Abstract
The regulation of vascular smooth muscle cell (VSMC) proliferation is an important issue because it has major implications for the prevention of pathological vascular conditions. Using microRNA array screen, we found the expression levels of 200 unique miRNAs in hyperplasic tissues. Among them, miR-200c expression substantially was down-regulated. The objective of this work was to assess the function of miR-200c and SUMOylated Krϋppel-like transcription factor 4 (KLF4) in the regulation of VSMC proliferation in both cultured cells and animal models of balloon injury. Under basal conditions, we found that miR-200c inhibited the expression of KLF4 and the SUMO-conjugating enzyme Ubc9. Upon PDGF-BB treatment, Ubc9 interacted with and promoted the SUMOylation of KLF4, which allowed the recruitment of transcriptional corepressors (e.g., nuclear receptor corepressor (NCoR) and HDAC2) to the miR-200c promoter. The reduction in miR-200c levels led to increased target gene expression (e.g., Ubc9 and KLF4), which further repressed miR-200c levels and accelerated VSMC proliferation. These results demonstrate that induction of a miR-200c-SUMOylated KLF4 feedback loop is a significant aspect of the PDGF-BB proliferative response in VSMCs and that targeting Ubc9 represents a novel approach for the prevention of restenosis.
Keywords: miR-200c, Ubc9, SUMOylation, KLF4, VSMC, Proliferation
1. Introduction
Vascular smooth muscle cells (VSMCs) play pivotal roles in a variety of diseases, including atherosclerosis [1], hypertension [2], cancer [3], asthma, and vascular aneurysms [4]. Major challenges for the field of vascular medicine have been identifying environmental cues, signaling pathways, and molecular mechanisms that normally control VSMC proliferation and determining how these are disrupted in disease states. A key to understanding the basis of VSMC proliferation and differentiation switching is identification of the mechanisms that regulate the transcription of proliferation-related genes. Among the known proliferation-related genes, p21WAF1/Cip1(p21) is known to play a particularly critical role. [5].
MicroRNAs (miRs) are processed from pre-miR hairpin structures by DICER1 and associate with Argonaute family members and other components to generate micro-ribonucleoproteins that can suppress or promote protein translation through regulatory elements within mRNAs [6]. It is well known that miR-145 [7,8], miR-221 [9], miR-146a [10], miR-31 [11], and miR-208 [12] are post-transcriptional regulators of genes that participate in VSMC proliferation and differentiation. We recently reported that miR-146a and KLF4 form a feedback loop to regulate each other’s expression, which is consistent with the notion that KLF4 can switch from a positive to a negative regulator of VSMC proliferation [10]. miR-200c is an important miRNA that participates in epithelial proliferation, esophageal cancer chemoresistance, and migration and invasion by breast cancer cells [13,14]. Although many studies on miR-200c have focused on its function in cancer, few have paid attention to its role in VSMC proliferation and differentiation.
Protein modification involving small ubiquitin-like modifier (SUMO) has been shown to have an important function in regulating the assembly and disassembly of protein complexes, as well as protein localization, stability, and function [1,15]. SUMO proteins are moieties that are enzymatically conjugated to lysine residues in a variety of target proteins. One of these proteins, the E2-conjugating enzyme Ubc9, is encoded by a single-copy gene and is ubiquitously expressed in all human organs and tissues [4]. Ubc9 affects a variety of cellular pathways, such as cell growth, proliferation, apoptosis, and chromatin remodeling pathways [9,16]. SUMO-specific SENP family proteases ensure that the SUMOylation reaction is reversible and highly dynamic [17]. Several cell-proliferation transcription factors are modified by SUMOylation, including PPAR-σ [18], KLF5 [18,19], C/EBPs, and SREBPs [20].
Krüppel-like factor 4 (KLF4), a zinc finger-containing transcription factor, is one of the four original factors known to induce formation of pluripotent stem cells via the reprogramming of somatic cells [21]. In pathological states, KLF4 plays a role in tumorigenesis [22] as well as in cardiovascular [23] and inflammatory disorders [24]. KLF4 has previously been identified as a proliferation–differentiation switch in VSMCs [25]. The overexpression of KLF4 inhibits neointimal hyperplasia induced by balloon injury, blocks platelet-derived growth factor (PDGF)-BB-PDGFR-MEK-ERK signaling, and reduces VSMC proliferation induced by the mitogen PDGF-BB [26]. Pidkovka et al. [27] reported that KLF4 inhibits proliferation induced by redox-sensitive signaling through the inducible expression of several negative cell-cycle regulatory genes, including p27, Mdm2, and retinoblastoma. Vascular injury promotes KLF4-dependent induction of p21 in concert with p53, thus counteracting pro-mitogenic stimuli in VSMCs [5]. Although KLF4 can induce p53 expression [5], it remains unclear how KLF4 functions in the transcriptional regulatory programs that govern cell proliferation.
Here, we determined that miR-200c was among the miRNAs whose expression was substantially changed both in hyperplasic tissues from a rat model of balloon injury and in VSMCs after stimulation with PDGF-BB. Extensive in silico analysis uncovered KLF4 and Ubc9 as putative miRNA-200c target genes. Using several molecular approaches and an in vivo model of balloon injury and carotid ligation-induced vascular inflammation, we identified some of the mechanisms of transcriptional control by miRNA-200c and the role that these molecular components have in the control of VSMC proliferation.
2. Methods
Details regarding the reagents, antibodies, construction of plasmid and adenovirus vectors, cell culture, oligonucleotide pull-down assay, ChIP assay, immunoprecipitation and Western blot analyses, animal models of balloon injury, and statistical analysis are provided in the Supplementary Online Data.
3. Results
3.1. miR-200c is downregulated in vascular tissue hyperplasia
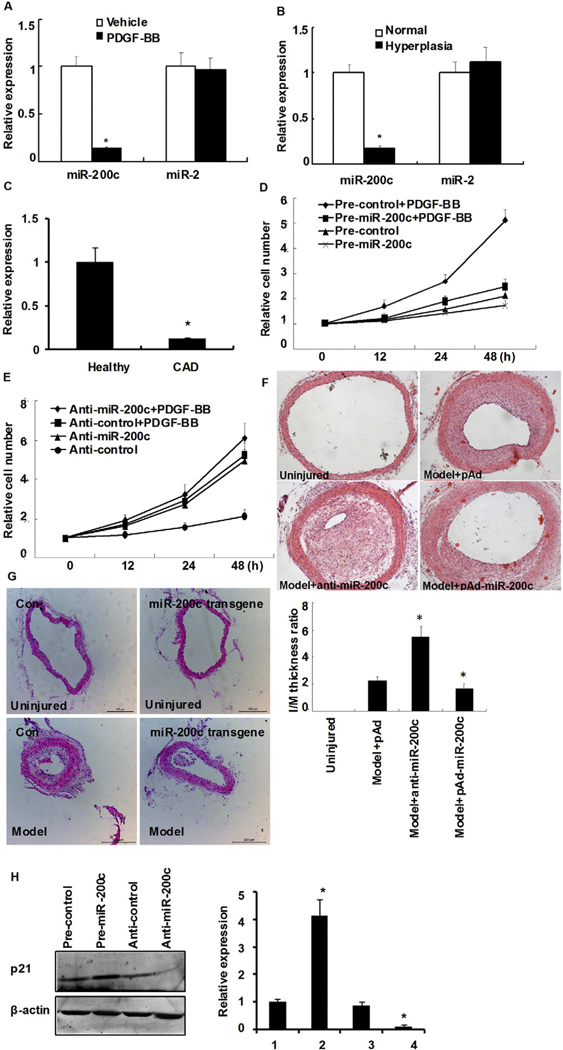
VSMC proliferation is a major adaptive mechanism of cardiovascular diseases, including hypertension and restenosis. To determine whether miRNA changes occur in human vascular tissue hyperplasia, a microRNA array screen was performed, whereby total RNA was extracted and used to determine the expression levels of 200 unique human miRNAs in their mature forms. The results showed that miR-200c was substantially down-regulated in hyperplasic tissues (Table 1). A portion of the RNA used for the microarray was then converted to cDNA and subjected to quantitative PCR (qPCR). Consistent with the microarray data, miR-200c levels in hyperplasic tissues were reduced to 14.9% of the control tissues. By contrast, the expression levels of miR-2 remained unchanged between both tissues (Fig. 1A). miR-200c levels decreased significantly in serum-starved human aortic smooth muscle cells (HASMCs)following cell stimulation with PDGF-BB (Fig. 1B). To investigate whether circulating miR-200c also decreased, twenty-four patients with angiographically documented coronary artery disease (CAD) and twenty-four individuals without evidence for CAD were selected. The clinical characteristics of the 2 study populations are summarized in Online Table I. As shown in Fig. 1C, circulating miR-200c decreased 1.74-fold in patients with CAD. These data demonstrate that miR-200c is probably a novel biomarker for CAD.
Table 1.
Comparison of array analyses of kidney vascular tissues.
| Name | Fold | Expression (hyperplasia vs control) |
|---|---|---|
| hsa-miR-548a-5p | 10.75 | Upregulated |
| hsa-miRPlus-F1050 | 54.13 | Upregulated |
| hsa-miR-10a* | 10.38 | Upregulated |
| hsa-miR-744 | 9.08 | Upregulated |
| hsa-miR-302c* | 15.25 | Upregulated |
| hsa-miR-30c-1* | 13.70 | Upregulated |
| hsa-miR-27a | 2.40 | Upregulated |
| hsa-miR-342-5p | 0.07 | Downregulated |
| hsa-miR-221 | 0.28 | Downregulated |
| hsa-miR-212 | 0.09 | Downregulated |
| hsa-miR-200c | 0.09 | Downregulated |
| hsa-miR-1915* | 0.10 | Downregulated |
| hsa-miR-222 | 0.26 | Downregulated |
| hsa-miR-564 | 0.02 | Downregulated |
| hsa-miR-143 | 0.44 | Downregulated |
| hsa-miR-130b* | 0.04 | Downregulated |
| hsa-miR-363* | 0.09 | Downregulated |
| hsa-miR-340* | 0.14 | Downregulated |
| hsa-miR-668 | 0.09 | Downregulated |
| hsa-miR-215 | 0.14 | Downregulated |
| hsa-miR-589 | 0.13 | Downregulated |
| hsa-miR-122* | 0.11 | Downregulated |
| hsa-miR-2113 | 0.10 | Downregulated |
Fig. 1.
miR-200c is down-regulated in vascular hyperplasia tissues and proliferating HASMCs. (A) miR-200c and miR-2 expression from normal and matched renal vascular hyperplasia tissues were detected by quantitative real-time PCR (qRT-PCR). Shown are representative of three specimen pairs. *P < 0.05 vs. normal tissue using Student’s t test. (B) HASMCs were treated with PDGF-BB (10 ng/mL) for 2 h followed by qRT-PCR analysis to detect miR-200c and miR-2 expression. Bars represent the means ± SEM from three independent experiments. *P < 0.05 vs. vehicle using Student’s t test. (C) A miR-29a expression in EDTA-plasma obtained from patients with CAD (n = 24) and healthy volunteers (HC; n = 24), as determined by TaqMan PCR. Bars represent the means ± SEM from three independent experiments. *P < 0.05 vs. healthy volunteers using Student’s t test. (D, E) VSMCs were transfected either with miR-200c (D) or anti-miR-200c (miR-200c inhibitor) (E) followed by incubation with or without PDGF-BB for 24 h. Cell proliferation was determined by MTT assay. Values represent fold increase relative to cells transfected with control oligonucleotides. Experiments were performed in triplicate and each value is the mean ± SEM of three independent experiments. *P < 0.05 vs. control using Student’s t test. (F) Neointima hyperplasia 14 days after balloon injury. Representative sections of hemotoxylin and eosin-stained arterial section from uninjured, model (control virus, pAd), anti-miR-200c (miR-200c inhibitor), and pAd-miR-200c-infected rats. Magnification, × 100. Neointimal formation was evaluated by calculating the I/M ratio *P < 0.05 vs. model group (n = 6 in each group). (G) Neointima hyperplasia 14 days after carotid ligation. Representative sections of hemotoxylin and eosin-stained arterial section from different vessels including unligated vessels, ligated vessels of miR-200c transgenic or control mice three time points (1.5 mm from ligation site). Magnification, × 200. Neointimal formation was evaluated by calculating the I/M ratio *P < 0.05 vs. control group (n = 3 in each group). (H) HASMCs were transfected either with miR-200c or anti-miR-200c (miR-200c inhibitor) and treated with PDGF-BB for 24 h. The expression of p21 protein was detected by Western blotting. Experiments were performed in triplicate and each value is the mean ± SEM of three independent experiments. *P < 0.05 vs. pre-control transfected cells.
3.2. miR-200c modulates HASMC proliferation in vitro
To investigate the effect of miR-200c on cell proliferation, gain-of-function and loss-of-function experiments were performed in HASMCs that were transfected with either miR-200c mimic or anti-miR-200c for 48 h and then stimulated with PDGF-BB. A random-sequence anti-miR molecule served as a negative control (pre-control). The overexpression of miR-200c markedly reduced PDGF-BB-mediated HASMC proliferation when compared with pre-control-transfected cells. In the absence of PDGF-BB, proliferation was only marginally affected by miR-200c overexpression (Fig. 1D). By contrast, under basal conditions, ectopic expression of anti-miR-200c led to greater HASMC proliferation, reaching the levels observed after PDGF-BB treatment of pre-control-transfected cells (Fig. 1E). To assess the effect of miR-200c in vivo, neointimal formation was made by balloon injury, and 14 days later the I/M thickness ratio was determined as a measure of VSMC proliferation. As shown in Fig. 1F, neointimal hyperplasia was abundant after balloon injury, with a significantly higher I/M ratio in anti-miR-200c animals vs. pAd controls (5.67 ± 0.45 vs. 3.72 ± 0.51; P < 0.01). Accordingly, a trend toward lower neointimal formation was found in animals overexpressing miR-200c vs. the pAd control group (2.84 ± 0.47 vs. 3.72 ± 0.51; P < 0.05). miR-200c expression was determined by real-time PCR (Online Fig. IA). To further corroborate miR-200c’s effects in vivo, we constructed miR-200c transgenic mice. Morphometric analysis showed that neointima accounted for more than 50% of the carotid arterial wall thickness (Fig. 1G, left panel). When compared with the ligated group, the miR-200c transgenic mice exhibited significantly thinner carotid arterial walls, lower I/M ratios and smaller intima areas(Fig. 1G, right panel). Western blot showed that overexpression of miR-200c markedly increased p21 expression in HASMCs treated with PDGF-BB. By contrast, anti-miR-200c transfection led to a decrease in p21 expression (Fig. 1H). These results suggest that miR-200c has a significant effect on VSMC growth both in vitro and in vivo.
3.3. KLF4 enhances, but SUMOylated KLF4 inhibits, miR-200c promoter activity
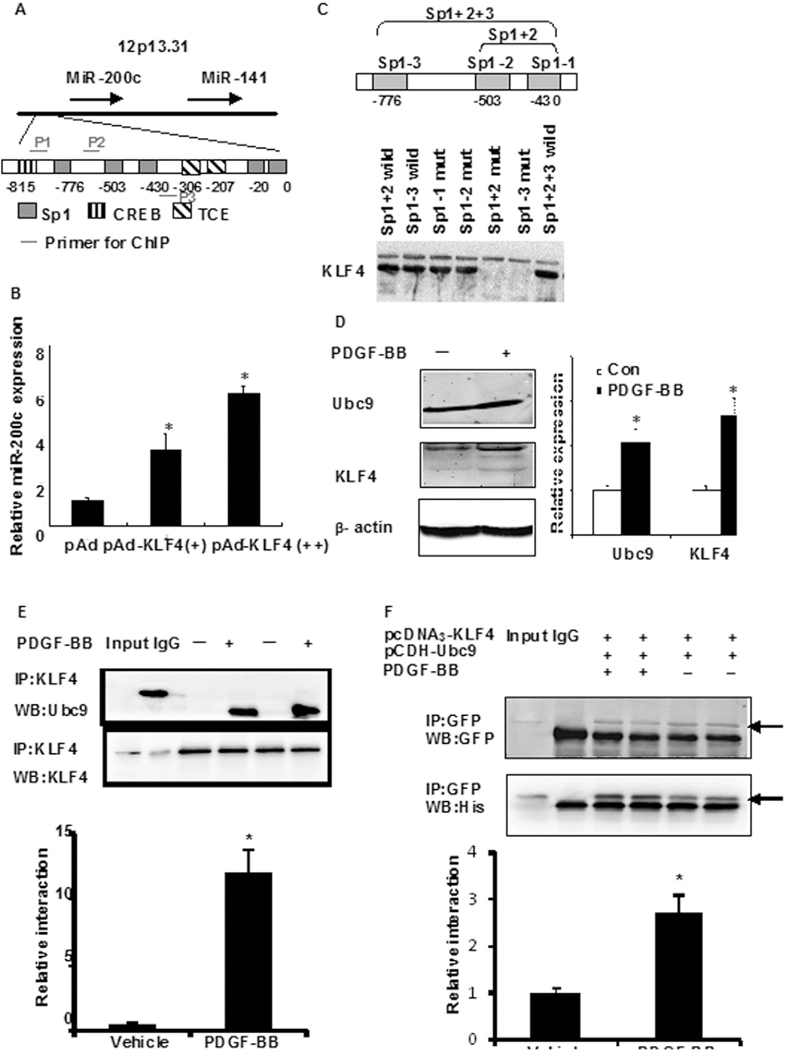
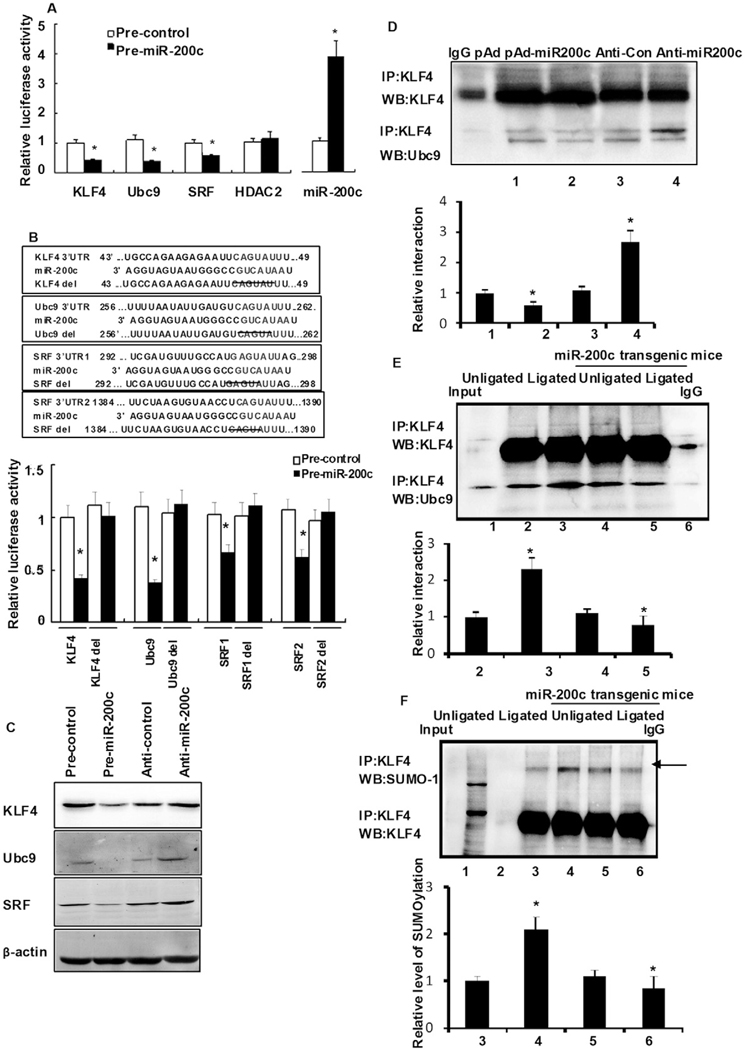
Next, we aimed to further determine the PDGF-BB-induced mechanism that decreased miR-200c expression, which might be related to activation of some transcriptional factors. Previous findings have indicated that PDGF-BB induces the expression of KLF4 [26,28], a transcription factor whose activity is regulated by post-translational modifications, including SUMOylation [18]. Therefore, the possibility exists that KLF4 is involved in the control of miR-200c levels. Two algorithms (TESS and PRPMO) predicted the binding of many transcription factors to the miR-200c promoter based on the presence of several GC boxes (Sp1-binding sites) (Fig. 2A), a typical KLF4-binding motif (CACCC) and its reverse orientation sequence (GGGTG). An oligonucleotide pull-down assay showed that KLF4 could bind to each of the three GC boxes (Fig. 2B) but not to the prototypical KLF4 binding site (data not shown). The relationship between KLF4 and miR-200c expression was investigated further by cloning a 1-kb regulatory sequence of the human miR-200c gene into the pGL3-luc reporter construct. Interestingly, the transfection of HASMCs with Ad-KLF4 increased miR-200c levels in a dose-dependent manner (Fig. 2C), which was contradictory to our presumption. Recent studies have also shown that transcription factors post-translationally conjugated with SUMO often inhibit transcription [18]. Thus, we hypothesized that miR-200c would be down-regulated by the SUMOylation of KLF4 in response to PDGF-BB challenge. First, we detected PDGF-BB-induced expression of KLF4 and Ubc9. As shown in Fig. 2D, HASMC cells stimulated with PDGF-BB for 24 h led to 2–2- and 3.1-fold increases in endogenous Ubc9 and KLF4 levels, respectively. Co-immunoprecipitation (co-IP) assays further found increased interactions between KLF4 and Ubc9 in response to PDGF-BB (Fig. 2E); similar results were observed in 293A cells transfected with plasmids encoding His-tagged KLF4 and GFP-tagged Ubc9 stimulated with PDGF-BB (Fig. 2F). The above data show that PDGF-BB stimulation induced KLF4:Ubc9 interactions. To further investigate whether the interaction of Ubc9 and KLF4 affected KLF4 modification, the SUMOylation of KLF4 was observed. As shown in Fig. 2G, the rapid accumulation of SUMOylated KLF4 in response to PDGF-BB was time dependent (Fig. 2G) and dose dependent (Online Figure IB) and largely reflected Ubc9-mediated SUMOylation events. Indeed, the transfection of Ubc9-DN (dominant negative mutant) abrogatedKLF4 SUMOylation compared with Ubc9-transfected cells (Online Figure IC). Moreover, an in vitro SUMOylation assay indicated that KLF4 was a SUMOylation target when incubated in the presence of SUMO1 and Ubc9 and that the deSUMOylating enzyme SENP1 efficiently reversed this reaction (Online Fig. ID, upper panel). Two KLF4 SUMOylation mutants (K275R and K225/K229/K275R) were constructed, and our data indicated that these residues were critical for Ubc9 modification (Online Fig. IE). The SUMOylation of KLF4 is completely offset by K225/K229/K275R mutation, suggesting K225/K229 mutation maybe affect the modification of SUMOylation. We next detected this interaction in human vascular tissue. SUMOylated KLF4 levels were higher in human renal vascular hyperplasia tissues than in normal vascular tissue (Fig. 2G and Online Fig. IF). Taken together, our findings indicate that Ubc9 and SUMOylated KLF4 play a key role in the process of vascular hyperplasia.
Fig. 2.
KLF4 enhances, but SUMOylated KLF4 inhibits, transactivation activity of miR-200c. (A) Partial sequence of the miR-200c promoter and Sp1-, CREB-, and TCE-binding motifs. The green underlined sequences are primers used for ChIP assay. (B) Oligonucleotide pull-down assay was performed with biotin-labeled, double-stranded oligonucleotides for the Sp1-binding sites and their mutants as probes. The DNA-bound proteins were collected with streptavidin-agarose beads and analyzed by Western blotting with KLF4 antibody. (C) VSMCs were transduced with Ad-KLF4 for 24 h, after which miR-200c expression levels were detected by qRT–PCR. *P < 0.05 compared with Ad-GFP control. All experiments were repeated three times. (D) VSMCs were treated with PDGF-BB for 2 h and the expression of Ubc9 and KLF4 proteins was detected by Western blotting. The right panel shows densitometric analyses from three independent experiments, one of which is shown on the left. *P < 0.05 vs. the respective control group. (E) HASMC were treated with PDGF-BB (10 ng/mL) for 2 h. Anti-KLF4 immunoprecipitates were immunoblotted with anti-Ubc9 and anti-KLF4 antibody. All experiments were repeated three times. (F) VSMCs cotransfected with pcDNA3-KLF4 (His-tagged) and pCDH-Ubc9 (GFP-tagged) were treated with or without PDGF-BB for 2 h. Anti-GFP immunoprecipitates were immunoblotted with anti-GFP (top) and anti-His (bottom) antibody. The bars represent the mean ± SEM from 3 independent experiments. *P < 0.05 compared with control treatment. (G) VSMCs were treated with PDGF-BB (10 ng/mL) for the time points indicated. Anti-KLF4 immunoprecipitates were immunoblotted with anti-SUMO1 (arrows indicate SUMOylated KLF4) and anti-KLF4 antibody. All experiments were repeated three times. Representative gel for SUMOylated KLF4 levels (upper) (Arrowhead). (H) Normal tissues and vascular hyperplasia specimens were prepared and subjected to anti-KLF4 immunoprecipitation followed by Western blot analysis. All experiments were repeated three times. Representative gel for SUMOylated KLF4 levels (upper) (arrowhead). (I) 293A cells were cotransfected with pGL3-miR-200c-luc together with either KLF4, wild-type Ubc9 or Ubc9-DN (dominant negative mutant) expression plasmid. Luciferase activity was measured 24 h later. *P < 0.05 compared with empty vector control. (J) VSMCs were treated with or without PDGF-BB for 2 h. ChIP assays were performed with anti-KLF4 or anti-Ubc9 antibody and the miR-200c promoter region containing Sp1-binding sites were amplified by qRT-PCR. The bars represent the mean ± SEM from 3 independent experiments. *P < 0.05 compared with vehicle. (K) VSMCs cotransfected with pcDNA3-KLF4 and pCDH-Ubc9 were treated with or without PDGF-BB for 2 h. Oligonucleotide pull-down assay was performed with biotin-labeled, double-stranded oligonucleotide probes corresponding to the Sp1-binding sites (Sp1 + 2 + 3) and their mutants. The DNA-bound proteins were collected with streptavidin-agarose beads and analyzed by Western blotting with His or GFP antibody. The bars represent the mean ± SEM from 3 independent experiments. *P < 0.05 compared with vehicle. (L) HASMCs were transfected with the indicated siRNAs for 24 h followed by PDGF-BB stimulation for 2 h. Chromatin fragments were immunoprecipitated with anti-KLF4 or anti-Ubc9 antibody, followed by a brief treatment with 0.5% SDS-containing buffer. The immunoprecipitated DNA was amplified by PCR using primers (P2, forward primer; P3, reverse primer) specific for the KLF4-binding region. IgG was used as a negative control. The bars represent the mean ± SEM from 3 independent experiments. *P < 0.05 compared with control siRNA. (M) HASMCs were transfected with KLF4 siRNA for 24 h followed by a 2-h treatment with PDGF-BB. miR-200c expression was detected by qRT-PCR. The bars represent the mean ± SEM from 3 independent experiments. *P < 0.05 compared with control siRNA.
To further investigate the effect of KLF4 and Ubc9 on miR-200c promoter activity, 293A cells were cotransfected with pGL3-miR-200c-luc and expression plasmids for KLF4 (pEGFP-KLF4), Ubc9 (pCDH-Ubc9) or pcDH-Ubc9 DN (dominant negative mutant). The results indicated that KLF4 overexpression significantly stimulated miR-200c promoter activity by ~2.46-fold (Fig. 2I). However, the ectopic expression of Ubc9 significantly reduced the KLF4-mediated activation of the miR-200c promoter (2.17 ± 0.39 vs. 0.39 ± 0.54-fold stimulation over control transfected cells; P < 0.05). ChIP assays indicated strong GC box binding of KLF4 and Ubc9 to the miR-200c promoter in response to PDGF-BB (Fig. 2J). However, transfection with pcDH-Ubc9 DN abrogated KLF4’s repressive effect on miR-200c expression, whereas mutation of the GC boxes reversed the negative actions of KLF4 and Ubc9 (Online Fig. IG). An oligonucleotide pull-down assay further indicated that PDGF-BB increased the binding of Ubc9 to the miR-200c promoter (Fig. 2K). To gain further insight into the roles of KLF4 and Ubc9 in the formation of transcriptional complexes, KLF4 or Ubc9 levels were down-regulated using siRNA techniques, after which ChIP assays were performed. As shown in Fig. 2L, silencing of KLF4 adversely affected the binding of Ubc9 to the miR-200c promoter in response to PDGF-BB. However, downregulation of Ubc9 levels did not affect KLF4 binding. The fact that the PDGF-BB-induced decrease in miR-200c expression was attenuated after KLF4 silencing (Fig. 2M) supports the notion that PDGF-BB reduces miR-200c levels via Ubc9-dependent SUMOylation of KLF4.
3.4. SUMOylated KLF4 interacts with the corepressors NCoR, HDAC2 and LSD1
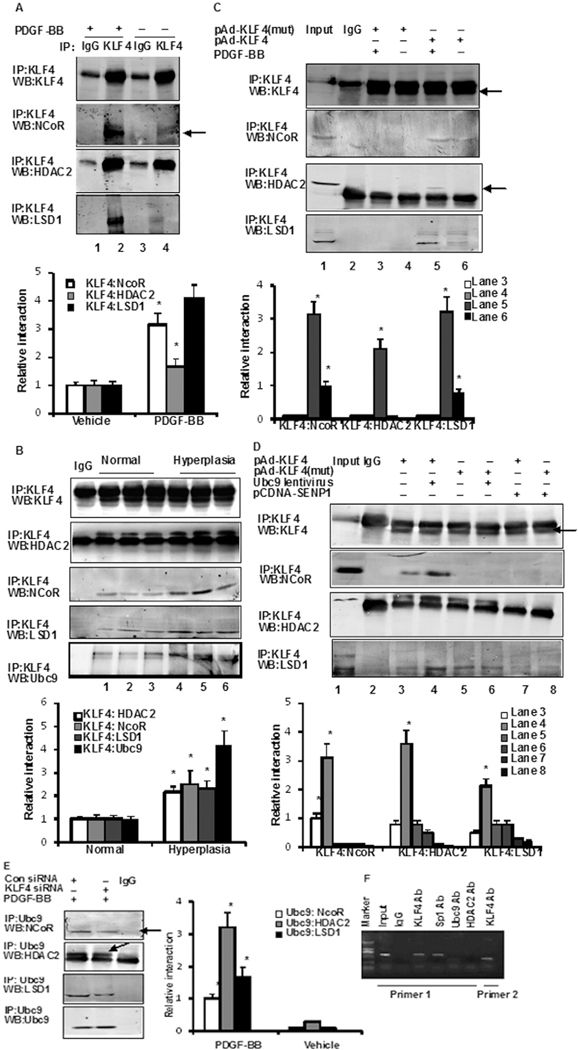
Previous studies have shown that the SUMOylation of KLF5 increases its affinity for the corepressor nuclear receptor corepressor (NCoR) [18]. Therefore, SUMOylated KLF4 may control the transcription of miR-200c through coregulator recruitment. As shown in Fig. 3A, KLF4 interactions with NCoR, histone deacetylase 2 (HDAC2), and (lysine-specific demethylase 1(LSD1) were clearly observed in co-IP assays using VSMCs treated for 2 h with PDGF-BB. Stronger interactions were observed between KLF4 and Ubc9, NCoR, LSD1, and HDAC2 corepressors in human vascular hyperplasia specimens than in normal vascular tissue samples (Fig. 3B). The SUMOylation-deficient KLF4 mutant was found to be a poor interactor in HASMCs (Fig. 3C). The binding of KLF4 with NCoR, HDAC2, and LSD1 was significantly weaker after deSUMOylation of KLF4 by the ectopic expression of SENP1; conversely, strong interactions were observed upon KLF4 SUMOylation with overexpressed Ubc9 (Fig. 3D). Moreover, the binding of KLF4 with these corepressors was markedly attenuated in KLF4 knockdown cells (Fig. 3E). None of the experimental conditions shown in Fig. 3A to I led to alterations in the expression of HDAC2, NCoR, and LSD1 (Online Fig. IIA–C). Collectively, these findings indicate that SUMOylation is key for proper interactions of KLF4 with NCoR, HDAC2, and LSD1. Finally, ChIP assays using the two primer pairs shown in Fig. 2A indicated that KLF4 binds to the three GC boxes of the miR-200c promoter (Fig. 3F). PDGF-BB increased the binding activity of Ubc9 and HDAC2 on the miR-200c promoter (Online Fig. IID). The SUMOylation-deficient KLF4 mutant bound poorly to the miR-200c promoter (Online Fig. IIE). Accordingly, the binding of KLF4 and Ubc9 on the miR-200c promoter was significantly lower after transfection with SENP1, and conversely, strong binding was observed when KLF4 SUMOylation was induced by Ubc9 overexpression (Online Fig. IIE). Thus, the reduction in miR-200c levels in response to PDGF-BB stemmed from the rapid SUMOylation of KLF4 and from the recruitment of corepressors.
Fig. 3.
Sumoylated KLF4 interacts with corepressors NCoR, HDAC2, and LSD1. (A) VSMCs were treated with PDGF-BB for 2 h, after which the interaction between KLF4 and NCoR, HDAC2 and LSD1 was examined in anti-KLF4 immunoprecipitates and Western blot analysis. The bars represent the mean ± SEM from 3 independent experiments. *P < 0.05 compared with vehicle. (B) Interaction between KLF4 and Ubc9, NCoR, HDAC2, and LSD1 was examined in normal tissues (n = 6) and vascular hyperplasia (n = 6) specimens. *P < 0.05 compared with normal tissues. Representative gel for interaction. (C) VSMCs were infected with pAd-KLF4 wild-type or pAd-KLF4-mut (pAd-KLF4-K225R/K229R/K279R) adenovirus for 24 h. Then, cells were treated or not with PDGF-BB for 2 h. Interaction between KLF4 and NCoR, HDAC2, and LSD1 was examined (Arrowhead). The bars represent the mean ± SEM from 3 independent experiments. *P < 0.05 compared with pAd-KLF4 (mut). (D) VSMCs were infected either with pAd-KLF4 wild-type or pAd-KLF4-mut in the presence of Ubc9 lentivirus or pcDNA-SENP1 for 24 h. Interaction between KLF4 and NCoR, HDAC2, and LSD1 was examined (arrowhead). The bars represent the mean ± SEM from 3 independent experiments. *P < 0.05 compared with pAd-KLF4 (mut). (E) VSMCs were transfected either with control siRNA or KLF4 siRNA for 24 h, and then treated with PDGF-BB for 2 h. Interaction between NCoR (Arrowhead), HDAC2 (Arrowhead), LSD1, and Ubc9 was examined in anti-Ubc9 immunoprecipitates by Western blot analysis. The bars represent the mean ± SEM from 3 independent experiments. *P < 0.05 compared with con siRNA. (F) ChIP assays in VSMCs were performed with the indicated antibodies, and the miR-200c promoter region containing Sp1-binding sites was amplified by PCR. Primer 1 (P1, forward primer; P3, reverse primer); Primer 2 (P2, forward primer; P3, reverse primer). All experiments were repeated three times. Representative gel for interaction.
3.5. PDGF-BB induces KLF4 SUMOylation via JNK and ERK pathways
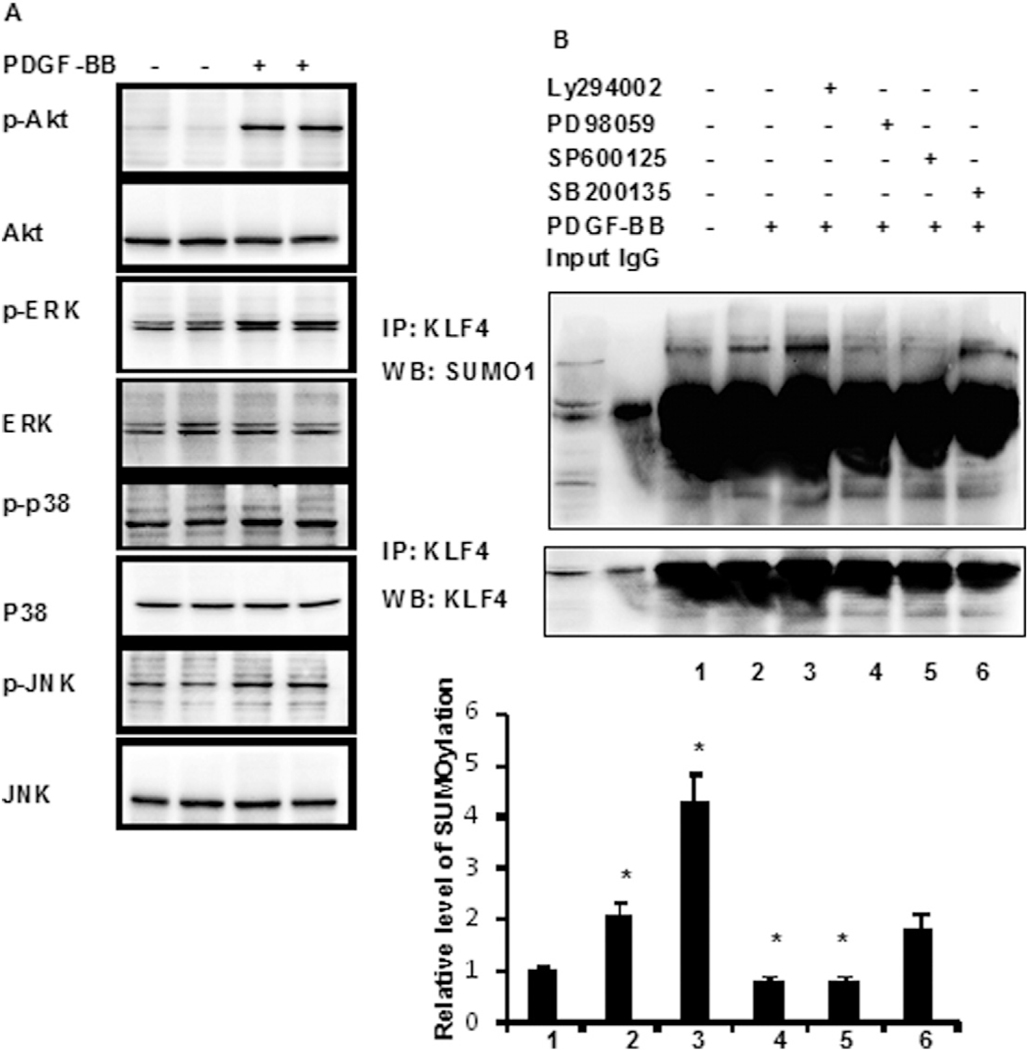
PDGF-BB increased KLF4 SUMOylation in a time-dependent manner (Fig. 2G). We next investigated which signaling pathway mediates KLF4 SUMOylation in response to PDGF-BB. Under these experimental conditions, PDGF-BB dramatically increased phospho-JNK, ERK, and JNK levels. However, the levels of phospho-p38 increased only slightly (Fig. 4A). These results suggest that multiple signaling pathways are involved in PDGF-BB-induced KLF4 SUMOylation in HASMCs. We then incubated HASMCs with the JNK inhibitor SP600125, the p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580, the ERK inhibitor PD98059, or the PI3K/Akt inhibitor LY294002 for 4 h before exposure to PDGF-BB. As shown in Fig. 4B, the inhibition of JNK or ERK blocked PDGF-BB-induced KLF4 SUMOylation, suggesting that PDGF-BB induces KLF4 SUMOylation via the JNK and ERK pathways.
Fig. 4.
PDGF-BB induces KLF4 SUMOylation via JNK and ERK pthway. (A) Phospho-JNK, phospho-p38, phospho-ERK, and phospho-Akt were analysed by western blotting with their respective antibodies. The total protein of JNK, p38, ERK, and Akt is also shown. (B) VSMCs were pretreated with SP600125, SB203580, PD98059, or LY294002 for 4 h and then treated with PDGF-BB for 2 h. SUMOylated KLF4 was determined by immunoprecipitation using anti-SUMO1 antibody and immunoblotting with anti-KLF4 antibody. The bars represent the mean ± SEM from 3 independent experiments. *P < 0.05 compared with Vehicle.
3.6. KLF4, Ubc9, and SRF are miR-200c targets
Next, we investigated the mechanism by which reduced miR-200c expression affected cell proliferation. The identification of miR-200c targets was first performed using different algorithms, including miRecords, miRanda, and TargetScan5.1. Significantly, the transcriptional regulators KLF4, Ubc9, HDAC2, and SRF, as well as the cell cycle-related factors cyclin E and cyclin-dependent kinase 2 (cdk2), were identified as putative miR-200c targets. These results suggest that miR-200c can regulate cell proliferation through many pathways. We investigated the functionality of miR-200c in 293A cells, in which a miR-200c plasmid was coexpressed with a luciferase reporter fused to the 3′ UTR (untranslated region) of KLF4, Ubc9, SRF, or HDAC2. The levels of reporter activity for KLF4, SRF, and Ubc9 were significantly lower in miR-200c-expressing cells, whereas HDAC2 activity was unaffected (Fig. 5A). To confirm the selectivity of miR-200c target regulation, a four-nucleotide deletion in the miR-200c seed sequence of KLF4, Ubc9, and SRF 3′ UTRs was performed. No inhibition of reporter activity was observed in the miR-200c-expressing cells (Fig. 5B, lower panel). Similar results were obtained in HASMCs transfected with miR-200c precursors (Online Figure IIIA). Thus, KLF4, Ubc9, and SRF are direct miR-200c targets. Ectopic expression of miR-200c also decreased the abundance of KLF4, Ubc9, and SRF proteins in HASMCs (Fig. 5C, lane 2 vs. 1); however, their levels were higher in anti-miR-200c-transfected cells than in cells transfected with an anti-miR control (Online Fig. IIIB, lane 4 vs. 3; P < 0.05).
Fig. 5.
Role of miR-200c targets in HASMC proliferation in vitro. (A) 293A cells were cotransfected with pre-miR-200c together with either pLuc-KLF4-3′ UTR, pLuc-Ubc9–3′ UTR, pLuc-SRF-3′ UTR, or pLuc-HDAC2-3′ UTR. Twenty-four hours later, cells were harvested for the determination of luciferase activities. Experiments were performed in triplicate and each value is the mean ± SEM of three independent experiments. *P < 0.05 vs. control, untransfected cells. (B) Schematic description of KLF4, Ubc9, and SRF 3′ UTR with putative binding sites for miR-200c (upper). Deletion analysis of the pLuc-KLF4–3′ UTR, pLuc-Ubc9–3′ UTR, and pLuc-SRF-3′ UTR was detected as above (lower). Values are averages of three separate experiments ± SEM. (C) HASMCs were transfected either with miR-200c or anti-miR-200c (miR-200c inhibitor). The expression of KLF4, Ubc9, and SRF proteins was detected by Western blotting. (D) HASMCs were transfected either with miR-200c or anti-miR-200c (miR-200c inhibitor). The interaction between KLF4 and Ubc9 was detected by Co-IP. Experiments were performed in triplicate and each value is the mean ± SEM of three independent experiments. *P < 0.05 vs. anti-con cells. #P < 0.05 vs. pAd cells. (E, F) Vascular tissue of unligated vessels, ligated vessels of miR-200c transgenic or control mice 7 days after carotid ligation were prepared and subjected to anti-KLF4 immunoprecipitation followed by Western blot analysis of Ubc9 (E) and SUMO-1 (F). The bars represent the mean ± SEM from 3 independent experiments. *P < 0.05 compared with ligated vessels of control mice.(G) HASMCs were transfected of 24 h with different vectors and cell proliferation was then detected by BrdU assay. Values represent fold increase relative to the control, untransfected cells. Experiments were performed in triplicate and each value is the mean ± SEM of three independent experiments. *P < 0.05 vs. control using one-way ANOVA for independent groups. (H) HASMCs were transfected with anti-miR-200c or induced by PDGF-BB for 24 h, followed by cell proliferation determination by BrdU assay. Values represent fold increase relative to controls. *P < 0.05 vs. the control using one-way ANOVA for independent groups. The expression of KLF4, Ubc9, and SRF were detected by Western blotting after a 24-h treatment with PDGF-BB. (I) Twenty-four hours after transfection with the indicated vectors, HASMCs were treated with PDGF-BB for 2 h. Anti-KLF4 immunoprecipitates were immunoblotted with anti-SUMO1 (arrows indicate SUMOylated KLF4) and anti-KLF4 antibody. The expression of Ubc9 and SRF were detected by Western blotting using the cell lysates. The bars represent the mean ± SEM from 3 independent experiments. *P < 0.05 compared with con siRNA.
The expression of KLF4, Ubc9, and SRF was further examined in the vascular tissues of animals infected with either pAd-miR-200c or pAd control. Immunohistochemical staining revealed that the percentage of cells positively stained for Ubc9, SRF, and KLF4 was lower in the pAd-miR-200c group (Online Fig. IIIC). Conversely, more intense staining for Ubc9, SRF, and KLF4 was observed after anti-miR-200c transfection (Online Fig. IIIC). After staining, positive cells were found primarily in the subintimal media. Collectively, these results show that Ubc9, SRF, and KLF4 transcripts are endogenous targets of miR-200c. Co-IP results further showed that the interaction between Ubc9 and KLF4 was lower in the anti-miR-200c group. By contrast, an increase in interactions between Ubc9 and KLF4 was observed after a pAd-miR-200c transfection (Fig. 5D). To further demonstrate the effect of miR-200c in vivo, SUMOylation of KLF4 and the interaction of KLF4 and Ubc9 were detected in the carotid arterial walls of miR-200c transgenic mice. The interaction between Ubc9 and KLF4 (Fig. 5E) and the SUMOylation of KLF4 (Fig. 5F) were higher in miR-200c transgenic mice at 7 days after vessel ligation. These data further revealed that miR-200c could regulate the SUMOylation of KLF4 and affect its function.
We next assessed the question of whether the rescue of KLF4, Ubc9 or SRF could prevent the inhibitory effects of miR-200c on HASMC growth. As shown in Fig. 5G, ectopic expression of Ubc9 blocked the anti-proliferative action observed in miR-200c-infected cells (lane 3 vs. lane 2), whereas overexpression of KLF4 synergistically enhanced the ability of miR-200c to inhibit VSMC proliferation (lane 4 vs. lane 2). Surprisingly, the combined overexpression of KLF4 and Ubc9 more than tripled HASMC proliferation in miR-200c-infected cells (lane 6 vs. lane 2), which is consistent with the notion that the combination of KLF4 and Ubc9 conferred protection against the suppressive action of miR-200c on VSMC growth. The role of SRF seemed quite distinct from that of KLF4 and Ubc9 because coexpression of SRF and Ubc9 did not affect the growth-inhibitory signals elicited by miR-200c (lane 7 vs. lane 2). The combined transfection of SRF and KLF4 markedly enhanced the anti-proliferative response of miR-200c (lane 8 vs. lane 2). Western blot analysis performed under these experimental conditions showed that reciprocal regulation occurred between cell proliferation and p21 protein levels (Online Fig. IIID).
siRNA techniques were used to examine the effect of KLF4, Ubc9, or SRF knockdown in HASMCs stimulated with PDGF-BB. The effect of anti-miR-200c expression under conditions where KLF4, Ubc9, and/or SRF were downregulated was also investigated. As shown in Fig. 5H, knockdown of Ubc9 alone markedly reduced cell growth induced by either anti-miR-200c or PDGF-BB (lane 4 vs. 2), whereas KLF4 silencing was not as effective (lane 3 vs. 4). The cotransfection of both Ubc9 and KLF4 siRNAs yielded a pattern of cell proliferation resembling that of KFL4 siRNA alone (lane 6 vs. 3). The reduction in SRF levels led to greater cell proliferation in response to anti-miR-200c expression, but the proliferative effect of PDGF-BB was significantly lower when compared to that in cells transfected with a non-silencing control siRNA (lane 5 vs. 2; P < 0.05). These data suggest that KLF4 and Ubc9 participated in miR-200c-induced growth arrest, and that the regulation of cell proliferation by PDGF-BB appears to be primarily under the control of Ubc9 rather than of SRF. Hence, the PDGF-BB-mediated increase in SUMOylated KLF4 levels was dependent on Ubc9 but not on SRF (Fig. 5I), and these levels were unaffected by anti-miR-200c (data not shown). Hence, KLF4/Ubc9 and miR-200c form a negative-feedback loop, but SRF/miR-200c does not. A reporter gene assay further showed that the reporter activity of p21 significantly increased (3.7-fold) with KLF4 overexpression (lane 3 vs. 1; P < 0.05) but decreased by 3.2-fold with Ubc9 and KLF4 overexpression (lane 3 vs. 1; P < 0.05) (Online Fig. IIIE). These results show that a miR-200c-SUMOylated KLF4 feedback loop is an important aspect of the PDGF-BB proliferative response in VSMCs.
4. Discussion
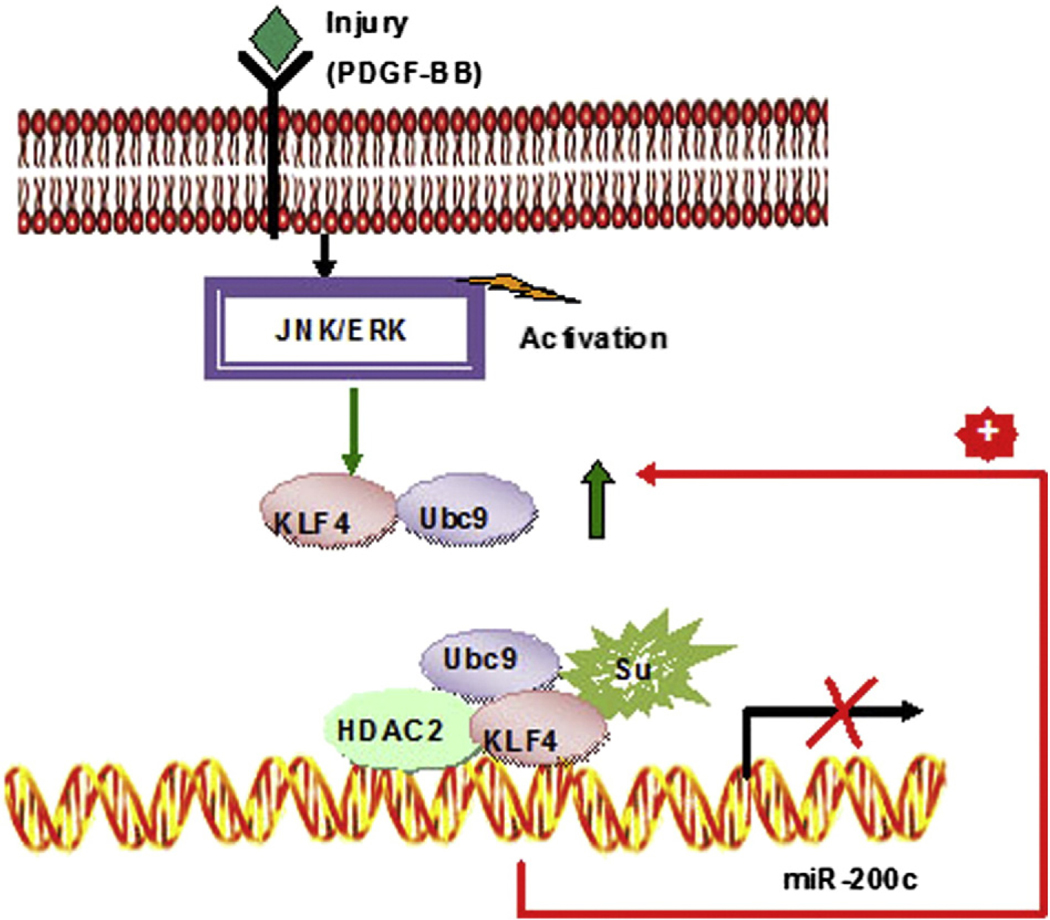
Cardiovascular disease is the largest single cause of mortality. The proliferation of VSMCs is a key event in the pathogenesis of various vascular diseases, including atherosclerosis and hypertension [29]. Many factors, such as KLFs [1,23,25,30], myocardin [31], and SRF [32], are involved in the abnormal proliferation of VSMCs associated with atherosclerosis and hypertension. As shown in other studies, miRNAs are post-transcriptional regulators of genes that participate in the proliferation and differentiation of VSMCs. We screened miRNAs expressed in human normal and vascular hyperplasia tissues and found that miR-200c expression decreased dramatically with hyperplasia. The function of miR-200c in cancer biology has been well established; however, its role during VSMC proliferation and differentiation is unknown. In this study, PDGF-BB treatment induced the interaction of Ubc9 with KLF4 and the promotion of KLF4 SUMOylation, which allowed the recruitment of transcriptional corepressors (e.g., NCoR and HDAC2) to the miR-200c promoter. Furthermore, lower miR-200c levels restored the expression of target genes (e.g., Ubc9 and KLF4), thereby accelerating VSMC proliferation through the formation of a KLF4-miR-200c positive-feedback loop.
In recent years, tremendous efforts have been made to use a miR-microarray screening approach to address the miR expression profiling of CAD in animal models and human patients. However, the roles of specific miRs in CAD are only emerging. In the present study, we found that miR-200c is relevant to CAD. miR-200c participates in epithelial proliferation, esophageal cancer chemoresistance, and invasion by breast cancer cells [13,14]. Rebustini et al. found that miR-200c regulates FGFR-dependent epithelial proliferation via Vldlr [33]. Liu identified that miR-200c inhibited melanoma progression by targeting Bmi-1 [34]. Hur et al. proved that miR-200c modulates epithelial-to-mesenchymal transition in human colorectal cancer metastasis [35]. However, little focus on its role in CAD. Therefore, we focused on elucidating the functional consequences of miR-200c in vivo and in vitro using gain-of-function and loss-of-function approaches. MTT assay showed that overexpression of miR-200c markedly reduced PDGF-BB-mediated HASMC proliferation in vitro (Fig. 1D). Accordingly, a trend toward lower neointimal formation was found in animals overexpressing pAd-miR-200c (Fig. 1F). Furthermore, the miR-200c transgenic mice exhibited significantly thinner carotid arterial walls, lower I/M ratios in vivo (Fig. 1G). It is well known that p21WAF1/Cip1 is a potent cyclin-dependent kinase inhibitor. Western blot showed that overexpression of miR-200c markedly increased p21WAF1/Cip1 expression in HASMCs. These data showed that miR-200c reduces PDGF-BB-induced VSMC proliferation, which may be related to p21 expression (Fig. 1). To further detect the mechanism of miR-200c activation, we analyzed the miR-200c promoter and obtained evidence for the presence of GC boxes and a KLF4-binding site. In fact, KLF4 regulates the transcription of many genes by binding to GC-rich sites or CACCC-boxes [7,23]. This finding prompted us to investigate whether KLF4 participates in the regulation of miR-200c. KLF4 has been reported to inhibit VSMC proliferation by upregulating several negative cell-cycle-regulatory genes, including p21WAF1/Cip1 [5], p27 [36], and Mdm2 [5], as well as by downregulating several positive cell-cycle-regulatory genes, such as cyclin D1 [37] and ODC [38]. However, our recent observations that PDGF-BB induced an increase in KLF4 expression [28] along with a concomitant surge in miR-200c levels appeared contradictory to these findings. Some mechanism must be involved in this regulation. Protein modification is known to have an important function in regulating gene expression. For example, SUMOylation of transcription factors leads to repression of transcription [18,19]. Importantly, we found that the expression of Ubc9 and KLF4 was unregulated by PDGF-BB. The roles of Ubc9 in protein SUMOylation and SUMOylation-mediated cellular pathways have been established [39]. Ubc9 is to be juxtaposed with its ability to enhance the activity of pleomorphic adenomagene like-2 (PLAGL2), a transactivator of surfactant protein-C, by a SUMOylation-independent mechanism [40]. Ubc9 SUMOylates Smad4, which promotes stability and nuclear accumulation. Notably, Ubc9 interacts with Vsx-1 [41], glucocorticoid receptors [42], and estrogen receptors [43] to inhibit gene expression. Furthermore, ectopically expressed Ubc9 inhibits GLUT4 degradation while promoting its targeting to a unique insulin-responsive storage compartment in muscle [44]. Therefore, we speculate that KLF4 may regulate miR-200c expression by SUMOylation.
Kawai-Kowase et al. [1] reported that the E3-ligase PIAS1 promotes TGFβ-induced activation of smooth muscle α-actin gene expression partly via KLF4 SUMOylation. The cotransfection of KLF4 and GFP-SUMO-1 expression vectors results in the SUMOylation of KLF4 [45]. In the present study, we found that PDGF-BB blocks KLF4-mediated induction of miR-200c by enhancing SUMOylation of KLF4 and subsequent recruitment of the corepressors NCoR, HDAC2 and LSD1 (Fig. 3), together with lower binding affinity for p300 (Fig. 2). Moreover, ChIP analyses indicated that PDGF-BB induces rapid changes in the assembly of transcriptional regulatory complexes to the endogenous miR-200c promoter. Furthermore, reporter gene assay showed that the reporter activity of p21 significantly increased with KLF4 overexpression, but decreased by 3.2-fold with Ubc9 and KLF4 overexpression (Online Fig. IIIE). These results show that a miR-200c-SUMOylated KLF4-p21 feedback loop is an important aspect of the PDGF-BB proliferative response in VSMCs.
These results indicate that SUMOylation enables KLF4 to have dual roles in transcriptional regulation in response to cellular and environmental cues, which in turn suggests that inducible SUMOylation of KLF4 allows exchange of the components of transcriptional regulatory complexes and chromatin remodeling.
Most interestingly, in the current study, we also identified KLF4, Ubc9, and SRF as miR-200c targets (Fig. 6). In fact, as an important transcriptional factor, downregulated expression of KLF4 may affect the expression of many genes, including p21, p53, SM-α actin, and SM-22α. Therefore, we propose that miR-200c and KLF4 form a feedback loop to regulate each other’s expression. However, the molecular mechanism by which the KLF4-miR-200c pair controls VSMC proliferation remains to be elucidated.
Fig. 6.
Model illustrating a molecular mechanism of VSMC proliferation induced by PDGF-BB. Upon PDGF-BB treatment, Ubc9 interacts with and promotes SUMOylation of KLF4, which allows recruitment of transcriptional corepressors (e.g., NCoR) to miR-200c promoter. Lower miR-200c level restores the expression of target genes (e.g., Ubc9 and KLF4), which further represses miR-200c levels thereby accelerating VSMC proliferation.
In our proposed model (Fig. 5), unSUMOylated KLF4 is associated with p300 under basal conditions and functions to activate miR-200c transcription. In response to PDGF-BB challenge, Ubc9 interacts with and promotes SUMOylation of KLF4, which allows recruitment of transcriptional corepressors (e.g., NCoR and HDAC2) to the miR-200c promoter. Furthermore, lower miR-200c levels restore the expression of target genes (e.g., Ubc9 and KLF4), which further repress miR-200c levels and accelerate VSMC proliferation through the formation of a KLF4-miR-200c positive-feedback loop. Because of the high baseline levels of miR-200c and its ability to reduce KLF4 expression, it remains unclear how miR-200c expression is controlled by KLF4. It would appear that SUMOylated KLF4 does not inhibit KLF4 expression but instead can be induced by forming a positive-feedback loop. The roles of other trans-acting factors in the regulation of KLF4 expression as well as in the formation of the KLF4-miR-200c positive-feedback loop cannot be discounted.
Supplementary Material
Funding
This work was supported by the National Natural Science Foundation of People’s Republic of China (No. 31271396, 31271224, 31301136), Fok Ying Tung Education Foundation (131037). This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Abbreviations:
- ATRA
all-trans retinoic acid
- cdk2
cyclin-dependent kinase 2
- HASMC
human aortic smooth muscle cell
- HDAC2
histone deacetylase 2
- KLF
Krüppel-like factor
- LSD1
lysine-specific demethylase 1
- miR
microRNA
- NCoR
nuclear receptor corepressor
- p21
p21WAF1/Cip1
- PDGF
platelet-derived growth factor
- qRT-PCR
quantitative real-time polymerase chain reaction
- SRF
serum response factor
- SUMO
small ubiquitin-like modifier
- Ubc9
E2-conjugatingenzymeUbc9
- UTR
untranslatedregions
- VSMCs
vascular smooth muscle cells
Footnotes
Disclosures
None.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.yjmcc.2015.03.011.
References
- [1].Kawai-Kowase K, Ohshima T, Matsui H, Tanaka T, Shimizu T, Iso T, et al. PIAS1 mediates TGFbeta-induced SM alpha-actin gene expression through inhibition of KLF4 function-expression by protein sumoylation. Arterioscler Thromb Vasc Biol 2009;29:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang C, Zhang Y, Yang Q, Yang Y, Gu Y, Wang M, et al. A novel cultured tissue model of rat aorta: VSMC proliferation mechanism in relationship to atherosclerosis. Exp Mol Pathol 2007;83:453–8. [DOI] [PubMed] [Google Scholar]
- [3].Coinu R, Chiaviello A, Galleri G, Franconi F, Crescenzi E, Palumbo G. Exposure to modeled microgravity induces metabolic idleness in malignant human MCF-7 and normal murine VSMC cells. FEBS Lett 2006;580:2465–70. [DOI] [PubMed] [Google Scholar]
- [4].Satoh K, Nigro P, Matoba T, O’Dell MR, Cui Z, Shi X, et al. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med 2009;15:649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wassmann S, Wassmann K, Jung A, Velten M, Knuefermann P, Petoumenos V, et al. Induction of p53 by GKLF is essential for inhibition of proliferation of vascular smooth muscle cells. J Mol Cell Cardiol 2007;43:301–7. [DOI] [PubMed] [Google Scholar]
- [6].Fabian MR, Sonenberg N. The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat Struct Mol Biol 2012;19:586–93. [DOI] [PubMed] [Google Scholar]
- [7].Davis-Dusenbery BN, Chan MC, Reno KE, Weisman AS, Layne MD, Lagna G, et al. Down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J Biol Chem 2011;286:28097–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res 2009;105:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res 2009;104:476–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sun SG, Zheng B, Han M, Fang XM, Li HX, Miao SB, et al. miR-146a and Kruppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep 2011;12:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu X, Cheng Y, Chen X, Yang J, Xu L, Zhang C. MicroRNA-31 regulated by the extracellular regulated kinase is involved in vascular smooth muscle cell growth via large tumor suppressor homolog 2. J Biol Chem 2011;286:42371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang Y, Wang Y, Wang X, Eisner GM, Asico LD, Jose PA, et al. Insulin promotes vascular smooth muscle cell proliferation via microRNA-208-mediated downregulation of p21. J Hypertens 2011;29:1560–8. [DOI] [PubMed] [Google Scholar]
- [13].Rokavec M, Wu W, Luo JL. IL6-mediated suppression of miR-200c directs constitutive activation of inflammatory signaling circuit driving transformation and tumorigenesis. Mol Cell 2012;45:777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008;10:593–601. [DOI] [PubMed] [Google Scholar]
- [15].Kiyan Y, Limbourg A, Kiyan R, Tkachuk S, Limbourg FP, Ovsianikov A, et al. Urokinase receptor associates with myocardin to control vascular smooth muscle cells phenotype in vascular disease. Arterioscler Thromb Vasc Biol 2012;32:110–22. [DOI] [PubMed] [Google Scholar]
- [16].Wu F, Zhu S, Ding Y, Beck WT, Mo YY. MicroRNA-mediated regulation of Ubc9 expression in cancer cells. Clin Cancer Res 2009;15:1550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Martin S, Nishimune A, Mellor JR, Henley JM. SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature 2007;447:321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Oishi Y, Manabe I, Tobe K, Ohsugi M, Kubota T, Fujiu K, et al. SUMOylation of Kruppel-like transcription factor 5 acts as a molecular switch in transcriptional programs of lipid metabolism involving PPAR-delta. Nat Med 2008;14:656–66. [DOI] [PubMed] [Google Scholar]
- [19].Du JX, Bialkowska AB, McConnell BB, Yang VW. SUMOylation regulates nuclear localization of Kruppel-like factor 5. J Biol Chem 2008;283:31991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sumoylation Khanna-Gupta A. and the function of CCAAT enhancer binding protein alpha (C/EBP alpha). Blood Cells Mol Dis 2008;41:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Papapetrou EP, Tomishima MJ, Chambers SM, Mica Y, Reed E, Menon J, et al. Stoichiometric and temporal requirements of Oct4, Sox2, Klf4, and c-Myc expression for efficient human iPSC induction and differentiation. Proc Natl Acad Sci U S A 2009;106:12759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gamper AM, Qiao X, Kim J, Zhang L, DeSimone MC, Rathmell WK, et al. Regulation of KLF4 turnover reveals an unexpected tissue-specific role of pVHL in tumorigenesis. Mol Cell 2012;45:233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang XH, Zheng B, Gu C, Fu JR, Wen JK. TGF-beta1 downregulates AT1 receptor expression via PKC-delta-mediated Sp1 dissociation from KLF4 and Smad-mediated PPAR-gamma association with KLF4. Arterioscler Thromb Vasc Biol 2012;32: 1015–23. [DOI] [PubMed] [Google Scholar]
- [24].Tetreault MP, Wang ML, Yang Y, Travis J, Yu QC, Klein-Szanto AJ, et al. Klf4 overexpression activates epithelial cytokines and inflammation-mediated esophageal squamous cell cancer in mice. Gastroenterology 2010;139:2124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yoshida T, Kaestner KH, Owens GK. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res 2008;102:1548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zheng B, Han M, Bernier M, Zhang XH, Meng F, Miao SB, et al. Kruppel-like factor 4 inhibits proliferation by platelet-derived growth factor receptor beta-mediated, not by retinoic acid receptor alpha-mediated, phosphatidylinositol 3-kinase and ERK signaling in vascular smooth muscle cells. J Biol Chem 2009;284:22773–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pidkovka NA, Cherepanova OA, Yoshida T, Alexander MR, Deaton RA, Thomas JA, et al. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ Res 2007;101:792–801. [DOI] [PubMed] [Google Scholar]
- [28].Yu K, Zheng B, Han M, Wen JK. ATRA activates and PDGF-BB represses the SM22alpha promoter through KLF4 binding to, or dissociating from, its cis-DNA elements. Cardiovasc Res 2011;90:464–74. [DOI] [PubMed] [Google Scholar]
- [29].Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res 2012;95:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shi JH, Zheng B, Chen S, Ma GY, Wen JK. Retinoic acid receptor alpha mediates All-trans-retinoic acid-induced Klf4 gene expression by regulating Klf4 promoter activity in vascular smooth muscle cells. J Biol Chem 2012;287:10799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yoshida T, Sinha S, Dandre F, Wamhoff BR, Hoofnagle MH, Kremer BE, et al. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res 2003;92:856–64. [DOI] [PubMed] [Google Scholar]
- [32].McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest 2006;116:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rebustiniv IT, Hayashi T, Reynolds AD, Dillard ML, Carpenter EM, Hoffman MP. miR-200c regulates FGFR-dependent epithelial proliferation via Vldlr during submandibular gland branching morphogenesis. Development 2012;139:20–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu S, Tetzlaff MT, Cui R, Xu X. miR-200c inhibits melanoma progression and drug resistance through down-regulation of Bmi-1. Am J Pathol 2012;181:1823–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hur K, Toiyama Y, Takahashi M, Balaguer F, Nagasaka T, Koike J, et al. MicroRNA200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut 2013;62:1315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wei D, Kanai M, Jia Z, Le X, Xie K. Kruppel-like factor 4 induces p27Kip1 expression in and suppresses the growth and metastasis of human pancreatic cancer cells. Cancer Res 2008;68:4631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shie JL, Chen ZY, Fu M, Pestell RG, Tseng CC. Gut-enriched Kruppel-like factor represses cyclin D1 promoter activity through Sp1 motif. Nucleic Acids Res 2000;28: 2969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen ZY, Shie JL, Tseng CC. Gut-enriched Kruppel-like factor represses ornithine decarboxylase gene expression and functions as checkpoint regulator in colonic cancer cells. J Biol Chem 2002;277:46831–9. [DOI] [PubMed] [Google Scholar]
- [39].Qin Y, Xu J, Aysola K, Begum N, Reddy V, Chai Y, et al. Ubc9 mediates nuclear localization and growth suppression of BRCA1 and BRCA1a proteins. J Cell Physiol 2011;226:3355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guo Y, Yang MC, Weissler JC, Yang YS. Modulation of PLAGL2 transactivation activity by Ubc9 co-activation not SUMOylation. Biochem Biophys Res Commun 2008;374:570–5. [DOI] [PubMed] [Google Scholar]
- [41].Kurtzman AL, Schechter N. Ubc9 interacts with a nuclear localization signal and mediates nuclear localization of the paired-like homeobox protein Vsx-1 independent of SUMO-1 modification. Proc Natl Acad Sci U S A 2001;98:5602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sun Y, Tao YG, Kagan BL, He Y, Jr SS. Modulation of transcription parameters in glucocorticoid receptor-mediated repression. Mol Cell Endocrinol 2008;295:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sentis S, Le Romancer M, Bianchin C, Rostan MC, Corbo L. Sumoylation of the estrogen receptor alpha hinge region regulates its transcriptional activity. Mol Endocrinol 2005;19:2671–84. [DOI] [PubMed] [Google Scholar]
- [44].Kampmann U, Christensen B, Nielsen TS, Pedersen SB, Orskov L, Lund S. GLUT4 and UBC9 protein expression is reduced inmuscle fromtype 2 diabetic patients with severe insulin resistance. PLoS One 2011;6:e27854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Du JX, McConnell BB, Yang VW. A small ubiquitin-related modifier-interacting motif functions as the transcriptional activation domain of Kruppel-like factor 4. J Biol Chem 2010;285:28298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.