Abstract
A membrane-associated, dye-linked formaldehyde dehydrogenase (DL-FalDH) was isolated from the obligate methylotroph Methylococcus capsulatus Bath. The enzyme was the major formaldehyde-oxidizing enzyme in cells cultured in high (above 1 μmol of Cu per mg of cell protein) copper medium and expressing the membrane-associated methane monooxygenase. Soluble NAD(P)+-linked formaldehyde oxidation was the major activity in cells cultured in low-copper medium and expressing the soluble methane monooxygenase (Tate and Dalton, Microbiology 145:159–167, 1999; Vorholt et al., J. Bacteriol. 180:5351–5356, 1998). The membrane-associated enzyme is a homotetramer with a subunit molecular mass of 49,500 Da. UV-visible absorption, electron paramagnetic resonance, and electrospray mass spectrometry suggest the redox cofactor of the DL-FalDH is pyrroloquinoline quinone (PQQ), with a PQQ-to-subunit stochiometry of approximately 1:1. The enzyme was specific for formaldehyde, oxidizing formaldehyde to formate, and utilized the cytochrome b559/569 complex as the physiological electron acceptor.
Formaldehyde is a key intermediate in the oxidation of methane to carbon dioxide by methanotrophic bacteria (1, 5, 12, 18, 34). In methanotrophs, the formaldehyde formed during the oxidation of methanol is either assimilated into cell carbon via the serine or ribulose monophosphate cycle or oxidized for energy to formate by the formaldehyde dehydrogenase (FalDH) (1, 18). The central role of formaldehyde in methanotrophic metabolism is reflected in the variety of different formaldehyde-oxidizing enzymes identified in this microbial group. Based on the nature of the electron acceptor, formaldehyde-oxidizing enzymes are divided into two groups, NAD(P)+-dependent and dye (cytochrome)-linked. The NAD(P)+-linked enzymes are further subdivided based on the need for the secondary cofactors, such as thiol compounds, tetrahydrofolate, methylene tetrahydromethanopterin, or modifier proteins (6, 10, 21, 30, 31, 41, 42, 46, 50, 51).
The NAD+-linked formaldehyde dehydrogenase has been isolated from Methylococcus capsulatus Bath (42, 46). This glutathione-independent FalDH was originally reported as a homodimer (42), but subsequently shown to be a homotetramer, with a subunit molecular mass of 63,615 Da (46). The substrate specificity and kinetics of the purified enzyme are regulated by a small (8,600 Da) heat-stable protein, modifin (46). In the presence of modifin, the enzyme is specific for formaldehyde; in the absence of modifin, the enzyme is a general class III alcohol/aldehyde dehydrogenase.
The structural gene and enzymatic properties of a class III alcohol dehydrogenase have also been reported in the marine methanotroph Methylobacter marinus A45. The NAD(P)+-dependent, glutathione-independent enzyme has a predicted subunit molecular mass of 46,000 Da and an isoelectric point of 4.8 (41). As in the methanol-utilizing bacterium Methylobacterium extorquens AM1, many methane-oxidizing bacteria also appear to utilize a tetrahydrofolate (H4F)- and/or methylene tetrahydromethopterin (H4MPT)-dependent formaldehyde-oxidizing pathway(s) (10, 28, 31, 38, 50, 51). The activities and structural genes for a methenyl H4MPT cyclohydrase, a methenyl H4MPT, and NADP+-dependent methylene H4MPT have also been identified in M. capsulatus Bath (51).
Compared to the other classes of formaldehyde oxidation systems in methanotrophs, the dye-linked formaldehyde dehydrogenases (DL-FalDH) have received little attention. The activity has been reported in a number of methanotrophs (1, 21, 37, 42), but the enzyme has only been isolated from Methylosinus trichosporium OB3b (37). The enzyme from M. trichosporium OB3b was a broad-substrate-range aldehyde (C1 to C10) dehydrogenase with a subunit molecular mass of 22,000 Da. Although the UV absorption spectra of the enzyme indicated the presence of a c-type heme, the heme-to-monomer concentration (approximately 0.1) indicated the heme component was a contaminating cytochrome (1).
Dye-linked formaldehyde oxidation has also been observed in a number of methylotrophic bacteria. In general, the activity in methylotrophic bacteria is associated with nonspecific aldehyde dehydrogenases, most of which are not induced or induced at low levels during growth on C1 compounds (1–3, 5, 6, 15, 17, 21, 26, 30, 31, 36, 37). Thus, most DL-FalDHs are not believed to be of physiological significance in formaldehyde metabolism. The exceptions to this rule are found in the dye-linked aldehyde dehydrogenases from Pseudomonas sp. strain RJ1 (33) and Hyphomicrobium zavarzinii ZV 580 (26). Both enzymes are induced in cells cultured on C1 compounds and show optimal activity with formaldehyde. The DL-FalDH has been isolated from Hyphomicrobium zavarzinii ZV 580 (26). The enzyme is a homotetramer with a subunit molecular mass of 54,000 Da. Although indirect, spectral and inhibitor studies provided strong evidence that the prosthetic group was a redox-active quinone cofactor, possibly pyrroloquinoline quinone (PQQ) covalently bound to the peptide chain (26).
This report describes the regulation, isolation, and initial characterization of a DL-FalDH from M. capsulatus Bath that utilizes a b-type cytochrome or a quinone as the physiological electron acceptor. The enzyme was induced in cells grown in high-copper medium, 5 μM CuSO4, and was the major FalDH in cells grown under these culture conditions. The DL-FalDH from M. capsulatus Bath is a membrane-associated PQQ containing protein, and this is the first report of a PQQ-containing FalDH that is coupled to the electron transport chain via a b-type cytochrome or quinone.
MATERIALS AND METHODS
Cultivation conditions and isolation of membrane fraction.
M. capsulatus Bath was grown in nitrate mineral salts medium (NMS) plus 5 μM CuSO4 and a vitamin mixture at 42°C for cells expressing the membrane-associated methane monooxygenase (pMMO) or in copper-deficient medium for cells expressing the soluble methane monooxygenase (sMMO) (54). Cells were harvested at late log phase (optical density at 600 nm [OD600] = 0.7 to 1.2) by centrifugation at 13,000 × g for 15 min at 4°C and resuspended (1:20, wt/vol) in 20 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer, pH 7.4 (buffer A). All purification procedures were performed at 0 to 4°C unless otherwise stated. Cells were lysed by three passes though a French pressure cell at 18,000 lb/in2. The homogenate was centrifuged at 12,000 × g for 20 min to remove unlysed cells, and the supernatant was centrifuged at 140,000 × g for 2 h.
Isolation of membrane-associated quinoprotein formaldehyde dehydrogenase. (i) Method I.
The 140,000 × g supernatant was discarded, and the pellet was resuspended with a Dounce homogenizer in buffer A plus 1 M KCl. The membrane fraction resuspended in buffer A plus 1 M KCl and centrifuged at 155,000 × g for 2 h, and the pellet was resuspended with a Dounce homogenizer in buffer A (washed membrane fraction). The washed membrane fraction was diluted to 20 mg of protein/ml in buffer A containing 6% Triton X-100 and 0.4% sodium cholate. The membrane suspension was then stirred at 4°C for 1 h and centrifuged at 155,000 × g for 2 h. The soluble fraction was collected and diluted in an equal volume of buffer A. The detergent-solubilized fraction was loaded on a DEAE-Sepharose FF (5 cm by 18 cm) and the unbound fraction (∼1,000 ml) was diverted to a second by phenyl-Sepharose CL-4B column (5 by 18 cm), and both columns were equilibrated with buffer A. The flowthrough fraction (∼1,100 ml) from the phenyl-Sepharose CL-4B column was pooled and concentrated on an ultrafiltration cell to a final volume of 100 ml. The sample was then dialyzed for 16 h against three changes of buffer A containing 0.05% dodecyl maltoside (buffer B) and loaded on a high-performance Resource Q column (1.25 by 60 cm) equilibrated in buffer B. The column was washed with 200 ml of buffer B and then developed with a linear gradient of buffer B plus 500 mM KCl (buffer C). Fractions exhibiting formaldehyde dehydrogenase activity eluted from the Resource Q column in a single peak at 5 to 9 mM KCl. These fractions were pooled and dialyzed for 16 h against three changes of buffer B.
In addition to collecting fractions exhibiting formaldehyde dehydrogenase activity, the flowthrough fraction, which contained cytochrome b559/569 complex with a purity of approximately 80% as estimated by densitometry on a denaturing sodium dodecyl sulfate (SDS) gel, was pooled and dialyzed for use in formaldehyde-cytochrome b559/569 oxidoreductase activity assays.
(ii) Method II.
The pellet from the washed membrane fraction was resuspended in buffer A plus 0.07% dodecyl-β-d-maltoside to a final detergent-to-protein concentration of 1 to 20 (wt/wt), stirred for 1 h and centrifuged at 155,000 × g for 2 h. The supernatant was discarded, and the pellet was resuspended in buffer A plus 1% dodecyl-β-d-maltoside to a final detergent-to-protein concentration of 1 to 7 (wt/wt). The sample was stirred for 1 h and centrifuged at 155,000 × g for 2 h. The supernatant was dialyzed against three changes of buffer A and loaded on a Resource Q column (1.25 by 60 cm) equilibrated with buffer B. The column was run as described for method I. The DL-FalDH fraction from the Resource Q column was dialyzed against buffer A and loaded on a Resource Q column (1.25 by 60 cm) equilibrated with buffer B, and the column was developed as described for method I.
13C-NMR spectroscopy.
Cell extracts, soluble cell extracts, and salt-washed (1 M KCl) cell membrane extracts were prepared in 20 mM PIPES buffer at a concentration of 35 mg of protein/ml. Samples were prepared for 13C nuclear magnetic resonance (NMR) spectroscopy by placing 2,000 μl of sample extract into a 10-ml serum vial fitted with a Teflon-silicone septum. The reaction was initiated by addition of 13CHOH (99%; Cambridge Isotope Laboratories, Andover, Mass.) to a final concentration of 15 mM. Reactions were stopped by addition of dimethyl-d6 sulfoxide (deuterium, 99.96%; Cambridge Isotope Laboratories) to a final concentration of 20% (vol/vol) and then frozen in an acetone-dry ice bath. Carbon dioxide generated by oxidation of formate by formate dehydrogenase was trapped internally with a microtube vial insert containing 100 μl of 6 M KOH. Samples were thawed to room temperature, placed in 5-mm NMR tubes, and analyzed by 13C-NMR spectroscopy. Control experiments to evaluated the reaction termination process showed that no detectable formaldehyde oxidation products were formed from formaldehyde following the addition of dimethyl sulfoxide and the freeze-thaw cycle.
Decoupled 13C-NMR spectra were acquired on a Bruker Instruments AC-250, operated at 62.898 MHz and a sweep width of 15,625.0 Hz. Peak assignments were made in reference to dimethyl-d6 sulfoxide (deuterium, 99.96%) and confirmed with commercially available standards.
Enzyme activity.
Dye-linked spectral assays were performed at ambient temperatures (∼20°C) on 1-ml reaction mixtures containing enzyme (2.5 μg), formaldehyde (0.03 to 20 mM), phenazine ethosulfate (PES; 360 μM), and dichlorophenol-indophenol (DCPIP; 20 μM) in 50 mM PIPES (pH 7.4). Absorbance change was monitored at a wavelength of 578 nm over an initial 0.5-min period in the absence of formaldehyde to establish a baseline and then for an additional 3-min period following the addition of formaldehyde. The amount of DCPIP reduced over the reaction period was calculated from the absorbance change using a molar absorption constant for DCPIP of ɛ578 = 13,000 M−1 cm−1 (30). For dye-linked assays using DCPIP as the electron acceptor, 1 U of enzyme activity is defined as the reduction of 1 μmol of DCPIP min−1.
NAD+- or NADP+-linked formaldehyde activity was measured at ambient temperatures according to the methods of Stirling and Dalton (42) and Pomper et al. (38), respectively. For NAD+- or NADP+-linked assays, 1 U of enzyme activity is defined as the reduction of μmol of NAD+ or NADP+ min−1.
Alternative substrates and inhibitors of dye-linked formaldehyde dehydrogenase activity were tested at concentrations of 0.1, 1, and 10 mM and include methanol (C1), acetaldehyde (C2), glyoxal (C2), butyraldehyde (C4), phenylhydrazine, acetylene, N-ethylmaleimide, potassium cyanide, sodium azide, Tris (pH 7.4), EDTA, CuCl2 · 2H2O, FeCl3 · 6H2O, NiCl2 · 6H2O, and ZnSO4 · 7H2O. The effect of pH on dye-linked formaldehyde dehydrogenase activity was completed over a pH range from 6 to 10 using 2-(N-morpholino) ethanesulfonic acid (MES, pH 6.0 to 6.7), PIPES (pH 6.5 to 7.5), 3-(N-morpholino) propanesulfonic acid (MOPS; pH 7.0 to 7.9), and 3-(cyclohexylamino)-1-propanesulfonic acid (CAPS; pH 9.7 to 10).
Formaldehyde-cytochrome b559/569 oxidoreductase activity was measured spectrophotometrically in a 1-ml reaction mixture containing formaldehyde dehydrogenase (2.5 μg), cytochrome b559/569 complex (12 μg), and 50 mM PIPES. The formaldehyde-dependent reduction of cytochrome b559/569 complex was recorded as the spectral difference between a reaction mixture containing 1 mM formaldehyde and a reaction mixture containing no formaldehyde. The absorbance spectrum for the reaction mixture containing formaldehyde was taken within 3 min from the addition of substrate.
Extraction of covalently bound prosthetic group from formaldehyde dehydrogenase.
Extraction of the prosthetic group from formaldehyde dehydrogenase was performed on a sample of purified enzyme (7.1 mg of protein) according to the hexanol extraction method described by Vander Meer et al. (47). Briefly, the enzyme (7.1 mg) was refluxed at 150°C for 1 h in a 15-ml solution containing 50% hexanol–water (vol/vol) and 3 M HCl. After 1 h of refluxing, the solution was cooled and diluted in an equal volume of water, and then the pH was adjusted to 5.5 with 0.5 M KOH. The hexanol was removed from the solution under vacuum, and the remaining aqueous material was passed through two Waters Sep-Pak Plus C18 cartridges connected in series. The C18 cartridges were washed with 50 ml of water, 50 ml of 5% methanol–water (vol/vol), and then eluted in 100% methanol-containing 10 mM HCl. The eluate changed from a brick red appearance on the stationary phase to a lighter red color as it eluted from the C18 column. The sample, approximately 5 ml, was diluted in a 100 ml of water, frozen in an acetone-dry ice bath, and then lyophilized overnight under vacuum (40 to 80 torr) to remove the methanol and HCl. Conversion of the putative PQQ-5,5 dihexyl ketal to PQQ was completed as previously described by Vander Meer et al. (47). The identity of reaction products and end products was based on the comparison of electrospray mass spectrometry data and absorption spectra to authentic standards (PQQ) isolated from the methanol dehydrogenase from M. capsulatus Bath (2, 13, 14).
HPLC ES-MS.
Chemical analysis of solid-phase extraction (SPE) eluates was performed by high-performance liquid chromatography electrospray ionization mass spectrometry (HPLC ES-MS) as described by Julian et al. (23). Methanol-solubilized analytes (50 μl) were separated on a Novapak C18 analytical column (3.9 by 100 mm) over a 30-min linear gradient from 2% methanol plus 6.5 mM ammonium acetate (pH 5.5) to 95% methanol plus 6.5 mM ammonium acetate (pH 5.5) at a flow rate of 1 ml min−1. The column effluent was split to a Finnigan Navigator (160 μl min−1) equipped with a Finnigan electrospray source and to a Sedex 55 evaporative light-scattering detector (840 μl min−1; Sedex, Alfortville, France) in order to provide qualitative and quantitative data, respectively. UV and visible absorption spectra (190 to 550 nm) were acquired with a series 1100 Hewlett Packard photodiode array spectrophotometer on column effluent prior to analysis by evaporative light scattering. The electrospray source was switched between positive and negative ion mode in 0.5-s intervals to acquire both positive and negative ion spectra. During data acquisition, the MS probe was maintained at 3.6 kV, cone voltage was maintained at 36 V, the source temperature was held at 179°C, and drying gas flow was set at 505 liters h−1. MS tuning was performed using m-cresol purple.
Purification of methanol dehydrogenase from M. capsulatus Bath.
Cell lysis and initial separation of methanol dehydrogenase from soluble c-type cytochromes were preformed as described by Bergmann et al. (7) and Zahn et al. (53).
Spectroscopy.
Optical absorption spectrophotometry was performed with an SLM Aminco DW-2000 spectrophotometer in the split-beam mode using quartz cuvettes with a 1-cm path length.
Electron paramagnetic resonance (EPR) spectra were recorded at X-band on a Bruker ER200D EPR spectrometer equipped with an Oxford Instruments ESR-900 liquid helium cryostat. Operating parameters during spectral acquisition were: modulation frequency, 100 kHz; modulation amplitude, 1.25 mT; microwave frequency, 9.422 GHz; receiver gain, 3.20 × 103; and time constant, 100 ms. Cryostat temperature was maintained at 8 K during spectral acquisition.
Other methods.
Protein concentration was determined by the method of Lowry et al. (29) using bovine serum albumin as the standard.
SDS-polyacrylamide gel electrophoresis was performed by the method of Laemmli (27) or on Tricine gels as specified by the manufacturer (Novex Experimental Technologies, San Diego, Calif.).
N-terminal amino acid sequencing was done by Edman degradation with an Applied Biosystems 477A protein sequencer coupled to a 120A analyzer. Amino acid sequencing was performed on samples of DL-FalDH that were electroblotted to polyvinylidene difluoride membranes.
Heme composition was assessed by the pyridine ferrohemochrome method as described previously (13).
Metal analyses (Mo, Zn, W, Ni, Cu, Fe, and Mn) were performed on microwave-digested samples of purified DL-FalDH (5.3 mg) using a Thermo Jarrel ASH model ICAP 61E inductively coupled plasma atomic emission spectrometer. Microwave digestions of samples were completed according to Environmental Protection Agency method SW 846-3015 (CEM Corp., 1996) in pressure vessels containing 0.1 M nitric acid.
Flavin content was assessed by comparison of absorption spectra against authentic standards of flavin adenine dinucleotide (FAD) (Sigma, St. Louis, Mo.) or flavin mononucleotide (FMN) (Sigma, St. Louis, Mo.) after precipitation of formaldehyde dehydrogenase in trichloroacetic acid or as described by Kilgour et al. (25).
RESULTS
Cellular location and electron donor preference in M. capsulatus Bath.
Previous studies have shown that cells of M. capsulatus Bath have a variety of formaldehyde-oxidizing enzyme systems (25, 28, 42, 46, 51). Studies by Dalton and coworkers indicated that the formaldehyde oxidation enzyme system in cells of M. capsulatus Bath expressing sMMO is catalyzed by an NAD+-dependent FalDH (42, 46). Since cell-free preparations of pMMO have been reported to utilize NADH + H+ as well as duroquinol as reductants (35, 40, 43–45, 54), an investigation was undertaken to evaluate the potential bioenergetic coupling of FalDH to pMMO through pyridine nucleotide mediators.
Although we were unsuccessful in coupling these two reactions, the change in growth conditions from sMMO to pMMO expression was observed to alter the cellular location and electron donor preference of the main formaldehyde dehydrogenase activity. In cells expressing the sMMO, 98% (4.8 ± 0.11 U per mg of soluble protein) of the total formaldehyde dehydrogenase activity in cell extracts was observed in the soluble fraction and was NAD(P)+ and dependent. In cells expressing the pMMO, 86% (1.4 ± 0.05 U per mg of membrane protein) of the formaldehyde dehydrogenase activity was membrane associated and dye linked. The dye-linked activity was resistant to acetylene treatment, indicating that the enzyme or enzymes catalyzing this activity were catalytically independent of the pMMO.
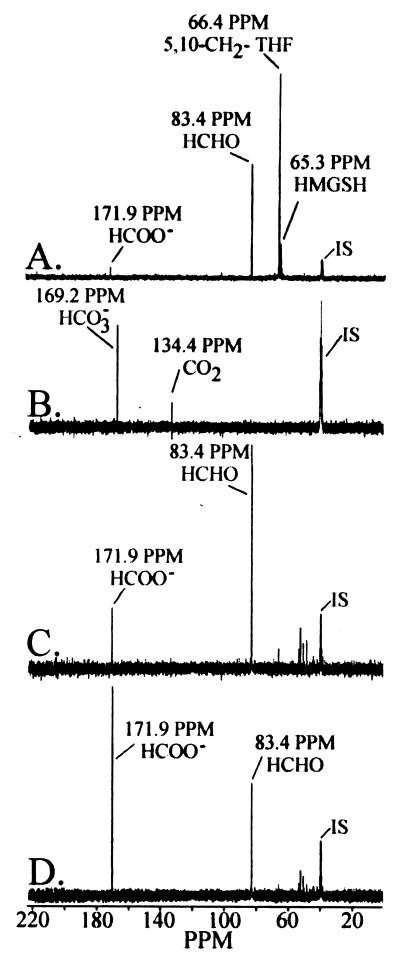
In an effort to characterize reaction substrates and products catalyzed by the dye-linked formaldehyde dehydrogenase, in situ labeling studies employing 13C-NMR were conducted to evaluate the fate of [13C] formaldehyde in whole-cell and cell-free samples from cells expressing the pMMO. Transformation of [13C] formaldehyde to [13C] carbon dioxide was observed in whole cells and in cell-free samples within 30 s from reaction initiation (Fig. 1A and B). [13C] formate was at or below the detection threshold of 0.1 mM in whole-cell or cell-free fractions. In addition to the reaction products associated with the dissimililatory pathway of formaldehyde oxidation, 13C-NMR spectra showed evidence for the assimilation of formaldehyde through 5,10-methylenetetrahydrofolate and a low concentration of S-(hydroxymethyl)glutathione.
FIG. 1.
Time course of metabolic fate profiles of [13C]formaldehyde in cell extracts from M. capsulatus Bath. 13C-NMR spectra of (A) a cell extract (34 mg of protein per ml) 10 min after the addition of [13C]formaldehyde and (B) 13C-NMR spectra of gaseous metabolites trapped from the headspace of the reaction vial in an insert that contained 6 M KOH. 13C-NMR spectra of (C) the washed membrane fraction (26 mg of protein per ml) 7 min after the addition of [13C]formaldehyde and (D) the same reaction mixture 14 min after the addition of [13C]formaldehyde. All peaks were normalized in reference to the internal standard (IS) dimethyl-d6 sulfoxide and confirmed by authentic standards. Assay conditions are described in Materials and Methods. Abbreviations: 5,10-CH2-THF, 5,10-methylenetetrahydrofolate; HCHO, formaldehyde; HMGSH, S-(hydroxymethyl)glutathione; HCOO−, formate; CO2, carbon dioxide; and HCO3−, bicarbonate.
Following separation of the cell-free fraction into soluble and membrane fractions, a time-dependent decrease in [13C] formaldehyde coupled with an increase in [13C] formate was observed in the washed membrane fraction (Fig. 1C and D). The results shown in Fig. 1 were performed on whole-cell, soluble, and membrane fractions stored aerobically on ice for approximately 12 h. Short-term storage on ice had no effect on DL-FalDH, NAD+-FalDH, or soluble formate dehydrogenase activity. However, under these conditions, no pMMO activity was detected in the washed membrane fraction (54). Inactivation of the pMMO in the washed membrane fraction resulted in the accumulation of formate and in the absence of 13CO2 or H13CO3 in these samples. In membrane samples containing active pMMO, formate would be oxidized to CO2.
The oxidation products of formaldehyde in the soluble fraction over a 1-h time period were below the detection limit, which was approximately 0.1 mM. The results indicate that in cells expressing the pMMO, the main formaldehyde dehydrogenase activity is located in the membrane fraction, while the formate dehydrogenase activity was associated in the soluble fraction.
Purification of formaldehyde dehydrogenase.
Washed cell membrane preparations exhibited reproducible levels of dye-linked formaldehyde dehydrogenase activity when grown in high-copper medium according to the procedure described in Materials and Methods. Purification of DL-FalDH from M. capsulatus Bath cells expressing the pMMO was performed by following formaldehyde-dependent DCPIP-PES reduction. The purification procedures used either a mixture of the nonionic and ionic detergents Triton X-100 and deoxycholate (method 1) or the nonionic detergent dodecyl-β-d-maltoside (method II) for solublization of the DL-FalDH activity from membrane preparations. Detergent solublization was followed by a ion-exchange chromatography step, a hydrophobic interaction chromatography step, and then a second ion-exchange chromatography step for method I or by two ion-exchange chromatography steps for method II. Results from a representative purification of DL-FalDH using method I are shown in Table 1.
TABLE 1.
Purification of DL-FalDH from M. capsulatus Bath by method I
| Step | Total protein (mg) | Total activity (U) | Sp act (U per mg of protein) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Membrane | 15,042 | 19.0 | 1.3 | 100 | 1 |
| Detergent extract | 14,892 | 18.9 | 1.3 | 100 | 1 |
| DEAE-Sepharose | 909 | 6.2 | 6.8 | 33 | 5 |
| Phenyl-Sepharose | 428 | 4.9 | 11.5 | 26 | 9 |
| Resource Q | 26 | 3.8 | 146.0 | 20 | 116 |
The physical and enzymatic properties of the purified DL-FalDH isolated by either method were indistinguishable. In addition to the simplicity of method II versus the increased detergent cost of this procedure, both isolation methods have certain advantages. A secondary benefit of method I was as a means of isolating partially purified cytochrome b559/569 complex. However, the dodecyl-β-d-maltoside solublization procedure of method II resulted in a fourfold-higher cytochrome b559/569 complex-to-DL-FalDH ratio during the terminal steps in the purification procedure. Thus, method II provided samples for studies evaluating the bioenergetics coupling between cytochrome b559/569 complex and DL-FalDH, while method I provided partially purified cytochrome b559/569 complex.
Physical properties.
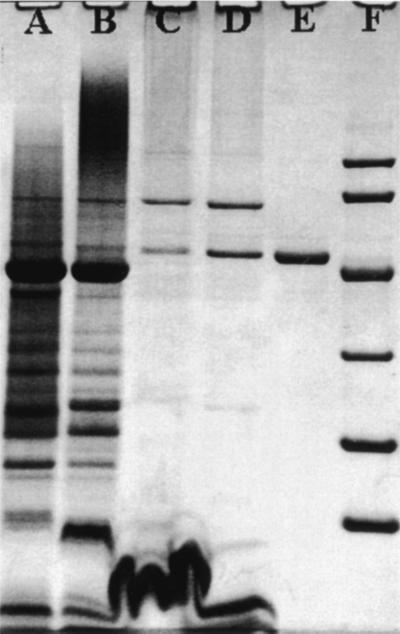
SDS denaturing gels of the purified DL-FalDH showed that the enzyme consisted of a single subunit with an apparent molecular mass of 49,500 Da (Fig. 2). The native mass of the enzyme was estimated by gel filtration chromatography on Sephadex G-150 in 20 mM PIPES (pH 7.5) plus 150 mM KCl and 0.2% dodecyl-β-d-maltoside to be approximately 210,000 Da, which indicated a tetrameric quaternary structure (α4) for the enzyme.
FIG. 2.
SDS-polyacrylamide slab gel electrophoresis of fractions during the purification of DL-FalDH by method I from M. capsulatus Bath. Washed membrane fraction (lane A), detergent extract (lane B), post-DEAE-Sepharose FF (lane C), post-phenyl-Sepharose (lane D), post-Resource Q (lane E), and low-range SDS Bio-Rad protein standards (lane F).
Absorption spectra of the native enzyme showed the presence of a weak chromophore with an absorbance of 408 nm with shoulders at 453 and 481 nm. These maxima were atypical of heme protein but showed some similarity to pyridine nucleotide-containing enzymes and to quinoproteins. An assay of noncovalently bound heme protein by the pyridine ferrohemochromagen assay or pyridine nucleotides by precipitation of the enzyme in trichloroacetic acid indicated the absence of either species in the purified sample. Spectral features of the enzyme were reduced in intensity upon reduction of the enzyme with dithionite or formaldehyde.
Absorption spectra and phenylhydrazine inhibition (see below) of formaldehyde dehydrogenase indicated the presence of a redox-active cofactor in DL-FalDH. However, metal analyses by inductively coupled atomic emission spectroscopy indicated that the cofactor was nonmetallic in nature. Attempts to establish the nature of the chromophore by treatment of the enzyme with solvents such as methanol and acetone or with protein denaturants such as trichloroacetic acid were not successful in removing the chromophore from the denatured polypeptide. Examination of the native enzyme by EPR spectroscopy showed a free radical signal near g = 2.0 in the substrate-free form of the enzyme that disappeared in the presence of formaldehyde (data not shown). The EPR properties for M. capsulatus Bath DL-FalDH were found to be identical to the uncharacterized redox-active quinone cofactor present in the DL-FalDH from the facultative methylotroph Hyphomicrobium zavarzinii (26) as well as the EPR spectrum from the PQQ-containing enzyme methanol dehydrogenase (14; unpublished data). UV-visible absorption spectra for the enzyme provided additional supporting evidence for the presence of a quinone cofactor in the enzyme, since these spectra were similar to optical spectra of PQQ (47).
In the oxidized state, the chromophore had absorption maxima at 269, 416, 453, and 480 nm. Upon reduction with dithionite, all absorption features were lost except for the 269-nm absorbance feature and a new absorption maximum at 418 nm. These spectral similarities to quinone cofactors, along with the observation that the chromophore could not be extracted by procedures that are commonly employed to release noncovalent prosthetic groups from enzymes, provided evidence that the prosthetic group in the M. capsulatus Bath DL-FalDH was a covalently bound quinone-like moiety. For this reason, the hexanol extraction procedure described by Vander Meer et al. (47) was employed in an attempt to remove the prosthetic group.
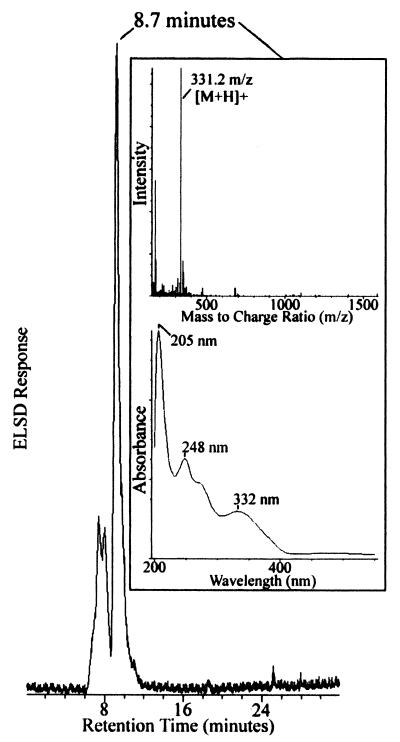
After 0.5 h of refluxing, a color change was observed in the solution from a pale yellow to a brick red color. The brick red appearance was consistent with the color observed for purified PQQ (15). Direct-injection electrospray mass spectrometry of the fraction gave a mass of 518 Da, which was consistent with the PQQ-5,5-dihexyl ketal form of PQQ. The putative PQQ-5,5-dihexyl ketal form of the isolated cofactor was subjected to the condensation conversion procedure of Vander Meer et al. (47), and the product was analyzed by HPLC ES-MS. Results of these experiments are shown in Fig. 3. The major product of the reaction eluted with a retention time of 8.7 min, had a molecular mass of 331.2 m/z [M + H]+, and absorption spectrum that was identical to samples of PQQ extracted from purified samples of methanol dehydrogenase from M. capsulatus Bath.
FIG. 3.
Purification and characterization of prosthetic group from DL-FalDH by HPLC ES-MS. The evaporative light-scattering profile of the prosthetic group following conversion of the ketal into putative PQQ through the hexoxy-PQ intermediate. The insets show the diode array wavelength spectrum (λmax = 205, 248, and 332 nm) and positive-ion electrospray mass spectrum ([M + H] + = 331.2 m/z) for the major species (>83% purity) of the conversion reaction that eluted at 8.7 min.
The N-terminal amino acid sequence of the DL-FalDH is shown in Fig. 4. The sequence from M. capsulatus Bath showed a high level of homology to the N-terminal region of a membrane-associated sulfide quinone reductase from Rhodobacter capsulatus (39) and Oscillatoria limnetica (4, 9). As in the case of the sulfide quinone reductases, the membrane-associated DL-FalDH contains sequence motifs for zinc as well as NAD(P)+/FAD-binding sites (16, 19, 52). The N-terminal sequence of the DL-FalDH scored a perfect score of 11, which should result in βαβ-fold and should provide an FAD or NAD+ binding site (52). However, none of these cofactors were associated with the purified protein. In addition, the purified enzyme did not bind NAD+, NADH, NADP+, NADPH, FAD, FADH2, or Zn. Finally, the addition of these metals and cofactors had no effect on the enzymatic properties of the purified enzyme, nor did they increase dye-linked formaldehyde oxidation rates in the washed membrane fraction.
FIG. 4.
N-terminal amino acid sequence of DL-FalDH from M. capsulatus (Mc) Bath, the sulfide quinone reductase from Oscillatoria limnetica (Ol) (4), and the sulfide quinone reductase from Rhodobacter capsulatus (Rc) (39). The fingerprint of the βαβ fold of the NAD(P)/FAD binding domain (16, 19, 52) is also shown. Amino acid residues matching the fingerprint of the βαβ fold of the NAD(P)/FAD binding domain are in boldface type.
To explore the possibility that the M. capsulatus Bath enzyme functioned as a quinone reductase, the activity was tested under aerobic and anaerobic conditions in the presence of decylubiquinone or ubiquinone-1 using formaldehyde, hydroxylamine, or sulfide as the initial electron donor. However, there was no indication in these experiments that the enzyme functioned as a quinone reductase using these electron acceptors or donors.
Catalytic properties.
13C-NMR experiments using the purified DL-FalDH showed that the enzyme catalyzed the oxidation of formaldehyde to formate in the presence of the redox mediator PES and the artificial electron acceptor DCPIP. Quantitation of [13C]formaldehyde and [13C]formate produced in time course experiments of the purified enzyme supported a 1:1 reaction stochiometry between substrate and product (data not shown). The purified form of the enzyme did not require exogenous glutathione to support enzyme activity. This observation indicated that the enzyme mechanism was independent of glutathione or that the enzyme was saturated with glutathione. 13C-NMR spectra of reaction products supported the conclusion that the DL-FalDH catalytic mechanism was independent of glutathione, since there was no evidence of S-(hydroxymethyl)glutathione in enzymatically active membrane fractions or in purified preparations of the enzyme (Fig. 1).
The substrate specificity of formaldehyde dehydrogenase was tested by substituting the formaldehyde in reaction mixtures with methanol (C1), acetaldehyde (C2), glyoxal (C2), or butyraldehyde (C4). These experiments indicated that the substrate specificity range for the DL-FalDH was extremely narrow, since formaldehyde was found to be the only substrate that supported enzyme-mediated reduction of DCPIP. Inhibitor series showed that DL-FalDH was insensitive to the metal chelator EDTA, to the sufhydryl-modifying agent N-ethylmaleimide, and to the respiratory inhibitors cyanide and azide. In contrast to whole-cell activity, the activity of the purified enzyme showed partial inhibition in the presence of Zn2+, Fe2+, and Cu2+.
Hydrazines have been noted as potent inhibitors of quinoproteins and have been used as active-site probes to establish the quinoprotein nature of enzymes (47). The mechanism of hydrazine inhibition in quinoproteins is believed to be the formation of reaction products from the oxidation of the hydrazine that react with an essential amino acid in the active site of the enzyme (26, 47). DL-FalDH activity was observed to be sensitive to phenylhydrazine in a manner similar to that observed for known quinoproteins (47). The results suggest that the prosthetic group in DL-FalDH may be PQQ or quinone-like in structure, which is consistent with the structural characterization described above.
Formaldehyde-cytochrome b559/569 oxidoreductase activity: evidence that cytochrome b559/569 complex is the initial electron acceptor to formaldehyde dehydrogenase.
Specific activity of the enzyme preparation was observed to increase nearly 13-fold following chromatography on Resource Q. Since it was unlikely that a change of this magnitude was due solely to enrichment of DL-FalDH by purification (Fig. 2), other mechanisms were considered, including the effect of detergent exchange into dodecyl-maltoside and/or the disruption of redox coupling between copurifying redox-active proteins. The effect of detergent exchange on specific activity was eliminated by purifying formaldehyde dehydrogenase in buffers containing 0.05% Triton X-100 and 0.01% deoxycholate versus results form purification method II. While Triton-containing fractions were more difficult to manipulate due to nondialyzable properties and protein assay interferences, no differences in specific activity change for this last step were found for Triton X-100/deoxycholate or for dodecyl-maltoside-containing preparations (12.1-fold and 12.7-fold, respectively).
Potential disruption of redox-coupling between copurifying redox-active proteins was investigated by first identifying redox-active protein components separated from DL-FalDH during chromatography on Resource Q. An evaluation of fractions from the Resource Q column showed that at least three redox-active proteins were separated from formaldehyde dehydrogenase at this step: cytochrome b559/569 complex (54), a monoheme cytochrome c553 (53), and cytochrome c556 peroxidase (53). As isolated, cytochrome b559/569 complex appears to be the cytochrome bc1 complex in M. capsulatus Bath minus the c-type cytochrome and the Rieske iron-sulfur protein. These cytochromes were isolated in the oxidized state and therefore were amenable without modification for use in spectral assays to evaluate their role as electron acceptors to the DL-FalDH.
Assays were performed in which individual redox-active proteins were individually reconstituted in the dye-linked spectrophotometric assay system in the absence of PES and DCPIP. Difference spectra for wavelength scans were recorded for reaction mixtures in the presence and absence of formaldehyde to assess the rate of reduction for heme proteins present in the reaction mixture. While there was no evidence for redox coupling between cytochrome c553 or cytochrome c556 peroxidase and DL-FalDH, rapid formaldehyde-dependent reduction of cytochrome b559/569 complex was observed in reaction mixtures containing formaldehyde, formaldehyde dehydrogenase, and cytochrome b559/569 complex. Kinetic rates for the reduction of cytochrome b559/569 complex by formaldehyde dehydrogenase (turnover number = 266 ± 28 mol of cytochrome b559/569 reduced per mol of FalDH per s) were similar to the rate of reduction observed for PES/DCPIP (turnover number = 290 ± 72 mol of DCPIP reduced per mol of FalDH per s). The preferential reduction of cytochrome b559/569 complex was demonstrated by the 13-fold decrease in DCPIP reduction rate upon addition of cytochrome b559/569 complex to assay mixtures containing purified DL-FalDH. These observations support the assignment of cytochrome b559/569 complex as the physiological electron acceptor to DL-FalDH.
DISCUSSION
This report describes the first membrane-associated dye- or cytochrome-linked formaldehyde dehydrogenase from a methanotroph. Although this activity has been observed in a number of obligate and facultative methylotrophs in the last 40 years, the rates of dye reduction were low and not considered physiologically significant (1). In contrast to these earlier reports, the results presented here demonstrate that, at least in M. capsulatus Bath, the cytochrome-linked FalDH can be physiologically significant depending on the growth or environmental conditions.
The difference between this and previous studies can be explained by the assay used to measure dye-linked formaldehyde activity. In samples containing cytochrome b559/569 complex, electrons will preferentially reduce cytochrome b559/569 complex before the dye (DCPIP) being used to monitor activity. Thus, in whole-cell or cell-free extracts, the electrons from formaldehyde oxidation bypass DCPIP and enter the respiratory chain at the cytochrome b/ubiquinone level. Correcting for the differential rates of DCPIP reduction by DL-FalDH in the presence and absence of cytochrome b559/569 complex, the rates of membrane-associated formaldehyde oxidation in cells expressing the pMMO were similar to the NAD(P)+-dependent FalDH oxidation rates observed in cells expressing the sMMO. In addition, the membrane-associated DL-FalDH described in this study is inhibited by Tris-HCl, which may also describe why this dye-linked activity was not detected in studies utilizing this buffer.
As described in a number of papers, the metabolic fate of formaldehyde in methylotrophic bacteria is complex, with most methylotrophs exhibiting more than one formaldehyde oxidation system (1, 10, 50, 51). The results presented here illustrate that the cytochrome-linked formaldehyde oxidation system may also be of physiological significance depending on the growth conditions.
The properties of the DL-FalDH from M. capsulatus Bath are summarized in Table 2. The enzyme showed a number of similarities to the soluble dye-linked formaldehyde dehydrogenase from the facultative methylotroph Hyphomicrobium zavarzinii (26). Both enzymes had similar holo- and subunit molecular masses and spectral (EPR and UV-visible absorption) properties and were inhibited by phenylhydrazine without the formation of hydrazone. Spectral and inhibitor studies by Klein et al. (26) provided evidence that the prosthetic group present in formaldehyde dehydrogenase from H. zavarzinii was a redox-active quinone cofactor, possibly PQQ, covalently bound to the peptide chain. The results of this study show that the prosthetic group in the DL-FalDH from M. capsulatus Bath is PQQ and provide evidence that a PQQ can be the redox-active component in the oxidation of formaldehyde to formate.
TABLE 2.
Physical and catalytic properties of DL-FalDH from M. capsulatus Bath
| Property | Value |
|---|---|
| Molecular mass (kDa) | |
| Enzyme | 210 ± 6.1 |
| Subunit | 49.5 |
| PQQ concn (mol/mol of enzyme) | 3.6 |
| Absorption maxima (nm) | 416, 453, 481 |
| EPR signals | 2.0 |
| Km (μM CHOH) | 143 ± 12 |
| Turnover number (mol of cytochrome b559/569 reduced per mol of enzyme per s) | 266 ± 28 |
| Turnover number (mol of DCPIP reduced per mol of enzyme per s) | 290 ± 72 |
| pH optimum (PIPES) | 7.4 |
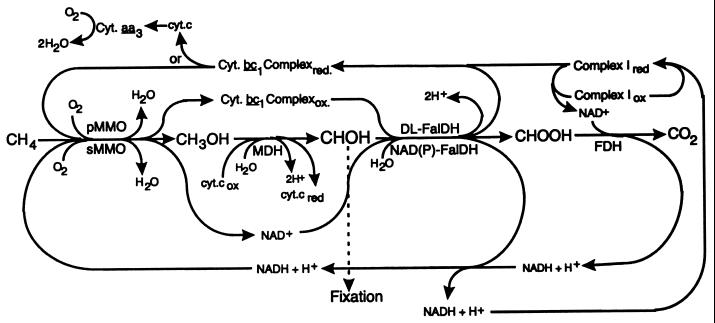
In contrast to the regulation of FalDHs by facultative methylotrophs, at least two of the FalDHs in M. capsulatus Bath are regulated by copper in a manner similar to that observed for the two methane monooxygenases. In facultative methylotrophs, the FalDHs are usually induced by growth on one-carbon compounds (1, 5, 6, 10, 18, 21, 48, 49, 50). In contrast, methanotrophic growth is restricted to one-carbon compounds but still uses multiple FalDHs. The results presented here may provide an explanation for why methanotrophs that can only be cultured on one-carbon compounds possess multiple FalDHs. As illustrated in Fig. 5, the cytochrome b559/569 complex (cytochrome bc1 complex) may function as the physiological reductant to the pMMO. Previous studies have indicated that pMMO is coupled to the electron transport chain at the quinone/semiquinone/cytochrome bc1 complex level (54). Electron transport inhibitor studies provided additional evidence that the pMMO is linked to the respiratory chain at the cytochrome bc1 complex level (1, 11, 45, 54, 55). The coinduction by copper of a cytochrome b-linked FalDH presented in this report provides additional evidence that the redox coupling between the respiratory chain and pMMO is at the level of the bc1 complex. Assuming that the cytochrome bc1 complex in M. capsulatus Bath is similar to studied mitochondrial and bacterial complexes (8, 20, 24, 32, 55), the results point to an electron flow from the cytochrome bc1 to pMMO, possibly via ubiquinone 8.
FIG. 5.
Proposed pathways of methane oxidation in M. capsulatus Bath. Membrane-associated proteins are shown above and soluble proteins below the substrate oxidation steps. With the exception of the methane oxidation by the pMMO, complex I, and the cytochrome bc1 complex, the enzymes and physiological electron acceptors/donors involved in the oxidation of methane to carbon dioxide have been characterized in a number of methanotrophs (1, 3, 22, 34, 44, 46, 51, 54). Abbreviations: complex I, NADH: quinone oxidoreductase; cyt., cytochrome; MDH, methanol dehydrogenase; NAD(P)-FalDH, NAD- and NADP-linked formaldehyde dehydrogenases; FDH, formate dehydrogenase.
ACKNOWLEDGMENTS
We acknowledge the I.S.U. Protein Facility and I.S.U. Department of Biochemistry and Biophysics for amino acid sequence analysis and use of the mass spectrometer.
This work was supported by Department of Energy grant 0296ER20237 (A.A.D.).
REFERENCES
- 1.Anthony C. The biochemistry of methylotrophs. London, England: Academic Press; 1982. [Google Scholar]
- 2.Anthony C. The microbial metabolism of C1 compounds: the cytochromes of Pseudomonas AM1. Biochem J. 1975;146:289–298. doi: 10.1042/bj1460289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anthony C. Quinoprotein-catalyzed reactions. Biochem J. 1996;320:697–711. doi: 10.1042/bj3200697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ariel B, Shahak Y, Taglicht D, Hauska G, Padan E. Purification and characterization of sulfide-quinone reductase, a novel enzyme driving anoxygenic photosynthesis in Oscillatoria limnetica. J Biol Chem. 1994;268:5705–5711. [PubMed] [Google Scholar]
- 5.Attwood M, Quayle J R. Formaldehyde as a central intermediary metabolite in methylotrophic metabolism. In: Crawford R L, Hanson R H, editors. Microbial growth on C1 compounds. Washington, D.C.: ASM Press; 1984. pp. 315–323. [Google Scholar]
- 6.Attwood M M. Formaldehyde dehydrogenases from methylotrophs. Methods Enzymol. 1990;188:314–324. [Google Scholar]
- 7.Bergman D J, Zahn J A, DiSpirito A A. High-molecular-mass multi-c-heme cytochromes from Methylococcus capsulatus Bath. J Bacteriol. 1999;181:991–997. doi: 10.1128/jb.181.3.991-997.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brand U, Trumpower B. The protonmotive Q cycle in mitochondria and bacteria. Biochem Mol Biol. 1994;29:165–197. doi: 10.3109/10409239409086800. [DOI] [PubMed] [Google Scholar]
- 9.Bronstein M, Schütz M, Hauska G, Padan E, Shahak Y. Cyanobacterial sulfide-quinone reductase: cloning and heterologous expression. J Bacteriol. 2000;182:3336–3344. doi: 10.1128/jb.182.12.3336-3344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chistoserdova L, Vorholt J A, Thauer R K, Lidstrom M E. C1 transfer enzymes and coenzymes linking methylotrophic bacteria and methanotrophic archaea. Science. 1998;281:99–102. doi: 10.1126/science.281.5373.99. [DOI] [PubMed] [Google Scholar]
- 11.Dalton H, Prior S D, Leak D J, Stanley S H. Regulation and control of methane monooxygenase. In: Crawford R L, Hanson R S, editors. Microbial growth on C1 compounds. Washington, D.C.: American Society for Microbiology; 1984. pp. 75–82. [Google Scholar]
- 12.Dijhuizen L, Levering P R, De Vries G E. The physiology and biochemistry of aerobic methanol-utilizing gram-negative and gram-positive bacteria. In: Murrell J C, Dalton H, editors. Methane and methanol utilizers. New York, N.Y: Plenum Press; 1992. pp. 149–181. [Google Scholar]
- 13.DiSpirito A A. Soluble cytochromes c from Methylomonas A4. Methods Enzymol. 1990;188:289–297. doi: 10.1016/0076-6879(90)88045-c. [DOI] [PubMed] [Google Scholar]
- 14.Duine J A, Frank J, Westerling J. Purification and properties of methanol dehydrogenase from Hyphomicrobium X. Biochim Biophys Acta. 1978;524:277–287. doi: 10.1016/0005-2744(78)90164-x. [DOI] [PubMed] [Google Scholar]
- 15.Duine J A, Frank J, Jongejan J A. PQQ and quinoprotein enzymes in microbial oxidations. FEMS Lett. 1986;32:165–178. [Google Scholar]
- 16.Eggink G, Engel H, Vriend G, Terpstra P, Witholt B. Rubredoxin Reductase of Pseudomonas oleovorans: structural relationship to other flavoprotein oxidoreductases based on one NAD and two FAD fingerprints. J Mol Biol. 1990;212:135–142. doi: 10.1016/0022-2836(90)90310-I. [DOI] [PubMed] [Google Scholar]
- 17.Groen B W, van Kleef A G, Duine J A. Quinohaemoprotein alcohol dehydrogenase apoenzyme from Pseudomonas testosteroni. Biochem J. 1986;234:611–615. doi: 10.1042/bj2340611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson R S, Hanson T E. Methanotrophic bacteria. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanukoglu H, Gutfinger T. cDNA sequence of adrenodoxin reductase: identification of NADP-binding sites in oxidoreductases. Eur J Biochem. 1989;180:479–484. doi: 10.1111/j.1432-1033.1989.tb14671.x. [DOI] [PubMed] [Google Scholar]
- 20.Jagow C V, Link T A. Use of specific inhibitors on the mitochondrial bc1 complex. Methods Enzymol. 1986;126:253–271. doi: 10.1016/s0076-6879(86)26026-7. [DOI] [PubMed] [Google Scholar]
- 21.Johnson P A, Quale J R. Microbial Growth on C-1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol grown Pseudomonas AM1. Biochem J. 1964;93:281–290. doi: 10.1042/bj0930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jollie D R, Lipscomb J D. Formate dehydrogenase from Methylosinus trichosporium OB3b. Methods Enzymol. 1990;188:331–334. doi: 10.1016/0076-6879(90)88051-b. [DOI] [PubMed] [Google Scholar]
- 23.Julian R K, Higgs R E, Gygi J D, Hilton M D. A method for quantitatively differentiating crude natural extracts using high-performance liquid chromatography-electrospray mass spectrometry. Anal Chem. 1998;70:3249–3254. doi: 10.1021/ac971055v. [DOI] [PubMed] [Google Scholar]
- 24.Junemann S, Heathcote P, Rich P R. On the mechanism of quinol oxidation in the bc1 complex. J Biol Chem. 1998;273:21603–21607. doi: 10.1074/jbc.273.34.21603. [DOI] [PubMed] [Google Scholar]
- 25.Kilgor G L, Fenton S P, Huennekens F M. Paper chromatography of flavins and flavin nucleotides. Flavin Nucleotides Flavoproteins. 1956;79:2254–2256. [Google Scholar]
- 26.Klein C R, Kesseler F P, Perreli C, Frank J, Duine J A, Schwartz A C. A novel dye-linked formaldehyde dehydrogenase with some properties indicating the presence of a protein-bound redox-active quinone cofactor. Biochem J. 1994;301:289–295. doi: 10.1042/bj3010289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Large E, Quayle J R. Enzyme activities in extracts of Pseudomonas AM1. Biochem J. 1963;87:386–396. doi: 10.1042/bj0870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowry O H, Rosebrough N R, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Marison I W, Attwood M M. Partial purification and characterization of a dye-linked formaldehyde dehydrogenase from Hyphomicrobium X. J Gen Microbiol. 1980;177:305–313. [Google Scholar]
- 31.Marison I W, Attwood M M. Possible alternative mechanism of the oxidation of formaldehyde to formate. J Gen Microbiol. 1982;128:1441–1446. [Google Scholar]
- 32.Matsumo-Yagi A, Hatefi Y. Ubiquinol cytochrome c oxidoreductase. Effects of inhibitors on reveres electron transfer from the iron-sulfur protein to cytochrome b. J Biol Chem. 1999;274:9383–9288. doi: 10.1074/jbc.274.14.9283. [DOI] [PubMed] [Google Scholar]
- 33.Mehta R J. A novel inducible formaldehyde dehydrogenase of Pseudomonas sp. (RJ1). Antonie van Leeuwenhoek J. Microbiol Serol. 1975;41:89–95. doi: 10.1007/BF02565039. [DOI] [PubMed] [Google Scholar]
- 34.Murrell J C, McDonald I R, Gilbert B. Regulation of expression of methane monooxygenases by copper ions. Trends Microbiol. 2000;8:221–225. doi: 10.1016/s0966-842x(00)01739-x. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen H H, Elliott T, Yip S J, Chan J H K, Chan S I. The particulate methane monooxygenase from Methylococcus capsulatus (Bath) is a novel copper-containing three-subunit enzyme. J Biol Chem. 1998;273:1957–7966. doi: 10.1074/jbc.273.14.7957. [DOI] [PubMed] [Google Scholar]
- 36.Patel R N, Hou C T, Felix A. Microbial oxidation of methane and methanol: purification and properties of a heme-containing aldehyde dehydrogenase from Methylomonas methylovora. Arch Microbiol. 1979;122:241–247. doi: 10.1007/BF00411286. [DOI] [PubMed] [Google Scholar]
- 37.Patel R N, Hou C T, Derelanko P, Felix A. Purification and properties of a heme-containing aldehyde dehydrogenase from Methylosinus trichosporium. Arch Biochem Biophys. 1980;203:654–662. doi: 10.1016/0003-9861(80)90223-4. [DOI] [PubMed] [Google Scholar]
- 38.Pomper B K, Vorholt A, Chistoserdova L, Lidstrom M E, Thauer R K. A methyenyl tetrahydromethanopterin cyclohydrolase and an methyl tetrahydrofolate cyclohydrolase in Methylobacterium extorquens AM1. Eur J Biochem. 1999;261:476–480. doi: 10.1046/j.1432-1327.1999.00291.x. [DOI] [PubMed] [Google Scholar]
- 39.Schutz M Y S, Padan E, Hauska G. Sulfide-quinone reductase from Rhodobacter capsulatus. J Biol Chem. 1997;272:9890–9894. doi: 10.1074/jbc.272.15.9890. [DOI] [PubMed] [Google Scholar]
- 40.Shiemke A K, Cook S A, Mily T, Singleton P. Detergent-solublization of membrane-bound methane monooxygenase requires plastoquinol analogues as electron donors. Arch Biochem Biophys. 1995;321:521–528. doi: 10.1006/abbi.1995.1413. [DOI] [PubMed] [Google Scholar]
- 41.Speer B L C, Lidstrom M E. Sequence of the gene for a NAD(P)-dependent formaldehyde dehydrogenase (class III alcohol dehydrogenase) from a marine methanotroph Methylobacter marinus A45. FEMS Lett. 1994;121:349–356. doi: 10.1111/j.1574-6968.1994.tb07125.x. [DOI] [PubMed] [Google Scholar]
- 42.Stirling D I, Dalton H. Purification and properties of an NAD(P)+-linked formaldehyde dehydrogenase from Methylococcus capsulatus (Bath) J Gen Microbiol. 1978;107:19–29. doi: 10.1099/00221287-107-1-19. [DOI] [PubMed] [Google Scholar]
- 43.Takeguchi I O. Role of iron and copper in particulate methane monooxygenase of Methylosunus trichosporium OB3b. Catalysis Surveys Jpn. 2000;4:51–63. [Google Scholar]
- 44.Takeguchi M K M, Okura I. Purification and properties of particulate methane monooxygenase from Methylosinus trichosporium OB3b. J Mol Catal A Chem. 1998;132:145–153. doi: 10.1023/a:1009278216452. [DOI] [PubMed] [Google Scholar]
- 45.Takeguchi T Y, Miyakawa M K, Okura I. Redox behavior of copper in particulate methane monooxygenase from Methylosinus trichosporium OB3b. BioMetals. 1999;12:27–33. doi: 10.1023/a:1009278216452. [DOI] [PubMed] [Google Scholar]
- 46.Tate S, Dalton H. A low-molecular-mass protein from Methylococcus capsulatus (Bath) is responsible for the regulation of formaldehyde dehydrogenase activity in vitro. Microbiology. 1999;145:159–167. doi: 10.1099/13500872-145-1-159. [DOI] [PubMed] [Google Scholar]
- 47.Vander Meer R A, Mulder A C, Jongejan J A, Duine J A. Determination of PQQ in quinoproteins with covalently bound cofactor and in PQQ-derivatives. FEBS Lett. 1989;254:99–105. [Google Scholar]
- 48.Van Ophem P W, Van Beeumen J, Duine J A. NAD-linked factor dependent formaldehyde dehydrogenase or trimeric, zinc-containing, long-chain alcohol dehydrogenase from Amycolatopsis methanolica. Eur J Biochem. 1992;206:511–518. doi: 10.1111/j.1432-1033.1992.tb16954.x. [DOI] [PubMed] [Google Scholar]
- 49.Van Ophem P W, Bystrykh L V, Duine J A. Dye-linked dehydrogenase activities for formate and formate esters in Amycolatopisis methanolica: characterization of a molybdoprotein enzyme active with formate esters and aldehydes. Eur J Biochem. 1992;206:519–525. doi: 10.1111/j.1432-1033.1992.tb16955.x. [DOI] [PubMed] [Google Scholar]
- 50.Vorholt J A, Chistoserdova L, Lidstrom M E, Thauer R K. The NADP-dependent methylene tetrahydromethanopterin dehydrogenase in Methylobacterium extorquens AM1. J Bacteriol. 1998;180:5351–5356. doi: 10.1128/jb.180.20.5351-5356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vorholt J A, Chistoserdova L, Stolyar S M, Thauer R K, Lidstrom M E. Distribution of tetrahydromethanopterin-dependent enzymes in methylotrophic bacteria and phylogeny of methenyl tetrahydromethanopterin cyclohydrolases. J Bacteriol. 1999;181:5750–5757. doi: 10.1128/jb.181.18.5750-5757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wierenga R K, Terpstra P, Hol W G. Prediction of the occurrence of the ADP-binding βαβ-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986;187:101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- 53.Zahn J A, Arciero D M, Hooper A B, DiSpirito A A. Cytochrome c peroxidase from Methylococcus capsulatus Bath. Arch Microbiol. 1997;168:362–372. doi: 10.1007/s002030050510. [DOI] [PubMed] [Google Scholar]
- 54.Zahn J A, DiSpirito A A. The membrane-associated methane monooxygenase from Methylococcus capsulatus Bath. J Bacteriol. 1996;178:1018–1029. doi: 10.1128/jb.178.4.1018-1029.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Z, Huang L, Shipmaster V M, Chi Y-I, Kim K K, Hung L-W, Croft A C, Berry E A, Kim S-H. Electron transfer by domain movement in cytochrome bc1. Nature. 1998;392:677–687. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Z, Huang L, Shipmaster V M, Chi Y-I, Kim K K, Hung L-W, Croft A C, Berry E A, Kim S-H. Electron transfer by domain movement in cytochrome bc1. Nature. 1998;392:677–687. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]