Abstract
Background
Although treatment-resistant depression (TRD) is a major public health problem that increases mortality due to suicides, a considerable percentage of patients do not respond adequately to variable treatments. Patients with TRD sometimes have comorbid cervical stiffness. This observational study aims to examine the association of local modulation of cervical muscles with TRD and to learn the involvement of the parasympathetic nervous system in the underlying mechanism.
Methods
A total of 1103 hospitalized patients with TRD who were resistant to outpatient care were enrolled between May 2006 and October 2021. All patients underwent local modulation of the cervical muscles by physical therapy during hospitalization. The presence or absence of TRD and whole-body disorders, such as headache, dazzling, cervical stiffness, and cardiovascular and gastrointestinal disorders, was determined by the patient’s subjectivity using the self-rated medical interview sheet at admission and discharge. Pupil light reflex parameters were also measured at admission and discharge using a binocular infrared pupilometer.
Results
The improvement rate of TRD during hospitalization was 72.1%, and did not differ significantly by sex, age, and hospitalization period. The improvement of TRD showed a strong association with those of cervical stiffness and dazzling, a pupil light reflex disorder (p < 0.001: odds ratios = 12.76 and 6.39, respectively), but not with those of headache or cardiovascular and gastrointestinal disorders (p > 0.05). In the TRD-improved patients, the pupil light reflex parameters representative of the parasympathetic nervous system function ameliorated: pupil diameter decreased, while constriction rate and velocity increased during hospitalization. In contrast, little amelioration of the parameters was seen in the TRD-unimproved patients.
Conclusions
Cervical muscle stiffness may be associated with TRD, possibly through dysfunction of the parasympathetic nervous system.
Trial registration
ID: UMIN000040590. First registration date: 30/05/2020.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-022-05860-2.
Keywords: Treatment-resistant depression (TRD), Physical therapy, Cervical muscle, Parasympathetic nervous system
Background
Although major depressive disorder is a representative public health problem, the diagnostic criteria have not yet been defined [1, 2]. Furthermore, despite a wide variety of treatments, including antidepressants, mood stabilizers, exercise, education, and psychotherapy, a significant percentage of patients with this disorder do not respond adequately to them [3, 4]. The patients who fail to respond to two or more treatments are diagnosed with treatment-resistant depression (TRD) [5–7], which increases mortality due to suicides and imposes an enormous burden on society [8–10].
TRD can be accompanied by indefinite disorders in the whole body [8, 11, 12]. In our clinical experience, we have observed a potential trend in indefinite whole-body disorders including TRD that occasionally coincide with cervical stiffness, and proposed a new medical concept called “cervical neuro-muscular syndrome” [13]. Our previous study of 1863 patients showed that about 60% of patients with cervical disorders have comorbid depression [14]. We have attempted local and physical modulation of the cervical stiffness to treat these indefinite whole-body disorders. Among physical therapies, low-frequency electrical stimulation [15, 16] and far-infrared irradiation [17] are reportedly effective at ameliorating the stiffness. The previous study showed that the combined application of these two physical therapies to the cervical muscles significantly decreased the number of indefinite whole-body disorders [14]. Also, our studies disclosed that the physical therapies to the cervical muscles were effective in patients with whiplash-associated disorders [18] and myalgic encephalomyelitis/chronic fatigue syndrome [19].
As the underlying mechanism, we proposed dysfunction of the parasympathetic nervous system which might possibly be caused by the cervical stiffness [14, 19]. The parasympathetic nervous system controls rest, recovery from stress, and maintaining homeostasis, while the sympathetic nervous system, the other autonomic nervous system, plays an excitatory role in a stressful situation. To evaluate parasympathetic nervous system function, the pupil light reflex is known to be a representative indicator; the constrictor muscle of the pupil decreases in diameter under the control of the ciliary ganglion, which is activated and innervated by a preganglionic autonomic nerve fiber [20, 21]. The pupil light reflex has been used to evaluate autonomic nerve dysfunction in patients with Parkinson’s disease, Alzheimer’s disease, and diabetes mellitus [22–24].
This study examined the association of improvement of TRD with those of whole-body disorders using physical therapies [15–17] as a method to ameliorate cervical stiffness. Furthermore, the involvement of the parasympathetic nervous system was investigated by comparing the changes in the pupil light reflex parameters during hospitalization between patients with and without TRD improvement.
Methods
Study design
This study is an observational study that compared patients with versus without improvement of TRD.
Patients
This study was conducted with the approval of the institutional review board of Tokyo Neurological Center and Matsui Hospital. Written informed consent was obtained from all participants or a parent or guardian for participants under 18 years old.
The presence or absence of symptoms was based on the patient’s subjectivity using the self-rated medical interview sheet, a part of which is shown as Supplementary Table 1. Since the diagnostic criteria of TRD have not yet been defined, we have diagnosed patients who reported all four representative symptoms; depressive condition, bedridden condition, lethargy, and uneasy feeling, as the major depressive disorder, based on previous reports [1, 2]. For all participants, the self-rated medical interview sheets documenting the presence or absence of the four representative symptoms were collected at admission and discharge. Also, the interview sheets documenting the presence or absence of representative five whole-body disorders; headache, dazzling, cervical stiffness, cardiovascular disorders, and gastrointestinal disorders, that have been reported to accompany TRD [8, 11, 12] were collected at admission and discharge. Patients who reported one or more symptoms among palpation, chest tightness, thermoregulation disorder and poor circulation were diagnosed with cardiovascular disorders, while those with nausea or stomachache and diarrhea or constipation were diagnosed with gastrointestinal disorders (Supplementary Table 1).
Among patients with major depressive disorder, who visited our institution between May 2006 and October 2021, we enrolled 1277 who were diagnosed with TRD due to failure to respond to two or more outpatient treatments [5–7] and were hospitalized. Outpatient treatments at our institution included antidepressants, mood stabilizers, exercise, education, and psychotherapy, but did not include physical therapy for cervical muscles. This is because the outpatient facility at our institution, like most hospitals in Japan, does not have equipment for the physical therapy, whereas the inpatient facility does.
The TRD patients who recovered from two or more symptoms among the four representative symptoms of the major depressive disorder were defined to be improved (Supplementary Table 1). Similarly, for patients with cardiovascular and gastrointestinal disorders, those who recovered from one or more symptoms were defined to be improved.
Hospitalization and discharge were decided with consent between patients and physicians without any objective criteria. Although our institution has had evidence that physical therapy decreased the number of indefinite whole-body disorders including depression [14], there have been no reports showing the success of physical therapy on TRD nor information of the effects on the patients. Hence, it is improbable that the decision of hospitalization or discharge was dependent on or biased by the patient’s prejudice. Although patients were allowed to stay until they felt improved, those hospitalized for 10–120 days were included in this study. Patients who refused the pupil light reflex test, those who were diagnosed with specific diseases in other organs after admission, or those who discharged themselves from the hospital based on their judgment for unknown reasons, were excluded.
Intervention
All patients underwent low-frequency electrical stimulation and far-infrared irradiation [15–17] applied to the cervical muscles for 15 minutes two times daily throughout hospitalization (Supplementary Fig. 1A). No other intervention, including medication, injection, cervical traction or fixation, exercise, education, or psychotherapy, was performed under the written informed consent of participants. The approval of this management by our institution was because the patients have already exhausted all possibilities of outpatient treatments. Also, our institution has had evidence that physical therapies without any intervention decreased the number of indefinite whole-body disorders including depression [14], suggesting a possibility of the effect.
Silver spike point (SSP; Nihon Medix, Chiba, Japan) and pain topra (LCF-30; Celcom, Inc., Fukuoka, Japan) were used for the low-frequency electrical stimulation and the far-infrared ray irradiation, respectively. The physical therapies were applied from all directions, at least to one point in the anterior, posterior, right, and left regions, depending on palpation by physicians to determine the muscle lesions [13] (Supplementary Fig. 1B).
Assessment of the pupil light reflex was performed at admission and discharge using a binocular infrared pupilometer (Iriscoder Dual C10641; Hamamatsu Photonics, Shizuoka, Japan) as previously reported [25, 26] (Supplementary Fig. 2). Briefly, a red-light stimulus was presented to the right eye, and three parameters were measured in the right eye: initial pupil diameter (mm) before the stimulus, percentage constriction rate ([pupil diameter before the light stimulus – minimum pupil diameter after the light stimulus]/[pupil diameter before the light stimulus] × 100), and the constriction velocity (mm/second).
Statistical analysis
Statistical analyses were performed using SPSS 16.0 J for Windows. Two-sided p-values less than 0.05 were considered statistically significant. As the sample size (n = 1103) was sufficient, the central limit theorem could be applied to confirm that the data were normally distributed and that violating the normality assumption would not cause major problems [27]. Hence, the paired Student’s t-test was used to examine the difference in the number of disorders at admission versus discharge, while the unpaired t-test was used to compare means between the improved and unimproved TRD groups. In the univariate and multivariate logistic regression analysis, all variables were force entered into the multivariate model. Forward stepwise multivariate logistic regression analysis was also performed. The best model was selected based on the likelihood ratio test results.
Results
Participant flow and backgrounds
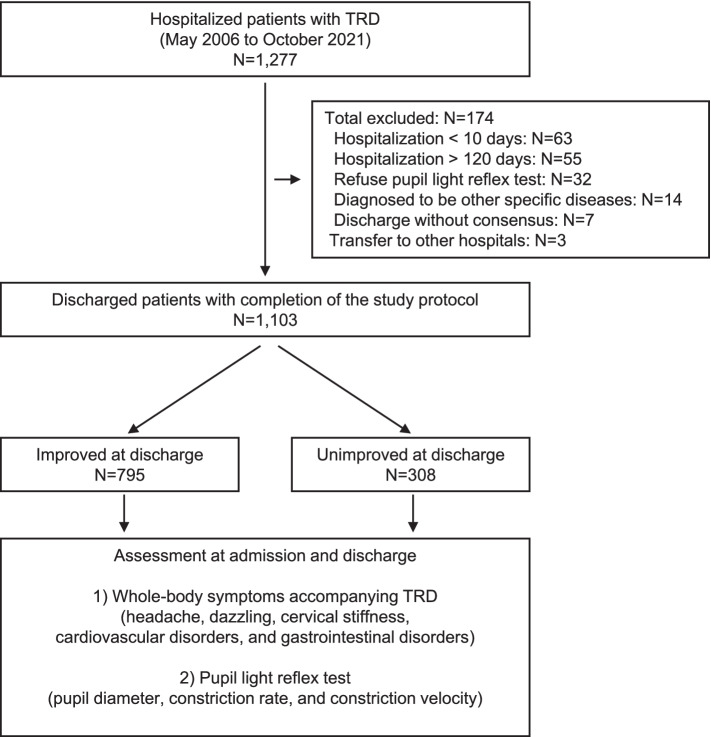
Figure 1 shows a flowchart of the patient enrollment process of the present study. A total of 1277 hospitalized patients diagnosed with TRD [5–7] were initially enrolled in this study. Of them, 174 were excluded after enrollment, including 63 who were discharged from the hospital after less than ten days, 55 who were hospitalized for more than 120 days, 32 who refused pupil light reflex test, 14 who were diagnosed with specific diseases in other organs after admission, seven who discharged themselves from the hospital based on their judgment with unknown reasons, and three who were transferred to other hospitals for treatment of specific diseases. After the removal of these patients, 1103 completed the study protocol. Among them, 795 (72.1%) were diagnosed as having improved from TRD at discharge, while 308 (27.9%) remained unimproved.
Fig. 1.
Flow chart of participant enrollment and study design
The presence or absence of whole-body disorders and the pupil light reflex parameters at admission and discharge were compared between the TRD-improved and -unimproved patients. Table 1 shows the baseline characteristics of the 1103 participants (383 men, 720 women) with a mean age of 49.5 ± 18.1 years (mean ± standard deviation) and a mean hospitalization period of 66.9 ± 25.4 days. The improvement rate was not significantly altered by sex (men versus women), age strata (< 50 versus ≥50 years), or hospitalization period (10–60 versus 61–120 days) (p > 0.05).
Table 1.
Baseline characteristics of the study participants with versus without TRD recovery
| Number (%) | Number (%) | Number (%) | |||
|---|---|---|---|---|---|
| Total | Improved | Unimproved | P-value | ||
| Variables | 1103 (100.0) | 795 (72.1) | 308 (27.9) | ||
| Sex | Men | 383 (34.7) | 287 (74.9) | 96 (25.1) | 0.213 |
| Women | 720 (65.3) | 508 (70.6) | 212 (29.4) | ||
| Age strata (years old) | < 50 | 632 (57.3) | 470 (74.4) | 162 (25.6) | 0.152 |
| ≥50 | 471 (42.7) | 325 (69.0) | 146 (31.0) | ||
| Hospitalization period (days) | 10–60 | 434 (39.3) | 306 (70.5) | 128 (29.5) | 0.631 |
| 61–120 | 669 (60.7) | 486 (72.6) | 183 (27.4) |
Relationship between TRD improvement and related disorders
Among the representative five disorders accompanying TRD [8, 11, 12], more than 75% of TRD patients reported headache and cervical stiffness at admission, while 45.4, 23.2, and 59.3% reported dazzling, cardiovascular disorders, and gastrointestinal disorders, respectively (Table 2). On the other hand, the improvement rates of these disorders in the total population were more than 70% in patients with dazzling and cervical stiffness, but less than 70% in those with headache, cardiovascular and gastrointestinal disorders (Table 2). This was reproducible in the TRD-improved population, suggesting a positive association of improvements of dazzling and cervical stiffness with that of TRD.
Table 2.
Number (percentage) of patients with representative five disorders accompanying TRD at admission and those with and without improvement at discharge
| Total (n = 1103) | TRD improved (n = 795; 72.1%) | TRD unimproved (n = 308; 27.9%) | |
|---|---|---|---|
| Headache at admission | 862 (78.2) | 618 (71.7) | 244 (28.3) |
| Improved at discharge | 451 (52.3) | 324 (52.4) | 127 (52.0) |
| Unimproved at discharge | 411 (47.7) | 294 (47.6) | 117 (48.0) |
| Dazzling at admission | 501 (45.4) | 358 (71.5) | 143 (28.5) |
| Improved at discharge | 378 (75.4) | 281 (78.5) | 97 (67.8) |
| Unimproved at discharge | 123 (24.6) | 77 (21.5) | 46 (32.2) |
| Cervical stiffness at admission | 839 (76.1) | 683 (81.4) | 156 (18.6) |
| Improved at discharge | 591 (70.4) | 580 (84.9) | 11 (7.1) |
| Unimproved at discharge | 248 (29.6) | 103 (15.1) | 145 (92.9) |
| Cardiovascular disorders at admission | 256 (23.2) | 195 (76.2) | 61 (23.8) |
| Improved at discharge | 162 (63.3) | 125 (64.1) | 37 (60.7) |
| Unimproved at discharge | 94 (36.7) | 70 (35.9) | 24 (39.3) |
| Gastrointestinal disorders at admission | 654 (59.3) | 340 (52.0) | 314 (48.0) |
| Improved at discharge | 423 (64.7) | 187 (55.0) | 236 (75.2) |
| Unimproved at discharge | 231 (35.3) | 153 (45.0) | 78 (24.8) |
The logistic regression analysis confirmed that the improvement (versus non-improvement) of dazzling and cervical stiffness showed a strong association with that of TRD (p < 0.001; odds ratios = 6.39 and 12.76, respectively), while headache and cardiovascular and gastrointestinal disorders did not (p > 0.05) (Table 3). These results demonstrate that TRD improvement during hospitalization is associated with the pupil light reflex which is impaired in the dazzling, as well as with cervical stiffness.
Table 3.
Odds ratio (95% CI) of the improvement (vs. non-improvement) of indefinite whole-body disorders to that of TRD by logistic regression analysis
| Number (%) of patients | Number (%) of improved patients | Odds ratio | 95% CI | P-value | |
|---|---|---|---|---|---|
| Headache | 862 (78.2) | 451 (52.3) | 2.60 | 0.87–4.51 | > 0.05 |
| Dazzling | 501 (45.4) | 378 (75.4) | 6.39 | 3.45–11.92 | < 0.001 |
| Cervical stiffness | 839 (76.1) | 591 (70.4) | 12.76 | 8.05–21.42 | < 0.001 |
| Cardiovascular disorders | 256 (23.2) | 162 (63.3) | 4.65 | 0.71–8.87 | > 0.05 |
| Gastrointestinal disorders | 654 (59.3) | 423 (64.7) | 3.79 | 0.93–6.90 | > 0.05 |
CI Confidence of interval
Pupil light reflex test
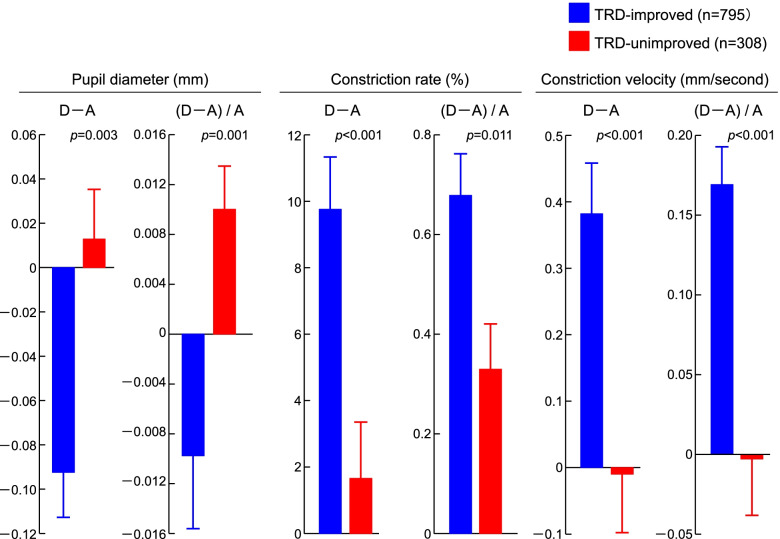
We further examined the possible involvement of parasympathetic nervous system function by comparing three pupil light reflex parameters between admission and discharge. We first confirmed that the changes (D–A) of pupil reflex parameters (pupil diameter, constriction rate, and constriction velocity) were not significantly different between shorter (10–60 days) and longer (61–120 days) hospitalization periods, just like the improvement rate of cervical stiffness (Supplementary Table 2). In the TRD-improved patients, both pupil diameter (D–A = –0.091 ± 0.021 mm; mean ± standard error) and the ratio adjusted by those at admission ([D–A]/A = –0.009 ± 0.006) decreased during hospitalization, while constriction rate (D–A = 9.82 ± 1.64%) and the ratio ([D–A]/A = 0.66 ± 0.09), as well as constriction velocity (D–A = 0.38 ± 0.08 mm/second) and the ratio ([D–A]/A = 0.162 ± 0.033) were increased (Fig. 2). These suggest that the physical therapy ameliorated pupil light reflex representing parasympathetic nervous system function during hospitalization. In the TRD-unimproved patients, however, amelioration of the pupil light reflex parameters; decrease in pupil diameter and increases in constriction rate and velocity, were not seen or were significantly lower than in the TRD-improved patients (p = 0.003, p < 0.001, and p < 0.001 in D–A; p = 0.001, p = 0.011, and p < 0.001 in [D–A]/A, respectively). Taken together, the improvement of TRD was positively associated with that of pupil light reflex and parasympathetic nervous system function.
Fig. 2.
Changes (D–A) in three parameters of the pupil light reflex test (pupil diameter, constriction rate, and constriction velocity) between admission and discharge, as well as the ratio adjusted by those at admission ([D–A]/A). Blue and red bars show the mean ± standard error of treatment-resistant depression (TRD)-improved patients and -unimproved patients, respectively, with the p-values of differences between them
Discussion
This study showed a strong association of the improvement of TRD with those of cervical stiffness and dazzling which is the disorder of pupil light reflex and possibly that of parasympathetic nervous system function. We, therefore, propose that cervical stiffness may be associated with TRD, possibly through dysfunction of the parasympathetic nervous system.
The canonical pathway that regulates the pupil light reflex is such that the ganglion cell axons project to the Edinger-Westphal nucleus in the midbrain, where the preganglionic parasympathetic neuron fibers in the oculomotor nerve which are activated and command the constrictor muscles of the pupil [20, 21]. Although the oculomotor nerve does not run through the cervical region, another non-canonical pathway via the afferent parasympathetic neuron fiber in the vagus nerve arising from the brainstem and running alongside cervical muscles down to the thoracic and abdominal viscera [28] may be involved in the regulation of the pupil light reflex. In fact, the cervical vagus nerve stimulation (VNS) therapy was approved by the U.S. Food and Drug Administration as a treatment for TRD with the advantages of sustained therapeutic response and favorable safety profile [29–32]. Considering that the cervical VNS is supposed to work through the parasympathetic nervous system, the present results may not be much novel or surprising. However, the advantage of the present physical therapy is that this is less invasive than VNS which needs a surgical procedure.
The causal relationship between improvements in cervical stiffness and the parasympathetic nervous system remains unclarified. Our analysis of the changes (D–A and [D–A]/A) of the pupil light reflex parameters showed that pupil diameter decreased while constriction rate and velocity increased in the cervical stiffness-improved patients (Supplementary Fig. 3). In the cervical stiffness-unimproved patients, amelioration of the pupil light reflex parameters was lower than that in the cervical stiffness-improved patients. These suggest that improvement of cervical stiffness was positively correlated with the amelioration of pupil light reflex and parasympathetic nervous system. However, the differences of the parameters between stiffness-improved and -unimproved patients, as shown by the p-values, were smaller than those between TRD-improved patients and -unimproved patients (Fig. 2). Hence, there might be other mechanisms underlying the improvements of parasympathetic nervous system and TRD, independent of that of the cervical stiffness. In fact, both electrical stimulation and far-infrared irradiation are reported to cause nerve regeneration and repair directly [33, 34]. Furthermore, the vagus nerve does not travel inside the cervical muscles, but runs along the carotid sheath in the neck. The present physical therapies were applied from all directions; anterior, posterior, and lateral to the cervical region (Supplementary Fig. 1B). Further studies on the relationship between the effect and the application site associated with the vagus nerve localization would possibly clarify whether the effect is direct to the vagus nerve or indirect through the cervical muscle modulation.
Most patients with TRD have substantial mental comorbidity and disorders related to muscle tension. Although there was a strong association of improvements between cervical stiffness and TRD (Table 3), a crucial limitation of this study is that it remains unclarified whether cervical stiffness is the cause or effect of TRD. In addition, the presence or absence of cervical stiffness was determined by the patient’s subjectivity using the self-rated medical interview sheet. More quantitative and objective measurements, e.g., ultrasound elastography technique used in oncology and now applied to measure the mechanical properties of the muscle in patients with multiple sclerosis spasticity [35], may be needed. A prospective study using shear-wave elastography [36], a representative ultrasound elastography technique, to measure cervical muscle stiffness is now underway.
Although about 80% of the TRD patients complained of headache at admission, the improvement of TRD was not significantly associated with that of headache (Tables 2 and 3), suggesting a different mechanism between TRD and headache underlying the effects of physical therapy. In addition to the upper cervical dysfunction of facet joints or occipital muscles, we speculate that the headache in this study may at least partly be the cervicogenic type whose diagnostic criteria are unilateral headache without side-shift and pain starting in the neck and spreading to the fronto-ocular area [37]. Since it is reported that upper cervical spine mobilization demonstrates more clinical benefits than massage therapy of the cervical muscles for this type of headache [38], it is expected that the present physical therapy for modulation of the cervical muscles is not effective for this headache.
This study is the first to show that physical therapy delivered to the cervical muscles improved more than 70% of the patients with TRD. However, we would like to emphasize that the present study is an observational study that examined the association of local modulation of cervical muscles with TRD, but not a prospective study aiming at the clinical application of specific physical therapies. In other words, we used physical therapies as a method for the local modulation of cervical muscles. Although the patients in this study did not receive any other interventions during the hospitalization, we cannot deny the possibility that TRD might improve through long-term rest or changing air. As a trial study aiming at the clinical application of the physical therapies to TRD treatment, a control group of patients who did not undergo the therapies to cervical muscles during hospitalization would be essential. However, physical therapy performed two times daily for a mean of 66.9 days per patient is too costly for health care providers and patients. Since we believe that local modulation of the cervical muscles by pharmacological intervention would also effectively treat TRD, we are now planning a clinical trial that examines the effects of more feasible treatments like a topical muscle-relaxant poultice or ointment in patients with TRD.
Conclusions
Cervical muscle stiffness may be associated with TRD, possibly through dysfunction of the parasympathetic nervous system, although the causality requires further studies.
Supplementary Information
Acknowledgments
The authors thank Mrs. Hiroki Fujii and Ryuji Mashima at our institution for their invaluable technical assistance.
Abbreviations
- TRD
Treatment-resistant depression
- SSP
Silver spike point
- VNS
Vagus nerve stimulation
Authors’ contributions
TM and KH conceived of the study idea. TM, KH, MI, YE, NS, and HK started the study. TM and HK obtained ethical approval. TM, MI, YE, NS, SH, HF, MM, and HK acquired the data. TM, KH, and HK interpreted the data. TM and HK performed the statistical analysis. TM and HK wrote the initial manuscript. All authors reviewed the manuscript and approved the submitted version for publication.
Funding
There was no external funding associated with this study. Since the present physical therapies are approved by the Japanese Ministry of Health, Labour and Welfare, the cost of treatment was borne partly by the patients and partly by the Japanese national insurance as a rightful medical fee without any external research funding.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted with the approval of the institutional review board of Tokyo Neurological Center and Matsui Hospital, following the STOBE guideline and the Declaration of Helsinki. Written informed consent was obtained from all participants or a parent or guardian for participants under 18 years old. All patients’ rights were protected against any kind of disadvantage and individual matters. Patient names were removed entirely from all text/figures/tables/images and cannot be identified in any way.
Consent for publication
Informed consent from all subjects and their legal guardian(s) can be obtained for publication of identifying information/images.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Takayoshi Matsui, Email: t.matsui@matsui-hp.com.
Kazuhiro Hara, Email: hara@med.kagawa-u.ac.jp.
Makoto Iwata, Email: maciwata@gmail.com.
Shuntaro Hojo, Email: shuntarojiji@gmail.com.
Nobuyuki Shitara, Email: nc-stra@jb3.so-net.ne.jp.
Yuzo Endo, Email: yuzoendo2003@yahoo.co.jp.
Hideoki Fukuoka, Email: hideoki.fukuoka@gmail.com.
Masaki Matsui, Email: makmmkpj@icloud.com.
Hiroshi Kawaguchi, Email: kawaguchi0126@gmail.com.
References
- 1.McKeever A, Agius M, Mohr P. A review of the epidemiology of major depressive disorder and of its consequences for society and the individual. Psychiatr Danub. 2017;29(Suppl 3):222–231. doi: 10.1177/070674370705200108. [DOI] [PubMed] [Google Scholar]
- 2.Martin-Key NA, Mirea DM, Olmert T, Cooper J, Han SYS, Barton-Owen G, Farrag L, Bell E, Eljasz P, Cowell D, Tomasik J, Bahn S. Toward an extended definition of major depressive symptomotology: digital assessment and cross-validation study. JMIR Form Res. 2021;28:2279078. doi: 10.2196/27908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oquendo MA, Malone KM, Ellis SP, Sackeim HA, Mann JJ. Inadequacy of antidepressant treatment for patients with major depression who are at risk for suicidal behavior. Am J Psychiatry. 1999;156:190–194. doi: 10.1176/ajp.156.2.190. [DOI] [PubMed] [Google Scholar]
- 4.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 5.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62:10–17. doi: 10.1177/070674370705200108. [DOI] [PubMed] [Google Scholar]
- 6.Berlim MT, Turecki G. Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatr. 2007;52:46–54. doi: 10.1177/070674370705200108. [DOI] [PubMed] [Google Scholar]
- 7.Gaynes BN, Lux L, Gartlehner G, Asher G, Forman-Hoffman V, Green J, Boland E, Weber RP, Randolph C, Bann C, Coker-Schwimmer E, Viswanathan M, Lohr KN. Defining treatment-resistant depression. Depress Anxiety. 2020;37:134–145. doi: 10.1002/da.22968. [DOI] [PubMed] [Google Scholar]
- 8.Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(Suppl 6):16–22. doi: 10.1177/070674370705200108. [DOI] [PubMed] [Google Scholar]
- 9.Hirschfeld RM, Keller MB, Panico S, Arons BS, Barlow D, Davidoff F, Endicott J, Froom J, Goldstein M, Gorman JM, Marek RG, Maurer TA, Meyer R, Philips K, Ross J, Schwenk TL, Sharfstein SS, Thase ME, Wyatt RJ. The national depressive and manic-depressive association consensus statement on the undertreatment of depression. JAMA. 1997;277:333–340. doi: 10.1001/jama.1997.03540280071036. [DOI] [PubMed] [Google Scholar]
- 10.Klein N, Sacher J, Wallner H, Tauscher J, Kasper S. Therapy of treatment resistant depression: focus on the management of TRD with atypical antipsychotics. CNS Spectr. 2004;9:823–832. doi: 10.1017/s1092852900002248. [DOI] [PubMed] [Google Scholar]
- 11.Lauden A, Geishin A, Merzon E, Korobeinikov A, Green I, Golan-Cohen A, Vinker S, Manor I, Weizman A, Magen E. Higher rates of allergies, autoimmune diseases and low-grade inflammation markers in treatment-resistant major depression. Brain Behav Immun Health. 2021;16:100313. doi: 10.1016/j.bbih.2021.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maes M. "Functional" or "psychosomatic" symptoms, e.g. a flu-like malaise, aches and pain and fatigue, are major features of major and in particular of melancholic depression. Neuro Endocrinol Lett. 2009;30:564–573. doi: 10.1177/070674370705200108. [DOI] [PubMed] [Google Scholar]
- 13.Matsui T, Ii K, Hojo S, Sano K. Cervical neuro-muscular syndrome: discovery of a new disease group caused by abnormalities in the cervical muscles. Neurol Med Chir. 2012;52:75–80. doi: 10.2176/nmc.52.75. [DOI] [PubMed] [Google Scholar]
- 14.Matsui T, Hara K, Kayama T, Iwata M, Shitara N, Hojo S, Endo Y, Fukuoka H, Yoshimura N, Kawaguchi H. Cervical muscle diseases are associated with indefinite and various symptoms in the whole body. Eur Spine J. 2020;29:1013–1021. doi: 10.1007/s00586-019-06233-5. [DOI] [PubMed] [Google Scholar]
- 15.Kang DH, Jeon JK, Lee JH. Effects of low-frequency electrical stimulation on cumulative fatigue and muscle tone of the erector spinae. J Phys Ther Sci. 2015;27:105–108. doi: 10.1589/jpts.27.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maneski LZ, Malešević NM, Savić AM, Keller T, Popović DB. Surface-distributed low-frequency asynchronous stimulation delays fatigue of stimulated muscles. Muscle Nerve. 2013;48:930–937. doi: 10.1002/mus.23840. [DOI] [PubMed] [Google Scholar]
- 17.Lai CH, Leung TK, Peng CW, Chang KH, Lai MJ, Lai WF, Chen SC. Effects of far-infrared irradiation on myofascial neck pain:a randomized, double-blind, placebo-controlled pilot study. J Altern Complement Med. 2014;20:123–129. doi: 10.1089/acm.2013.0122. [DOI] [PubMed] [Google Scholar]
- 18.Matsui T, Iwata M, Endo Y, Shitara N, Hojo S, Fukuoka H, Hara K, Kawaguchi H. Effect of intensive inpatient physical therapy on whole-body indefinite symptoms in patients with whiplash-associated disorders. BMC Musculoskelet Disord. 2019;20:251–258. doi: 10.1186/s12891-019-2621-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsui T, Hara K, Iwata M, Hojo S, Shitara N, Endo Y, Fukuoka H, Matsui M, Kawaguchi H. Possible involvement of the autonomic nervous system in cervical muscles of patients with myalgic encephalomyelitis / chronic fatigue syndrome (ME/CFS) BMC Musculoskelet Disord. 2021;22:419. doi: 10.1186/s12891-021-04293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bremner F. Pupil evaluation as a test for autonomic disorders. Clin Auton Res. 2009;19:88–101. doi: 10.1007/s10286-009-0515-2. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Zekveld AA, Naylor G, Ohlenforst B, Jansma EP, Lorens A, Lunner T, Kramer SE. Parasympathetic nervous system dysfunction, as identified by pupil light reflex, and its possible connection to hearing impairment. PLoS One. 2016;11:e0153566. doi: 10.1371/journal.pone.0153566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giza E, Fotiou D, Bostantjopoulou S, Katsarou Z, Karlovasitou A. Pupil light reflex in Parkinson's disease: evaluation with pupillometry. Int J Neurosci. 2011;121:37–43. doi: 10.3109/00207454.2010.526730. [DOI] [PubMed] [Google Scholar]
- 23.Fotiou DF, Stergiou V, Tsiptsios D, Lithari C, Nakou M, Karlovasitou A. Cholinergic deficiency in Alzheimer's and Parkinson's disease: evaluation with pupillometry. Int J Psychophysiol. 2009;73:143–149. doi: 10.1016/j.ijpsycho.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Lanting P, Heimans JJ, Reulen JP, Nauta J, van der Veen EA. Pupil light reflex and quantitative sensory and motor neural function tests in diabetic patients. J Neurol. 1988;235:245–247. doi: 10.1007/BF00314357. [DOI] [PubMed] [Google Scholar]
- 25.Narita A, Shirai K, Kubota N, Takayama R, Takahashi Y, Onuki T, Numakura C, Kato M, Hamada Y, Sakai N, Ohno A, Asami M, Matsushita S, Hayashi A, Kumada T, Fujii T, Horino A, Inoue T, Kuki I, Asakawa K, Ishikawa H, Ohno K, Nishimura Y, Tamasaki A, Maegaki Y, Ohno K. Abnormal pupil light reflex with chromatic pupillometry in Gaucher disease. Ann Clin Transl Neurol. 2014;1:135–140. doi: 10.1002/acn3.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishikawa H, Onodera A, Asakawa K, Nakadomari S, Shimizu K. Effects of selective-wavelength block filters on pupil light reflex under red and blue light stimuli. Jpn J Ophthalmol. 2012;56:181–186. doi: 10.1007/s10384-011-0116-1. [DOI] [PubMed] [Google Scholar]
- 27.Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab. 2012;10:486–489. doi: 10.5812/ijem.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bianca R, Komisaruk BR. Pupil dilatation in response to vagal afferent electrical stimulation is mediated by inhibition of parasympathetic outflow in the rat. Brain Res. 2007;1177:29–36. doi: 10.1016/j.brainres.2007.06.104. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter LL, Friehs GM, Price LH. Cervical vagus nerve stimulation for treatment-resistant depression. Neurosurg Clin N Am. 2003;14:275–282. doi: 10.1016/s1042-3680(02)00121-3. [DOI] [PubMed] [Google Scholar]
- 30.Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, George MS, Charney DS, Brannan SK. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31:1345–1355. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- 31.Park MC, Goldman MA, Carpenter LL, Price LH, Friehs GM. Vagus nerve stimulation for depression: rationale, anatomical and physiological basis of efficacy and future prospects. Acta Neurochir (Suppl) 2007;97:407–416. doi: 10.1007/978-3-211-33081-4_46. [DOI] [PubMed] [Google Scholar]
- 32.Vlaicu A, Bustuchina VM. Vagus nerve stimulation for treatment-resistant depression: is this therapy distinct from other antidepressant treatments? Int J Psychiatry Clin Pract. 2020;24:349–356. doi: 10.1080/13651501.2020.1779751. [DOI] [PubMed] [Google Scholar]
- 33.Gordon T. Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics. 2016;13:295–310. doi: 10.1007/s13311-015-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang JL, Lin YC, Young TH, Chen MH. Far-infrared ray radiation promotes neurite outgrowth of neuron-like PC12 cells through AKT1 signaling. J Formos Med Assoc. 2019;118:600–610. doi: 10.1016/j.jfma.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Illomei G. Muscle elastography in multiple sclerosis spasticity. Neurodegener Dis Manag. 2016;6:13–16. doi: 10.2217/nmt-2016-0048. [DOI] [PubMed] [Google Scholar]
- 36.Paluch Ł, Noszczyk BH, Walecki J, Osiak K, Kiciński M, Pietruski P. Shear-wave elastography in the diagnosis of ulnar tunnel syndrome. J Plast Reconstr Aesthet Surg. 2018;71:1593–1599. doi: 10.1016/j.bjps.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Antonaci F, Ghirmai S, Bono G, Sandrini G, Nappi G. Cervicogenic headache: evaluation of the original diagnostic criteria. Cephalalgia. 2001;21:573–583. doi: 10.1046/j.0333-1024.2001.00207. [DOI] [PubMed] [Google Scholar]
- 38.Youssef EF, Shanb AS. Mobilization versus massage therapy in the treatment of cervicogenic headache: a clinical study. J Back Musculoskelet Rehabil. 2013;26:17–24. doi: 10.3233/BMR-2012-0344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.