Structured Abstract
Objective:
To demonstrate the use of Ulipristal acetate (UPA), as a long-term medical treatment for uterine fibroids, does not increase the risk of endometrial hyperplasia.
Design:
Case Study
Setting:
National Institutes of Health, 2006-2012
Patients:
Patient with history of benign metastasizing leiomyoma undergoing medical treatment for five years
Interventions:
Ulipristal acetate (UPA), a selective progesterone receptor modulator (SPRM), given to reduce uterine fibroid volume and growth.
Main Outcome Measures:
Endometrial biopsies were performed at established intervals to monitor for intraepithelial neoplasia or progesterone receptor modulator associated endometrial changes (PAEC). Two pathologists reviewed a hysterectomy specimen for evidence of endometrial pathology.
Results:
The patient tolerated UPA therapy and there was no evidence of endometrial hyperplasia or proliferative changes.
Conclusions:
Ulipristal acetate may be a potential option for long-term medical treatment of uterine fibroids in patients who are not surgical candidates.
Keywords: Ulipristal acetate, progesterone receptor, selective progesterone receptor modulator, SPRM, uterine fibroid, leiomyoma, endometrial hyperplasia
Capsule
The selective progesterone receptor modulator ulipristal acetate is a potential, long-term medical treatment for uterine fibroids. A patient demonstrated no adverse endometrial effects after prolonged treatment.
Introduction
Selective progesterone receptor modulators, SPRMs, have been investigated for the treatment of uterine leiomyomas for almost two decades with encouraging results. Initially mifepristone and asoprisnil both demonstrated a decrease in leiomyoma size and fibroid-associated symptoms after three to six months of therapy (1-5). Ulipristal acetate (UPA), the subject of this case report, is a SPRM that is currently approved in Europe for pre-surgical treatment of fibroid-related symptoms (Esmya®) and the United States as an emergency contraceptive (Ella-One®) (6). Studies of UPA in women with uterine leiomyomas demonstrate that it effectively reduces fibroid volume, induces amenorrhea and improves quality of life after three months of therapy (7, 8). In a double-blind non-inferiority trial, UPA and the gonadotropin releasing hormone analogue (GnRHa) leuprolide acetate, had similar efficacy in controlling uterine bleeding while UPA achieved amenorrhea on average two weeks faster (9). Another significant benefit of UPA therapy is the absence of hypo-estrogenic side effects associated with GnRHa. Patients treated with SPRMs usually do not exhibit hot flushes, and maintain mid-follicular estradiol levels. The latter is likely protective against adverse effects on bone mineral density (10).
Short-term (<6 months) therapy with SPRMs has proven to be an effective way to treat uterine leiomyoma without increasing the risk of developing endometrial hyperplasia or neoplasia (15-17) and studies of proliferation markers in patients exposed to SPRMs also have shown no evidence concerning for increased endometrial proliferation (18-20). However, an earlier concern with SPRMs was the development, in some women, of endometrial histology with an unclear prognosis (11). Retrospective studies demonstrated a small risk of developing endometrial hyperplasia after two to nine year treatments with mifepristone for management of meningioma (12, 13). A panel of experts, convened at the NIH, considered the endometrial histology after SPRM therapy to be a separate clinical entity from endometrial hyperplasia, that is specific to the compound class with its own individual characteristics. The consensus group termed these novel findings as “progesterone receptor modulator associated endometrial changes (PAEC)” (14).
Nevertheless, long-term utilization of SPRMs in the treatment of gynecologic conditions raises the question of endometrial safety. Currently, data are limited to interventional trials lasting less than six months (7-9, 15). In trials reporting on endometrial pathology after less than 6 months of UPA therapy, four out of twenty-one evaluated patients developed PAEC and none demonstrated atypical hyperplasia (7, 8). In studies comparing UPA to leuprolide and placebo, one patient developed simple endometrial hyperplasia after 13 weeks of UPA and one patient developed atypical hyperplasia after receiving placebo. However, approximately 60% of patients receiving UPA developed non-physiologic endometrial changes (9, 15). Evaluation of endometrial thickness demonstrated that the number of patients with a concerning endometrial thickness of > 16 mm was similar in patients taking UPA, leuprolide or placebo after 13 weeks of therapy (9, 15).
Here we present a case of a patient with benign metastasizing leiomyoma (BML) treated for UPA for over five years under a compassionate use exemption to protocol 06-CH-0090 Evaluation of Whether the Selective Progesterone Receptor Modulator CDB-2914 Can Shrink Leiomyomata (NCT number 00290251). The medical records of the patient undergoing medical management of BML at the National Institute of Health (NIH) from 2006-2012 were reviewed. We report the responses to therapy and effect on the endometrium as evaluated by endometrial biopsy and surgical pathology.
Case
The patient is a 41 year-old Gravida zero, Caucasian female diagnosed with BML at age 33 after presenting for an abdominal myomectomy during which incidental pelvic and omental masses were discovered. She subsequently developed dyspnea and was diagnosed with a pleural effusion. She was treated with leuprolide, with subsequent resolution of the pleural effusion and dyspnea. However, there was little improvement in the pelvic tumor burden. Two years later, she developed acute abdominal pain due to small bowel obstruction requiring exploratory laparotomy and a small bowel resection. The patient continued to have abdominal discomfort and postoperatively was started on ganirelix acetate. She had a minimal response in the pelvic tumor burden. At that time therapeutic resection of BML lesions was not considered an option secondary to concern for the tumor burden and the potential morbidity for surgery. Thus, her therapy was changed to UPA, 20 mg/day in September 2007.
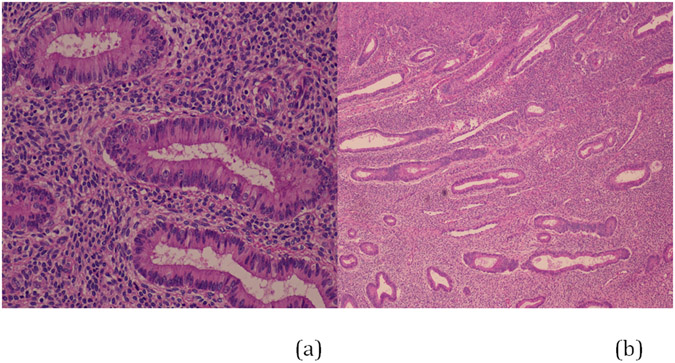
After 3 months of UPA therapy, the patient’s symptoms significantly improved. Radiographic imaging revealed enlargement of the dominant mass with marked degenerative changes. Endometrial evaluation at that time demonstrated superficial endometrial cells without evidence of hyperplasia or neoplasia. Repeat endometrial sampling after 6 months of therapy showed atrophic, superficial fragments without PAEC, hyperplasia or neoplasia. From the initiation of UPA therapy in 2007 to the discontinuation of therapy in 2012 before surgery, the rate of growth of the dominant mass was 2.4 cm a year, which is consistent with the rate of growth of benign leiomyoma (21). Although the rate of growth was consistent with a benign process and the patient was asymptomatic, in September of 2011 consultation with Gynecologic Oncology was obtained secondary to an increase in size of the dominant mass from 14 to 17 cm. The Gynecologic Oncology consultant recommended referral to Interventional Radiology, and embolization of the vasculature supplying her multiple tumors was performed. Because the size of the dominant mass did not decrease appreciably after embolization (and with continued UPA treatment), histologic assessment of the dominant mass was recommended. In 2012 the patient underwent exploratory laparotomy with total abdominal hysterectomy, bilateral salpingoophorectomy, rectosigmoid colon resection and tumor debulking by the Gynecologic Oncologic service. Visually complete tumor cytoreduction was achieved. UPA was discontinued after surgery. Her post-operative course was unremarkable. The patient has made a complete recovery and is currently asymptomatic. Endometrial pathology of the hysterectomy demonstrated atrophic endometrium measuring < 1 mm in thickness without polyps (Figure 1). There was no evidence of hyperplasia or proliferative changes.
Figure 1.
Hysterectomy specimen. Endometrium at 20X (a) and at low power (b) magnification after five years of therapy with ulipristal acetate.
The patient tolerated UPA therapy well and had no adverse effect of treatment. Her monitoring blood work demonstrated no evidence of hepatic, adrenal or renal dysfunction. She maintained follicular phase estradiol levels during therapy with a minimum of 43.3 pg/ml. Endometrial surveillance was performed at approximately six months intervals for a total of 11 procedures, with some being repeated for insufficient tissue obtained. None of the samples demonstrated PAEC, hyperplasia or evidence of endometrial intraepithelial neoplasia.
Discussion
There is no definitive medical therapy for uterine fibroids. GnRHa decrease leiomyoma size, but are often associated with significant symptoms and complications such as osteoporosis that preclude prolonged therapy. Currently, the only FDA approved medical therapy is leuprolide acetate use as a pre-operative adjunct to improve hematologic parameters before surgery and decrease operative blood loss (22). Recent randomized controlled trials in the U.S. and Europe demonstrated that UPA was non-inferior to leuprolide with none of the associated hypoestrogenic side effects that impact patient compliance (9, 15, 23). These findings have led to the approval of UPA (5mg Esmya®) in Europe for three months of preoperative use in patients with uterine leiomyoma-related symptoms.
This case report documents the longest continuous treatment with UPA to date. Despite concerns of a deleterious effect on the endometrium, none of the surveillance endometrial biopsies or the final surgical specimen demonstrated endometrial hyperplasia or PAEC in this patient. Furthermore, the hysterectomy specimen was consistent with the most common endometrial finding demonstrated in patients treated with UPA, which is an atrophic endometrium. It is notable that this patient received a daily dose of 20 mg, higher than the 5 mg approved for treatment of fibroids, lending further assurance regarding safety. However, despite promising results, long-term SPRM effects on the endometrium remain a theoretical concern and the natural history of PAEC remains unknown. This report provides limited evidence that long term UPA therapy, in this case five years of treatment, is not associated with endometrial hyperplasia or neoplasia.
References
- 1.Feng C, Meldrum S, Fiscella K. Improved quality of life is partly explained by fewer symptoms after treatment of fibroids with mifepristone. Int J Gynaecol Obstet 2010;109:121–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiscella K, Eisinger SH, Meldrum S, Feng C, Fisher SG, Guzick DS. Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: a randomized controlled trial. Obstet Gynecol 2006;108:1381–7. [DOI] [PubMed] [Google Scholar]
- 3.Engman M, Granberg S, Williams AR, Meng CX, Lalitkumar PG, Gemzell-Danielsson K. Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Hum Reprod 2009;24:1870–9. [DOI] [PubMed] [Google Scholar]
- 4.DeManno D, Elger W, Garg R, Lee R, Schneider B, Hess-Stumpp H et al. Asoprisnil (J867): a selective progesterone receptor modulator for gynecological therapy. Steroids 2003;68:1019–32. [DOI] [PubMed] [Google Scholar]
- 5.Chwalisz K, Larsen L, Mattia-Goldberg C, Edmonds A, Elger W, Winkel CA. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertil Steril 2007;87:1399–412. [DOI] [PubMed] [Google Scholar]
- 6.Jadav SP, Parmar DM. Ulipristal acetate, a progesterone receptor modulator for emergency contraception. J Pharmacol Pharmacother 2012;3:109–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levens ED, Potlog-Nahari C, Armstrong AY, Wesley R, Premkumar A, Blithe DL et al. CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstetrics and gynecology 2008;111:1129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieman LK, Blocker W, Nansel T, Mahoney S, Reynolds J, Blithe D et al. Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: a randomized, double-blind, placebo-controlled, phase IIb study. Fertility and sterility 2011;95:767–72 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnez J, Tomaszewski J, Vazquez F, Bouchard P, Lemieszczuk B, Baro F et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. The New England journal of medicine 2012;366:421–32. [DOI] [PubMed] [Google Scholar]
- 10.Kettel LM, Murphy AA, Morales AJ, Ulmann A, Baulieu EE, Yen SS. Treatment of endometriosis with the antiprogesterone mifepristone (RU486). Fertil Steril 1996;65:23–8. [DOI] [PubMed] [Google Scholar]
- 11.Chabbert-Buffet N, Pintiaux-Kairis A, Bouchard P. Effects of the progesterone receptor modulator VA2914 in a continuous low dose on the hypothalamic-pituitary-ovarian axis and endometrium in normal women: a prospective, randomized, placebo-controlled trial. J Clin Endocrinol Metab 2007;92:3582–9. [DOI] [PubMed] [Google Scholar]
- 12.Spitz IM, Grunberg SM, Chabbert-Buffet N, Lindenberg T, Gelber H, Sitruk-Ware R. Management of patients receiving long-term treatment with mifepristone. Fertil Steril 2005;84:1719–26. [DOI] [PubMed] [Google Scholar]
- 13.Grunberg SM, Weiss MH, Russell CA, Spitz IM, Ahmadi J, Sadun A et al. Long-term administration of mifepristone (RU486): clinical tolerance during extended treatment of meningioma. Cancer investigation 2006;24:727–33. [DOI] [PubMed] [Google Scholar]
- 14.Mutter GL, Bergeron C, Deligdisch L, Ferenczy A, Glant M, Merino M et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2008;21:591–8. [DOI] [PubMed] [Google Scholar]
- 15.Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. The New England journal of medicine 2012;366:409–20. [DOI] [PubMed] [Google Scholar]
- 16.Eisinger SH, Bonfiglio T, Fiscella K, Meldrum S, Guzick DS. Twelve-month safety and efficacy of low-dose mifepristone for uterine myomas. J Minim Invasive Gynecol 2005;12:227–33. [DOI] [PubMed] [Google Scholar]
- 17.Ioffe OB, Zaino RJ, Mutter GL. Endometrial changes from short-term therapy with CDB-4124, a selective progesterone receptor modulator. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2009;22:450–9. [DOI] [PubMed] [Google Scholar]
- 18.Williams AR, Critchley HO, Osei J, Ingamells S, Cameron IT, Han C et al. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Hum Reprod 2007;22:1696–704. [DOI] [PubMed] [Google Scholar]
- 19.Heikinheimo O, Vani S, Carpen O, Tapper A, Harkki P, Rutanen EM et al. Intrauterine release of progesterone antagonist ZK230211 is feasible and results in novel endometrial effects: a pilot study. Hum Reprod 2007;22:2515–22. [DOI] [PubMed] [Google Scholar]
- 20.Williams AR, Bergeron C, Barlo DH, Ferenczy A. Endometrial Morphology After Treatment of Uterine Fibroids with the Selective Progesterone Receptor Modulator, Ulipristal Acetate. Int J Gynecol Pathol 2012. [Epub] [DOI] [PubMed] [Google Scholar]
- 21.Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL et al. Growth of uterine leiomyomata among premenopausal black and white women. Proceedings of the National Academy of Sciences of the United States of America 2008;105:19887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy G, Hill MJ, Beall S, Zarek SM, Segars JH, Catherino WH. Leiomyoma: genetics, assisted reproduction, pregnancy and therapeutic advances. J Assist Reprod Genet 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croxtall JD. Ulipristal acetate: in uterine fibroids. Drugs 2012;72:1075–85. [DOI] [PubMed] [Google Scholar]