Abstract
Vitreoretinal mechanics plays an important role in retinal trauma and many sight-threatening diseases. In age-related pathologies, such as posterior vitreous detachment and vitreomacular traction, lingering vitreoretinal adhesions can lead to macular holes, epiretinal membranes, retinal tears and detachment. In age-related macular degeneration, vitreoretinal traction has been implicated in the acceleration of the disease due to the stimulation of vascular growth factors. Despite this strong mechanobiological influence on trauma and disease in the eye, fundamental understanding of the mechanics at the vitreoretinal interface is limited. Clarification of adhesion mechanisms and the role of vitreoretinal mechanics in healthy eyes and disease is necessary to develop innovative treatments for these pathologies. In this review, we evaluate the existing literature on the structure and function of the vitreoretinal interface to gain insight into age- and region-dependent mechanisms of vitreoretinal adhesion. We explore the role of vitreoretinal adhesion in ocular pathologies to identify knowledge gaps and future research areas. Finally, we recommend future mechanics-based studies to address the critical needs in the field, increase fundamental understanding of vitreoretinal mechanisms and disease, and inform disease treatments.
Keywords: vitreous, retina, vitreoretinal adhesion, vitreoretinal mechanics, posterior vitreous detachment
1. Introduction
The mechanics of adhesion at the vitreoretinal interface (VRI) contribute to the progression of many diseases that lead to visual impairment or blindness. For example, strong focal vitreoretinal adhesions can prevent complete posterior vitreous detachment (PVD) from occurring. These adhesions can exert traction forces on the retina, leading to macular holes and epiretinal membranes in the macula, and retinal tears and detachment in the periphery (Escoffery et al., 1976; Jackson et al., 2013; Patronas et al., 2009). In age-related macular degeneration (AMD), persistent focal attachments between the vitreous cortex and the macula are more prevalent in exudative AMD than non-exudative AMD (Jackson et al., 2013; Krebs et al., 2007) and may be an important risk factor for the disease.
Despite its involvement in these debilitating visual diseases, the mechanisms and mechanics of vitreoretinal adhesion are not well understood. The protein composition at the VRI has been investigated (Jerdan et al., 1989; Kohno et al., 1987; Russell et al., 1991), but has not been explicitly linked to the mechanics of the VRI. Further, vitreoretinal adhesion has not been directly measured until very recently (Creveling et al., 2018). There is a critical need for research focused on elucidating the mechanics of the VRI to address these limitations. Such research could identify region-dependent multiscale mechanisms across the VRI and evaluate how those mechanisms change or weaken with age. The strength of adhesion could be connected to the microstructure and offer insight into the best methodology to eliminate adhesion without compromising the underlying architecture of the retina. Further, a better understanding of the adhesion mechanisms could lead to imaging biomarkers to identify those at risk for lingering vitreoretinal adhesion.
In this review, we prepare for the development of this new field of research by evaluating existing literature on the VRI from the perspective of mechanics. We first evaluate the well-characterized microstructure and composition of the VRI and identify how each component contributes to structural integrity and mechanical adhesive strength. Then, we discuss the many pathologies affected by vitreoretinal adhesion and identify areas where knowledge of adhesion mechanics could accelerate the discovery of treatments for those diseases. Next, we explore microstructural mechanisms of adhesion in non-ocular biological tissues to identify potential mechanisms of adhesion at the VRI in light of existing experimental VRI data. Finally, we end the review with a discussion of the critical needs in the field and recommend future mechanics-based studies to address those needs.
2. Ocular Anatomy, Microstructures, and Compositions Related to Vitreoretinal Adhesion
2.1. Vitreous
The vitreous occupies 80% of the ocular volume, making it the largest structure in the eye (Sebag, 1998). The vitreous is between 98–99.7% water with a three-dimensional network of heterotypic collagen fibrils maintaining the gel-like structure (Bishop, 1996). These fibrils are randomly spaced and held apart by hyaluronan, otherwise known as hyaluronic acid (HA). HA is a glycosaminoglycan (GAG) that is hydrophilic and acts to inflate the vitreous, providing resistance to compression. The vitreous contains other proteins and GAGs (Bishop, 2000), but the mechanical properties and strength of the vitreous are primarily dictated by the concentrations and interactions of collagen and HA (Lee et al., 1994a, 1994b).
Vitreous collagen fibrils are heterotypic, composed primarily of type II collagen (80%) and type IX collagen (20%), with negligible amounts of type V/XI collagen (Bishop et al., 1994a). Bishop (1996) proposed a structure of vitreous collagen fibrils based on knowledge of the constituent collagen molecules (Figure 1). In this model, the typical vitreous collagen fibril is composed of a type V/XI collagen core, with surrounding type II and type IX collagen. Type V/XI collagen is a hybrid collagen molecule that is responsible for nucleating collagen fibril formation and regulating fibril diameter (Wenstrup et al., 2004b, 2004a). Type II collagen is a fibril-forming collagen bound to the outside of the type V/XI collagen core. Type IX collagen does not typically form fibrils, but can be found on the surface of fibrils of other collagens (Bishop et al., 2004), potentially mediating interactions between collagen fibrils. N-propeptides of type II and type v/XI collagen protrude from the surface of the fibril (Smith and Birk, 2012), which may allow the fibril to interact with the proteoglycans found in the vitreous (Sebag, 1998; Zhidkova et al., 1995).
Figure 1:
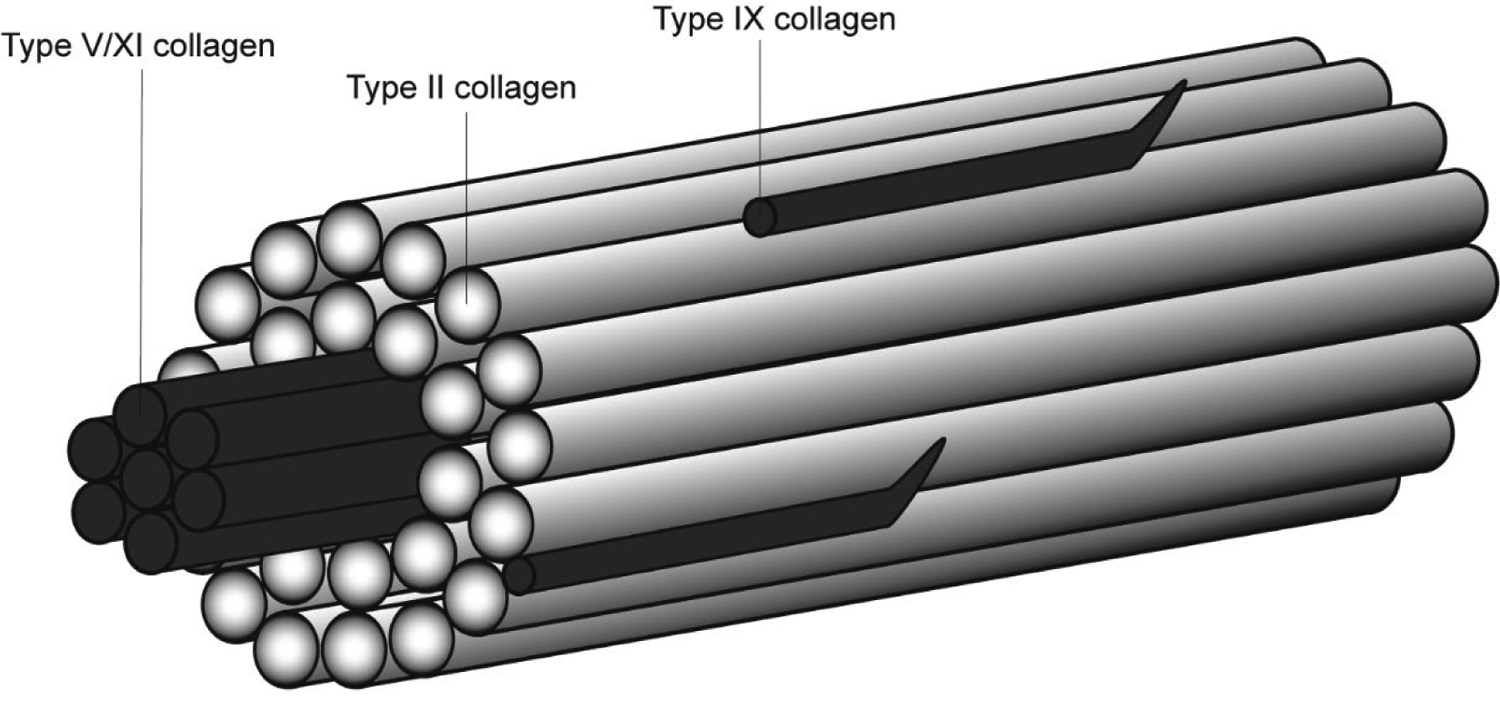
Proposed structure of vitreous collagen fibrils based on knowledge of the constituent collagen fibrils. This figure was adapted from Bishop (1996).
Evidence suggests that the total amount of vitreous collagen is constant through development, increases slightly in adolescence, and remains relatively constant into adulthood and advanced age (Balazs and Denlinger, 1984). Some believe vitreal collagen genesis ceases with age (Balazs and Denlinger, 1982), but there is evidence in adult bovine and human eyes to suggest vitreous collagen turnover continues into adulthood and is actively synthesized through aging (Bishop, 1996; Bishop et al., 1994b; Los et al., 2003; Ponsioen et al., 2005). If collagen is not actively synthesized in advanced age, collagen structure will naturally degrade and weaken the mechanics of the vitreous. However, if collagen is actively synthesized, the vitreal mechanics will remain fairly stable as long as the collagen network structure is unchanged. Therefore, clarification on this debate will be important to understanding and simulating changes in vitreous mechanics with age.
Collagen, however, is not the sole contributor to the mechanics of the vitreous. Vitreous collagen fibrils interact with HA to form a network characterized by widely separated fibrils. When the collagen fibrils are removed, a viscous solution remains (Comper and Laurent, 1978). If HA is removed from the network, the fibrils lose their spacing, and the vitreous shrinks and liquefies (Bishop et al., 1999). Changes in the material properties of the vitreous have been observed when HA is injected into (Schulz et al., 2019) or exuded from (Nickerson et al., 2008) the vitreous.
In a multi-part study, the rheological mechanical properties of the anterior, central, and posterior vitreous were quantified in bovine, porcine, and human eyes (Lee et al., 1994a, 1994b, 1992). Mechanical properties across the three species were compared to species-specific differences in concentrations of collagen and HA. It was concluded that the differences in collagen and HA among bovine, porcine, and human eyes did not explain the mechanical differences in rheological properties. However, when evaluating each species independently, regional trends in collagen and HA concentration correlated with regional trends in rheology. For example, in bovine and human vitreous, increasing HA concentration from anterior to posterior vitreous correlated with increasing viscosity from the anterior to posterior vitreous. In porcine eyes, increasing collagen concentration correlated with increasing porcine viscosity. No statistics were performed, but these data indicate that both collagen and HA contribute to the complex, rate-dependent, regional material properties of the vitreous.
Colter et. al. (2015) examined the age-dependent viscoelastic material properties of the vitreous in a sheep model, specifically quantifying the storage modulus, loss modulus, and tan δ. The storage modulus is a measurement of stored energy in a viscoelastic material and represents the material’s elastic response. The viscous portion of the material’s response representing lost energy during deformation is quantified by the loss modulus, and tan δ is the ratio of the loss modulus to the storage modulus. In adult eyes, the storage modulus decreased and tan δ increased compared to infant eyes, suggesting reduced elasticity with age. Similar changes have been noted when comparing adults younger than 60 years of age to those older than 60 years of age (Tram and Swindle-Reilly, 2018). More importantly, similar changes in viscoelasticity measurements have been reported following enzymatic degradation of vitreous collagen (Filas et al., 2014), suggesting that collagen network degradation may be a more important factor in age-related changes of the vitreous’ dynamic response than changes in HA. However, the contribution of collagen and HA to the material response were not measured directly. Future studies that directly measure the contribution of HA and collagen, and their interaction, to the mechanical properties of vitreous throughout development and aging will be valuable in designing vitreous substitutes or identifying strategies to prevent adverse effects of PVD.
2.2. Retina
The retina is a multi-layered structure, of which the inner limiting membrane (ILM) is the most superficial layer (Figure 2). The ILM forms the retinal portion of the VRI and is likely the most important retinal structure involved in vitreoretinal adhesion mechanics. To understand the mechanics of adhesion at the VRI, however, one must also understand the connections of the ILM to the underlying retinal structure, specifically to the Müller cells in the nerve fiber layer.
Figure 2:

The layers of the retina include the inner limiting membrane (ILM), the nerve fiber layer (NFL), the ganglion cell layer (GCL), the inner plexiform layer (IPL), the inner nuclear layer (INL), the outer plexiform layer (OPL), the outer nuclear layer (ONL), the outer limiting membrane (OLM), the photoreceptor layer (PL), and the retinal pigment epithelium (not shown).
Müller cell bodies reside within the inner nuclear layer of the retina. Their processes extend superficially to interact with the ILM as well as deeper into the retina to interact with the photoreceptors in the outer limiting membrane (Hoon et al., 2014). It is believed that Müller cells act like springs, providing essential tensile strength to hold the neural retina together. In vivo, the cells have been shown to be under tension and recoil upon being cut (Macdonald et al., 2015). The end feet of the Müller cells contain integrin cell surface receptors that mediate adhesion with the overlying ILM, or potentially with vitreous collagen fibrils that penetrate through the ILM, as reported in the vitreous base (Sarthy and Ripps, 2001; Wang et al., 2003a). This retinal organization results in a mechanosensing mechanism where tangential and normal stresses applied to the ILM can be relayed to other retina structures. To date, intracellular calcium, extracellular signal-regulated kinase, c-Fos, and basic fibroblast growth factor have been correlated to mechanical stimulation of Müller cells (Lindqvist et al., 2010). However, it’s likely that the expression of other Müller cell-derived proteins, such as vascular endothelial growth factor (VEGF), are altered as a function of mechanical loading and may have implications in the progression and treatment of diseases such as diabetic retinopathy, retinopathy of prematurity, and AMD. Further research in this area is needed to clarify the effect of vitreoretinal traction on the mechanotransduction of Müller cells.
2.3. Vitreoretinal Interface: Inner Limiting Membrane
The VRI consists of the vitreous cortex and the retina ILM. The ILM is a 3-layered structure resembling a typical basement membrane (Heegaard, 1997; Matsumoto et al., 1984), and is often considered the basement membrane of the Müller cells (Sebag, 1992). The ILM consists of a lamina rara externa, which borders the vitreous cortex. The middle layer is the lamina densa. The lamina densa is an electron dense matrix composed primarily of collagen, which appears as a dark, thick line in transmission electron microscopy (TEM) images. The third layer is the lamina rara interna, or lamina lucida, adjacent to the endfeet of Müller cells. There is some debate as to whether the lamina lucida is an actual layer of the ILM or an artifact of microscopy preparation. Basement membranes in the trachea and epidermis have been shown to have lamina lucida when rapid dehydration was used and no lamina lucida when slow dehydration was used (Chan et al., 1993). A similar result has been observed in muscle fibers (Goldberg and Escaig-Haye, 1986). Heegaard (1997) reported that the lamina rara interna appeared only sporadically in his TEM images of non-human primate and human ILM, which supports the theory that this third layer is a microscopy artifact.
The vitreous side of the ILM is typically smooth, while the retinal side of the ILM conforms to the underlying Müller cells and contains irregular undulations. The undulations become increasingly irregular in the posterior pole, leading to a greater variation in ILM thickness (Figure 3) (Creveling, 2021; Foos, 1972). The ILM provides a physical barrier to macromolecules (Jackson et al., 2003) and is thought to anchor the vitreous cortex to the retina through mechanisms that are not known (Balazs, 1973). In the vitreous base, scanning electron microscopy of the VRI shows vitreous collagen penetrating through the ILM (Wang et al., 2003a). These penetrations visually appear as thick braids of fibrils that splay out beneath the ILM to form a web of thin fibrils. In younger eyes, these splays are relatively sparse. With age, the splays progress into a dense mat beneath the ILM. The penetrating and splaying fibers likely provide structural resistance to vitreoretinal separation. It is unknown if there are chemical adhesive components or additional mechanisms that contribute to the strong vitreoretinal adhesion at the vitreous base.
Figure 3:

TEM images of the equator (A) and posterior pole (B) retina from a 78-year-old donor. The ILM (red bracket) is relatively uniform in the equator, while the posterior ILM displays characteristic undulations and irregularities. Here, the lamina lucida (cyan arrows) appears in the equator, but not in the posterior pole.
Focal densities on the surface of Müller cells, known as ‘attachment plaques,’ are another potential mechanism of adhesion between the vitreous and the retina. TEM has illustrated dark spots beneath the ILM at the surface of Müller cells (Figure 4) (Foos, 1972; Heegaard, 1997; Sarthy and Ripps, 2001; Sebag, 1991). These attachment plaques are present in the vitreous base and equator, but are absent from the posterior pole except in the fovea (Foos, 1972). Attachment plaques in the vitreous base appear to coincide with an increased number of delicate fibrils traversing the ILM (Foos, 1972), but this has not been observed in the equator. The ultrastructure of these plaques, and their location at the surface of Müller cells where integrin receptors exist (Elner and Elner, 1996; Guidry et al., 2003), suggests they may be hemidesmosomes. Hemidesmosomes are known for their role in adhesion in basement membranes and contribute to the adhesion of the corneal epithelium to the stroma (Chi and Trinkaus-Randall, 2013; Gipson, 1992; Madigan and Holden, 1992; Theocharis et al., 2016; Zagon et al., 2001). While the functionality of attachment plaques in the retina has not been explicitly studied, their association with strong regions of vitreoretinal adhesion in the eye suggests they may indeed be hemidesmosomes and contribute to regional differences in adhesion at the VRI.
Figure 4:

TEM image of a retina from the equator of a 69-year-old donor with multiple attachment plaques (cyan arrows).
In addition to the regional variations in the number of attachment plaques, regional variations with respect to ILM thickness have been observed that may have implications for vitreoretinal adhesion (Creveling, 2021; Foos, 1972; Heegaard, 1997). The ILM is thinnest at the vitreous base and thickens towards the posterior pole. The ILM thins again in the fovea and at the optic nerve (Foos, 1972). Creveling (2021) captured this trend using TEM. Specifically, he found that the thickness of the ILM in the posterior pole (1165.3±597.6 nm) was 3.8 times greater than in the equator (304.8±151.3 nm). The variability of ILM thickness was greater in the posterior pole due to the increasingly undulated ILM as it conforms to the uneven surface of the Müller cells on its retinal side. In both the equator and the posterior pole, the ILM was significantly thinner in donors older than the age of 60 compared to younger than the age of 60 (Creveling, 2021). These age-related thickness measurements reflect other previously reported trends in or near the macula (Heegaard, 1997), but differ from measurements made superior to the optic nerve in the peripapillary region (Candiello et al., 2010). There are distinct structural differences in the ILM between the macular sub-regions and the peripapillary regions (Matsumoto et al., 1984), which likely influence ILM thickness changes with age. Importantly, these sub-regional differences in ILM structure and thickness highlight potential variability in adhesion mechanisms and mechanics within the sub-regions of the posterior pole.
2.4. Vitreoretinal Interface: Vitreous Cortex
Darkfield slit illumination of whole vitreous reveals collagen fibers running predominantly in an antero-posterior direction (Sebag and Balazs, 1989). This suggests that vitreous fibers are generally perpendicular to the retinal surface in the vitreous base and macula, and parallel to the retinal surface in the equator. However, the VRI consists of a dense packing of collagen fibrils, called the vitreous cortex, which encapsulates the vitreous and interfaces with the ILM. The vitreous cortex has an organizational pattern unique from the fibers in the vitreous body. On high magnification TEM, collagen fibrils at the vitreous base can be described as numerous and often oriented perpendicular to the ILM (Foos, 1972). However, at lower TEM magnifications, collagen fibers have been described as primarily parallel to the VRI (Wang et al., 2003a). To our knowledge, quantification of the fiber angles in the vitreous base has not been performed at any magnification.
Similar discrepancies in the literature exist surrounding the collagen fiber orientation in the equator and posterior pole. Some studies have reported that fibers run primarily parallel to the ILM in the equator (Foos, 1972), but other studies have observed no differences in fibril angle relative to the ILM in the equator or posterior pole (Creveling, 2021; Heegaard, 1997). In the only quantitative study to measure fibril angle, there were no significant differences between the equator and posterior pole quantified by segmentation of TEM images from human donors (Creveling, 2021). Specifically, collagen fibrils in the posterior pole were normally distributed about 30° with a range of 9–60° and collagen fibrils in the equator had a mean of 29° with a range of 13–71° (Figure 5).
Figure 5:
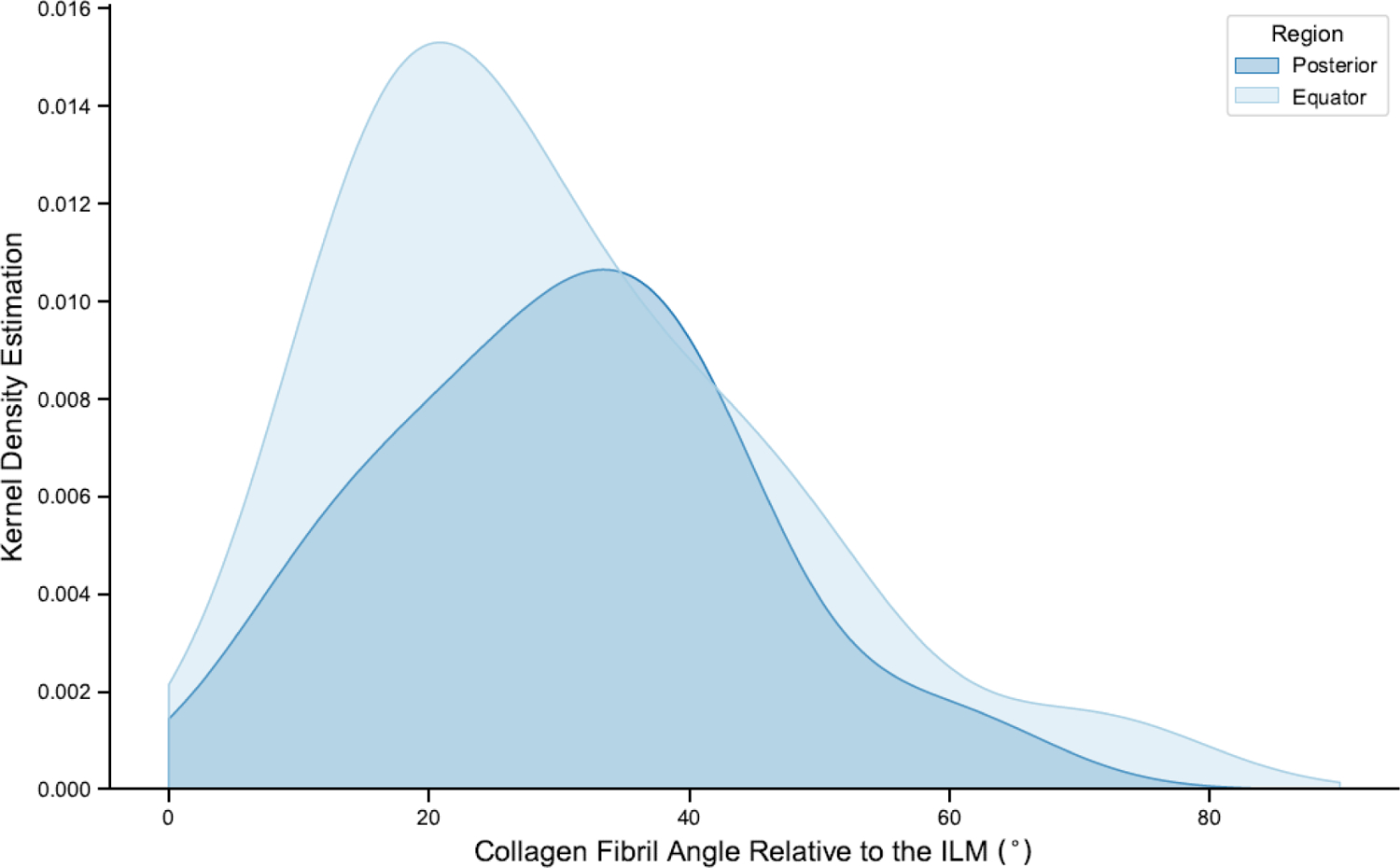
Creveling (2021) reported no significant difference between collagen fibril orientation in the posterior pole and equator.
2.5. Similarities and Differences with Species
The vitreous of all mammalian species is composed of the same protein and GAG components, but the relative concentrations of HA and collagen vary (Table 1) (Sebag, 1998). One would expect these relative component concentration variations to account for the differences in rheologic properties between species; however, as previously discussed, no significant correlation between HA or collagen concentration with rheological properties across cow, pig, and human vitreous was found (Lee et al., 1994a, 1994b, 1992). Still, within each species, decreased collagen or HA concentrations generally correlated with decreased viscosities. These data suggest that there may be other chemical or structural differences between species that contribute to differences in material properties between species.
Table 1:
HA and collagen concentration in vitreous for humans and common animal models.
| Species | HA Concentration (μg/ml) | Collagen Concentration (μg/ml) | Source |
|---|---|---|---|
| Human | 100–400 | 40–120 | (Balazs and Denlinger, 1984) |
| Sheep | 100–400 | 67–82 | (Balazs and Denlinger, 1984) |
| Rabbit | 20–50 | 75–900 | (Balazs and Denlinger, 1984) |
| Cat | 20–40 | - | (Balazs and Denlinger, 1984) |
| Pig | 90±4 | 150±8 | (Noulas et al., 2002) |
| Cattle | 110–1070 | 52–112 | (Balazs and Denlinger, 1984) |
HA is not uniformly distributed across the vitreous in humans or other mammals. Specifically, higher concentrations of HA are found in the posterior pole (Bishop, 1996; Lee et al., 1994b) compared to the equator or vitreous base. Additionally, HA concentration does not remain constant throughout life (Balazs et al., 1959). In humans, HA concentration increases with age until 20 years, then remains relatively constant until 70 years, where it again increases, possibly as a result of the shrinking vitreous due to aging (Balazs and Denlinger, 1982). Collagen concentration is not uniformly distributed in humans either, with the highest concentrations found in the vitreous base and decreasing towards the central and posterior vitreous (Bishop, 1996).
All vertebrates share the same fundamental components and functions of the retina (Hoon et al., 2014), and the human ILM has a similar arrangement to that of most animals (Heegaard, 1997). Primates are the only species to mimic the inter-regional differences of ILM thickness and undulations in the macula of humans (Heegaard, 1997). Species-specific structural differences, such as ILM thickness and undulation, likely affect vitreoretinal mechanics. For example, in humans, peeling the ILM around the macula as a continuous sheet is feasible, but attempts to remove the ILM as a single sheet in the macula of a cat model was unsuccessful and resulted in intraretinal bleeding (Gandorfer et al., 2005). Similarly, vitreoretinal adhesion measurements in young adult sheep eyes (human age equivalence of 28–36 years old, 16.7±7.5 mN) were on average 2.3 times greater than in human young adult donor eyes (30–39 years old, 7.2±4.1 mN) (Creveling et al., 2018). Despite the species-related differences in magnitudes of adhesion, the regional trends within each species were the same. Specifically, in both human and sheep eyes, the vitreoretinal adhesion in the equator was double the adhesion in the posterior pole. This suggests that sheep eyes may be useful to make comparisons by region.
3. Pathologies Affected by Vitreoretinal Adhesion
3.1. Posterior Vitreous Detachment (PVD)
With age, the vitreous gel begins to liquefy and separate from the retina. Vitreous liquefaction first appears in human eyes as early as four years of age (Balazs and Denlinger, 1982). At least half of the vitreous is liquefied in most people over 70 years of age. Accelerated vitreous liquefaction may lead to adverse outcomes, and may occur in people with myopia (Stirpe and Heimann, 1996), aphakia (Harocopos et al., 2004), intraocular inflammation (Hogan, 1975), retinal vein occlusion (Ma et al., 2005), and following ocular trauma (Tolentino, 1987). There is some debate over the cause of vitreous liquefaction. Bishop (2000) proposed that the vitreous liquefies due to a loss of interaction between HA and collagen, allowing HA to diffuse away from the collagen network and pool, creating liquefied pockets. This theory is supported by the chemical composition of the liquefied pockets in vitreous, which consist predominantly of HA (Balazs and Denlinger, 1984). Los et al. (2003) found evidence of collagen fibril breakdown into smaller fragments surrounding the liquefied pockets, which may further contribute to the liquefaction process. The molecular changes that initiate liquefaction are still unclear. Clarification of the mechanisms of liquefaction will improve understanding of PVD and other disorders affected by vitreoretinal traction.
As the vitreous gel begins to shrink and liquefy, one of two events may occur. The vitreous begins to detach from the retina, starting in the mid-periphery (the area between the posterior pole and equator) and progressing toward the posterior pole (Koller et al., 2021; Tsukahara et al., 2018). Nearly all healthy individuals have vitreoretinal separation in the mid-periphery by age 40 (Tsukahara et al., 2018), and 80% of healthy individuals have vitreoretinal separation from the posterior pole by age 70 (Itakura and Kishi, 2013). However, when vitreous liquefaction occurs before sufficient weakening of vitreoretinal adhesion, the vitreous does not separate completely from the posterior retina, resulting in localized traction on the retina from lingering adhesions that can induce retinal hemorrhages, tears, and macular holes (Balazs, 1973; Foos, 1972).
It is not clear why vitreoretinal adhesion weakens with aging or why some lingering adhesions persist long after the vitreous has separated. The contact area of lingering vitreoretinal adhesions affects the risk for adverse outcomes. Specifically, a smaller area of lingering adhesion in the fovea may result in greater stress and deformation, and increased risk of retinal damage (Spaide et al., 2002). A better understanding of the adhesion mechanisms and mechanics could provide insight into why lingering adhesions occur and lead to novel strategies to prevent adverse outcomes during PVD.
3.2. Age-related Macular Degeneration (AMD)
AMD is the leading cause of irreversible blindness in developed countries in people 50 years of age or older (Eagle Jr., 2016). The initial stage of AMD is called non-exudative AMD, or dry AMD, and consists of atrophy of the retinal pigment epithelium (RPE) beneath the fovea and photoreceptor degeneration. The disease progresses to exudative AMD, or wet AMD, characterized by choroidal neovascularization. Choroidal neovascularization, the leaking of fluid, lipids, and blood, ultimately results in fibrous scarring of the macula and vision loss (Krebs et al., 2007; Lim et al., 2012). Lingering vitreomacular adhesions due to partial PVD are more prevalent in wet AMD patients than dry AMD patients (Jackson et al., 2013; Krebs et al., 2007). Therefore, lingering vitreomacular adhesions may exacerbate the progression of AMD from non-exudative to exudative.
Vitreomacular adhesions have been shown to negatively affect the outcome of anti-VEGF treatments (Krishnan et al., 2015). This may be due to the release of VEGF in response to mechanical stimulation from the adhesion, counteracting the anti-VEGF treatment. Cyclic stretching of rodent retinal pigment epithelial cells in vitro led to a significant increase in VEGF expression compared to controls after one hour of 15% pulsatile stretching, and after 24 hours of 10% pulsatile stretching (Seko et al., 1999). These data support the hypothesis that vitreomacular adhesion may promote exudative AMD by increasing VEGF expression. Understanding the mechanics of adhesion in this population and eliminating localized vitreomacular adhesions may be a solution to mitigate escalation from dry to wet AMD (Furashova and Engelmann, 2020; Mojana et al., 2008).
3.3. Vitreomacular Traction
In addition to its potential role in the progression of AMD, vitreomacular traction has many other clinical implications. Tractional forces exerted on the macula can cause decreased vision, metamorphopsia, photopsia, and micropsia (Maumenee, 1967). Vitreomacular traction has been associated with other pathologies, including cystoid macular edema, epiretinal membranes, and macular holes (Bottós et al., 2012b). Two patterns of pathology have been identified in vitreomacular traction syndrome (Gandorfer et al., 2002). The first pattern consists of a cellular monolayer covering the vitreal side of the ILM and no detectable epiretinal membrane. The second consists of fibrocellular tissue separated from the ILM by a layer of native vitreous collagen resembling the clinical features of an idiopathic epiretinal membrane. Symptoms of vitreomacular traction syndrome typically develop slowly, resulting from chronic tractional effects (Bottós et al., 2012a). In many cases, the symptoms are minor and require no treatment, or they may resolve spontaneously with the onset of PVD (Errera et al., 2018). A better understanding of the region-specific mechanisms of vitreoretinal adhesion may guide bio-imaging studies to identify patients at risk for traction-related disorders.
3.4. Other Vitreoretinal Pathologies
Many other vitreoretinal pathologies, such as diabetic macular edema, retinal vein occlusion, and macular holes are negatively affected by vitreoretinal adhesions either directly through traction or indirectly through the potential release of VEGF as a result of traction (Jackson et al., 2013; Patronas et al., 2009; Seko et al., 1999). Macular holes may be considered a variant of vitreomacular traction syndrome and develop when anteroposterior traction on the retina creates a full-thickness neurosensory hole (Gass, 1995). Additionally, epiretinal membranes may form as a result of vitreomacular traction as well as retinal vascular disease, retinal breaks and detachments, inflammation, and surgical interventions. Epiretinal membranes are associated with the pathogenesis of many other interface diseases, but are typically asymptomatic.
In summary, several of the most severely impacting visual disorders are created or worsened through lingering vitreoretinal adhesion. There is a definitive knowledge gap in the underlying mechanisms and mechanics of vitreoretinal adhesion in multiple regions of the eye and at different stages of life that may be inhibiting the advancement of innovative treatments for these diseases. Quantifying the contribution and interaction of key proteins in the mechanics of adhesion will assist in establishing imaging biomarkers suggestive of strong adhesion and lead to more targeted therapeutic strategies to eliminate lingering adhesions.
4. Experimental and Computational Studies of Vitreoretinal Mechanics
4.1. Experimental Measurement of Vitreoretinal Adhesion
Very little data has been collected to measure the strength of adhesion in different regions of the eye and at different ages. Sebag (1991) peeled the retina from the ILM in the vitreous base and posterior pole in 59 human eyes (ages 33 weeks gestation to 94 years of age) using forceps. After peeling, retina samples were evaluated using TEM to visualize the failure and estimate strength. In the posterior pole, all eyes from individuals 21 years of age and older failed cleanly between the ILM and the posterior vitreous cortex. Most of the eyes tested in this age group came from individuals older than 41 years of age. In 40% of eyes from individuals aged 20 years or younger, the retina failed within the Müller cells adjacent to the VRI. The inner portions of these cells were found adherent to the ILM, which remained attached to the vitreous cortex. Sebag suggested the deeper failure location indicated that the adhesive strength was greater in the younger eyes than the older ones.
A recent study by Creveling et al. (2018) directly measured vitreoretinal adhesion in the equator and posterior pole in sheep and human eyes of different ages. They assessed ILM damage by light microscopy. As previously observed qualitatively (Sebag, 1987), the maximum peel force in human eyes was significantly greater in the equator (7.2 ± 4.1 mN) than in the posterior pole (4.1 ± 2.0 mN), a trend that was especially evident in eyes from donors 30 to 39 years old. Regional maximum peel forces in adult sheep mimicked their human counterparts and had nearly double the amount of adhesion force in the equator (16.7 ± 8.5 mN) than the posterior pole (8.5 ± 2.4 mN).
Adhesion between the neurosensory layer and RPE is thought to be weaker than vitreoretinal adhesion in young, healthy eyes. To date, there are no quantitative measurements of RPE adhesion in human eyes to compare to the vitreoretinal adhesion data. Kita and Marmor (1992) performed subretinal injections to estimate RPE adhesion in young adult primates. They report average posterior RPE adhesive forces of 140±3 dynes/cm. Without knowing the bleb circumference, it is challenging to compare their measurements to Creveling et al. However, Kita and Marmor state that adhesive forces were 140% greater than their previous study in rabbits (Kita et al., 1990). Therefore, we estimate the posterior RPE adhesion in primates was 2.8±0.3 mN, which is nearly 1.5 times lower than average posterior adhesion in humans reported by Creveling. These reported values provide confidence in the vitreoretinal measurements, but may not be directly comparable to humans.
Creveling et al. report ILM disruption in the posterior pole, similar to Sebag. However, the ILM disruption was not correlated to the measured adhesive strength. Specifically, the ILM in the posterior pole was consistently disrupted and torn, but had lower adhesion forces than the equator, which had smooth separation between the ILM and vitreous cortex (Figure 7). Therefore, failure characteristics are likely a function of the mechanism of adhesion, and not necessarily correlated to the strength of the adhesion.
Figure 7:

Light microscopy images from the peeled retina of a 63-year-old human donor. (A) In the posterior pole, peeling resulted in ILM disruption. (B) In the equator, peeling resulted in a smooth, intact ILM. Magnification 20x.
Researchers have described strong adhesion in the vitreous base (Sebag, 1987), macula (Grignolo, 1952), and the optic disc (Foos, 1973; Grignolo, 1952), all regions with relatively thin ILM. Sebag hypothesized that these thin ILM regions have stronger adhesion than other regions because a thicker ILM may interfere with Müller cells’ ability to synthesize components critical for maintaining adhesion integrity (Sebag, 1991). While Creveling et al. did not measure adhesion directly in the macula, around the optic nerve, or in the vitreous base, this hypothesis matches the measurements made by Creveling et al. that adhesion in the equator was greater than in the posterior pole, and the ILM is thinner in the equator than in the posterior pole.
The regional dependence of vitreoretinal adhesion and the varying structure in those regions suggests that the mechanism of adhesion may be unique to each region. In addition to variation in the vitreous base, equator, and posterior pole, there are likely additional variations in adhesion in the small foveal, parafoveal, and perifoveal subregions of the macula. Therefore, future studies will likely need to rely on microscale, or even nanoscale, experimental testing such as atomic force microscopy (Strasser et al., 2007; Van Der Rijt et al., 2006), or optical or magnetic tweezers (Aermes et al., 2020; Herath et al., 2017; Shawky and Davidson, 2015; Sun et al., 2004) to capture the heterogeneity of vitreoretinal adhesion across the retina. An in-depth evaluation of these methods is outside the scope of this review, but future experimental challenges will include the creation of innovative methods for gripping collagen fibers within the gel-like vitreous and validation of methods for estimating stress distribution on the ILM. Further, interpretation of data collected will likely rely on a basic understanding of how adhesion is formed in these microscale interactions.
4.2. Computational Studies of the Vitreoretinal Interface
In addition to experimental studies evaluating vitreoretinal adhesion, mathematical and computational models of the eye have led to improved understanding of mechanics of the vitreous, retina and their interface. Examples include models evaluating saccadic movement (David et al., 1998), eye rotations (Bonfiglio et al., 2015; Repetto et al., 2004), retinal detachment (Bottega et al., 2013; Lakawicz et al., 2015), and eye trauma (Bhardwaj et al., 2014; Rangarajan et al., 2009; Rossi et al., 2011; Yoshida et al., 2014). The paucity of data of vitreoretinal adhesion mechanics during development of these models has resulted in the general assumption that the vitreous is rigidly or uniformly attached to the retina. For some models, such as vitreous flow during eye motion, this assumption may be allowable. However, for others, such as those simulating eye trauma or retinal detachment due to vitreous contraction, this assumption may limit the results.
To date, very few computational studies exist that focus on the mechanics of the VRI. Di Michele et al. (2020) created a computational framework to model visually realistic results of an anomalous PVD and infer the intensity of traction from the shape of the detached vitreous. A dimensionless parameter representing the strength of vitreoretinal adhesion was varied in the absence of experimental data. It was found that the higher strengths of adhesion resulted in higher stresses experienced at the VRI, and the shape of the detached vitreous provided an indication of the magnitude of vitreomacular stress.
In development of a method to represent regional non-rigid adhesion of the vitreous to the retina, Creveling (2021) used cohesive element modeling to optimize three-dimensional adhesion modulus (Ks, Kt, and Kn), failure stress (ts, tt, and tn), and fracture energy against experimental peel measurements. The vitreoretinal adhesion modulus was insensitive to region and age-related differences in adhesion. This insensitivity is likely because each model was tuned to match vitreous elasticity prior to initiation of damage and this elasticity likely encompassed both vitreous and adhesive elasticity. Normal failure stress did not significantly correlate to maximum peel force, but both shear failure stresses (ts and tt) had positive correlations with maximum peel force and were 594.02 ± 398.20 Pa on average. While thresholds tended to decrease in simulations of eyes from donors greater than 63 years of age, no statistically significant age and region effects were found. The fracture energy was the failure parameter that most significantly correlated with maximum vitreoretinal adhesion for each region and age. Specifically, simulations of peel tests performed in the equator with eyes from donors less than 60 years of age had higher fracture energies (2.2±0.2 × 10−4 J) than the posterior pole and eyes greater than 60 years of age (9.6±15.2 × 10−6 J).
These studies highlight the potential of computational modeling to provide significant insights into vitreoretinal disorders and injuries. However, more experimental data is needed to improve the biofidelity and advance the predictive capability of the models. Once that data is made available, mathematical and numerical analyses will be able to contribute substantially to the advancement and understanding of PVD, AMD, vitreomacular adhesion, trauma and many other vitreoretinopathies.
5. Potential Mechanisms of Vitreoretinal Adhesion
The above experimental and computational studies illustrate age and region-specific differences in vitreoretinal adhesion and failure. However, the precise mechanisms of adhesion at the VRI remain unclear. Several possibilities emerge when considering the biochemistry of the interface and adhesion mechanisms in other tissues. Interfacial adhesion in biological tissues is achieved mainly through complex networks of fibrous proteins, elastin, fibronectin, laminins, glycoproteins, proteoglycans, and GAGs called the extracellular matrix (ECM). The ECM can be divided into two types: the interstitial matrix, characterized by cells dispersed within the matrix, and the pericellular matrix, which comes into contact with a cell or tissue layer (Theocharis et al., 2016). Basement membranes are multi-layered membranes found at tissue interfaces and are considered pericellular matrices.
Basement membranes, such as the ILM, are composed of a laminin network that adheres to the underlying cells and a type IV collagen network that stabilizes the matrix structure. Other proteins and proteoglycans, such as fibronectin, agrin, and perlecan have also been identified in the ILM (Candiello et al., 2007; Halfter et al., 2008, 2000; Kohno et al., 1987). Laminins are large, heterotrimeric proteins that interact with other laminins, cells, or ECM components, such as collagen to contribute to the organization and mechanical strength of the tissue (Durbeej, 2010). Laminin has been shown to have a high affinity for type IV collagen (Terranova et al., 1980), which is prominent in the ILM (Ponsioen et al., 2008). Fibronectin is a glycoprotein dimer that serves as a linker in the ECM and binds to cells via integrin receptor domains (Pankov and Yamada, 2002). Fibronectin has been shown to have a high affinity for binding to type II and VI collagen (Engvall et al., 1978; Kuo et al., 1997; Ruoslahti et al., 1982), found in the vitreous and ILM, respectively (Bu et al., 2015).
Laminin and fibronectin are commonly implicated in vitreoretinal adhesion, particularly in the posterior pole and equator, and exhibit unique regional and age-related distributions that may correspond with the region and age-related distributions of adhesion. In the posterior pole of young (<40 years of age) donor eyes, laminin and fibronectin are distributed throughout the thickness of the ILM (Kohno et al., 1987). Given the high affinity of fibronectin for type II collagen, one proposed hypothesis for vitreoretinal adhesion in this region is that type II collagen fibers from the vitreous cortex penetrate into the ILM to bind to fibronectin. TEM images have shown individual vitreal collagen fibrils penetrating the ILM (Creveling, 2021; Foos, 1972; Heegaard, 1997), but no studies have directly evaluated fibril penetrations as a mechanism of adhesion. The penetration of fibrils into the ILM may explain the observations of ILM disruption following vitreoretinal separation in the posterior pole (Creveling et al., 2018; Sebag, 1991).
In the equator of young (<40 years of age) donor eyes fibronectin is absent. Laminin, however, is strongly present on the surface of the ILM. This supports the theory of equatorial adhesion being formed by a type of ‘ECM glue’ between the vitreous cortex and ILM, and may result in strong adhesion through increased contact area compared to collagen fibril penetration. It also corresponds with the smooth surface separation between the ILM and vitreous cortex upon peeling (Creveling et al., 2018). Laminin, however, likely requires a mediator to bind strongly to type II collagen. Le Goff and Bishop (2008) proposed opticin and heparan sulphate as potential mediators, but no studies have evaluated the role of these proteoglycans in vitreoretinal adhesion.
The role of fibronectin and laminin in vitreoretinal adhesion in the equator and posterior pole is supported further by Gandorfer et al. (2001). In this study, plasmin, an enzyme known to hydrolyze fibronectin and laminin, was injected into pig eyes using different concentrations and incubated for different periods of time. Eyes injected with the smallest dose of plasmin (1 U) and incubated for the shortest amount of time (30 minutes) only had remnants of cortical vitreous remaining in the posterior pole and the equator. The vitreous base did not appear affected by the plasmin. These data suggest laminin and fibronectin facilitate adhesion in the posterior pole and equator, but not in the vitreous base. However, the degree of enzymatic reaction between the plasmin and the laminin/fibronectin was not quantified, so it is not explicitly known how fibronectin and laminin were altered in each region when plasmin was injected. A later study was conducted by the same group in human eyes, and similar results were found (Gandorfer et al., 2002). A careful investigation of the how plasmin alters laminin and fibronectin when injected into the eye is important to understand the contributions of each protein to adhesion the equator and posterior pole.
Vitreoretinal adhesion in the vitreous base is less ambiguous than adhesion in the posterior pole and equator. SEM and TEM studies have illustrated that thick collagen fiber bundles from the cortex penetrate through the ILM in the vitreous base and splay underneath (Gloor and Daicker, 1975; Wang et al., 2003b). The penetrating and splayed fiber bundles likely anchor the vitreous to the retina, similar to adhesion in amniotic epithelium (Ockleford et al., 2013). The protein interactions that bind collagen fiber bundles to the retina are unknown, but data suggests that laminin and fibronectin do not play a substantial role. In the ILM at the vitreous base, laminin is present and fibronectin absent. With age, laminin decreases and fibronectin increases (Kohno et al., 1987). Despite these changes with age, adhesion in the vitreous base appears to remain strong. Further, injection of plasmin did not alter the VRI in the vitreous bases as it did in the equator and posterior pole (Gandorfer et al., 2002).
All aspects of vitreoretinal mechanics are important for developing computational models and understanding vitreoretinal pathology and disease progression. However, understanding the mechanisms of adhesion in the posterior pole and equator may have the most immediate clinical impact. Specifically, they can lead to strategies to modulate problematic adhesion such as that found in vitreomacular traction or PVD. In addition, proactive modulation of adhesion could be beneficial to inhibit disease progression, such as in AMD where VEGF production may be stimulated by vitreoretinal traction. Any studies investigating the modulation of adhesion, however, would need to be coupled with mechanical testing to verify successful modulation.
6. Conclusion and Future Studies
From the available experimental data, we conclude that mechanisms of vitreoretinal adhesion are region-dependent. The primary adhesion mechanisms in the posterior pole appear to be different from those in the equator and vitreous base. It is likely that the macula also has a unique adhesion mechanism, but there is no data to date to verify this statement or suggest what that mechanism may be. The specific region-dependent adhesion mechanisms need to be clarified to understand the regional variations in vitreoretinal adhesive strength measured by Creveling et al. (2018). In the basal zone, vitreous collagen fiber bundle penetration likely plays an important role in the strong adhesive strength. In the equator, we hypothesize that sheet-like laminin networks provide the primary source of adhesion between the vitreous cortex and ILM, but other proteins may mediate that adhesion. In the posterior pole, the presence of fibronectin throughout the ILM may suggest that collagen penetrates the ILM. This collagen penetration may explain why Creveling et al. observed increased disruption of the ILM in the posterior pole after mechanical peeling. In contrast, traction in the equator may peel the laminin networks from the ILM, resulting in mostly smooth separation.
There are minimal quantitative data available to elucidate vitreoretinal adhesion mechanics with age. Data by Creveling et al. (2018) confirms that adhesion in the equator decreases with age. Creveling et al. did not find a significant decrease in adhesive strength in the posterior pole with age, but noted increased ILM disruption. Bilamination of laminin and fibronectin within the ILM in the posterior pole may contribute to the disruption and weakening of the ILM with age. Microscale studies are needed to evaluate and further clarify failure mechanisms at the VRI, identify weak links within the architecture, and quantify how the strength of all the components varies with age.
The cause of lingering adhesions in PVD remains unclear. Kohno et al. (1987) found no significant difference in the distribution of laminin and fibronectin in age-matched eyes with and without PVD. The mechanism of vitreoretinal separation or failure in eyes with and without lingering adhesions during PVD will be necessary to identify microstructural differences. This effort will require a combination of microscale imaging and mechanical testing to correlate the structural and mechanical differences, respectively.
Once the mechanisms of adhesion, or lingering adhesion, are known, new opportunities for identifying the risk of lingering vitreoretinal adhesion and treating it, can be developed. For example, understanding the microstructure of lingering vitreoretinal adhesion can lead to imaging biomarkers to identify patients at risk for lingering vitreoretinal adhesion. Closer follow-up or treatment can be implemented to prevent retinal tears or detachment. Physical or pharmacological treatments targeting the specific proteins driving lingering adhesions can be developed to effectively eliminate adhesions with minimal disruption to the underlying retina. Finally, understanding adhesion mechanisms will allow focused and realistic experimental efforts to link traction forces to the upregulation of VEGF or other proteins that exacerbate diseases such as AMD or lead to epiretinal membranes. With careful collaboration between clinical scientists and researchers, vitreoretinal mechanics is necessary to achieve all these goals and make a significant contribution to reducing debilitating vision loss in patients.
Acknowledgments
Research reported in this review was supported by the National Eye Institute of the US National Institutes of Health under award number R21EY025813. The authors would like to acknowledge the Utah Lions Eye Bank for human tissue donation and the Dr. Kurt Albertine Lab for sheep eye donation. Electron microscopy was performed at the University of Utah Electron Microscopy Core Laboratory. Financial support for authors E. Hwang and D. Morgan during generation of this review article include the National Institutes of Health Core Grant (EY014800), an unrestricted grant from Research to Prevent Blindness, the Alsam Foundation, and the Knights Templar Eye Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of these supporting organizations.
Footnotes
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aermes C, Hayn A, Fischer T, Mierke CT, 2020. Environmentally controlled magnetic nano-tweezer for living cells and extracellular matrices. Sci. Rep 10, 1–17. 10.1038/s41598-020-70428-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs EA, 1973. The Vitreous. Int Opthalmol Clin 13, 169–187. [PubMed] [Google Scholar]
- Balazs EA, Denlinger JL, 1984. The Vitreus, in: Davson H (Ed.), The Eye. Academic Press, London, pp. 533–589. [Google Scholar]
- Balazs EA, Denlinger JL, 1982. Aging Changes in the Vitreus, in: Sekuler R, Kline D, Dismukes K (Eds.), Aging and Human Visual Function. pp. 45–57. [Google Scholar]
- Balazs EA, Laurent TC, Laurent UBG, 1959. Studies on the structure of the vitreous body VI. Biochemical changes during development. J. Biol. Chem 234, 422–430. [PubMed] [Google Scholar]
- Bhardwaj R, Ziegler K, Seo JH, Ramesh KT, Nguyen TD, 2014. A computational model of blast loading on the human eye. Biomech. Model. Mechanobiol 13, 123–140. 10.1007/s10237-013-0490-3 [DOI] [PubMed] [Google Scholar]
- Bishop P, 1996. The biochemical structure of mammalian vitreous. Eye 10, 664–670. 10.1038/eye.1996.159 [DOI] [PubMed] [Google Scholar]
- Bishop PN, 2000. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog. Retin. Eye Res 19, 323–344. [DOI] [PubMed] [Google Scholar]
- Bishop PN, Crossman MV, McLeod D, Ayad S, 1994a. Extraction and characterization of the tissue forms of collagen types II and IX from bovine vitreous. Biochem. J 299, 497–505. 10.1042/bj2990497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop PN, Holmes DF, Kadler KE, McLeod D, Bos KJ, 2004. Age-related changes on the surface of vitreous collagen fibrils. Investig. Ophthalmol. Vis. Sci 45, 1041–1046. 10.1167/iovs.03-1017 [DOI] [PubMed] [Google Scholar]
- Bishop PN, Mcleod D, Reardon A, 1999. Effect of Hyaluranon Lyase, Hyaluronidase, and Chondroitin ABC Lyase on Mammalian Vitreous Gel. Investig. Ophthalmol. Vis. Sci 40, 2173–2177. [PubMed] [Google Scholar]
- Bishop PN, Reardon AJ, Mcleod D, Ayad S, 1994b. Identification of Alternatively Spliced Variants of Type II Procollagen in Vitreous. Biochem. Biophys. Res. Commun 203, 289–295. 10.1006/BBRC.1994.2180 [DOI] [PubMed] [Google Scholar]
- Bonfiglio A, Lagazzo A, Repetto R, Stocchino A, 2015. An experimental model of vitreous motion induced by eye rotations. Eye Vis. 2, 1–10. 10.1186/s40662-015-0020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottega WJ, Bishay PL, Prenner JL, Fine HF, 2013. On the mechanics of a detaching retina. Math. Med. Biol 30, 287–310. 10.1093/imammb/dqs024 [DOI] [PubMed] [Google Scholar]
- Bottós JM, Elizalde J, Arevalo JF, Rodrigues EB, Maia M, 2012a. Vitreomacular traction syndrome. J Ophthalmic Vis Res 7, 148–161. 10.1590/S1679-45082015AI2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottós JM, Elizalde J, Rodrigues EB, Maia M, 2012b. Current concepts in vitreomacular traction syndrome. Curr. Opin. Ophthalmol 23, 195–201. 10.1097/ICU.0b013e328352404c [DOI] [PubMed] [Google Scholar]
- Bu SC, Kuijer R, Van Der Worp RJ, Li XR, Hooymans JMM, Los LI, 2015. The ultrastructural localization of type II, IV, and VI collagens at the vitreoretinal interface. PLoS One 10, 1–23. 10.1371/journal.pone.0134325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiello J, Balasubramani M, Schreiber EM, Cole GJ, Mayer U, Halfter W, Lin H, 2007. Biomechanical properties of native basement membranes. FEBS J. 274, 2897–2908. 10.1111/j.1742-4658.2007.05823.x [DOI] [PubMed] [Google Scholar]
- Candiello J, Cole GJ, Halfter W, 2010. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 29, 402–410. 10.1016/j.matbio.2010.03.004 [DOI] [PubMed] [Google Scholar]
- Chan FL, Inoue S, Leblond CP, 1993. The basement membranes of cryofixed or aldehyde-fixed, freeze-substituted tissues are composed of a lamina densa and do not contain a lamina lucida. Cell Tissue Res. 273, 41–52. 10.1007/BF00304610 [DOI] [PubMed] [Google Scholar]
- Chi C, Trinkaus-Randall V, 2013. New insights in wound response and repair of epithelium. J. Cell. Physiol 228, 925–929. 10.1002/jcp.24268 [DOI] [PubMed] [Google Scholar]
- Colter J, Williams A, Moran P, Coats B, 2015. Age-related changes in dynamic moduli of ovine vitreous. J. Mech. Behav. Biomed. Mater 41, 315–324. 10.1016/j.jmbbm.2014.09.004 [DOI] [PubMed] [Google Scholar]
- Comper WD, Laurent TC, 1978. Physiological Function of Connective Tissue Polysaccharides. Physiol. Rev 58, 255–315. [DOI] [PubMed] [Google Scholar]
- Creveling CJ, 2021. Characterization and modeling of vitreoretinal adhesion in the eye. The University of Utah. [Google Scholar]
- Creveling CJ, Colter J, Coats B, 2018. Changes in vitreoretinal adhesion with age and region in human and sheep eyes. Front. Bioeng. Biotechnol 6, 1–11. 10.3389/fbioe.2018.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David T, Smye S, Dabbs T, James T, 1998. A model for the fluid motion of vitreous humour of the human eye during saccadic movement. Phys. Med. Biol 43, 1385–1399. 10.1088/0031-9155/43/6/001 [DOI] [PubMed] [Google Scholar]
- Di Michele F, Tatone A, Romano MR, Repetto R, 2020. A mechanical model of posterior vitreous detachment and generation of vitreoretinal tractions. Biomech. Model. Mechanobiol 10.1007/s10237-020-01360-1 [DOI] [PubMed] [Google Scholar]
- Durbeej M, 2010. Laminins. Cell Tissue Res 339, 259–268. 10.1016/B978-0-12-809847-9.00029-5 [DOI] [PubMed] [Google Scholar]
- Eagle RC Jr., 2016. Eye pathology: an atlas and text, Third. ed. Wolters Kluwer Health. [Google Scholar]
- Elner SG, Elner VM, 1996. The integrin superfamily and the eye. Investig. Ophthalmol. Vis. Sci 37, 696–701. [PubMed] [Google Scholar]
- Engvall E, Ruoslahti E, Miller EJ, 1978. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med 147, 1584–1595. 10.1084/jem.147.6.1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errera MH, Liyanage SE, Petrou P, Keane PA, Moya R, Ezra E, Charteris DG, Wickham L, 2018. A Study of the Natural History of Vitreomacular Traction Syndrome by OCT. Ophthalmology 125, 701–707. 10.1016/j.ophtha.2017.10.035 [DOI] [PubMed] [Google Scholar]
- Escoffery RF, Okun E, Boniuk I, 1976. Vitreoretinal pathology. Int. Ophthalmol. Clin 16, 45–62. [DOI] [PubMed] [Google Scholar]
- Filas BA, Zhang Q, Okamoto RJ, Shui YB, Beebe DC, 2014. Enzymatic degradation identifies components responsible for the structural properties of the vitreous body. Investig. Ophthalmol. Vis. Sci 55, 55–63. 10.1167/iovs.13-13026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foos RY, 1973. Anatomic and pathologic aspects of the vitreous body. Trans Am Acad Ophthalmol Otolaryngol 77, 171–183. [PubMed] [Google Scholar]
- Foos RY, 1972. Vitreoretinal juncture; topographical variations. Invest. Ophthalmol 11, 801–808. [PubMed] [Google Scholar]
- Furashova O, Engelmann K, 2020. To peel or not to peel: Pars plana vitrectomy with macular membrane peel in eyes with abnormalities of vitreomacular interface and coexisting dry age-related macular degeneration. Clin. Ophthalmol 14, 389–396. 10.2147/OPTH.S240480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandorfer Arnd, Priglinger S, Schebitz K, Hoops J, Ulbig M, Ruckhofer J, Grabner G, Kampik A, 2002. Vitreoretinal morphology of plasmin-treated human eyes. Am. J. Ophthalmol 133, 156–159. 10.1016/S0002-9394(01)01252-1 [DOI] [PubMed] [Google Scholar]
- Gandorfer A, Putz E, Welge-Lüßen U, Grüterich M, Ulbig M, Kampik A, 2001. Ultrastructure of the vitreoretinal interface following plasmin assisted vitrectomy. Br. J. Ophthalmol 85, 6–10. 10.1136/bjo.85.1.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandorfer A, Rohleder M, Charteris DG, Sethi C, Kampik A, Luthert P, 2005. Staining and Peeling of the Internal Limiting Membrane in the Cat Eye. Curr. Eye Res 30, 977–987. 10.1080/02713680500320745 [DOI] [PubMed] [Google Scholar]
- Gandorfer A, Rohleder M, Kampik A, 2002. Epiretinal pathology of vitreomacular traction syndrome. Br. J. Ophthalmol 86, 902–909. 10.1136/bjo.86.8.902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JDM, 1995. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am. J. Ophthalmol 119, 752–759. 10.1016/S0002-9394(14)72781-3 [DOI] [PubMed] [Google Scholar]
- Gipson IK, 1992. Adhesive Mechanisms of the Corneal Epithelium. Acta Ophthalmol. 70, 13–17. 10.1111/j.1755-3768.1992.tb02162.x [DOI] [PubMed] [Google Scholar]
- Gloor PB, Daicker BC, 1975. Pathology of the vitreo-retinal border structures. Trans Ophthalmol Soc U K. 95, 387–390. [PubMed] [Google Scholar]
- Goldberg M, Escaig-Haye F, 1986. Is the lamina lucida of the basement membrane a fixation artefact? Eur. J. Cell Biol 42, 365–368. [PubMed] [Google Scholar]
- Grignolo A, 1952. Fibrous components of the vitreous body. AMA Arch Ophthalmol 47, 760–774. [DOI] [PubMed] [Google Scholar]
- Guidry C, Bradley KM, King JL, 2003. Tractional force generation by human Müller cells: Growth factor responsiveness and integrin receptor involvement. Investig. Ophthalmol. Vis. Sci 44, 1355–1363. 10.1167/iovs.02-0046 [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Dong A, Eller AW, Nischt R, 2008. Origin and turnover of ECM proteins from the inner limiting membrane and vitreous body. Eye 22, 1207–1213. 10.1038/eye.2008.19 [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Schurer B, Osanger A, Schneider W, Ruegg M, Cole GJ, 2000. Composition, synthesis, and assembly of the embryonic chick retinal basal lamina. Dev. Biol 220, 111–128. 10.1006/dbio.2000.9649 [DOI] [PubMed] [Google Scholar]
- Harocopos GJ, Shui YB, McKinnon M, Holekamp NM, Gordon MO, Beebe DC, 2004. Importance of Vitreous Liquefaction in Age-Related Cataract. Investig. Ophthalmol. Vis. Sci 45, 77–85. 10.1167/iovs.03-0820 [DOI] [PubMed] [Google Scholar]
- Heegaard S, 1997. Morphology of the Vitreoretinal Border Region. Acta Ophthalmol. Scand 75, 7–31. 10.1111/j.0954-6820.1971.tb01590.x [DOI] [PubMed] [Google Scholar]
- Herath SCB, Sharghi-Namini S, Du Y, Wang D, Ge R, Wang QG, Asada H, Chen PCY, 2017. A Magneto-Microfluidic System for Investigating the Influence of an Externally Induced Force Gradient in a Collagen Type I ECM on HMVEC Sprouting. SLAS Technol. 22, 413–424. 10.1177/2211068216680078 [DOI] [PubMed] [Google Scholar]
- Hogan MJ, 1975. Inflammation and its effect on the vitrteous. Trans Ophthalmol Soc U K. 95, 378–81. [PubMed] [Google Scholar]
- Hoon M, Okawa H, Della Santina L, Wong ROL, 2014. Functional Architecture of the Retina: Development and Disease. Prog. Retin. Eye Res 42, 44–84. 10.1016/j.preteyeres.2014.06.003.Functional [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura H, Kishi S, 2013. Evolution of vitreomacular detachment in healthy subjects. JAMA Ophthalmol. 131, 1348–1352. 10.1001/jamaophthalmol.2013.4578 [DOI] [PubMed] [Google Scholar]
- Jackson TL, Antcliff RJ, Hillenkamp J, Marshall J, 2003. Human retinal molecular weight exclusion limit and estimate of species variation. Investig. Ophthalmol. Vis. Sci 44, 2141–2146. 10.1167/iovs.02-1027 [DOI] [PubMed] [Google Scholar]
- Jackson TL, Nicod E, Angelis A, Grimaccia F, Prevost AT, Simpson ARH, Kanavos P, 2013. Vitreous attachment in age-related macular degeneration, diabetic macular edema, and retinal vein occlusion: A systematic review and metaanalysis. Retina 33, 1099–1108. 10.1097/IAE.0b013e31828991d6 [DOI] [PubMed] [Google Scholar]
- Jerdan JA, Pepose JS, Michels RG, Hayashi H, De Mustros S, Sebag M, Glaser BM, 1989. Proliferative vitreoretinopathy membranes: an immunohistochemical study. Opthalmology 96, 801–810. [DOI] [PubMed] [Google Scholar]
- Kita M, Marmor MF, 1992. Retinal adhesive force in living rabbit, cat, and monkey eyes: Normative data and enhancement by mannitol and acetazolamide. Investig. Ophthalmol. Vis. Sci 33, 1879–1882. [PubMed] [Google Scholar]
- Kita M, Negi A, Kawano S, Honda Y, Maegawa S, 1990. Measurement of retinal adhesive force in the in vivo rabbit eye. Investig. Ophthalmol. Vis. Sci 31, 624–628. [PubMed] [Google Scholar]
- Kohno T, Sorgente N, Ishibashi T, Goodnight R, Ryan SJ, 1987. Immunofluorescent studies of fibronectin and laminin in the human eye. Investig. Ophthalmol. Vis. Sci 28, 506–514. [PubMed] [Google Scholar]
- Koller EC, Kraker JA, Hwang ES, 2021. Progression of Partial Posterior Vitreous Detachment Over Time. Retin. J. Retin. Vitr. Dis 41, 1396–1402. 10.1097/IAE.0000000000003039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs I, Brannath W, Glittenberg C, Zeiler F, Sebag J, Binder S, 2007. Posterior Vitreomacular Adhesion: A Potential Risk Factor for Exudative Age-related Macular Degeneration? Am. J. Ophthalmol 144, 741–746. 10.1016/j.ajo.2007.07.024 [DOI] [PubMed] [Google Scholar]
- Krishnan R, Arora R, De Salvo G, Stinghe A, Severn PS, Pal B, Goverdhan S, 2015. Vitreomacular traction affects anti-vascular endothelial growth factor treatment outcomes for exudative age-related macular degeneration. Retina 35, 1750–1756. 10.1097/IAE.0000000000000714 [DOI] [PubMed] [Google Scholar]
- Kuo HJ, Maslen CL, Keene DR, Glanville RW, 1997. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J. Biol. Chem 272, 26522–26529. 10.1074/jbc.272.42.26522 [DOI] [PubMed] [Google Scholar]
- Lakawicz JM, Bottega WJ, Prenner JL, Fine HF, 2015. An analysis of the mechanical behaviour of a detaching retina. Math. Med. Biol 32, 137–161. 10.1093/imammb/dqt023 [DOI] [PubMed] [Google Scholar]
- Le Goff MM, Bishop PN, 2008. Adult vitreous structure and postnatal changes. Eye 22, 1214–1222. 10.1038/eye.2008.21 [DOI] [PubMed] [Google Scholar]
- Lee B, Litt M, Buchsbaum G, 1994a. Rheology of the vitreous body: Part 2. Viscoelasticity of bovine and porcine vitreous. Biorheology 31, 327–338. [DOI] [PubMed] [Google Scholar]
- Lee B, Litt M, Buchsbaum G, 1994b. Rheology of the vitreous body: Part 3. Concentration of electrolytes, collagen, and hyaluronic acid. Biorheology 31, 339–351. [DOI] [PubMed] [Google Scholar]
- Lee B, Litt M, Buchsbaum G, 1992. Rheology of the vitreous body: Part 1. Viscoelasticity of human vitreous. Biorheology 29, 521–533. [DOI] [PubMed] [Google Scholar]
- Lim LS, Mitchell P, Seddon JM, Wong TY, 2012. Age-related macular degeneration. Lancet 379, 1728–1738. 10.1016/S0140-6736(18)31550-2 [DOI] [PubMed] [Google Scholar]
- Lindqvist N, Liu Q, Zajadacz J, Franze K, Reichenbach A, 2010. Retinal Glial (Müller) Cells: Sensing and responding to tissue stretch. Investig. Ophthalmol. Vis. Sci 51, 1683–1690. 10.1167/iovs.09-4159 [DOI] [PubMed] [Google Scholar]
- Los LI, Van der Worp RJ, Van Luyn MJA, Hooymans JMM, 2003. Age-related liquefaction of the human vitreous body: LM and TEM evaluation of the role of proteoglycans and collagen. Investig. Ophthalmol. Vis. Sci 44, 2828–2833. 10.1167/iovs.02-0588 [DOI] [PubMed] [Google Scholar]
- Ma J, Ke Y, Wu D, Gu X, Gao R, 2005. Experimental study on relationship between retinal vein occlusion and loss of vitreous gel mass. Mol. Vis 11, 744–748. [PubMed] [Google Scholar]
- Macdonald RB, Randlett O, Oswald J, Yoshimatsu T, Franze K, Harris WA, 2015. Müller glia provide essential tensile strength to the developing retina. J. Cell Biol 210, 1075–1083. 10.1083/jcb.201503115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan MC, Holden BA, 1992. Reduced epithelial adhesion after extended contact lens wear correlates with reduced hemidesmosome density in cat cornea. Investig. Ophthalmol. Vis. Sci 33, 314–323. [PubMed] [Google Scholar]
- Matsumoto B, Blanks JC, Ryan SJ, 1984. Topographic Variations in the Rabbit and Primate Internal Limiting Membrane. Investig. Ophthalmol. Vis. Sci 25, 71–82. [PubMed] [Google Scholar]
- Maumenee AE, 1967. Further Advances in the Study of the Macula. Arch. Ophthalmol 78, 151–165. 10.1001/archopht.1967.00980030153008 [DOI] [PubMed] [Google Scholar]
- Mojana F, Cheng L, Bartsch DUG, Silva GA, Kozak I, Nigam N, Freeman WR, 2008. The Role of Abnormal Vitreomacular Adhesion in Age-related Macular Degeneration: Spectral Optical Coherence Tomography and Surgical Results. Am. J. Ophthalmol 146. 10.1016/j.ajo.2008.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson CS, Park J, Kornfield JA, Karageozian H, 2008. Rheological properties of the vitreous and the role of hyaluronic acid. J. Biomech 41, 1840–1846. 10.1016/j.jbiomech.2008.04.015 [DOI] [PubMed] [Google Scholar]
- Noulas AV, Theocharis AD, Feretis E, Papageorgakopoulou N, Karamanos NK, Theocharis DA, 2002. Pig vitreous gel: Macromolecular composition with particular reference to hyaluronan-binding proteoglycans. Biochimie 84, 295–302. 10.1016/S0300-9084(02)01389-5 [DOI] [PubMed] [Google Scholar]
- Ockleford CD, McCracken SA, Rimmington LA, Hubbard ARD, Bright NA, Cockcroft N, Jefferson TB, Waldron E, D’Lacey C, 2013. Type VII collagen associated with the basement membrane of amniotic epithelium forms giant anchoring rivets which penetrate a massive lamina reticularis. Placenta 34, 727–737. 10.1016/j.placenta.2013.06.002 [DOI] [PubMed] [Google Scholar]
- Pankov R, Yamada KM, 2002. Fibronectin at a glance. J. Cell Sci 115, 3861–3863. 10.1242/jcs.00059 [DOI] [PubMed] [Google Scholar]
- Patronas M, Kroll AJ, Lou PL, Ryan EA, 2009. A review of vitreoretinal interface pathology. Int. Ophthalmol. Clin 49, 133–143. 10.1097/IIO.0b013e3181924b3e [DOI] [PubMed] [Google Scholar]
- Ponsioen TL, Van Der Worp RJ, Van Luyn MJA, Hooymans JMM, Los LI, 2005. Packages of vitreous collagen (type II) in the human retina: An indication of postnatal collagen turnover? Exp. Eye Res 80, 643–650. 10.1016/j.exer.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Ponsioen TL, Van Luyn MJA, Van Der Worp RJ, Van Meurs JC, Hooymans JMM, Los LI, 2008. Collagen distribution in the human vitreoretinal interface. Investig. Ophthalmol. Vis. Sci 49, 4089–4095. 10.1167/iovs.07-1456 [DOI] [PubMed] [Google Scholar]
- Rangarajan N, Kamalakkannan SB, Hasija V, Shams T, Jenny C, Serbanescu I, Ho J, Rusinek M, Levin AV, 2009. Finite element model of ocular injury in abusive head trauma. J. AAPOS 13, 364–369. 10.1016/j.jaapos.2008.11.006 [DOI] [PubMed] [Google Scholar]
- Repetto R, Ghigo I, Seminara G, Ciurlo C, 2004. A simple hydro-elastic model of the dynamics of a vitreous membrane. J. Fluid Mech 503, 1–14. 10.1017/S0022112003007389 [DOI] [Google Scholar]
- Rossi T, Boccassini B, Esposito L, Iossa M, Ruggiero A, Tamburrelli C, Bonora N, 2011. The pathogenesis of retinal damage in blunt eye trauma: Finite element modeling. Investig. Ophthalmol. Vis. Sci 52, 3994–4002. 10.1167/iovs.10-6477 [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher M, Engvall E, Oldberg A, Hayman EG, 1982. Molecular and biological interactions of fibronectin. J. Invest. Dermatol 79, 65–68. 10.1038/jid.1982.12 [DOI] [PubMed] [Google Scholar]
- Russell SR, Shepherd JD, Hageman GS, 1991. Distribution of glycoconjugates in the human retinal internal limiting membrane. Investig. Ophthalmol. Vis. Sci 32, 1986–1995. 10.1016/j.ssc.2008.05.013 [DOI] [PubMed] [Google Scholar]
- Sarthy V, Ripps H, 2001. The Retinal Müller Cell: Structure and Function. Kluwer Academic / Plenum Publishers. [Google Scholar]
- Schulz A, Wahl S, Rickmann A, Ludwig J, Stanzel BV, Von Briesen H, Szurman P, 2019. Age-related loss of human vitreal viscoelasticity. Transl. Vis. Sci. Technol 8. 10.1167/tvst.8.3.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebag J, 1998. Macromolecular structure of the corpus vitreus. Prog. Polym. Sci 23, 415–446. [Google Scholar]
- Sebag J, 1992. Anatomy and pathology of the vitreo-retinal interface. Eye 6, 541–552. 10.1038/eye.1992.119 [DOI] [PubMed] [Google Scholar]
- Sebag J, 1991. Age-related differences in the human vitreoretinal interface. Arch. Ophthalmol 109, 966–971. [DOI] [PubMed] [Google Scholar]
- Sebag J, 1987. Age-related changes in human vitreous structure. Graefe’s Arch. Clin. Exp. Ophthalmol 225, 89–93. 10.1177/0047287517717350 [DOI] [PubMed] [Google Scholar]
- Sebag J, Balazs EA, 1989. Morphology and Ultrastructure of Human Vitreous Fibers. Investig. Ophthalmol. Vis. Sci 30, 1867–1871. [PubMed] [Google Scholar]
- Seko Yuko, Seko Yoshinori, Fujikura H, Pang J, Tokoro T, Shimokawa H, 1999. Induction of vascular endothelial growth factor after application of mechanical stress to retinal pigment epithelium of the rat in vitro. Investig. Ophthalmol. Vis. Sci 40, 3287–3291. [PubMed] [Google Scholar]
- Shawky JH, Davidson LA, 2015. Tissue mechanics and adhesion during embryo development. Dev. Biol 401, 152–164. 10.1016/j.ydbio.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Birk DE, 2012. Focus on Molecules: Collagens V and XI. Exp. Eye Res 98, 105–106. 10.1016/j.exer.2010.08.003.Focus [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide RF, Wong D, Fisher Y, Goldbaum M, 2002. Correlation of vitreous attachment and foveal deformation in early macular hole states. Am. J. Ophthalmol 133, 226–229. 10.1016/S0002-9394(01)01377-0 [DOI] [PubMed] [Google Scholar]
- Stirpe M, Heimann K, 1996. Vitreous changes and retinal detachment in highly myopic eyes. Eur. J. Ophthalmol 6, 50–58. [DOI] [PubMed] [Google Scholar]
- Strasser S, Zink A, Janko M, Heckl WM, Thalhammer S, 2007. Structural investigations on native collagen type I fibrils using AFM. Biochem. Biophys. Res. Commun 354, 27–32. 10.1016/j.bbrc.2006.12.114 [DOI] [PubMed] [Google Scholar]
- Sun YL, Luo ZP, Fertala A, An KN, 2004. Stretching type II collagen with optical tweezers. J. Biomech 37, 1665–1669. 10.1016/j.jbiomech.2004.02.028 [DOI] [PubMed] [Google Scholar]
- Terranova VP, Rohrbach DH, Martin GR, 1980. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell 22, 719–726. 10.1016/0092-8674(80)90548-6 [DOI] [PubMed] [Google Scholar]
- Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK, 2016. Extracellular matrix structure. Adv. Drug Deliv. Rev 97, 4–27. 10.1016/j.addr.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Tolentino FI, 1987. The vitreous in ocular trauma, in: Schepens CL, Neetens A (Eds.), The Vitreous and Vitreoretinal Interface. New York, NY. [Google Scholar]
- Tram NK, Swindle-Reilly KE, 2018. Rheological Properties of Age-Related Changes of the Human Vitreous Humor. Front. Bioen. Biotech 4:199. 10.3389/fbioe.2018.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara M, Mori Keiko, Gehlbach PL, Mori Keisuke, 2018. Posterior Vitreous Detachment as Observed by Wide-Angle OCT Imaging. Ophthalmology 125, 1372–1383. 10.1016/j.ophtha.2018.02.039 [DOI] [PubMed] [Google Scholar]
- Van Der Rijt JAJ, Van Der Werf KO, Bennink ML, Dijkstra PJ, Feijen J, 2006. Micromechanical testing of individual collagen fibrils. Macromol. Biosci 6, 697–702. 10.1002/mabi.200600063 [DOI] [PubMed] [Google Scholar]
- Wang J, McLeod D, Henson DB, Bishop PN, 2003a. Age-dependent changes in the basal retinovitreous adhesion. Investig. Ophthalmol. Vis. Sci 44, 1793–1800. 10.1167/iovs.02-0802 [DOI] [PubMed] [Google Scholar]
- Wang J, McLeod D, Henson DB, Bishop PN, 2003b. Age-dependent changes in the basal retinovitreous adhesion. Investig. Ophthalmol. Vis. Sci 44, 1793–1800. 10.1167/iovs.02-0802 [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE, Biochem DEJC, 2004a. Type V Collagen Controls the Initiation of Collagen Fibril Assembly*. J. Biol. Chem 279, 53331–53337. 10.1074/jbc.M409622200 [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Cole WG, Willing MC, Birk DE, 2004b. Reduced Type I Collagen Utilization : A Pathogenic Mechanism in COL5A1 Haplo-Insufficient Ehlers – Danlos Syndrome. J. Cell. Biochem 92, 113–124. 10.1002/jcb.20024 [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yamazaki J, Mizunuma H, 2014. A finite element analysis of the retinal hemorrhages accompanied by shaken baby syndrome/abusive head trauma. J. Biomech 47, 3454–3458. 10.1016/j.jbiomech.2014.09.014 [DOI] [PubMed] [Google Scholar]
- Zagon IS, Sassani JW, Ruth TB, McLaughlin PJ, 2001. Epithelial adhesion complexes and organ culture of the human cornea. Brain Res. 900, 205–213. 10.1016/S0006-8993(01)02296-X [DOI] [PubMed] [Google Scholar]
- Zhidkova NI, Justice SK, Mayne R, 1995. Alternative mRNA processing occurs in the variable region of the pro-α1(XI) and pro- α2(XI) collagen chains*. J. Biol. Chem 270, 9486–9493. [DOI] [PubMed] [Google Scholar]


