Abstract
Background
Lung cancer mortality is reduced via low-dose computed tomography screening and treatment of early-stage disease. Evidence-based smoking cessation treatment in the lung screening setting can further reduce mortality. We report the results of a cessation trial from the National Cancer Institute’s Smoking Cessation at Lung Examination collaboration.
Methods
Eligible patients (n = 818) aged 50-80 years were randomly assigned (May 2017-January 2021) to the intensive vs minimal arms (8 vs 3 phone sessions plus 8 vs 2 weeks of nicotine patches, respectively). Bio-verified (primary) and self-reported 7-day abstinence rates were assessed at 3, 6, and 12 months post random assignment. Logistic regression analyses evaluated the effects of study arm. All statistical tests were 2-sided.
Results
Participants reported 48.0 (SD = 17.2) pack-years, and 51.6% were not ready to quit in less than 30 days. Self-reported 3-month quit rates were statistically significantly higher in the intensive vs minimal arm (14.3% vs 7.9%; odds ratio [OR] = 2.00, 95% confidence interval [CI] = 1.26 to 3.18). Bio-verified abstinence was lower but with similar relative differences between arms (9.1% vs 3.9%; OR = 2.70, 95% CI = 1.44 to 5.08). Compared with the minimal arm, the intensive arm was more effective among those with greater nicotine dependence (OR = 3.47, 95% CI = 1.55 to 7.76), normal screening results (OR = 2.58, 95% CI = 1.32 to 5.03), high engagement in counseling (OR = 3.03, 95% CI = 1.50 to 6.14), and patch use (OR = 2.81, 95% CI = 1.39 to 5.68). Abstinence rates did not differ statistically significantly between arms at 6 months (OR = 1.2, 95% CI = 0.68 to 2.11) or 12 months (OR = 1.4, 95% CI = 0.82 to 2.42).
Conclusions
Delivering intensive telephone counseling and nicotine replacement with lung screening is an effective strategy to increase short-term smoking cessation. Methods to maintain short-term effects are needed. Even with modest quit rates, integrating cessation treatment into lung screening programs may have a large impact on tobacco-related mortality.
Lung cancer screening with computed tomography and treatment of early-stage disease can lower lung cancer mortality by 20%-24% (1-3). An estimated 14.5 million Americans are eligible for lung screening, and nearly one-half currently smoke cigarettes (3–5). To realize the maximum benefit of lung screening, individuals undergoing screening who smoke need to receive evidence-based smoking cessation treatment (6-8). As part of the National Cancer Institute’s Smoking Cessation at Lung Examination collaboration (9), the goal of the Georgetown Lung Screening, Tobacco, and Health (LSTH) trial was to conduct a scalable and cost-effective phone-based cessation intervention for future implementation in lung screening settings.
The LSTH trial built on previous work (10-13) and was guided by the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) Framework (14), a model developed to increase the reach and effectiveness of health promotion interventions. Proactive telephone counseling for smoking cessation treatment is well suited to the lung screening setting because its effectiveness has been demonstrated with older (50+) adults (15-18), those not ready to quit (19-22), and those not seeking treatment (21,23). It is also intensive enough to provide tailored support to assist individuals who are ready to quit (23–27). Further, as a remotely delivered intervention, telephone counseling can reach people during the teachable moment that may be provided by lung screening (28-32) as well as counteract the reduced motivation that can follow a normal screening result (33). The LSTH trial personalized tobacco-related health risks within an evidence-based cessation intervention and maximized generalizability with broad inclusion criteria, including the large proportion undergoing lung screening who were not ready to quit (22).
We hypothesized that an intensive intervention would yield improved cessation outcomes relative to a minimal intervention while maintaining the potential for widespread implementation. Moreover, we expected that an intensive (vs minimal) intervention would be superior among individuals who may have more difficulty quitting (ie, less ready to quit, higher nicotine dependence, a normal lung screening result). The results can guide the evaluation of the costs and population impact of these approaches for implementation at a nationwide scale (34–37).
Methods
Overview
The LSTH trial accrued participants in partnership with 8 lung screening sites located in geographically diverse community-based hospitals and academic medical centers (Table 1). Each site had a thoracic tumor board and provided diagnostic work-ups and treatment as needed. The study was approved by the Georgetown University Medical Center Oncology IRB (IRB of Record) and the Lahey Hospital and Medical Center IRB. Clinicaltrials.gov registration is NCT03200236 (38). Study enrollment (May 2017-January 2021) and the 3-, 6-, and 12-month follow-up outcomes are described here. The study design and methods were described previously (39).
Table 1.
Baseline characteristics
| Characteristicsa | Intensive arm No. (%) | Minimal arm No. (%) | Total No. (%) |
|---|---|---|---|
| Total No. | 409 | 409 | 818 |
| Demographic and clinical characteristics | |||
| Age | |||
| Mean (SD), y | 63.6 (5.87) | 63.7 (5.84) | 63.6 (5.86) |
| 50-54 y | 6 (1.5) | 2 (0.5) | 8 (1.0) |
| 55-59 y | 116 (28.4) | 111 (27.1) | 227 (27.8) |
| 60-69 y | 212 (51.8) | 219 (53.5) | 431 (52.7) |
| 70-80 y | 75 (18.3) | 77 (18.8) | 152 (18.6) |
| Sex | |||
| Female | 212 (51.8) | 218 (53.3) | 430 (52.6) |
| Male | 197 (48.2) | 191 (46.7) | 388 (47.4) |
| Race | |||
| African American | 34 (8.3) | 31 (7.6) | 65 (7.9) |
| Other (American Indian, Asian, not reported) | 11 (2.7) | 13 (3.2) | 24 (2.9) |
| White | 364 (89.0) | 365 (89.2) | 729 (89.1) |
| Ethnicity | |||
| Hispanic origin | 23 (5.6) | 28 (6.9) | 51 (6.2) |
| Non-Hispanic origin | 386 (94.4) | 380 (93.1) | 766 (93.8) |
| Language | |||
| English | 402 (98.3) | 404 (98.8) | 806 (98.5) |
| Spanish | 7 (1.7) | 5 (1.2) | 12 (1.5) |
| Marital status | |||
| Married/living as married | 207 (50.7) | 198 (48.6) | 405 (49.7) |
| Not married | 201 (49.3) | 209 (51.4) | 410 (50.3) |
| Missing | 1 | 2 | 3 |
| Education level | |||
| High school/GED or less | 143 (35.1) | 143 (35.2) | 286 (35.2) |
| Associate degree/vocational school | 165 (40.5) | 162 (39.9) | 327 (40.2) |
| Bachelor’s degree or more | 99 (24.3) | 101 (24.9) | 200 (24.6) |
| Missing | 2 | 3 | 5 |
| Health insurance | |||
| Private | 193 (53.6) | 201 (56.5) | 394 (55.0) |
| Public (Medicare, Medicaid) | 137 (38.1) | 117 (32.9) | 254 (35.5) |
| Combined (public and private) | 18 (5.0) | 23 (6.5) | 41 (5.7) |
| None | 12 (3.3) | 15 (4.2) | 27 (3.8) |
| Missing/refused | 49 | 53 | 102 |
| Tobacco-related comorbidities | |||
| Mean (SD) | 1.6 (1.02) | 1.6 (1.05) | 1.6 (1.03) |
| 0 | 62 (15.6) | 74 (18.4) | 136 (17.0) |
| 1 | 134 (33.8) | 119 (29.6) | 253 (31.7) |
| 2 | 105 (26.4) | 113 (28.1) | 218 (27.3) |
| 3+ | 96 (24.2) | 96 (23.9) | 192 (24.0) |
| Missing | 12 | 7 | 19 |
| First-degree relative with lung cancer | |||
| No/does not apply | 302 (77.6) | 303 (78.9) | 605 (78.3) |
| Yes | 87 (22.4) | 81 (21.1) | 168 (21.7) |
| Missing | 20 | 25 | 45 |
| Lung cancer screening-related variables | |||
| Screening result, no. (%) | |||
| Lung-RADS® 1 | 125 (30.6) | 116 (28.4) | 241 (29.5) |
| Lung-RADS® 2 | 245 (59.9) | 250 (61.1) | 495 (60.5) |
| Lung-RADS® 3 | 23 (5.6) | 24 (5.9) | 47 (5.7) |
| Lung-RADS® 4 | 16 (3.9) | 19 (4.6) | 35 (4.3) |
| Follow-up procedures recommended | |||
| No | 360 (88.0) | 358 (87.5) | 718 (87.8) |
| Yes | 49 (12.0) | 51 (12.5) | 100 (12.2) |
| Lung cancer screening site | |||
| Anne Arundel Medical Center (E. Maryland) | 11 (2.7) | 10 (2.4) | 21 (2.6) |
| Baptist Hospital of Miami (S. Florida) | 38 (9.3) | 38 (9.3) | 76 (9.3) |
| Georgetown Univ. Medical Center (DC) | 17 (4.2) | 16 (3.9) | 33 (4.0) |
| Hackensack Univ. Medical Center (New Jersey) | 45 (11.0) | 39 (9.5) | 84 (10.3) |
| Hartford Hospital (Connecticut) | 18 (4.4) | 19 (4.6) | 37 (4.5) |
| Lahey Hospital and Medical Center (Massachusetts) | 179 (43.8) | 185 (45.2) | 364 (44.5) |
| MedStar Shah Medical Group (S. Maryland) | 15 (3.7) | 15 (3.7) | 30 (3.7) |
| UnityPoint Health (W. Illinois) | 86 (21.0) | 87 (21.3) | 173 (21.1) |
| NCCN group | |||
| Group 1 (55-80 y, 30+ pack-y) | 391 (95.6) | 392 (95.8) | 783 (95.7) |
| Group 2 (50-80 y, 20+ pack-y + risk factor) | 18 (4.4) | 17 (4.2) | 35 (4.3) |
| Annual vs baseline LDCT screening | |||
| Annual | 233 (57.0) | 240 (58.7) | 473 (57.8) |
| Baseline | 176 (43.0) | 169 (41.3) | 345 (42.2) |
| Cigarette smoking-related characteristics | |||
| Pack-years | |||
| Mean (SD) | 48.2 (17.29) | 47.8 (17.05) | 48.0 (17.16) |
| 20-29 | 9 (2.2) | 8 (2.0) | 17 (2.1) |
| 30-39 | 107 (26.2) | 113 (27.6) | 220 (26.9) |
| 40-49 | 155 (37.9) | 157 (38.4) | 312 (38.1) |
| 50+ | 138 (33.7) | 131 (32.0) | 269 (32.9) |
| Cigarettes per d | |||
| Mean (SD) | 17.0 (9.50) | 16.9 (8.60) | 16.9 (9.06) |
| Median (range) | 15.0 (1–60) | 17.0 (1–45) | 16.0 (1–60) |
| Missing | 3 | 1 | 4 |
| Mean age started smoking cigarettes daily (SD), y | 17.0 (4.0) | 17.3 (4.2) | 17.1 (4.1) |
| Time to first cigarette, no. (%) | |||
| Within 5 min | 120 (29.7) | 126 (31.1) | 246 (30.4) |
| 6 to 30 min | 166 (41.1) | 170 (42.0) | 336 (41.5) |
| 31 to 60 min | 72 (17.8) | 54 (13.3) | 126 (15.6) |
| After 60 min | 46 (11.1) | 55 (13.6) | 101 (12.5) |
| Refused/missing | 5 | 4 | 9 |
| Fagerstrom test for nicotine dependencea | |||
| Mean (SD) | 4.4 (2.1) | 4.4 (2.1) | 4.4 (2.1) |
| Missing/refused | 36 | 43 | 79 |
| Lives with current smoker | |||
| No | 271 (66.4) | 286 (70.3) | 557 (68.3) |
| Yes | 137 (33.6) | 121 (29.7) | 258 (31.7) |
| Missing | 1 | 2 | 3 |
| Readiness to quit | |||
| Not considering quitting (1–5) | 131 (32.0) | 131 (32.0) | 262 (32.0) |
| Next 6 mo (6) | 78 (19.1) | 82 (20.0) | 160 (19.6) |
| Next 30 d (7–10) | 200 (48.9) | 196 (47.9) | 396 (48.4) |
| Motivation to quit (1 = low, 10 = high) | |||
| Mean (SD) | 6.7 (2.32) | 6.7 (2.25) | 6.7 (2.28) |
| Median | 7.0 | 7.0 | 7.0 |
| Missing | 4 | 4 | 8 |
| Confidence to Quit (1 = low, 10 = high) | |||
| Mean (SD) | 5.9 (2.51) | 5.8 (2.58) | 5.8 (2.54) |
| Median | 6.0 | 6.0 | 6.0 |
| Missing | 12 | 8 | 20 |
| 24-h quit attempt in past 7 d | |||
| No | 366 (89.9) | 363 (88.8) | 729 (89.3) |
| Yes | 41 (10.1) | 46 (11.2) | 87 (10.7) |
| Missing | 2 | 0 | 2 |
| Evidence-based treatment in past 7 days | |||
| No | 364 (89.0) | 355 (86.8) | 719 (87.9) |
| Yes | 45 (11.0) | 54 (13.2) | 99 (12.1) |
| Health and substance usec,d | |||
| Health Index Scale (0 = worst/100 = best) | |||
| Mean (SD) | 69.2 (20.29) | 70.7 (18.7) | 69.98 (19.53) |
| Missing/refused | 5 | 4 | 9 |
| Alcohol frequency (past year) | |||
| Never | 115 (28.4) | 106 (26.2) | 221 (27.3) |
| Monthly or less | 89 (22.0) | 104 (25.7) | 193 (23.8) |
| 2-4 times per mo | 66 (16.3) | 68 (16.8) | 134 (16.5) |
| 2-3 times per wk | 58 (14.3) | 61 (15.1) | 119 (14.7) |
| 4+ times per wk | 77 (19.0) | 66 (16.3) | 143 (17.7) |
| Refused/missing | 4 | 4 | 8 |
| Intervention engagement and satisfaction | |||
| Median days from lung scan to random assignment: median (range) | 13 (2–155) | 14 (2–79) | 13 (2–155) |
| Median days from random assignment to Call #1 (range) | 8 (1–62) | 9 (1–81) | 9 (1–81) |
| Counseling session engagement | |||
| Mean (SD) | 5.0 (3.04) | 1.9 (1.2) | 3.5 (2.8) |
| Median | 6.0 | 3.0 | 3.0 |
| None/low: intensive (0–5); minimal (0–2) | 184 (45.0) | 200 (48.9) | 384 (46.9) |
| High: intensive (6–8); minimal (3) | 225 (55.0) | 209 (51.1) | 434 (53.1) |
| NRT engagement, No. of wkd | |||
| Mean (SD) | 4.2 (3.06) | 1.5 (0.89) | 1.4 (1.3) |
| Median | 4 | 2 | 1 |
| Intensive: (2–8 wk); minimal: (2 wk) | 333 (81.4) | 299 (73.1) | 632 (77.3) |
| None: (0 wk) | 76 (18.6) | 110 (26.9) | 186 (22.7) |
| Participant satisfaction with counseling | |||
| Not at all satisfied | 15 (6.8) | 20 (12.3) | 35 (9.2) |
| A little satisfied | 15 (6.8) | 25 (15.4) | 40 (10.5) |
| Somewhat satisfied | 61 (27.9) | 43 (26.5) | 104 (27.3) |
| Very satisfied | 128 (58.4) | 74 (45.7) | 202 (53.0) |
| Participant satisfaction with NRT | |||
| Not at all satisfied | 2 (0.7) | 8 (2.9) | 10 (1.8) |
| A little satisfied | 10 (3.4) | 15 (5.5) | 25 (4.4) |
| Somewhat satisfied | 35 (11.9) | 64 (23.4) | 99 (17.5) |
| Very satisfied | 247 (84.0) | 186 (68.1) | 433 (76.4) |
The FTND total score was not used in the analyses because 10% were missing 1 or more items that make up the total score. Lung-RADS®= Lung Imaging Reporting and Data System; NCCN = National Comprehensive Cancer Network; LDCT = low-dose computed tomography; FTND = Fagerstrom test for nicotine dependence; NRT = nicotine replacement therapy.
Supplementary Table 5 (available online).
Supplementary Table 6 (available online).
A box of NRT contains 14 patches (2-week supply).
Study Participants
Inclusion was based on the National Comprehensive Cancer Network’s broad eligibility criteria for lung screening (40): 1) aged 50-80 years and 2) 20+ pack-year smoking history. Additional criteria included 3) enrolled before undergoing lung screening; 4) smoked cigarettes, cigarillos, or little cigars within the past 7 days; and 5) English- or Spanish-speaking. Exclusion criteria were history of lung cancer and hearing or cognitive impairment preventing study engagement. Previous lung screening, current cessation treatment, and readiness to quit were not exclusion criteria.
Study Procedures
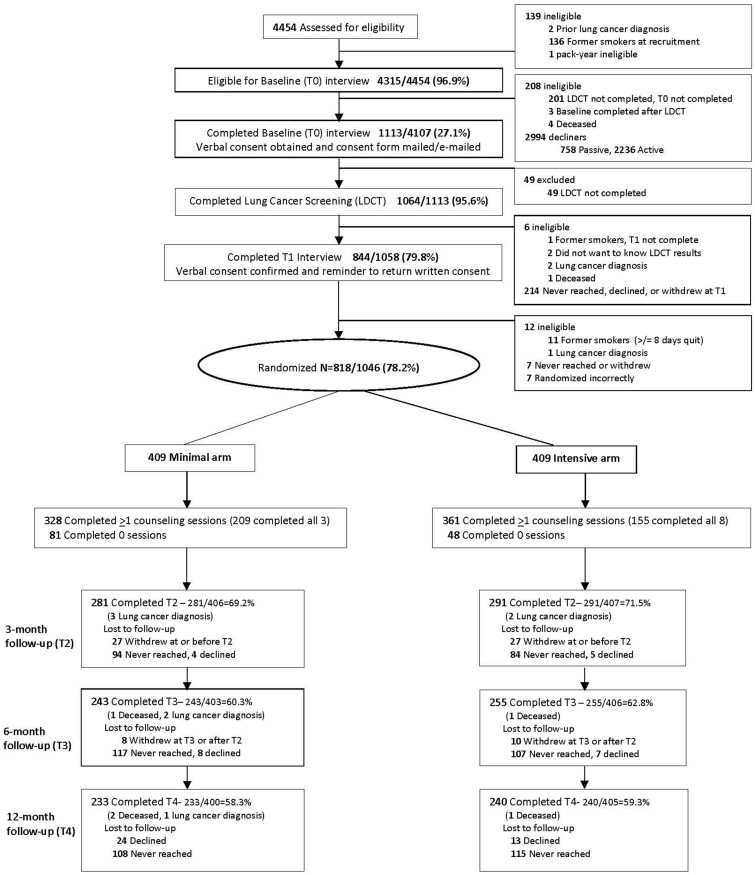
We conducted manualized staff training at each site to recruit and consent participants (Supplementary Methods, available online). We reviewed enrollment procedures monthly with each site. Site coordinators made up to 10 calls to eligible individuals with a scheduled lung screening exam (Figure 1) to assess eligibility, obtain verbal consent, and complete the 15-minute baseline (T0) assessment before their screening exam. Following enrollment, Georgetown staff mailed or e-mailed the consent and HIPAA forms for signature. Individuals who declined were approached for participation once more at their next annual screen. The denominator used to calculate reach was all trial-eligible individuals who underwent lung screening during the study enrollment period at each of the lung screening programs (Figure 1). Sites communicated the Lung-RADS® (Lung Imaging Reporting and Data System) results (41) via phone, letter, or in-person consultation. Referring providers contacted patients with results suspicious for lung cancer to discuss follow-up procedures. We offered the intervention (intensive arm) to those diagnosed with lung cancer (n = 10) but excluded them from the trial.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram. LDCT = Low-dose computed tomography.
Georgetown tobacco treatment specialists (TTSs) made up to 10 attempts to complete the 20-minute postscreening phone assessment (T1). Participants who had quit smoking for at least 8 days were excluded before random assignment and withdrawn from the trial (N = 11).
Following the postscreening assessment (T1), using a password-protected program, a Georgetown TTS conducted 1:1 random assignment in blocks of 4, stratified by site, readiness to quit (next 30 days vs next 6 months or not considering quitting), lung screening result (Lung-RADS® 1 or 2 vs 3 or 4), and language (English or Spanish). The TTS then provided brief advice to quit and encouraged participants to return the consent form. Randomly assigned participants who did not sign the consent (after 10 reminders) were ineligible for the intervention but remained in the intent-to-treat analyses (Figure 1). The same TTS conducted the T1 assessment and all counseling calls.
Georgetown research assistants completed the 15-minute telephone follow-up assessments at 3 (T2), 6 (T3), and 12 months (T4) post random assignment (42) and were blinded to study arm. Participants received a $15 gift card (increased to $25 during the study) after completion of each assessment.
Within 2 weeks of self-reported 7-day point-prevalence smoking abstinence, participants completed bio-verification using a carbon monoxide (CO) test (43) conducted at the lung screening site, a mailed NicAlert (44) or NicoTest (45) saliva strip (for persons using marijuana or unable to use the iCO during COVID-19), or a mailed iCO (46) remote device to measure CO. The standard abstinence cutoffs were less than 30 ng/mL for NicAlert and NicoTest and less than or equal to 6 ppm for CO (47). Parking costs and a $25 gift card were provided. The equivalence between NicAlert and CO has been demonstrated (43,45).
The TTSs received training at an Association for the Treatment of Tobacco Use and Dependence–accredited program (48), weekly supervision for protocol adherence, and monthly supervision from a motivational interviewing expert. All calls were audio-recorded. Protocol adherence, assessed by coding a random selection of 10% of the intervention calls (49), was very high in both arms: M = 94.5% (88–100) in the intensive arm and M = 95.5% (89–100) in the minimal arm. Interrater reliability, calculated for 20% of the coded calls, showed high overall agreement: M = 95.0% (80–100) (Supplementary Methods, available online).
We mailed letters to referring providers notifying them of their patients’ study enrollment and 6-month smoking status. The letters also reminded providers to discuss smoking at each visit, consider pharmacological aids when appropriate, and provide support for relapse prevention.
Intervention Procedures
Both arms included empirically validated behavioral and motivational interviewing intervention methods (15,23,50,51). Core components included discussion of smoking-related goals, nicotine patch use, strategies to address smoking triggers, readiness to quit, and confidence and motivation to quit (Supplementary Methods, available online). These elements used motivational interviewing informed open-ended questions and reflections in a nonjudgmental atmosphere (52). Participants set a quit date only once they were ready. Those who quit focused on relapse prevention during the remaining calls.
To capitalize on the screening result as a potential teachable moment (6,29), the intervention began shortly post random assignment (Table 1). TTSs proactively called participants for all sessions (scheduled at participants’ convenience), which were completed within 3 months post random assignment. Participants received educational materials for use during and outside of the calls.
Participants were offered free nicotine replacement therapy (NRT; NicoDerm CQ 21-mg, 14-mg, and 7-mg patches) express-mailed in 2-week batches to interested participants. Participants not interested in patches were encouraged to discuss other FDA-approved pharmacological aids with their provider (Supplementary Methods, available online).
The Intensive Arm
This arm included eight 20-minute phone sessions and 8 weeks of nicotine patches. During the first 3 calls, the TTS initiated a discussion of the screening results and any follow-up procedures to address thoughts that reflected minimization of the need to quit and/or the lung screening process as a potential motivator to stop smoking (Supplementary Methods, available online) (29). To encourage counseling engagement, each 2-week supply of NRT was mailed only after completion of subsequent calls.
The Minimal Arm
This arm included three 20-minute phone sessions and one 2-week supply of nicotine patches designed to emulate what was currently offered by state quitlines (53). TTSs did not initiate a discussion of lung screening results in the minimal arm.
Measures
Table 2 describes the electronic health record data provided by the lung screening sites, measures included in the baseline and follow-up telephone assessments, and the process data regarding the intervention delivery (39). We also measured intervention delivery costs to evaluate cost-effectiveness; those data are the subject of another report (34).
Table 2.
Summary of measures and assessment points
| Measuresa | Screening site and EHR | T0, T1 (baseline) | T2, T3, T4 (3, 6, 12 mo) |
|---|---|---|---|
| Demographic and clinical information | |||
| Age, sex, language, date of birth, insurance coverage (64,65) | Yes | No | No |
| Race and ethnicity (another race include Asian, American Indian, Alaska Native, Native Hawaiian) (64,65) | Yes | Yes | No |
| Marital status and education level (64,65) | No | Yes | No |
| Tobacco-related comorbid illnesses (COPD, stroke, heart attack, high blood pressure, diabetes, asthma, chronic bronchitis) (1,64,65) | Yes | Yes | No |
| Family history of lung cancer (first-degree relative) (1,64,65) | No | Yes | No |
| Lung cancer screening | |||
| CT scan results (EHR) (41,64,65) | Yes | No | No |
| Recommended follow-up procedures (lung biopsy, sputum cytology, bronchoscopy, follow-up CT scan in 3, 6, or 12 mo, follow-up PET scan, appointment with pulmonologist or PCP) (64,65) | Yes | Yes | Yes |
| Final diagnosis: lung cancer, other cancer, nondiagnostic, alternate benign diagnosis (64,65) | Yes | No | No |
| Smoking and cessation history | |||
| Cigarettes per d (64-66) | No | Yes | Yes |
| Other tobacco/nicotine use (pipes, cigars, smokeless, e-cigarettes) (64-66) | No | Yes | Yes |
| Fagerstrom test for nicotine dependence (64,65,67) | No | Yes | Yes |
| Pack years (no. of years smoked × packs per d) (64,65) | Yes | No | No |
| Smoking/tobacco outcomes | |||
| Readiness to quitb, (64,65,68) | No | Yes | Yes |
| Confidence and motivation to quit (10-point scale, 0 = least confident or motivated, 10 = most confident or motivated) (64,65,69) | No | Yes | Yes |
| 24-h quit attempts within past 7 d (64,65) | No | Yes | Yes |
| Evidence-based treatment in past 7 d (pharmacotherapy, counseling, or electronic interventions) | No | Yes | Yes |
| Self-reported 7-d abstinence (42,55,64,65,70) | No | Yes | Yes |
| Biochemical verification: NicAlert and NicoTest saliva test, expired CO (in person) and iCO (remote test) (42,55,64,65,70) | No | No | Yes |
| Health and substance use | |||
| Alcohol use frequency (never, monthly or less, 2-4 times per mo, 2-3 times per wk, 4+ times per wk) (71) | No | Yes | No |
| Health Index Scale (0 = worst, 100 = best health) (64,65,72) | No | Yes | Yes |
| Treatment engagement and satisfaction | |||
| Engagement (no. of counseling sessions completed and amount of NRT requested (box contains 2-week supply of patches) | No | No | Yes |
| Satisfaction with telephone counseling and nicotine patches (not at all satisfied, a little satisfied, somewhat satisfied, very satisfied) (64,65) | No | No | Yes |
CO = carbon monoxide; COPD = chronic obstructive pulmonary disease; CT = computed tomography; EHR = electronic health record; NRT = nicotine replacement therapy; PCP = primary care provider; PET = positron emission tomography.
1-10 scale; 10 = already quit smoking, 9 = made changes in smoking but need to keep working , 8 = begun to make changes in smoking, 7 = plan to quit in the next 30 d, 6 = plan to quit in next 6 mo, 5 = often think about quitting but have no plans yet, 4 = sometimes think about quitting and have no plans yet, 3 = rarely think about quitting and have no plans to quit, 2 = do not think about quitting, 1 = decided to continue smoking.
Statistical Analyses
All analyses were based on the intent-to-treat principle. The outcomes of those lost to follow-up were imputed as continuing to smoke. All statistical tests were 2-sided. We used descriptive statistics and bivariate analyses (t tests and χ2 tests) to describe the associations of baseline characteristics with the outcomes and potential moderators and to evaluate those lost to follow-up.
We used logistic regression models to compare the study arms on bio-verified (primary) and self-reported abstinence (7-day point prevalence) at 3, 6, and 12 months. We conducted separate logistic regression models to assess the hypothesized moderators (readiness to quit, screening result, engagement with treatment, and nicotine dependence) at 3 months. All analyses controlled for baseline demographic and clinical characteristics that were statistically significantly associated with the outcome. Finally, we conducted sensitivity analyses to determine if the lung screening site with the largest number of study participants had an undue influence on the results.
As a result of COVID-19, screening sites were closed for several months and we were unable to randomly assign the planned number (1,200, 600 per arm) despite an additional 8 months of accrual beyond the intended end date (Supplementary Table 1, available online). At a statistical significance level of .05, with 403 per arm (after accounting for attrition), we had at least 80% power to detect differences in bio-verified abstinence rates for planned comparisons at 3, 6, and 12 months, ranging from 4% to 8% (when the minimal arm abstinence rate ranged from 1% to 15%). All analyses were conducted using SAS version 9.4 (54).
Results
Descriptive Characteristics
On average, participants were 63.6 years old, had 48 pack-years, and currently smoked 16.9 cigarettes per day (SD = 9.06; Table 1). Most participants were White (89%), smoked within 30 minutes of waking (71.9%), and were not ready to stop smoking in 30 days or less (51.6%). Only 2.1% had 20-29 pack years and 0.98% were 50-54 years old; thus, the sample closely matched those eligible for screening under the 2013 United States Preventive Services Task Force (USPSTF) guidelines.
Counseling engagement was similar by study arm, with 55% completing 6-8 of the intensive arm sessions and 51.1% completing all 3 sessions in the minimal arm. NRT use was proportional to study arm: 81.4% and 73.1% in the intensive arm vs minimal arm, respectively. Satisfaction with the intervention was high (Table 1).
Figure 1 presents the reach and retention rates (see also Supplementary Table 2, available online). In univariate analyses, we found that compared with White participants, African American participants were statistically significantly more likely to enroll and to be retained in the trial.
Cessation Outcomes
At 3 months, the intensive arm had statistically significantly higher quit rates compared with the minimal arm for self-reported (14.3% vs 7.9%, respectively; odds ratio [OR] = 2.00, 95% confidence interval [CI] = 1.26 to 3.18) and bio-verified rates (9.1% vs 3.9%, respectively; OR = 2.70, 95% CI = 1.44 to 5.08). At 6 and 12 months, the study arms no longer differed statistically significantly (Table 3). Exploratory analyses suggested that repeated point-prevalence abstinence (55) was higher in the intensive (vs the minimal) arm when comparing assessment points (Supplementary Figure 1, available online).
Table 3.
Biochemically verified and self-reported 7-day point prevalence abstinence rates
| Smoking abstinence among all randomly assigned participants (intention-to-treat analysis) | Intensive armaNo. (%) | Minimal armaNo. (%) | OR (95% CI) |
|---|---|---|---|
| Biochemically verifiedb,c | |||
| 3 mo | 37 (9.1) | 16 (3.9) | 2.7 (1.44 to 5.08) |
| 6 mo | 29 (7.1) | 24 (5.95) | 1.2 (0.68 to 2.11) |
| 12 mo | 34 (8.4) | 25 (6.3) | 1.4 (0.82 to 2.42) |
| Self-reportedd | |||
| 3 mo | 58 (14.3) | 32 (7.9) | 2.0 (1.26 to 3.18) |
| 6 mo | 42 (10.3) | 38 (9.4) | 1.1 (0.70 to 1.76) |
| 12 mo | 49 (12.1) | 40 (10.0) | 1.3 (0.82 to 2.00) |
The total numbers for each arm at each assessment differ because of the exclusion of patients diagnosed with lung cancer or deceased before the assessment (intensive arm: n = 407, 3 months; n = 406, 6 months; n = 405, 12 months; minimal arm: n = 406, 3 months; n = 403, 6 months; n = 400, 12 months). CI = confidence interval; OR = odds ratio.
Methods of verification: NicAlert, NicoTest, expired carbon monoxide (CO) conducted in person, expired CO using iCO remote device.
Covariates included for biochemically verified abstinence rates at: 3 months = recommended follow-up lung biopsy and computed tomography (CT) scan in 3 months, number of tobacco-related comorbid conditions; 6 months = recommended follow-up CT scan in 3 months and number of tobacco-related comorbid conditions; 12 months = race.
Covariates included for self-reported abstinence rates at: 3 months = number of tobacco-related comorbid conditions and the age when first started smoking cigarettes every day; 6 months = no covariates; 12 months = race and number of cigarettes per day at T1.
Regarding hypothesized moderators (Table 4), compared with the minimal arm, the intensive arm was more effective at 3 months among those with normal screening results (OR = 2.58, 95% CI = 1.32 to 5.05), greater nicotine dependence (OR = 3.47, 95% CI = 1.55 to 7.76), high engagement in counseling (OR = 3.03, 95% CI = 1.50 to 6.14), and receipt of NRT (OR = 2.81, 95% CI = 1.39 to 5.68). Because of small cell sizes in the minimal arm, there was suggestive evidence that readiness to quit moderated the intervention (OR = 10.54, 95% CI = 2.42 to 46.01). The site contributing the largest sample had similar bio-verified quit rates as the other sites combined (Table 4). Quit rates among those who completed at least 1 counseling session and those who completed the follow-up assessments were slightly higher compared with the entire sample (Supplementary Tables 3 and 4, available online).
Table 4.
Moderation effects at 3 months for biochemically verified 7-day point prevalence abstinencea
| Moderators | Intensive arm (n = 407) No. (%) | Minimal arm (n = 406) No. (%) | Adjusted OR (95% CI) |
|---|---|---|---|
| Lung screening result | |||
| Lung-RADS® 1-2 | 32 (8.6) | 14 (3.8) | 2.58 (1.32 to 5.03) |
| Lung-RADS® 3-4 | 5 (13.5) | 2 (5.0) | 3.85 (0.56 to 26.45) |
| Readiness to quit (T1) | |||
| Next 30 d | 18 (9.1) | 14 (7.2) | 1.44 (0.67 to 3.10) |
| Next 6 mo/not considering quitting | 19 (9.1) | 2 (0.9) | 10.54 (2.42 to 46.01) |
| Time to first cigarette (T1) | |||
| ≤30 min | 26 (9.1) | 9 (3.1) | 3.47 (1.55 to 7.76) |
| >30 min | 10 (8.6) | 7 (6.4) | 1.50 (0.52 to 4.31) |
| Engagement with phone counseling sessions | |||
| None/lowb | 3 (1.6) | 3 (1.5) | 1.15 (0.23 to 5.83) |
| Highc | 34 (15.2) | 13 (6.3) | 3.03 (1.50 to 6.14) |
| Engagement with NRT | |||
| None (0 wk) | 4 (5.3) | 3 (2.7) | 1.82 (0.39 to 8.56) |
| Any NRTd | 33 (10.0) | 13 (4.4) | 2.81 (1.39 to 5.68) |
| Site | |||
| Largest site (Lahey) | 16 (9.0) | 7 (3.8) | 3.12 (1.19 to 8.24) |
| Other 7 sites combined | 21 (9.2) | 9 (4.1) | 2.41 (1.05 to 5.54) |
| Baseline vs annual scan | |||
| Baseline | 13 (7.4) | 7 (4.1) | 2.01 (0.76 to 5.36) |
| Annual | 24 (10.3) | 9 (3.8) | 3.31 (1.44 to 7.62) |
The logistic regression analyses adjusted for recommended follow-up procedures for lung biopsy, recommended CT scan at 3 months, and number of tobacco-related comorbid conditions. CI = confidence interval; CT = computed tomography; Lung-RADS® = Lung Imaging Reporting and Data System; NRT = nicotine replacement therapy; OR = odds ratio; T1 = postscreening assessment.
Intensive: 0-5 sessions; Minimal: 0-2 sessions.
Intensive: 6-8 sessions; Minimal: 3 sessions.
Intensive: 2-8 wk; Minimal: 2 wk.
Discussion
This randomized clinical trial provides evidence to support the value of integrating smoking cessation treatment with lung cancer screening programs. The results demonstrated that intensive telephone counseling and NRT statistically significantly increased short-term quit rates compared with minimal telephone counseling and NRT. The intensive treatment was especially effective among those with higher nicotine dependence, normal lung screening results, and individuals who were not ready to quit.
This trial extends the evidence for the efficacy of the combined cessation treatments of telephone counseling and NRT (23) to older adults in the lung cancer screening setting. Proactive telephone counseling is compatible with the lung screening setting, because both telephone counseling and nicotine replacement can reach people quickly, within or outside the radiology clinic, and during the time when individuals may be most receptive to engaging in treatment (28–33). Although the effectiveness of phone counseling has been demonstrated with similar populations, including older adults (15–18), those not ready to quit (19–22), those not seeking treatment (21,23), and those who are ready to quit (23–27), we are aware of only 1 published phone-based trial conducted in any setting that included all these important trial components (56). The authors reported 14% (intervention arm) vs 12.6% (control arm) self-reported point-prevalence abstinence at 12 months (56), comparable with the LSTH self-reported 12-month rates (12.1% and 10.0%, respectively). The LSTH trial adds to this literature by using broad inclusion criteria and a standard cessation intervention, increasing the likelihood of greater reach and implementation in other lung screening settings.
Engagement with telephone counseling and NRT use was robust, suggesting that participants considered these treatment modalities to be beneficial. Further, those who were highly engaged with either intervention had statistically significantly higher abstinence rates than those who were less engaged. Despite the promising findings at the 3-month assessment, there was no statistically significant difference between arms at 6 or 12 months. Additional research is needed to assess potential methods of maintaining short-term effects, such as the use of booster sessions, particularly for those less motivated to quit (26), the addition of video-based phone interventions (57), or longer-term combined NRT or other pharmacotherapies (58). Further, a stepped care approach (ie, if NRT does not result in quitting) may be appropriate given the greater expense and expertise required for prescription medications. These issues may also be addressed by ongoing Smoking Cessation at Lung Examination trials (9).
Further, although the intensive arm was statistically significantly more efficacious than the minimal arm at 3 months, the bio-verified quit rates were, as expected, somewhat lower than self-reported rates (11). The low quit rates may be partially explained by the fact that this trial was designed to reach a broader and more heterogeneous sample than is recruited in most smoking cessation trials (eg, trials recruiting highly motivated volunteers) (26). Thus, the intensive intervention was not equally effective in all participants, such as those with lower nicotine dependence, normal screening results, less readiness to quit, and undergoing their baseline scan. Improving the tailoring and targeting of interventions to better assist these groups is particularly important because they represent a large proportion of individuals undergoing lung cancer screening (5).
Several caveats should be considered in evaluating our trial results. First, despite the broad inclusion criteria, such as those not currently ready to quit, a lack of diversity remained among participants at disproportionate risk for lung cancer, such as lower socioeconomic groups. This limitation is largely a reflection of the current population undergoing lung screening, and efforts are needed to reach more diverse patients (59,60). Importantly, the percentage of eligible African American participants who enrolled and who were retained was greater than among African Americans who declined or dropped out, respectively, indicating the potential for similar interventions to have an impact among African Americans (Supplementary Table 2, available online). The identification and referral of lung screening–eligible individuals must become more widely integrated in primary care to increase participant diversity along with opt-out methods for cessation treatment that are known to improve reach to historically underserved patients (61,62). Further limitations include lower than anticipated study enrollment, which was worsened by COVID-19. As a result, the moderation analyses should be interpreted with caution because of limited cell sizes. Retention rates at follow-up and bio-verification completion rates were also lower than expected.
This study has several strengths, including the large, geographically diverse sample and the wide inclusion criteria that provide generalizability to the broad population of patients eligible for lung screening who smoke. We did not exclude participants based on their motivational readiness to quit, behavioral health diagnoses, or concurrent smoking cessation treatment. Second, the study enrollment rate was based on the denominator that included all trial-eligible patients who underwent lung screening at each site during the recruitment period. This population-based approach is important when considering implementation on a broader scale. Third, the necessity of bio-verification of smoking status was confirmed for populations in which follow-up may be difficult and when high-risk status may impact self-report (42). Other strengths include the rigorous randomized design, bio-verification, and TTSs’ excellent protocol adherence.
Overall, this trial provides important evidence about an efficacious, scalable approach to deliver smoking cessation to older individuals undergoing lung cancer screening, including those who may not be ready to quit or who may not be seeking cessation treatment (16–21,25,26). Telephone counseling with nicotine replacement addresses the behavioral and dependence aspects of cessation treatment at a time when individuals may be amenable to receiving support for quitting. Our approach considers feasibility, because telephone counseling is provided outside of busy lung cancer screening settings that typically have limited staffing. Remote interventions are critical because Centers for Medicare & Medicaid Services (CMS) does not require screening practices to provide cessation counseling within the radiology clinic (8). Remote telephone counseling and mailed nicotine replacement is an efficient modality for intervention implementation. Telehealth approaches can provide broad reach and are likely to remain an important feature of cessation interventions going forward (57).
Because the intensive arm was statistically significantly more effective than the minimal arm only in the short term, it will be important to address relapse prevention in the intensive arm and to determine if the added costs are offset by the higher quit rates and long-term effects on mortality. In a separate article, we report the costs associated with intervention delivery and a cost-effectiveness analysis to guide future implementation and maintenance of cessation programs in the lung screening setting (34). Even with modest quit rates, the long-term population impact of effective cessation interventions delivered with lung cancer screening can be substantial (35,63).
Funding
This study was supported by the National Cancer Institute at the National Institutes of Health (grant numbers R01CA207228 to KT and R01CA207228-S1 to KT).
Notes
Role of the funder: The funder had no involvement in the design of the study, analysis of the data, the writing of the manuscript, and the decision to submit the manuscript for publication.
Disclosures: The authors have no conflicts of interest to declare. JM, a JNCI Associate Editor and coauthor on this article, was not involved in the editorial review or decision to publish this manuscript.
Author contributions: Conceptualization—KLT, CS, RN, DA. Data curation—RMW, LS, TL. Formal analysis—GL, TL, RMW, LS. Funding acquisition- KLT, GL, JM, RM, EDA. Investigation—LS TL. Methodology—KLT, CS, RN, DA. Project administration—KLT, RMW, LS. Supervision—KLT, KMD, RMW, LS. Validation—RMW, LS. Visualization—LS, RMW. Writing—original draft: KLT, RMW, LS, GL, TL. Writing—review & editing: KLT, RMW, TL, GL, LS, KMD, TL, CS, RN, DA, JM, JJ, RM, JJ, PC, EDA.
Acknowledgements: The authors gratefully acknowledge the contributions of the Lung Screening, Tobacco, and Health (LSTH) trial collaborators (alphabetical order): Ryan Anderson, BS, Shacoria Anderson, MPH, Juan Batlle, MD, Chavalia J. Breece, NP, Claudia Campos, MA, Lisa Charles, BS, Marisa Cordon, MPH, Danielle E. Deros, MS, Ellen Dornelas, PhD, Daisy Dunlap, BS, Joanne Ebner, BSN, OCN, NCTTP, Ellie Eyestone, MPS, Shelby Fallon. MPH, Jennifer Frey, PhD, Julia Friberg, BS, Lucia Galleno, Ph.D., Maria M. Geronimo, RN, Darilyn Gould, BA, Charlotte Hagerman, PhD, Harry Harper, MD, Melissa Harris, Judith Howell, RN, Sarah Hutchison, Jen-Yuan Christine Kao, Emily Kim, BS, Andrea Borondy-Kitts, MS, MPH, Yamile Leon, MSN, RN, Andrea McKee, MD, Brady McKee, MD, Vicky Parikh, MD, Margaret Pless, MS, Michael Ramsaier, BA, Shawn Regis, PhD, Nicolas Rojas, BA, Diana Ruiz, RN, Andrew Salner, MD, Jennifer Stephens, MS, and Felice Yang, MPH.
Supplementary Material
Contributor Information
Kathryn L Taylor, Cancer Prevention and Control Program, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
Randi M Williams, Cancer Prevention and Control Program, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
Tengfei Li, Department of Biostatistics, Bioinformatics, and Biomathematics, Georgetown University Medical Center, Washington, DC, USA.
George Luta, Department of Biostatistics, Bioinformatics, and Biomathematics, Georgetown University Medical Center, Washington, DC, USA.
Laney Smith, Cancer Prevention and Control Program, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
Kimberly M Davis, Cancer Prevention and Control Program, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
Cassandra A Stanton, Behavioral Health, Westat, Rockville, MD, USA.
Raymond Niaura, School of Global Public Health, New York University, New York, NY, USA.
David Abrams, School of Global Public Health, New York University, New York, NY, USA.
Tania Lobo, Cancer Prevention and Control Program, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
Jeanne Mandelblatt, Cancer Prevention and Control Program, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
Jinani Jayasekera, Cancer Prevention and Control Program, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
Rafael Meza, Department of Epidemiology, University of Michigan, Ann Arbor, MI, USA.
Jihyoun Jeon, Department of Epidemiology, University of Michigan, Ann Arbor, MI, USA.
Pianpian Cao, Department of Epidemiology, University of Michigan, Ann Arbor, MI, USA.
Eric D Anderson, Department of Pulmonary and Sleep Medicine, Georgetown University Medical Center, Washington, DC, USA.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author. Please see https://doi.org/10.7910/DVN/0O31JY for a description of the available data and to request access.
References
- 1. Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van der Aalst CM, de Koning HJ, van den Bergh KAM, Willemsen MC, van Klaveren RJ. The effectiveness of a computer-tailored smoking cessation intervention for participants in lung cancer screening: a randomised controlled trial. Lung Cancer Amst Neth. 2012;76(2):204–210. doi: 10.1016/j.lungcan.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 3. US Preventive Services Task Force. Screening for lung cancer: US preventive services task force recommendation statement. JAMA. 2021;325(10):962–970. doi: 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 4. Lozier JW, Fedewa SA, Smith RA, Silvestri GA. Lung cancer screening eligibility and screening patterns among Black and White adults in the United States. JAMA Netw Open. 2021;4(10):e2130350.doi: 10.1001/jamanetworkopen.2021.30350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Landy R, Young CD, Skarzynski M, et al. Using prediction-models to reduce persistent racial/ethnic disparities in draft 2020 USPSTF lung-cancer screening guidelines. J Natl Cancer Inst. 2021;113(11):1590–1594. doi: 10.1093/jnci/djaa211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol . 2011;6(11):1841–1848. doi: 10.1097/JTO.0b013e31822e59b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Villanti AC, Jiang Y, Abrams DB, Pyenson BS. A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS One. 2013;8(8):e71379.doi: 10.1371/journal.pone.0071379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Decision Memo for Screening for Lung Cancer with Low Dose Computer Tomography. Centers for Medicare and Medicaid Services (CMS; ); 2022. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304. Accessed July 28, 2022. [Google Scholar]
- 9. Joseph AM, Rothman AJ, Almirall D, et al. Lung cancer screening and smoking cessation clinical trials. SCALE (smoking cessation within the context of lung cancer screening) collaboration. Am J Respir Crit Care Med. 2018;197(2):172–182. doi: 10.1164/rccm.201705-0909CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deros DE, Hagerman CJ, Kramer JA, et al. Change in amount smoked and readiness to quit among patients undergoing lung cancer screening. J Thorac Dis. 2021;13(8):4947–4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taylor KL, Hagerman CJ, Luta G, et al. Preliminary evaluation of a telephone-based smoking cessation intervention in the lung cancer screening setting: a randomized clinical trial. Lung Cancer. 2017;108:242–246. doi: 10.1016/j.lungcan.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hagerman CJ, Tomko CA, Stanton CA, et al. Incorporating a smoking cessation intervention into lung cancer screening programs: preliminary studies. J Psychosoc Oncol. 2015;33(6):703–723. doi: 10.1080/07347332.2015.1082171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tammemägi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst. 2014;106(6):dju084. doi: 10.1093/jnci/dju084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health. 2013;103(6):e38–e46. doi: 10.2105/AJPH.2013.301299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fiore MC, Jaen C, Baker TB, Bailey W, Benowitz N, Curry S, Treating Tobacco Use and Dependence: 2008 Update. Washington DC: US Department of Health and Human Services; 2008. [Google Scholar]
- 16. Joyce GF, Niaura R, Maglione M, et al. The effectiveness of covering smoking cessation services for Medicare beneficiaries. Health Serv Res. 2008;43(6):2106–2123. doi: 10.1111/j.1475-6773.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morgan GD, Noll EL, Orleans CT, Rimer BK, Amfoh K, Bonney G. Reaching midlife and older smokers: tailored interventions for routine medical care. Prev Med. 1996;25(3):346–354. doi: 10.1006/pmed.1996.0065. [DOI] [PubMed] [Google Scholar]
- 18. Tait RJ, Hulse GK, Waterreus A, et al. Effectiveness of a smoking cessation intervention in older adults. Addiction. 2007;102(1):148–155. doi: 10.1111/j.1360-0443.2006.01647.x. [DOI] [PubMed] [Google Scholar]
- 19. Tzelepis F, Paul CL, Wiggers J, et al. A randomised controlled trial of proactive telephone counselling on cold-called smokers’ cessation rates. Tob Control. 2011;20(1):40–46. doi: 10.1136/tc.2010.035956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Curry SJ, McBride C, Grothaus LC, Louie D, Wagner EH. A randomized trial of self-help materials, personalized feedback, and telephone counseling with nonvolunteer smokers. J Consult Clin Psychol. 1995;63(6):1005–1014. doi: 10.1037//0022-006x.63.6.1005. [DOI] [PubMed] [Google Scholar]
- 21. Emmons KM, Puleo E, Mertens A, Gritz ER, Diller L, Li FP. Long-term smoking cessation outcomes among childhood cancer survivors in the partnership for health study. J Clin Oncol. 2009;27(1):52–60. doi:10.1200/J Clin Oncol.2007.13.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ali A, Kaplan CM, Derefinko KJ, Klesges RC. Smoking cessation for smokers not ready to quit: meta-analysis and cost-effectiveness analysis. Am J Prev Med. 2018;55(2):253–262. doi: 10.1016/j.amepre.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matkin W, Ordóñez‐Mena JM, Hartmann‐Boyce J. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2019;5(5):CD002850. doi: 10.1002/14651858.CD002850.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lichtenstein E, Zhu SH, Tedeschi GJ. Smoking cessation quitlines: an underrecognized intervention success story. Am Psychol. 2010;65(4):252–261. doi: 10.1037/a0018598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ellerbeck EF, Mahnken JD, Cupertino AP, et al. Effect of varying levels of disease management on smoking cessation: a randomized trial. Ann Intern Med. 2009;150(7):437–446. doi: 10.7326/0003-4819-150-7-200904070-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tzelepis F, Paul CL, Walsh RA, McElduff P, Knight J. Proactive telephone counseling for smoking cessation: meta-analyses by recruitment channel and methodological quality. JNCI J Natl Cancer Inst. 2011;103(12):922–941. doi: 10.1093/jnci/djr169. [DOI] [PubMed] [Google Scholar]
- 27. Zhu SH, Stretch V, Balabanis M, Rosbrook B, Sadler G, Pierce JP. Telephone counseling for smoking cessation: effects of single-session and multiple-session interventions. J Consult Clin Psychol. 1996;64(1):202–211. doi: 10.1037//0022-006x.64.1.202. [DOI] [PubMed] [Google Scholar]
- 28. Taylor KL, Cox LS, Zincke N, Mehta L, McGuire C, Gelmann E. Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer Amst Neth. 2007;56(1):125–134. doi: 10.1016/j.lungcan.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 29. McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- 30. Poghosyan H, Kennedy Sheldon L, Cooley ME. The impact of computed tomography screening for lung cancer on smoking behaviors: a teachable moment? Cancer Nurs. 2012;35(6):446–475. doi: 10.1097/NCC.0b013e3182406297. [DOI] [PubMed] [Google Scholar]
- 31. Piñeiro B, Simmons VN, Palmer AM, Correa JB, Brandon TH. Smoking cessation interventions within the context of low-dose computed tomography lung cancer screening: a systematic review. Lung Cancer. 2016;98:91–98. doi: 10.1016/j.lungcan.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 32. Williams RM, Cordon M, Eyestone E, et al. Improved motivation and readiness to quit shortly after lung cancer screening: evidence for a teachable moment. Cancer. 2022;128(10):1976–1986. doi: 10.1002/cncr.34133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Slatore CG, Baumann C, Pappas M, Humphrey LL. Smoking behaviors among patients receiving computed tomography for lung cancer screening. Systematic review in support of the U.S. Preventive Services Task Force. Ann Am Thorac Soc. 2014;11(4):619–627. doi: 10.1513/AnnalsATS.201312-460OC. [DOI] [PubMed] [Google Scholar]
- 34. Cao P, Smith L, Mandelblatt JS, et al. Cost-effectiveness of a telephone-based smoking cessation randomized trial in the lung cancer screening setting. JNCI Cancer Spectr. 2022. pkac048. doi: 10.1093/jncics/pkac048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meza R, Jeon J, Toumazis I, et al. Evaluation of the benefits and harms of lung cancer screening with low-dose computed tomography: modeling study for the US Preventive Services Task Force. JAMA. 2021;325(10):988–997. doi: 10.1001/jama.2021.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holford TR, Meza R, Warner KE, et al. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964-2012. JAMA. 2014;311(2):164–171. doi: 10.1001/jama.2013.285112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jeon J, Holford TR, Levy DT, et al. Smoking and lung cancer mortality in the United States from 2015 to 2065: a comparative modeling approach. Ann Intern Med. 2018;169(10):684–693. doi: 10.7326/M18-1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Georgetown University. Integrating evidence-based smoking cessation interventions into lung cancer screening programs: a randomized trial. clinicaltrials.gov; 2021. https://clinicaltrials.gov/ct2/show/NCT03200236. Accessed February 6, 2022.
- 39. Taylor KL, Deros DE, Fallon S, et al. Study protocol for a telephone-based smoking cessation randomized controlled trial in the lung cancer screening setting: the lung screening, tobacco, and health trial. Contemp Clin Trials. 2019;82:25–35. doi: 10.1016/j.cct.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lung Cancer Screening, Version 3.2018. NCCN clinical practice guidelines in oncology. J Natl Compr Canc Net. 2018;16. https://jnccn.org/view/journals/jnccn/16/4/article-p412.xml. Accessed February 7, 2022. [DOI] [PMC free article] [PubMed]
- 41. American College of Radiology. Lung CT Screening Reporting and Data System (Lung-RADS). https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-. Accessed November 11, 2021.
- 42. Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob Res. 2020;22(7):1086–1097. doi: 10.1093/ntr/ntz132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marrone GF, Paulpillai M, Evans RJ, Singleton EG, Heishman SJ. Breath carbon monoxide and semiquantitative saliva cotinine as biomarkers for smoking. Hum Psychopharmacol. 2010;25(1):80–83. doi:10.1002/hup. 1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cooke F, Bullen C, Whittaker R, McRobbie H, Chen MH, Walker N. Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine Tob Res. 2008;10(4):607–612. doi: 10.1080/14622200801978680. [DOI] [PubMed] [Google Scholar]
- 45. Murray RP, Connett JE, Istvan JA, Nides MA, Rempel-Rossum S. Relations of cotinine and carbon monoxide to self-reported smoking in a cohort of smokers and ex-smokers followed over 5 years. Nicotine Tob Res. 2002;4(3):287–294. doi: 10.1080/14622200210141266. [DOI] [PubMed] [Google Scholar]
- 46. Smokerlyzer Industries - coVita. https://www.covita.net/smokerlyzer-industries/. Accessed November 9, 2021.
- 47. Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addict Abingdon Engl. 2005;100(2):159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- 48. University of Medicine and Dentistry of New Jersey (UMDNJ), Rutgers-- Tobacco Dependence Program. Certified tobacco treatment specialist training. http://www.tobaccoprogram.org/index.php?src=news&refno=1&category=default. Accessed November 11, 2021.
- 49. Catley D, Goggin K, Harris KJ, et al. A randomized trial of motivational interviewing: cessation induction among smokers with low desire to quit. Am J Prev Med. 2016;50(5):573–583. doi: 10.1016/j.amepre.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Graham AL, Papandonatos GD, DePue JD, et al. Lifetime characteristics of participants and non-participants in a smoking cessation trial: implications for external validity and public health impact. Ann Behav Med Publ Med. 2008;35(3):295–307. doi: 10.1007/s12160-008-9031-1. [DOI] [PubMed] [Google Scholar]
- 51. Stead LF, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev. 2015;(10):CD009670. doi: 10.1002/14651858.CD009670.pub3. [DOI] [PubMed] [Google Scholar]
- 52. Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. The Guilford Press; 2002: xx, 428. [Google Scholar]
- 53. Rudie M. Results from the 2017 NAQC Annual Survey of Quitlines. North American Quitline Consortium. 2017. https://www.naquitline.org/page/2017survey. Accessed January 6, 2022.
- 54. SAS Software. Cary, NC: SAS Institute Inc.; 2013. [Google Scholar]
- 55. Piper ME, Bullen C, Krishnan-Sarin S, et al. Defining and measuring abstinence in clinical trials of smoking cessation interventions: an updated review. Nicotine Tob Res. 2020;22(7):1098–1106. doi: 10.1093/ntr/ntz110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tremblay A, Taghizadeh N, Huang J, et al. A randomized controlled study of integrated smoking cessation in a lung cancer screening program. J Thorac Oncol Off Oncol. 2019;14(9):1528–1537. doi: 10.1016/j.jtho.2019.04.024 [DOI] [PubMed] [Google Scholar]
- 57. Rigotti NA, Taylor KL, Beneventi D, et al. Telehealth delivery of tobacco cessation treatment in cancer care: an ongoing innovation accelerated by the COVID-19 pandemic. J Natl Compr Canc Netw. 2021;19(suppl 1):s21–s24. doi: 10.6004/jnccn.2021.7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5–e31. doi: 10.1164/rccm.202005-1982ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kee D, Wisnivesky J, Kale MS. Lung cancer screening uptake: analysis of BRFSS 2018. J Gen Intern Med. 2021;36(9):2897–2899. doi: 10.1007/s11606-020-06236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Williams RM, Li T, Wang M, et al. Lung cancer screening utilization and implications of varying eligibility criteria by race and ethnicity: 2019 behavioral risk factor surveillance system data. Cancer. 2022;128(9):1812–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baker TB, Berg KM, Adsit RT, et al. Closed-loop electronic referral from primary care clinics to a state tobacco cessation quitline: effects using real-world implementation training. Am J Prev Med. 2021;60(3 suppl 2):S113–S122. doi: 10.1016/j.amepre.2019.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Creswell PD, McCarthy DE, Trapskin P, et al. Can inpatient pharmacists move the needle on smoking cessation? Evaluating reach and representativeness of a pharmacist-led opt-out smoking cessation intervention protocol for hospital settings. Am J Health-Syst Pharm. 2021;79(12):969–978. doi: 10.1093/ajhp/zxab488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cao P, Jeon J, Levy DT, et al. Potential impact of cessation interventions at the point of lung cancer screening on lung cancer and overall mortality in the United States. J Thorac Oncol. 2020;15(7):1160–1169. doi: 10.1016/j.jtho.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. National Cancer Institute. Smoking Cessation at Lung Examination: The SCALE Collaboration | BRP | DCCPS/NCI/NIH. https://cancercontrol.cancer.gov/brp/tcrb/scale-collaboration.html. Accessed August 13, 2020.
- 65. National Cancer Institute. Smoking cessation at lung examination (SCALE) collaboration special collection (NCI). https://www.gem-measures.org/Public/wsoverview.aspx?wid=33&cat=8. Accessed September 28, 2020.
- 66. Sample adult tobacco document 2015. National Health Interview Survey; 2016:44-66. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Survey_Questionnaires/NHIS/2015/english/qcancer.pdf. Accessed July 28, 2022.
- 67. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 68. Apodaca TR, Abrantes AM, Strong DR, Ramsey SE, Brown RA. Readiness to change smoking behavior in adolescents with psychiatric disorders. Addict Behav. 2007;32(6):1119–1130. doi: 10.1016/j.addbeh.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Latimer-Cheung AE, Fucito LM, Carlin-Menter S, et al. How do perceptions about cessation outcomes moderate the effectiveness of a gain-framed smoking cessation telephone counseling intervention? J Health Commun. 2012;17(9):1081–1098. doi: 10.1080/10810730.2012.665420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tobacco Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 71. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA; for the Ambulatory Care Quality Improvement Project (ACQUIP). The AUDIT Alcohol Consumption Questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 72. EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy Amst Neth. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author. Please see https://doi.org/10.7910/DVN/0O31JY for a description of the available data and to request access.