Abstract
Background & objectives:
The COVID-19 disease profile in Indian patients has been found to be different from the Western world. Changes in lymphocyte compartment have been correlated with disease course, illness severity and clinical outcome. This study was aimed to assess the peripheral lymphocyte phenotype and subset distribution in patients with COVID-19 disease from India with differential clinical manifestations.
Methods:
Percentages of peripheral lymphocyte subsets were measured by flow cytometry in hospitalized asymptomatic (n=53), mild symptomatic (n=36), moderate and severe (n=30) patients with SARS-CoV-2 infection, recovered individuals (n=40) and uninfected controls (n=56) from Pune, Maharashtra, India.
Results:
Percentages of CD4+Th cells were significantly high in asymptomatic, mild symptomatic, moderate and severe patients and recovered individuals compared to controls. Percentages of Th memory (CD3+CD4+CD45RO+), Tc memory (CD3+CD8+CD45RO+) and B memory (CD19+CD27+) cells were significantly higher in the recovered group compared to both asymptomatic, mild symptomatic patient and uninfected control groups. NK cell (CD56+CD3-) percentages were comparable among moderate +severe patient and uninfected control groups.
Interpretation & conclusions:
The observed lower CD4+Th cells in moderate+severe group requiring oxygen support compared to asymptomatic+mild symptomatic group not requiring oxygen support could be indicative of poor prognosis. Higher Th memory, Tc memory and B memory cells in the recovered group compared to mild symptomatic patient groups might be markers of recovery from mild infection; however, it remains to be established if the persistence of any of these cells could be considered as a correlate of protection.
Keywords: Asymptomatic, lymphocyte subset, mild symptomatic, moderate + severe patients, recovered individuals, SARS-CoV-2
Patients with SARS-CoV-2 infection show a complex profile with varied clinical presentations1,2. Based on the worldwide hospitalized patient data, approximately 80 per cent of infections were mild or asymptomatic, 15 per cent were severe and five per cent are critical infections requiring ventilation3. Asymptomatic infections have been reported to have the same infectivity as symptomatic infections4. An important public health concern is the extent to which immunity in asymptomatic patients may confer protection from re-infection and whether its breadth is lesser than/similar to that observed in symptomatic patients. In a proportion of patients, the infection results in moderate-to-severe acute respiratory distress syndrome (ARDS), requiring invasive mechanical ventilation resulting in damage of internal organs, multiple organ failure and sometimes death5,6. It is, therefore, important to identify the key differentiating host factors/immune molecules responsible for the differential clinical manifestation and outcome.
Studies in hospitalized patients infected with novel coronavirus pneumonia from Wuhan, China, have indicated a decrease in peripheral lymphocytes; however, overall alteration in the subsets remains underexplored7,8,9. It would be worthwhile to study the immunological profile in Indian patients with SARS-CoV-2 infection with severe disease with or without ARDS and compare it with mild patients. Thus, in the present study, the percentages of peripheral lymphocyte subsets were measured by flow cytometry in hospitalized asymptomatic (n=53), mild symptomatic (n=36), moderate and severe (n=30) SARS-CoV-2-infected patients, clinically recovered individuals (n=40), and in uninfected control individuals (n=56) from India.
Material & Methods
This study carried out between March and June 2020, consisted of the patients whose nasal/throat swabs were tested positive for SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) at ICMR-National Institute of Virology, Pune or at Dr. D.Y. Patil Medical College, Hospital and Research Centre, Pune, India. RT-PCR screening, identification and quarantine of close contacts of index cases and asymptomatic patients were made during the stated period10. Asymptomatic cases were defined by an RT-PCR positive test but without any relevant clinical symptoms. The mild symptomatic patients were defined by an RT-PCR positive test with mild symptoms10. The patients with severe/moderate illness were those who required critical care and required oxygen support. In brief, the severe patients had oxygen saturation (SpO2) of ≤90 per cent, mostly required supplement oxygen of 4-20 l and non-invasive ventilation or high-flow oxygen. The moderate patients had oxygen saturation (SpO2) of 90-94 per cent, mostly required supplement oxygen of 2-6 l and did not require non-invasive ventilation. A total of 53 asymptomatic, 36 mild symptomatic, 30 severe/moderate patients [in the acute phase during hospitalization (≤6 days for mild and ≤2 days for severe/moderate patients)] and 40 recovered individuals, having a previous history of symptomatic/asymptomatic SARS-CoV-2 infection and negative for SARS-CoV-2 RT-PCR were included in the study. Following the revised guidelines on clinical management of COVID-19 provided by the Government of Maharashtra, the COVID-19 patients were discharged at 10-14 days post diagnosis11. Sampling for recovered individuals was done between 45 and 60 days post diagnosis.
Fifty six age and sex matched healthy individuals from blood donation camps organized in Pune in January 2020 were recruited as uninfected controls for phenotype comparison. All uninfected controls were negative for anti-SARS-CoV-2 IgG antibody (COVID Kavach-Anti-SARS-CoV-2 IgG Antibody Detection ELISA, M/s Cadila Healthcare Limited, Ahmedabad).
The study protocol was approved by the Institutional Ethical Committee for Research on Humans, based on the guidelines set by the Indian Council of Medical Research, New Delhi. Informed written consent was obtained from all study participants.
Flowcytometric analysis
Natural Killer (NK) and NKT cells, T-cells and subsets, B and B memory cells, T regulatory cells (Tregs) enumeration: Freshly isolated peripheral blood mononuclear cells (PBMCs) (0.5 X 105) from all study participants were stained with antibodies specific for NK and NKT, T and subsets, B and B memory, T regulatory (Tregs) cells using anti-human CD3PerCP (cloneSK7), CD56APC (cloneNCAM16.2), CD4PE (cloneRPAT4), CD8APCH7 (cloneRPAT8), CD19PECy7 (cloneSJ25C1), CD27APCH7 (cloneM-T271), CD45ROAPC (cloneUCHL1) monoclonal antibodies and Treg cocktail [CD127Alexa Fluor 647 (clone HIL-7R), CD25PE-Cy7, CD4FITC (clone7RM21|2A3|SK3)] as previously described12,13,14,15,16. PE-Cy™7 Mouse IgG1 κ Isotype Control (BD Biosciences, San Jose, CA, USA) was used as negative control. The gating strategy is described in Figure 1. The phenotypes of B (CD19+), memory B cell (CD19+CD27+) subsets and Tregs were not assessed in the severe/moderate patients.
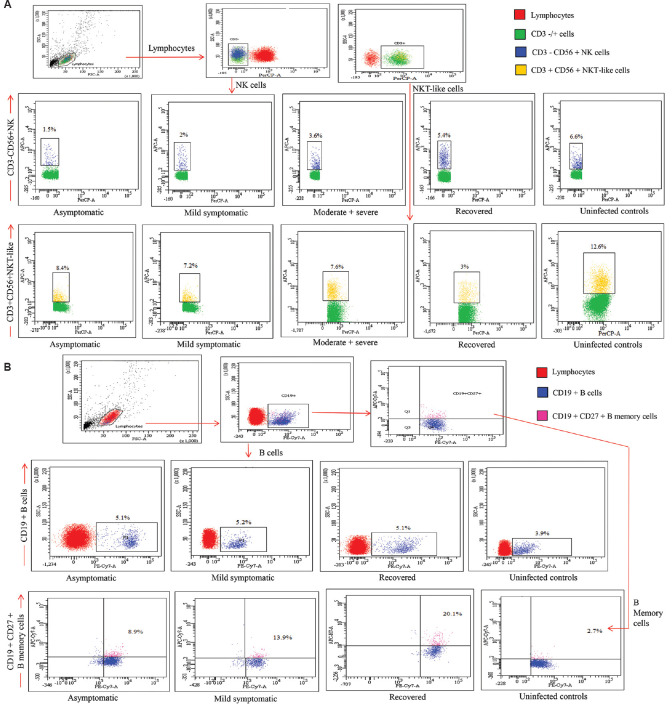
Fig. 1.
(A) Gating strategy for the enumeration of natural killer (NK) and natural killer T-like (NKT-like) cells in different study groups. Lymphocyte gate was recognized by forward scatter (FSC) & side scatter (SSC). Further, the CD3 negative cells from this population were screened for CD56+ expression to identify the NK cells and were identified as CD3-CD56+ and NKT-like (NKT-like) cells were CD3+CD56+. (B) Gating strategy for the enumeration of B and memory B cells in different study groups. B cells from lymphocytes were identified with the help of CD19 expression. For further differentiation, expression of CD27 was considered as memory B cells.
PBMCs isolation, surface staining and acquisition in flow cytometer: It was carried out following the methods described previously12,13,14,15,16. Data were analyzed using FACS Diva software (Version 8.14 BD Biosciences, San Jose, CA, USA) and results are expressed as the percentage of positive cells in the gated population (Fig. 1).
Fig. 1.
(C) Gating strategy for the enumeration of T helper and T cytotoxic cells in different study groups. Lymphocyte populations were identified based on forward and side scatter properties of PBMCs (peripheral blood mononuclear cells). The CD3 positive population was further discriminated to identify CD4 and CD8 T cells. T cell subsets differentiate into T helper cells (CD3+CD4+CD8-) and T cytotoxic cells (CD3+CD4-CD8+). (D) Gating strategy for the enumeration of memory Th and memory Tc cells in different study groups. T cell subsets differentiation into T helper (CD3+CD4+CD8-) and T cytotoxic (CD3+CD4-CD8+) cells from CD3+ cells. Further screening of memory Th cells (CD3+CD4+CD8-CD45RO+) and memory Tc cells (CD3+CD4-CD8+CD45RO+) were done.
Statistical analysis: The statistical analyses were performed on IBM SPSS software version 25 (SPSS Inc. Chicago, IL, USA). All data are expressed as mean (range). Mann–Whitney U test was used for the comparison among patient groups. Bonferroni’s correction was applied to consider multiple comparisons for each cell type. Thus, the conventional cut-off for P=0.05 was lowered down by dividing it by the number of comparisons made for each cell type. Bonferroni’s corrected cut-off for P value was considered significant as follows (NK and NKT-like cell <0.004, B cell <0.006, T cell profile <0.004, memory T cell <0.006 and Tregs profile <0.006). The dot plots were generated on Graphpad Prism software version 8 (GraphPad, San Diego, CA, USA).
Results
Table I depicts the characteristics of the study groups. The study investigated 89 patients with SARS-CoV-2 infection, including 53 asymptomatic and 36 mild symptomatic patients with relevant clinical symptoms (category a), 30 patients with SARS-CoV-2 infection requiring critical care, that included 15 moderate and 15 severe patients (category b), 40 recovered individuals from asymptomatic/ mild symptomatic SARS-CoV-2 infection (category c), and 56 anti-SARS-CoV2 IgG negative uninfected controls (category d). The patient in category (a) had uneventful recovery, whereas 11 out of 30 patients from category (b) succumbed. Ten of 11 non-survivors belonged to severe category, while one was from moderate category. Seven of 11 non-survivors had ARDS and six had septic shock. All patients sampled in the acute phase of infection (categories a and b) were positive for SARS-CoV-2 by quantitative RT-PCR, while the recovered individuals belonging to category (c) had reported a history of SARS-CoV-2 infection. In the study asymptomatic+mild symptomatic patients were clubbed together as patients not requiring oxygen support, while moderate+severe patients were clubbed together as patients requiring oxygen support.
Table I.
Demographic and baseline characteristics of patients with SARS-CoV-2 infection and uninfected controls
| Parameters Characteristics | Study participants | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Asymptomatic | Mild symptomatic | Moderate+severe | Recovered | Uninfected controls | |||
| Study population (n) | 53 | 36 | 30 | 40 | 56 | ||
| Male | 31 | 24 | 19 | 23 | 34 | ||
| Female | 22 | 12 | 11 | 17 | 22 | ||
| Sex ratio (male: female) | 1.4 | 2 | 1.7 | 1.35 | 1.54 | ||
| Age (yr), median (range) | 32 (12-79) | 38 (15-75) | 51 (29-74) | 38 (18-70) | 27 (18-47) | ||
| Parameters Signs and symptoms | NA | Positive cases | 95% CI (range) | Positive cases | 95% CI (range) | NA | NA |
|
| |||||||
| Fever (97.1-101 F) | 21 | 58.33 (42-74) | 28 | 93 (84-100) | |||
| Cough | 22 | 61.11 (45-77) | 22 | 73 (57-89) | |||
| Sore throat | 13 | 36.11 (20-51) | 10 | 33 (16-50) | |||
| Breathing difficulty | 7 | 19.44 (6-32) | 28 | 93 (84-100) | |||
| Body ache | 6 | 16.67 (4-28 | 22 | 73 (57-89) | |||
| Headache | 1 | 2.78 (0-8) | 12 | 4 (22-57) | |||
| Diarrhoea | 1 | 2.78 (0-8) | 4 | 13 (1.1-25) | |||
| Nasal discharge | 9 | 25 (10-39) | 0 | 0 | |||
| Nausea vomiting | 0 | 0 | 8 | 26 (10-42) | |||
| Dysuria | 0 | 0 | 2 | 6 (0-15) | |||
| Abdominal pain | 0 | 0 | 3 | 10 (0-20) | |||
| Anosmia | 0 | 0 | 4 | 13 (1-25) | |||
| Loss of smell and taste | 0 | 0 | 9 | 30 (13-43) | |||
| Post onset days of illness, median (range) | NA | 7 (0-17) | 6 (1-15) | 45-60* | NA | ||
| Chronic medical illness/comorbidities | NA | NA | Positive/total number of cases | 95% CI (range) | NA | NA | |
|
| |||||||
| Condition tuberculosis | 1 | 3 (0-9) | |||||
| Diabetes | 12 | 40 (22-57) | |||||
| Hypertension | 14 | 46 (28-64) | |||||
| Heart disease | 1 | 3 (0-9) | |||||
| Cancer | 0 | 0 | |||||
| COPD | 0 | 0 | |||||
| Liver failure | 0 | 0 | |||||
| Dialysis | 0 | 0 | |||||
| HIV | 0 | 0 | |||||
| Kidney disease | 0 | 0 | |||||
| Sickle cell disease or thalassaemia | 0 | 0 | |||||
| Sepsis | 6 | 20 (5-34) | |||||
| ARDS | 7 | 23 (8-38) | |||||
| ARDS with AKI | 1 | 3.33 (0-9) | |||||
* recovered from mild symptomatic infection; # not applicable. ARDS: acute respiratory distress syndrome; AKI: acute kidney injury; NA: not available; COPD: Chronic obstructive pulmonary disease: Human Immunodeficiency Virus
Immunophenotyping in asymptomatic, mild symptomatic, (moderate + severe) patients
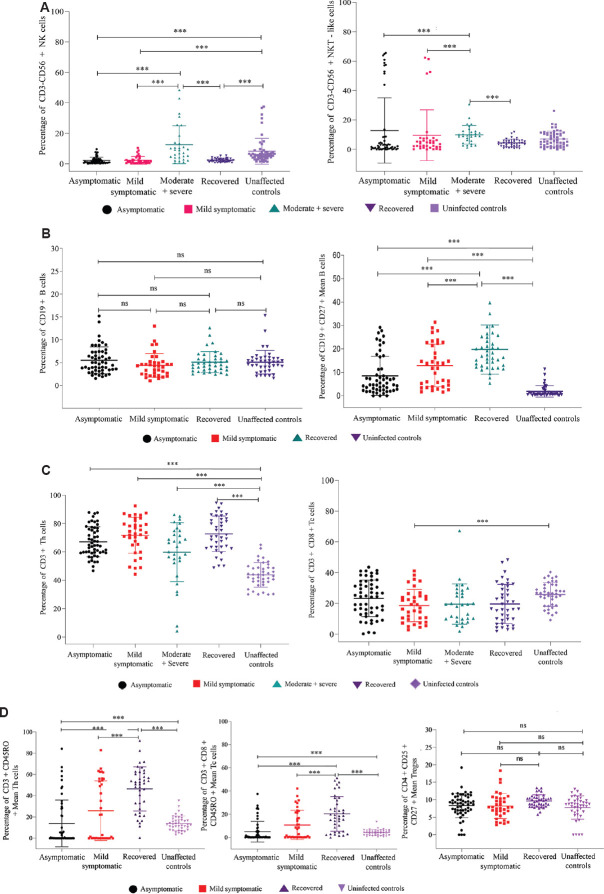
NK (CD56+CD3−) and NKT-like (CD56+CD3+) cell subsets: The percentages of NK cells were significantly lower in both asymptomatic, mild symptomatic patients and recovered individuals compared to uninfected controls [NK cells: (asymptomatic 2.16 (0.10-9.6), mild symptomatic 2.27 (0.2-10.4), recovered 2.38 (0.7-5.4) uninfected controls 8.25 (1.4-37.6), P<0.004 in each]. However, the percentages of NK cells were comparable among the moderate+severe patient and uninfected control groups (Table II). The percentages of NK cells were comparable among asymptomatic and mild symptomatic patients and recovered individuals. Percentages of NK cells were significantly higher in moderate+severe patient group compared to asymptomatic, mild symptomatic, recovered individuals (P<0.004 in each).
Table II.
Comparison of immune cell profiles among patients with asymptomatic, mild symptomatic, severe+moderate infection, recovered individuals from severe acute respiratory syndrome-coronavirus-2 infection and uninfected controls
| Cell type | Asymptomatic (n=53) | Mild symptomatic (n=40) | Moderate+severe (n=30) | Asymptomatic+mild symptomatic (n=89) | Recovered (n=40) | Uninfected controls (n=56) |
|---|---|---|---|---|---|---|
| NK and NKT-like cell profile | ||||||
| CD3-CD56+NK cells | 2.16 (0.1-9.6) | 2.27 (0.2-10.4) | 12.5 (0.5-48.4)*$ | 2.21 (0.1-10.4) | 2.38 (0.7-5.4)@ | 8.25 (1.4-37.6)# |
| CD3+CD56+NKT-like cells | 12.7 (0-65.6) | 9.59 (0-62.4) | 9.93 (1.2-30.7)*$ | 11.4 (0-65) | 4.31 (0.7-12)@ | 7.09 (0-26.14) |
| B cell profile | ||||||
| CD19+B cells | 5.49 (1.5-15.20) | 4.40 (1-13) | NA | 5.05 (1-15.2) | 5.09 (2.4-12.6) | 5.09 (1.6-15.30) |
| CD19+CD27+memory B cells | 8.5 (0-29.2) | 12.9 (1.6-31.4) | NA | 10.3 (0-31.4)#, † | 19.7 (5.4-65.8)*,$ | 1.91 (0.2-11.4)# |
| T cell profile | ||||||
| CD3+CD4+Th cells | 67.0 (46.7-87.8)† | 71.7 (44.3-92.4)† | 59.7 (4.19-86.2)† | 68.9 (44.3-92.4)† | 72.6 (48.8-94.5) | 43.8 (30-65)# |
| CD3+CD8+TcCells | 23.3 (0.2-43.6) | 18.6 (2.9-41.1)† | 19.5 (1.9-67.2) | 21.4 (0.2-43.6) | 19.6 (1.9-48.3) | 25.8 (9.2-40.4) |
| Memory T cell profile | ||||||
| CD3+CD4+CD45RO+memTh | 13.8 (0-84.10)† | 25.8 (0-82.9) | 70.5 (36.1-83.3) | 18.7 (0-84.1)# | 46.4 (0.4-91.7)*,$ | 13.9 (3.4-35.2)# |
| CD3+CD8+CD45RO+mem Tc | 4.95 (0-37.5)† | 10.7 (0-42) | 67.8 (5.57-89.0) | 7.28 (0-42)# | 20.3 (0-51.2)*,$ | 4.15 (0.5-13.5)# |
| Treg profile | ||||||
| CD4+CD25+CD127- | 8.35 (0-19.2) | 8.21 (2.8-18.3) | NA | 8.2 (0-19.2) | 9.65 (5.8-13.4) | 8.62 (3.8-13) |
Percentage of each cell type are represented as mean (range) P values: * P<0.006 compared to asymptomatic; $ P<0.006 compared to mild symptomatic; @ P<0.006 compared to moderate+severe, # P<0.006 compared to recovered; † P<0.006 compared to uninfected controls. Bonferroni’s corrected cut off for P values: NK and NKT-like cell profile-(P<0.004), B cell profile-(P<0.006), T cell profile-(P<0.004), Memory T cell profile-(P<0.006), Tregs profile-(P<0.006) are considered significant. NA: not available
The percentages of NKT-like cells were comparable in asymptomatic, mild symptomatic, moderate+severe patient and in recovered groups compared to uninfected control group. Percentages of NKT-like cells were significantly higher in asymptomatic patient group compared to mild symptomatic moderate +severe and recovered groups. It was significantly lower in patient group compared to moderate+severe patient group (P<0.004 in each) (Table II and Fig. 2).
Fig. 2.
Flowcytometric analysis of NK/NKT-like, B and memory B cells, T cell subsets. PBMCs from 53 asymptomatic, 36 mild symptomatic, 30 (Moderate+Severe) COVID-19 patients, 40 recovered individuals and 56 uninfected controls were stained and acquired on flowcytometer. Vertical scatter plots denote the comparisons of frequencies of immune cells and their subpopulation among different study groups: (A) NK cells and NKT-like cells profile, (B) B and memory B cells profile, (C) T-helper and T-cytotoxic cells profile, (D) Memory Th and Tc cells, Tregs profile. Data are presented as percentage of immune cells out of lymphocytes. The dots represent individual values and bars represent Mean+SD values (P *** <0.0001). ns, not significant
Flowcytometric analysis of NK/NKT-like, B and memory B cells, T cell subsets. PBMCs from 53 asymptomatic, 36 mild symptomatic, 30 (Moderate+Severe) COVID-19 patients, 40 recovered individuals and 56 uninfected controls were stained and acquired on flowcytometer. Vertical scatter plots denote the comparisons of frequencies of immune cells and their subpopulation among different study groups: (A) NK cells and NKT-like cells profile, (B) B and memory B cells profile, (C) T-helper and T-cytotoxic cells profile, (D) Memory Th and Tc cells, Tregs profile. Data are presented as percentage of immune cells out of lymphocytes. The dots represent individual values and bars represent Mean+SD values (P *** <0.0001). ns, not significant
Th (CD3+CD4+) and Tc (CD3+CD8+) cells subsets: The percentages of Th cells were significantly higher in asymptomatic, mild symptomatic, moderate+severe patient groups and in recovered individuals compared to uninfected controls. However, the percentages of Th cells were comparable among asymptomatic, mild symptomatic, moderate+severe and recovered individuals (Table II).
The percentages of Tc cells were significantly lower in the mild symptomatic patient group compared to the uninfected control group ( P<0.004). The percentages of Tc cells were comparable among the asymptomatic, moderate+severe, recovered and uninfected control groups. Further, percentages of Tc cells were comparable among moderate+severe and mild symptomatic patient groups. Th memory (CD3+CD4+CD45RO+) and Tc memory (CD3+CD8+CD45RO+) cell were significantly high in the recovered group compared to both asymptomatic, mild symptomatic patient groups and uninfected control group (Table II and Fig. 2).
B (CD19+) and memory B (CD19+CD27+) cells subsets: Percentages of B cells were comparable among asymptomatic, mild symptomatic patients, recovered individuals and uninfected controls. The percentages of B memory cells were significantly higher in mild symptomatic, asymptomatic patients and in recovered individuals compared to the uninfected controls (P<0.006 in each). Among the patient categories, the percentage of B memory cells was comparable among mild symptomatic and asymptomatic groups. However, the percentages of B memory cells were significantly higher in the recovered group compared to both asymptomatic and mild symptomatic groups (Table II and Fig. 2)].
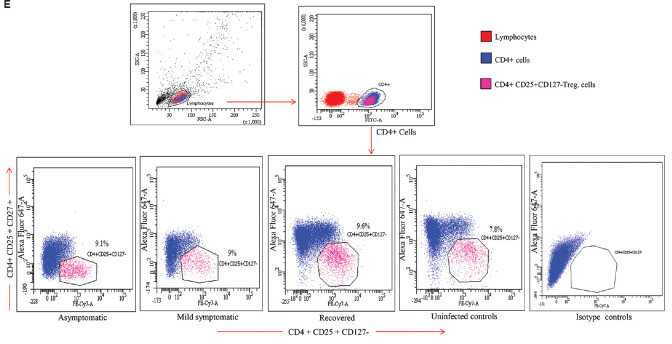
CD4+CD25+CD127- Treg cells: The percentages of Tregs were comparable among asymptomatic, mild symptomatic patients, recovered individuals and uninfected controls (Table II and Fig. 2).
Immunophenotyping in moderate + severe cases and asymptomatic + mild symptomatic cases
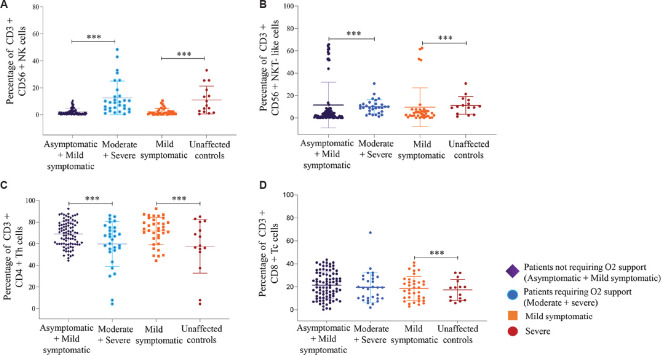
NK (CD56+CD3−) and NKT-like (CD56+CD3+) cells subsets: Percentages of NK cells were significantly higher in the patients requiring oxygen support compared to patients not requiring oxygen support [NK cells: patients requiring oxygen support 12.5 (0.5-48.4), patients not requiring oxygen support 2.21 (0.1-10.4), P<0.05]. Percentages of NKT-like cells were significantly lower in the patients requiring oxygen support group compared to those not requiring oxygen support (Table III and Fig. 3)].
Table III.
Comparison of immune cell profiles among severe, mild symptomatic, patients not requiting O2 support (asymptomatic+mild symptomatic) and patients requiting O2 support (moderate + severe) patients with SARS-CoV-2 infection
| Cell type | Patients not requiting O2 support (asymptomatic + mild symptomatic [n=89]) | Patients requiting O2 support (moderate + severe [n=30]) | Mild symptomatic (n=36) | Severe (n=15) |
|---|---|---|---|---|
| NK and NKT-like cell profile | ||||
| CD3-CD56+NK cells | 2.21 (0.1-10.4) | 12.5 (0.5-48.4)* | 2.27 (0.2-10.4) | 10.9 (0.57-33)$ |
| CD3+CD56+NKT-like cells | 11.4 (0-65) | 9.93 (1.2-30.7)* | 9.59 (0-62.4) | 11.0 (1.2-30.7)$ |
| T cell profile | ||||
| CD3+CD4+Th cells | 68.9 (44.3-92.4) | 59.7 (4.19-86.2)* | 71.7 (44.3-92.4) | 57.6 (4.1-85.1)$ |
| CD3+CD8+TcCells | 21.4 (0.2-43.6) | 19.5 (1.9-67.2) | 18.6 (2.9-41.1) | 17.3 (5.8-32.2) |
Percentage of each cell type are represented as mean (range). P* <0.05 compared to patients not requiring O2 support; P$ <0.05 compared to mild symptomatic
Fig. 3.
Flow cytometric analysis of NK/NKT-like, T cell subsets. PBMCs from 89 patients not requiring O2 support (Asymptomatic+ Mild symptomatic), 30 patients requiring O2 support (Moderate+ Severe), 36 mild symptomatic & 15 severe patients were stained and acquired on flowcytometer. Vertical scatter plots denote the comparisons of frequencies of immune cells and their subpopulation among different study groups: (A) CD3-CD56+ NK cells (B) CD3+CD56+ NKT-like cells (C) CD3+CD4+Th (D) CD3+CD8+Tc cells Data are presented as percentage of immune cells out of lymphocytes. The dots represent individual values and bars represent Mean+SD values. (P ***<0.0001).
Th (CD3+CD4+) and Tc (CD3+CD8+) cells subsets: Significantly lower percentages of Th cells were observed in the patients requiring oxygen support group compared to patients not requiring oxygen support group (P<0.05). However, percentages of Tc cells were comparable among the patient categories (Table III and Fig. 3).
Fig. 1.
(E) Gating strategy for the enumeration of T regulatory (Treg) cells (CD4+CD25+ CD127-) in different study groups. Lymphocyte gate was recognized by FSC & SSC. Further, the CD4 positive cells from this population were screened for CD25+CD127- expression to identify (Treg) cells.
Comparison of immunophenotypes among severe and mild symptomatic patients: The percentages of Th cells were significantly lower in the severe patients (n=15) compared to mild symptomatic patients (n=36). The percentages of Tc cells were lower among severe patients compared to mild symptomatic patients (P<0.05). Percentages of NK and NKT-like cells were significantly higher in the severe patients (n=15) compared to the mild symptomatic patients (n=36) (P<0.05) (Table III and Fig. 3).
Discussion
Evidence suggest that the coronavirus mainly acts on lymphocytes, especially T lymphocytes. Two studies compared between patients with severe and non-severe SARS-CoV-2 infection and demonstrated that the number of T cells significantly decreased and were more impaired in severe cases with both Th cells and Tc cells below normal levels16,17. Lower percentages of CD3+CD4+Th cells in the moderate+severe group requiring oxygen support of the present study compared to asymptomatic+ mild symptomatic group not requiring oxygen support goes in line with the report of Song et al18 demonstrating decreased peripheral CD4+ T-cells in the severe SARS-COV-2 infected patients. In view of the reported observation that a low CD4+ T-cell count in HIV infection increases the risk of opportunistic infections and lowers antiviral immune surveillance, more attention need to be paid to moderate+severe category of patients having low CD3+CD4+Th cells18.
Reduced CD4+and particularly CD8+T lymphocyte subtypes in patients with moderate and severe COVID-19 infection has been associated with deficiency of the adaptive immune response19,20 Our study demonstrated comparable CD3+CD8+Tc cells in asymptomatic patients and moderate+severe patient group and also in recovered individuals. Since T-cells and T-cell responses against SARS-CoV-2 are important for the recognition and killing of infected cells, closely monitoring the T and T cell subsets during the course of the disease may yield meaningful information21.
Decreased helper T-cells of memory phenotype in severe cases indicated that the immune system in the severe infection was impaired more severely16. Higher percentages of Th and Tc memory cells in the recovered individuals may be a prognostic observation and further supports a role for T-cells in COVID-19 and probably in the immunological memory that forms following recovery22.
Although the reasons for reduced frequency of peripheral Tregs in the severely ill COVID-19 patients compared with mild patients are not completely understood, one of the possibilities is that Tregs might have migrated to the lungs to prevent tissue damage2. Therefore, higher levels of Th cells and normalized levels of Tregs observed in the our mild patient population could probably be associated with their prognostic outcome.
Although CD19+B cells were similar among the non-severe patient population in the early phase of infection, recovered individuals and healthy controls, but, CD19+CD27+ B memory cell population was significantly higher in the recovered group at 45-60 days post-infection compared to non-severe patient population and uninfected controls. Our data could be useful in establishing co-correlates of protection in ongoing vaccine studies.
Based on the reduced percentages of NK cells in COVID-19 patients and the well-known antiviral role of NK cells, it has been speculated that the restoration of NK cells and their function may be a near-future solution for COVID-19 patients1,15,18,23. In our study asymptomatic, mild symptomatic patients and recovered individuals demonstrated a similar low NK cell profile, consistent with other reports. Li et al24 have demonstrated a similar scenario where cytotoxic CD3-CD56dimCD16+ cell population was significantly decreased, while the CD3-CD56dimCD16- part was significantly increased in severe COVID-19 patients, thus making it essential to understand the mechanism behind the NK cell loss/gain in COVID-19 disease. Higher percentage of NK cells in the patients group requiring oxygen support compared to patients group not requiring oxygen support group further suggests the need for measuring the NK cells, its subsets and their effector function at an early stage of infection.
The absence of longitudinal follow up data of patient population and small sample size in the moderate and severe categories of patients were the limitations of our study.
Higher levels of Th memory, Tc memory and B memory cells in the recovered individuals compared to mild symptomatic patient groups could be put forward as markers of recovery from mild infection; it remains to be established if the persistence of any of these cells could be considered as a correlate of protection.
In conclusion, higher percentages of B memory cells, Th and Tc memory cells in the recovered individual may be considered a prognostic observation. The mechanism of immune evasion by SARS-CoV-2 in non-severe group could be put forward to a reduced NK cell population against a higher CD4+ T cell population. Lower CD4+Th cells in the moderate+severe group requiring oxygen support compared to asymptomatic+ mild symptomatic group not requiring oxygen support could be an indicative of poor prognosis, though needs validation in a larger patient population.
Acknowledgment:
The authors thank Servshree K.D. Ramaiah, Bipin Tilekar and P.D. Sarje for their help in clinical sample collection from the study participants. and acknowledge Shri A.M. Walimbe for his statistical suggestions.
Footnotes
Financial support & sponsorship: Financial support provided by the Indian Council of Medical Research, New Delhi, is duly acknowledged.
Conflicts of Interest: None.
References
- 1.Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221:1762–9. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen MC, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75:1564–81. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rokni M, Ghasemi V, Tavakoli Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran:Comparison with SARS and MERS. Rev Med Virol. 2020;30:e2107. doi: 10.1002/rmv.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Z, Xu Y, Sun C, Wang X, Guo Y, Qiu S, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2021;54:12–6. doi: 10.1016/j.jmii.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiskopf D, Schmitz KS, Raadsen MP, Grifoni A, Okba NM, Endeman H, et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd2071. eabd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao Z, Zheng Z, Wu K, Junhua Z. Immune environment modulation in pneumonia patients caused by coronavirus:SARS-CoV, MERS-CoV and SARS-CoV-2. Aging (Albany NY) 2020;12:7639–51. doi: 10.18632/aging.103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urra JM, Cabrera CM, Porras L, Ródenas I. Selective CD8 cell reduction by SARS-CoV-2 is associated with a worse prognosis and systemic inflammation in COVID-19 patients. Clin Immunol. 2020;217:108486. doi: 10.1016/j.clim.2020.108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China:A descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tripathy AS, Vishwakarma S, Trimbake D, Gurav YK, Potdar VA, Mokashi ND, et al. Pro-inflammatory CXCL-10, TNF-α, IL-1β, and IL-6:Biomarkers of SARS-CoV-2 infection. Arch Virol. 2021;166:3301–10. doi: 10.1007/s00705-021-05247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medical Education and Drugs Department, Government of Maharashtra. Compendium of guidelines, instruction and standard operative procedures for COVID-19. [accessed on January 21, 2021]. Available from: https://www.maharashtramedicalcouncil.in/Files/MEDD%20Compendium%204th%20Edition%20Volume%204.pdf .
- 12.Thanapati S, Das R, Tripathy AS. Phenotypic and functional analyses of NK and NKT-like populations during the early stages of chikungunya infection. Front Microbiol. 2015;6:895. doi: 10.3389/fmicb.2015.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathod SB, Das R, Thanapati S, Arankalle VA, Tripathy AS. Suppressive activity and altered conventional phenotype markers/mediators of regulatory T cells in patients with self-limiting hepatitis E. J Viral Hepat. 2014;21:141–51. doi: 10.1111/jvh.12125. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni SP, Sharma M, Tripathy AS. Antibody and memory B cell responses in hepatitis E recovered individuals, 1-30 years post hepatitis E virus infection. Sci Rep. 2019;9:1–9. doi: 10.1038/s41598-019-40603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rathod SB, Tripathy AS. Hepatitis E rORF2p stimulated and unstimulated peripheral expression profiling in patients with self-limiting hepatitis E infection. J Immunol Res. 2014;2014:565284. doi: 10.1155/2014/565284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–8. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song JW, Zhang C, Fan X, Meng FP, Xu Z, Xia P, et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun. 2020;11:3410. doi: 10.1038/s41467-020-17240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ni M, Tian FB, Xiang DD, Yu B. Characteristics of inflammatory factors and lymphocyte subsets in patients with severe COVID-19. J Med Virol. 2020;92:2600–6. doi: 10.1002/jmv.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID-19:Current state of the science. Immunity. 2020;52:910–41. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bobcakova A, Petriskova J, Vysehradsky R, Kocan I, Kapustova L, Barnova M, et al. Immune profile in patients with COVID-19:Lymphocytes exhaustion markers in relationship to clinical outcome. Front Cell Infect Microbiol. 2021;11:646688. doi: 10.3389/fcimb.2021.646688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20:529–36. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed F, Jo DH, Lee SH. Can natural killer cells be a principal player in anti-SARS-CoV-2 immunity? Front Immunol. 2020;11:586765. doi: 10.3389/fimmu.2020.586765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, Guo W, Dong Y, Wang X, Dai D, Liu X, et al. Elevated exhaustion levels of NK and CD8+ T cells as indicators for progression and prognosis of COVID-19 disease. Front Immunol. 2020;11:580237. doi: 10.3389/fimmu.2020.580237. [DOI] [PMC free article] [PubMed] [Google Scholar]