Abstract
Background
Women treated for breast cancer (BC) often suffer genitourinary syndrome of menopause. These symptoms may be alleviated by vaginal estrogen therapy (VET) or menopausal hormone therapy (MHT). However, there are concerns of risks of recurrence of BC and death following treatment.
Methods
Our study included longitudinal data from a national cohort of postmenopausal women, diagnosed 1997-2004 with early-stage invasive estrogen receptor–positive nonmetastatic BC, who received no treatment or 5 years of adjuvant endocrine therapy. We ascertained prescription data on hormone therapy, VET or MHT, from a national prescription registry. We evaluated mortality and risk of recurrence associated with use of VET and MHT vs non-use using multivariable models adjusted for potential confounders.
Results
Among 8461 women who had not received VET or MHT before BC diagnosis, 1957 and 133 used VET and MHT, respectively, after diagnosis. Median follow-up was 9.8 years for recurrence and 15.2 years for mortality. The adjusted relative risk of recurrence was 1.08 (95% confidence interval [CI] = 0.89 to 1.32) for VET (1.39 [95% CI = 1.04 to 1.85 in the subgroup receiving adjuvant aromatase inhibitors]) and 1.05 (95% CI = 0.62 to 1.78) for MHT. The adjusted hazard ratios for overall mortality were 0.78 (95% CI = 0.71 to 0.87) and 0.94 (95% CI = 0.70 to 1.26) for VET and MHT, respectively.
Conclusions
In postmenopausal women treated for early-stage estrogen receptor–positive BC, neither VET nor MHT was associated with increased risk of recurrence or mortality. A subgroup analysis revealed an increased risk of recurrence, but not mortality, in patients receiving VET with adjuvant aromatase inhibitors.
Breast cancer (BC) survivors often experience symptoms of declining estrogen levels. Adjuvant endocrine therapy, in particular aromatase inhibitors (AIs), may aggravate these symptoms (1,2). The urogenital system is particularly sensitive to estrogen deprivation, and genitourinary syndrome of menopause (GSM) often develops, including vaginal dryness, itchiness, burning, overactive bladder, and urinary incontinence (3). The American Endocrine Society concluded that level A evidence exists regarding the effectiveness of systemic or local hormone therapy in GSM (4). Vaginal estrogen therapy (VET) is well-tolerated and indicated for isolated GSM among healthy women (5). The use of menopausal hormone therapy (MHT) has been cautioned in BC survivors following demonstration of an increased risk of recurrence in the HABITS (Hormonal Replacement After Breast Cancer – is it Safe?) trial (6) and in the Livial Intervention following Breast cancer: Efficacy, Recurrence, And Tolerability Endpoints trial (7), though this association was not reproduced in the results published from the Stockholm trial (8). A small cohort study and a nested case-control study including a total of 340 women with early BC investigated the risk of BC recurrence associated with the use of VET (9,10). Neither showed an increased risk. However, the studies had several limitations, including small sample size and short follow-up. Therefore, it remains unclear whether VET or MHT is safe in women treated for BC (11,12).
Among women without a history of BC, a meta-analysis from the Collaborative Group on Hormonal Factors in Breast Cancer reported increased risk of primary BC among women treated with MHT compared with never-users, whereas VET was not associated with an increased risk of BC (13,14).
The objective of this observational cohort study was to determine the association of use of hormonal treatment (VET and MHT) with the risk of BC recurrence and mortality in a large population-based cohort of Danish postmenopausal women treated for early-stage estrogen receptor–positive (ER+) BC.
Methods
Setting
We conducted this study in Denmark, where all citizens have unfettered access to tax-funded health care at public hospitals. A unique civil personal registration number is assigned to all citizens at birth or immigration, enabling individual-level data linkage across all registries, including the Danish Breast Cancer Group (DBCG) clinical database, the Danish National Prescription Database, the Danish National Patient Registry, and the Danish Civil Registration System.
Ethics
The study was approved by the Danish Data Protection Agency (2012–58-0018/2008–58-0035, 16/43717) and was conducted in accordance with the General Data Protection Regulation (GDPR). This registry-based observational study did not need approval from the region ethics committee according to Danish legislation.
Study Population
The study cohort included postmenopausal Danish women, aged 35-95 years, diagnosed with invasive early-stage nonmetastatic, ER+ BC from 1997 through 2004 registered in the DBCG clinical database and who did not receive chemotherapy (15). In accordance with national treatment guidelines during the study period, all patients were allocated either to 5 years of tamoxifen, an AI, or both treatments in sequence (women with tumor size >2 cm, node-positive disease, or malignancy grade 2-3) or to no endocrine treatment. The cohort has previously been described in detail (15,16) as well as the organization of the DBCG (17).
Data Collection and Follow-up
The clinical follow-up of each woman began on the date of primary BC surgery and continued until a first event (recurrence of BC, diagnosis of a secondary malignancy, or death) or a maximum of 10 years, which was mandatory follow-up.
Diagnostic information was entered prospectively by Danish treatment units into the DBCG database on all women with early-stage BC at surgery. Subsequently, detailed clinical information concerning definitive surgery, radiotherapy, systemic treatment, and follow-up was consecutively registered in the database. From the DBCG database, we ascertained the following data for each patient: age, date and type of surgery, pathoanatomic features, adjuvant therapy (radiation therapy and duration and type of endocrine therapy), recurrence, second malignancy, and death, if any. A patient was classified as adherent following continuation of endocrine therapy (tamoxifen or AI) for at least 4.5 years in the absence of a BC event, or otherwise as nonadherent (15). Regarding adjuvant treatment, women who received both an AI and tamoxifen were categorized as AI users from the date of initiation of AI treatment.
For a complete follow-up on vital status, DBCG data were linked with the Danish Civil Registration System, thus recording death from any cause. Overall survival (OS) in the study cohort was determined from surgery until the date of death, emigration, or December 31, 2016, whichever came first. Each patient’s medical history registered in the Danish National Patient Registry for a period of 10 years before her BC diagnosis was summarized via the Charlson Comorbidity Index (CCI) (18).
VET and MHT Treatment
Since 1995, the Danish National Prescription Database has recorded all prescriptions dispensed at Danish pharmacies, including the medication dispensed (classified by the Anatomical Therapeutic Chemical Classification system), dosage, number of packages, defined daily dose, and route of administration (19) (see Supplementary Material for Anatomical Therapeutic Chemical Classification codes).
Each woman was categorized as VET, MHT, or never-user according to hormone therapy prescription. Users of both VET and MHT were considered MHT users; thus, MHT users used either MHT solely or MHT and VET. Users were defined as individuals who redeemed at least 2 prescriptions after the diagnosis of BC. In a sensitivity analysis, we defined users as women who redeemed 1 or more prescriptions. Use of VET or MHT was classified as a time-varying dichotomous variable updated daily during follow-up and lagged by 1 year.
Patients with records of prediagnostic use of VET or MHT were excluded from the main analysis. Registered prediagnostic use was based on prescriptions preceding BC diagnosis for up to 2 years before diagnosis.
Statistical Analysis
We examined the frequency and proportion of BC patients according to use of VET and MHT after the BC diagnosis.
Follow-up time was quantified in terms of a Kaplan-Meier estimate of potential follow-up. For recurrence and competing risk analyses, the Fine and Gray proportional subdistribution hazards model and cumulative incidences were used (20). The cumulative incidence of recurrence was determined as the interval from surgery to any first event of local or regional invasive recurrence or distant recurrence, whereas other first events were considered competing risk events. The Cox regression model was used to assess OS (15).
Poisson regression was used to compute the standardized mortality ratio (SMR) and associated 95% confidence intervals (CIs) based on the assumption that the observed number of deaths followed a Poisson distribution (21). SMR were computed as the ratio of the observed to the expected number of deaths and served as an estimate of relative risk of death. The number of deaths expected was calculated by applying age- and calendar year–specific female mortality figures of the general Danish population and the corresponding person-years of follow-up for the respective cohort members.
Crude and adjusted hazard ratios (HRs) and relative risk estimates together with associated 95% confidence intervals according to exposure to VET and MHT were estimated. Use of VET and MHT were included simultaneously in the models, with the reference group being patients without prescriptions. The multivariable analyses included the following covariates: age at surgery, tumor size, nodal status, histological type and grade, ER, progesterone receptor, lymphovascular invasion, loco-regional therapy, CCI, and, as time-dependent variables, use of tamoxifen, use of AIs, and noncompliance for endocrine therapy (22). To comply with the proportional hazards’ assumption, ER status and lymphovascular invasion at surgery were included with a time-dependent component (15).
The Wald test was used to evaluate interaction with type of concurrent adjuvant endocrine therapy. Statistical analysis was conducted using SAS v 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Study Population
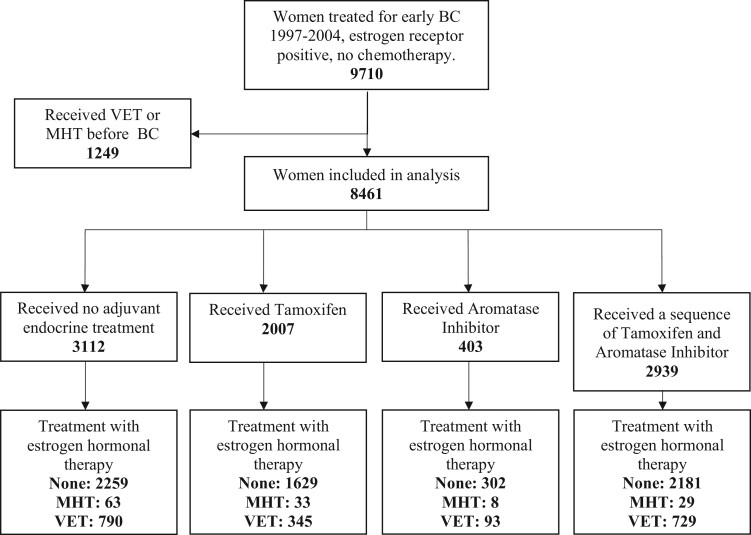
Between January 1997 and December 2004, 9710 postmenopausal women underwent a complete resection for incident invasive ER+ BC and were registered in the clinical database of the DBCG. All patients were allocated to either 5 years of endocrine treatment or no adjuvant systemic treatment according to treatment guidelines (Figure 1). Overall, 1249 women (13%) were prescribed VET or MHT before BC diagnosis and were excluded from the main analysis. Among the remaining 8461 patients, 6371 were not prescribed any hormonal treatment, 1957 (23%) were prescribed VET, and 133 (2%) were prescribed either MHT solely or both MHT and VET (Table 1).
Figure 1.
Use of menopausal hormone therapy (MHT) or vaginal estrogen therapy (VET) in a cohort of Danish women treated for early-stage breast cancer (BC) according to adjuvant endocrine therapy. Use of MHT, VET, or no hormonal treatment (none) among patients with early-stage BC 1997-2004 according to use of adjuvant endocrine treatment.
Table 1.
Basic characteristics of women with early breast cancer 1997-2004 according to use of no hormonal treatment (none), MHT, or VET
| Characteristic of participants | All participants (N = 8461) | None, No. (%)(n = 6371) | MHTa, No. (%) (n = 133) | VET, No. (%) (n = 1957) |
|---|---|---|---|---|
| Age | ||||
| <65 y | 5505 | 4074 (64) | 104 (78) | 1327 (68) |
| ≥65 y | 2956 | 2297 (36) | 29 (22) | 630 (32) |
| Tumor size | ||||
| ≤2 cm | 5616 | 4098 (64) | 96 (72) | 1422 (73) |
| >2 cm | 2845 | 2273 (36) | 37 (28) | 535 (27) |
| Nodal status | ||||
| Node negative | 4856 | 3575 (56) | 87 (65) | 1194 (61) |
| 1-3 positive lymph nodes | 2378 | 1796 (28) | 32 (24) | 550 (28) |
| ≥4 positive lymph nodes | 1227 | 1000 (16) | 14 (11) | 213 (11) |
| Histological type | ||||
| Ductal | 6528 | 4966 (78) | 88 (66) | 1474 (75) |
| Lobular | 1299 | 959 (15) | 29 (22) | 311 (16) |
| Other or unknown | 634 | 446 (7) | 16 (12) | 172 (9) |
| Malignancy grade (Elston) | ||||
| 1 | 3317 | 2481 (39) | 54 (41) | 782 (40) |
| 2 | 2865 | 2174 (34) | 35 (26) | 656 (34) |
| 3 | 785 | 629 (10) | 6 (5) | 150 (8) |
| Missing/nonductal/lobular | 1494 | 1087 (17) | 38 (29) | 369 (19) |
| ER status | ||||
| 10%-89% positive | 2906 | 2130 (33) | 55 (41) | 721 (37) |
| 90%-99% positive | 2146 | 1638 (26) | 33 (25) | 475 (24) |
| 100% positive | 3124 | 2397 (38) | 37 (28) | 690 (35) |
| ≥10% positiveb | 285 | 206 (3) | 8 (6) | 71 (4) |
| PgR status | ||||
| Absent | 1203 | 883 (14) | 12 (9) | 308 (16) |
| Present | 3344 | 2502 (39) | 52 (39) | 790 (40) |
| Unknown | 3914 | 2986 (47) | 69 (52) | 859 (44) |
| Local treatment | ||||
| Mx/radiotherapy | 1567 | 1232 (19) | 16 (12) | 319 (16) |
| Mx/no radiotherapy | 3543 | 273 (42) | 65 (49) | 775 (40) |
| BCS/radiotherapy | 3351 | 2436 (38) | 52 (39) | 863 (44) |
| Adjuvant endocrine therapy | ||||
| None | 3112 | 2259 (35) | 63 (47) | 790 (40) |
| Tamoxifen | 2007 | 1629 (26) | 33 (25) | 345 (18) |
| AI | 403 | 302 (5) | 8 (6) | 93 (5) |
| Seq. of Tamoxifen and AI | 2939 | 2181 (34) | 29 (22) | 729 (37) |
| Charlson Comorbidity | ||||
| CCI 0 | 7064 | 5289 (83) | 112 (84) | 1663 (85) |
| CCI 1 | 919 | 696 (11) | 11 (8) | 212 (11) |
| CCI ≥2 | 478 | 386 (6) | 10 (8) | 82 (4) |
Included all patients using MHT, whether solely or with VET. AI = aromatase inhibitor; BCS = breast conserving surgery; CCI = Charlson Comorbidity Index; ER = estrogen receptor; MHT = menopausal hormone therapy; Mx = mastectomy; PgR = progesterone receptor; VET = vaginal estrogen treatment.
In a subgroup of patients, the ER level was only reported as positive (10%-100%).
The median age of patients was 61 years (range = 35-95 years); 77% had an invasive ductal carcinoma and 57% were node negative. Non-users of hormonal treatment were older, had larger tumors, and were more likely to have lymph node metastasis. There was no difference in the CCI at the time of surgery among patients who later received VET or MHT compared with non-users of VET or MHT. Adherence to adjuvant endocrine therapy (recorded through the regular clinical follow-up) was 88% in users of VET and 90% in never-users.
Risk of Recurrence
During an estimated median of 9.8 years of potential follow-up, 1333 patients (16%) had a BC recurrence. A total of 111 patients who experienced a recurrence had received VET, 16 had received MHT, and 1206 did not receive either treatment. Women who received VET had an adjusted risk of recurrence similar to never-users (HR = 1.08, 95% CI = 0.89 to 1.32) (Table 2). After stratifying by adjuvant endocrine therapy, the use of VET initiated during adjuvant treatment among patients who received AI was associated with an elevated risk of recurrence (HR = 1.39, 95% CI = 1.04 to 1.85). For women receiving MHT, the adjusted relative risk of recurrence was 1.05 (95% CI = 0.62 to 1.78) compared with never-users of hormonal treatment. The absolute 10-year cumulative incidence of recurrence was 19.2% in never-users of VET or MHT, 15.4% in VET users, and 17.1% in users of MHT (Figure 2). A sensitivity analysis with users categorized to only 1 or more redeemed prescriptions did not change the risk estimates (data not shown).
Table 2.
Risk of recurrence among patients with early-stage breast cancer 1997-2004 according to use of MHT or VET compared with non-users (none)a
| Hormonal treatment | No. at risk | Unadjusted HR (95% CI) | Adjusted HR 95% CI | P heterogeneity |
|---|---|---|---|---|
| None | 8461 | Reference | Reference | |
| Menopausal hormone therapyb | 117 | 1.05 (0.64 to 1.74) | 1.05 (0.62 to 1.78) | |
| VET | 1222 | 0.99 (0.82 to 1.21) | 1.08 (0.89 to 1.32) | |
| Adjuvant treatment | ||||
| None | 662 | 1.02 (0.74 to 1.41) | 1.04 (0.75 to 1.46) | .03c |
| TAM | 305 | 0.55 (0.34 to 0.90) | 0.64 (0.39 to 1.06) | .01d |
| AI or AI and TAM in sequence | 443 | 1.28 (0.96 to 1.70) | 1.39 (1.04 to 1.85) |
Risk of recurrence among patients with early-stage breast cancer 1997-2004 according to use of MHT or VET in terms of subdistribution hazard ratios from the Fine Gray proportional subdistribution hazards model. AI = aromatase inhibitors; CCI = Charlson Comorbidity Index; CI = confidence interval; HR = hazard ratio; MHT = menopausal hormone therapy; TAM = tamoxifen; VET = vaginal estrogen treatment.
Including all patients using MHT, whether solely or with VET.
Adjusted for age at surgery, year of diagnosis, tumor size, nodal status, histologic type and grading, estrogen receptor, progesterone receptor, lymphovascular invasion, loco-regional therapy, CCI, and as time-dependent variables use of tamoxifen, AIs, and noncompliance for endocrine treatment. Number at risk does not add up due to patients shifting group according to treatment. Pinteraction = “none” vs TAM vs AI ± TAM.
TAM vs AI ± TAM.
Figure 2.
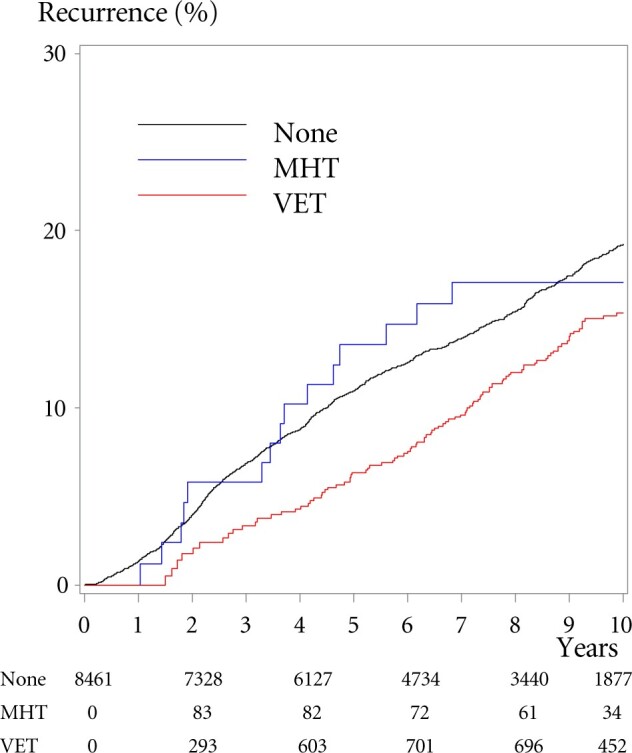
Cumulative incidence of recurrence of breast cancer according to use of menopausal hormone therapy (MHT) or vaginal estrogen therapy (VET). Cumulative incidence of breast cancer recurrence following treatment among patients with early-stage breast cancer 1997-2004 according to use of MHT, VET, or no hormonal treatment (none). Numbers indicate numbers at risk from baseline and after 2, 4, 6, 8, and 10 years; the numbers diminish as patients shift to another group, have an event, or are censored for other reason.
Mortality and SMR
Of the 8461 women in the study cohort, 3370 (40%) died before January 1, 2017 (estimated median follow-up = 15.2 years). A total of 497 of the patients who died in the follow-up period had received VET, 47 had received MHT, and 2826 did not receive either treatment.
The adjusted hazard ratio for OS for users of VET compared with never-users in the cohort was 0.78 (95% CI = 0.71 to 0.87) (Table 3). The analyses stratified by adjuvant endocrine therapy did not reflect an increased mortality according to the use of AIs (adjusted HR = 0.94, 95% CI = 0.70 to 1.26). For women prescribed MHT, the adjusted hazard ratio for OS compared with never-users was 0.94 (95% CI = 0.70 to 1.26). The SMR estimates correspond to the described OS. Never-users of VET or MHT had an absolute 10-year OS of 73.8% compared with 79.5% and 80.5% among the women who used VET or MHT, respectively (Figure 3).
Table 3.
Hazard ratios of OS and SMR according to use of MHT or VET in women treated for early breast cancer compared with non-users (none)a
| Hormonal treatment | No. at risk | OS |
SMR |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
||||||
| HR (95% CI) | P heterogeneity | HR (95% CI) | P heterogeneity | RR (95% CI) | P heterogeneity | RR (95% CI) | P heterogeneity | ||
| None | 8461 | 1 (Referent) | 1 (Referent) | 1 (Referent) | 1 (Referent) | ||||
| MHTb | 133 | 0.85 (0.63 to 1.14) | 0.94 (0.70 to 1.26) | 1.04 (0.78 to 1.40) | 0.93 (0.69 to 1.24) | ||||
| VET | 1971 | 0.79 (0.72 to 0.88) | 0.78 (0.71 to 0.87) | 0.81 (0.74 to 0.90) | 0.83 (0.75 to 0.91) | ||||
| Adjuvant treatment | |||||||||
| None | 1411 | 0.86 (0.77 to 0.97) | .19c | 0.80 (0.70 to 0.91) | .90c | 0.89 (0.79 to 1.10) | .14c | 0.88 (0.77 to 0.99) | .29c |
| TAM | 305 | 0.65 (0.50 to 0.86) | .23d | 0.76 (0.58 to 0.99) | .94d | 0.69 (0.53 to 0.90) | .48d | 0.78 (0.55 to 0.94) | .60d |
| AI or AI and TAM in sequence | 443 | 0.80 (0.66 to 0.97) | 0.94 (0.70 to 1.26) | 0.77 (0.64 to 0.94) | 0.78 (0.64 to 0.94) | ||||
AI = aromatase inhibitors; CCI = Charlson Comorbidity Index; CI = confidence interval; HR = hazard ratio; MHT = menopausal hormone therapy; OS = overall survival; RR = relative risk; TAM = tamoxifen; SMR = standardized mortality ratio; VET = vaginal estrogen treatment. OS and SMR in patients with early-stage breast cancer 1997–2004 according to use of MHT or VET in terms of hazard ratios for OS and relative risks of SMR.
Including all patients using MHT, whether solely or with VET.
Adjusted for age at surgery, year of diagnosis, tumor size, nodal status, histologic type and grading, estrogen receptor, progesterone receptor, lymphovascular invasion, loco-regional therapy, CCI, and as time-dependent variables use of tamoxifen, AIs, and noncompliance for endocrine treatment. Number at risk does not add up due to patients shifting group according to treatment. Pinteraction = “none” vs TAM vs AI ± TAM.
TAM vs AI ± TAM.
Figure 3.
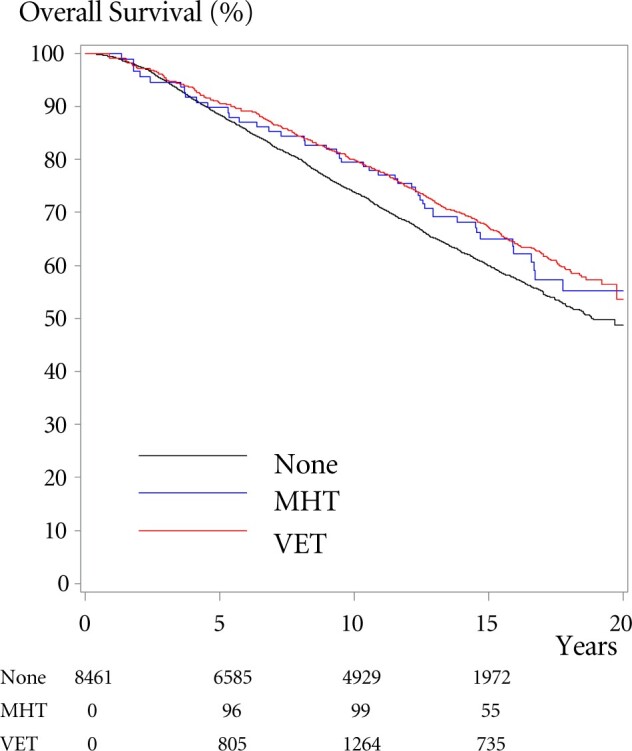
Overall survival (OS) according to use of menopausal hormone therapy (MHT) or vaginal estrogen therapy (VET). OS among patients with early-stage breast cancer 1997-2004 according to use of MHT, VET, or no hormonal treatment (none). Numbers indicate numbers at risk from baseline and after 5, 10, and 15 years, the numbers diminish as patients shift to another group, have an event, or are censored for other reason.
Discussion
Findings from this nationwide, prospective study of postmenopausal women treated for early-stage ER-positive BC suggested that the use of VET was associated with an increased risk of recurrence among postmenopausal BC patients treated with adjuvant AIs. There was no evidence of an increased risk of recurrence among patients treated with tamoxifen or those who did not receive adjuvant endocrine therapy. We did not observe an increased risk of recurrence in the MHT-treated patients compared with non-users. Neither VET nor MHT was associated with an increased overall mortality or increased SMR, irrespective of the receipt of AIs or tamoxifen.
Our study is, to our knowledge, the first to report a potential increased risk of recurrence in patients receiving AIs treated with VET. Few studies have previously investigated the risk of recurrence following VET in patients treated for early BC. In a cohort study, Dew et al. (9) reported that the use of VET was not associated with an increased risk of recurrence in the 69 treated patients compared with 1403 non-users, with a median follow-up of 5.5 years.
A nested case-control study by Le Ray et al. (10) reported no evidence of increased risk of recurrence among 271 patients treated with VET compared with patients in the follow-up period of 3.5 years. These estimates were not affected by the use of adjuvant tamoxifen.
Concern regarding the use of VET was emphasized in a study by Kendall et al. (23) that demonstrated elevated estrogen levels in the blood of BC patients treated with AIs who received VET. Although the elevated estrogen was temporary, the authors hypothesized that vaginal application of estrogens might pass through the epithelium and enter the blood stream. A meta-analysis by Pavlovic et al. (24), however, concluded that current evidence does not suggest that treatment with VETs is associated with considerable systemic absorption in postmenopausal women with a history of BC treated with AIs. The recently updated systematic review by Santen and colleagues (25) reported that estradiol absorption varies by dose, formulation, and placement in the vagina. Researchers of ongoing studies are measuring blood levels of estrogen in BC patients receiving adjuvant AIs and treated with VET (26). However, only 1 of these studies has BC recurrence as an endpoint.
In a recent systematic review concerning safety of VET, Crandall et al. (27) suggested that slightly elevated serum estrogen levels may be observed within the first 12 weeks following VET. AIs lower or nearly eliminate estrogen. As such, even a modest increase in circulating estrogens may have contributed to our observed increased risk of recurrence in the AI-treated subgroup of BC survivors who received VET. In contrast, tamoxifen competes with estrogen for binding the ER (28). A modest elevation of the very low serum estrogen levels in AI-treated women is not assumed to counteract the receptor blockade. The increased risk of recurrence in patients receiving AIs and VET in our study was, however, not accompanied by increased mortality. Therefore, we urge researchers to assess in future studies whether there is an increased mortality associated with VET use in BC survivors, particularly in patients treated with AIs.
The increasing use of adjuvant AIs raises 2 other important issues related to side effects: the impact on quality of life and the risk of noncompliance. The impact on sexual behavior is important; at least one-half of women on AIs report major problems, and up to 24% cease sexual activity (1). Regarding adherence to adjuvant endocrine therapy, discontinuation rates in the range of 15%-30% have been found (29). Early discontinuation and nonadherence are associated with increased mortality (30). The GSM problems may play an important role in this discontinuation, ultimately compromising the effectiveness of AIs and affecting survival. Nonetheless, we did not observe an increase in adherence to endocrine therapy among BC survivors who received VET vs no treatment.
In BC survivors, the HABITS randomized clinical trial reported an increased risk of recurrence, with a hazard ratio of 2.4 (95% CI = 1.3 to 4.2) in the MHT group compared with the non-MHT group, prompting the discontinuation of the trial (6). In the Stockholm trial—another RCT comparing MHT with no MHT—the risk of recurrence of BC at 10-year follow-up in the MHT group was 1.3 (95% CI = 0.9 to 1.9) and the relative risk of death was 1.1 (95% CI = 0.6 to 2.0) compared with the placebo group (8). An increased risk of recurrence with a hazard ratio of 1.64 (95% CI = 0.99 to 2.72) among users of tibolone (a synthetic steroid with estrogenic, androgenic, and progestogenic activity) was also reported in the Livial Intervention following Breast cancer: Efficacy, Recurrence, And Tolerability Endpoints study comparing tibolone with symptomatic treatment in BC patients (7). Our findings contrast somewhat with these studies, because we did not observe an increased risk of recurrence in BC survivors treated with MHT compared with non-users.
The risk of primary BC has been associated with the use of MHT in a meta-analysis by the Collaborative Group on Hormonal Factors in Breast Cancer from 2019 (13). The risk increased with increased length of use, and certain types of MHT (estrogen plus progestogen > progestogen > estrogen) were associated with an increased risk. The risk was said to persist for several years after termination of treatment. MHT has also been associated with increased risk of BC death, as observed in the Million Women Study (31). However, we did not observe an increased risk of mortality among the 133 MHT-treated patients in our study. This does not allow us to make any firm conclusion due to the small number of MHT-treated patients, the potential for selection bias due to unknown confounding factors, and the potential safety associated with use of MHT in BC survivors is still debateable (32).
The strengths of this study are the large nationwide cohort of patients uniformly treated according to national guidelines and the long-term follow-up. Further, the validity of exposure was presumably high because users of MHT or VET were classified based on at least 2 redeemed prescriptions, thus bypassing recall bias and increasing the probability that redeemed prescriptions reflected actual use.
The major limitation of the study is its nonrandomized nature. Though many factors are included in the adjusted analyses, our findings may be prone to residual confounding from differences in lifestyle factors, such as nutrition, physical activity, and adiposity. The treatment of GSM in BC survivors remains a challenge. Nonhormonal substances may relieve symptoms (33). If insufficient, VET may be considered after thorough discussion of the pros and cons. Because we did not observe increased risk of recurrence in VET-treated patients receiving tamoxifen, switching to tamoxifen after 2 to 3 years of an AI may be considered for women initiating VET.
In postmenopausal women treated for early-stage ER+ BC, use of VET or MHT was not associated with increased risk of recurrence or mortality. In patients treated with VET and adjuvant AIs, we observed an increased risk of recurrence but not mortality. This association was not observed among women who received tamoxifen or in those who did not receive adjuvant endocrine therapy. In the small subset receiving hormone replacement treatment, no increased risk of recurrence or mortality was observed. For early-stage BC patients receiving adjuvant AIs, vaginal estrogen therapy should be used with caution.
Funding
This work was supported by Breast Friends, a part of the Danish Cancer Society.
Notes
Role of the funder: The funder of the study had no role in study design, data collection, data analysis, data interpretation, writing of the manuscript, or the decision to submit the manuscript for publication.
Disclosures: SC has received support from Breast Friends for the present manuscript; MJ has received institutional grants from Samsung BIOEPIS, Nanostring Technologies and Oncology Venture and has received support for attending scientific meeting from Novartis; PC has received honoraria and support for attending scientific meeting from Roche Denmark; BE has received institutional grants from AstraZeneca, Pfizer, Eli Lilly, MSD, Roche, Novartis, Samsung BIOEPIS, Nanostring Technologies and Oncology Venture and has received support for attending scientific meeting from MSD; FC and DCF declared no conflicts of interest. All authors had full access to all the data in the study and approved to submit for publication.
Author contributions: Conceptualization: SC, MJ and BE. Data curation: BE and PC. Formal analysis: MJ and SC. Funding acquisition: SC. Methodology: SC, MJ, DCF, BE and FC. Project administration: SC and FC. Writing—original draft: SC and FC. Writing—review & editing: SC, MJ, DCF, PC, BE and FC.
Prior presentations: Preliminary analysis of parts of the included data has been presented at the European Breast Cancer Conference, Vienna 2015 (32).
Data Availability
The data supporting all the figures and tables in the published article are not publicly available because of restrictions. The Danish Data Protection Board approved the study, and data were accessed through a secure server at Statistics Denmark (https://www.dst.dk) and only Danish research environments are granted authorization. The authors do not have permission to share the data.
The lead author (SC) affirms that this manuscript is honest, accurate, and transparent account of the study being reported; that no important aspects of the study has been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supplementary Material
Contributor Information
Søren Cold, Department of Oncology, Odense University Hospital, Odense, Denmark.
Frederik Cold, Department of Oncology, Odense University Hospital, Odense, Denmark.
Maj-Britt Jensen, Danish Breast Cancer Group, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark.
Deirdre Cronin-Fenton, Department of Clinical Epidemiology, Department of Clinical Medicine, Aarhus University and Aarhus University Hospital, Aarhus, Denmark.
Peer Christiansen, Department of Plastic and Breast Surgery, Aarhus University Hospital, Aarhus, Denmark.
Bent Ejlertsen, Danish Breast Cancer Group, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark; Department of Oncology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark.
References
- 1. Schover LR, Baum GP, Fuson LA, et al. Sexual problems during the first 2 years of adjuvant treatment with aromatase inhibitors. J Sex Med. 2014;11(12):3102-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frechette D, Paquet L, Verma S, et al. The impact of endocrine therapy on sexual dysfunction in postmenopausal women with early stage breast cancer: encouraging results from a prospective study. Breast Cancer Res Treat. 2013;141(1):111-117. [DOI] [PubMed] [Google Scholar]
- 3. Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063-1068. [DOI] [PubMed] [Google Scholar]
- 4. Santen RJ, Allred DC, Ardoin SP, et al. ; Endocrine Society. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95(7 Suppl 1):s1-s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888-902. [DOI] [PubMed] [Google Scholar]
- 6. Holmberg L, Iversen OE, Rudenstam CM, et al. ; HABITS Study Group. Increased risk of recurrence after hormone replacement therapy in breast cancer survivors. J Natl Cancer Inst. 2008;100(7):475-482. [DOI] [PubMed] [Google Scholar]
- 7. Bundred NJ, Kenemans P, Yip CH, et al. Tibolone increases bone mineral density but also relapse in breast cancer survivors: LIBERATE trial bone substudy. Breast Cancer Res. 2012;14(1):R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fahlen M, Fornander T, Johansson H, et al. Hormone replacement therapy after breast cancer: 10 year follow up of the Stockholm randomised trial. Eur J Cancer. 2013;49(1):52-59. [DOI] [PubMed] [Google Scholar]
- 9. Dew JE, Wren BG, Eden JA. A cohort study of topical vaginal estrogen therapy in women previously treated for breast cancer. Climacteric. 2003;6(1):45-52. [PubMed] [Google Scholar]
- 10. Le Ray I, Dell’Aniello S, Bonnetain F, et al. Local estrogen therapy and risk of breast cancer recurrence among hormone-treated patients: a nested case-control study. Breast Cancer Res Treat. 2012;135(2):603-609. [DOI] [PubMed] [Google Scholar]
- 11. Shapiro M. What should guide our patient management of vulvovaginal atrophy? Climacteric. 2019;22(1):38-43. [DOI] [PubMed] [Google Scholar]
- 12. Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):e93-e96. [DOI] [PubMed] [Google Scholar]
- 13. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394(10204):1159-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. 2018;25(1):11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ejlertsen B, Jensen MB, Mouridsen HT; for the Danish Breast Cancer Cooperative Group. Excess mortality in postmenopausal high-risk women who only receive adjuvant endocrine therapy for estrogen receptor positive breast cancer. Acta Oncol. 2014;53(2):174-185. [DOI] [PubMed] [Google Scholar]
- 16. Christiansen P, Bjerre K, Ejlertsen B, et al. Danish Breast Cancer Cooperative Group. Mortality rates among early-stage hormone receptor-positive breast cancer patients: a population-based cohort study in Denmark. J Natl Cancer Inst. 2011;103(18):1363-1372. [DOI] [PubMed] [Google Scholar]
- 17. Christiansen P, Ejlertsen B, Jensen MB, et al. Danish Breast Cancer Cooperative Group. Clin Epidemiol. 2016;8:445-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 19. Lokkegaard E, Lidegaard O, Moller LN, et al. Hormone replacement therapy in Denmark, 1995-2004. Acta Obstet Gynecol Scand. 2007;86(11):1342-1351. [DOI] [PubMed] [Google Scholar]
- 20. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 21. Taylor P. Standardized mortality ratios. Int J Epidemiol. 2013;42(6):1882-1890. [DOI] [PubMed] [Google Scholar]
- 22. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403-410. [DOI] [PubMed] [Google Scholar]
- 23. Kendall A, Dowsett M, Folkerd E, et al. Caution: vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol. 2006;17(4):584-587. [DOI] [PubMed] [Google Scholar]
- 24. Pavlovic R, Jankovic SM, Milovanovic JR, et al. The safety of local hormonal treatment for vulvovaginal atrophy in women with estrogen receptor-positive breast cancer who are on adjuvant aromatase inhibitor therapy: meta-analysis. Clin Breast Cancer. 2019;19(6):e731-e740. doi:10.1016/j.clbc.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 25. Santen RJ, Mirkin S, Bernick B, et al. Systemic estradiol levels with low-dose vaginal estrogens. Menopause. 2020;27(3):361-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sulaica E, Han T, Wang W, et al. Vaginal estrogen products in hormone receptor-positive breast cancer patients on aromatase inhibitor therapy. Breast Cancer Res Treat. 2016;157(2):203-210. [DOI] [PubMed] [Google Scholar]
- 27. Crandall CJ, Diamant A, Santoro N. Safety of vaginal estrogens: a systematic review. Menopause. 2020;27(3):339-360. [DOI] [PubMed] [Google Scholar]
- 28. Riggs BL, Hartmann LC. Selective estrogen-receptor modulators -- mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618-629. [DOI] [PubMed] [Google Scholar]
- 29. Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chirgwin JH, Giobbie-Hurder A, Coates AS, et al. Treatment adherence and its impact on disease-free survival in the breast international group 1-98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol. 2016;34(21):2452-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beral V, Peto R, Pirie K, et al. Menopausal hormone therapy and 20-year breast cancer mortality. Lancet. 2019;394(10204):1139. [DOI] [PubMed] [Google Scholar]
- 32. Ugras SK, Layeequr Rahman R. Hormone replacement therapy after breast cancer: yes, no or maybe? Mol Cell Endocrinol. 2021;525:111180. [DOI] [PubMed] [Google Scholar]
- 33. Sussman TA, Kruse ML, Thacker HL, et al. Managing genitourinary syndrome of menopause in breast cancer survivors receiving endocrine therapy. J Oncol Pract. 2019;15(7):363-370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting all the figures and tables in the published article are not publicly available because of restrictions. The Danish Data Protection Board approved the study, and data were accessed through a secure server at Statistics Denmark (https://www.dst.dk) and only Danish research environments are granted authorization. The authors do not have permission to share the data.
The lead author (SC) affirms that this manuscript is honest, accurate, and transparent account of the study being reported; that no important aspects of the study has been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.