in a second strategy, that it could also be synthesized by an Ugi-4CR using o-iodobenzaldehyde, benzoic acid, isocyanides, and cyanamide followed by radical cyclization of the N-acylcyanamide moiety since the cyano group is a well-established radical acceptor that has been involved in the build of various heterocycles and carbocycles.13 We were the first to report cyanamide as an acid component in the Ugi reaction, reacting with enamines and isocyanides in the presence of Lewis acids to give the medicinally important scaffold α-amino-N-cyanoamidines.14 Here, we report for the first time cyanamide to react as an amine component in the Ugi reaction.
Scheme 1. Representatives and Synthesis of Polycyclic Quinazolinones.
Results and Discussion
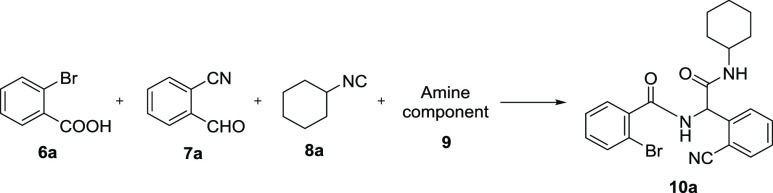
As the starting point of our work to elaborate the first strategy, the model Ugi-4CR with 2-bromobenzoic acid (6a), 2-cyanobenzaldehyde (7a), cyclohexyl isocyanide (8a), and NH4Cl (9a) was performed in MeOH/H2O (3:1) under room temperature for 12 h (entry 1) as shown in Table 1.15 The precipitated Ugi product 10a formed, and the solid was filtered and washed with diethyl ether giving a 33% yield. We then used MeOH as the solvent (entry 2) or increased the temperature to 55 °C (entry 3), giving yields of 60% and 33%, respectively. Not satisfied, we tried to use ammonia (7 N in MeOH, 9c) instead of NH4Cl in 2,2,2-trifluoroethanol (TFE) at 55 °C in a closed vial for 12 h (entry 5).16 No precipitated Ugi product was observed, but a yield of 62% was isolated after flash chromatography purification. Thereafter, the reactions were carried out under room temperature in TFE (entry 6) or MeOH (entry 7), which both formed the precipitated Ugi product with yields of 75% and 60%, respectively. When ammonia (in water, 9b) was used (entry 4), a trace amount of Ugi product was formed. Though 2,4-dimethoxylbenzylamine (entry 8) as an ammonia surrogate showed good performance in this reaction, two steps with a total yield of 59% were not competitive. Finally, the optimized reaction condition was concluded to be ammonia (in MeOH, 9c) in TFE at room temperature for 12 h (entry 6).
Table 1. Optimization of Ugi-4CR Conditions.
| entrya | amine component | solvent | T (°C) | yieldb |
|---|---|---|---|---|
| 1 | NH4Cl (9a) | MeOH/H2O = 3:1 | r.t. | 33% |
| 2 | NH4Cl | MeOH | r.t. | 41% |
| 3 | NH4Cl | MeOH/H2O = 3:1 | 55 °C | 33% |
| 4 | ammonia in water (9b) | TFE | r.t. | trace |
| 5 | ammonia in MeOH (9c) | TFE | 55 °C | 62% |
| 6 | ammonia in MeOH | TFE | r.t. | 75% |
| 7 | ammonia in MeOH | MeOH | r.t. | 60% |
| 8c | 2,4-dimethoxybenzylamine (9d) | TFE | r.t. | 59% |
Reaction conditions: 6a (1 mmol), 7a (1 mmol), 8a (1 mmol), 9 (1 mmol), solvent (1 mL).
Isolated yields.
Followed by HCl-catalyzed cleavage.
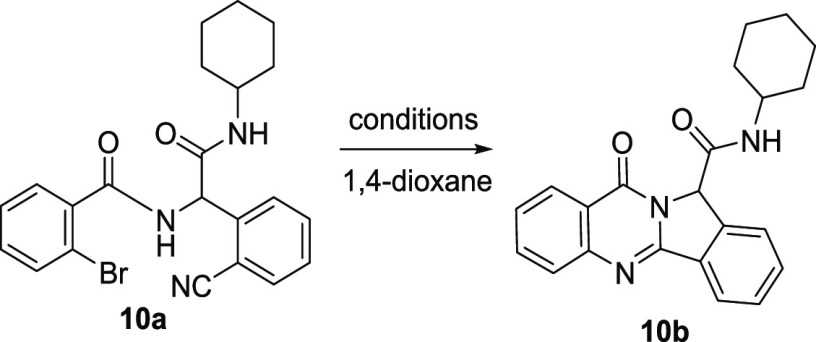
With optimized conditions for the Ugi-4CR in hand, we next investigated the subsequent palladium-catalyzed annulation step by exploring varied catalysts, ligands, and bases (Table 2). When the reaction was performed in the presence of 5 mol % Pd(OAc)2, 10 mol % 1,1′-bis(diphenylphosphino)ferrocene (dppf), and 2 equiv of K3PO4 in 1,4-dioxane at reflux for overnight, the desired product 10b was obtained in 65% yield (entry 9). When PdCl2 (entry 10) or PdO (entry 11) has been used as the catalyst, the yield decreased to 16% and 20%, respectively. Replacing the dppf with Xantphos resulted in a lower yield (33%, entry 12). To our delight, the desired product 10b was formed in 81% yield by using K2CO3 (entry 15) as the base, compared with K3PO4 (65%, entry 9) and Cs2CO3 (72%, entry 14). Notably, microwaves did not facilitate the reaction as we expected (42%, entry 13). Furthermore, we tried different organometallic complexes giving no product formation or low yields (entry 16–19). Finally, the optimized condition was concluded to be the Ugi adduct 10a, Pd(OAc)2, dppf, and K2CO3 in 1,4-dioxane at reflux overnight (entry 15).
Table 2. Optimization of [Pd]-Catalyzed Annulation.
| entrya | catalyst | ligand | base | yieldc |
|---|---|---|---|---|
| 9 | Pd(OAc)2 | dppf | K3PO4 | 65% |
| 10 | PdCl2 | dppf | K3PO4 | 16% |
| 11 | PdO | dppf | K3PO4 | 20% |
| 12 | Pd(OAc)2 | Xantphos | K3PO4 | 33% |
| 13b | Pd(OAc)2 | dppf | K3PO4 | 42% |
| 14 | Pd(OAc)2 | dppf | Cs2CO3 | 72% |
| 15 | Pd(OAc)2 | dppf | K2CO3 | 81% |
| 16 | PdCl2(PPh3)2 | K3PO4 | n.dd | |
| 17 | Pd(dppf)Cl2 | K3PO4 | 34% | |
| 18 | [(C6H5)3P]2Pd(CH2C6H5)Cl | K3PO4 | trace | |
| 19 | PdCl2·(CH3CN)2 | K3PO4 | trace | |
Reaction conditions: 10a (0.5 mmol), catalyst (5 mol %), ligand (10 mol %), base (1 mmol), 1,4 dioxane (5 mL), reflux, overnight.
Microwave, 120 °C, 30 min.
Isolated yields.
Not detected.
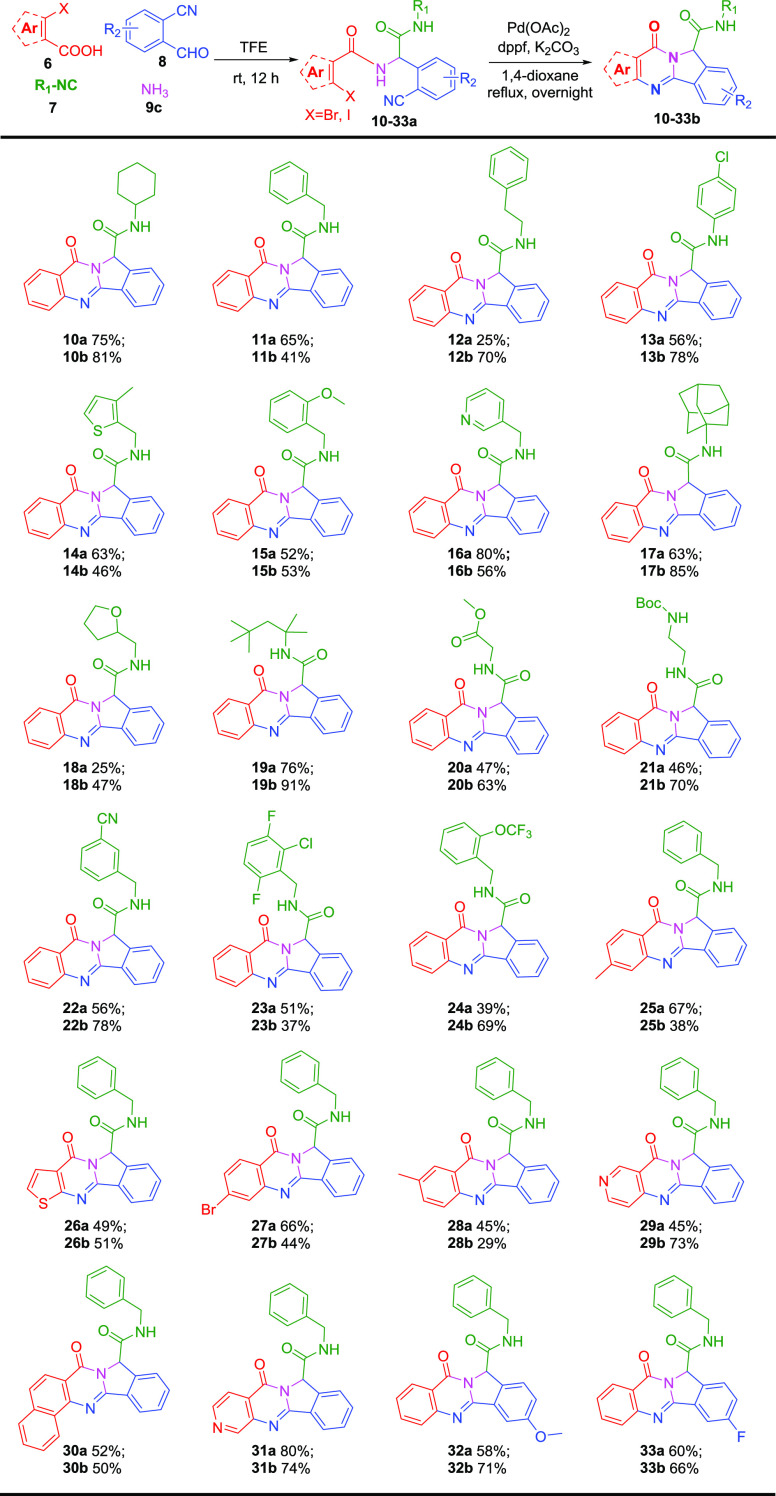
With the optimal conditions in hand, we then set out to explore the scope and limitation of these tandem reactions. A set of Ugi-4CR adducts 10a–33a were efficiently synthesized in moderate to good yields without column purification required for most cases. Aromatic/aliphatic isocyanides, versatile o-bromobenzoic acids, and substituted o-cyanobenzaldehydes were all tolerated well in this ammonia Ugi four-component reaction. Then, all the Ugi adducts were examined to determine the scope of the tandem reaction to furnish the corresponding products 10b–33b.
As can be seen in Scheme 2, all the Ugi adducts led to the expected polycyclic quinazolinones. We initially replaced cyclohexyl isocyanide with various aromatic isocyanides, such as benzyl (11b, 41%), phenethyl (12b, 70%), phenyl (13b, 78%), thienylmethyl (14b, 46%), and pyridylmethyl (16b, 56%), all giving good yields. Benzyl isocyanides bearing diverse functional groups also performed well in this reaction, as examples, methoxy (15b, 53%), nitrile (22b, 78%), halide (23b, 37%), and trifluoromethoxy (24b, 69%). Further, aliphatic isocyanides 1-adamantyl isocyanide (17b, 85%), tetrahydrofuran-2-ylmethyl isocyanide (18b, 47%), tert-octyl isocyanide (19b, 91%), methyl isocyanoacetate (20b, 63%), and tert-butyl (2-isocyanoethyl)carbamate (21b, 70%) gave good yields as well. This is meaningful since the previously reported methods either have the limited type of substitutions or are non-substituted in this position.9−11 Meanwhile, by using our strategy, we opened the substitution possibilities at this position for all available isocyanides providing the opportunity for various further chemical modifications.
Scheme 2. Substrate Scope in the [Pd]-Catalyzed Synthesis of Polycyclic Quinazolinones–
Acid, isocyanide, aldehyde, and ammonia components are depicted with red, green, blue, and pink color, respectively.
Ugi reaction was carried out using 6 (1 mmol), 7 (1 mmol), 8 (1 mmol), and 9c (1 mmol) in TFE (1 M) for 12 h at r.t.
[Pd]-catalyzed reaction conditions: 10–34a (0.2 mmol), Pd(OAc)2 (5 mol %), dppf (10 mol %), K2CO3 (1 mmol), 1,4 dioxane (2 mL), reflux, overnight.
Yield refers to purified products.
Subsequently, we examined the ability of these tandem reactions to incorporate a diverse panel of carboxylic acids and aldehydes components. All the used acid and aldehyde components were well-tolerated in Ugi-4CR, giving good yields ranging from 45% to 80%. For the subsequent [Pd]-catalyzed annulation, thiophenecarboxylic acid (26b), nicotinic acids (29b, 31b), and naphthoic acid (30b) proceeded smoothly. Nicotinic acids significantly increased yields when compared with benzoic acid (11b), which may probably benefit from the electron-withdrawing effect of the nitrogen atom. Additionally, different functional groups at the aromatic ring of o-bromobenzoic acid were well tolerated, such as methyl (25b, 27b) and bromine (27b). The relatively lower yields have been observed when the methyl group attached may be due to its electron-donating effect, making the acid less reactive. In addition, good substrate tolerance was also achieved for the aldehyde component. 2-Cyano-4-methoxybenzaldehyde (32b) and 4-fluoro-3-cyanobenzaldehyde (33b) yielded 71% and 66% of products. A plausible mechanism of this reaction is that the cyano group is first activated by a transition metal to form a σ-coordination, which will facilitate further nucleophilic addition. Then, the following intramolecular cyclization might be executed by the metal-catalyzed SNAr reaction.
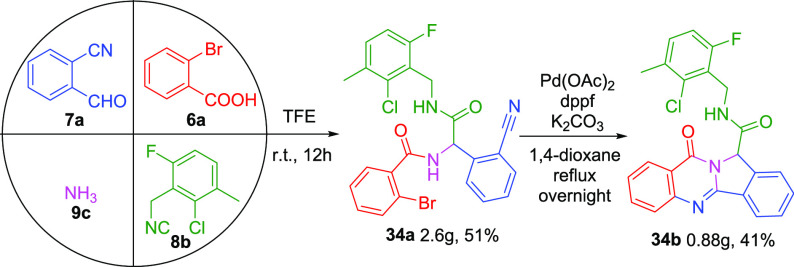
Furthermore, the scalability of this method was investigated. A four-component reaction of 2-bromobenzoic acid (6a), 2-cyanobenzaldehyde (7a), 2-chloro-3-methyl-6-fluorobenzyl isocyanide (8b), and ammonia (9c) was conducted on a 10 mmol scale. The participate was filtered and washed with diethyl ether, affording 2.6 g of Ugi adduct 34a (51%). Then, Ugi adduct 34a yielded 0.88 g of the final cyclized product 34b (41%) through silica gel flash chromatography as shown in Scheme 3.
Scheme 3. Scale-up Synthesis.
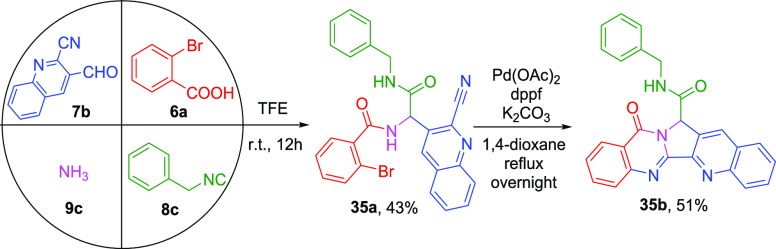
luotonins A, B, and E (1–3) are naturally occurring cytotoxic alkaloids, being demonstrated as human DNA topoisomerase I (hTopI) poisons.17 The current synthesis of luotonin derivatives may suffered from multiple synthetic procedures and/or a limited substrate scope.9−11,18Scheme 4 illustrates our initial success of luotonin A derivative 35b (51%) synthesis of which the Ugi adduct 35a (43%) was assembled from the appropriate 2-bromobenzoic acid (6a), 2-quinolinecarbonitrile (7b), ammonia (9c), and benzyl isocyanide (8c). Despite this, the Passerini three-component (P-3CR) byproduct was observed in Ugi-4CR. Our strategy could incorporate a variety of functionalities from largely available isocyanides into the luotonin scaffold.
Scheme 4. Synthesis of luotonin Derivatives.
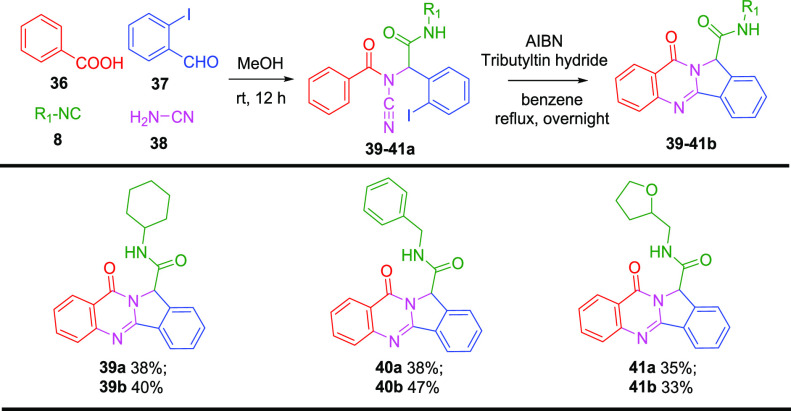
Encouraged by our successful development, we continued to explore the second approach via a cyanamide-Ugi-4CR followed by an AIBN/tributyltin hydride-induced radical cyclization. The reaction started with benzoic acid (36), 2-iodobenzaldehyde (37), cyanamide (38), and three different isocyanides (Scheme 5). The reactions afforded the corresponding Ugi products 39a–41a with moderate yields because P-3CR byproducts were isolated with a nearly 1:1 ratio from each reaction. The P-3CR product is often observed when less nucleophilic amines are employed in the Ugi-4CR, which can be explained by a low formation of the iminium intermediates in the reaction mixture.19 To optimize the reaction, we tried several reaction conditions: (i) using 2 equiv of cyanamide; (ii) heating to 60 °C; (iii) using microwaves (100 °C, 30 min); (iv) changing the solvent; (v) adding Lewis acids as the catalyst, such as ZnCl2, TiCl4, HClO4, scandium(III) triflate, ytterbium(III) triflate.20 Unfortunately, they failed to improve the yields.
Scheme 5. Various Isocyanides in the Synthesis of Polycyclic Quinazolinones via Radical Cyclization–
Acid, aldehyde, isocyanide, and cyanamide components are depicted with red, blue, green, and pink color, respectively.
Ugi reaction was carried out using 36 (1 mmol), 37 (1 mmol), 8 (1 mmol), and 38 (1 mmol) in TFE (1 M) for 12 h at r.t.
Radical cyclization conditions: 39–41a (0.15 mmol), tributyltin hydride (0.3 mmol), AIBN (0.15 mmol), benzene (7 mL), reflux, overnight.
Yield refers to purified products.
Despite achieving moderate yields, we were able to isolate sufficient quantities of the Ugi products to examine the subsequent step. The reactions were performed between Ugi adducts, tributyltin hydride, and AIBN in benzene at reflux overnight. To our delight, all the three Ugi adducts yielded the corresponding products 39–41b, giving yields of 40%, 47%, and 33%, respectively. Therefore, we have successfully introduced cyanamide into the Ugi-4CR as the amino component that generated the stable N-acylcyanamide moiety, which could undergo further cyclization leading to the desired polycyclic quinazolinones. A plausible mechanism for this radical cascade is that the trapped aromatic radical on the cyanamide triple bond could further cyclize on an aryl substituent. This cyclization undergoes direct addition to form the hexadienyl radical, which further goes with rearomatization.21
Conclusions
Two orthogonal Ugi-4CR-based synthesis strategies for fully substituted polycyclic quinazolinone derivatives have been developed. Alternative Ugi-4CRs were instrumental in this approach for introducing the critical cyano group, leading to potentially bioactive polycyclic quinazolinones through two different cyclization mechanisms. For this, we introduced cyanamide for the first time as an amine component in the Ugi-4CR. Remarkably, cyanamide is thus the first amphoteric building block that can function as an acid and amine component in Ugi-type transformations. Significantly, the successfully synthesized N-acylcyanamide moiety, as an efficient amide-iminyl radical, opens the door for the rapid and straightforward synthesis of pharmaceutically important heterocycles by Ugi-4CR. Diverse isocyanides with versatile functional groups can be introduced into the polycyclic quinazolinone scaffold allowing the modification of physicochemical properties, further chemical manipulations, and so on. Our protocol is outperforming all known strategies toward this important scaffold in terms of utilization and accessibility of commercially available starting materials, mild conditions, and simple purification work-up and is of excellent maneuverability and efficiency. Work aiming at the optimization of cyanamide involved Ugi-4CR as well as extending the scope of the subsequent annulation is in progress and will be reported in due course.
Experimental Section
General Information
Nuclear magnetic resonance spectra were recorded on a Bruker Avance 500 spectrometer. Chemical shifts for 1H NMR were reported relative to TMS (δ 0 ppm) or the internal solvent peak (CDCl3 δ 7.26 ppm, DMSO-d6 δ 2.50 ppm or CD3OD δ 3.31 ppm) and coupling constants were in hertz (Hz). The following abbreviations were used for spin multiplicity: s = singlet, d = doublet, t = triplet, dt = double triplet, ddd = doublet of double doublet, m = multiplet, and br = broad. Chemical shifts for 13C NMR reported in ppm relative to the solvent peak (CDCl3 δ 77.23 ppm, DMSO δ 39.52 ppm, CD3OD δ 49.00 ppm). Flash chromatography was performed on a Grace Reveleris X2 using Grace Reveleris Silica columns (12 g) and a gradient of petroleum ether/ethyl acetate (0–100%) or dichloromethane/methanol (0–10%) was applied. Thin-layer chromatography was performed on Fluka precoated silica gel plates (0.20 mm thick, particle size 25 μm). Reagents were available from commercial suppliers and used without any purification unless otherwise noted. All isocyanides were made in house by performing the Ugi procedure. Other reagents were purchased from Sigma Aldrich, ABCR, Acros, Fluorochem, and AK Scientific and were used without further purification. High-resolution mass spectra (HRMS) were recorded using a QTOF Bruker Maxis Plus, mass range 100–1500 m/z, spectra rate 2.00 Hz. Yields given refer to chromatographically purified and spectroscopically pure compounds unless otherwise stated.
General Experimental Procedure of Ammonia-Ugi-4CR
To a stirred solution of aldehyde (1 mmol, 1 equiv) in 2,2,2-trifluoroethanol (1 mL) was added ammonia solution (7 N in methanol, 1 mmol, 1 equiv) and stirred for 10 min followed by the addition of isocyanide (1 mmol, 1 equiv) and carboxylic acid (1 mmol, 1 equiv). The reaction was allowed to stir at room temperature in a close screwed vial for 12 h. The precipitate was filtered and washed with diethyl ether, affording the corresponding Ugi adduct. Otherwise, the Ugi adduct was purified by silica gel flash chromatography using either PE/EA or DCM/MeOH as the eluent.
General Experimental Procedure of [Pd]-Catalyzed Annulation
The Ugi adduct (0.15–0.4 mmol, 1 equiv), Pd(OAc)2 (0.0075–0.02 mmol, 0.05 equiv), dppf (0.015–0.04 mmol, 0.1 equiv), and K2CO3 (0.3–0.8 mmol, 2 equiv) were added to a 5 mL (or 10 mL) reaction tube equipped with a magnetic stir bar, and 1.5–4 mL of 1,4-dioxane was added. The mixture was heated to reflux in a metal heating block overnight. After the reaction was completed, the solvent was removed under reduced pressure, and the residue was purified by silica gel flash chromatography using either PE/EA or DCM/MeOH as the eluent.
Scale-up Synthesis of 34b
A 50 mL flask equipped with a magnetic stirrer bar was charged with 2-formylbenzonitrile (10 mmol, 1.3 g, 1 equiv) and a calculated amount of 7 N ammonia solution (in methanol, 10 mmol, 1.4 mL, 1 equiv) in 2,2,2-trifluoroethanol (10 mL). We sealed the reaction flask immediately and allowed it to stir for 10 min. Then, 2-chloro-4-fluoro-3-(isocyanomethyl)-1-methylbenzene (10 mmol, 1.8 g, 1 equiv) and 2-bromobenzoic acid (10 mmol, 2.0 g, 1 equiv) were added to the solution and the reaction mixture was stirred at room temperature for 12 h. The precipitate was filtrated and washed by diethyl ether, affording the pure Ugi adduct 34a with a yield of 51%. Then, the Ugi adduct (5 mmol, 2.6 g, 1 equiv) was added to Pd(OAc)2 (0.25 mmol, 56 mg, 0.05 equiv), dppf (0.5 mmol, 277 mg, 0.1 equiv), and K2CO3 (10 mmol, 1.4 g, 2 equiv) in 1,4-dioxane (50 mL) in a 200 mL round flask with a magnetic stirrer bar. The reaction mixture was heated to reflux in an oil bath overnight. After the reaction was completed, the solvent was removed under reduced pressure, and the crude product was purified by column chromatography (silica gel, petroleum ether/ethyl acetate = 2:1) to afford the product 34b (0.88 g, 41% yield).
Synthesis of Luotonin Derivative 35b
To a stirred solution of 2-quinolinecarbonitrile (1 mmol, 154 mg, 1 equiv) in 2,2,2-trifluoroethanol (1 mL) was added ammonia solution (7 N in methanol, 1 mmol, 140 μL, 1 equiv) and stirred for 10 min followed by the addition of benzyl isocyanide (1 mmol, 117 mg, 1 equiv) and 2-bromobenzoic acid (1 mmol, 201 mg, 1 equiv). The reaction was allowed to stir at room temperature in a close screwed vial for 12 h. The reaction was concentrated and purified by column chromatography (silica gel, petroleum ether/ethyl acetate as eluent) to afford the corresponding Ugi product 35a. Subsequently, the Ugi adduct (0.15 mmol, 75 mg, 1 equiv), Pd(OAc)2 (0.0075 mmol, 1.7 mg, 0.05 equiv), dppf (0.015 mmol, 8.3 mg, 0.1 equiv), and K2CO3 (0.3 mmol, 41.5 mg, 2 equiv) were added to a 5 mL reaction tube equipped with a magnetic stir bar, and 1.5 mL of 1,4-dioxane was added. The mixture was heated to reflux in a metal heating block overnight. After the reaction was completed, the solvent was removed under reduced pressure, and the residue was purified by silica gel flash chromatography using PE/EA as the eluent.
General Experimental Procedure of Cyanamide-Ugi-4CR
To a stirred solution of aldehyde (1 mmol, 1 equiv) in MeOH (1 mL) was added cyanamide (1 mmol, 1 equiv) and stirred for 30 min followed by the addition of isocyanide (1 mmol, 1 equiv) and carboxylic acid (1 mmol, 1 equiv). The reaction was allowed to stir at room temperature for 12 h. The reaction was concentrated and purified by column chromatography (silica gel, petroleum ether/ethyl acetate = 2:1) to afford the corresponding Ugi product.
General Experimental Procedure of Radical Cyclization
The Ugi adduct (0.15 mmol, 1 equiv), tributyltin hydride (0.3 mmol, 2 equiv), and azobisisobutyronitrile (AIBN, 0.15 mmol, 1 equiv) were added to a 25 mL round flask equipped with a magnetic stir bar, and 7 mL of benzene was added. The mixture was heated to reflux overnight. After the reaction was completed, NaOH (aq, 1 M, 10 mL) was added to the reaction mixture and stirred for 30 min. The organic phase was extracted with ethyl acetate (2 × 20 mL), dried over MgSO4, and concentrated under vacuum. The residue was purified by silica gel flash chromatography using PE/EA as the eluent.
2-Bromo-N-(1-(2-cyanophenyl)-2-(cyclohexylamino)-2-oxoethyl)benzamide (10a)
Obtained from a 1 mmol reaction as a white solid, 330 mg, yield 75%; 1H NMR (500 MHz, DMSO-d6) δ 9.30 (d, J = 7.7 Hz, 1H), 8.18 (d, J = 7.8 Hz, 1H), 7.87 (d, J = 8.8 Hz, 1H), 7.72 (td, J = 7.7, 1.5 Hz, 1H), 7.65 (d, J = 8.0 Hz, 1H), 7.60 (d, J = 7.8 Hz, 1H), 7.52 (td, J = 7.5, 1.2 Hz, 1H), 7.49–7.42 (m, 2H), 7.37 (td, J = 7.8, 2.0 Hz, 1H), 5.82 (d, J = 7.7 Hz, 1H), 3.67–3.53 (m, 1H), 1.82–1.62 (m, 4H), 1.59–1.51 (m, 1H), 1.31–1.22 (m, 3H), 1.20–1.06 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 167.3, 167.3, 141.9, 138.5, 133.6, 133.5, 133.2, 133.0, 131.5, 129.7, 128.9, 127.8, 119.5, 117.8, 112.7, 55.9, 48.5, 32.6, 25.6, 24.8. HRMS (ESI) m/z calculated for C22H23BrN3O2 [M + H]+: 440.0974, found [M + H]+: 440.0967.
N-(2-(benzylamino)-1-(2-cyanophenyl)-2-oxoethyl)-2-bromobenzamide (11a)
Obtained from a 1 mmol reaction as a white solid, 290 mg, yield 65%; 1H NMR (500 MHz, chloroform-d) δ 7.74–7.69 (m, 3H), 7.64 (td, J = 7.6, 1.3 Hz, 1H), 7.60 (dt, J = 7.6, 1.5 Hz, 2H), 7.45 (td, J = 7.6, 1.1 Hz, 1H), 7.36 (td, J = 7.5, 1.2 Hz, 1H), 7.32–7.26 (m, 4H), 7.18–7.14 (m, 2H), 6.47 (t, J = 5.6 Hz, 1H), 5.96 (d, J = 6.1 Hz, 1H), 4.54–4.39 (m, 2H). 13C{1H} NMR (126 MHz, CDCl3) δ 167.8, 166.5, 141.4, 136.9, 136.2, 133.6, 133.5, 133.2, 131.8, 130.1, 128.8, 128.8, 128.0, 127.7, 127.6, 127.5, 119.5, 117.9, 111.6, 56.5, 44.1. HRMS (ESI) m/z calculated for C23H19BrN3O2 [M + H]+: 448.0661, found [M + H]+: 448.0654.
2-Bromo-N-(1-(2-cyanophenyl)-2-oxo-2-(phenethylamino) ethyl)benzamide (12a)
Obtained from a 1 mmol reaction as a light yellow solid, 95 mg, yield 25%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, chloroform-d) 1H NMR (500 MHz, chloroform-d) δ 7.77 (d, J = 6.0 Hz, 1H), 7.71–7.59 (m, 5H), 7.46 (td, J = 7.4, 1.6 Hz, 1H), 7.38 (td, J = 7.5, 1.2 Hz, 1H), 7.32 (td, J = 7.6, 1.8 Hz, 1H), 7.26–7.17 (m, 3H), 7.06–7.01 (m, 2H), 6.16 (t, J = 5.9 Hz, 1H), 5.86 (d, J = 5.9 Hz, 1H), 3.70–3.60 (m, 1H), 3.53–3.43 (m, 1H), 2.83–2.73 (m, 2H). 13C{1H} NMR (126 MHz, CDCl3) δ 167.7, 166.4, 141.6, 138.0, 136.2, 133.5, 133.5, 133.2, 131.7, 130.0, 128.7, 128.6, 128.6, 127.9, 127.6, 126.6, 119.5, 117.8, 111.5, 56.3, 41.2, 35.2. HRMS (ESI) m/z calculated for C24H21BrN3O2 [M + H]+: 462.0817, found [M + H]+: 462.0812.
2-Bromo-N-(2-((4-chlorophenyl)amino)-1-(2-cyanophenyl)-2-oxoethyl)benzamide (13a)
Obtained from a 1 mmol reaction as a yellow solid, 260 mg, yield 56%; 1H NMR (500 MHz, DMSO-d6) δ 10.63 (s, 1H), 9.49 (d, J = 7.1 Hz, 1H), 7.93 (dd, J = 7.7, 1.4 Hz, 1H), 7.74 (td, J = 7.7, 1.4 Hz, 1H), 7.71–7.64 (m, 3H), 7.62–7.51 (m, 3H), 7.47 (td, J = 7.5, 1.2 Hz, 1H), 7.43–7.36 (m, 3H), 6.00 (d, J = 7.1 Hz, 1H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 167.5, 167.4, 140.7, 138.4, 138.1, 133.8, 133.7, 133.2, 133.1, 131.6, 129.8, 129.2, 129.2, 127.7, 121.5, 121.4, 119.5, 117.7, 112.8, 56.7. HRMS (ESI) m/z calculated for C22H16BrClN3O2 [M + H]+: 468.0114, found [M + H]+: 468.0109.
2-Bromo-N-(1-(2-cyanophenyl)-2-(((3-methylthiophen-2-yl) Methyl)amino)-2-oxoethyl)benzamide (14a)
Obtained from a 1 mmol reaction as a light yellow solid, 294 mg, yield 63%; 1H NMR (500 MHz, chloroform-d) δ 7.76–7.57 (m, 6H), 7.47 (td, J = 7.6, 1.3 Hz, 1H), 7.42–7.37 (m, 1H), 7.32 (td, J = 7.8, 1.8 Hz, 1H), 7.12 (d, J = 5.1 Hz, 1H), 6.80 (d, J = 5.1 Hz, 1H), 6.37 (s, 1H), 5.95 (d, J = 6.1 Hz, 1H), 4.65–4.50 (m, 2H), 2.18 (s, 3H). 13C{1H} NMR (126 MHz, CDCl3) δ 167.4, 166.5, 141.2, 136.2, 135.4, 133.5, 133.2, 132.1, 131.8, 131.0, 130.2, 130.1, 128.9, 128.1, 127.6, 123.6, 119.4, 117.9, 111.6, 56.4, 37.0, 13.5. HRMS (ESI) m/z calculated for C22H19BrN3O2S [M + H]+: 468.0381, found [M + H]+: 468.0375.
2-Bromo-N-(1-(2-cyanophenyl)-2-((2-methoxybenzyl)amino)-2-oxoethyl)benzamide (15a)
Obtained from a 1 mmol reaction as a brown solid, 248 mg, yield 52%; 1H NMR (500 MHz, chloroform-d) δ 7.84–7.77 (m, 1H), 7.74–7.68 (m, 2H), 7.65–7.58 (m, 3H), 7.44 (td, J = 7.6, 1.1 Hz, 1H), 7.38 (td, J = 7.5, 1.3 Hz, 1H), 7.34–7.29 (m, 2H), 7.18 (dd, J = 7.3, 1.7 Hz, 1H), 6.92–6.86 (m, 2H), 6.80 (t, J = 5.9 Hz, 1H), 5.91 (d, J = 5.8 Hz, 1H), 4.55–4.37 (m, 2H), 3.85 (s, 3H). 13C{1H} NMR (126 MHz, CDCl3) δ 167.3, 166.3, 157.5, 141.9, 136.3, 133.5, 133.4, 133.0, 131.7, 130.0, 129.6, 129.2, 128.6, 127.6, 127.5, 124.9, 120.5, 119.5, 117.8, 111.6, 110.2, 56.4, 55.2, 40.7. HRMS (ESI) m/z calculated for C24H21BrN3O3 [M + H]+: 478.0766, found [M + H]+: 478.0760.
2-Bromo-N-(1-(2-cyanophenyl)-2-oxo-2-((pyridin-3-ylmethyl)amino)ethyl) Benzamide (16a)
Obtained from a 1 mmol reaction as a yellow solid, 359 mg, yield 80%; 1H NMR (500 MHz, chloroform-d) δ 8.50 (dd, J = 4.9, 1.6 Hz, 1H), 8.45 (d, J = 2.3 Hz, 1H), 7.77 (d, J = 6.3 Hz, 1H), 7.74–7.69 (m, 2H), 7.65 (td, J = 7.7, 1.4 Hz, 1H), 7.63–7.56 (m, 3H), 7.47 (td, J = 7.6, 1.1 Hz, 1H), 7.37 (td, J = 7.5, 1.2 Hz, 1H), 7.34–7.29 (m, 1H), 7.27–7.23 (m, 1H), 7.08 (t, J = 6.1 Hz, 1H), 6.04 (d, J = 6.4 Hz, 1H), 4.54–4.43 (m, 2H). 13C{1H} NMR (126 MHz, CDCl3) δ 168.2, 166.7, 148.6, 141.0, 136.1, 135.7, 133.6, 133.4, 131.8, 131.2, 130.6, 130.0, 129.0, 128.2, 127.6, 127.1, 123.7, 119.4, 117.9, 111.6, 56.4, 41.5. HRMS (ESI) m/z calculated for C22H18BrN4O2 [M + H]+: 449.0613, found [M + H]+: 449.0606.
N-(2-(((3S,5S,7S)-Adamantan-1-yl)amino)-1-(2-cyanophenyl)-2-oxoethyl)-2-bromobenzamide (17a)
Obtained from a 1 mmol reaction as a white solid, 310 mg, yield 63%; 1H NMR (500 MHz, chloroform-d) δ 7.74–7.67 (m, 3H), 7.66–7.59 (m, 3H), 7.43 (td, J = 7.6, 1.3 Hz, 1H), 7.37 (td, J = 7.6, 1.2 Hz, 1H), 7.30 (td, J = 7.7, 1.8 Hz, 1H), 5.82 (s, 1H), 5.79 (d, J = 6.0 Hz, 1H), 2.05 (t, J = 3.2 Hz, 3H), 2.00–1.90 (m, 6H), 1.69–1.61 (m, 6H). 13C{1H} NMR (126 MHz, CDCl3) δ 166.4, 166.2, 142.2, 136.2, 133.6, 133.5, 133.1, 131.7, 130.2, 128.5, 127.6, 127.6, 119.5, 112.3, 111.2, 56.9, 53.1, 41.3, 36.1, 29.3. HRMS (ESI) m/z calculated for C26H27BrN3O2 [M + H]+: 492.1287, found [M + H]+: 492.1283.
2-Bromo-N-(1-(2-cyanophenyl)-2-oxo-2-(((tetrahydrofuran-2-yl)methyl)amino)ethyl) Benzamide (18a)
Obtained from a 1 mmol reaction as a white solid, 110 mg, yield 25%; eluent: VDCM/VMeOH = 20:1; 1H NMR (500 MHz, chloroform-d) δ 7.75 (t, J = 6.6 Hz, 1H), 7.71–7.66 (m, 2H), 7.62 (dt, J = 7.7, 1.8 Hz, 1H), 7.60–7.56 (m, 2H), 7.43 (td, J = 7.6, 1.2 Hz, 1H), 7.34 (td, J = 7.5, 1.2 Hz, 1H), 7.30–7.25 (m, 1H), 6.46–6.37 (m, 1H), 5.90 (s, 1H), 3.97–3.86 (m, 1H), 3.85–3.62 (m, 2H), 3.54–3.42 (m, 1H), 3.35–3.23 (m, 1H), 1.96–1.82 (m, 2H), 1.81–1.62 (m, 1H), 1.57–1.29 (m, 1H). 13C{1H} NMR (126 MHz, CDCl3) δ 167.9, 166.5, 141.6, 136.3, 133.5, 133.3, 131.7, 130.0, 128.8, 128.3, 128.2, 127.5, 119.5, 117.7, 111.6, 76.9, 68.2, 56.5, 43.4, 28.4, 25.8. HRMS (ESI) m/z calculated for C21H21BrN3O3 [M + H]+: 442.0766, found [M + H]+: 442.0759.
2-Bromo-N-(1-(2-cyanophenyl)-2-oxo-2-((2,4,4-trimethylpentan-2-yl)amino)ethyl) Benzamide (19a)
Obtained from a 1 mmol reaction as a light yellow solid, 356 mg, yield 76%; 1H NMR (500 MHz, DMSO-d6) δ 9.18 (d, J = 7.8 Hz, 1H), 7.84 (dd, J = 7.7, 1.4 Hz, 1H), 7.71 (td, J = 7.7, 1.4 Hz, 1H), 7.68–7.61 (m, 3H), 7.50 (td, J = 7.6, 1.2 Hz, 1H), 7.45–7.42 (m, 2H), 7.39–7.35 (m, 1H), 5.81 (d, J = 7.9 Hz, 1H), 1.79 (d, J = 14.6 Hz, 1H), 1.64 (d, J = 14.6 Hz, 1H), 1.32 (d, J = 7.8 Hz, 6H), 0.92 (s, 9H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 167.2, 167.1, 142.1, 138.6, 133.7, 133.3, 133.2, 133.0, 131.5, 129.7, 128.8, 127.9, 119.4, 118.0, 112.5, 56.3, 55.1, 50.6, 31.7, 29.5, 29.4. HRMS (ESI) m/z calculated for C24H29BrN3O2 [M + H]+: 470.1443, found [M + H]+: 470.1429.
Methyl (2-(2-bromobenzamido)-2-(2-cyanophenyl)acetyl)glycinate (20a)
Obtained from a 1 mmol reaction as a white solid, 200 mg, yield 47%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, chloroform-d) δ 7.77–7.70 (m, 3H), 7.67 (td, J = 7.7, 1.4 Hz, 1H), 7.65–7.61 (m, 2H), 7.49 (td, J = 7.6, 1.3 Hz, 1H), 7.39 (td, J = 7.5, 1.2 Hz, 1H), 7.32 (td, J = 7.7, 1.8 Hz, 1H), 6.67 (t, J = 5.4 Hz, 1H), 6.02 (d, J = 6.0 Hz, 1H), 4.15–4.02 (m, 2H), 3.75 (s, 3H). 13C{1H} NMR (126 MHz, CDCl3) δ 169.2, 168.1, 166.5, 141.0, 136.1, 133.6, 133.3, 131.8, 130.1, 129.0, 128.3, 127.6, 119.4, 117.8, 111.7, 56.3, 52.5, 41.6. HRMS (ESI) m/z calculated for C19H17BrN3O4 [M + H]+: 430.0402, found [M + H]+: 430.0392.
tert-Butyl (2-(2-(2-bromobenzamido)-2-(2-cyanophenyl)acetamido)ethyl) Carbamate (21a)
Obtained from a 1 mmol reaction as a white solid, 230 mg, yield 46%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, chloroform-d) δ 7.76–7.69 (m, 3H), 7.68–7.61 (m, 3H), 7.47 (t, J = 7.4 Hz, 1H), 7.39 (t, J = 7.4 Hz, 1H), 7.35–7.30 (m, 1H), 6.86 (s, 1H), 5.91 (d, J = 5.5 Hz, 1H), 4.92 (s, 1H), 3.54–3.45 (m, 1H), 3.41–3.34 (m, 1H), 3.30–3.24 (m, 2H), 1.43 (s, 9H). 13C{1H} NMR (126 MHz, CDCl3) δ 168.2, 166.6, 141.3, 136.2, 133.5, 133.5, 133.4, 131.7, 130.1, 128.8, 128.7, 127.6, 119.5, 117.8, 111.6, 79.8, 56.6, 41.3, 40.0, 28.3. HRMS (ESI) m/z calculated for C23H26BrN4O4 [M + H]+: 501.1137, found [M + H]+: 501.1132.
2-Bromo-N-(2-((3-cyanobenzyl)amino)-1-(2-cyanophenyl)-2-oxoethyl)benzamide (22a)
Obtained from a 1 mmol reaction as a yellow solid, 264 mg, yield 56%; 1H NMR (500 MHz, chloroform-d) δ 7.81–7.72 (m, 3H), 7.69 (td, J = 7.7, 1.4 Hz, 1H), 7.65–7.60 (m, 2H), 7.55–7.50 (m, 2H), 7.46–7.43 (m, 1H), 7.42–7.36 (m, 3H), 7.35–7.30 (m, 1H), 7.05 (t, J = 6.2 Hz, 1H), 6.09 (d, J = 6.4 Hz, 1H), 4.55–4.45 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 168.8, 167.5, 141.3, 141.3, 138.4, 133.5, 133.1, 132.5, 131.6, 131.0, 129.9, 129.6, 129.4, 129.1, 128.9, 127.8, 127.5, 119.5, 119.2, 117.8, 112.7, 111.6, 56.04 42.2. HRMS (ESI) m/z calculated for C24H18BrN4O2 [M + H]+: 473.0613, found [M + H]+: 473.0607.
2-Bromo-N-(2-((2-chloro-3,6-difluorobenzyl)amino)-1-(2-cyanophenyl)-2-oxoethyl) Benzamide (23a)
Obtained from a 1 mmol reaction as a white solid, 263 mg, yield 51%; 1H NMR (500 MHz, chloroform-d) δ 7.73 (dd, J = 7.8, 1.4 Hz, 1H), 7.71–7.57 (m, 5H), 7.47 (td, J = 7.6, 1.4 Hz, 1H), 7.38 (td, J = 7.6, 1.3 Hz, 1H), 7.32 (td, J = 7.7, 1.8 Hz, 1H), 7.15–7.07 (m, 1H), 7.00 (td, J = 8.9, 4.1 Hz, 1H), 6.56 (t, J = 6.2 Hz, 1H), 5.94 (d, J = 6.0 Hz, 1H), 4.77–4.58 (m, 2H). 13C{1H} NMR (126 MHz, CDCl3) δ 167.6, 166.5, 164.3 (d, J = 279.3 Hz), 163.7 (d, J = 281.6 Hz), 141.1, 136.1, 133.6, 133.6 (d, J = 2.5 Hz), 133.2, 131.8, 130.0, 128.9, 128.0, 127.6, 124.0 (d, J = 6.0 Hz), 119.4, 117.9, 116.4 (dd, J = 32.0, 3.1 Hz), 114.9 (dd, J = 37.0, 7,5 Hz) , 111.5, 56.3, 35.6. HRMS (ESI) m/z calculated for C23H16BrClF2N3O2 [M + H]+: 518.0082, found [M + H]+: 518.0078.
2-Bromo-N-(1-(2-cyanophenyl)-2-oxo-2-((2-(trifluoromethoxy)benzyl)amino)ethyl) Benzamide (24a)
Obtained from a 1 mmol reaction as a white solid, 207 mg, yield 39%; 1H NMR (500 MHz, chloroform-d) δ 7.73–7.67 (m, 3H), 7.63 (td, J = 7.7, 1.4 Hz, 1H), 7.61–7.58 (m, 2H), 7.46 (td, J = 7.6, 1.4 Hz, 1H), 7.39–7.27 (m, 4H), 7.24–7.19 (m, 2H), 6.45 (t, J = 5.7 Hz, 1H), 5.96 (d, J = 6.0 Hz, 1H), 4.59–4.48 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 170.1, 168.4, 167.1, 146.1, 140.8, 138.0, 131.3, 130.1 (q, J = 254.5 Hz), 129.2, 128.8, 128.6, 127.4, 127.0, 120.6, 119.1, 118.6, 117.4, 112.3, 55.6, 37.1. HRMS (ESI) m/z calculated for C24H18BrF3N3O3 [M + H]+: 532.0484, found [M + H]+: 532.0479.
N-(2-(benzylamino)-1-(2-cyanophenyl)-2-oxoethyl)-2-bromo-4-methylbenzamide (25a)
Obtained from a 1 mmol reaction as a white solid, 310 mg, yield 67%; 1H NMR (500 MHz, chloroform-d) δ 7.81 (d, J = 6.2 Hz, 1H), 7.76–7.70 (m, 2H), 7.66 (td, J = 7.7, 1.4 Hz, 1H), 7.56 (d, J = 7.8 Hz, 1H), 7.50–7.43 (m, 2H), 7.34–7.28 (m, 3H), 7.19 (dt, J = 7.9, 2.1 Hz, 3H), 6.47 (t, J = 5.9 Hz, 1H), 5.98 (d, J = 6.0 Hz, 1H), 4.56–4.42 (m, 2H), 2.38 (s, 3H). 13C{1H} NMR (126 MHz, CDCl3) δ 167.9, 166.4, 142.6, 141.6, 137.0, 134.0, 133.6, 133.2, 132.9, 130.2, 128.8, 128.8, 128.4, 128.0, 127.7, 127.5, 119.3, 118.0, 111.5, 56.5, 44.1, 20.9. HRMS (ESI) m/z calculated for C24H21BrN3O2 [M + H]+: 462.0817, found [M + H]+: 462.0813.
N-(2-(benzylamino)-1-(2-cyanophenyl)-2-oxoethyl)-2-bromothiophene-3-carboxamide (26a)
Obtained from a 1 mmol reaction as a white solid, 220 mg, yield 49%; 1H NMR (500 MHz, chloroform-d) 1H NMR (500 MHz, chloroform-d) δ 8.29 (d, J = 5.8 Hz, 1H), 7.71–7.66 (m, 2H), 7.62 (t, J = 7.7 Hz, 1H), 7.43 (t, J = 7.6 Hz, 1H), 7.35 (d, J = 5.8 Hz, 1H), 7.31–7.21 (m, 4H), 7.18–7.12 (m, 2H), 6.43 (t, J = 6.7 Hz, 1H), 5.93 (d, J = 5.7 Hz, 1H), 4.53–4.39 (m, 2H). 13C{1H} NMR (126 MHz, CDCl3) δ 168.0, 161.0, 141.7, 137.0, 134.6, 133.7, 133.2, 129.6, 128.8, 128.7, 127.9, 127.7, 127.4, 126.2, 117.9, 113.7, 111.5, 56.3, 44.1. HRMS (ESI) m/z calculated for C21H17BrN3O2S [M + H]+: 454.0225, found [M + H]+: 454.0219.
N-(2-(benzylamino)-1-(2-cyanophenyl)-2-oxoethyl)-4-bromo-2-iodobenzamide (27a)
Obtained from a 1 mmol reaction as a yellow solid, 377 mg, yield 66%; 1H NMR (500 MHz, chloroform-d) δ 8.01 (d, J = 1.9 Hz, 1H), 7.73–7.68 (m, 2H), 7.61 (td, J = 1.5 Hz, 1H), 7.59 (d, J = 6.2 Hz, 1H), 7.50 (dd, J = 8.2, 1.9 Hz, 1H), 7.45 (td, J = 7.7, 1.2 Hz, 1H), 7.41 (dd, J = 8.1, 1.9 Hz, 1H), 7.32 (d, J = 8.3 Hz, 1H), 7.30–7.27 (m, 1H), 7.26–7.23 (m, 1H), 7.15 (dd, J = 7.7, 1.9 Hz, 2H), 6.60 (t, J = 5.9 Hz, 1H), 5.95 (d, J = 6.2 Hz, 1H), 4.51–4.36 (m, 2H). 13C{1H} NMR (126 MHz, CDCl3) δ 167.7, 167.5, 142.2, 141.0, 139.3, 136.9, 133.5, 133.4, 131.4, 129.7, 129.0, 128.7, 128.3, 127.8, 127.5, 124.9, 117.8, 111.6, 92.9, 56.3, 44.1. HRMS (ESI) m/z calculated for C23H18BrIN3O2 [M + H]+: 573.9627, found [M + H]+: 573.9622.
N-(2-(benzylamino)-1-(2-cyanophenyl)-2-oxoethyl)-2-bromo-5-methylbenzamide (28a)
Obtained from a 1 mmol reaction as a white solid, 203 mg, yield 45%; 1H NMR (500 MHz, chloroform-d) δ 7.71 (d, J = 9.0 Hz, 3H), 7.64 (td, J = 7.7, 1.4 Hz, 1H), 7.49–7.40 (m, 3H), 7.32–7.25 (m, 3H), 7.19–7.14 (m, 2H), 7.10 (dd, J = 8.3, 2.3 Hz, 1H), 6.43 (s, 1H), 5.95 (d, J = 6.0 Hz, 1H), 4.55–4.39 (m, 2H), 2.31 (s, 3H). 13C{1H} NMR (126 MHz, CDCl3) δ 167.8, 166.6, 141.5, 137.8, 136.9, 135.7, 133.6, 133.3, 133.2, 132.6, 130.8, 128.8, 128.8, 128.0, 127.7, 127.5, 117.9, 116.0, 111.6, 56.5, 44.1, 20.7. HRMS (ESI) m/z calculated for C24H21BrN3O2 [M + H]+: 462.0817, found [M + H]+: 462.0808.
N-(2-(benzylamino)-1-(2-cyanophenyl)-2-oxoethyl)-4-bromonicotinamide (29a)
Obtained from a 1 mmol reaction as a white solid, 200 mg, yield 45%; 1H NMR (500 MHz, chloroform-d) δ 8.72 (s, 1H), 8.40 (d, J = 5.3 Hz, 1H), 8.08 (d, J = 6.1 Hz, 1H), 7.71 (d, J = 8.9 Hz, 1H), 7.64 (td, J = 7.7, 1.4 Hz, 1H), 7.54 (d, J = 5.3 Hz, 1H), 7.50–7.43 (m, 1H), 7.31–7.24 (m, 3H), 7.15 (dd, J = 7.6, 1.9 Hz, 2H), 6.76 (t, J = 5.9 Hz, 1H), 6.01 (d, J = 6.2 Hz, 1H), 4.51–4.37 (m, 2H). 13C{1H} NMR (126 MHz, CDCl3) δ 167.6, 164.1, 151.6, 150.3, 141.1, 136.9, 133.6, 133.3, 132.2, 130.3, 129.0, 128.7, 128.3, 127.9, 127.7, 127.4, 117.8, 111.7, 56.3, 44.1. HRMS (ESI) m/z calculated for C22H18BrN4O2 [M + H]+: 449.0613, found [M + H]+: 449.0606.
N-(2-(benzylamino)-1-(2-cyanophenyl)-2-oxoethyl)-1-bromo-2-naphthamide (30a)
Obtained from a 1 mmol reaction as a light yellow solid, 260 mg, yield 52%; 1H NMR (500 MHz, chloroform-d) δ 8.36 (d, J = 8.5 Hz, 1H), 7.89–7.83 (m, 2H), 7.78 (dd, J = 8.0, 1.2 Hz, 1H), 7.75 (dd, J = 7.7, 1.4 Hz, 1H), 7.71–7.66 (m, 3H), 7.65–7.59 (m, 1H), 7.54 (d, J = 8.4 Hz, 1H), 7.49 (td, J = 7.7, 1.2 Hz, 1H), 7.37–7.27 (m, 3H), 7.22–7.17 (m, 2H), 6.62 (t, J = 6.0 Hz, 1H), 6.07 (d, J = 6.3 Hz, 1H), 4.55–4.42 (m, 2H). 13C{1H} NMR (126 MHz, CDCl3) δ 167.8, 167.7, 141.3, 137.0, 134.9, 134.8, 133.6, 133.3, 131.9, 128.9, 128.8, 128.4, 128.3, 128.3, 128.2, 127.9, 127.8, 127.7, 127.5, 125.1, 120.27, 117.9, 111.6, 56.4, 44.1. HRMS (ESI) m/z calculated for C27H21BrN3O2 [M + H]+: 498.0817, found [M + H]+: 498.0811.
N-(2-(benzylamino)-1-(2-cyanophenyl)-2-oxoethyl)-3-bromoisonicotinamide (31a)
Obtained from a 1 mmol reaction as a yellow solid, 360 mg, yield 80%; 1H NMR (500 MHz, DMSO-d6) δ 9.64 (d, J = 7.4 Hz, 1H), 8.93 (t, J = 5.9 Hz, 1H), 8.81 (s, 1H), 8.65 (d, J = 4.8 Hz, 1H), 7.91 (dd, J = 7.7, 1.4 Hz, 1H), 7.74 (td, J = 7.7, 1.4 Hz, 1H), 7.62 (dd, J = 7.9, 1.2 Hz, 1H), 7.56 (td, J = 7.6, 1.2 Hz, 1H), 7.53 (d, J = 4.9 Hz, 1H), 7.36–7.23 (m, 5H), 5.94 (d, J = 7.4 Hz, 1H), 4.44–4.34 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 168.2, 165.6, 152.1, 149.0, 145.2, 141.1, 139.3, 133.6, 129.3, 128.9, 128.8, 128.7, 127.7, 127.3, 123.8, 117.8, 117.7, 112.7, 55.8, 43.0. HRMS (ESI) m/z calculated for C22H18BrN4O2 [M + H]+: 449.0613, found [M + H]+: 449.0605.
N-(2-(benzylamino)-1-(2-cyano-4-methoxyphenyl)-2-oxoethyl)-2-bromobenzamide (32a)
Obtained from a 1 mmol reaction as a light yellow solid, 277 mg, yield 58%; 1H NMR (500 MHz, chloroform-d) δ 7.69 (d, J = 6.0 Hz, 1H), 7.64–7.59 (m, 3H), 7.38 (td, J = 7.5, 1.2 Hz, 1H), 7.35–7.29 (m, 4H), 7.23–7.15 (m, 4H), 6.41 (t, J = 5.8 Hz, 1H), 5.91 (d, J = 6.0 Hz, 1H), 4.55–4.41 (m, 2H), 3.86 (s, 3H). 13C{1H} NMR (126 MHz, CDCl3) δ 168.1, 166.5, 159.4, 137.0, 136.3, 133.5, 133.5, 131.7, 130.0, 129.5, 128.7, 127.7, 127.6, 127.5, 120.0, 119.4, 117.7, 117.6, 112.4, 55.9, 55.7, 44.1. HRMS (ESI) m/z calculated for C24H21BrN3O3 [M + H]+: 478.0766, found [M + H]+: 478.0760.
N-(2-(benzylamino)-1-(2-cyano-4-fluorophenyl)-2-oxoethyl)-2-bromobenzamide (33a)
Obtained from a 1 mmol reaction as a white solid, 273 mg, yield 60%; 1H NMR (500 MHz, chloroform-d) δ 7.74–7.68 (m, 2H), 7.61–7.56 (m, 2H), 7.41–7.37 (m, 1H), 7.37–7.31 (m, 2H), 7.31–7.27 (m, 3H), 7.27–7.26 (m, 1H), 7.17 (dd, 2H), 6.46 (t, J = 5.9 Hz, 1H), 5.92 (d, J = 6.0 Hz, 1H), 4.54–4.37 (m, 2H). 13C{1H} NMR (126 MHz, CDCl3) δ 167.6, 166.5, 161.7(d, J = 252.9 Hz), 137.6 (d, J = 4.0 Hz), 136.8, 136.0, 133.6, 131.9, 130.3(d, J = 8.7 Hz), 130.1, 128.8, 127.7 (d, J = 26.4 Hz), 121.3(d, J = 21.2 Hz), 119.9 (d, J = 24.8 Hz), 119.4, 55.9, 44.2. HRMS (ESI) m/z calculated for C23H18BrFN3O2 [M + H]+: 466.0566, found [M + H]+: 466.0559.
2-Bromo-N-(2-((2-chloro-6-fluoro-3-methylbenzyl)amino)-1-(2-cyanophenyl)-2-oxoethyl)benzamide (34a)
Obtained from a 10 mmol reaction as a light yellow solid, 2.6 g, yield 51%; 1H NMR (500 MHz, chloroform-d) δ 7.71–7.68 (m, 1H), 7.68–7.65 (m, 1H), 7.63–7.58 (m, 1H), 7.58–7.55 (m, 1H), 7.54–7.49 (m, 1H), 7.43 (td, J = 7.6, 1.3 Hz, 1H), 7.35 (td, J = 7.5, 1.2 Hz, 1H), 7.28 (td, J = 7.8, 1.9 Hz, 1H), 7.25–7.13 (m, 2H), 6.90 (t, J = 8.7 Hz, 1H), 6.47 (t, J = 5.5 Hz, 1H), 5.89 (d, J = 6.0 Hz, 1H), 4.80–4.47 (m, 2H), 2.32 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 170.3, 167.8, 166.8, 160.0 (d, J = 246.5.4 Hz), 143.6, 141.0, 138.1, 134.7 (d, J = 5.7 Hz), 133.0, 132.4, 132.1 (d, J = 3.7 Hz), 131.1, 128.5 (d, J = 32.6 Hz), 126.9, 123.1, 123.0, 119.0, 118.6, 117.3, 113.8 (d, J = 23.7 Hz), 112.4, 55.3, 35.1, 19.7. HRMS (ESI) m/z calculated for C24H19BrClFN3O2 [M + H]+: 514.0333, found [M + H]+: 514.0326.
N-(2-(benzylamino)-1-(2-cyanoquinolin-3-yl)-2-oxoethyl)-2-bromobenzamide (35a)
Obtained from a 1 mmol reaction as a yellow solid, 214 mg, yield 43%; eluent: VPE/VEA = 1:1; 1H NMR (500 MHz, DMSO-d6) δ 9.45 (d, J = 7.3 Hz, 1H), 8.95 (t, J = 5.9 Hz, 1H), 8.50 (s, 1H), 8.18 (d, J = 8.5 Hz, 1H), 8.10 (d, J = 8.1 Hz, 1H), 7.97 (t, J = 7.7 Hz, 1H), 7.84 (t, J = 7.6 Hz, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.57 (dd, J = 7.6, 1.8 Hz, 1H), 7.47 (t, J = 7.5 Hz, 1H), 7.39 (td, J = 7.7, 1.8 Hz, 1H), 7.35–7.29 (m, 4H), 7.27–7.20 (m, 1H), 6.10 (d, J = 7.3 Hz, 1H), 4.56–4.35 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 168.1, 167.5, 147.0, 139.3, 138.3, 137.2, 137.1, 134.6, 133.3, 133.1, 132.2, 131.7, 130.5, 129.8, 129.3, 128.7, 128.5, 127.8, 127.4, 119.5, 116.6, 54.5, 43.1. HRMS (ESI) m/z calculated for C26H20BrN4O2 [M + H]+: 499.0770, found [M + H]+: 499.0764.
N-Cyano-N-(2-(cyclohexylamino)-1-(2-iodophenyl)-2-oxoethyl)benzamide (39a)
Obtained from a 1 mmol reaction as a white solid, 135 mg, yield 38%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, chloroform-d) δ 8.14–8.09 (m, 2H), 7.87 (dd, J = 8.0, 1.2 Hz, 1H), 7.63–7.56 (m, 2H), 7.45 (t, J = 7.8 Hz, 2H), 7.39 (td, J = 7.6, 1.3 Hz, 1H), 7.05 (td, J = 7.7, 1.7 Hz, 1H), 6.44 (s, 1H), 6.12 (d, J = 8.3 Hz, 1H), 3.91–3.78 (m, 1H), 2.02–1.95 (m, 1H), 1.88–1.81 (m, 1H), 1.73–1.54 (m, 3H), 1.43–1.30 (m, 2H), 1.28–1.22 (m, 1H), 1.20–1.10 (m, 2H). 13C{1H} NMR (126 MHz, CDCl3) δ 166.4, 165.2, 139.9, 138.5, 133.6, 130.6, 129.9, 129.3, 129.1, 128.7, 128.5, 98.9, 78.9, 48.5, 32.8, 32.7, 25.4, 24.6. HRMS (ESI) m/z calculated for C22H23IN3O2 [M + H]+: 488.0835, found [M + H]+: 488.0828.
N-(2-(benzylamino)-1-(2-iodophenyl)-2-oxoethyl)-N-cyanobenzamide (40a)
Obtained from a 1 mmol reaction as a white solid, 188 mg, yield 38%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, chloroform-d) δ 7.98 (dd, J = 8.0, 1.3 Hz, 1H), 7.92–7.88 (m, 2H), 7.62 (t, J = 7.5 Hz, 1H), 7.54 (dd, J = 7.8, 1.7 Hz, 1H), 7.50 (t, J = 7.8 Hz, 2H), 7.42 (td, J = 7.6, 1.3 Hz, 1H), 7.37–7.32 (m, 2H), 7.32–7.30 (m, 1H), 7.28–7.26 (m, 2H), 7.16 (td, J = 7.7, 1.6 Hz, 1H), 6.24 (t, J = 5.9 Hz, 1H), 6.11 (s, 1H), 4.62–4.47 (m, 2H). 13C{1H} NMR (126 MHz, CDCl3) δ 168.5, 166.4, 140.4, 137.2, 134.7, 133.4, 131.9, 131.0, 130.4, 129.2, 129.0, 128.8, 128.6, 127.8, 127.7, 108.9, 102.1, 66.5, 44.1. HRMS (ESI) m/z calculated for C23H19IN3O2 [M + H]+: 496.0522, found [M + H]+: 496.0513.
N-Cyano-N-(1-(2-iodophenyl)-2-oxo-2-(((tetrahydrofuran-2-yl)methyl)amino)ethyl) Benzamide (41a)
Obtained from a 1 mmol reaction as a white solid, 171 mg, yield 35%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 8.94–8.86 (m, 1H), 8.12–8.07 (m, 1H), 7.89–7.79 (m, 2H), 7.72 (t, J = 7.5 Hz, 1H), 7.61 (t, J = 7.8 Hz, 2H), 7.52 (t, J = 7.6 Hz, 1H), 7.41–7.35 (m, 1H), 7.24 (t, J = 7.6 Hz, 1H), 5.98 (s, 1H), 3.97–3.87 (m, 1H), 3.82–3.71 (m, 1H), 3.67–3.59 (m, 1H), 3.53–3.30 (m, 1H), 3.30–3.15 (m, 1H), 1.99–1.87 (m, 1H), 1.86–1.74 (m, 2H), 1.61–1.49 (m, 1H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 168.5, 166.9, 140.4, 136.1, 133.9, 131.9, 131.0, 130.9, 129.3, 129.2, 128.8, 109.6, 103.5, 77.3, 67.7, 66.0, 43.5, 28.9, 25.6. HRMS (ESI) m/z calculated for C21H21IN3O3 [M + H]+: 490.0628, found [M + H]+: 490.0621.
N-Cyclohexyl-10-oxo-10,12-Dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (10b)
Obtained from a 0.2 mmol reaction as a white solid, 58 mg, yield 81%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 8.82 (d, J = 7.9 Hz, 1H), 8.21 (d, J = 8.9 Hz, 1H), 8.11 (d, J = 7.6 Hz, 1H), 7.92–7.87 (m, 1H), 7.84 (d, J = 7.8 Hz, 1H), 7.78–7.65 (m, 3H), 7.58 (t, J = 7.4 Hz, 1H), 6.00 (s, 1H), 3.55 (m, 1H), 1.90–1.81 (m, 1H), 1.78–1.66 (m, 3H), 1.62–1.52 (m, 1H), 1.40–1.15 (m, 5H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 164.6, 159.3, 155.5, 149.5, 141.1, 135.0, 133.2, 132.4, 130.0, 127.7, 127.0, 126.4, 123.4, 123.1, 121.2, 64.1, 48.6, 48.5, 32.7, 24.8. HRMS (ESI) m/z calculated for C22H22N3O2 [M + H]+: 360.1712, found [M + H]+: 360.1705.
N-Benzyl-10-oxo-10,12-Dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (11b)
Obtained from a 0.2 mmol reaction as a white solid, 30 mg, yield 41%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 9.38 (t, J = 5.8 Hz, 1H), 8.25 (d, J = 7.9 Hz, 1H), 8.13 (d, J = 7.6 Hz, 1H), 7.91 (td, J = 7.7, 6.9, 1.5 Hz, 1H), 7.87–7.84 (m, 1H), 7.79–7.73 (m, 2H), 7.69 (td, J = 7.1, 6.3, 1.8 Hz, 1H), 7.59 (t, J = 7.5 Hz, 1H), 7.38–7.26 (m, 5H), 6.09 (s, 1H), 4.45–4.31 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 165.8, 159.5, 155.4, 149.5, 140.8, 139.1, 135.0, 133.2, 132.4, 130.1, 128.8, 127.7, 127.6, 127.4, 127.0, 126.5, 123.5, 123.4, 121.2, 64.2, 42.9. HRMS (ESI) m/z calculated for C23H18N3O2 [M + H]+: 368.1399, found [M + H]+: 368.1391.
10-oxo-N-Phenethyl-10,12-Dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (12b)
Obtained from a 0.2 mmol reaction as a light yellow solid, 40 mg, yield 70%; eluent: VDCM/VMeOH = 20:1; 1H NMR (500 MHz, DMSO-d6) δ 8.93 (t, J = 5.5 Hz, 1H), 8.23 (dd, J = 7.9, 1.6 Hz, 1H), 8.11–8.08 (m, 1H), 7.93–7.88 (m, 1H), 7.84 (dd, J = 8.2, 1.2 Hz, 1H), 7.71–7.63 (m, 2H), 7.59 (ddd, J = 8.2, 7.0, 1.3 Hz, 1H), 7.51 (d, J = 6.9 Hz, 1H), 7.34–7.29 (m, 2H), 7.26–7.22 (m, 3H), 5.98 (s, 1H), 3.47–3.36 (m, 2H), 2.78 (t, J = 7.1 Hz, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 169.5, 165.6, 159.4, 155.4, 149.5, 140.8, 139.6, 134.9, 133.1, 132.3, 131.1, 130.0, 129.2, 128.8, 127.7, 127.0, 126.6, 126.5, 123.4, 121.2, 119.0, 64.2, 41.0, 35.2. HRMS (ESI) m/z calculated for C24H20N3O2 [M + H]+: 382.1556, found [M + H]+: 382.1548.
N-(4-Chlorophenyl)-10-oxo-10,12-dihydroisoindolo[1,2-b] Quinazoline-12-carboxamide (13b)
Obtained from a 0.15 mmol reaction as a white solid, 45 mg, yield 78%; eluent: VDCM/VMeOH = 20:1; 1H NMR (500 MHz, chloroform-d) δ 10.14 (s, 1H), 8.41 (d, J = 7.7 Hz, 1H), 8.14 (d, J = 7.6 Hz, 1H), 7.89–7.78 (m, 3H), 7.67 (td, J = 7.6, 1.3 Hz, 1H), 7.63–7.58 (m, 1H), 7.56–7.49 (m, 1H), 7.44–7.39 (m, 2H), 7.12–7.07 (m, 2H), 6.33 (s, 1H). 13C{1H} NMR (126 MHz, CDCl3) δ 163.6, 161.5, 154.5, 149.5, 139.1, 138.2, 136.2, 135.1, 133.6, 132.8, 132.1, 130.0, 128.7, 127.8, 127.0, 126.7, 123.7, 121.0, 120.4, 65.0. HRMS (ESI) m/z calculated for C22H15ClN3O2 [M + H]+: 388.0853, found [M + H]+: 388.0849.
N-((3-Methylthiophen-2-yl)methyl)-10-oxo-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (14b)
Obtained from a 0.2 mmol reaction as a white solid, 46 mg, yield 46%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 9.35 (t, J = 5.6 Hz, 1H), 8.22 (dd, J = 7.9, 1.6 Hz, 1H), 8.11 (d, J = 7.5 Hz, 1H), 7.90 (td, J = 7.5, 6.8, 1.6 Hz, 1H), 7.85 (d, J = 8.1 Hz, 1H), 7.78–7.65 (m, 3H), 7.59 (t, J = 7.5 Hz, 1H), 7.33 (d, J = 5.1 Hz, 1H), 6.86 (d, J = 5.0 Hz, 1H), 6.04 (s, 1H), 4.45 (d, J = 5.6 Hz, 2H), 2.17 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 165.4, 159.4, 155.4, 149.5, 140.7, 135.0, 134.8, 134.6, 133.1, 132.4, 130.4, 130.2, 130.0, 127.7, 126.9, 126.5, 123.9, 123.5, 121.2, 64.0, 36.2, 13.7. HRMS (ESI) m/z calculated for C22H18N3O2S [M + H]+: 388.1120, found [M + H]+: 388.1115.
N-(2-methoxybenzyl)-10-oxo-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (15b)
Obtained from a 0.17 mmol reaction as a white solid, 35 mg, yield 53%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, chloroform-d) δ 8.36 (dd, J = 7.9, 1.6 Hz, 1H), 8.13 (dd, J = 6.8, 1.5 Hz, 1H), 7.87–7.78 (m, 2H), 7.74 (d, J = 7.6 Hz, 1H), 7.66–7.57 (m, 2H), 7.51 (td, J = 7.5, 6.8, 1.5 Hz, 1H), 7.24–7.17 (m, 2H), 6.95 (t, J = 6.0 Hz, 1H), 6.85 (td, J = 7.5, 1.1 Hz, 1H), 6.78 (d, J = 8.7 Hz, 1H), 5.88 (s, 1H), 4.54–4.39 (m, 2H), 3.67 (s, 3H). 13C{1H} NMR (126 MHz, CDCl3) δ 165.2, 157.4, 149.3, 139.5, 136.3, 134.7, 132.6, 132.0, 129.7, 129.7, 129.0, 127.6, 126.7, 126.7, 125.2, 123.9, 123.5, 120.8, 120.6, 118.9, 110.2, 64.5, 55.0, 40.2. HRMS (ESI) m/z calculated for C24H20N3O3 [M + H]+: 398.1505, found [M + H]+: 398.1495.
10-Oxo-N-(pyridin-3-ylmethyl)-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (16b)
Obtained from a 0.29 mmol reaction as a brown solid, 60 mg, yield 56%; eluent: VDCM/VMeOH = 20:1; 1H NMR (500 MHz, chloroform-d) δ 8.41 (s, 1H), 8.39 (d, J = 3.3 Hz, 1H), 8.20 (d, J = 8.1 Hz, 1H), 7.99 (d, J = 7.6 Hz, 1H), 7.79–7.68 (m, 4H), 7.64–7.57 (m, 2H), 7.53 (t, J = 7.5 Hz, 1H), 7.41 (ddd, J = 8.1, 6.4, 1.8 Hz, 1H), 7.16 (dd, J = 7.8, 4.8 Hz, 1H), 5.88 (s, 1H), 4.53–4.39 (m, 2H). 13C{1H} NMR (126 MHz, CDCl3) δ 166.0, 160.7, 154.5, 149.2, 148.9, 148.9, 139.1, 135.5, 134.8, 133.3, 132.8, 131.9, 129.9, 127.5, 126.9, 126.7, 123.7, 123.6, 123.5, 120.6, 64.3, 41.3. HRMS (ESI) m/z calculated for C22H17N4O2 [M + H]+: 369.1352, found [M + H]+: 369.1342.
N-((3S,5S)-Adamantan-1-yl)-10-oxo-10,12-dihydroisoindolo-[1,2-b]quinazoline-12-Carboxamide (17b)
Obtained from a 0.19 mmol reaction as a white solid, 80 mg, yield 85%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, chloroform-d) δ 8.35 (dd, J = 7.9, 1.4 Hz, 1H), 8.12 (d, J = 7.0 Hz, 1H), 7.84–7.76 (m, 2H), 7.69 (d, J = 7.5 Hz, 1H), 7.61 (td, J = 7.5, 1.3 Hz, 1H), 7.59–7.55 (m, 1H), 7.49 (ddd, J = 8.1, 6.7, 1.5 Hz, 1H), 6.40 (s, 1H), 5.76 (s, 1H), 2.08–2.03 (m, 3H), 2.03–1.99 (m, 6H), 1.64 (t, J = 3.1 Hz, 6H). 13C{1H} NMR (126 MHz, CDCl3) δ 164.2, 160.6, 154.7, 149.4, 139.8, 134.5, 132.5, 132.2, 129.6, 127.6, 126.7, 126.6, 123.5, 123.4, 120.8, 65.1, 52.9, 41.3, 36.2, 29.4. HRMS (ESI) m/z calculated for C26H26N3O2 [M + H]+: 412.2025, found [M + H]+: 412.2016.
10-Oxo-N-((tetrahydrofuran-2-yl)methyl)-10,12-dihydroisoindolo-[1,2-b]quinazoline-12-carboxamide (18b)
Obtained from a 0.18 mmol reaction as a white solid, 31 mg, yield 47%, dr ratio = 1:1; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, chloroform-d) δ 8.33 (ddd, J = 7.9, 4.2, 1.5 Hz, 1H), 8.14 (d, J = 8.4 Hz, 1H), 7.85–7.71 (m, 3H), 7.65–7.56 (m, 2H), 7.48 (ddd, J = 8.2, 6.9, 1.4 Hz, 1H), 6.80 (dt, J = 74.6, 5.7 Hz, 1H), 5.88 (d, J = 17.3 Hz, 1H), 4.04–3.89 (m, 1H), 3.81–3.62 (m, 2H), 3.62–3.43 (m, 1H), 3.40–3.23 (m, 1H), 1.96–1.78 (m, 3H), 1.64–1.48 (m, 1H). 13C{1H} NMR (126 MHz, CDCl3) δ 165.7, 160.4, 154.6, 149.3, 139.5, 134.6, 132.6, 132.1, 129.8, 127.6, 126.7, 126.6, 123.6, 123.4, 120.8, 77.5, 68.2, 64.4, 43.3, 28.3, 25.8. HRMS (ESI) m/z calculated for C21H20N3O3 [M + H]+: 362.1505, found [M + H]+: 362.1499.
10-Oxo-N-(2,4,4-trimethylpentan-2-yl)-10,12-dihydroisoindolo-[1,2-b]quinazoline-12-carboxamide (19b)
Obtained from a 0.2 mmol reaction as a white solid, 85 mg, yield 91%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 8.46 (s, 1H), 8.22 (dd, J = 8.0, 1.5 Hz, 1H), 8.09 (d, J = 7.6 Hz, 1H), 7.88 (td, J = 7.6, 6.9, 1.6 Hz, 1H), 7.82 (d, J = 7.0 Hz, 1H), 7.74 (d, J = 4.2 Hz, 2H), 7.68–7.63 (m, 1H), 7.56 (t, J = 7.4 Hz, 1H), 6.02 (s, 1H), 1.86 (d, J = 14.6 Hz, 1H), 1.57 (d, J = 14.6 Hz, 1H), 1.38 (s, 3H), 1.30 (s, 3H), 1.01 (s, 9H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 164.2, 159.2, 155.5, 149.5, 141.1, 134.9, 133.0, 132.5, 129.9, 127.7, 126.9, 126.6, 123.4, 121.2, 64.7, 55.4, 50.9, 31.6, 29.5, 29.0. HRMS (ESI) m/z calculated for C24H28N3O2 [M + H]+: 390.2182, found [M + H]+: 390.2174.
Methyl (10-Oxo-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carbonyl)glycinate (20b)
Obtained from a 0.2 mmol reaction as a white solid, 44 mg, yield 63%; eluent: VPE/VEA = 1:1; 1H NMR (500 MHz, DMSO-d6) δ 9.42 (t, J = 6.0 Hz, 1H), 8.21 (d, J = 8.0 Hz, 1H), 8.11 (d, J = 7.6 Hz, 1H), 7.90 (td, J = 7.6, 7.0, 1.5 Hz, 1H), 7.84 (d, J = 8.3 Hz, 2H), 7.78 (t, J = 7.0 Hz, 1H), 7.69 (t, J = 7.5 Hz, 1H), 7.58 (t, J = 7.3 Hz, 1H), 6.12 (s, 1H), 4.10–3.95 (m, 2H), 3.63 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 170.3, 166.3, 159.3, 155.4, 149.5, 140.6, 135.0, 133.1, 132.3, 130.1, 127.7, 127.0, 126.5, 123.9, 123.4, 121.1, 63.8, 52.3, 41.1. HRMS (ESI) m/z calculated for C19H16N3O4 [M + H]+: 350.1141, found [M + H]+: 350.1131.
tert-Butyl-(2-(10-oxo-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamido)ethyl)-carbamate (21b)
Obtained from a 0.2 mmol reaction as a white solid, 58 mg, yield 70%; eluent: VPE/VEA = 1:1; 1H NMR (500 MHz, DMSO-d6) δ 8.87 (t, J = 5.7 Hz, 1H), 8.15 (d, J = 8.0 Hz, 1H), 8.05 (d, J = 7.6 Hz, 1H), 7.84 (td, J = 7.6, 6.9, 1.4 Hz, 1H), 7.78 (d, J = 8.2 Hz, 1H), 7.73–7.64 (m, 2H), 7.62 (t, J = 7.3 Hz, 1H), 7.52 (td, J = 7.5, 6.9, 1.3 Hz, 1H), 6.78 (t, J = 5.7 Hz, 1H), 5.94 (s, 1H), 3.26–3.18 (m, 1H), 3.09–3.01 (m, 1H), 2.99–2.93 (m, 2H), 1.33 (s, 9H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 165.9, 159.4, 156.0, 155.4, 149.5, 140.8, 135.0, 133.2, 132.3, 130.1, 127.7, 127.0, 126.5, 123.6, 123.4, 121.2, 78.2, 64.2, 28.7, 28.6. HRMS (ESI) m/z calculated for C23H25N4O4 [M + H]+: 421.1876, found [M + H]+: 421.1868.
N-(3-Cyanobenzyl)-10-oxo-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (22b)
Obtained from a 0.2 mmol reaction as a light purple solid, 61 mg, yield 78%; eluent: VDCM/VMeOH = 20:1; 1H NMR (500 MHz, DMSO-d6) δ 9.44 (t, J = 6.0 Hz, 1H), 8.25 (dd, J = 7.9, 1.5 Hz, 1H), 8.14 (d, J = 7.6 Hz, 1H), 7.91 (td, J = 7.6, 6.9, 1.6 Hz, 1H), 7.86 (d, J = 8.2 Hz, 1H), 7.78–7.73 (m, 4H), 7.72–7.69 (m, 1H), 7.65 (d, J = 7.9 Hz, 1H), 7.62–7.55 (m, 2H), 6.11 (s, 1H), 4.49–4.38 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 166.3, 159.6, 155.3, 149.5, 141.0, 140.6, 135.0, 133.3, 132.4, 132.3, 131.1, 130.9, 130.2, 130.0, 127.8, 127.1, 126.5, 123.6, 123.4, 121.2, 119.2, 111.8, 64.2, 42.2. HRMS (ESI) m/z calculated for C24H17N4O2 [M + H]+: 393.1352, found [M + H]+: 393.1342.
N-(2-Chloro-3,6-difluorobenzyl)-10-oxo-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (23b)
Obtained from a 0.2 mmol reaction as a white solid, 32 mg, yield 37%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 9.38 (t, J = 5.1 Hz, 1H), 8.21 (dt, J = 7.6, 1.1 Hz, 1H), 8.10 (d, J = 7.1 Hz, 1H), 7.92–7.87 (m, 1H), 7.84 (d, J = 8.1 Hz, 1H), 7.73 (t, J = 6.9 Hz, 1H), 7.69–7.62 (m, 2H), 7.61–7.55 (m, 1H), 7.50 (td, J = 8.9, 4.6 Hz, 1H), 7.36 (td, J = 9.1, 4.1 Hz, 1H), 6.03 (s, 1H), 4.58–4.42 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 165.1, 158.9, 157.0 (dd, J = 240.1, 2.1 Hz), 154.9, 154.1 (dd, J = 243.0, 2.5 Hz), 149.0, 140.2, 132.0, 129.7, 126.6, 126.1 (d, J = 11.8 Hz), 125.0 (d, J = 20.0 Hz), 123.0 (dd, J = 20.6, 6.5 Hz), 121.4 (dd, J = 20.0, 5.4 Hz), 120.7, 63.3, 35.0. HRMS (ESI) m/z calculated for C23H15ClF2N3O2 [M + H]+: 438.0821, found [M + H]+: 438.0814.
10-Oxo-N-(2-(trifluoromethoxy)benzyl)-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (24b)
Obtained from a 0.2 mmol reaction as a white solid, 62 mg, yield 69%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 9.42 (t, J = 5.8 Hz, 1H), 8.25 (dd, J = 7.9, 1.5 Hz, 1H), 8.13 (dd, J = 7.6, 1.0 Hz, 1H), 7.90 (ddd, J = 8.4, 6.7, 1.6 Hz, 1H), 7.85 (dd, J = 8.1, 1.2 Hz, 1H), 7.76 (d, J = 4.0 Hz, 2H), 7.73–7.67 (m, 1H), 7.59 (d, J = 7.4 Hz, 1H), 7.54 (dd, J = 6.8, 2.5 Hz, 1H), 7.47–7.39 (m, 2H), 7.40–7.35 (m, 1H), 6.12 (s, 1H), 4.50–4.37 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 165.8, 159.1, 154.9, 149.1, 146.1, 140.2, 133.7 (q, J = 241.0 Hz), 132.0, 131.0, 129.4, 129.0, 127.5, 126.6, 126.1, 123.1, 120.8, 120.6, 119.2, 63.8, 37.2. HRMS (ESI) m/z calculated for C24H17F3N3O3 [M + H]+: 452.1222, found [M + H]+: 452.1216.
N-Benzyl-7-methyl-10-oxo-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (25b)
Obtained from a 0.2 mmol reaction as a white solid, 29 mg, yield 38%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 9.36 (t, J = 6.0 Hz, 1H), 8.16–8.08 (m, 2H), 7.79–7.72 (m, 2H), 7.72–7.64 (m, 2H), 7.41 (dd, J = 8.2, 1.6 Hz, 1H), 7.38–7.34 (m, 2H), 7.33–7.30 (m, 2H), 7.30–7.25 (m, 1H), 6.07 (s, 1H), 4.43–4.32 (m, 2H), 2.48 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 165.9, 159.4, 155.4, 149.6, 145.5, 140.8, 139.2, 132.5, 128.8, 128.4, 127.6, 127.5, 127.4, 126.4, 126.3, 123.5, 123.4, 123.3, 118.9, 64.0, 42.9, 21.8. HRMS (ESI) m/z calculated for C24H20N3O2 [M + H]+: 382.1556, found [M + H]+: 382.1550.
N-Benzyl-4-oxo-4,6-dihydrothieno[2′,3′:4,5]pyrimido[2,1-a]isoindole-6-carboxamide (26b)
Obtained from a 0.2 mmol reaction as a white solid, 38 mg, yield 51%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 9.37 (t, J = 6.0 Hz, 1H), 8.09 (d, J = 7.6 Hz, 1H), 7.78–7.73 (m, 2H), 7.71–7.67 (m, 1H), 7.65 (d, J = 5.8 Hz, 1H), 7.51 (d, J = 5.7 Hz, 1H), 7.39–7.30 (m, 4H), 7.30–7.25 (m, 1H), 6.07 (s, 1H), 4.46–4.30 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 165.5, 165.2, 156.2, 156.1, 140.9, 139.1, 133.0, 131.8, 130.2, 128.8, 127.6, 127.4, 124.6, 123.3, 123.0, 122.3, 122.2, 64.2, 43.0. HRMS (ESI) m/z calculated for C21H16N3O2S [M + H]+: 374.0963, found [M + H]+: 374.0957.
N-Benzyl-7-bromo-10-oxo-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (27b)
Obtained from a 0.2 mmol reaction as a white solid, 39 mg, yield 44%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 9.36 (t, J = 5.9 Hz, 1H), 8.16 (d, J = 8.5 Hz, 1H), 8.12 (d, J = 7.5 Hz, 1H), 8.06 (d, J = 1.9 Hz, 1H), 7.82–7.73 (m, 3H), 7.71 (t, J = 7.4 Hz, 1H), 7.39–7.25 (m, 5H), 6.09 (s, 1H), 4.45–4.30 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 170.3, 165.1, 158.6, 156.2, 150.3, 140.6, 138.7, 133.2, 133.1, 131.6, 129.7, 129.5, 128.3, 128.1, 127.2, 127.0, 123.2, 123.0, 119.9, 63.9, 42.5. HRMS (ESI) m/z calculated for C23H17BrN3O2 [M + H]+: 446.0504, found [M + H]+: 446.0498.
N-Benzyl-8-methyl-10-oxo-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (28b)
Obtained from a 0.2 mmol reaction as a white solid, 22 mg, yield 29%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, chloroform-d) δ 8.12 (s, 1H), 8.05 (d, J = 7.6 Hz, 1H), 7.77 (dd, J = 7.6, 1.0 Hz, 1H), 7.69 (d, J = 8.2 Hz, 1H), 7.66–7.54 (m, 3H), 7.29–7.26 (m, 1H), 7.25–7.18 (m, 4H), 6.93 (t, J = 5.9 Hz, 1H), 5.89 (s, 1H), 4.54–4.42 (m, 2H), 2.51 (s, 3H). 13C{1H} NMR (126 MHz, CDCl3) δ 165.7, 160.7, 153.8, 147.2, 139.2, 137.4, 137.1, 136.2, 132.4, 132.1, 129.8, 128.6, 127.6, 127.5, 127.3, 126.2, 123.8, 123.3, 120.4, 64.4, 43.9, 21.3. HRMS (ESI) m/z calculated for C24H20N3O2 [M + H]+: 382.1556, found [M + H]+: 382.1549.
N-Benzyl-12-oxo-10,12-dihydropyrido[4′,3′:4,5]pyrimido[2,1-a]isoindole-10-carboxamide (29b)
Obtained from a 0.15 mmol reaction as a white solid, 40 mg, yield 73%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 9.39 (t, J = 4.7 Hz, 2H), 8.91 (d, J = 5.7 Hz, 1H), 8.17 (d, J = 7.6 Hz, 1H), 7.85–7.81 (m, 1H), 7.80–7.70 (m, 3H), 7.38–7.33 (m, 2H), 7.33–7.26 (m, 3H), 6.13 (s, 1H), 4.45–4.31 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 165.3, 159.5, 158.9, 154.7, 154.0, 149.9, 141.5, 139.0, 134.2, 131.8, 130.4, 128.8, 127.6, 127.5, 124.2, 123.5, 121.0, 116.9, 64.6, 43.0. HRMS (ESI) m/z calculated for C22H17N4O2 [M + H]+: 369.1352, found [M + H]+: 369.1343.
N-Benzyl-7-oxo-7,9-dihydrobenzo[h]isoindolo[1,2-b]quinazoline-9-carboxamide (30b)
Obtained from a 0.3 mmol reaction as a light pink solid, 63 mg, yield 50%; eluent: VDCM/VMeOH = 20:1; 1H NMR (500 MHz, DMSO-d6) δ 9.42 (t, J = 5.9 Hz, 1H), 9.20–9.13 (m, 1H), 8.30 (d, J = 7.4 Hz, 1H), 8.19 (d, J = 8.7 Hz, 1H), 8.14–8.09 (m, 1H), 8.02 (d, J = 8.7 Hz, 1H), 7.86–7.82 (m, 2H), 7.81–7.74 (m, 3H), 7.40–7.31 (m, 4H), 7.31–7.26 (m, 1H), 6.17 (s, 1H), 4.45–4.34 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 165.7, 159.5, 155.8, 147.9, 140.9, 139.1, 136.2, 133.3, 132.6, 130.2, 129.9, 129.7, 128.8, 128.5, 127.6, 127.4, 127.0, 125.2, 123.7, 123.6, 121.8, 117.4, 64.5, 43.0. HRMS (ESI) m/z calculated for C27H20N3O2 [M + H]+: 418.1556, found [M + H]+: 418.1548.
N-Benzyl-5-oxo-5,7-dihydropyrido[3′,4′:4,5]pyrimido[2,1-a]isoindole-7-carboxamide (31b)
Obtained from a 0.2 mmol reaction as a white solid, 54 mg, yield 74%; eluent: VDCM/VMeOH = 20:1; 1H NMR (500 MHz, DMSO-d6) δ 9.37 (t, J = 5.9 Hz, 1H), 9.24 (s, 1H), 8.74 (d, J = 5.1 Hz, 1H), 8.17 (d, J = 7.6 Hz, 1H), 8.09 (dd, J = 5.2, 0.9 Hz, 1H), 7.85–7.69 (m, 3H), 7.38–7.33 (m, 2H), 7.32–7.25 (m, 3H), 6.12 (s, 1H), 4.44–4.31 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 165.4, 158.6, 157.2, 150.9, 146.4, 144.2, 140.8, 139.1, 133.8, 131.9, 130.3, 128.8, 127.6, 127.4, 126.2, 123.8, 123.4, 118.8, 64.5, 43.0. HRMS (ESI) m/z calculated for C22H17N4O2 [M + H]+: 369.1352, found [M + H]+: 369.1346.
N-Benzyl-3-methoxy-10-oxo-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (32b)
Obtained from a 0.2 mmol reaction as a white solid, 56 mg, yield 71%; eluent: VDCM/VMeOH = 20:1; 1H NMR (500 MHz, DMSO-d6) δ 9.31 (t, J = 5.9 Hz, 1H), 8.24 (dd, J = 7.9, 1.5 Hz, 1H), 7.90 (td, J = 6.9, 1.5 Hz, 1H), 7.85 (m, 1H), 7.65–7.56 (m, 3H), 7.38–7.34 (m, 2H), 7.34–7.30 (m, 3H), 7.29–7.27 (m, 1H), 5.99 (s, 1H), 4.37 (t, J = 5.7 Hz, 2H), 3.93 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 169.5, 166.0, 161.0, 159.4, 155.3, 149.4, 139.8, 139.2, 134.9, 133.8, 133.1, 131.1, 128.8, 127.6, 127.4, 126.5, 121.3, 121.1, 106.3, 63.6, 56.2, 42.9. HRMS (ESI) m/z calculated for C24H20N3O3 [M + H]+: 398.1505, found [M + H]+: 398.1501.
N-Benzyl-3-fluoro-10-oxo-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (33b)
Obtained from a 0.15 mmol reaction as a white solid, 38 mg, yield 66%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 9.36 (t, J = 5.9 Hz, 1H), 8.25 (dd, J = 7.9, 1.5 Hz, 1H), 7.95–7.90 (m, 2H), 7.86 (d, J = 8.1 Hz, 1H), 7.79–7.75 (m, 1H), 7.65–7.58 (m, 2H), 7.38–7.33 (m, 2H), 7.33–7.26 (m, 3H), 6.07 (s, 1H), 4.44–4.30 (m, 2H). 13C{1H} NMR (126 MHz, CDCl3) δ 165.2, 162.9 (d, J = 246.4 Hz), 158.9, 154.1(d, J = 4.0 Hz), 148.8, 138.6, 136.3, 134.1(d, J = 9.8 Hz), 127.3 (d, J = 20.2 Hz), 127.1 (d, J = 21.9 Hz), 126.1, 120.9, 63.4, 42.5. HRMS (ESI) m/z calculated for C23H17FN3O2 [M + H]+: 386.1305, found [M + H]+: 386.1299.
N-(2-chloro-6-fluoro-3-methylbenzyl)-10-oxo-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (34b)
Obtained from a 5 mmol reaction as a white solid, 0.88 g, yield 41%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 9.27 (t, J = 5.0 Hz, 1H), 8.22 (d, J = 8.0 Hz, 1H), 8.10 (d, J = 7.6 Hz, 1H), 7.89 (t, J = 7.4 Hz, 1H), 7.84 (d, J = 8.1 Hz, 1H), 7.73 (t, J = 7.5 Hz, 1H), 7.69–7.62 (m, 2H), 7.59 (t, J = 7.3 Hz, 1H), 7.41 (t, J = 7.3 Hz, 1H), 7.20 (t, J = 8.9 Hz, 1H), 6.04 (s, 1H), 4.59–4.40 (m, 2H), 2.36 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 165.0, 159.5 (d, J = 246.7 Hz), 158.9, 154.9, 149.0, 140.3, 134.7, 132.2 (d, J = 3.4 Hz), 132.0, 131.1, 129.7 (d, J = 27.2 Hz), 126.6 (d, J = 20.3 Hz), 126.0, 123.0, 122.8, 120.7, 113.9 (d, J = 22.8 Hz), 63.5, 35.4, 19.7. HRMS (ESI) m/z calculated for C24H18ClFN3O2 [M + H]+: 434.1072, found [M + H]+: 434.1067.
N-Benzyl-11-oxo-11,13-dihydroquinolino[2′,3′:3,4]pyrrolo[2,1-b]quinazoline-13-carboxamide (35b)
Obtained from a 0.2 mmol reaction as a white solid, 42 mg, yield 51%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 9.48 (t, J = 5.9 Hz, 1H), 8.71 (s, 1H), 8.34–8.29 (m, 2H), 8.25 (d, J = 8.1 Hz, 1H), 8.03–7.94 (m, 3H), 7.82 (t, J = 7.5 Hz, 1H), 7.68 (ddd, J = 8.2, 5.9, 2.3 Hz, 1H), 7.36–7.32 (m, 4H), 7.30–7.25 (m, 1H), 6.27 (s, 1H), 4.48–4.31 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 168.1, 167.5, 147.0, 139.3, 138.3, 137.2, 137.1, 134.6, 133.3, 133.1, 132.2, 131.7, 130.5, 129.8, 129.3, 128.7, 128.5, 127.8, 127.4, 119.5, 116.6, 54.5, 43.1. HRMS (ESI) m/z calculated for C26H19N4O2 [M + H]+: 419.1508, found [M + H]+: 419.1500.
N-Cyclohexyl-10-oxo-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (39b)
Obtained from a 0.15 mmol reaction as a white solid, 21 mg, yield 40%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 8.78 (d, J = 7.8 Hz, 1H), 8.20 (dd, J = 7.9, 1.5 Hz, 1H), 8.09 (d, J = 7.6 Hz, 1H), 7.88 (ddd, J = 8.5, 6.9, 1.6 Hz, 1H), 7.83 (d, J = 6.9 Hz, 1H), 7.77–7.70 (m, 2H), 7.66 (t, J = 7.4 Hz, 1H), 7.56 (ddd, J = 8.1, 7.0, 1.3 Hz, 1H), 5.99 (s, 1H), 3.60–3.50 (m, 1H), 1.88–1.81 (m, 1H), 1.78–1.67 (m, 3H), 1.59–1.53 (m, 1H), 1.38–1.20 (m, 5H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 164.6, 159.3, 155.5, 149.5, 141.1, 135.0, 133.2, 132.4, 130.0, 127.7, 127.0, 126.5, 123.4, 123.2, 121.2, 64.1, 48.5, 32.6, 25.6, 24.7. HRMS (ESI) m/z calculated for C22H22N3O2 [M + H]+: 360.1712, found [M + H]+: 360.1706.
N-Benzyl-10-oxo-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (40b)
Obtained from a 0.15 mmol reaction as a white solid, 26 mg, yield 47%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 9.37 (t, J = 5.9 Hz, 1H), 8.24 (dd, J = 7.9, 1.5 Hz, 1H), 8.13 (d, J = 7.6 Hz, 1H), 7.90 (ddd, J = 8.5, 7.0, 1.6 Hz, 1H), 7.85 (d, J = 8.1 Hz, 1H), 7.79–7.73 (m, 2H), 7.69 (ddd, J = 8.6, 6.5, 2.0 Hz, 1H), 7.59 (ddd, J = 8.0, 7.0, 1.3 Hz, 1H), 7.38–7.30 (m, 4H), 7.30–7.25 (m, 1H), 6.09 (s, 1H), 4.45–4.29 (m, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 165.9, 159.5, 155.4, 149.5, 140.8, 139.1, 135.0, 133.3, 133.1, 132.4, 130.2, 128.8, 127.6, 127.4, 127.0, 126.6, 126.5, 123.5, 121.2, 64.1, 42.9. HRMS (ESI) m/z calculated for C23H18N3O2 [M + H]+: 368.1399, found [M + H]+: 368.1393.
10-Oxo-N-((tetrahydrofuran-2-yl)methyl)-10,12-dihydroisoindolo[1,2-b]quinazoline-12-carboxamide (41b)
Obtained from a 0.15 mmol reaction as a white solid, 18 mg, yield 33%; eluent: VPE/VEA = 2:1; 1H NMR (500 MHz, DMSO-d6) δ 9.04 (q, J = 5.8 Hz, 1H), 8.21 (d, J = 7.9 Hz, 1H), 8.11 (d, J = 7.7 Hz, 1H), 7.90 (td, J = 7.5, 6.9, 1.6 Hz, 1H), 7.84 (d, J = 8.0 Hz, 1H), 7.78–7.72 (m, 2H), 7.70–7.66 (m, 1H), 7.58 (t, J = 7.4 Hz, 1H), 6.09 (s, 1H), 3.92–3.80 (m, 2H), 3.72–3.62 (m, 1H), 3.32–3.17 (m, 2H), 1.94–1.78 (m, 4H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 165.9, 159.3, 155.4, 149.5, 141.0, 134.9, 133.1, 132.4, 130.1, 127.7, 127.0, 126.5, 123.5, 123.4, 121.2, 77.5, 67.8, 64.1, 43.3, 28.7, 25.8. HRMS (ESI) m/z calculated for C21H20N3O3 [M + H]+: 362.1505, found [M + H]+: 362.1495.
Acknowledgments
R.X., Z.W., Q.Z. are supported by the Chinese Scholarship Council Grant.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.2c01561.
Characterization data and copies of NMR spectra (PDF)
Author Contributions
‡ R.X. and Z.W. contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- a Hameed A.; Al-Rashida M.; Uroos M.; Ali S. A.; Arshia; Ishtiaq M.; Khan K. M. Quinazoline and quinazolinone as important medicinal scaffolds: a comparative patent review (2011–2016). Expert Opin. Ther. Pat. 2018, 28, 281–297. 10.1080/13543776.2018.1432596. [DOI] [PubMed] [Google Scholar]; b Auti P. S.; George G.; Paul A. T. Recent advances in the pharmacological diversification of quinazoline/ quinazolinone hybrids. RSC Adv. 2020, 10, 41353–41392. 10.1039/D0RA06642G. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Biological activity of quinazolinones; Radwan A. A., Alanazi F. K. Eds.; Books on Demand: London, 2020. [Google Scholar]
- a Shakhidoyatov K. M.; Elmuradov B. Z. Tricyclic quinazoline alkaloids: isolation, synthesis, chemical modification, and biological activity. Chem. Nat. Compd. 2014, 50, 781–800. 10.1007/s10600-014-1086-6. [DOI] [Google Scholar]; b Cagir A.; Jones S. H.; Gao R.; Eisenhauer B. M.; Hecht S. M. Luotonin A. A naturally occurring human DNA topoisomerase I poison. J. Am. Chem. Soc. 2003, 125, 13628–13629. 10.1021/ja0368857. [DOI] [PubMed] [Google Scholar]; c Joshi B. K.; Gloer J. B.; Wicklow D. T.; Dowd P. F. Sclerotigenin: a new antiinsectan benzodiazepine from the sclerotia of Penicillium sclerotigenum. J. Nat. Prod. 1999, 62, 650–652. 10.1021/np980511n. [DOI] [PubMed] [Google Scholar]; d Tian K. M.; Li J. J.; Xu S. W. Rutaecarpine: a promising cardiovascular protective alkaloid from Evodia rutaecarpa (Wu Zhu Yu). Pharmacol. Res. 2019, 141, 541–550. 10.1016/j.phrs.2018.12.019. [DOI] [PubMed] [Google Scholar]
- a Dömling A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]; b Dömling A.; Ugi I. Multicomponent reactions with isocyanides. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. . [DOI] [PubMed] [Google Scholar]
- a Dömling A.; Wang W.; Wang K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Younus H. A.; Al-Rashida M.; Hameed A.; Uroos M.; Salar U.; Rana S.; Khan K. M. Multicomponent reactions (MCR) in medicinal chemistry: a patent review (2010-2020). Expert Opin. Ther. Pat. 2021, 31, 267–289. 10.1080/13543776.2021.1858797. [DOI] [PubMed] [Google Scholar]; c Rotstein B. H.; Zaretsky S.; Rai V.; Yudin A. K. Small heterocycles in multicomponent reactions. Chem. Rev. 2014, 114, 8323–8359. 10.1021/cr400615v. [DOI] [PubMed] [Google Scholar]
- Wang C.; Lai Z.; Xie H.; Cui S. Triazenyl Alkynes as Versatile Building Blocks in Multicomponent Reactions: Diastereoselective Synthesis of β-Amino Amides. Angew. Chem., Int. Ed. 2021, 60, 5147–5151. 10.1002/anie.202014686. [DOI] [PubMed] [Google Scholar]
- a Marcaccini S.; Torroba T. The use of the Ugi four-component condensation. Nat. Protoc. 2007, 2, 632–639. 10.1038/nprot.2007.71. [DOI] [PubMed] [Google Scholar]; b Ugi I. The α-addition of immonium ions and anions to isonitriles accompanied by secondary reactions. Angew. Chem., Int. Ed. 1962, 1, 8–21. 10.1002/anie.196200081. [DOI] [Google Scholar]
- a Semple J. E.; Wang P. C.; Lysenko Z.; Joullie M. M. Total synthesis of (+)-furanomycin and stereoisomers. J. Am. Chem. Soc. 1980, 102, 7505–7510. 10.1021/ja00545a018. [DOI] [Google Scholar]; b Brown A. L.; Churches Q. I.; Hutton C. A. Total Synthesis of Ustiloxin D Utilizing an Ammonia–Ugi Reaction. J. Org. Chem. 2015, 80, 9831–9837. 10.1021/acs.joc.5b01519. [DOI] [PubMed] [Google Scholar]
- a Wang Q.; Mgimpatsang K. C.; Li X.; Dömling A. Isoquinolone-4-Carboxylic Acids by Ammonia-Ugi-4CR and Copper-Catalyzed Domino Reaction. J. Org. Chem. 2021, 86, 9771–9780. 10.1021/acs.joc.1c01170. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wang Y.; Patil P.; Kurpiewska K.; Kalinowska-Tluscik J.; Dömling A. Diverse Isoquinoline Scaffolds by Ugi/Pomeranz–Fritsch and Ugi/Schlittler–Müller Reactions. Org. Lett. 2019, 21, 3533–3537. 10.1021/acs.orglett.9b00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Sahoo S.; Pal S. Copper-Catalyzed One-Pot Synthesis of Quinazolinones from 2-Nitrobenzaldehydes with Aldehydes: Application toward the Synthesis of Natural Products. J. Org. Chem. 2021, 86, 18067–18080. 10.1021/acs.joc.1c02343. [DOI] [PubMed] [Google Scholar]; b Wang C.; Qian P. C.; Chen F.; Cheng J. Rhodium-catalyzed [4+1] annulation of sulfoxonium ylides: Sequential ortho-CH functionalization/carbonyl α-amination toward polycyclic quinazolinones. Tetrahedron Lett. 2020, 61, 152441. 10.1016/j.tetlet.2020.152441. [DOI] [Google Scholar]; c Ju Y.; Liu F.; Li C. Palladium-catalyzed sequential cyanation/N-addition/N-arylation in one-pot: Efficient synthesis of Luotonin A and its derivatives. Org. Lett. 2009, 11, 3582–3585. 10.1021/ol901305q. [DOI] [PubMed] [Google Scholar]; d Diener M. E.; Metrano A. J.; Kusano S.; Miller S. J. Enantioselective synthesis of 3-arylquinazolin-4 (3 H)-ones via peptide-catalyzed atroposelective bromination. J. Am. Chem. Soc. 2015, 137, 12369–12377. 10.1021/jacs.5b07726. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Yoon H.; Galls A.; Rozema S. D.; Miller S. J. Atroposelective Desymmetrization of Resorcinol-Bearing Quinazolinones via Cu-Catalyzed C–O Bond Formation. Org. Lett. 2022, 24, 762–766. 10.1021/acs.orglett.1c04266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Servais A.; Azzouz M.; Lopes D.; Courillon C.; Malacria M. Radical Cyclization of N-Acylcyanamides: Total Synthesis of Luotonin A. Angew. Chem., Int. Ed. 2007, 46, 576–579. 10.1002/anie.200602940. [DOI] [PubMed] [Google Scholar]; b Sun B.; Huang P.; Yan Z.; Shi X.; Tang X.; Yang J.; Jin C. Self-catalyzed phototandem perfluoroalkylation/cyclization of unactivated alkenes: synthesis of perfluoroalkyl-substituted quinazolinones. Org. Lett. 2021, 23, 1026–1031. 10.1021/acs.orglett.0c04225. [DOI] [PubMed] [Google Scholar]
- Rasapalli S.; Sammeta V. R.; Murphy Z. F.; Huang Y.; Boerth J. A.; Golen J. A.; Savinov S. N. Synthesis of C-Ring-Substituted Vasicinones and Luotonins via Regioselective Aza-Nazarov Cyclization of Quinazolinonyl Enones. Org. Lett. 2019, 21, 9824–9828. 10.1021/acs.orglett.9b03586. [DOI] [PubMed] [Google Scholar]
- a Hsieh J. C. Transition-Metal-Catalyzed Addition/Cyclization Reactions of the C-N Multiple Bonds Containing Species. Chem. Rec. 2021, 21, 3370–3381. 10.1002/tcr.202100008. [DOI] [PubMed] [Google Scholar]; b Hsieh J. C.; Su H. L. Synthesis of N-heterocycles via transition-metal-catalyzed tandem addition/cyclization of a nitrile. Synthesis 2020, 52, 819–833. [Google Scholar]
- a Sun K.; Lv Q. Y.; Lin Y. W.; Yu B.; He W. M. Nitriles as radical acceptors in radical cascade reactions. Org. Chem. Front. 2021, 8, 445–465. 10.1039/D0QO01058H. [DOI] [Google Scholar]; b Larraufie M. H.; Ollivier C.; Fensterbank L.; Malacria M.; Lacôte E. Radical synthesis of guanidines from N-acyl cyanamides. Angew. Chem., Int. Ed. 2010, 49, 2178–2181. 10.1002/anie.200907237. [DOI] [PubMed] [Google Scholar]; c Prabhath M.; Williams L.; Bhat S.; Sharma P. Recent advances in cyanamide chemistry: Synthesis and applications. Molecules 2017, 22, 615. 10.3390/molecules22040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dömling A.; Herdtweck E.; Heck S. Cyanamide in isocyanide-based MCRs. Tetrahedron Lett. 2006, 47, 1745–1747. 10.1016/j.tetlet.2006.01.032. [DOI] [Google Scholar]
- Patil P.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. Ammonia-promoted one-pot tetrazolopiperidinone synthesis by Ugi reaction. ACS Comb. Sci. 2017, 19, 343–350. 10.1021/acscombsci.7b00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Tuinhof J.; Mgimpatsang K. C.; Kurpiewska K.; Kalinowska-Tluscik J.; Dömling A. Copper-Catalyzed Modular Assembly of Polyheterocycles. J. Org. Chem. 2020, 85, 9915–9927. 10.1021/acs.joc.0c01238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Chavan S. P.; Sivappa R. A short and efficient general synthesis of luotonin A, B and E. Tetrahedron 2004, 60, 9931–9935. 10.1016/j.tet.2004.08.025. [DOI] [Google Scholar]; b Rao K. R.; Mekala R.; Raghunadh A.; Meruva S. B.; Kumar S. P.; Kalita D.; Laxminarayana E.; Prasad B.; Pal M. A catalyst-free rapid, practical and general synthesis of 2-substituted quinazolin-4 (3 H)-ones leading to luotonin B and E, bouchardatine and 8-norrutaecarpine. RSC Adv. 2015, 5, 61575–61579. 10.1039/C5RA10928K. [DOI] [Google Scholar]; c Yang G. Z.; Zhang J.; Peng J. W.; Zhang Z. J.; Zhao W. B.; Wang R. X.; Ma K. Y.; Li J. C.; Liu Y. Q.; Zhao Z. M.; Shang X. F. Discovery of luotonin A analogues as potent fungicides and insecticides: Design, synthesis and biological evaluation inspired by natural alkaloid. Eur. J. Med. Chem. 2020, 194, 112253. 10.1016/j.ejmech.2020.112253. [DOI] [PubMed] [Google Scholar]
- Wu F.; Dong W.; Fan S.; Yuan Y.; Liang C.; Chen A.; Yin Z.; Zhang Z. Rapid Synthesis of Luotonin A Derivatives via Synergistic Visible-Light Photoredox and Acid Catalysis. J. Org. Chem. 2022, 87, 1302–1312. 10.1021/acs.joc.1c02617. [DOI] [PubMed] [Google Scholar]
- Ugi I.; Offermann K. Isonitrile, XIX. Die Kondensation von Carbonsäuren, Aldehyden und Isonitrilen mit primären aliphatischen Aminen, die einen abspaltbaren Alkyl-oder Alkenyl-Rest tragen. Chem. Ber. 1964, 97, 2996–3007. 10.1002/cber.19640971105. [DOI] [Google Scholar]
- a Boltjes A.; Dömling A. The Groebke-Blackburn-Bienaymé Reaction. Eur. J. Org. Chem. 2019, 42, 7007–7049. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Godet T.; Bonvin Y.; Vincent G.; Merle D.; Thozet A.; Ciufolini M. A. Titanium catalysis in the Ugi reaction of α-amino acids with aromatic aldehydes. Org. Lett. 2004, 6, 3281–3284. 10.1021/ol048850x. [DOI] [PubMed] [Google Scholar]; c Okandeji B. O.; Gordon J. R.; Sello J. K. Catalysis of Ugi four component coupling reactions by rare earth metal triflates. J. Org. Chem. 2008, 73, 5595–5597. 10.1021/jo800745a. [DOI] [PubMed] [Google Scholar]
- Beaume A.; Courillon C.; Derat E.; Malacria M. Unprecedented Aromatic Homolytic Substitutions and Cyclization of Amide Iminyl Radicals: Experimental and Theoretical Study. Chem. – Eur. J. 2008, 14, 1238–1252. 10.1002/chem.200700884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.