Abstract
Background
Both schizophrenia (SZ) and overweight/obesity (OWB) have shown some structural alterations in similar brain regions. As higher body mass index (BMI) often contributes to worse psychiatric outcomes in SZ, this study was designed to examine the effects of OWB on gray matter volume (GMV) in patients with SZ.
Methods
Two hundred fifty subjects were included and stratified into four groups (n = 69, SZ patients with OWB, SZ-OWB; n = 74, SZ patients with normal weight, SZ-NW; n = 54, healthy controls with OWB, HC-OWB; and n = 53, HC with NW, HC-NW). All participants were scanned using high-resolution T1-weighted sequence. The whole-brain voxel-based morphometry was applied to examine the GMV alterations, and a 2 × 2 full factorial analysis of variance was performed to identify the main effects of diagnosis (SZ vs HC), BMI (NW vs OWB) factors, and their interactions. Further, the post hoc analysis was conducted to compare the pairwise differences in GMV alterations.
Results
The main effects of diagnosis were located in right hippocampus, bilateral insula, rectus, median cingulate/paracingulate gyri and thalamus (SZ < HC); while the main effects of BMI were displayed in right amygdala, left hippocampus, bilateral insula, left lingual gyrus, and right superior temporal gyrus (OWB < NW). There were no significant diagnosis-by-BMI interaction effects in the present study, but the results showed that both SZ and OWB were additively associated with lower GMV in bilateral insula. Moreover, mediation analyses revealed the indirect effect of BMI on negative symptom via GMV reduction in bilateral insula.
Conclusion
This study further supports that higher BMI is associated with lower GMV, which may increase the risk of unfavourable disease courses in SZ.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-022-04285-4.
Keywords: Schizophrenia, Obesity, Gray matter, Insula, Negative symptom
Introduction
Schizophrenia (SZ) is a highly disabling mental disorder with a lifetime prevalence of approximately 1% [1], imposing an enormous burden on public health. Until now, the pathogenesis and development of SZ remains unelucidated, and current treatment approaches supported by evidence-based medicine are limited [2]. Hence, it is important to clarify the etiology and neurobiological mechanisms of SZ from a more in-depth perspective.
With the development of magnetic resonance imaging (MRI) technology, researchers have identified multiple brain structural alterations associated with SZ, the most commonly observed of which are the frontal, temporal, insular, and mesostriatal brain regions [3, 4]. Nevertheless, the location and direction of these brain regions often vary across studies, and the reasons for this heterogeneity remain unknown. They may be related to premorbid neurodevelopmental differences or the presence of certain clinical characteristics [5]. As such, a better understanding of the impacts of key clinical factors on brain alterations is critical for interpreting these inconsistent findings, and it is also the first step towards their management. Of note, excess body weight, such as being overweight or obese, may be a potential source of heterogeneity in brain structural alterations.
Clinically, overweight/obesity is particularly prevalent in SZ, with nearly 40–60% of patients with SZ being overweight or obese, a rate significantly higher than that in the general population (20–40%) [6–9]. Among the factors contributing to this high prevalence, a complex interplay between the adverse effects of antipsychotics, unhealthy lifestyle, and pathophysiological mechanisms inherent in SZ, have been the most cited explanations [7]. Notably, SZ patients with overweight/obesity tend to have poorer cognitive performance and physical condition, as well as shorter life expectancy than their lean counterparts [10]. In light of this, weight gain abnormalities are also considered one of the strongest contributors to adverse psychiatric outcomes in SZ [11]. In addition to these observations, researchers have also demonstrated that being overweight or obese is related to altered brain structure in the general population [12]. Specifically, overweight/obesity-related brain structural alterations have been identified in a wide range of areas involved in emotion and memory (e.g., the amygdala, hippocampus), cognitive control (e.g., cingulate, prefrontal cortex), reward (e.g., the striatum, insula, orbitofrontal cortex), and regulation of energy homeostasis (e.g., the hypothalamus) [13–15]. Notably, all these brain regions, as well as the associated neural circuits and functions are frequently disrupted in SZ [16]. Indeed, obesity has been suggested to share some common pathophysiological mechanisms with various mental disorders, including SZ [17, 18]. Overlapping pathoetiologies include chronic low-grade inflammation, increased oxidative stress, neurotransmitter imbalances, and hypothalamic–pituitary–adrenal axis disturbances [17, 18], which might be related to the common neuroimaging abnormalities and high prevalence of obesity in patients with SZ.
As aforementioned, both SZ and overweight/obesity showed structural alterations in similar brain regions. Since the prevalence of overweight/obesity is remarkably increased in patients with SZ, previous findings on brain structural changes in SZ may have been confounded by the effects of weight gain abnormalities. Accordingly, future study investigating brain morphometric alterations in SZ should carefully stratify the groups according to BMI. Moreover, comorbidity with overweight/obesity often contributes to an unfavorable course of disease [6, 19], suggesting that similar brain structural changes might have mediated the adverse influence of overweight/obesity on clinical outcomes in SZ. Thus, the relationship between BMI, brain structural alterations, and clinical symptoms should be clarified. Therefore, in the present study, we performed (1) a 2 × 2 full factorial analysis of variance (ANOVA) to examine the effects of diagnosis, BMI, and their interactions on GMV using a whole-brain voxel-based morphometry (VBM) approach, (2) a post hoc analysis to determine the pairwise comparison in brain regions with significant GMV alterations, and (3) association and mediation analyses to investigate the underlying relationship among BMI, GMV alterations, and clinical symptoms.
To our knowledge, while the effects of overweight/obesity on brain structure are of great concern in SZ, few studies have adopted such a design to examine overweight/obesity-related brain structural alterations and whether these directly overlap with brain regions associated with SZ [20]. Therefore, our research may address this knowledge gap. We hypothesized that both SZ and overweight/obesity will be negatively related to some GMV alterations in similar brain regions, and that the relationship between BMI and clinical symptoms may be mediated by GMV alterations.
Methods
Participants
In total, 250 Chinese Uyghur participants were included into this study, including 69 SZ patients with overweight or obesity (SZ-OWB), 74 SZ patients with normal weight (SZ-NW), 54 healthy controls with OWB (HC-OWB), and 53 healthy controls with NW (HC-NW). The healthy participants were recruited from physical examination center and local communities through advertising. The SZ patients were recruited from the Psychiatry Department of the Affiliated Kashi Hospital of Sun Yat-Sen University, Kashi, Xinjiang, China. The BMI category are as follows: normal weight as 18.50 ≤ BMI < 25.00 kg/m2, overweight or obesity as BMI ≥ 25.00 kg/m [21].
Inclusion criteria for SZ patients: (1) met the diagnostic criteria for SZ according to the International Classification of Disease 10th Revision (ICD-10); (2) age between 18 and 55; (3) right handedness; (4) had been educated for at least six years.
Inclusion criteria for HC: (1) no personal or family history of mental disorder refer to mini-SCID; (2) age between 18 and 55; (3) right handedness; (4) had been educated for at least six years.
Exclusion criteria: (1) history of other mental disorder, including anxiety, depression, bipolar disorder, mental retardation, eating disorders, etc.; (2) significant brain lesions, including cerebral infarction, hemorrhage, or intracranial masses; (3) alcohol or substance addiction; (4) metabolic related diseases such as hypertension, diabetes, hypothyroidism or hyperthyroidism; (5) any MRI contraindications, such as metallic implants and claustrophobia; (6) pregnant or lactating women.
Psychological and clinical assessments
The SZ was diagnosed by a chief psychiatrist based on the ICD-10 diagnostic criteria [22]. The positive and negative syndrome scale (PANSS) was used to quantify the severity of symptoms in SZ patients. The Montreal cognitive assessment (MoCA) was adopted to measure the cognitive performance in all participants. The MoCA is a cognitive screening test, which provided scores for seven cognitive domains: visuospatial/constructional abilities, naming, language, attention, abstraction, delayed memory, orientation [23].
Additionally, all the participants were asked to fill in a designed questionnaire, including sex, age, ethnicity, education background, duration of illness, age of onset, number of hospitalizations, antipsychotics history (type, dose and duration), and lifestyle (diet, smoking, and drinking habits). The body parameters including height, weight as well as waist and hip circumferences of all participants were measured and recorded by the same nurse. All the psychological and clinical assessments were conducted within three days after MRI scanning.
Imaging acquisition
The MRI data was obtained on 3.0 T magnetic resonance scanner (Magnetom Skyra, Siemens, Erlangen, Germany) equipped with a 32-channel head coil. Participants lied on the scanner in the supine position with eyes closed, and foam pads were used to minimize head motion. The high-resolution sagittal T1-weighted images were acquired using a T1-weighted magnetization-prepared-rapid-acquisition-gradient-echo (MP-RAGE) sequence (repetition time = 2000.0 ms, echo time = 2.22 ms, inversion time = 900 ms, flip angle = 8°, field of view = 256 × 256 mm2, voxel size = 0.9 × 0.9 × 0.9 mm3, and 176 slices). The total scan time was 3 min and 54 s.
Voxel-based morphometry (VBM) analysis
The structural data were preprocessed using optimized VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) for SPM8 (http://www.fil.ion.ucl.ac.uk/spm) under a MATLAB (version R2013b, MathWorks, MA, USA) environment. Briefly, the main steps include: a) discarded the images with visible artifacts; b) format conversion and corrected the image origin (reorientation); c) bias field correction; d) structural images were segmented into three tissue components: gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF); e) the segmented images of all subjects were normalized to the Montreal Neurological Institute (MNI) space using the DARTEL deformations (resliced into 1.5mm3); f) the resulting GM images ( modulated GMV) were smoothed using an isotropic Gaussian kernel of 8 mm FWHM.
At the secondary level of analysis, we applied a whole-brain voxel-wise general linear model with a 2 (diagnosis: SZ vs HC) × 2 (BMI category: OWB vs NW) full factorial ANOVA, controlling for age, sex, education and total intracranial volume (ICV), to determine the main effects of diagnosis and BMI factors, as well as their interaction effects. The voxel-wise threshold was set at p < 0.001, and corrected by a cluster-level Familywise error (FWE) correction. For reporting purposes, the masks of significant cluster were defined using the Automated Anatomical Labeling (AAL) atlas [24]. Then, the brain regions with significant difference were saved as masks, and the mean GMV values within each significant cluster were extracted for all subjects, For each significant cluster, the region of interest (ROI)-based post hoc analyses was applied to compare the pairwise differences among the four groups (HC-NW vs SZ-NW; HC-OWB vs SZ-OWB; HC-NW vs HC-OWB; SZ-NW vs SZ-OWB) while controlling for age, sex, education and total ICV (p < 0.001, FWE corrected).
Statistical analyses
For the demographic and clinical data, the statistical analysis was performed using the SPSS 25.0 (IBM Corp., Armonk, NY), with a threshold of p < 0.05 being considered as statistically significant. The continuous variables were summarized by means ± standard deviations, and analysed by independent t-test or one-way ANOVA. The categorical variables were summarized using frequencies, and analysed by Chi-square test. To further test the “diagnosis × BMI” interaction effects on GMV, we performed the multivariate analysis of covariance with diagnosis and BMI as explanatory variables, and regional GMV as the dependent variables, while controlling for age, sex, education and ICV as covariates of no interest. To increase the sensitivity, we also repeated the analyses with BMI as a continuous variable. Correlation analyses of significant GMV with clinical symptoms and cognitive test scores were conducted using Pearson’s partial correlation coefficient. Age, sex, education, duration of illness, BMI and ICV were included as covariates in the partial correlation analysis within the SZ group. Age, sex, education, BMI and ICV were included as covariates in the partial correlation analysis within the overweight/obese group.
To determine whether the effects of BMI on psychiatric symptoms (PANSS) were mediated by altered GMV, the mediation analysis was conducted by employing a model 4 of the PROCESS software (http://www.processmacro.org) implanted in SPSS, controlling for age, sex, education, ICV and duration of illness. The Bonferroni-correction with q < 0.05 was applied to correct for multiple comparisons, resulting in a statistical threshold of p < 0.0167 (0.05/3). The direct effects and total effects were considered significant when the p < 0.0167 or the 95% confidence intervals (CI) did not include zero in the 5,000 bootstrap samples corrected. The indirect effects were considered significant when the CI did not include zero in the 5,000 bootstrap samples corrected [25]. The mediation effects exist when the indirect effects were statistically significant.
Results
Demographic and clinical comparisons
As shown in Table 1, there were no significant differences in age, sex, education, and smoking and drinking rates among the four groups. The two subgroups of patients with SZ did not show significant differences in duration of illness, age of onset, antipsychotic medication use, and PANSS total and subscale scores. As expected, the patients with SZ showed lower scores in the MoCA total and six subscale scores compared to the HC. Compared with SZ patients with normal weight, patients with overweight/obesity displayed lower scores in attention and delayed memory (Table S1); compared with the HC with normal weight, the HC with overweight/obesity displayed lower score in visuospatial/constructional abilities (Table S2).
Table 1.
Demographic and clinical characteristics for all participants (n = 250)
| SZ | HC | F/t/2 | p vaue | |||
|---|---|---|---|---|---|---|
| OWB (n = 69) | NW (n = 74) | OWB (n = 54) | NW (n = 53) | |||
| Age (years)a | 33.88 ± 8.85 | 31.86 ± 8.70 | 33.31 ± 7.91 | 31.42 ± 7.07 | 1.248 | 0.293 |
| Sex (male/female) | 32/37 | 31/43 | 23/31 | 23/30 | 0.327 | 0.955 |
| Education (years) | 10.28 ± 3.67 | 10.41 ± 3.30 | 10.83 ± 3.01 | 11.55 ± 3.34 | 1.710 | 0.165 |
| BMI (kg/m2) | 28.61 ± 2.70 | 21.67 ± 2.44 | 28.19 ± 2.62 | 21.63 ± 2.33 | 149.234 | < 0.001 |
| Age of onset (years) | 26.32 ± 8.92 | 24.76 ± 7.62 | N/A | N/A | 1.125 | 0.263 |
| Duration of illness (years) | 7.81 ± 5.63 | 7.10 ± 5.32 | N/A | N/A | 0.772 | 0.441 |
| Antipsychotic medications (%) | 58 (84.1) | 57 (77.0) | N/A | N/A | 0.719 | 0.397 |
| CPeq (mg/day) | 349.6 ± 170.4 | 374.7 ± 253.9 | N/A | N/A | -0.697 | 0.487 |
| Smoking regularly (%) | 26 (37.7) | 20 (27.0) | 15 (27.8) | 11 (20.8) | 3.327 | 0.344 |
| Drinking regularly (%) | 11 (15.9) | 6 (8.1) | 7 (13.0) | 3 (5.7) | 4.166 | 0.244 |
| PANSS total scores | 81.62 ± 15.06 | 78.45 ± 17.91 | N/A | N/A | 1.144 | 0.255 |
| Positive symptom | 21.38 ± 5.33 | 21.53 ± 6.10 | N/A | N/A | -0.156 | 0.876 |
| Negative symptom | 21.25 ± 5.59 | 20.26 ± 4.90 | N/A | N/A | 1.128 | 0.610 |
| General psychopathology | 39.00 ± 10.11 | 36.66 ± 11.76 | N/A | N/A | 1.271 | 0.206 |
| MoCA total scores | 19.46 ± 5.44 | 20.26 ± 6.02 | 24.76 ± 3.06 | 24.57 ± 3.96 | 19.477 | < 0.001 |
| Visuospatial/constructional | 2.81 ± 1.53 | 2.86 ± 1.55 | 3.22 ± 1.19 | 3.75 ± 1.37 | 5.391 | 0.001 |
| Naming | 2.43 ± 0.67 | 2.31 ± 0.72 | 2.63 ± 0.52 | 2.62 ± 0.63 | 3.590 | 0.014 |
| Attention | 3.52 ± 1.56 | 4.23 ± 1.57 | 5.31 ± 0.93 | 4.92 ± 1.41 | 19.115 | < 0.001 |
| Language | 2.25 ± 0.91 | 2.05 ± 1.01 | 2.43 ± 0.84 | 2.40 ± 0.91 | 2.190 | 0.090 |
| Abstraction | 1.25 ± 0.85 | 1.22 ± 0.75 | 1.28 ± 0.63 | 1.28 ± 0.66 | 0.113 | 0.952 |
| Delayed memory | 1.97 ± 1.64 | 2.68 ± 1.52 | 3.46 ± 1.40 | 3.17 ± 1.65 | 10.895 | < 0.001 |
| Orientation | 4.57 ± 1.37 | 4.26 ± 1.70 | 5.81 ± 0.48 | 5.87 ± 0.44 | 29.577 | < 0.001 |
Significant p-values are shown in bold cases
SZ Schizophrenia, HC Healthy controls, BMI Body mass index, CPeq Chlorpromazine-equivalent doses, PANSS Positive and Negative Syndrome Scale, MoCA Montreal Cogntive Assessment
aAge at the time of MR imaging
GMV alterations
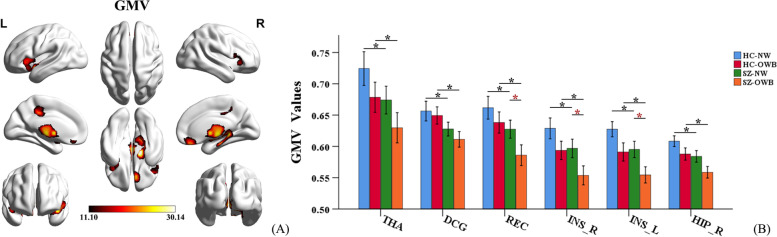
There were significant main effects of diagnosis in the right hippocampus, bilateral insula, rectus, median cingulate/paracingulate gyri and thalamus, with reduced GMV in patients with SZ relative to HC (p < 0.001, FWE corrected) (Fig. 1A and Table 2). The post hoc analysis showed that both SZ-NW and SZ-OWB patients had significant GMV reduction in the aforementioned brain regions relative to that of the HC-NW and HC-OWB subjects, respectively (p < 0.001, FWE corrected). Meanwhile, SZ-OWB patients also showed significant GMV reduction in the bilateral insula and rectus relative to that of SZ-NW patients (p < 0.001, FWE corrected). No significant differences were observed in the aforementioned brain regions between HC-OWB and HC-NW participants (p > 0.001, FWE corrected) (Fig. 1B).
Fig. 1.
Brain regions showing significant main effects of diagnosis (p < 0.001, FWE corrected). A Results in the whole-brain VBM analysis: the red/orange indicates brain regions with lower GMV in SZ compared to HC group (SZ < HC), the color scales represent F-values. B A post hoc ROI analysis shows the pairwise comparisons in GMV, error bars indicate the standard error of the mean. *p < 0.001, FWE corrected. Abbreviations: SZ = schizophrenia; HC = healthy controls; L = left; R = right; HIP = hippocampus; INS = insula; REC = gyrus rectus; DCG = median cingulate and paracingulate gyri; THA = thalamus
Table 2.
Brain regions showing significant main effects of diagnosis and BMI in VBM analysis*
| Brain region | Cluster size (voxels) | F value | MNI coordinate | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Main effects of diagnosis | ||||||
| Hippocampus | R | 1307 | 30.1 | 16.5 | -12 | -12 |
| Insula | L | 2511 | 28.3 | -45 | 4.5 | -7.5 |
| Insula | R | 1031 | 19.1 | 54 | 21 | 1.5 |
| Rectus | B | 1110 | 22.9 | 7.5 | 37.5 | -21 |
| Cingulum-Mid | B | 1224 | 21.1 | -7.5 | -31.5 | 42 |
| Thalamus | B | 1934 | 25.3 | 1.5 | -18 | -3 |
| Main effects of BMI | ||||||
| Amygdala | R | 1982 | 24.8 | 37.5 | -1.5 | -5.5 |
| Hippocampus | L | 4339 | 27.2 | -30 | 4.5 | -34.5 |
| Insula | L | 1626 | 19.7 | -24 | 22.5 | -6 |
| Insula | R | 668 | 19.2 | 40.5 | 30 | 4.5 |
| Lingual | L | 1383 | 26.0 | -24 | -63 | -1.5 |
| Temporal_sup | R | 1254 | 23.9 | 67.5 | -13.5 | 4.5 |
SZ Schizophrenia, OWB Overweight or obesity, L Left, R Right, B Bilateral, Cingulum-Mid Median cingulate and paracingulate gyri, Temporal_sup Superior temporal gyrus, MNI Montréal neurological institute
*p < 0.001, FWE corrected
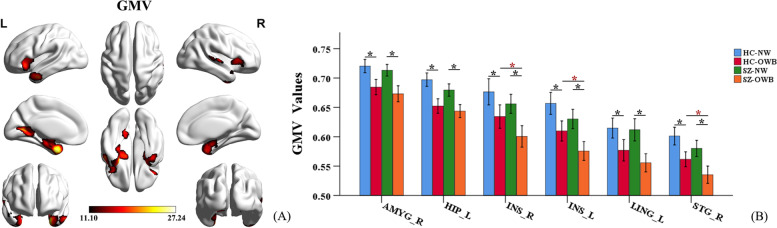
In addition, there were significant main effects of BMI in the right amygdala, left hippocampus, bilateral insula, left lingual gyrus, and right superior temporal gyrus, with decreased GMV in OWB participants compared to NW individuals (p < 0.001, FWE corrected) (Fig. 2A and Table 2). The post hoc analyses showed that both the HC-OWB and SZ-OWB participants had significant GMV reduction in the aforementioned brain regions relative to that of the HC-NW and SZ-NW participants, respectively (p < 0.001, FWE corrected). Meanwhile, SZ-OWB patients also showed significant GMV reduction in the bilateral insula and right superior temporal gyrus relative to that of the HC-OWB patients (p < 0.001, FWE corrected). No significant differences were observed in the aforementioned brain regions between SZ-NW and HC-NW subjects (p > 0.001, FWE corrected) (Fig. 2B).
Fig. 2.
Brain regions showing significant main effects of BMI (p < 0.001, FWE corrected). A Results in the whole-brain VBM analysis: the red/orange indicates brain regions with lower GMV in OWB compared to NW group (OWB < NW), the color scales represent F-values. B A post hoc ROI analysis shows the pairwise comparisons in GMV, error bars indicate the standard error of the mean. *p < 0.001, FWE corrected. Abbreviations: OWB = overweight or obesity; NW = normal weight; L = left; R = right; AMYG = amygdala; HIP = hippocampus; INS = insula; LING = lingual gyrus; STG = superior temporal gyrus
There were no significant diagnosis-by-BMI interaction effects in both whole-brain VBM analysis and multivariate analysis of covariance. The results of multivariate analysis of covariance are summarized in Table S3. Notably, the results showed that both SZ and OWB were additively associated with lower GMV in bilateral insula (Fig. S3).
Partial correlation analysis
The relationship between GMV alterations and clinical symptoms is presented in Table 3. The brain regions displaying significant main effects of diagnosis are shown in Table 3a. In the SZ group, both the GMV values in the left insula and right insula were positively correlated with attention (r = 0.250, p = 0.003), and negatively correlated with PANSS negative scores (r = -0.184, p = 0.032; r = -0.214, p = 0.012, respectively). In addition, the GMV values in the rectus showed a positive relationship with abstraction (r = 0.210, p = 0.014), but a negative relationships with PANSS negative scores (r = -0.180, p = 0.035) and general psychopathology scores (r = -0.222, p = 0.009). Additionally, both the GMV in the thalamus and right hippocampus were positively correlated with visuospatial/constructional abilities (r = 0.197, p = 0.021and r = 0.191, p = 0.026, respectively), and the GMV in the thalamus was positively correlated with attention (r = 0.256, p = 0.003) in patients with SZ.
Table 3.
Pearson’s partial correlations between brain GMV and clinical symptoms
| 3a: Brain regions showing significant main effects of diagnosisa | ||||||
|
Cingulum_Mid (r, p) |
Insula-L (r, p) |
Insula-R (r, p) |
HIP-R (r, p) |
Rectus (r, p) |
Thalamus (r, p) |
|
| PANSS total | -0.001, 0.991 | -0.036, 0.678 | -0.106, 0.218 | 0.005, 0.957 | 0.041, 0.634 | -0.035, 0.685 |
| PANSS positive | -0.061, 0.481 | 0.042, 0.627 | -0.037, 0.671 | -0.022, 0.801 | -0.143, 0.094 | 0.046, 0.591 |
| PANSS negative | -0.141, 0.100 | -0.254, 0.003 | -0.301, 0.000 | -0.071, 0.412 | -0.180, 0.035 | -0.139, 0.105 |
| PANSS general psychopathology | 0.097, 0.260 | 0.045, 0.600 | 0.002, 0.978 | 0.052, 0.548 | 0.222, 0.009 | -0.010, 0.904 |
| MoCA total | 0.082, 0.342 | 0.160, 0.062 | 0.192, 0.025 | 0.142, 0.099 | 0.114, 0.184 | 0.137, 0.110 |
| Visuospatial/constructional | 0.044, 0.611 | 0.148, 0.084 | 0.163, 0.057 | 0.191, 0.026 | 0.098, 0.253 | 0.197, 0.021 |
| Naming | -0.012, 0.890 | 0.039, 0.654 | 0.015, 0.866 | 0.087, 0.310 | 0.031, 0.723 | 0.090, 0.294 |
| Attention | 0.154, 0.072 | 0.250, 0.003 | 0.250, 0.003 | 0.157, 0.067 | 0.151, 0.078 | 0.256, 0.003 |
| Language | 0.006, 0.948 | 0.053, 0.540 | 0.112, 0.193 | 0.044,0.609 | 0.009, 0.917 | -0.014, 0.874 |
| Abstraction | -0.098, 0.252 | -0.019, 0.823 | 0.015, 0.865 | -0.045,0.599 | 0.210, 0.014 | -0.074, 0.391 |
| Delayed memory | -0.008, 0.929 | 0.116, 0.177 | 0.114, 0.184 | 0.102, 0.234 | -0.025, 0.770 | 0.026, 0.759 |
| Orientation | 0.117, 0.174 | -0.015, 0.864 | 0.059, 0.496 | 0.069, 0.420 | 0.024, 0.777 | 0.053, 0.539 |
| 3b: Brain regions showing significant main effects of BMIb | ||||||
|
HIP_L (r, p) |
Insula-L (r, p) |
Insula-R (r, p) |
LING_L (r, p) |
AMYG_R (r, p) |
STG_R (r, p) |
|
| MoCA total | 0.012, 0.900 | 0.180, 0.052 | 0.118, 0.201 | 0.013, 0.889 | 0.362, 0.000 | 0.360, 0.000 |
| Visuospatial/constructional | 0.050, 0.591 | 0.058, 0.532 | 0.038, 0.684 | -0.151,0.103 | 0.134, 0.149 | 0.184, 0.046 |
| Naming | -0.106, 0.254 | -0.028, 0.762 | -0.101, 0.276 | 0.072, 0.436 | 0.284, 0.002 | 0.087, 0.347 |
| Attention | 0.066, 0.479 | 0.253, 0.006 | 0.260, 0.004 | 0.158, 0.087 | 0.287, 0.002 | 0.302, 0.001 |
| Language | -0.143, 0.123 | 0.090, 0.332 | -0.059, 0.529 | -0.168, 0.069 | 0.087, 0.351 | 0.116, 0.212 |
| Abstraction | -0.043, 0.643 | -0.027, 0.768 | -0.059, 0.523 | -0.068, 0.465 | 0.066, 0.478 | 0.099, 0.287 |
| Delayed memory | 0.019, 0.836 | 0.117, 0.206 | 0.100, 0.284 | 0.029, 0.758 | 0.307, 0.001 | 0.219, 0.017 |
| Orientation | 0.059, 0.523 | 0.081, 0.385 | 0.049, 0.595 | 0.105, 0.256 | 0.208, 0.024 | 0.294, 0.001 |
Significant p-values are shown in bold cases
SZ Schizophrenia, OWB Overweight or obesity, PANSS Positive and Negative Syndrome Scale, MoCA Montreal Cogntive Assessment, L Left, R Right, HIP Hippocampus, AMYG Amygdala, LING Lingual gyrus, STG Superior temporal gyrus, BMI Body mass index, ICV Total intracranial volume
aCorrelations between brain GMV and clinical symptoms (PANSS and MoCA) in the SZ group, controlling for age, sex, education, illness duration, BMI and ICV
bCorrelations between brain GMV and clinical symptoms (MoCA) in OWB group, controlling for age, sex, education, BMI and ICV
The brain regions that showed significant main effects of BMI are shown in Table 3b. Among the participants with overweight/obesity, both the GMV values in the left insula and right insula were positively correlated with attention (r = 0.253, p = 0.006; r = 0.260, p = 0.004, respectively), while the GMV alterations in the right amygdala and superior temporal gyrus had positive relationships with both the MoCA total and subscale scores (including visuospatial/constructional abilities, naming, attention, delayed memory, and orientation) (all p < 0.05).
Mediation analysis
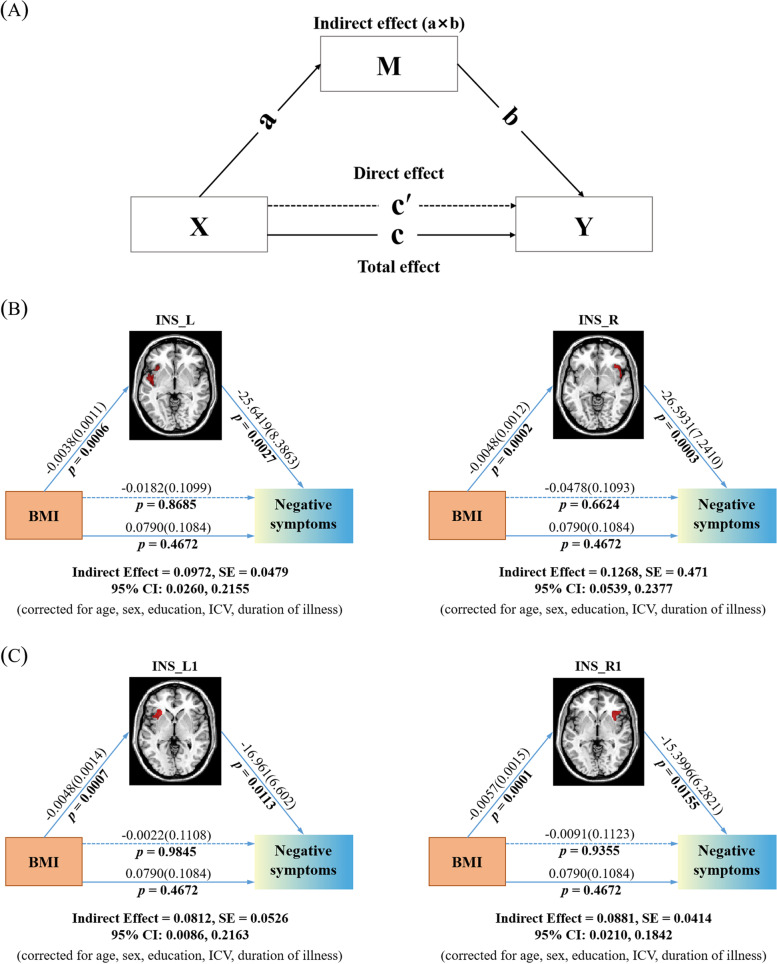
In the SZ group, both the BMI and negative symptom scores showed a negative relationship with the bilateral insula (Figs. S1 and S2). To determine whether the effect of BMI on negative symptom was mediated by altered GMV in the bilateral insula, a mediation analysis was conducted in the SZ group. Mediation analysis revealed an indirect effect of BMI on negative symptom via GMV alterations in the bilateral insula (Fig. 3); however, the total and direct effects of BMI on negative symptom were not significant, indicating that inhibitory effects existed [26], and the association between BMI and negative symptom became statistically significant only when the GMV in the bilateral insula was included in the models. Hence, the lower GMV in the bilateral insula mediates the effect of higher BMI on negative symptom severity.
Fig. 3.
Mediation analysis testing whether GMV in bilateral insula mediates the relationship between BMI and negative symptoms in SZ. The age, sex, education, ICV, and duration of illness were included as covariates. A Standard 3-variable path model of mediation analysis. Path c is the total effect of X on Y; path c′ is the direct effect of X on Y after controlling for M; the product of paths a and b (a × b) is the indirect effect of X on Y through M. B Results from the bilateral insula identified in main effects of diagnosis: INS_L (left) and INS_R (right). C Results from the bilateral insula identified in main effects of BMI: INS_L1 (left) and INS_R1 (right). Unstandardized path coefficients are displayed along with standard errors in parentheses, and significance levels (ie, p value) underneath them. The total effects, direct effects, and indirect effects were considered significant when the p < 0.05 or 95% CIs did not contain zero. Note: GMV, gray matter volume; INS, insula; L, left; R, right; SZ, schizophrenia; OWB, overweight/obesity; ICV, total intracranial volume; BMI, body mass index; SE, standard errors; CIs, confidence intervals
Specifically, for the bilateral insula identified in main effects of diagnosis, BMI had significant indirect effects on negative symptom severity via GMV in left the insula (a × b = 0.0972, 95% CI: 0.0260, 0.2155) or right insula (a × b = 0.1268, 95% CI: 0.0539, 0.2377) and had no significant direct effect on negative symptom severity (c′ = -0.0182, p = 0.8685; c′ = -0.0478, p = 0.6624; respectively) (Fig. 3B). In addition, similar results were found for the bilateral insula identified in main effects of BMI, BMI had significant indirect effects on negative symptom severity via GMV in the left insula (a × b = 0.0812, 95% CI: 0.0086, 0.2163) or right insula (a × b = 0.0881, 95% CI: 0.0210, 0.1842) and had no significant direct effect on negative symptom severity (c′ = -0.0022, p = 9845; c′ = -0.0091, p = 0.9355; respectively) (Fig. 3C).
Discussion
In this study, we investigated the impact of the diagnosis and BMI on brain GMV, and we demonstrated that lower GMV in the right hippocampus, bilateral insula, rectus, median cingulate/paracingulate gyri, and thalamus were associated with SZ, while lower GMV in the right amygdala, left hippocampus, bilateral insula, left lingual gyrus, and right superior temporal gyrus were associated with being overweight/obesity independent of SZ diagnosis. There were no significant diagnosis-by-BMI interaction effects in this study, but the results showed that both SZ and OWB were additively associated with lower GMV in the bilateral insula. In SZ, mediation analysis revealed an indirect effect of BMI on negative symptom via GMV reduction in the bilateral insula. Altogether, these findings provide further evidence that higher BMI is associated with lower GMV, which could increase the risk of unfavorable disease course in SZ.
We identified significant main effects of SZ diagnosis in the frontal, temporal, insular, and thalamus regions, which are considered the most replicated regions observed in SZ [3, 4]. The rectus and median cingulate/paracingulate gyri are integral parts of the frontal cortex [27], structural abnormalities in these two regions are responsible for impulsive behavior in SZ [28, 29]. Additionally, both the rectus (part of the orbitofrontal cortex proper) and insula are major components of the reward circuit [30]. Disrupted reward circuit has been proposed as the neuropathological basis of negative symptoms, manifested as apathy and social withdrawal in SZ [31]. This hypothesis is further supported by findings from the present study that lower GMV in rectus and insula were related to worsen negative symptom in patients with SZ. However, it is worth noting that insula abnormalities have also been reported to be associated with positive symptoms [32], so future studies may need to disentangle (or map) the abnormal structure, function and connectivity of insular subregions to symptoms, behavior and physiology. The thalamus is a major information hub implicated in multiple conscious integration processes, including cognition, emotion, and sensorimotor information processing [33, 34]. In support of this, our study also showed that a lower GMV in the thalamus was associated with cognitive decline (including visuospatial/constructional abilities and attention) in patients with SZ.
Moreover, the current study also identified lower GMV in temporal and insular regions in overweight/obese subjects, regardless of the SZ diagnosis. Temporal regions are associated with many cognitive processes [35, 36]. Substantial evidence suggests that gray matter atrophy in the temporal regions is associated with poor cognitive performance in obesity [15, 37], which is consistent with findings of our study that GMV loss in the amygdala, hippocampus and superior temporal gyrus were related with multiple cognitive impairments. Further, hippocampal-dependent learning and memory mechanisms have significant impacts on the regulation of food intake, mainly through the integration of external sensory information (olfactory and visuospatial) and internal cues (gustatory, endocrine, and gastrointestinal interoceptive stimuli) [38, 39]. In support are findings that hippocampal lesions lead to increased food intake and subsequent obesity in both animal and human studies [40, 41]. Similarly, the amygdala is known for detecting salient stimuli [42], which shows consistent functional activation in response to food-related stimuli and has consistently been observed in research on appetitive behavior [43, 44]. In addition, the insular cortex is predominantly involved in empathy, self-awareness, and interoception [45]. Previous studies have detected activation of the insula using balloon distention to mimic gastric dilation, thus indicating its role in the perception of fullness [46]. There is also evidence that blunt self-awareness of satiety signals may cause obese individuals to increase their food intake in response to interoceptive cues [47]. Along with these findings from previous and our study, we suppose that the brain structural deficits in the temporal and insular regions may underlie the dysfunction of appetite regulation in overweight/obese subjects.
Another obesity-affected region in the present study was the left lingual gyrus, a key part of visual information processing [48]. Previous studies have detected robust activation of the occipital cortex in response to visually presented food cues [49]. Recently, Stopyra et al. [50] revealed that increased activation of the lingual gyrus is strongly associated with a decrease in food craving. In the field of obesity, researchers have revealed a reduced activation of the occipital cortex in obese individuals [51]. Based on these evidence, it is possible that GMV loss in the left lingual gyrus may underlie the reduced brain activity in this area, thus leading to a stronger increase in food craving in overweight/obese subjects.
In the present study, most of the SZ-related and overweight/obesity-related brain regions were identified in the limbic regions associated with reward information processing, i.e., bilateral insula (sensory processing), amygdala-hippocampus (reward learning and memory), rectus and median cingulate/paracingulate gyri (reward value appraisal, executive control and decision-making) [27, 32, 42]. Indeed, appropriate responses to external salient stimuli, especially rewarding cues, require constant updating and learning to adjust behaviors according to new data [52]. This process requires coordination of all the above brain regions, thus structural deficits in any of these regions may lead to abnormalities in expressed behaviors. In line with this, theories of SZ indicate that dysfunction of the reward circuit is associated with negative symptom, primarily through disruption of reward processing and promotion of false integration of the inner sensory perception with salient stimuli [30]. Similarly, theories of obesity suggest that both hyporesponsivity to rewards and high levels of trait reward sensitivity cause individuals to consume more high-fat and high-calorie foods, thus increasing their vulnerability to overeating and obesity [53]. These theories support the role of neural reward circuits in both SZ and obesity. This similarity in the GMV deficit pattern may also serve as a potential explanation for the high prevalence of overweight or obesity in patients with SZ.
Notably, despite the relatively large sample size of this study (N = 250), we were not powered to detect a significant interaction effect in a whole-brain analysis having to correct for multiple (voxel-by-voxel) comparisons. However, the results did show that both SZ and overweight/obesity were additively related to lower GMV in bilateral insula. As a paralimbic structure, the insula has been implicated in a wide range of conditions and behaviors, from interoception of body sensations and movement to selective attention and salience detection [45]. GMV reduction in this region has been reported to be linked to negative symptom (e.g., anhedonia and amotivation) in SZ, which may be related to deficient insular detection of salience in reward processing [54]. The insula is a key node of the reward circuit, which is also involved in reward-related eating behaviors such as craving, feeding, and satiety [55]. Blunted activation of the insula is thought to result in overeating and subsequent obesity [56]. Conversely, overeating or obesity can further reduce the neural response to food cues, especially in hypothalamus and insular cortex, thereby leading to a vicious cycle [57, 58]. Thus, the common GMV deficits in bilateral insula may represent a key intersection point for both SZ and overweight/obesity.
Furthermore, the bilateral insula identified in both SZ and OWB was associated with attention deficits in the respective illnesses. In addition, mediation analysis revealed an indirect effect of BMI on negative symptom via GMV alterations in bilateral insula. These results are highly clinically relevant, as both SZ and OWB are responsible for worse cognitive performance and comorbidity with overweight/obesity often contribute to worse psychiatric outcomes in patients with SZ [59, 60]. As such, our findings suggest a critical role of the bilateral insula in the neuropathology of SZ as a potential adverse consequence of overweight/obesity that might contribute to an unfavorable course of disease. Moreover, both negative symptom and cognitive deficits tend to be resistant to antipsychotic treatments, and the presence of negative symptom early in the illness is closely related to a worsening course of the disease and greater lifelong disability [61]. Thus, identifying the mechanisms underlying such brain structural deficits in the bilateral insula may yield preventative and therapeutic targets for both SZ and OWB. Of relevance, longitudinal studies in the general population have shown that overweight/obesity-related brain structural alterations might be preventable or even reversible with lifestyle/medication/surgical interventions focused on weight management, particularly in teenagers and young adults [62–64]. Consistent with this evidence, lifestyle interventions for weight loss have shown positive effects in improving functional outcomes in recurrent depression [65], which are related to increases in brain volume [66, 67]. Therefore, efforts to foster weight loss may help address some common but currently intractable clinical outcomes such as negative symptom and attention deficits in SZ.
Finally, a previous study with a similar design reported that both SZ and overweight/obesity were associated with overlapped GMV alterations in the cerebellum [20], a region deeply connected to the cerebrum and had been proven to have potential functions in cognitive and mood regulation [68]. However, our study failed to identify similar results in the cerebellum. One explanation is that the effects of SZ on brain structural alterations may have been confounded by prolonged exposure to antipsychotics in our study, since long-term antipsychotic treatment have potential effects on brain structure [69]. Other factors, such as differences in the underlying methodology, as well as differences in the power and clinical characteristics of the study samples, might have also contributed to this inconsistent finding. Therefore, future studies are required to substantiate these findings in unmedicated patients with first-episode psychosis.
Limitations
This study has some limitations. First, this study had a cross-sectional design, which precluded causal inferences, thus longitudinal design is needed to determine whether GMV loss occurred before or after the weight gain abnormalities in patients with SZ. Second, the results of the mediation analysis should be interpreted with caution, as it is challenging to discuss causality in a mediation analysis. Future longitudinal research should be conducted to clarify the causal relationship between BMI and negative symptoms. Third, we did not collect information on the factors that might influence the correlation between BMI and brain structure, such as chronic stressors, diet, and exercise. Future studies will benefit from this consideration. Finally, the SZ participants in this study mainly consisted of chronic patients taking medications, thus failing to eliminate the confounders of illness chronicity and prolonged exposure to antipsychotics on brain alterations. However, to minimize the effect of these factors, the two SZ groups were matched for duration of illness and use of antipsychotic medications. To further increase homogeneity, we did not include participants with metabolic comorbidities, such as diabetes.
Conclusion
In summary, the present study provides further evidence that both SZ and OWB adversely affect similar brain regions associated with reward processing, in which the common brain structural deficits in the bilateral insula may represent a key intersecting point. This similarity in the morphometric deficit pattern may serve as a potential explanation for the higher prevalence of overweight/obesity in patients with SZ. Moreover, this study provides preliminary support for the role of the bilateral insula in mediating the indirect effect of BMI on negative symptoms, which further indicates a critical role of the bilateral insula in the neuropathology of SZ. Taken together, these findings suggest that higher BMI is associated with lower GMV, and more consideration should be given to the insula pathophysiology, which may serve as a potential novel target for the treatment of both SZ and OWB.
Supplementary Information
Additional file 1: Fig. S1. Partial correlation analysis between BMI and bilateral insula in SZ group, controlling for sex, age and education. Fig. S2. Partial correction analysis between negative symptom and bilateral insula in SZ group, controlling for sex, age and education. Fig. S3. Results of bilateral insula in the whole-brain VBM analyses. Red indicates the bilateral insula in the main effects of diagnosis (SZ<HC); Yellow indicates bilateral insula in the main effects of BMI (OWB<NW); color between yellow and red indicate overlapping brain regions (orange). Results are displayed superimposed on the ch2bet template. Fig. S4. Mediation analysis testing whether GMV in bilateral insula mediates the relationship between BMI and negative symptoms in SZ. The age, sex, education, and ICV were included as covariates. Table S1. Demographic and clinical characteristics for SZ patients. Table S2. Demographic and clinical characteristics for HC participants. Table S3. The multivariate analysis of covariance. Table S4. Pearson’s partial correlations between brain GMV and clinical symptoms, controlling for age, sex, education, BMI and ICV.
Acknowledgements
We are grateful for all members of the Departments of Psychiatry and Radiology at the Affiliated Kashi Hospital of Sun Yat-Sen University in completing this study, and we also thank all participants for their cooperation.
Authors’ contributions
Author contributions included conception and study design (HW, YW and XW), carrying out the investigation and data collection (HW, MA, JT and KZ), statistical analysis (HW, GD, XW), interpretation of results (HW, GD, MA, YW and XW), drafting the manuscript (HW, GD), revising the manuscript critically for important intellectual content (YW and XW) and approval of final version to be published (All authors).
Funding
This study was funded by the Natural Science Foundation of Guangdong Province (2020A1515011288) and the National Key Research and Development Program of China (2016YFC1306900).
Availability of data and materials
The datasets generated in this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Ethics Committee of the First People’s Hospital of Kashi Prefecture approved this study protocol (no. 2021–05), and all participants provided the written informed consent before any procedures were performed. All methods were performed in accordance with the relevant guidelines and regulations and it conformed to the provisions of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
There is no conflict of interest in relation to this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hui Wu and Guochao Dai contributed equally to this work.
Contributor Information
Yuhua Wu, Email: 2384310071@qq.com.
Xiaoli Wu, Email: wuxiaoli@mail.sysu.edu.cn.
References
- 1.McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-An Overview. JAMA Psychiatry. 2020;77(2):201–210. doi: 10.1001/jamapsychiatry.2019.3360. [DOI] [PubMed] [Google Scholar]
- 2.Stępnicki P, Kondej M, Kaczor AA. Current Concepts and Treatments of Schizophrenia. Molecules. 2018;23(8):2087. doi: 10.3390/molecules23082087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietsche B, Kircher T, Falkenberg I. Structural brain changes in schizophrenia at different stages of the illness: A selective review of longitudinal magnetic resonance imaging studies. Aust N Z J Psychiatry. 2017;51(5):500–508. doi: 10.1177/0004867417699473. [DOI] [PubMed] [Google Scholar]
- 4.Shah C, et al. Common pattern of gray-matter abnormalities in drug-naive and medicated first-episode schizophrenia: a multimodal meta-analysis. Psychol Med. 2017;47(3):401–413. doi: 10.1017/S0033291716002683. [DOI] [PubMed] [Google Scholar]
- 5.Moser DA, et al. Multivariate Associations Among Behavioral, Clinical, and Multimodal Imaging Phenotypes in Patients With Psychosis. JAMA Psychiat. 2018;75(4):386–395. doi: 10.1001/jamapsychiatry.2017.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian Y, et al. Prevalence of obesity and clinical and metabolic correlates in first-episode schizophrenia relative to healthy controls. Psychopharmacology. 2021;238(3):745–753. doi: 10.1007/s00213-020-05727-1. [DOI] [PubMed] [Google Scholar]
- 7.Manu P, et al. Weight gain and obesity in schizophrenia: epidemiology, pathobiology, and management. Acta Psychiatr Scand. 2015;132(2):97–108. doi: 10.1111/acps.12445. [DOI] [PubMed] [Google Scholar]
- 8.Strassnig M, et al. Twenty-year progression of body mass index in a county-wide cohort of people with schizophrenia and bipolar disorder identified at their first episode of psychosis. Bipolar Disord. 2017;19(5):336–343. doi: 10.1111/bdi.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell AJ, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders–a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306–318. doi: 10.1093/schbul/sbr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bora E, Akdede BB, Alptekin K. The relationship between cognitive impairment in schizophrenia and metabolic syndrome: a systematic review and meta-analysis. Psychol Med. 2017;47(6):1030–1040. doi: 10.1017/S0033291716003366. [DOI] [PubMed] [Google Scholar]
- 11.Minichino A, et al. Investigating the link between drug-naive first episode psychoses (FEPs), weight gain abnormalities and brain structural damages: Relevance and implications for therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:9–22. doi: 10.1016/j.pnpbp.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Yau PL, et al. Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity (Silver Spring) 2014;22(8):1865–1871. doi: 10.1002/oby.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekkers IA, Jansen PR, Lamb HJ. Obesity, Brain Volume, and White Matter Microstructure at MRI: A Cross-sectional UK Biobank Study. Radiology. 2019;291(3):763–771. doi: 10.1148/radiol.2019181012. [DOI] [PubMed] [Google Scholar]
- 14.García-García I, et al. Neuroanatomical differences in obesity: meta-analytic findings and their validation in an independent dataset. Int J Obes (Lond) 2019;43(5):943–951. doi: 10.1038/s41366-018-0164-4. [DOI] [PubMed] [Google Scholar]
- 15.Willette AA, Kapogiannis D. Does the brain shrink as the waist expands? Ageing Res Rev. 2015;20:86–97. doi: 10.1016/j.arr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, et al. Disruption of brain anatomical networks in schizophrenia: A longitudinal, diffusion tensor imaging based study. Schizophr Res. 2016;171(1–3):149–157. doi: 10.1016/j.schres.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Lopresti AL, Drummond PD. Obesity and psychiatric disorders: commonalities in dysregulated biological pathways and their implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:92–99. doi: 10.1016/j.pnpbp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Morris G, et al. Shared pathways for neuroprogression and somatoprogression in neuropsychiatric disorders. Neurosci Biobehav Rev. 2019;107:862–882. doi: 10.1016/j.neubiorev.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Li Q, et al. The prevalence, risk factors and clinical correlates of obesity in Chinese patients with schizophrenia. Psychiatry Res. 2017;251:131–136. doi: 10.1016/j.psychres.2016.12.041. [DOI] [PubMed] [Google Scholar]
- 20.Kolenič M, et al. Higher Body-Mass Index and Lower Gray Matter Volumes in First Episode of Psychosis. Front Psychiatry. 2020;11:556759. doi: 10.3389/fpsyt.2020.556759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurisu S, et al. Frontal QRS-T angle and World Health Organization classification for body mass index. Int J Cardiol. 2018;272:185–188. doi: 10.1016/j.ijcard.2018.08.060. [DOI] [PubMed] [Google Scholar]
- 22.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22–33. [PubMed] [Google Scholar]
- 23.Nasreddine ZS, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 24.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 25.Duan X, et al. Reduced Hippocampal Volume and Its Relationship With Verbal Memory and Negative Symptoms in Treatment-Naive First-Episode Adolescent-Onset Schizophrenia. Schizophr Bull. 2021;47(1):64–74. doi: 10.1093/schbul/sbaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci. 2000;1(4):173–181. doi: 10.1023/A:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudebeck PH, Rich EL. Orbitofrontal cortex. Curr Biol. 2018;28(18):R1083–r1088. doi: 10.1016/j.cub.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoptman MJ, et al. Cortical thinning, functional connectivity, and mood-related impulsivity in schizophrenia: relationship to aggressive attitudes and behavior. Am J Psychiatry. 2014;171(9):939–948. doi: 10.1176/appi.ajp.2014.13111553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barry AB, Koeppel JA, Ho BC. Impulsive decision making, brain cortical thickness and familial schizophrenia risk. Schizophr Res. 2020;220:54–60. doi: 10.1016/j.schres.2020.03.072. [DOI] [PubMed] [Google Scholar]
- 30.Gradin VB, et al. Salience network-midbrain dysconnectivity and blunted reward signals in schizophrenia. Psychiatry Res. 2013;211(2):104–111. doi: 10.1016/j.pscychresns.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, et al. Grey matter reduction in the caudate nucleus in patients with persistent negative symptoms: An ALE meta-analysis. Schizophr Res. 2018;192:9–15. doi: 10.1016/j.schres.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt A, et al. Dysfunctional insular connectivity during reward prediction in patients with first-episode psychosis. J Psychiatry Neurosci. 2016;41(6):367–376. doi: 10.1503/jpn.150234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giraldo-Chica M, et al. Prefrontal-Thalamic Anatomical Connectivity and Executive Cognitive Function in Schizophrenia. Biol Psychiatry. 2018;83(6):509–517. doi: 10.1016/j.biopsych.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolff M, Vann SD. The Cognitive Thalamus as a Gateway to Mental Representations. J Neurosci. 2019;39(1):3–14. doi: 10.1523/JNEUROSCI.0479-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knierim JJ. The hippocampus. Curr Biol. 2015;25(23):R1116–R1121. doi: 10.1016/j.cub.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 36.Berron D, et al. Medial temporal lobe connectivity and its associations with cognition in early Alzheimer’s disease. Brain. 2020;143(4):1233–1248. doi: 10.1093/brain/awaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farruggia MC, Small DM. Effects of adiposity and metabolic dysfunction on cognition: A review. Physiol Behav. 2019;208:112578. doi: 10.1016/j.physbeh.2019.112578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones S, Hyde A, Davidson TL. Reframing appetitive reinforcement learning and reward valuation as effects mediated by hippocampal-dependent behavioral inhibition. Nutr Res. 2020;79:1–12. doi: 10.1016/j.nutres.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanoski SE, Grill HJ. Hippocampus Contributions to Food Intake Control: Mnemonic, Neuroanatomical, and Endocrine Mechanisms. Biol Psychiatry. 2017;81(9):748–756. doi: 10.1016/j.biopsych.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davidson TL, et al. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19(3):235–252. doi: 10.1002/hipo.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevenson RJ, Francis HM. The hippocampus and the regulation of human food intake. Psychol Bull. 2017;143(10):1011–1032. doi: 10.1037/bul0000109. [DOI] [PubMed] [Google Scholar]
- 42.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517(7534):284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nieuwpoort ICV, et al. Food-Related Brain Activation Measured by fMRI in Adults with Prader-Willi Syndrome. J Clin Med. 2021;10(21):5133. doi: 10.3390/jcm10215133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li G, et al. Resting activity of the hippocampus and amygdala in obese individuals predicts their response to food cues. Addict Biol. 2021;26(3):e12974. doi: 10.1111/adb.12974. [DOI] [PubMed] [Google Scholar]
- 45.Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 46.Wang GJ, et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage. 2008;39(4):1824–1831. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Livneh Y, et al. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature. 2017;546(7660):611–616. doi: 10.1038/nature22375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huerta CI, et al. Neural bases of food perception: coordinate-based meta-analyses of neuroimaging studies in multiple modalities. Obesity (Silver Spring) 2014;22(6):1439–1446. doi: 10.1002/oby.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stockburger J, et al. The impact of hunger on food cue processing: an event-related brain potential study. Neuroimage. 2009;47(4):1819–1829. doi: 10.1016/j.neuroimage.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 50.Stopyra MA, et al. Homeostasis and food craving in obesity: a functional MRI study. Int J Obes (Lond) 2021;45(11):2464–2470. doi: 10.1038/s41366-021-00920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nummenmaa L, et al. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS ONE. 2012;7(2):e31089. doi: 10.1371/journal.pone.0031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagy GA, et al. Reward Network Modulation as a Mechanism of Change in Behavioral Activation. Behav Modif. 2020;44(2):186–213. doi: 10.1177/0145445518805682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farr OM, Li CR, Mantzoros CS. Central nervous system regulation of eating: Insights from human brain imaging. Metabolism. 2016;65(5):699–713. doi: 10.1016/j.metabol.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SH, et al. Initial and Progressive Gray Matter Abnormalities in Insular Gyrus and Temporal Pole in First-Episode Schizophrenia Contrasted With First-Episode Affective Psychosis. Schizophr Bull. 2016;42(3):790–801. doi: 10.1093/schbul/sbv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69(4):664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kotkowski E, et al. A neural signature of metabolic syndrome. Hum Brain Mapp. 2019;40(12):3575–3588. doi: 10.1002/hbm.24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Livneh Y, Andermann ML. Cellular activity in insular cortex across seconds to hours: Sensations and predictions of bodily states. Neuron. 2021;109(22):3576–3593. doi: 10.1016/j.neuron.2021.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kerem L, et al. Modulation of neural fMRI responses to visual food cues by overeating and fasting interventions: A preliminary study. Physiol Rep. 2021;8(24):e14639. doi: 10.14814/phy2.14639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pugazhenthi S, Qin L, Reddy PH. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1037–1045. doi: 10.1016/j.bbadis.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jauch-Chara K, Oltmanns KM. Obesity–a neuropsychological disease? Systematic review and neuropsychological model. Prog Neurobiol. 2014;114:84–101. doi: 10.1016/j.pneurobio.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Harvey PD, et al. Negative symptoms and cognitive deficits: what is the nature of their relationship? Schizophr Bull. 2006;32(2):250–258. doi: 10.1093/schbul/sbj011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michaud A, et al. Neuroanatomical changes in white and grey matter after sleeve gastrectomy. Neuroimage. 2020;213:116696. doi: 10.1016/j.neuroimage.2020.116696. [DOI] [PubMed] [Google Scholar]
- 63.Mueller K, et al. Physical exercise in overweight to obese individuals induces metabolic- and neurotrophic-related structural brain plasticity. Front Hum Neurosci. 2015;9:372. doi: 10.3389/fnhum.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mansur RB, et al. Treatment with a GLP-1R agonist over four weeks promotes weight loss-moderated changes in frontal-striatal brain structures in individuals with mood disorders. Eur Neuropsychopharmacol. 2017;27(11):1153–1162. doi: 10.1016/j.euroneuro.2017.08.433. [DOI] [PubMed] [Google Scholar]
- 65.Goracci A, et al. Development, acceptability and efficacy of a standardized healthy lifestyle intervention in recurrent depression. J Affect Disord. 2016;196:20–31. doi: 10.1016/j.jad.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 66.Firth J, et al. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage. 2018;166:230–238. doi: 10.1016/j.neuroimage.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 67.Woodward ML, et al. Hippocampal volume and vasculature before and after exercise in treatment-resistant schizophrenia. Schizophr Res. 2018;202:158–165. doi: 10.1016/j.schres.2018.06.054. [DOI] [PubMed] [Google Scholar]
- 68.Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64(2):81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lesh TA, et al. A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiat. 2015;72(3):226–234. doi: 10.1001/jamapsychiatry.2014.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Partial correlation analysis between BMI and bilateral insula in SZ group, controlling for sex, age and education. Fig. S2. Partial correction analysis between negative symptom and bilateral insula in SZ group, controlling for sex, age and education. Fig. S3. Results of bilateral insula in the whole-brain VBM analyses. Red indicates the bilateral insula in the main effects of diagnosis (SZ<HC); Yellow indicates bilateral insula in the main effects of BMI (OWB<NW); color between yellow and red indicate overlapping brain regions (orange). Results are displayed superimposed on the ch2bet template. Fig. S4. Mediation analysis testing whether GMV in bilateral insula mediates the relationship between BMI and negative symptoms in SZ. The age, sex, education, and ICV were included as covariates. Table S1. Demographic and clinical characteristics for SZ patients. Table S2. Demographic and clinical characteristics for HC participants. Table S3. The multivariate analysis of covariance. Table S4. Pearson’s partial correlations between brain GMV and clinical symptoms, controlling for age, sex, education, BMI and ICV.
Data Availability Statement
The datasets generated in this study are available from the corresponding author on reasonable request.