Abstract
Two-pore channels are ancient members of the voltage-gated ion channel superfamily that are expressed predominantly on acidic organelles such as endosomes and lysosomes. Here we review recent advances in understanding how TPCs are activated by their ligands and identify five salient features: (1) TPCs are Ca2+-permeable non-selective cation channels gated by NAADP. (2) NAADP activation is indirect through associated NAADP receptors. (3) TPCs are also Na+-selective channels gated by PI(3,5)P2. (4) PI(3,5)P2 activation is direct through a structurally-resolved binding site. (5) TPCs switch their ion selectivity in an agonist-dependent manner.
Keywords: TPCN1, TPCN2, TPCN3, TPC1, TPC2:TPC3, JPT2, LSM12, Lysosomes, Endosomes, Ca2+
1. Introduction
Ion channels are ubiquitous proteins that exploit ionic gradients to move Ca2+, Na+, K+, Cl− and other ions across biological membranes to drive diverse physiological processes [1]. Ion channels are located on both the cell surface as well as organelles. Lysosomes and related acidic organelles are no exception and express a growing family of Ca2+-permeable channels [2]. Endo-lysosomal two-pore channels (TPCs) in particular have attracted much attention over the last decade or so since their identification as target channels for the Ca2+ mobilizing messenger NAADP (Fig. 1A) [3–5]. But TPCs also function as Na+ channels gated by the phosphoinositide, PI(3,5)P2 (Fig. 1B) as well as voltage [6, 7]. Structurally, TPCs are dimers where each protomer contains two shaker-like channel domains (I and II) each comprising a voltage sensor (S1-S4) and pore (S5-S6) (Fig. 1C) [8–11]. They therefore display pseudo-four-fold symmetry typical of other members of the voltage-gated ion channel superfamily, and likely represent key evolutionary intermediates in the transition from one- to four-domain channels [12]. TPCs are ancient proteins with homologs identifiable in unicellular organisms including parasitic protists [13] but they have undergone substantial lineage-specific loss and duplication through their evolutionary journey [2]. Humans possess two isoforms, TPC1 and TPC2.
Fig. 1. Structural basis underpinning TPC activation.
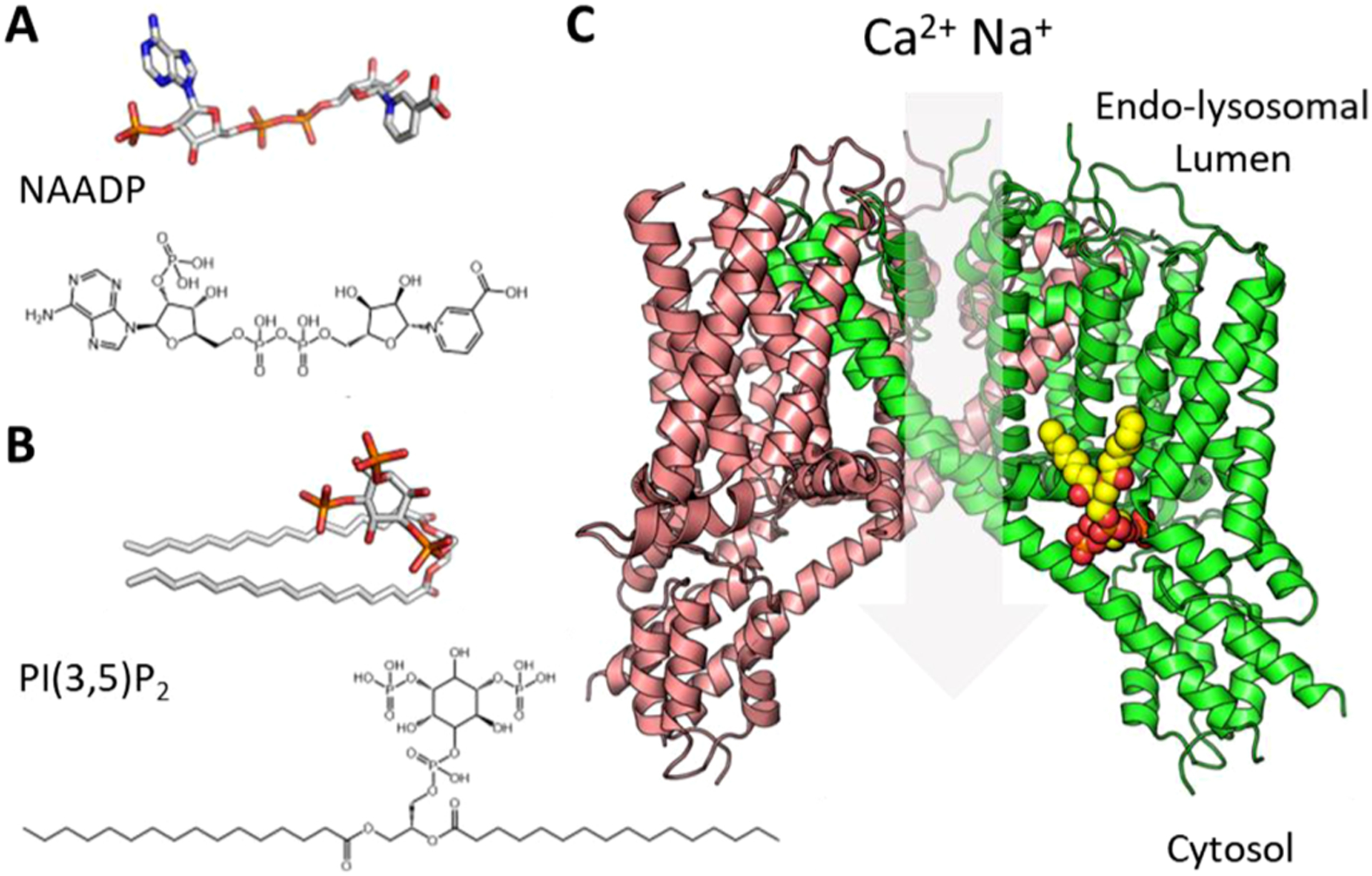
Structures of NAADP (A) and PI(3,5)P2 (B), C, Structure of human TPC2 based on pdb 6NQ0 where each protomer is represented by the coloured ribbons and bound PI(3,5)P2 by the ball and sticks.
Functionally, TPCs regulate numerous cellular processes from those anticipated from analysis of NAADP signaling e.g. differentiation [14, 15] through to new un-anticipated ones e.g. viral entry [16]. In particular, much attention has been devoted to dissecting their role in membrane traffic (reviewed recently in [17]). Given such widespread functionality, it is not so surprising that TPCs are increasingly linked to diseases such as liver dysfunction [18], neurodegeneration [19] and cancer [20, 21]. And related to this, there has been a push of late to identify drugs to target TPCs [22–24]). In this review, we focus on the mechanisms underlying activation of TPCs by NAADP and PI(3,5)P2. We review the evidence showing TPCs are NAADP-gated Ca2+-permeable channels including recent work identifying long sought NAADP receptors. We also review apparently contrary evidence showing TPCs are PI(3,5)P2-gated Na+-selective channels and the structural underpinnings. Finally, we discuss how agonist-dependent switching of ion selectivity of TPCs reconciles conflicting evidence relating to gating and permeability. We do so in the context of five key take home messages.
1. TPCs are Ca2+-permeable non-selective cation channels gated by NAADP
NAADP (Fig. 1A) is nearly identical to the co-enzyme NADP, differing only by the presence of a hydroxyl group in place of an amide. NAADP was shown to release Ca2+ from intracellular stores in the 1990’s [25]. Since then it has emerged as a second messenger produced in response to diverse stimuli, functioning to drive numerous Ca2+-dependent processes [26, 27] stemming from early work in the nervous system [28, 29]. Unlike inositol trisphosphate and cyclic ADP-ribose, NAADP releases Ca2+ primarily from acidic organelles and not the ER [30]. Three independent studies converged on TPCs as the long-sought target channels for NAADP responsible for this release focusing on TPC1 [3] and TPC2 [4, 5]. This evidence was based on the localization of TPCs to the endo-lysosomal system and functional studies upon TPC overexpression, knockdown, knockout and mutagenesis [3–5]. An essential requirement for TPCs in NAADP action was soon confirmed in multiple follow-up studies (reviewed in [31]) and such a requirement continues to attract attention in varied contexts from smooth muscle [32] to stem cells [33].
TPCs were cloned two decades ago from both animal [34] and plant [35] sources. Analyses of animal TPCs followed work in plants identifying TPC as the ‘slow vacuolar’ (SV) channel [36]. The SV channel had been characterized electro-physiologically since the 1980’s [37] but its molecular identity was not known. Electrophysiological analyses of animal TPCs followed soon after their identification as NAADP targets. The intracellular location of TPCs presents significant access issues compared to channels in the plasma membrane. Nevertheless, analysis of TPC2 by the ‘planar’ patch clamp method [38], in artificial lipid bilayers [39] or in the plasma membrane upon re-routing by mutation of an endo-lysosomal targeting motif [40] provided the first insight. These studies, as well as others with TPC1 [41, 42] and TPC2 [43] confirmed that they were gated by NAADP and permeable to Ca2+ and other divalent as well as monovalent cations. In particular, bilayer analysis upon knockdown of TPC1 [41] and ‘vacuolar’ patch clamp analysis upon knockout of TPC2 [18] confirmed NAADP action on endogenous TPCs. These results were congruent with the non-selective nature of cation currents through plant TPCs [44]. Intriguingly, sensitivity of TPC1 to NAADP was regulated by voltage [41].
In sum, both cell biology and biophysics converged on TPCs as the long-sought target channels for NAADP.
2. NAADP activation is indirect through associated NAADP receptors
With TPCs established as the targets for NAADP action, this discovery should have enabled resolution of the NAADP binding site within the TPC structure. However, these data were not forthcoming, leaving a gap in our understanding of how NAADP activates TPCs. Clues to solving this puzzle came from observations that TPC overexpression only modestly increased levels of 32P-NAADP binding [4] and results from photo-affinity labeling (PAL) assays using NAADP-derived photoprobes [45, 46]. Application of PAL probes crucially revealed that NAADP selectively binds to soluble proteins much smaller than TPCs (~23 kDa in mammalian cells [46–48]) that persist in TPC knockout samples [44, 46]. Therefore, this insight established the concept of NAADP binding proteins (NAADP-BPs) as distinct entities from the channel itself, acting as independent protein partners within a larger TPC complex [31, 47, 49].
While conceptually appealing - for example, to explain how NAADP sensitivity may be absent in broken cell preparations through loss of cytoplasmic NAADP-BPs [31] - their molecular identity remained elusive for over a decade following definition of TPCs as the targets of NAADP action. However, two different mammalian NAADP-BPs have recently been identified [50–52], both candidates fulfilling the criteria of correct size (~23 kDa), selective binding of NAADP, ability to associate with TPCs and necessity for NAADP-evoked Ca2+ release. These proteins are (i) Jupiter Microtubule Associated Homolog-2 (JPT2 [50]), also known as HN1L [51]) and ‘like-Sm’ protein 12 (LSM12 [52]) - a member of the ‘like-Sm’ RNA-binding protein family [53]. Both these NAADP-BPs had not previously been implicated in NAADP action, cytoplasmic Ca2+ signaling, or were well represented in TPC proteomic studies.
Existing literature for both proteins is currently small. JPT2 is implicated in cancer and viral infection, which is intriguing given the role of TPCs in both conditions [54]. The role of LSM12 as an RNA-binding protein is also unexpected, potentially extending the reach of NAADP and TPC signaling into new aspects of cell biology.
In short, the recent unmasking of two NAADP-BPs provides new impetus to resolve the mechanistic basis for NAADP binding, the choreography of NAADP-BP association with TPCs, and how these processes are entwined with physiological signaling and pathogenesis.
3. TPCs are also Na+-selective channels gated by PI(3,5)P2
Despite the substantial evidence for TPCs as NAADP-gated Ca2+-permeable channels, a body of work suggested otherwise. Vacuolar patch clamp measurements showed that TPCs were not Ca2+-permeable and instead Na+-selective, and that they were not NAADP-sensitive but instead activated/regulated by PI(3,5)P2. PI(3,5)P2 (Fig. 1B) is a minor phosphoinositide that is enriched on the endo-lysosomal system and heavily implicated in membrane traffic [55]. PI(3,5)P2 also regulates endo-lysosomal TRP mucolipin (TRPML) channels [56]. But the biophysical signatures for TPCs and TRMLs are quite distinct, and endogenous PI(3,5)P2-induced Na+ currents reminiscent of those evoked through TPC2 in macrophages were absent in knockout mice lacking both TPC isoforms [6]. Independent studies confirmed an action of PI(3, 5)P2 on TPCs, its Na+ selectivity and its NAADP insensitivity [7, 9, 57–59].
Regulation of TPCs by PI(3,5)P2 was first reported for mammalian TPC2 [6]. PI(3,5)P2 but not related lipids activated the channel. Interestingly, PI(4,5)P2 inhibited PI(3,5)P2 activation of Xenopus TPC2 [60]. Mammalian TPC1 is also regulated by PI(3,5)P2 but not other isomers [7]. For TPC1, PI(3,5)P2 appears to be essential for voltage activation [9]. Regulation of TPC3 by phosphoinositides is less clear. This isoform is present in most animals but unusual as it has undergone striking lineage-specific loss being present in primates but not humans and rodents but not mice [61, 62]. Zebrafish TPC3 encodes a reportedly purely voltage-gated ion channel in the plasma membrane when expressed heterologously [60, 63] but is also active in lysosomes where it appears insensitive to PI(3,5)P2 [60]. Xenopus TPC3 also appears to function similarly as a voltage-gated ion channel in the absence of exogenous phosphoinositides [60]. Xenopus TPC3 over-expressed homologously in oocytes however was modestly activated by PI(3,5)P2 and also more robustly by PI(3,4)P2 [64], a phosphoinositide enriched in the plasma membrane [65].
PI(3,5)P2 levels reportedly increase to stimuli such as hypertonic stress [55], so it is possible that TPC activity could be regulated acutely much like by NAADP. But how widespread stimulus-evoked PI(3,5)P2 production is in animal cells is unclear at present. Rather, PI(3,5)P2 might instead act as a cofactor perhaps stabilizing the channel. As Na+ channels, TPCs have been shown to be tonically inhibited by mTOR and only become activated during nutrient stress [66]. Alternatively, TPCs may regulate the resting endo-lysosomal membrane potential [6] or even underlie endo-lysosomal action potentials [7]. A recent study has identified a novel role for PI(3,5)P2-mediated Na+ fluxes primarily through TPC1 in maintaining endocytic volume during fluid uptake [67]. Xenopus TPC3 likely encodes a cell surface Na+ channel previously characterized in oocytes based on similar biophysical properties including an unusual increase in activity following prolonged depolarization [60]. Interestingly, PI(3,4)P2 levels demonstrably increase upon depolarization [64] providing a mechanism coupling excitability to TPC3 activation and perhaps corresponding to the so called fertilization potential induced by sperm.
In sum, a body of literature, mostly biophysical, indicates that TPCs can function as Na+ channels regulated by phosphoinositides in an isoform- and species-specific manner.
4. PI(3,5)P2 activation is direct through a structurally resolved binding site
In contrast to NAADP, which gates TPCs indirectly through associated binding proteins (Section 2), the effects of PI(3,5)P2 are direct. Structural work, first for mouse TPC1 [9] and then human TPC2 [11] resolved the PI(3,5)P2 binding site. In both channels, the negatively charged phosphorylated inositol head group interacted with positively charged arginine (TPC1) or lysine (TPC2) residues in the linker region connecting S4 and S5 in the first channel domain. This site is very different to that in TRPML1 [68]. Rather, the TPC binding site resembles better that of the putative activating lipid binding site in TRPV1 [69], TRPV6 [70] and Polycystin-2 [71]. Predictions by molecular dynamics simulations for TPC2 published prior to the structural work proved remarkably accurate [72]. The PI(3,4)P2 binding site for TPC3 has also been modelled based on the PI(3,5)P2 binding site in TPC1.
Additional interactions of PI(3,5)P2 with residues in the S6 extension of domain I provides a link to the channel gate and a structural basis for channel opening. In TPC1, a key lysine residue (K331) acts as the ‘bridge’ that straightens the S6 helix in the presence of P(I3,5)P2 [9]. This together with an upward movement of the voltage-sensor in domain II upon depolarization and consequent structural rearrangements of the S6 helix conspire to open the gate. In TPC2, the corresponding residue based on sequence alignment (S322) together with an arginine (R329) residue are the key residues in the S6 helix of domain I that engage P(I3,5)P2 [11]. TPC2 is voltage-insensitive and it is structural ordering of the S4-S5 linker in domain II which is coupled to channel opening. In TPC3, it is suggested that PI(3,4)P2 activation involves more direct interactions between residues in the S6 helicies of domain I and Domain II [64] but this awaits structural confirmation.
Molecular dynamic simulations of free and PI(3,5)P2-bound TPC1 failed to recapitulate Na+ flux through the TPC1, although metadynamic simulations did capture fluctuations of the gate and Na+ flux in a partially dehydrated state in response to PI(3,5)P2 [73]. Molecular dynamic simulations of Na+ flux through TPC2 suggest that accumulation of Na+ in the cavity between the selectivity filter and the gate regulates ion flow by acting as a ‘reservoir’ [74]. But details of the Na+ transport through the selectivity filter are currently lacking.
Benefiting from the resolution revolution, atomic structures of TPCs indicate that P(I3,5)P2 directly gates TPCs.
5. TPCs switch their ion selectivity in an agonist-dependent manner
If the biophysical signatures of TPCs activated by NAADP and PI(3,5) P2 were presented blindly to an electrophysiologist, then they would naturally conclude that they were associated with two different channels. So how do we rationalize the very different reported behavior of TPC2? It was the outcome of a recent high throughput, Ca2+-based screen for small molecule activators of TPC2 that provided an explanation [75]. In a single screen, two structurally distinct agonists of TPC2, TPC2-A1-N and TPC2-A1-P were identified [75, 76]. These results alone confirmed the Ca2+-permeability of TPC2 consistent with the actions of NAADP on TPC2.
TPC2-A1-N induced more robust Ca2+ signals than TPC2-A1-P [75]. This was particularly pronounced when TPC2 was expressed in its native lysosomal environment. Electrophysiological analysis showed that TPC2-A1-N induced both Ca2+ and Na+ currents whereas TPC2-A1-P induced only Na+ currents. The Na+-selective nature of the TPC2-A1-P current is consistent with the actions of PI(3,5)P2 on TPC2. Parallel electrophysiological analysis of TPC2 in the presence NAADP and PI(3, 5)P2 confirmed that TPC2-A1-N is an NAADP-mimetic whereas TPC2-A1-P is a PI(3,5)P2 mimetic. In other words, the ion selectivity of TPC2 depends on the activating ligand thereby uniting apparently conflicting results.
In hindsight, the above finding was obvious given the clear distinction in properties when TPC2 is activated by NAADP or PI(3,5)P2. The problem was the limited evidence where both NAADP and PI(3,5)P2 were compared under the same experimental setting. Either PI(3,5)P2 was not tested in NAADP-centric experiments or TPC2 was insensitive to NAADP in PI(3,5)P2-centric experiments. The loss of soluble NAADP receptors (Section 2) could readily explain the latter. But in studies where dual agonist sensitivity was retained in some form, it is notable that i) PI(3,5)P2 appeared not to gate TPC1 in bilayers but increased the permeability to Na+ (and more modestly H+) relative to Ca 2+ upon activation with NAADP [42] ii) lysosomal TPC2 currents induced by NAADP relative to PI(3,5)P2 were significantly larger using Ca2+ as the major permeant ion compared to Na+ [32]. Both these studies are not inconsistent with the idea that the ion selectivity of TPCs is agonist-selective.
An independent screen focusing on approved drugs also identified a number of tricyclic anti-depressants as TPC agonists [77]. Although the screen was Ca2+-based like the one that identified TPC2-A1-N and TPC2-A1-P, electrophysiological analysis inexplicably revealed that the agonists induced Na+-selective currents through TPC2. Some also had effects on TPC1 causing either activation (e.g. Clomipramine) or inhibition (e.g. Chlorpromazine) of inward currents. Unlike TPC1, TPC2 is not sensitive to voltage due to the absence of critical arginine in S4 of domain II [11] but intriguingly, several of the agonists revealed voltage-sensitivity in TPC2 and the reversed the voltage-dependence of TPC1. This complex interaction between agonist and voltage is reminiscent of the voltage-dependence of NAADP action at TPC1 [41]. An unrelated drug (riluzole) was also identified in a second (unspecified) screen as a TPC2 agonist that operated in a voltage-insensitive manner [77]. Mutation of K204 which contributes to PI(3,5)P2 activation [11] inhibited TPC2-A1-P [75] but not TPC2-A1-N [75] or riluzole [77] action at TPC2 suggesting independent drug binding sites.
In sum, identification of TPC agonists has provided key insight into multi-modal activation of TPC2 linking gating mechanism with ion selectivity and thereby uniting disparate biophysical data sets. Fig. 2
Fig. 2. Ion selectivity of TPCs is agonist dependent.
Schematic showing how NAADP, and PI(3,5)P2 alter the Ca 2+ and Na+ permeability of TPC2. Created with BioRender.
6. Conclusions and open questions
Here we have attempted to reduce our current understanding of how TPCs are activated by their ligands to five take home messages. But expectedly there remain gaps in our knowledge. The identification of TPC-associated NAADP-binding proteins raises questions as to how binding of NAADP to its receptors is coupled to opening of its target channels (discussed in [54]). We now have agonists that can mimic the actions of NAADP and PI(3,5)P2 on TPC2 but what are their mechanisms of action, and how do these relate to their endogenous counterparts? TPC1 and the lesser studied TPC3 are regulated by voltage. Is their interplay between voltage and ligand activation? And while TPC activation has been considered primarily in the context of Ca2+ and Na+ signals what about permeability to other ions, in particular H+?, Is such permeability agonist selective? And how might we go about teasing apart the various modalities in the context of TPC-dependent function?
Stay tuned.
Acknowledgements
Supported by grants from the BBSRC (BB/T015853/1 to SP) and NIH (GM088790 to JSM and SP).
Footnotes
CRediT authorship contribution statement
Sandip Patel: Writing – original draft, Funding acquisition. Yu Yuan: Writing – review & editing, Visualization. Gihan S. Gunaratne: Writing – original draft, Writing – review & editing. Taufiq Rahman: Writing – review & editing, Visualization. Jonathan S. Marchant: Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest
References
- [1].Hille B, Ionic Channels of Excitable Membranes, 1 ed., Sinauer Associates, Sunderland, Massachusets, 1984. [Google Scholar]
- [2].Patel S, Cai X, Evolution of acid Ca2+ stores and their resident Ca2+-permeable channels, Cell Calcium 57 (2015) 222–230. [DOI] [PubMed] [Google Scholar]
- [3].Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, Patel S, Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling, J. Cell Biol 186 (2) (2009) 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX, NAADP mobilizes calcium from acidic organelles through two-pore channels, Nature 459 (7246) (2009) 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rotzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C, The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores, Pflugers Arch. 458 (5) (2009) 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, Zhu MX, Clapham DE, Ren D, Xu H, TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes, Cell 151 (2) (2012) 372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cang C, Bekele B, Ren D, The voltage-gated sodium channel TPC1 confers endolysosomal excitability, Nat. Chem. Biol 10 (6) (2014) 463–469. [DOI] [PubMed] [Google Scholar]
- [8].Patel S, Penny CJ, Rahman T, Two-pore channels enter the atomic era. Structure of plant TPC revealed, Trends Biochem. Sci 41 (2016) 475–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].She J, Guo J, Chen Q, Zeng W, Jiang Y, Bai XC, Structural insights into the voltage and phospholipid activation of the mammalian TPC1 channel, Nature 556 (7699) (2018) 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Patel S, Two-pore channels open up, Nature 556 (7699) (2018) 38–40. [DOI] [PubMed] [Google Scholar]
- [11].She J, Zeng W, Guo J, Chen Q, Bai XC, Jiang Y, Structural mechanisms of phospholipid activation of the human TPC2 channel, Elife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rahman T, Cai X, Brailoiu GC, Abood ME, Brailoiu E, Patel S, Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels, Sci. Signal 7 (352) (2014) ra109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li ZH, King TP, Ayong L, Asady B, Cai X, Rahman T, Vella SA, Coppens I, Patel S, Moreno SNJ, A plastid two-pore channel essential for inter-organelle communication and growth of Toxoplasma gondii, Nat. Commun 12 (1) (2021) 5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brailoiu E, Churamani D, Pandey V, Brailoiu GC, Tuluc F, Patel S, Dun NJ, Messenger-specific role for NAADP in neuronal differentiation, J. Biol. Chem 281 (2006) 15923–15928. [DOI] [PubMed] [Google Scholar]
- [15].Aley PK, Mikolajczyk AM, Munz B, Churchill GC, Galione A, Berger F, Nicotinic acid adenine dinucleotide phosphate regulates skeletal muscle differentiation via action at two-pore channels, Proc. Natl. Acad. Sci. U. S. A 107 (46) (2010) 19927–19932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sakurai Y, Kolokolstov AA, Chen CC, Tidwell MW, Bauta WE, Klugbauer N, Grimm C, Wahl-Schott C, Biel M, Davey RA, Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment, Science 347 (6225) (2015) 995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vassileva K, Marsh M, Patel S, Two-pore channels as master regulators of membrane trafficking and endocytic well-being, Current Opinion in Physiol. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Grimm C, Holdt LM, Chen CC, Hassan S, Muller C, Jors S, Cuny H, Kissing S, Schroder B, Butz E, Northoff B, Castonguay J, Luber CA, Moser M, Spahn S, Lullmann-Rauch R, Fendel C, Klugbauer N, Griesbeck O, Haas A, Mann M, Bracher F, Teupser D, Saftig P, Biel M, Wahl-Schott C, High susceptibility to fatty liver disease in two-pore channel 2-deficient mice, Nat. Commun 5 (2014) 4699. [DOI] [PubMed] [Google Scholar]
- [19].Hockey LN, Kilpatrick BS, Eden ER, Lin-Moshier Y, Brailoiu GC, Brailoiu E, Futter CE, Schapira AH, Marchant JS, Patel S, Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition, J. Cell. Sci 128 (2) (2015) 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nguyen ON, Grimm C, Schneider LS, Chao YK, Atzberger C, Bartel K, Watermann A, Ulrich M, Mayr D, Wahl-Schott C, Biel M, Vollmar AM, Two-pore channel function is crucial for the migration of invasive cancer cells, Cancer Res. 77 (6) (2017) 1427–1438. [DOI] [PubMed] [Google Scholar]
- [21].Patel S, Kilpatrick BS, Two-pore channels and disease, Biochimica et biophysica acta. Mol. Cell Res (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gunaratne GS, Johns ME, Hintz HM, Walseth TF, Marchant JS, A screening campaign in sea urchin egg homogenate as a platform for discovering modulators of NAADP-dependent Ca(2+) signaling in human cells, Cell Calcium 75 (2018) 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Penny CJ, Vassileva K, Jha A, Yuan Y, Chee X, Yates E, Mazzon M, Kilpatrick BS, Muallem S, Marsh M, Rahman T, Patel S, Mining of Ebola virus entry inhibitors identifies approved drugs as two-pore channel pore blockers, Biochimica et biophysica acta, Mol. Cell Res 1866 (7) (2019) 1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jin X, Zhang Y, Alharbi A, Hanbashi A, Alhoshani A, Parrington J, Targeting two-pore channels: current progress and future challenges, Trends Pharmacol. Sci 41 (8) (2020) 582–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lee HC, Aarhus R, A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose, J. Biol. Chem 270 (1995) 2152–2157. [DOI] [PubMed] [Google Scholar]
- [26].Lee HC, Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization, J. Biol. Chem 287 (38) (2012) 31633–31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Galione A, A primer of NAADP-mediated Ca signalling: from sea urchin eggs to mammalian cells, Cell Calcium 58 (2015) 27–47. [DOI] [PubMed] [Google Scholar]
- [28].Brailoiu E, Miyamoto MD, Dun NJ, Nicotinic acid adenine dinucleotide phosphate enhances quantal neurosecretion at the frog neuromuscular junction: possible action on synaptic vesicles in the releasable pool, Mol. Pharmacol 60 (2001) 718–724. [PubMed] [Google Scholar]
- [29].Brailoiu E, Patel S, Dun NJ, Modulation of spontaneous transmitter release from the frog neuromuscular junction by interacting intracellular Ca2+ stores: critical role for nicotinic acid-adenine dinucleotide phosphate (NAADP), BJ 373 (2003) 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A, NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs, Cell 111 (2002) 703–708. [DOI] [PubMed] [Google Scholar]
- [31].Patel S, Function and dysfunction of two-pore channels, Sci. Signal 8 (2015) re7. [DOI] [PubMed] [Google Scholar]
- [32].Ogunbayo OA, Duan J, Xiong J, Wang Q, Feng X, Ma J, Zhu MX, Evans AM, mTORC1 controls lysosomal Ca(2+) release through the two-pore channel TPC2, Sci. Signal 11 (525) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moccia F, Zuccolo E, Di Nezza F, Pellavio G, Faris PS, Negri S, De Luca A, Laforenza U, Ambrosone L, Rosti V, Guerra G, Nicotinic acid adenine dinucleotide phosphate activates two-pore channel TPC1 to mediate lysosomal Ca (2+) release in endothelial colony-forming cells, J. Cell Physiol 236 (1) (2021) 688–705. [DOI] [PubMed] [Google Scholar]
- [34].Ishibashi K, Suzuki M, Imai M, Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels, Biochem. Biophys. Res. Commun 270 (2) (2000) 370–376. [DOI] [PubMed] [Google Scholar]
- [35].Furuichi T, Cunningham KW, Muto S, A putative two pore channel AtTPC1 mediates Ca2+ flux in Arabidopsis leaf cells, Plant Cell Physiol. 42 (9) (2001) 900–905. [DOI] [PubMed] [Google Scholar]
- [36].Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D, The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement, Nature 434 (7031) (2005) 404–408. [DOI] [PubMed] [Google Scholar]
- [37].Hedrich R, Neher E, Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles, Nature 329 (1987) 833–836. [Google Scholar]
- [38].Schieder M, Rotzer K, Bruggemann A, Biel M, Wahl-Schott CA, Characterization of two-pore channel 2 (TPCN2)-mediated Ca2+ currents in isolated lysosomes, J. Biol. Chem 285 (28) (2010) 21219–21222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pitt SJ, Funnell T, Sitsapesan M, Venturi E, Rietdorf K, Ruas M, Ganesan A, Gosain R, Churchill GC, Zhu MX, Parrington J, Galione A, Sitsapesan R, TPC2 is a novel NAADP-sensitive Ca2+-release channel, operating as a dual sensor of luminal pH and Ca2+, J. Biol. Chem 285 (2010) 24925–24932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brailoiu E, Rahman T, Churamani D, Prole DL, Brailoiu GC, Hooper R, Taylor CW, Patel S, An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals, J. Biol. Chem 285 (49) (2010) 38511–38516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rybalchenko V, Ahuja M, Coblentz J, Churamani D, Patel S, Kiselyov K, Muallem S, Membrane potential regulates NAADP dependence of the pH and Ca2 + sensitive organellar two-pore channel TPC1, J. Biol. Chem 287 (2012) 20407–20416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pitt SJ, Lam AK, Rietdorf K, Galione A, Sitsapesan R, Reconstituted human TPC1 is a proton-permeable ion channel and is activated by NAADP or Ca2+, Sci. Signal 7 (326) (2014) ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lee CS, Tong BC, Cheng CW, Hung HC, Cheung KH, Characterization of two-pore channel 2 by nuclear membrane electrophysiology, Sci. Rep 6 (2016) 20282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ruas M, Davis LC, Chen CC, Morgan AJ, Chuang KT, Walseth TF, Grimm C, Garnham C, Powell T, Platt N, Platt FM, Biel M, Wahl-Schott C, Parrington J, Galione A, Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells, EMBO J. 34 (2015) 1743–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Walseth TF, Lin-Moshier Y, Jain P, Ruas M, Parrington J, Galione A, Marchant JS, Slama JT, Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide 2′-phosphate (NAADP) proteins in sea urchin egg, J. Biol. Chem 287 (2012) 2308–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lin-Moshier Y, Walseth TF, Churamani D, Davidson SM, Slama JT, Hooper R, Brailoiu E, Patel S, Marchant JS, Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells, J. Biol. Chem 287 (4) (2012) 2296–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Marchant JS, Lin-Moshier Y, Walseth TF, Patel S, The molecular basis for Ca2+ signalling by NAADP: two-pore channels in a complex? Messenger 1 (2012) 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Walseth TF, Lin-Moshier Y, Weber K, Marchant JS, Slama JT, Guse AH, Nicotinic acid adenine dinucleotide 2′-phosphate (NAADP) binding proteins in T-lymphocytes, Messenger (Los. Angel.) 1 (1) (2012) 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Guse AH, Linking NAADP to ion channel activity: a unifying hypothesis, Sci. Signal 5 (221) (2012) e18. [DOI] [PubMed] [Google Scholar]
- [50].Gunaratne GS, Brailoiu E, He S, Unterwald EM, Patel S, Slama JT, Walseth TF, Marchant JS, Essential requirement for JPT2 in NAADP-evoked Ca(2 +) signaling, Sci. Signal 14 (675) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Roggenkamp HG, Khansahib I, Hernandez CL, Zhang Y, Lodygin D, Krüger A, Gu F, Möckl F, Löhndorf A, Wolters V, Woike D, Rosche A, Bauche A, Schetelig D, Werner R, Schlüter H, Failla AV, Meier C, Fliegert R, Walseth TF, Flügel A, Diercks BP, Guse AH, HN1L/JPT2: a signaling protein that connects NAADP generation to Ca(2+) microdomain formation, Sci. Signal 14 (675) (2021). [DOI] [PubMed] [Google Scholar]
- [52].Zhang J, Guan X, Shah K, Yan J, Lsm12 is an NAADP receptor and a two-pore channel regulatory protein required for calcium mobilization from acidic organelles, Nat. Commun 12 (1) (2021) 4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lekontseva NV, Stolboushkina EA, Nikulin AD, Diversity of LSM family proteins: similarities and differences, Biochemistry (Mosc) 86 (Suppl 1) (2021) S38–S49. [DOI] [PubMed] [Google Scholar]
- [54].Marchant JS, Gunaratne GS, Cai X, Slama JT, Patel S, NAADP binding proteins find their identity, Trends Biochem. Sci (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hasegawa J, Strunk BS, Weisman LS, PI5P and PI(3,5)P(2): minor, but essential phosphoinositides, Cell Struct. Funct 42 (1) (2017) 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H, PI(3,5)P2 controls membrane traffic by direct activation of mucolipin Ca2+ release channels in the endolysosome, Nat. Commun 1 (4) (2010) 38, pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Boccaccio A, Scholz-Starke J, Hamamoto S, Larisch N, Festa M, Gutla PV, Costa A, Dietrich P, Uozumi N, Carpaneto A, The phosphoinositide PI(3,5)P(2) mediates activation of mammalian but not plant TPC proteins: functional expression of endolysosomal channels in yeast and plant cells, Cell Mol. Life Sci 71 (21) (2014) 4275–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lagostena L, Festa M, Pusch M, Carpaneto A, The human two-pore channel 1 is modulated by cytosolic and luminal calcium, Sci Rep 7 (2017) 43900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Guo J, Zeng W, Jiang Y, Tuning the ion selectivity of two-pore channels, Proc. Natl. Acad. Sci. U.S.A 114 (5) (2017) 1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cang C, Aranda K, Ren D, A non-inactivating high-voltage-activated two-pore Na (+) channel that supports ultra-long action potentials and membrane bistability, Nat. Commun 5 (2014) 5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Brailoiu E, Hooper R, Cai X, Brailoiu GC, Keebler MV, Dun NJ, Marchant JS, Patel S, An ancestral deuterostome family of two-pore channels mediate nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles, J. Biol. Chem 285 (2010) 2897–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cai X, Patel S, Degeneration of an intracellular ion channel in the primate lineage by relaxation of selective constraints, Mol. Biol. Evol 27 (10) (2010) 2352–2359. [DOI] [PubMed] [Google Scholar]
- [63].Dickinson MS, Myasnikov A, Eriksen J, Poweleit N, Stroud RM, Resting state structure of the hyperdepolarization activated two-pore channel 3, Proc. Natl. Acad. Sci. U.S.A 117 (4) (2020) 1988–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Shimomura T, Kubo Y, Phosphoinositides modulate the voltage dependence of two-pore channel 3, J. Gen. Physiol 151 (8) (2019) 986–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Balla T, Phosphoinositides: tiny lipids with giant impact on cell regulation, Physiol. Rev 93 (3) (2013) 1019–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cang C, Zhou Y, Navarro B, Seo YJ, Aranda K, Shi L, Battaglia-Hsu S, Nissim I, Clapham DE, Ren D, mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state, Cell 152 (4) (2013) 778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Freeman SA, Uderhardt S, Saric A, Collins RF, Buckley CM, Mylvaganam S, Boroumand P, Plumb J, Germain RN, Ren D, Grinstein S, Lipid-gated monovalent ion fluxes regulate endocytic traffic and support immune surveillance, Science 367 (6475) (2020) 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Fine M, Schmiege P, Li X, Structural basis for PtdInsP(2)-mediated human TRPML1 regulation, Nat. Commun 9 (1) (2018) 4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gao Y, Cao E, Julius D, Cheng Y, TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action, Nature 534 (7607) (2016) 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].McGoldrick LL, Singh AK, Saotome K, Yelshanskaya MV, Twomey EC, Grassucci RA, Sobolevsky AI, Opening of the human epithelial calcium channel TRPV6, Nature 553 (7687) (2018) 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wang Q, Corey RA, Hedger G, Aryal P, Grieben M, Nasrallah C, Baronina A, Pike ACW, Shi J, Carpenter EP, Sansom MSP, Lipid interactions of a ciliary membrane TRP channel: simulation and structural studies of polycystin-2, Structure 28 (2) (2020) 169–184, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kirsch SA, Kugemann A, Carpaneto A, Bockmann RA, Dietrich P, Phosphatidylinositol-3,5-bisphosphate lipid-binding-induced activation of the human two-pore channel 2, Cell Mol. Life Sci (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Milenkovic S, Bodrenko IV, Lagostena L, Gradogna A, Serra G, Bosin A, Carpaneto A, Ceccarelli M, The mechanism and energetics of a ligand-controlled hydrophobic gate in a mammalian two pore channel, Phys. Chem. Chem. Phys 22 (27) (2020) 15664–15674. [DOI] [PubMed] [Google Scholar]
- [74].Milenkovic S, Bodrenko IV, Carpaneto A, Ceccarelli M, The key role of the central cavity in sodium transport through ligand-gated two-pore channels, Phys. Chem. Chem. Phys 23 (34) (2021) 18461–18474. [DOI] [PubMed] [Google Scholar]
- [75].Gerndt S, Chen CC, Chao YK, Yuan Y, Burgstaller S, Scotto Rosato A, Krogsaeter E, Urban N, Jacob K, Nguyen ONP, Miller MT, Keller M, Vollmar AM, Gudermann T, Zierler S, Schredelseker J, Schaefer M, Biel M, Malli R, Wahl-Schott C, Bracher F, Patel S, Grimm C, Agonist-mediated switching of ion selectivity in TPC2 differentially promotes lysosomal function, Elife 9 (2020) e54712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gerndt S, Krogsaeter E, Patel S, Bracher F, Grimm C, Discovery of lipophilic two-pore channel agonists, Febs J. 287 (24) (2020) 5284–5293. [DOI] [PubMed] [Google Scholar]
- [77].Zhang X, Chen W, Li P, Calvo R, Southall N, Hu X, Bryant-Genevier M, Feng X, Geng Q, Gao C, Yang M, Tang K, Ferrer M, Marugan JJ, Xu H, Agonist-specific voltage-dependent gating of lysosomal two-pore Na(+) channels, Elife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


