Abstract
In many filamentous cyanobacteria, vegetative cells can differentiate into heterocysts, cells that are specialized for aerobic fixation of N2. Synthesis of the heterocyst envelope polysaccharide is dependent on the gene hepA in Anabaena sp. strain PCC 7120. In search of genes that are involved in the regulation of hepA, we transposon mutagenized strain DR1069, which bears a chromosomal hepA::luxAB fusion. One resulting mutant, designated HNL3, grows normally in medium with nitrate and shows poor induction of hepA in response to nitrogen deprivation. In HNL3, transposon Tn5-1058 is inserted within gene hcwA, a constitutively expressed open reading frame whose predicted product resembles N-acetylmuramoyl-l-alanine amidases. Reconstruction of the mutation confirmed that the mutant phenotype resulted from the insertion of the transposon. The induction of hepA in HNL3 is partially restored upon recombination of HNL3 with plasmid-borne, wild-type hcwA. Moreover, HcwA expressed in Escherichia coli exhibits wall-lytic activity. These results suggest that the degradation, or possibly reconstruction, of the cell peptidoglycan layer is a prerequisite for heterocyst maturation.
Certain filamentous cyanobacteria, such as Anabaena spp., adapt to deprivation of fixed nitrogen by forming heterocysts, differentiated cells in which nitrogenase reduces atmospheric dinitrogen to ammonia. Because nitrogenase is highly sensitive to oxygen, the interior of the heterocyst must be rendered microaerobic. Microaerobic conditions result from (i) inactivation of the oxygen-producing photosystem II in developing heterocysts; (ii) synthesis, outside the cell wall, of a layer of polysaccharide and, within that layer, a layer of glycolipids that is little permeable to O2; and (iii) reduction of residual permeant O2 to H2O by respiration (37).
A number of genes involved in the biosynthesis and organization of the polysaccharide and glycolipid layers of the heterocyst envelope have been identified. Mutation of these genes leaves heterocysts unable to fix nitrogen in the presence of oxygen (referred to as the Fox− phenotype [14]) but able to fix N2 anaerobically. How glycolipids and polysaccharides are transported and deposited through a preexisting wall remains unclear. Diverse Fox− mutants with abnormalities of envelope deposition have been isolated. For example, a mutation in hepA prevents the formation of envelope polysaccharides (34). The predicted HepA is a member of the family of ATP-binding cassette (ABC) inner membrane transport proteins. hepA is first activated 4 to 7 h after nitrogen stepdown, several hours before morphological differentiation of heterocysts first becomes apparent by light microscopy (6, 17, 36). By 10 h after nitrogen stepdown, about when differentiation becomes morphologically evident by transmission light microscopy, hepA is induced transcriptionally over 10-fold (6, 36). The induction depends on activation of hetR several hours earlier (4). Recent studies have shown that two regions upstream from the transcriptional start site of hepA are required for the expression of hepA in nitrogen-free medium; at least the proximal region is a binding site for particular proteins (39). By mutagenesis of hepA::luxAB strain DR1069 with transposon Tn5-1058, additional genes that regulate the expression of hepA in response to nitrogen stepdown were sought. One such mutant, in which a hepC::Tn5-1058 fusion led to constitutive expression of hepA independent of the presence of fixed nitrogen, was earlier described (39). Here we report the characterization of a similarly identified gene that is, however, required for the induction of hepA in response to nitrogen stepdown and whose predicted product shows extensive similarity to N-acetylmuramoyl-l-alanine amidases.
MATERIALS AND METHODS
Culture conditions and transformation of strains of Anabaena sp.
Anabaena sp. strain PCC 7120 and its derivatives (Table 1) were grown at 30°C in the light (ca. 3,500 ergs cm−2 s−1) on a rotary shaker in AA/8 medium (18) supplemented with 5 mM nitrate in 125-ml Erlenmeyer flasks. Cultures of derivatives of PCC 7120 were supplemented with appropriate antibiotics at the concentrations described by Khudyakov and Wolk (19). Plasmids (Table 1) were introduced into Anabaena sp. strain PCC 7120 by conjugation (11), and single or double recombinants were selected as described earlier (4, 5). To induce heterocyst formation, portions of actively growing cultures were washed three times with AA/8, suspended in a volume of AA/8 without antibiotics equal to the original volume, and incubated under growth conditions. Filaments were examined by microscopy 24 to 72 h following nitrogen stepdown. Samples were prepared for electron microscopy (2) and micrographed by S. Burns (MSU Center for Electron Optics).
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Derivation and/or relevant characteristicsa | Source or reference |
|---|---|---|
| Anabaena sp. strains | ||
| PCC 7120 | Wild type | R. Haselkorn |
| CPB7084 | Bmr Nmr Smr Spr; hcwA::Tn5-1058 hepA::luxAB-aadA, formed by double reciprocal recombination of pRL 1992 with DR1069 | This study |
| CPB8089 and CPB8090 | Bmr Emr Nmr Smr Spr; hcwA::Tn5-1058 hepA::luxAB-aadA, with pRL2431 integrated in the chromosome of HNL3 by single-crossover homologous recombination (Fig. 1 and 6) | This study |
| DR1069b | Smr Spr; luxAB-aadA cassette fused at NruI site of hepA | 4 |
| DR1992b | Bmr Nmr Smr; hcwA::Tn5-1058 | This study |
| HNL3 | Bmr Nmr Smr Spr; hcwA::Tn5-1058 hepA::luxAB-aadA | This study |
| Plasmids | ||
| pBS-SK(+) | Apr; cloning vector | Stratagene |
| pIC19H, pIC19R and pIC20H | Apr; cloning vectors | 23 |
| pJRD184 | Apr Tcr; cloning vector | 15 |
| pRL59HE | Apr; cloning vector | 19 |
| pRL148 | Apr Cmr; cloning vector | 10 |
| pRL453 | Apr Smr; cloning vector | 10 |
| pRL660 | Apr; 3′ end of hcwA, as a ClaI-MfeI fragment of pRL2130, inserted between the ClaI and EcoRI sites of pIC20H | This study |
| pRL663 | Apr; SalI fragment of a PCR product that bears the 5′ terminus of hcwA, inserted in the XhoI site of pRL660 | This study |
| pRL669a | Apr Cmr Emr; Cmr Emr cassette C.CE3 (Y. Cai and C. P. Wolk, unpublished data) in the BamHI site of pRL59HE (Ptac→Pcat→cat→erm) | This study |
| pRL677 | Apr Smr Spr Tcr; aadA from the omega interposon (25) excisable with various enzymes, including BsaBI | This study |
| pRL687 | Apr Smr Spr; BsaBI-aadA-BsaBI transferred from pRL677 to the unique unmethylated BsaBI site in Dam-methylated pRL2130 | This study |
| pRL691 | Apr Smr Spr; pIC20H::(hcwA::aadA) | This study |
| pRL838 | Cmr Emr; BAC vector (GenBank AF403425) based on pBAC108L (27) | Tabata et al., unpublished data |
| pRL1058 | Bmr Kmr Smr; bears transposon Tn5-1058 | 35 |
| pRL1977 | Bmr Kmr Smr; ClaI recovery of Tn5-1058 and contiguous DNA from HNL3 | This study |
| pRL1992 | Bmr Cmr Emr Kmr Smr; pRL1977, cut with ClaI and ligated to the sacB-bearing AsuII fragment of pRL1075 (4) | This study |
| pRL2130 | Apr; clone, in the SalI site of pUC19 (29) of the 12-kb insert, bearing orf1 and the 3′ terminal portion of hcwA, from a λEMBL3 phage | This study |
| pRL2138 | Apr; a 6-kb ClaI fragment of pRL2130 in the ClaI site of pIC19H | This study |
| pRL2168 | Apr; PstI-SpeI fragment of pRL2138 in PstI-XbaI hole of pIC19H | This study |
| pRL2174 | Apr; a 5.1-kb, ClaI-SpeI subclone of the insert of pRL2138, blunted with the Klenow fragment, inserted in the SmaI site of pIC19H | This study |
| pRL2332 | Apr; a 790-bp PstI-EcoRI subclone of pRL2174 in the PstI-EcoRI hole of pBS-SK(+) | This study |
| pRL2333 | Apr; a 573-bp PstI-DraI fragment of pRL2168 cloned between the PstI and SmaI sites of pBS-SK(+) | This study |
| pRL2430 | Cmr Emr Smr Spr; BAC vector::hcwA::aadA (see Materials and Methods) | This study |
| pRL2431 | Cmr Emr; BsaBI deletion product of pRL2430; BAC vector bearing a fragment of DNA that contains hcwA and a region 5′ from that gene, including only the 3′ end of orf2 | This study |
| pRL2509a | Apr Cmr Emr Smr Spr; pRL59HE::(hcwA::aadA), Ptac→amidase | This study |
| pRL2509b | Apr Cmr Emr Smr Spr; pRL59HE::(hcwA::aadA), Ptac→←amidase | This study |
| pRL2510 | Cmr Emr; BAC vector (see Materials and Methods) whose sole BsaBI site is Dam-methylatable | This study |
Ap, ampicillin; Bm, bleomycin; Cm, chloramphenicol; Em, erythromycin; Km, kanamycin; Nm, neomycin; superscript r, resistance; Sm, streptomycin; Sp, spectinomycin.
DRx refers to a product of double reciprocal recombination of plasmid pRLx with the genome of PCC 7120, as confirmed by Southern hybridization.
DNA manipulation.
Recombinant DNA procedures were performed according to established standards (26). Enzymes were purchased from New England BioLabs, Beverly, Mass., or occasionally from other suppliers and used as recommended.
Transposon Tn5-1058 and contiguous chromosomal DNA was excised from HNL3 genomic DNA with ClaI, self-ligated, and transferred to Escherichia coli DH10B (Life Technologies, Inc., Gaithersburg, Md.) by electroporation, yielding plasmid pRL1977. A 0.55-kb fragment, derived from pRL1977, extending from the ClaI site in hcwA to the Psp1406I site ca. 90 bp from the end of IS50R in Tn5-1058, was used as a probe to identify λEMBL3 clones bearing corresponding wild-type DNA (3). Automated sequencing (Applied Biosystems Inc., Foster City, Calif.) was performed on both strands of the DNA by primer walking.
Database comparisons and alignments of the translated sequences were performed by using the default settings of the algorithm developed by Altschul et al. (1), using the BLAST network service at the National Center for Biotechnology Information, and the Genetics Computer Group Pileup program (Program manual for the Wisconsin sequence analysis package; Genetics Computer Group, Madison, Wis., 1994).
Transposon mutagenesis and reconstruction of mutant HNL3.
Mutagenesis of strain DR1069 with transposon Tn5-1058 was performed as described by Wolk et al. (35). Plasmid pRL1992, a derivative of pRL1977, was transferred to strain DR1069 by conjugation. Several independently isolated neomycin-resistant, erythromycin-sensitive, sucrose-resistant clones were shown by Southern hybridization to be double recombinants.
Construction of a complementing plasmid.
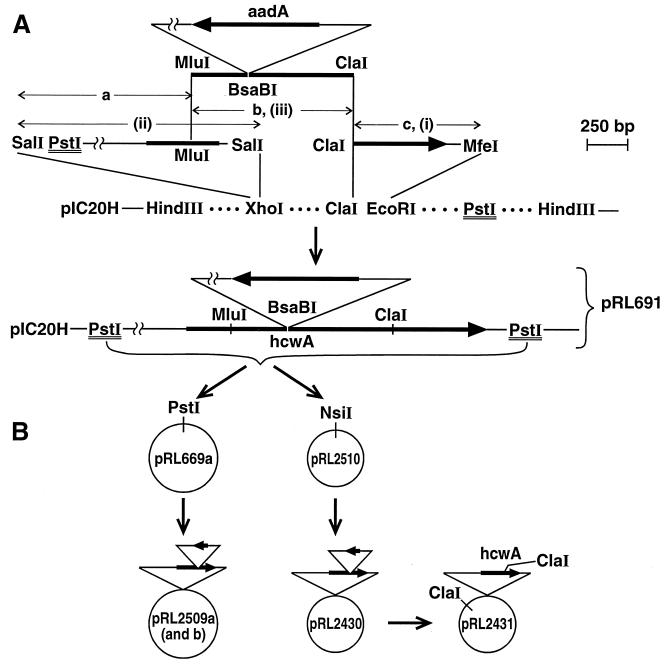
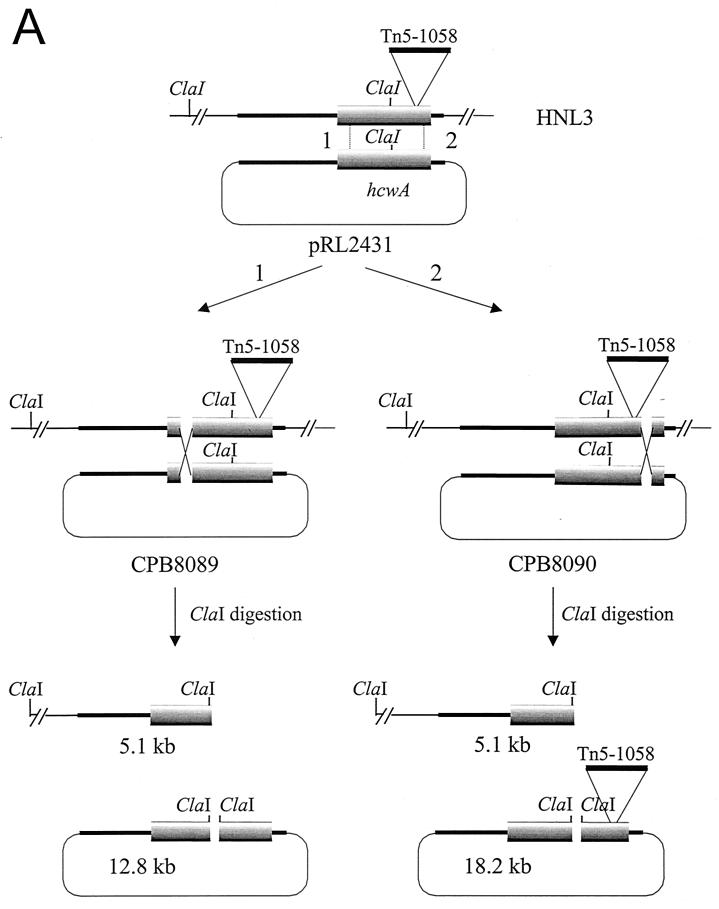
Because the putative protein encoded by the open reading frame (ORF) intercepted in mutant HNL3 may be related to the synthesis or turnover of the heterocyst cell wall (see below), we designated the ORF hcwA. Our initial attempts to complement the mutation in HNL3 having foundered on our inability to subclone the entirety of hcwA in a pUC-based vector, we first synthesized pRL691 (Fig. 1), which bears an interrupted form of hcwA (long heavy arrow). pRL691 is a derivative of pIC20H (23) whose insert consists of fragments a, b, and c. Fragment a, from XhoI (destroyed) to MluI, is most of a 2.6-kb PCR product of a λEMBL3 clone (designated C2) used as a template, with primers CPW117 (5′-CTCGTCCGAGAATAACGAGTGG-3′) from within the right arm of λEMBL3 and CPW118 (5′-GGTACGTCGACAATCCATCGCCTGTAACTCGTA-3′; this is based on a sequence that is 3′ from the MluI site in hcwA). Fragment b, from MluI to ClaI, is derived from pRL2130, a subclone from a different, 12-kb λEMBL3 clone that begins between the initiation codon of hcwA and its MluI site and continues beyond its downstream ORF, which we designated orf1. Within the BsaBI site of fragment b is inserted a BsaBI-aadA-BsaBI cassette, derived from the omega interposon (25) that is bracketed by inverted repeats consisting of portions of the polylinkers from pJRD184 (15), pRL148 (10) and pRL453 (10). The aadA gene (short heavy arrow) confers resistance to streptomycin (Sm) and spectinomycin (Sp). In all of the Smr Spr constructs described, only these two BsaBI sites can be cut by BsaBI when the constructs are isolated from dam+ E. coli. Fragment c, from ClaI of fragment b to the EcoRI site (destroyed) of pIC20H, is the ClaI-MfeI fragment from pRL2130 that contains the 3′ end of hcwA.
FIG. 1.
Synopsis of derivation of plasmids for attempted complementation of the mutation in strain HNL3. (A) Successive steps in the construction of pRL691. The 678-bp ClaI-MfeI fragment from pRL2130, bearing the 3′ end of hcwA, was inserted between the ClaI and EcoRI sites of pIC20H, yielding pRL660 (i). A 2.3-kb PCR product from λEMBL3 clone C2 containing the 5′ end of hcwA was trimmed with SalI and inserted in the XhoI site of pRL660, yielding pRL663 (ii). The 2.95-kb MluI-ClaI fragment of pRL687 bearing, within its BsaBI site, an aadA-containing cassette, was transferred between the MluI and ClaI sites of pRL663, yielding pRL691 (iii). The figure emphasizes the relationship between sites, not distances in base pairs. Series of dots indicate additional sites in the pIC20H polylinker. (B) hcwA::aadA, bracketed between PstI sites that are double underlined in panel A, was transferred to the PstI site of pRL669a and the (compatible) NsiI site of pRL2510, yielding, respectively, pRL2509a (and in opposite orientation, pRL2509b) and pRL2430. Only the latter, cut with BsaBI, religated, and plated at 30°C (not 37°C) yielded pRL2431 transformants bearing uninterrupted hcwA.
The insert in pRL691 can be excised with PstI: the PstI site at one end is derived from the right arm of λEMBL3; the other PstI site is from the pIC20H polylinker. pRL669a consists of RSF1010-based plasmid pRL59HE (19) into the BamHI site of which, downstream from Ptac, was introduced the Cmr Emr cassette C.CE3 (Pcat → cat → erm) (Y. Cai and C. P. Wolk, unpublished data). The PstI-bracketed insert from pRL691 was transferred to the PstI site of pRL669a, generating plasmids pRL2509a, with Ptac 5′ from the 5′ end of hcwA, and pRL2509b, with hcwA inverted. BAC vector pRL838 is a Cmr Emr derivative of pBAC108L (27), with unique NsiI and NruI sites and a single BsaBI site that can be cut coming from a dam+ strain. pRL2510 has the structure of pRL838, but with the two corresponding BsaBI/NruI fragments inverted (and those sites destroyed). Insertion of the 5.9-kb, PstI-bracketed fragment from pRL691 into the NsiI site of pRL2510 generated pRL2430. pRL2431 was generated from pRL2430 by restriction with BsaBI and religation, deleting the aadA-bearing fragment. The ClaI sites in the insert and the vector are separated by 3.6 kb.
Luciferase assays.
Luminescence of colonies on filters was assessed as described previously (35). Luciferase activity of suspensions, measured with an ATP photometer (Turner Designs, Sunnyvale, Calif. [12]), was normalized to the concentration of chlorophyll in the sample, which was measured in methanolic extracts (22).
Northern blot hybridizations.
The hcwA probe consisted of a digoxigenin (DIG)-labeled antisense RNA generated from pRL2333 with a DIG RNA Labeling Kit (Boehringer Mannheim GmbH, Mannheim, Germany) using T3 RNA polymerase according to the instructions of the manufacturer. Similarly, the orf1 probe consisted of a DIG-labeled single-stranded RNA generated from pRL2332 using T7 RNA polymerase.
Total RNA (5 or 10 μg per lane, as indicated) was extracted from 50-ml cultures of Anabaena sp. strain PCC 7120 that were grown and treated as described under Materials and Methods and then harvested and washed with 10 mM Tris-HCl–0.1 mM EDTA (pH 7.5). To approximately 400 μl of resuspended cells was added an equal volume of phenol-chloroform, 0.2% sodium dodecyl sulfate (SDS) (final concentration) and 150 μl of glass beads (diameters, 212 to 300 μm). The cells were broken by vortexing at the maximum speed of a Vortex-Genie 2 mixer (Fisher Scientific, Pittsburgh, Pa.) for four cycles of 1 min on and 1 min off (on ice). The supernatant solution from a 10-min centrifugation at 14,900 × g and 4°C was ethanol precipitated. The resulting pellet was dissolved in 100 μl of RNase-free water and combined with 350 μl of RLT buffer, a guanidinium isothiocyanate-containing lysis buffer (RNeasy Kit; Qiagen GmbH, Hilden, Germany). Purification then proceeded with the RNeasy kit as described by Qiagen.
Total RNA suspended in 20 μl of RNA loading buffer was heated at 65°C for 5 min, chilled on ice for 1 min, and loaded onto gels of 1.2% agarose in 1× MOPS buffer (20 mM 3-N-morpholinepropanesulfonic acid [MOPS], 5 mM sodium acetate, 2 mM EDTA [pH 7.0]) containing 2% formaldehyde. The electrophoresis buffer contained 1× MOPS buffer and 1.33% (final concentration) formaldehyde. After electrophoresis, the gels were washed in 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 10 min twice and RNA transferred downwards (8) to a Nytran membrane (Schleicher & Schuell, Dassel, Germany) with 20× SSC as transfer solution. RNA was cross-linked to the membrane with a UV-Stratalinker (Stratagene, La Jolla, Calif.).
Hybridization, washing, and detection of chemiluminescence were based on the instructions in Boehringer Mannheim's manual (The DIG system user's guide for filter hybridizations), modified according to Engler-Blum et al. (13) to achieve a higher sensitivity with the DIG nonradioactive hybridization system. Briefly, prehybridization and hybridization were performed overnight at 68°C in 250 mM sodium phosphate (pH 7.2)–1 mM EDTA–20% SDS–0.5% blocking reagent without and with 2.5 ng of DIG-labeled probe per ml, respectively.
Reverse transcription (RT)-PCR.
Total RNA was prepared from Anabaena sp. strain PCC 7120 as described in the Northern blot procedure above. Two-microgram aliquots of each RNA preparation were reverse-transcribed by Omniscript Reverse Transcriptase (Qiagen) from random hexamer primers (Promega, Madison, Wis.) in a reaction volume of 20 μl. Two microliters from each cDNA pool was used as a PCR template. Thermal cycling conditions were as follows: 94°C for 2 min, followed by 18 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s. Samples of the same cDNAs were subjected to 18 cycles of PCR with a primer pair specific to rnpB, a constitutively expressed gene from PCC 7120 (30), used as a loading control. The PCR primers used were hepA forward (5′-ACATGATTTTGGCTGCTGATGC-3′), hepA reverse (5′-GCTAAAACAACTTTGATAGCTGC-3′), rnpB forward (5′-GGCGTTGGCGGTTGCAGACC-3′), and rnpB reverse (5′-AGTTGGTGGTAAGCCGGGTTC-3′).
Expression of HcwA in E. coli.
Plasmid pRL2431 or pRL2510 was introduced into E. coli strain BL21 by transformation. Transformants were grown in Luria broth supplemented with 0.2% maltose and 100 μg of ampicillin per ml to an optical density at 600 nm of 0.6 to 1.0. MgSO4 and λCE6 (Novagen, Madison, Wis.) were added to final concentrations of 10 mM and 2 × 109 to 4 × 109 PFU/ml, respectively. The infected cells were grown for 3 h and harvested by centrifugation. Cells were disrupted by cavitation, on ice, with a Vibra cell sonicator (Sonics & Materials, Inc., Danbury, Conn.) equipped with a 2-mm tip, at 40% of maximum power, for 10 s, and extract was collected by centrifugation (13,000 × g, 5 min, 4°C).
Detection of lytic activity in SDS-polyacrylamide gel electrophoresis (PAGE) gels.
For isolation of cell wall material from PCC 7120, cells were harvested from 500 ml of stationary-phase culture and disrupted with a French press. The crude cell wall material was collected by centrifugation (30,000 × g, 60 min, 4°C) and boiled in 4% SDS for 15 min. The suspension was centrifuged (45,000 × g, 30 min, 20°C), and the sedimented material was washed six times with twice-distilled H2O.
Protein-containing extracts of E. coli were heated at 95°C for 2 min in Laemmli sample buffer and loaded onto an SDS-polyacrylamide gel (10% acrylamide) containing 0.2% (wt/vol) SDS-treated cell walls from PCC 7120. SDS-PAGE was carried out at 4°C. After electrophoresis, the gels were incubated for 12 to 16 h at 37°C in 500 ml of 25 mM Tris-HCl, pH 8.0, containing 1% Triton X-100 to permit protein renaturation. The visibility of transparent bands of lysis in the translucent gel was enhanced by staining with 0.1% methylene blue in 0.01% KOH prior to photography.
Nucleotide sequence accession number.
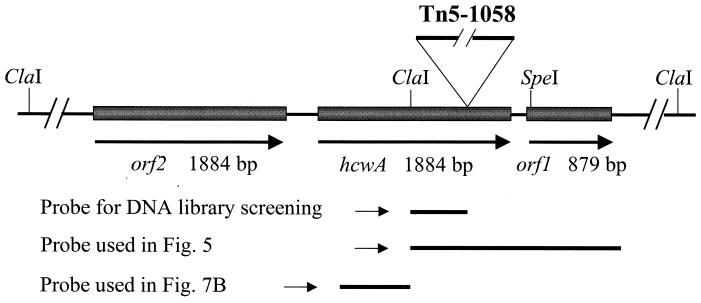
The sequence of hcwA and orf1 reported in this paper has been submitted to GenBank under accession no. AF216288. The presence of orf2, shown in Fig. 2, the sequence of Anorf2, shown in Fig. 3, and predicted lengths of restriction fragments that extend beyond those reported in AF216288 are based in whole or in part on sequence data that subsequently became available at the website http://www.kazusa.or.jp/cyano /anabaena/distribute.html (Kazusa DNA Research Institute, Chiba, Japan).
FIG. 2.
Organization of the orf2-hcwA-orf1 region of the genome of Anabaena sp. strain PCC 7120. The site of insertion of Tn5-1058 in hcwA is shown (IS50R is to the left), as are the chromosomal portion of the 0.55-kb ClaI-Psp1406I (the latter site lies within the transposon) fragment used as a probe for library screening and the fragments used as probes for Fig. 5 and 7B.
FIG. 3.
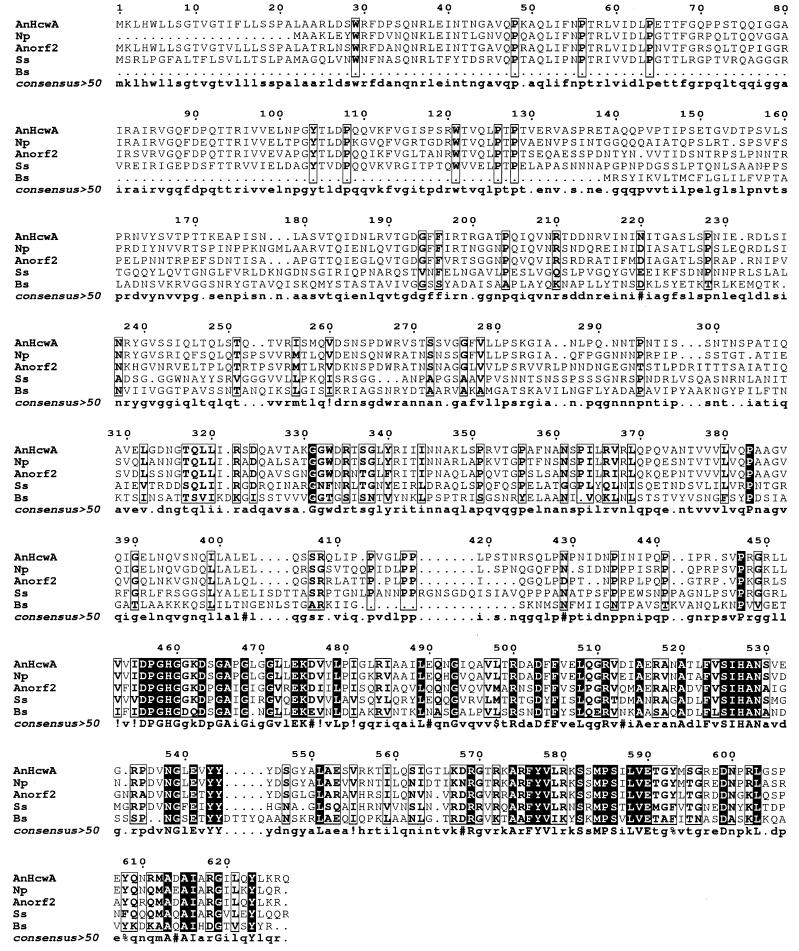
Alignment of the deduced sequences of HcwA (AnHcwA; GenBank accession no. AF216288) and Orf2 (Anorf2; Kazusa contig C365, bp 77324 to 75444) from Anabaena sp. strain PCC 7120 with the deduced sequences of similar proteins from N. punctiforme (Np; JGI contig 566, bp 29874 to 27982) and Synechocystis sp. strain PCC 6803 (Ss; GenBank D90909, bp 21195 to 23144) and of CwlB (Bs; GenBank accession no. M81324 M61747) from B. subtilis. This alignment was generated with the Genetics Computer Group Pileup program. Black, identical amino acids in all five strains; bold, extensive overall similarity, with at least two amino acids identical at any position.
RESULTS
Isolation of mutant HNL3.
Mutant HNL3 was isolated as a transposon Tn5-1058-derivative of hepA::luxAB strain DR1069, which upon nitrogen stepdown showed greatly decreased luminescence (35) compared with strain DR1069 (Table 2). Tn5-1058 mutant HNL3 grew normally in nitrate-containing liquid medium. As shown by electron microscopy, the immature-appearing heterocysts that differentiated upon nitrogen stepdown of HNL3, like the heterocysts of its parental strain DR1069 (32), formed a laminated layer of glycolipids but no envelope polysaccharide layer (data not shown).
TABLE 2.
Effect of the HNL3 mutation on expression of hepA::luxAB
| Strain | Luminescence valuea after nitrogen stepdown at time:
|
|
|---|---|---|
| 0 h | 10 h | |
| DR1069 | 1.6 ± 0.1 | 210.4 ± 1.8 |
| HNL3 | 0.3 ± 0.1 | 6.7 ± 0.3 |
| CPB7084 | 0.4 ± 0.1 | 11.0 ± 0.6 |
Values presented are means ± standard errors of the means of relative units of luminescence from triplicate readings from one of three experiments, all of which produced similar results.
Cloning of hcwA and orf1
A 10.5-kb ClaI fragment containing the transposon and contiguous Anabaena sp. strain DNA was recovered, as pRL1977, from HNL3. The flanking DNA, including a nearby SpeI site, was sequenced using primers extending outward from the transposon. Clones of fragments of wild-type DNA were isolated from a λEMBL3 phage library of Anabaena sp. strain PCC 7120 DNA (3) using the flanking DNA as probe. Sequence analysis of both strands of a region surrounding the SpeI site showed the presence of three parallel, neighboring ORFs (Fig. 2). The central ORF, into which Tn5-1058 had inserted, predicts a 68.0-kDa protein of 627 amino acids that shares 70.2 and 36.8% identity with presumptive N-acetylmuramoyl-l-alanine amidases of Nostoc punctiforme (JGI website http://spider.jgi-psf.org/JGI_microbial/html /nostoc_homepage.html) and Synechocystis sp. strain PCC 6803, respectively, and 16.7% identity with CwlB of Bacillus subtilis, as well as 59% identity and 73% similarity with the 627-amino acid protein predicted by the upstream ORF, which we designated orf2 (Fig. 2 and 3). (Despite this extensive amino acid similarity, there is extensive nucleotide disparity, so that the Northern blot hcwA probe that shares 578 bp of nucleotide identity with hcwA has a longest string of 20 nucleotides identical with orf2.)
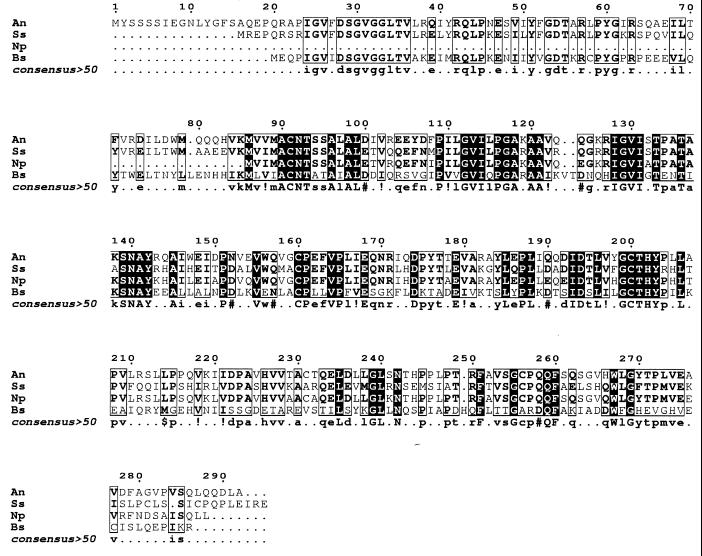
The downstream ORF, orf1, predicts a protein of 292 amino acids that shares 56.2 and 60.3% identity with presumptive glutamate racemases of N. punctiforme and Synechocystis sp. strain PCC 6803, and 35.3% identity with Glr of B. subtilis (Fig. 4). In Synechocystis sp. strain PCC 6803, which does not form heterocysts, the putative amidase and racemase genes are closely linked and oriented as in PCC 7120. Such amidases hydrolyze bacterial peptidoglycan, whereas glutamate racemases catalyze the formation, or initiate the degradation, of d-glutamic acid, one of the essential components of bacterial cell wall peptidoglycan.
FIG. 4.
Alignment of the deduced sequences of Orf1 from Anabaena sp. strain PCC 7120 (An; GenBank accession no. AF216288), similar proteins from N. punctiforme (Np; JGI contig 556, bp 27783 to 26992) and Synechocystis sp. strain PCC 6803 (Ss; SwissProt P73737), and Glr (Bs; SwissProt accession no. 082826) from B. subtilis. This alignment was generated and annotated as for Fig. 3.
Reconstruction of the mutation in mutant HNL3.
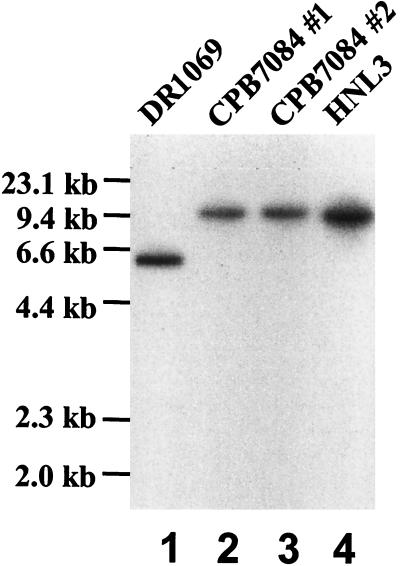
To determine whether the phenotype of HNL3 was the result of insertion of the transposon, pRL1992 was introduced into strain DR1069, resulting in replacement of hcwA with hcwA::Tn5-1058. The structure of the double recombinants was confirmed by Southern hybridization to ClaI-digested DNA from strains DR1069, HNL3, and CPB7084 (Fig. 5). Heterocysts in these strains have the same appearance in the microscope, and the expression of hepA is greatly reduced in CPB7084, as in HNL3 (Table 2). When the DR1992 mutation was constructed in wild-type PCC 7120, the resulting strain grew normally in medium with fixed nitrogen. In response to nitrogen deprivation, it formed what appeared to be normal heterocysts with only slight defects in envelopes sometimes seen by bright-field microscopy, but it was nonetheless Fox− (data not shown).
FIG. 5.
Reconstruction of mutant HNL3. Southern analysis of total genomic DNA of strain DR1069 and of derivatives of it, digested with ClaI. DNA samples were hybridized to a 32P-labeled, PCR-amplified 1.93-kb fragment of DNA that extends from ClaI within hcwA to a position 3′ from orf1. Lanes 2 and 3 are from independently isolated colonies.
Expression of hcwA and orf1
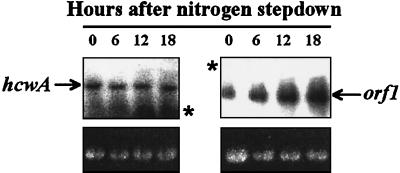
Total RNA was isolated from PCC 7120 cultures grown with fixed nitrogen or deprived of fixed nitrogen for 6, 12, or 18 h. Using DNA fragments specific for hcwA and orf1 as probes, Northern hybridization (Fig. 6) indicated that both genes had basal expression in medium with fixed nitrogen. After nitrogen stepdown, transcription of hcwA was not significantly altered. However, transcription of orf1 increased substantially. The hcwA and orf1 probes hybridized to RNA fragments of ca. 2.3 and 1.0 kb, respectively, lengths which are ca. 15 to 20% greater than those of the coding regions of the corresponding genes.
FIG. 6.
Transcripts of hcwA and orf1 in Anabaena sp. strain PCC 7120. In the upper left panel, Northern hybridization with the hcwA probe (see Materials and Methods) shows a ca. 2.3-kb mRNA transcript in Anabaena sp. PCC 7120 grown in the presence of combined nitrogen (0 h) and then subjected to nitrogen stepdown for 6, 12, and 18 h. All lanes contain 10 μg of total RNA. In the upper right panel, Northern hybridization with the orf1 probe (see Materials and Methods) shows a ca. 1-kb mRNA transcript in Anabaena sp. PCC 7120 that increases in abundance after 6 h of deprivation for combined nitrogen (two right lanes). All lanes contain 5 μg of total RNA. Transcript sizes were determined relative to the sizes of Nicotiana tabacum rRNA (data not shown). The lower panels show 1,489-nucleotide 16S rRNA (21) in the same gels as the upper panels, ethidium bromide stained, as loading standards; asterisks in the upper panels mark the positions of that RNA. Transcripts of larger size are higher in the lanes.
Complementation of the HNL3 mutation.
To determine whether the phenotype of the HNL3 mutation is due to a polar effect on the expression of the orf1 gene, we attempted complementation of the original mutation. Removal of the aadA-bearing BsaBI fragment from within hcwA (Fig. 1) by restriction (which appeared complete) and religation of high-copy-number plasmid pRL691 or lower-copy-number, RSF1010-based plasmids pRL2509a and pRL2509b, produced no spectinomycin-sensitive transformants. When BAC-based plasmid pRL2430 was digested with BsaBI and religated, Cmr Sms Sps transformants (bearing pRL2431) of E. coli were obtained only at 30°C, not at 37°C, whereas E. coli bearing pRL2430 grows well at 37°C. Thus, cloning of intact hcwA proved possible only in a very-low-copy-number, F-plasmid-based vector, and then only at 30°C, not 37°C, implying that hcwA is expressed in E. coli and that its product is toxic to the bacterial host.
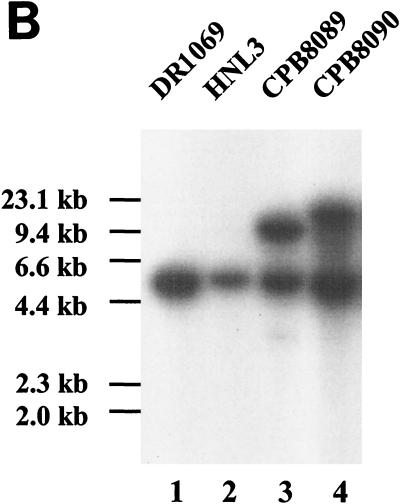
Rather than try to complement the mutation in trans by adding a replicon functional in Anabaena sp. to plasmid pRL2431, pRL2431 was introduced into strain HNL3 through single recombination. As confirmed by Southern hybridization (Fig. 7B), recombination took place either 5′ (strain CPB8089) or 3′ (strain CPB8090) from the site of insertion of the transposon within strain HNL3, producing one intact copy and one interrupted copy of hcwA. Only in CPB8090 was orf1 downstream from an intact copy of hcwA. However, both tested recombinants partially restored the induction of hepA::luxAB (Table 3). Because, in addition, the transcripts appeared independent (Fig. 6), it is unlikely that the blocking of hepA expression is due to a polar effect, on orf1, of the insertion of the transposon.
FIG. 7.
Recombination of pRL2431 with the genome of mutant HNL3. (A) Single recombinants, CPB8089 and CPB8090, that would result from the recombination of pRL2431 with HNL3 to the left (1) or to the right (2) of Tn5-1058, as illustrated. Only those ClaI sites that can be digested by ClaI in DNA isolated from a dam+ host (24) are shown. Linearized ClaI fragments that would hybridize with the probe labeled in panel B are shown. (B) Southern hybridization of recombinants. Total genomic DNA was isolated and digested with ClaI. DNA samples were hybridized to a PCR-amplified, 32P-labeled, 0.81-kb DNA fragment (template, pRL2130; primers, 5′-TCAGAAACCGGAGTGGACAC-3′ and 5′-GGGTGGTAAACCTACTGGTG-3′) that lies between the 5′ end of hcwA and its ClaI site. The bands observed are consistent (see bottom of panel A) with the expected sizes of 5.1 kb (all lanes), plus 12.8 kb (lane 3) or 18.2 kb (lane 4).
TABLE 3.
Complementation of HNL3 by pRL2431
| Strain | Luminescence valuea after nitrogen stepdown at time:
|
|
|---|---|---|
| 0 h | 10 h | |
| DR1069 | 1.9 ± 0.1 | 430.0 ± 21.8 |
| HNL3 | 0.6 ± 0.2 | 11.6 ± 3.3 |
| CPB8089 | 1.1 ± 0.3 | 83.3 ± 9.5 |
| CPB8090 | 0.7 ± 0.4 | 83.0 ± 16.3 |
Values presented are means ± standard errors of the mean of relative units of luminescence from triplicate readings from one of three experiments, all of which produced similar results.
Expression of hepA in hcwA mutant.
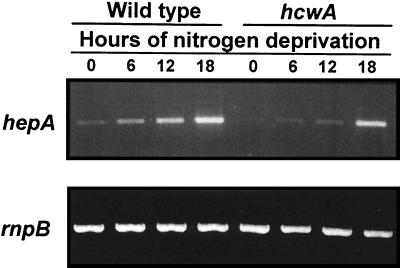
To test the possibility that the blocked induction of hepA::luxAB in strain HNL3 was influenced by the loss of hepA itself, the effect of hcwA on hepA was explored in a genetic background with an intact hepA gene. Plasmid pRL1992 was introduced into PCC 7120, generating hcwA-knockout mutant DR1992. The expression of hepA in response to nitrogen stepdown was detected by semiquantitative RT-PCR. In wild-type PCC 7120, induction of hepA was detected as early as 6 h after nitrogen deprivation, and the hepA transcript continued to accumulate. In the hcwA mutant, in contrast, expression of hepA did not exceed wild-type background levels at 6 and 12 h after stepdown but became comparable with that in the wild type at 18 h (Fig. 8). This experiment indicates that the loss of hcwA decreased activation of hepA early in heterocyst development, corroborating the result of Table 2 without reliance on a lux reporter.
FIG. 8.
hepA transcripts of PCC 7120 and hcwA mutant DR1992 in response to nitrogen deprivation. hepA mRNA levels were detected by semiquantitative RT-PCR. rnpB RNA levels were examined as a control for equal loading of the lanes.
Expression of HcwA in E. coli and characterization of its lytic activity.
In pRL2431, gene hcwA is preceded by a T7 promoter. Expression of HcwA was induced in BL21 by infecting the cells with λCE6, a source of T7 RNA polymerase. Plasmid pRL2510, the parental BAC vector without an hcwA insert, was used as a negative control. The lytic activity of HcwA was visualized in a zymogram. Protein extracts of BL21 (pRL2431) were separated in an SDS-PAGE gel containing cell walls from PCC 7120. The zymogram showed that the recombinant E. coli produces a lytic enzyme with an apparent molecular mass of ca. 74 kDa (Fig. 9). E. coli harboring pRL2510 expressed no detectable lytic activity.
FIG. 9.
Determination of the cell wall lytic activity of HcwA in a zymogram assay. A renaturation gel assay was performed with HcwA extracted from BL21(pRL2431) (lane 3). Protein extract from BL21(pRL2510) was used as a control (lane 2). The sizes of molecular mass standards (lane 1) are shown in kilodaltons.
DISCUSSION
The cell walls of cyanobacteria, outside of the cytoplasmic membrane, consist of a peptidoglycan layer, an outer membrane, and often a sheath (16, 33). These cell walls are qualitatively similar to those of gram-negative bacteria. However, the thickness of the peptidoglycan layer, together with its degree of cross-linking and the presence of covalently linked polysaccharide, is more characteristic of the walls of gram-positive cells (31).
Heterocysts exhibit structural differences from vegetative cells. Outside a wall that appears similar to that of vegetative cells, heterocysts are enveloped, except where they are attached to vegetative cells, by a layer of glycolipids (32) and a layer of polysaccharide (7). The interpretation that the underlying cell wall of heterocysts is similar to that of vegetative cells is based in part on low-resolution electron micrographs and in part on evidence that suggests that hexosamine is incorporated into the wall early during the differentiation process and that slow synthesis or turnover of N-acetylglucosamine, a constituent of peptidoglycan, occurs throughout the process (9). Defective synthesis of lipopolysaccharide, a constituent of the wall, leads to apparently defective deposition of the heterocyst envelope and to a Fox− phenotype (38). Heterocyst envelope glycolipids and polysaccharides synthesized within and at the plasmalemma must traverse the cell wall before they are deposited at the outside surface of differentiating heterocysts. Therefore, rearrangement, perforation, or partial degradation of the peptidoglycan layer may be required for assembly of the glycolipid or polysaccharide layer of the heterocyst envelope. It is germane that 1 of at least 12 putative penicillin-binding proteins encoded by Anabaena sp. strain PCC 7120 is required for aerobic nitrogen fixation, although its encoding gene appears to be expressed equally strongly in the presence of nitrate (20).
While searching for genes whose products control the expression of hepA, we found that a mutation in hcwA, which putatively encodes an N-acetylmuramoyl-l-alanine amidase, extensively reduces the induction of hepA upon nitrogen deprivation. The expression of hepA is restored, albeit incompletely, by recombination with an intact copy of hcwA. Incompleteness of restoration may be due to competition between intact and truncated copies of HcwA for binding to substrate or to other proteins. Alternatively, the increased copy number of sequences 5′ from hcwA might indirectly affect the expression of hepA, perhaps as a result of titration of effectors by increased binding to upstream regulatory sequences. The transcript of a neighboring gene, orf1, differs in size from that of hcwA and appears to be an independent transcript, suggesting that the effect of the mutation in hcwA is not a polar effect on the transcription of orf1. hcwA appears, both by similarity (Fig. 3) and by its lytic activity on walls of Anabaena sp. strain PCC 7120 (Fig. 9), to encode a metabolic enzyme similar to an autolysin. It therefore seems unlikely that HcwA directly regulates the expression of hepA.
In wild-type PCC 7120, the expression of hcwA remains relatively constant upon nitrogen deprivation, and hcwA mutants grow well in medium with fixed nitrogen. Similarly, B. subtilis genes that encode autolysins that function during spore germination are transcribed at an earlier stage of development (28).
We conjecture that HcwA increases the permeability of the peptidoglycan layer of the cell wall of the developing heterocyst, thereby facilitating the penetration of glycolipids and polysaccharides. We suggest that in mutant HNL3, an external signal that regulates hepA may fail to reach the inside of developing heterocysts. Alternatively, components of the heterocyst envelope may accumulate inside those cells and negatively regulate the expression of hepA.
ACKNOWLEDGMENTS
We thank Xiaoqiong Qin for a sample of tobacco rRNA.
This work was supported by the U.S. Department of Energy under grant DOE-FG02-91ER20021 and by NSF grant MCB 9723193.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black K, Buikema W J, Haselkorn R. The hglK gene is required for the localization of heterocyst-specific glycolipids in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1995;177:6440–6448. doi: 10.1128/jb.177.22.6440-6448.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black T A, Wolk C P. Analysis of a Het− mutation in Anabaena sp. strain PCC 7120 implicates a secondary metabolite in the regulation of heterocyst spacing. J Bacteriol. 1994;176:2282–2292. doi: 10.1128/jb.176.8.2282-2292.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black T A, Cai Y, Wolk C P. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol Microbiol. 1993;9:77–84. doi: 10.1111/j.1365-2958.1993.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 5.Cai Y, Wolk C P. Use of a conditionally lethal gene in Anabaena sp. strain PCC 7120 to select for double recombinants and to entrap insertion sequences. J Bacteriol. 1990;172:3138–3145. doi: 10.1128/jb.172.6.3138-3145.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Y, Wolk C P. Anabaena sp. strain PCC 7120 responds to nitrogen deprivation with a cascade-like sequence of transcriptional activations. J Bacteriol. 1997;179:267–271. doi: 10.1128/jb.179.1.267-271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardemil L, Wolk C P. The polysaccharides from heterocyst and spore envelopes of a blue-green alga. Structure of the basic repeating unit. J Biol Chem. 1979;254:736–741. [PubMed] [Google Scholar]
- 8.Chomczynski P. One-hour downward alkaline capillary transfer for blotting of DNA and RNA. Anal Biochem. 1992;201:134–139. doi: 10.1016/0003-2697(92)90185-a. [DOI] [PubMed] [Google Scholar]
- 9.Dunn J H, Simon R D, Wolk C P. Incorporation of amino sugars into walls during heterocyst differentiation. Dev Biol. 1971;26:159–164. doi: 10.1016/0012-1606(71)90115-1. [DOI] [PubMed] [Google Scholar]
- 10.Elhai J, Wolk C P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988;68:119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- 11.Elhai J, Wolk C P. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 1988;167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- 12.Elhai J, Wolk C P. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 1990;9:3379–3388. doi: 10.1002/j.1460-2075.1990.tb07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engler-Blum G, Meier M, Frank J, Müller G A. Reduction of background problems in nonradioactive Northern and Southern blot analyses enables higher sensitivity than 32P-based hybridizations. Anal Biochem. 1993;210:235–244. doi: 10.1006/abio.1993.1189. [DOI] [PubMed] [Google Scholar]
- 14.Ernst A, Black T, Cai Y, Panoff J-M, Tiwari D N, Wolk C P. Synthesis of nitrogenase in mutants of the cyanobacterium Anabaena sp. strain PCC 7120 affected in heterocyst development or metabolism. J Bacteriol. 1992;174:6025–6032. doi: 10.1128/jb.174.19.6025-6032.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heusterspreute M, Thi V H, Emery S, Tournis-Gamble S, Kennedy N, Davison J. Vectors with restriction site banks. IV. pJRD184, a 3793-bp plasmid vector having 43 unique cloning sites. Gene. 1985;39:299–304. doi: 10.1016/0378-1119(85)90327-0. [DOI] [PubMed] [Google Scholar]
- 16.Hoiczyk E, Hansel A. Cyanobacterial cell walls: news from an unusual prokaryotic envelope. J Bacteriol. 2000;182:1191–1199. doi: 10.1128/jb.182.5.1191-1199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland D, Wolk C P. Identification and characterization of hepA, a gene that acts early in the process of morphological differentiation of heterocysts. J Bacteriol. 1990;172:3131–3137. doi: 10.1128/jb.172.6.3131-3137.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu N-T, Thiel T, Giddings T H, Wolk C P. New Anabaena and Nostoc cyanophages from sewage settling ponds. Virology. 1981;114:236–246. doi: 10.1016/0042-6822(81)90269-5. [DOI] [PubMed] [Google Scholar]
- 19.Khudyakov I, Wolk C P. Evidence that the hanA gene coding for HU protein is essential for heterocyst differentiation in, and cyanophage A-4(L) sensitivity of, Anabaena sp. strain PCC 7120. J Bacteriol. 1996;178:3572–3577. doi: 10.1128/jb.178.12.3572-3577.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lázaro S, Fernández-Piñas F, Fernández-Valiente E, Blanco-Rivero A, Leganés F. pbpB, a gene coding for a putative penicillin-binding protein, is required for aerobic nitrogen fixation in the cyanobacterium Anabaena sp. strain PCC7120. J Bacteriol. 2001;183:628–636. doi: 10.1128/JB.183.2.628-636.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ligon P J B, Meyer K G, Martin J A, Curtis S E. Nucleotide sequence of a 16S rRNA gene from Anabaena sp. strain PCC 7120. Nucleic Acids Res. 1991;19:4553. doi: 10.1093/nar/19.16.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackinney G. Absorption of light by chlorophyll solutions. J Biol Chem. 1941;140:315–322. [Google Scholar]
- 23.Marsh J L, Erfle M, Wykes E J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984;32:481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- 24.Padhy R N, Hottat F G, Coene M M, Hoet P P. Restriction analysis and quantitative estimation of methylated bases of filamentous and unicellular cyanobacterial DNAs. J Bacteriol. 1988;170:1934–1939. doi: 10.1128/jb.170.4.1934-1939.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prentki P, Binda A, Epstein A. Plasmid vectors for selecting IS1-promoted deletions in cloned DNA: sequence analysis of the omega interposon. Gene. 1991;103:17–23. doi: 10.1016/0378-1119(91)90385-o. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Shizuya H, Birren B, Kim U J, Mancino V, Slepak T, Tachiiri Y, Simon M. Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc Natl Acad Sci USA. 1992;89:8794–8797. doi: 10.1073/pnas.89.18.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith T J, Blackman S A, Foster S J. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology. 2000;146:249–262. doi: 10.1099/00221287-146-2-249. [DOI] [PubMed] [Google Scholar]
- 29.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 30.Vioque A. Analysis of the gene encoding the RNA subunit of ribonuclease P from cyanobacteria. Nucleic Acids Res. 1992;20:6331–6337. doi: 10.1093/nar/20.23.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weckesser J, Jürgens U J. Cell walls and external layers. Methods Enzymol. 1988;167:173–188. [Google Scholar]
- 32.Winkenbach F, Wolk C P, Jost M. Lipids of membranes and of the cell envelope in heterocysts of a blue-green alga. Planta. 1972;107:69–80. doi: 10.1007/BF00398015. [DOI] [PubMed] [Google Scholar]
- 33.Wolk C P. Physiology and cytological chemistry of blue-green algae. Bacteriol Rev. 1973;37:32–101. doi: 10.1128/br.37.1.32-101.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolk C P. Heterocyst formation in Anabaena. In: Brun Y V, Shimkets L, editors. Prokaryotic development. Washington, D.C.: ASM Press; 1999. pp. 83–104. [Google Scholar]
- 35.Wolk C P, Cai Y, Panoff J-M. Use of a transposon with luciferase as a reporter to identify environmentally responsive genes in a cyanobacterium. Proc Natl Acad Sci USA. 1991;88:5355–5359. doi: 10.1073/pnas.88.12.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolk C P, Elhai J, Kuritz T, Holland D. Amplified expression of a transcriptional pattern formed during development of Anabaena. Mol Microbiol. 1993;7:441–445. doi: 10.1111/j.1365-2958.1993.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 37.Wolk C P, Ernst A, Elhai J. Heterocyst metabolism and development. In: Bryant D, editor. Molecular genetics of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 769–823. [Google Scholar]
- 38.Xu X, Khudyakov I, Wolk C P. Lipopolysaccharide dependence of cyanophage sensitivity and aerobic nitrogen fixation in Anabaena sp. strain PCC 7120. J Bacteriol. 1997;179:2884–2891. doi: 10.1128/jb.179.9.2884-2891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J, Kong R, Wolk C P. Regulation of hepA of Anabaena sp. strain PCC 7120 by elements 5′ from the gene and by hepK. J Bacteriol. 1998;180:4233–4242. doi: 10.1128/jb.180.16.4233-4242.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]