Abstract
Neuropeptides are signaling molecules that regulate almost all physiological processes in animals. Around 50 different genes for neuropeptides have been described in insects. In Coleoptera, which is the largest insect order based on numbers of described species, knowledge about neuropeptides and protein hormones is still limited to a few species. Here, we analyze the neuropeptidomes of two closely related tenebrionid beetles: Tenebrio molitor and Zophobas atratus—both of which are model species in physiological and pharmacological research. We combined transcriptomic and mass spectrometry analyses of the central nervous system to identify neuropeptides and neuropeptide-like and protein hormones. Several precursors were identified in T. molitor and Z. atratus, of which 50 and 40, respectively, were confirmed by mass spectrometry. This study provides the basis for further functional studies of neuropeptides as well as for the design of environmentally friendly and species-specific peptidomimetics to be used as biopesticides. Furthermore, since T. molitor has become accepted by the European Food Safety Authority as a novel food, a deeper knowledge of the neuropeptidome of this species will prove useful for optimizing production programs at an industrial scale.
Keywords: mass spectrometry, peptidomics, transcriptome, differential processing, Coleoptera
Introduction
Neuropeptides are among the most ancient and diverse signaling molecules in metazoans, regulating several physiological functions, such as behavior, development, and environmental stress response.1−5 Endogenous peptides may act both as hormones in the circulatory system and neuromodulators in the peripheral and central nervous systems.6 Neuropeptides exert their functions mostly by activating specific cell membrane receptors, such as G-protein-coupled receptors (GPCRs).7 For these reasons, neuropeptides and their receptors co-evolved and show conserved evolutionary features.8 Neuropeptides are mostly produced in neurons, interneurons, and neurosecretory cells within the central nervous system (CNS),6,9 in the peripheral nervous system, and in the endocrine cells of the intestine.6 After prohormone processing, a single or multiple copies (paracopies) of mature peptides are released across axonal pathways in the circulatory system and in the CNS or stored and released from neurohemal organs. The majority of paracopies produced from a single neuropeptide precursor share a similar or identical C-terminus and activate the same receptor, while in a few cases, the mature products of a neuropeptide precursor may activate different GPCRs. The latter is the case for genes such as capa and pk/pban (pyrokinin/pheromone biosynthesis activating neuropeptide), as demonstrated in Drosophila melanogaster.10−12
The identification of neuropeptide precursors has accelerated during the last decade thanks to the availability of genomic and transcriptomic data.13 In insects, there are currently around 50 recognized genes encoding for neuropeptides, putative neuropeptides, and protein hormones (for a review, see Nässel and Zandawala14). Using a combination of transcriptomic and peptidomic analyses, it is possible to simultaneously identify expressed neuropeptide genes and examine the processing of the respective putative bioactive neuropeptides in several species.7,15−19 Until recently, most neuropeptide genes and precursors had been described in hemimetabolan insect orders, such as Blattodea, Orthoptera, and Hemiptera, and in a few holometabolan orders, such as Lepidoptera, Diptera, and Coleoptera.20 The almost complete set of predicted neuropeptide precursors in Coleoptera was recently described in silico by two comparative studies based on publicly available databases.8,21 Detailed neuropeptidomes have been studied using mass spectrometry in the large pine weevil Hylobius abietis (Polyphaga: Curculionidae)22 and in ground beetles of the genus Carabus (Adephaga: Carabidae).18 One of the main findings of these studies was the loss of several neuropeptide genes, especially in Polyphaga species, commonly found in other insect groups, such as insect kinins (only present in Adephaga), allatostatin a, corazonin, and allatostatin ccc.8,18,22−24
Many phytophagous Coleoptera species are recognized worldwide as pests capable of causing serious losses both in cultivated and forest areas. Over the years, there has been an increasing use of insecticides25 to reduce damage caused by pests.25 Besides the negative environmental effects of broad-spectrum insecticides on beneficial species (e.g., pollinators and natural predators of pests), beetles are known to develop resistance against insecticides and xenobiotics (www.pesticideresistance.org). During the last decade, neuropeptide mimetic analogues have been proposed as candidates for the development of species-specific insecticides that target the key physiological functions of the pests only, without harming beneficial species.18,22,26−28 In order to apply this strategy, it is necessary to obtain comprehensive knowledge of the neuropeptidomes of both the target pest species (or group of species) and the beneficial species to be protected.18 Until now, the insecticidal effects of several neuropeptide analogues were successfully tested in aphids, in moths, and in T. castaneum.29−34 Currently, the main limitations of this approach are the stability of peptides against endogenous peptidases and the penetration through the gut or cuticle into the hemolymph.35
Beetle species belonging to the Tenebrionidae family are commonly employed as models in life science research, such as in medicine, physiology, and agronomy.25T. castaneum is widely used in genetics, immunology, and developmental biology,36−38 but it is not the best candidate for physiological bioassays due to its small size. Alternative beetle model organisms for physiological experiments are the larger Tenebrio molitor and Zophobas atratus, which have been successfully used for studying tissue- or organ-specific effects of different biologically active molecules, such as neurohormones or alkaloids, as well as in pharmacological studies.25,39−42T. molitor is currently one of the most cited Coleoptera species on PubMed, with more than 300 publications during the past three years, partly due to the fact that this species was recently accepted by the European Food Safety Authority as a novel food source.43
Until now, peptidomic studies have been limited in T. molitor and Z. atratus. In T. molitor, the peptidome of the cerebral ganglia and retrocerebral complex was investigated with matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF)44 using the deduced neuropeptide precursor sequences of T. castaneum to identify evolutionarily conserved neuropeptides between these two tenebrionid species. Four neuropeptides from the cerebral ganglia and corpora cardiaca-corpora allata (CC-CA) were reported with this approach.44 In Z. atratus, nine neuropeptides were identified using a similar approach.45 More recently, the products and distribution of capa and pk genes in the CNS of Z. atratus were investigated using MALDI-TOF46 and the almost complete set of predicted neuropeptide precursors of T. molitor was included in a comparative study among Coleoptera.8 The aim of the present study was to identify neuropeptides and neuropeptide-like and protein hormones and their distribution in the nervous systems of T. molitor and Z. atratus. The information obtained will expand our knowledge of processed neuropeptides in beetles and will be useful for the design of peptide mimetic analogues to be tested as biopesticides. At the same time, increased knowledge of T. molitor neuropeptidomes could turn out to be useful for optimizing the production of larvae at an industrial scale. To achieve this goal, we combined the information obtained by transcriptome and mass spectrometry analyses of the nervous systems of T. molitor and Z. atratus. We also took advantage of recently available T. molitor genome assemblies47,48 to solve ambiguities or missing information of our transcriptome. Overall, we identified around 50 neuropeptide precursors in both species, the majority of which confirmed by a combination of two mass spectrometry approaches: one based on MALDI-TOF direct profiling of CNS tissues and the second based on extracts of the complete CNS analyzed with LC coupled to an Orbitrap mass spectrometer.
Materials and Methods
Insects and Rearing Conditions
Adults of T. molitor (Linnaeus, 1758) and Z. atratus (Fabricius, 1776) were obtained from a culture maintained at the Department of Animal Physiology and Developmental Biology, Adam Mickiewicz University, Poznań, Poland. T. molitor were reared as described previously,49 whereas Z. atratus, according to the procedure described in Quennedey et al.50
RNA Extraction
Total RNA was extracted from the brain and retrocerebral complex of 10 adults of each species using TRIzol (Invitrogen, Grand Island, NY, USA), following the manufactory instructions of the Beijing Genomics Institute (BGI, China). RNA quality parameters were estimated using RNA concentration (ng/μL) and the RNA integrity number as implemented in an Agilent 2100 Bioanalyzer.
Library Construction and Transcriptome Sequencing
Libraries were sequenced using an Illumina Truseq RNA sample preparation kit (Illumina, San Diego, USA) at BGI, as already described in ref (51). Briefly, once total RNA was extracted, magnetic beads with Oligo (dT) were used to isolate mRNA and mixed with buffer to fragment the mRNA into short fragments. The cDNA was synthesized using these mRNA fragments as templates. First-strand cDNA was generated using random hexamer-primed reverse transcription, followed by second-strand cDNA synthesis, double-stranded cDNA purification and resolution using elution buffer for end reparation, and Poly (A) tail addition. Finally, after ligating the Illumina sequencing paired end (PE) adaptors, products were purified on Tris–acetate–ethylenediamine tetraacetic acid agarose gel and suitable fragments were selected for PCR amplification in order to enrich the purified cDNA template. Quality control of the cDNA library was performed using an Agilent 2100 Bioanalyzer. Finally, the resulting cDNA library was sequenced using an Illumina HiSeqTM4000 with strategies of 100 bp PE at BGI.
De Novo Assembly of Nucleotide Sequences and Quality Control
Raw data were filtered by removing adapters and low quality reads at BGI. The resulting filtered RAW reads were submitted to NCBI (Sequence Read Archives (SRA): SRR11184806 and SRR11358229, BioProject PRJNA608239 for T. molitor and SRR11178058 and SRR11178059, BioProject PRJNA608269 for Z. atratus). Filtered reads were de novo assembled by means of Trinity 2.2.0, using the trinity read normalization option. Trinity transcriptome assemblies were submitted to the NCBI Transcriptome Shotgun Assembly (TSA) database. The T. molitor assembly has been deposited at DDBJ/EMBL/GenBank under accession number GIPG00000000. The version described in this paper is the first version, GIPG01000000. The Z. atratus assembly has been deposited at DDBJ/EMBL/GenBank under accession number GIPJ00000000. The version described in this paper is the first version, GIPJ01000000. In addition to our transcriptome data, we also used the recently published genome of T. molitor (BioProject: PRJNA579236; BioProject: PRJEB44755)47,48 to complete neuropeptide ambiguities or missing neuropeptide precursors.
Compiling of Precursor Sequences
For identification of precursor sequences, we performed a search by homology (tBLASTn) using sequences of known insect neuropeptide precursors as reference queries,8,16,18,22,52 on a local computer as implemented in BLAST+.53 Positive hits within the transcriptome assembly were translated into proteins using the ExPASy translate tool54 (web.expasy.org/translate/). Signal peptides for the putative precursors were predicted using the SignalP 5.0 and 6.0 servers55 (www.cbs.dtu.dk/services/SignalP/). Cleavage sites were initially manually assigned based on known cleavage sites in homologous precursors from other species. Missing neuropeptide precursors were also searched in the raw data using the BLAST+ algorithm.
Tissue Preparation for MALDI-TOF MS
Animals were kept at −4 °C for 15 min before dissecting the CNS in insect saline solution (NaCl 126 mM, KCl 5.4 mM, NaH2PO4 0.17 mM, KH2PO4 0.22 mM; pH 7.4).56 We carefully dissected the antennal lobe, the neurohemal organs from the abdominal and thoracic ganglia and the retrocerebral complex and the frontal and terminal ganglia as previously described.18
Tissue Preparation for Orbitrap MS
Dissected tissues were briefly washed in a drop of distilled water to remove salt contaminations and immediately transferred to a 30 μL extraction solution (Buffer 1: 90% methanol, 1% formic acid [FA]). Tissues were dissociated in an ultrasonic bath (Transonic 660/H, Elma Schmidbauer GmbH, Hechingen, Germany) at 4 °C for 5 min and with an ultrasonic probe three times for 2 s (Bandelin Sonopuls HD 200, Bandelin electronic GmbH, Berlin, Germany), respectively. Extracts were then centrifuged for 20 min at 15,000 rpm at 4 °C. Supernatants were transferred to a fresh tube (Eppendorf, Hamburg, Germany), and methanol was evaporated in a vacuum concentrator (Hetovac VR-1, Heto Lab Equipment, Roskilde, Denmark).
Reduction of Disulfide Bonds, Carbamidomethylation of Cysteines, and Protein Digestion
To analyze protein hormones we used a fraction of the extracts previously prepared as described in ref (18). Protein extracts were quantified using a Direct Detect infrared spectrometer (Merck KGaA, Darmstadt, Germany) for subsequent protein digestion. We used 10 μg of proteins for each sample. Extracts were subjected to disulfide reduction by adding dithiothreitol (DTT) to a final concentration of 20 mM at 37 °C for 1 h. Subsequently, samples were immediately subjected to carbamidomethylation by adding iodoacetamide to a final concentration of 40 mM for 30 min in darkness. The reaction was quenched by adding DTT at a final concentration of 40 mM. For protein digestion, we first added endoproteinase Lys-C (Lys-C; Wako, Richmond, VA, U.S.A) at 0.5 μg/μL for 4 h at 37 °C, followed by trypsin at 1 μg/μL (Sigma-Aldrich, Steinheim, Germany), and incubated it at 37 °C for 12 h. Both enzymes were added at an enzyme/substrate ratio of 1:75 following the manufacturer’s instructions. Enzymatic digestion was stopped by adding FA to a final concentration of 1%.
Quadrupole Orbitrap MS
Before being injected into the nanoLC system, all samples were desalted using self-packed Stage Tip SDB-RPS (IVA Analysentechnik e. K., Meerbusch, Germany) spin columns as described previously.57 Peptides were first separated with an EASY nanoLC1000 UPLC system (Thermo Fisher Scientific) using in-house packed 50 cm RPC18 columns (fused Silica tube with ID 50 μm ± 3 μm, OD 150 μm ± 6 μm; Reprosil 1.9 μm, pore diameter 60 Å; Dr. Maisch GmbH, Ammerbuch-Entringen, Germany) and a binary buffer system (A: 0.1% FA; B: 80% ACN, 0.1% FA). The UPLC was coupled to a Q-Exactive Plus (Thermo Fisher Scientific) mass spectrometer. Running conditions were as described in ref (56). Mass spectrometry proteomics data were deposited at the ProteomeXchange Consortium via the PRIDE58 partner repository under dataset identifiers PXD032947 (doi:10.6019/PXD032947) for T. molitor and PXD033000 (doi:10.6019/PXD033000) for Z. atratus.
Raw data were analyzed using PEAKS Studio 10 (BSI, ON, Canada).59 Samples not subjected to enzymatic digestion were matched against an internal database containing the transcriptome-derived precursor sequences of T. molitor and Z. atratus, as well as the six frame translation of the complete transcriptomes. For analyses with PEAKS, the same set of post-translational modifications was used, and peptides were searched against the same internal databases with a parent error mass tolerance of 10 ppm and fragment mass error tolerance of 0.05 Da. No enzyme mode was selected. Variable post-translational modifications included in the searches were oxidation at methionine, acetylation at the N-terminus, amidation of C-terminal glycine, pyroglutamate from glutamine, pyroglutamate from glutamic acid, and disulfide bridges. The false discovery rate (FDR) was determined by a decoy database search implemented in PEAKS 10 and set below 1%. To provide the accurate monoisotopic mass of a peptide, Q Exactive Orbitrap RAW data were corrected prior to the analysis (precursor mass correction only). Fragment spectra with a peptide score (−10lgP) equivalent to a P-value of about 1% were manually reviewed. Peptide spectrum matches with an FDR of 0.1% (approximately −10logP values higher than 30) were subsequently manually checked. Samples that were enzymatically treated with LysC and trypsin were analyzed using the same parameters but with enzyme mode set to trypsin and carboxymethylation as a fixed modification.
MALDI-TOF MS
For direct tissue profiling, small parts of the nervous system, including the antennal lobe and neurohemal organs, were carefully dissected, washed in a drop of distilled water, transferred on a MALDI-TOF sample plate, and allowed to air-dry. Dried tissues were covered with 0.3–0.4 μL drops of matrix. Matrices used in this study were 2,5-dihydroxybenzoic acid (DHB, Sigma-Aldrich) at a final concentration of 10 mg/mL in 1% aqueous FA containing 20% acetonitrile (ACN) (v/v) and α-cyano-4-hydroxycinnamic acid (α-CHCA Sigma- Aldrich) at 10 mg/mL in 60% ethanol, 36% ACN, 4% water (stock solution) and dissolved in 50% methanol/water (2:1 50% methanol/CHCA stock solution). Mass fingerprint (MS1) and ion fragmentation (LIFT mode – MS2) spectra were acquired using two instruments: an UltrafleXtreme MALDI-TOF mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) and a MALDI TOF ABI 4800 Proteomics Analyzer (Applied Biosystems Framingham, MA). The UltrafleXtreme was mostly used for MS1 under manual control in reflectron-positive ion mode and for MS2 experiments with LIFT technology without CID for both DHB- and α-CHCA-coated samples, while the MALDI TOF ABI 4800 was used exclusively for MS2 in gas off mode with α-CHCA-coated samples. MS2 spectra were manually reviewed by comparing with theoretical ion fragments (prospector.ucsf.edu/prospector/mshome.htm). For external calibration, a mixture of proctolin, Drosophila melanogaster short neuropeptide 212−19 (sNPF-212−19), Periplaneta americana (Pea-FMRFa-12), Locusta migratoria periviscerokinin (Lom-PVK), Pea-SKN, and glucagon for the lower mass range (m/z 600–4000) and a mixture of bovine insulin, glucagon, and ubiquitin for the higher mass range (m/z 3000–10,000) were used. Spectra were analyzed using flexAnalysis 3.4 (Bruker Daltonik) and Data Explorer v. 4.3 (Applied Biosystems). MALDI raw data were deposited at the Zenodo (doi: 10.5281/zenodo.6913565) for T. molitor and PXD033000 (doi: 10.5281/zenodo.6948001) for Z. atratus.
Results and Discussion
Transcriptome Identifications
The numbers of identified neuropeptide, neuropeptide-like, and protein hormone precursors in T. molitor and Z. atratus were 60 and 59, respectively (Table 1). In the T. molitor transcriptome, we found several splice forms for CAPA, neuropeptide F1 (NPF1), orcokinin-like (Ork-like), agatoxin-like peptide (ALP), ion transport peptide (ITP), arthropod insulin-like growth factor (aIGFa-b),52 and corticotropin-releasing factor-like diuretic hormone CRF-DH (Table 1; Figures S1 and S2). Moreover, we found a few precursors that did not match the ones described in Veenstra.8 Most differences arose in proctolin, orcokinin, prothoracicotropic hormone (PTTH), and calcitonin-like diuretic hormone (CT-DH) precursors. We assembled two proctolin genes, which have been fully confirmed in the T. molitor genome. The second gene (proctolin 2) differs considerably at the precursor N-terminal from the one described in Veesntra.8 In the genome of T. molitor, we also found a second allele for proctolin 2 (Figure S3). Orcokinin and orcomyotropin peptides were originally described in Crustacea,60,61 and two orcokinin genes were later described in Daphnia.62 Insect orcokinins are encoded by a single gene with two splice variants (a and b).63 In tenebrionids, the pre-propeptides of orcokinin transcripts a and b do not contain any sequence with a typical orcokinin (NXDEIDR) or orcomyotropin (FDAFTTGF) motif.64 We therefore named the two splice variants orcokinin-like transcripts a and b (OK-likea and OK-likeb). In transcript a, we identified two splice forms (named OK-likea1 and OK-likea2; Figure S2A), both of which contain a different signal peptide from the one described in Veenstra.8 In our transcriptome, we could detect only a small fragment of the OK-likeb precursor containing four peptides (SLDGIGGGNLV-NH2; SLDRIGGGNLV-NH2; STDGIDGDLI-NH2; and SLARTNKLN-NH2). In order to complete the OK-likeb precursor, we combined genomic and transcriptomic data and found at least 30 amidated peptides similar to those described in T. castaneum (Figure S2B). The PTTH-like precursor described in Veenstra8 contains several gaps in the central region of the precursor. Finally, Veenstra8 described two additional splice forms of the calcitonin-like diuretic hormone, but we did not include them in our precursor list as they do not encode any CT-DH.
Table 1. Precursors for Neuropeptides and Neuropeptide-Like and Protein Hormones Identified in the Transcriptomes of T. molitor and Z. atratusa.
|
T. molitor |
Z. atratus |
||||||
|---|---|---|---|---|---|---|---|
| designation | abbreviations | accession | amino acids | MS | accession | amino acids | MS |
| neuropeptides | |||||||
| adipokinetic hormone 1 | AKH | ON086791 | 72 | + | ON155921 | 71 | + |
| adipokinetic hormone/corazonin-related peptide | ACP | ON086792 | 82 | + | ON155922 | 88 | + |
| allatostatin C | AstC | ON086793 | 103 | + | ON155923 | 104 | + |
| allatostatin CC | AstCC | ON086794 | 135 | + | ON155924 | 134 | + |
| allatotropin | AT | ON086795 | 101 | + | ON155925 | 104 | + |
| antidiuretic factor b-1 | AFB-1 | ON110494 | 137 | + | ON155926 | 129 | + |
| antidiuretic factor b-2 | AFB-2 | – | – | – | ON155927 | 165 | + |
| antidiuretic factor b-3 | AFB-3 | – | – | – | ON155928 | 114 | – |
| antidiuretic factor b-4 | AFB-4 | – | – | – | ON155929 | 144 | – |
| calcitonin 1 | calcitonin 1 | ON110495 | 120 | – | ON155930 | 121 | – |
| calcitonin 2 | calcitonin 2 | ON110496 | 115b | – | – | – | – |
| calcitonin-like diuretic hormone | CT-DH | ON110497 | 119 | + | ON155931 | 119 | + |
| CAPA transcript a | CAPAa | ON110498 | 174 | + | ON155932 | 154 | + |
| CAPA transcript b | CAPAb | ON110499 | 158 | + | – | – | – |
| CCHamide1 | CCHa-1 | ON110500 | 159 | + | ON155933 | 155 | + |
| CCHamide2 | CCHa-2 | ON110501 | 113 | + | ON155934 | 112 | + |
| CNMamide | CNMa | ON110502 | 141 | – | ON155935 | 95c | – |
| corticotropin-releasing factor-like diuretic hormone (DH37) | CRF-DH37 | ON110503 | 125 | + | ON155936 | 128 | + |
| corticotropin-releasing factor-like diuretic hormone (DH47) | CRF-DH47 | ON110504 | 154 | + | ON155937 | 157 | + |
| crustacean cardioactive peptide | CCAP | ON110505 | 143 | + | ON155938 | 143 | + |
| ecdysis triggering hormone | ETH | ON110506 | 139 | – | ON155939 | 77c | – |
| elevenin | elevenin | ON110507 | 126 | + | ON155940 | 83c | – |
| FMRFamide-related peptides | FMRF | ON110508 | 207 | + | ON155941 | 200 | + |
| HanSolin | HanSolin | ON110509 | 121 | + | ON155942 | 117 | – |
| IDL-containing | IDL | ON110510 | 204 | + | ON155943 | 204 | + |
| inotocin (vasopressin-like) | inotocin | ON110511 | 151 | + | ON155944 | 152 | + |
| insect parathyroid hormone | IPH | ON110512 | 110 | + | ON155945 | 110 | – |
| myoinhibitory peptide | MIP | ON110513 | 187 | + | ON155946 | 185 | + |
| myosuppressin | MS | ON110514 | 91 | + | ON155947 | 102 | + |
| natalisin | natalisin | ON110515 | 163 | + | ON155948 | 165 | + |
| neuropeptide F1a | NPF1a | ON110516 | 85 | + | ON155949 | 82 | – |
| neuropeptide F1b | NPF1b | ON110517 | 123 | – | ON155950 | 120 | – |
| neuropeptide F2 | NPF2 | ON110518 | 90 | + | ON155951 | 89 | + |
| orcokinin-like transcript a1 | OK-likea1 | ON155962 | 171 | + | ON155952 | 170 | + |
| orcokinin-like transcript a2 | OK-likea2 | ON155961 | 146 | – | – | – | – |
| orcokinin-like transcript b | OK-likeb | ON155963 | 439b | – | – | – | – |
| pigment dispersing factor | ON110519 | 99 | + | ON155953 | 100 | + | |
| proctolin 1 | proctolin 1 | ON155964 | 83 | + | ON155954 | 83 | + |
| proctolin 2 allele 1 | proctolin 21 | ON155965 | 77 | + | – | – | – |
| proctolin 2 allele 2 | proctolin 22 | ON155966 | 77b | + | – | – | – |
| pyrokinin | PK | ON110520 | 163 | + | ON155955 | 168c | + |
| RFLamide | RFLa | ON110521 | 185 | – | ON155956 | 185 | – |
| RYamide | RYa | ON110522 | 128 | + | ON155957 | 130 | + |
| short neuropeptide F | sNPF | ON125379 | 97 | + | ON155958 | 100 | + |
| SIFamide | SIFa | ON125380 | 75 | + | ON155959 | 75 | + |
| sulfakinin | SK | ON125381 | 116 | + | ON155960 | 115 | + |
| tachykinin-related peptide | TKRP | ON125382 | 272 | + | ON155969 | 273 | + |
| trissin | trissin | ON125383 | 100 | – | ON155970 | 99 | – |
| neuropeptide-like | |||||||
| agatoxin-like peptide a | ALPa | ON125384 | 108 | + | ON155971 | 108 | + |
| agatoxin-like peptide b | ALPb | ON125385 | 99 | + | ON155972 | 99 | + |
| neuropeptide-like precursor 1 | NPLP1 | ON125386 | 423 | + | ON155973 | 422 | + |
| NVP-like | NVP | ON125387 | 322 | + | ON155974 | 317 | + |
| Periplaneta neuropeptide-like precursor | Pea-NPLP | ON125388 | 691 | + | ON155975 | 679 | + |
| protein hormones | |||||||
| bursicon alpha | Burs-α | ON155991 | 160b | – | ON155976 | 167 | – |
| bursicon beta | Burs-ß | ON155992 | 135 | + | ON155977 | 133c | – |
| eclosion hormone 1 | EH 1 | ON125389 | 81 | – | ON155978 | 82 | – |
| eclosion hormone 2 | EH 2 | ON125390 | 77 | – | ON155979 | 77 | – |
| glycoprotein hormone alpha 2 | GPA2 | ON125391 | 122 | + | ON155980 | 122 | + |
| glycoprotein hormone beta 5 | GPB5 | ON125392 | 155 | + | ON155981 | 155 | + |
| arthropod insulin-like growth factora | aIGF | ON155967 | 162 | – | – | – | – |
| arthropod insulin-like growth factorb | aIGF | ON125395 | 162 | – | ON155984 | 179 | – |
| insulin-like peptide 1 | ILP 1 | ON125393 | 125 | + | ON155982 | 125 | + |
| insulin-like peptide 2 | ILP 2 | ON125394 | 133 | – | ON155983 | 125 | – |
| insulin-like peptide 3 | ILP 3 | ON155968 | 121c | + | – | – | – |
| insulin-like peptide 4 (allele 1) | ILP 41 | ON125396 | 104 | + | – | – | – |
| insulin-like peptide 4 (allele 2) | ILP 42 | ON125397 | 104 | + | – | – | – |
| relaxin | relaxin | ON125398 | 145 | – | ON155985 | 83c | – |
| ion transport peptide-likea | ITPa | ON125399 | 136 | + | ON155986 | 136 | + |
| ion transport peptide-likeb | ITPb | ON125400 | 120 | + | ON155987 | 120 | – |
| ITG-like | ITG | ON125401 | 214 | + | ON155988 | 214 | + |
| neuroparsin | neuroparsin | ON125402 | 107 | + | ON155989 | 109 | + |
| prothoracicotropic hormone | PTTH | ON125403 | 179c | – | ON155990 | 184 | + |
Sequences are listed in the Supporting Information S1. Different transcripts are marked with subscript characters and alleles with subscript numbers.
Completed with BioProject: PRJNA579236; BioProject: PRJEB44755PRJNA646689.
Incomplete.
Our precursor list also includes five additional precursors known across insects: the antidiuretic factor ADF;65 IDL-like; neuropeptide F1;66 and two recently described precursors, the insect parathyroid hormone (iPTH)67 and Periplaneta neuropeptide-like (Pea-NPLP = PaOGS36577).68 The latter is homologous to a precursor described in the ant Cataglyphis nodus with the name of Fliktin.69 Finally, we detected an additional precursor of insulin-like peptide (ILP) in the T. molitor transcriptome (Table1; Supporting Information S1).
In Z. atratus, we identified only three splice forms in alp, npf1, and itp (Figure S4). We could not find any sequence for OK-likeb and calcitonin 2, most likely because these two genes are expressed in the midgut tissue, which was not covered in our transcriptome.64,70
Based on the investigated transcriptomes and genomes, we further confirmed the absence of allatostatin a, corazonin, insect kinins, and allatostatin ccc genes in tenebrionids. Moreover, we could not identify the recently described genes for smyamide, a paralog of sifamide,71gonadulin,72 and carausius-like neuropeptide.16,19
Mass Spectrometry Identifications
Overall, we confirmed the presence of 50 and 40 neuropeptide precursor products and transcripts across the CNS of T. molitor and Z. atratus, respectively (Table 1). The neuropeptide products that were not detected in either species using mass spectrometry were calcitonins 1 and 2, CNM, ecdysis triggering hormone (ETH), OK-likeb, RFLa, and trissin. Calcitonins and OK-likeb are mostly expressed in the midgut and could be less abundant in our preparations.63,64,70 Of the protein hormones, EH, relaxin, ILP-2, and aIGFa-b were not detected in either species. EH and ETH have key functions in regulating ecdysis behavior in insects and therefore were likely not expressed in the CNS of larvae and adults.73 In Z. atratus, we could not confirm ADF-b3, ADF-b4, HanSolin, iPTH, NPF1, and RYa precursors.
The two mass spectrometry approaches yielded similar results except for inotocin, NPF-1, and NPF-2, which were detected only in the MALDI-TOF fingerprints, and ADF, HanSolin, iPTH, natalisin, and OK-likea, which were confirmed only by Orbitrap analyses in T. molitor. Similarly, in Z. atratus, Orbitrap analyses could detect two ADF peptides and CCHa2, whereas in MALDI-TOF, we confirmed ALP, CRF-DH37, crustacean cardioactive peptide (CCAP), inotocin, and RYa peptides.
Using protein tryptic digestion, we detected several fragments of neuropeptides and neuropeptide-like precursor peptide (PP) and digested fragments of large protein hormones such as bursicon (Burs-β), glycoprotein hormone alpha 2 (GPA2), glycoprotein hormone beta 5 (GPB5), ITG-like (ITG), and neuroparsin (Supporting Information S1, Tables S1 and S2).
Neuropeptide Distribution across the CNS
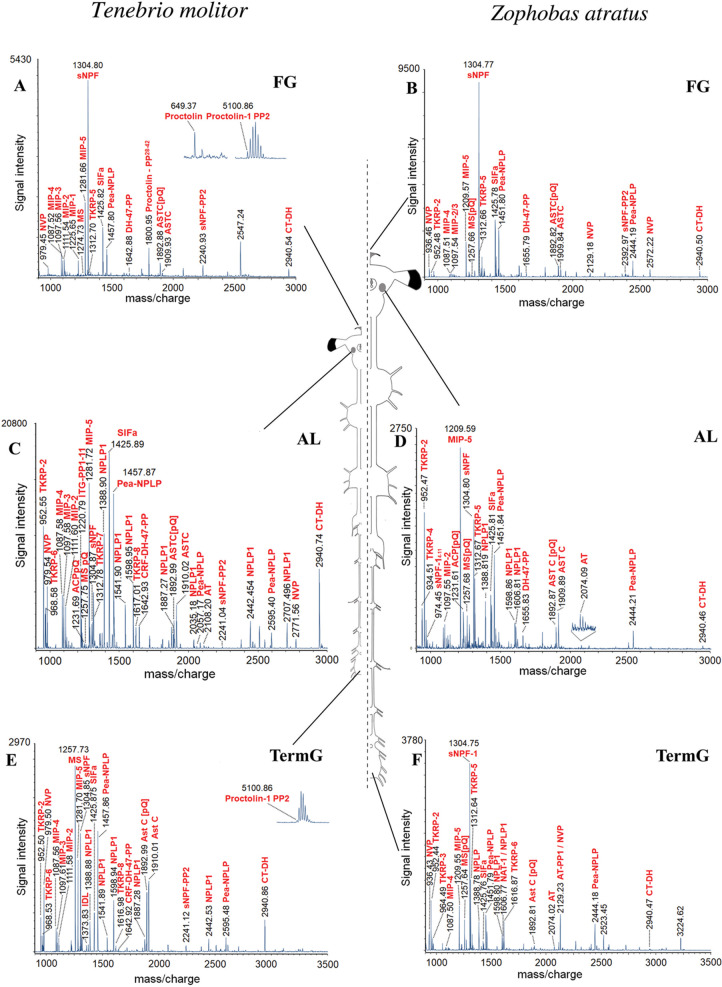
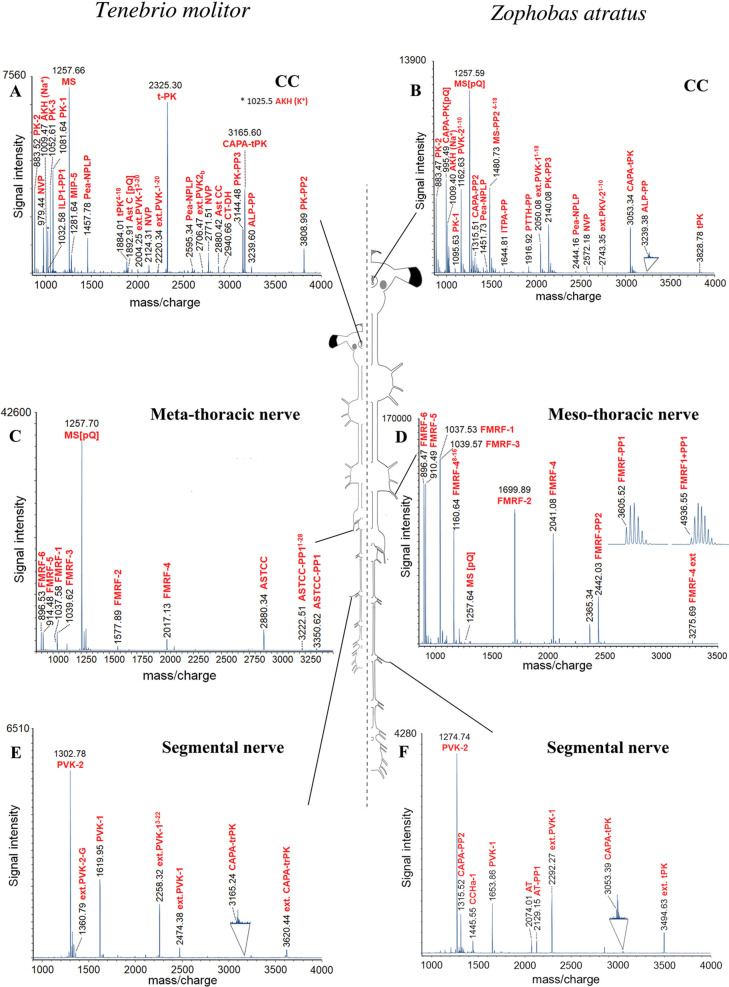
Direct tissue profiling offers the possibility to investigate the distribution of neuropeptides in the CNS and the differential processing of neuropeptide precursors.46 For these purposes, we analyzed two neuropil regions within the CNS (the antennal lobe and posterior terminal ganglion), the frontal ganglion and the major neurohemal organs of tenebrionids (corpora cardiaca, thoracic segmental nerves, and abdominal segmental nerves). For an overview of neuropeptide distribution, see Figures 1 and 2, Table 2, and S3.
Figure 1.
MALDI-TOF MS1 spectra obtained by direct tissue profiling of frontal ganglia and neuropile regions of the CNS of T. molitor (left panel) and Z. atratus (right panel). The mass spectra illustrate a tissue-specific distribution of neuropeptides across the CNS. (A) Mass spectrum of T. molitor FG preparation with prominent ion signals for sNPF, SIFa, Proctolin 1, and Pea-NPLP (m/z 800–3000). (B) Mass spectrum of Z. atratus FG preparation with particularly prominent ion signals for sNPF, SIFa, and Pea-NPLP (m/z 800–3000). (C) Mass spectrum of T. molitor AL with prominent ion signals for SIFa, ITG, NPLP1, MIP, TKRP, and Pea-NPLP (m/z 800–3000). (D) Mass spectrum of Z. atratus AL with prominent ion signals for MIP, TKRP, sNPF, and Pea-NPLP (m/z 800–3000). (E) Mass spectrum of T. molitor terminal ganglion (TermG) preparation with predominant ion signals of MS, sNPF, proctolin, and Pea-NPLP (m/z 900–3500). (F) Mass spectrum of Z. atratus TermG preparation with predominant ion signals of sNPF and TKRP-5 (m/z 900–3500).
Figure 2.
MALDI-TOF MS1 spectra obtained by direct tissue profiling of neurohemal organs of T. molitor (left panel) and Z. atratus (right panel). (A) Mass spectrum of T. molitor corpus cardiacum (CC) preparation with prominent ion signals for MS, AKH, PK, and CAPA peptides (m/z 800–4000). (B) Mass spectrum of Z. atratus CC preparation with prominent ion signals for MS (including the PP MS-PP24−18), AKH, PK, and CAPA peptides (CAPA-PK and tryptoPK), including truncated forms of periviscerokinins (PVK-1 and 2) (m/z 800–4000). (C) Mass spectrum of T. molitor meta-thoracic SN preparation with predominant ion signal for MS and FMRF peptides. Additionally, ion signals for ASTCC (including the intermediate precursor peptides ASTCC-PP11−28 and ASTCC-PP1) were detected (m/z 800–3500). (D) Mass spectrum of Z. atratus meso-thoracic SN preparation with ion signals for FMRF (m/z 900–3500). (E) Mass spectrum of a T. molitor abdominal SN with ion signals of CAPA peptides (m/z 900–4000). (F) Mass spectrum of the Z. atratus abdominal SN with ion signals of CAPA peptides (m/z 900–4000).
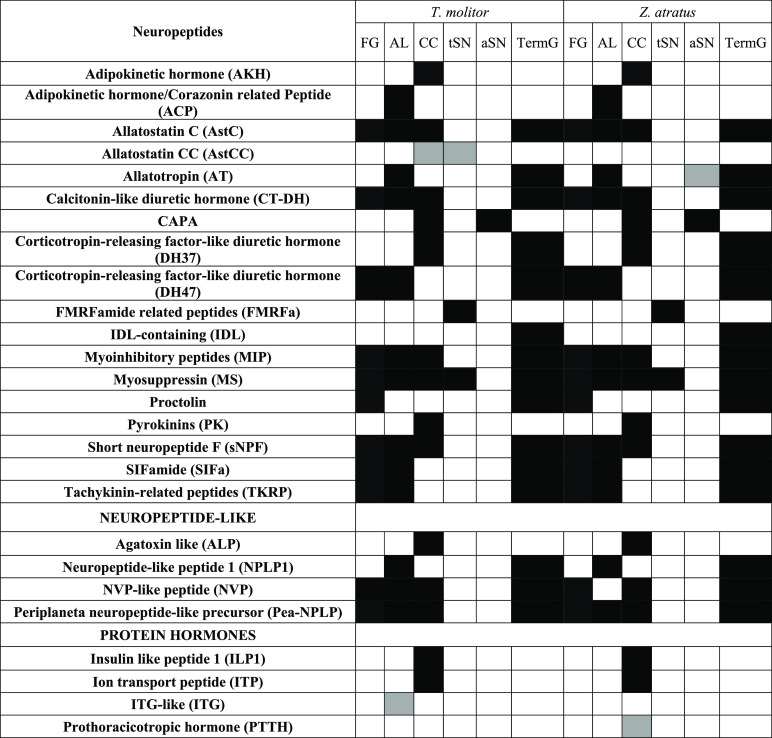
Table 2. Distribution of Neuropeptide Precursor Products throughout the Nervous Systems of T. molitor and Z. atratus Detected in MALDI-TOF MS1 Spectraa.
The presence of a peptide is indicated in black when shared by both species or in gray when not observed in both species. If a neuropeptide is not detected in the listed tissues (i.e., pigment dispersing factor is present only in pars intercerebralis), the precursor is not mentioned in the table. FG, frontal ganglion; AL, antennal lobe; CC, corpora cardiaca; tSN, thoracic segmental nerve; aSN, abdominal segmental nerve; and TermG, terminal ganglia.
The frontal ganglion (FG) is part of the stomatogastric nervous system (SNS) and is involved in the neural regulation of foregut activity.74 In beetles, it is also well connected to both the tritocerebrum and the retrocerebral complex (RCC). In both analyzed species, the most abundant neuropeptides in the FG were the short neuropeptides F (sNPF), myoinhibitory peptides (MIP), Pea-NPLP, and SIFamide (SIFa), followed by allatostatin C (AstC), CT-DH, myosuppressin (MS), NVP-like peptide (NVP), proctolin, and tachykinin-related peptides (TKRP) (Figure 1A,B). In addition to the above-mentioned neuropeptides, we also detected a PP of the CRF-DH47 transcript in the FG of both species.
The antennal lobes (AL) are a portion of the deutocerebrum mostly involved in the elaboration of olfactory information and contain several neuropeptides expressed in the cerebral ganglia.75 In the AL of T. molitor, we could confirm products of the adipokinetic hormone/corazonin-related peptide (ACP), AstC, allatotropin (AT), CRF-DH-47, CT-DH, ITG, NVP, MS, MIP, NPLP1, Pea-NPLP, SIFa, sNPF, and TKRP (Figure 1C), whereas we could not confirm ITG and NVP in the Z. atratus AL (Figure 1D), even though they were detected in other regions of the cerebral ganglia such as the pars intercerebralis.
The terminal ganglion (TermG) contains neuropeptides that are potentially involved in contractions of the hindgut and share several neuropeptides with the FG, such as AstC, CT-DH, MIP, MS, NVP, proctolin, sNPF, SIFa, and TKRP (Figure 1E,F). We also detected AT, IDL-containing (IDL), Pea-NPLP, and NPLP1 in the terminal ganglion of the two tenebrionids.
The RCC is the main neuroendocrine organ of insects and is connected to the cerebral ganglia, hypocerebral ganglion, FG, and gnathal ganglion. This organ consists of a pair of corpora cardiaca (CC), which are fused posteriorly to a pair of corpora allata. The CC store and release hormones and, in the glandular part, produce the adipokinetic hormone (AKH). Direct tissue profiling of the CC detected mostly AKH, MS, MIP, Pea-NPLP, pyrokinin (PK), and lower intensity signals matching with AstC, CT-DH, and NVP (Figure 2A,B). We also detected signals belonging to the CAPA precursor, such as CAPA-tPK, CAPA-PK, and the N-terminal truncated forms of PVK-1 and -2. In addition, we detected peptides below 10,000 Da matching with PPs of protein hormones such as ILP, ITP, and PTTH.
The thoracic and abdominal neurohemal organs of tenebrionids are different from those of other insects and other Coleoptera such as Carabus.18,46 The posterior lateral cells (PLCs) of the thoracic ganglia and the Va cells of the abdominal ganglia have axons projecting into segmental nerves (SNs), reaching paired neurohemal areas where neuropeptides are normally stored and released.46 The products of the PLCs entering the SN are mostly FMRFamide-related peptides (FMRF) (Figure 2C,D), whereas the Va cells produce CAPA peptides (Figure 2E,F). In the thoracic segmental neurohemal area of T. molitor, we also detected an abundant signal of MS and AstCC (Figure 2C).
Neuropeptide Precursor Processing Based on MALDI-TOF Direct Tissue Profiling
Below, we report only the main findings regarding processed neuropeptides in comparison with other Coleoptera species.
Adipokinetic Hormone
Mature AKH neuropeptide was detected in the corpus cardiacum in the typical N-terminal blocked form only with Na+ and K+ adducts. Several intermediates of AKH have been described in other insect species containing the amidation signal (GK)76−78 and the dibasic cleavage motif (GKR),76,77 none of which we could confirm here.
Allatostatin C and CC
In holometabolan insects, allatostatin C and CC18,24 are the only two confirmed paralogs. AstC was detected with both the N-terminal blocked and not blocked forms, mostly in neuropil regions (see Figure 1). Previous immunochemical examination of the T. molitor CNS also showed the presence of AstC in the brain, the corpora allata, and the ventral nerve cord.79
Allatotropin
In both species, the consensus C-terminus of AT is typical for polyphagous Coleoptera (TARGYamide), i.e., contains a Tyr residue. Ion signals matching those of AT were detected in several preparations in the CNS of the two tenebrionids, especially in abdominal transverse nerves. In MALDI-TOF spectra, the ion signal intensities of the mature AT were often of relatively low abundance.
Calcitonin-Like Diuretic Hormone
The only precursor that was confirmed by mass spectrometry across several CNS tissues was the typical precursor containing CT-DH. Veenstra8 reported two additional splicing variants for CT-DH (=DH31) in T. molitor that were not detected in our mass spectrometry analyses.
CAPA
The capa gene encodes for periviscerokinins (PVKs), PKs and tryptopyrokinins (tPK) and is typically expressed by neurosecretory cells of the abdominal ganglia.80 Differential processing of this gene in the giant mealworm beetle Z. atratus was recently reported. The capa gene is expressed both in the neurosecretory cells of the gnathal ganglion, processing mostly CAPA-tPK and CAPA-PK, and in Va cells of the abdominal ganglia, in which mostly PVKs are processed.46 The differential processing of capa has also been reported in other insects, such as T. castaneum,81 the tobacco hawk moth Manduca sexta,82 the kissing bug Rhodnius prolixus,83 and ground beetles of the genus Carabus.18 In T. molitor, we could confirm the differential processing of the CAPA precursor in the gnathal ganglion with ion signals matching with mature CAPA-tPK and the CAPA-PK in CC preparations (Figure 2A). The latter peptide, which represents the third putative receptor ligand encoded by the CAPA precursor, was less abundant in T. molitor CC than in Z. atratus across several preparations (Figure 2A,B). The two PVKs of the CAPA precursors were both processed as predicted in the abdominal SNs, including additional N-terminally extended forms of PVK-1 (Figure 2E,F). The differential processing of the CAPA precursor is likely associated with mechanisms of inactivation of CAPA-tPK in abdominal Va cells46 and PVKs in CAPA cells of the gnathal ganglion.18 The C-terminally extended CAPA-tPK (ext. CAPA-tPK) was the most abundant form in the abdominal SNs. As far as the C-terminal consensus sequence of this peptide is not fully processed, it is likely inactive in abdominal ganglia (Figure 2E,F). Similarly, PVKs were detected in CC preparations only with a truncated C-terminus and thus likely inactive (Figure 2A,B). The inactivation of PVKs in CAPA cells of the gnathal ganglion by the truncation of the C-terminus had already been reported in Carabus(18) and in D. melanogaster,84 suggesting that this is a common feature, at least in holometabolan insects.
CCHamide
The CCHa-1 has two peptides resulting from a differential cleavage of the precursor signal peptide. The two peptides have a similar mass to peptides of the NPLP1 precursor and were sequence-confirmed only in preparations of the terminal ganglia nerves.
Corticotropin-Releasing Factor-Like Diuretic Hormone
The two transcripts8 of this gene were confirmed, and the shorter PP (CRF-DH-47-PP) of this gene was detected in several preparations across the CNS, especially in AL (Figure 1C,D) and FG (Figure 1A,B).
FMRFamide-Related Peptides
The precursor sequences for both species are currently complete, including the signal peptide of T. molitor, and mature FMRF neuropeptides were mostly detected in the neurohemal areas of the thoracic ganglia. FMRF-1 and -4 in Z. atratus were the only ones detected with an N-terminal extended form, which is the result of less efficient cleavage at the Arg residues. Among the six mature neuropeptides, the relative signal intensities were similar across different preparations, suggesting that potentially all six FMRF paracopies are equally expressed.
Myoinhibitory Peptide
Mature products of the mip gene were detected across the whole CNS, except ventral neurohemal areas, with MIP-5 being the peptide with the highest signal intensity in the MALDI-TOF spectra of both species. Immunohistochemical examination using antibodies against Drome-MIP also detects MIP immunoreactive neurons in the brain, along the ventral nerve cord and in the RCC of T. molitor.79
Myosuppressin
The sequence of the mature myosuppressin neuropeptide is one of the most conserved across insects, while the rest of the precursors (signal peptide and precursor peptide) are more variable, as shown in Polyneoptera insects.85 In tenebrionids, there is an additional cleavage site (Arg–Arg) along the precursor, suggesting the presence of at least two PPs. Moreover, in the Z. atratus precursor, there is an insertion of eight amino acids in the second PP, absent in T. molitor and T. castaneum. Myosuppressin was mostly detected in its pyroglutamate-blocked N-terminus form in both species across different tissues (Figure 1) in the MALDI-TOF spectra. We also sequenced a pyroglutamate-blocked form truncated at the C-terminus (MS1−9[pQ]), with similar mass match to MIP-2, and an N-terminal truncated form without the pyroglutamate-blocked Gln (MS2−10), both of which are from TermG preparations of T. molitor (Table S1). The second PP, where the insertion of eight amino acids is located, was confirmed only in Z. atratus but surprisingly was cleaved at a single Arg residue (Table S2) and not at the Arg–Arg site, producing a shorter than expected PP (MS-PP24−18), abundant in preparations of the CC and FG (Figure 1; Table S2). The Arg–Arg cleavage site of tenebrionids is possibly not processed due to the presence of an aliphatic amino acid (Val) in the plus one position.86 Notwithstanding that we did not detect the homologous second PP in T. molitor, we could confirm a truncated PP cleaved at the single Arg residue (MS-PP1−42), suggesting that the same cleavage site was used in T. molitor (Table S1).
Pigment Dispersing Factor
The pdf gene was only recently described and confirmed by mass spectrometry in Coleoptera.8,18 In Carabus, the PDF was detected both in cerebral ganglia pars lateralis closer to the optic lobes and in the RCC.18 In tenebrionids, we could confirm the PDF only in cerebral ganglia preparations, but we could not detect any ion signal in the RCC.
Proctolin
In T. molitor, two genes encode for a single, not amidated peptide well conserved across insects, which is typically abundant in the SNS. In T. molitor preparations of both FG and TermG, we could confirm the proctolin peptide and the complete PP of proctolin 1, including a truncated form cleaved at unexpected residue (proctolin 1 – PP228−42) (Figure 1F, Table S1). The proctolin 2 allele 1 (PP2) was only confirmed by MS1, whereas using enzymatic digestion, we could also detect few peptides matching with fragments of the PP of both proctolin 2 alleles 1 and 2 (Supporting Information S1).
Periplaneta Neuropeptide-Like Precursor (=PaOGS36577)
This neuropeptide-like precursor was detected in FG, AL, RCC, and TermG preparations of both species (Figures 1 and 2), similar to those already reported in P. americana(68) and in C. nodus.69
Pyrokinin
The pk/pban gene of Z. atratus now includes the nearly complete signal peptide and tPK peptide (Table S2).18,22,46 tPK typically have the consensus sequence of MWFXPRLamide, with the characteristic constant presence of Trp at position 6 from the C-terminus followed by the pentapeptide FXPRL motif typical of PKs.70 In Z. atratus, the C-terminus consensus sequence of tPK is derived compared to other beetles including tenebrionids,8 with the Phe5 replaced by a Pro and the Arg2 replaced by a Lys. Using mass spectrometry, we could confirm the atypical tPK of Z. atratus and all processed T. molitor neuropeptides. Interestingly, the presence of both tPK was confirmed in Z. atratus CC, one encoded by the capa gene, having a typical C-terminus (...MWFGPRL-NH2), and one with the atypical C-terminus encoded by the pk/pban gene (...VWPSPKL-NH2). As these two tPK have relatively different C-terminus consensus sequences, it would be interesting to test if they also differ in ligand–receptor interactions.
Short Neuropeptide F
This was one of the most abundant peptides detected in our preparations, especially in the FG and TermG. Similarly to what was reported in Carabus,18 the internal Arg residue, which is used as an internal cleavage site in several insects,19,76,87 is not efficient in cleaving the mature peptide.
Protein Hormones
Large proteins are rarely detected in MALDI-TOF direct tissue profiling because they are outside of the mass range used to analyze neuropeptides and neuropeptide-like hormones. In our MALDI-TOF spectra, we detected few short PPs, which suggest the presence of these large proteins mostly in preparations of the RCC, such as ILP, ITP, and PTTH (Tables S1 and S2; Figure 3). ILP peptides had already been reported in other insects,18,88 whereas we detected an ITP precursor peptide (Figure 2B) matching with a predicted alternative cleavage site of the signal peptide; in the case of PTTH, we detected a peptide (Figure 2B) cleaved at two Lys–Lys cleavage sites.
Figure 3.
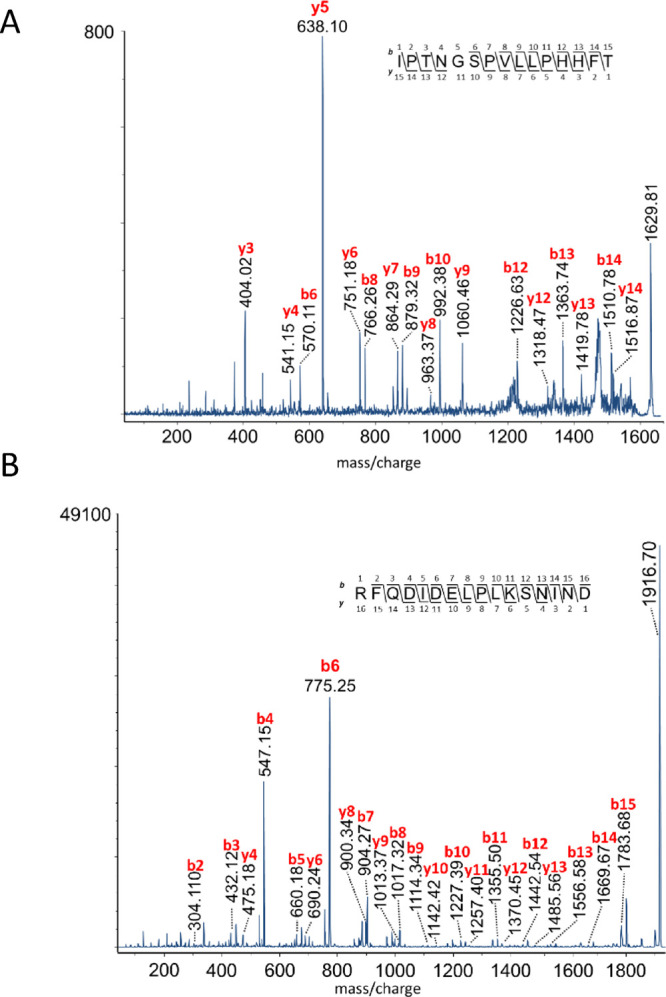
MALDI-TOF MS2 spectrum of (A) T. molitor ITP-PP1 and (B) Z. atratus PTTH-PP2, both from preparations of the RCC. Ion signals of b- and y-type fragment ions are labeled.
Conclusions
Using transcriptomic and mass spectrometry analyses of two tenebrionid species, we were able to obtain a comprehensive list of neuropeptide, neuropeptide-like, and protein hormones and their distributions across the CNS of T. molitor and Z. atratus. The present study is one of the most detailed neuropeptidomic surveys within Tenebrionidae and Polyphaga beetles. The neuropeptidomes of the two studied species are similar, and the information obtained about the distribution of neuropeptides in the CNS should be similar in closely related tenebrionid species commonly used in genetics, immunology, and developmental biology, such as T. castaneum. This study also provides the basis for functional studies of neuropeptides in these two model organisms for physiology. For instance, FMRF-6 is identical between these two species and has already been tested in both species showing myostimulatory activity of visceral muscles (i.e., heart, hindgut, and oviduct) in a dose-dependent manner.89 Similarly, myosuppressin was shown to modulate muscle contractility in different insects, including beetles,90−92 and to regulate digestive system function.93
During recent years, neuropeptide analogues of insect kinin, TKRP, PKs, and sulfakinins have been successfully tested as greener insecticides.30−35,94 Based on the current list of mass spectrometrically confirmed neuropeptides, it would be possible to design and test additional neuropeptide analogues targeting key physiological and behavioral mechanisms of selected species in order to control pests in cultivated fields and grain storage without affecting nontargeted beneficial species. At the same time, since T. molitor larvae have been accepted as novel food, increased knowledge on their genomes, genes, and neuropeptidomes could prove useful to optimize programs for the production of this species, as well as other tenebrionids, at the industrial scale.47,48
Acknowledgments
Special thanks to Reinhard Predel for supporting this study and for his comments on the first draft of this paper and to Heinrich Dircksen (Stockholm University, Dept. Zoology) for precious comments. We would also like to thank two anonymous reviewers for their constructive comments. We thank Stefan Müller, Astrid Wilbrand-Hennes, Jan Krueger, and Ursula Cullman (CECAD Cologne Proteomics Facility) for the Orbitrap MS analyses and Tobias Schulz (Biocenter Cologne) for IT support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jproteome.1c00694.
Table S1. Mature neuropeptides and additional precursor peptides (PPs) of T. molitor identified by mass spectrometry. Table S2. Mature neuropeptides and additional precursor peptides (PPs) of Z. atratus identified by mass spectrometry. Supporting information S1: List of neuropeptide precursors, neuropeptide-like precursors and protein hormone precursors from T. molitor and Z. atratus. Figure S1: Splice forms in T. molitor. Figure S2: Orcokinin-like precursor alignments of T. castaneum, T. molitor and Z. atratus. Figure S3: Proctolin 2 alleles alignment of T. molitor. Figure S4: Splice forms in Z. atratus(95) (PDF)
This study was financially supported by the European Union’s Horizon 2020 Research and Innovation programme (grant number 634361 to Reinhard Predel). P.M. was supported by National Science Center Poland (project number NCN 2013/09/D/NZ3/00002).
The authors declare no competing financial interest.
Supplementary Material
References
- Grimmelikhuijzen C. J. P.; Leviev I. K.; Carstensen K. Peptides in the nervous systems of cnidarians: Structure, function, and biosynthesis. Int. Rev. Cytol. 1996, 167, 37–89. 10.1016/s0074-7696(08)61345-5. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I. Neurobiology of the Caenorhabditis elegans genome. Science 1998, 282, 2028–2033. 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- Nassel D. R. Functional roles of neuropeptides in the insect central nervous system. Naturwissenschaften 2000, 87, 439–449. 10.1007/s001140050756. [DOI] [PubMed] [Google Scholar]
- Schoofs L.; De Loof A.; Van Hiel M. B. Neuropeptides as Regulators of Behavior in Insects. Annu. Rev. Entomol. 2017, 62, 35–52. 10.1146/annurev-ento-031616-035500. [DOI] [PubMed] [Google Scholar]
- Takahashi T.; Takeda N. Insight into the Molecular and Functional Diversity of Cnidarian Neuropeptides. Int. J. Mol. Sci. 2015, 16, 2610–2625. 10.3390/ijms16022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassel D. R. Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Prog. Neurobiol. 2002, 68, 1–84. 10.1016/S0301-0082(02)00057-6. [DOI] [PubMed] [Google Scholar]
- Caers J.; Verlinden H.; Zels S.; Vandersmissen H. P.; Vuerinckx K.; Schoofs L. More than two decades of research on insect neuropeptide GPCRs: an overview. Front. Endocrinol. (Lausanne) 2012, 3, 151. 10.3389/fendo.2012.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra J. A. Coleoptera genome and transcriptome sequences reveal numerous differences in neuropeptide signaling between species. Peerj 2019, 7, e7144 10.7717/peerj.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassel D. R.; Homberg U. Neuropeptides in interneurons of the insect brain. Cell Tissue Res. 2006, 326, 1–24. 10.1007/s00441-006-0210-8. [DOI] [PubMed] [Google Scholar]
- Cazzamali G.; Torp M.; Hauser F.; Williamson M.; Grimmelikhuijzen C. J. P. The Drosophila gene CG9918 codes for a pyrokinin-1 receptor. Biochem. Biophys. Res. Commun. 2005, 335, 14–19. 10.1016/j.bbrc.2005.07.038. [DOI] [PubMed] [Google Scholar]
- Iversen A.; Cazzamali G.; Williamson M.; Hauser F.; Grimmelikhuijzen C. J. P. Molecular cloning and functional expression of a Drosophila receptor for the neuropeptides capa-1 and-2. Biochem. Biophys. Res. Commun. 2002, 299, 628–633. 10.1016/S0006-291X(02)02709-2. [DOI] [PubMed] [Google Scholar]
- Rosenkilde C.; Cazzamali G.; Williamson M.; Hauser F.; Sondergaard L.; DeLotto R.; Grimmelikhuijzen C. J. P. Molecular cloning, functional expression, and gene silencing of two Drosophila receptors for the Drosophila neuropeptide pyrokinin-2. Biochem. Biophys. Res. Commun. 2003, 309, 485–494. 10.1016/j.bbrc.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Lee J. E. Neuropeptidomics: Mass Spectrometry-Based Identification and Quantitation of Neuropeptides. Genomics Inform. 2016, 14, 12–19. 10.5808/GI.2016.14.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassel D. R.; Zandawala M. Recent advances in neuropeptide signaling in Drosophila, from genes to physiology and behavior. Prog. Neurobiol. 2019, 179, 101607 10.1016/j.pneurobio.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Clynen E.; Schoofs L. Peptidomic survey of the locust neuroendocrine system. Insect Biochem. Mol. Biol. 2009, 39, 491–507. 10.1016/j.ibmb.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Liessem S.; Ragionieri L.; Neupert S.; Buschges A.; Predel R. Transcriptomic and Neuropeptidomic Analysis of the Stick Insect, Carausius morosus. J. Proteome Res. 2018, 17, 2192–2204. 10.1021/acs.jproteome.8b00155. [DOI] [PubMed] [Google Scholar]
- Predel R.; Neupert S.; Derst C.; Reinhardt K.; Wegener C. Neuropeptidomics of the Bed Bug Cimex lectularius. J. Proteome Res. 2018, 17, 440–454. 10.1021/acs.jproteome.7b00630. [DOI] [PubMed] [Google Scholar]
- Ragionieri L.; Predel R. The neuropeptidome of Carabus (Coleoptera, Adephaga: Carabidae). Insect Biochem. Mol. Biol. 2020, 118, 103309 10.1016/j.ibmb.2019.103309. [DOI] [PubMed] [Google Scholar]
- Ragionieri L.; Verdonck R.; Verlinden H.; Marchal E.; Vanden Broeck J.; Predel R. Schistocerca neuropeptides - An update. J. Insect Physiol. 2022, 136, 104326 10.1016/j.jinsphys.2021.104326. [DOI] [PubMed] [Google Scholar]
- Yeoh J. G. C.; Pandit A. A.; Zandawala M.; Nassel D. R.; Davies S. A.; Dow J. A. T. DINeR: Database for Insect Neuropeptide Research. Insect Biochem. Mol. Biol. 2017, 86, 9–19. 10.1016/j.ibmb.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Pandit A. A.; Davies S. A.; Smagghe G.; Dow J. A. T. Evolutionary trends of neuropeptide signaling in beetles - A comparative analysis of Coleopteran transcriptomic and genomic data. Insect Biochem. Mol. Biol. 2019, 114, 103227 10.1016/j.ibmb.2019.103227. [DOI] [PubMed] [Google Scholar]
- Pandit A. A.; Ragionieri L.; Marley R.; Yeoh J. G. C.; Inward D. J. G.; Davies S. A.; Predel R.; Dow J. A. T. Coordinated RNA-Seq and peptidomics identify neuropeptides and G-protein coupled receptors (GPCRs) in the large pine weevil Hylobius abietis, a major forestry pest. Insect Biochem. Mol. Biol. 2018, 101, 94–107. 10.1016/j.ibmb.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Li B.; Predel R.; Neupert S.; Hauser F.; Tanaka Y.; Cazzamali G.; Williamson M.; Arakane Y.; Verleyen P.; Schoofs L.; Schachtner J.; Grimmelikhuijzen C. J.; Park Y. Genomics, transcriptomics, and peptidomics of neuropeptides and protein hormones in the red flour beetle Tribolium castaneum. Genome Res. 2008, 18, 113–122. 10.1101/gr.6714008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra J. A. Allatostatins C, double C and triple C, the result of a local gene triplication in an ancestral arthropod. Gen. Comp. Endocrinol. 2016, 230-231, 153–157. 10.1016/j.ygcen.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Adamski Z.; Bufo S. A.; Chowanski S.; Falabella P.; Lubawy J.; Marciniak P.; Pacholska-Bogalska J.; Salvia R.; Scrano L.; Slocinska M.; Spochacz M.; Szymczak M.; Urbanski A.; Walkowiak-Nowicka K.; Rosinski G. Beetles as Model Organisms in Physiological, Biomedical and Environmental Studies - A Review. Front. Physiol. 2019, 10, 319. 10.3389/fphys.2019.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. nEUROSTRESSPEP: Novel biocontrol agents for insect pests from neuroendocrinology. Int. Pest Control 2017, 59, 164–165. 10.3030/634361. [DOI] [Google Scholar]
- Gade G.; Goldsworthy G. J. Insect peptide hormones: a selective review of their physiology and potential application for pest control. Pest Manag. Sci. 2003, 59, 1063–1075. 10.1002/ps.755. [DOI] [PubMed] [Google Scholar]
- Scherkenbeck J.; Zdobinsky T. Insect neuropeptides: structures, chemical modifications and potential for insect control. Bioorg. Med. Chem. 2009, 17, 4071–4084. 10.1016/j.bmc.2008.12.061. [DOI] [PubMed] [Google Scholar]
- Smagghe G.; Mahdian K.; Zubrzak P.; Nachman R. J. Antifeedant activity and high mortality in the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidae) induced by biostable insect kinin analogs. Peptides 2010, 31, 498–505. 10.1016/j.peptides.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Qu Y.; Wu X.; Song D.; Ling Y.; Yang X. Design, synthesis and aphicidal activity of N-terminal modified insect kinin analogs. Peptides 2015, 68, 233–238. 10.1016/j.peptides.2014.07.028. [DOI] [PubMed] [Google Scholar]
- Nachman R. J.; Mahdian K.; Nassel D. R.; Isaac R. E.; Pryor N.; Smagghe G. Biostable multi-Aib analogs of tachykinin-related peptides demonstrate potent oral aphicidal activity in the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidae). Peptides 2011, 32, 587–594. 10.1016/j.peptides.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Nachman R. J.; Kaczmarek K.; Zabrocki J.; Denlinger D. L. Disruption of insect diapause using agonists and an antagonist of diapause hormone. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 16922–16926. 10.1073/pnas.1113863108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Nachman R. J.; Kaczmarek K.; Kierus K.; Zabrocki J.; Denlinger D. L. Development of neuropeptide analogs capable of traversing the integument: A case study using diapause hormone analogs in Helicoverpa zea. Insect Biochem. Mol. Biol. 2015, 67, 87–93. 10.1016/j.ibmb.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Yu N.; Benzi V.; Zotti M. J.; Staljanssens D.; Kaczmarek K.; Zabrocki J.; Nachman R. J.; Smagghe G. Analogs of sulfakinin-related peptides demonstrate reduction in food intake in the red flour beetle, Tribolium castaneum, while putative antagonists increase consumption. Peptides 2013, 41, 107–112. 10.1016/j.peptides.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Nachman R. J.Insect Gpcrs and Development of Mimetic Analogs of the Insect Kinin, Pyrokinin-Like, and Sulfakinin Neuropeptide Classes as Pest Management Tools. Advances in Invertebrate (NEURO) Endocrinology 2020, 325 −373.
- Joop G.; Vilcinskas A. Coevolution of parasitic fungi and insect hosts. Zoology 2016, 119, 350–358. 10.1016/j.zool.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Rylee J. C.; Sinlard D. J.; Doucette K.; Zentner G. E.; Zelhof A. C. Expanding the genetic toolkit of Tribolium castaneum. PLoS One 2018, 13, e0195977 10.1371/journal.pone.0195977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ott U.; Lynch J. A. Emerging developmental genetic model systems in holometabolous insects. Curr. Opin. Genet. Dev. 2016, 39, 116–128. 10.1016/j.gde.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Lubawy J.; Marciniak P.; Kuczer M.; Rosinski G. Myotropic activity of allatostatins in tenebrionid beetles. Neuropeptides 2018, 70, 26–36. 10.1016/j.npep.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Marciniak P.; Urbanski A.; Kudlewska M.; Szymczak M.; Rosinski G. Peptide hormones regulate the physiological functions of reproductive organs in Tenebrio molitor males. Peptides 2017, 98, 35–42. 10.1016/j.peptides.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Peng Y. C.; Wang K. X.; Fu W. X.; Sheng C. W.; Han Z. J. Biochemical Comparison of dsRNA Degrading Nucleases in Four Different Insects. Front. Physiol. 2018, 9, 624. 10.3389/fphys.2018.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventrella E.; Marciniak P.; Adamski Z.; Rosinski G.; Chowanski S.; Falabella P.; Scrano L.; Bufo S. A. Cardioactive properties of Solanaceae plant extracts and pure glycoalkaloids on Zophobas atratus. Insect Sci. 2015, 22, 251–262. 10.1111/1744-7917.12110. [DOI] [PubMed] [Google Scholar]
- Turck D.; Castenmiller J.; De Henauw S.; Hirsch-Ernst K. I.; Kearney J.; Maciuk A.; Mangelsdorf I.; McArdle H. J.; Naska A. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343 10.2903/j.efsa.2021.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. J.; Audsley N. Neuropeptides of the beetle, Tenebrio molitor identified using MALDI-TOF mass spectrometry and deduced sequences from the Tribolium castaneum genome. Peptides 2008, 29, 168–178. 10.1016/j.peptides.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Marciniak P.; Audsley N.; Kuczer M.; Rosinski G. Identification of myotropic neuropeptides from the brain and corpus cardiacum-corpus allatum complex of the beetle, Zophobas atratus. J. Insect Sci. 2010, 10, 156. 10.1673/031.010.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert S.; Marciniak P.; Kohler R.; Nachman R. J.; Suh C. P. C.; Predel R. Different processing of CAPA and pyrokinin precursors in the giant mealworm beetle Zophobas atratus (Tenebrionidae) and the boll weevil Anthonomus grandis grandis (Curculionidae). Gen. Comp. Endocrinol. 2018, 258, 53–59. 10.1016/j.ygcen.2017.08.026. [DOI] [PubMed] [Google Scholar]
- Eriksson T.; Andere A. A.; Kelstrup H.; Emery V. J.; Picard C. J. The yellow mealworm (Tenebrio molitor) genome: a resource for the emerging insects as food and feed industry. J. Insects Food Feed 2020, 6, 445–455. 10.3920/JIFF2019.0057. [DOI] [Google Scholar]
- Eleftheriou E.; Aury J.-M.; Vacherie B.; Istace B.; Belser C.; Noel B.; Moret Y.; Rigaud T.; Berro F.; Gasparian S.; Labadie-Bretheau K.; Lefebvre T.; Madoui M. A. Chromosome-scale assembly of the yellow mealworm genome. Open Res. Europe 2021, 1, 94. 10.12688/openreseurope.13987.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinski G.; Pilc L.; Obuchowicz L. Effect of Hydrocortisone on Growth and Development of Larvae Tenebrio-Molitor. J. Insect Physiol. 1978, 24, 97–99. 10.1016/0022-1910(78)90017-3. [DOI] [PubMed] [Google Scholar]
- Quennedey A.; Aribi N.; Everaerts C.; Delbecque J. P. Postembryonic Development of Zophobas-Atratus Fab (Coleoptera, Tenebrionidae) under Crowded or Isolated Conditions and Effects of Juvenile-Hormone Analog Applications. J. Insect Physiol. 1995, 41, 143–152. 10.1016/0022-1910(94)00091-T. [DOI] [Google Scholar]
- Ragionieri L.; Ozbagci B.; Neupert S.; Salts Y.; Davidovitch M.; Altstein M.; Predel R. Identification of mature peptides from pban and capa genes of the moths Heliothis peltigera and Spodoptera littoralis. Peptides 2017, 94, 1–9. 10.1016/j.peptides.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Veenstra J. A. Arthropod IGF, relaxin and gonadulin, putative orthologs of Drosophila insulin-like peptides 6, 7 and 8, likely originated from an ancient gene triplication. Peerj 2020, 8, e9534 10.7717/peerj.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C.; Coulouris G.; Avagyan V.; Ma N.; Papadopoulos J.; Bealer K.; Madden T. L. BLAST plus : architecture and applications. BMC Bioinformatics 2009, 10, 421. 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artimo P.; Jonnalagedda M.; Arnold K.; Baratin D.; Csardi G.; de Castro E.; Duvaud S.; Flegel V.; Fortier A.; Gasteiger E.; Grosdidier A.; Hernandez C.; Ioannidis V.; Kuznetsov D.; Liechti R.; Moretti S.; Mostaguir K.; Redaschi N.; Rossier G.; Xenarios I.; Stockinger H. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. 10.1093/nar/gks400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H. Predicting Secretory Proteins with SignalP. Methods Mol. Biol. 2017, 1611, 59–73. 10.1007/978-1-4939-7015-5_6. [DOI] [PubMed] [Google Scholar]
- Rubakhin S. S.; Sweedler J. V. Characterizing peptides in individual mammalian cells using mass spectrometry. Nat. Protoc. 2007, 2, 1987–1997. 10.1038/nprot.2007.277. [DOI] [PubMed] [Google Scholar]
- Rappsilber J.; Mann M.; Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007, 2, 1896–1906. 10.1038/nprot.2007.261. [DOI] [PubMed] [Google Scholar]
- Perez-Riverol Y.; Bai J.; Bandla C.; Garcia-Seisdedos D.; Hewapathirana S.; Kamatchinathan S.; Kundu D. J.; Prakash A.; Frericks-Zipper A.; Eisenacher M.; Walzer M.; Wang S.; Brazma A.; Vizcaino J. A. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. 10.1093/nar/gkab1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Xin L.; Shan B. Z.; Chen W. W.; Xie M. J.; Yuen D.; Zhang W. M.; Zhang Z. F.; Lajoie G. A.; Ma B. PEAKS DB: De Novo Sequencing Assisted Database Search for Sensitive and Accurate Peptide Identification. Mol. Cell Proteomics 2012, 11, M111.010587 10.1074/mcp.M111.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dircksen H.; Burdzik S.; Sauter A.; Keller R. Two orcokinins and the novel octapeptide orcomyotropin in the hindgut of the crayfish Orconectes limosus: Identified myostimulatory neuropeptides originating together in neurones of the terminal abdominal ganglion. J. Exp. Biol. 2000, 203, 2807–2818. 10.1242/jeb.203.18.2807. [DOI] [PubMed] [Google Scholar]
- Stangier J.; Hilbich C.; Burdzik S.; Keller R. Orcokinin - a Novel Myotropic Peptide from the Nervous-System of the Crayfish, Orconectes-Limosus. Peptides 1992, 13, 859–864. 10.1016/0196-9781(92)90041-Z. [DOI] [PubMed] [Google Scholar]
- Dircksen H.; Neupert S.; Predel R.; Verleyen P.; Huybrechts J.; Strauss J.; Hauser F.; Stafflinger E.; Schneider M.; Pauwels K.; Schoofs L.; Grimmelikhuijzen C. J. P. Genomics, Transcriptomics, and Peptidomics of Daphnia pulex Neuropeptides and Protein Hormones. J. Proteome Res. 2011, 10, 4478–4504. 10.1021/pr200284e. [DOI] [PubMed] [Google Scholar]
- Sterkel M.; Oliveira P. L.; Urlaub H.; Hernandez-Martinez S.; Rivera-Pomar R.; Ons S. OKB, a novel family of brain-gut neuropeptides from insects. Insect Biochem. Mol. Biol. 2012, 42, 466–473. 10.1016/j.ibmb.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Jiang H. B.; Kim H. G.; Park Y. Alternatively spliced orcokinin isoforms and their functions in Tribolium castaneum. Insect Biochem. Mol. Biol. 2015, 65, 1–9. 10.1016/j.ibmb.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenheer R. A.; Wiehart U. M.; Nicolson S. W.; Schoofs L.; Schegg K. M.; Hull J. J.; Schooley D. A. Isolation, identification and localization of a second beetle antidiuretic peptide. Peptides 2003, 24, 27–34. 10.1016/S0196-9781(02)00273-5. [DOI] [PubMed] [Google Scholar]
- Nassel D. R.; Wegener C. A comparative review of short and long neuropeptide F signaling in invertebrates: Any similarities to vertebrate neuropeptide Y signaling?. Peptides 2011, 32, 1335–1355. 10.1016/j.peptides.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Xie J.; Sang M.; Song X. W.; Zhang S. S.; Kim D.; Veenstra J. A.; Park Y.; Li B. A new neuropeptide insect parathyroid hormone iPTH in the red flour beetle Tribolium castaneum. PLoS Genet. 2020, 16, e1008772 10.1371/journal.pgen.1008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H. C.; Qin Y. R.; Du E. X.; Wei Q. L.; Li Y.; Huang D. Y.; Wang G. R.; Veenstra J. A.; Li S.; Li N. Genomics- and Peptidomics-Based Discovery of Conserved and Novel Neuropeptides in the American Cockroach. J. Proteome Res. 2021, 20, 1217–1228. 10.1021/acs.jproteome.0c00596. [DOI] [PubMed] [Google Scholar]
- Habenstein J.; Schmitt F.; Liessem S.; Ly A.; Trede D.; Wegener C.; Predel R.; Rossler W.; Neupert S. Transcriptomic, peptidomic, and mass spectrometry imaging analysis of the brain in the ant Cataglyphis nodus. J. Neurochem. 2021, 158, 391–412. 10.1111/jnc.15346. [DOI] [PubMed] [Google Scholar]
- Veenstra J. A. The contribution of the genomes of a termite and a locust to our understanding of insect neuropeptides and neurohormones. Front. Physiol. 2014, 5, 454. 10.3389/fphys.2014.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra J. A. The neuropeptide SMYamide, a SIFamide paralog, is expressed by salivary gland innervating neurons in the American cockroach and likely functions as a hormone. Peptides 2021, 136, 170466 10.1016/j.peptides.2020.170466. [DOI] [PubMed] [Google Scholar]
- Veenstra J. A.; Leyria J.; Orchard I.; Lange A. B. Identification of Gonadulin and Insulin-Like Growth Factor From Migratory Locusts and Their Importance in Reproduction in Locusta migratoria. Front. Endocrinol. (Lausanne) 2021, 12, 693068 10.3389/fendo.2021.693068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakane Y.; Li B.; Muthukrishnan S.; Beeman R. W.; Kramer K. J.; Park Y. Functional analysis of four neuropeptides, EH, ETH, CCAP and bursicon, and their receptors in adult ecdysis behavior of the red flour beetle, Tribolium castaneum. Mech. Dev. 2008, 125, 984–995. 10.1016/j.mod.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Ayali A. The insect frontal ganglion and stomatogastric pattern generator networks. Neurosignals 2004, 13, 20–36. 10.1159/000076156. [DOI] [PubMed] [Google Scholar]
- Shipley M.; Puche A.. Olfactory Glomeruli: Structure and Circuitry. In Encyclopedia of Neuroscience; Elsevier Ltd. 2009, 119 −127. [Google Scholar]
- Predel R.; Wegener C.; Russell W. K.; Tichy S. E.; Russell D. H.; Nachman R. J. Peptidomics of CNS-associated neurohemal systems of adult Drosophila melanogaster: A mass spectrometric survey of peptides from individual flies. J. Comp. Neurol. 2004, 474, 379–392. 10.1002/cne.20145. [DOI] [PubMed] [Google Scholar]
- Zoephel J.; Reiher W.; Rexer K. H.; Kahnt J.; Wegener C. Peptidomics of the Agriculturally Damaging Larval Stage of the Cabbage Root Fly Delia radicum (Diptera: Anthomyiidae). PLoS One 2012, 7, e41543 10.1371/journal.pone.0041543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. M.; Neupert S.; Predel R. Neuropeptidomics of the Australian sheep blowfly Lucilia cuprina (Wiedemann) and related Diptera. Peptides 2013, 41, 31–37. 10.1016/j.peptides.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Lubawy J.; Marciniak P.; Rosinski G. Identification, Localization in the Central Nervous System and Novel Myostimulatory Effect of Allatostatins in Tenebrio molitor Beetle. Int. J. Mol. Sci. 2020, 21, 3510. 10.3390/ijms21103510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predel R.; Wegener C. Biology of the CAPA peptides in insects. Cell. Mol. Life Sci. 2006, 63, 2477–2490. 10.1007/s00018-006-6187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert S.; Derst C.; Sturm S.; Predel R. Identification of two capa cDNA transcripts and detailed peptidomic characterization of their peptide products in Periplaneta americana. EuPA Open Proteom. 2014, 3, 195–205. 10.1016/j.euprot.2014.02.005. [DOI] [Google Scholar]
- Neupert S.; Huetteroth W.; Schachtner J.; Predel R. Conservation of the function counts: homologous neurons express sequence-related neuropeptides that originate from different genes. J. Neurochem. 2009, 111, 757–765. 10.1111/j.1471-4159.2009.06361.x. [DOI] [PubMed] [Google Scholar]
- Neupert S.; Russell W. K.; Russell D. H.; Predel R. Two capa-genes are expressed in the neuroendocrine system of Rhodnius prolixus. Peptides 2010, 31, 408–411. 10.1016/j.peptides.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Wegener C.; Reinl T.; Jansch L.; Predel R. Direct mass spectrometric peptide profiling and fragmentation of larval peptide hormone release sites in Drosophila melanogaster reveals tagma-specific peptide expression and differential processing. J. Neurochem. 2006, 96, 1362–1374. 10.1111/j.1471-4159.2005.03634.x. [DOI] [PubMed] [Google Scholar]
- Blaser M.; Predel R. Evolution of Neuropeptide Precursors in Polyneoptera (Insecta). Front. Endocrinol. (Lausanne) 2020, 11, 197. 10.3389/fendo.2020.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra J. A. Mono- and dibasic proteolytic cleavage sites in insect neuroendocrine peptide precursors. Arch. Insect Biochem. Physiol. 2000, 43, 49–63. . [DOI] [PubMed] [Google Scholar]
- Neupert S.; Fusca D.; Schachtner J.; Kloppenburg P.; Predel R. Toward a single-cell-based analysis of neuropeptide expression in Periplaneta americana antennal lobe neurons. J. Comp. Neurol. 2012, 520, 694–716. 10.1002/cne.22745. [DOI] [PubMed] [Google Scholar]
- Sturm S.; Ramesh D.; Brockmann A.; Neupert S.; Predel R. Agatoxin-like peptides in the neuroendocrine system of the honey bee and other insects. J. Proteomics 2016, 132, 77–84. 10.1016/j.jprot.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Marciniak P.; Witek W.; Szymczak M.; Pacholska-Bogalska J.; Chowanski S.; Kuczer M.; Rosinski G. FMRFamide-Related Peptides Signaling Is Involved in the Regulation of Muscle Contractions in Two Tenebrionid Beetles. Front. Physiol. 2020, 11, 456. 10.3389/fphys.2020.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski A.; Lubawy J.; Marciniak P.; Rosinski G. Myotropic activity and immunolocalization of selected neuropeptides of the burying beetle Nicrophorus vespilloides (Coleoptera: Silphidae). Insect Sci. 2019, 26, 656–670. 10.1111/1744-7917.12569. [DOI] [PubMed] [Google Scholar]
- Marciniak P.; Kuczer M.; Rosinski G. New physiological activities of myosuppressin, sulfakinin and NVP-like peptide in Zophobas atratus beetle. J. Comp. Physiol. B 2011, 181, 721–730. 10.1007/s00360-011-0563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson M.; McCormick J.; Mispelon M.; Paisley K.; Nichols R. Structure-activity and immunochemical data provide evidence of developmental- and tissue-specific myosuppressin signaling. Peptides 2012, 36, 272–279. 10.1016/j.peptides.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. J.; Seike H.; Nagata S. Function of myosuppressin in regulating digestive function in the two-spotted cricket, Gryllus bimaculatus. Gen. Comp. Endocrinol. 2019, 280, 185–191. 10.1016/j.ygcen.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Gui S. H.; Taning C. N.; De Schutter K.; Yang Q.; Chen P.; Hamshou M.; Nachman R. J.; Pandit A. A.; Dow J. A.; Davies S.; Smagghe G. Assessment of insecticidal effects and selectivity of CAPA-PK peptide analogues against the peach-potato aphid and four beneficial insects following topical exposure. Pest Manag. Sci. 2020, 76, 3451–3458. 10.1002/ps.5971. [DOI] [PubMed] [Google Scholar]
- Katoh K.; Standley D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.